STUDIES ON
OZONOLYSIS OF OLEANOLIC ACID IN THE EPICUTICULAR WAXES OF GRAPES UNDER AMBIENT CONDITIONS
INTRODUCTION
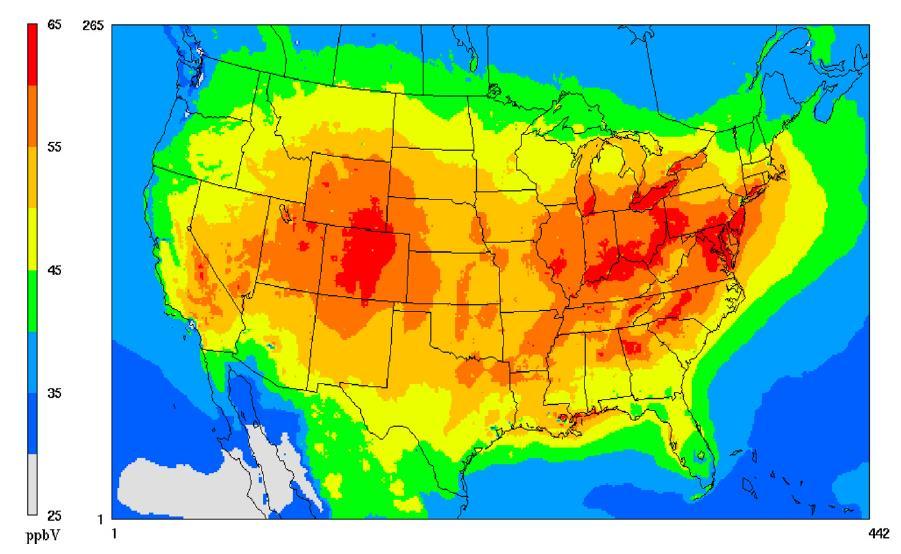
Ozone is a highly reactive gas in our atmosphere known to impact agriculture and crop production. McGrath et al. found that increasing levels of ozone in the troposphere are relevant because ozone damages crops and agricultural life, reducing crop yields.[1] Tropospheric ozone is formed from photochemical reactions between volatile organic compounds (VOCs) and NOx (nitrogen oxide) gases.[2] Ozone levels are often very high over agricultural areas as shown in the map of ozone concentrations over the US in 2009.[3] Note that levels are particularly high over the Central Valley of California and over the Inland Empire (where the University of Redlands is located).
Given the significant impacts tropospheric ozone has on human and plant health, it is essential to understand how ozone concentrations change over time (ozone flux) to help predict ozone concentrations and develop better regulatory practices. To model ozone flux, scientists must be able to model sources of ozone (such as NO gases and VOCs [2]) and sinks which are mechanisms in which ozone gets stored or used. The accurate representation of where ozone goes in the troposphere is important for determining the best policies to mitigate tropospheric ozone. Plants are known to be ozone sinks and contribute to ozone flux by stomatal and non-stomatal means.[4-6] Stomatal flux occurs when ozone is taken up into the plant via stomata, which are chambers on plant leaf surfaces where gas exchange takes place. Ozone deposition by non-stomatal means may occur through reactions between ozone and compounds in the soil, water, and air near plants as well as directly with plant surfaces, primarily the epicuticular waxes on plants and fruit. While reactions of ozone with volatile compounds around plants or produced by plants are well-studied,[4-6] very little work has been done on the reaction of ozone with non-volatile compounds in the epicuticular waxes of plants. In one of the few studies exploring the direct reaction of ozone with compounds in the epicuticular waxes of plants, Jud et al. quantified the number of volatile compounds produced from plant surface reactions with ozone.[7] While their research focused on the volatile products of reactions between ozone and compounds in plant waxes, the authors explicitly stated that reactions that produce non-volatile products could be significant contributors to ozone deposition models. The epicuticular waxes of plants and fruit are composed mostly of non-volatile organic compounds such as oleanolic acid.[8] Wax crystals function as a barrier that protects against uncontrolled water loss and other environmental factors like unwanted pathogens or herbivore attacks.[9] However, if crystals are damaged because of surface reactions with ozone, that may inhibit a plant's ability to protect itself. Studies of the direct reaction of ozone with non-volatile compounds in epicuticular waxes of plants and fruits to produce non-volatile products would contribute to a more complete understanding of non-stomatal contributions to ozone flux, which in turn will better inform policies to mitigate tropospheric ozone levels.
EPICUTICULAR WAXES OF GRAPES & OZONE
Oleanolic acid is a prevalent component of a variety of plant and fruit species, making it a significant part of wax structures.[8] Oleanolic acid also has well-known solution-phase chemistry with ozone, forming a lactone that is non-volatile.[10,11] These factors make oleanolic acid an excellent compound to explore ozone deposition on plants via the ozonolysis of non-volatile compounds to form non-volatile products. The epicuticular waxes of grapes have been shown to contain high levels of oleanolic acid, making grape waxes ideal samples.[12,13] In addition, grapes are an important agricultural product, are easy to acquire and are relatively inexpensive.
In order to confirm that ozonolysis of oleanolic acid in the solid state produces the same lactone as solution-phase ozonolysis, Smith treated solid oleanolic acid with ozone, purified the product, and identified the product as lactone using NMR and high performance liquid chromatography-mass spectrometry (LC-MS).[14] Navarro exposed epicuticular waxes stripped from grapes and olive leaves to ozone, analyzed the exposed waxes using LC-MS, and confirmed that oleanolic acid in the epicuticular waxes was converted into lactone under exposure to high ozone concentrations.[15] Ramirez did further work to develop a method that quantified oleanolic acid and lactone levels in grape wax. [16] Ramirez stripped the wax from about 250 green table grapes using HPLC grade chloroform, distributed the solution among four petri dishes, and allowed the chloroform to evaporate. She kept one plate in the refrigerator as a control and exposed the other three to Redlands ambient air during the summer of 2020 She repeated the process with red table grapes. For the samples exposed to ambient air over the summer, 2% to 4% of the oleanolic acid by mass converted to lactone. These results confirmed that ambient ozone deposits onto the epicuticular wax of grape skins via reaction with oleanolic acid. However, only six samples over one summer are not sufficient to provide accurate results of non-volatile compounds’ contribution to non-stomatal flux. Further investigation is required to confirm that the conversion of oleanolic acid to lactone is reproducible and could be an important contribution to non-stomatal flux.

TO AMBIENT OZONE


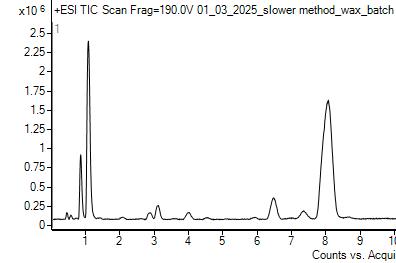
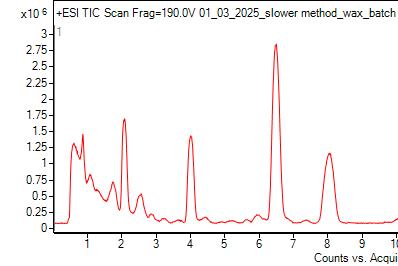
The chromatograms above are typical for wax that was kept in the fridge so there was no reaction with ozone (left) and for wax that was exposed to Redlands ambient air over the summer (right). This comparison demonstrates that the lactone peak is below the quantification limit in the control wax while also demonstrating that the production of lactone from ozone exposure can be detected using LC-MS. The same chromatogram result was shown in Ramirez’ work[16], verifying that we can reproducibly demonstrate that oleanolic acid in plant waxes exposed to ambient air forms lactone.
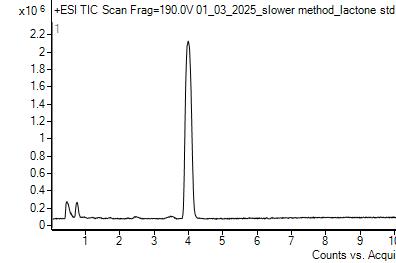
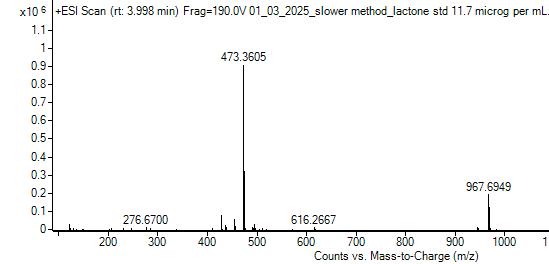
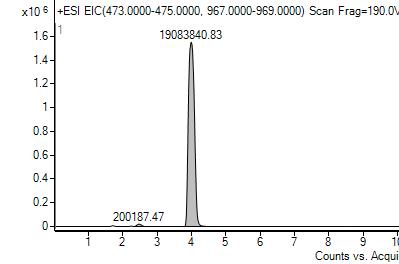
Wax samples were analyzed using LC-MS along with standards of oleanolic acid and lactone. There are two methods used to integrate chromatogram peaks using our instrument: extracted ion chromatogram (EIC) and total ion chromatogram (TIC). For TIC, the software integrates the entire area under a peak. For EIC, the mass spectrum is used to determine the masses of the principal ions for the compound of interest and the software uses those masses to obtain the area under a peak due to just those masses With TIC, the software algorithm must estimate a baseline, which tended to produce deviations in the areas of the lactone peaks in the samples. The software does not need to approximate baselines for EIC. EIC results on average had less standard deviation, making that the preferred integration method.
On average a slightly higher mass percent of oleanolic acid converted into lactone in 2024 compared to 2020. In order to determine if differing ozone levels were responsible for that increase in conversion, we looked at daily ozone concentration data for Redlands from the Air Quality Management District. [18] Average daily ozone levels for the time of exposure for 2020 and
are shown in the
These
show that there were not significantly higher ozone levels in 2024 compared to
Therefore,
levels are probably not the reason for the increased conversion of oleanolic acid to lactone from
to
The one difference in the setup of the plate samples for exposure is that in 2020 the plates were placed in a region shaded from sun exposure, while in 2024 the plates were placed in an area that was shaded for only part of the day. The increase in sun exposure could explain the increase in conversion. Additional work must be done to determine if sun exposure is a factor in the increased conversion of oleanolic acid to lactone
The results from this study show that conversion of oleanolic acid to lactone upon exposure to ambient air in Redlands is reproducible from year to year. We determined that EIC is the best method for analyzing the chromatograms of wax samples. We saw slightly higher mass percent of oleanolic acid converted to lactone in 2024 versus 2020. Since the AQMD data show similar daily averages of ozone concentration in 2020 and 2024, we need to explore other reasons for the higher conversion. While the percent by mass of oleanolic acid converted to lactone seems small, oleanolic acid is only one of many non-volatile organic compounds in the crystalline wax structure of grapes. Taken as a group, the reaction of nonvolatile compounds in plant waxes with ozone to form nonvolatile products could play a significant role in non-stomatal flux. As previously mentioned, one more summer of study is required. Half of the outside samples will be left in the sun while the other half will be left in the shade. The data from these samples will be used to confirm the impact of sunlight on the amount of oleanolic acid converted to lactone by ambient ozone.
Pinter, K.; Weidinger, T. An Attempt to Partition Stomatal and Non-stomatal Ozone Deposition Parts on a Short Grassland. Boundary Layer Meterol 2018 167 303-326.
[6] Fares, S.; Weber, R.; Park, J. H.; Gentner, D.; Karlik, J.; Goldstein, A.H. Ozone deposition to an orange orchard: Partitioning between stomatal and non-stomatal sinks. Environmental Pollution 2012 169, 258-266.
[7] Jud, W.; Fischer, L.; Canaval E.; Wohlfahrt, G.; Tissier, A.; Hansel, A. Plant surface reactions: an opportunistic ozone defence mechanism impacting atmospheric chemistry. Atmos. Chem. Phys. 2016, 16 277-292.
[8] Vrancheva R.; Ivanov, I.; Dincheva I.; Badjakov I.; Pavlov, A. Triterpenoids and Other Non-Polar Compounds in Leaves of Wild and Cultivated Vaccinium Species. Plants 2021 10 (1), 94. https://doi.org/10.3390/plants10010094
[9] Markstädter C.; Federle, W.; Jetter, R.; Riederer, M.; Hölldobler B. Chemoecology 2000 10 (1), 33–40.
[10]Csuk, R.; Siewert, B. Tetrahedron Let. 2011, 52, 6616-6618.
[11] Hochstetler, A.R. J. Org. Chem., 1975, 40, 1536.
[12] Casado, C.G.; Heredia, A.; Structure and dynamics of reconstituted cuticular waxes of grape berry cuticle (Vitis vinifera L.) Journal of Experimental Botany 1999 50 (331), 175-182
[13] N. Orbán et al. LC-MS method development to evaluate major triterpenes in skins and cuticular waxes of grape berries. Int. J. Food Sci. Technol. 2009 44 869-873.
[14] Smith, Caitlin. The Effects of Ozone on the Representative Molecular Components in EpicuticularWaxes of Leaves: Oleanolic and Ferulic Acid. B.S. Thesis, University of Redlands, Redlands, CA, 2017.
[15] Huerta-Navarro, Vanessa The Effects of Ozone on Oleanolic Acid and Ferulic Acid in Leaf Epicuticular Waxes. B.S. Thesis, University of Redlands, Redlands, CA, 2018.
[16] Ramirez, B. The Direct Reaction of Tropospheric Ozone with Oleanolic Acid in the Epicuticular Wax of Grape Skins. B.S. Thesis, University of Redlands, Redlands, CA, 2021.
[17] Line Fire | CAL FIRE. Ca.gov. https://www.fire.ca.gov/incidents/2024/9/5/line-fire (accessed 2025-02-05).
[18] Current Air Quality Data www.aqmd.gov https://www.aqmd.gov/home/air-quality/current-air-quality-data (accessed 2025-0205).