Urinary Bladder Matrix [UBM] Technology
How Does Cytal® Wound Matrix Work?
Wound Matrix supports wound healing and tissue deposition by providing a temporary collagen scaffold that facilitates cellular infiltration, supports angiogenesis1,3-5 and promotes rapid revascularization6-9
Provides a structural scaffold: Cytal® Wound Matrix stems from porcine UBM, which is rich in collagen – a key structural protein in the human body. When applied to a wound, this threedimensional scaffold acts as a temporary framework where cells can adhere to the collagen VII of the matrix, migrate through the open, porous structure, and support the deposition of new tissue3,10,11
Supports a shift in the inflammatory response: Cytal® Wound Matrix provides a solution that enables a transition in wound environment from a pro-inflammatory state towards a proremodeling state1,2,12 to facilitate the formation of site-appropriate tissue.
Mitigates the complexity of reconstructive surgery:6,7 Cytal® Wound Matrix facilitates rapid primary closure,6-8 may be used over exposed avascular structures6-8 and may support limb salvage6,8,13.
In contaminated wounds that have been cleaned and/or debrided, Cytal® Wound Matrix products have facilitated successful wound management14-16
Figure 1: Cytal® Wound Matrix
Extracellular Matrix (ECM) Products
Cytal® Wound Matrix
Urinary Bladder Matrix [UBM] Technology
What Types of Wounds are Suitable?
Cytal® Wound Matrix is indicated for the management of wounds including: partial and full-thickness wounds, pressure ulcers, venous ulcers, diabetic ulcers, chronic vascular ulcers, tunneled/undermined wounds, surgical wounds (donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence), trauma wounds (abrasions, lacerations, second-degree burns, skin tears), and draining wounds.
Warnings, Precautions, Storage, Complications
■ Chronic Wound Management
Cytal® Wound Matrix has been shown to be effective in the treatment of chronic wounds, such as diabetic foot ulcers and venous leg ulcers. These wounds often fail to heal due to underlying conditions like poor blood circulation and chronic inflammation. Cytal® Wound Matrix allows for rapid progression of the wound healing process by providing a scaffold for cellular infiltration and tissue deposition10
■ Acute Wound Healing
Cytal® Wound Matrix can be used in the management of acute wounds such as surgical wounds, traumatic injuries (abrasions, lacerations, second-degree burns, and skin tears), donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence. Its ability to support quick tissue deposition makes it ideal for situations where quick wound closure is desired.
■ Reconstructive Surgery
In reconstructive surgery, Cytal® Wound Matrix is used to support the healing of soft tissue defects and to facilitate the integration of grafts. It can be used in conjunction with other collagen-based devices, such as Integra’s MicroMatrix® Dermal Repair Powder, to support wound closure in complex reconstructive procedures.
■ Irregular wound beds
In conjunction with Integra’s MicroMatrix® Dermal Repair Powder it helps to address contour challenges (e.g. uneven, tunneled, undermined wounds).
■ Exposure to contaminated or infected field can lead to rapid breakdown of device.
■ If active infection is present, treat patient to resolve infection prior to device application.
■ Do not use if cracked, broken, or otherwise damaged.
■ Once package is opened, do not reseal. Delayed use of remnant or resealed material could increase the likelihood of exposure to excess moisture or product contamination, increasing the risk of adverse reaction or infection.
■ Always use aseptic technique when handling device.
■ Complications and reactions are possible with any soft tissue repair, including but not limited to: infection, increased chronic inflammation, allergic reaction, unexplained fever or chills, excessive redness, pain, or swelling.
■ Do not use on patients with known sensitivity or allergy to porcine materials.
■ Do not use on third-degree burns.
■ This device is intended for one-time use.
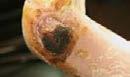
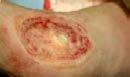
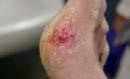
Figure 3: Examples of clinical wounds suitable for Cytal® Wound Matrix
A: Necrotic diabetic ulcer to the medial aspect of the patient's right foot
B: Debridement in the operating room down to the joint capsule
C: Cytal® Wound Matrix technology secured to wound bed with sutures
D: Full-thickness wound healed 13 weeks from the date of surgery
Kimmel H, Rahn M, Gilbert TW. The Journal of the American College of Certified Wound Specialists. 2010 Nov. Images reprinted with permission from Elsevier.
Masterclass GUIDES
What is the Evidence?
Extracellular Matrix (ECM) Products
Cytal® Wound Matrix
Urinary Bladder Matrix [UBM] Technology
The clinical efficacy of Cytal® Wound Matrix is supported by several studies and case reports, demonstrating its effectiveness in various wound healing and tissue regeneration scenarios.
Inflammatory Response
■ Cytal® Wound Matrix provides an environment where the body is more likely to exhibit a higher ratio of M2 (pro-remodeling) to M1 (pro-inflammatory) macrophages1,2.
■ In a prospective case control study, wound management with UBM resulted in a decrease in M1:M2 scores for both diabetic (DM) and non-diabetic (non-DM) patients. This was a statistically significant decrease for DM patients. These decreased M1:M2 scores correlated with the rate of wound area reduction2
■ Application of UBM was associated with a statistically significant wound size reduction in both groups: DM SUBJECTS 35% (±14%) and NON-DM SUBJECTS 43% (±18%)1,2.
Comprehensive Wound Management
■ Cytal® Wound Matrix offers a wound management solution for different wound sizes or etiologies. In conjunction with Integra’s MicroMatrix® Dermal Repair Powder, it can be used to treat irregular, tunneled, or undermined wounds.
■ Cytal® Wound Matrix offers quick tissue formation to support wound closure by permitting cell infiltration and neovascularization1,3,4,5
Cost
■ Cytal® Wound Matrix provides a cost-effective alternative for the management of complex wounds17
■ Cytal® Wound Matrix allowed for healing of complex wounds at lower costs due to requiring less material and employee hours than NPWT with comparable healing times16
For more information or to place an order, please contact Sales & Marketing EMEA, Integra LifeSciences Services (France) SAS, Immeuble Séquoia 2 97 allée Alexandre Borodine, Parc technologique de la Porte des Alpes, 69800 Saint Priest FRANCE, Phone: +33 (0)4 37 47 59 00.
Availability of these products might vary from a given country or region to another, as a result of specific local regulatory approval or clearance requirements for sale in such country or region.
Non contractual document. The manufacturer reserves the right, without prior notice, to modify the products in order to improve their quality. Warning: Applicable laws restrict these products to sale by or on the order of a physician. Consult product labels and inserts for any indications, contraindications, hazards, warnings, precautions, and instructions for use.
Products mentioned in this document are CE class III devices. Please contact Integra customer service should you need any additional information on devices classification. All the medical devices mentioned on this document are CE marked in accordance with the applicable European laws, unless specifically identified as “NOT CE MARKED”. Integra and the Integra logo are registered trademarks of Integra LifeSciences in the United States and/or other countries. Cytal and MicroMatrix are registered trademarks of Integra LifeSciences Corporation or its subsidiaries in the United States and/or other countries. ©2025 Integra LifeSciences Corporation. All rights reserved. Last revision date: 07/2025. 5509683-1-EN.
Cytal® Wound Matrix
INDICATIONS
Cytal Wound Matrix is intended for the management of wounds including: partial and full-thickness wounds, pressure ulcers, venous ulcers, diabetic ulcers, chronic vascular ulcers, tunneled/undermined wounds, surgical wounds (donor sites/ grafts, post-Moh’s surgery, post-laser surgery, podiatric, wound dehiscence), trauma wounds (abrasions, lacerations, second-degree burns, skin tears), and draining wounds. The device is intended for one-time use.
CONTRAINDICATIONS
1. Patients with known sensitivity or allergy to porcine materials.
2. Third-degree burns.
Extracellular Matrix (ECM) Products
Cytal® Wound Matrix
Urinary Bladder Matrix [UBM] Technology
Key Points
■ Cytal® Wound Matrix is a versatile and clinically proven product that can make a difference in wound healing and new tissue deposition. Its collagen-based scaffold supports cellular infiltration, angiogenesis1,3-5 and site-appropriate tissue formation1,18-23, making it an effective solution for a wide range of clinical applications.
■ Cytal® Wound Matrix provides an environment where the body is more likely to exhibit a higher ratio of M2 (pro-remodeling) to M1 (pro-inflammatory) macrophages1,3. A lower M1 to M2 ratio has been associated with constructive tissue remodeling, whereas a higher M1 response is associated with an encapsulation or integration response1,2
■ The clinical efficacy of Cytal® Wound Matrix is supported by several studies and case reports that demonstrate its effectiveness in various wound healing and tissue regeneration scenarios.
■ Cytal® Wound Matrix is available in multiple layering configurations and sizes with varying resorption profiles that allow for tailored management of a wide variety of wounds.
References
1. Brown BN et al., Acta Biomaterialia. 2012. In animal.
2. Paige JT et al. Regen Med. 2019.
3. Sadtler et al, Seminars in Immunology 29 (2017).
4. Song J et al. Journal of Regenerative Medicine and Tissue Engineering. 2014.
5. Remlinger NT et al. Organogenesis. 2013. In animal.
6. Geiger SE et al. Wounds. 2016
7. Kraemer BA et al. Wounds. 2016
8. Valerio IL et al. Regenerative Medicine. 2015
9. Lecheminant J et al. Journal of Wound Care. 2012
10. Agrawal V et al. Proceedings of the National Academy of Sciences of the USA. 2010. In animal
11. Sicari BM et al. Science Translational Medicine. 2014
12. Nassiri S, et al. Relative expression of pro-inflammatory and anti-inflammatory genes reveals differences between healing and nonhealing human chronic diabetic foot ulcers. Journal of Investigative Dermatology, 2015. 135: 1700-1703
13. Parry et al. Journal of Orthopaedic Trauma, 2022.
14. Underwood P et al. Am Surg. 2020.
15. Lanier ST et al. Curr Orthop Pract. 2018.
16. Sasse KC et al. OA Surgery. 2013.
17. Frykberg et al., Journal of Wound Care, 2016.
18. Gilbert TW et al, J Surg Res., 2008.
19. Nieponice A et al, Ann Thorac Surg. 2006.
20. Boruch AV et al, J Surg Res. 2010.
21. Agrawal V et al, J Tissue Eng Regen Med. 2009.
22. Young DA et al, Regen Med. 2018.
23. Young DA et al, Regenerative Medicine. 2018.
Use your device to scan the QR code for more information about Cytal® Wound Matrix®: Click here to visit the Integra® website: https://www.integralife.com/
How to Cite this Article
Masterclass Guide: Extracellular Matrix (ECM) Products Cytal® Wound Matrix: Urinary Bladder Matrix [UBM] Technology. Wound Masterclass. Volume 4. No #. May 2025
Useful Links