Drug-coated balloons (DCBs) and stents were not associated with reduced risk of amputation or improved quality of life compared with uncoated devices in the SWEDEPAD 1 and 2 trials. In addition, higher five-year mortality with drug-coated devices in patients with intermittent claudication was noted, leading researchers to stress that the safety of paclitaxel-coated devices is an “ongoing discussion”.
SWEDEPAD 1 and 2 were pragmatic, participant-blinded, registry-based randomised trials that set out to determine the clinical impact of drug-coated technology on patients with peripheral arterial disease (PAD). Late-breaking findings from the trial were presented at the 2025 European Society of Cardiology (ESC) congress (29 August–1 September, Madrid, Spain) and simultaneously published in The Lancet Explaining the rationale for the trials at ESC, co-principal investigator Joakim Nordanstig (University of Gothenburg, Gothenburg, Sweden), said: “Drug-coated balloons and stents have been shown to reduce restenosis and the need for reinterventions in the endovascular treatment of PAD. However, there are uncertainties regarding whether drug-coated devices
improve outcomes that are meaningful to patients, quality of life and reducing amputations, and there are some concerns over safety. We investigated these and other endpoints in two trials in PAD—one in chronic limb-threatening ischaemia [CLTI] and one in intermittent claudication—comparing drug-coated and uncoated devices.”
In SWEDEPAD 1, 2,355 patients with CLTI (Rutherford stage 4–6) undergoing infrainguinal endovascular treatment were randomised 1:1 to drug-coated or uncoated balloons or stents. In nearly all of the drug-coated devices implanted, the drug delivered was paclitaxel (>99%). There was no significant difference in the primary endpoint of time to ipsilateral aboveankle amputation with drug-coated versus uncoated devices (hazard ratio [HR] 1.05; 95% confidence interval [CI] 0.87–1.27) over five years of follow-up. Target vessel reinterventions were reduced in the drug-coated group during the first year (HR 0.81; 95% CI 0.66–0.98), but this difference disappeared with longer follow-up. There was no difference in all-cause mortality or in quality of life (as assessed using the VascuQoL-6 questionnaire).
In SWEDEPAD 2, 1,155 patients with intermittent claudication (Rutherford stage 1–3) undergoing infrainguinal endovascular treatment were randomised 1:1 after successful guidewire crossing to receive either drug-coated or uncoated balloons or stents. All drug-coated devices implanted delivered paclitaxel. There was no difference in the primary efficacy endpoint of quality of life between the drug-coated and uncoated groups at 12 months (mean difference in VascuQoL-6 scores: –0.02; 95% CI –0.66–0.62). Target vessel reintervention rates were not different at one year or over a median followup of 6.2 years. All-cause mortality did not differ over 7.1 years (HR 1.18; 95% CI 0.94–1.48), although higher five-year mortality was noted with drug-coated versus uncoated devices (HR 1.47; 95% CI 1.09–1.98).
Summarising the findings, co-principal investigator Mårten Falkenberg (Sahlgrenska University Hospital and the University
Closer to the goal: Shoring up data behind
ANDRES SCHANZER
(University of Massachusetts [UMass], Worcester, USA) peers down the lens of an if-not radiationfree complex aortic procedural future, then one in which the methods of today will look positively quaint.
“I say this a lot,” says Schanzer, “but I think there is a time within the next five or 10 years when we’ll be talking to medical students and they will say to us, kind of confused, ‘What do you mean you stepped on a pedal the entire time you were doing one of these cases.’”
He is reflecting on the advance of imaging technologies designed to dramatically reduce exposure to radiation, particularly during lengthy complex aortic endovascular aneurysm repair procedures. Several modalities, loosely grouped together under the banner of “radiation-free,” have emerged in this space in recent years. The “radiation-free” tag represents a lofty goal. Or “a very high bar,” Schanzer says, explaining: “I think what we have all come to realise as we have explored these new technologies is that hopefully we can get closer and closer to that goal. But certainly, within the short term, we are not going to get to a complete radiation-free repair.”
Short of that high bar, significant strides forward have been made. And now, those advances have given way to a randomised controlled trial (RCT) designed to test one of these technologies. Playing on the radiation-free moniker, the RadFree unblinded, multicentre, international study places LumiGuide (Philips), powered by Fiber Optic RealShape (FORS), in a head-to-head with conventional X-ray in a bid to
Continued on page 5
The year might be drawing to a close, but the vascular calendar is in full swing. Ahead of the upcoming Vascular InterVentional Advances (VIVA; 2–5 November, Las Vegas, USA) and VEITHsymposium (18–22 November, New York, USA) gatherings, this issue highlights the abundance of new data presented—and the ensuing discussions—in recent months.
One of the most talked about presentations since our last issue has been that of the registry-based, randomised SWEDEPAD 1 and 2 trials. The results were first shared at the 2025 European Society of Cardiology (ESC) congress (29 August–1 September, Madrid, Spain) and simultaneously published in The Lancet. The full results are outlined in this issue’s cover story, alongside a Q&A with Athanasios Saratzis (University of Leicester, Leicester, UK), who shares his initial reaction to the results and highlights a pressing need to offer all patients the chance to take part in a randomised controlled trial (RCT).
Of note, SWEDEPAD 2 showed higher five-year mortality with drug-coated devices in patients with intermittent claudication. Coincidentally, published in the European Heart Journal at the same time as SWEDEPAD 2 was the final report from SAFE-PAD, which is also highlighted in this issue and tells a different story. SAFEPAD followed more than 150,000 US patients treated with drug-coated and non-coated devices and found no difference in survival through more than seven years of follow-up.
Elsewhere in this issue, we present some highlights from several of the recent US regional vascular surgery meetings. These include a presentation on real-world outcomes of transcatheter arterialisation of the deep veins at the 2025 annual meeting of the Eastern Vascular Society (EVS; 4–7 September, Nashville, USA), and on the findings of a new comparative study of the Gore Excluder iliac branch endoprosthesis (IBE; Gore) in both the investigational device exemption (IDE) and GREAT registry studies of the device at the 49th annual meeting of the Midwestern Vascular Surgical Society (MVSS; 18–20 September, Cincinnati, USA).
On the topic of US regional meetings, the newly elected president of the New England Society for Vascular Surgery (NESVS), Alik Farber, is profiled in this issue. Farber discusses his life and career in vascular surgery, reflecting on the landmark BEST-CLI RCT and considering some of the biggest challenges currently facing vascular surgery.
The optimal trial type is a wider theme of this issue, as explored in two feature articles on the topic. We cover a session that took place at the 39th European Society for Vascular Surgery (ESVS) annual meeting (23–26 September, Istanbul, Türkiye) on RCTs and registries, which
looked at the benefits and drawbacks of both. The session also considered the pros and cons of registry-based RCTs such as SWEDEPAD. The suitability of randomised trials in vascular access is the subject of another of this issue’s feature articles.
Among several other items, this issue also features randomised trial data from CLARIFY IMA and SWHSI-2, on inferior mesenteric artery (IMA) embolisation during endovascular aneurysm repair (EVAR) and negative pressure wound therapy for surgical wounds healing by secondary intention, respectively, as well as late-breaking data presented at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) annual congress (13–17 September, Barcelona, Spain).
Furthermore, the issue includes a number of interviews. Andres Schanzer (University of Massachusetts, Worcester, USA) speaks on an upcoming RCT to assess Philips’ breakthrough imaging technology, Palma Shaw (SUNY Upstate Medical University, Syracuse, USA) shares the latest from the the World Federation of Vascular Societies (WFVS), and Baris Ozdemir (North Bristol NHS Trust, Bristol, UK) discusses the European Venous Registry.
We hope you enjoy the issue and look forward to seeing many of you at the upcoming autumn meetings.
SAFE-PAD followed more than 150,000 US patients treated with drug-coated and noncoated devices and found no difference in survival through more than seven years of follow-up.”


ERIC SECEMSKY is director of vascular intervention and an interventional cardiologist at Beth Israel Deaconess Medical Center and associate professor of medicine at Harvard Medical School (Boston, USA).
Editorial board: Ross Milner, Erin Murphy, Eric Secemsky and Bart Dolmatch | Publisher: Stephen Greenhalgh
Editor: Jocelyn Hudson Jocelyn@bibamedical.com | Contributing editor: Bryan Kay
Editorial contribution: Jamie Bell, Will Date and Éva Malpass
Design: Terry Hawes, Josh Lyon and David Reekie
Advertising: Nathalie Fortin Nathalie@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
Published by: BIBA News, which is a subsidiary of BIBA Medical Ltd
BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788 BIBA Medical, North America, 155 North Wacker Drive, Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323
Printed by: Buxton Press. Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2025. All rights reserved.
THE LATEST STORIES FROM THE VASCULAR WORLD
n VASCULAR ACCESS:
Optimal trial design for vascular access studies went under the microscope at the Vascular Access Society of Britain and Ireland (VASBI) 2025 annual scientific meeting (25–26 September, Bournemouth, UK), where speakers highlighted some of the challenges facing researchers in conducting randomised controlled trials within this space.

For more on this story go to page 8.
n PRIZE-WINNING CLTI PAPER:
Researchers have shown that quiescentinflow single-shot (QISS) magnetic resonance imaging (MRI) is able to identify more below-the-knee vessel segments than digital subtraction angiography (DSA) in patients with chronic limb-threatening ischaemia (CLTI). Taking first prize for best abstract, Alexander Crichton (Houston, USA and Birmingham, UK) shared this and other findings at the 39th European Society for Vascular Surgery (ESVS) annual meeting (23–26 September, Istanbul, Türkiye).

For more on this story go to page 16.
n WOUND CARE RANDOMISED TRIAL: “How did an ineffective and costly intervention become routine care in the NHS [National Health Service]?” Ian Chetter (Hull, UK) posed at ESVS 2025 following a presentation highlighting the SWHSI-2 trial results. SWHSI-2—data from which were first shared at the 2024 Vascular Society of Great Britain and Ireland (VSGBI) annual scientific meeting (VSASM; 27–29 November, Brighton, UK) and recently published in The Lancet— suggests that negative pressure wound therapy (NPWT) should not be first-line treatment for open surgical wounds.
For more on this story go to page 20.

Scan the QR code to subscribe
If you have comments on this issue or suggestions for upcoming editions write to jocelyn@bibamedical.com
SWEDEPAD reopens paclitaxel safety discussion, finds drug-coated devices do not improve outcomes
devices were not effective in preventing amputation in chronic limb-threatening ischaemia or improving quality of life in intermittent claudication. Given the signal of increased mortality with intermittent claudication, clinicians should carefully evaluate the potential risks and benefits when considering these expensive devices. Devices incorporating antiproliferative agents other than paclitaxel warrant further investigation in PAD.”
Paclitaxel mortality issue
“an ongoing discussion”
In a press conference at ESC, Nordanstig commented on how the newly presented SWEDEPAD data compare to other trials in the PAD space. He noted that the results are “a bit different” to those of previously conducted pivotal trials and meta-analyses on drug-coated technology in patients with PAD, which
“consistently demonstrated” reduced reintervention rates. “The big difference here I think,” Nordanstig said, “is this is a strategy trial rather than a single device trial, and [the SWEDEPAD findings] might be what is happening when broadly introducing these therapies in a more everyday patient population.”
Nordanstig also touched on the finding in SWEDEPAD 2 that higher five-year mortality was noted with drug-coated versus uncoated devices, stressing that the safety of paclitaxel-coated devices is “still an ongoing discussion” following the identification in 2018 by Katsanos et al of a late mortality signal. He remarked: “It’s hard for us to ignore the fact that it seems that we mirrored that signal in SWEDEPAD 2, but not in SWEDEPAD 1.”
Closing the press conference, session chair Dan Atar (Oslo University Hospital Ulleval, Oslo, Norway) commended the researchers for their use of “hard” endpoints in the SWEDEPAD trials. “That’s a very interesting approach to showing outcomes,” he said.
Speaking to Vascular News at the 39th European Society for Vascular Surgery (ESVS; esvs.org) annual meeting (23–26 September, Istanbul, Türkiye), one month following initial presentation and publication of SWEDEPAD, Nordanstig reflected on initial reactions to the trial. “I think, so far, it has been very well received,” he said. “I think everyone has been interested in the data.”
“Obviously there has been interest regarding this potential safety signal in SWEDEPAD 2, that we were very surprised about as well, and that raises certain questions we need to address, both as trialists but also as clinicians,” he said. “As we wrote in the Lancet papers, I think at this stage it’s very reasonable to say that we need to carefully consider when we use these devices and for what reasons.”
Nordanstig also shared plans for future research. “More data will come out of this and a very high priority for us now is to scrutinise the mortality signal in SWEDEPAD 2,” he revealed. “We are in a good position to study cause-specific mortality, which I think is a new piece in this intriguing puzzle.”
“No evidence” of long-term mortality risk in SAFE-PAD
The conversation around the paclitaxel mortality signal continues with the publication of the final report from SAFE-PAD, which differs in findings from SWEDEPAD. The study—which was commissioned by the US Food and Drug Administration (FDA) and helped inform the reversal of regulatory warnings against routine use of drugcoated devices—was published in the European Heart Journal (EHJ).
Findings from SAFE-PAD show no evidence of long-term mortality risk associated with drug-coated devices used for femoropopliteal revascularisation.
“All patients need to be offered the chance to take part in a randomised controlled trial”
Speaking to Vascular News at the 39th European Society for Vascular Surgery (ESVS) annual meeting (23–26 September, Istanbul, Türkiye), Athanasios Saratzis (University of Leicester, Leicester, UK) shared his initial reactions to the recently published SWEDEPAD findings. Stressing an urgent and continuing need to recruit patients to peripheral arterial disease (PAD) trials, Saratzis highlighted two UK-based randomised studies that are set to commence in 2026.
What is your initial reaction to the results from the SWEDEPAD trials?
SWEDEPAD 1 and 2 were very well conducted studies, and I congratulate the investigators. I was not surprised by the results. What we are realising following the publication of the SWEDEPAD trials is the fact that we don’t know what we don’t know, which is a major problem when you are a clinician. The only way to address that is by funding and delivering more trials like SWEDEPAD, driven by clinical/cost-effectiveness. From now on, I hope that surgeons, radiologists and all vascular physicians will realise that their patients need to be offered the chance to take part in a randomised controlled trial (RCT) of this nature. We must take the time to present ongoing studies to our patients in a balanced way and let them make up their minds as to whether they want to take part.
Are there any PAD trials in particular you would encourage vascular specialists to get involved with?
We have recently received funding for two major RCTs that will take place across the UK National Health Service (NHS), which we hope will answer what I think are two of the most important questions in PAD at present. One of them is called RAF and will look at open versus endovascular treatment for common femoral artery disease. It is a pragmatic trial, and any NHS site can take part. We are looking at a composite of amputation-free survival and reintervention as a primary
In their EHJ paper, authors Robert M Kim and colleagues at Beth Israel Deaconess Medical Center (Boston, USA), including senior author Eric A Secemsky, note that SAFE-PAD was a retrospective cohort study of 168,553 Medicare fee-for-service beneficiaries aged 66 years and older who underwent femoropopliteal artery revascularisation between 2015 and 2018.
The authors share that device exposure to either drug-coated devices or nondrug-coated devices was identified using Medicare claims data, with the primary outcome being all-cause mortality. Secondary outcomes included all-cause hospitalisations, repeat revascularisation, major amputation, and cardiovascular medication use.
Kim and colleagues report that, at a median follow-up of 4.3 years, drugcoated device use was not associated with increased mortality, meeting a prespecified 5% non-inferiority relative margin. They add that secondary outcomes showed similar hospitalisation and amputation rates between groups but an increase in repeat revascularisation with drug-coated devices.
“These real-world findings indicate no long-term mortality risk with paclitaxelcoated devices,” Kim et al write as their take-home message. “These findings have informed regulatory guidance, and highlight the utility of large-scale observational research.”
outcome measure and plan to conduct a full health economic analysis. The second trial is called RAVE. It was commissioned by the UK government and will look at vessel preparation in any type of PAD, any anatomy, as long as there is calcium present, with a focus on intravascular lithotripsy. Patients will be randomised to treatment either with or without intravascular lithotripsy. Clinicians will be able to perform whatever in-flow and out-flow procedures they choose, and whatever subsequent therapy they want to deliver after vessel preparation. They can even choose to not use anything for vessel preparation, depending on randomisation arm.

Both of these trials are starting in 2026 across the NHS.
What is your message to clinicians regarding recruitment to these and similar trials?
What we are realising [...] is the fact that we don’t know what we don’t know.”
We need to make sure that every patient is offered the chance to take part in a high-quality randomised trial that aims to address questions around their care. That’s the main message from me in light of the SWEDEPAD findings. There are a number of ongoing PAD trials that are struggling and we need people to take this seriously. It should be part of clinicians’ daily practice. The final thing that I will say is that, last year, the UK General Medical Council updated its clinical practice guidance to state that it is your duty as a healthcare professional to offer every patient that you see the chance to take part in an RCT in the NHS, regardless of where that patient is seen in the NHS. It is your duty to signpost patients to the right trial.
Researchers have reported comparable five-year outcomes of the Gore Excluder iliac branch endoprosthesis (IBE; Gore) in both the investigational device exemption (IDE) and GREAT registry studies of the device.
MEGHAN BARBER (UNIVERSITY OF Chicago, Chicago, USA) shared this main finding from a new comparative study at the 49th annual meeting of the Midwestern Vascular Surgical Society (MVSS; 18–20 September, Cincinnati, USA).
Barber began by sharing the researchers’ hypothesis that there would be no difference in outcomes between the IDE study of the IBE device and the IBE component of the GREAT registry, representing “ideal” and “real-world” scenarios, respectively.
In order to test their hypothesis, the researchers compared the five-year outcomes of patients included in both the IDE study and the GREAT registry in the period 2013–2016. “We looked at all-cause mortality, aortic-related mortality, aortic rupture, reintervention, fracture, migration, endoleak and aneurysm growth,” Barber tells Vascular News, reporting that the only differences identified between the two groups were that more patients were followed out to five years in the GREAT registry and that there was “slightly higher” all-cause mortality in the GREAT registry. “Otherwise,” she says, “there was no difference between the two sets of patients.”
Barber comments that these results are “really good” for the IBE device. She notes: “The good outcomes we were able to demonstrate in the controlled setting of an IDE trial translated very well to the patients that were in the GREAT registry.”
Going into more detail about the two studies, senior author Ross Milner (University of Chicago, Chicago,
USA) explains that the IBE component of GREAT comprises the final group of patients included in the registry. He explains: “When the IBE device got FDA [US Food and Drug Administration] approval, it was actually fairly close to the time that we had enrolled the 5,000 patients we were looking for, so we actually have more patients in the registry than the IDE study.” Milner goes on to note that there were roughly 60 patients included in the IDE study compared to around 90 in the IBE component of GREAT.
Milner summarises that the ability to use an iliac branch device for internal iliac artery preservation was “equally effective” in both the IDE study and the GREAT registry. He adds that those who perform endovascular aneurysm repair (EVAR) with an IBE can be “confident” their outcomes will mimic those seen in the setting of a clinical trial.
From a strategy standpoint, Milner continues, “most people would prefer to use an internal iliac branch when possible, for preservation”. He does stress,
Most people would prefer to use an internal iliac branch when possible, for preservation.”
Tara Mastracci (Barts Health NHS Trust, London, UK) advocated a comprehensive, three-armed approach to the future of personalised aortic dissection management at the recent Interdisciplinary Aortic Dissection Symposium (IADS; 11 September; London, UK).
OPENING HER TALK, Mastracci defined personalised care at a specialist level as “empowering patients with the specific tools that will help them treat their disease alongside their clinical team”.
The presenter shared that the aortic team at Barts has adopted a threepronged personalised care strategy, focused on genotype, phenotype, and a factor she dubbed ‘digitype’. She told the IADS audience: “Many vascular surgeons focus on genotype and phenotype, and these things are really important, but there’s a third arm that revolves around the patient’s behaviour and interaction with the clinical team, which more and more requires digital tools.”
Starting with genotype, the presenter homed in on the need to look for genetic variants in aortic patients to inform personalised management.
“Genetic testing is really important,” she said, highlighting it as a key part of the aortic dissection pathway at Barts. Looking ahead, Mastracci focused on improving the speed at which results from such testing become available. “Wouldn’t you love to have these diagnostics faster?” she asked.
“Wouldn’t it be great to have mutationguided therapy for biologics or gene therapy for dissection?” Mastracci urged the audience to “stay involved in this conversation” as the field progresses.
Regarding phenotype, Mastracci’s
however, that both the IDE study and IBE portion of GREAT are US centric, which “obviously makes it a little bit harder to extrapolate to the rest of the world”.
On the next steps for GREAT, Milner notes that there are now long-term follow-up data available, referencing 10-year results on some of the patients treated with standard EVAR using the Excluder abdominal aortic aneurysm (AAA) endoprosthesis (Gore). “That’s the predominant patient population in the registry,” he says, noting that almost 3,300 patients out of 5,000 in GREAT underwent EVAR with the Excluder.

Gore Excluder iliac branch endoprosthesis
Milner notes that one of the limitations of GREAT, however, is the lack of core-lab imaging. “We’re completely dependent on the sites for recognition of problems,” he says. In order to address this, Milner explains that Gore has now established the TOGETHER registry, which will feature core-lab imaging and a “more in-depth understanding of patient selection”.
“The goal is to collect 150 IBE patients,” Milner shares, highlighting the main goal of TOGETHER as being to assess branch technology, with the Gore IBE set to be a “big component” of the study. The pair also consider the Gore IBE as part of the wider treatment landscape. While acknowledging that changes in how patients are treated are inevitable, Barber emphasises that the IBE is “still a fairly unique device”. She remarks: “I don’t think that much has changed in the last five years at least, but in the next 10 years that may be a different story.”
focus was on high-risk anatomy and risk factors for aortic dissection. The presenter explained that, at Barts, every aortic dissection patient gets an echocardiogram to look at the bicuspid aortic valve, after which the team uses the Society of Thoracic Surgeons (STS) classification to discuss the true anatomy of the dissection. Mastracci shared her prediction that future care with regard to phenotype will involve changing the surveillance cadence depending on high-risk factors and focusing more on volume instead of diameter, citing the increasing availability of artificial intelligence (AI) tools to assess imaging.
Finally, Mastracci considered the ‘digitype’ aspect of personalised care for aortic dissection, focusing on the patient’s interaction with the disease and with the team through the example of blood pressure control.
Mastracci noted that blood pressure control post-discharge is “really important,” linking it to fewer acute aortic events in the short term. At Barts, Mastracci and team have built a remote post-dissection blood pressure protocol that involves patients providing blood pressure readings via an app on their smartphone. Readings are fed back to the Barts team from the community on a regular basis.
“We have 213 people in this virtual ward so far,” Mastracci reported, specifying “moderate compliance” with the app among a heavily deprived patient population.
Looking ahead, Mastracci said there is a need to start “gamifying” follow-up for patients. She explained: “I think that this is going to be a lot more fun for patients if we make it a game.” The presenter also underscored the importance of addressing the social determinants of health digital exclusion, especially among patients from lower socioeconomic backgrounds.
We have to make sure we take care of the whole patient.”
“At the end of the day, I think personalised care for dissection is what we’re all already doing,” Mastracci said, closing her presentation. “But surgery can’t be the only intervention we test as surgeons. We’re bigger than that. We have to make sure we take care of the whole patient.”
Closer to the goal: Shoring up data behind breakthrough imaging technology that plots a radiation-free future in new RCT
Continued from page 1
establish that it requires less average fluoroscopy time. Several patients have already been randomised, with the first receiving treatment using LumiGuide recently carried out by Darren Schneider at the University of Pennsylvania (Philadelphia, USA).
Schanzer is a principal investigator in this 11-site RCT. He has been on the radiation-busting trail for some time. His UMass team was involved in some of the earliest cases of FORS in the USA, reporting a 75% decrease in fluoroscopy use while carrying out a fenestrated endovascular aneurysm repair (FEVAR) of a thoracoabdominal aortic aneurysm (TAAA). In the period since the technology first received US Food and Drug Administration (FDA)
approval in 2020, they have been at the centre of studies probing its safety and effectiveness, Schanzer details. “We’ve had the opportunity to be involved in several institutional studies looking at our own data and some multicentre studies with other collaborators from the US and around the world, and what we have learned in these early experiences is that use of LumiGuide seems to decrease radiation time, decrease radiation dose, and the hope is that by doing a randomised controlled trial we can definitively show whether or not there is a benefit associated with using this technology.”
The rationale is beyond question. The sheer length of a FEVAR or branched EVAR (BEVAR) can expose care teams to extensive amounts of radiation. Initial concerns around radiation exposure rightly focused on the patient, Schanzer continues. But, all things considered, patients might expect to undergo one or perhaps two procedures. So thoughts soon turned to the fact that providers might take part in several over the course of any given week.
“If you think about it, it’s crazy that we’re standing right up against an image intensifier that is emitting radiation for procedures that can be several hours long, and we’re taking that radiation and exposing the entire care team to it, day in and day out,”
Cumulative radiation exposure from imaging systems used to monitor patients who have undergone thoracic endovascular aortic repair (TEVAR) may be associated with the development of malignant cancers in the long term.
THIS IS ACCORDING TO THE FINDINGS OF A single-centre retrospective analysis of more than 500 patients to have undergone TEVAR at the University of Freiburg (Freiburg, Germany), presented at the 2025 annual meeting of the European Association for Cardio-Thoracic Surgery (EACTS, 8–11 October, Copenhagen, Denmark). Joseph Kletzer (University of Freiburg, Freiburg, Germany) reported that over the 13-year span of the study, 19 of the 542 patients followed were found to have developed some form of malignancy.
“With the implementation of TEVAR, both intraoperative management and postoperative monitoring rely heavily on advanced imaging, particularly CT [computed tomography] angiography [CTA]. Whilst these tools are essential, repeated radiation exposure is an unavoidable consequence, raising justifiable concerns about the potential for induced malignancies over time,” Kletzer said. Capturing long-term clinical data and imaging
says Schanzer. “So, this is a complete paradigm shift, and it has the potential to really be transformative if the results bear out and show that there is a significant reduction in radiation time and dose. Moreover, I’m sure that, as we continue to iterate on the generations of this technology, it will be able to be used for more and more steps of the procedure. It will be an adjunct to radiation use, but I think that we could potentially cut the dose of radiation more than in half by embracing technologies like this.”
The UMass team has completed more than 100 cases using LumiGuide’s FORS technology. Schanzer believes the UMass data and that accumulated elsewhere in the USA and in Europe suggests wider use, but only an RCT like RadFree can provide
I think that we could potentially cut the dose of radiation more than in half by embracing technologies like this.”
histories, Kletzer and colleagues were able to assess the possible relationship between the cumulative radiation dose and cancer amongst patients undergoing TEVAR at their centre.
They found that patients who developed malignancies had undergone substantially more CT angiograms during follow-up. In his presentation of the results, Kletzer detailed a “consistent association” between the number of CT angiograms and cancer emergence, meaning patients who received a more frequent follow-up protocol were more likely to develop malignancies compared to those who received imaging less often.
The association held true after adjusting for confounding factors, Kletzer said, adding: “These data suggest that radiation exposure from imaging postoperatively has a measurable oncogenic risk, demanding a careful balance between the aortic events and the potential for late harm.”
Somewhat counterintuitively, factors such as a history of smoking or age did not show any significant association with the occurrence of malignancy within the study, whilst higher periprocedural X-ray times seemed to reduce the hazard of malignancy.
“This might be because of survivorship bias; more complicated TEVAR leads to lower life expectancy, leading to a lower signal of malignancies in these patients, when in fact it is just because of a high rate of death,” said Kletzer.
Additionally, the analysis showed that there was a significant association between the average frequency of postoperative CT scans and subsequent cancer risk in patients receiving TEVAR. Using one CTA per year as a reference point, the investigators observed that increasing imaging beyond this threshold was
the sort of robust evidence needed to do so. “The initial investigators that started using and testing LumiGuide have been working with Philips for several years now, and really pushing that we need level-one data to support a larger, broad rollout of this technology,” Schanzer says. Such technologies tackle some of the most radiation-intensive steps in complex aortic procedures. “There are certainly many technologies that have come along that have helped decrease the radiation necessary with the more modern, hybrid room setups,” Schanzer continues. “But even as we practice good radiation safety behaviour, there still is a significant amount of radiation used for these complex procedures. Where we are with this technology is we can now take a lot of the radiationintense steps and decrease the use of radiation, and eliminate it for several of these steps.” A case in point: cannulation of the vessels during FEVAR. “This is something that can be done entirely— safely and effectively—using LumiGuide,” he adds.

linked with a rise in malignancy.
“Patients who underwent two CTAs per year, as opposed to one, faced a hazard for developing malignancy that was more than three times higher than those with only one annual scan,” Kletzer commented, added that this increase remained robust even after adjusting for confounding variables.
“These findings underscore the importance of a prudent and vigilant individualised approach to postoperative surveillance. While vigilant followup is critical to detect aortic complications early, we must also remain mindful of the cumulative risk we introduce our patients to,” he said. “Our results support guideline strategies that limit routine imaging to one CT angiography per year whenever clinically feasible, ensuring we protect patients from acute aortic events and unnecessary long-term harm.”
Discussing the research following Kletzer’s presentation, Florian Schoenhoff (University Hospital Bern, Bern, Switzerland) drew parallels with a JAMA Internal Medicine paper from May 2025 in which it was modelled that CT scans may be linked to as many as 5% of cancers in the USA every year.
“As aortic surgeons we have become better at the lifetime management of the aorta, but we all know that we are not very good at the lifetime management of the radiation burden to our patients,” Schoenhoff commented. However, he questioned whether the size of the cohort or the overall length of follow-up were enough to draw firm conclusions about the potential link between CT imaging and cancer in aortic disease.
“I don’t believe this study captures the whole effect of CTA scans on malignancy development, but I think this study is mainly to show an important signal. It shows there is a direction, it shows us that yes, even in aortic patients with this rather limited follow-up there is a signal of cancer,” Kletzer said in response.
“may not be effective” in EVAR
Results from a multicentre randomised controlled trial (RCT) indicate that pre-emptive inferior mesenteric artery (IMA) embolisation during endovascular aneurysm repair (EVAR) does not significantly reduce aneurysm sac volume or rates of type II endoleak.
THE CLARIFY IMA STUDY—WHICH the authors note is the first multicentre RCT to provide data on the effectiveness of pre-emptive IMA embolisation during EVAR—was recently published in the European Journal of Vascular and Endovascular Surgery (EJVES).
Opening their paper, Shigeo Ichihashi (Nara Medical University, Nara, Japan) and colleagues write that persistent type II endoleak following EVAR has been identified as a cause of aneurysm sac expansion, which they note can lead to reintervention and aneurysm rupture.
They go on to state that the role of pre-emptive IMA embolisation to prevent type II endoleak and sac expansion “remains controversial,” with the present study aiming to evaluate the influence of IMA embolisation on aneurysm sac change after EVAR.
In the CLARIFY IMA study, which was conducted at 24 centres in Japan, patients with a fusiform abdominal aortic aneurysm (AAA) were randomised to undergo EVAR either with or without pre-emptive IMA embolisation. The primary outcome was defined as the percentage change in computed tomography (CT)-assessed aneurysm sac volume at 12 months, with secondary outcomes including sac diameter changes, prevalence of type II endoleak, freedom from reintervention, and overall survival at six, 12,
and 24 months.
Ichihashi and colleagues share that 138 patients with AAA (mean age, 76 years; 117 men) were randomised to either the IMA embolisation (n=70) or the control group (n=68). Of the 70 patients included in the former, the authors report that IMA embolisation was successful in 63 (90%) patients.
The authors add that, at 12 months, there was no statistically significant difference in aneurysm sac volume change between the embolisation group and control group. Furthermore, no statistically significant differences were observed in sac diameter change, rate of type II endoleak, freedom from reintervention, and overall
The findings indicate that IMA embolisation does not significantly reduce aneurysm sac volume or rates of type II endoleak.”
“What I’m hoping we get out of this is not a debate to say that one is better than another, but to get people actually to do these things, because there are advantages and disadvantages of both.” So said Jacob Budtz-Lilly (Aarhus University, Aarhus, Denmark), opening a session at the 39th European Society for Vascular Surgery (ESVS) annual meeting (23–26 September, Istanbul, Türkiye) on randomised controlled trials (RCTs) and observational registries.
‘RCTS ARE SUPERIOR TO registries’ was the title of the opening talk, delivered by Katharine McGinigle (University of North Carolina, Chapel Hill, USA). “This title does not leave a lot of room for nuance,” she began. While acknowledging that RCTs “are the gold standard,” the presenter then focused on a specific type of RCT: the sequential multiple assignment randomised trial, or SMART. She explained that this is a type of clinical trial where participants may be randomised more than once.
“SMARTs are going to be smarter than registries,” McGinigle concluded, “and I’m very hopeful that SMARTs can better reflect the real-world clinical care that we’re doing over time with our patients.”
Subsequently, Alison Halliday
(University of Oxford, Oxford, UK) also spoke on RCTs, arguing that ‘there is no superior way to find answers,’ as per the title of her talk. “Systematic error is inevitable in observational studies, even if you increase the size of the registry,” she said in conclusion, adding that bias is “sneaky and powerful,” and can be avoided by randomisation.
“The play of chance will actually reduce by getting large numbers of participants and hence of course the conclusion that there is a need for large, simple RCTs,” the presenter said in closing.
On the topic of RCTs, Anna Pouncey (St George’s, University of London, London, UK) then highlighted the need for more randomised trials on women.
“What we need to do is move beyond the bikini line,” the presenter argued,
survival at any follow-up time point.
“Unlike previous single-centre RCTs, the findings indicate that IMA embolisation does not significantly reduce aneurysm sac volume or rates of type II endoleak,” Ichihashi and colleagues conclude. “These results suggest that while safe and technically feasible, pre-emptive IMA embolisation may not be effective in EVAR procedures, prompting further investigation into alternative methods for reducing endoleak and sac expansion in clinical practice.”
Ichihashi presented these findings at the 39th European Society for Vascular Surgery (ESVS) annual meeting (23–26 September, Istanbul, Türkiye). Following his talk, one question from the audience probed possible reasons behind the results.
“Can I ask why you think you did not show a difference?” Richard Bulbulia (University of Oxford, Oxford, UK) posed. “Was your study too small, or was your study too short, or perhaps both were true?”
“I believe two years is long enough,” Ichihashi responded. In their EJVES paper, the authors elaborate on the length of follow-up during discussion of several study limitations. They acknowledge that the follow-up period of 24 months, “while sufficient to observe initial trends,” may not have captured sac behaviour or aneurysmrelated death in the long term.
Among other limitations, the authors highlight the fact that the COVID-19 pandemic prevented them from recruiting as many patients to the trial as planned, the incomplete data regarding type II endoleak during follow-up for approximately onethird of the participants who underwent only plain CT, and the possibility that the sample size “may not have been large enough to detect smaller but clinically meaningful differences between the groups”.
highlighting the “misconception that sex is actually only really related to reproductive medicine”. In reality, Pouncey stressed, “cardiovascular disease in particular is affected by sex from the molecular level to the population level”. Pouncey highlighted the WARRIORS RCT, which will look at whether women with small aneurysms might benefit from early endovascular aneurysm repair (EVAR) compared to standard treatment.
of this study type. This newly published two-arm RRCT, he said, “showcases that registry-based RCTs are possible to do in vascular surgery” and represents “the best of two worlds” when it comes to research.

Attention then turned to registries, with Arun Pherwani (University Hospitals of North Midlands NHS Trust, Stoke-on-Trent, UK) focusing on the ‘superiority’ of vascular registries when it comes to finding ‘real clinical solutions’.
Pherwani underlined several challenges associated with developing RCTs—including “huge” ethical concerns for patients, the difficulties associated with securing funding and recruiting patients, and questions around the generalisability of RCT results to current patient populations. He also called into question the value of metaanalyses of results. “You cannot take a bunch of lemons and make a melon out of it,” he quipped.
Kevin Mani (Uppsala University, Uppsala, Sweden) then put forward the case for registry-based RCTs, or RRCTs, using SWEDEPAD as a recent example
A wide-ranging discussion following the five talks saw audience member Sandip Nandhra (Newcastle University, Newcastle, UK) ask about the pressing matter of getting clinicians interested in the topic at hand. He asked the panel: “What are we going to do about the people who aren’t in this room who maybe have biases one way or the other, whether that’s to recruit to trials or even engage with registries?”
Halliday, who mentioned extensive experience trying to recruit UK vascular surgeons to several carotid RCTs, noted the importance of trying to find the “grey areas” in clinicians’ biases.
Nandhra also highlighted the importance of ensuring trial endpoints take patient preference into consideration, mentioning quality-of-life outcomes in particular. Mani noted this is easier to do in RRCTs than RCTs, with Pherwani stressing that patient-reported outcome measures (PROMs) are “a very difficult thing to collect in vascular surgery” given that many treatments are preventative and make patients feel worse in the short term.









Optimal trial design for vascular access studies went under the microscope at the Vascular Access Society of Britain and Ireland (VASBI) 2025 annual scientific meeting (25–26 September, Bournemouth, UK), where speakers highlighted some of the challenges facing researchers in conducting randomised controlled trials within this space.
Speakers at the two-day conference—which brings together specialists in vascular access surgery, interventional radiology, nephrology and other professionals involved in dialysis access from the UK and further afield—discussed whether they could be best served using more data from retrospective studies or real-world registries to guide practice, as a way of overcoming some of the limitations of randomised trials which are seen as the gold standard of scientific research, but can be costly and complicated to administer.
Michael Robson, a consultant nephrologist at King’s College London (London, UK) set out the extent of the challenge facing trialists in the vascular access space, describing it as a “difficult job” to design and execute a trial. Offering details of the ongoing PAVE-2 trial, a multicentre double-blind randomised controlled trial to determine the efficacy of additional paclitaxel-coated or sirolimus-coated balloons for treating stenosis in arteriovenous fistulas (AVF)—for which he is the lead investigator— Robson outlined the scale of the task of bringing the trial from conception in late 2021 to its first patient enrolment in June 2024, months later than anticipated.
The trial, which has been funded by the National Institute for Health and Care Research (NIHR), is a three-armed trial aiming to include over 600 patients from 20 centres around the UK, assessing both the IN.PACT AV (Medtronic) and MagicTouch (Concept Medical) drug-coated balloons (DCBs) compared to a control group, with a primary endpoint of time to end of treatment segment primary patency.
“For PAVE-2, we wanted to try and confirm the findings of the IN.PACT AV access trials which showed that the Medtronic IN.PACT paclitaxel-coated balloon was effective, whereas other trials, the PAVE trial and the Lutonix trials using a different balloon had given a negative result,” Robson detailed. “We also wanted to look at sirolimus-coated balloons in the same trial and we really wanted to provide a definitive answer as to whether drug-coated balloons were a good thing, so we wanted a big sample.”
Recruiting the size of population needed for the trial to provide meaningful evidence has been a
particular challenge, as has organisation of the supply and payment for the devices used in the study. These were major factors pushing back the commencement of recruitment beyond both the anticipated mid-2023 start date, and the officially planned January 2024 start date. Robson said that the trial will need to enrol in the region of 20 patients per month to reach its recruitment targets, and NIHR are actively reviewing progress.
“Trials, studies and research come with different levels of evidence; systematic reviews are at the top of the pyramid, and randomised controlled trials are basically the gold standard,” commented Nicholas Inston (University Hospitals Birmingham, Birmingham, UK), a renal transplant and vascular access surgeon, offering a view on the ideal trial design for vascular access surgery research. Much of the evidence that underpins practice in the vascular access field, he said, is derived from what is perceived to be lower quality evidence which includes cohort studies and case series. “If you look at the guidelines there is very little that is led by randomised controlled trials and a lot of that is expert opinion,” he noted.
There is a learning curve. Often these will be new devices, so what do you do with the first five cases?”
Inston offered an appraisal on the design, delivery and interpretation of randomised trials and questioned whether this level of evidence should necessarily be viewed as the default when it comes to shaping care that is most relevant to patients requiring vascular access interventions.
Factors such as operator variability can make direct statistical comparison challenging within a surgical setting, Inston commented, noting that issues such as a surgeon’s experience with certain types of procedure may also colour outcomes. “Different surgeons will do different procedures, and so it is very difficult to standardise this stuff,” he said. “There is a learning curve. Often these will be new devices, so what do you do with the first five cases? Do you do a training set so that people do five new grafts before we start collecting data?”
representative of real-world practice. “Trials are going to have inclusion and exclusion criteria; many trials will exclude old patients, younger patients, patients with previous access, patients with certain comorbidities. This doesn’t apply to the real world, and the control group is actually pushed up because these patients are in a trial and are being looked at more rigorously and picked out,” he said, commenting that, instead, greater utilisation of real-world data in the form of registries is something that “shouldn’t be dismissed”.
Following Inston, interventional radiologist Robert Jones (Queen Elizabeth Hospital, Birmingham, UK) detailed what he saw as the key studies needed in the endovascular management of vascular access. “There are many unanswered questions both within the AV [arteriovenous] access maintenance space, and more recently within the AV access creation space with the advent of endoAVF,” he said.
According to Jones, an area that has been well studied is the application of covered stents for the treatment of AV access stenosis, which has level-one evidence for both grafts and fistulas demonstrating superiority over angioplasty alone. Jones, however, questioned whether trials in this area adopt a “onesize-fits-all” approach, which, he said, may miss some of the nuance of patient-specific factors that could impact outcomes.
“Covered stent may not always be appropriate, for example in the inflow segment. We know stenosis is the enemy of vascular access, and each stenosis is, to some extent, fairly unique, both in terms of its pathophysiology and its response to angioplasty. Lots of trials and studies that are being designed tend to adopt a one-size-fits all approach to stenosis,” he said.
Jones called for a “lesion-specific” approach to answer questions in this arena, in order to draw out “the right treatment for the right lesion”.
“As a community we are very committed to work on improvement of the treatment of our patients, but if we compare ourselves with the people of cardiology, we do a rather poor job in terms of performing trials,” Joris Rotmans (Leiden University Medical Centre, Leiden, the Netherlands), chair of the session, commented during discussion that followed the presentations. Rotmans said that patients are not solely undergoing an intervention, but there is also very intensive use of the AV access afterwards, for which there is a lot of practice variation. “That is, to some extent, hampering the execution of these kinds of trials,” he commented.
Rotmans asked the panelists how best to deal with practice variation, in light of their comments, and how to minimise its impact on the outcome of trials.


Inston also questioned whether, with a heavily selected population of patients, randomised trials can be considered truly

“All surgeons will do something differently unless you have someone peering over their shoulder,” responded Inston, who commented that results can sometimes differ between trials depending on how they are funded. “Sometimes there is influence where you have got that industry rep behind you saying ‘do it like this’,” he said.
Jones commented that a lot of variation in practice can be attributed to how aware clinicians are of latest data. “Even in my own centre not everyone is aware of the latest stent graft data for example—a lot of it boils down to lack of awareness of what the data say,” he noted.
Robson opined that trials can be designed to minimise the impact of bias and to lessen the influence of operator variability on results. “The design of a trial to try and minimise bias is really important and blinding is part of that; how the patients are allocated and randomised is another thing,” he said. “In terms of practice variation, that is part of life, and you can account for that if you stratify or minimise according to centre, so that you make sure every centre has the same number of patients in each treatment arm.”




“Despite greater initial treatment costs, intervention in acute and subacute lower extremity deep vein thrombosis (DVT) with mechanical thrombectomy is cost effective in the UK.” This is according to an article in press in the European Journal of Vascular and Endovascular Surgery (EJVES).
AUTHORS STEPHEN BLACK
(King’s College London, London, UK) and colleagues write that interventional methods—such as mechanical thrombectomy and catheter-directed thrombolysis—offer an alternative strategy for managing DVT that may reduce the incidence of subsequent complications, such as post-thrombotic syndrome (PTS). However, the authors note that such methods incur higher initial treatment costs compared with the standard of care, namely anticoagulation, and it is unclear whether those higher costs are mitigated by the reduction in PTS. It was the aim of the present study, therefore, to evaluate the lifetime health utility of mechanical thrombectomy plus anticoagulation
compared with anticoagulation alone in the UK.
The researchers implemented a combined decision tree and Markov model to evaluate complications from a healthcare payer’s perspective over a lifetime horizon in patients with acute and subacute (i.e. symptom duration of less than or equal to four weeks) iliofemoral DVT treated with either mechanical thrombectomy or anticoagulation.
Black and colleagues specify that resource utilisation and cost estimates were sourced from published literature and were evaluated in terms of inflation-adjusted 2021 British pound sterling. They add that complication rates, PTS health state transition probabilities, and health utilities were
established from a published cohort of 164 pairs of propensity-score matched patients treated with mechanical thrombectomy or anticoagulation from the CLOUT registry and ATTRACT trial, respectively.
In EJVES, the authors report that mechanical thrombectomy resulted in lower lifetime costs (£20,889 vs. £26,193) and greater mean lifetime quality-adjusted life years (QALYs; 15.4 vs. 14.3) compared with anticoagulation.
“Thus, mechanical thrombectomy was dominant compared with the standard of care, with a net monetary benefit of £27,904 at a willingnessto-pay threshold of £20,000,” Black and colleagues write. In addition, they note that results of univariate and probabilistic sensitivity analyses were consistent with the deterministic result, “suggesting robustness of the model results”.
Based on their findings, Black and colleagues conclude that mechanical thrombectomy is cost effective in the UK, regardless of higher initial treatment costs.
Their results “suggest that patient benefit is realised over a lifetime horizon by reducing PTS incidence and associated costs,” the authors state, going on to stress that randomised
“Things are going well”: European Venous Registry secretary reflects on progress at one-year mark
Speaking to Vascular News at this year’s European Society for Vascular Surgery (ESVS) annual meeting (23–26 September, Istanbul, Türkiye), European Venous Registry (EVeR) secretary Baris Ozdemir (North Bristol NHS Trust, Bristol, UK) reflects on the year since the launch of the registry’s pilot phase in September 2024.
DEVELOPED AND HOSTED BY THE ESVS, EVeR is designed to illuminate the real-world outcomes of deep venous intervention over a 10-year timeframe and is set to be an international repository of deep venous treatment data.
“We have treatments that seem to work very well, but also some uncertainties about who they should and shouldn’t be applied to,” Ozdemir shares, outlining the rationale behind the registry. EVeR is the culmination of several discussions amongst a group of stakeholders who are “interested in addressing the same questions,” he continues— including physicians, industry, and patients.
With the registry now in full swing, Ozdemir reflects that “things are going well”. “Going live was a big deal,” he says, specifying that the main aim of the registry thus far has been “working out the finer details” with regard to recruitment and data analysis.
Ozdemir details that, to date, 114 centres have shown interest in the registry. Of those, he notes, over 40 have received clinical approval and over 30 are now actively recruiting patients. “We’ve recruited over 60 patients,” he says. Ozdemir is confident about the future too. “We hope that if we keep this pace up, we’re going to grow fast.”
Ozdemir reflects that it was “very difficult” getting the first patient involved. “But now it actually seems like not a big deal, it’s easy. You kind of get familiar with the software.”
Looking ahead, Ozdemir shares that the registry recruitment software is set to be updated to make it “more user-friendly” and to allow centres to “gather more data, faster”. He adds that the registry team is currently working on an annual report to outline progress thus far.
Despite the overall “good progress” being made, Ozdemir also reflects on some setbacks. “It’s been challenging because we have to pass different ethical criteria in every country that we recruit to,” he shares, noting that the team has had “great support” from the ESVS to help navigate this obstacle.
Ozdemir adds that the team has had to “tweak” the registry to match projects that are running in parallel. “In addition to EVeR, we have published a core outcome set for deep venous disease and we are
We have treatments that seem to work very well, but also some uncertainties about who they should and shouldn’t be applied to.”
controlled trials will validate these potential clinical and economic benefits.
Black presented these findings at the 39th European Society for Vascular Surgery (ESVS) annual meeting (23–26 September, Istanbul, Türkiye), closing his talk with the remark: “I think we can quite confidently now say [...] that intervention does not cost you money.”
“We do need better longterm data”
During discussion time following Black’s presentation, one audience member asked about mean follow-up time in the study.
Black shared that, for the modelling, the team had two-year follow-up data from the ATTRACT trial available.
“There are some data points that we included that give us out to five years of follow-up on post-thrombotic syndrome,” he added, before noting that the data “start to become weaker” at that stage.
“But what we see is that patients will probably progress to severe [PTS], and unless you account for that long-term progression in the untreated arm, you lose the QALY gained, particularly in a young cohort of patients who have 30 years to live,” he added. “So we do need better long-term data.”
about to submit a core descriptive set for the same group of patients” he explains, noting that the idea is for the data collected in EVeR to then be “mergeable” and fit to be analysed with other registries and randomised trials.
At this juncture, Ozdemir is keen to reiterate the importance of the registry and of recruiting patients to it. “Essentially, this is an area where we have relatively novel treatments that have been shown to work in case series, both in acute and chronic patients, but there are still question marks in the wider vascular community about who derives benefits from these interventions and frankly there aren’t any long-term data in a real-world setting,” he says. “We have long-term data from certain centres of excellence from a few parts of the world but not in the real-world setting, and we hope over the next decade we’re going to acquire that real-world data.”
Ozdemir points out that an important element of the registry is that it is recruiting not only patients who are undergoing intervention, but also those who are not. “This will allow us to compare what happens to the patients in these two groups,” he explains. “It’s not just a procedural registry, we want it to be a patient registry that includes those who choose to and those who choose not to have an intervention.”
Ozdemir also stresses that venous disease affects a particularly young population, with EVeR set to answer questions for a cohort of patients who will live to see the effects of an intervention—or lack thereof—for many years to come. “As physicians, we don’t understand the burden of disease. It’s almost like we just see a young patient who seems okay and we say, ‘are you sure you want to have a stent?’ But actually, for them it can be positively life changing. On the flip side of that there’s a lot of patients with extensive DVT [deep vein thrombosis] who never get problems, and we’re hoping the registry will help us to start working out who’s going to fall into which group.”



The field of embolisation has seen an increasing number of devices enter the market, raising an important question: how much does the choice of embolic device influence outcomes? The Impede embolisation plug (Shape Memory Medical), comprised of a shape memory polymer (SMP), or smart polymer, has garnered significant attention in recent years, appearing frequently in the scientific and clinical discourse. To better understand what differentiates this technology, Vascular News spoke with two of the scientists driving its development—Marziya Hasan and Landon Nash—who both hold PhDs from Texas A&M University (College Station, USA).
Background
SMP has emerged as a novel material and is currently employed in the Impede embolisation plug family of devices, indicated for peripheral vascular embolisation. These devices incorporate Shape Memory Medical’s proprietary polymer—a porous, radiolucent material which allows for enhanced visualisation of the surrounding anatomy during and after the procedure. Crimped for catheter introduction, the polymer self-expands to its original shape upon contact with the warm, aqueous environment of the blood vessel. This expansion creates a conformable, high-volume fill that facilitates stable thrombus formation and rapid, durable occlusion.
The Impede embolisation plug family is
How does shape memory polymer differ from other polymer technology in terms of its bioresorbability?
body, but the severity and duration of the tissue response is dependent on many chemical and physical factors unique to every device, the materials of construction, and targeted therapeutic use.5
For example, embolic devices composed of platinum are comparatively biologically inert. In some cases, they may not generate a sufficient signal to fully activate the body’s healing cascade. In contrast, a transient inflammatory response can be beneficial serving as a signal to initiate tissue repair. The critical factor is ensuring that this inflammation resolves quickly, allowing the implant site to transition smoothly from the acute inflammation phase to longterm healing.


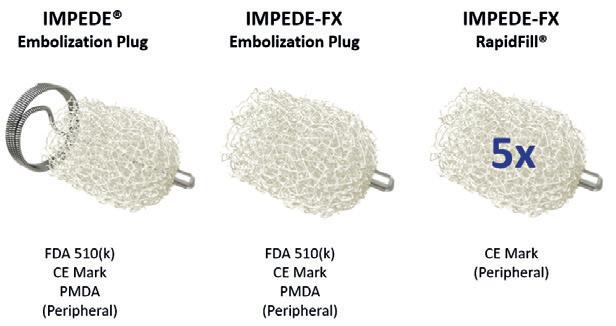
commercially available in more than 30 countries across Asia, the Middle East, Europe, the Americas, and Australia, reflecting its growing global adoption. Recently, Shape Memory Medical announced the completion of the EMBO-postmarket surveillance registry, a prospective, multicentre study evaluating real-world outcomes with the Impede and ImpedeFX embolisation plugs as well as the Impede-FX RapidFill device in peripheral vascular embolisation.
What distinguishes the chemistry of shape memory polymer?
MH and LN: Shape Memory Medical’s proprietary polymer is an ultra-low-density, bioresorbable polyurethane foam engineered with high volume recovery. The material is manufactured in an expanded state and subsequently crimped to allow catheter-based delivery. Once deployed, it returns to its original form, enabling high-volume space filling. While polyurethanes are widely used in blood-contacting medical devices, the use of a bioresorbable polyurethane in this context represents a novel approach not commonly seen in commercially available embolic technologies.
MH and LN: The bioresorption of SMP is driven by a cell-mediated mechanism rather than the more common hydrolytic pathway. The polymer network is designed to degrade at specific functional net points under oxidative conditions.1 This occurs through the action of reactive oxygen species (ROS) produced by macrophages—immune cells that play a central role in clearing foreign materials. In this setting, macrophage-generated ROS initiate the breakdown of the polymer, leading to gradual bioresorption. By contrast, many commercially available bioresorbable materials rely on hydrolytic degradation. For example, ester-based polymers degrade in the presence of water, generating carboxylic acids that lower local pH and accelerate breakdown. This hydrolysis-driven mechanism is largely passive, whereas SMP’s cellmediated oxidative degradation represents a more active and biologically responsive process.
What is the effect of bioresorption of occlusion durability?

Preclinical and clinical experience to date has demonstrated the unique biological response of shape memory polymer, the material used in the Impede embolisation plug family of devices, which facilitates stable clot formation, supports remodelling, undergoes gradual bioresorption, and is ultimately replaced with organised collagen scar tissue. This tissue response not only stabilises the implant site but may also influence long-term vessel remodelling.
Building on this foundation, Shape Memory Medical is advancing the AAA-SHAPE investigational device exemption (IDE) trial—a prospective, multicentre, randomised, open-label study designed to evaluate whether the Impede-FX RapidFill device can improve aneurysm sac behaviour and potentially enhance rates of aneurysm shrinkage when used alongside elective endovascular aneurysm repair (EVAR). Enrolment is underway and will continue through 2025. Looking further ahead, the company is also exploring novel applications, and developing a large-diameter device comprised of SMP specifically engineered for false lumen embolisation in aortic dissection. These efforts underscore a broader vision: harnessing biomaterials that actively engage the body’s biological response to shape the future of embolisation therapy.
MH and LN: The bioresorption of SMP occurs gradually, based on preclinical in vivo studies.2,3 Importantly, as the polymer resorbs, it is replaced by the body’s own native tissue. During this process, myofibroblasts actively deposit collagen—a type of scar tissue—ensuring that the initial scaffold progressively remodels rather than leaving voids. This mechanism distinguishes SMP from hydrolytically degradable polymers, as SMP degrades in direct response to the body’s immune and healing processes. It is only resorbed when cells actively remodel the implant site, with the polymer acting as a temporary scaffold that transitions to durable, collagenous connective tissue.
Preclinical studies have further demonstrated that SMP is biocompatible.3 Rather than compromising durability, the material supports a natural healing response that stabilises the site over time.
Clinically, the initial prospective safety study on the Impede embolisation plug evaluated 10 patients undergoing peripheral vascular embolisation. Longterm imaging follow-up in this cohort demonstrated no evidence of recanalisation, underscoring the device’s durable embolic performance.4
Is an inflammatory response associated with SMPs and other polymers? MH and LN: Yes. Immune cells recognise and respond to all foreign materials introduced into the
Disclaimers: The Impede and Impede-FX embolisation plugs and Impede-FX RapidFill are CE-mark approved. The Impede and Impede-FX embolisation plugs are approved in Japan and cleared for use in the USA. In the USA, the Impede embolisation plug is indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature, and the Impede-FX embolisation plug is indicated for use with the Impede embolisation plug to obstruct or reduce the rate of blood flow in the peripheral vasculature. In the USA, Impede-FX RapidFill is an investigational device, limited by Federal (or US) law to investigational use. For more information, visit www.shapemem.com
References
1. Weems AC et al. Shape memory polyurethanes with oxidation-induced degradation: In vivo and in vitro correlations for endovascular material applications. Acta Biomater. 2017 Sep 1; 59: 33–44.
2. Horn J et al. Comparison of shape memory polymer foam versus bare metal coil treatments in an in vivo porcine sidewall aneurysm model. J Biomed Mater Res B Appl Biomater. 2017 Oct; 105(7): 1892–1905.
3. Jessen SL et al. Microscopic assessment of healing and effectiveness of a foam-based peripheral occlusion device. ACS Biomater Sci Eng 2020 May 11; 6(5): 2588–2599.
4. Holden A, Hill AA, Buckley BT. Shape memory polymer technology in peripheral vascular embolization. Vascular. 2024 Oct; 32(5): 1137–1142.
5. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008 Apr; 20(2):86–100.








Alik Farber (Boston, USA) speaks to Vascular News about his life and career in vascular surgery. The surgeon-in-chief at Boston Medical Center and James Utley professor and chair of surgery at Boston University Chobanian and Avedisian School of Medicine talks BEST-CLI, his plans for the New England Society for Vascular Surgery (NESVS) as its recently elected president, and the importance of multispecialty collaboration.
Why did you choose a career in medicine and what drew you to vascular surgery in particular?
My parents and I emigrated from Moldova to the USA when I was 10 years old. My father was a surgeon, but he never practised in the USA, instead working multiple jobs—as a butcher, a nurse’s aide, and a newspaper salesman. We had a rough immigration experience, but from an early age, I wanted to be a physician, perhaps to follow in my father’s footsteps. I loved science and I knew that I wanted to help others.
When I was a sophomore at Brown University, I volunteered in the emergency room at Rhode Island Hospital, and after that experience, I was determined to go to medical school. As a third-year medical student at Harvard Medical School, I fell in love with surgery during my clerkship at the Massachusetts General Hospital (MGH) and went on to match there for my residency. Toward the end of my residency, my path to vascular surgery was clear. At the time, my attendings at the MGH were placing endovascular grafts and I found that emerging technology exciting. Being able to do both open surgery and endovascular therapy sealed the deal for me.
Who were your career mentors?
I had a number of mentors. I can’t list all of them, but the ones that come to mind include Richard Paul Cambria, Jack Cronenwett, and Rick Powell.
Rich Cambria was a consummate surgeon who performed thoracic abdominal aneurysm repairs, which are some of the biggest operations that one can perform on a human being. He was a great teacher and an even better speaker.
Jack Cronenwett is one of the most intelligent and knowledgeable surgeons that I’ve ever met. He was an incredible leader and was always focused on improving everyone around him. He was never insecure, always on point, always mentoring, and always reaching for the true north. I’ve tried to model him, not always successfully, in many of my endeavours.
Rick Powell is an incredibly talented open surgeon, a gifted endovascular specialist, a kind human being, the epitome of calm and control. He has been a great mentor and, over the years, became a friend.
You served as co-principal investigator for the landmark BEST-CLI trial. Three years on from the publication of the main findings, have you noticed changes in practice patterns in the management of
chronic limb-threatening ischaemia (CLTI)?
The whole BEST-CLI journey is an incredible thing that I still can’t believe actually happened. It started out with an idea that my good friend Matt Menard and I had about answering an important question in our field, and it ended up in us getting the funds necessary to run and then actually execute the trial. BEST-CLI was very hard to complete for many reasons, one being that we were comparing bypass surgery, an invasive procedure, with minimally invasive endovascular therapy. There were all sorts of obstacles, incredible trials and tribulations, but, in the end, with the help of Kenny Rosenfield, who joined us as third national principal investigator, we were successful. Of course, it was not a perfect trial. However, we had very smart people across multiple specialties working together to agree on the best possible protocol. In the end, we got some really interesting answers and added to the badly needed evidence base in the CLTI space.
Our main finding was that, if a patient is at an acceptable risk for open surgery, has complex infrainguinal occlusive disease, an adequate distal target and has good great saphenous vein, they should be considered for infrainguinal bypass. At the time, this suggestion was something of an anathema. It was the wrong thing to say because everybody was moving towards the endovascular-first and, really, endovascular-only approach to treating patients with CLTI.
As to how the trials findings are being implemented, that is still hard to assess accurately. I do hope that vascular specialists are using the available evidence base in guiding best treatment for their patients. We, certainly, have implemented the findings at our centre by changing our practice so that when a patient comes into our hospital with CLTI, they will get vein mapping before they get an angiogram or a computed tomography angiography (CTA) to see whether there’s good vein available or not, and that will drive our treatment decision. How it’s being implemented elsewhere is not yet clear. That is a very important question and, in the near future, we hope to answer it.
What do you think is the most important research paper that has been published in the last 12 months?
I would have to say LIFE-BTK. This study looked at the effect of a drug-eluting resorbable scaffold versus angioplasty in patients who had infrapopliteal disease. The tibial arteries have been the final frontier of intervention for patients with CLTI and
CURRENT APPOINTMENTS
2025–present: Chief of surgery, Boston Medical Center Health System (Boston, USA)
2024–present: Surgeon-in-chief, Boston Medical Center (Boston, USA)
2024–present: James Utley professor and chair of surgery, Boston University Chobanian and Avedisian School of Medicine (Boston, USA)
2015–present: Professor, Departments of Surgery and Radiology, Boston University Chobanian and Avedisian School of Medicine
EDUCATION
2019: MBA, Heller School for Social Policy and Management, Brandeis University (Boston, USA)
1992: MD, Harvard Medical School (Boston, USA)
1987: BS (Biology), Brown University (Providence, USA)
SOCIETY POSITIONS (SELECTED)
2025–2026: President, New England Society for Vascular Surgery
2025–2026: Vice president, Society for Clinical Vascular Surgery
2024–present: Governor, American College of Surgeons
2024–present: Secretary, Boston Surgical Society
really the only thing that’s been standard of care is angioplasty alone, which we know doesn’t work that well. This study is exciting because it showed the scaffold, which elutes everolimus, to work better than angioplasty. I think the positive results of this trial are going to generate further innovation in the tibials.
What are your initial thoughts on the SWEDEPAD results?
I was not surprised. The results mirror what we found in a sub-analysis of BEST-CLI. I think that at the end of the day, we’re all going to move away from paclitaxel towards the limus family of drugs.
You have just been elected president of the New England Society for Vascular Surgery (NESVS). What are your plans for the year ahead?
The NESVS was the first regional vascular society in the USA. It was founded in 1973 by Robert Linton and R Clement Darling of the MGH and Ralph A Deterling Jr of the New England Medical Center. I remember attending the first meeting of the NESVS as a fellow at Dartmouth Hitchcock Medical Center, and I was instantly impressed with the breadth and depth of the science that was presented. The NESVS is a wonderful, close-knit organiation that hosts an outstanding scientific meeting. I plan to survey our current members to understand what’s important to them and then address these needs. I also aim to grow our membership, even beyond New England, and tweak our meeting to more deeply involve both trainees and allied professionals.
What are the biggest challenges currently facing vascular surgery, and what do you think could be done to address these?
There are several challenges. First is the way the health system is set up, at least in the USA. Due to the fee-for-service model, physicians are incentivised to perform procedures resulting in their overuse. That’s a big problem because, in the end, patients suffer. Frankly, it is not about office-based laboratories (OBLs) versus hospitals, or vascular surgeons versus cardiologists, it’s about having a workforce that is incentivised to do more vascular procedures rather than do the right thing by the patient. That’s a huge problem that we need to tackle, and I believe that it’s up to the vascular surgery, cardiology and radiology communities to collaborate to fix this issue.
The second topic is the relationship between specialists. While the relationships between cardiologists and vascular surgeons is much better than it was 20 years ago, it’s still not as

good as it can be. I think there’s an opportunity for all of us to work more closely together to take the best care of our patients.
Thirdly, because of the rise of endovascular technology and growth of endovascular procedures, open vascular surgery is at risk of not being taught and learned as well as it needs to be. There still is and will be a role for open surgery, and, for us to have the best trained vascular workforce, we must be very careful that our vascular surgery training programmes teach both open surgical skills as well as endovascular skills to our trainees.
Could you outline one of your most memorable cases?
One case comes to mind of a middle-aged woman who had an internal carotid aneurysm. This aneurysm was treated by a flow-diverting stent, and the stent pulled out from its distal attachment in the distal internal carotid artery. The interventionalist who was managing this patient wanted to go back and deploy another stent. I was consulted and thought that this was
not the right thing to do. We took the patient to the operating room and, because the aneurysm was very high in the neck, the oral surgeons had to split the mandible to provide access for us to get to the internal carotid artery at the base of the skull. We were able to control the aneurysm, and when we opened it, we found the flow-diverting stent attached proximally, but distally waving around in the aneurysm sac. The stent was filled with thrombus. If that patient had gone on to have another stent, she would have certainly embolised this thrombus to her brain. I harvested a piece of saphenous vein and used it to bypass the internal carotid artery. The patient did well.
What advice would you give to someone looking to start a career in medicine generally, or vascular surgery specifically?
I love being a physician and vascular surgeon and as a result, I don’t view going to work every day as working. My advice for those who are starting their careers is to find
“I think that at the end of the day, we’re all going to move away from paclitaxel.”
something you are truly passionate about. Vascular surgery a wonderful field. It provides a tremendous amount of variety and a tremendous degree of satisfaction. Our patient population is getting older and the demand for vascular surgeons is rising. It’s rewarding to be able to have the skillset to take care of patients in the office medically, but then also to treat them in the endovascular suite or operating room using endovascular or open surgical techniques.
What are your hobbies and interests outside of vascular surgery?
My hobby is biking. My wife and I have taken many biking trips across the world, and we really enjoy doing that. It’s a wonderful way to see different countries while exercising. I also love history and in particular ancient Roman and medieval British history. On a recent trip to the UK I was able to visit Battle Abbey, which is the location where the Battle of Hastings took place. This was a fascinating experience.
Researchers have shown that quiescentinflow single-shot (QISS) magnetic resonance imaging (MRI) is able to identify more below-the-knee vessel segments than digital subtraction angiography (DSA) in patients with chronic limb-threatening ischaemia (CLTI). Taking first prize for best abstract, Alexander Crichton (Houston Methodist Hospital, Houston, USA and University of Birmingham, Birmingham, UK) shared this and other findings at the 39th European Society for Vascular Surgery (ESVS) annual meeting (23–26 September, Istanbul, Türkiye).
“IMAGING PATIENTS WITH BELOW-THEknee CLTI is challenging,” Crichton, who was presenting the research on behalf of Trisha Roy and colleagues at Houston Methodist, began. He cited the rise in diabetes and end-stage renal failure as two
of the key reasons behind this, noting both cause calcified arterial disease that can “significantly” affect computed tomography (CT) and ultrasound imaging.
“Is digital subtraction angiography—our gold standard of current imaging—showing us what we need to see? Is it similarly affected by this calcium?” Crichton posed to the ESVS audience.
Regarding the available literature on the topic, the presenter referenced a study from the 1990s showing that non-contrast MRI could identify more patent vessel segments than DSA. He noted that this finding changed the researchers’ management of patients in 17% of cases. The problem was, however, that the technique was slow and marred by imaging artifacts. “It never really caught on,” Crichton remarked.
The presenter stated that clinicians now commonly use contrast-enhanced MRI. “But in 2025, do we need to?” he asked, citing newer, non-contrast MRI techniques, such as QISS MRI, that are fast, less affected by artifacts and “give a fantastic image”.
At Houston Methodist, Crichton shared, clinicians offer patients who require an MRI of their lower limbs a QISS MRI followed by a contrast MRI. In the present study, the researchers compared these patients’ QISS MRIs to their DSAs across 14 vessel segments.
The primary outcome of the study was to assess whether QISS MRI could identify more patent vessel segments than DSA, with the secondary outcome being the effect of QISS MRI versus DSA on disease severity scores, namely Trans-Atlantic Inter-Society
The first post-approval multicentre analysis of so-called ‘nooption’ chronic limb-threatening ischaemia (CLTI) patients treated using a pioneering transcatheter arterialisation of the deep veins (TADV) device lays out a set of real-world outcomes that align with those achieved in the PROMISE II pivotal trial of the device, the authors report.
RESEARCHERS FROM Northwell Health in New Hyde Park, USA, MedStar Health in Washington, DC, USA, and UT Southwestern in Dallas, USA, revealed limb salvage rates of 76.1% at six months and 71.7% at one year, respectively, among an 80-patient cohort with a median followup of 184 days after TADV (LimFlow, acquired by Inari Medical, now part of Stryker). Furthermore, the study showed survival of 93.1% and 84.7% at six months and one year, respectively. Likewise, the research team reported amputation-free survival rates of 74.2% and 63.6% at the same time points.
The data were revealed during the 2025 annual meeting of the Eastern Vascular Society (EVS; 4–7 September, Nashville, USA) by presenting author Yana Etkin (Hempstead, USA).
Etkin pointed to six-month results from PROMISE II showing a limb salvage rate of 76%, survival of 87.1% and amputation-free survival of 66.1%.
“This first real-world, multicentre retrospective study of LimFlow showed outcomes consistent with PROMISE II,” she told those gathered at EVS
2025. “[TADV] achieves favourable outcomes with a high rate of limb salvage up to one year.”
In an interview with Vascular News ahead of presenting the data, Etkin spoke of the device’s role in the fight against diabetes-driven peripheral arterial disease (PAD) across the USA.
“PAD is on rise in the US, specifically because of the really high rates of diabetes,” she says. “Every 11th person in our country has diabetes—that is now the no. 1 risk for PAD—and almost four million people have PAD. So, the rate of limb loss continues to increase, and probably about 10% of patients who have PAD have what we call ‘no option’ for revascularisation.”
TADV has long been sought after as an alternative means of providing an option for this high-risk CLTI population of patients, Etkin relates.
“For years, we have been talking about what to do with these patients, and we’ve tried DVA, where we connect the arteries to the veins, to arterialise the veins, and it has been tried with variable success,” she explains. “Open deep vein arterialisations [DVAs] have
Consensus (TASC) and Global Limb Anatomic Staging System (GLASS) scores.
In total, the researchers compared QISS MRI to DSA across 752 vessel segments in 56 patients.
Crichton reported that the median time from QISS MRI to DSA was short, at around four days, and that the number of patent vessel segments on QISS MRI was an average of 10% higher than that on DSA, which he detailed was a statistically significant difference.
In addition, Crichton revealed that the difference in the number of visible vessels on QISS MRI compared to DSA became more pronounced the further down the limb the imaging was used. He cited a statistically significant difference of over 30% at the level of the dorsalis pedis artery, for example.
The presenter also noted a “knock-on effect” on both TASC and infrapopliteal GLASS scores. “The mean TASC score and the mean infrapopliteal GLASS score were significantly downgraded when we used QISS MRI to evaluate these arteries,” he said. “This is really important, because this is our language to classify disease severity. It’s what we’ve used in the most recent SWEDEPAD trial.”
Sharing his take-home message with the ESVS 2025 audience, Crichton remarked on the future potential of QISS MRI. “I truly believe that this could change how we manage our patients,” he said. “This is easy to integrate into our practice, and I think we could use it a lot more.”
been described, and percutaneous ones as well, but the percutaneous ones were done randomstance, involving specialists using devices off the shelf, self-made, in very few small series, so not well reported.”
Then along came the LimFlow device, Etkin continues. Approved by the US Food and Drug Administration (FDA) in 2023, LimFlow rolled out into the real-world environment off the back of the six-month results’ publication in the New England Journal of Medicine (NEJM). “But the trial is the trial,” Etkin says, “the perfect kind of environment. We started using [LimFlow] in our system when it got approved, and so the idea behind this project was to look at real-world data. Very commonly, after the trials are over, when the devices get to the general population, they don’t perform as well, and then, of course, there is a learning curve.”
With a median follow-up of six months among the 80 patients in the real-world analysis, Etkin points to data showing that of the patients who did not die or lose a limb, stent patency was 70%, with 94% of patients exhibiting either healed or improved wounds. In terms of learning curve of the LimFlow procedure, Etkin can claim 30 of those 80 patients as experience. In that vein, one of the routes to good results involved completing cases together with Northwell vascular surgeon colleague, Jeffrey
EVS 2025
Silpe, she explains. “The first few cases took a long time, an average of five to six hours,” Etkin says. “But now, after doing these for over a year, we are down to about one-and-a-half to two hours. So, there is definitely a learning curve in probably the first 10 cases.”
She continues: “It is more than just a technical procedure. There is a lot of thought that needs to go into the planning, the intraoperative decisionmaking. It’s not a straightforward procedure where you have an occluded artery and try to open it up. You’re creating a fistula, and there is a risk that you could steal the blood flow from the native circulation into this fistula, and this fistula is not going to provide enough blood flow for the first six weeks or so because it has to mature. So, you can actually make things worse if you’re not carefully planning and looking at the imaging.”
The procedure is “not going to save everyone,” Etkin says. “This is the first option for these patients who truly have no option.” But, with limb loss associated with “really high mortality,” by saving legs “you’re actually saving people’s lives”.

Work to more fully understand who the right patients are for the TADV procedure continue, Etkin adds. “I think all the results—PROMISE, our results—supports that there is something here that people should really consider including in their treatment algorithm. This is not the first option; this is clearly for when you have exhausted all the options.”
decade of new
The Society for Vascular Surgery (SVS) recently published a comprehensive update to its clinical practice guidelines (CPGs) for the management of intermittent claudication (IC), urging clinicians to prioritise conservative treatment strategies and patient-centred care.
THE NEW RECOMMENDATIONS, which appeared in the August issue of the Journal of Vascular Surgery (JVS), incorporate nearly a decade of new evidence and mark the first time the SVS has included a formal patient panel in the guideline development process. The IC guidelines writing group, led by first author Michael Conte (University of California San Francisco, San Francisco, USA), produced 12 formal recommendations and two best practice statements, focusing on antithrombotic therapy, exercise interventions and revascularisation procedures.
The update is intended to provide clinicians with the best available contemporary data on optimal medical therapy (OMT), exercise and interventions to promote an evidencebased framework for the management of IC. The guidelines reaffirm that firstline treatment should include patient
education, smoking cessation, risk factor control, optimal medical therapy and structured exercise programmes. Revascularisation—whether surgical or endovascular—is recommended only for patients with lifestyle-limiting symptoms who do not respond to conservative therapy.
The guideline development process included input from a panel of patient advisors with lived experience of peripheral arterial disease (PAD) and claudication. Their feedback emphasised the importance of clear communication, individualised treatment goals and transparency about risks and benefits.
Despite advances in pharmacotherapy and endovascular technology, the SVS identified several gaps in the evidence base, including limited data on longterm outcomes and the effectiveness of home-based exercise programmes.
“The areas selected for focus concern the role of therapeutic interventions for patients with IC,” Conte et al write. “Within the domain of medical therapies, we focused on antithrombotic management because of important new evidence in this arena directly relevant to the patient with IC.”
The updated guidelines aim to shift clinical practice toward a more thoughtful, individualised model of care, encouraging clinicians to weigh treatment options in the context of each patient’s overall health, preferences and life goals.
the update.
In an editorial accompanying the guidelines, also published in JVS, Britt Tonnessen (Yale Medicine, New Haven, USA) and Marc Schermerhorn (Beth Israel Deaconess Medical Center, Boston, USA) say that such SVS guidelines provide “in-depth, evidence-based updates without recapping every aspect of a vascular condition.”

“In addition to the 2015 SVS guideline document on IC, the reader should refer to other relevant multispecialty guidelines on general cardiovascular risk management and preoperative evaluation for patients with PAD and IC to supplement this update,” Conte and colleagues add in
Conte
“The SVS has a responsibility to remain steadfast in the sharing of evidence that helps our patients and members,” they write. “This latest CPG on intermittent claudication is rooted in quality and safety, and encourages a patientcentred approach to the management of intermittent claudication—we would encourage everyone to read and consider which parts may best benefit their patients.”
Within the domain of medical therapies, we focused on antithrombotic management because of important new evidence in this arena directly relevant to the patient with IC.”
A research study testing video follow-ups instead of returning to hospital among vascular surgery patients in Hull, UK, has been shortlisted for a national healthcare award. The project showed carbon emissions could be cut by up to 89% per patient, while maintaining safe, high-quality care.
THE PROJECT, TITLED ‘REMOTE-FIRST recovery: Cutting carbon, not care’, has been named as a contender in the ‘Towards Net Zero’ category at the 2025 Health Service Journal (HSJ) Awards, placing Hull University Teaching Hospitals NHS Trust (HUTH) on the national stage. The initiative was led by the Academic Vascular Surgical Unit (AVSU) at HUTH. Rather than a permanent change in service, the project was run as a structured research study. Patients who could be reviewed remotely had secure video consultations, while those needing a physical examination continued to be seen face-to-face. The study showed that smarter use of digital tools could cut unnecessary travel, lower costs, and reduce environmental impact—without compromising outcomes.
Ross Lathan (HUHT, Hull, UK), National Institute for Health and Care Research (NIHR) academic clinical fellow and lead researcher, explained: “The key lesson is that environmental sustainability doesn’t mean higher costs or reduced standards. With careful planning, research can uncover new ways of working that deliver excellent care, real savings, and major environmental benefits.”
Lathan has since been awarded the National Health Service (NHS) England Sustainability Fellowship, recognising his potential as a national leader in greener healthcare research. His next project, working with Dan Carradice (HUTH, Hull, UK), the National Institute for Health and Care Excellence (NICE), and the Universities of Hull, York and Leeds, will explore how sustainability can be built into the NHS’s formal evaluation of new treatments and technologies.
For senior leaders at HUTH, the HSJ Awards shortlist is a reflection not only of innovative research, but also of a broader culture of sustainability.
Tom Myers, group director of Estates, Facilities and
If we can cut carbon without cutting care, everyone wins—patients, staff, and future generations.”
Ross Lathan
Development said: “We are honoured to be finalists. This is a proud moment for our AVSU team, and recognition of their hard work. But it also reflects our wider commitment to becoming a greener, more sustainable health service.”
A press release notes that the Trust has already secured £68 million in funding for sustainability projects, introduced solar panels and heat pumps, installed new insulation, and replaced all fluorescent lighting with energy-saving LEDs. By the end of the year, the release continues, 100% of HUTH’s lighting will be eco-friendly, and its hospitals will generate 10MW of solar power across their sites.
Myers added: “Sustainability is not about compromise. This research shows that greener care can also mean smarter care—and that’s the direction we want the whole NHS to move in.”
The HSJ Awards recognise excellence and innovation across the UK’s National Health Service (NHS) and its partners. A press release details that this year more than 1,250 entries were received, with just 245 projects and individuals making the final shortlist.
HSJ editor Alastair McLellan said: “All of the applications represent the very best of the NHS. They showcase innovation, dedication, and impact. Hull’s research stands out as an excellent example of how evidence can shape the future of care.”
The winners will be announced at a ceremony on 20 November 2025 at Evolution London, where leaders from across the health service will come together to share best practice and celebrate success.
For the team at HUTH, being shortlisted is not just about recognition, but about demonstrating how research and innovation can help the NHS tackle its biggest challenge: delivering high-quality care while protecting the planet. As Lathan put it:
“If we can cut carbon without cutting care, everyone wins—patients, staff, and future generations.”
Positive 12-month outcomes for the Selution sustained limus release (SLR; Cordis) drug-eluting balloon (DEB) were observed, with consistent haemodynamic, functional and clinical improvements irrespective of lesion location or Rutherford classification in both above-the-knee (ATK) and below-the-knee (BTK) arteries.
MICHAEL LICHTENBERG (Klinikum Hochsauerland, Arnsberg, Germany), presenting on behalf of the SUCCESS PTA study research team at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) annual congress (13–17 September, Barcelona, Spain), stated that these results support the utility of the Selution DEB in everyday clinical practice for a “wider spectrum of patients and peripheral arterial disease [PAD] presentations”.
“With this stratified analysis we can now conclude that patients with complex BTK lesions have a very good outcome after sirolimus technology usage—these are the first data to prove that,” said Lichtenberg.
In the trial, investigators included the full SUCCESS PTA cohort of 720 patients, stratified by ATK or BTK lesions: 666 patients to ATK and 156 to BTK. Among the overall cohort,
74% were identified with claudication and 26% with chronic limb-threatening ischaemia (CLTI). Within these subgroups, 78% of claudicants had ATK lesions compared with 54% with BTK lesions; 22% of patients in the ATK group were identified with CLTI compared with 46% in the BTK group.
The patient population had an average age of 70.7 years, 37.9% had diabetes mellitus and 16.8% had renal failure or impairment, Lichtenberg highlighted.
Concerning procedural characteristics, Lichtenberg emphasised that 98.6% of ATK and 99.3% of BTK patients received lesion preparation, largely using plain balloon angioplasty or, to a lesser extent, atherectomy/ thrombectomy.
Device success was reported in 99% of ATK lesions and 99.1% of BTK lesions, with procedural success rates of 98% and 97.6%, respectively. At 12 months, freedom from clinically
SAVE trial reports higher efficacy for Selution SLR DEB in failing AVF
A first report from the SAVE prospective, multicentre, single-blinded randomised controlled trial of the Selution sustained limus release (SLR; Cordis) drug-eluting balloon (DEB) in patients with failing arteriovenous fistula (AVF) undergoing haemodialysis has demonstrated “significantly higher efficacy” compared with plain balloon angioplasty, with a higher target lesion primary patency (TLPP) at six-month follow-up.
KONSTANTINOS KATSANOS (PATRAS University Hospital, Rion, Greece) presented these data during the FIRST@CIRSE session at this year’s Cardiovascular and Interventional Radiological Society of Europe (CIRSE) annual congress (13–17 September, Barcelona, Spain).
A total of 84 patients were randomised to either standard high-pressure balloon angioplasty followed by local application of Selution SLR, or high-pressure balloon angioplasty with no further lesion treatment. The primary safety endpoint was defined as freedom from any serious adverse event involving the patient’s arteriovenous access at 30 days. The primary efficacy endpoint was TLPP, defined as freedom from clinically driven target lesion revascularisation (CD-

CIRSE 2025
driven target lesion revascularisation (CD-TLR) was achieved in 91.4% of ATK lesions and 89.3% of BTK lesions. “This is very convincing data for patients,” Lichtenberg commented, also underlining the 1.7% and 5.2% rates of target limb amputation in the ATK and BTK groups, respectively.
Following his presentation, a question from the audience focused on the nearidentical CD-TLR rates between ATK and BTK lesions, the latter of which are generally assumed to have inferior outcomes. Lichtenberg responded that this discrepancy may have arisen due to the lack of emphasis placed on “proper lesion preparation” in previous datasets concerning BTK disease.
TLR) or access-circuit thrombosis at six months.
Patients were enrolled across three sites in Greece and Singapore. Eligibility criteria included at least one previous successful dialysis access session, stenosis >50% at the outflow vein with clinical circuit dysfunction, and lesions ≤7mm in diameter and ≤80mm in length. Patients had an average age of 63.8 years in the DEB arm and 65 years in the plain balloon angioplasty arm. The number of pretreatment devices used was 1.5±0.7 in the DEB group and 1.3±0.5 in the plain balloon angioplasty group.
“All of these randomised, prospective DEB or paclitaxel trials didn’t use proper preparation strategies, and I can show you here that a lot of colleagues and centres [in the SUCCESS PTA dataset] used BTK lesion preparation strategies. I think this is an important aspect here in combination with DEB technology and this is a shift we need to concentrate on,” he said.
He went on to add that plain balloon angioplasty is “not the way to go” for lesion preparation in future, highlighting instead that new strategies using intravascular lithotripsy, atherectomy or scoring devices in combination with DEBs will produce “much better outcomes” in BTK lesions.
Using Kaplan–Meier curves, Katsanos added that, at 180 days, an 82.5% TLPP rate was observed in the DEB arm compared with 67.5% in the plain balloon angioplasty group (p=0.036).

Reporting results, Katsanos noted 100% device success in both groups. In the DEB arm, a 95.2% rate of clinical success was observed compared with 92.9% in the plain balloon angioplasty arm.
For the primary efficacy endpoint, a 78% TLPP rate was achieved in the Selution SLR group at six months, compared with 58.5% in the plain balloon angioplasty group (p=0.026), with an odds ratio (OR) of 3.1 (95% confidence interval [CI]: 1.1–8.9).
In all cases you must achieve the best angioplasty effect first and then decide whether to upgrade to the DCB.”
Freedom from serious adverse events at 30 days was 92.9% in the DEB arm and 100% in the plain balloon angioplasty arm (p=0.08). CDTLR occurred in 17.1% of patients in the DEB arm compared with 31.7% in those treated with plain balloon angioplasty (p=0.03).
“Selution SLR showed significantly higher efficacy versus uncoated angioplasty at six-month follow-up,” Katsanos underlined.
“This was also associated with improved angiographic follow-up but, most importantly, with improved physiological function, as reflected by the higher minimal lumen diameter and the significantly higher volume flow rate of the fistula at six months.” Volume flow rate was reported as 1,061.7ml/min with Selution SLR versus 823.7ml/ min in the control group (p=0.03).
In the discussion, Katsanos was asked whether lesion preparation had any bearing on the results of the SAVE trial, to which he responded: “Yes— you first prepare the lesion by adequate balloon angioplasty and then deliver sirolimus with Selution SLR.”
“In all cases you must achieve the best angioplasty effect first and then decide whether to upgrade to the DCB. In this study, we performed high-pressure balloon angioplasty with balanced diameter sizing in both study arms, and then applied Selution SLR in the active intervention group,” he explained. “It is imperative to obtain a very good angioplasty effect to begin with.”
Three vascular surgeons discuss how and when they deploy the transformative Shockwave Javelin first-of-its-kind Forward Intravascular Lithotripsy (IVL) Platform in cases of heavily calcified peripheral vascular occlusive disease.
Viewpoints on when and how to use Shockwave Javelin vary, but one insight unites them: prior to its emergence there were limited options available to get through the sorts of severely calcified lesions the device opens up. Or, as Sung Yup Kim (Mount Sinai Health System, New York, USA) puts it: “In the past we have used balloons, we have used orbital atherectomy, cutting balloons with no major success, nothing would track in these areas, and there were cases where we just had to abort and think about an open option. Shockwave Javelin allows us to deliver endovascular therapy for these patients.”
Paul Foley
“At the outset, how we thought we were initially going to use Shockwave Javelin is not how it has turned out to be.” The words of Paul Foley (Doylestown Hospital, Doylestown, USA) as he assesses the evolution in his use of the Shockwave Javelin, from initial study in the FORWARD PAD investigational device exemption (IDE) trial, through limited market release and, earlier this year, the launch of the platform in the USA. Understanding now what the device can do, Foley sees Shockwave Javelin as a routine IVL delivery device in tibial vessels and below the ankle, which is also able to tackle some of the most challenging disease.
That broader canvas for the Shockwave Javelin platform includes use as a primary IVL modality. In the limited market release phase of Shockwave Javelin, the conventional wisdom went that “if you had a boulder of calcium that you couldn’t get across, this was going to be the savior device,” Foley explains. “And, certainly, that’s one piece of Shockwave Javelin.”
However, after more experience Foley has found Shockwave Javelin’s role to be more nuanced and depends on the vessel bed, he says. In the femoropopliteal space, the Shockwave Javelin works best as facilitator, “modifying calcium to facilitate
When I look at a calcified, highly stenotic tibial vessel, Javelin is now my knee-jerk device.”
Paul Foley
the next step”. In the tibial vessels, Foley continues, a good outcome is defined as successfully crossing the lesion while delivering pulses. “I don’t see Shockwave Javelin simply as a method of crossing the uncrossable anymore; I see it as way more than that. When I look at a calcified, highly stenotic tibial vessel, Shockwave Javelin is now my knee-jerk device.” Below the ankle, Foley says, the Shockwave Javelin is breaking new ground by effectively crossing through vessels previously unpassable by any other method. Shockwave Javelin is proving to be a multi-tool for



patients with CLTI, he adds. While vessel bed may vary, the success of the product lies in modifying plaque while achieving luminal gain and a reduction in diameter stenosis—and doing so safely without a high risk of angiographic complications, perforation or distal embolisation.1
For Kenneth Tran (Stanford Health Care, Stanford, USA), Shockwave Javelin first and foremost has proven an important precursor in complex cases. “It’s not intended to be used as the primary IVL technology—I think of it as enabling me to do my next step in my treatment algorithm, where I can’t deliver the device I’m trying to deliver,” he says. In below-theknee (BTK) lesions, Tran sees its use as often initial vessel prep, such as to advance an intravascular ultrasound (IVUS) catheter or Shockwave E8 IVL balloon. However, he recognises instances in which Shockwave Javelin has a role to play as the go-to IVL catheter. “For isolated lesions that are very small, I have had success using the Shockwave Javelin as the sole lithotripsy device,” says Tran.
On the other hand, Kim sees Shockwave Javelin make the biggest difference in his practice in cases involving the femoropopliteal vessel bed. These patients will tend to have either a low segmental chronic total occlusion (CTO) or a couple of focal areas where no devices will track, he explains.
“These are cases where we already have a wire through, there is a rock sitting there, and, with Shockwave Javelin on that spot, we crack open the area, apply some forward pressure, and then try to crack the calcium distal to that. We maintain Shockwave Javelin for one or two cycles in one spot that is really, really calcified and heavy. Then, in the next few cycles, we are moving
forward with Shockwave Javelin.”
Kim doesn’t see the platform as a crossing device. “It is not a case of when you can’t go through a CTO, and you use Shockwave Javelin and try to tunnel a channel through severe calcium,” he says. “I don’t think that’s the purpose of Shockwave Javelin.” Kim tends to encounter trouble with femoropopliteal lesions most often at the Hunter’s canal, where the artery sometimes bends at the popliteal facia. “If you have a severe calcium there, the bend is a killer with a rock-hard calcium,” Kim says.
Step forward Shockwave Javelin: in this small portion of cases, too, the device has proven successful, he adds.
Views on optimal access vary. While Kim prefers a contralateral approach for precision to traverse tough lesions, both Foley and Tran err toward an antegrade access.

“If there is no inflow disease, no disease in the common femoral artery, no disease in the femoropopliteal region that I think is significant, so that going in there is going to be a high likelihood I’m doing a below-knee or a tibial or even a pedal artery intervention, I approach all of those cases from an antegrade approach,” says Foley. “And I don’t have any hesitation to do that because I believe that, with an antegrade approach for below the knee, especially for calcified lesions, really stenotic or at least occlusive lesions, you have a much better chance of getting across them. You get much better pushability and tactile feedback than you would if you were going up and over from a contralateral approach. “But in any patient in whom I’m doing an antegrade approach where I know I’m going to be working below the knee, I always have the foot prepped out so that I have a low threshold of approaching from a retrograde pedal access as well.”



b b b

a) Completion angiogram of AT b) Completion angiogram of DP a
and
Shockwave Javelin provides more flexibility in terms of the level of support available while across a lesion, Tran says. In the BTK space, the antegrade approach allows for more pushability, but some anatomically inappropriate patients enforce the up-andover access of the contralateral approach, he adds.
The platform is a unique tool that can be used to modify calcium previously beyond what was available in his PAD toolkit, Tran says. “This has enabled treatment of more complex tibial lesions from an up-and-over approach. It has also allowed us to be able to treat more complex lesions more thoroughly with larger profile devices.”
Reference 1. Corl J et al. FORWARD PAD IDE/feasibility studies: Primary endpoint analysis of a novel non–balloon-based peripheral IVL catheter. J AmColl Cardiol Intv. 2025 Feb, 18 (3) 398–399.
Updated safety information for the Shockwave advertorial: In the US: Rx Only. Prior to use, please reference Instructions For Use for information on indications, contraindications, warnings, precautions, and adverse events. www.shockwavemedical.com/IFU
Dr. Foley, Dr. Kim and Dr. Tran are paid consultants of Shockwave Medical, the views expressed are of their own opinions, reflect their daily medical practice and do not necessarily represent Shockwave Medical. SPL-78322 Rev. A
“How did an ineffective and costly intervention become routine care in the NHS [UK National Health Service]?” Ian Chetter (Hull York Medical School, University of Hull, Hull, UK) posed at the 39th European Society for Vascular Surgery (ESVS) annual meeting (23–26 September, Istanbul, Türkiye) following a presentation highlighting the SWHSI-2 trial results. SWHSI-2—data from which were first shared at the 2024 Vascular Society of Great Britain and Ireland (VSGBI) annual scientific meeting (VSASM; 27–29 November, Brighton, UK) and recently published in The Lancet—suggested that negative pressure wound therapy (NPWT) should not be first-line treatment for open surgical wounds.
“Negative pressure doesn’t seem to have any benefit whatsoever.” This was Chetter’s key conclusion to be drawn from the “long-awaited” results of the SWHSI-2 trial in his initial presentation of the results at VSASM 2024. For Chetter, the data underscore the severity of the clinical issue at hand, highlight a pressing need for research aimed at accelerating wound healing, and raise questions around how this technology—commonly used in the NHS—was accepted into clinical practice without the robust evidence to back it.
Providing context to the trial at the UK meeting, co-investigator Catherine Arundel (York Trials Unit, University of York, York, UK) first noted the “common” nature of secondary wounds healing by secondary intention (SWHSI) and the “prolonged and complex” nature of their healing. Arundel also underlined the costly nature of treatment, “particularly considering that the use of complex, expensive treatments such as [NPWT] has been and continues to be increasing”.
Against this backdrop, SWHSI-2 was initiated by Chetter, Arundel and colleagues and funded by National Institute for Health and Care Research funding (NIHR HTA 17/42/94). This was a pragmatic, multicentre, two-arm, parallel-group randomised controlled trial designed to assess NPWT versus usual care in patients with open surgical wounds. The primary outcome was wound healing and time to wound healing. Across 28 UK sites, 686 patients were enrolled, with 349 allocated to the negative pressure intervention group and 337 to usual care.
Chetter then provided an overview of patient characteristics, noting that 75% of the trial cohort were men, the majority of whom were white and had diabetes. He added that 90% of patients were vascular patients. “Although we tried to recruit across the different surgical specialties,” said Chetter, “this ended up being a vascular surgical, diabetic foot wound trial.”
Regarding wound characteristics, Chetter shared that the wounds included in the trial were large—26cm2 on average—with the majority on either the foot or the leg.
Sharing the results of SWHSI-2 for the first time at VSASM 2024, Chetter reported that 42% of patients had unhealed wounds at 12 months, a result which “shocked” him. “Importantly,” he continued,” there was no difference in the number of healed wounds between patients randomised to negative pressure or usual care.”
Chetter added that the median time to wound healing was 187 days (NPWT)/195 (standard dressings), or six months, and that there was no difference between negative pressure and usual care. “There’s no evidence that negative pressure accelerates healing in these patients,” the presenter told the VSASM audience, going on to state that the primary result was consistent throughout various subgroup analyses.
The researchers also looked at secondary outcomes, finding high rates of hospital readmission, reoperation, and wound infection, as well as a high number of patients receiving antibiotics for their surgical wound infections, with no statistically significant difference between the two groups. “Negative pressure doesn’t reduce wound infection, doesn’t reduce your risk of reoperation, and doesn’t reduce your risk of requiring
antibiotics,” Chetter summarised.
Furthermore, on the topic of secondary outcomes, Chetter underlined that 10% of patients ended up with an amputation and 10% of patients died.
Quality of life was also measured. “With both treatments,” Chetter reported, “the scores improved— so wound healing improved, and pain improved with both treatments over time—but there’s no statistically significant difference between the two groups. So, negative pressure doesn’t improve wound pain and doesn’t improve your quality of life related to the wound.”
Finally, Arundel shared the results of a cost-effectiveness analysis. “Within the trial,” she summarised, “we can say that negative pressure was more costly, but not more effective than usual care.”
The researchers also considered the longer-term cost-effectiveness of negative pressure, employing a decision analytic model that allowed the team to use both evidence from the trial as well as external evidence.
rates of 10% among the patients included in the trial. Here, Chetter queried how the SWHSI-2 findings might impact his clinical decision-making moving forward. “If I was faced with a patient […] and I could predict that that patient would be in the 40% that didn’t heal or be in the 10% that ended up with an amputation, anyway, would we together—me and the patient—make a different decision about how we manage that foot problem?” he asked.
Closing his presentation, Chetter restated the severity of the problem at the centre of the SWHSI-2 trial. “These wounds are horrendous,” he stressed. “They severely affect our patients.” As a result, Chetter’s forward-looking message was clear: “We need to continue research to try and find a way to accelerate wound healing. Patients are desperate to get these wounds to heal and the quicker we can help them heal these wounds, the better.”
Chetter also highlighted the fact that the data raise wider questions about evidence-based practice. “I think we need to contemplate how technology gets into the NHS,” he posited, citing that NPWT is “widely used” and “widely advocated for” in the UK.
“It was kind of putting the cart before the horse,” the presenter analogised, detailing that NPWT was accepted into UK practice without “robust” clinical and cost-effectiveness data being available.

“There are very small gains in quality-adjusted life years over a longer-term model,” Arundel shared with the VSASM audience. “There were lower healthcare costs for negative pressure compared to usual care, but higher intervention costs, and the incremental costs are therefore higher for negative pressure, but not significantly.”
“There was no gain in terms of quality of life, and no real gain in terms of cost either,” Arundel concluded at this point during the dedicated SWHSI-2 trial session.
It’s a level-one, high-quality trial— the best evidence you can get. Why would you go against that?”
Following the presentation of these first-time results, Chetter shared his key take-home messages.
“These wounds are a chronic, disabling problem,” the presenter stressed, citing again the proportion of wounds in the trial that did not heal being “much higher” than he expected and the length of time it took for those wounds that did heal being “much longer” than anticipated.
Chetter continued that the indications for negative pressure are “minimal”. It “doesn’t accelerate healing, doesn’t improve your quality of life, and doesn’t reduce those secondary outcome measures that we looked at,” he listed. In addition, the presenter reiterated that negative pressure does not reduce a patient’s chance of their wound getting an infection, does not reduce their chance of needing another operation or being readmitted to hospital, and does not reduce their chance of needing antibiotics. Chetter also referenced again the “scary” amputation and mortality
Initial reactions
Before opening the floor to questions, session co-chair Matt Brown (King’s College London, London, UK) commented on the significance of the SWHSI-2 results for vascular practice: “This is incredibly important, to debunk some of these things we do routinely because we think they work.”
Subsequently, one audience member asked for Chetter’s advice to encourage units who might rely on their positive experiences regarding negative pressure to change their practice based on the SWHSI-2 data.
“My answer is: the data’s there,” Chetter replied. “It’s a level-one, high-quality trial—the best evidence you can get. Why would you go against that?”
Manj Gohel (Cambridge University Hospitals
NHS Foundation Trust, Cambridge, UK) praised the “incredible achievement” of Chetter, Arundel and colleagues in completing the trial. “This is why we do randomised trials—to challenge those dogmas,” he remarked, adding that the results were “going to take a while to sink in”.
Gohel went on to ask whether there are any subgroups in which there is still a role for negative pressure. Chetter replied in the affirmative, with the caveat that there are far fewer than previously thought. One example he mentioned was as a wound management strategy. “Save the money,” was Chetter’s main piece of advice here. “Spend it on something more useful, like more nurses, more research nurses, to get that research delivery problem sorted out.”
At ESVS 2025, where Chetter presented the SWHSI-2 findings during the Janet Powell session on latebreaking news in clinical trials, a question from Ian Loftus (St George’s University Hospitals NHS Foundation Trust, London, UK) probed whether the results will translate into clinical practice. Loftus cited anecdotal evidence that he has not seen a reduction in the use of NPWT at his centre in the months following Chetter and Arundel’s initial presentation of the findings in November 2024.
Chetter noted that the SWHSI-2 team has ongoing plans to disseminate the results of the trial widely, and subsequently to conduct a survey to assess their real-world impact. He also shared that the team plans to look at Hospital Episode Statistics (HES) data to see whether NPWT prescriptions are falling.








The CORE-MD—Coordinating Research and Evidence for highrisk Medical Devices—consortium has published new consensus recommendations in The Lancet Regional Health Europe, setting out scientifically robust methodologies for clinical investigations of high-risk medical devices.
THE RECOMMENDATIONS respond directly to a request from the European Commission to provide expert advice on trial design, addressing a longstanding gap in guidance for the evaluation
of high-risk technologies such as cardiovascular implants, orthopaedic devices, and systems for managing diabetes.
Unlike medicines, which must demonstrate safety and efficacy before
market entry, high-risk medical devices in Europe are required to provide only “sufficient clinical evidence”—a standard that is not defined in detail. The CORE-MD analyses confirm that many devices have entered the market without robust evidence from randomised trials, often with little or no data in the public domain.
Key recommendations from COREMD include a four-stage framework for clinical investigations, from initial studies through to long-term follow-up; greater use of randomised controlled trials, including sham-controlled trials with appropriate ethical safeguards; efficient large-scale trials embedded in registries to accelerate evidence generation; mandatory transparency of study design, protocols, and results; and, tailored approaches for
The World Federation of Vascular Societies (WFVS) secretary general details how the organisation has evolved in recent years, seeing gains in representation and access to education among members from underserved regions.
“THE WHOLE MANTRA OF THE WFVS IS the haves help the have nots,” Palma Shaw (SUNY Upstate Medical University, Syracuse, USA), the WFVS secretary general in question tells Vascular News she gears up to attend the latest WFVS symposium in South Africa. “We’re here to pool our resources together and we’re committed to that. We’ve spent a lot of time trying to re-establish the federation as an impactful entity. We’ve put on a lot of different sessions to increase the federation’s visibility, and we have really worked to bring together resources that are accessible.”
The WFVS is an umbrella organisation that unites societies across the globe; a collaborative forum to advance vascular science, education and patient care worldwide; and a network to share expertise and establish global standards of care. In 2021, the federation was revamped with bylaw updates, a new website, and the SVS taking over day-today management.
Shaw says those founding WFVS goals have seen some significant advances in recent times. One of the most prominent: the Global Training Initiative is now established with the aim of bringing together trainees and young surgeons from across the world in accessible programming. “We have representatives from each member society who come together every two months to try to discuss ways we can help them fill in gaps in education,” explains Shaw. “Some have more exposure to different areas of vascular surgery than others. The website is a great enabler of this, helping provide access to trainees and young surgeons in any country, from Africa to South America.” Resources now available include contributions from the SVS, the European Society for Vascular Surgery (ESVS) and the Japanese Society for Vascular Surgery (JSVS).
In person, the WFVS has broadened its footprint at national and international conferences and meetings, which has included WFVS-dedicated sessions at
the SVS Vascular Annual Meeting, the VEITHsymposium, and Charing Cross (CX) International Symposium. And this month sees the WFVS stage its own annual symposium during the Vascular Society of South Africa (VASSA) Congress in Cape Town, the event for which Shaw is preparing as she outlines the federation’s progress.
breakthrough or orphan devices, requiring post-market confirmatory studies.
Alan Fraser (University Hospital of Wales, Cardiff, UK), coordinator of the CORE-MD project, said: “These recommendations provide a clear scientific foundation for the evaluation of high-risk medical devices in Europe. Unlike the European Medicines Agency or the US Food and Drug Administration, which utilise regulatory science, the EU framework has relied in the past on legal interpretation rather than clinical principles. By setting out a hierarchy of methodologies, we can now help developers, regulators and clinicians implement strategies that deliver robust, transparent, and patientrelevant evidence.”
Australia and New Zealand Society for Vascular Surgery (ANZSVS) on the Global Vascular Companionship, a mentorship-based programme to create sustainable vascular units in underserved regions. “This is aimed at trying to help specific nations that have the capacity to build vascular units back home, but who just need that education,” Shaw details, pointing to examples of general surgeons in Barbados and Fiji being mentored by vascular surgeons to get units up and running. “We are endorsing and expanding the reach across the world.

Cost is a limitation to expanding the Global Training Initiative, which has in part spurred the development of WFVS’ digital platform and access to its repository of educational resources. But a couple of key face-to-face opportunities have also emerged.
“We now have WFVS International Fellowships, which are part of a visiting scholar programme, to help those from underserved regions who need more hands-on, advanced training,” Shaw continues. One is hosted by Jikei University in Tokyo, Japan, in which scholars spend three months under the leadership of Takao Ohki. The other is a 12-month program at Houston Methodist DeBakey Heart & Vascular Center in Houston, USA, under the guidance of Alan Lumsden.
WFVS is also working with Iman Bayat (The Northern Hospital, Melbourne, Australia) from the
We’ve put on a lot of different sessions to increase the federation’s visibility, and we have really worked to bring together resources that are accessible.”
“We also worked with the Rouleaux Club, the UK and Ireland’s vascular trainee association, who put on the Worrying Foot Competition at CX in London,” says Shaw. “The problem is funding for these trainees to do anything. The Worrying Foot Competition provided an opportunity to attend. But if you’re not in Europe, it’s an expensive trip so how do you get that support?”
Further WFVS efforts are afoot to plug the gaps. A collaboration with the Journal of Vascular Surgery-Vascular Insights saw the development of a special edition focused on rare vascular diseases, which is available online and due to be published in print. A multi-societal effort to bring together experience with uncommon vascular diseases, Shaw says the edition is aimed at providing perspectives from different parts of the world “you may not get if you’re always coming from the US or Europe”. In the same vein, the WFVS is collaborating with the Vascular Low Frequency Disease Consortium to expand global access to data on rare diseases. Meanwhile, the WFVS council has been expanded. From two per society, the body now includes an additional representative from each constituent member society with an executive leader. “The goal was again to try to have more representation rather than less,” Shaw added. That ecumenical approach extends to helping women vascular surgeons advance, including in areas of the world where barriers endure. “Speaking to colleagues in different parts of the world, it is clear there is less opportunity for exposure, especially women,” she says. “There are fewer women vascular surgeons in places like Africa, for example and, even in Romania, some of the female vascular surgeons may train but they don’t get to work independently, ending up as surgical assists. So, there are countries where I think that maybe we can help women advance and spread knowledge and experience.”



“True patient-centred care demands more than interpreting scans and assessing flow,” Adam Talbot (Manchester, UK) writes, arguing that vascular surgeons must broaden their definition of limb function beyond the realm of mechanics when it comes to the management of patients with chronic limbthreatening ischaemia (CLTI).
IN VASCULAR SURGERY, WE ARE TRAINED to act decisively to save the leg. For patients with CLTI, the rationale for revascularisation is often framed around preserving ambulation and independence. Yet when a limb is perceived as non-functional, for example due to neurological impairment or immobility, decisions about intervention can shift. Amputation may be viewed as the pragmatic or inevitable choice.
However, such reasoning risks reducing the limb to nothing more than a mechanical structure, valued only for its ability to enable
walking. In doing so, we overlook what a limb often represents to patients: a source of stability, a tool for transfers, and a contributor to cardiovascular health. Beyond physical roles, limbs also carry deep psychological and symbolic meaning, tied to body image, identity, and dignity. A limb can remain profoundly meaningful even when its motor function is limited.
When decisions are made solely on the basis of perceived usefulness, we risk falling into the trap of ableist bias, assuming that a life without walking is inherently less valuable, or that limb preservation is not worthwhile. Such assumptions can unconsciously influence multidisciplinary team (MDT) discussions, particularly when the patient’s lived experience is not fully appreciated.

True patient-centred care demands more than interpreting scans and assessing flow. It requires seeing the person behind the limb: understanding how they use it, what it represents, and what matters most to them. Direct
clinical engagement and involving patients in shared decisionmaking provides context that imaging and physiological data alone cannot. Clinicians who spend time with patients are often best placed to advocate for these broader considerations within the MDT. We must also reflect on how our language shapes our decisions.
Describing a limb as non-functional can prematurely close off treatment pathways, while framing it as functionally important in non-ambulatory ways keeps options open. Revascularisation should not be judged solely by its potential to restore walking. Instead, we must broaden our definition of function to include the ways a limb supports independence, self-esteem, and quality of life.
As vascular clinicians, our challenge is to look beyond perfusion and anatomy to the human meaning behind each decision. A limb is more than just function. It is part of the person who lives with it, and our care should reflect that truth.
ADAM TALBOT is a specialty trainee in vascular surgery based in Manchester, UK, and education representative for the Rouleaux Club—the vascular trainees’ association for Great Britain and Ireland.


FastWave Medical appoints principal investigators for IVL pivotal trial FastWave Medical has announced the appointment of its principal investigators and steering committee for the upcoming investigational device exemption (IDE) pivotal trial of Artero, the company’s peripheral electric intravascular lithotripsy (E-IVL) system.
Sahil Parikh, director of endovascular services at Columbia University Irving Medical Center in New York, USA and Venita Chandra, clinical professor of surgery at Stanford Health Care in Stanford, USA, will serve as co-principal investigators. They are joined by Eric Secemsky, director of vascular intervention at Beth Israel Deaconess Medical Center in Boston, USA; Marc Bonaca, vascular medicine and cardiology doctor at the University of Colorado in Boulder, USA; and Daniel Clair, professor and chair of the department of vascular surgery at Vanderbilt University Medical Center in Nashville, USA, who will help guide the trial’s clinical strategy.
The study will evaluate the safety and effectiveness of the Artero IVL system in treating peripheral arterial disease (PAD).
“Calcified lesions aren’t just a technical challenge—the condition remains one of the most significant barriers to successful peripheral interventions,” said Parikh. “FastWave’s pivotal trial gives us a chance to assess whether an advanced IVL system can meaningfully improve the lives of patients who suffer from this difficult disease.”
“The key question isn’t just whether a device works, but whether it makes procedures more efficient and provides physicians with a more predictable tool for treating patients with complex arterial disease,” said Chandra. “I’m excited to see how the promise of FastWave’s peripheral IVL system plays out in this study.”
In a recent press release, the company have stated that Artero delivers circumferential sonic pressure waves at 4Hz—twice the speed of legacy IVL devices—through a oneclick, hands-free system. Its ruptureresistant balloon and low crossing profile are engineered to streamline procedures and maintain reliable performance, even in complex disease.
“With Artero, one of our primary goals is to give physicians a system they can use with more confidence
and predictability—combining speed, simplicity, and reliability to treat all forms of calcified lesions,” said Scott Nelson, chief executive officer of FastWave Medical.
This announcement comes as FastWave advances both of its IVL platforms, including its coronary feasibility study of the Sola laser-based IVL system, which commenced in May 2025.
Shape Memory Medical completes enrolment in EMBO-PMS registry
Shape Memory Medical recently announced the completion of patient enrolment in the EMBO postmarket surveillance (EMBO-PMS) registry, the company’s prospective, multicentre registry of the Impede and ImpedeFX embolisation plugs, and ImpedeFX RapidFill device when used for peripheral vascular embolisation.
A press release details that the Impede embolisation plug family of devices utilises Shape Memory Medical’s proprietary shape memory polymer—a porous, radiolucent embolic material that is crimped for catheter delivery and self-expands to its original shape upon exposure to the warm, aqueous environment of a blood vessel.
According to Shape Memory Medical, EMBO-PMS represents the first prospective, multicentre study designed to systematically evaluate the real-world application of the novel shape memory polymer devices in peripheral vascular embolisation procedures. The study encompasses a diverse spectrum of arterial and venous applications, including visceral aneurysms, pre-endovascular aneurysm repair (EVAR) branch vessel occlusion, pelvic venous disorders, and vascular anomalies. To date, sustained occlusion has been observed at early follow-up, with no serious adverse events related to the study devices.
“Metallic embolisation coils and plugs have long been effective tools, but their use can be limited by imaging interference and artifact on follow-up CT [computed tomography] scans,” said Robert Morgan (St George’s University Hospitals NHS Foundation Trust, London, UK), principal investigator for EMBO-PMS UK. “The radiolucency of shape memory polymer presents a promising advantage by enhancing visualisation of surrounding anatomy during the procedure and improving clarity in follow-up imaging.”
Götz Martin Richter (Klinikum Stuttgart, Stuttgart, Germany), principal investigator for EMBO-PMS Germany, added: “It’s been highly encouraging to see the broad utility of the Impede devices across multiple endovascular applications. We’re eager to see the one-year follow-up data, which will provide important insight into the durability of the embolotherapy.”
Microbot Medical secures patent in Japan for Liberty endovascular robotic system
Microbot Medical recently announced that the Japanese Patent Office has granted the company its first patent, which covers the core Liberty system technology—a compact robotic device for driving and manipulating movement of at least one elongate surgical tool.
In addition to this news, the company has also revealed that it recently received patents in the USA, China and Israel.
The company announced marketing clearance for the Liberty system by the US Food and Drug Administration (FDA) on 8 September 2025 and has accelerated its commercial launch plans as it targets an estimated 2.5 million peripheral endovascular procedures performed in the USA, annually.
A recent press release made by the company states that as it evaluates other future global markets that have historically accepted FDA-cleared devices, the successful implementation of its global intellectual property strategy is expected to protect and allow it to monetise its technology.
Abbott’s Esprit BTK receives Health Canada approval for treating below-the-knee CLTI Health Canada has authorised the Esprit BTK everolimus-eluting resorbable scaffold system for the treatment of below-the-knee (BTK) chronic limb-threatening ischaemia (CLTI), Abbott has revealed in a press release.
Until this recent Health Canada

approval for the Esprit BTK system, Abbott notes that the standard of care has been balloon angioplasty. However, the company notes that treatment with balloon angioplasty alone can have poor short- and long-term results, and—in many instances—the vessels do not remain patent, requiring additional treatment.
Abbott describes the Esprit BTK system as a first-of-its-kind stent that is designed to keep arteries patent and deliver everolimus to support vessel healing prior to completely dissolving.
At the 2024 Vascular InterVentional Advances (VIVA) conference (3–6 November, Las Vegas, USA), Abbott released late-breaking two-year results from the LIFE-BTK trial. The trial demonstrated that, compared to balloon angioplasty, the Esprit BTK system results in improved patient outcomes and 48% fewer repeat procedures over the study period.
“Health Canada’s approval of Abbott’s Esprit BTK system marks a significant milestone in our fight against peripheral arterial disease below the knee and represents a new era of improved outcomes for people worldwide,” said Brian DeRubertis (New York Presbyterian-Weill Cornell Medical Center, New York, USA), one of the principal investigators of the LIFE-BTK trial.
He continued: “Abbott is changing the landscape of CLTI therapy by introducing a treatment option that is superior to balloon angioplasty.”
Xeltis poised to commercialise aXess vascular access conduit following pivotal trial results Xeltis has announced clinical data from the aXess EU pivotal trial, the prospective study investigating the patency, safety, and performance of its restorative vascular access conduit— aXess—in adult patients with end-stage renal disease requiring vascular access to start or maintain haemodialysis. The results from the trial, which was conducted in 18 centres across Europe, showed that aXess delivered improvements on all key clinical metrics compared to the standard of care, Xeltis has said in a press release. aXess demonstrated superior sustained patency across both primary and secondary outcomes, compared to other arteriovenous grafts (AVG), while requiring fewer interventions and showing a lower reintervention rate compared to standard of care and high infection resistance compared to grafts. Many of the patients in the trial had a history of failed AVGs or fistulas for dialysis or had previously relied on central venous catheters (CVC), which further underscores the significance of the results, despite the patients’ serious health challenges, the company press release states.
An De Vriese (AZ Sint-Jan, Brugge, Belgium), coordinating investigator of the aXess EU pivotal trial, commented: “Xeltis’ technology is truly transformative, delivering superior sustained patency along with fewer interventions and complications, such as infections. This is unprecedented in our industry and heralds a new dawn in sustainable treatment options for patients.”
Developed through Xeltis’ proprietary Endogenous Tissue Restoration (ETR) platform technology, aXess is gradually replaced over time by patients’ own living tissue, dissolving completely over the course of the process. With only one cannulation infection-related (partial) explant across the full 120 patient population, Xeltis notes that aXess is also highly infection resistant, and can deliver near-immediate cannulation, with a below 0.02% rate of bleeding complications observed across over 15,000 dialysis sessions.

Terumo Aortic and Bentley announce collaboration on US FEVAR study
Terumo Aortic and Bentley recently announced their partnership in a clinical study in the USA. The objective is to obtain US Food and Drug Administration (FDA) investigational device exemption (IDE) approval for Terumo Aortic’s fenestrated Treo device, in combination with Bentley’s BeFlared fenestrated endovascular aneurysm repair (FEVAR) stent graft system for FEVAR procedures. The IDE submission is planned for the first half of 2026.
Through this collaboration, Terumo Aortic and Bentley note in a press release that they aim to bring their FEVAR solutions to benefit US patients suffering from complex abdominal aortic aneurysms, namely juxtarenal and suprarenal aneurysms. Following the completion of the joint clinical study and FDA approval, both companies plan to independently market their products in the USA.
Terumo Aortic shares that its fenestrated Treo device offers a patient-specific solution tailored to the anatomical conditions and clinical needs for treating aortic aneurysms. The company details that, to ensure perfusion of vital organs, the device can be designed to include up to five fenestrations, requiring reliable bridging stents to maintain perfusion to the target vessels.
Erik Pomp, chief executive officer (CEO) of Terumo Aortic, commented: “Bentley’s BeFlared, the world’s first-to-market dedicated bridging stent, is a great fit for our fenestrated Treo platform. With its innovative features, this stent has the potential to become the gold standard in FEVAR procedures, thus complementing our fenestrated Treo platform.”
Conference calendar
29 October–1 November Egyptian Vascular and Endovascular Society (EVES) Summit Cairo, Egypt evesonline.org/2025-event
30 October–1 November Venous Symposium Europe Athens, Greece venoussymposiumeurope.com
Bentley adds that BeFlared is the world’s first bridging stent specifically developed for FEVAR procedures. The company notes that the stent is crimped on a specially designed stepped balloon, which will reduce the number of steps that are needed for optimal deployment. Additionally, a third radiopaque marker aids in optimal positioning of the stents in the fenestration. BeFlared has received CE certification from TÜV Süd and the first-in-human procedure was performed by Stéphan Haulon (Université Paris-Saclay and Hôpital Marie Lannelongue, GHPSJ, Paris, France) in November 2024.
“By partnering with Terumo Aortic, we’re advancing BeFlared’s role as the go-to bridging stent for FEVAR. Already available in most key markets, its value is being demonstrated through strong outcomes led by KOLs [key opinion leaders]. This study will further solidify its impact in treating complex aortic aneurysms. Terumo Aortic’s fenestrated Treo platform is an excellent complement, and together, we are setting new standards in patient care,” said Sebastian Büchert, CEO of Bentley.
“The combination of fenestrated Treo and BeFlared is designed to set a new benchmark for FEVAR procedures by offering both ease of use and reduced complications for patients,” the press release reads. “Together, Terumo Aortic and Bentley are driving innovation in the field of aortic disease treatment.”
Jupiter Endovascular secures funds to continue development of TFX platform
Jupiter Endovascular has closed a series B financing round, surpassing its US$40 million target, with proceeds to be used to complete the ongoing SPIRARE II pivotal clinical trial, prepare for commercialisation, and develop new clinical applications for its Transforming Fixation (TFX) platform technology.
The financing comes between two major milestones; US Food and Drug Administration (FDA) 510(k) clearance of the Vertex catheter incorporating TFX, and the presentation of first-inhuman results for the Vertex pulmonary embolectomy system with TFX from
1–2 November The VEINS (Venous Endovascular Interventional Strategies) Las Vegas, USA viva-foundation.org
2–5 November VIVA (Vascular InterVentional Advances) Las Vegas, USA viva-foundation.org
the SPIRARE I trial at the 2025 TCT meeting (25–28 October, San Francisco, USA).
The round was led by Sonder Capital, with participation from Senvest Management, LB Investment, and a new strategic corporate investor.
“Closing this oversubscribed series B financing round reflects strong conviction in our mission to rewrite the rules of endovascular medicine using TFX technology,” said Carl J St Bernard, chief executive officer of Jupiter Endovascular. “We’re energised by the support of this distinguished syndicate of investors as we tackle one of the greatest unmet needs in transcatheter interventions—the lack of stability and control physicians face while operating in complex cardiovascular anatomies—starting with pulmonary embolism.”
SPIRARE I is a prospective, single-

arm, multicentre study which enrolled 10 subjects with acute, intermediaterisk pulmonary embolism (PE) treated with the Vertex pulmonary embolectomy system at two sites in Europe. The results from SPIRARE I will be presented at the TCT 2025 meeting by Irene Lang (Medical University of Vienna, Vienna, Austria), the trial’s principal investigator.
The company is also currently enrolling patients in SPIRARE II, a prospective, single-arm, multicentre pivotal trial which will enrol up to 145 patients with acute, intermediate-risk PE treated with the Vertex pulmonary embolectomy system at up to 25 sites in the USA and Europe.
Trial endpoints for both SPIRARE I and SPIRARE II will characterise the procedural and clinical benefits of PE treatment with TFX using the Vertex system, across measures of safety, right heart function, and clinical improvement from the time of the procedure to 30 days post-procedure.
The company’s TFX platform
technology, integrated into the Vertex system, is designed to bring stability and control to a variety of catheter interventions while protecting the cardiovascular anatomy, with the goal of enabling interventionalists to treat anatomical sites that are not safely or easily accessible via conventional endovascular approaches.
The Vertex device is delivered in a flexible, relaxed state over a guidewire to a target location in the vasculature, rapidly pressurised with saline to fix it in a stable position for intervention, then returned to its relaxed state to navigate to another target location or for removal.
“Since the earliest days of endovascular medicine, the inability to maintain catheter control has been the single greatest barrier to the wider adoption of transcatheter therapies,” said Deborah Kilpatrick, partner at Sonder Capital. “Jupiter is addressing this vast unmet need with a revolutionary advance that can provide stability in even the most tortuous anatomies, unlocking new possibilities for the millions of patients who are being underserved with today’s intravascular approaches. We are proud to lead this round and to partner with Carl and the Jupiter team to bring this technological breakthrough to physicians and patients worldwide.”
Interventional Systems and Siemens Healthineers partner to expand access to robotics in the angiography suite
Interventional Systems and Siemens Healthineers recently announced a co-marketing agreement to promote the benefits of robot-assisted percutaneous procedures in the Siemens Healthineers angiography suites.
A press release notes that this collaboration leverages the strengths of both companies to address the growing demand for enhanced precision and efficiency in image-guided therapies. Interventional Systems details that its Micromate product is a miniature robotic platform cleared for any 2D and 3D image-guided, needle-based procedures that delivers submillimetre accuracy to reduce radiation exposure to operating room (OR) staff and patients and speed up intervention times. Siemens Healthineers shares that its Artis systems offer high-resolution real-time imaging.
18–22 November VEITHsymposium New York, USA veithsymposium.org
26–28 November The Vascular Societies’ Annual Scientific Meeting Hull, UK vascularsociety.org.uk/events/57/ vascular-society-annual-scientificmeeting-2025
4–5 December 14th Munich Vascular Conference (MAC) Munich, Germany mac-conference.com
4–6 December VERVE Symposium Sydney, Australia vervesymposium.com
11–13 December Paris Vascular Insights Course (PVIC) Paris, France paris-vascular-insights.com
27–30 January Leipzig Interventional Course (LINC) Leipzig, Germany hmpglobalevents.com/linc