

A guide to Breaking seed dormancy of common wetland plant species
Samantha R. Kurkowski, Rae Robinson, Sandra E. Johnston, Montana M. Horchler, Adrienne E. Ernst, Margaret D. Haney, and Karin M. Kettenring
Wetland Ecology and Restoration Laboratory, Utah State University




Preface
Restoration of native plants in degraded wetlands is essential to recover native plant communities and the critical ecosystem services they provide. These services include supporting biodiversity, improving water quality, providing wildlife habitat, sequestering carbon, and mitigating drought, flooding, and wildfire impacts (Kettenring & Galatowitsch, 2007a). Seeds are commonly used in restoration due to their broader availability, cost effectiveness, and greater scalability than plants. However, seed dormancy can challenge seed-based restoration of wetlands.
Seed dormancy is an evolutionary trait that prevents seeds from germinating under otherwise suitable conditions (Baskin & Baskin, 2014; Willis et al., 2014) Dormancy helps regulate the timing of seed germination to foster seedling establishment and survival. The specific environmental conditions required to break dormancy vary widely by species. For example, some species’ seeds require a period of cold, moist conditions, such as seeds might experience during winter underneath snow or leaves, to break dormancy and germinate once conditions become favorable in the spring.
Knowing how to break dormancy for different species is an important aspect of restoration. While it is not inherently detrimental to sow dormant seeds sometimes it is even advantageous, breaking seed dormancy before sowing is desirable when high, rapid germination is needed for the restoration (Kildisheva et al., 2020). For example, in highly invasion-prone sites, rapidly establishing native plant communities is essential.
This guide was developed from a series of seed dormancy break and germination experiments conducted over 4 years with 41 species The purpose of this guide is to synthesize the information we learned through these experiments and present the findings for those interested in breaking seed dormancy, such as land managers, restoration practitioners, and wetland researchers. Ultimately, we hope this guidance catalyzes further wetland restoration throughout the Intermountain West. As information is developed for new species, the guide will be updated with new information (as indicated by the edition number).
Acknowledgments
This guide was made possible with support from the Environmental Protection Agency; Utah Agricultural Experiment Station; Utah Department of Agriculture and Food; Utah Division of Forestry, Fire and State Lands; Utah Division of Wildlife Resources; and Utah State University Extension.
Recommended citation
Kurkowski, S. R., Robinson, R., Johnston, S. E., Horchler, M. M., Ernst, A. E., Haney, M. D., & Kettenring, K. M. (2025) A guide to breaking seed dormancy of common wetland plant species. Utah State University’s Wetland Ecology and Restoration Laboratory and Utah State University Extension.

Background information
What is dormancy?
Dormancy is an innate property of seeds that prevents them from germinating even when environmental conditions are favorable for germination including the appropriate temperature, moisture, and light conditions (Baskin & Baskin, 2014; Willis et al., 2014). It is a period of low metabolic activity for seed that is mediated by environmental conditions and plant hormones (Willis et al., 2014) Germination is the expansion of the embryo through the seed coat to begin the growth of the seedling, marked by the protrusion of the radicle (the embryonic root). Interestingly, the conditions required to break dormancy are distinct from the conditions required for germination (Thompson et al., 2003). If a seed has dormancy, both the dormancy breaking conditions and favorable germination conditions must be met before the seed will germinate. Treatments that can break dormancy include cold stratification, chemical or physical scarification, and dry heat, each of which will be discussed later in this guide (Baskin & Baskin 2014).
Evolutionarily, seeds benefit from having dormancy because it can prevent them from germinating in unfavorable environmental conditions when seedling survival would likely be low (Baskin & Baskin, 2014; Finch-Savage & Leubner-Metzger, 2006). Timing is often an important part of seed germination, because seedlings are highly vulnerable to unfavorable conditions. In general, seeds have a higher likelihood of survival if they germinate at the beginning of the growing season as opposed to the end (Lu et al., 2016).
Dormancy also helps species withstand harsh or unfavorable conditions. By preventing germination until conditions are likely suitable for seedlings, species are more resilient to disturbance and extreme conditions (Schütz & Milberg, 1997). Resilience is also facilitated by the development of a seed bank, which is made up of dormant and nondormant seeds (Schütz & Milberg, 1997; Brock et al., 2003, Thompson et al., 2003). Dormant seeds are often buried in the soil, sometimes remaining there for long periods of time (even years to decades), allowing species’ persistence at the site until more favorable conditions for germination and establishment return (Satterthwaite, 2009; Willis et al., 2014; Long et al., 2014).
It is important to note that not all species have seed dormancy. Some species have “non-dormant” seeds where germination occurs readily if the appropriate germination conditions are met. For those that do have dormancy, there are five main types as defined by Baskin and Baskin (2014).
• Physiological dormancy: Seeds readily imbibe (uptake) water and have fully developed embryos but are slow to germinate because of a chemical or physical barrier. The embryos of physiologically dormant seeds also have low growth potential, or “push power,” that contributes to the prevention of germination. This


type of dormancy can often be overcome with cold and moist conditions (i.e., cold stratification). Physiologically dormant seeds can cycle between dormant and non-dormant states over growing seasons. As seeds transition out of dormancy (i.e., are partially dormant) with increasing exposure to dormancy breaking conditions, the range of suitable conditions for germination widens and seeds germinate more readily.
• Morphological dormancy: Seeds have small embryos that are underdeveloped and/or undifferentiated (i.e., lacking a radicle and cotyledon embryonic root and leaves, respectively). The seed coat is water permeable and there are no physical or chemical barriers. Seeds need more time under favorable conditions to break this type of dormancy.
• Physical dormancy: The embryo is fully developed, but the seed coat is impermeable to water, which prevents water uptake and germination. Physical dormancy is often broken with mechanical or chemical scarification that removes the barrier to water imbibition and sometimes heat broken by boiling seeds or placing them in an oven at low temperatures
• Morphophysiological dormancy: A combination of morphological and physiological dormancy.
• Combinational dormancy: A combination of physical and physiological dormancy.
Why is breaking dormancy important?
While dormancy is an important evolutionary trait of many seeds, sowing dormant seeds during restoration projects can lead to initially low germination due to the delay that occurs while seed dormancy is naturally broken in the field over weeks, months, or years (Espeland et al., 2017; Kildisheva et al., 2020). A dormant seeding can also result in lower germination percentages because there will be some (sometimes a little, sometimes a lot) seed mortality due to predation, desiccation, and loss of seed reserves in the period between when dormancy is broken and germination occurs (Kildisheva et al., 2020). By breaking seed dormancy prior to seed sowing, higher germination can occur if the restoration conditions are suitable for supporting seed germination and seedling establishment (Kettenring & Galatowitsch, 2007a). Quickly establishing native plants by breaking dormancy also makes it possible to limit the establishment of invasive species and increase cover of the sown species to achieve other restoration goals like erosion control (Kettenring & Galatowitsch, 2007a).
Understanding seed dormancy also allows restoration practitioners to purposely sow dormant seeds, potentially waiting out periods of unfavorable conditions or taking a bethedging approach by strategically leaving some sown seed dormant to build up the seed bank while achieving some native plant cover with non-dormant seeds (Long et al., 2014; Kildisheva et al., 2020) Thus, determining dormancy breaking techniques for species used in restoration offers managers and restoration practitioners a valuable tool for restoration efforts (Kettenring & Galatowitsch 2007b). Of course, when plants are
used in restoration and research, it is often necessary to grow them from seeds, so understanding species’ dormancy behavior is essential.
Dormancy breaking techniques for physiologically dormant seeds
The species included in this guide have physiological seed dormancy, the most common type of dormancy in plants including those found in wetlands (Baskin & Baskin, 2004; Baskin & Baskin, 2014). We focus on three commonly used techniques for breaking dormancy of physiologically dormant seeds: (1) cold stratification, (2) chemical scarification, and (3) physical scarification We focus first on breaking dormancy in small batches of seed, followed by a section on working with large seed batches.
It is common for multiple techniques to be used in tandem, such as placing seeds into cold stratification following chemical seed scarification. It is also common for multiple techniques to work equally (or nearly equally) well to one another. In this guide, we will recommend a preferred method of breaking dormancy and any alternative methods of breaking dormancy that may also be acceptable or necessary given logistical constraints
1. Cold stratification: small seed batches
Cold stratification mimics moderate winter conditions in the soil cold and moist and mechanically or physically weakens the seed coat. The optimal duration of cold stratification varies widely between species, with a minimum of 2–4 weeks but sometimes requiring 12 weeks or more.
Supplies
● Container to hold sand and in which to bury seeds.
○ The container should have small holes drilled into the bottom for draining excess water (for moist but not saturated conditions).
● Quartz sand: both 4075 and 4095 grit are acceptable.
● Sphagnum moss to ensure some moisture retention within the cold stratification chamber and to keep mold grow to a minimum with its acidic properties.
● Mesh fabric for wrapping seeds.
○ Organza fabric from any craft store or paint strainers work well.
● Filter paper or paper towel to wrap extremely small seeds.
● Colored ribbon for tying seed packets if species need to be differentiated during cold stratification.
● Duct tape for sealing the containers.
● 2 °C – 4 °C cold storage space, such as a refrigerator or large, walk-in cooler.

Protocol
Prepare seed packets
1. Cut organza fabric into squares large enough to fit all the seeds, but not larger than necessary.
○ For larger batches of seed, paint strainers work well as a large mesh bag to hold the seeds.
2. Place seeds into the mesh organza fabric squares.
3. For extremely small seeds (e.g., seeds in the Juncaceae family), first place seeds in filter paper or paper towel inside the mesh. This approach will prevent the tiny seeds from falling through the mesh fabric.

Single seed packet tied with ribbon.
4. If needed, tie the seed packet tightly with colored ribbon The color of the ribbon will serve as a label while the seeds are in cold stratification.
○ The more types of seed packets being put into cold stratification, the more ribbon colors you will need
○ Make sure your ribbon colors are colorfast if wet (i.e., synthetic, not cotton).
○ Be sure to write down which colors of ribbon correspond with which seeds are in each packet.
5. It can be helpful to tie an additional long string to each packet (that reaches the edge of the container) to make the packets easier to find and retrieve if they are buried for cold stratification.
6. IMPORTANT: Soak seed packets in water for 24 hours to ensure the seeds are fully imbibed before going into cold stratification
7. Soak the sphagnum moss in water at this time (for 24 hours) to ensure it is also saturated before going into cold stratification.

Seed packets tied with various ribbon colors to enable identification after cold stratification.


Bury seed packets into layers of sand and moss
1. If your container does not already have holes in the bottom, use a drill or a sharp tool to create holes that will let water drain but will keep sand from escaping the container. The holes should ideally be <0.5 cm in diameter.
2. To avoid mold issues, bleach and thoroughly rinse your container prior to use.
3. Place the cold stratification container into a large sink or on a work table outside (this could get messy!)
4. Layer sand about 5 cm deep into the bottom of the container. Wet the sand thoroughly until saturated and allow the water to drain from the holes in the bottom of the container.
5. Dig small holes throughout the sand layer and bury the seed packets into the sand Be sure to press the seeds into the sand so that the whole packet has good contact with the moist sand.
○ Hang the long strings attached to the seed packets outside of the container as you bury the packets.
6. Cover seed packets with another 5 cm layer of sand.
7. Spread a layer of wet sphagnum moss on top of the sand a few centimeters thick.
8. If your container is very deep and you have lots of seeds to stratify, you can repeat steps 3–6 and create multiple layers of sand, seed packets, and moss in the container.
9. Put a lid on the container and duct tape the edges tightly so that water does not easily evaporate out.
10. Approximately every 3 weeks, open the container and check to ensure the moss on top is still moist. If needed, add more water, allow the water to drain, duct tape the lid, and return to cold stratification.
○ It is helpful to write the date the seeds were placed into cold stratification on the container. This step will ensure that it is known how long the seeds have been in cold stratification.

Burying the seed packets in moist sand

Sand covered with wet sphagnum moss

Containers sealed with duct tape to retain moisture.

○ If the container is placed in a shared cooling space, be sure to write your name, phone number, and the dates that the container should remain in the cooling space.
11. When the container reaches the recommended length of time in cold stratification, take the container out of the cooling space, and remove the seed packets from the sand.
○ The sand and moss should be discarded and not reused to minimize mold growth.
Duration
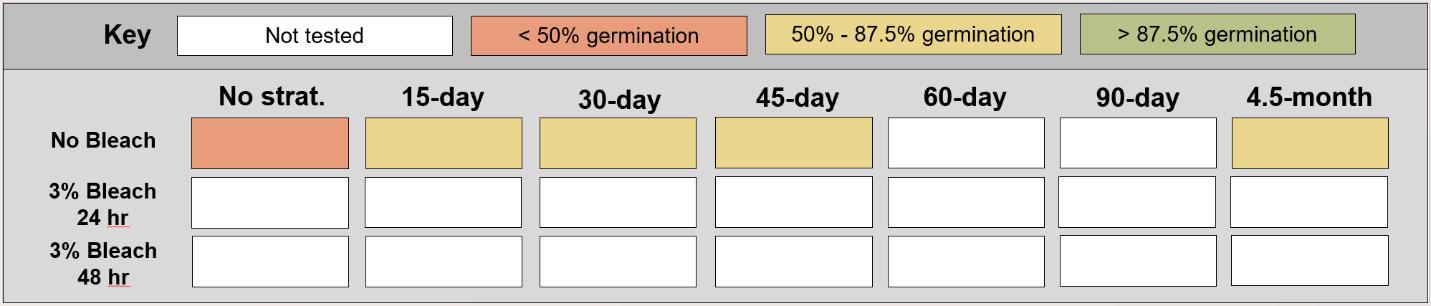
The length of cold stratification investigated and described in this document ranges from 15 days to 4.5 months. Cold stratification can be done for any length of time that is desired or best fits a species’ needs. The range included in this guide is simply the range that we tested and does not reflect the limits of cold stratification duration.
2. Chemical scarification: small seed batches
Chemical scarification is another way to break physiological seed dormancy and is used for seeds that have deeper dormancy or thicker seed coats. This process includes soaking the seeds in a basic solution such as sodium hypochlorite (bleach) or an acid solution like sulfuric or hydrochloric acid. These harsh chemicals break down the seed coat enough to allow the embryo to push through it. This method of dormancy breaking mimics seed abrasion in mineral soil and also animal digestion, with the weakening of the seed coat by digestive enzymes, common among many wetland species consumed by birds. However, this method can be risky. If seeds are soaked for too long, the embryo can be destroyed.
Supplies
• Bleach concentrate
o Most bleach concentrate comes in 6% or 7.5% bleach solution.
o Avoid “no splash” bleach or any other bleach that contains additives.
o Most generic bleach lists its ingredients and formula percentage.
• Water to mix the bleach solution (tap water is fine)
• Bleach-safe container (glass or high-density polyethylene [HDPE]), used to soak seeds in the bleach
o This container could be small or large depending on the number of seeds used.
• Graduated glass cylinder to measure liquid quantities to ensure the appropriate concentration of bleach is reached.
• Seeds to be bleached.

Protocol
1. Mix a 1% or 3% bleach solution (specified for each species), diluted properly to account for bleach concentration being used. See below for bleach mixing conversions.
○ Mix the appropriate amount of bleach with tap water to get the desired concentration.
○ Be sure to look at the concentration on the label of the bleach bottle to know how much it needs to be diluted.
○ The amount of bleach solution needed will depend on the number of seeds being soaked, so scale solution quantity appropriately. It is best to have just enough solution to submerge the seeds to avoid creating extra chemical waste.
2. Prepare seed packets according to the protocol above in the “cold stratification” section. For bleaching seeds, it is important to use as wide a mesh as possible to ensure the seeds are thoroughly rinsed once the bleaching process is complete while avoiding any seeds escaping through the holes in the mesh.
3. Soak seeds in the bleach solution for the length of time specified for each species (e.g., 24 hours).
○ The seed bags will likely float. Weigh down the seed bags until they are fully submerged with something heavy that will not be damaged by the bleach solution, such as another plastic container filled with water.

Seed packet submerged in bleach, held down by another cup filled with water.

Rinse the seed packet thoroughly under running water, massaging the packet with your fingers.

4. After soaking the seeds for the appointed time, rinse seeds thoroughly (potentially many times) with tap water to remove any bleach residue.
○ The easiest way to thoroughly rinse the seed packets is to run each packet under a faucet and massage the packet with your fingers. You may need to run water through the packet for over a minute to fully rinse the bleach off the seeds.
○ A good way to check that the bleach is fully rinsed is to smell the seed packet. Bleach has a strong smell, and if there is still bleach on the seeds, you will likely smell it.
5. Once done rinsing the seeds, you may need to cold stratify seeds in addition to chemical scarification. See instructions later in this guide for each species on which techniques to use and the “Cold stratification” section for instructions on how to perform this treatment.
Mixing ratios to make 3% bleach
If using 6% bleach concentrate…
● To create 100 mL, mix 50 mL of 6% bleach with 50 mL of tap water
● To create 250 mL, mix 125 mL of 6% bleach with 125 mL of tap water
● To create 500 mL, mix 250 mL of 6% bleach with 250 mL of tap water
If using 7.5% bleach concentrate…
● To create 100 mL, mix 40 mL of 7.5% bleach with 60 mL of tap water.
● To create 250 mL, mix 100 mL of 7.5% bleach with 150 mL of tap water.
● To create 500 mL, mix 200 mL of 7.5% bleach to 300 mL of tap water.
3. Physical scarification: small seed batches
Physical scarification serves a similar purpose as chemical scarification, but it uses tools like sandpaper to break down hard seed coats instead of an abrasive chemical. This approach mimics natural erosive forces that the seed experiences on the soil surface or in the soil with the mineral soil component
Soaking seeds in boiling water is another method of physical scarification, but it is not commonly used. None of the species included in this guide benefited from physical scarification as a breaking dormancy method in our trials. However, if you are having difficulty getting seeds to germinate, physical scarification is a viable breaking dormancy method to try.

Supplies
• Two or more sheets of sandpaper (60 grit is a good starting point but adapt to your species’ needs)
• Tray or bowl to hold seeds
• Wooden block for grinding the seeds into the sandpaper.
Protocol
1. Place one piece of sandpaper into the bottom of the tray.
2. Pour seeds into the tray on top of the sandpaper.
3. Use the second piece of sandpaper to gently rub the seeds between the two sheets of sandpaper.

Sandpaper taped to the bottom of a bowl and on the inside of a potato chip can. The seeds can be poured in and scarified with another piece of sandpaper. To scarify seeds inside the potato chip can, sandpaper was glued to a wooden dowl.
○ Do not to apply too much pressure at first, or you may damage the seeds.
○ Start gently and gradually add more pressure until the seed coat is visibly roughened.
4. Continue grinding the seeds between sheets of sandpaper until you can see abrasions digging through the seed coat. This process usually takes between 2–6 minutes depending on the thickness of the seed coat If there are not set protocols for your species, you will need to do some trial and error to determine how much abrasion is required for your species’ dormancy break.

Seeds before scarification; they are shiny and darker in color.

Seeds after scarification; they are duller and have light-colored abrasions on the seed coat.
4. Large batches: cold stratification and chemical scarification
When working with large batches of seed, the same processes can be followed for cold stratification and chemical scarification as with small batches of seed, but some adjustments need to be made to accommodate larger seed volumes. This section describes the techniques used by wetland managers around Great Salt Lake to break dormancy on large batches of seed (usually in 50 lb. bags) that are then sown using large wetland equipment such as a Marsh Master.
Supplies
• Large (e.g., 10-gallon) woven polypropylene bags (or similar large mesh bags)
• Rope or twine to tie bags closed.
• Plastic 50-gallon garbage cans.
Protocol
Bleaching, if applicable:
1. Fill mesh bag with 50 pounds of seed and tie closed. Repeat for entire seed lot.
2. Mix bleach solution (see small batch bleach protocol for bleach to water ratios).
3. Fill plastic garbage cans with bleach solution.
4. Soak seeds in the bleach solution for the length of time specified for each species (e.g., 24 hours).

5. After soaking the seeds for the appointed time, rinse seeds thoroughly (potentially many times) with tap water to remove any bleach residue.
o A good way to check that the bleach is fully rinsed out is to smell the seeds. Bleach has a strong smell and if there is still bleach on the seeds you will likelybe able to smell it.
6. Once done rinsing the seeds, you may need to cold stratify seeds in addition to chemical scarification. See instructions later in this guide for each species on which techniques to use. See the large batch “Cold stratification” section below for instructions on how to perform this treatment.


Large mesh bag partially filled with seeds.
Mesh bags tied shut, soaking in bleach.
Cold stratification
1. Fill mesh bag with 50 pounds of seed and tie closed. Repeat for entire seed lot.
2. If seeds were not already bleach treated, soak seed bags in water for 24 hours to ensure the seeds are fully saturated (imbibed) going into cold stratification. Otherwise, the treatment may not be successful.
3. Bleach and thoroughly rinse your seed storage container to avoid mold issues.
o If your container does not already have holes in the bottom, use a drill or a sharp tool to create holes that will allow water drain but will keep sand from escaping the container. They should ideally be <0.5 cm in diameter.
4. Put a lid on the container and duct tape the edges tightly so that water does not easily evaporate out.
o Label the container with the seed mix information and also write the date the seeds were placed into cold stratification on the container to track cold stratification length.
5. Place the containers in a large walk-in cooler set at 2 °C –4 °C.
6. When the containers reach the recommended length of time in cold stratification, take them out of the cooling space, and remove the bags


Totes with seed bags in the cooler for cold stratification. Totes are wrapped in plastic to maintain moisture and transported on pallets.

Mesh bags filled with seed, soaking in water in their totes before being put into cold stratification.

Results described in this guide
The dormancy break recommendations and seed germination data described in this guide are a result of 3 years of experiments on seeds from a wide variety of native wetland plant species. This section will describe the nature of the experiments carried out and the different dormancy breaking treatments that were tested. We also describe the way we displayed the data and how it can best be interpreted.
Experiment setup
Four experiments were conducted to produce the data described in this guide. Three were carried out sequentially in 2022 to test a variety of cold stratification and chemical scarification treatments, and an additional experiment was conducted in 2024 testing additional species under various cold stratification and germination temperature treatments. All experiments were conducted in four CONVIRON A1000 growth chambers that can be set to various temperature, humidity, photoperiod, and light level conditions. Growth chamber lights consisted of F39W/T5/841/ECO fluorescent lamps with 4100 K color bulbs. Light levels were maintained at the highest level throughout these experiments, reaching 350–450 µm m-2 sec-1
In each experiment, each treatment was replicated 3 times (one replicate germination dish per growth chamber), and germination dishes were placed randomly within each growth chamber. Replicates were made up of about 100 viable seeds of a given species. Viable seed estimates were based on laboratory tetrazolium viability tests that were conducted prior to the experiments, and the number of seeds per germination dish was increased proportionally to account for species with <100% viability
Procedures for cold stratification and chemical scarification treatments that were used in these experiments are described above in the “Dormancy breaking techniques” section. Treatments and growth chamber conditions applied in each experiment can be seen below in the “Experiment treatments” section. Experiments with multiple sets of treatments (such as cold stratification and bleach) were full factorial experiments where every combination of treatments was tested.
Data collection
Once dormancy break treatments were complete, seeds were laid out in a grid in 11 × 11 × 3.5 cm germination dishes and placed in the growth chambers for 21–60 days to track germination. Germination dishes were partially filled with 4075 industrial quartz sand, which was fully saturated (but without standing water), and a piece of filter paper was placed on top to prevent the seeds from getting buried in the sand.

Germination dish with 100 seeds gridded out to facilitate counting for data collection
Every 2–4 days during the experiments, seeds were examined under the microscope, and germinating seeds were counted. Germination was confirmed when the radicle or cotyledon of the seed protruded at least 1 mm from the seed coat. Germinated seeds were then removed from the germination dish and discarded.
Experiment treatments
Experiment 1
Growth chamber conditions represented spring wetland soil temperatures in the Intermountain West: 32 °C during the day and 15 °C at night. Lights were set to full brightness for a 14-hour photoperiod
Dormancy break treatments:
• 0-day cold stratification, 24-hour water soak, or
• 4.5-month cold stratification at 2 °C.
Experiment 2
Growth chamber conditions represented spring wetland soil temperatures in the Intermountain West: 32 °C during the day and 15 °C at night. Lights were set to full brightness for a 14-hour photoperiod.
Dormancy break treatments:
• 0-day cold stratification, 24-hour water soak,
• 15-day cold stratification at 2 °C,
• 30-day cold stratification at 2 °C, or
• 45-day cold stratification at 2 °C.
Experiment 3
Growth chamber conditions represented spring wetland soil temperatures in the Intermountain West: 32 °C during the day and 15 °C at night. Lights were set to full brightness for a 14-hour photoperiod. Bleach treatment seeds were placed in a 3%


sodium hypochlorite solution for 0, 24, or 48 hours and thoroughly rinsed before being placed into stratification.
Cold stratification treatments:
• 0-day cold stratification, 24-hour water soak,
• 30-day cold stratification at 2 °C, or
• 90-day cold stratification at 2 °C.
Bleach treatments:
• No bleach,
• 24-hour soak in 3% bleach solution, or
• 48-hour soak in 3% bleach solution
Experiment 4
Germination temperatures in the growth chambers varied, representing wetland soil temperatures in the Intermountain West during different summer months. Lights were set to full brightness for a 14-hour photoperiod.
Cold stratification treatments:
• 0-day cold stratification, 24-hour water soak,
• 30-day cold stratification at 2 °C,
• 60-day cold stratification at 2 °C, or
• 30-day warm dry; seeds were placed in a sealed glass jar in a growth chamber set to 40 °C with a 16-hour photoperiod
Germination condition treatments:
• May: 28 °C during the day, 10 °C at night,
• June: 32 °C during the day, 15 °C at night, or
• July: 36 °C during the day, 20 °C at night.
Graphing and data interpretation
Germination count data were graphed with experiment day along the x-axis and cumulative germination proportion (i.e., the number of seeds that had germinated by that day) along the y-axis. Viability of the experimental seed lot was also graphed to indicate the highest potential germination percentage for each species. Viability values were obtained from lab tetrazolium tests completed before the experiment. When maximum germination values exceeded the viability obtained from the tetrazolium tests (due to the inherent variability in tetrazolium testing), the graphed viability was set to the maximum germination value reached in the experiment.

How to use this guide
Below is an example of the content and format you will find on the following pages for each species.
Scientific name
Common name
Family name
Methods(s): List the dormancy breaking techniques that will be necessary for the given species.
Recommended protocol: Most optimal dormancy break method.
The top photo is of seeds taken with a microscope camera. Measurements are in millimeters (mm).
The bottom photo will show the plant and its defining features, such as flowers, seeds, or leaves.
Alternative protocol: Second most optimal dormancy break method.


Notes: Discussion about the difference in effectiveness between the two protocols above. This section also includes other comments regarding dormancy break and germination outcomes, other considerations, or data availability.
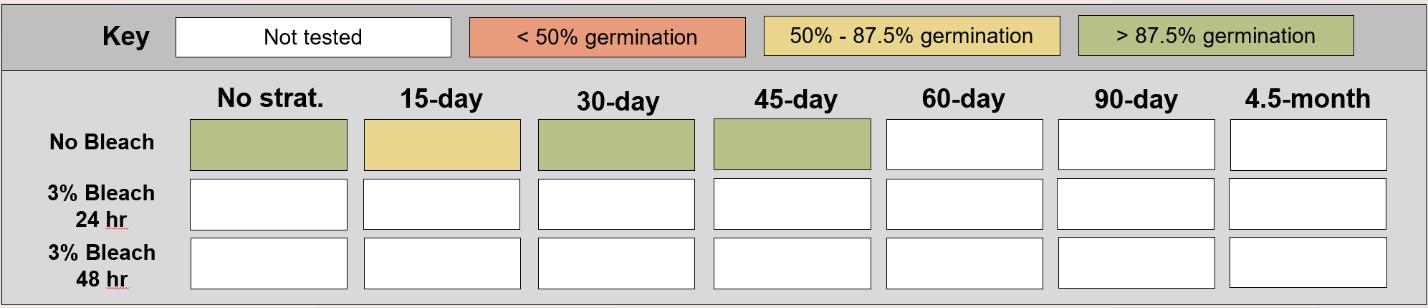
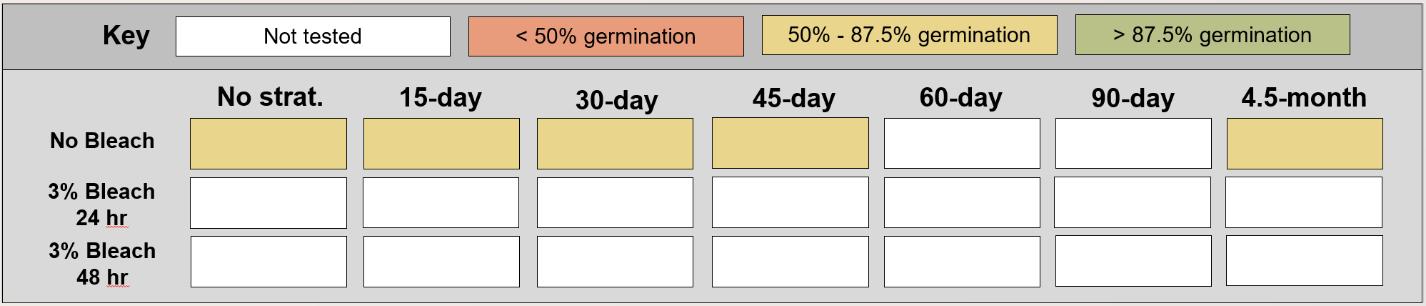
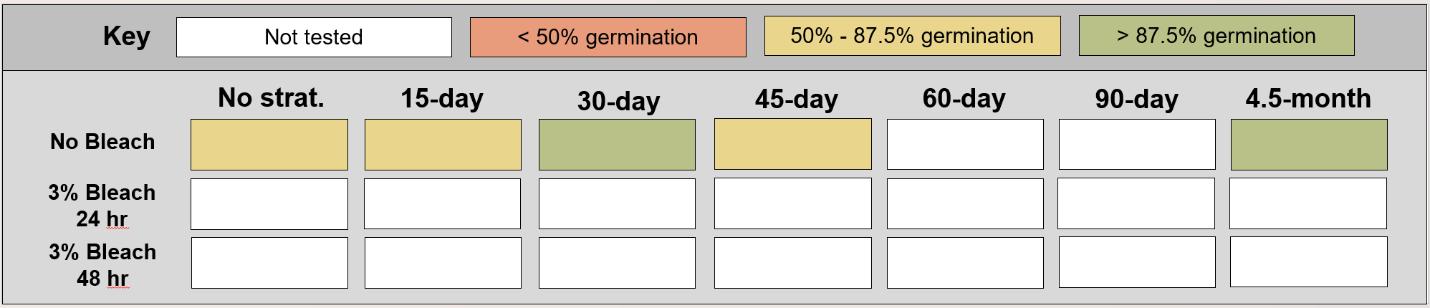
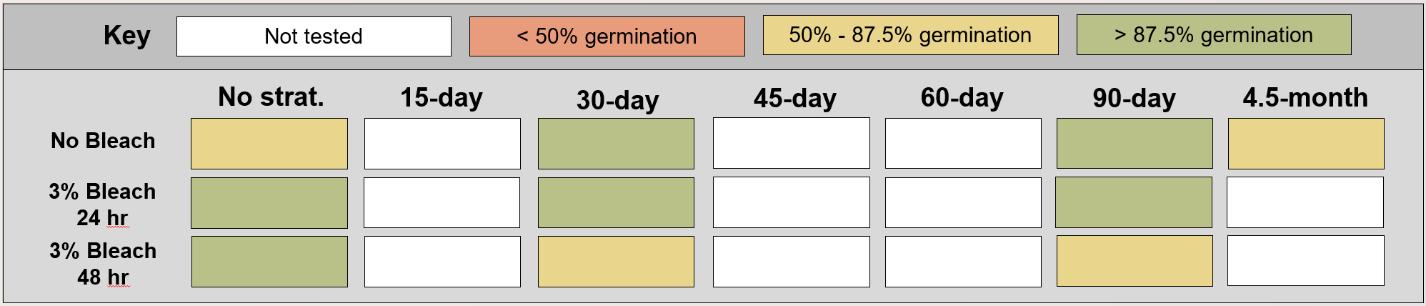
Table displaying the proportion of seeds germinated for each treatment, categorized and color-coded.
Graphs displaying raw data from up to two experiments that the species was included in.
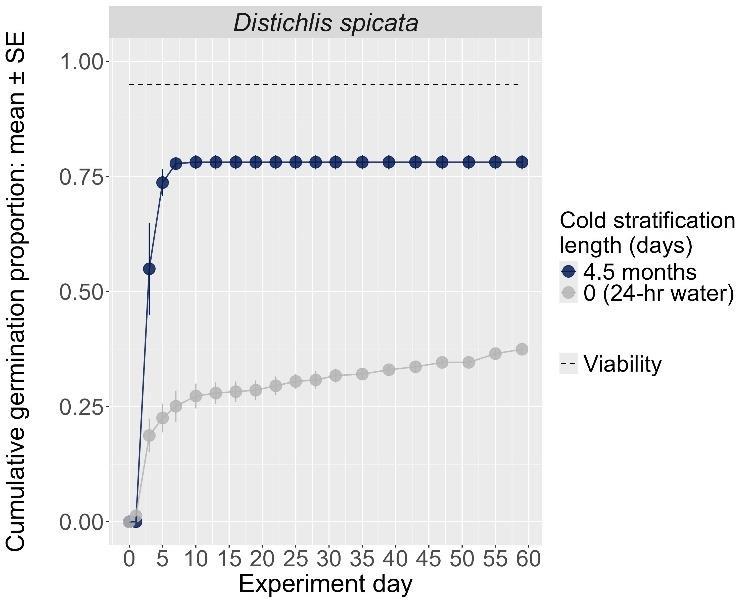
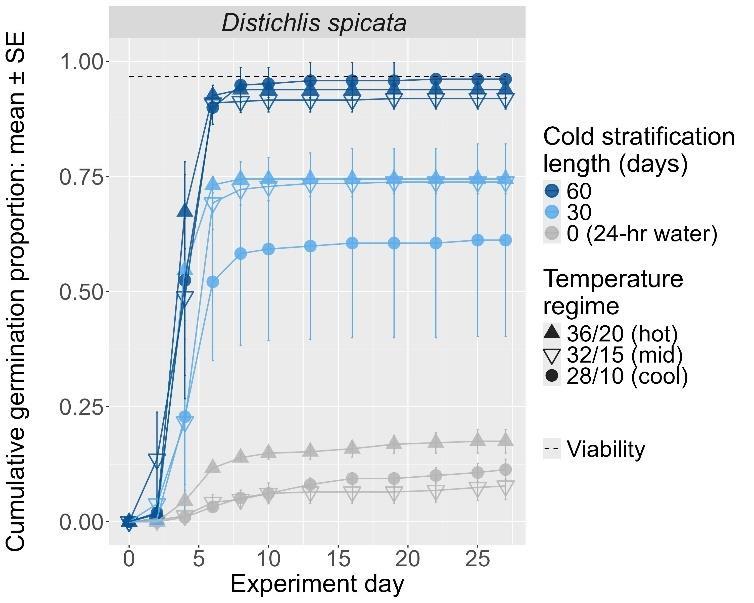
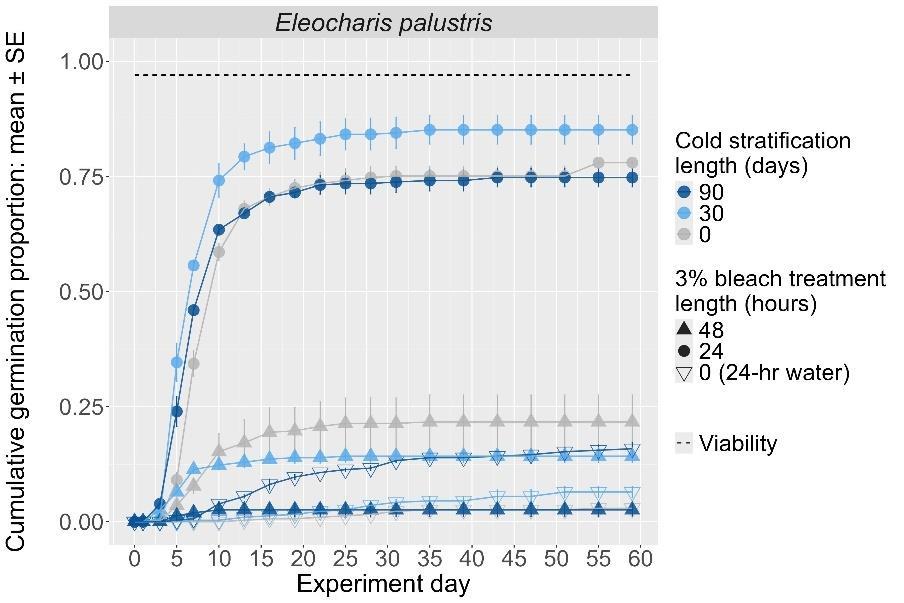
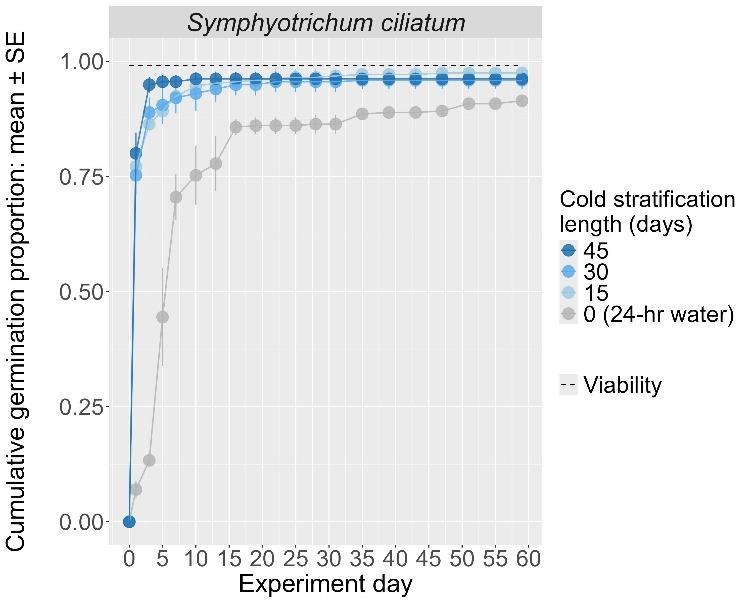
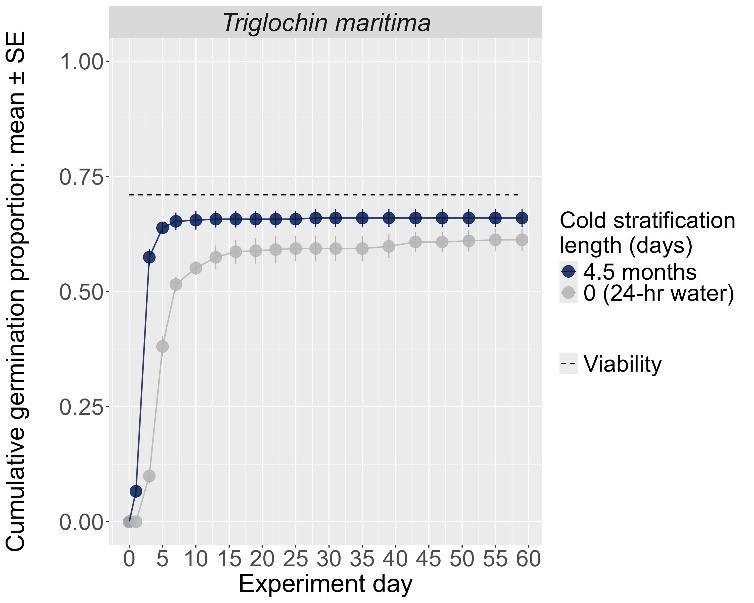
The y-axis displays the percent of viable seeds that germinated, and the x-axis displays the experiment day. Cold stratification treatments were differentiated by color, and bleach or germination temperature regime treatments were differentiated by shape. The horizontal black dashed line indicates the viability of the seed lot from tetrazolium tests.

Asclepias speciosa
Showy milkweed
Apocynaceae
Method(s): Chemical scarification
Recommended protocol: Soak seeds in 3% bleach for 24 hours without any cold stratification.
Alternative protocol: N/A
Notes: Germination was never high (~65%) for this species, regardless of treatment. Further research is needed to optimize dormancy break methods. No graphs were made due to the nature of the data from this experiment.



Units are in mm

Allenrolfea occidentalis
Iodinebush
Amaranthaceae
Method(s): Cold stratification
Recommended protocol: 60-day cold stratification
Alternative protocol: 30-day cold stratification
Notes: Both the recommended and alternative protocols did well (95%–100%) for this species, although 60-day resulted in slightly higher and faster germinations. Dormancy break treatments performed consistently between germination temperature regimes.

Units are in mm


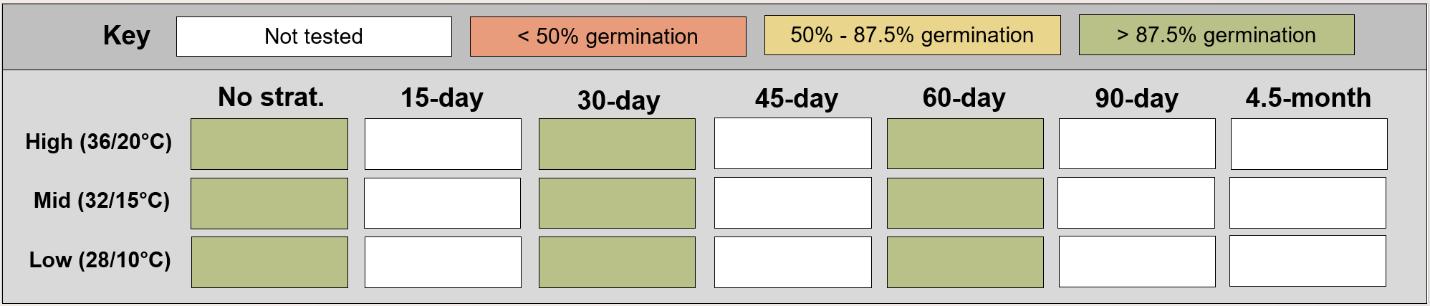

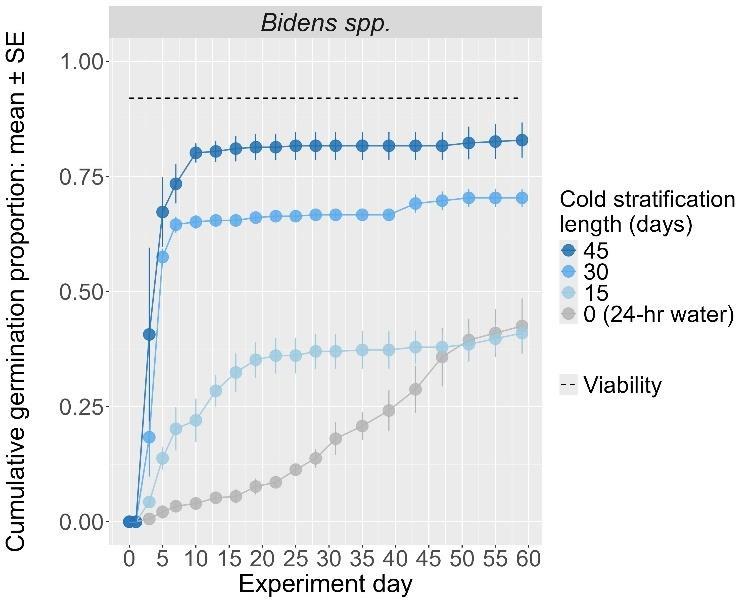
Bidens species
Nodding beggarstick
Asteraceae
Method(s): Cold stratification
Recommended protocol: 4.5-month cold stratification
Alternative protocol: 30-day or 45-day cold stratification
Notes: Alternative protocols will yield fast but lower germinations (10%–25% lower) but could be acceptable if there is a short timeline for dormancy break.


spp https ://wik i.cre ative com mon s.org /wiki/ Reco mme nded _pra ctice s_for _attri butio n#Th is_is _a_g ood_

Units are in mm



spp

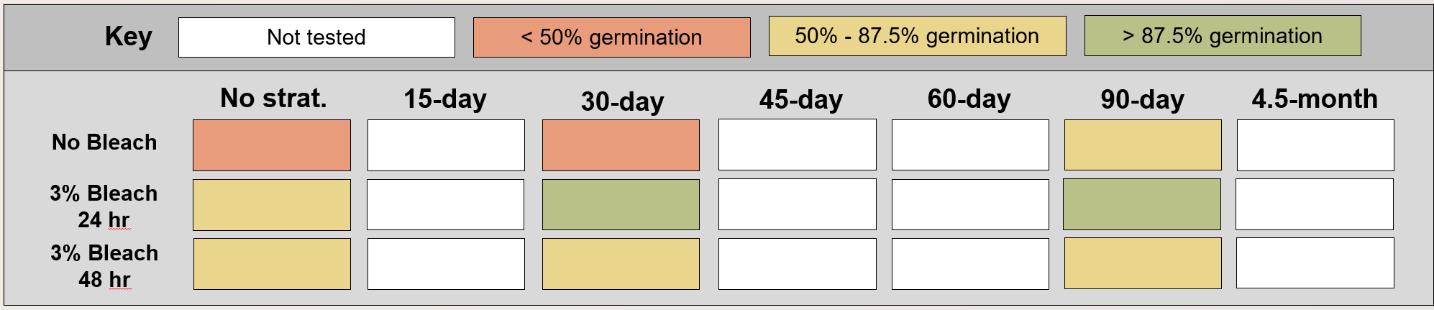
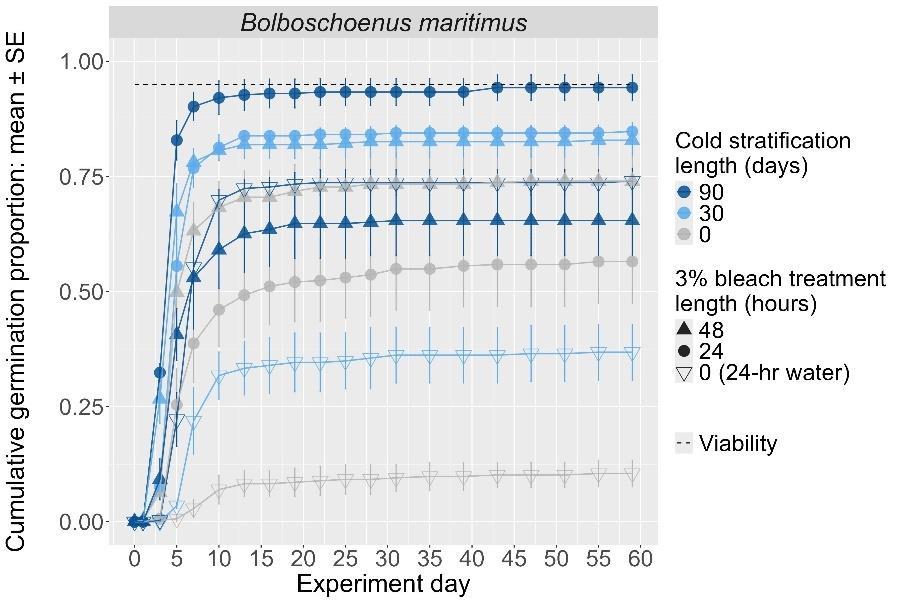
Bolboschoenus maritimus
Alkali bulrush
Cyperaceae
Method(s): Chemical scarification + cold stratification
Recommended protocol: Soak seeds in 3% bleach for 24 hours, then cold stratify for 90 days.
Alternative protocol: Soak seeds in 3% bleach for 24 hours, then cold stratify for 30 days.
Notes: Alternative protocol will yield lower germination (~10% lower) at a similar speed but could be acceptable if there is a short timeline for dormancy break.



Units are in mm
Carex nebrascensis
Nebraska sedge
Cyperaceae
Method(s): Cold stratification
Recommended protocol: 30-day cold stratification
Alternative protocol: 0-day, 90-day, or 4.5-month cold stratification
Notes: The alternative protocols will yield similar germination (0%–5% lower) at a similar speed. Seeds that experienced bleach before cold stratification were negatively affected.





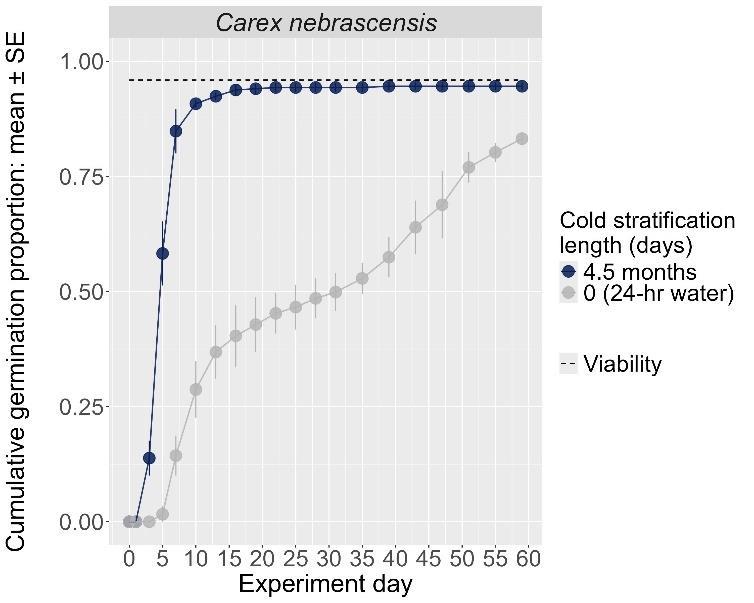
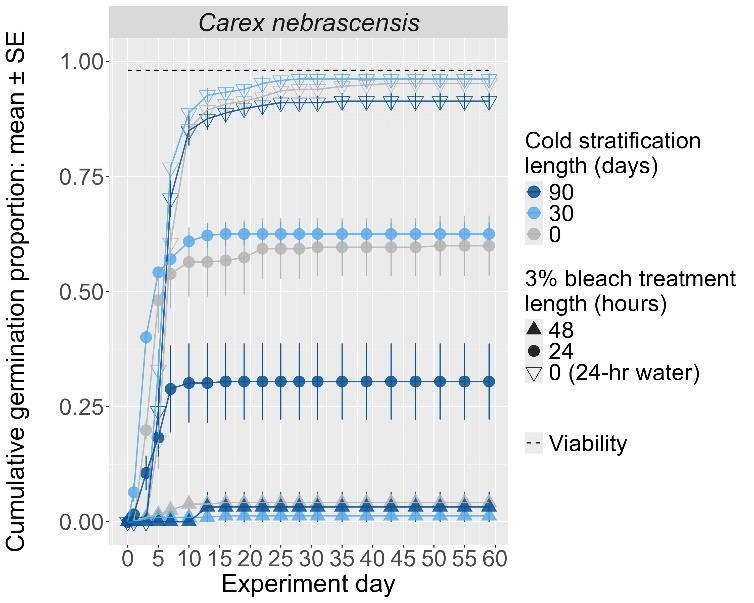

Units are in mm
Photo: Max Licher1
Seeds within perigynia (top)
Carex praegracilis
Clustered field sedge
Cyperaceae
Method(s): Chemical scarification
Recommended protocol: Soak seeds in 3% bleach for 24 hours without any cold stratification.
Alternative protocol: Soak seeds in 3% bleach for 24 hours, then cold stratify for 30 days
Notes: The alternative protocol will yield similar germination (0%–5% lower) at a similar speed. Seeds that experienced bleach for 48 hours before cold stratification were negatively affected.



Seeds without perigynia (bottom); units are in mm



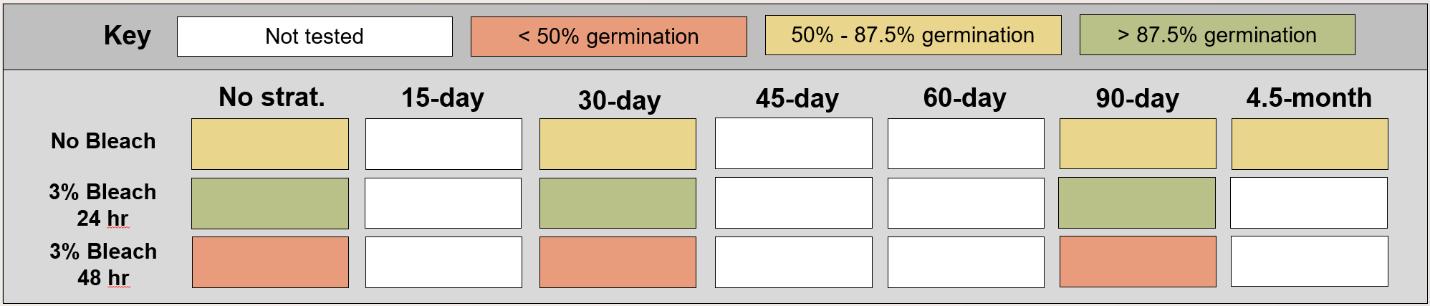

Photo: Max Licher2
Carex simulata
Analogue sedge
Cyperaceae
Method(s): Cold stratification
Recommended protocol: 4.5-month cold stratification
Alternative protocol: Soak seeds in 3% bleach for 24 hours without any cold stratification or cold stratify for 90 days
Notes: The recommended protocol had high total germination (99%) but was relatively slow. The alternate protocols had lower germination (20%–25% lower); 24-hour bleach was fast and 90-day cold stratification was much slower but had higher overall germination. Seeds that experienced bleach for 48 hours before cold stratification were negatively affected.








Units are in mm
Photo: Max Licher3
Seeds enclosed in the aril (top)
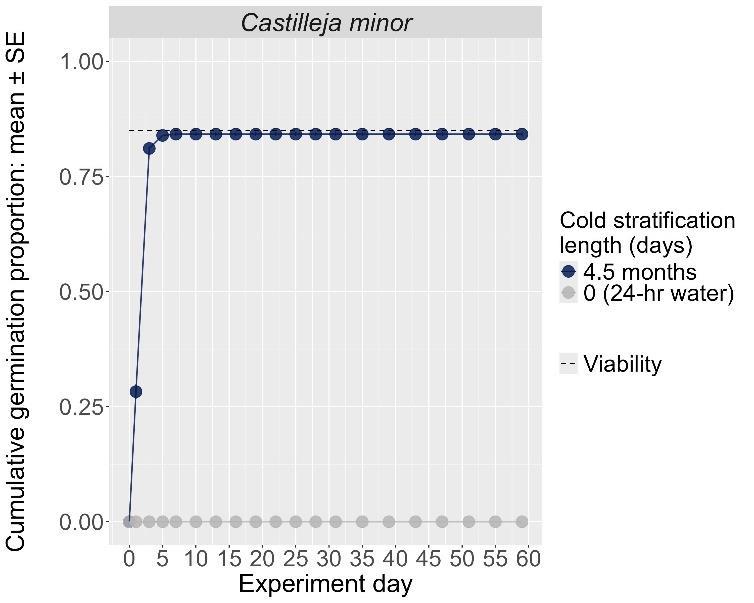
Castilleja minor
Lesser Indian paintbrush
Orobanchaceae
Method(s): Cold stratification
Recommended protocol: 4.5-month cold stratification
Alternative protocol: At least 45-day cold stratification
Notes: The recommended and alternative protocols result in high and fast germination (90%–100%). Seeds that experienced ≤ 30-day cold stratification had very low germination.
Seeds without the aril (bottom); units are in mm






Distichlis spicata
Saltgrass
Poaceae
Method(s): Cold stratification
Recommended protocol: 60-day cold stratification
Alternative protocol: 30-day cold stratification
Notes: The alternative protocol will yield lower germination (10%–15% lower) at a similar speed. Seeds that did not experience cold stratification had very low germination. Dormancy break treatments performed consistently between germination temperature regimes.
Seeds within the palea and lemma (top)



Seeds without the palea and lemma (bottom); units are in mm




Eleocharis palustris
Common spikerush
Cyperaceae
Method(s): Chemical scarification + cold stratification
Recommended protocol: Soak seeds in 3% bleach for 24 hours, then cold stratify for 30 days.
Alternative protocol: Soak seeds in 3% bleach for 24 hours without any cold stratification or cold stratify for 90 days
Notes: The alternative protocols will yield slightly lower germination (10%–15% lower) at a similar speed. Seeds that were not bleached or bleached for 48 hours before cold stratification were negatively affected.

are on the right of each seed





Tubercles
Units are in mm
Photo: Gerald Carr4

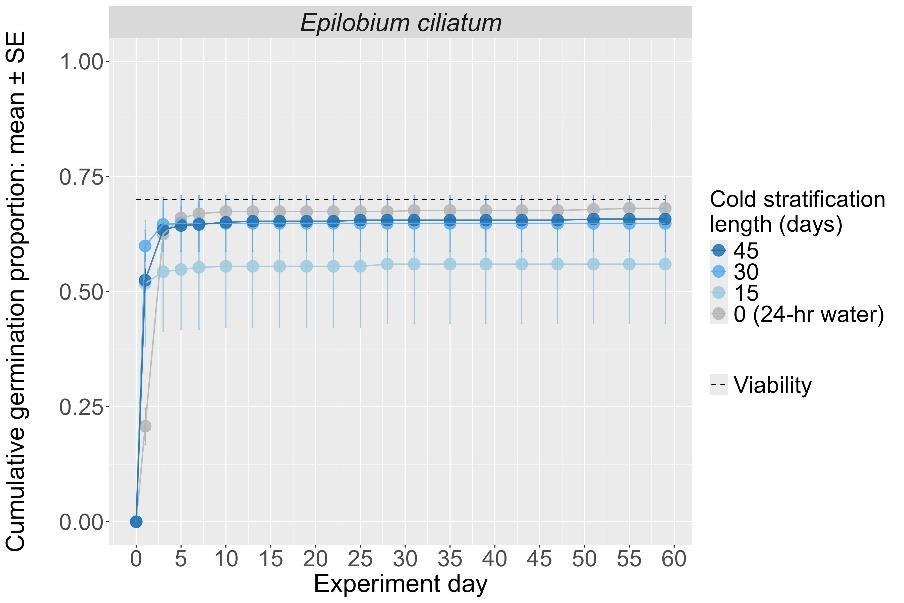
Epilobium ciliatum
Fringed willowherb
Onagraceae
Method(s): 24-hour water soak
Recommended protocol: No cold stratification
Alternative protocol: 30-day and 45-day cold stratification
Notes: The recommended and alternative protocols will both yield high and fast germination (90%–100%). Units




Euthamia occidentalis
Western goldentop
Asteraceae
Method(s): 24-hour water soak
Recommended protocol: No cold stratification
Alternative protocol: 4.5-month cold stratification
Notes: The recommended and alternative protocols will both yield high and fast germination (95%–100%).

Units are in mm




Eutrochium maculatum
Spotted Joe Pye weed
Asteraceae
Method(s): Cold stratification
Recommended protocol: 4.5-month cold stratification
Alternative protocol: 45-day cold stratification
Notes: The alternative protocol will yield slightly lower germination (15% lower) at a similar speed.








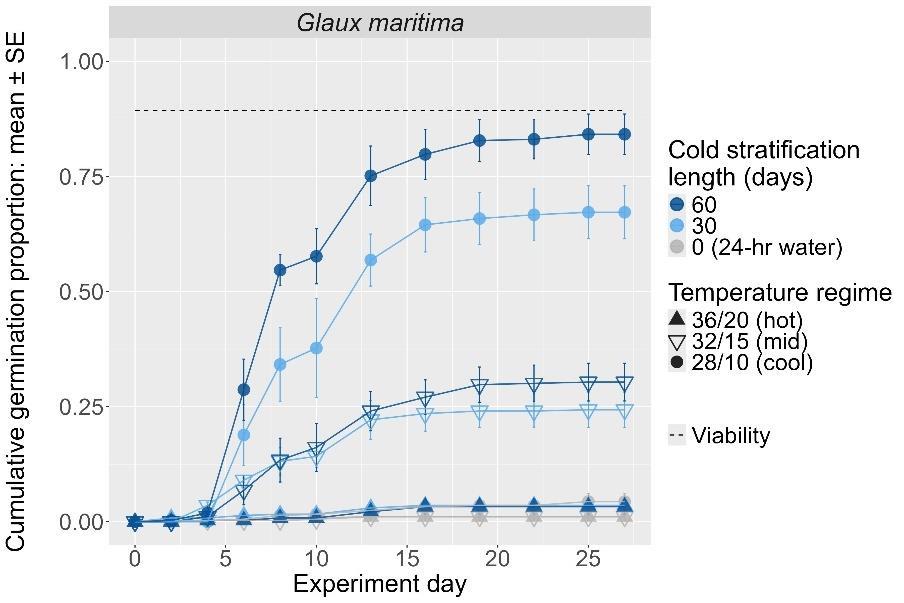
Glaux maritima
Sea milkwort
Primulaceae
Method(s): Cold stratification
Recommended protocol: 60-day cold stratification
Alternative protocol: 30-day cold stratification
Notes: The alternative protocol will yield lower germination (25% lower) at a similar speed. Lower temperatures support higher germination of this species.




Units are in mm

Grindelia squarrosa
Curlycup gumweed
Asteraceae
Method(s): 24-hour water soak
Recommended protocol: No cold stratification
Alternative protocol: 15-day, 30-day, or 45-day cold stratification
Notes: The alternative protocols will yield slightly lower germination (5%–15% lower) at a similar speed.






Helianthus annuus
Common Sunflower
Asteraceaee
Method(s): Cold stratification
Recommended protocol: 30-day cold stratification
Alternative protocol: 15-day or 45-day cold stratification
Notes: The alternative protocols will yield slightly lower germination (5% lower) at a similar speed. Germination was low for this species, regardless of treatment. Further research is needed to optimize dormancy break methods.





Units are in mm
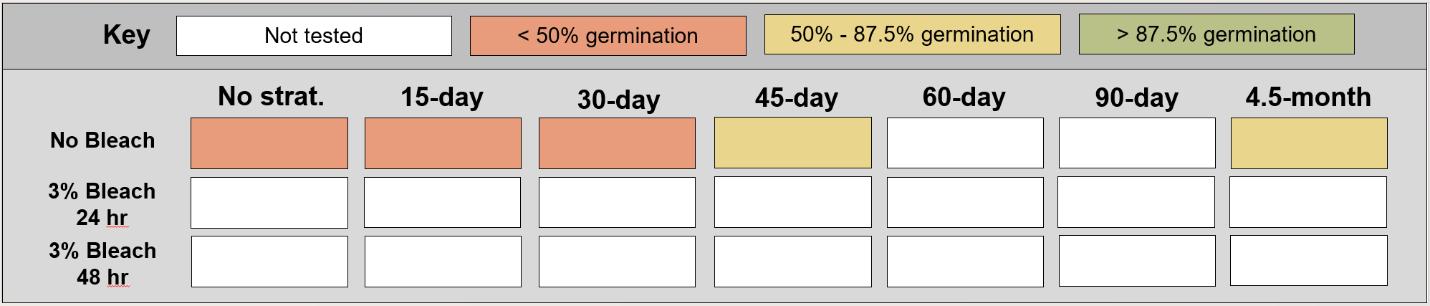
Helianthus nutallii
Nuttall’s sunflower
Asteraceae
Method(s): Cold stratification
Recommended protocol: 4.5-month cold stratification
Alternative protocol: 45-day cold stratification
Notes: The alternative protocol will yield lower germination (25%–30% lower) at similar speeds but could be acceptable if there is a short timeline for dormancy break. Seeds that experienced ≤ 30-day cold stratification had very low germination






Units are in mm

Juncus arcticus
Mountain rush
Juncaceae
Method(s): Cold stratification
Recommended protocol: 4.5-month cold stratification
Alternative protocol: No cold stratification
Notes: The alternative protocol will yield slightly lower germination (5% lower) at a slower speed but could be acceptable if there is a short timeline for dormancy break.





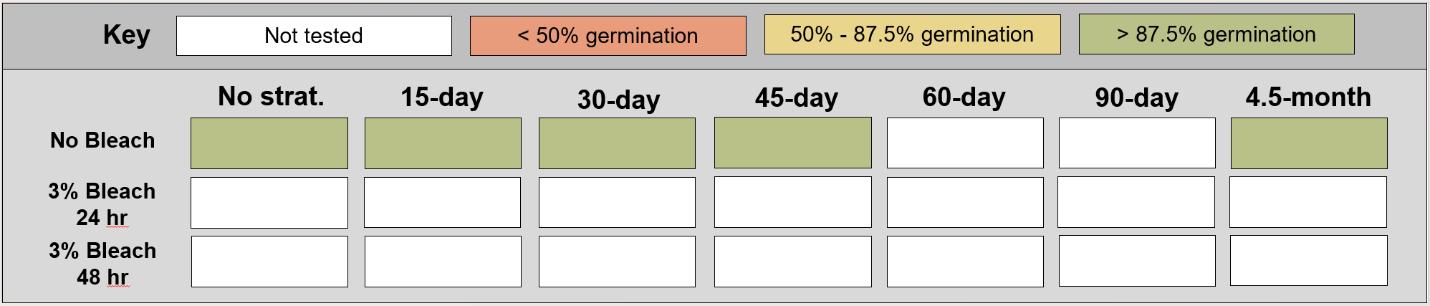
Juncus gerardii
Saltmeadow rush
Juncaceae
Method(s): Cold stratification
Recommended protocol: 45-day cold stratification
Alternative protocol: 0-day, 15-day, or 30-day cold stratification
Notes: The alternative protocols will yield similar germination (0%–5% lower) at a slightly slower speed.




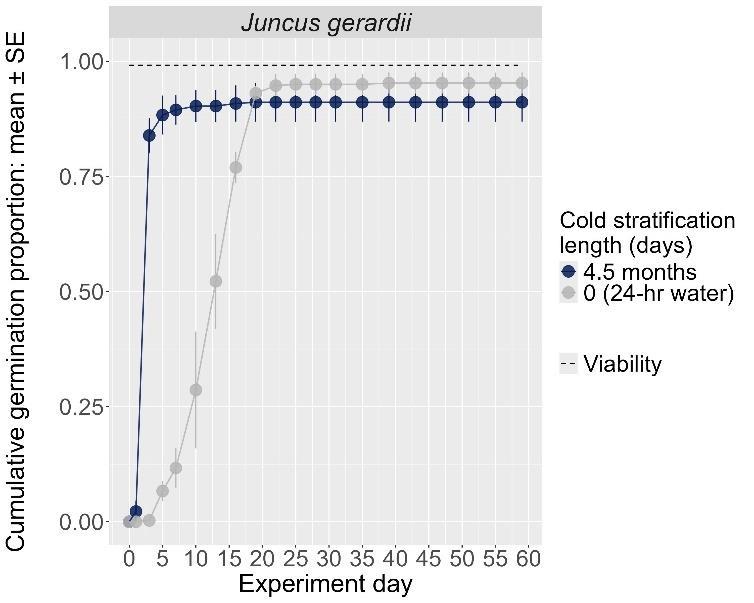


Units are in mm
Photo: Jim Brighton5
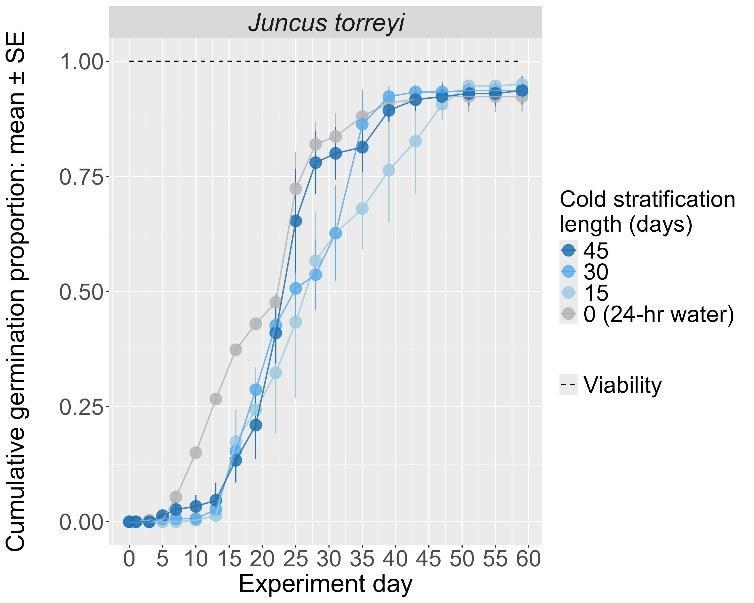
Juncus torreyi
Torrey’s rush
Juncaceae
Method(s): 24-hour water soak
Recommended protocol: No cold stratification
Alternative protocol: 15-day, 30-day, and 45-day cold stratification
Notes: The alternative protocols will yield similar germination (0%–5% lower) at a slightly slower speed. All protocols have relatively slow germination.



Units are in mm



Mentha arvensis
Wild mint
Lamiaceae
Method(s): Cold stratification
Recommended protocol: 45-day cold stratification
Alternative protocol: 30-day or 4.5-month cold stratification
Notes: The alternative protocols will yield slightly lower germination (10% lower) at a slightly slower speed. Germination was moderate (70%–80%) for this species, regardless of treatment. Further research is needed to optimize dormancy break methods.



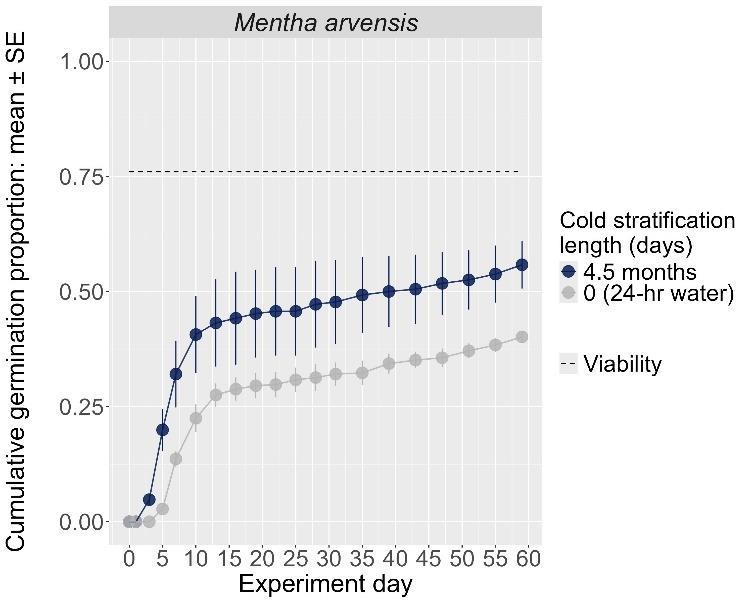


Units are in mm
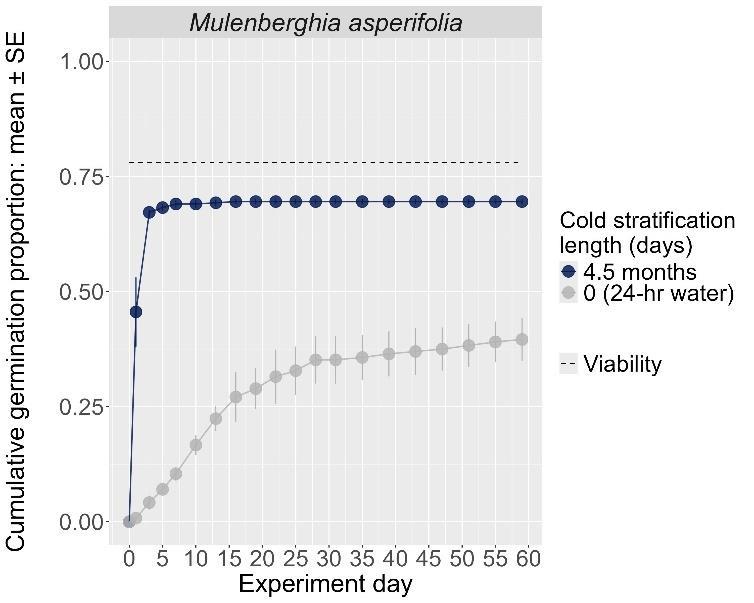
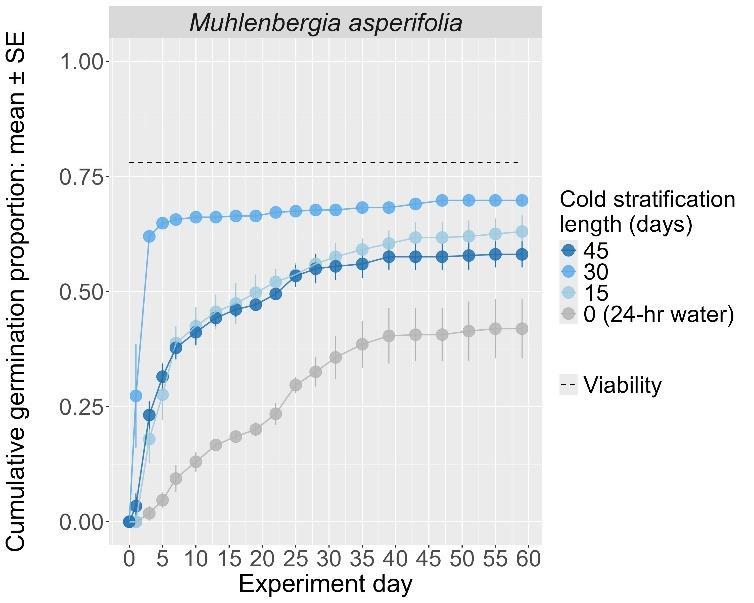
Muhlenberghia asperifolia
Scratchgrass
Poaceae
Method(s): Cold stratification
Recommended protocol: 30-day cold stratification
Alternative protocol: 4.5-month cold stratification
Notes: The alternative protocol will yield similar germination (0%–5% lower) at a similar speed. Seeds that experienced no cold stratification had much lower germination.
Seeds within the palea and lemma (top)


Seeds without the palea and lemma (bottom); units are in mm


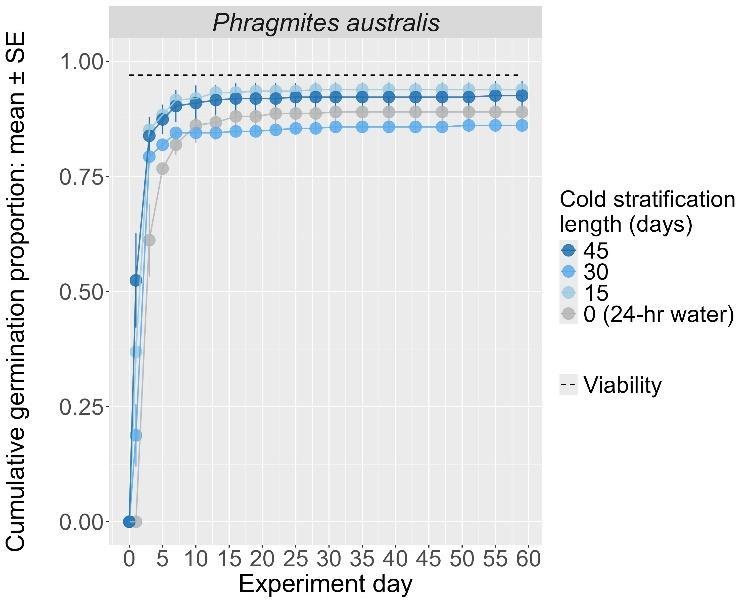
Phragmites australis
Common reed
Poaceae
Method(s): 24-hour water soak
Recommended protocol: No cold stratification
Alternative protocol: 15-day, or 45-day cold stratification
Notes: The alternative protocol will yield similar germination (0%–5% lower) at a similar speed. Seeds that experienced longer cold stratification (4.5 months) were negatively affected.






Units are in mm

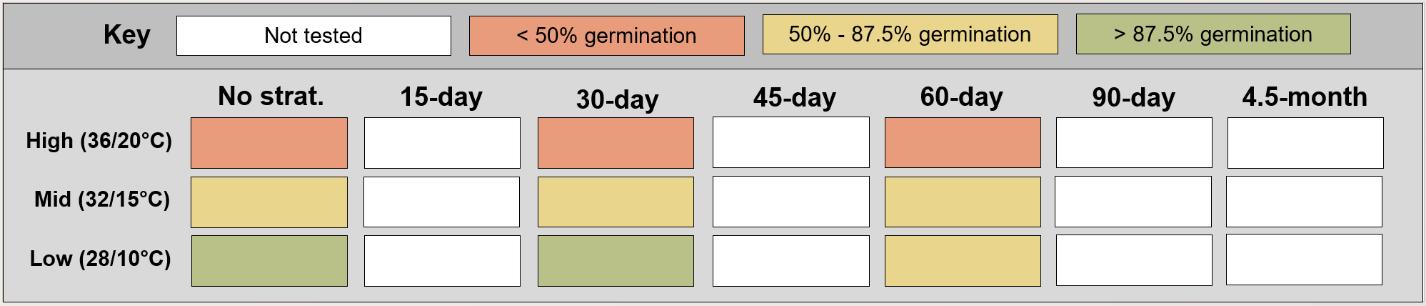
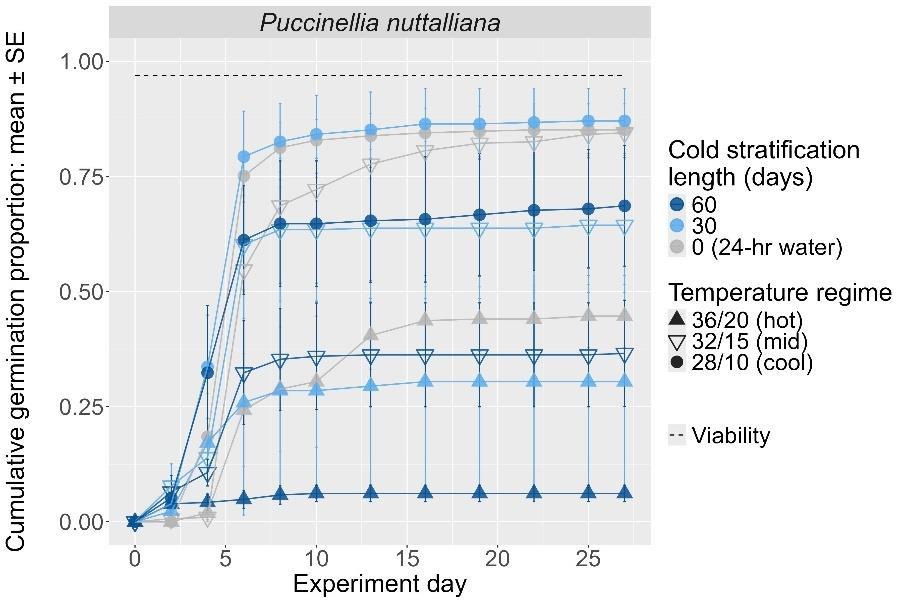
Puccinellia nuttalliana
Nuttall’s alkaligrass
Poaceae
Method(s): Cold stratification
Recommended protocol: 30-day cold stratification
Alternative protocol: No cold stratification
Notes: The alternative protocol will yield similar germination (0%–5% lower) at a slightly slower speed. Effectiveness of dormancy break treatments varied with germination temperature regime.
Seeds within the palea and lemma (top)

Seeds without the palea and lemma (bottom); units are in mm


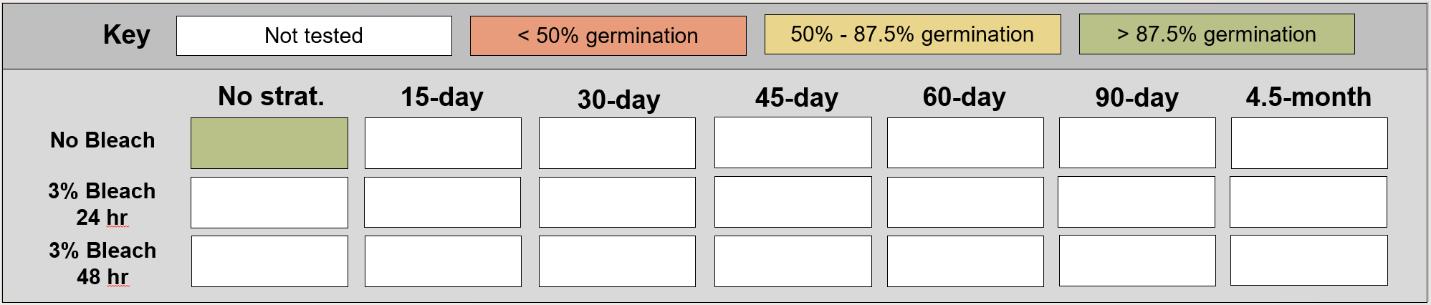
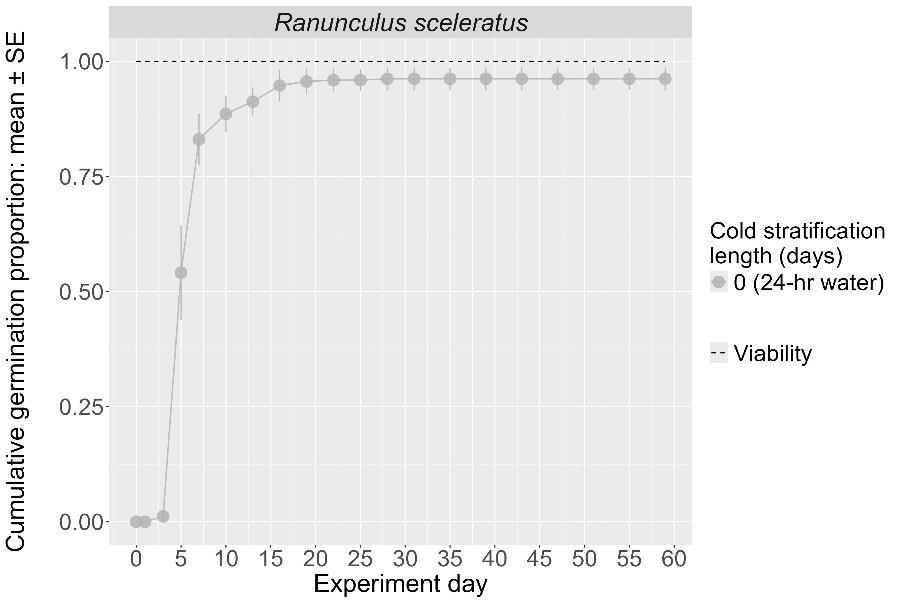
Ranunculus sceleratus
Cursed buttercup
Ranunculaceae
Method(s): 24-hour water soak
Recommended protocol: No cold stratification
Alternative protocol: N/A


Notes: The recommended protocol will yield high and fast germination. No other treatments were tested for this species. Units are in mm


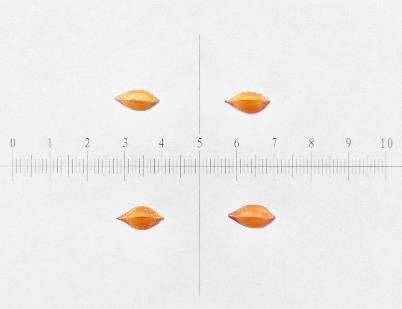
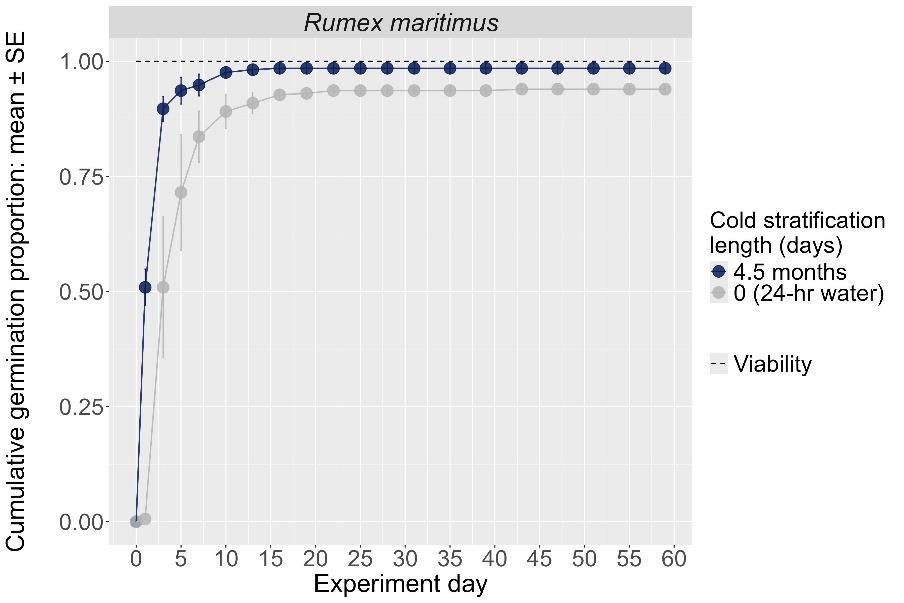
Rumex maritimus
Golden dock
Polygonaceae
Method(s): Cold stratification
Recommended protocol: 4.5-month cold stratification
Alternative protocol: No cold stratification
Notes: The alternative protocol will yield slightly lower germination (5% lower) at a similar speed but could be acceptable if there is a short timeline for dormancy break.




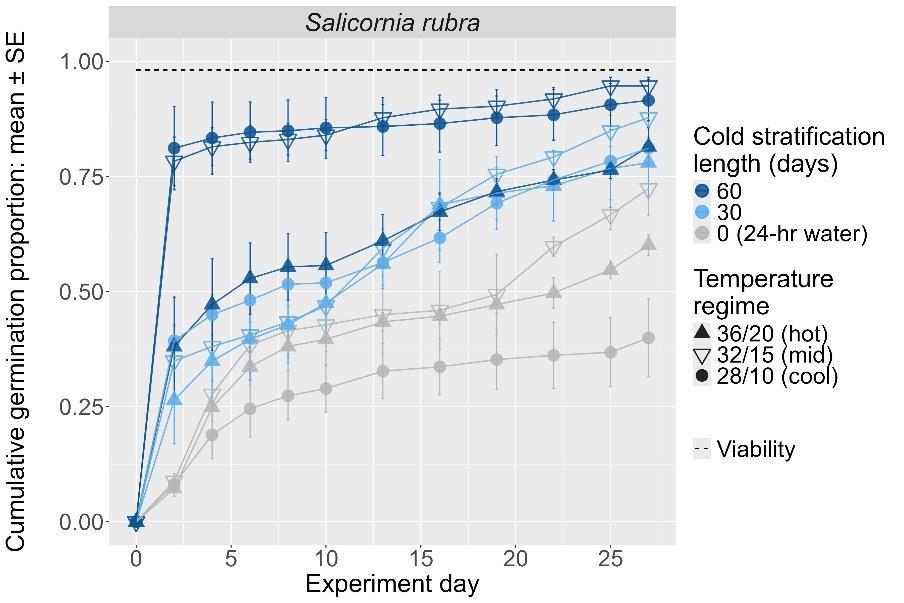
Salicornia rubra
Pickleweed
Amaranthaceae
Method(s): Cold stratification
Recommended protocol: 60-day cold stratification
Alternative protocol: 30-day cold stratification
Notes: The alternative protocol will yield slightly lower germination (5%–10% lower) at a much slower speed but could be acceptable if there is a short timeline for dormancy break. Effectiveness of dormancy break treatments varied with germination temperature regime.




Units

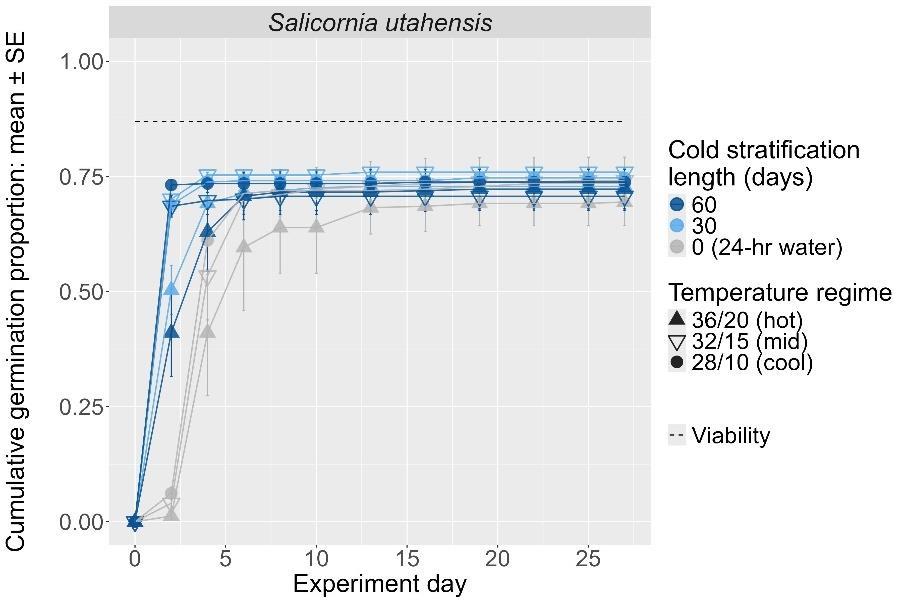
Salicornia utahensis
Utah swampfire
Amaranthaceae
Method(s): Cold stratification
Recommended protocol: 30-day cold stratification
Alternative protocol: 60-day cold stratification
Notes: The alternative protocol will yield similar germination (1%–5% lower) at a similar speed. Dormancy break treatments performed consistently between germination temperature regimes.
Seeds with chaff attached (top)

Seeds without the chaff (bottom); units are in mm




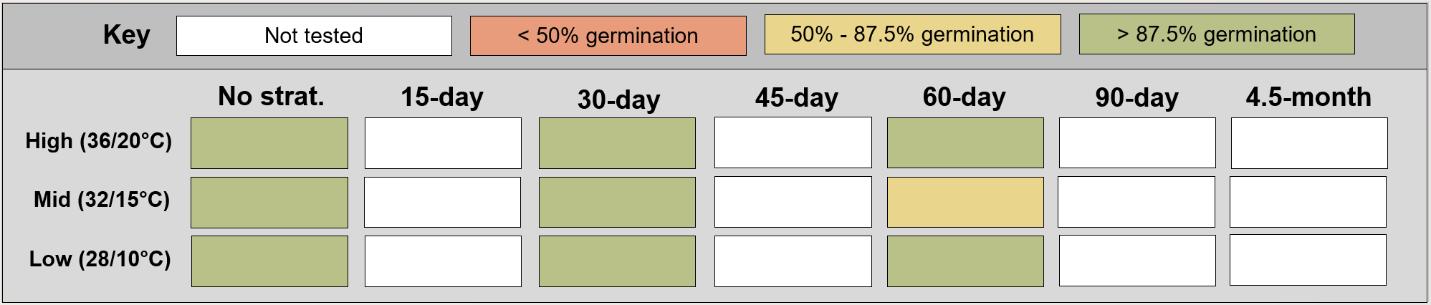
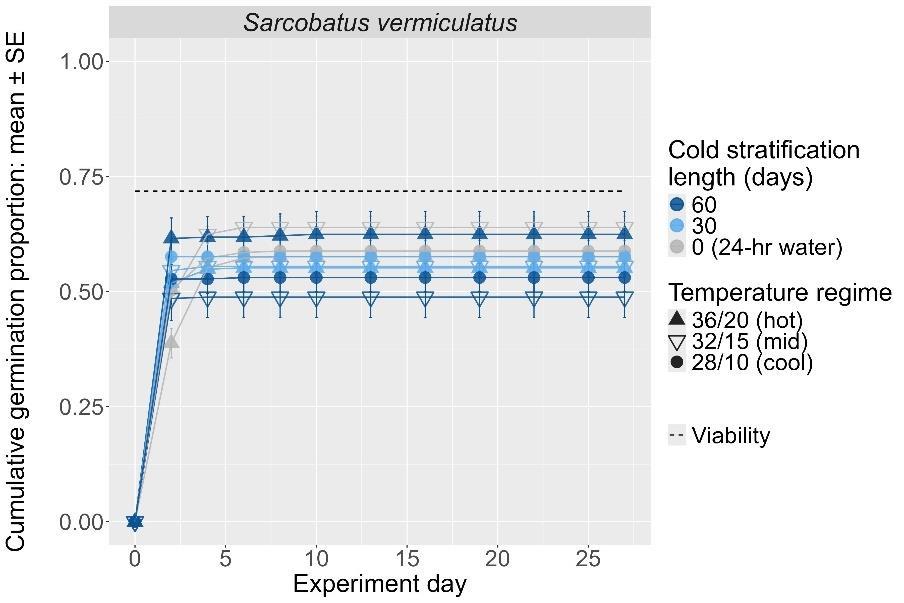
Sarcobatus vermiculatus
Greasewood
Chenopodiaceae
Method(s): 24-hour water soak
Recommended protocol: No cold stratification
Alternative protocol: 60-day cold stratification
Notes: The alternative protocol will yield similar germination (0%–5% lower) at a similar speed. Effectiveness of dormancy break treatments varied with germination temperature regime.
Seeds within papery pericarp (top)

Seeds without the pericarp (bottom); units are in mm

Schoenoplectus acutus
Hardstem bulrush
Cyperaceae
Method(s): Chemical scarification + cold stratification
Recommended protocol: Soak seeds in 3% bleach for 24 hours, then cold stratify for 90 days.
Alternative protocol: Soak seeds in 3% bleach for 24 hours, then cold stratify for 30 days.
Notes: The alternative protocol will yield slightly lower germination (5% lower) at a similar speed but could be acceptable if there is a short timeline for dormancy break. No treatments reached above 75% germination. Further research is needed to optimize dormancy break methods.



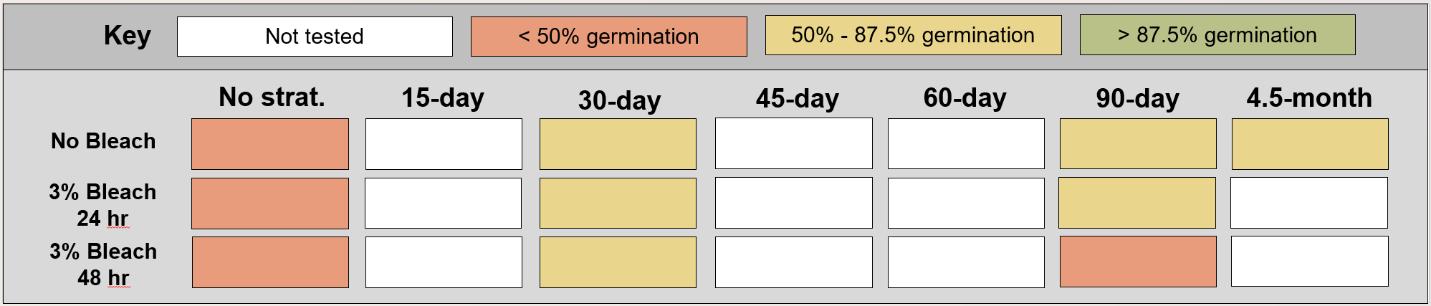
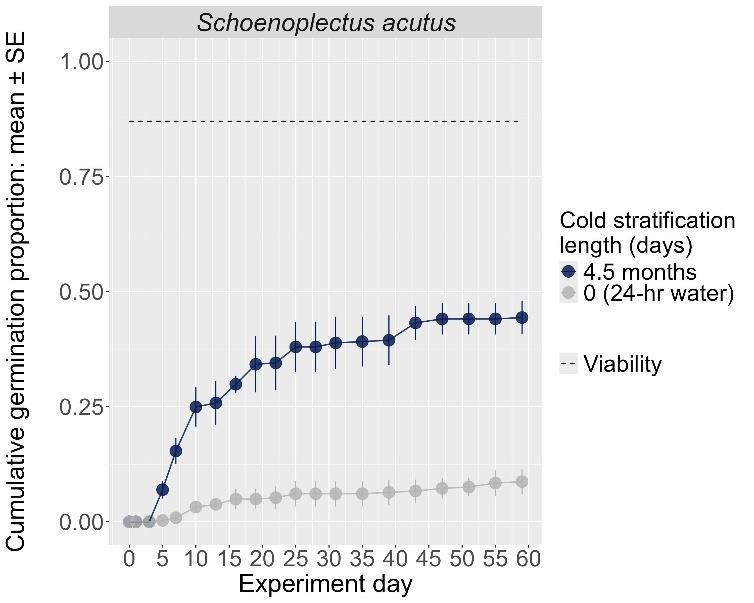
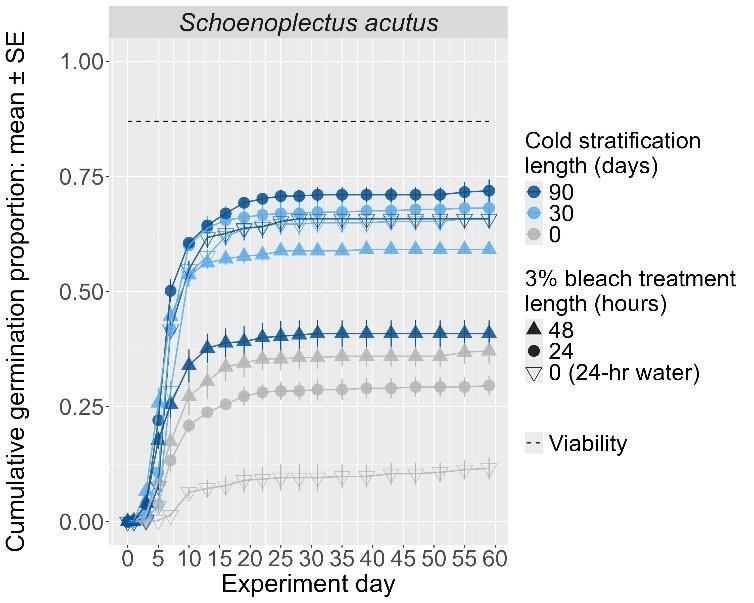

Units are in mm
Schoenoplectus americanus
Chairmaker’s bulrush
Cyperaceae
Method(s): Chemical scarification + cold stratification
Recommended protocol: Soak seeds in 3% bleach for 24 hours, then cold stratify for 90 days.
Alternative protocol: Soak seeds in 3% bleach for 24 hours, then cold stratify for 30 days.
Notes: The alternative will yield slightly lower germination (5% lower) at a similar speed but could be acceptable if there is a short timeline for dormancy break. Seeds that experienced no bleach treatment had lower germination. No treatments reached above 75% germination. Further research is needed to optimize dormancy break methods.




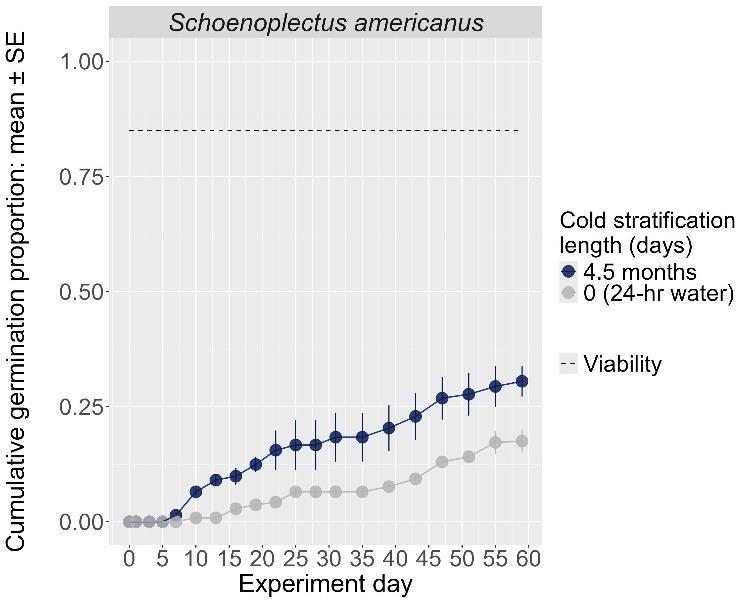
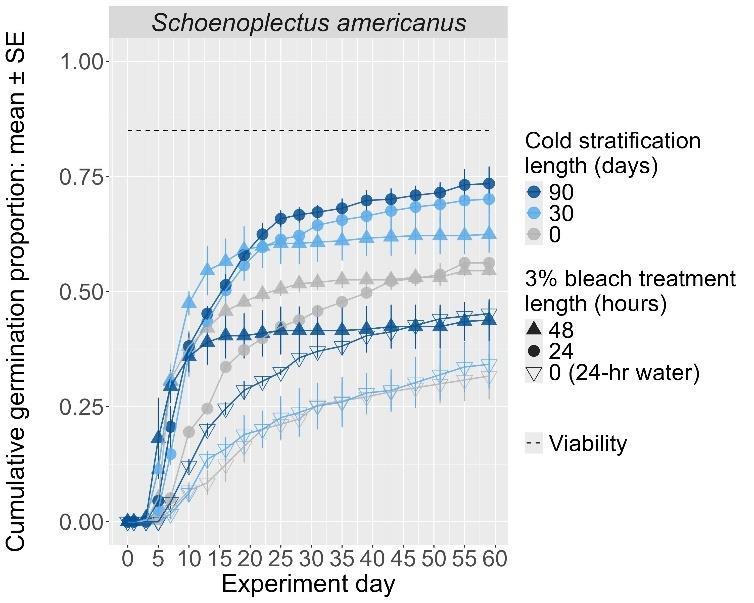

Units are in mm
Schoenoplectus pungens
Common threesquare bulrush
Cyperaceae
Method(s): Cold stratification
Recommended protocol: 90-day cold stratification
Alternative protocol: Soak seeds in 3% bleach for 24 hours, then cold stratify for 30 days.
Notes: The alternative protocol will yield similar germination (0%–5% lower) at a similar speed.




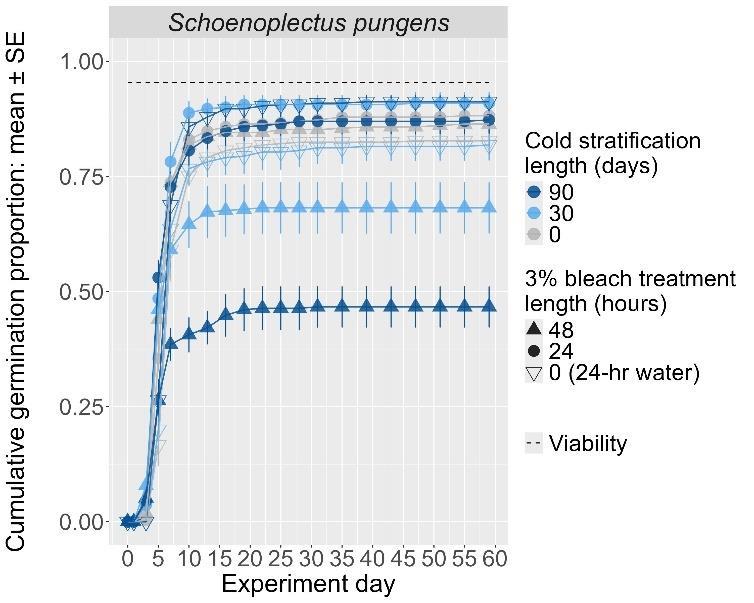

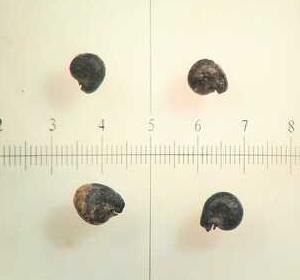
Units are in mm
Photo: Steve Matson6

Senecio hydrophilus
Water groundsel
Asteraceae
Method(s): Cold stratification
Recommended protocol: 45-day cold stratification
Alternative protocol: 0-day, 15-day, or 30-day cold stratification
Notes: The alternative protocols will yield similar germination (0%–5% lower) at slower speeds.





Sesuvium verrucosum
Verrucose seapurslane
Aizoaceae
Method(s): 24-hour water soak
Recommended protocol: No cold stratification
Alternative protocol: 30-day cold stratification
Notes: The alternative protocol will yield lower germination (5%–15% lower) but will germinate at a faster speed. Dormancy break treatments performed consistently between germination temperature regimes.




Units are in mm

Solidago canadensis
Canada goldenrod
Asteraceae
Method(s): Cold stratification
Recommended protocol: 15-day cold stratification
Alternative protocol: No cold stratification
Notes: The alternative protocol will yield similar germination (1%–5% lower) at a similar speed.





Sporobolus airoides
Alkali sacaton
Poaceae
Method(s): Cold stratification
Recommended protocol: 60-day cold stratification
Alternative protocol: 30-day cold stratification
Notes: The alternative protocol will yield lower germination (10%–15% lower) at a slightly slower speed but could be acceptable if there is a short timeline for dormancy break. Dormancy break treatments performed consistently between germination temperature regimes.
Seeds within the palea and lemma (top)

Seeds without the palea and lemma (bottom); units are in mm





Suaeda calceoliformis
Pursh seepweed
Chenopodiaceae
Method(s): Cold stratification
Recommended protocol: 30-day cold stratification
Alternative protocol: No cold stratification
Notes: The alternative protocol will yield similar germination (0%–5% lower) at a slightly slower speed. Lower and moderate germination temperatures support higher germination of this species.
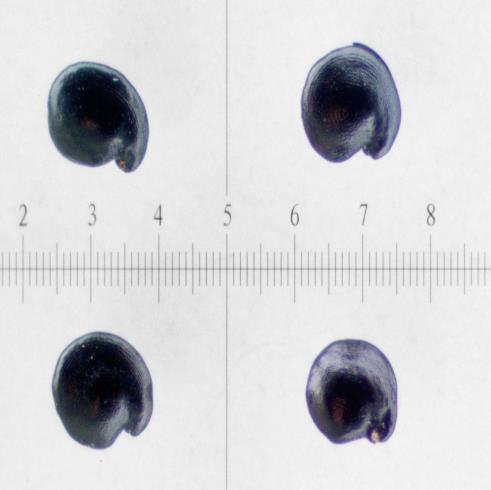



Symphyotrichum ciliatum
Rayless alkali aster
Asteraceae
Method(s): Cold stratification
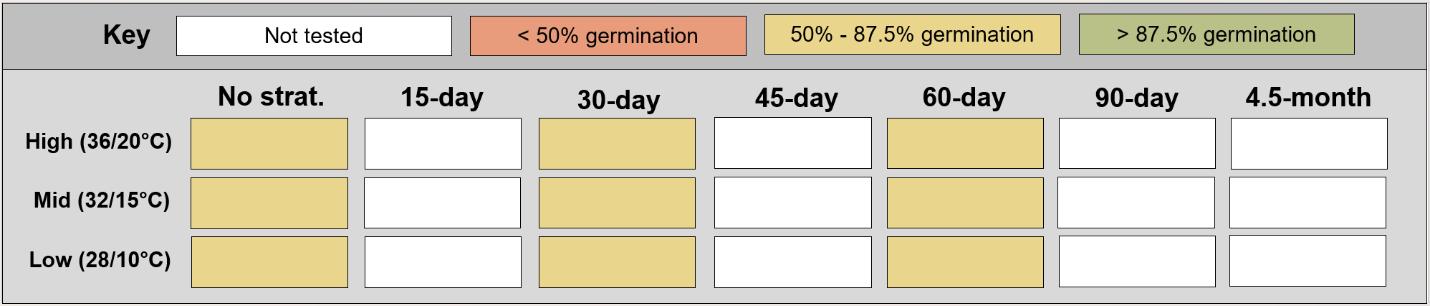
Recommended protocol: 15-day, 30-day, 45day, or 4.5-month cold stratification
Alternative protocol: No cold stratification
Notes: Any cold stratification improves germination. The alternative protocol will yield similar germination (0%–5% lower) at a slower speed.






Units are in mm
Triglochin maritima
Seaside arrowgrass
Juncaginaceae
Method(s): Cold stratification
Recommended protocol: 15-day, 30-day, 45day, or 4.5-month cold stratification
Alternative protocol: No cold stratification
Notes: Any cold stratification improves germination. The alternative protocol will yield similar germination (0%–5% lower) at a slightly slower speed.

Seeds are within the tan mericarps




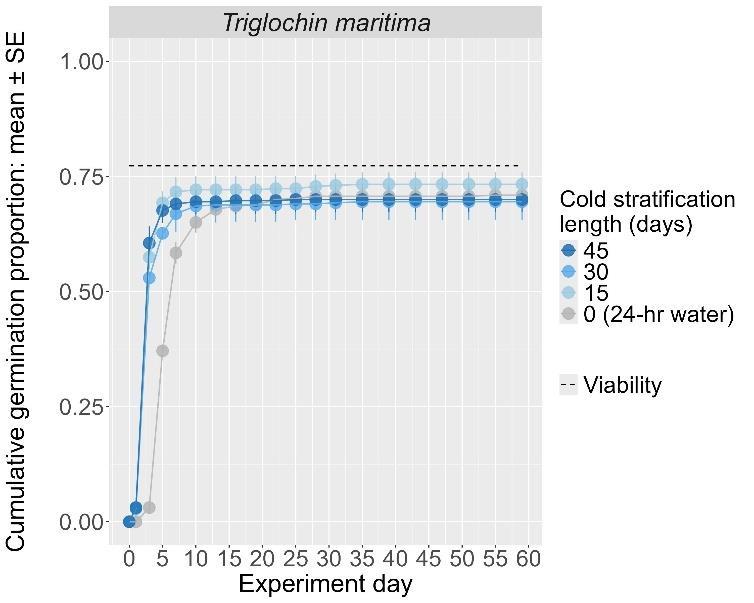

Units are in mm
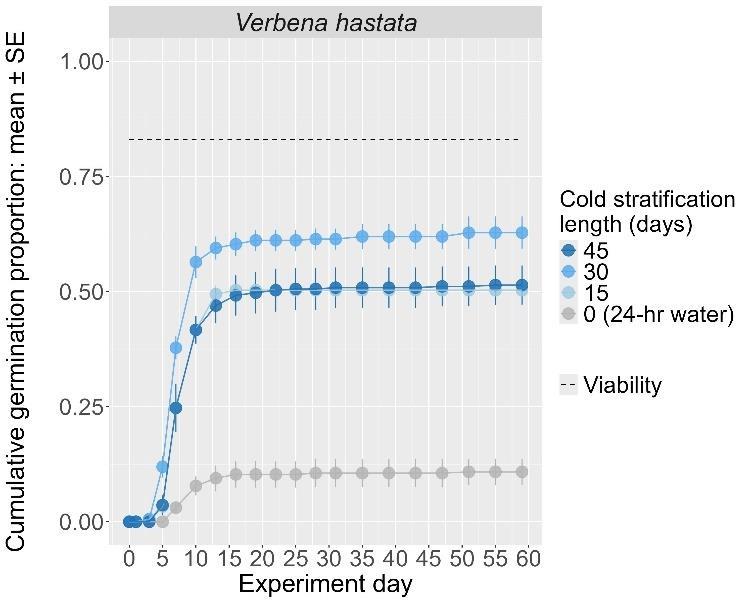
Verbena hastata
Swamp verbena
Verbenaceae
Method(s): Cold stratification
Recommended protocol: 4.5-month cold stratification
Alternative protocol: 30-day cold stratification
Notes: The alternative protocol will yield lower germinations (10%–15% lower) at a slightly slower speed but could be acceptable if there is a short timeline for dormancy break. Units are in






Veronica anagallisaquatica
Water speedwell
Plantaginaceae
Method(s): 24-hour water soak
Recommended protocol: No cold stratification
Alternative protocol: 4.5-month cold stratification
Notes: The alternative protocol will yield slightly lower germinations (5%–10%) at a slower speed. Units are in




References
Narrative references
Baskin, J. M., & Baskin, C. C. (2004). A classification system for seed dormancy. Seed Science Research, 14(1), 1–16. https://doi.org/10.1079/SSR2003150
Baskin, C. C., & Baskin, J. M. (2014). Seeds: Ecology, biogeography, and evolution of dormancy and germination (2nd ed.) Academic Press https://doi.org/10.1016/C2013-000597-X
Brock, M. A., Nielsen, D. L., Shiel, R. J., Green, J. D., & Langley, J. D. (2003). Drought and aquatic community resilience: the role of eggs and seeds in sediments of temporary wetlands. Freshwater Biology, 48(7), 1207–1218. https://doi.org/10.1046/j.13652427.2003.01083.x
Espeland, E. K., Emery, N. C., Mercer, K. L., Woolbright, S. A., Kettenring, K. M., Gepts, P., & Etterson, J. R. (2017). Evolution of plant materials for ecological restoration: Insights from the applied and basic literature. Journal of Applied Ecology, 54(1), 102–115. https://doi.org/10.1111/1365-2664.12739
Finch-Savage, W. E. & Leubner-Metzger, G. (2006). Seed dormancy and the control of germination. New Phytologist, 171(3), 501–523. https://doi.org/10.1111/j.14698137.2006.01787.x
Kettenring, K. M., & Galatowitsch, S. M. (2007a). Temperature requirements for dormancy break and seed germination vary greatly among 14 wetland Carex species. Aquatic Botany, 87(3), 209–220. https://doi.org/10.1016/j.aquabot.2007.06.003
Kettenring, K. M., & Galatowitsch, S. M. (2007b). Tools for Carex revegetation in freshwater wetlands: Understanding dormancy loss and germination temperature requirements. Plant Ecology, 193, 157–169. https://doi.org/10.1007/s11258-006-9255-8
Kildisheva, O. A., Dixon, K. W., Silveira, F. A. O., Chapman, T., Sacco, A. D., Mondoni, A., Turner, S. R., & Cross, A. T. (2020). Dormancy and germination: Making every seed count in restoration. Restoration Ecology, 28(S3), S256–S265. https://doi.org/10.1111/rec.13140
Long, R. L., Gorecki, M. J., Renton, M., Scott, J. K., Colville, L., Goggin, D. E., Commander, L. E., Westcott, D. A., Cherry, H., & Finch-Savage, W. E. (2014). The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise. Biological Reviews, 90(1), 31–59. https://doi.org/10.1111/brv.12095
Lu, J. J., Tan, D. Y., Baskin, C. C. & Baskin, J. M. (2016) Effects of germination season on life history traits and on transgenerational plasticity in seed dormancy in a cold desert annual. Scientific Reports, 6(25076). https://doi.org/10.1038/srep25076

Satterthwaite, W. H. (2009). Competition for space can drive the evolution of dormancy in a temporally invariant environment. Plant Ecology, 208, 167–185. https://doi.org/10.1007/s11258-009-9696-y
Schütz, W., & Milberg, P. (1997). Seed dormancy in Carex canescens: regional differences and ecological consequences. Oikos, 78(3), 420–428. https://doi.org/10.2307/3545604
Thompson, K., Ceriani, R. M., Bakker, J. P., & Bekker, R. M. (2003) Are seed dormancy and persistence in soil related? Seed Science Research, 13(2), 97–100. https://doi.org/10.1079/SSR2003128
Willis, C. G., Baskin, C. C., Baskin, J. M., Auld, J. R., Venable, D. L., Cavender-Bares, J., Donohue, K., Rubio de Casas, R., & The NESCent Germination Working Group. (2014). The evolution of seed dormancy: Environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytologist, 203(1), 300–309. https://doi.org/10.1111/nph.12782
Photo references
Authors provided photos, including the cover page, unless otherwise noted.
1 Licher, M. (2011). Carex nebranscensis Dewey [Photograph, licensed CC BY-SA 4.0]. SEINet Portal Network. Accessed April 28, 2025, from https://www.swbiodiversity.org/seinet/imagelib/imgdetails.php?imgid=21627989
2 Licher, M. (n.d.). Carex praegracilis W. Boott [Photograph, licensed CC BY-SA 4.0]. SEINet Portal Network Accessed April 28, 2025, from https://www.swbiodiversity.org/seinet/imagelib/imgdetails.php?imgid=216332.
3 Licher, M. (n.d.). Carex simulata Mack [Photgraph, licensed CC BY-SA 4.0] SEINet Portal Network. Accessed April 28, 2025, from https://www.swbiodiversity.org/seinet/imagelib/imgdetails.php?imgid=216365.
4 Carr, G. (2011). Eleocharis palustris Common spikerush [Photograph, cropped from original, licensed CC BY-NC 4.0] Calflora Accessed April 28, 2025, from https://www.calflora.org/entry/occdetail.html?seq_num=po83269
5 Brighton, J. (2021). Saltmeadow rush in Dorchester Co., Maryland [Photograph, cropped from original, licensed CC BY-NC 4.0] Accessed April 28, 2025, from https://www.marylandbiodiversity.com/species/1870.
6 Matson, S. (2008). Schoenoplectus pungens var. longispicatus [Photograph, cropped from original, licensed CC BY-NC 3.0]. CalPhotos Accessed April 28, 2025, from https://calphotos.berkeley.edu/cgi/img_query?enlarge=0000+0000+0508+0732

Photo Licenses
Attribution-ShareAlike 4.0 International (CC BY-SA 4.0)
Attribution-NonCommercial 3.0 Unported (CC BY-NC 3.0)
Attribution-NonCommercial 4.0 International (CC BY-NC 4.0)
In its programs and activities, including in admissions and employment, Utah State University does not discriminate or tolerate discrimination, including harassment, based on race, color, religion, sex, national origin, age, genetic information, sexual orientation, gender identity or expression, disability, status as a protected veteran, or any other status protected by University policy, Title IX, or any other federal, state, or local law. Utah State University is an equal opportunity employer and does not discriminate or tolerate discrimination including harassment in employment including in hiring, promotion, transfer, or termination based on race, color, religion, sex, national origin, age, genetic information, sexual orientation, gender identity or expression, disability, status as a protected veteran, or any other status protected by University policy or any other federal, state, or local law. Utah State University does not discriminate in its housing offerings and will treat all persons fairly and equally without regard to race, color, religion, sex, familial status, disability, national origin, source of income, sexual orientation, or gender identity. Additionally, the University endeavors to provide reasonable accommodations when necessary and to ensure equal access to qualified persons with disabilities. The following office has been designated to handle inquiries regarding the application of Title IX and its implementing regulations and/or USU’s non-discrimination policies: The Office of Equity in Distance Education, Room 400, Logan, Utah, titleix@usu.edu, 435-797-1266. For further information regarding non-discrimination, please visit equity.usu.edu, or contact: U.S. Department of Education, Office of Assistant Secretary for Civil Rights, 800-4213481, ocr@ed.gov or U.S. Department of Education, Denver Regional Office, 303-8445695 ocr.denver@ed.gov. Issued in furtherance of Cooperative Extension work, acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, Kenneth L. White, Senior Vice President for Statewide Enterprise and Utah State University Extension.

