Science and Technology of Liquid Metal Coolants in Nuclear Engineering 1st Edition - eBook PDF download
https://ebookluna.com/download/science-and-technology-of-liquidmetal-coolants-in-nuclear-engineering-ebook-pdf/
Environmental Science and Engineering 1st EditioneBook PDF
https://ebookluna.com/download/environmental-science-andengineering-ebook-pdf/
Ethanol : science and engineering 1st Edition- eBook PDF
https://ebookluna.com/download/ethanol-science-and-engineeringebook-pdf/
Chain Mobility and Progress in Medicine, Pharmaceuticals, and Polymer Science and Technology 1st edition - eBook PDF
https://ebookluna.com/download/chain-mobility-and-progress-inmedicine-pharmaceuticals-and-polymer-science-and-technologyebook-pdf/
Simultaneous Mass Transfer and Chemical Reactions in Engineering Science: Solution Methods and Chemical Engineering Applications 1st Edition- eBook PDF
https://ebookluna.com/download/simultaneous-mass-transfer-andchemical-reactions-in-engineering-science-solution-methods-andchemical-engineering-applications-ebook-pdf/
Structure in the Sea: The Science, Technology and Effects of Purpose-Built Reefs and Related Surfaces 1st edition - eBook PDF
https://ebookluna.com/download/structure-in-the-sea-the-sciencetechnology-and-effects-of-purpose-built-reefs-and-relatedsurfaces-ebook-pdf/
ScienceandTechnology ofLiquidMetalCoolants inNuclearEngineering FormerlyAssociateDirector,ChemistryGroup, IndiraGandhiCentreforAtomicResearch, Kalpakkam,TamilNadu,India
T.Gnanasekaran
WoodheadPublishingisanimprintofElsevier 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates TheBoulevard,LangfordLane,Kidlington,OX51GB,UnitedKingdom
Copyright © 2022ElsevierLtd.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans, electronicormechanical,includingphotocopying,recording,oranyinformationstorage andretrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhowto seekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandour arrangementswithorganizationssuchastheCopyrightClearanceCenterandthe CopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightby thePublisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthis fieldareconstantlychanging.Asnewresearchand experiencebroadenourunderstanding,changesinresearchmethods,professional practices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgein evaluatingandusinganyinformation,methods,compounds,orexperimentsdescribed herein.Inusingsuchinformationormethodstheyshouldbemindfuloftheirownsafety andthesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,or editors,assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatter ofproductsliability,negligenceorotherwise,orfromanyuseoroperationofanymethods, products,instructions,orideascontainedinthematerialherein.
ISBN:978-0-323-95145-6
ForinformationonallWoodheadPublishingpublicationsvisit ourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: CharlotteCockle
EditorialProjectManager: RupinderK.Heron
ProductionProjectManager: PremKumarKaliamoorthi
CoverDesigner: VickyPearsonEsser
TypesetbyTNQTechnologies
Dedicatedtomybelovedparents.
3.5Chemicalcharacterizationofliquidmetalcoolants
3.9Regenerationofcoldtrapsofsodiumsystems
4Pumpsandinstrumentsforliquidmetalcoolantcircuits239
4.1Introduction
5Corrosionandmasstransferinliquidmetalsystems385
5.1Introduction
5.2Generalfactorsthatdeterminecorrosionandmasstransferof structuralmaterialsinliquidmetalsystems
5.3Corrosionandmasstransferinliquidsodiumsystems
5.4Corrosionandmasstransferinliquidlithiumsystems
5.5Corrosioninliquidleadandlead-bismutheutecticsystems
5.6Corrosioninliquidlead-lithiumeutecticsystems
5.7Wettingofstructuralmaterialsbyliquidmetals
Epilogue437
Annexure1Radialdensityfunction441
Annexure2Ferromagneticmaterials447
Annexure3Basicsofheattransferbyliquidmetals455
Annexure4Thermochemicalaspectsofdissolutionofsolutesinliquid metals465
Annexure5Kineticsofprecipitationofsolutesfromsolutions473
Annexure6Magneticeffectsofcurrent,inductance,andeddycurrents481
Annexure7Propagationofsoundwavesthroughmatter491
Annexure8Piezoelectricityandpiezoelectricmaterials499
Annexure9Solidelectrolytes507 Index523
ForewordbyAnilKakodkar
Nuclearenergywouldplayasignifi cantroleinmeetingtheenergyneedsandclimate challengesbecauseofitsimmenseenergypotentialandlowcarbonfootprint.Inadditiontorapiddeploymentofcurrentnuclearreactorsystemstomeetthenetzerodeadlines,newandinnovativenuclearreactorsandtransmutationsystemsneedtobe developedtomeetthisrequirementonasustainablebasis.Inadditiontoadvanced thermalreactors,hightemperaturegas-cooledreactorsandmolten-salt-basedbreeder reactors,liquidmetal cooledfastbreederreactors(FBRs),andaccelerator-drivensystems(ADS)occupyimportantpositionsinthisdevelopmentstrategy.WhileliquidsodiumischosenasacoolantforpresentgenerationFBRs,liquidleadisacandidatefor futurereactors.Liquidleadorlead-bismutheutecticalloyalsoservesascoolantcum spallationtargetinADS.Fusionreactors,whichareexpectedtocontributesignificantlytothelongertermenergysecurity,couldalsouseeitherliquidlithiumor lead-lithiumalloyascoolantandforgenerationoftritium.Thus,liquidmetalswould playanimportantroleinthesefuturenuclearenergysystems.
Understandingofpropertiesandperformanceofliquidmetalsiscrucialtodevelopmentandengineeringofsystemsthatarebasedonthem.Althoughtherehavebeen earlierpublicationscoveringthisdomain,therewasafeltneedforabookthatis moreuptodateandcomprehensive,coversallaspectsofbehaviorandpropertiesof liquidmetalsincludingtheirmicroscopicorigins,anddealswithinterfacebetween liquidmetalsandequipment/environmentinwhichtheywork.Thisbookwould addressthatneed.Entry-levelscientistsorengineersstartingtheirworkinvolving liquidmetalswould fi ndthisbookparticularlyhelpfulingettingstartedwiththeir work.
Thisbookprovidesauniquecoverageonallpropertiesandtechnologicalaspectsof liquidmetalcoolants:thermophysical,nuclearandchemicalproperties,handling,puri fication, firehazards,andmethodsfortheirsafedisposal,instrumentationrequiredin heattransfercircuits,andcorrosionandmasstransferaspects.Startingfromtheir manufacture,methodstohandlethemsafelyinthelaboratory,andtheheattransfercircuitsthatleveragethemareoutlined.Pumpsandvariousinstrumentsneededinthe heattransfercircuitaredealtindetail.Theiroperatingprincipleswithdescriptionof theirbasicdesign,capabilities,andlimitsarediscussed.Corrosionandmasstransfer processesobservedintheliquidmetalcircuitsandthemechanismsinvolvedare discussed.
IcongratulateDr.T.Gnanasekaranonthisimportantinitiativethatwillprovidea uniquesourceofinformationforallresearchersandtechnologistswhowouldbe
ForewordbyChristianLatgé Advancednuclearsystemssuchasfastbreederreactorsandfusionreactorsarepowerintensivesystemsandnecessitateliquidcoolantswithhighheattransferproperties. Liquidmetalcoolantsareideallysuitedforthispurpose.Athoroughunderstanding ofthepropertiesofliquidmetals,improvementofexistingtechnologiesandequipment,anddevelopmentofnewonesisessentialforoperatingsafelywithhighefficiencythecoolantcircuitswiththeliquidmetals.Liquidsodiumhasbeenthe coolantofchoiceforthefastbreederreactorsrightfromtheearlyyearsandhence lotofexperiencehasbeengeneratedwithliquidsodium.Liquidlithiumandleadlithiumeutecticalloyarechosenfortritiumbreedingandascoolantforfusionreactors. Liquidleadandlead-bismutheutecticalloyarethecandidatecoolantscumneutron multipliersforaccelerator-drivensystemsandarealsoconsideredascoolantsfor somefuturefastbreederreactors.
O.J.Foustpublisheda five-volumehandbookonalkalimetalsinthe1970s.Inthe 1980s,twobookswerepublishedbothdealingwiththechemistryofliquidalkali metals.OnewasbyC.C.AddisonandtheotherwasbyH.U.BorgstedtandC.K.Mathews.Afterthesepublications,enormousamountofresearchworkhasbeencarriedout aspartofliquidmetalfastreactorsandfusionreactorstechnologiesdevelopment. Morerecently,acollaborativeworkisgoingtobepublishedbyIAEA(Tecdocs) focusedonphysicalandchemicalproperties,heattransfer,andpressuredropcorrelations,onlyforsodium.However,theinformationgeneratedbytheresearchliesscatteredasjournalpapers,reports,patents,andotherpublications,andnowherethesedata areconsolidated.Inthepast25years,severalstudieshavebeencarriedoutonliquid leadandlead-bismutheutecticalloyalso.Ahandbookprovidingthedataonleadand lead-bismutheutecticalloy(HandbookonLead-bismuthEutecticAlloyandLead Properties,MaterialsCompatibility,ThermalhydraulicsandTechnologies)hasbeen publishedin2015.However,thishandbookdoesnotdiscussthedetailsofvariousinstrumentsusedintheheavyliquidmetalsandtheirprinciples.
Thereisdefi nitelyaneedforacomprehensivebookdiscussingallthepropertiesof liquidmetalsincludingtheirmicroscopicoriginsandalsodiscussingthebasic principlesandoperationalexperienceofspecialinstrumentsandequipmentneeded foroperatingliquidmetalsystems.ThisbookbyT.Gnanasekaranamplyful fillsthe requirement.
Inthisbook,thethermophysicalpropertiesofliquidmetalsincludingtheircorrelationwiththestrengthoftheirmetallicbond,therelevantnuclearpropertiesofthe candidatecoolants,andtheirchemicalpropertiesarediscussedindetail.Besidesthe
above,themethodsofthemanufactureofthecoolants,theirpurificationandsafe handlinginlaboratoryaswellasinheattransfercircuits,andthe firesafetyaspects andsafedisposalofthesematerialshavealsobeenextensivelycoveredinthis book.Detaileddiscussiononthepumpsandvariousinstrumentsneededintheheat transfercircuitincludestheiroperatingprinciples,theirbasicdesign,capabilities, andlimits.Corrosionandmasstransferphenomenaandwettingofstructuralmaterials byliquidmetalsaretheothertopicsdiscussedinthisbookwhichareofimportance fromthetechnologydevelopmentpointofview.
Itisindeedveryrareto findabookthatcoverssuchawiderangeoftopicsright fromthebasicpropertiesofliquidmetalsuptothecorrosionaspects,andhence,I amcertainthisbookwillbecomeasourcebookonliquidmetalcoolantstothescientistsorengineersattheentrylevelstartingtheirworkinvolvingliquidmetalsandalso forpracticingtechnologists.
Dr.ChristianLatgé CEACadarache(France)
Preface Advancednuclearsystemssuchasfastbreederreactors,fusionreactorfacilitiesand accelerator-drivensystemsgeneratehighpowerandrequirecoolantswithveryefficientheattransportproperties.Liquidmetalspossessexcellentheattransfercharacteristicsandarechosenforuseinthesesystems.Whileliquidsodiumisthecoolantof choiceforthecurrentgenerationfastbreederreactors,liquidlithiumandleadlithiumeutecticalloysarecandidatecoolant-cum-tritiumbreedersinfusionreactor systems.Liquidleadandlead-bismutheutecticalloysarebeinginvestigatedforuse ascoolant-cum-neutronmultipliersinaccelerator-drivensystemsandalsoasacoolant forfuturefastbreederreactors.Thetechnologyofliquidalkalimetalshasbeenunder intensivedevelopmentsincethe1950s.Similarinvestigationsonheavyliquidmetal coolantsarebeingcarriedoutoverthepast20 30years.Severalsystemsandcomponentsneededforreliableoperationoftheseliquidmetalcircuitsareundervarious stagesofdevelopment:pumps(mechanicalandelectromagnetictypes),instruments tomeasurethe flowandlevelsoftheliquidmetals,andforviewingcomponents immersedintheliquidmetals,whichareopaque;chemicalandphysicalsensorsfor on-linesurveillanceoftheconcentrationofthedissolvednon-metallicimpuritiesin theliquidmetalsetc.
Manyoftheinstrumentsdevelopedforuseinliquidmetalcircuitsexploitthethermophysicalandchemicalpropertiesofliquidmetals.Methodologiesforsafehandling ofliquidmetals,particularlytheveryreactiveliquidalkalimetals,havenowbecomea routine.Techniquesforonlinepuri ficationoftheliquidmetalsintheheattransfercircuitsandmaintainingthematdesiredpuritylevelsareatdifferentstagesofmaturity. Structuralmaterials,whichpossessrequiredmechanicalpropertiesatthehighoperatingtemperaturesandradiationenvironments,andwhicharechemicallycompatible withthecandidateliquidmetalcoolant,arealsobeingdeveloped.The finalchoice amongthecandidatestructuralmaterialsisbasedontheoutcomeofthelongterm corrosionandmasstransferstudiesundertheintendedoperatingconditionsinthe liquidmetalcircuit.Detailsofmostofthesetechnologicaladvancesareavailableas publicationsinjournals,reports,conferenceproceedingsandcertainbookchapters inascatteredform.Foranefficientdesignandfortheoperationandutilizationofa liquidmetalsystem,anunderstandingofallthesetechnologiesstartingfromthe manufactureofliquidmetalsisrequired.Also,basicknowledgeofthethermophysical andchemicalpropertiesofliquidmetalsandthecorrelationsamongthemisalso needed.
Thefourthchapterdealswiththecomponentsandinstrumentsrequiredforthesafe andreliableoperationoftheheattransfercircuitswithliquidmetals.Centrifugaland electromagneticpumpsusedforcirculatingliquidmetalinthesecircuitsaredescribed indetail.Instrumentsfordetectingthelevelsand fl owofliquidmetals,aswellasultrasonictransducersforviewingcomponentsimmersedinliquidmetalsaredescribed indepth.Methodstodetectliquidalkalimetalleaksandforonlinemonitoringofimpuritiesinliquidmetal,circuitsarealsodiscussedindetail.Theoreticalprinciplesof operationofthesepumpsandinstrumentsareexplained.
The fifthchapterdwellsoncorrosionandmasstransferprocessesthatoccurinthe liquidmetalcoolantcircuits.It firstdealswiththedrivingforcesforthiscorrosionand masstransferphenomenon.Sincethestructuralmaterialsusedaredependentonthe coolantemployed,thecorrosionandmasstransferphenomenaareuniqueforeachsystem.Hence,theobservedcorrosionphenomenonineachliquidmetal structuralmaterialsystemandtheunderlyingmechanismsaredescribed.Thischapteralsodeals withtheexperimentallyobservedcharacteristicsofwettingofsurfacesofthestructural materialsbyliquidmetals,whichistheprerequisiteforcorrosionandmasstransfer processesinthesystemtosetin.
Thebookhasnineannexures.Beingabookdealingwithmulti-disciplinarysubjects andprimarilyintendedforthoseembarkingonliquidmetaltechnologyatentry-level, theannexuresdiscussthebasicsciencebehindrelevantphenomena/processesdealt withinthemaintext.Thiswouldfacilitateeasyreadingofthemaintext.Annexures providedareon(1)radialdistributionfunctionforabetterunderstandingofthestructureofliquidmetalsbasedontheiratomicarrangements,(2)ferromagneticmaterials, whichareusedinsometypesof flowmetersandelectromagneticpumps,(3)basicsof heattransfer,todescribetheheattransfercoeffi cientandthecorrelationsusedfor liquidmetals,(4)thermochemicalaspectsofdissolutionofsolutesinliquidmetals, (5)kineticsofprecipitationofsolutesfromaliquidmetal,(6)magneticeffectsofcurrent,whichareneededtounderstandtheoperationofleveldetectorsand flowmetersin liquidmetalcircuits,(7)propagationofsoundwavesthroughmattertounderstandthe operatingprinciplesandlimitationsoftheultrasonictransducersusedforviewing componentsimmersedinliquidmetals,(8)piezoelectricityandpiezoelectricmaterials tounderstandtheprinciplesofgenerationofultrasonicwavesandoperationofultrasonictransducersand(9)basicsofsolidelectrolytematerials,whichareusedinchemicalsensorsemployedforon-linemonitoringofdissolvedimpuritiesinliquidmetals.
TheEpiloguelistssomeoftheunsolvedproblemsandthedevelopmentsrequiredin the fieldofliquidmetalcoolants.
Thispageintentionallyleftblank
Acknowledgments IamindebtedtomymentorsDr.C.K.Mathews(Ex-Director,ChemistryGroup,Indira GandhiCentreforAtomicResearch,Kalpakkam,India)andDr.H.U.Borgstedt (formerlywithNuclearResearchCentre,Karlsruhe,Germany)forintroducingmeto theexciting fieldofliquidmetalcoolants.Theirbook “AppliedChemistryofAlkali Metals” wastheinspirationformetoventureintothepresentbook.Iamalsograteful tomyseniorcolleagueDr.G.Periaswami,whoencouragedmeinallofmyendeavors intheareaofliquidmetalsresearch.IthankmyfriendsfromAustria,Prof.Herbert IpserandProf.AdolfMikula,whohadencouragedmetowritethisbookandprovided mewithmanyvaluablereferences.IamgratefultotheDepartmentofAtomicEnergy, GovernmentofIndia,forprovidingmethe “RajaRamannaFellowship” aftermysuperannuationduringwhichIcouldpreparethemajorcontentsofthebook.Iamalso thankfultotheDepartmentforgrantingpermissiontopublishthebookinitspresent form.MyspecialthanksgoestoDr.P.R.VasudevaRao,FormerDirectorofIGCAR, Kalpakkam,andcurrentlyViceChancelloroftheHomiBhabhaNationalInstitute,India,forhisvaluableguidanceandveryhelpfulsuggestionsduringvariousstagesof writingandpublishingthebook.
Ithanktheanonymousreferees,whomadeusefulcommentsbasedonadetailed outlineforthebook.IamalsothankfultomanyoftheexpertsandcolleaguesatIGCAR fortheirusefuldiscussionsandforofferingsuggestions/commentsonthecontentsofthe book.MyspecialthanksgoestoDr.G.Vaidyanathan,Dr.K.K.Rajan,Dr.Christian Latge(CEA,France),Mr.M.Rajan,Dr.K.Swaminathan,Dr.B.K.Nashine,Mr.PrasantSharma,Mr.Hemanath,Mr.K.C.Srinivas,Dr.K.Nagarajan,Dr.K.V.Govindan Kutty,Dr.K.S.Viswanathan,Dr.V.Jayaraman,Dr.RajeshGanesan,Dr.K.I.Gnanasekar,Dr.S.Anthonysamy,andDr.T.S.LakshmiNarasimhanforstudyingdifferent partsofthebookandprovidingvaluablesuggestionsandcommentstoenrichitscontents.IextendmythankstoDr.R.KumarandDr.G.V.S.AshokKumarfortheir helpincollatingthedataonradioactivenuclideslistedin Tables1.15 and 3.1.Iam verythankfultoMr.V.SureshKumar,Mr.B.ArulKumarandM.Janarthanamfortheir helpinpreparingmanyofthe figuresincludedinthebook.Iamthankfultothemembers oftheElsevierteam,whowereextremelyhelpfulduringpublicationofthebook. Lastbutnottheleast,IamthankfultomywifeMalliga,daughterArunaandher family,andsonBharani,forbeingsounderstandingandprovidingmeconstantsupportandencouragementduringtheyearsofpreparationofthebook.
Igratefullyacknowledgethepermissionsforreuseofthefollowing figuresfrom previouspublications:
FigureNo.ReferencesourceUsedwithpermissionfrom Chapter4
Fig.4.2Fig.7ofRef.6IOPPublishingLtd.(CCBY3.0)
Fig.4.5Fig.6inRef.11Elsevier
Fig.4.7Fig.2.4inRef.8Elsevier
Fig.4.13Fig.3inRef.20Elsevier
Fig.4.18Fig.12inRef.39Elsevier
Fig.4.19Fig.11inRef.39Elsevier
Fig.4.21Fig.2inRef.48Elsevier
Fig.4.22Fig.5inRef.48Elsevier
Fig.4.23Fig.3inRef.56Elsevier
Fig.4.27Fig.3inRef.69Elsevier
Fig.4.28Fig.8inRef.45Elsevier
Fig.4.30Fig.1inRef.75SpringerNature
Fig.4.37Fig.1inRef.105Elsevier
Fig.4.39Fig.7inRef.113TaylorandFrancisLtd., © AmericanNuclearSociety
Fig.4.40Fig.15inRef.106Elsevier
Fig.4.46Fig.2inRef.141Elsevier
Fig.4.49Fig.1inRef.162TaylorandFrancisLtd.,
Fig.4.50AFig.8inRef.161Elsevier
Fig.4.50BFig.2inRef.162TaylorandFrancisLtd.,
Fig.4.51Fig.1inRef.163ArgonneNationalLaboratory, USA
Fig.4.56Fig.2inRef.183Elsevier
Fig.4.62Fig.2inRef.209Elsevier
Fig.4.63Fig.8inRef.208Elsevier Chapter5
Fig.5.14Fig.5inRef.135Elsevier
Annexure-1
Fig.A1.1Fig.4.8inRef.1JohnWileyandSons
Fig.A1.2Fig.4.8inRef.1JohnWileyandSons
Fig.A1.3Fig.4.8inRef.1JohnWileyandSons
Fig.A1.4Fig.4.8inRef.1JohnWileyandSons
Annexure-7
Fig.A7.4Fig.2inRef.1OfficeoftheScientificand TechnicalInformation(OSTI), DepartmentofEnergy(DOE), USA
Annexure-9
Fig.A9.3Fig.8.22inRef.1JohnWileyandSons
Fig.A9.8Fig.7inRef.4,andFig.2in Ref.5 IOPPublishingLtd.,
Author
Thispageintentionallyleftblank
Thermophysicalandnuclear propertiesofliquidmetal coolants 1 Chapteroutline 1.1Introduction2
1.2Metallicbondingandthestateofliquidmetals3
1.3Cohesiveenergyofliquidmetals7
1.4Structureofliquidmetals13
1.5Surfaceenergyofliquidsandthephenomenonofwetting17
1.5.1Surfaceenergiesofliquidmetalsandalloys19
1.5.2Surfaceenergyofmetallicsolids25
1.5.3Wettingphenomenoninliquidmetalsystems27
1.5.3.1Inertwetting29
1.5.3.2Reactivewetting31
1.6Viscosityofliquidmetals32
1.7Electricalconductivityofmetallicmaterials37
1.7.1Electricalconductivityofcrystallinemetals38
1.7.2Electricalconductivityofamorphoussolidandliquidmetals48
1.8Thermalconductivityofmaterials53
1.8.1Thermalconductivityofsolid-statematerials53
1.8.2Thermalconductivityofliquidmetals59
1.9Molarheatcapacityofliquidmetals62
1.10Vaporpressureofliquidmetals67
1.11Magneticpropertiesofmetals70
1.11.1Magneticpropertiesofsolid-statematerials70
1.11.1.1Diamagnetism73
1.11.1.2Paramagnetism74
1.11.1.3Ferromagnetism77
1.11.2Magneticpropertiesofmoltenmetals78
1.12Nuclearpropertiesoftheliquidmetalcoolants80
1.13Choiceofcoolantforadvancednuclearsystems80
1.13.1Fastbreederreactors80
1.13.2Tokamakfusionreactor choiceoflithiumandPb-Lieutecticalloyascoolant andtritiumbreeder91
1.13.3AcceleratordrivensystemandchoiceofleadandLBEasspallationtargetand coolant94
References97
1.2Metallicbondingandthestateofliquidmetals1 Majorityofthemetals( >90%)intheirsolidstateexisteitherasbodycenteredcubic(BCC)orasclosepackedcrystallinestr uctures(facecenteredcubicorhexagonal),althoughsomemetalstakeotherstructuresalso. Table1.1 liststhecrystal structureofsomeofthemetallicelements.Coordinationnumbersofatomsinthe BCCandcubic/hexagonalclosepackedstructuresare8and12,respectively.The crystalstructureadoptedbyametalisinfl uencedbyvariousfactorssuchas (a)theatomicradiioftheconstituentmetal atoms,(b)cohesivee nergiesresulting frommetallicbonding,and fi nally(c)theinevitablethermodynamicdrivetoattain thelowestenergystate.
Accordingtothefreeelectrontheoryofbondinginmetals,electronsfromthe valenceshelloftheatomsarefreeanddelocalized.Theioniccoresformaperiodic structure(lattice)andthedelocalizedelectronspropagatefreelyinthisperiodiclattice. Thephysicalpropertiesofthemetalsaredictatedbytheioniccoresinthelatticeand thedelocalizedelectrons.Thedelocalizedelectronsactaschargecarriersfortheconductionofelectricity.Theyalsoactasenergycarriersfortheconductionofheatand hencemetalsexhibitveryhighelectricalandthermalconductivities.Itisthisdelocalizationoftheelectronsovertheentirelatticethatrendersthemetallicbondingtobe nondirectional.Whenastressisapplied,ioniccorescanrollovereachotherwhile themobileelectronscontinuetoholdthestructuretogether.Thismakesthemetals malleableandductile.Thestrengthofthemetallicbonddependsonthenatureof themetal,thetypeofcrystalstructureadoptedbyit,thechargeandsizeofthemetal ionsinthecore,andthenumberofelectronscontributedbyeachatomtothedelocalized “sea” ofelectrons. Table1.2 showsthecomparisonofmechanicalstrengthsof somepuremetalsandascanbeseen,thesepropertiesdifferwidelydependingon thedegreeofmetallicbondinginthem.
Atagivenpressure,solidandliquidphasesofasubstancecancoexistwitheach otheronlyatitsmeltingpoint.Column3of Table1.1 showsthemeltingpointsof someofthemetalsanditisseenthatthevaluesvaryoveraverywiderange.Melting occursatthetemperaturewherethethermalenergyoftheatomsofthecrystalline metalisenoughtoovercometheforcesholdingtheminlatticepositions.Forexample, alkalimetalshavelowermeltingpointsthanthealkalineearthmetals.Thisisbecause (i)atomsofalkalimetalscontributeonlyoneelectroneachtothedelocalizedseaof electronscomparedtoalkalineearthmetalatomswhichcontributetwoelectrons each,(ii)alkalineearthmetalscrystallizeinclosepackedstructure(packing efficiency ¼ 74%)whilealkalimetalscrystallizeinbodycanteredcubicstructure (packingeffi ciency ¼ 68%)and(iii)atomicradiiofalkalimetalsarehigherresulting inthedelocalizedelectronstobefarawayfromthenucleiithaninthecaseofalkaline earthmetals.Onthesamecount,transitionmetals(whicharethealloyingcomponents
1 Theterm “liquidmetal” wouldbeusedtorefertoallmetalsintheirliquidstateinthisbook,althoughit shouldstrictlyrefertothosewithmeltingpointbelow660.2 C.
Table1.1 Structuralpropertiesofsometypicalmetalsinsolidandliquidstatesa.
Li6.941180.54.6BCC(He)2s1
Na22.989897.82.64BCC(Ne)3s1
K39.098363.62.4BCC(Ar)4s1
Mg24.30506508.8HCP(Ne)2s2
Ca40.0788428.4FCC(Ar)4s2 0.06311.1
Sr87.6277710.0FCC(Kr)5s2 0.06011.1
Cu63.5461084.6213.0FCC(Ar)3d104s1 0.05711.3
Ag107.8682961.7811.09FCC(Kr)4d10 5s1 0.05611.3
Au196.96651064.1812.76FCC(Xe)4f14 5d10 6s1 0.05910.9
Cr51.9961190720.9BCC(Ar)3d5 4s1 0.04311.2
Fe55.847153813.77BCC(Ar)3d6 4s2 0.05110.6
Co58.9332149515.48HCP(Ar)3d7 4s2 0.05311.4
Ni58.6934145517.15FCC(Ar)3d8 4s2 0.05211.6
Ti47.88166814.6HCP(Ar)3d2 4s2 0.05610.9
Mo95.94262335.6BCC(Kr)4d5 5s1 0.078
Ta180.9479301724.7BCC(Xe)4f14 5d3 6s2 0.065
Al26.9815660.3210.46FCC(Ne)3s2 3p1 11.5
Zn65.39419.537.28HCP(Ar)3d10 4s2 0.03910.5
Cd112.411321.076.4HCP(Kr)4d10 5s2 0.03810.3
In114.818156.63.26Tetragonal(Kr)4d10 5s2 5p1 0.04511.6
Sn118.710231.937.07Tetragonal(Kr)4d10 5s2 5p2 0.03310.9
Pb207.2327.45.12FCC(Xe)4f14 5d10 6s2 6p2 0.05010.9
Bi208.9804271.410.48Rhombohedral(Xe)4f14 5d10 6s2 6p3 0.0408.8
aDataonM.P.andmolarenthalpyoffusionforLi,Na,K,Pb,BiaretakenfromRef.[7]andthecorrespondingdataforallothersarefromRef.[8].
Thermophysicalandnuclearpropertiesofliquidmetalcoolants5
Table1.2 Mechanicalpropertiesofsomepuremetalsunderannealedconditionatroom temperature[12].
Metal
Ultimatetensile strength(MPa)
Modulusof elasticity(GPa)
Brinellhardness (MPa)
Al5069 72150
Cu215110 130
Ag140 18072 83.5500
Au15078 83220
Fe290195 205800
Cr300280 3151000
Ni400200 220800
Mo670300 3301800
Ta5001901250 1400
Ti250110600
Pb14 1814 18
Bi5 203290
Zn125110 130
Cd7550 5375
instructuralmaterialsforliquidmetalcoolantsystems)haveveryhighmeltingpoints because(i)electronsfromtheird-orbitalsalsoparticipateinthedelocalizationinadditiontothosefrom s- and p-orbitalsand(ii)theiratomicradiiaresmall. Lindemannproposedamodeltopredictthemeltingpointsofcrystallinesolids basedonthecharacteristicsofvibrationsofatomsinthelattice[9].Hesuggested thatacrystallinematerialwouldmeltwhentheamplitudeofthethermalvibration oftheatomexceedsacriticalfractionoftheaverageinteratomicdistanceinthelattice. Ifweconsidertheatomsinametallicsolidtobehaveasharmonicoscillators,each atominthesolidwouldvibrateindependentoftheneighborsatafrequencyvwhere v ¼ 1 2p k mr ,misthemassoftheatom,andkistheforceconstantoftheoscillator.2 If [x istheamplitudeofthevibrationoftheatominthex-directionfromitsequilibrium positioninthelattice,thesumofpotentialandkineticenergiesassociatedwiththis vibrationisgivenby3:
2 Insteadofreducedmassofthesysteminvolvingitsneighbors,onlymassoftheatomisusedforsimplicity.
3 Notethat [x isthepointofmaximumamplitudewhere all theenergyispotentialandhencethepotential energy 1 2 kð[x Þ2 becomesthetotalenergy.
Thermophysicalandnuclearpropertiesofliquidmetalcoolants7
Bydeducingtheforceconstantkfromexpression Eq.(1.4) andsubstitutingitin Eq.(1.3),wegetthefollowingexpressionforthecriticalfractionfcri (alsocalledas Lindemannfraction)as:
Theinteratomicspacinginametalcanbeestimatedfromitsdensity d andgram atomicmassM:
whereNA istheAvogadro’snumber.Usingthedataontypicalmetals[10,11],the Lindemannfractionsarecalculatedandlistedin Table1.1.ItisseenthattheLindemannfractionsofthetypicalmetalsareintherangeof0.04 0.08inspiteoftheir variedstructuresandlargedifferencesintheirelectronicconfiguration.
Meltingpointofthemetalsandthemolarenthalpyoffusionaresystematically relatedandthisisexpressedbyRichard’srule[13]:
where DSmfusion and DHmfusion arethemolarentropyandmolarenthalpyoffusion, respectively.Entropyoffusionispositiveandisassociatedwithincreasein randomnessonmelting.Meltingpointandmolarenthalpyoffusionofsometypical metalsaregivenin Table1.1
Whenacrystallinemetalmelts,almostallofitsphysicalpropertieschangesharply by fi niteamounts.Amongthem,thechangeinresistancetoshearstressisverylarge andsubstantial.Whentherigidcrystaltransformsintoaliquid,viscositychangesbya factorofabout1020.
1.3Cohesiveenergyofliquidmetals Classicaldefinitionofcohesiveenergy(orcohesiveenthalpy)ofacrystallinesolidis theenergythatmustbeaddedtothecrystaltoseparateitscomponentsintoneutral,free atomsatrest,atinfiniteseparationwiththesameelectronicconfiguration[11].Forone g-atomofametalat0Kand1barpressure,thiscanberepresentedas:
Sincemolarheatcapacityofanidealgascontainingmonomericspecies,Cg;ideal P ¼ (5/ 2)R, Eq.(1.11) canbewrittenas:
Cohesiveenergyofametalismainlyduetotheelectrostaticattractiveforcesbetweentheioniccoresandthedelocalizedvalenceelectronsthatcontributetothe metallicbond.Cohesionofatomsinaliquidmetalbecomesweakerwithincrease intemperaturesincethethermaloscillations/vibrationsanddiffusionofthemetal atoms(ioniccores)intheliquidphaseincreasewithincreaseintemperatureresulting inanincreaseinthemolarenthalpyoftheliquidphaseHT [ (see Eq.(1.9)).Considering theBorn-Habercyclein Fig.1.1,thecohesiveenergyoftheliquidatanytemperature T(T > Tm)canberepresentedintermsofmolarheatcapacityandcohesiveenergyof theliquidatitsmeltingpoint.Sincethesumoftheenthalpychangesinsteps5and6is equaltothesumoftheenthalpychangesinsteps7and8,
Asmentionedabove,themolarheatcapacityofanidealgascontainingmonomeric species,Cg;ideal P ¼ (5/2)R.Hence, Eq.(1.14) canbewrittenas:
;coh
Correlationofenthalpyofvaporizationofaliquidmetalatitsnormalboilingpoint tothecohesiveenergyofcrystallinemetallicsolidisshowninRef.[8]andtherelation betweentwoisgivenbythefollowingexpression:
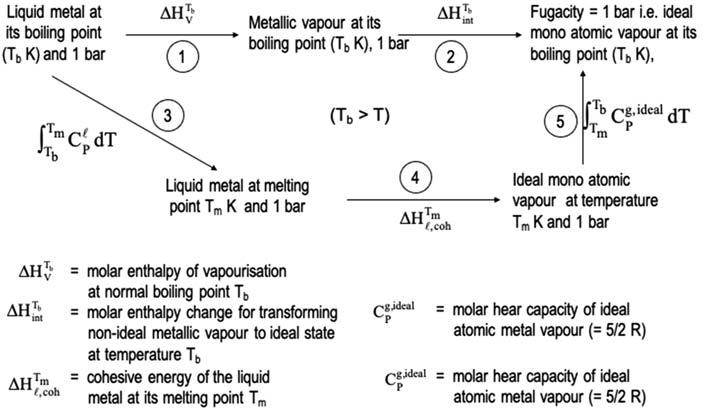
Enthalpyofvaporizationatthenormalboilingpointistakenasameasureofits cohesiveenergyatthemeltingpointalso[15].Theenergeticsandapproximations involvedinthiscanbeexaminedbyconsideringtheBorn-Habercyclegivenin Fig.1.2.EnthalpyofvaporizationattheboilingpointTb,i.e., DHTb V corresponding Thermophysicalandnuclearpropertiesofliquidmetalcoolants9
D
10ScienceandTechnologyofLiquidMetalCoolantsinNuclearEngineering
Figure1.2 Born-Habercycleforcalculationofcohesiveenergyofaliquidmetalfromits enthalpyofvaporization.
tostep1istheenthalpychangeintransforming1g-atomofaliquidmetalatTb tothe gaseousphaseat1barandatthesametemperature.Sincethevaporphaseiscondensableandisinequilibriumwiththepureliquidphase,thespeciesinvaporphasewould exhibitinteractionsamongthemanditsfugacitywouldbe < 1.Theenthalpychangein step2 ¼ DHTbint istheenthalpychangeinbringingthisnonidealmetalvaportothe statewithfugacity ¼ 1(idealstate)andthiswouldbeanendothermicprocess.While thisisthecasewhenthevaporphasecontainsonlymonomeric(atomic)species,additionalenthalpytermsneedtobeincorporatedwhenthevaporphasecontainspolymeric species.Vaporphaseofmetals,particularlythoseofalkalimetals,containspolymeric speciessuchasdimer,trimer,tetramer,etc.(e.g.,Na2,Na3,Na4)inadditiontothemajormonomericspecies[16].Dissociationofthesepolymericspeciestotheatomicspeciesalsowouldbeinvolvedinstep2.Thisisalsoanendothermicprocessandwould needtobeincorporatedin DHTbint 6 Thesumoftheenthalpiesinvolvedinsteps3,4,and 5isequaltothesumofthoseinvolvedinsteps1and2.Wecanrepresentthesameas:
6 Althoughitispossibletogettheenthalpychangeforthedissociationofpolymericspeciestoatomic speciesbyknowingthevaporphasecompositionandthebondenthalpies,theenthalpychangeinvolvedin changingfromnonidealtoidealstate(DHTb int )isdifficulttoestimate.