General features of mycotoxin biosynthetic gene clusters
In fungi, biosynthetic gene clusters (BGCs) are groups of genes that control mycotoxin production, confirming that the pathway functions in a coordinated manner, while polyketide synthase (PKS) acts as a backbone gene for most mycotoxins (Proctor et al., 2008; Caceres et al., 2020; Liew et al., 2023)
These clusters also include pathwayspecific regulators and multiple transcription factors.
They contain transporters responsible for exporting toxins out of the cell to reduce self-toxicity.
BGCs are also highly flexible, displaying gene losses or rearrangements that account for variations in toxin production among species and strains (Sultana et al., 2019).
Biosynthetic genes by mycotoxin types
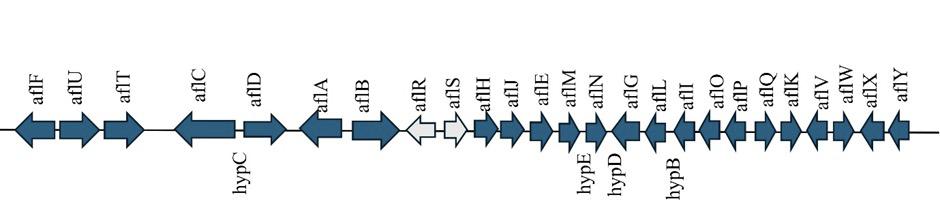
In Aspergillus species, the production of aflatoxins is controlled by two key genes, aflR and aflS, which encode a zinc-finger transcription factor that activates signaling pathway genes and a co-activator that enhances aflR function, respectively (Meyers et al., 1998).
Recent findings show that aflS, apart from its co-activator role, also helps to stabilize AflR–DNA interactions, and aflatoxin production can be affected by its loss (Caceres et al., 2020).
The ratio between aflS and aflR varies in response to environmental factors such as water activity and temperature, contributing to changes in toxin levels and the types of aflatoxins produced (Medina et al., 2017) (Figure 1).
KEY GENES RESPONSIBLE FOR AFLATOXINS (aflR & aflS)
Figure 1. Representation of the aflatoxin biosynthetic cluster in Aspergillus flavus. The arrows indicate the direction of gene transcription. Grey arrows represent regulatory genes, while blue arrows indicate structural genes (Serna et al., 2020).
KEY GENES RESPONSIBLE FOR FUMONISINS (FUM GENES 1,6,8,21)
Fumonisins are among the major mycotoxins secreted by Fusarium species, representing common contaminants of most cereals and grains (Dass et al., 2007; Sreenivasa et al., 2011).
The FUM gene cluster regulates the biosynthesis of these toxins, with FUM1, FUM6, FUM8, and FUM21 as key genes (Glenn et al., 2008) (Figure 2).
FUM1 encodes a polyketide synthase responsible for backbone assembly.
FUM6 and FUM8 mediate hydroxylation and methylation, both essential for toxin activity.
FUM21 acts as a regulatory transcription factor.
Disruption of these genes significantly reduces or eliminates fumonisin production.
Figure 2. Representation of the fumonisin biosynthetic gene cluster in Fusarium verticillioides. Arrows indicate the position and transcriptional orientation of genes. The grey arrow represents the regulatory gene. In Fusarium proliferatum and Fusarium oxysporum, the order and orientation of genes are similar, although the distances between them can vary (Alexander et al., 2009).
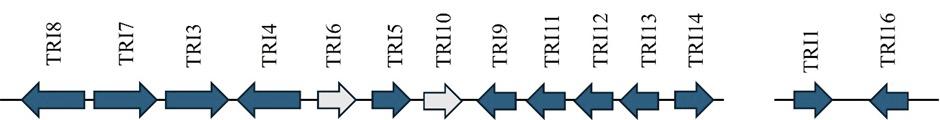
KEY GENES RESPONSIBLE FOR TRICHOTHECENES (TRI CLUSTERS, TRI5, TRI4, TRI6, TRI7, TRI8, TRI11, AND TRI13)
Trichothecenes are toxic sesquiterpenoid mycotoxins produced by Fusarium species that pose a serious food contamination risk.
Trichothecene biosynthesis is controlled by the TRI gene cluster.
TRI5 encodes trichodiene synthase, initiating trichothecene formation.
TRI4 catalyzes multiple oxygenation steps.
TRI6 is a regulatory gene that governs cluster expression.
TRI7, TRI8, TRI11 and TRI13 encode enzymes that modify the trichothecene backbone through reactions such as hydroxylation, acetylation, and epoxidation, producing diverse analogs (Proctor et al., 2008; Caceres et al., 2020)
Collectively, these genes coordinate the biosynthetic process and result in the secretion of type A and type B trichothecenes (Figure 3).
Figure 3. Representation of the trichothecene biosynthetic cluster and the TRI1–TRI16 cluster in Fusarium, which leads to the formation of T-2 toxin. Grey arrows represent regulatory genes, while the other arrows indicate the position and transcriptional orientation of genes (Alexander et al., 2009).
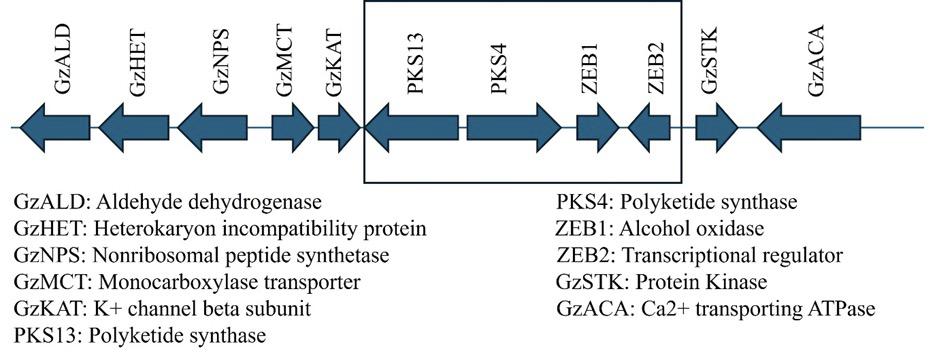
KEY GENES RESPONSIBLE FOR ZEARALENONE
(PKS 4, 13 AND ZEB1, ZEB2)
Zearalenone (ZEN) is an estrogenic mycotoxin produced by Fusarium species that contaminates cereals and their derived products (Yu et al., 2022)
Zearalenone biosynthesis depends on polyketide synthase (PKS) genes and regulatory transcription factors.
PKS4 and PKS13 catalyze the assembly of the polyketide backbone from acetyl-CoA units, forming the precursor of ZEN (Perincherry et al., 2019).
The expression of these genes is regulated by the key transcription factors ZEB1 and ZEB2, which coordinate secondary metabolism for efficient ZEN production (Villani et al., 2019).
Controlled expression of PKS4, PKS13, ZEB1, and ZEB2 plays a vital role in minimizing ZEN contamination in food, feed, and other agricultural products (Figure 4).
Figure 4. Representation of the ZEN gene cluster showing its genomic organization and flanking regions in Fusarium graminearum. The direction of the arrows indicates the predicted position and orientation of transcription for each gene/ORF. The boxed area comprises the genes required for ZEN biosynthesis in F. graminearum (Nahle et al., 2021).
KEY GENES RESPONSIBLE FOR OCHRATOXIN A (AcOTApks, PKS GENES)
Aspergillus and Penicillium species secrete ochratoxin A (OTA), which is responsible for the contamination of cereals and other agricultural products.
Ochratoxin biosynthesis involves a complex, coordinated series of enzymatic processes in which the polyketide synthase, AcOTApks, plays a fundamental role (Wang et al., 2016).
This gene encodes a PKS enzyme responsible for initiating the condensation of acetate and malonate units, resulting in the dihydroisocoumarin core of OTA (Gallo et al., 2012). The process is further anchored by the incorporation of L-phenylalanine and subsequent chlorination to complete the OTA molecule (El Khoury & Atoui, 2010)
OTA biosynthesis is also regulated by non-ribosomal peptide synthases and chlorinating enzymes, although the complete mechanism has not yet been fully elucidated.
Current findings provide insight into the complex genetic and enzymatic network required for OTA production (Figure 5)
Figure 5. Representation of the ochratoxin biosynthetic cluster in Aspergillus carbonarius. The grey arrow represents the regulatory gene, while the other arrows indicate the direction of gene transcription (Serna et al., 2020).
hal: Halogenase; bZIP: basic region/leucine zipper (Transcription factor); p450: cytochrome p450 monooxygenase; NRPS: non-ribosomal peptide synthetase; PKS: polyketide synthase
Applications and future prospects
Food safety is a major global concern and largely depends on developing countermeasures against toxin production by fungi.
In this regard, biosynthetic gene clusters (BGCs), responsible for producing toxins such as aflatoxins, fumonisins, trichothecenes, and zearalenones, have become a key target for developing strategies to improve food safety management.
The adoption of cutting-edge technologies such as CRISPR-based genome editing and RNA interference (RNAi) can effectively silence toxin gene expression. At the same time, host- or spray-delivered RNA molecules are emerging as promising and practical crop protection strategies (Stakheev et al., 2024; Chen et al., 2025).
Global regulators such as LaeA and the velvet complex, along with chromatin modifications, represent additional prospects for suppressing entire gene clusters (Zhang et al., 2024)
In the near future, integrating gene-based diagnostics, RNA technologies, and biocontrol agents with multi-omics surveillance may pave the way for long-lasting and safe mycotoxin mitigation (Liew et al., 2023).
References
1. Adithi, G., Somashekaraiah, R., Divyashree, S., Shruthi, B., & Sreenivasa, M. Y. (2022). Assessment of probiotic and antifungal activity of Lactiplantibacillus plantarum MYSAGT3 isolated from locally available herbal juice against mycotoxigenic Aspergillus species. Food Bioscience, 50, 102118.
2. Caceres, I., Al Khoury, A., El Khoury, R., Lorber, S., P. Oswald, André El Khoury, Ali Atoui, Olivier Puel, and Jean-Denis Bailly. (2020). Aflatoxin biosynthesis and genetic regulation: A review. Toxins, 12(3), 150.
3. Chang, P. K., Cary, J. W., Bhatnagar, D. E. E. P. A. K., Cleveland, T. E., Bennett, J. W., Linz, J. E., ... & Payne, G. A. (1993). Cloning of the Aspergillus parasiticus apa-2 gene associated with the regulation of aflatoxin biosynthesis. Applied and Environmental Microbiology, 59(10), 3273-3279.
4. Chen, C., Imran, M., Feng, X., Shen, X., & Sun, Z. (2025). Spray-induced gene silencing for crop protection: recent advances and emerging trends. Frontiers in Plant Science, 16, 1527944.
5. Deepa N, Charith Raj A P and M Y Sreenivasa. (2016). Nested PCR method for the early detection of fumonisin producing Fusarium verticillioides in pure cultures, cereal samples and plant parts. Food Biotechnology. 30(1): 18-29.
6. El Khoury, A., & Atoui, A. (2010). Ochratoxin A: General overview and actual molecular status. Toxins, 2(4), 461-493.
7. Ferrara, M., Haidukowski, M., Logrieco, A. F., Leslie, J. F., & Mulè, G. (2019). A CRISPR-Cas9 system for genome editing of Fusarium proliferatum. Scientific reports, 9(1), 19836.
8. Gallo, A., Bruno, K. S., Solfrizzo, M., Perrone, G., Mulè, G., Visconti, A., & Baker, S. E. (2012). New insight into the ochratoxin A biosynthetic pathway through deletion of a nonribosomal peptide synthetase gene in Aspergillus carbonarius. Applied and environmental microbiology, 78(23), 8208–8218.
9. Gil-Serna, J., Vázquez, C., & Patiño, B. (2020). Genetic regulation of aflatoxin, ochratoxin A, trichothecene, and fumonisin biosynthesis: A review. International Microbiology, 23(1), 89-96.
10. Glenn, A. E., Zitomer, N. C., Zimeri, A. M., Williams, L. D., Riley, R. T., & Proctor, R. H. (2008). Transformation-mediated complementation of a FUM gene cluster deletion in Fusarium verticillioides restores both fumonisin production and pathogenicity on maize seedlings. Molecular plant-microbe interactions, 21(1), 87-97.
11. Gomes, A. S. D. L. P. B., Weber, S. H., & Luciano, F. B. (2024). Resistance of Transgenic Maize Cultivars to Mycotoxin Production— Systematic Review and Meta-Analysis. Toxins, 16(8), 373.
12. Liew, M. X. X., Nakajima, Y., Maeda, K., Kitamura, N., & Kimura, M. (2023). Regulatory mechanism of trichothecene biosynthesis in Fusarium graminearum. Frontiers in Microbiology, 14, 1148771.
13. Majumdar, R., Rajasekaran, K., & Cary, J. W. (2017). RNA interference (RNAi) as a potential tool for control of mycotoxin contamination in crop plants: concepts and considerations. Frontiers in plant science, 8, 200.
14. Medina, A., Gilbert, M. K., Mack, B. M., OBrian, G. R., Rodriguez, A., Bhatnagar, D., ... & Magan, N. (2017). Interactions between water activity and temperature on the Aspergillus flavus transcriptome and aflatoxin B1 production. International journal of food microbiology, 256, 36-44.
15. Meyers, D. M., Obrian, G., Du, W. L., Bhatnagar, D., & Payne, G. A. (1998). Characterization of aflJ, a gene required for conversion of pathway intermediates to aflatoxin. Applied and Environmental Microbiology, 64(10), 3713-3717.
16. Nahle, S., El Khoury, A., & Atoui, A. (2021). Current status on the molecular biology of zearalenone: its biosynthesis and molecular detection of zearalenone-producing Fusarium species. European Journal of Plant Pathology, 159(2), 247-258.
17. Perincherry, L., Lalak-Kańczugowska, J., & Stępień, Ł. (2019). Fusarium-produced mycotoxins in plant-pathogen interactions. Toxins, 11(11), 664.
18. Proctor, R. H., Busman, M., Seo, J. A., Lee, Y. W., & Plattner, R. D. (2008). A fumonisin biosynthetic gene cluster in Fusarium oxysporum strain O-1890 and the genetic basis for B versus C fumonisin production. Fungal Genetics and Biology, 45(6), 1016-1026.
19. Regina Sharmila Dass, M.Y. Sreenivasa and G.R. Janardhana (2007). High incidence of Fusarium verticillioides in animal and poultry feed mixtures produced in Karnataka, India. Plant Pathology Journal, 6(2): 174- 178.
20. Seong, K. Y., Pasquali, M., Zhou, X., Song, J., Hilburn, K., McCormick, S., Dong, Y., Xu, J. R., & Kistler, H. C. (2009). Global gene regulation by Fusarium transcription factors Tri6 and Tri10 reveals adaptations for toxin biosynthesis. Molecular microbiology, 72(2), 354–367.
21. Sreenivasa M.Y., Regina Sharmila Dass, A. P. Charith Raj and G. R. Janardhana. (2011). Mycological evaluation of Maize grains produced in Karnataka (India) for the post-harvest fungal contamination. World Applied Sciences Journal, 13(4), 688 – 692.
22. Stakheev, A. A., Taliansky, M., Kalinina, N. O., & Zavriev, S. K. (2024). RNAi-Based Approaches to Control Mycotoxin Producers: Challenges and Perspectives. Journal of fungi (Basel, Switzerland), 10(10), 682.
23. Sultana, S., Kitajima, M., Kobayashi, H., Nakagawa, H., Shimizu, M., Kageyama, K., & Suga, H. (2019). A Natural Variation of Fumonisin Gene Cluster Associated with Fumonisin Production Difference in Fusarium fujikuroi. Toxins, 11(4), 200.
24. Villani, A., Proctor, R. H., Kim, H. S., Brown, D. W., Logrieco, A. F., Amatulli, M. T., ... & Susca, A. (2019). Variation in secondary metabolite production potential in the Fusarium incarnatum-equiseti species complex revealed by comparative analysis of 13 genomes. BMC genomics, 20(1), 314.
25. Wang, Y., Wang, L., Liu, F., Wang, Q., Selvaraj, J. N., Xing, F., Zhao, Y., & Liu, Y. (2016). Ochratoxin A Producing Fungi, Biosynthetic Pathway and Regulatory Mechanisms. Toxins, 8(3), 83.
26. Yu, J., Chang, P. K., Ehrlich, K. C., Cary, J. W., Bhatnagar, D., Cleveland, T. E., ... & Bennett, J. W. (2004). Clustered pathway genes in aflatoxin biosynthesis. Applied and environmental microbiology, 70(3), 1253-1262.
27. Zhang, Y., Yu, W., Lu, Y., Wu, Y., Ouyang, Z., Tu, Y., & He, B. (2024). Epigenetic Regulation of Fungal Secondary Metabolism. Journal of fungi (Basel, Switzerland), 10(9), 648.