
UF BME researchers are advancing biomechanics and orthopaedics through innovations in regenerative medicine, device design, AI, and more to restore and enhance mobility.


UF BME researchers are advancing biomechanics and orthopaedics through innovations in regenerative medicine, device design, AI, and more to restore and enhance mobility.

The J. Crayton Pruitt Family Department of Biomedical Engineering honors the memory of Dr. John Crayton Pruitt Jr., a distinguished cardiothoracic surgeon and beloved family man who passed away on Oct. 21, 2024. Known as “Crayton,” he carried forward the legacy of innovation and generosity begun by his father, Dr. J. Crayton Pruitt Sr., whose support led to the department’s naming in 2006. A UF College of Medicine graduate, Crayton trained in cardiothoracic surgery at the University of Utah and built a respected career in Clearwater, Florida. He was admired for his surgical skill, dedication to patients, and compassionate nature. Outside the operating room, Crayton lived life to the fullest—boating, climbing, building guitars, and embracing every challenge with curiosity and passion. His legacy continues to inspire the UF BME community.


Welcome to the 12th issue of CrossLink, a magazine dedicated to the research and education activities of the J. Crayton Pruitt Family Department of Biomedical Engineering at the University of Florida.
As chair of this distinguished department, I am proud to share with you the 12th edition of CrossLink. At a time when questions arise about our academic institutions, it is important to convey how the unique university ecosystem produces broad and transformative impacts. This edition highlights the dedication of our faculty, students, staff, and alumni in contributing to our community through education, innovation, entrepreneurship, and advocacy.
As biomedical engineers, we are uniquely suited to translate discoveries to improve the human condition. In this issue of CrossLink, we highlight a key aspect in maintaining quality of life: mobility. This issue showcases research approaches that seek answers to complex questions, including:
• Decoding how repetitive brain trauma leads to neurodegeneration
• Unraveling the complexity of pain and joint dysfunction
• Identifying ways to regenerate damaged tendons and joints
• Engineering precision drug delivery and immune reprogramming
• Designing next-generation braces, prosthetics, and mobility aids
We are excited to spotlight our inaugural STEM Outreach Day, which welcomed local students to explore biomedical engineering through hands-on activities led by our faculty and students. Events like this reflect our commitment to inspiring the next generation of engineers and expanding our impact beyond campus.
We also welcome two new faculty members, as well as highlight the new leadership roles for our instructional faculty. These efforts will continue to expand our research and educational impacts.
This issue further highlights the continued successes of our alumni, whose mentorship and leadership strengthen our department, as well as the accomplishments of our faculty and students, whose contributions are at the forefront of the field and bring new ideas and technologies to the clinic.
Looking ahead, we remain committed to expanding opportunities for our students, investing in research that addresses critical health needs, and continuing to grow as leaders in the field of biomedical engineering. As always, our department is not just defined by its strong academic programs and impactful research but by the people who make it possible. I am so grateful to be part of this community.
We sincerely appreciate your support of our mission, and please reach out if there are ways we can collaborate more. Together, we are shaping a healthier future.
A publication of the J. Crayton Pruitt Family Department of Biomedical Engineering at the University of Florida FALL 2025
INTERIM DEAN, HERBERT WERTHEIM COLLEGE OF ENGINEERING
Warren Dixon
DEPARTMENT CHAIR
Cherie Stabler
EDITORS
Candi Crimmins
Emily Jackson Cherie Stabler
CREATIVE DIRECTOR
Candi Crimmins
CONTRIBUTING WRITERS
Yelaine Aguilar
Melissa Lutz Blouin
Candi Crimmins
Megan Howard
Grace Huff
Dave Schlenker
UF BME STAFF:
Ismael Arroyo Engineer II
Julienne Barber Administrative Assistant
Janie Barnes Accounting Specialist
Candi Crimmins Marketing & Communications
Kimberly Depue Academic Assistant & Events Coordinator
Kaitlynn Gravely IT Professional
Kelley Hines Manager of Core & Laboratory Infrastructure
Mae Howell Fiscal Assistant
Emily Jackson Assistant to Department Chair
Ade Kumuyi Graduate Advisor
Francis Lai Undergraduate Advisor
Hollie Martin Human Resources Generalist
Britt Moore Fiscal Assistant
Natalie Roman Associate Director of Administrative Services
Jenna Ross Research Administrator
Obed Santana Research Administrator
Matt Taylor
BMS Building Manager
Jessica Williams Fiscal Assistant
Khaalil Williams-Frierson Research Administrator Assistant
Ann Wlordarski Research Administrator

At the University of Florida, our Biomedical Engineering ecosystem connects discovery to delivery— through education, innovation, entrepreneurship, and policy—amplifying our impact on health and society.

From immersive summer research programs to graduate training in cross-disciplinary research, UF BME empowers students through hands-on learning. For example:
Dr. Lee Murfee runs an NIH R25-funded summer program providing undergraduates with immersive clinical experiences.
Dr. Benjamin Keselowsky co-directs an NIH T32 training program supporting collaborative research in engineering and diabetes.
Engineering the Next Big Leap
Breakthroughs at UF BME leverage biotechnology and AI with a vision to translate discovery into impact for humankind. For example:
Dr. Gregory Hudalla designs new drug delivery materials that improve shelf-life and reduce costs.
Dr. Ruogu Fang develops AI-driven tools to advance equitable, precision diagnostics for neurological conditions.
UF is one of six universities in the country with colleges of medicine, veterinary medicine, engineering, law and agriculture all on one contiguous campus.

UF BME fosters a culture of innovation where discoveries move from the lab to life. For example:
Dr. Christine Schmidt’s regenerative technologies have helped tens of thousands of people recover from traumatic injuries.
Dr. Aysegul Gunduz’s neurotech research is optimizing devices to improve life for patients with Parkinson’s, Tourette syndrome, and other neurological conditions.
UF ranks #20 worldwide for U.S. utility patents granted (National Academy of Inventors).
UF BME faculty shape standards in research, policy, and education across the globe. For example:
Dr. Wesley Bolch serves as the U.S. delegate to the United Nations Scientific Committee on the Effects of Atomic Radiation.
Dr. Parisa Rashidi has provided expert testimony in congressional briefings on AI in healthcare, helping inform national policy and investment.

Dr. Wesley Bolch, fifth from left, at a Program Area Committee meeting of the National Council on Radiation Protection and Measurements, where he served as vice president.
of UF BME full professors are elected fellows of the American Institute for Medical and Biological Engineering (AIMBE), which recognizes leaders and shapes policy in science and engineering.
UF BME students lead the Policy Advocacy in Science and Engineering (PASE) organization, amplifying student voices in national conversations.
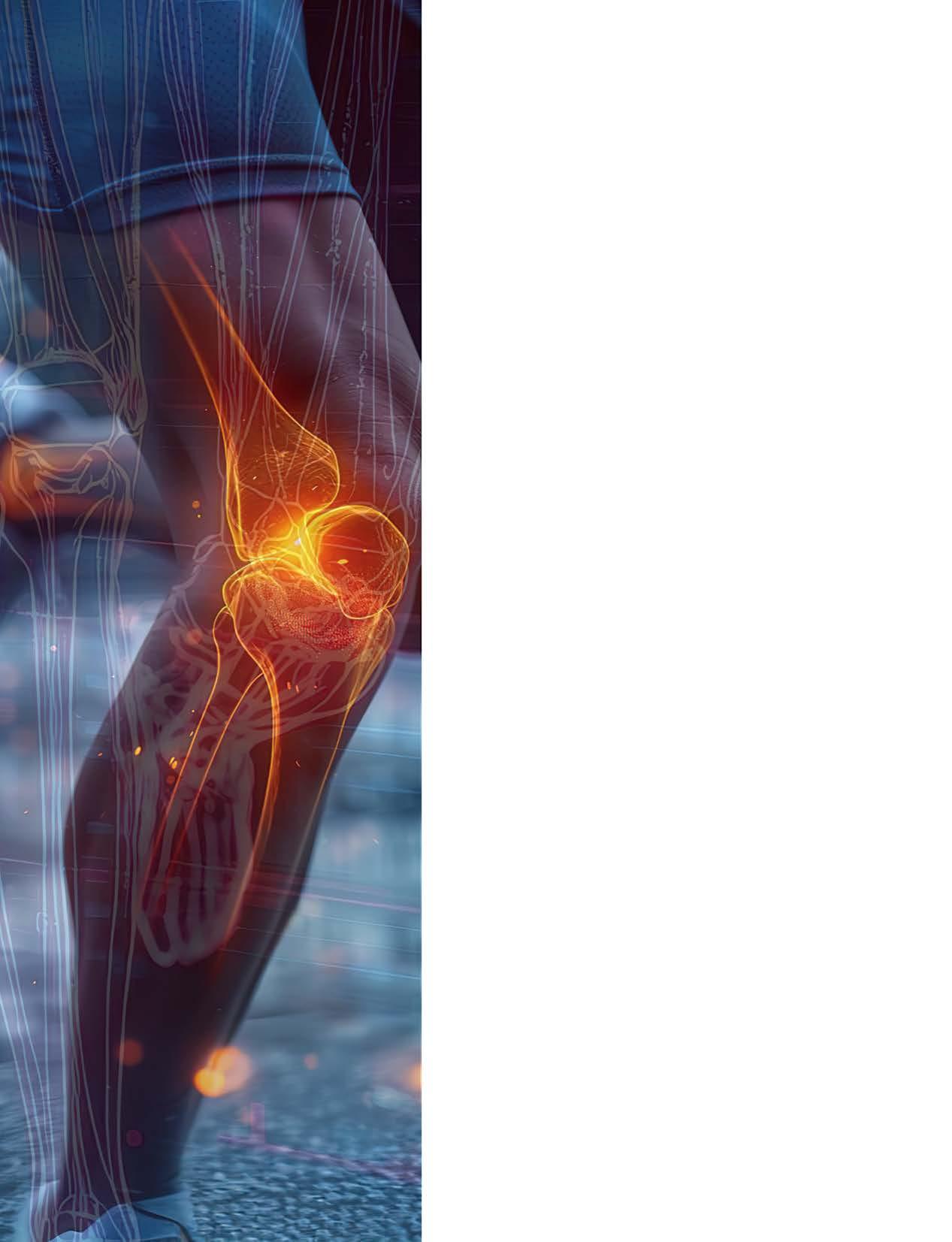
By: Melissa Lutz Blouin
From lifting groceries to sprinting down a soccer field, human movement relies on a finely tuned interplay of muscles, bones, joints and connective tissues. When even one part of the system breaks down from injury, age or disease, freedom of movement can slip away.
At the University of Florida’s J. Crayton Pruitt Family Department of Biomedical Engineering, faculty are engineering solutions that restore and enhance mobility across the lifespan, advancing the science of movement from molecules to machines.
UF’s biomechanics and orthopaedic research spans computational modeling, regenerative medicine, device design and artificial intelligence, with numerous faculty and research groups tackling these challenges in collaboration with clinicians, industry and national research networks. Their work is supported by competitive funding from the National Institutes of Health, the National Science Foundation and the Department of Defense, and is integrated with UF’s nationally ranked programs in engineering and medicine.
Together, these teams are:
Decoding how repetitive brain trauma leads to neurodegeneration
Unraveling the complexity of pain and joint dysfunction
Regenerating damaged tendons and joints
Engineering precision drug delivery and immune reprogramming
Designing next-generation braces, prosthetics and mobility aids

Recent news stories have highlighted the increased risk of long-term brain diseases, like chronic traumatic encephalopathy (CTE) and Alzheimer’s, in athletes who play high-contact sports. However, scientists still have many unanswered questions about what happens in the brain during an injury and how the severity and frequency of impacts influence the development of these diseases.
Dr. Lakiesha N. Williams, a professor, and her team are investigating the molecular and tissue changes that occur in the brain following trauma. “We’re trying to answer questions like: What exactly happens in the brain during a traumatic injury? How does the damage start? What factors influence how the injury develops and is there a point where recovery becomes impossible?” Williams said. “These are complex questions that keep our team engaged as we seek to uncover new insights.”
Their laboratory is examining how memory, behavior, and cellular signals change after an injury, providing insights into how damage progresses over time. The team is also exploring specific genetic factors to investigate why some people experience lasting cognitive or behavioral changes after an injury, while others recover quickly. In parallel, Williams’s team is investigating how tissues are affected during an injury. They are particularly interested in the blood-brain barrier, the brain’s protective shield, which can be compromised even after a mild trauma. Another area of interest is the cranial dura mater, a thick tissue layer that protects the brain. Often overlooked, Williams believes this layer plays a more dynamic role in brain health.
“Historically, cranial dura has been treated a bit like paint on a car, seen as a protective outer layer but not essential to brain function,” she said. “But we want to know the role of dura in supporting brain health. What happens when it’s damaged, whether from trauma, surgery, or other causes?”
By mapping the molecular pathways and tissues involved in trauma, Williams’s team aims to identify key targets for therapeutic interventions, such as delivering nanoparticles across the blood-brain barrier for real-time imaging. They are also pursuing engineered replacement tissues that not only protect the brain but also help it heal after injury.

While Williams focuses on brain injury and its link to neurodegeneration, other UF engineers are addressing pain and disability that originate in the joints.
“THE ULTIMATE GOAL OF OUR LAB IS TO TRANSLATE OUR DISCOVERIES INTO CLINICAL SOLUTIONS FOR TRAUMATIC BRAIN INJURY AND NEURODEGENERATION, AND MOST IMPORTANTLY, TO HELP THOSE SUFFERING FROM THESE PERSISTENT AND LIFE-ALTERING CONDITIONS.”
Lakiesha Williams
Kyle Allen, Ph.D., professor, is exploring a baffling phenomenon in osteoarthritis: some patients endure severe pain with minimal joint damage, while others show extensive degeneration with little discomfort.
“The damage often doesn’t match the symptoms,” Allen said. “Radiographs alone can’t guide treatment.”
To unravel this mystery, Allen’s lab uses both preclinical and clinical models to map the biological and behavioral aspects of pain. They examine nerve and immune cell changes, sleep patterns, coping mechanisms, biomechanical forces, and more.
“There’s no map to follow, so we look at everything,” Allen said. “It’s like a Monet painting, with thousands of tiny dots. We don’t know which dots matter, so we are attempting to capture as much as we can.”
In the clinic, Allen’s team partners with UF’s Pain Research and Intervention Center of Excellence. Before undergoing joint surgery, patients undergo sensory testing, which involves responding to heat, cold, touch, and emotional stress. Then, the gathered tissue are analyzed for changes in innervation, blood vessels, and metabolic and genetic alterations using advanced microscopy and mass spectroscopy. This approach seeks to better align tissue damage with patients’ pain experiences.
“Once you’re in pain, it’s hard to get you out of it,” Allen said. “We want to understand how tissue damage and/or degradation correlates with a person’s sense of not only pain, but their environment. Understanding this could help us create more personalized and effective treatments.”
They also leverage preclinical models to facilitate a more comprehensive investigation into pain and mobility. By combining high-speed video of gait, heart rate monitoring, and quantitative sensory testing assessments within controlled environments and pathology, a more holistic picture of the correlation between pain and mobility can emerge. Using these models, they are
uncovering the impact of the environment on mobility and pain, where changes in enrichment activities alter the presentation of pain.

By gathering comprehensive data, Allen’s team is creating more precise metrics for evaluating therapies, from medications and rehabilitation to bioelectric stimulation and nutrition. Outcomes can not only be beneficial to humans but also to alleviating mobility issues in animals.
“We’re the test bed that can help get these things into the clinic,” he said.
Allen’s search for more precise pain metrics aligns with Jennifer Nichols’ efforts to turn biomechanical data into predictive tools that can guide treatment decisions in real time.

“ONCE YOU’RE IN PAIN, IT’S HARD TO GET YOU OUT OF IT.”
“WE WANT TO UNDERSTAND HOW TISSUE DAMAGE AND/OR DEGRADATION CORRELATES WITH A PERSON’S SENSE OF NOT ONLY PAIN, BUT THEIR ENVIRONMENT. UNDERSTANDING THIS COULD HELP US CREATE MORE PERSONALIZED AND EFFECTIVE TREATMENTS.”
Kyle Allen
In the past, doctors had to make orthopaedic decisions based on limited clinical data and qualitative patient discussions. Now, robust datasets of movement are easily captured; however, they are hard to contextualize into clear clinical guidelines. Jennifer Nichols, Ph.D., an associate professor, seeks to address these challenges by combining real-world data, captured via 3D motion analysis, EMG, and imaging, with UF’s HiPerGator supercomputer to create robust artificial intelligence (AI) models that enable real-time, patient-specific prognostics in a doctor’s office.
One age-old question her team is exploring is: does pain alter how we move, or do changes in our movement lead to pain?
“When you’re in pain, you move differently,” Nichols explained. “But with conditions like osteoarthritis, do you start moving differently first, causing pain and degeneration? Or does bone degeneration start first, causing altered movement?”
To investigate this question, her laboratory is collecting data from individuals with specific biomechanical pathologies, such as thumb osteoarthritis and rotator cuff tears. Feeding this data into their customized predictive computer simulations, they can query why some people with visible joint damage are pain-free, while others continue to experience pain even after the damage is repaired.
Another project focuses on building personalized computer models that can predict outcomes for individual patients. Nichols’ team collects extensive datasets from people with and without specific joint pathologies to generate robust predictive models. Using UF’s HiPerGator supercomputer, Nichols’ lab can then create custom simulations of hand function in seconds, compared to the months it would take using traditional methods.
In another research area, Nichols’ team is investigating ankle syndesmosis injuries, where even small misalignments during surgery can significantly impact recovery, resulting in reduced stride length, altered muscle strength, and impaired ankle rotation.

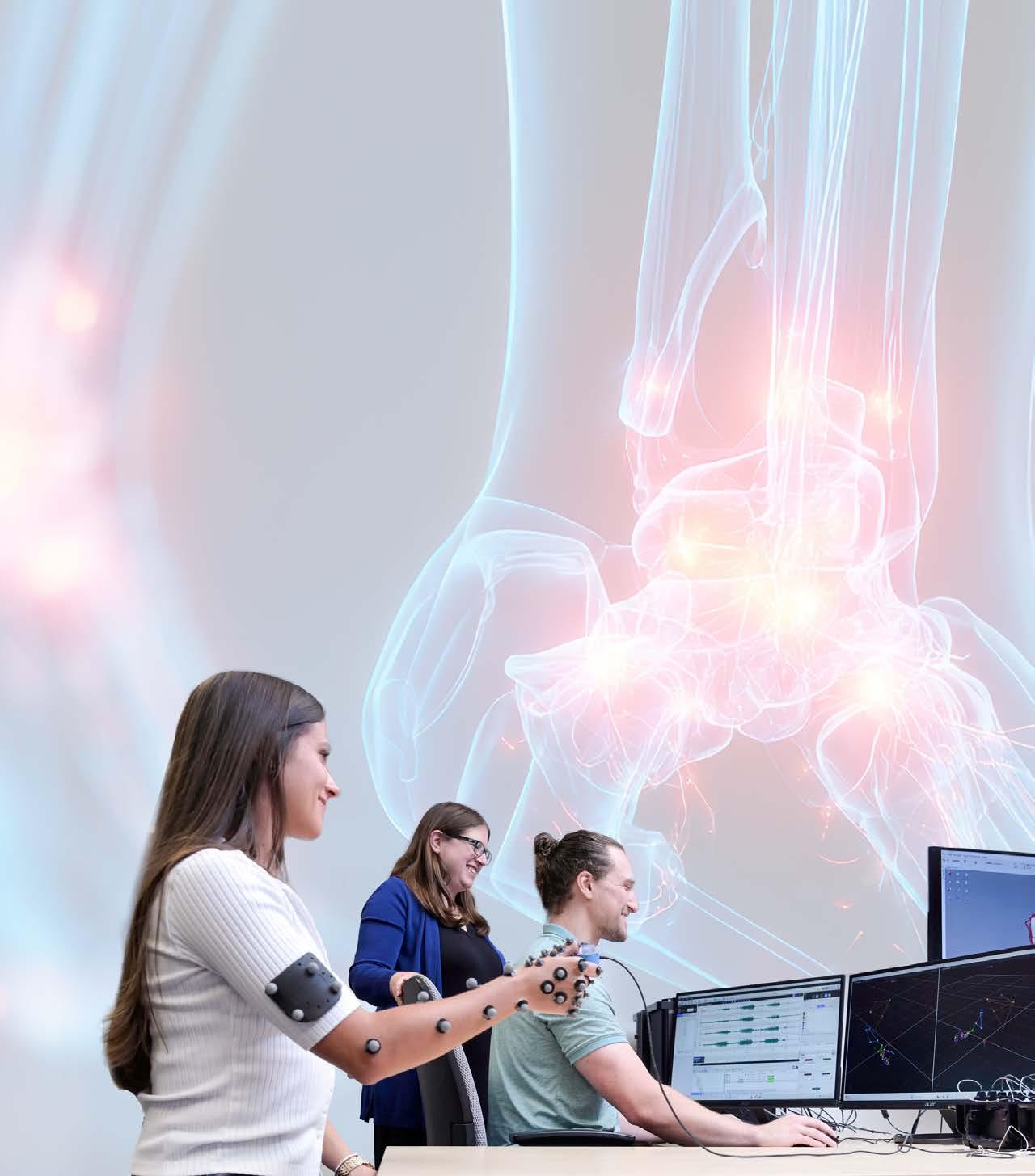
“According to the literature, orthopaedic surgeons who are putting screws in can be off by a few millimeters about 5 to 52 percent of the time,” she said. “Does 2 millimeters matter, biomechanically? This is where computer modeling comes in.”
Nichols’ team is collecting preoperative imaging, surgeon eye tracking, and postoperative gait analysis data to create models that can better understand the biomechanics of surgical outcomes. Outcomes can then drive better guidelines for these surgeries.
The predictive insights from Nichols’ computer models complement work by Blanka Sharma, who is developing targeted treatments that can be delivered directly to the damaged joint.
“WHEN YOU’RE IN PAIN, YOU MOVE DIFFERENTLY,” NICHOLS EXPLAINED. “BUT WITH CONDITIONS LIKE OSTEOARTHRITIS, DO YOU START MOVING DIFFERENTLY FIRST, CAUSING PAIN AND DEGENERATION? OR DOES BONE DEGENERATION START FIRST, CAUSING ALTERED MOVEMENT?”
Jennifer Nichols
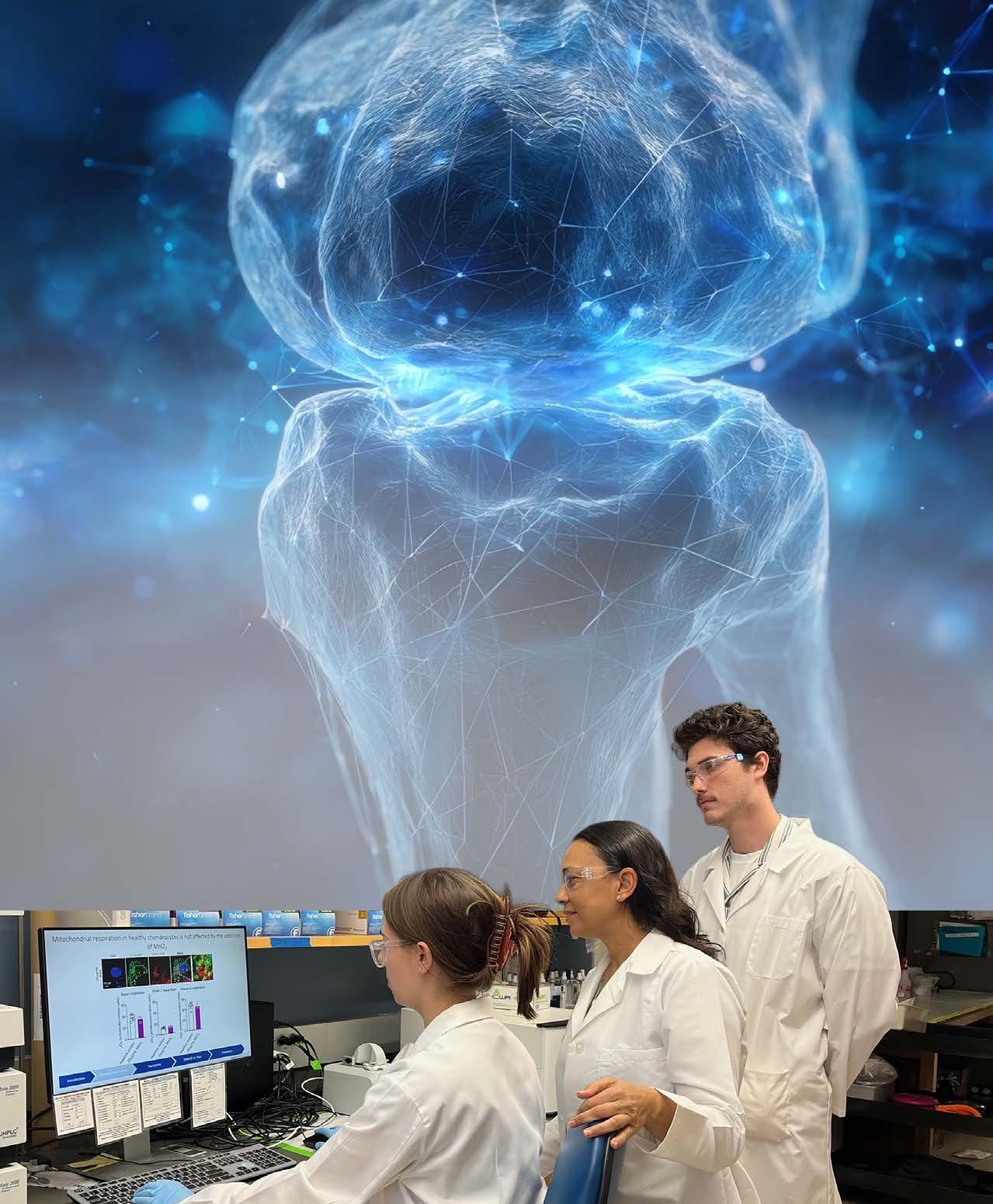
People currently living with osteoarthritis are keenly aware of the limitations of current treatments. Most medications serve to temporarily alleviate inflammation and pain, while falling short of halting or reversing joint damage. Blanka Sharma, Ph.D., associate professor, is devising more targeted and multi-pronged approaches to more effectively treat this condition.
“Osteoarthritis is a complex disease, not likely to be fixed by a single drug,” Sharma said. “Our philosophy in my lab is that in a complex disease with multiple disease mechanisms, each mechanism needs to be addressed holistically.”
Once a promising agent is identified, another part of the problem is delivery, as most drug candidates fail in clinical trials because they do not reach the right joint tissues in sufficient amounts.
Sharma’s lab addresses these challenges from multiple angles: creating benchtop models that can efficiently mimic complex tissue interactions and engineering novel particles to deliver treatments with greater precision.
To more effectively identify key targets and characterize the delivery and efficacy of a drug, Sharma’s lab is creating benchtop human joint tissues that include the complex features of cartilage, synovium and nerves. This shared culture system can more accurately replicate the joint’s internal communication.
“There is crosstalk between the various types of tissue in a joint,” Sharma said. “We want to capture this by growing these tissues together. This also allows us to test therapeutics more holistically.”

In collaboration with the University of Central Florida, Sharma’s lab aims to use this model to efficiently screen compounds from molecular libraries and assess their therapeutic potential. This approach can not only identify new targets but could also provide insight into how different drugs engage in this complex microenvironment to improve their delivery.
In parallel, Sharma and her team are engineering nanoparticles labeled with iron oxide, designed to penetrate joint tissues and deliver drugs. These engineered particles help drugs reach deep into cartilage and synovium cells by adjusting the particles’ size, shape and surface chemistry to match the target tissue.
But knowing whether the particles reach their mark requires visibility.
“One challenge is how to get carriers from the circulation system into joint tissue,” Sharma said. “Also, how can we track these particles? Where do we want them to go?”
To answer that, Sharma is leveraging Magnetic Particle Imaging (MPI), an emerging imaging technique that tracks the real-time distribution of iron oxide tracers. This work is in collaboration with Carlos Rinaldi-Ramos, Ph.D., professor.
In parallel with Sharma’s regenerative strategies, Jamal Lewis is reprogramming the immune system to stop the inflammation that drives joint destruction.
“OSTEOARTHRITIS IS A COMPLEX DISEASE, NOT LIKELY TO BE FIXED BY A SINGLE DRUG. OUR PHILOSOPHY IN MY LAB IS THAT IN A COMPLEX DISEASE WITH MULTIPLE DISEASE MECHANISMS, EACH
Blanka Sharma
Rheumatoid arthritis is a result of an autoimmune condition, where a person’s own immune cells turn against them. Researchers hypothesize that dendritic cells, which typically signal the presence of bacteria and viruses, mistakenly attack joints. This triggers an all-out immune assault, degrading cartilage and bone and, in late stages, leading to systemic inflammation and osteoporosis.
Current treatments aim to halt this damage by suppressing the entire immune system’s function. This leaves the patient susceptible to infections. Jamal Lewis, Ph.D., associate professor, is developing an alternative approach that doesn’t weaken the immune system. Instead of creating a drug that seeks to kill the aberrant immune cells, his team is engineering particles that seek to reeducate them. Lewis’s approach involves using nanoparticles and microparticles to deliver corrective signals directly to dendritic cells, teaching them that joint tissue is not the enemy.
These particles are designed to resemble bacteria or viruses, thereby attracting dendritic cells. Once inside, the nanoparticles deliver targeted instructions that tell these immune cells to stand down. As dendritic cells also play a key role in directing the activity of other immune cells, this process can also create additional downstream benefits.
“By putting into signals within particles that instruct the dendritic cell that specific joint proteins are good, it turns off their attack,” Lewis said. “These dendritic cells can then further propagate this favorable signal by reeducating other key immune cells, like T-cells and B-cells, to also not destroy the joints.”
In pre-clinical models, their innovative approach reversed established rheumatoid arthritis without impairing the function of healthy cells. Lewis’ team is now creating a newer generation,
with the goal of translating it for use in the clinic.

“We showed with prior systems that we can completely reverse the disease,” Lewis said. “I’m hopeful that in a year or two, we’ll have a solid model that will propel us forward in a translational direction.”
By harnessing and not destroying the abnormal cells, this approach could lead to more lasting impacts. Furthermore, the modularity of this approach allows for customization to other autoimmune joint diseases.
Beyond joints, tendon injuries present another major barrier to mobility. Brittany Taylor is taking an inside-out approach to help these critical connectors heal without scarring.


“WE SHOWED WITH BOTH SYSTEMS THAT WE CAN COMPLETELY REVERSE THE DISEASE. I’M HOPEFUL THAT IN A YEAR OR TWO, WE’LL HAVE A SOLID MODEL THAT WILL PROPEL US FORWARD IN A TRANSLATIONAL DIRECTION.”
Jamal Lewis
Tendons, which connect muscles to bones, are often overshadowed in discussions about biomechanical issues or arthritis treatments. Yet, their health is crucial for maintaining mobility and living pain-free. Brittany Taylor, Ph.D., assistant professor, and her team are pioneering new ways to treat tendon injuries, aiming to restore strength and reduce pain by making sure the healing process repairs them properly.
“Tendons heal through fibrotic scar formation, which reduces mobility and integrity and causes dysfunction and pain,” Taylor said. “This affects everyone, from athletes to workers to older adults, and can severely impact their quality of life.”
Current treatments address symptoms but do not stop the underlying scarring process. Taylor’s lab is taking a different approach, studying tendon healing at the cellular level to understand how to encourage regeneration instead of scarring. Her team builds engineered models of tendon microenvironments, using nanomaterials to replicate healthy and diseased conditions. They study how tendon cells respond to changes in collagen structure, inflammation, and mechanical loading, factors that influence healing outcomes. Their research has shown that tendons respond differently depending on how quickly or slowly they are loaded, offering insights to improve rehabilitation protocols.
Taylor’s lab also explores how immune cells influence tendon healing. For example, they have found that exposing tendon tissues to immune cells increases the levels of neuropeptides
associated with pain. Also, in diseased tissue, the regular communication between cells breaks down, contributing to disorganized healing.

A major focus of her laboratory’s research is how extracellular vesicles (EVs), which are tiny packages cells use to communicate, impact tendon healing. In diseased tendons, these EVs carry signals that promote fibrosis and inflammation.
“We believe this is driving the scar formation,” Taylor said. Her team validates their findings in pre-clinical models of tendon injury and repair. Of particular interest to her team is investigating the mechanisms of healing in models that do not scar. By identifying molecular and cellular differences in scarring and scar-free models, new therapeutic targets for novel drugs that promote scar-free tendon regeneration may be identified.
“In five to ten years, I hope we will have identified the key mechanisms that lead to disorganized scar tissue and pain, and that we will have developed therapeutics and rehabilitative strategies to improve tendon function by reducing scarring and mitigating pain, leading to a better quality of life for all,” she said.
While Taylor focuses on repairing the body’s natural connective tissues, Daniel Ferris is creating wearable technologies that restore mobility when the body alone cannot.

“IN FIVE TO TEN YEARS, I HOPE WE WILL HAVE IDENTIFIED THE KEY MECHANISMS THAT LEAD TO DISORGANIZED SCAR TISSUE AND PAIN, AND THAT WE WILL HAVE DEVELOPED THERAPEUTICS AND REHABILITATIVE STRATEGIES TO IMPROVE TENDON FUNCTION BY REDUCING SCARRING AND MITIGATING PAIN, LEADING TO A BETTER QUALITY OF LIFE FOR ALL.”
Brittany Taylor

While wearable technologies and devices can significantly improve mobility, they are often limited in their efficacy and accessibility in the real world. Daniel Ferris, Ph.D., a professor, seeks to reduce these barriers by developing human-machine systems, such as artificial limbs and braces, that function effectively in complex environments and across a wide range of activities and age groups. Ferris’s team also seeks to further our understanding of the neural connection between the device and the person.
“Much of our research looks at the relationship of biomechanics to the brain, and back from the brain to biomechanics,” he said. In one approach, they are developing advanced control systems for bionic limbs that combine electrical signals from muscles and nerves with the mechanical movement of prosthetics. Now working on fourth-generation devices, Ferris and his team are testing these limbs in real-world conditions, with participants of all ages walking across campus to fine-tune the technology.
“We talk with the individuals who would benefit from the device, create it, and then get feedback from the users and redesign,” Ferris said. “It takes time, but getting to the end goal of helping someone makes it all worthwhile.”
Second, Ferris is designing powered braces for children with conditions like cerebral palsy that limit leg control. Because children grow quickly, the braces must be lightweight, adaptable, and scalable. Using 3D printing, his team creates braces from carbon fiber and other materials, allowing new parts to be printed as the child grows.
“We’ve designed the first and second generations, and we have feedback on the second generation, but we haven’t yet tested it in the field,” Ferris said.
Lastly, his lab is addressing a challenge that affects nearly everyone with age: loss of balance. Ferris studies how older adults walk on uneven terrain and what contributes to balance loss, whether it is declining strength, sensory feedback, cognition, or vision.

“A lot of our research has been to separate out these candidates,” Ferris said.
In the lab, participants walk on narrow balance beams while wearing electrodes that track muscle activity and brain responses. This work demonstrates that balance relies on all three systems working in tandem: muscles, senses, and brain function.
“We can look at the process in your brain and how fast you react,” he said. “Our research has shown that it comes down to all three areas.”
To translate these findings, his team is developing training programs that combine muscle-strengthening exercises with electronic glasses that adjust visual cues, helping people improve balance and prevent falls.

“WE TALK WITH THE INDIVIDUALS WHO WOULD BENEFIT FROM THE DEVICE, CREATE IT, AND THEN GET FEEDBACK FROM THE USERS AND REDESIGN. IT TAKES TIME, BUT GETTING TO THE END GOAL OF HELPING SOMEONE MAKES IT ALL WORTHWHILE.”
Daniel Ferris

By: Yelaine Aguilar

4 Rotating Tours of Labs 4 Faculty Research Talks

“WE HAD KIDS DOING NEUROSCIENCE AND BIOELECTRONICS. I THINK THAT’S REALLY CUTTING-EDGE SCIENCE, AND WE MADE IT INTO

Associate Professor
If you ask an elementary schooler who attended the UF Biomedical Engineering STEM Outreach Day to explain what a brain looks like, he or she might compare the skull to an Oreo, the blood to Jello and the dura mater to red candy strips.
The candy brain was one of many activities at Saturday’s event in the J. Wayne Reitz Union Rion Ballroom aimed to educate K-12 students in the world of biomedical engineering and related disciplines.
More than 350 attendees flocked to the University of Florida for a science-filled day.
The event is the first of its kind for the UF J. Crayton Pruitt Family Department of Biomedical Engineering, offering activities from labs and science clubs across campus, public lectures from UF professors and guided tours of the Herbert Wertheim Laboratory for Engineering Excellence and the Neuromechanics of Mobility Lab.
“We had kids doing neuroscience and bioelectronics,” said Edward Phelps, UF Associate professor, referencing how the Dr. May Mansy’s Instrumentation Lab brought in an electrocardiogram that let kids monitor their heartbeat. “I think that’s really cutting-edge science, and we made it into activities we could do in a public setting.”
Phelps, founder of the Pancreatic Cellular and Biomaterials Engineering Lab at UF, represented his research in type 1 diabetes with a booth where kids could experiment with hydrogels.
He came up with the idea for a STEM Outreach Day after taking his kids to outreach events from the geology and math departments on campus. He felt the need to do the same for biomedical engineering. Twenty-eight activities represented the field and covered every research area from the department, as well as from other engineering disciplines doing biomedical-related work, Phelps said.
Jennifer Nichols, UF associate professor and director of the Musculoskeletal Biomechanics Lab, set up a table where kids made licorice models of the different muscles in the body. She hoped kids would see the broad and wide-reaching applications of biomedical engineering.
“If we inspire kids to pay a little bit more attention in science class or go find an area of biomedical engineering that they want to pursue,” Nichols said, “that would be great.” Nichols has contributed to breakthrough research at the Musculoskeletal Biomechanics Lab. In her public lecture across from the Rion Ballroom, she talked about
using artificial intelligence to make personalized models of how people move. According to Nichols, using AI to automate the creation of biomechanics models would speed up the process from three months to mere minutes. The next step is to use these models on patients with chronic diseases.

“As soon as you can model hand osteoarthritis or a neurodegenerative disease, you can actually put that in a clinic and have it help inform personalized care,” Nichols said.
UF Athletics applies these same technologies to protect student athletes from injuries on and off the playing field.
Austin Kratish, a homeschooled seventh grader accompanied by his mother Lorena Kratish, enjoyed the Ferris Lab’s booth that connected physical strength to biomechanics. He said can see himself making a difference in the world of biomechanical athletics.
“I like to play football and work out a lot, so it’s interesting,” Kratish said. “I could find new ways to help with injuries in sports.”
Stefanie Marquez, a research and development scientist at Axogen, Inc, came from Tampa with her husband and two young children. She wanted to give her kids the opportunity to explore possibilities in STEM.
“It’s important to spark creativity and curiosity in younger people,” Marquez said. She added that she hopes the event happens every year.
Phelps shared Marquez’s sentiments. While this year’s event was supported by a gift from a private foundation, he is looking for funding to make the outreach day an annual experience.
“My favorite thing was to see the smiles on everyone’s faces and how engaged and how much fun everyone was having with science,” Phelps said. “This was something that will create lasting memories in these kids that hopefully inspire them going forward.”

With newly expanded roles, UF BME’s instructional faculty are launching bold educational initiatives that put students at the center of real-world learning.
Director of Professional Experiences

Vision: I aspire to build a vibrant culture where student involvement sparks leadership, fuels personal growth, and drives community impact. By nurturing skills beyond the classroom, I want to empower students to become influential leaders who shape their professions and positively impact the world around them.
Initiatives:
Supports and advises student organizations to promote leadership, collaboration, and community building.
Leads initiatives that connect students with extracurricular and co-curricular opportunities that enhance their academic and professional journeys.
Designs and facilitates leadership development programming tailored to biomedical engineering students.
Promotes involvement in competitions, conferences, and campus-wide engagement to broaden student experience and visibility.
Provides guidance on professional skills development, including communication, teamwork, and personal branding.
Builds partnerships with campus units and alumni to enrich the professional pathways available to UF BME students.
Director of Design Innovation & Program Assessment

Vision: My vision is to transform the educational journey into a launchpad for meaningful, impactful careers. By connecting students with real-world experiences, industry partnerships, and innovative design challenges, I strive to prepare graduates who lead with creativity, adaptability, and purpose in a rapidly-evolving world.
Initiatives:
Leads efforts to expand access to internships, co-ops, and industrysponsored design projects that give students real-world experience.
Develops and maintains strong relationships with industry partners to create meaningful engagement opportunities for students.
Hosts networking events, alumni panels, and employer visits to connect students with professionals in biomedical engineering and related fields.
Integrates career readiness and professional development modules into core and elective courses.
Guides program-wide assessment efforts to ensure continuous improvement in student learning outcomes and experiential offerings.
Director of Peer Learning & Mentoring

Vision: I envision a learning community where every student feels empowered to take ownership of their education, mastering not only course content but also the critical skills needed to adapt, lead, and support others. By fostering peer-driven learning and resilience, I aim to cultivate confident, lifelong learners who uplift those around them.
Initiatives:
Developed and leads a structured peer mentoring program to foster academic confidence and belonging among undergraduate students.
Designs and delivers workshops on learning strategies, study habits, and time management tailored for engineering students.
Trains peer mentors and undergraduate learning assistants in evidence-based pedagogy and inclusive teaching practices.
Provides individualized and group academic coaching sessions for students seeking to strengthen soft skills and study techniques.
Collaborates with faculty and staff to integrate mentorship and academic support into key courses and co-curricular experiences.
Introducing experts in tissue regeneration and student engagement

Lesley W. Chow, Ph.D.
Associate Professor

Areas of Research: Biomaterials for Regenerative Medicine, Musculoskeletal Tissue Engineering, and 3D Printing
Dr. Chow comes to UF from Lehigh University, where she led a research program focused on designing advanced biomaterials for tissue engineering and regenerative medicine. Her interdisciplinary approach merges supramolecular design, biomaterial fabrication, and techniques such as 3D printing and molecular self-assembly to create scaffolds that replicate the complexity of native tissues.
Her work centers on developing spatially organized scaffolds that guide cell behavior and tissue regeneration, particularly at musculoskeletal interfaces like the osteochondral region between bone and cartilage. The Chow Lab has established a versatile biomaterials platform that integrates modular molecular building blocks and fabrication strategies to control biochemical, physical, and mechanical cues—enabling precise tuning of cell–material interactions for improved regeneration.
Dr. Chow’s path into biomaterials began as a University of Florida undergraduate in Materials Science and Engineering, where she discovered the potential of polymers in biomedical research. She earned her Ph.D. at Northwestern University, advancing supramolecular biomaterials for vascularization and transplant success, and completed postdoctoral training at Imperial College London developing spatially functionalized scaffolds.
With innovative work that bridges fundamental biomaterials science and clinical translation, Dr. Chow brings expertise that strengthens UF BME’s mission to advance health through engineering, while also mentoring and training the next generation of biomedical engineers.

Roza Vaez Ghaemi, Ph.D.
Instructional
Assistant Professor
Areas of Research: Biomaterials, Tissue Engineering, Engineering Education, Problem/Project Based Learning, Student Engagement
Dr. Roza Vaez Ghaemi is an instructional assistant professor in the J. Crayton Pruitt Family Department of Biomedical Engineering at the University of Florida. She leads the cell and tissue coursework, mentors the next generation of biomedical engineers, and contributes to the department’s evolving teaching infrastructure, including hands-on makerspaces and lab innovation.
She earned her Ph.D. in biomedical engineering from the University of British Columbia in 2022, where her research explored the biomechanics of stem cell-derived in vitro tissues such as neurospheres, providing new insights into how organoid systems respond to mechanical environments. Her teaching-focused postdoctoral work in engineering education at Michigan State University in 2023 led to the creation of the IDEAL (Interactive Digital Experience as an Alternative Lab) model, which uses immersive digital platforms and classroom analytics to enhance student engagement and performance in biomechanics.
In addition to her work in engineering education, Dr. Vaez Ghaemi has advanced a biomaterial-based approach to treat corneal blindness. Her development of a smart membrane capable of regulating oxygen, glucose, and ion transport through an external alternating magnetic field shows promise for restoring vision.
With her expertise at the intersection of research and pedagogy, Dr. Vaez Ghaemi brings an innovative perspective that strengthens UF BME’s commitment to experiential learning, translational innovation, and preparing students to become leaders in biomedical engineering.
At the J. Crayton Pruitt Family Department of Biomedical Engineering, mentorship is more than just guidance—it’s a powerful bridge between classroom learning and real-world impact. Through the newly revamped BMEntor Program, our Alumni Advisory Board (AAB) plays a vital role in shaping the next generation of biomedical engineers by mentoring students as they prepare for diverse careers in industry, academia, and beyond.
The BMEntor Program now features a structured, evidence-informed framework that emphasizes purposeful pairing, goal setting, and growth. In addition to one-on-one mentorship with accomplished UF BME alumni, participants benefit from a custom-designed mentorship training module that supports both mentors and mentees in building effective, responsive, and reflective mentoring relationships. This self-paced training includes educational videos, practical tools, and a library of resources aligned with best practices in mentoring. With BMEntor, UF BME students are gaining not just direction—but confidence, clarity, and community as they shape their futures.
Program Highlights
• Personalized and automated mentor-mentee matching based on shared interests, skills, and goals
• Expert guidance on career pathways, graduate school, and industry opportunities
• Networking connections with leaders across biomedical engineering and related fields
• Hands-on advice for resumes, interviews, and professional development
• A supportive, growing mentorship community within the Gator Nation
• Training to develop and enhance mentoring skills


Clinical Affairs Leader, Gore Medical Products
Clark, a UF Mechanical Engineering alum, leads global clinical research as the Clinical Affairs Leader for the Peripheral Business Group at W. L. Gore & Associates, advancing therapies that improve patient outcomes. She sees joining the BME Alumni Advisory Board as a way to give back,


BS
Employer: Oregon Health & Science University
Lindsay DeWeese, Ph.D., is a medical physicist and associate professor in the Department of Radiology at Oregon Health & Science University (OHSU). She is a triple-degree Gator with a BS and MS in nuclear engineering sciences and Ph.D. in medical physics.

Employer: Yale University School of Medicine
Christopher Tien is an associate professor of therapeutic radiology at the Yale University School of Medicine. Tien was able to graduate with his Ph.D. in medical physics from UF at 25, funded by the prestigious UF Alumni Graduate Fellowship. After stints at Brown University for residency and Chicago for private practice, he has been at Yale since 2016.
® Tissue Bank
The University of Florida’s Department of Biomedical Engineering has launched a new collaboration with LifeLink Tissue Bank, a division of the globally-recognized LifeLink Foundation. Through UF BME’s Industry Partners Program, the partnership will offer students and faculty hands-on experience in senior design, research, mentorship, and campus engagement. Led by LifeLink leaders including Brad Bassler, who joins UF BME’s External Advisory Board, the collaboration builds on a shared mission to advance healthcare innovation. With deep UF ties—LifeLink founder Dr. Dana Shires helped develop Gatorade in 1965—the Tissue Bank has grown into the Southeast’s largest nonprofit provider of lifesaving grafts. Together, UF BME and LifeLink will honor donor legacies and shape the next generation of biomedical innovators.





We are proud to welcome the newest members of our External Advisory Board—leaders and changemakers across academia, industry, and healthcare who bring a wealth of insight, experience, and passion. Their guidance helps propel our department forward, ensuring we remain at the forefront of biomedical engineering education, research, and impact.
Faculty and board members come together for the 2025 External Advisory Board Meeting at UF BME.
Timothy E. Allen, Ph.D. Professor, Biomedical Engineering, University of Virginia
Stacy Arnold Vice President, Product Development & Clinical Research, Axogen, Inc
Brad Bassler, M.B.A. Vice President of Operations, Associate Executive Director, LifeLink Tissue Bank
Karen Christman, Ph.D. Professor, Bioengineering; Associate Dean for Faculty, Jacobs School of Engineering, University of California San Diego
INDUSTRY PARTNERS:
OUR COMMON BOND IS MORE THAN A RALLYING CRY. IT’S A CALLING.
At the University of Florida’s J. Crayton Pruitt Family Department of Biomedical Engineering, excellence means more than discovery — it’s about impact. By supporting the BME Excellence Fund, you empower our students and faculty to push the boundaries of research, develop life-changing technologies, and prepare the next generation of biomedical innovators. With your partnership, we can accelerate progress and transform lives. HOW CAN YOU MAKE AN IMPACT?




Karen Gallen Vice President of Engineering, Arthrex
Lori Setton, Ph.D.
Department Chair, Lucy & Stanley Lopata Distinguished Professor of Biomedical Engineering, Washington University in St Louis
Jason Smith, Ph.D. Senior Director of Research & Development, Evergen
Andrew Stricklin, Assistant Director of Engineering KLS Martin







CHRISTINE SCHMIDT
Elected to National Academy of Medicine & Biomedical Engineering Society (BMES)
Athanasiou Medal of Excellence in Translational Bioengineering

PARISA RASHIDI
Presidential Early Career Award for Scientists and Engineers (PECASE) & 2025 Biomedical Engineering Society (BMES) Fellow
CELEBRATING YOUNG INNOVATORS AWARDED NIH HONORS FOR BREAKTHROUGH RESEARCH



XIAO FAN
Innovator Award (DP2): Fan will harness language models to decode DNA as a ‘biological language,’ aiming to uncover genetic patterns that could revolutionize our understanding of human diseases.
IVANA PARKER
Pioneer Award High Risk, High Reward (DPI): Parker will investigate Bacterial Vaginosis and its impact on HIV risk, paving the way for personalized treatment solutions.
ANA MARIA PORRAS
Maximizing Investigators’ Research Award (MIRA): Porras is exploring groundbreaking interactions between the human microbiome and the extracellular matrix to uncover new insights into tissue maintenance and inflammation.









RUOGU FANG
HWCOE Doctoral Dissertation Advisor/ Mentoring Award & UF Pioneering Research HiPerGator Award
AYSEGUL GUNDUZ
2024 Biomedical Engineering Society (BMES) Fellow
JAMAL LEWIS
UF Tech Licensing’s Invention of the Year
WALTER LEE MURFEE
HWCOE Excellence Award in Leadership, AIMBE Fellow & 2025 Biomedical Engineering Society (BMES) Fellow
IVANA PARKER
Keystone Symposia Fellow
ANA MARIA PORRAS
UF Excellence Award for Assistant Professors, UF Center for Teaching Excellence Rising Star Award, Biomedical Engineering Society (BMES) Social Impact Lecture Award on behalf of LatinXinBME
LAKIESHA WILLIAMS
2024 Biomedical Engineering Society (BMES) Fellow
GREGORY HUDALLA
Promoted to Professor
WESLEY BOLCH
Congratulations on serving 30 years to UF!










Kyle Allen
Professor
Ph.D., Rice University
Novel strategies to diagnose and treat joint disease that scale from preclinical models to equine or human patients
Wesley E. Bolch
Distinguished Professor & UF Term Professor
Ph.D., University of Florida
Dosimetry, computational medical physics and radiation dose assessment
Markia Bowe
Instructional Assistant Professor
Ph.D., University of Florida
Biomechanics of bone, 3D imaging, inclusive design and engineering education research
Lesley W. Chow
Associate Professor Ph.D., Northwestern University
Biomaterials for regenerative medicine, musculoskeletal tissue engineering, and 3D printing
Mingzhou Ding
Distinguished Professor & J. Crayton Pruitt Family Professor
Ph.D., University of Maryland
Cognitive neuroscience, signal processing and neural imaging
Xiao Fan
Assistant Professor Ph.D., University of Alberta
Computational approaches to study genetic architecture of rare diseases and interpretation of genetic variants
Ruogu Fang
Associate Professor & J. Crayton
Pruitt Family Term Fellow
Ph.D., Cornell University
Artificial intelligence (AI), brain dynamics and medical image analysis
Meghan Ferrall-Fairbanks
Assistant Professor
Ph.D., Georgia Institute of Technology
Quantitative systems biology, mathematical modeling, cancer heterogeneity and evolutionary dynamics
Daniel Ferris
Robert W. Adenbaum Professor
Ph.D., University of California, Berkeley
Biomechanics, neuromechanical control, locomotion, mobile brain imaging, robotic exoskeletons and bionic prostheses
Sarah Furtney
Director of Experiential Learning, Herbert Wertheim College of Engineering
Ph.D., Clemson University
Cell and tissue engineering and engineering education research










Chris Geiger
Instructional Associate Professor
Ph.D., Northwestern University
Senior Design and engineering education research
Kuang Gong
Assistant Professor
Ph.D., University of California at Davis
Deep learning, medical imaging, and data science
Aysegul Gunduz
Professor & Fixel Brain Mapping
Professor
Ph.D., University of Florida
Human brain mapping, neuromodulation and neural interfacing
Gregory A. Hudalla
Professor, Integra
LifeSciences Term Professor
Ph.D., University of Wisconsin
Molecular engineering for immunotherapies and immune modulation
Benjamin G. Keselowsky
Professor
Ph.D., Georgia Institute of Technology
Biomaterials and Immune Engineering
Jamal Lewis
Associate Professor & Graduate
Coordinator
Ph.D., University of Florida
Biomaterials, drug delivery and immunoengineering
May Mansy
Instructional Assistant Professor
Ph.D., University of Florida
Bio-signals & systems, bio-instrumentation lab and engineering education
Walter Lee Murfee
Professor & Associate Chair for Undergraduate Studies
Ph.D., University of Virginia
Cell dynamics, microcirculation, angiogenesis, lymphangiogenesis and neurogenesis
Jennifer A. Nichols
Associate Professor & J. Crayton
Pruitt Family Term Fellow
Ph.D., Northwestern University
Biomechanics, musculoskeletal modeling, predictive simulation, medical imaging and machine learning
Ivana Parker
Assistant Professor
Ph.D., Georgia Institute of Technology
Trained immunity, systems biology, HIV/TB, host-pathogen interactions and applied proteomics










Edward A. Phelps
Associate Professor
Ph.D., Georgia Institute of Technology
Biomaterials, islet biology, diabetes, and immune engineering
Ana Maria Porras
Assistant Professor
Ph.D., University of Wisconsin
Cell and tissue engineering to study human-microbe interactions, and evidence-based public engagement with science
Parisa Rashidi
Professor, UF Research Foundation
Professor & IC3 Co-Director
Ph.D., Washington State University
Medical artificial intelligence (AI) and pervasive health
Carlos Rinaldi-Ramos
Professor
Ph.D., MIT
Nanomedicine and magnetic nanoparticles
Christine E. Schmidt
Distinguished Professor & J. Crayton Pruitt Family
Endowed Chair
Ph.D., University of Illinois
Biomaterials for neural tissue regeneration and neural interfacing
Blanka Sharma
Associate Professor & Associate Chair for Graduate Studies
Ph.D., Johns Hopkins University
Nanomedicine, biomaterials, targeted drug/gene delivery and immunoengineering
Cherie Stabler
Professor & Department Chair, J. Crayton Pruitt Family & UF
Foundation Preeminence Professor
Ph.D., Georgia Institute of Technology
Biomaterials, controlled release, regenerative medicine and diabetes
Brittany Taylor
Assistant Professor
Ph.D., Rutgers University
Nanomedicine, regenerative rehabilitation, biomaterials, and tendon injury and disease
Roza Vaez Ghaemi
Instructional Assistant Professor
Ph.D., University of British Columbia
Biomaterials, tissue engineering, engineering education, and student engagemen
Lakiesha N. Williams
Professor
Ph.D., Mississippi State University
Traumatic brain injury, Alzheimer’s disease and related dementias and soft tissue biomechanics
By: Grace Huff

Artificial Intelligence (AI) is revolutionizing healthcare, and at the heart of this transformation are foundation models (FMs), large-scale deep learning systems trained on massive datasets and a more general form of ChatGPT beyond text. In their recent paper, A Comprehensive Survey of Foundation Models in Medicine accepted by IEEE Reviews in Biomedical Engineering (RBME), researchers Ruogu Fang, Associate Professor and Pruitt Family Endowed Faculty Fellow in the J. Crayton Pruitt Family Department of Biomedical Engineering, along with her Postdoc Associate Wasif Khan, provide an in-depth exploration of these models, their applications, and the challenges they present in modern medicine.
FMs have shifted the way researchers approach AI, evolving beyond specialized, task-specific algorithms to generalizable systems capable of performing multiple functions with minimal additional training. While large language models (LLMs) such as ChatGPT and Gemini have taken center stage, foundation models extend beyond text, spanning fields such as medical imaging, genomics, drug discovery, and clinical decision support.
“Foundation models are transforming our perception of AI and the way we interact with different modalities of data such as satellite images, neuroimaging, genomics, and social networks, just like chatGPT changed the way of interaction with text in late 2022” says Ruogu Fang.
At their core, foundation models are large-scale neural networks that use self-supervised learning techniques to generalize knowledge across different tasks. This enables them to be adapted for applications in clinical natural language processing (NLP), medical imaging, biological data analysis, and more. By learning from diverse datasets, these models improve diagnostic accuracy, predictive modeling, and personalized medicine.
“Foundation models serve as a versatile base for a wide range of downstream tasks across different domains, including medicine,” explains Wasif Khan. “Their significance in healthcare stems from their ability to generalize across multiple applications, such as clinical NLP, medical image analysis, omics research, and decision support systems.”
The authors highlight several key areas where FMs are poised to revolutionize medicine:
Clinical Decision Support – Enhancing diagnostics, treatment recommendations, and patient management.
Medical Image Analysis – Improving radiology and pathology workflows by automating segmentation, classification, and anomaly detection.
Natural Language Processing (NLP) in Healthcare – Enabling improved chatbot-based virtual assistants, medical documentation automation, and enhanced information retrieval from clinical texts.
Drug Discovery and Precision Medicine – Accelerating new treatment development and personalizing patient care based on genetic data.
Telemedicine and Digital Health – Integrating multimodal data for remote healthcare delivery and patient engagement.
“FMs have the potential to revolutionize medicine due to their ability to learn from diverse and large-scale medical datasets,” says Fang. “Their robustness enables generalization across multiple applications through zero-shot or few-shot learning, which substantially reduces the need for time-consuming and expensive human labeling of medical data.”
“Interpretability is one of the biggest concerns,” explains Khan. “Clinicians need to trust the outputs of these models before integrating them into their workflows. We are seeing progress in developing explainable AI techniques, but there is still work to be done.”
Despite these challenges, the future of FMs in medicine is promising. “I hope to see more lightweight, efficient, and multimodal foundation models integrated into clinical workflows over the next five to ten years,” says Fang. “These advancements could bridge the gap between multimodal medical data, the findings, and disease indications, the three different segments of knowledge that are quite siloed in today’s biomedical and clinical research and practice.”
The authors also emphasize the need for interdisciplinary collaboration: “AI researchers, clinicians, and policymakers should work together to ensure the ethical and responsible deployment of

these models,” adds Khan. “Interdisciplinary research and regulatory oversight will be key in guiding the future of AI in healthcare.”
FMs are not just reshaping medicine—they are laying the groundwork for a new era of AI-driven healthcare. As researchers continue to refine these models, their ability to improve diagnostics, streamline workflows, and personalize treatments will only grow. However, addressing the challenges of bias, interpretability, and accessibility will be critical in determining how these models ultimately impact patients and healthcare providers worldwide.
“We provide a clear picture of the current development and taxonomy of FMs since their dawn in 2022,” concludes Fang, “and a roadmap for the larger AI and healthcare communities to see the direction forward.”
For those looking to leverage FMs in medicine, Fang advises: “Prioritize explainability. Developing transparent models will be critical for widespread clinical adoption. Additionally, focus on multi-modal integration, as combining text, images, and omics data can unlock the full potential of FMs in medicine.”
Artificial Intelligence (AI) is transforming medicine, and foundation models (FMs) are leading the way. These powerful AI systems can analyze medical images, assist with diagnoses, and even help discover new drugs.
In their paper, A Comprehensive Survey of Foundation Models in Medicine, researchers Wasif Khan and Ruogu Fang explain how
FMs work and their potential impact. Unlike traditional AI, FMs can handle multiple tasks with minimal training, making them valuable in clinical decision support, medical imaging, and drug discovery.
“These models have the potential to revolutionize medicine,” says Khan. However, challenges remain, including trust, fairness, security, and cost. “Clinicians need to trust AI outputs before integrating them into their workflows,” adds Fang. For AI to truly succeed, researchers, doctors, and policymakers must collaborate to ensure ethical and responsible use.
Fang also credits her mentees, students, and collaborators –Seowung Leem (BME PhD student), Kyle B. See (recently graduated BME PhD student and now postdoc at UF), and Joshua K. Wong, M.D. (Assistant Professor and clinical collaborator at UF Neurology and Norman Fixel Institute for Neurological Diseases).
“The greatest joy comes from working with brilliant minds at the forefront of AI!” Fang said.
“FOUNDATION MODELS ARE TRANSFORMING OUR PERCEPTION OF AI AND THE WAY WE INTERACT WITH DIFFERENT MODALITIES OF DATA SUCH AS SATELLITE IMAGES, NEUROIMAGING, GENOMICS, AND SOCIAL NETWORKS, JUST LIKE CHATGPT CHANGED THE WAY OF INTERACTION WITH TEXT IN LATE 2022.”
Ruogu Fang

By: Dave Schlenker and Candi Crimmins
Osteoarthritis is a debilitating and degenerative joint disease with no current cure or effective long-term therapy. Our research seeks to overcome the significant challenges in delivering therapeutics directly to the site of disease, which has hindered the success of many promising treatments.
-Blanka Sharma

Equipped with a cutting-edge nanoparticle imaging technology, federal funds and a quest to quash pain, researchers at the University of Florida are working to revolutionize osteoarthritis treatments.
Central to the research is the Magnetic Particle Imaging (MPI) technology, a scanner with unparalleled precision in tracking nanoparticles in the body. With intravenously administered nanoparticles, the team uses the scanner to track how well the particles – with potential as diagnostic and therapeutic agents – reach inflamed joints.
“Osteoarthritis is a debilitating and degenerative joint disease with no current cure or effective long-term therapy,” said Blanka Sharma, Ph.D., an associate professor in the J. Crayton Pruitt Family Department of Biomedical Engineering and co-lead on the project. “Our research seeks to overcome the significant challenges in delivering therapeutics directly to the site of disease, which has hindered the success of many promising treatments.”
Funded by a grant from the National Institutes of Health’s Institute of Arthritis and Musculoskeletal and Skin Diseases, this research is a partnership between UF’s Biomedical and Chemical Engineering departments. Sharma’s co-principal investigator is Carlos Rinaldi-Ramos, professor and former chair of UF’s Chemical Engineering department. The team also is working with the University of Central Florida on this project.
The MPI scanner has been a transformative research tool at UF for years. It is one of five Magnetic Insight MPI scanners in North America, Rinaldi-Ramos said. And while the team is knee-deep in the osteoarthritis research, they hope the scanner will, one day, also track how well therapies and medications reach cancerous tumors.
With the osteoarthritis project, the test subjects will be mice with knee injuries. In this model, if the nanoparticle scanner can show the arrival of intravenously administered nanoparticles to the joint, then the researchers will seek to develop novel methods to monitor disease progression and deliver therapies without the need for direct injection into the joint.
“This technology helps inform the clinician on the best strategies to treat a patient,” Rinaldi-Ramos said.
Affecting more than 33 million adults in the United States, osteoarthritis is the most common type of arthritis, according to the U.S. Centers for Disease Control and Prevention. It causes joint pain, stiffness, and swelling in the hands, hips, back, or knees.
“It’s highly prevalent, and we have no therapy currently that is actually disease-modifying,” Sharma said. “Everything now is symptom management until someone needs a total joint replacement. Joint replacements are great for certain populations – the elderly and those strong enough to undergo the surgery – but it is not ideal for younger patients because of the lifespan of the implants. They also do not fully replicate the function of your joints.
Read Full Story
Drs. Carlos Rinaldi-Ramos and Blanka Sharma demonstrate how to load a sample into the Magnetic Particle Imaging nanoparticle scanner. The two are using the scanner to explore better ways to treat osteoarthritis.

By: Megan Howard

Twelve times a day for 14 years – starting at 3 a.m. – Cameron Crouse checked his blood sugar with finger pricks from a glucometer.
Diagnosed a month shy of his third birthday with type 1 diabetes in 2003, Crouse said administering 12 pricks a day became second nature. But for many people, especially those diagnosed later in life, managing diabetes often presents substantial physical, emotional and financial challenges.
“I consider myself fortunate because I was so young that I don’t have any memories of being diagnosed with type 1 diabetes,” said Crouse, 24, a University of Florida Ph.D. student and biomedical engineering major in the Herbert Wertheim College of Engineering.
The Alabama native no longer pricks his finger each day, now using a glucose monitor with a continuous subcutaneous drip of insulin to track and correct changes in his blood sugar. These pieces of biomedical technology are able to “talk to each other,” Crouse said –an advancement that has made a huge difference for diabetics.
But Crouse contends that technology for diabetes treatment should not end there.
A year after Crouse was diagnosed, he and his family dove into diabetes research initiatives through the advocacy organization Breakthrough T1D. Crouse has been involved ever since, sharing his story often and seeking support from local and national politicians.
“I advocate, I fundraise and I do research to make their [diabetics’] lives easier. That makes it all worthwhile,” Crouse said. “I’m passionate about this cause because not only do I live with it and know people who live with it, I know how tough it is to live with this disease in a fortunate situation.”
In fact, Breakthrough T1D honored Crouse in May for his advocacy work in Alabama, which includes a campaign called Promise to Remember Me that connects young diabetics with their state representatives to raise awareness for legislation and funding initiatives.
“I owe it to my parents for being amazing people with amazing ideas and amazing resolve and willpower,” Crouse said.
His parents served on the Breakthrough T1D board and were honored in 2009 for spearheading large-scale fundraisers and advocacy throughout Alabama and Washington, D.C. Michelle and Hans Crouse immersed themselves in diabetes advocacy partly to remind their son that his voice can make a difference.
“He’s been all in and we’re so proud of him,” his mother said. “He probably wouldn’t be in a diabetes lab right now if he didn’t live with it.”
I advocate, I fundraise and I do research to make their [diabetics’] lives easier. That makes it all worthwhile, Crouse said.
I’m passionate about this cause because not only do I live with it and know people who live with it, I know how tough it is to live with this disease in a fortunate situation.
- Cameron Crouse
Crouse completed his undergraduate work in tissue engineering at Clemson University and spent an undergraduate summer in Boston at the Joslin Diabetes Center, pursuing tissue research in diabetes.
For his graduate studies, he was inspired by UF professor and J. Crayton Pruitt Family Department of Biomedical Engineering Chair Cherie Stabler, Ph.D., who has been researching diabetes for 20 years. Like Crouse, Stabler is driven by translational research and wants her work to make a direct impact on the lives of people with diabetes.
“He is a brilliant researcher,” Stabler said about Crouse, who has been working in her lab. “He’s exceptionally hard-working. He’s very creative, very dexterous.”
Crouse wants to utilize biomaterials in islet cell transplantation to treat type 1 diabetes, Stabler said. Challenges with this approach include immune system rejection and oxygen support for transplanted cells, she added.
Such challenges only inspire Crouse to apply his background in tissue engineering – and experiences as a patient – to improve islet viability and function after transplant, Stabler said.
But Crouse is up to the challenge.
“Ph.D. is very difficult,” Crouse said. “There are days I want to pull my hair out, but it’s all working toward improving the field, improving the treatments available to people who live with type 1.”





UF








INTERNSHIP HIGHLIGHTS

Internship: FeelTect
Completed a 10-week R&D internship with FeelTect, contributing to improvements for its wearable compression therapy platform. He developed a personalized pressure prediction model using statistical analysis and Python, and built a machine learning pipeline for activity recognition from accelerometer data. His work gave him valuable exposure to device testing, regulatory processes, and medical product development in an international setting.

Internship: Medtronic
Completed an 11-week internship with Medtronic’s Research & Technology team in the Cardiac Rhythm Management unit. She initiated the development of a Pugh Matrix for material selection, designed new testing methods, and conducted market assessments. Jennifer also contributed to knowledge-sharing platforms and even participated in a hackathon, leaving with stronger technical, professional, and cross-disciplinary communication skills.

Natalia Munetones Martinez Internship: Edwards Lifesciences
Finished a 7-month co-op with Edwards Lifesciences, working with the PASCAL Clinical Development team. She designed a fixture to aid physicians, led process improvements that boosted yield quality, and gained experience in device characterization testing and data analysis. Collaborating with cross-functional partners, she strengthened her skills in engineering design and clinical development.

Arabella Readey Internship: Cook Medical
Spent 12 weeks with Cook Medical’s Endoscopy division in Winston-Salem, NC, where she created tools to reduce manufacturing nonconformances and addressed CAPA investigations. She also contributed to testing components for an upcoming hemostasis product. Her hands-on work included benchtop testing, machine shop collaboration, and production line support, strengthening her technical and teamwork skills.

Lauren Valdes
Internship: Skeletal Dynamics
Completed a 12-week R&D internship with Skeletal Dynamics in Miami, collaborating with the elbow team on new product design and development. She strengthened her skills in SolidWorks, GD&T, and design controls while gaining hands-on experience with machining, welding, and 3D printing. Lauren also expanded her knowledge of patent systems and medical databases, leaving with a strong foundation in both design and regulatory processes.

I began my career as a Biomedical Engineering student at the University of Florida, where I had the opportunity to gain research experience in multiple laboratories. In Dr. Aaron Mickle’s lab, I studied inflammation by analyzing H&E-stained skin samples and later examined fMRI imaging to confirm the activation of nociceptive pathways caused by bladder inflammation. These early experiences sparked my interest in both neural engineering and cell and tissue engineering.
During a summer research internship at the University of Florida, I joined Dr. Benjamin Keselowsky’s lab, where I worked on a hydrogel platform for localized immunomodulation, analyzing inflammatory cytokine levels through ELISA and investigating gene expression using qPCR. This experience deepened my interest in regenerative medicine and motivated me to pursue this research path further. I was fortunate to present this work at the BMES Annual Conference, where I learned the value of sharing research with the broader community and building connections across disciplines.
I chose to continue my Ph.D. studies at UF because of the collaborative environment, strong expertise in regenerative medicine, and the opportunity to learn from faculty whose research closely aligns with my interests. Now as a Ph.D. student, I am excited to further explore these research areas through rotations with Dr. Christine Schmidt and Dr. Ivana Parker. Looking ahead, my goal is to continue advancing hydrogel platforms to support nerve regeneration and contribute to therapies that restore function and quality of life for patients.

My path to UF has been shaped by a consistent drive to understand how cellular and molecular mechanisms translate into human disease, particularly within populations most affected by health disparities. As an undergraduate, I first explored these questions in Dr. Alexa Mattheyses’ lab, where I used fluorescence microscopy to study clathrin-mediated endocytosis. That experience taught me how engineering approaches can illuminate biological processes and inspired me to apply these tools to more clinically relevant questions. I was recognized as an Honors College Presidential Summer Fellow, Diversity Champion and Yale BioMed/Amgen Scholar. Later, as an American Heart Association Scholar at Yale School of Medicine, I investigated how metabolic stress influences vascular biology, deepening my commitment to studying public health issues and their disproportionate impact on marginalized communities.
At UF, my research builds on this trajectory by investigating how metabolic dysfunction contributes to disease progression. I am excited by UF’s strengths in biomedical science and the collaborative environment across engineering and medicine, which provide both the tools and mentorship to tackle these complex questions.
I chose UF because of its emphasis on interdisciplinary training, its strong community of cardiovascular researchers such as Dr. Kyle Allen and Dr. Ana Maria Porras, and its commitment to supporting young investigators. I see my time here as the foundation for my long-term goal of becoming a physician-scientist who bridges engineering, biology and equity to advance medicine.
With passion and persistence, our students are redefining what’s possible in biomedical engineering.

Ashleigh Abbott
Lab: Kyle Allen

Joseph Luper
Tsenum
Lab: Xiao Fan

Jessica Molina
Lab: Jennifer Nichols
From hosting startup panels to fostering regional partnerships, Ashleigh is gaining real-world experience at the intersection of science, leadership, and venture creation.
Ashleigh Abbott, a Ph.D. candidate in Dr. Kyle Allen’s lab with a passion for entrepreneurship and technology development, is carving out a unique path that blends scientific discovery with innovation and leadership. As part of her career development, Ashleigh has taken an active role in building networks, gaining training, and showcasing her expertise in the entrepreneurial ecosystem. She has shared her insights as a panelist at BioFlorida x Johnson & Johnson in Tampa, hosted and emceed multiple Nucleate Florida events—including the IGNITE Student Entrepreneurship & Networking Night and Fueling Early Stage Growth in partnership with UF Innovate and Excedr—and represented Nucleate Florida at BioFlorida’s 20th Anniversary Celebration of Biotechnology in Alachua.
Her involvement extends beyond speaking roles, with hands-on engagement in advancing opportunities for women in science and entrepreneurship. Ashleigh attended the Embarc Collective x ReliaQuest Glaring Gap Summit in Tampa, where she joined conversations on women’s leadership in venture capital, and she played a key role in planning the Women Leading Life Sciences Lunch alongside industry leaders Karen Zaderej and Dr. Jackie von Salm. By actively participating in conferences such as the Florida Innovation Conference in Orlando, Ashleigh continues to expand her entrepreneurial knowledge while fostering collaborations that strengthen Florida’s biotech and innovation landscape.
From pioneering AI-driven drug discovery to presenting alongside Nobel Laureates, Joseph is expanding the reach of biomedical innovation worldwide.
Joseph Luper Tsenum, a Ph.D. student in Dr. Xiao Fan’s lab with a passion for computational discovery and translational medicine, is charting a global path that merges cutting-edge research with international collaboration and leadership. As part of his training and career development, Joseph has embraced opportunities to present his work, engage with leaders across disciplines, and strengthen his skills as a communicator and innovator. He has shared his expertise on the role of generative AI in drug design, demonstrating how machine learning can accelerate the creation of new molecules, and has contributed to panels highlighting the impact of artificial intelligence in biomedicine.
This year, Joseph was selected as one of only a few young scientists representing the United States at the 74th Lindau Nobel Laureate Meeting in Chemistry, June 29 – July 4, 2025, in Lindau, Germany. The meeting will connect him with Nobel Laureates, global researchers, and influential figures in science, government, and industry. Leading up to the event, Joseph also participated in the Pre-Lindau Scholars Program hosted by Texas A&M University, where 27 of the nation’s most promising scientists came together for workshops and networking. By engaging in these international forums, Joseph is advancing his scholarship while raising the profile of UF BME, exemplifying how the department’s students are shaping the future of science and medicine on a global stage.
Through research, clinical training, and community engagement, Jessica is advancing biomechanics to improve movement and rehabilitation strategies.
.
Jessica E. Molina is an MD/PhD student and current PhD candidate in Dr. Jennifer Nichols’ lab. She has a strong passion for understanding movement, influenced by her personal limitations and lifelong career in dance. Recently, Jessica received a National Institutes of Health (NIH) Ruth L. Kirschstein National Research Service Award (NRSA) Individual Fellowship (Parent F30) for her project titled, “Identifying Phenotypes in Carpometacarpal Osteoarthritis through Pain, Biomechanics, and Machine Learning Analyses.” Her clinical and research interests align with her aspiration to become a Physical Medicine and Rehabilitation (PM&R) physician specializing in biomechanics and personalized rehabilitation strategies. Jessica has fully immersed herself in the field of biomechanics and has taken on various leadership roles both within her institution and the broader community. For the past year, she has served on the executive board of the University of Florida’s American Society of Biomechanics (ASB) student chapter, moving into the Vice President position this year. She has helped lead multiple outreach events aimed at introducing the next generation of scientists to the field. More recently, she joined the Communications Committee for the national ASB organization, where her contributions have helped increase engagement within the biomechanics community at large.
Jessica’s dedication to understanding movement extends beyond her research and academic work; she also volunteers with a local adaptive gymnastics team, Balance 180. There, she coaches athletes with disabilities year-round in preparation for Special Olympics and HUGS competitions. Jessica has been volunteering for the past two years and has developed a strong connection with the athletes, constantly striving to help them become the best they can be!


UF BME’s GRiP group joined Beautifully Made Community at Boston Children’s Hospital, gifting 40 adapted toys created at their annual Adapt-a-thon to children with limb differences and their families.
UF

National Biomechanics Day: Middle school students explored biomechanics through interactive, hands-on stations at the University of Florida. From testing sports performance tools to examining rehabilitation technologies, they engaged with more than 10 demonstrations led by UF faculty and students. The activities highlighted the real-world impact of biomechanics in engineering, sports, and health, sparking curiosity about science and STEM careers.
EmpowerHer Event: Middle and high school students from Johannesburg’s Soweto community take part in hands-on STEM activities with the Parker Lab, exploring biomechanics and biomedical engineering research in action.
Valentina Muriel Ruiz of the Porras Lab helps organize events throughout the semester and introduces K–12 students to the world of engineering.




UF Marching Band

BIOMEDICAL SCIENCES BUILDING JG56
1275 CENTER DRIVE, P.O. BOX 116131
GAINESVILLE, FL 32611-6131
WWW.BME.UFL.EDU

Engineering graduates light up the arena as they sing along to Tom Petty’s “I Won’t Back Down” during the Spring 2025 University of Florida commencement ceremony — a beloved Gator tradition.