
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 11 Issue: 04 | Apr 2024 www.irjet.net p-ISSN:2395-0072


International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 11 Issue: 04 | Apr 2024 www.irjet.net p-ISSN:2395-0072
Momtazul Haquea , O.S Bhatiab Lovneesh Sharmac
aM.Tech Scholar, Department of Mechanical Engineering, Universal Institute of Engineering & Technology, Lalru
bProfessor, Department of Mechanical Engineering, Universal Institute of Engineering & Technology, Lalru
c Assistant Professor, Department of Civil Engineering, Universal Institute of Engineering & Technology, Lalru
Abstract:Thefabricationofthealloyswascarriedoutthroughameltingandcastingmethodunderanargonatmosphereto ensure the preservation of their inherent properties. The characterization of these samples was conducted using X-ray diffraction (XRD) and Differential Scanning Calorimetry (DSC). Vickers hardness testing apparatus was utilized to assess the mechanicalpropertiesofthealloys.TheobjectivebehindmodifyingtheAl-12Sialloycompositionwastodecreasethebrazing filler's melting point, a hypothesis that was later confirmed through DSC analysis. Subsequently, the alloys' resistance to corrosion was evaluated using an electrochemical potentiostat in environments containing 3.5 wt% NaCl and 0.1 M H2SO4. This allowed for a comprehensive comparison of corrosion behaviors across the various alloy compositions, focusing specificallyonmetricssuchascorrosioncurrentdensity(icorr),corrosionpotential(Ecorr),andtheoverallrateofcorrosion.
Keywords: Brazingfiller,X-raydiffraction,Opticalmicroscopy,Differentialscanningcalorimetry,Corrosion
Introduction:
In the fabrication of thermoelectric modules, brazing is identified as the predominant technique for joining components, leveraging a bonding agent with a melting point below those of the components being bonded. This method necessitates elevating the assembly's temperature above the bonding agent's melting point specifically, temperatures must surpass 450°Ctodistinguishbrazingfromsoldering,whichemploysfillersmeltingbelowthistemperature[1-4].
Recentinvestigationshaveaimedatcurtailingtheformationofintermetalliccompoundsbyintroducinghighentropyalloysas innovative brazing fillers. Such approaches have previously facilitated the effective bonding of nickel-based superalloys and solid oxide fuel cells (SOFCs) [5,6]. The high entropy concept posits that an alloy comprising multiple components in equal proportionscanreducetheemergenceofcomplexphases,favoringthedevelopmentofarandomsolidsolutionwherealloying elementsareevenlydistributed[7,8].ThisnotionwasfurtherillustratedbyYehetal.,whodemonstratedthatahighentropy valuepromotestheformationofarandomsolidsolution,enhancingthealloy'shomogeneity[9,10].
Particularly,theequimolarhighentropyalloyAl-Si-Sn-Zn-Cuwasexploredasafillermaterialforvacuumbrazingapplications, especiallyforjoiningAl-basedsuperalloys.Remarkably,theliquidustemperatureofthisalloystandsat1346degreesCelsius, significantly exceeding the solution treatment temperature range for Mar-M 247, typically between 1080 and 1170 degrees Celsius [11]. The initiative to modify the Al-12Si alloy aimed at reducing the brazing filler's melting point has been substantiatedthroughDSCanalysis.Furthermore,thecorrosionresistanceofthedevelopedalloyswasassessedina3.5wt% NaClsolution,enablingthecomparisonofcorrosionparametersacrossthevariousalloysproduced.

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 11 Issue: 04 | Apr 2024 www.irjet.net p-ISSN:2395-0072

Material and Methods:
2.1. Materials:
For this study, the initial step involved utilizing the received powders of aluminum, copper, cobalt, molybdenum, silicon, tin, andzinctopreparethealloysamples.Theserawmaterialswerecarefullymeasuredoutin precise proportions to matchthe targetedcompositionsforthealloysynthesis.(Table1).
Table1Compositionofalloys(wt.%)
Firstandforemost,thepowdersweretakenandcombinedintothemortarinaccordancewiththecompositions.Then,usinga 15mmdiameterdiemountedinuni-axialcompactionmachinery,thepowders werecompactedtoproducethefinalproduct. The pellets weremadein thismanner and wereoffive distinctcompositions.Inthenextstep,the pelletswere insertedinto thecoppermold,andtherequisitevacuumlevelwasobtained,afterwhichinertargon gaswaspurgedfromthecoppermold. Finally,thepellets were melted five times witha spark, thestrength of whichmay becontrolled by varyingthe current flow rateinthecircuit.Byimpactingelectronswithalargeamountofkineticenergy,thetemperatureofthematerialwillrisevery quicklyveryquickly.

2395-0056
Volume: 11 Issue: 04 | Apr 2024 www.irjet.net p-ISSN:2395-0072
Extensive phase investigation of aluminum-based alloy samples was carried out using XRD, which operates on the basis of Bragg's Law. Equipment using Cu radiation was used to conduct XRD experiments on a variety of various compositions. The samples were put on a silicon wafer and agglomerated with acetone after being soaked for many hours. The analysis was carriedoutinthe2orangeof20o-90ousinga10secondscananda2ºstepsizeof0.04ºinthe2ºrangeof20º-90º.AllXRD peaks were fitted using peak fitting to get the peak locations and full width at half maxima, which were then computed. The XRDdatawasalsousedtodeterminethelatticeparametersofthecrystal.DifferentialScanningisakindofscanninginwhich two or more images are compared side by side. For thermal examination of materials in which phase transitions such as melting, glass transitions, or exothermic decompositions are investigated, the calorimetry method is utilized. Both the reference sample and the sample on which thermal analysis is to be performed are necessary for this experiment to be completed.Thereferencesampleshouldhaveawell-definedheatcapacityacrossthetemperaturerangethatwillbe scanned inordertobeuseful.
Fortheopticalmicroscopy,thesamplewerepolishedwithSiCabrasivepaperswithgritsize220,400,600,800,and1000ina series, after which polishing was done using velvet cloth having alumina suspension diluted with water on a double disk polishing machine. For the hardnessofthesamples, thesamples waskeptundertheindenter ofhardnesstestingapparatus, and3kgfloadwillbeappliedfor15secondsofdwelltime.Afterusingloadandkeepingthatloadforthedecideddwelltime, theindenterwillbereleased. Forthepurposeofensuringrepeatabilityinthecorrosiontests,thetestswerecarriedoutthree times on eachsample, repeatedly.The experiment beganwiththe plotofthe OCP (Opencircuit potential),andaftera stable conditionhadbeenattained,thepolarizationcurveswereconstructedusingthedatafromtheplot.Thevaluesofecorr andicorr werefoundafterTafel fitting.
3.1. X-Ray Diffraction of Al-based alloys
We performed XRD analysis on each and every one of the aluminum-based alloys we tested in order to determine which phaseswerepresentinthealloys.Figure2showscrystallinepeaksoftwoseparatephasesofaluminumandsiliconthatmay be distinguished. According to certain theories, the strength of the Si peak has been weakening as a consequence of the creationofsupersaturatedSisolutionsintheAl,aswellastherapidcoolingratesthatwereseenthroughoutthe meltingand castingprocesses.ForeachXRDpatternillustratedbelow,thevaluesof(hkl)planes,dspacingforthecorrespondingelement, andangle2thetaarecomputedandtabulated.BecausethealloyingelementsCo,Mo,Sn,Zn,andCuwerecompletelydissolved andasolidsolutionproduced,it isnotfeasibletoobservepeaksfortheseelementsintheresultinganalysis infigure2ofC1 When comparing the other alloying elements, including alloys, to the single Si-containing alloy, the intensity of the Al and Si peaksintheotheralloyingelements,includingalloys,issubstantiallydecreasedincomparisontothesingleSi-containingalloy (C0).
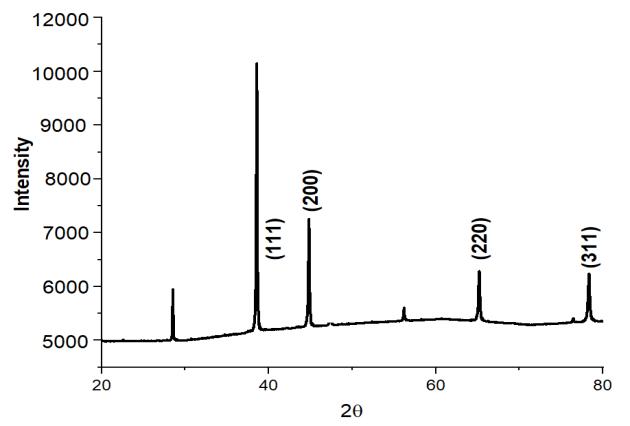
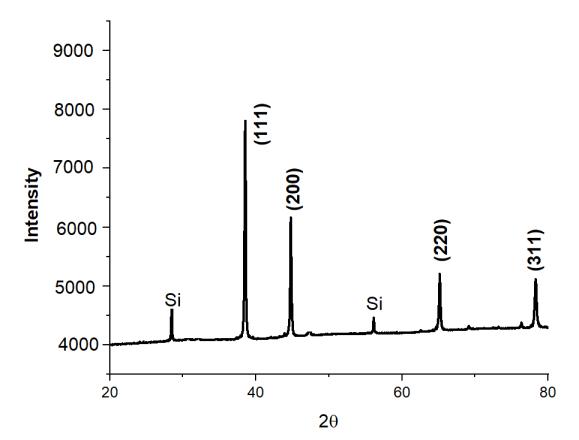

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 11 Issue: 04 | Apr 2024 www.irjet.net p-ISSN:2395-0072
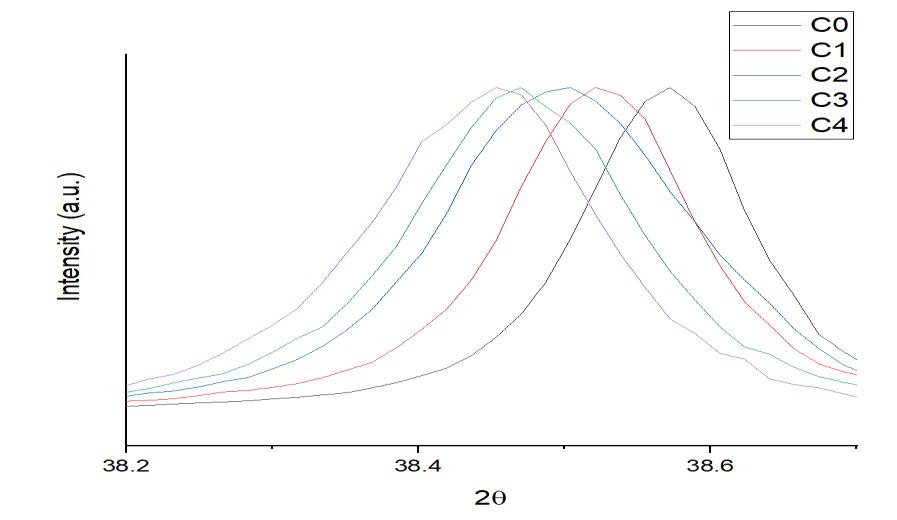
This migration of the most prominent XRD peak of alloys from its original location to its left side indicates that the lattice expansionofAl atomshashappenedasa consequenceofsolidsolutionformationwithalloyingelements(Co,Mo,Cu,Sn,Zn, Si).AstheconcentrationofCuinthesolutionrose,thelatticestructurecontinuedtogrowasseeninfigure4
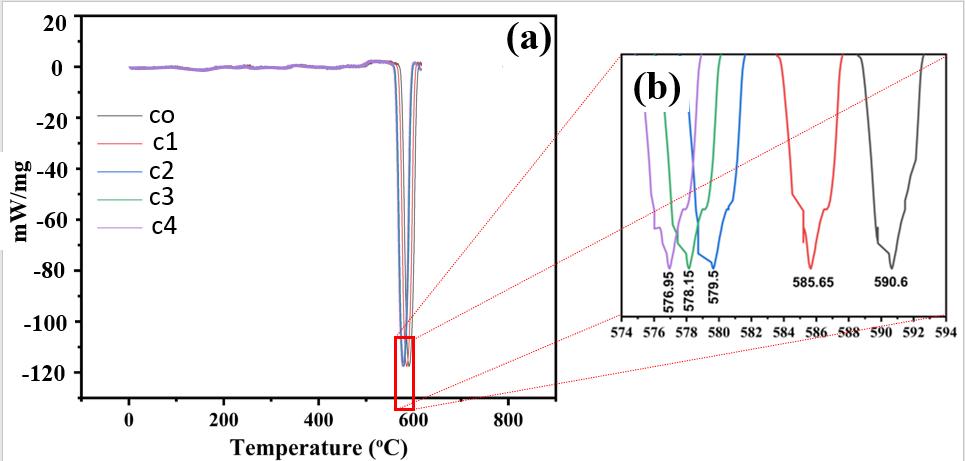
TheDSCstudywascarriedoutonalloftheAl-basedalloysthatarepresentlybeingsynthesized.Allalloysweresubmittedto DSCtestingupto700degreesCelsius.Ingeneral,themeltingpointofAl-basedalloysdeclinedastheamountofCuinthealloy rose,asseeninFigure5.570ºCwasreachedbytheC0alloybeforetheAlSieutecticreactiontookplace,indicatingthatthefirst phase transition had been achieved. 590.6 degrees Celsius was revealed to be the melting point of C0 alloy, according to research. The melting point of the metal decreased when alloying was introduced. Furthermore, when the Cu concentration grew,themeltingpointdecreased.Becausefillermaterialsshouldhavethelowestmeltingpointpossible,itmeansthatthe Cu additionmakesthealloymoresuitableforuseasafiller.TheopticalmicrographsofallthealloysareshowninFigure6.

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 11 Issue: 04 | Apr 2024 www.irjet.net p-ISSN:2395-0072
Afterthepolishedsamplesofaluminum-basedalloyswerelinkedwithcopperwireandsubsequentlymountedinepoxyresin, just one surface with a surface area of one centimeter square (cm2) was subjected to corrosive conditions. The sample was usedastheworkingelectrode,anditwasplacedinathree-electrodeglasscellwithacounterelectrode(platinummesh)anda referenceelectrodetoconducttheexperiment(SCE).Eachexperimentstartedwithanhourofocp(opencircuitpotential)to allow the sample to stabilize in the corrosive liquid before continuing. The potentiodynamic polarizaton curve was then generatedbyscanningtheelectrodesatarateof0.5mV/s.Usingthetafelfit,itwaspossibletodeterminetheelectrochemical parameters of the sample, such as the corrosion potential (Ecorr) and the corrosion current density (icorr). Because the corrosion rate (CR) is closely related to the corrosion current density (icorr), the value of icorr was used to compute the corrosion rate (CR). In order to compute CR, it was required to take into account two more factors: density and equivalent weight.Figure7showsthepotentiodynamicpolarizationcurvesofallofthealloys,whichwerecreatedina3.5weightpercent NaCl solution in order to highlight the influence of Cu on corrosion behavior. The drop in corrosion potential implies a decreaseinthesusceptibilitytocorrode,andthedecreaseincurrentdensityindicatesadecreaseintherateofcorrosion.

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
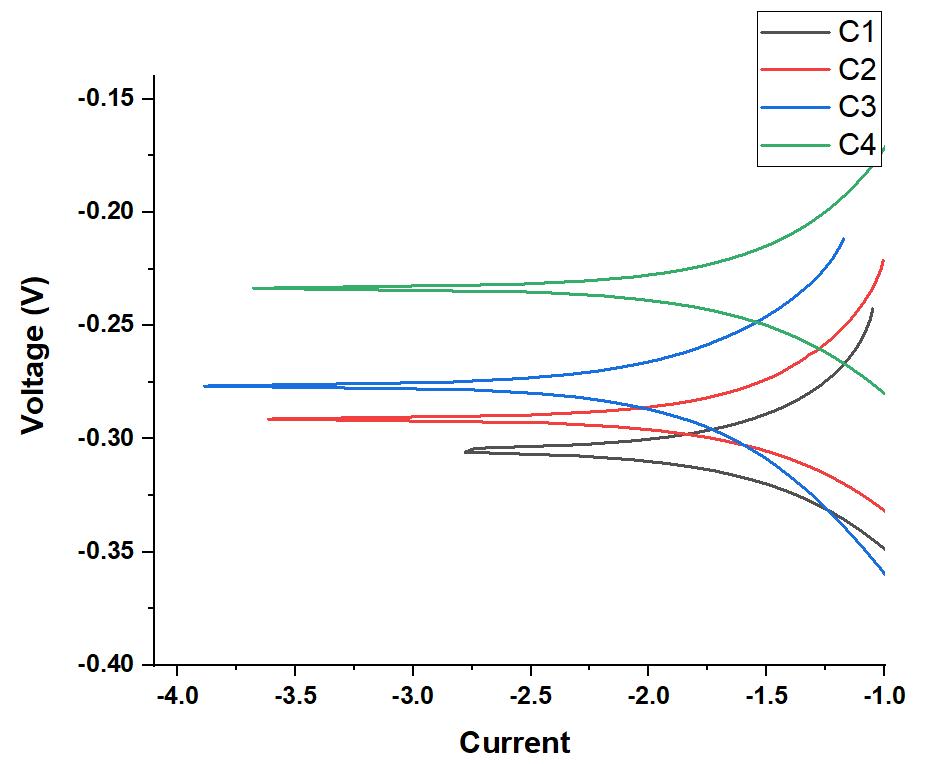
Figure7.Potentiodynamicpolarizationcurvesofsamplesin3.5wt%NaClaqueoussolution
Conclusion: Thefabricationofaluminum-basedalloyswasachievedthroughaprocessofmeltingandcasting.Introduction of various alloying elements, and notably copper, led to a reduction in the alloys' melting points. This reduction was particularlymarkeduptoaspecificlevelofcopperaddition.Asthecopperconcentrationwaselevated,therewasanoticeable increase in the alloys' densities, although these measured densities were marginally below the calculated theoretical values, likely due to the presence of porosity within the materials. Enhancements in the hardness of the alloys were observed in correlation with rising copper percentages. Among the compositions evaluated, the C3 alloy exhibited outstanding corrosion resistanceinasalinesolutionof3.5%NaCl,outperformingtheotheralloyssubjectedtothesameconditions.
Conflict of Interest: Authorsdeclarenoconflictofinterest
References:
Volume: 11 Issue: 04 | Apr 2024 www.irjet.net p-ISSN:2395-0072 © 2024, IRJET | Impact Factor value: 8.226 | ISO 9001:2008 Certified Journal | Page2467
1. T. Onzawa, A. Suzumura, and M. Ko, “Brazing of titanium using low-melting-point Ti-based filler metals,” Welding Journal,vol.462,1990.
2. A.E.ShapiroandY.A.Flom,“Brazingoftitaniumattemperaturesbelow800°C:reviewandprospectiveapplications,” DVSBerichte,vol.243,p.254,2007.
3. B.S.Murty,J.W.Yeh,.;S.Ranganathan,”High.EntropyAlloys,1sted.”;Butterworth-Heinemann:London,UK,2014.
4. M. Way, J. Willingham, R. Goodall, Brazing filler metals, Int. Mater. Rev. 0 (2019) 1–29. https://doi.org/10.1080/09506608.2019.1613311
5. Tillmann W, Wojarski L, Manka M et al. (2018) Eutectic high entropy alloys a novel class of materials for brazing applications, Proceedings from the International Brazing & Soldering Conference, 15th to 18th April 2018, New Orleans,pp142–148
6. Hardwick L, Rodgers P, Pickering EJ et al. (2019) Development of novel nickel-based brazing alloys, utilising alternative melting point depressants and high entropy alloy concepts, Proceedings from Brazing, high temperature brazinganddiffusionbonding,12thInternationalConference,21stto23rd May2019,Aachen,pp7–17

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 11 Issue: 04 | Apr 2024 www.irjet.net p-ISSN:2395-0072
7. TillmannW,WojarskiL,UlitzkaTetal.(2019)Brazing ofhightemperature materialsusingmeltingrangeoptimized filler metals based, Proceedings from Brazing, high temperature brazing and diffusion bonding, 12th International Conference,21stto23rdMay2019,Aachen,pp1–6
8. CantorB,ChangITH,KnightPetal(2004)Microstructuraldevelopment inequiatomicmulticomponentalloys.Mater SciEngA375-377:213–218.https://doi.org/10.1016/j.msea.2003.10.257
9. ZhangLX,ShiJM,LiHWetal(2016)InterfacialmicrostructureandmechanicalpropertiesofZrB2SiCCceramicand GH99 superalloy joints brazed with a Ti-modified FeCoNiCrCu high-entropy alloy. Mater Des 97:230–238. https://doi.org/10.1016/j.matdes 2016.02.055
10. Yeh J-W (2013) Alloy design strategies and future trends in highentropy alloys. JOM 65(12):1759–1771. https://doi.org/10.1007/s11837-013-0761-6
11. Baldan R, da Rocha RLP, Tomasiello RB et al (2013) Solutioning and aging of MAR-M247 nickel-based superalloy. J MaterEngPerform22(9):2574–2579.
© 2024, IRJET | Impact Factor value: 8.226 | ISO 9001:2008 Certified Journal | Page2468