



























































































As your behind-the-scenes partner, our mission is to ensure your greatest MedTech ambitions become a reality. That’s why Resonetics is laser-focused on the details—ensuring every aspect of your critical components is engineered, prototyped, and manufactured to the highest specifications. And with our unmatched dedication, we’re able to move rapidly from design to full-scale production so you can bring exciting developments to market. Speak to an engineer by visiting resonetics.com.



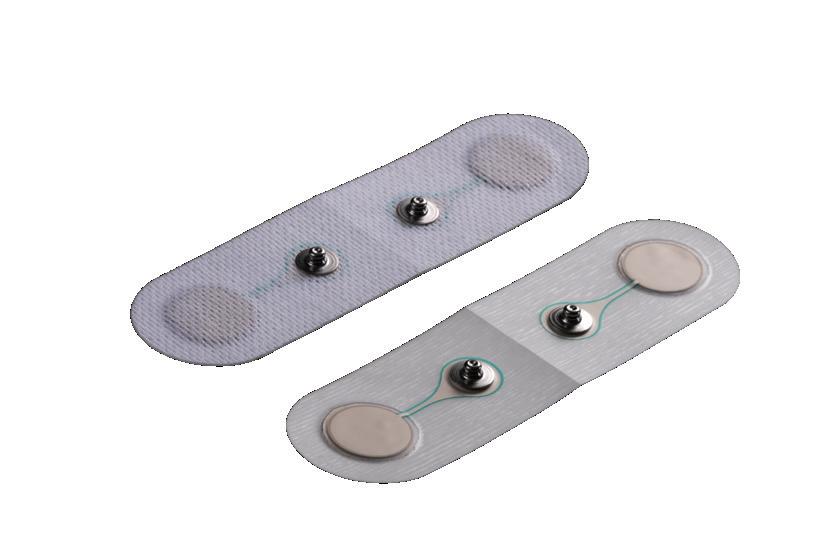
ᘩ High-force with significant travel
ᘩ Offers spring rate flexibility
ᘩ Produced from one piece of wire for consistent load properties
ᘩ Ideal for replacing stacks of Belleville washers
• Elimination of improper installation
• Saves on manual labor
• Reduces inventory needs

Nestawave Replaces Belleville Stack


Technical Specifications
ᘩ 6.5 mm to 510 mm diameter
ᘩ Custom-made for your specific needs
ᘩ Available in standard and exotic materials
ᘩ Shim ends available
ᘩ Actuators
ᘩ Bearing Preloads
ᘩ Compressors
ᘩ Clamping Tools
ᘩ Valves
ᘩ Vibration Dampeners Applications








































Medical Design & Outsourcing is excited to release the winners of our annual Leadership in Medical Technology program. Since we announced the nominees online in January 2025, our user community has voted on what companies they feel best exemplify medical technology leadership in 17 categories. We are happy to celebrate the winners here and at www.medicaldesignandoutsourcing.com.












EDITORIAL
Editor in Chief Chris Newmarker cnewmarker@wtwhmedia.com
Managing Editor Jim Hammerand jhammerand@wtwhmedia.com
Senior Editor Sean Whooley swhooley@wtwhmedia.com
Editorial DirectorDeviceTalks Tom Salemi tsalemi@wtwhmedia.com
Managing EditorDeviceTalks Kayleen Brown kbrown@wtwhmedia.com
Editor in ChiefR&D World Brian Buntz bbuntz@wtwhmedia.com
SALES
Ryan Ashdown 216.316.6691 rashdown@wtwhmedia.com
Jami Brownlee 224.760.1055 jbrownlee@wtwhmedia.com


CREATIVE SERVICES
VP, Creative Director Matthew Claney mclaney@wtwhmedia.com
DIGITAL MARKETING
VP, Operations Virginia Goulding vgoulding@wtwhmedia.com
Digital Marketing Manager Taylor Meade tmeade@wtwhmedia.com
AUDIENCE DEVELOPMENT
Director, Audience Growth Rick Ellis rellis@wtwhmedia.com
Audience Growth Manager Angela Tanner atanner@wtwhmedia.com
Mary Ann Cooke 781.710.4659 mcooke@wtwhmedia.com
Mike Francesconi 630.488.9029 mfrancesconi@wtwhmedia.com

PRODUCTION SERVICES
Customer Service Manager Stephanie Hulett shulett@wtwhmedia.com
Customer Service Rep Tracy Powers tpowers@wtwhmedia.com
Customer Service Rep JoAnn Martin jmartin@wtwhmedia.com
Customer Service Rep
Renee Massey-Linston renee@wtwhmedia.com
Customer Service Rep
Trinidy Longgood tlonggood@wtwhmedia.com
LEADERSHIP TEAM
CEO Matt Logan mlogan@wtwhmedia.com
Jim Powers 312.925.7793 jpowers@wtwhmedia.com
Brian Toole 267.290.2386 btoole@wtwhmedia.com














Medtech pros know there are some things you just can’t find with a Google search or by asking ChatGPT.
Sure, those generative AI responses sound confident. But anyone who has queried a large language model on a topic in which they’re an expert quickly realizes these chatbots don’t actually know anything and can’t reason.
In medtech, you need real expertise from people who have been there before and succeeded. More than ever, we at Medical Design & Outsourcing are committed to serving you with that authoritative, authentic expertise and being transparent about our sources.
This latest version of our annual Medical Device Handbook offers tech tips and actionable advice from medtech pros with a track record of success at device OEMs and their partners for raw materials, components, contract development and manufacturing, and other outsourced services.
No one has more AI-enabled medical devices on the FDA’s list of marketing authorizations than GE HealthCare, and our cover story explores its use of artificial intelligence, its vision for the technology and Chief AI Officer
Parminder Bhatia’s tips for other device developers to take advantage of the latest advances.
Is there a company with more cardiac implant experience than Medtronic? In another feature, Dr. Jonathan Piccini offers lessons from Medtronic’s Define AFib study of implantable cardiac monitors.
And in our final feature, we share the latest on pulsed-field ablation system safety from Boston Scientific’s Dr. Brad Sutton and Abbott’s Dr. Christopher Piorkowski as they blaze new trails for this minimally invasive technology.
This issue also features advice from other medical device developers and experts in materials, regulatory matters and cybersecurity.
It’s the kind of expertise we strive to offer in every magazine we print, every newsletter we send, every webinar we host and every special report we publish.
We’re only able to do this thanks to the willingness of medtech experts to share what they’re doing and what they’ve learned with our audience of medical device professionals. We want to inspire and enable you to advance the medtech mission.
As always, I hope you enjoy this edition of Medical Design & Outsourcing. Thank you for reading.





Customize Interpower® North American and international hospital-grade cord sets to arrive in the specialized lengths you need. Do you want those cords coiled and tied, or hanked? Want bagged cords with labels on them, and labels and barcodes on your boxes sending them straight to the shelf in your warehouse? Besides providing the correct amperages and voltages for medical equipment such as portable CT scanners, X-ray machines, and surgical robots, all Interpower hospital-grade conductors inside the plug are secured by a stainless steel ring ensuring electrical continuity when AC power is vital.
North American and Japanese hospital-grade plugs and receptacles bear the green dot, signifying the plugs have passed the rigorous UL 817 Abrupt Removal Test (UL 817, 18.2.4.1) requirements for hospital-grade cords. Interpower offers value-added options such as ring and spade terminals, heat-shrink tubing, reinforced cord clips molded onto the cord to take up slack, ferrites, ferrules— and an array of value-added services.
• Same-day shipping on stocked products
• Blanket and Scheduled orders available
• No minimum orders











































HERE’S WHAT WE SEE:
Medical device development tips for professionals, from professionals
ELECTRONICS:
Why cleaning medical PCBAs is harder than ever — and what to do about it
IMAGING:
Reimagining cardiovascular care through the lens of OCT and AI
MATERIALS:
Vulcanizing and customizing silicone for medical device applications; Benefits of thermoplastic polyurethane films for wearable devices
NITINOL:
Nitinol machining and finishing for medical devices
PRODUCT DEVELOPMENT:
How Aktiia developed the first FDAcleared OTC cuffless blood pressure monitor; A J&J MedTech leader offers advice for device innovation, including an unusual method in the OR; How Abbott developed the firstof-its-kind Infinity DBS system
REGULATORY:
Why smarter FDA pre-submissions matter more than ever
SOFTWARE:
3 surprising cybersecurity risks in medical device software
AD INDEX


















Chief AI Officer Parminder Bhatia discusses GE HealthCare’s vision for artificial intelligence in medtech and what’s needed to get there.
Medtronic‘s study of Linq implantable cardiac monitor (ICM) devices in atrial fibrillation (AFib) patients offers important lessons for other device developers.
The chief medical officer of Boston Scientific’s Atrial Solutions Business discusses coronary spasm, hemolysis, microbubbles, tissue contact and force during pulsed-field ablation procedures.
From high-resolution printing to life-saving devices, our advanced ow technology delivers smooth, turbulencefree performance where predictability is everything. Find your perfect pump solution. Contact us today!



































Tubing,
Swiss-
Femtosecond
Electropolishing
PVD


Why cleaning medical PCBAs is harder than ever — and what to do about it
Vapor degreasing is well-suited for modern medical electronics used in implantable devices, diagnostic tools, imaging equipment and wearables.



By Elizabeth Norwood MicroCare
As medical electronics become smaller, more powerful and more complex, cleaning their PCBAs has grown increasingly difficult.
Traditional methods often fall short, and contamination risks device performance and patient safety. Adapting cleaning strategies to meet these new challenges is essential for success.
The complexity behind the clean
Medical devices are shrinking in size while expanding in capability. To support this evolution, designers rely on high-density layouts and hybrid material assemblies that balance electrical, mechanical, and thermal performance. Common materials in modern medical PCBAs include PTFE laminates for signal integrity, ceramicfilled substrates for heat dissipation, polyimide flex circuits for wearables, and nanocomposites for precision performance in compact spaces
These materials enable innovations like faster diagnostics, more accurate
monitoring, and smaller implantables, but they also introduce unique cleaning challenges. That’s because many of these substrates have surface characteristics that resist wetting or react negatively to certain chemistries or temperatures.
At the same time, complex board architecture makes it harder to reach all surfaces. Common design features such as bottom-termination components, fine-pitch BGA and QFN packages and stacked assemblies and tight standoffs create cleaning blind spots.
Microscopic residues from flux, solder paste, or even handling oils can hide in those inaccessible areas. If left behind, these residues can corrode traces, interfere with electrical conductivity, or lead to total device failure.
Why traditional cleaning falls short
Many manufacturers still rely on aqueous cleaning systems, which use water and detergents to remove residues. >>

But aqueous cleaning has real limitations when it comes to medical-grade PCBAs.
• Material incompatibility: Some advanced substrates absorb moisture, delaminate, or warp when exposed to water or heat.
• Limited penetration: Water may not fully reach under low-standoff or bottom-terminated components.
• Drying challenges: Thorough drying is crucial, but complex geometries make it difficult, leading to trapped moisture or water spots.
• Residue risks: Detergents and additives must be completely rinsed off to avoid residue, which isn’t always guaranteed.
In high-reliability applications like medical electronics, these risks are often unacceptable. That’s where vapor degreasing offers a safer, smarter alternative.
Vapor degreasing is a better fit for modern medical electronics
Vapor degreasing is a closed-loop cleaning method that uses engineered, low-boiling cleaning fluids in a vapor state to clean surfaces without water, scrubbing or aggressive mechanical action. It’s especially well-suited to complex and sensitive assemblies because it penetrates tight spaces and under components with ease, using fluids specifically formulated for different fluxes, pastes and materials. It’s non-aqueous, reducing the risk of water-related issues, and requires no drying cycle, with components emerging clean, dry, and residue-free. Finally, it’s repeatable and controllable, making it easier to validate and scale for production.
These attributes make vapor degreasing ideal for cleaning PCBAs used in implantable devices, diagnostic tools, imaging equipment, wearable monitors, and anywhere else precision and reliability are non-negotiable.
Collaboration is critical
One of the biggest missteps in medical device manufacturing is treating cleaning as a last-minute consideration. But by that point, the choices in board layout, materials and assembly may limit what’s possible. The solution? Early collaboration. Involving your contract electronics manufacturer (CEM) and cleaning experts at the start of the design
process pays dividends later. This early engagement allows teams to assess material compatibility with cleaning fluids, identify potential cleaning trouble spots before they become issues, and tailor processes for specific contaminants, components and devices
Early collaboration streamlines validation, reduces risk, and helps ensure devices meet regulatory and performance requirements without rework or redesign.
Thorough testing and validation are non-negotiable in medical device manufacturing. Cleaning processes must be evaluated for effectiveness (contaminant removal), material compatibility (component alteration, damage, or degradation) and biocompatibility (ensuring no harmful residues were left behind).
This typically involves testing cleaning performance on sacrificial boards, starting with mild fluids and moving up to stronger
solutions only if needed. Each test should assess cleaning success across multiple PCBA zones: topside, underside, and under-component areas.
Once validated, the cleaning process must be continually audited to catch drift, equipment wear, or environmental changes that could impact performance. Documentation and traceability are crucial, especially when meeting FDA, ISO, and other medical compliance standards.
Cleanability should be considered during the design phase, not after. From board layout to flux type, every decision can affect how easy or difficult cleaning will be.
For instance, no-clean fluxes may reduce steps but become problematic if cleaning is later required. Highperformance substrates may demand specialized chemistries to avoid damage. Component placement affects how fluids
can reach and remove residues. By designing with cleaning in mind, manufacturers avoid costly late-stage changes and ensure that cleanliness and functionality go hand-in-hand.
As medical electronics advance, cleaning their complex, high-performance PCBAs has become more challenging. Traditional methods often fall short. Vapor degreasing offers a reliable, material-safe solution. With early planning, collaboration, and validation, manufacturers can ensure performance, compliance, and patient safety.
Elizabeth Norwood is a senior chemist at MicroCare, which offers precision cleaning solutions. Norwood researches, develops and tests cleaning-related products and has been in the industry for more than 25 years, holding a B.S. in chemistry from the University of St. Joseph.

The combination of optical coherence tomography and artificial intelligence is improving cardiovascular interventions and transforming the future of care.
Heart disease continues to be the No. 1 cause of death for men and women worldwide, which makes advancements in care critical.

By Dr. Ethan Korngold Abbott
Over the years, interventional treatments and medical technologies have continued to evolve to address heart-related issues.
However, the introduction and integration of artificial intelligence (AI) is transforming the care landscape and shifting how physicians are approaching treatment options and delivering care.
The powerful combination of OCT and AI One of the most transformative pairings today is the integration of optical coherence tomography (OCT) and AI. OCT is an intravascular imaging technology that uses light

waves to capture high-resolution, layered images of coronary arteries.
These images enable cardiologists to assess plaque characteristics, vessel size, and stent placement with greater detail than traditional imaging solutions like angiography. By providing a realtime, three-dimensional view of the inside of the artery, OCT improves procedural planning and outcomes in coronary interventions.
While OCT alone is very useful, OCT and AI together enhance and streamline physicians’ workflows and ensure that critical details are accounted for. AI can visually overlay and provide quantitative metrics, giving physicians an intuitive, data-driven roadmap for making treatment decisions both before and during procedures.
For example, Abbott’s Ultreon 2.0 software leverages OCT imaging in conjunction with AI allowing for fast and efficient decision-making for percutaneous coronary intervention (PCI). As a result, individuals experience fewer repeat procedures, better outcomes, and faster recovery times.
Abbott’s Ultreon 2.0 intravascular imaging software combines optical coherence tomography and artificial intelligence for better percutaneous coronary intervention outcomes.
Image courtesy of Abbott
How to prepare your cath lab
As AI-driven OCT becomes integral to cardiovascular treatment, interventionalists who proactively embrace these technologies will be better positioned to deliver more precise, data-driven care that results in improved outcomes.
So, how can physicians accurately prepare their cath labs? Here are my three recommendations:
Invest in training and evaluate readiness: Physicians should seek hands-on training in OCT interpretation and AI-powered platforms. Learning to interpret AI-generated overlays, measurements, and guidance ensures physicians remain in control while benefiting from AI insights. At the same time, cath labs should assess whether current imaging systems support AIenabled software. Upgrading platforms with real-time analytics, image storage, and EHR integration can ease the transition to advance workflows.
Foster collaboration: Integrating AI into the cath lab is not just a technical decision. It requires collaboration across IT, radiology, data security, and cardiovascular teams. Establishing partnerships early can help improve adoption, address data governance, and promote shared ownership of clinical improvements.
Participate in research and pilot programs: Participating in OCT- and
AI-related research or early-access programs provide real-world exposure to new technologies and contribute to the broader evidence base. These efforts often include direct support from technology developers and into future capabilities.
The combination of OCT and AI is not just improving today’s interventions. It’s transforming the future of care to help combat cardiovascular disease. We see this with Ultreon 2.0 and how it is currently helping people living with coronary artery disease (CAD). By empowering physicians with faster insights, predictive intelligence, and seamless personalized guidance, OCT and AI are making it possible to treat heart disease more proactively and precisely than ever before.
The key to using AI effectively is ensuring it enhances a physician’s ability to make the best decisions for their patients. As technologies continue to advance, the next
frontier lies in making AI-driven imaging not just faster and more accurate, but also predictive, personalized, and seamlessly integrated into each step of the treatment process. Machine learning models will soon analyze patient-specific imaging data alongside historical outcomes to help identify plaques at high risk of rupture, allowing for treatment before a major cardiovascular event occurs.
Fully automated decision-support systems are on the horizon. These platforms will offer guidance on stent sizing, placement, and post-deployment optimization, potentially standardizing care across varied clinical environments. So whether an individual is in a rural hospital or a leading medical facility in a large city, they will still receive the best cardiovascular treatment and care possible.
Dr. Ethan Korngold is chief medical officer and divisional vice president of medical affairs for Abbott’s vascular business.
















Medtech manufacturers are using advanced silicone processing techniques to enhance device performance, assembly and visibility.

SBy Matt Pagel Lubrizol
mart processing techniques let manufacturers customize silicone for what matters most: secure assembly, clear imaging, and markings that enhance usability. By refining how silicone is processed, manufacturers can streamline assembly, move away from liquid adhesives, and design easier-to-use devices.
It’s all part of how today’s silicone-based solutions are enabling better performance, efficiency and patient outcomes.
Vulcanized or unvulcanized?
Vulcanized silicone sheeting is delivered fully cured, meaning it is already strong and durable. It may be cut into a final shape (for example, a suture tab) and various 2D shapes may be assembled to create a 3D device.
As many bakers know, silicone sheets and parchment paper coated with silicone are often used to prevent sticking. Because cured silicone has low surface energy, this makes it difficult for other materials to adhere to it. Fortunately, for the purpose of device fabrication, unvulcanized silicone sheeting can be vulcanized to form a strong bond with already vulcanized silicone components.
Unvulcanized sheeting is soft and tacky until heat is applied to cure it. Once heated, it acts like doubled-sided tape — perfect for bonding vulcanized sheets or attaching components, giving manufacturers a clean, consistent assembly process without the hassle of liquid adhesives. It’s cut to the exact shape you need, placed where adhesion is required, and then heat-cured to create a precise bond. >>
For example, you might use unvulcanized sheeting to attach a suture tab to a device, or to bond two vulcanized sheets together around their perimeter to create something fillable, or a sealed device in other cases. Vulcanized sheeting gives you the stability needed for the structural parts, while unvulcanized provides an efficient way to assemble those parts.
Taking silicone’s flexibility a step further, laminated sheeting combines vulcanized and unvulcanized silicone
professionals instantly differentiate between things like instrument types, implant sizes and material stiffness.
Standardized color schemes further allow medical device manufacturers to create a recognizable product line while ensuring compliance with industry safety and identification rules.
Importantly, these pigments do not alter the material. The only thing that changes with pigment is the color. Silicone’s toughness, flexibility and body-friendly properties stay the same.

in a single material. Imagine a singlesided tape, with the vulcanized layer adding strength and the unvulcanized side creating adhesion. This type of sheeting may be used to seal or patch a dip cast balloon or shell or to encapsulate a metal component (e.g., a circuit) to protect it from the body while simultaneously protecting the body from whatever has been encapsulated.
Silicone is great on its own, but with a few smart enhancements, customized silicone becomes an even better fit for all kinds of devices. With the right additives, it can do even more to enhance product perceptibility and identification.
In a high-stakes medical environment, color differentiation makes devices more efficient, intuitive and safer to use. Distinct colors help healthcare
Silicone is naturally invisible under imaging techniques, but radiopaque additives make it easy to track and monitor inside the body. For devices that require precise placement and regular check-ups, these agents make silicone clearly visible on X-rays and scans.
Surgeons and medical teams rely on this capability to get a clear view whether they’re placing an implant, guiding a catheter or using a surgical tool. For implantable drug systems and neurological electrodes, adding radiopacity ensures that providers can monitor placement and fine-tune positioning over time. Devices like hydrocephalus shunts and implantable infusion pumps also benefit from radiopacity, allowing for easy X-ray visibility and periodic monitoring without invasive inspections.
Specialized inks let medical-grade silicone hold onto permanent, highcontrast markings so they’ll stay readable for years, whether it’s for function, branding or instructions.
Think about printed measurement scales that guide insertion depth in catheter systems, or wearable medical devices like continuous glucose monitors and ECG electrodes that may feature instructions or alignment indicators. These markings play multiple critical roles in many devices. Inks must also be formulated to withstand fading or degrading, especially where there is prolonged bodily contact, without releasing harmful substances or causing irritation.
Silicone’s versatility in medical applications isn’t just about the material itself. It’s also about how it’s processed and put together. The mix of vulcanized and unvulcanized silicone streamlines manufacturing without sacrificing durability. Add pigments, radiopaque agents and medical-grade inks, and the customization potential is huge.
Designing with silicone? Whether you need it to be stronger, softer or more visible, a medical materials specialist can help you get it just right.
Matt Pagel is a new product development engineer at Lubrizol‘s specialty silicone manufacturing plant in Montana. He has more than 15 years of experience in silicone product design and manufacturing.
















COMPREHENSIVE EXPERTISE: From early-stage product development to biocompatibility testing, we provide a full spectrum of engineering and testing services under one roof, ensuring a seamless process and faster time-to-market.
CUTTING-EDGE CAPABILITIES: With advanced equipment and industry know-how, we offer specialized testing in drug stability, device verification, and biocompatibility services that few labs can match and few medical device manufacturers have access to.
TAILORED SOLUTIONS: Whether you're a startup or a Fortune 500 company, we offer customized support, guiding your product through its entire lifecycle with precision and care. Custom Injection Molding R&D Processing Development






By Timothy Frisch Covestro

and Justin Spitzer Covestro
TPU films offer a combination of flexibility, stretchability, biocompatibility and manufacturability for next-generation printed electronics.
nnovations in medical wearable solutions are revolutionizing healthcare delivery, portability, user comfort and patient outcomes. Materials like thermoplastic polyurethane (TPU) films are enabling devices that are both more durable and more comfortable for longterm wear, improving patient acceptance and compliance.
TPU films offer a wide range of benefits that make them an excellent choice for medical and wearable technology. They have multiple uses in medical wearables, from adhesive mountings to top cover layers to electronic substrates. Outer TPU layers provide a soft hand feel and can be color customized, while inner layers provide electronic functionality and user comfort contributing to the overall user experience of products made with these materials.
TPU films are flexible, stretchable and conformable TPU films are known for their exceptional mechanical properties. Their flexibility, stretchability, and conformability enable devices to dynamically move and conform to complex body shapes, ensuring comfort and effective adhesion over extended periods. TPU’s inherent resistance to tearing, puncturing, and abrasion contributes to wearable durability and longevity. Their high abrasion and wear resistance ensures they can withstand physical stress and mechanical wear in applications like clothing and hospital bedding.
Compliant over a wide range of temperatures, TPU films exhibit good low-temperature flexibility and impact strength. Some varieties remain flexible even at sub-freezing temperatures, allowing for a broad range of indoor and outdoor uses.
The films’ stretchable nature, paired with a stretchable conductive ink, enables the creation of conductive circuit layers that can flex and stretch without compromising functionality.
Biocompatibility and engineered adaptability (multifunctional performance)
Another key feature of TPU films in medical applications is their ability to be formulated free of solvents and/ or plasticizers, making them safe for prolonged skin contact.
This biocompatibility is complemented by their breathability, which allows for air and moisture exchange to prevent skin irritation. The films can be engineered to provide an excellent balance of blocking liquids while remaining highly permeable to water vapor in applications like wound coverings.
Antifungal or antimicrobial formulations can provide a key advantage in minimizing bacterial and viral transfer and growth in medical settings. TPU can also handle sterilization methods such as ethylene oxide (EtO), gamma radiation and electron beam (e-beam) radiation.
Electrical properties of TPU
TPU films exhibit key electrical properties that make them optimal electronic substrates. TPU films can be produced to exhibit optimal surface topographies for printed circuits, and their inherent insulating properties make them an ideal material in protecting wearers from shock, electrostatic discharge and

other electrical intrusions or leaks. The stable dielectric constant is important when using TPU films in applications that may include electrodes, antennas or capacitive circuits. These properties collectively make TPU films a reliable choice for electronics, enabling the creation of flexible and stretchable circuit assemblies.
Wearable technologies are often subjected to varied and uncontrolled environments, and key properties of TPU films are well-suited for environmental protection. Aliphatic TPU chemistries exhibit excellent resistance to ultraviolet (UV) radiation, preventing degradation and maintaining structural integrity when exposed to sunlight.
TPU films can withstand a wide range of temperatures, maintaining flexibility and strength even in extreme cold or heat, making them ideal for outdoor user applications where temperature fluctuations are common.
Polyether-based TPU films in particular demonstrate strong resistance to hydrolysis when exposed to sweat, body fluids, humidity, moisture and even submersion, making them an excellent choice for protecting electronics in showers and bathing. Additionally, TPU films are resistant to many chemicals, including
oils, greases, cleaning fluids and solvents, helping them maintain performance and durability in challenging environments.
From a manufacturing perspective, TPU films can be seamlessly integrated into existing high-volume printed electronics manufacturing processes, making them a practical choice for large-scale production. New TPU film offerings, some with a stabilizing carrier, are designed for high printing quality, excellent processability, and handling. These properties facilitate easier printing, curing, and converting processes for printed electronics.
TPU films can be further shaped by thermo-forming. And finally, TPU films can be bonded using thermal, radio frequency (RF) or ultrasonic methods to additional layers of TPU or other substrates without additional adhesives, offering unique solutions for sealing, assembly, reduced overall layer count and increased flexibility.
TPU films represent a valuable enhancement for wearable medical devices. Their unique combination of flexibility, stretchability, biocompatibility and manufacturability makes them an optimal choice for the next generation of printed electronics in healthcare.

As the demand for more sophisticated, comfortable, and reliable wearable medical devices grows, TPU films will continue to shape the future of remote patient monitoring and personalized healthcare.
courtesy of Covestro
As the demand for more sophisticated, comfortable, and reliable wearable medical devices grows, TPU films will continue to shape the future of remote patient monitoring and personalized healthcare.
Timothy Frisch is the innovation manager for specialty films at Covestro. He has worked for over 10 years in product development, application development, and manufacturing of polycarbonate and thermoplastic polyurethane resin and film products.
Justin Spitzer has over 25 years of experience in flexible printed electronics focusing on application development, market strategy, and product development. He has successfully managed business development, sales teams, and strategic initiatives across various technology sectors in the Americas region and globally.
Electrocardiogram (ECG) sensor patches made with thermoplastic polyurethane (TPU) film

These stages can make or break medical-grade nitinol used in cutting-edge devices.

itinol machining and finishing are some of the final steps on this nickel-titanium alloy’s journey from the earth’s crust into the hands of doctors and bodies of patients as
We’ve previously covered how medical nitinol is mined and melted into raw material and then processed into nitinol wires, tubes and sheets for device manufacturers to use in medical devices, components or parts.
Laser cutting nitinol tubes and sheets is the most common way to manufacture nitinol medical devices, components and parts. Laser cutting is used for common products, including stents, heart valves, inferior vena cava (IVC) filters and most other nitinol implants.
More advanced than fiber lasers, femtosecond lasers can cut medical grade nitinol with ultra precision without heating the alloy, which is sensitive to heat changes.
Other nitinol machining and manufacturing methods include:
• Electric discharge machining (EDM): Used to turn large nitinol bars or plates into a shape such as a bone staple for orthopedics
• Chemical etching: Corrosive chemicals to eliminate material, for example to make blades for surgical equipment
• Wire winding: Nitinol wires are wound by hand or machine one at a time to make nitinol shape memory springs.
• Wire braiding: Similar to wire winding, except nitinol braiding weaves or braids multiple nitinol wires together by hand or machine for devices such as clot retrievers, stents and flow diverters
• Computer numerical control (CNC) machining: Conventional CNC machining operations — turning, milling, lathing, etc. — are possible with nitinol, but very difficult without specialized techniques for this particular alloy and its properties.
• Joining: Nitinol can be joined with nitinol or other metals such as stainless using welding, soldering, coining or riveting.

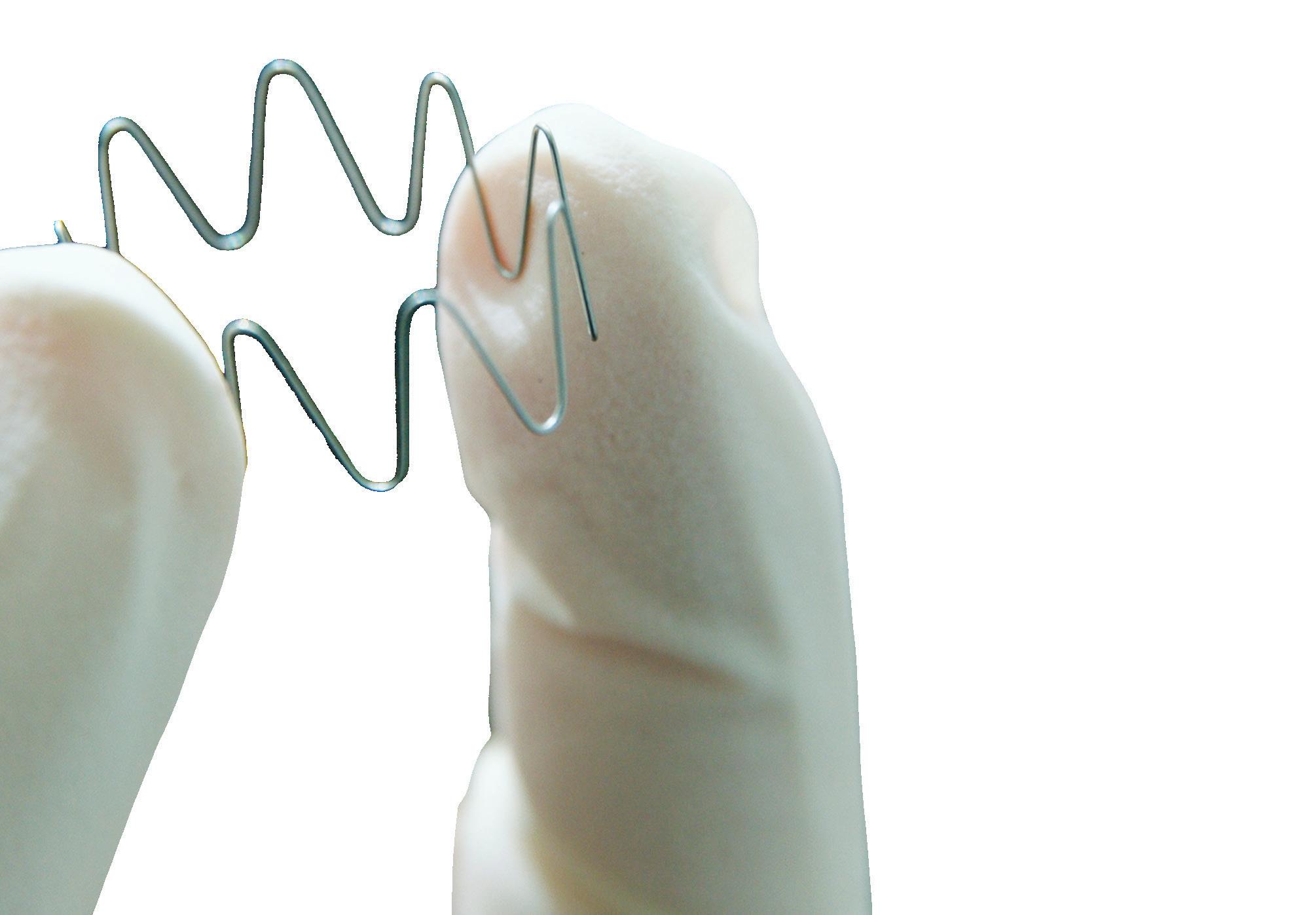
• Laser ablation: Similar to laser cutting, laser ablation removes much smaller bits of material but leaves the bulk of the material, similar to Swiss turning for small, precision parts.
• Grinding: Profile and centerless grinding are good methods of nitinol manufacturing because nitinol maintains its straightness and can be ground with precision, resulting in components like guidewires.
• 3D printing: Additive manufacturing methods with nitinol such as laser sintering are in very early stages.
Medical nitinol shape setting and finishing After laser cutting — or an alternative machining process — comes deburring (if needed) and then shape setting.
Nitinol shape setting uses a tooling fixture to compress or expand the nitinol into the desired final form, and heat from a salt bath, fluidized bed or furnace to treat the nitinol so it maintains that shape.
The heat applied in shape setting also affects the special shape memory and superelastic/pseudoelastic properties of nitinol. Different heat treatment temperatures and times will yield different properties, giving a stent radial force to resist crushing, for example, or allowing a minimally invasive implant to compress down into a catheter for delivery and then expand when placed inside the patient. The nitinol’s fatigue durability is another property that is affected by heat.
Shape setting is also a stage where nitinol can be given different colors and shades (like you might get by anodizing titanium) with different applications of time, temperature and atmosphere in the furnace. A traditional anodization
process can also be used to give nitinol a colored finish, similar to what you might do with aluminum.
After shape setting, surface finishing for oxide removal is a necessary step for biocompatibility. This step prevents the harmful effects of long-term nickel exposure to the human body. There are benefits and drawbacks to different surface finishing treatments for nitinol.
Electropolishing and mechanical polishing can ensure biocompatibility and also remove blemishes that could become fatigue nucleation sites.
Electropolishing is a great way to achieve biocompatibility by removing surface oxide while softening sharp edges and creating smooth surfaces. Electropolishing doesn’t have the fatigue drawbacks of pickling or chemical polishing.
Pickling (or etching) uses acid to clean the surface, removing the oxide.
Pickling can be used in a batch process by dipping parts into an acid bath to remove oxide for biocompatibility. However, pickling will leave microscopic changes to the surface topography that could introduce the risk of fatigue cracking. But that same surface topography could also enhance adhesion of polymer coatings on devices such as guide wires.
Chemical polishing is a similar acid bath batch process. It leaves a smoother surface than pickling for better fatigue resistance, but still offers some topography for coating applications.
Finally, visual and dimensional inspections verify the quality of the finished nitinol parts, components or devices before assembly and/or packaging, sterilization and shipping. Go to wtwh.me/nitinol for more nitinol know-how from Medical Design & Outsourcing.






How Aktiia developed the first FDA-cleared OTC cuffless blood pressure monitor
Aktiia co-founder and CTO Josep Solà discusses the Hilo Band’s technology, development challenges and potential applications.

his year, Aktiia won the first FDA 510(k) clearance for over-thecounter use of a cuffless blood pressure monitor, the G0 Blood Pressure Monitoring System (branded
“Hypertension affects over 1.3 billion people and is still the leading risk factor for cardiovascular disease, yet most cases go undiagnosed or are poorly managed. That has to change,” Aktiia co-founder and CTO Josep Solà said in a Medical Design & Outsourcing interview.
“The future of medtech is moving toward continuous, seamless and invisible monitoring, especially at home,” he continued. “We need technologies that fit into people’s lives without asking them to become medical experts. That means smarter algorithms, better signal processing, fully automated workflows, and user interfaces that are intuitive for everyone, not just tech-savvy users.”
We asked Solà about the technology that makes the Hilo Band possible, product development challenges and solutions, and other applications of the technology.
“At Aktiia, we believe cuffless, effortless blood pressure monitoring is the foundation,” he said. “But it’s just the beginning. As we integrate this into more wearable form factors and combine it with other vital signs and AI-driven insights, we can move from occasional diagnosis to proactive, personalized hypertension management on a global scale.”
MDO: What’s the big advance that makes your monitoring technology possible?
Solà: “The big advance is twofold: it’s about automation and nextgeneration algorithms.
“On the automation side, we’ve fully integrated the measurement and calibration process into a seamless flow. The user doesn’t need to push buttons, follow complex steps, or even think about how the system works. It just works. That includes signal quality checks, measurement timing, and even calibration reminders, all managed in the background. It’s designed to be completely lay-user proof, which is essential for OTC use. >>
“On the technology side, we’ve developed a new generation of pulse wave analysis algorithms that simply didn’t exist before. They’re trained and validated on data from over 130,000 users across Europe, giving us a depth and diversity that’s unmatched. These algorithms adapt to real-world conditions far beyond what traditional methods could handle. It’s not just more data; it’s a fundamentally smarter way of interpreting the optical signal.
“That combination — automated user experience and truly adaptive algorithms — is what makes cuffless, OTC blood pressure monitoring finally possible.”
What was the biggest technical challenge in the development of this device/system, and what was the solution?
Solà: “The biggest technical challenge was calibration, specifically how to automate the calibration of a cuffless system without breaking the user experience or relying on external hardware.
“Our solution was to fully integrate an oscillometric cuff into the product itself, not as an accessory, but as part of the system’s core workflow. That allowed us to handle calibration silently in the background, without asking the user to switch devices or follow complicated instructions. It made the entire process seamless, consistent and invisible to the end user, which is essential for OTC use.

“In short, we had to reinvent how calibration works, rethink how to validate it, and build the data foundation to make it real.”
Does that have potential applications for other devices, systems or conditions?
Solà: “Absolutely. While this first FDA clearance is focused specifically on our own over-the-counter blood pressure monitor, the core technology is deviceagnostic. It’s designed to work with any wearable that includes a PPG sensor: smartwatches, rings, patches, and so on.
mitigations ranging from ensuring that the signal is taken when the patient is not moving to incorporating noise filtration in our algorithm. We’ve even ensured that the optical light exposure from the PPG sensor has passed the applicable safety standards.
“But that wasn’t enough on its own. Existing clinical standards weren’t designed for this type of hybrid device. So we had to design new types of clinical studies, going beyond current publications, to demonstrate both accuracy and usability in realworld, unsupervised settings. That meant working closely with the FDA to align on methods that had never been reviewed before.
“The third piece was data. To train the underlying machine learning algorithms, we needed high-quality, diverse, real-world data, not just from lab environments. We’ve now collected data from over 130,000 users across Europe, which has been critical for building and validating a new generation of pulse wave analysis algorithms that can truly adapt to people’s lives, not just test benches.
“The calibration automation, signal processing pipeline, and the AI algorithms we’ve developed are modular. They can be integrated into other platforms and adapted to new use cases, whether that’s longterm cardiovascular risk monitoring, hypertension management, or even early detection of other conditions.
“So while this clearance is just the beginning, the underlying technology is already built to scale across multiple devices and clinical applications.”
What are the safety risks of this device, and how does your design minimize those risks?
Solà: “The amazing aspect of our device is that it was designed to be an inherently low-risk device. It is not required to be sterile and is not an implantable. It’s a simple wearable worn on the user’s wrist, similar to a smart watch. The primary risk is associated with the device providing inaccurate blood pressure values. We have mitigated this through multiple design
“With any electronics device, there is always the risk of electrical shock and EMC interference. This has been mitigated through standard IEC 60601 safety testing.
“Lastly, with any continuously worn device, there is always the risk of skin irritation, which has been mitigated through biocompatibility testing.”
Are you working on the next generation of this technology?
Solà: “Of course, but we cannot disclose this yet. Have a look at our European offering today to understand how far away we are planning to go.”



Johnson
& Johnson MedTech’s Sandeep Makkar discusses customer empathy and determining user needs for device design and development.


know what they really need.

Deep insights for device design and development come from customer empathy, Johnson & Johnson MedTech Worldwide President of Endomechanical & Energy Sandeep Makkar said in a Medical Design & Outsourcing interview. He offered advice for other device developers, including a unique way his team identified an innovation opportunity out of complete silence.
“Most device manufacturers look at a point of care, almost like a transaction within a procedure: an anesthesia machine looking at a point of time, or an energy device looking for a sealing or a transection moment, etc.” he said. “The successful companies or the successful technologies are those that zoom out and look at the workflow. My guidance would be to have empathy for the surgical teams — not just the surgeon but the surgical teams and the
surrounding ecosystem — and looking at the patient more as end-to-end care.”
“You have standard questionnaires, you have usability, then concept and use,” he later continued. “So you have a certain concept — first paper concepts, and then prototypes — then you have them use it … and as the prototypes evolve, you have them give feedback. That’s one element. But that’s after the solution has been identified. One step before, when you’re just trying to think through what solutions would you put in play, sometimes your end user doesn’t know what to ask for. And in this case, sometimes it’s just being in the operating rooms and observing workflows — and there are formal methods to capture that.”
When developing the Harmonic Focus ultrasonic shears for thyroidectomies, his team put heart
rate monitors on surgeons, monitored the audio in their operating rooms and measured the exchange of instruments during the procedures.
“The surgeon may not be able to articulate sometimes, but when we were observing, the devices got near the recurrent laryngeal nerve and the room would go quiet,” he said. “Instruments are being exchanged, the room goes quiet, heart rate goes up. That’s when you know.”
That tension and silence in the room indicated an energy device could add value by minimizing the number of instruments being exchanged and by reducing the risk of injury to that nerve.
“That’s exactly what that device did,” Makkar said. “We created a device that reduced the number of instruments exchanged and reduced the risk for electrical energy transmitting into the nerves, and that device has been very successful for us even decades later now.”
If they had asked what the need was, they might have thought the need was for a better dissector to transect the tissue — and they would have missed the opportunity.
“It’s really reading the workflow of the room, which is why I’m a big believer,” he continued. “… We studied the workflow — [the] entire team’s workflow, then you zoom in to the job to be done so you can create a technology that simplifies the operating room experience end-to-end while delivering on its primary mandate, whether it’s a tissue effect that you desire or a hemostasis solution, or whatever that need is.
“How are you improving workflows through your device? Are you making it simple to use, simple to access, simple to use, simple to dispose? Is your device connected to the other components of the workflow seamlessly? … Our technologies must reduce the cognitive burden for our end user, helping them make better decisions intraoperatively or post-op follow-ups or plan their surgeries better, improve ease of use, learning curves, effectiveness and safety, all of that.
“It’s common sense, but as you get into the design process, sometimes technology takes over,” he continued. “That’s why we keep talking about: Make your technology more human, care more adaptable, and people more connected with your technologies.”

Harness our leading-edge vacuum technology . . . because lives depend on it.


Your high-value medical parts need special treatment. Solar’s leading-edge vacuum heat treating technology produces clean, bright, consistent results. From annealing to age hardening, rest assured knowing your life-critical parts were vacuum heat treated to your exact specs.
For your prosthetics, guide wires, stents, surgical tools, device and battery cases, hypodermics and hypodermic tubing, brazements for analytical devices...and more, trust Solar Atmospheres to provide you with uncompromising quality.












































The Abbott Liberta rechargeable neurostimulator (left) compared to Abbott Infinity neurostimulators Image courtesy of Abbott


Nearly a decade after the FDA approved the Infinity deep brain stimulation (DBS) system, it’s still the smallest nonrechargable device of its kind on the market.
Months after St. Jude Medical won that initial indication for treating Parkinson’s disease and essential tremor — making it the first nonrechargable eight-channel DBS system, the first DBS system with directional leads and the first upgradable DBS platform for movement disorders — Abbott bought the company and its technology in 2017.
Since then, Abbott has added new features such as a smartphone app and won expanded indications to target all the major parts of the brain for treating movement disorders.
The device developer is now using the Infinity system to study DBS as a therapy for treatment-resistant depression, with an eye on potential applications for chronic pain and anxiety-driven disorders like obsessive-compulsive disorder.
How Abbott developed the firstof-its-kind Infinity DBS system
Abbott’s Rebecca Wilkins looks back at the Infinity DBS system’s development and offers advice to help device developers better understand unmet needs and meet them in innovative ways.
Abbott Neuromodulation R&D Divisional VP Rebecca Wilkins has been working with the Infinity system the entire time, joining St. Jude Medical in 2006 as an electrical engineer.
“I’ve always found the technology fascinating. We’re still learning about how the brain and the nervous system work, and being part of a cycle where you’re creating technology that’s advancing that understanding and then creates better technology is a dream job,” she said. “My mom has Parkinson’s and our products, so I have a personal motivation to continue to innovate and help people with motion disorders live full lives, and I think I have a uniquely empathetic view of what our patients go through.”
In a Medical Design & Outsourcing interview, Wilkins discussed the system’s development and the technical challenges of miniaturization, power optimization and industry firsts like the use of Bluetooth Low Energy for smartphone pairing.
“Infinity is a unique story of innovation, because … we were looking to solve a big problem with our motion disorder patients and our pain patients, which is that people hate charging the device,” she said. “It’s very burdensome and hard to do, and if you think about motion disorder patients, sometimes they have mobility issues. We wanted a recharge-free option that reduced that burden.”
Infinity DBS design and engineering
“The trade-off of having a nonrechargeable system is that sometimes they can be bigger, but we had a lot of really good engineering innovation to keep the power consumption down,” Wilkins said. “… We were very, very disciplined about every microamp we were using that wasn’t going to therapy.”
The implants needed custom chips and boards for miniaturization and manufacturability, including a tri-fold board that Wilkins described as (continued on page 35)








CGI Motion standard products are designed with customization in mind. Our team of experts will work with you on selecting the optimal base product and craft a unique solution to help di erentiate your product or application. So when you think customization, think standard CGI assemblies.

Connect with us today to explore what CGI Motion can do for you.









(continued from page 33)
“a flex circuit folded many different ways, kind of an accordion-looking thing.”
“It was a lot of fitting things in, simplifying, making sure we weren’t adding things that we didn’t need — because sometimes if you don’t stay focused, it can start to grow legs a little bit — and working closely with our manufacturing partners,” she said.
In another industry first, Abbott used Bluetooth Low Energy to connect to a patient’s smartphone to let them manage their therapy and let their doctor to remotely adjust the neuromodulation
“Motion disorder patients want discreetness,” Wilkins said. “They may already have some sensitivity around how they’re viewed because of their disease, so being able to adjust their therapy without pulling out something that’s clearly a medical device was top of mind for us.”
Infinity’s directional leads allow for focused stimulation to specific targets in the brain to maximize the therapeutic effects and minimize side effects on a patient’s speech or gait.
“There’s full rings on the ends, and then the middle rings are broken up into three segments so you can partition simulation and pick a number of combinations,” Wilkins said. “You ultimately have eight different contacts on that lead that you can create a field with.”

Abbott
Neuromodulation
R&D Divisional VP Rebecca Wilkins
“We make a lot of decisions around what do we do that’s custom versus what do we use an off-the-shelf product for and why, because you may get a much smaller custom chip, but if you lack the mass to support that or you don’t have the volumes, there’s trade offs,” she continued. “It’s complicated. There’s no perfect answer. It’s always trade-offs. … Those can be tough decisions. As a leader, a lot of the time I spend is understanding the why behind the decision-making.”
Abbott used what it achieved through its Infinity effort to inform the design of its Liberta rechargeable DBS system, making charging less burdensome and less frequent at ten times per year compared to weekly for some competing devices.
With Medtronic winning the first FDA approval for adaptive DBS (aDBS) closed-loop neuromodulation to treat Parkinson’s, we asked whether Abbott is working on aDBS.
“I like to start meetings with,’What are we trying to do here? What’s the problem we’re trying to solve?’ And if I see a lot of confusion or I don’t see a cohesive statement, I know we need to step back and get a more organized about what we’re trying to do and reiterate the importance of the work,” she said. “Because when you’re in the trenches and you’re working on the minutia of something, you forget the big picture. I like to remind people why we’re here, why we’re doing what we’re doing. We could be anywhere. We’re here. There’s a reason why we’re here. Don’t forget that. It’s connection to our purpose.”
When considering the problem, she advised staying away from solutions early in the process, including offering potential solutions to users.
“Spend focused and disciplined time on a problem statement, understanding the problem, prioritizing the problems, and then getting out in the field and talking and viewing,” she said. “We have a lot of opportunities to map out a process flow and see things and then ask questions, but I encourage people not to ask questions that have integrated the solution in the question at first. … If you ask the wrong question, you get the wrong answer back and it can lead the team off. Asking really objective, neutral questions that take away biases is really important. ”
“IT WAS A LOT OF FITTING THINGS IN, SIMPLIFYING, MAKING SURE WE WEREN’T ADDING THINGS THAT WE DIDN’T NEED — BECAUSE SOMETIMES IF YOU DON’T STAY FOCUSED, IT CAN START TO GROW LEGS A LITTLE BIT.”
Power transfer for implantable devices requires optimization of three conflicting factors for performance, convenience and safety: charging range, time and heat.
“There’s things you can do with the energy transfer in terms of the frequency band you select. That might have some optimizations in power transfer, but then you have all these different radio requirements in different geographies you have to consider, especially if it’s a worldwide product,” she said. “You may want to have a large range, but maybe don’t want a huge coil outside the body, so you optimize the coil design with the range. And then usability, how long do you need to charge your device, things like that.”
“We’re interested in understanding how to close the loop and what the right biomarker is, but we want to make sure that it’s solving clinical problems,” Wilkins said. “What we see right now is there’s a lot of interest in local field potential, so we’re watching that, but we want to understand does that bring better clinical benefits, because the cost is now you’re processing data at the implant level, which goes back to size and power consumption and battery life.”
Advice for other medical device professionals Wilkins urged device designers and engineers to “go solve the biggest problems that haven’t been solved yet.”
And remember that those patients aren’t always like you or your medtech colleagues.
“Think about how you’re asking questions to a really diverse candidate pool that represents your patient population,” she said. “They might not have the same technical capabilities, they might not have different things that we take for granted. Getting users in to walk through and live their lives so we can understand their lives through their eyes is super important.
“Anytime we think about a solution, thinking about how we’re increasing access to care — what are we doing that’s making this more available to everyone — is hugely important for us,” she continued. ” … At Abbott, we’re really focused on solving these important patient needs. We love being industry first at things, and you’re going to keep seeing some really interesting innovation come out from us.”
From components and finished devices to the next generation of medical technologies, now more than ever, you can count on us.



































































































Strengthen your innovation with our rapid prototyping and clinical know-how to take you seamlessly from concept to production





Get to market faster by utilizing our platform technologies and market-ready products that provide differentiated performance and portfolio expansion
Extend your reach through our global R&D and manufacturing capabilities to consistently achieve design requirements, quality standards, and on-time delivery
















































limited their access to consults and tools, and the number of submissions per reviewer has nearly doubled. Submissions without a clear narrative or organized structure make it harder for reviewers to engage.
The result? Vague feedback, delays, and missed opportunities. Wellstructured, reviewer-friendly submissions with clear goals have a clear advantage: enabling reviewers to respond effectively and build trust.
Start with the questions, not the data Many companies compile data without first asking what they want to learn from the FDA. This often produces irrelevant paperwork and limited feedback.
Focused submissions flip the script: What decisions are we trying to derisk? What do we need from the FDA? Purpose matters more than volume. The FDA’s 2025 Q-sub guidance emphasizes a question-driven structure and encourages sponsors to work backward from the desired feedback. A submission built around clear questions will more likely yield helpful instruction.
Two ways submissions miss the mark Submissions often fail due to too little detail, which causes disengagement or vague feedback like, “can’t offer an opinion,” and may paint an inaccurate picture about the product to reviewers.
The other extreme is including everything: irrelevant data, full QMS, or cybersecurity plans that do not address the regulatory question of whether the product is safe, effective and similar to its predicate. Data dumps obscure what matters and increase the risk of irrelevant feedback.
A strong pre-submission is a strategic tool. When reviewers are stretched thin, submission quality shapes the experience.
The FDA’s 2023 guidance on realworld data is clear: it must be relevant, reliable, and directly tied to the question.
The description is not marketing
Product descriptions are one of the most misunderstood sections of a submission and a common source of irrelevant or insufficient information. Many companies copy investor or sales materials, but what the FDA really needs is a detailed, functional walkthrough of how the product works, who uses it, and each step in its use.
A strong product description lays the foundation for meaningful regulatory conversation. For the FDA to comment on regulations, reviewers must understand what the product is. A vague summary won’t cut it. A well-written description can also be reused for future pre-submissions and market clearance processes if the product remains unchanged over time.
Say what you mean
Overly technical language slows and complicates reviews. Dense submissions may push reviewers to rely on AI tools to parse them. Plain language supports efficient, AI-assisted review, helping reviewers use tools they already have. Plain language isn’t dumbing it down. It’s smart regulatory design.
Clarity is also about telling a story. Build a case that’s easy to follow, making it simple for reviewers to draw on every piece of information needed to answer your question.
De-risk your submission
Several tools can help mitigate risk in premarket submissions. Informational Q-sub meetings are helpful for novel tech, allowing informal input before full submission and clarifying FDA priorities.
Predetermined change control plans (PCCPs) are valuable for AIenabled devices. Rather than rolling future changes into a 510(k) and risking rejection, sponsors can outline their evolution plan and seek early feedback. With few cleared PCCPs, work with the FDA before including one in a submission. For the right product, they can reduce future delays and submission costs.
The FDA’s new Regulatory Accelerator offers resources for sponsors, including guidance about requesting an early orientation meeting during a premarket submission. These meetings let sponsors demonstrate their product to assigned reviewers and answer early questions. Understanding a product g is central to success, so sponsors should take every opportunity to help reviewers understand.
Avoid the ‘I can’t comment’ trap Few things are more frustrating than waiting 70 days for FDA feedback only to hear, “I am not able to comment at this time.”
This usually results from unclear questions or poor context, as the FDA can only respond to what’s clearly asked and supported. If the product description is vague or the regulatory question poorly framed, reviewers are limited in their response. Once written feedback is issued, the follow-up meeting can only address those same topics, leaving little room to reframe and get a full answer.
A strong pre-submission is a strategic tool. When reviewers are stretched thin, submission quality shapes the experience. Thoughtfully structured documents signal regulatory fluency and help the FDA work efficiently, eliciting more valuable feedback, higher success rates, stronger trust and a smoother experience for everyone.










Cyberattackers are well aware of medtech software weaknesses. Are you?

Healthcare cybersecurity conversations typically focus on hospital IT networks and patient data protection. What’s less talked about is the risks in the software powering medical devices themselves.
According to a 2025 report from Claroty, 93% of healthcare organizations have confirmed known exploited vulnerabilities (KEVs) in their Internet of Medical Things (IoMT) devices. The FDA’s recent requirements on postmarket cybersecurity and the growing role of software bill of materials (SBOMs) signal that regulators are increasingly aware of these issues and are making a push for greater software security.
What are ways to improve device security and address widespread problems? The following three risks in medical device software are a strong place to start.
Risk 1: IoMT devices and insecure internet connectivity
IoMT has reshaped healthcare delivery with improved operational efficiencies and capabilities for patient care. However, insecure internet connectivity is a risk to everything from imaging systems to patient devices.
Major weak spots for IoMT devices are the vulnerabilities and connectivity issues in underlying operating systems, particularly for devices running on legacy Windows and Linux platforms.
We saw this clearly with URGENT/11, a set of 11 critical vulnerabilities in the TCP/IP stack (IPnet) of VxWorks, a real-time operating system (RTOS) deployed in over 2 billion embedded devices, including MRI machines, infusion pumps, and patient monitors. The vulnerabilities stemmed from memory corruption flaws that allowed remote code execution, as well as vulnerabilities that led to information leaks or logic flaws. By
exploiting these vulnerabilities, attackers could potentially manipulate infusion pump dosing, falsify emergency alerts on monitors, or otherwise manipulate devices remotely.
URGENT/11 highlights how connectivity risks can turn into widespread problems and allow attackers to move laterally across healthcare networks.
While third-party components and open source code offer efficiency advantages for development teams, they introduce risks to medical device software that require proactive management. Ultimately, device developers and manufacturers are responsible for all aspects of their software, including components that come from elsewhere. SBOMs, particularly those generated at build time, provide visibility into the software supply chain, including any open source code or vendor-supplied components. SBOMs allow for early identification of vulnerabilities and provide a comprehensive list of issues that need mitigation to meet FDA submission timelines. SBOMs also support postmarket security efforts by providing immediate access to a complete component inventory when vulnerabilities are discovered down the line.
Importantly for development teams, SBOMs reveal common, recurring vulnerabilities in software, allowing organizations to address them systematically through secure coding practices or specialized security solutions focused on areas of greatest risk.
Perhaps the most persistent challenge in medical device security is patching vulnerabilities in deployed devices. (continued on page 41)













(continued from page 39)
Patching medical devices is timeconsuming and difficult for many reasons. Not patching, however, leaves devices exposed.
Forescout Vedere Labs found that 32% of DICOM workstations have critical unpatched vulnerabilities, while 26% of pump controllers have critical unpatched vulnerabilities, with 20% having extreme exploitability.
As seen in the URGENT/11 example, unpatched vulnerabilities can have farreaching consequences across healthcare environments. One significant way to reduce risk is by focusing on removing entire classes of vulnerabilities from device software. For example, memorybased vulnerabilities are common in medical devices and software written in C/C++. By embedding runtime security solutions into the software development process, manufacturers can effectively eliminate attackers’ ability to exploit memory-based flaws, even in devices that cannot be easily updated.
Next steps for device security
Medical device software issues are a risk to patient health and safety. The risks outlined above also directly affect product development, regulatory approval processes, and overall healthcare security.
To address challenges, there are several areas to consider:
• Enhanced device-level security protocols: Incorporate authentication measures, encrypted communications, and runtime protection directly into development processes.
• Secure software supply chain practices: Implement rigorous vendor assessments and open source component review processes.
• Comprehensive SBOM monitoring: Integrate SBOM generation and analysis into the software development lifecycle to catch vulnerabilities before they reach production and to align with FDA expectations.
• Address entire classes of vulnerabilities: Consider security solutions that reduce software risk by eliminating the ability to exploit common vulnerabilities across codebases.
Attackers are well aware of the software weaknesses in medical devices. By pinpointing the greatest areas of risk, device developers and manufacturers can take the upper hand and protect products and the patients who depend on them.
Joe Saunders is the founder and CEO of RunSafe Security, a pioneer in cyberhardening technology for embedded systems across critical infrastructure. Saunders has built and scaled technology for both private and public sector security needs in his 25 years in national security and cybersecurity, and aims to transform the field by challenging outdated assumptions and disrupting hacker economics.
















GE HealthCare Chief AI Officer
Parminder Bhatia


CHIEF AI OFFICER PARMINDER BHATIA DISCUSSES GE HEALTHCARE’S VISION FOR ARTIFICIAL INTELLIGENCE IN MEDTECH AND WHAT’S NEEDED TO GET THERE.
BY JIM HAMMERAND MANAGING EDITOR
GEHealthCare has had more AI-enabled medical devices on the FDA’s list of marketing authorizations than anyone else for four straight years.
GE HealthCare now has 100 products on the agency’s list, which the agency says is nonexhaustive but includes devices with 510(k) clearances, De Novo classifications and premarket approvals.
GE HealthCare — the world’s sixthlargest medical device company according to Medical Design & Outsourcing‘s 2025 Medtech Big 100 ranking by revenue — says it intends to have 200 AI-powered authorizations by 2028.

To help other device developers make the most of the artificial intelligence opportunity, we asked GE HealthCare Chief AI Officer Parminder Bhatia to share how his company is using AI and what he and his team have learned.
The following has been lightly edited for clarity and space.
MDO: The term AI is being thrown around a lot right now from algorithms to LLMs, so can you share how you define artificial intelligence, what specific kinds of AI you think have the most potential in medtech, and which do you think have the least potential in medtech?
Bhatia: “That’s a great question, because not all AI is created equal. It’s important to be precise about what kind of AI a technology is using and what problem it’s solving. The reality is that AI has been used in healthcare for years with proven success. A prime example is imaging, where deep learning tools help care teams capture clearer images more efficiently and support more confident diagnostics. Our AIR Recon DL technology, for instance, has enabled radiologists to achieve sharper images faster. It has been estimated that more than 50 million patients have been scanned since its launch in 2020. >>

“What’s different today is the emergence of generative AI and foundation models. We see enormous potential here because most of healthcare’s data is unstructured, ranging from medical images and clinical notes to audio recordings and device signals. Traditional analytics and narrow machine-learning approaches struggle to make sense of this diversity. Generative and multimodal AI models are uniquely capable of integrating across these data types, opening new possibilities for workflow automation, decision support, and personalized care. That’s why GE HealthCare is investing in healthcare-specific foundation models and pioneering solutions that embed generative and agentic AI capabilities.
“On the flip side, the kinds of AI that are least impactful in medtech are those that remain siloed or brittle, tools that cannot adapt across modalities, lack explainability, or don’t meaningfully fit into the clinical workflow. In healthcare, AI has to be more than a proof of concept. It has to be scalable, safe, and trustworthy to deliver real impact.”
What’s GE HealthCare’s vision for AI in medtech?
Bhatia: “AI-enabled solutions have the potential to address the toughest challenges our customers are facing, including care team shortages and burnout, rising costs and inefficient workflows.
“Our goal is to transform these challenges into opportunities for faster, more accessible, and more personalized care. In practice that means using AI not just as an add-on, but as an enabler of systemic change. For example, in maternal and infant care, our Centricity Perinatal Software and the Mural Clinical Intelligence Suite already help clinicians monitor mothers and babies in near real-time by integrating fetal strips, EMR data and decision support into a single view. We are exploring how AI could help in this context to reduce the documentation burden and flag risk earlier with the goal of ultimately giving time back to clinicians to focus more on the mother and child.
“With generative and multimodal AI, we can unlock insights from the 97% of healthcare data that currently goes unused. With agentic AI, we see the potential to create proactive systems that collaborate like virtual care teams, anticipating patient needs instead of simply reacting to them. And with Responsible AI principles guiding us — safety, fairness, explainability — we are designing these solutions to be trustworthy and clinically meaningful.
“This matters because the stakes are global. Nearly 4.5 billion people still lack access to essential health services. By making care smarter, more scalable, and more precise, AI can help bridge this gap and bring the benefits of modern medicine to more people, everywhere.”

“OUR GOAL IS TO TRANSFORM THESE CHALLENGES INTO OPPORTUNITIES FOR FASTER, MORE ACCESSIBLE, AND MORE PERSONALIZED CARE. IN PRACTICE THAT MEANS USING AI NOT JUST AS AN ADD-ON, BUT AS AN ENABLER OF SYSTEMIC CHANGE.”
What do you need to get there in terms of infrastructure and nextgeneration tech like better imaging, for example, or components like sensors?
Bhatia: “Healthcare-specific foundation models, which will be fundamental to developing generative AI-powered solutions, are going to be instrumental to helping the industry take a big leap forward. That’s because these models adeptly handle multi-modal data, from images to clinical records to EKG traces to sequenced genes, to power applications that are aiming at improving imaging accuracy or guidance, providing better visual diagnostics, and automating clinical workflows from screening, to diagnosis, to treatment, to monitoring.
“We have been investing in this area and are developing pioneering research including industry-first research foundation models, for example

SonoSAM Track for ultrasound, full-body 3D MRI, and full-body X-ray.
“Additionally, based on what we’ve seen published, GE HealthCare is the first to investigate how multi-agentic AI can be applied to the challenges facing the healthcare industry with Project Health Companion, where multiple AI agents are designed to collaborate much like a tumor board to synthesize complex data and proactively generate recommendations.
“And because infrastructure is more than algorithms, we’re partnering with Amazon Web Services (AWS), Nvidia, and leading academic centers to combine sensors, imaging hardware, cloud, and compute at scale. This is how we turn vision into reality.”
How do you think about outsourcing versus developing in-house expertise?
Bhatia: “We take a mix of both, and lean on external vendors to give us scale and expertise that doesn’t make sense for us to build-up in-house. We are pioneering academic research with leading institutions including Mass General Brigham, Vanderbilt University, and the University of California San Francisco (UCSF). Our collaboration with AWS is designed to help GE HealthCare accelerate and scale our development of purpose-built foundation models and speed the development of cloud- and AI-enabled healthcare solutions. We are also collaborating with Nvidia on the development of pioneering innovation
FAR LEFT: GE HealthCare says its Mural Clinical Intelligence Suite “aggregates near realtime data from multiple systems and devices and conveniently displays it on one screen.”
LEFT: This image compares a conventional MRI scan (left) to a AIR Recon DL scan.
Photo courtesy of GE HealthCare
in autonomous imaging, beginning with autonomous X-ray technologies and autonomous applications within ultrasound.
“We’re also working with the Bill & Melinda Gates Foundation to use AI for maternal and fetal health, showing how partnerships can extend the reach of innovation to underserved communities globally.
“The way I see it, it’s not an ‘either/ or’ choice. It’s about working backward from the customer need, deciding what we must own to differentiate, and then collaborating to scale the rest. That’s how we move faster, responsibly, and with greater impact for patients.”
What are regulators looking for when reviewing AI in medtech, and do you have any unique advice to help device developers meet the bar?
Bhatia: “Regulators play an important role, and we share their focus on patient safety. That’s why we incorporate our Responsible AI principles at every stage of our product development, which include a focus on safety, validity, transparency, explainability, and fairness. Be intentional at every step in layering in test, checks, and safeguards. You can’t retrofit safety. It has to be there from the start.”
How do you expect regulations around medtech AI will change in the coming years?
Bhatia: “Oversight is essential, and I expect we’ll see regulations around medtech AI continue to be honed over the coming years. Regulators are already working closely with industry experts to fully understand both the opportunities and the complexities that come with technologies like generative and agentic AI. As part of that, I expect that we’ll see more emphasis on transparency, explainability, and lifecycle monitoring, >>
GE HealthCare says its Centricity Perinatal Software “facilitates real-time fetal strip analysis, helping streamline communication and decision-making with key features including data viewing, annotation review, and seamless HIS/EMR integration.”
Photo courtesy of GE HealthCare
















Let Argon Medical Custom Product Solutions team up with you today.
as well as mechanisms for continuous validation in real-world use. At GE HealthCare, we welcome that direction because it aligns with how we already work. Our Responsible AI principles — safety, validity, transparency, explainability, and fairness — are built in from the very beginning of product development. That’s how we ensure our innovations not only meet today’s requirements but are prepared for the regulatory expectations of tomorrow.”
How can medical device developers build trust in AI?
Bhatia: “There are two big things. The first is making sure the solutions are safe and effective, built with responsible AI principles in mind. The second is education, and ensuring care teams understand how the solutions work, including usage but also explainability and the safeguards.”
Do you have any mantras or mottos or often repeated phrases you use with your teams or partners when talking about AI and digital tech?
Bhatia: “There are a few guiding phrases I repeat often. One is: ‘Start with the problem, not the technology.’ At GE HealthCare, we work backward from the toughest challenges our customers face, whether it’s reducing clinician burnout, improving workflow efficiency, or expanding access to care. Technology is a means to an end, not the end itself.
“Another is: ‘This is healthcare’s iPhone moment.’ Just as the smartphone unlocked entirely new ways of interacting with technology, I believe foundation models and agentic AI will redefine how care teams use data, making care more proactive, personalized, and accessible.
“For example, access is one of the biggest global challenges. With handheld devices like our Vscan Air combined with Caption AI, a nurse in a rural clinic can capture diagnostic-quality ultrasound images without waiting for a specialist. That’s what it means to start with the customer problem — limited access to expertise — and then use technology as the enabler to close that gap.”
What have you learned about AI that might help other device developers innovate or succeed with their own projects?
Bhatia: “One of the most important lessons I’ve learned about AI is that innovation takes time, persistence, and iteration. Early in my career, during graduate school, I worked on developing advanced MRI technology. At the time, it felt like we were building something far ahead of where the industry was, but that experience taught me that groundbreaking ideas often take years before they find the right moment and infrastructure to scale.
“That perspective is very relevant to AI today. Device developers should know that success isn’t about building the flashiest algorithm. It’s about creating something clinically meaningful, validating it rigorously and being patient enough to see it through.
“I’ve also learned the importance of collaboration. The MRI work I did was not in isolation. It was part of a larger community of researchers, clinicians, and engineers. The same holds true with AI in healthcare. No single person or company has all the answers, but when we bring together expertise across academia, industry, and clinical practice, that’s when real breakthroughs happen.”
Medtronic‘s study of Linq implantable cardiac monitor (ICM) devices in atrial fibrillation (AFib) patients offers important lessons for other device developers, says the cardiac electrophysiologist who chaired the Define AFib clinical study steering committee.
MDO: What’s the significance of the Define AFib study for patients and for the technology?
Piccini: “There are a lot of different ways you can think about Define AFib. On one hand, you could say it’s a study about implantable loop recorders and the information they
routine and not under the auspices or in the settings of dedicated research visits where we intermittently collect information. Define AFib was very unusual in that patients who got one of these implantable loop recorders as part of their regular clinical care were invited to participate, and once they

BY JIM HAMMERAND MANAGING EDITOR

In this case, they were in a brick-andmortar facility for that original implant, but everything after that was really all remote. As a result it had as minimal a burden as possible on patients. More than that, the study was designed largely through patient feedback. The study team reached out to patients and said, ‘You have this implantable monitor. What type of information do you find helpful for managing your illness?’ The entire app that the patient had displayed information in a very patient-centered way.”
What about this study made it different than more traditional clinical trials?
Piccini: “The concept was wearables are becoming more and more common. They’re collecting a significant amount of information on patient health. Smartphones are becoming more and more common. They too are collecting lots of information, not just necessarily health information, but also information like someone’s location. So if someone goes into a healthcare facility, does that mean they’ve been hospitalized? There’s so many different pieces of innovation. One was making sure that patients were informed and that when they agreed to participate, they understood what information the study was going to use to help us understand the disease better.
“WE OFTEN HEAR CONCERNS ABOUT INFORMATION OVERLOAD, BUT BY AND LARGE THE FEEDBACK WE RECEIVED WAS THE ADDITIONAL INFORMATION WAS HELPFUL AND NOT OVERLY BURDENSOME.”
For example, part of this geofencing was that if a patient provided permission and their phone was in a healthcare facility for a certain period of time, that launched a query to see if the patient had been hospitalized. There were quality of life surveys that if a patient’s device picked up atrial fibrillation at a certain interval, it would send a patient a questionnaire that assessed how their quality of life activity levels were, those types of things. There’s so many things we learned, and it would be hard to go through all of them, but some of the highlights are that we definitely did see

We often hear concerns about information overload, but by and large the feedback we received was the additional information was helpful and not overly burdensome.”
What was the major takeaway for AFib from this study?
Piccini: “The main scientific finding presented at the AF Symposium is when you take basic information about someone’s atrial fibrillation combined with information about their medical conditions — do they have heart failure, do they have diabetes, do they have high blood pressure — and you combine that with continuous information about their heart rhythm and how often they’re in atrial fibrillation, how long the episodes of atrial fibrillation are, all of that information together, you can begin to be able to predict how likely someone is to need care for their atrial fibrillation. Why is that important? When they need care, won’t they just show up for it? That’s one view you could take. But a much better way to deliver healthcare is to make sure the right treatment gets to the right patient at the right time. So it would be much better if we can anticipate that someone’s going to need a change in their medication so we can avoid them needing to come in for an urgent last-minute clinic visit or avoid them going to the emergency room or being hospitalized.”
Medtronic’s Linq II implantable cardiac monitor (featuring AccuRhythm artificial algorithms) is for long-term heart monitoring. Image courtesy of Medtronic

and then asks you to answer some questions for the study, are you really going to do it? We were very pleasantly surprised. Our survey response rates were very high throughout the study. We think that’s a reflection of the fact that these are patients who really wanted to help improve our knowledge of atrial fibrillation so in the future we get even better at treating the illness. That was one pleasant surprise. I wouldn’t say it was a disappointment, but something I think we need to continue to work on is that the vast majority of individuals have a smart device, but they may not have the specific smart device that a given study is using. In the future we need to focus on interoperability so no matter what type of device someone has, they can still participate in the study. And it is true that remote studies still have barriers. If you have a hard time navigating electronic enrollment or setting up things on your phone, those can be barriers in these types of studies. No matter what type of information you’re collecting, you still want to be able to validate things in individuals medical records. If a patient says they have this condition or that condition or they went to the emergency room, you want to be able to verify that and the technologies we have to do that are

still very labor intensive. Having ways to quickly validate things electronically is something else we’re going to need to pay close attention to in the future.”
How did you achieve such a high response rate?
Piccini: “There’s three reasons. The first is we really leaned heavily into patient engagement. The study team aggressively sought out patient opinion, asking, ‘Will this be burdensome? Do you think your peers will react favorably to this?’ There was a lot of engagement to get advice from patients in the very beginning. The second reason is we used validated surveys that are designed to collect the maximal amount of information while avoiding survey fatigue to every extent possible. And then the third reason is — and this is something we often don’t talk about — these are patients who said, ‘I want to help.’ They were highly motivated to make sure the study could learn as much about atrial fibrillation as possible. And we have just an incredible amount of data, thousands and thousands and thousands of observations and data points from the study participants that we hope is going to continue to contribute to the body of knowledge around AFib for a very long time to come.”





























BY JIM HAMMERAND MANAGING EDITOR
Pulsed-field ablation (PFA) is a nonthermal therapy for atrial fibrillation that’s red-hot right now thanks to its ability to kill targeted heart tissue without damaging nearby nerve cells.
But there’s still much to learn about PFA as device developers move past the first generation of this high-voltage technology, refining the systems and addressing safety risks.
We spoke with Dr. Brad Sutton, the chief medical officer of Boston Scientific’s Atrial Solutions Business, to learn more about PFA system safety, including coronary spasm, hemolysis and microbubbles.
Go to wtwh.me/sutton for more from this interview, including a first look at the next-gen Faraflex catheter, why he thinks Boston Scientific has a PFA advantage, how he thinks about minimally invasive medtech, and advice he shared for other device developers.
The following has been lightly edited for clarity and space.
MDO: What are the biggest safety issues with PFA?

CORONARY SPASM, HEMOLYSIS, MICROBUBBLES, TISSUE CONTACT AND FORCE DURING PULSED-FIELD ABLATION PROCEDURES.

Sutton: “You’ve got a high output electrical field with PFA. If you’re in close proximity to a coronary artery, it can cause reactive spasm. Coronary spasm is one risk we’ve been addressing. The way we’ve mitigated that risk is if we know we’re anatomically approaching an area near a coronary artery, let’s say … in the right side of the heart for atrial flutter, we give prophylactic nitroglycerin. We’ve identified and developed a protocol and validated that now in Advantage Phase 2. We used our Farapoint bipolar, biphasic PFA catheter for adjunctive cavo-tricuspid isthmus (CTI) line for atrial flutter ablations, and that was done with prescriptive nitro prophylaxis protocol. All PFA, I think, has some inherent spasm risk, and it probably varies depending on how much energy you’re putting out there, how close you are to the coronary, etc. The other concern that people talk a lot about and we’re learning more about is hemolysis. >>
PHOTO ON PAGE 51: Boston
Scientific’s Farapulse PFA system includes the nitinol-enabled Farawave ablation catheter, which expands inside a patient and takes the shape of a flower (shown) or basket for energy delivery. Image courtesy of Boston Scientific

This breakdown of red blood cell risk seems to be a function, again, of how much energy, how much surface area of electrodes is exposed to the blood pool and not in tissue contact, and how many times you hit it. … People came out of the gate saying, ‘PFA is the safest thing we’ve ever seen. You can ablate with impunity.’ And there was this early tendency to do more in some places, some centers, some operators. When you do two or three times the recommended dose, the hemolysis risk does go up. I think it’s rare that it’s clinically meaningful, but it happens occasionally, and in those scenarios where you feel like you need to do a bit more work, we’ve rallied around this idea of hydration through IV fluids to flush out some of the hemoglobin and mitigate the risk of kidney damage. We’ve seen that with every system to varying degrees, and the risk seems to go up as you do more and more and more ablation.”
Can you explain what microbubbles are, how they form and how to avoid them — and even if we need to worry about them?
Sutton: “There’s a bit we don’t know yet. Microbubbles are tiny bubbles that form when you deliver the energy. You can see them on intracardiac ultrasound as this burst of bubbles. That’s another one where the systems are very different. Some have a lot, some have a little. What do they mean?
Are they clinically meaningful? You’ve seen people do transcranial dopplers and brain MRIs to try to understand what the clinical implications are. For the most part, they don’t seem to be associated with adverse clinical events. There probably is a threshold, though, above which you’re more worried, and we don’t know enough to quantify that. Our commercial iteration of Farawave and the waveform we’re using has an essentially unnoticeable amount of microbubbles. I think that’s very different, depending on which PFA system you’re talking about. And they’re not all the same there. Do we need to avoid them completely? I don’t know the answer to that. I think everybody would say it’s good if we can avoid them completely, but I don’t know how much net clinical benefit there is from having zero versus a little bit or a lot.”
How much does tissue contact matter in PFA, and what about contact force?
Sutton: “Contact matters for a couple reasons. One, you need good tissue coupling to deliver the energy effectively to the tissue itself. But if you don’t have good contact, you’ve got blood pool on either surface of the catheter and the electrodes, and that can increase the risk of things like homolysis. So contact is important from a safety standpoint and from an efficacy standpoint. There’s a lot of ways you can get contact. You can validate contact through impedance

“CONTACT IS IMPORTANT FROM A SAFETY STANDPOINT AND FROM AN EFFICACY STANDPOINT. THERE’S A LOT OF WAYS YOU CAN GET CONTACT. YOU CAN VALIDATE CONTACT THROUGH IMPEDANCE TRACKING OR MAGNETIC TRACKING OF YOUR CATHETER IF IT’S FULLY INTEGRATED INTO YOUR MAPPING SYSTEM.”
tracking or magnetic tracking of your catheter if it’s fully integrated into your mapping system. You can validate contact with intracardiac ultrasound and literally watch the contact happen on ultrasound, which is an easy way to do it. This notion of force comes from the radiofrequency point catheter days. It was this idea that if you could integrate a force sensor and identify grams of force, you’d know you have good contact and avoid perforation risk. The data really never supported the fact that it improved safety, but it became a pretty standard of care approach, and so contact force for point RF catheters was table stakes. The catheters that we’re using right now are not point catheters. Faraflex is not a point catheter. Farawave’s not a point catheter. Neither is Varipulse or PulseSelect or Omnypulse or Affera. They’re just different form factors, I think less prone to perforation than a point catheter would be. So there’s no good data that says force is necessary here. If you have a point catheter form factor, is force going to deform the tissue? You probably get a bit more surface area of the tissue around the catheter, and that may ultimately make a deeper lesion. We’ve shown that you don’t need force to get very deep lesions with our PFA point catheter. So contact, yes. Force, not necessary. Nice to have in a point catheter, but I think largely unnecessary in a wide focal or these other form factors.”
DO PFA MICROBUBBLES MATTER? WE ASKED ABBOTT’S
BY JIM HAMMERAND MANAGING EDITOR
As electrophysiologists increasingly adopt pulsed-field ablation (PFA) devices to treat atrial fibrillation, the microbubbles that can be created during these minimally invasive catheter procedures remain a bit of a mystery.
Are they harmful? Do we need to eliminate them, reduce them — or do we need to worry about them at all?
“That’s the $10,000 question,” said Dr. Christopher Piorkowski, the chief medical officer of Abbott’s electrophysiology division, during an interview with Medical Design & Outsourcing in which he discussed the Volt PFA system’s development.
Microbubbles aren’t a new phenomenon. Radiofrequency ablation can also cause microbubbles, and they can be seen using intracardiac echocardiography (ICE) during RF ablation and PFA procedures alike.

“It’s the amount of microbubbles that matters, and what is even more important are questions on potential clinical implications,” Piorkowski said. “And I think on that, the books are not scientifically closed.
“Everybody is talking about silent cerebral emboli, and with first-generation PFA technologies, there are some indicators that silent cerebral emboli on MRI scans can be pretty frequent,” he continued. “And when you see these publications, there’s always the discussion of where does it come from, are those microbubbles or is this potentially air that is being drawn through the sheath based on sheath management, so nothing related to the PFA delivery itself? That is never really settled.”
Silent cerebral emboli are a potential outcome of microbubbles, but it’s not yet clear whether there are risks of other adverse events like thromboembolisms. >>

Abbott
Electrophysiology Chief Medical Officer and Division VP Dr. Christopher Piorkowski



Superior Quality, Experience and Integrity You Can Rely On

We work alongside you to transform your innovative medical device ideas into successful products. Some say partners, we say co-creators. With Surface Solutions Group, you get fast, on-time delivery, consistent and repeatable quality, and unequaled customer service and collaboration.


“What I can say for our development, it goes back to the testing required by regulatory bodies that we needed to comply to and that we needed to fulfill,” Piorkowski said. “We tested to show that the microbubble creation that we see with Volt in terms of number of microbubbles but also in terms of volume of microbubbles is not inferior to established RF technologies. And I think that’s a pretty strong message in terms of safety that we have for the device today.”
Microbubble formation is influenced by a PFA system’s waveform, he said. A PFA waveform can be thought of as a device developer’s secret recipe for the high-voltage energy their system delivers, with ingredients including varying phases, frequencies, durations, repetitions and pauses.



“That was very surprising to me … how much the microbubble performance changes depending on the waveform,” Piorkowski said.
Waveform also influences char formation during PFA. Char is when coagulum forms on an ablation catheter electrode from burned blood or tissue.
“If you are on the left side of the heart, such coagulum has a risk to embolize into the brain, into the kidney, to make strokes or other adverse events on the arterial side of the circulation,” Piorkowski said.
That risk led to the development of catheter irrigation for RF ablation to flush the electrodes and mitigate char formation.
“In the PFA world, that risk also needs to be mitigated, and it needs to be mitigated through — again — waveform design and it needs to be mitigated through the way the PFA energy is delivered and the catheter itself is designed,” Piorkowski said. “There are many factors … that influence this risk of char formation. It’s a question how the electrode is designed, how the field expands around the electrode, field densities, etc. All of these topics need to go into the engineering approach.”



COMPLETE MAILING ADDRESS OF KNOWN OFFICE OF PUBLICATION WTWH Media, 1111 Superior Ave, Suite 1120, Cleveland, OH 44114
COMPLETE MAILING ADDRESS OF PUBLISHER’S HEADQUARTERS
WTWH Media, 1111 Superior Ave, Suite 1120, Cleveland, OH 44114
FULL NAMES AND COMPLETE MAILING ADDRESSES OF PUBLISHER, EDITOR, AND MANAGING EDITOR
PUBLISHER: Courtney Nagle, WTWH Media, 1111 Superior Ave, Suite 1120, Cleveland, OH 44114
EDITOR: Chris Newmarker, WTWH Media, 1111 Superior Ave, Suite 1120, Cleveland, OH 44114
MANAGING EDITOR: Jim Hammerand, WTWH Media, 1111 Superior Ave, Suite 1120, Cleveland, OH 44114
OWNER COMPLETE MAILING ADDRESS
WTWH Media, LLC, 1111 Superior Ave, Suite 1120, Cleveland, OH 44114
Mountaingate Capital, 1225 17th St, Suite 2575, Denver, CO 80202 KNOWN BONDHOLDERS, MORTGAGEES, AND OTHER
OF TOTAL AMOUNT OF BONDS, MORTGAGES, OR OTHER SECURITIES
Statement of
Ken Gradman (signed), CFO Filed on: 9/22/25



















