
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 05 | May 2025 www.irjet.net p-ISSN:2395-0072


International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 05 | May 2025 www.irjet.net p-ISSN:2395-0072
Dr. Girish K B1 , Dr. Manjunath S H1 , Sudeepu L A2 , Hemanth J3 , Madhuchandra K M4 , Chethana G C5
Professor1, Student2,3,4 , Research Scholar5 , Mechanical Engineering, BGS Institute of Technology, ADICHUNCHANAGIRI UNIVERSITY, B G Nagara ***
Abstract: Dental models are essential for education, research,andclinicalpractice,yettraditionalfabrication methods like plaster casting lack flexibility and costefficiency. This study explores 3D printing of test teeth using a Polylactic Acid (PLA)-Calcium composite via Fused Deposition Modelling (FDM) to create realistic, durable,andcost-effectivedentalmodels.Thecomposite enhances PLA’s mechanical properties to mimic natural teeth, with calcium additives improving hardness and biocompatibility. The methodology involves CAD modelling, FDM printing with varied infill parameters, and mechanical testing (tensile, compression, and impact). Results show that a 50% infill density with a triangular pattern achieves optimal tensile strength (2.4921 MPa) and compressive strength (35.91 MPa), whilean80%infillmaximizesimpacttoughness(1.7914 kJ/m²). The study concludes that PLA-Calcium composites offer a viable alternative for dental model fabrication, supporting sustainable and customizable solutionsfordentaleducationandpreclinicaltesting.
Keywords: 3D Printing, PLA-Calcium Composite, Fused Deposition Modelling, Dental Models, Mechanical Testing
Introduction
Dental models are critical tools in dental education, surgical planning, and material testing, traditionally produced using plaster or resin. These methods are labour-intensive, costly, and limited in design flexibility. Additivemanufacturing,particularly3Dprinting,offersa transformative approach by enabling rapid, precise, and customizable fabrication of dental structures. Fused Deposition Modelling (FDM), an economical 3D printing technique, is widely adopted for its accessibility and compatibility with biocompatible materials like PolylacticAcid(PLA).
Thisstudyfocuseson3DprintingtestteethusingaPLACalcium composite, where calcium additives enhance mechanical strength and mimic the mineral content of natural teeth. The objectives are to design anatomically accurate tooth models, optimize FDM printing parameters, and evaluate the composite’s mechanical performance through tensile, compression, and impact tests. The outcomes aim to provide cost-effective, durable dental models for educational and preclinical
applications, addressing the limitations of traditional methods.
The demand for accurate dental models has driven advancementsinadditivemanufacturing.[1]highlighted that PLA, a biodegradable thermoplastic, is biocompatiblebutlacksthehardnessrequiredfordental applications.Incorporatingcalcium-basedfillers,suchas hydroxyapatite (HA) or calcium carbonate, improves stiffness and bioactivity [2]. [3] reported that PLA-HA composites significantly enhance tensile strength, while [4] demonstrated bone-like properties in PLA-HA scaffolds,supportingosteogenicdifferentiation.
FDM printing enables precise control over infill densityandpatterns,impactingmechanicalperformance [5]. Studies by [6] and [7] emphasize the hierarchical structure of natural teeth, with enamel (96% HA, hardness 3–6GPa) and dentin (elastic modulus 15–21GPa) requiring materials that balance hardness and toughness.PLA-Calciumcompositesaddresstheseneeds byemulatingtooth-likeproperties,makingthemsuitable fordentaltrainingmodels.
While PLA-Calcium composites show promise, optimizing infill parameters and achieving microscale accuracy in FDM-printed dental models remain not explored. This study addresses these gaps by systematically evaluating the effects of infill density and patterns on mechanical properties, aiming to produce realistic test teeth for educational and research purposes.
The methodology involves designing, fabricating, and testing 3D-printed test teeth using PLA-Calcium compositeviaFDM.Thestepsareoutlinedbelow:
1. Material Selection: PLA-Calcium composite filament (1.75 mm diameter) was chosen for its biocompatibility, printability, and enhanced mechanical properties due to calcium additives (e.g., calciumcarbonate).

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 05 | May 2025 www.irjet.net p-ISSN:2395-0072

2. CAD Modelling: Tooth models were designed using CATIA, referencing high-resolution dental scans to ensureanatomicalaccuracy.Modelswereexportedas STLfiles.
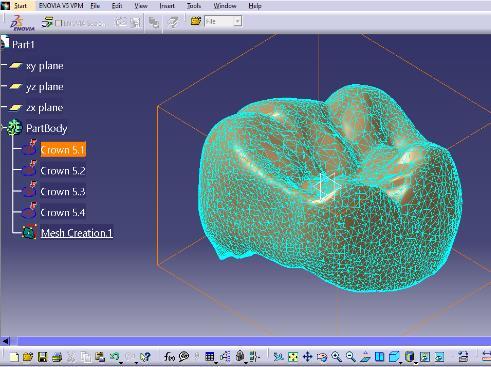
3. FDM Printing: An FDM printer was used with PLACalciumfilament.Printingparametersincluded:
• Infilldensities:40%,50%,80%
• InfillPatterns:Triangular,Octo-gram

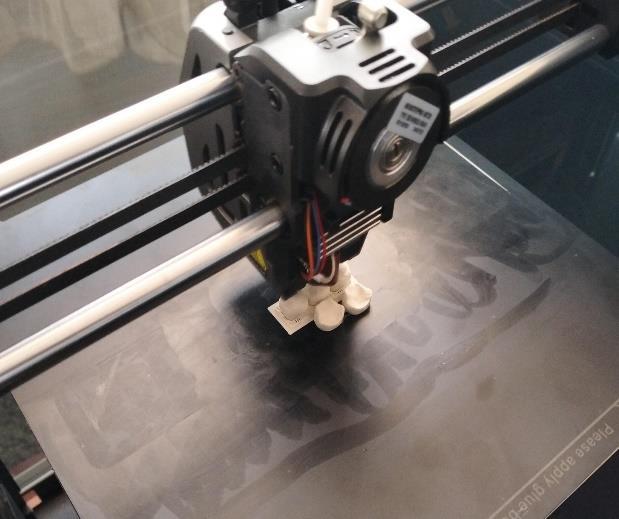

• LayerHeight:0.2mm
• NozzleTemperature:200°C
• BedTemperature:60°C

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 05 | May 2025 www.irjet.net p-ISSN:2395-0072

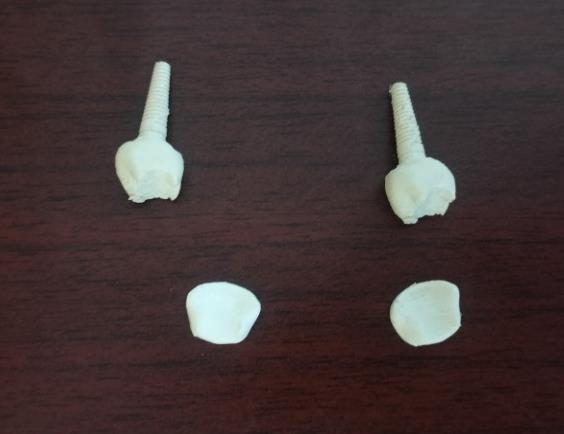
4. Post-Processing: Printed models were cleaned using isopropyl alcohol and ultrasonic baths, followed by airandvacuumdryingtoensuresurfacequality.
5. MechanicalTesting:
• TensileTesting:ConductedperASTMD638onTypeI specimens(165×13×3.2mm)at4mm/min.
• Compression Testing: Performed per ASTM D695 on specimens(12.7×12.7×25.4mm).
• Impact Testing: Executed per ASTM D256 on specimens (63.5 × 12.7 × 3.2 mm) using a pendulum tester.
6. Statistical Analysis: Analysis of Variance (ANOVA) was used to assess the significance of infill parametersonmechanicalproperties.
Results and Discussion
Chemical and Physical Properties
The PLA-Calcium composite exhibited biocompatibility and enhanced hardness due to calcium carbonate, mimickingthemineralcontentofenamel(96%HA).The filament’s printability was consistent, with no nozzle cloggingat200°C.

Tensile tests (Table 1) showed that triangular infill at 50% density achieved the highest tensile strength (2.4921 MPa) and elongation (1.9262%). Octo-gram infill at 50% density also performed well (2.2439 MPa), but higher densities (80%) reduced elongation due to increasedbrittleness.
Table 1: Tensilestrengthresults

Figure 9: TensileStrengthGraphInfillDensityV/S TensileStrength

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 05 | May 2025 www.irjet.net p-ISSN:2395-0072
2. Impact Strength

Figure 10: ImpacttestSpecimen
Impact tests (Table 2) revealed that octo-gram infill at 80%densityyieldedthehighestimpactstrength(1.7914 kJ/m²), indicating better resistance to sudden forces. Triangular infill at 80% density also performed well (1.6278kJ/m²).
Table 2: Impactstrengthresults
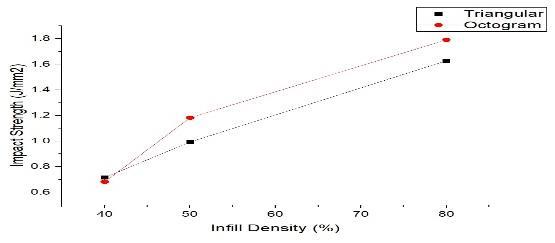
Figure 11:
3. Compression Strength

Figure 12: CompressiontestSpecimen
Compressiontests(Table3)showedthattriangularinfill at 80% density achieved the highest compressive strength (43.34 MPa) with minimal length reduction (2.36%). Octo-gram infill at 80% density was slightly lower (37.41 MPa), indicating triangular infill’s superior load-bearingcapacity.
Table 3: compressionstrengthresults
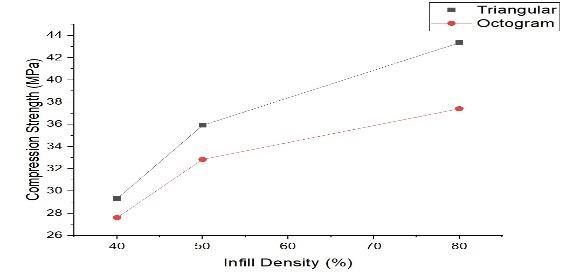
Figure 13: CompressiveStrengthGraphInfillDensity

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 05 | May 2025 www.irjet.net p-ISSN:2395-0072
Discussion
The results indicate that infill density and pattern significantly affect mechanical performance. A 50% triangular infill optimizes tensile strength and elongation, suitable for simulating dentin’s elasticity (15–21GPa). An 80% octo-gram infill maximizes impact toughness, mimicking enamel’s resistance to fracture (0.6–1.5 MPa·m05). Compressive strength peaks at 80% triangular infill, aligning with dentin’s compressive strength (100–150 MPa). However, the composite’s tensile strength (1.7–2.5 MPa) is lower than natural teeth, suggesting the need for further calcium optimizationoralternativefillerslikeHA.
ANOVA analysis confirmed that infill density has a statisticallysignificanteffect(p<0.05)onallmechanical properties, with pattern influencing impact toughness more than tensile or compressive strength. The PLACalcium composite’s biocompatibility and printability make it ideal for educational models, but its mechanical propertiesrequireenhancementforclinicalapplications.
• Mechanical Properties: The PLA-Calcium composite’s tensile strength is lower than natural teeth, limiting itsuseinload-bearingapplications.
• Microarchitecture:FDMstrugglestoreplicateenameldentininterfacesand nanoscaleHAcrystals, affecting anatomicalrealism.
• Biodegradability: Controlling degradation rates to matchdentaltissueregenerationischallenging.
• Scalability: Producing uniform models at scale is time-intensiveduetopost-processingrequirements.
• Hurdles: Clinical adoption requires rigorous testing forbiocompatibilityandlong-termstability.
Futureresearchshouldfocuson:
• Incorporating higherHAcontentorbioactiveglassto enhancemechanicalstrengthandbioactivity.
• Using high-resolution printing techniques (e.g., SLA) forfinermicroarchitecture.
• IntegratingAIforautomateddesignoptimizationand predictivemodelling
• Conducting long-term studies on degradation and oralenvironmentinteractions.
• Exploring bioprinting with cell-laden materials for regenerativedentistry.
This study demonstrates that 3D printing test teeth using PLA-Calcium composite via FDM is a viable approach for producing cost-effective, customizable dental models. Optimal parameters include 50% triangular infill for tensile strength (2.4921 MPa) and 80% infill for compressive strength (43.34 MPa) and impact toughness (1.7914 kJ/m²). While the composite mimics some properties of natural teeth, its mechanical strength requires improvement for clinical applications. ThefindingssupporttheuseofPLA-Calciumcomposites in dental education and preclinical testing, contributing tosustainableandinnovativedentalmodelfabrication.
[1] Backes, E.H., et al., “Polylactic Acid Composites for DentalApplications,”J.Mater.Sci.,2020.
[2] Plamadiala, P., et al., “Calcium-Reinforced PLA for 3DPrintingDentalModels,”Addit.Manuf.,2025.
[3] Custodio, R., et al., “Mechanical Enhancement of PLA-HAComposites,”Biomaterials,2021.
[4] Bernardo, A., et al., “PLA-HA Scaffolds for Bone Regeneration,”J.Biomed.Mater.Res.,2022.
[5] Kamio, T., et al., “Infill Optimization in FDM Dental Models,”Dent.Mater.,2024.
[6] Delgado-Ruiz,R.,Romanos,G.,“ToothStructureand MechanicalProperties,”J.Prosthodont.,2018.
[7] He, L.H., Swain, M.V., “Nano-Mechanics of Teeth,” J. Mech.Behav.Biomed.Mater.,2007.
[8] Hull, C.W., “Apparatus for Production of ThreeDimensional Objects by Stereolithography,” Google Patents,1986.
[9] Nyberg, et al., “3D-Printing Technologies for Craniofacial Rehabilitation,” Ann. Biomed. Eng., 45(1),2017.
[10] Murphy, S.V., Atala, A., “3D Bioprinting of Tissues andOrgans,”Nat.Biotechnology,32(8),2014.