Assisted Reproduction Techniques
Laparoscopic Oviductal Artificial Insemination (LO-AI)
The standard protocol for artificial insemination in non-domestic feline species that produces the best chance of successful pregnancy requires exogenous gonadotropin treatment to synchronize follicular growth and ovulation with insemination procedures [45-46]. Because ocelots are induced ovulators (i.e., requiring mating to stimulate ovulation), exogenous hormones are necessary when natural mating does not occur. High pregnancy percentages (~70%) have been obtained in domestic cats using a LO-AI approach with low sperm numbers in females treated with gonadotropins before the procedure [47]. Porcine luteinizing hormone (pLH) has proven effective in inducing ovulation in feline species when compared to other gonadotropins and reduces the formation of unwanted secondary ovarian structures that could disrupt the endocrine environment and oviductal transport [47-50].
For felines, several variations of artificial insemination exist, including placement of sperm into the vagina, uterine horn, uterine body, and/or the oviduct [24, 51-57]. However, many of these techniques require the use of high sperm numbers [24, 51-57], limiting their application to many feline species. LO-AI presents a technique that can bypass anatomical barriers, which and reduces the need for both high sperm numbers [32-33, 51] and sperm transport to the site of fertilization in the ampulla [51, 58-59]. Furthermore, multiple LO-AI procedures can be performed with a single ejaculate [51]
Since 1995, in zoo-based ocelots in the United States, there have been three successful pregnancies using artificial insemination with frozen semen collected from zoo-based males, and 6 pregnancies using non-frozen semen from zoo-based males [W. Swanson, Lindner Center for the Conservation and Research of Endangered Wildlife, personal communication]. Since 2019, there have been eight artificial insemination procedures conducted in zoo-based female ocelots using semen collected from free-ranging male ocelots trapped in Texas. None of the eight procedures resulted in a pregnancy, likely due to a combination of low-quality sperm collected from male ocelots in Texas and further damage by cryopreservation [A. Reeves, East Foundation, unpublished data]. Continued assessment of artificial insemination techniques in ocelots using non-frozen sperm and/or sperm of higher quality could make this technique more successful in the ocelot breeding program.
Ovarian Synchronization and Ovulation Induction
[32,60]
For ocelots, oral progestin (Regumate; 0.044 mg/kg BW) is fed daily for 30 consecutive days and then discontinued during a 7-day withdrawal period. On the 8th day of withdrawal, 400 IU of equine chorionic gonadotropin (eCG) is administered intramuscularly (IM). Approximately 82 hours post eCG injection, 3000 IU of porcine luteinizing hormone (pLH) is administered IM ~ 38 hours prior to the laparoscopic oviductal artificial insemination (LO-AI) procedure.
Semen Thawing Procedure
For thawing of straw-frozen semen samples, the straw is removed from the liquid nitrogen canister and held in room air for 10 seconds, then transferred to a 37°C water bath for 30 seconds for thawing. The straw is removed, wiped dry, and the sample placed into an Eppendorf tube. FOCM-Hepes medium (100 μL) is slowly added to each straw sperm sample and all straw samples are combined into one Eppendorf tube. Initial postthaw motility is assessed under light microscopy at 400X, and then an aliquot is spread across a slide and allowed to air dry for later post-thaw acrosome assessment. A small aliquot is also diluted (1:400) in water in an
Edperdorf tube for a concentration count under light microscopy. The semen sample is split equally into two tubes and centrifugated for 8 minutes at 300g. The supernatant is removed, each pellet is measured, and, if necessary, resuspended to 15-20 μL total for insemination. The entire volume of one pellet is placed onto a sterile petri dish and aspirated into the sterile AI needle. The AI needle is inserted through an 18-gauge catheter into the oviduct for LO-AI (below). Residual sperm is flushed from the AI needle to assess final motility.
LO-AI
Procedure [22, 32, 51-52, 61]
The female’s hair is clipped on her ventral abdomen (from xiphoid to pubis), the surgical field is prepped with betadine and alcohol alternating 3 repetitions, a sterile drape placed over the surgical field, and the drape cut to expose the clipped area. The Verres needle is placed in the right caudal abdomen approximately 1 inch caudal and lateral to the umbilicus by tenting the skin and using manual force to enter the abdomen. A hand pump is attached to the Verres needle to insufflate the abdominal cavity with room air to a uniform tautness. An ~ 1 cm incision is made ~ 2-3 cm cranial to the umbilicus and the surgical table is tilted at a 20-30° angle. The skin caudal to the incision is grasped and the trocar-cannula assembly is inserted at a 60° angle to the ventral abdomen with a sharp thrusting motion.
The trocar is removed, and the laparoscope, with a camera attached, is inserted and attached by a fiberoptic cable to a light source. The laparoscope (10 mm diameter) is used to visualize the ovaries, oviduct, and/or uterus for various procedures, using the Verres needle to manipulate abdominal contents as necessary. Both ovaries are examined for follicles, corpus luteum, corpus albicans, and cysts. The uterine horns are assessed for tone, symmetry, and size. The oviducts are examined for distinctness and presence of adequate fimbrial tissue for grasping. The Verres needle is removed, and the accessory trocar is inserted in the same location into the abdomen for placement of the grasping forceps. The grasping forceps are inserted through the accessory cannula and the oviductal tissue picked up using the grasping forceps to lift the bursa laterally.
An 18-gauge (18 g, 3.2 cm length; Terumo Medical Corporation, Elkton, MD, USA) catheter is placed into the abdomen lateral and caudal to the ovary on the left side. The needle is removed from the catheter and a blunted, artificial insemination needle (22 g, 6.8 cm length), derived from the stylet within an i.v. catheter (20 g, 5.0 cm length; Sherwood Medical Co.), is attached to a 1-mL syringe and placed through the catheter. The AI needle is inserted into the ampulla via the oviductal opening and the sperm injected as the insemination needle is retracted from the oviduct. The same procedure is performed on the right ovary. Following the AI procedure, surgical closure of each skin incision requires 1–2 sutures and a small amount of tissue adhesive. Cats typically return to normal activities shortly following anesthetic recovery.
Sources (Who) – Options for LO-AI
1) Captive-born ocelot females with wild-born fresh or frozen-thawed male ocelot semen
2) Captive-born females with captive-born fresh or frozen-thawed male ocelot semen
3) Wild-born females with captive-born fresh or frozen-thawed male ocelot semen
4) Wild-born females with wild-born fresh or frozen-thawed male ocelot semen
Advantages of LO-AI
● Selecting the parents of the offspring without having to account for behavioral mismatches in breeding pairs.
● Use frozen-thawed sperm collected from wild or captive ocelots to increase genetic diversity in the breeding program.
● For both male and females, procedures are minimally invasive and virtually no recovery period is needed.
● The opportunity to use freshly collected sperm obtained from a nearby wild-born (or captive-born) ocelot could increase pregnancy success.
Disadvantages of LO-AI
● For AI of wild females, the procedure requires manipulation of the ovarian cycle to induce follicular growth and ovulation in time with the scheduled AI procedure without the advantage of ovarian synchronization (using Regumate treatment)
● If performing an AI procedure on wild-born ocelots, females must be maintained in a captive environment for ~1 week to induce follicular growth and ovulation before AI
● Stress from maintenance in captivity may affect ovarian response or pregnancy success in wild-born females
Laparoscopic Oviductal-Embryo Transfer (LO-ET)
Embryo transfer (ET) has shown great promise for allowing propagation of non-domestic feline species [45,6263]. Live offspring have been produced following ET in ten cat species (domestic cat, wild cat, tiger, ocelot, serval, caracal, fishing cat, black-footed cat, sand cat, cheetah) [33,67-69]. Most of the earlier ET procedures required intra-abdominal laparotomy to gain access to the reproductive structures [33]. The ability to perform these techniques laparoscopically has helped to alleviate animal welfare concerns and health concerns during intra-abdominal reproductive procedures [64]. Laparoscopic oocyte collection has provided a minimally invasive technique to collect oocytes from numerous feline species [45,52,65] and has allowed the creation of embryos for more than 20 felid species following in vitro fertilization. Additionally, techniques to access the oviduct via laparoscopic approaches have been developed to conduct LO-ET, similar to laparoscopic oviductal artificial insemination (LO-AI) [46,47, 66]. The success of this procedure, like LO-AI, depends on the use of exogenous gonadotropins to induce ovulation for the timed-ET.
Over the last few decades, the Lindner Center for the Conservation and Research of Endangered Wildlife (CREW) has investigated the use of LO-ET in domestic cats for potential application to the propagation of nondomestic feline species [33]. This approach has been successfully used for the production of multiple pregnancies and viable offspring in the ocelot and sand cat (Felis margarita) [33]. A total of 5 pregnancies and 5 term kittens have been born from 10 LO-ET attempts with frozen-thawed In-Vitro Fertilization (IVF) embryos in ocelots in the United States and Brazil [33]. Additionally, one pregnancy leading to the birth of two sand cat kittens born has resulted from 4 attempts of LO-ET with non-frozen embryos [33].
Oocyte collection [70]
Females are treated intramuscularly first with equine chorionic gonadotropin (eCG) (Sigma Chemical Co. or Sioux Biochemical Inc., Sioux Center, IA), and then 84-86 hours later, with human chorionic gonadotropin (Sigma Chemical Co. or Sioux Biochemical Inc.). At 24-47 hours after hCG administration, in vivo-matured oocytes are collected using techniques for laparoscopic oocyte recovery [20, 71]. Mature follicles (≥2 mm) are aspirated using a 22-gauge needle with aspiration pressure (~ 1.5 mm Hg) provided by a vacuum pump, and follicular contents are collected in a sterile glass tube containing FOCMH with 40 units/mL of heparin (ElkinsSinn Inc., Cherry Hill, NJ). Only oocytes with dark homogeneous cytoplasm that are surrounded by expanded cumulus cells (grade 1) are used for IVF.
In-Vitro Fertilization (IVF) [72-74]
For IVF, spermatozoa (~ 1 X 106 mL-1) are co-incubated with oocytes in 5% CO2 in air at 38°C under mineral oil (#4008, Sage BioPharma, Bedminster, NJ, USA) in Tyrode’s solution containing 6 mg/mL BSA, 15 mM NaHCO3, 0.36 mM pyruvate, 2.2 mM calcium lactate, 1.0 mM glutamine and 50 µg/mL gentamicin (IVF medium 8). At 5-6 hours post-insemination, oocytes are rinsed and cultured in 500 µl of Tyrode’s solution that contains supplements used for IVF along with 1% MEM non-essential amino acids (NEAA) and BSA reduced to 3 mg/mL (IVC(in-vitro culture)-1 medium) in an atmosphere of 5% CO2, 5% O2, 90% N2 at 38°C. Embryos are cultured in a three-step system: 1) culture in IVC-1 medium until day 2; (2) culture in fresh IVC-1 medium containing 1% EAA (IVC-1A) until day 5; (3) culture in IVC-2 medium until cryopreservation or transfer to a recipient.
Embryo cryopreservation and thawing for transfer [72-73, 75]
On IVC day 5, if embryos are to be cryopreserved, embryos are equilibrated in a cryoprotectant solution consisting of 1.4 M propylene glycol, 0.125 M sucrose, 10% dextran 70 and 10% FBS in HeTy (CPS) and cooled at a slow, controlled rate to 30°C before storage in liquid nitrogen. Embryos are warmed by holding the straw in air for 2 min and CPS is removed in five steps of 3 min each. Then, embryos are cultured in IVC-2 medium until transferred to a recipient within 2–24 h.
LO-ET Procedure [33]
The female is clipped from xiphoid to pubis, the surgical field is prepped with betadine and alcohol alternating 3 repetitions, a sterile drape placed over the surgical field, and the drape cut to expose the clipped area. The Verres needle is placed in the right caudal abdomen approximately 1 inch caudal and lateral to the umbilicus by tenting the skin and using manual force to enter the abdomen. A hand pump is attached to the Verres needle to insufflate the abdominal cavity with room air to a uniform tautness. An ~ 1 cm incision is made ~ 2-3 cm cranial to the umbilicus and the surgical table is titled at a 20-30° angle. The skin caudal to the incision is grasped and the trocar-cannula assemble is inserted at a 60° angle to the ventral abdomen with a sharp thrusting motion. The trocar is removed, the laparoscope is inserted and attached by a fiberoptic cable to a light source. The laparoscope (10 mm diameter) is used to visualize the ovaries, oviduct, and/or uterus for various procedures, using the Verres needle to manipulate abdominal contents as necessary. Both ovaries are examined for follicles, corpus luteum, corpus albicans, and cysts. The uterine horns are assessed for tone, symmetry, and size. The oviducts are examined for distinctness and presence of adequate fimbrial tissue for grasping. The camera is attached to the end of the scope. The Verres needle is removed, and the accessory trocar placed in the same location in the abdomen for the grasping forceps. The grasping forceps are placed through the trocar opening and the oviductal tissue picked up using the grasping forceps and rolling the bursa laterally. A polypropylene i.v. catheter (20 g, 5 cm length; Sherwood Medical Co. St. Louis, MO, USA) is placed through the ventral abdominal wall dorsal to the ovary and inserted through the oviductal ostium into the distal oviduct. Polyethylene tubing (25 cm length, PE10; Bectin Dickinson Co Sparks, MD, USA), attached to a blunted 30 g (1.25 cm) needle and 1 ml syringe, is passed through the catheter and, with continued forward pressure on the tubing, the catheter is completely withdrawn from the oviduct. Embryos (n=3-7, depending on species), contained in 5-10 μL of culture medium at the distal end of the transfer tubing, are expelled deep into the oviductal lumen with slight air pressure from the syringe. Surgical closure of each skin incision requires 1–2 sutures and a small amount of tissue adhesive, and cats typically return to normal activities shortly following anesthetic recovery.
1) Wild-born female oocytes with captive or wild-born male semen, and the embryo is placed into captive-born or wild-born females
2) Captive-born female oocytes with wild-caught semen and embryo placed into wild-born females
Advantages of LO-ET
● LO-ET could allow use of additional source of female oocytes to increase genetic diversity in the breeding program
● Embryos can be frozen for subsequent transfer into a different female. For example, use of wild-born ocelot female eggs could be used to produce embryos for transfer into captive females.
● Fertilization is assured since the fertilization process is conducted in vitro.
Disadvantages of LO-ET
● Involves multiple additional steps compared to other techniques and options
● Involves additional sedation events for females to collect oocytes and transfer embryos
● Involves substantial lab techniques to properly fertilize and culture embryos prior to transfer or freezing
● Embryo freezing can compromise viability relative to non-frozen embryos
Semen collection and cryopreservation
Although various techniques and technologies are utilized for semen collection in different species, there are two collection techniques of interest in ocelots: electroejaculation and urethral catheterization. Electroejaculation has been the long-accepted standard for many species and while effective, utilization requires advanced skill and equipment. Urethral catheterization is a relatively new technique (established in 2008 in domestic cats) that requires less equipment and expertise and is most adaptable to field use. Catheterization has been studied in wild ocelot populations in southern Texas since 2019, but many samples collected so far have proved nonviable due to urine contamination during sampling and low sperm recovery [A. Reeves, East Foundation, unpublished data]. Potential ways to improve semen recovery by catheter must be further investigated to mitigate urine contamination for optimal sperm recovery. Meanwhile, when collecting samples by electroejaculation, the inclusion of seminal fluids creates a potential buffer against the negative impacts of urine on seminal quality. The seminal fluids are either not present or are present in small amounts in semen samples collected by catheterization. Semen collection opportunities for wild male ocelots are limited to those occurring at the time of capture, and an individual ocelot may be captured only once in its lifetime. Given its efficiency and reliability, electroejaculation is currently the preferred method for semen collection in wild ocelots.
When cryopreserving semen samples, straw freezing is a complicated and time-consuming technique, but results suggest that this technique may produce superior post-thaw sperm traits and be more effective for insemination procedures in ocelots compared to ultra-rapid freezing (URF, A. Reeves, East Foundation, unpublished data). The combination of electroejaculation and URF has not been studied at this time but could be an additional approach to field semen recovery and cryopreservation. The simplification of the semen collection and cryopreservation process may be particularly valuable for banking semen samples in other countries (e.g., Mexico) under less-than-optimal field conditions.
Semen Collection Techniques
Specific pharmaceutical agents are typically used to successfully collect semen in many species. Pharmacological methods have been studied with success in species such as the horse [1-3], domestic felids [4], domestic canids [5], and white rhinoceros [6]. When conducting field-based studies on wild felids, immobilization of the animal can be achieved using many combinations of pharmaceuticals, especially alphaadrenergic agonists (α2 agonists). Alpha-2 adrenergic agonists are reported to influence erection [6,7], and the ejaculatory reflex [7] and induce action at the level of the vas deferens [8]. Medetomidine, a potent α2 agonist, is thought to induce smooth muscle contraction of the vas deferens, which then forces semen into the pelvic urethra [9]. An alternative method for semen collection that was recently developed and reported to be successful in domestic felids is urethral catheterization of male cats after they have been sedated with medetomidine [4]. This technique has allowed for the recovery of high sperm numbers in domestic cats [4], jungle cats (Felis chaus) [10], Amur leopard cats (Prionailarus bengalensis euptilurus) [11], lions (Panthera leo) [12], and other wild felids. Urethral catheterization under medetomidine sedation could provide a costeffective and simplified technique that could be valuable for imperiled felid conservation globally.
One confounding factor recently observed in semen collection in ocelots is urine contamination of cathetercollected samples [A. Reeves, East Foundation, unpublished data]. Due to the lack of seminal fluids in catheter samples and the high osmolarity, the acidity of the urine damages the sperm and decreases its viability, even if immediately diluted and centrifuged to remove urine. Samples collected by electroejaculation include more seminal fluids and thus have higher alkalinity when compared to catheter samples. The seminal fluids may provide a better buffer against urine contamination. Spermatozoa characteristics of frozen-thawed semen samples do not differ between the catheterization and electroejaculation methods [4]. Overall, electroejaculation provides another opportunity to obtain a semen sample if the initial catheter sample is compromised.
Electroejaculation has been performed successfully in domestic cats and virtually all wild felid species [13-16] maintained under human care in zoos, ranging in body size from the tiger (Panthera tigris) [16] to the blackfooted cat (Felis nigripes) [15]. During electroejaculation, a lubricated rectal probe, varying in diameter based on species size, and an electro stimulator are used to deliver 80 -140 electrical stimuli (voltage range 2-6 V) divided into 3 to 5 separate series [13-16]. During collection, cats typically extend their hind limbs due to electrical stimulation of peripheral motor nerves. Rarely, they may vocalize, but because cats are anesthetized with a dissociative anesthetic, they do not have any conscious perception of muscular stimulation or experience any associated discomfort. The electroejaculation semen collection method has been used in cats for over 40 years and has been applied to thousands of domestic and wild felids by CREW and by other investigators. Safety and health concerns for felids are almost nonexistent assuming proper techniques are used. Scientists from the CREW lab have conducted more than 200 electroejaculation procedures in ocelots within zoos without any reported adverse effects. In ocelots, electroejaculation remains the most effective approach for recovering semen. In a recent study in ocelots (n=7) assessing semen collection with urethral catheterization followed by immediate electroejaculation (during the same anesthetic event), it was determined that electroejaculation produced significantly greater seminal volume and more than doubled the total sperm numbers collected across males [16]. These findings suggest that high numbers of residual sperm may be safely collected from ocelots by conducting electroejaculation immediately after urethral catheterization.
Electroejaculation (EEJ) has been used extensively as part of captive propagation and reintroduction programs for other wildlife species, such as the black-footed ferret [17,18]. EEJ in ferrets requires four sets of electrostimulations (20-30 stimuli per set over a 2-5 V range) using a 6 mm diameter rectal probe [18]. As in ocelots, no negative impacts on the animal’s health have been reported [17,18]. The black-footed ferret is one of
the most intensively managed mammals in North America and, with the use of EEJ and artificial insemination, the black-footed ferret reintroduction program has created a model for applying assisted reproduction to address challenges posed by a small number of founders available to support species reintroduction programs [17]. Much like ocelots, ferret populations are challenged by population declines due to genetic restriction, and the black-footed ferret was listed as endangered in 1967 [14]. Since then, 30 generations of successful ferret kit births and more than 9,100 ferrets have been produced in the ex-situ breeding program [19], including >100 offspring produced from artificial insemination with freshly collected and frozen-thawed semen. Use of EEJ procedures in free-ranging ocelots in Texas has the potential to improve success in obtaining viable semen samples from this imperiled population and ultimately supporting breeding of ocelots for reintroduction.
Urethral Catheterization Procedure [4]
Males are immobilized and maintained at a light anesthetic plane for semen collection. The anesthetic protocol consists of an injectable combination of ketamine hydrochloride with medetomidine or dexmedetomidine followed by partial reversal with atipamezole. Approximately 25-40 minutes after anesthetic injection, the penis is extruded with manual manipulation and sterile gloves. Debris on the penis and in the preputial cavity is removed with water-soaked gauze. A 3.5- or 5- French urinary catheter (dependent on age, size, and species) is advanced approximately 15 cm into the urethra, left in place for 30 seconds and slowly removed. The sample is placed into an Eppendorf vial using a one -mL syringe and a small amount of air.
Electroejaculation Procedure [13, 20, 21]
A lubricated probe is gently inserted into the rectum with the electrodes directed ventrally. A warmed, sterile collection cup is placed over the end of the penis. The electro ejaculator is turned on (after ensuring that the voltage rheostat is turned to zero). A series of 1 to 3 electrical stimulations will occur, beginning at 2 volts and progressing to 5 or 6 volts (Series 1, ~ 30 stimulations; Series 2, ~ 30 stimulations; Series 3, ~ 20 stimulations) with 10 stimulations at each voltage and 3–5-minute rests between series. Initial electrical stimulation is applied by slowly increasing voltage from 0 to 2 volts, pausing momentarily, and then abruptly returning to 0 volts. This stimulation is repeated 10 times, then voltage is increased to 3 volts for another 10 stimulations, and then voltage occurs at 4 volts for another 10 stimulations. When the series is completed, the electro ejaculator is switched off. The cup is removed from the penis and any additional liquid adhering to the penis is collected with a sterile pipettor. The probe is removed from the rectum. The total semen volume is measured and transferred into a sterile, warm Eppendorf tube.
Semen Cryopreservation Techniques
Previous semen cryopreservation efforts in other feline species have used either sperm pelleting on indentations in dry ice [22-25] or straw freezing over liquid nitrogen vapor [23, 26-28] to preserve semen samples for later use. Despite fairly substantial acrosome damage to semen post-thaw after pelleting on dry ice, frozen-thawed spermatozoa in ocelots may be functionally competent [22], similar to findings in fishing cats (Prionailurus viverrinus) [29]. In an earlier study, one nulliparous ocelot female treated with exogenous gonadotropins and inseminated with thawed spermatozoa, previously frozen by pelleting, conceived and gave birth to a healthy kitten 78 days later [22]. However, compared to freshly collected inseminates used for artificial insemination in ocelots, the amount of frozen-thawed sperm for artificial insemination must be increased to compensate for the acrosome damage to frozen-thawed sperm [22]. In another study comparing freshly collected and frozen-thawed ocelot semen, frozen-thawed spermatozoa showed similar values for progressive motility status but had decreased percentages of normal sperm morphology and lower percentages of intact acrosomes [13]. However, higher numbers of spermatozoa were bound to fertilized domestic cat oocytes when using the pelleted dry ice
treatment compared to straw freezing of samples over liquid nitrogen and storage in a dry shipper [13]. Furthermore, semen samples frozen by pelleting on dry ice exhibit higher motility or viability immediately postthaw compared to samples frozen in straws over liquid nitrogen [13].
A newer sperm cryopreservation approach, ultra-rapid freezing or URF, offers simplicity and minimal equipment needs because it requires only URF-specific medium and liquid nitrogen [30,31]. The URF process involves extending the spermatozoa into a soy-lethicin-based medium with 0.2 M sucrose, equilibrating, and directly pipetting into an open container of liquid nitrogen. Then, the sperm pellets are transferred into labeled cryovials and stored until thawing. A comparative study in domestic cats involving catheter-recovered sperm samples frozen by URF and by conventional straw freezing reported no difference in post-thaw motility and acrosome status of URF-catheter samples over time when compared to straw-frozen samples [9]. Preliminary in-vitro fertilization (IVF) results indicated that URF-catheter sperm is capable of fertilizing oocytes in vitro, and fertilization success with URF sperm for all inseminated oocytes (30%) did not differ from success observed with straw-frozen samples (57%). However, based on mature oocytes (M2 cell or cleaving), fertilization success with URF sperm (35%) was slightly lower than that of sperm frozen in straws (65%) [9].
With laparoscopic oviductal artificial insemination (LO-AI), sperm function and motility over time are not as critical as with intravaginal or intrauterine AI. High pregnancy rates (70-80%) have been obtained with LO-AI using low sperm numbers (~ 1 million motile/oviduct) for insemination, including semen that was frozen using standard straw cryopreservation methods [32-34].
From 2019 to 2022, semen samples were collected by catheter collection from free-ranging ocelots in Texas to compare the URF and straw techniques for sperm freezing (A. Reeves, East Foundation, unpublished data). Preliminary findings suggested similar results between the two freezing techniques (URF and straw) for motility and forward progression of sperm over time. However, heterologous IVF results suggested that the straw technique was superior to URF for fertilization success.
In the study, additional analysis and sample collection further explored the results of semen collection and cryopreservation methods. While there was not a significant difference in pre- and post-thaw parameters and quality of semen, most catheter-collected semen samples were contaminated by urine and were not of adequate quality for cryopreservation. Although the catheter-collection/ URF cryopreservation combination provides a simple field technique for collecting and storing sperm from free-ranging ocelots using minimal equipment, it is not recommended as a first-line technique for semen collection, especially when the capture of a wild male ocelot could be a one-time occurrence. Further assessment of the catheterization combination is needed to mitigate urine contamination and ensure best sample collection practices before this technique is used in field settings While straw freezing is a more complicated and time-consuming semen cryopreservation technique, results suggest this technique may produce superior post-thaw sperm traits and be more effective for insemination procedures in ocelots (A. Reeves, East Foundation, unpublished data). Additionally, the use of electroejaculation yielded semen samples of higher quality for cryopreservation when employed in the field and therefore, has become the preferred method to be utilized in field collections.
Straw freezing
For straw freezing, the raw semen is diluted 1:5 with FOCM-Hepes medium. The diluted straw sample is centrifuged at 600Xg for 8 minutes and the resulting sperm pellet is resuspended in soy lethicin with 4% glycerol to a concentration of 50 X 106 motile sperm/mL and loaded into 0.25 mL straws (30-50 μL/straw). The ends of the straws are heat sealed, transferred into a sealable plastic bag, submerged in room-temperature water (100 mL) within a glass container, and cooled in a refrigerator to 4°C for a minimum of 2 hours in a refrigerator. Straws are then frozen using a modified two-step protocol [29, 35]. In this protocol, two metal racks are placed in a polystyrene foam container partially filled with liquid nitrogen (LN2). Cooled straws are placed on the top rack (7.5 cm above the LN2 surface) for one minute and then transferred to the bottom rack (2.5 cm above the LN2 surface) for one minute before plunging directly into liquid nitrogen for storage until thawing.
Ultra-rapid freezing (URF)
For URF, the raw semen is diluted 1:5 with soy-lethicin 0.2 M sucrose medium and allowed to equilibrate at room temperature for 5 minutes. The diluted URF sample is cryopreserved using a micropipette, pipetting one ~ 20 μL drop at a time directly into liquid nitrogen and allowing the pellet to sink to the bottom before the next drop is added to the LN2 container. This process is repeated for the entirety of the volume. The pellets are recovered using forceps and placed into a labeled cryovial for storage in liquid nitrogen until thawing.
Pregnancy Monitoring
Fecal samples are collected three days per week beginning two months prior to an assisted reproduction or natural breeding procedure, with sample collection continued for 85 days after assisted reproduction or natural breeding Fecal samples are placed into labeled (name, studbook number, institution name, date) plastic bags and immediately frozen (-20°C) for storage until processing. Samples are then lyophilized via a freeze dryer (Labconoco Corp., Kansas City, MO, USA) in their plastic bags, pulverized into a fine powder, and then weighed (250± 5 mg) into labeled 15 mL-polypropylene conical tubes. Each sample is then extracted by adding 2.5 mL of 90% ethanol (or a 1:10 weight:volume) overnight on a mechanical rocker (≥12 hours). Extracted samples are centrifuged (1000g, 15 min, Eppendorf, Enfield, CT, USA), supernatants are removed, and samples are stored in 2.0-mL cryovials at -20 °C until analysis.
Procedures for enzyme immunoassays (EIAs) used by Herrick et al. (2010) [76] and Bateman et al. (2009) [77] are recommended for assessing pregnancy status. Arbor Assays progesterone mini-kit (ISWE003, Arbor Assays, Ann Arbor, MI, USA) will be used to determine progestogens (this kit included both antibody and horseradish peroxidase) and been previously used in the CREW endocrine laboratory and validated for ocelots Similarly, fecal estrogen metabolites can be quantified using specific EIAs to monitor natural or induced ovarian follicular activity [76]. EIAs may be conducted in endocrine laboratories to be established at the Ocelot Conservation Facility or at partnered institutions.
References
[1] McDonnell SM. 2001. Oral imipramine and intravenous xylazine for pharmacologically-induced ex copula ejaculation in stallions. Animal Reproduction Science 68: 153-159.
[2] Calvero TMS, Papa FO, Schmith RAM, Scheeren VFC, Canuto LEF, Gobato MLM, Rodrigues LT, Freitas-Dell’aqua CP. 2019. Protocols using detomidine and oxytocin induce ex copula ejaculation in stallions. Theriogenology 140: 93-98.
[3] Card CE, Manning ST, Bowman P, Leibel T. 1997. Pregnancies from imipramine and xylazine-induced ex copula ejaculation in a disabled stallion. Canadian Veterinary Journal 38(3): 171-174.
[4] Zambelli D, Prait F, Cunto M, Iacono E, Merlo B. 2008/ Quality and in vitro fertilizing ability of cryopreserved cat spermatozoa obtained by urethral catheterization after medetomidine administration. Theriogenology 69(4): 485-490.
[5] Kuczmarski AH, Alves de Barros M, Souza de Lima LF, Motheo TF, Bento HJ, Iglesias GA, Sonego DA, Rodrigues da Paz RC. 2020. Urethral catheterization after pharmacological induction for semen collection in dog. Theriogenology 153: 34-38.
[6] Silinski S, Walzer C, Schwarzenberger F, Slotta-Bachmayr L, Stolla R. 2002. Pharmacological methods of enhancing penile erection for ex-copula semen collection in standing white rhinoceros (Ceratotherium simum simum). European Association of Zoo and Wildlife Veterinarians (EAZWV) 4th Scientific Meeting
[7] Hafez ES. 2013. Anatomy of male reproduction. In: Hafez B, Hafez ESE, editors. Reproduction in Farm Animals Maryland. Lippincot Williams & Wilkins; 3-11.
[8] MacDonald A, McGrath JC. 1980. The distribution of adrenoceptors and other drug receptors between the two ends of the rat vas deferens as revealed by selective agonists and antagonists. British Journal of Pharmacology 71:696-701.
[9] Swanson WF, Bateman HL, Vansandt LM. 2017 Urethral catheterization and sperm vitrification for simplified semen banking in felids. Reproduction in Domestic Animals 52(Suppl 2): 255-260.
[10] Kheirkhah MS, Mollapour sisakht M, Mohammadsadegh M, Moslemi HR. 2017. Sperm evaluation of Jungle Cat (Felis chaus) obtained by urethral catheterization (CT) after medetomidine administration Theriogenology 91: 17-20.
[11] Jeong DH, Kim JH, Na KJ. 2018. Characterization and cryopreservation of Amur leopard cats (Prionailarus bengalensis euptilurus) semen collected by urethral catheterization. Theriogenology 119: 91-5.
[12] Lueders I, Luther I, Scheepers G, van der Horst G. 2012. Improved semen collection method for wild felids: urethral catheterization yields high sperm quality in African lions (Panthera leo). Theriogenology 78(3): 696-701.
[13] Stoops MA, Bond JB, Bateman HL, Campbell MK, Levens GP, Bowsher TR, Ferrell ST, Swanson WF. 2007. Comparison of different sperm cryopreservation procedures on post-thaw quality and heterologous in vitro fertilization success in the ocelot (Leopardus pardalis). Reproduction, Fertility and Development 19: 685-694.
[14] Swanson WF, Johnson WE, Cambre RC, Citino SB, Quigley KB, Brousset DM, Morais RN, Moreira N, O’Brien SJ, Wildt DE. 2003. Reproductive Status of Endemic Felid Species in Latin American Zoos and Implications for Ex Situ Conservation. Zoo Biology 22: 421-441.
[15] Vansandt LM, Bateman HL, Miller AG, Herrick JR, Moresco A, Gonzalez R, Iwaniuk ME, Swanson WF. 2021 Cross-species efficacy of a chemically defined, soy lecithin-based cryomedium for semen banking in imperiled wild felids. Theriogenology 159: 108-115.
[16] Swanson WF, Vansandt LM, Citino S, Larsen RS, Cole GA, Moresco A. 2017. Addressing the Challenge of Do-It-Yourself (DIY) Semen Banking in Wild Felids. Abstract in 2017 49th AAZV Annual Conference Proceedings
[17] Santymire RM, Lonsdorf EV, Lynch SM, Wildt DE, Marinari PE, Kreeger JS, Howard JG. 2019. Inbreeding causes decreased seminal quality affecting pregnancy and litter size in the endangered black-footed ferret. Animal Conservation 22: 331-340. © 2018 The Zoological Society of London.
[18] Howard JG, Lynch C, Santymire RM, Marinari PE, Wildt DE. 2016. Recovery of gene diversity using long-term cryopreserved spermatozoa and artificial insemination in the endangered black-footed ferret. Animal Conservation 19: 102-111.
[19] Marinari P. 2017. North American regional black-footed ferret studbook. Front Royal: Smithsonian National Zoological Park and Conservation Biology Institute.
[20] Herrick JR, Bond JB, Magarey GM, Bateman HL, Krisher RL, Dunford SA, Swanson WF. 2007. Development of a feline optimized culture medium: effects of ions, carbohydrates, essential amino acids, vitamins and serum on the development and metabolism of IVF-derived feline embryos relative to embryos grown in vivo Biology of Reproduction 76: 858-870.
[21] Howard JG, Bush M, Wildt DE. 1986. Semen collection, analysis and cryopreservation in nondomestic mammals. In: Morrow D, editor. Current therapy in theriogenology Philadelphia, PA: WB Saunders Co. 1047-53.
[22] Swanson WF, Howard JG, Roth TL, Brown JL, Alvarado T, Burton M, Starnes D, Wildt DE. 1996a. Responsiveness of ovaries to exogenous gonadotrophins and laparoscopic artificial insemination with frozen–thawed spermatozoa in ocelots (Felis pardalis). Journal of Reproduction and Fertility 106(1): 87-94.
[23] Swanson WF, Roth TL, Blumer E, Citino SB, Kenny D, Wildt DE. 1996b. Comparative cryopreservation and functionality of spermatozoa from the normospermic jaguar (Panthera onca) and teratospermic cheetah (Acinonyx jubatus). Theriogenology 45(1): 241.
[24] Platz CC, Wildt DE, Seager SW. 1978. Pregnancy in the domestic cat after artificial insemination with previously frozen spermatozoa. Journal of Reproduction and Fertility 52(2): 279-282.
[25] Donoghue AM, Johnston LA, Seal US, Armstrong DL, Simmons LG, Gross T, Tilson RL, Wildt DE. 1992. Ability of thawed tiger (Panthera tigris) spermatozoa to fertilize conspecific eggs and bind and penetrate domestic cat eggs in vitro. Journal of Reproduction and Fertility 96: 555-564.
[26] Zambelli D, Caneppele B, Castagnetti C, Belluzzi S. 2002. Cryopreservation of Cat Semen in Straws: Comparison of Five Different Freezing Rates Reproduction in Domestic Animals 37(5): 310-313.
[27] Hay MA, Goodrowe K. 1993. Comparative cryopreservation and capacitation of spermatozoa from epididymides and vasa deferentia of the domestic cat. Journal of Reproduction and Fertility 47: 297-305.
[28] Byers AP, Hunter AG, Seal US, Binczik GA, Graham EF, Reindl NJ, Tilson RL. 1989. In-vitro induction of capacitation of fresh and frozen spermatozoa of the Siberian tiger (Panthera tigris). Journal of Reproduction and Fertility 86(2): 599-607.
[29] Thiangtum K, Swanson WF, Howard J, Tunwattana W, Thongthainan D, Wichasilpa W, Patumrattanathan P, Pinyopoommintr T. 2006. Assessment of basic seminal characteristics, sperm cryopreservation and heterologous in vitro fertilisation in the fishing cat (Prionailurus viverrinus). Reproduction, Fertility, and Development 18(3): 373-82.
[30] Rosato MP, Iaffaldano N. 2013. Cryopreservation of rabbit semen: Comparing the effects of different cryoprotectants, cryoprotectant-free vitrification, and the use of albumin plus osmoprotectants on sperm survival and fertility after standard vapor freezing and vitrification. Theriogenology 79(3): 508-516.
[31] Isachenko E, Isachenko V, Weiss JM, Kreienberg R, Katkov II, Schulz M, Lulat AG-MI, Ridopatron MJ, Sanchez R. 2008. Acrosomal status and mitochondrial activity of human spermatozoa vitrified with sucrose. Reproduction (Cambridge, England) 136(2): 167.
[32] Conforti VA, Bateman HL, Schook MM, Newsom J, Lyons LA, Grahn RA, Deddens JA, Swanson WF. 2013. Laparoscopic oviductal artificial insemination improves pregnancy success in exogenous gonadotropin-treated domestic cats as a model for endangered felids. Biology of Reproduction 89: 1-9.
[33] Swanson WF. 2012. Laparoscopic Oviductal Embryo Transfer and Artificial Insemination in Felids – Challenges, Strategies and Successes. Reproduction in Domestic Animals 47(S6): 136-140.
[34] Swanson WF, Newsom J, Lyons LA, Grahn RA, Bateman HL. 2014. Ovarian Down-Regulation with Oral Progestin for FixedTime Laparoscopic Oviductal Artificial Insemination with Freshly-Collected and Frozen-Thawed Spermatozoa in Domestic Cats. Reproduction, Fertility, and Development 26(1): 143.
[35] Crosier AE, Pukazhenthi BS, Henghali JN, Howard J, Dickman AJ, Marker L, Wildt DE. 2006. Cryopreservation of spermatozoa from wild-born Namibian cheetahs (Acinonyx jubatus) and influence of glycerol on cryosurvival. Cryobiology 52: 169-181.
[36] Griffith B, Scott JM, Carpenter JW, Reed C. 1989. Translocation as a species conservation tool: status and strategy. Science 245: 477-480.
[37] Wilson SM. 2018. Lessons Learned from Past Reintroduction and Translocation Efforts with an Emphasis on Carnivores. Action A.4: Elaboration of plans for reinforcement of the Dinaric – SE Alpine population and for creation of new “stepping stone” population – Guidelines for Lynx Reinforcement in Preventing the extinction of the Dinaric-SE Alpine lynx population through reinforcement and long-tern conservations. p 1-43.
[38] Wolf CM, Griffith B, Reed C, Temple S. 1996. Avian and mammalian translocations: update and reanalysis of 1987 survey data. Conservation Biology 10: 1142–1154.
[39] Fischer J, Lindenmayer DB. 2000. An assessment of the published results of animal relocations. Biological Conservation 96: 111.
[40] Jule KR, Leaver LA, Lea SEG. 2008. The effects of captive experience in reintroduction survival in carnivores: a review and analysis. Biological Conservation 141: 355-363.
[41] Beldon RC, McCown JW. 1996). Florida panther reintroduction feasibility study. Final Report, Study no. 7507. Tallahassee, FL: Florida Game and Fresh Water Fish Commission.
[42] IUCN/SSC. 2013. Guidelines for Reintroductions and other Conservation Translocations. Version 1.0, Gland, Switzerland: IUCN Species Survival Commission, viii + 57pp.
[43] Macdonald DW. 2009. Lessons Learnt and Plans Laid: Seven Awkward Questions for the Future of Reintroductions. Pgs. 411448. In M.W. Hayward and M. Somers, editors. Reintroduction of Top-Order Predators. John Wiley & Sons, Incorporated. Hoboken, USA.
[44] Mellen JD. 1993. A comparative analysis of scent-marking, social and reproductive behavior in 20 species of small cats (Felis). American Zoologist 33, 151–166.
[45] Howard JG, Wildt DE. 2009. Approaches and efficacy of artificial insemination in felids and mustelids. Theriogenology 71:130148.
[46] Lambo CA, Grahn RZ, Lyons LA, Bateman HL, Newsom J, Swanson WF. 2012. Comparative fertility of freshly collected vs frozen-thawed semen with laparoscopic oviductal artificial insemination in domestic cats. Reproduction in Domestic Animals 47: 284288.
[47] Conforti VA, Bateman HL, Vick MM, Lyons LA, Grahn RA, Deddens JW, Swanson WF. 2011. Improved fertilization success using laparoscopic oviductal artificial insemination with low sperm numbers in domestic cats. Proceedings of the Society for the Study of Reproduction 40 [abstract no. 173).
[48] Swanson WF, Wolf BA, Brown JL, Martin-Jiminez T, Riviere JE, Roth TL, Wildt DE. 1997. Pharmokinetics and ovarian stimulatory effects of equine and human chorionic gonadotropins administered singly and in combinations in the domestic cat. Biology of Reproduction 57: 295-302.
[49] Graham LH, Swanson WF, Birchard GF, Brown JL. 2000. Chorionic gonadotropin administration in domestic cats causes an abnormal endocrine environment which disrupts oviductal embryo transport. Theriogenology 54: 1117-1131.
[50] Magarey GM, Bond JB, Herrick JR, Stoops MA, Swanson WF. 2005. Improved recipient synchronization protocol for embryo transfer in the domestic cat (Felis silvestris catus) using equine chorionic gonadotropin (eCG) and porcine luteinizing hormone (pLH). Proceedings of the 38th Society for the Study of Reproduction 91 [Abstract no. 48].
[51] Swanson WF. 2019. Practical application of laparoscopic oviductal artificial insemination for the propagation of domestic cats and wild felids. Reproduction, Fertility and Development 31: 27-39.
[52] Howard JG, Barone MA, Donoghue AM, Wildt DE. 1992.The effect of preovulatory anesthesia on ovulation in laparoscopically inseminated domestic cats. Journal of Reproduction and Fertility 96: 175-186.
[53] Tsutsui T, Tanaka A, Takagi K, Nakagawa K, Fujimoto Y, Murai M, Anzai M, Hori T. 2000. Unilateral intrauterine horn insemination of fresh semen in cats. Journal of Veterinary Medical Science 62: 1241-1245.
[54] Tsutsui T, Tanaka A, Hori T. 2001. Intratubal insemination with fresh semen in cats. Journal of Reproduction and Fertility Supplement 57: 347-351. PMID: 11787173.
[55] Toyonaga M, Sato Y, Sasaki A, Kaihara A, Tsutsui T. 2011. Artificial insemination with cryopreserved sperm from feline epididymides at 4℃. Theriogenology 76: 532-537.
[56] Zambelli D, Cunto M. 2005. Transcervical artificial insemination in the cat. Theriogenology 64: 698-705.
[57] Chatdarong K, Axner E, Manee-In S, Thuwanut P, Linde-Forsberg C. 2007). Pregnancy in the domestic cat after vaginal or transcervical insemination with fresh and froze semen. Theriogenology 68: 1326-1333.
[58] Chatdarong K, Lohachit C, Linde-Forsberg C. 2004. Distribution of spermatozoa in the female reproductive tract of the domestic cat in relation to ovulation induced by natural mating. Theriogenology 62: 1027-1041.
[59] Coy P, Garcia-Vazquez FA, Viaconti PE, Aviles M. 2012. Roles of the oviduct in mammalian fertilization. Reproduction 144: 64-660.
[60] Swanson WF, Howard JG, Roth TL, Brown JL, Alvarado T, Burton M, Starnes D, Wildt DE. 1996. Responsiveness of ovaries to exogenous gonadotropins and laparoscopic artificial insemination with frozen-thawed spermatozoa in ocelots (Felis pardalis). J. Reproduction and Fertility 106 :87-94.
[61] Lambo C, Conforti V, Goff D, Gyimesi Z, Campbell M, Lewandowski A, Limoges MJ, Bateman H, Swanson WF. 2011. Improving pregnancy success with laparoscopic artificial insemination in Brazilian ocelots (Leopardus pardalis mitis). Proceedings of the American Association of Zoo Veterinarians 76-78.
[62] Pope CE, Gomez MC, Dresser BL. 2006. In vitro production and transfer of cat embryos in the 21st century. Theriogenology 66: 59–71.
[63] Swanson WF. 2006. Application of assisted reproduction for population management in felids: the potential and reality for conservation of small cats. Theriogenology 66: 49–58.
[64] Wildt DE, Kinney GM, Seager SWJ. 1977. Laparoscopy for direct observation of internal organs in the domestic cat and dog. American Journal of Veterinary Research 38: 1429–1432.
[65] Goodrowe KL, Wall RJ, O’Brien SJ, Schmidt PM, Wildt DE. 1988. Developmental competence of domestic cat oocytes after fertilization in vitro. Biology of Reproduction 39: 355–372.
[66] Swanson WF, Bond JB, Steinetz B, McRae MA. 2001. Fetal and neonatal development of domestic cats produced from in vitro fertilization and laparoscopic oviductal embryo transfer versus natural mating. Theriogenology 55: 371.
[67] Pope CE, Gómez MC, Dresser BL. 2006a. In vitro embryo production in domestic and nondomestic cats. In Special issue of Theriogenology: Proceedings of the Fifth International Symposium of Canine and Feline Reproduction, v.66, 1518-1524
[68] Pope CE, Gómez MC, Galiguis J, Dresser BL. 2012. Applying embryo cryopreservation technologies to the production of domestic and black-footed cats. Reproduction in Domestic Animals v.47(Suppl. 6), 125-129
[69] Pope CE. 2019. Forty years of assisted reproduction research in non-domestic, wild and endangered mammals. Anais do XXIII Congresso Brasileiro de Reprodução Animal (CBRA-2019); Gramado, RS, 15 a 17 de maio de 2019.
[70] Herrick JR, Campbell M, Levens G, Moore T, Benson K, D’Agostino J, West G, Okeson DM, Coke R, Portacio SC, Leiske K, Kreider C, Polumbo PJ, Swanson WF. 2010/ In vitro fertilization and sperm cryopreservation in the black-footed cat (Felis nigripes) and sand cat (Felis margarita). Biology of Reproduction 82: 552-562.
[71] Howard JG. 1998. Assisted reproductive techniques in nondomestic carnivores. In: Fowler ME, Miller RE (eds.) Zoo and Wild Animal Medicine, IV. Philadelphia: WB Saunders; 449-457.
[72] Pope CE, Gómez MC, Cole A, Dumas C, Dresser BL. 2006b. Oocyte recovery, in vitro fertilization and embryo transfer in the serval (Leptailurus serval). Reproduction, Fertility, and Development, v.18, p.223 (Abstract).
[73] Pope CE, Gómez MC, Dresser BL. 2006c In vitro production and transfer of cat embryos in the twenty-first century. In: Special Issue of Theriogenology: Feline Reproduction, v.66, p.72-81.
[74] Pope CE. 2004. In vitro fertilization and embryo transfer in felids. In: Schatten H, editor. Methods in molecular biology germ cell protocols molecular embryo analysis live imaging transgenesis and cloning, vol. 2. Totowa, NJ: The Humana Press, Inc.; 227–44.
[75] Gomez MC, Pope CE, Harris R, Mikota S, Dresser BL. 2003. Development of in vitro matured, in vitro fertilized domestic cat embryos following cryopreservation, culture and transfer. Theriogenology 60: 239–51.
[76] Herrick JR, Bond JB, Campbell M, Levens G, Moore T, Benson K, D’Agostino J, West G, Okeson DM, Coke R, Portacio SC, Leiske K, Kreider C, Polumbo PJ, Swanson WF. 2010. Fecal endocrine profiles and ejaculate traits in black-footed cats (Felis nigripes) and sand cats (Felis margarita). General and Comparative Endocrinology 165: 204-214.
[77] Bateman HL, Bond J, Campbell M, Barrie M, Riggs G, Snyder B, Swanson W 2009. Characterization of basal seminal traits and reproductive endocrine profiles in North American river otters and Asian small-clawed otters. Zoo Biology 28: 107-126.
General Health Monitoring, Preventive Medicine, Pathogen Surveillance, and Quarantine
All general health monitoring data captured in ocelots at the Ocelot Conservation Facility and in free-ranging ocelots in the wild in Texas will be used to inform necessary veterinary care for ocelots held at the Ocelot Conservation Facility and reintroduced to the wild. Health monitoring will also be required for ocelots entering the Ocelot Conservation Facility’s quarantine facility to assure that they do not introduce health concerns to ocelots already held at the Ocelot Conservation Facility. Finally, all ocelots considered for release to the wild should have a health assessment and be tested for pathogens. Results will be used to determine if an ocelot is
Photo courtesy Caesar Kleberg Wildlife Research Institute
suitable for release, and to provide baseline health information should the individual be captured in the wild in the future and reevaluated.
General Health Monitoring and Preventative Medicine
Visual examinations of ocelots will be performed periodically in individual ocelots present in the Ocelot Conservation Facility. Meanwhile, physical examinations of adult ocelots in the Ocelot Conservation Facility will take place under full anesthesia periodically (Tables 1-2), and in all cases prior to an ocelot’s transfer to a reintroduction site. Additionally, ocelots captured in the wild for monitoring of wild populations will undergo similar general health monitoring. Physical exams will include blood draws for health and pathogen testing, and assessment of body condition, weight, and specific body systems described below. General health monitoring may occur during regularly occurring exams or when otherwise warranted, such as if an individual is ill.
Tables 1 and 2 provide general recommendations for health monitoring and preventative medicine procedures and their frequency of performance based on information provided by Felid Taxon Advisory Group (TAG) veterinarians and other zoo veterinarians working at Association of Zoos and Aquariums (AZA) institutions that currently care for ocelots. Table 1 is for “individuals that will not be released” and Table 2 for “individuals that will be considered for release.”
Table 1. For ocelots to be human-managed and not considered for release, recommendations for general health monitoring and preventative medicine.
Physical Examination
Vaccination
Fecal Exam
Neonate 8, 12, 16 weeks
Adult Q2-3years or when warranted
FVRCP Neonate 8, 12, 16 weeks
Adult 1 annual; then Q3 years
RABIES Neonate 16 weeks
Adult 1 annual; then Q3 years
FeLV Neonate 12 and 16 weeks
Adult Annually if using recombinant; Q3 if using killed
Intestinal Parasite Screening Q6 months
Hormone Testing When warranted
Ectoparasites During examination
Urinalysis
CBC/CHEM/Bank Samples
Q2-3Years or when warranted
Neonate exam; Annual for 3 years; then Q2-3 years or when warranted
Disease Testing Situational
Dental Prophy
Q2-3 years
Ultrasound Exam (M/F) Annual/ when warranted
Heartworm Antigen Testing Annual
Ivermectin Monthly
Deworming
Radiographs
Q6 months/ when warranted
Q2-3 years
Table 2. For ocelots considered for release, recommendations for health monitoring and preventative medicine
Physical Examination
Vaccination
Fecal Exam
Neonate 8, 12, 16 weeks
Adult Annual until release
FVRCP Neonate 8, 12, 16 weeks
Adult Annual until release
RABIES Neonate 16 weeks
Adult Annual until release
FeLV Neonate 12 and 16 weeks
Adult Annual booster if warranted before release; recommend killed vaccine due to longevity of rebooster
Intestinal Parasite Screening Q6 months
Hormone Testing When warranted
Ectoparasites During examination
Urinalysis
CBC/CHEM/Bank Samples
Annual until release/ when warranted
Neonate exam/ Annual until release/ when warranted
Disease Testing 30 days prior to release/ when warranted
Dental Prophy Never unless warranted
Reproductive Ultrasound Exam (M/F)
Heartworm Antigen Testing
Annual until release/ when warranted
Annual until release
Ivermectin Monthly until release (tentative)
Deworming Q6 months until release/ when warranted
Radiographs At least once prior to release
Preventative Medicine
Vaccinations of zoo-managed non-domestic felids are based on both recommendations made by the American Association of Feline Practitioners (AAFP) and on the specific risks to non-domestic species. These vaccinations are divided into core (recommended for all felids) and non-core (optional vaccinations where use
is dependent on risk factors, Table 3). In domestic cats, studies have shown protection for 3 years with certain vaccines: killed rabies and killed and modified live combination (MLV) vaccines (panleukopenia, calicivirus, herpesvirus) [236-237]. However, specific information relating to vaccines for non-domestic species is generally lacking, and therefore, most AZA institutions recommend a frequency of vaccination every 1-3 years for core vaccines [238]. There have been several cases of disease in non-domestic species after the administration of MLV vaccines, such as feline herpesvirus (FHV), feline calicivirus (FCV), and canine distemper virus (CDV), As such, MLV vaccines are not recommended in cubs or kittens [239]. It is recommended to always record the site of injection for each vaccine to document any reactions that may occur.
Table 3: Core and non-core vaccines for felines as recommended by the American Association of Feline Practitioners.
Vaccines
Core
Rabies
Vaccine Type
Killed (Imrab 3®, Merial); recombinant canarypox-vectored (PureVax Rabies®, Merial)
Feline panleukopenia, calicivirus, herpesvirus (FVRCP) Killed (Fel-O-Vax®, Elanco)
Non-Core
Canine distemper virus (CDV)
Recombinant canarypox-vectored (PureVax Ferret Distemper®, Merial); MODIFIED LIVE NOT RECOMMENDED
FeLV Killed; Recombinant (PureVax®, Merial)
General Anesthesia
General anesthesia for physical exams or other veterinary procedures will consist of medication combinations used frequently in feline species and specifically designed for ocelots (Appendix 5). Current protocols include a combination of ketamine (200 mg/mL), medetomidine (10 mg/ mL), midazolam (5 mg/ mL), and/or butorphanol (10 mg/ mL). Many combinations of these medications have been utilized successfully in ocelots at varying dosages, depending on individual physiology and veterinarian preference. Other anesthetic medications within similar drug classes may also be utilized at the veterinarian’s discretion, and anesthetic medications are not limited to the above medication list. Medication use will be identified by the attending veterinarian and may be informed by resources provided by the zoo community or other researchers. Resources may include but are not limited to, unpublished zoo records housed in zoological institutions, published anesthetic regimes, and direct conversation with other researchers or managers of captive ocelots
Physical examinations
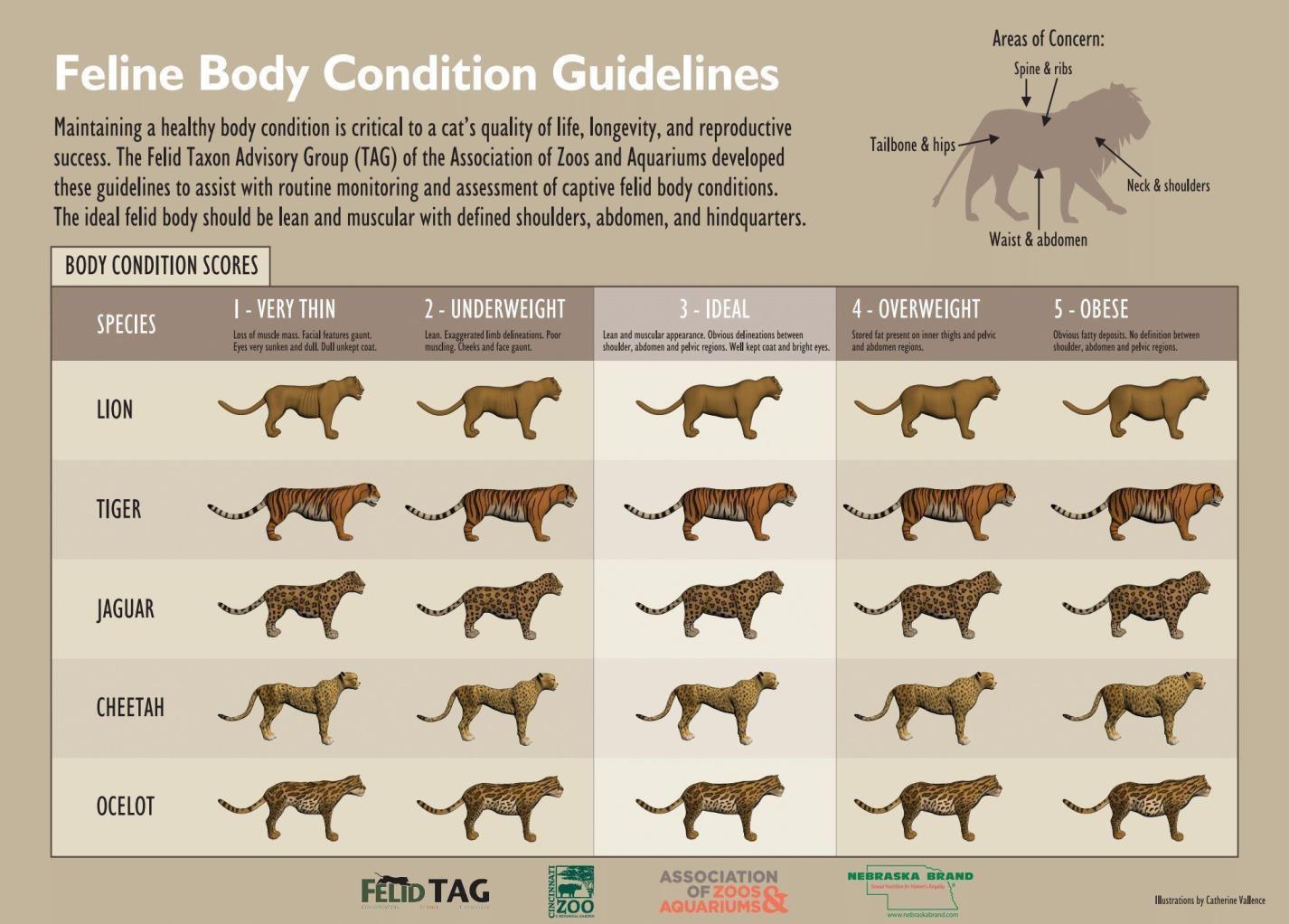
Physical examinations of ocelots at the Ocelot Conservation Facility will be performed under general anesthesia assessing the following body systems/parameters: general appearance, respiratory system, eyes/ears/nose/throat, peripheral lymph nodes, nervous, musculoskeletal, and body condition score (Figure 1). Additional items to assess include:
Gastrointestinal: Fecal samples assessed for intestinal parasites and fecal consistency
Genitourinary: For males, palpation of the testes for tumors or abnormalities, collection of sperm sample for assessment of seminal parameters, assessment of penis for spine development and abnormalities (persistent frenulum, etc.). For females, vulvar and mammary examinations for tumors, assessment of discharge/discoloration and lactation status
Cardiac: Echocardiogram (ECG) for arrhythmias. Indirect blood pressure should be measured with a cuff, sized at 30-40% of the limb circumference, and placed either on the forelimb (below or above the elbow), hind limb (above the hock), or at the base of the tail. Hypotension will be defined as a systolic arterial pressure (SAP) of less than 90 mmHg, mean arterial pressure (MAP) less than 70 mmHg and/or diastolic arterial pressure (DAP) less than 50 mmHg [17-19].
Dentition: Tooth wear, discoloration, fractures, missing teeth; aging [20]. Dental radiographs should be taken at sedation events when possible. (Table 4)
Integument: Skin scraping, or hair culture as indicated by skin lesions or patterns.
Figure 1. Feline Body Condition Guidelines by the Felid Taxon Advisory Group.
Table 4 Dentition and aging information for ocelots.
Parameter PDF Reference
Dentition/Aging https://link.springer.com/article/10.1007/s10344-018-1198-6
http://www.toothvet.ca/PDFfiles/Cat_Chart.pdf
http://www.toothvet.ca/PDFfiles/Cat_Deciduous_Chart.pdf
Hematology Profile
A complete blood count (CBC) evaluates the cells that circulate within the blood, including red blood cells (RBCs), white blood cells (WBCs), and platelets [1]. The results describe the number, size, and shape of each cell type [1]. Elevated white blood cell counts indicate the presence of inflammation or infection, while a decrease in red blood cells and platelets can indicate the presence of anemia or decreased clotting abilities [2]. A blood chemistry profile evaluates internal organ function and electrolyte status and can inform about disease processes affecting the internal organs of that individual [3-4]. In combination, these two analyses can provide information regarding the health of an individual and a population.
Minimal baseline hematology (Table 5) and biochemical parameters (Table 7) exist for ocelots currently, with one set originating from free-ranging ocelots in Brazil [5] and another from zoo-managed ocelots included in the Species360 online database [6]. Baseline hematology (Table 6) and biochemical parameters (Table 8) have also been collected for free-ranging ocelot populations in Texas [A. Reeves, East Foundation, unpublished data, n=24 ocelots]. Blood samples should continue to be collected in free-ranging ocelots and captive-held ocelots in the Ocelot Conservation Facility to establish a reliable health monitoring system and to assess the health of captive-held ocelots. Blood parameters may vary between free-ranging and captive populations [7-11], between sex [8, 12-14], and age of the individual at time of collection [7-8, 13-16].
Sample Type and Collection Method
Whole blood (2-3 mL) will be collected into an Ethylenediaminetetraacetic acid (EDTA) blood tube (typically with a lavender top). For best results, this sample should either be tested the same day of collection or kept on ice/placed into the refrigerator until testing is possible. Four blood smears will be created per individual using the EDTA whole blood, including one for differential white blood cell count and platelet estimation, one for disease testing, and two for sample archive or other use. See Table 9.
Sample Testing
Complete Blood Count analysis will be either tested in laboratory space at the Ocelot Conservation Facility using the IDEXX Procyte® Hematology Analyzer (IDEXX Laboratories, Inc., Westbrook, Maine USA) using whole blood in EDTA or will be sent to a testing laboratory at the discretion of the veterinarian.
If white blood cell differential counts are performed at the Ocelot Conservation Facility, the following procedures will take place (http://www.vspn.org/Library/Misc/VSPN_M02362.html):
1) A single blood smear will be stained with Diff-Quik Stain (Jorgensen Laboratories, Inc. Loveland, CO, USA) and a differential white blood cell count will be performed under 1000X oil immersion using a biological compound light microscope. One hundred white blood cells (neutrophils, lymphocytes,
macrophages, eosinophils, basophils) will be counted and a percentage of 100 will be reported. Additionally, abnormal cells and/or pathogen inclusion bodies will be noted.
2) Platelet counts will be performed under 1000X oil immersion using a biological compound light microscope. The smears will be assessed for platelet number by taking the average number of platelets in 5 microscopic fields and multiplying them by 15,000 and 20,000 for an estimated platelet range (http://www.vspn.org/Library/Misc/VSPN_M02362.htm ).
Table 5 Available hematology parameters of free-ranging ocelots, captive ocelots, and domestic felines [5-6, 21].
MCV=mean cell volume; MCH=mean cell hemoglobin; MCHC=mean cell hemoglobin concentration
* Student’s t-test: statistically significant differences (P<0.05) between means for wild and captive ocelots.
Table 6 Available hematology parameters for free-ranging ocelots in Texas) [A. Reeves, East Foundation, unpublished data, n= 24 ocelots]
Free-Ranging Texas Ocelots
RI, reference interval; LRL, lower reference limit; URL, upper reference limit; ALT; RBC= red blood cell; Conc.= concentration; MCV=mean cell volume; MCH=mean cell hemoglobin; MCHC=mean cell hemoglobin concentration; WBC= white blood cell.
*90% confidence interval around the 95% LRL and URL are shown these columns
Sample Type and Collection Method
Whole blood (4-6 mL minimum) will be collected into a plain red top serum tube or a serum separator tube. The serum tube will be allowed to clot for a minimum of 20 minutes and centrifuged for 10 minutes at ≥ 2000g and the serum removed for serum chemistry analysis. Serum will be maintained in a refrigerator until analysis can be performed on the day of collection or stored in the refrigerator for a maximum of 7 days prior to shipping to a partner laboratory for completion. This sample should not be frozen prior to biochemical profile completion.
Sample Testing
Chemistry analysis may be conducted on-site using the IDEXX Catalyst One® Chemistry Analyzer and a Chem 17 CLIP with electrolytes (IDEXX Laboratories, Inc., Westbrook, Maine USA) or may be sent to a testing laboratory at the discretion of the veterinarian. The CHEM 17 clip contains albumin (ALB), albumin/globulin ratio (ALB/GLOB), globulins (GLOB), alkaline phosphatase (ALKP), alanine transaminase (ALT), amylase (AMYL), cholesterol (CHOL), glucose (GLU), blood urea nitrogen (BUN), creatinine (CREA), blood urea nitrogen/ creatinine ratio (BUN/CREA), lipase (LIPA), gamma-glutamyl transferase (GGT), phosphate (PHOS), total bilirubin (TBIL), calcium (Ca), and total protein (TP) (IDEXX Laboratories, Inc., Westbrook, Maine USA).
The electrolyte clip contains chloride (Cl), potassium (K), sodium (Na), and sodium/potassium ratio (Na/K) (IDEXX Laboratories, Inc., Westbrook, Maine USA).
Sodium/Potassium ratio
ALKP= alkaline phosphatase; ALT=alanine aminotransferase; GGT=gamma-glutamyl transferase; BUN= blood urea nitrogen; TBIL= total bilirubin
* Student’s t-test: statistically significant differences (P<0.05) between means for wild and captive ocelots.
Table 8 Available values for biochemical parameters of free-ranging ocelots in Texas [A. Reeves, East Foundation, unpublished data, n=24 ocelots]
Free-Ranging Texas Ocelots
RI, reference interval; LRL, lower reference limit; URL, upper reference limit; ALT, alanine transaminase. *90% confidence interval around the 95% LRL and URL are shown in these columns.
Table 9. Recommended blood collection volume by ocelot weight (kg) and use of blood for testing based on weight. 1% of kg weight is the maximum volume of blood that may be collected from an animal, but collection of that volume rarely necessary. This upper limit will be most important for young kittens and small individuals.
Urine Diagnostics
The use of urinalysis in felids can help detect or characterize renal (kidney) disease; monitoring kidney disease once diagnosed; detecting diabetes and complications of undiagnosed or unregulated diabetes; or identifying signs of malnourishment, starvation, urinary tract disorder, or other pathologies [23-25]. Further examination of urine sediment after centrifugation can reveal crystals, renal casts, bacteria, and abnormal cells which can identify diet-related issues, urinary tract infections, kidney damage and certain urogenital and renal neoplasia (cancers) [23-24]. This information can be coupled with the complete blood count and biochemical analysis to characterize and/or add to information about organ function [24].
Sample Collection Method
During anesthesia, urine of ocelots at the Ocelot Conservation Facility can be collected by cystocentesis. A 1.5inch, 22-gauge needle will be attached to a 3 to 6 mL syringe and passed through the abdominal wall into the bladder, where 3 to 6 mL of urine will be aspirated into the syringe. A second approach to collect urine is to place a pan in an area of consistent elimination and attempt to capture urine voluntarily.
Sample Testing (Table 10)
Testing of urine may be performed by sending samples to a partner lab or by performing the analysis at the Ocelot Conservation Facility. If sending a sample to another laboratory, collection in a non-additive, sterile container (white top tube) is preferred, though urine can also be collected and stored in a red top (non-additive) tube or urine sample cup. This sample should be shipped chilled on ice packs the same day it is collected or stored in the refrigerator to be shipped within 7 days for routine testing [10].
IDEXX UATM Strip Test: 1 drop of sample is placed onto each pad, left to develop for the specified duration for a particular pad, and then evaluated based on the strip test results provided on the package. The duration for each pad and evaluation results will differ for each company in which the strip tests are purchased. Follow package instructions that accompany each strip pad test.
Urine Specific Gravity (Refractometer): 1 drop is placed onto the window of the calibrated refractometer and the upper window closed to spread the drop. The refractometer is held toward a light and a blue line guides the concentration of the urine. There are multiple readings on the refractometer depending on the type purchased. Instructions will be provided as to which reading coincides with specific gravity.
Urine Sediment Examination [24]: The rest of the urine sample is centrifuged for 5-10 minutes at ~250g, the supernatant poured off, and the remaining pellet is mixed in the bottom of the container. A drop is placed onto a microscope slide (with or without urine sediment stain, dependent on personnel) and read on a compound light microscope using the 10X and 40X objectives. Number of cells, bacteria, and other abnormalities are recorded as described in resources provided in Table 10.
Table 10: Urinalysis Test Assessments and Supplemental Information [23-25]
Urine Test Assessment Guide Screens for:
Visual Assessment Color, turbidity
Urine Specific Gravity Concentrating ability of the kidneys
Urine Sediment Crystals, white blood cells, red blood cells, bladder epithelial cells, sperm, kidney casts, neoplastic cells
https://eclinpath.com/urinalysis/visual-features/ Infection, toxicity
https://www.bristol.ac.uk/medialibrary/sites/vetscience/documents/clinicalskills/Urinalysis%20Specific%20Gravity.pdf
https://eclinpath.com/urinalysis/cell-quick-quide/
https://eclinpath.com/urinalysis/cellular-constituents/
https://eclinpath.com/urinalysis/crystals/
https://eclinpath.com/urinalysis/crystal-quick-guide/
https://eclinpath.com/urinalysis/casts/
https://eclinpath.com/urinalysis/infectious-agents/
Kidney disease (acute or chronic)
Infection, toxicity, kidney disease (acute or chronic), neoplasia, muscular injury, nutrition, parasites
Urine Strip Test Organ function and urine metabolites
Vaginal
(https://www.idexx.com/files/urine-sedimentguide.pdf)
Cytology, Abdominal Ultrasonography, and Hormone Analysis
blood, myoglobin, renal disease, toxicity, others
Vaginal cytology can be a useful tool for staging the current phase of a female’s reproductive cycle through microscopic examination of stained vaginal epithelial cells. Combining findings gained from cytology, abdominal ultrasonography, hormone assessment will provide relevant information regarding ovarian cyclicity, pregnancy status, and measurement of fetal parameters. These techniques may also provide insight into causes of reduced fertility and reproductive failures. Standardized methods to evaluate vaginal cells and to perform progesterone testing relative to the stage of the ovarian cycle are useful for detecting estrus in wild ocelots or captive-held ocelots who may be used for artificial insemination procedures.
Currently, there is no standardized stain recommended for application with felid vaginal cytology slides. Although Wright-Giemsa stain (Rapidiff Fixative®, Clinical Sciences Diagnostics CC, South Africa) has been assessed in lions [26], this has not been compared to other types of stains. In humans, Papanicolaou (Pap) stain has long been used for assessing vaginal epithelial cells and provides an approved standard stain for these types of cells [27]. This stain has multiple steps for completion and can be technically complicated (https://www.biovision.com/documentation/datasheets/K1440.pdf), whereas the Rapidiff stain is a simple, three step process (https://vetlabsupplies.co.uk/assets/Rapi-diff-Instructions-For-Use.pdf). Comparing the use of the Pap stain to the Wright-Giemsa stain in ocelots could be informative for improving vaginal cytology staining.
Meanwhile, ultrasonography of the abdomen is used for many animals to confirm pregnancy, approximate fetal age, and assess the reproductive tract. In canids and felids, measurements such as biparietal diameter, thoracic diameter, and gestation sac diameter have been used to evaluate stages of fetal development relative to specific time points during pregnancy [28-30]. Currently, there is no standard fetal measurement tool used for nondomestic felids, though it could be beneficial for future pregnancy evaluations in the field or other situations when the date of mating/conception is unknown. Measuring these parameters at different time points throughout
an ocelot’s gestation (i.e., 79-85 days) [31] relative to known breeding/artificial insemination date or parturition date could standardize methods for pregnancy detection and estimations of gestational ages. This information would be helpful when preparing for births or for recognizing delayed parturition or dystocia.
Various methods for monitoring endocrine activity in serum, feces, and urine have been assessed in domestic cats [32-33] and many non-domestic cat species [34-41]. Monitoring steroid hormones in fecal samples is preferred over monitoring in urine samples because steroid hormones are almost exclusively excreted in the feces [41-45] and this method provides the most non-invasive method of sample collection. Fecal hormone assessment can also be used to assess responses to ovarian synchronization protocols, ovulation, and pregnancy following artificial insemination procedures [40]. Progesterone and estrogen (and their metabolites in fecal samples) are the primary hormones of interest for assessing feline reproductive cycles. In ocelots, fecal estrogen and progesterone metabolite profiles have been described in captive ocelots throughout their reproductive cycle [40, 45] (Figure 2). In studies of wild ocelots in Texas and captive ocelots, the primary interest for hormone analysis is for tracking fecal progesterone metabolites during cycle manipulations for artificial insemination and during pregnancy. Hormone monitoring also could provide valuable information about normal and/or abnormal cyclicity in study females. In non-domestic felids, fecal progestin levels are indistinguishable between pregnant and non-pregnant luteal phases; however, non-pregnant luteal phases are characterized by a shorter duration of progestin elevation (~1/3 to 1/2 of the gestation period) than pregnant luteal phases [36, 44, 46-49]. In ocelots, the non-pregnant luteal phase lasts for ~45 days post-ovulation compared to a typical 79-day to 85-day gestation length after natural breeding.
Sample Collection Method
Vaginal cytology: When an ocelot is under anesthesia, a sterile, moistened cotton-tipped applicator will be passed through the vulva and into the vagina, rotated 360°, and withdrawn. The end of the applicator would be rolled onto two microscope slides to recover vaginal cells for staining and the swab stored in a plain tube.
Abdominal ultrasonography: When an ocelot is under anesthesia, an ultrasound probe will be placed on the abdomen using gel and alcohol to improve visualization of the reproductive tract. Dating of fetal parameters can be initiated for the ocelot by using the following guidelines (https://www.imv-imaging.es/educacion/educacionanimales-pequeno/tracto-reproductivo/canine-and-feline-foetal-ageing/) and establishing fetal measurements for this species based on known gestation dates and time points
Hormone analysis: Feces (~1 gram) will be collected after natural voiding or, if necessary and for anesthetized ocelots, via a lubricated rectal loop. Feces will stored in a cryovial or plastic specimen bag at -20°C or lower until analysis. In ocelots at the Ocelot Conservation Facility, fecal samples for hormone analysis should be collected three times weekly (every other day) by non-invasive methods of collection (from the enclosure they reside in) either (1) continually for a year to assess ovarian cyclicity (normal versus abnormal) or (2) beginning one month prior to gonadotropin treatment until at least 85 days after insemination. Serum (0.5 mL) will be stored in a cryovial at -20°C or lower until analysis.
Sample Testing
Vaginal cytology: The assessment of vaginal epithelial cells has not been standardized for ocelots. A recent study with lions (Panthera leo) [26] established vaginal cytology parameters as one component of their slide evaluation methods, and these can be useful for assessing vaginal cytology of other felid species [50]. These parameters can be viewed in Table 11. Examples of epithelial cell classifications in domestic cats have been
described [50]. Detailed measurements of epithelial cells and standardization of cell sizes for ocelots may be possible using methods described for lions [26].
Table 11. Vaginal Cytology Evaluation Parameters [26, 50]
Mucus/Cellular
Debris (40x and 200x)
0-1 minimal quantity/no debris
1-2.5 small quantity/debris
2.5-4.5 moderate quantity/debris
4.5-6 large quantity/debris
Cellular distribution (40x and 200x)
0 single epithelial cells
1 small clusters of epithelial cells
2 moderate clusters of epithelial cells
3 large clusters or piles of epithelial cells
Epithelial Cell Classification (200 cells on 1000x)
Basal
Epithelial Cell Quantity (40x and 200x) PMN’s (1000x)
0-20% small
Parabasal 20-50% moderate
Small- 0-2/100 epithelial cells
Moderate- 20-100/100 epithelial cells
Intermediate 50-100% large Large- 100-400/100 epithelial cells
Superficialnucleated/enucleat ed
Very large- >400/100 epithelial cells
Microbial Presence (1000x)
Minimal- <10 per field
Small/few- 10-25/ field
Moderate- 20100/field
Large- >5/ field
Hormone analysis: Fecal and serum samples can be utilized for estrogen and/or progesterone hormone analysis. However, because serum sample collection requires anesthesia, few serum samples are expected to be available for analysis. If serum is collected, analysis of serum samples for progesterone (and/or estrogen) can be performed at a partnered endocrine laboratory or at the Ocelot Conservation Facility, possibly after validating progesterone testing using an in-house IDEXX analyzer. Fecal samples will be stored at -20°C in a cryovial or specimen bag labeled with the date, animal ID, and location or institution. Samples shipped to a partnered laboratory should be shipped (Monday to Wednesday only) on dry ice (~5-6 pounds/shipment) using next-day delivery An example ocelot endocrine profile is in Figure 2.
Figure 2: Ocelot fecal endocrine profile [40]. Longitudinal profile of fecal estrogens (open triangle) and progestogens (closed circle) in a singleton ocelot.
Fecal parasite evaluation
Since 2019, fecal samples have been collected from free-ranging ocelots in Texas to assess parasitic prevalence. In wild ocelots in Texas, the most common intestinal parasites identified were the roundworm, Toxascaris leonina, a protozoon, Cystoisospora felis, and an unknown species of capillarid [A. Reeves, East Foundation, unpublished data]. Any released ocelots will likely be exposed to these parasites upon release to the wild. Parasites found in the wild or in ocelots held in the Ocelot Conservation Facility should be taken into consideration when employing deworming practices.
Sample Collection Method
Fecal samples will be collected from ocelots at the Ocelot Conservation Facility by obtaining it from their enclosures, and samples will be collected from wild ocelots caught in a box trap (if available). If a wild ocelot does not defecate in the trap, a sample may be obtained from the ocelot once anesthetized by passing a lubricated fecal loop (approximately 3/4 inch in diameter) approximately 2 to 4 inches into the rectum, rotating it, and removing the loop. Digital collection from the rectum of an anesthetized ocelot is also an option. Three cryovials will be stored at -20°C or lower for intestinal parasite sequencing (and fecal hormone analysis as described previously). Additionally, 1 gram of feces will be placed into formalin for future fecal flotation and intestinal parasite identification and load if fecal flotation cannot be performed on the day of collection. A fresh fecal sample also may be sent to a partnered laboratory for flotation and intestinal parasite identification.
Sample Testing
Testing will be conducted via fecal flotation by centrifugation (Table 12). Fecal direct smears will be made by mixing a small amount of fecal material with saline and placing a coverslip over the mixture. Fecal direct examination and flotation will be evaluated at 100X, 400X, and/or 500X magnification using light microscopy, and intestinal parasite ova identified based on literature (Table 12). Further speciation can be confirmed by polymerase chain reaction (PCR) on frozen feces.
Table 12: Intestinal parasite testing and identification references.
Test PDF Reference
Fecal Centrifugation
Intestinal Ova Identification
https://www.jorvet.com/wp-content/uploads/2017/05/LITCentrifuge-Fecal-Protocal.pdf
https://www.midamericaagresearch.net/documents/Internal%20Parasite%20Manual%20for%20dogs.pdf
https://veteriankey.com/reference-to-common-parasite-ova-and-forms-seen-in-veterinary-medicine/
https://www.jorvet.com/wp-content/uploads/2011/12/Fecal-Study-Gastrointestinal-Parasites-Guide.pdf
Ectoparasites
Since 2019, ectoparasites were collected from free-ranging ocelots in Texas to assess parasite prevalence. In this population, the most common ectoparasites identified were the tick species Dermacentor variabilis and an unknown species of Pulex flea [A. Reeves, East Foundation, unpublished data]. Any ocelots released to the reintroduction site will likely be exposed to these parasites upon release. Monitoring of ectoparasites and related pathologies in free-ranging ocelots and ocelots held at the Ocelot Conservation Facility is necessary.
Sample Collection Method
While an ocelot is sedated, ectoparasites (ticks, fleas, lice, etc.) will be collected with forceps and placed into a cryovial containing alcohol for preservation and future identification.
Sample Testing
Ectoparasites will be identified to species level (Table 13) under a dissecting scope in collaboration with a partnered parasitology laboratory. If an individual ocelot tests positive for a vector-borne pathogen, the collected vectors (if present at the time of positive test) can be tested for the pathogen as well. Depending on the pathogen of interest, storing ectoparasites in a cryovial without alcohol at -20 ℃ is recommended [266]. It is recommended to identify best methods of storage based on the specific pathogen of interest.
Table 13. Ectoparasite identification references
Test PDF Reference
Ectoparasite
Identification
https://vlm.ub.ac.id/pluginfile.php/46102/mod_resource/content/1/Veterinary%20Ectoparasites%20%20Biology%2C%20Pathology%20and%20Control%20%28VetBooks.ir%29.pdf
https://todaysveterinarypractice.com/feline-arthropods/
https://todaysveterinarypractice.com/parasitology-expertise-from-the-ncvp-parasitology-expertise-from-thencvp-feline-tick-borne-diseases/
https://www.fleatickrisk.com/sites/fleatickrisk/files/docs/Under%20the%20microscope.pdf
Pathogen Surveillance
A variety of pathogens may affect ocelots at the Ocelot Conservation Facility as well as ocelots or other freeranging carnivores in the wild. It is important to conduct routine pathogen testing using samples collected during health monitoring events to assess health risks for ocelots, prepare any necessary veterinary responses, and be aware of possible health risks to humans working on the ocelot reintroduction program Additionally, some pathogens potentially impacting ocelots may be transmissible to or by humans who contact ocelots. To support the health and safety of both people and ocelots, personnel working with ocelots should follow the most updated and available guidance from the Center for Disease Control and Association of Zoos and Aquariums to avoid transmission of the diseases between personnel and ocelots.
Pathogen surveillance in ocelots is necessary to inform veterinary treatment of ill ocelots as well as the use of vaccines or other preventative medicines. Additionally, pathogen testing must occur for all ocelots prior to transfer into the Ocelot Conservation Facility or into the wild to avoid introducing health risks to ocelot populations in the Ocelot Conservation Facility or in the wild. The following are pathogens that ocelots could be screened for. Use of specific pathogen screenings will be dependent on the risk assessments of particular pathogens on ocelots in the Ocelot Conservation Facility and the wild. In all cases of pathogen testing, if the testing will be performed within 7 days of collection, the samples can remain refrigerated (~4℃). If the testing will be performed after 7 days of collection, the samples should be frozen and stored at -40℃ until testing will be completed. When working with new partner laboratories, pathogens or testing methods, communication with the partnered laboratory on the preferred method of storage and preservation is recommended.
Feline Immunodeficiency Virus (FIV)
FIV is a lentivirus that has variable prevalence in cat populations across the world [51]. FIV is primarily transmitted through bite wounds with infected saliva entering the blood, though it can be spread in the absence of fighting in immunosuppressed individuals or from dam to kitten [52]. Although felids can contract this disease at all ages, infection is likely to be the most severe in kittens due to the immaturity of their immune systems. Typically, FIV causes a decline, rebound, and repeated decline of CD4 and CD8 T-lymphocytes, leading to a decline in cell-mediated immunity, which acutely can cause fever, lymphadenopathy, and lymphopenia [52]. FIV is a prevalent disease in wild felid populations [53-56], with most infectious strains being considered species-specific [57] and tending to group geographically rather than in relation to the affected species [58]. Although cross-species transmission has been reported [58-59], it has rarely been documented in managed-care settings where risk of contact is higher [58].
Testing for FIV is important when planning movement of individuals between populations. While FIV could be endemic in one population, movement of individuals from other populations may introduce a strain of FIV that could cause detrimental effects in the recipient population. All ocelots should be tested for FIV when determining suitability for transfer into the Ocelot Conservation Facility or transfer to the wild.
Feline Leukemia Virus (FeLV)
FeLV has not been identified in free-ranging Texas ocelots studied since 2019 (n=24, A. Reeves, East Foundation, unpublished data), and a select number of ocelot samples from South Texas and dated from 1985 to 2012 (n=9) [Reeves et al., unpublished data, in prep.] also were negative for FeLV Though FeLV is not currently present in Texas ocelot populations, it is important to continue screening for FeLV in free-ranging ocelot populations in Texas and at the Ocelot Conservation Facility given the potential health impacts of FeLV.
FeLV is a gammaretrovirus [60] that can be transmitted vertically to kittens and horizontally among other adult cats [52]. Once infected, FeLV spreads from local lymphoid tissues, where it eventually infects the bone marrow [52] and can cause tumors, bone marrow suppression syndromes, and increased susceptibility to secondary infections due to suppression of the immune system [61]. FeLV infections have been categorized as abortive, regressive, progressive, or focal based on how well the virus is contained during the early stage of infection [52, 61]. Kittens are more susceptible to infections becoming progressive, leading to a greater chance of associated disease and death [52]. Approximately 2-4% of domestic cats test positive for FeLV in the United States [52, 62]. There is currently a vaccination protocol for domestic felines, based on their level of risk, established by the American Association of Feline Practitioners [52].
FeLV is rarely noted in non-domestic felines [55] and most cases have been reported in captive individuals that have been previously exposed to infected domestic cats [63-64]. The only wild species known to have endemic FeLV in their populations is the European wildcat (Felis silvestris) [65]. Additionally, FeLV has been found in other non-domestic free-ranging feline populations including Florida panthers (Puma concolor coryii) [66-67], and Iberian lynx (Lynx pardinus) [68]. While most non-domestic felids experience no adverse effects or just the immunosuppressive effects of FeLV as seen in domestic cats [69], there have been reported outbreaks leading to mortality indicating the existence of a pathogenic form of the disease [66-68] and a strain causing significant disease in Florida panthers documented cross-species infection from domestic felines in the area [66].
For ocelots in southern Texas, the high number of feral domestic cats surrounding current populations presents concern for transmission of FeLV from domestic cats into ocelot populations. If FeLV infection produces
decreased immune fitness in ocelots, it could impact population health or present the possibility of increased susceptibility to other pathogens. Additionally, decreasing genetic diversity documented in other species (Iberian lynx and Florida panther) show that lack of a robust immune system may increase the potential risk of introduction of disease, cross-species transmission of pathogens, and mortality.
Sample Type and Testing (FIV/FeLV)
Testing for FeLV and FIV will use the IDEXX SNAP Feline Leukemia Virus Antigen-Feline Immunodeficiency Virus Antibody Test Kit (IDEXX Laboratories, Inc., Westbrook, Maine USA). The IDEXX SNAP FIV/FeLV Combo test has a sensitivity and specificity of 96-100% for domestic cats [70]. Whole blood can produce false positive results for FIV, so it is recommended to use serum for testing. For individuals under 6 months of age, retesting should take place after 6 months of age as maternal antibodies against FIV can cause a false positive result (https://catvets.com/public/PDFs/PracticeGuidelines/RetrovirusGLS-Summary.pdf ; https://www.vet.cornell.edu/departments-centers-and-institutes/cornell-feline-health-center/healthinformation/feline-health-topics/feline-immunodeficiency-virus-fiv). Additionally, confirmatory testing is recommended for FeLV positive snap tests, as more information about the type of infection can be identified (https://www.idexx.com/files/updates-diagnosis-management-felv.pdf ). Furthermore, PCR testing for FeLV will be performed to identify potential regressive infections in ocelots in the Ocelot Conservation Facility or in the wild in Texas. PCR testing for FeLV can be performed on whole blood through many laboratories. “Realtime PCR (FeLV RealPCR™ Test) detects proviral DNA and, therefore, is useful to confirm the presence of FeLV infections that have progressed to the bone marrow” [71-72]. Three drops of serum and four drops of conjugate (anti-FELV/FIV Ag: HRPO conjugate) are mixed in a vial and poured into the sample well. When the solution reaches the activation circle, the activator is pushed firmly until flush with the device body and the test is read in 10 minutes. Positive, negative, and invalid test results are interpreted by the parameters set forth in Figure 3 Confirmation of a positive test requires additional testing methods such as a Western Blot. (https://www.idexx.com/files/fiv-diagnostic-algorithm.pdf; https://www.dvm360.com/view/a-practical-guideto-feline-retrovirus-testing ).
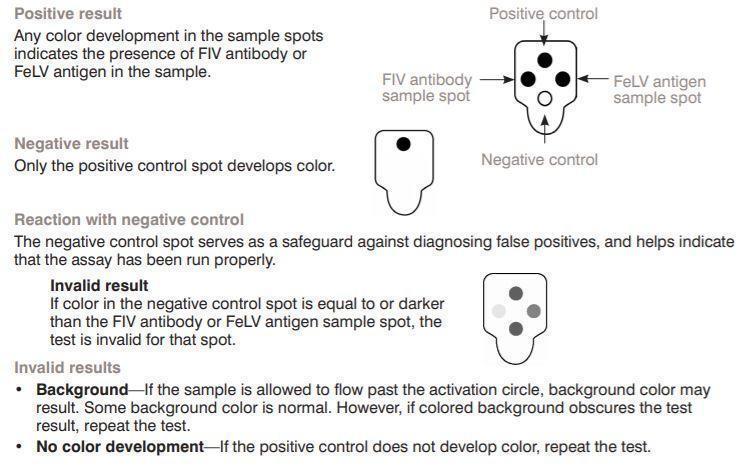
Figure 3: FeLV/FIV Snap Test IDEXX Laboratories, Inc., Westbrook, Maine USA Snap Combo Package Insert https://www.idexx.com/files/SNAP_COMBO_package_insert_032917.pdf
Toxoplasma gondii
Since 2019, Toxoplasma gondii antibodies were identified in free-ranging Texas ocelots (9/22; 40.9%) [A. Reeves, East Foundation, unpublished data] as well as in a select number of samples dating from 1985 to 2012 (4/9; 44.4 %) [A. Reeves et al., unpublished data, in prep.]. This pathogen is consistently present within freeranging ocelot populations and reintroduced ocelot populations are likely to be exposed to the parasite. While previous research suggests low concern for either negative side effects or population level impacts, T. gondii should be continually monitored for in ocelots at the Ocelot Conservation Facility and in the wild to identify possible pathology due to infection.
Toxoplasmosis is a zoonotic disease caused by infection with the protozoan parasite T. gondii. Its definitive host is all members of the family Felidae, and the primary intermediate hosts are rodent prey species, but any warmblooded animal, including humans, may serve that role [73-74]. Transmission of T. gondii can occur horizontally through ingestion of bradyzoites encysted in undercooked animal tissue, or sporulated oocysts from cat feces that have contaminated water or food supplies [75-76]. Transmission can also occur vertically through transplacental and lactogenic infection by tachyzoites [74-75].
In members of the family Felidae, such as bobcats (Lynx rufus) and ocelots, horizontally transmitted toxoplasmosis most commonly causes either mild clinical signs such as diarrhea or no clinical signs at all in individuals who are otherwise healthy [77]. If there is concurrent immunosuppression or decreased fitness, more severe clinical signs might occur [77-79]. These clinical signs could include fever, cough, dyspnea, jaundice, neurologic signs, ocular signs, and even death [77]. Vertically transmitted toxoplasmosis is usually asymptomatic in kittens but has the potential to result in more severe signs in otherwise healthy, definitive host species [77]. In bobcat kittens specifically, clinical signs have included myocarditis, hepatitis, and encephalitis, eventually leading to death [80].
In healthy intermediate hosts, specifically in humans, horizontal transmission can result in flu-like symptoms, decrease reaction times to outside stimuli, or cause no symptoms at all [74]. However, immunosuppression can make symptoms much worse. The most common clinical sign of toxoplasmosis in immunosuppressed humans is toxoplasmic encephalitis [76]. Vertical transmission in intermediate hosts, such as humans, has been reported to lead to a variety of clinical signs depending on the time during gestation that the infection occurred [81-82]. In humans, the risk of transplacental transmission increases as the length of gestation increases [81-82]. However, the consequences of congenital infection are more severe the earlier the transmission occurs in gestation, meaning the severity of disease is inversely correlated with the risk of transmission [81-82]. Symptoms in humans due to congenital transmission can vary but tend to target the eyes and brain [83-85]. Abortion is also possible with congenitally transmitted toxoplasmosis [74].
Due to the threat that toxoplasmosis poses to inbred, non-domestic feline populations, understanding the prevalence of toxoplasmosis in ocelot populations in Texas is important for the reintroduction program as well as the protection of the humans and wild or domestic animals who interact with them directly or indirectly. Currently, there is published information on T. gondii antibody prevalence in bobcat populations in Minnesota, Mississippi, and Pennsylvania, all of which indicate that T. gondii has high prevalence in these populations [8688]. One study of T. gondii prevalence in ocelot populations in Mexico found that this population had high prevalence of antibodies as well [89].
Sample Type and Testing
Toxoplasma gondii titers will be evaluated using the Toxoplasma gondii MAT test Kit (TgMAT Kit; University of Tennessee Research Foundation) for detection of anti-Toxoplasma IgG antibodies in serum [90-91]. The antigen used in the test kit is formalin-treated Toxoplasma tachyzoites. The presence of anti-Toxoplasma IgG antibodies in the samples will cause the suspension to form a diffuse cellular mat in the bottom of the well, whereas a negative sample will produce a smaller pellet on the well bottom. Methods will be performed and interpreted according to the recommended protocol for MAT testing provided by the University of Tennessee (https://volweb.utk.edu/~csu1/MATprotocol.pdf; https://volweb.utk.edu/~csu1/TgMATModified_Agglutination_Test.html) or another laboratory of the veterinarian’s choosing. A titer equal to or greater than 1:25 is considered a positive result.
Trypanosoma cruzi
Two non-domestic cat species, ocelots and pumas (Felis concolor) have been reported to be serologically positive for Trypanosoma cruzi [99-101]. In pumas, T. cruzi infection rates were shown to increase significantly with the number of vertebrate species present in their diet. Similarly, ocelots with a high dietary presence of a diverse assemblage of mammalian species were shown to be consistently infected as well [99], and more recently T. cruzi was identified in 3/21 (14.3%) of ocelots in a southern Texas population [102]. This study identified PCR-positive samples from muscle, heart tissue, and a blood clot from ocelot carcasses collected from roadway mortalities from 2010-2017 around Laguna Atascosa National Wildlife Refuge, with one individual showing anti-T. cruzi antibodies [102]. Although the parasite was identified, the clinical significance remains unknown. Over the last three years, T. cruzi antibodies were not identified in free-ranging Texas ocelots (A. Reeves, East Foundation, unpublished data, n=24) and a select number of samples dating from 1985 to 2012 (n=9) [A. Reeves et al., unpublished data, in prep]. Continuing to screen for Trypanosoma cruzi and potential negative side effects pre-mortem and post-mortem in ocelots could enhance the understanding of the clinical significance of T. cruzi in ocelots and dictate any necessary management of this disease.
T. cruzi, a zoonotic parasite, causes Chagas disease in humans and other mammalian species. The vector, a triatomine bug, must take a blood meal to ingest the trypomastigotes in the bloodstream of a given mammal, which was initially infected by oral ingestion of the infected bug’s feces, contaminated food products, or by consuming an infected insect or animal [92]. This parasite is endemic in the southern half of the US, including southern Texas, with well-established zoonotic cycles [93]. Although there is little known about T. cruzi in felines, a seroprevalence of ~ 30 % is reported in domestic cats in Mexico and Argentina [94-97]. In southern Texas, there are reports of 11.4% seropositivity in domestic cats [98]; however, little is known about the clinical progression of this disease in cats and further studies are needed to determine the clinical impact of this disease in feline species.
Sample Type and Testing
One mL of serum is transferred into a non-additive tube and placed on ice for shipping overnight to a partnered laboratory. If shipping is not performed on the same day, this sample should remain in the refrigerator. Antibodies to Trypanosoma cruzi are detected by indirect fluorescent antibody testing (IFA) with serial dilutions (https://tvmdl.tamu.edu/tests/trypanosoma-cruzi-ifa/).
Cytauxzoon felis
Cytauxzoon felis is the causative agent of the protozoal disease, cytauxzoonosis, which is transmitted among felids by ticks [103]. The schizogenous phase, or tissue phase, occurs after the tick has fed on a reservoir host (e.g., bobcats) and bites a new, uninfected host [104]. This phase develops in macrophages within the lungs, spleen, liver, and lymph nodes of an infected individual [105], and can progress to a chronic, erythrocytic (red blood cell) piroplasm phase and become established in the bloodstream of the individual [104-105]. The American dog tick, Dermacentor variabilis, and the lone star tick, Amblyomma americanum, have been confirmed as competent vectors of this disease in a laboratory setting, although the lone star tick is considered the primary vector of Cytauxzoon felis [104]. The range of infection of C. felis includes south-central, midcentral, and mid-Atlantic regions of the United States, which correlates with the range of the lone star tick [104]
Cytauxzoon felis was first reported in 1976 in a domestic cat in Missouri and since then, cases have been reported in other states, including Texas [105]. Infected domestic cats are known to suffer from severe and often fatal illness due to large amounts of parasitic replication, leading to obstructive blood flow, organ damage, and disseminated intravascular coagulation, making them a dead-end host [104, 106]. In non-domestic felids in the United States, C. felis infection has been reported in pumas [103], Florida panthers (Puma concolor coryi) [103], and bobcats [104], while outside of the United States, the parasite is reported in Iberian lynx [103] and other wild felid populations including ocelots, pumas, and jaguars (Panthera onca) [103]. Infected bobcats usually undergo a brief illness and then recover, becoming lifelong carriers and reservoir hosts, although there are reports of rare fatalities [104]. As the ocelot populations share much of their habitat in southern Texas with bobcats and may come into contact with feral domestic cats as well, investigation of the prevalence of infection in ocelots, the effect on their health, and possible sources of transmission would benefit ocelot conservation and health.
Sample Type and Testing
EDTA whole blood (minimum 0.5 mL) is used to create a blood smear for staining using the Wright-Giemsa Stain Solution (Thermo Scientific “Richard Allan Scientific”, Kalamazoo, Michigan, USA) that is examined using standard light microscopy at 400x, 500x, and 1000x magnification with oil for intracytoplasmic and intranuclear inclusions of parasitic origin. PCR testing will be performed using whole blood at a partnered laboratory
Coronavirus
Feline coronavirus (FCoV) is a common virus among domestic cat populations. Transmission is via the fecaloral route and is more common with a higher density of cats [107]. Lower prevalence has been found in stray and feral cats compared to companion animals due to lower population densities and less exposure to contaminated fecal material [107-111]. Cats are most likely to spread FCoV during the primary stage of infection (7-18 months) in which the level of viral shedding is highest [112]. Following the primary stage of infection, cats can become intermittent shedders (70-80%), consistent shedders (10-15%), or non-shedders (<5%) [112-114].
FCoV is divided into two biotypes: common asymptomatic or mild feline enteric coronavirus (FECV) and the possibly fatal feline infectious peritonitis virus (FIPV) [115-118]. FECV and FIPV are nearly identical antigenically and genetically dependent on the body system affected, but differ from other geographic isolates, leading to the assumption that FIPV arises from the FECV within an individual [115, 117-122]. With the FECV
biotype, there may be vomiting and diarrhea in a small proportion of adult cases and kittens [123]; however, most individuals remain asymptomatic. Cats infected with FCoV have a 5-12% chance of developing feline infectious peritonitis (FIP) which is a highly lethal systemic immune-mediated disease, characterized by the depletion of T-lymphocytes [107, 109, 124-127]. Of these, sexually intact males and young cats are at the greatest risk of developing FIP [128]. In addition, certain cat lineages [129-131], individuals with preexisting immunosuppressive conditions such as FIV or FeLV [125, 132-133], and those under stress are at greater risk [134].
There are two forms of FIP: non-effusive/dry form and an effusive/wet form [135-136]. The effusive/wet form presents abundant clear, protein-rich, straw-colored peritoneal effusions [137] and large amounts of thick exudative fluid abundant in fibrin, which causes a distended abdomen, and a perivascular inflammatory reaction [107,130]. The non-effusive/dry form has little to no effusion but is marked by perivascular granulomatous lesions with or without vasculitis in multiple organs [107]. As certain feline lineages have shown to have an increase in mutation of FECV to FIP, monitoring of ocelot populations as it pertains to the individual, lineage, and population should be considered.
In non-domestic felids, FCoV testing has been performed on multiple species, including cheetahs (Acinonyx jubatus), leopards, tigers (Panthera tigris), lions, lynx, ocelots, jaguars, and bobcats by fecal RT/nPCR and serum FCoV-specific antibodies [138]. Of the 75 individual felids tested, 24 of 72 (32%) were positive for FCoV using RT/nPCR and 29 of 63 (46%) were seropositive [138]. Of the species tested in this study, none of the ocelots tested positive by either test. However, because bobcats were among the positive individuals, FCoV should be monitored in ocelots in the wild and at the Ocelot Conservation Facility since bobcats live in proximity to ocelots in Texas, and theoretically, could share infectious agents with ocelots. Additionally, FIP has been described in multiple non-domestic feline species [139-148], and FCoV antibodies have been reported in two, free-ranging ocelots in Brazil [149-150].
Sample Type and Testing
For screening of cats that may be shedding the virus in feces, 2-5 grams of fresh feces of five consecutive fecal samples are submitted for reverse transcription polymerase chain reaction (RT-PCR) screening. For clinical cases, RT-PCR can be performed on abdominal effusion, whole blood, plasma, serum, or fresh tissues (if necropsy is being performed). The amount of fluid submitted should be 1-2 mL (abdominal effusion, whole blood, serum, or plasma) or 1-2 grams of fresh tissues. It is important to note that a positive test will diagnose infection with FCoV but does not differentiate between FIP and FCoV. The cat must be exhibiting signs of FIP to identify it. A positive FCoV test without clinical signs of FIP diagnoses a FCoV infection only. (https://www.vet.cornell.edu/animal-health-diagnostic-center/veterinary-support/disease-information/felinecoronavirus). Additionally, there are two types of coronaviruses (I and II) and the only laboratory that tests for antibodies against both virus types is the University of Tennessee (http://www.vet.utk.edu/diagnostic/virology/index.php). If the above samples are not available, a rectal swab can be substituted for testing.
Feline Calicivirus (FCV)
FCV is a highly contagious virus causing mild to severe respiratory infections and oral ulcers in domestic cats that occurs most commonly in shelters or areas of high density [151-152]. All members of the Felidae family are susceptible to FCV infection [153]. Most domestic cats will recover from a calicivirus infection, but in rare cases this disease can be fatal [151]. Several strains circulate in domestic and wild cat species and mutate
readily, leading to new strains with varying severity of disease that may compromise the effectiveness of vaccines [151, 154]. Rare outbreaks of mutant strains of FCV (e.g., FCV-associated virulent systemic disease or FCV-VSD) have been reported and cause serious disease with multi-system organ failure and death [151].
FCV spreads through direct contact with saliva, nasal mucus, ocular discharge, and aerosol droplets [151]. Shedding lasts around 2-3 weeks after infection, but some individuals will become long-term carriers and continue to shed on and off for months [151]. This virus is very hardy in the environment and can be spread by objects and personnel working with infected cats [151]. Transplacental infection has not been documented, but FCV has been isolated from aborted fetuses [155]. Symptoms can range from a mild cold (sneezing, nasal congestion, fever, drooling) to oral ulceration, lethargy, lameness, inappetence and even pneumonia [151]. Symptoms of FCV-VSD can be more severe including swelling of the head and legs, crusting sores, hair loss, and liver damage causing jaundice [151]. Severity of clinical signs vary depending on age, exposure route, concurrent infections, immune status of the host, and vaccination history [156].
Although serological detection has been reported in many wild cat species [65, 150, 157-160], including ocelots [161], RT-PCR detection of FCV was unsuccessful in 21 ocelot samples from 1999-2011 in Brazil [162]. However, serological detection of FCV was reported in areas where domestic cats were not present, suggesting FCV to be endemic in ocelot populations and not acquired from domestic cats in that region [160]. Clinically, there were no reports of physical ailments as it pertains to an infection with FCV or FCV-VSD in ocelots.
Given the population density of ocelots held at the Ocelot Conservation Facility and wild ocelots surviving in limited habitat space, the monitoring of this pathogen is important for future assessment of transmission and the health impact it may have on current or future populations. Given present difficulties in detection using RTPCR, identification of new sequences for detection should be explored or considered. Additionally, concurrent serological testing should be performed to differentiate between exposure and infection.
Sample Type and Testing
A Feline Calicivirus- RT-PCR may be conducted using a conjunctival or oral swab, or 1 g fresh tissue (biopsy, lesion) (https://tvmdl.tamu.edu/tests/feline-calicivirus-rtpcr/). Detection of antibodies requires 1 mL of serum (https://tvmdl.tamu.edu/tests/feline-calicivirus-vn/). It is important to note that commercial testing cannot distinguish mild strains of FCV from FCV-VSD. Detection of antibodies can be performed on serum, but presence of antibodies only confirms exposure, and not active infection [156] and should be performed with concurrent RT-PCR testing.
Feline Herpesvirus (FHV)
FHV is the agent of feline viral rhinotracheitis and although only one serotype is described, virulence can differ between strains [163]. In domestic felids, FHV replicates in the epithelial cells of the upper respiratory tract, neurons, and conjunctiva [164]. Neuronal infection allows the virus to establish a lifelong latency in the trigeminal ganglia after a primary infection [164]. This latent infection can undergo intermittent reactivation, creating viral shedding in oronasal and conjunctival secretions, which is mainly associated with stressful events [164]. Although related to other herpesviruses known to infect other species, cross-species transfer is not known to occur [165]. The domestic cat is the main host of FHV, but it has been documented in other felids such as cheetahs, lions, and pumas [164].
FHV is shed by cats with an acute infection or a latent infection that has been reactivated [166]. Although transplacental infection has not been documented, queens with a latent infection may transmit FHV to their
offspring as parturition and lactation create stress within the queen, leading to viral reactivation and subsequent shedding [164]. In domestic cats, risk is higher in shelters where close quarters and high numbers of cats can make disinfection and separation of viral shedders difficult. Once the virus has entered the nasal/oral cavity or conjunctiva, it can spread throughout the rest of the respiratory tract causing a transient viremia [163, 167], and viral excretion, beginning ~ 24 hours after infection and lasting one to three weeks [164].
Acute disease typically resolves in 10 to 14 days; however, some individuals may develop chronic lesions in the upper respiratory tract and/or ocular tissues [164]. Conjunctivitis can be associated with corneal ulcers, chronic sequestrum, stromal keratitis, and damage to the nasal turbinate’s leading to chronic rhinitis [163]. Clinical signs are associated with the disease type and many types have been described: classical acute disease (cytolytic), atypical acute disease, chronic immune-mediated disease, and possible FHV-related diseases [164]. Clinical signs can range from a mild upper respiratory infection with nasal discharges, sneezing, corneal ulcers, and dermatitis to pneumonia, coughing, fading kitten syndrome, and blindness, with disease typically more severe in kittens [164].
Most kittens acquire immunity via colostrum ingestion; however, this protection will wane between 6 to 10 weeks of age [168-169]. Additionally, natural infection does not result in complete immunity, protecting against disease but not infection, and clinical signs can return with re-infection, although generally mild [170]. The FHV vaccine for domestic cats provides protection against clinical signs and reduces viral shedding within a week of administration [171] but does not provide full protection [163]. Most vaccines are combined with FCV or additional agents in both modified-live and inactivated parenteral (injectable) vaccines; subunit FHV and modified intranasal vaccines also are available in many countries [164]. Two vaccinations, an initial injection and booster, are recommended for domestic feline kittens starting around nine weeks of age with a four-week interval, followed by annual boosters [164]. Other protocols from the American Association of Feline Practitioners recommend vaccinations beginning between 6 to 10 weeks with boosters every 3-4 weeks until 16 to 20 weeks for age (i.e., 8, 12 and 16 weeks)
Among non-domestic felids, cheetahs are particularly susceptible to FHV-1 (alpha herpesvirus) [172-174] and although clinical signs can be like those described previously, some infections can cause severe clinical disease and death, especially in young animals [175-177]. In studies of other non-domestic cat species, FHV is reported to be endemic in East African free-ranging lions [160] but was not detected in ocelots in the Bolivian Chaco [161].
Sample Type and Testing
The preferred method of detection is PCR (conventional, nested, and real-time) testing of conjunctival, corneal, or oropharyngeal swabs, corneal scraping, aqueous humor, corneal sequestra, blood or biopsy specimens [178187]. This test should be interpreted given observed clinical signs and is important to note that PCR tests can detect FHV DNA in modified-live vaccines [188]. A positive PCR result may indicate low level shedding or viral latency but does not necessarily correlate clinical signs with the actual viral infection [167]. When using quantitative real-time PCR [185], the amount of virus detected can suggest active replication and involvement of the virus in the present clinical signs. Recommended laboratories for serology (serum neutralization) testing is the Veterinary Diagnostic Laboratory at Cornell University (www.diaglab.vet.cornell.edu) and for PCR, the University of Tennessee College of Veterinary Medicine (http://www.vet.utk.edu/diagnostic/virology/index.php).
Canine Distemper Virus (CDV)
CDV is a highly contagious paramyxovirus seen in dogs worldwide [189]. Although domestic dogs are the reservoir hosts, many species are susceptible to infection, including large felids [189]. The main route of spread is via aerosol droplet secretions shed by infected animals for several months [189]. Clinically, a canine infected with CDV will present with a fever, leukopenia, gastrointestinal and respiratory signs, pneumonia, and neurologic signs [189]. Classic neurologic signs include muscle twitching, convulsions, salivation, and chewing movements of the jaw, and may include circling, a head tilt, nystagmus, paresis to paralysis, and seizures [189]. Nasal discharge is serous with an associated mucopurulent ocular discharge, lethargy, and anorexia [189]. If an animal survives the infection, it may have hyperkeratosis of the footpads and nasal planum and enamel hypoplasia of incompletely erupted teeth [189]. Since there is no curative treatment and only supportive care can be attempted, prevention of canine distemper is necessary by widespread vaccination of domestic dogs.
CDV has been reported in both wild and captive populations of nondomestic felids [190-193] and recent identification of this virus in wild populations of Amur tigers (Panthera tigris altaica) present conservation concerns for this endangered felid [194-196]. CDV vaccination is currently effective at managing infection in domestic dogs and other species [192, 197], and is recommended for tigers in captive settings [198]. The PureVax Ferret Distemper vaccine, a monovalent canarypox-vectored canine distemper vaccine has been demonstrated to be effective and safe in several wild carnivore species [199-201]. Although parenteral vaccination may be the most effective way to manage this virus in captive animals, another vaccine delivery method may be more effective for widespread vaccination of wild animals. Similar to the oral-transmucosal rabies vaccination method used for raccoon rabies in the eastern United States, a recent study sought to examine the immune response of tigers following parenteral and oral-transmucosal vaccination. Their results showed a poor serologic response to this vaccine using both administration routes [202]. Similarly, other vaccines have shown similar poor serological responses in tigers [203]. Both papers suggest these results could be due to a dose-dependent effect when comparing tigers to domestic cats as they were given the same standard vaccine dose across highly differing body weights [202]. The best vaccination route for CDV prevention in the wild is still being explored in wild cats, with large felids apparently the main species of Felidae that are susceptible to this virus.
In ocelots, a few studies have assessed the prevalence of CDV in free-ranging populations by various methods: serology of 10 ocelots reported 7/10 samples were seropositive for CDV (70%) in the Bolivian Chaco [161], serology of two free-ranging ocelots reported 0/2 seropositive samples (0%) in Brazil [204], and 3/71 freeranging ocelots in Costa Rica tested positive by fecal RT-PCR [205]. However, there were no reports of clinical illness associated with the positive tests where the individual underwent a concurrent physical examination. Small cats are not thought to be susceptible to disease caused by CDV, but some may seroconvert once infected [206]. Although unlikely to cause illness in small to medium-sized felids, this virus has the potential to cause detrimental effects to populations. Additionally, a decline in genetic diversity and heterozygosity could present a situation in which a typically asymptomatic disease could impact an immunocompromised individual or population. Screening for this virus is recommended to assess the potential for introduction into a potentially naïve population.
Sample Type and Testing
One mL of serum in a plain red top tube should be submitted for serology using the serum neutralization assay for CDV. Cornell Diagnostic Laboratory has utilized the laboratory assay to diagnose CDV in felids and multiple other species. Additionally, Canine Distemper PCR can be performed to detect acute infection in
individuals with clinical disease. This test recommends using 5 mL of urine, 1 gram of tissue, or a swab of nasal or ocular discharge. The swab must be placed into a non-additive tube with a few drops of sterile saline to prevent desiccation (www.diaglab.vet.cornell.edu).
COVID-19
COVID-19 (SARS-CoV-2) is a recently identified coronavirus that is differentiated from the canine coronavirus (CCoV) or feline coronavirus (FCoV) [207]. Although humans can infect cats and dogs with the same coronavirus that causes COVID-19 in humans, there is little evidence that animals can infect humans with this same virus [207], except for transmission from mink (Neovison vison) to humans reported in the Netherlands and Denmark [208-209]. However, there is no evidence that dogs and cats can infect humans at this time. Dogs infected with this virus do not appear to show clinical signs of illness; however, some domestic cats have demonstrated mild illness with respiratory and gastrointestinal symptoms [207]. However, many other diseases can cause similar symptoms, such as fever, coughing, difficulty breathing, lethargy, sneezing, nasal discharge, ocular discharge, vomiting and/or diarrhea [210]. At this time, routine testing of companion animals is not recommended; however, testing may be recommended on a case-by-case basis [207].
Non-domestic animals and wildlife species that have been reported to be infected with the virus include several cat species [211]. Most of these cases were reported following contact with people infected with COVID-19, including owners, caretakers, or others in close proximity to the cats [211]. Additionally, cats have been shown to be able to transmit this virus among other individuals of the same species [211]. In the United States, several large cat species have become infected with SARS-CoV-2 including tigers, lions, Amur leopards (Panthera pardus orientalis), snow leopards (Panthera uncia), pumas and a fishing cat (Prionailurus viverrinus) after being exposed to people with COVID-19 [212]. At this time, SARS-CoV-2 fatalities have been confirmed in a lion and several snow leopards [212]. The Felid Taxon Advisory Group (TAG) supports vaccination of all nondomestic felids with the new Zoetis vaccine specifically formulated for vaccinating at-risk non-domestic species in zoological collections [212]; however, efficacy of this vaccine has not been established due to its recent availability.
While most cases of SARS-CoV-2 infection in cats (domestic and non-domestic) have been mild and most have recovered with supportive care, the risk of transmission and significant disease remains. The startling deaths of multiple snow leopards and increase in infections at captive facilities raises concern for ocelots in captive settings, although infection of ocelots with SARS-CoV-2 is yet to be documented. Monitoring ocelots in the Ocelot Conservation Facility for infection with this virus, as well as transmission from personnel, will be vital to ensure viruses are not shared among species or introduced into the wild, where supportive care would not be possible.
Sample Type and Testing [208]
Concurrent antibody and PCR testing are recommended to determine exposure versus active infection. PCR testing can be performed on swabs of the respiratory tract (nasal swab or oropharyngeal swab) and/or rectal swabs. Antibody testing can be performed on serum.
Panleukopenia
Feline panleukopenia virus (FPLV; also known as feline distemper or feline parvo) is a highly contagious parvovirus of cats (domestic and non-domestic) [213-214]. Although historically the leading cause of death in cats, the availability and use of effective vaccines have made the disease uncommon today [213]. Kittens are
most affected by the virus, as it kills cells that are rapidly growing and dividing, such as bone marrow, intestinal cells, and developing fetuses [213]. This virus is present in the environment, and virtually all felines (adult and kittens) are exposed at some point in their lives [213]. Kennels, pet shops, shelters, and unvaccinated feral cat colonies are the main reservoirs of FPLV, and it is seen more commonly in high density areas [213].
This virus is shed in the urine, stool and nasal secretions of infected cats and can transmit rapidly when another uninfected individual encounters these infected secretions [213]. Although shedding is short-lived (one to two days), the virus can survive up to a year in the environment, infecting individuals without direct contact with another infected cat [213, 215]. The virus causes damage to the intestinal lining, bone marrow and lymph nodes resulting in decreases in all red and white blood cell types and causing panleukopenia and anemia [213, 216]. Clinical signs include depression, loss of appetite, high fever, lethargy, vomiting, severe diarrhea, nasal discharge, and dehydration [213, 216]. In pregnant females, abortion can occur or kittens may have cerebellar damage causing incoordination (cerebellar ataxia) presenting as severe tremors or shaking [213, 216]. There are no current medications to successfully kill this virus, so the mainstay of treatment is supportive care while the individual’s immune system fights the virus, as with many other viral infections. Strict isolation from other cats is required due to the high transmissibility of this virus.
FPLV-like viruses have been documented in many non-domestic felid species including the cheetah [217], European wild cat [218], lion [219], African wild cat (Felis lybica), ocelot, puma, Amur tiger and southern tigrina (Leopardus tigrinus) [150], and Eurasian lynx (Lynx lynx) [243], bobcat [241], and Iberian lynx [242243] in which mortality events were linked to FPLV infection. Although strains can differ between species as this virus can mutate within the body of the host, an outbreak of FPLV in cheetah cubs involved a previously unreported strain of FPLV that was also found in a deceased lion, African black footed cat (Felis nigripes), caracal (Caracal caracal), ocelot and serval (Leptailurus serval) [214]. The likelihood of mutation and transmission increases concern over introductions into wild populations from captive felines and vice versa. Detection of this pathogen can be challenging for diagnosis due to the short-lived shedding and identical presentation to other viral diseases. Typically, a presumptive diagnosis is made from clinical signs, blood work abnormalities and lack of vaccination, prior to a laboratory diagnosis.
Sample Type and Testing
Diagnosis is typically made based on clinical signs, leukopenia on the complete blood count, and lack of vaccination. Fecal antigen and/or PCR testing is the recommended test for this pathogen, but the virus is only detectable for a short time and false negatives are common. Additionally, false positives can occur if vaccinated within 5-12 days of the test. An antibody test is available; however, it only documents exposure and may result in a false positive due to vaccination.
Canine heartworm
Dirofilaria immitis (canine heartworm) antibody testing may be performed prior to release of an ocelot as well as opportunistically during other physical exams to screen for this pathogen. Monthly preventative for canine heartworm is not possible, so infection with canine heartworm must be tested for. For captive individuals that will not be released, heartworm tests can be performed with the general blood screening conducted during physical examinations or sooner if warranted.
Highly Pathogenic Avian Influenza Virus
Influenza viruses, including Highly Pathogenic Avian Influenza Virus (HPAI), have been reported in many domestic and non-domestic species. In domestic and captive cats, the World Health Organization (WHO) reported HPAI H5N1 strain in domestic cats in Thailand in 2004 and in at least one case the cat was known to be in contact with deceased chickens (see https://www.who.int/.) Laboratory studies confirmed domestic cat susceptibility though experimental inoculation, through feeding of birds infected with H5N1 avian influenza viruses, or through horizontal transmission between cats [244-246]. Not all infections caused severe disease or death, and some subclinical infections were apparent [247]. In laboratory studies, challenging cats with an attenuated strain of H5N6 low pathogenic avian influenza virus (LPAI) resulted in subclinical infections and viral shedding and further protected them against HPAI H5N1 viruses [248-249], suggesting a level of crossstrain protection [250]). In the United States, an outbreak occurred in 2016 among shelter cats in New York [251-252]. This virus most closely matched the avian H7N2 virus that has circulated in New York historically, and compared to the avian lineage, the feline isolate showed improved replication in mammalian cell lines [253]. Disease in these cases were mild [254].
In non-domestic cat species, further cases in Thailand in 2004 were documented in captive tigers and leopards who were fed infected chickens [255] although some tigers became infected even without consuming chickens, suggesting cat-cat transmission [256]. Many other species have been observed with influenza within the order Carnivora, including ferrets [257-258], minks [259-261], and seals and related Pinnipeds [262-264].
In 2022 and 2023, HPAI began emerging in many wild carnivore species in the United States, including many wild cats. In the United States, there have been cases reported in Amur leopards (n=1; NY), Amur tigers (n=1; NE), bobcats (n=6; WI, WA, CA, CO), and mountain lions (n=16; NE, CO, WY, CA, MT, UT) (https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/avian/avianinfluenza/hpai-2022/2022-hpai-mammals; https://www.cdc.gov/flu/avianflu/spotlights/2022-2023/h5n1technical-report.htm#infections-among-mammals). In December 2022, a bobcat’s remains were collected in California and cause of death was confirmed as a result of HPAI H5N1 (https://wildlife.ca.gov/News/avianinfluenza-detected-in-deceased-bobcat#gsc.tab=0). Although two mountain lions found deceased in December 2022 and January 2023 tested positive for the pathogen (https://wildlife.ca.gov/News/avian-influenza-detectedin-deceased-mountain-lions#gsc.tab=0), HPAI has not yet been ruled the sole cause of death. Although reports of infection have been reported in many cat species thus far, these cases are the most recent reports of death caused by the current outbreak. Currently, infection of wild mammals with HPAI appears to be rare with periodic detections throughout the United States. The main route of infection in mammals appears to be through infected prey items, specifically wild birds. The monitoring and testing of bird prey items is recommended where direct ingestion could be possible. Where direct ingestion is not possible, aerosolized transmission from birds has not yet been shown to be an effective mode of HPAI transmission.
Sample Type and Testing
In poultry prey items, the detection of HPAI can be monitored using the Applied Biosystems™ VetMAX™Gold AIV Detection Kit. The Thermo Fisher Scientific USDA-licensed avian influenza diagnostic kit is widely available for testing and provides a qualitative, one-step, real-time RT-PCR assay to detect AI virus (AIV) in RNA isolated from individual poultry oropharyngeal/tracheal swab samples.
In felines, it is recommended to collect antemortem nasal and oropharyngeal swabs for testing. In the event of mortality, nasal, oropharyngeal, tracheal, intestinal, rectal, and tissue swabs should be collected and placed in viral transport media and tissue samples should be stored refrigerated or frozen were fixed in 10% neutral
buffered formalin [265]. Samples can be submitted to the National Veterinary Services Laboratory (NVSL) in Ames, Iowa. Sample collection and storage should be in accordance with NVSL guidelines: https://www.aphis.usda.gov/animal_health/lab_info_services/downloads/WIAV0020.pdf.
Other Vector Borne Pathogens
(Ehrlichia, Babesia, Rickettsia rickettsia, Hepatozoon felis, Anaplasma spp)
Various vector-borne pathogens have been identified in several mammalian species, with few causing disease [220]. Although they may not cause significant disease in certain animals, many vector-borne pathogens carry a zoonotic concern for humans, specifically A. phagocytophilum, Ehrlichia chaffeensis, Ehrlichia ewingii, and Rickettsia conorii, ricketsii, felis, and typhi [220] Anaplasma, Ehrlichia and Rickettsia genera are vector-born members of the Rickettsiales order infecting humans and many domestic and non-domestic animals worldwide [221]. Among these pathogens, little information is available on the pathogenesis of these agents in cats, with Anaplasma phagocytophilum being the most important pathogen for cats [220]. Table 14 shows current areas where these pathogens have been noted in cats around the world [220, 222]. Although many of these pathogens have not been detected in cats in the United States, the table only lists published reports, and it is likely these pathogens exist in countries that have not published the data or are not currently testing for the pathogens.
Most tick-borne illnesses will present with similar clinical signs so testing for the pathogen is the only way to discern the causative agent. Little information is available on the pathogenesis of these agents in cats; however, mild reductions in white blood cell counts, red blood cell counts, and liver values have been detected [223]. Other domestic cat cases with vector-borne infections, following experimental exposure, only showed a transient lymphopenia during 13 weeks of observation after tick infestation with a normal general appearance, appetite, body temperature and cell blood count otherwise reported [224]. Other clinical signs reported in cats can range from thrombocytopenia, joint swelling, fever, anorexia, anemia, dehydration, lethargy, epistaxis, and pain on abdominal palpation [225-231]. The main indication for diagnosis of a Rickettsial disease is a febrile cat exposed to ticks in an endemic area of the pathogen, especially outdoor or free-ranging felids that cannot be protected by ectoparasiticides. Certain antibiotics, such as doxycycline and similar compounds, are the recommended therapy for treating these infections [220]. Otherwise, supportive care is indicated.
Hepatozoonosis of domestic cats has been reported in many countries, however infection is often subclinical. Although the vector for domestic cats is unknown, it is suspected to be the same vector as documented in canines (Table 8) and the infection has been described in the same regions where canine infection is documented [222]. Transplacental transmission of H. felis has been suggested and could be an important route of transmission [232]. At this time, the pathogenesis has not been described in cats. Although the infection of myocardial and skeletal muscle is common, the infection does not lead to significant inflammatory reactions, so clinical signs are rarely noted [232-234]. No specific treatment is recommended but a case in a domestic cat has been successfully treated with imidocarb and doxycycline [222, 235]. Babesia is not currently reported in cats in the United States; however, although the vector is unknown, it is assumed to be transmitted by the same tick species as canine Babesiosis (Dermacentor reticulatus; Rhipicephalus sanguineus and Haemaphysalis leachi).
Although most of the vector-borne pathogens discussed previously do not produce clinical disease in cats or have not been documented in the United States, screening of these pathogens intermittently for their arrival provides important information regarding the spread of emerging pathogens and diseases. This testing becomes especially important when translocating individuals from a country with endemic disease to a country with no known detection of such disease, pathogen, or vector of causative agents.
Since 2019 Babesia spp. and Rickettsia spp. (PCR, gel electrophoresis) were not identified in free-ranging ocelots in Texas (n=22, A. Reeves, East Foundation, unpublished data) nor a select number of samples dating from 1985 to 2012 (n=9) while Hepatozoon spp. (5/22; 22.7%) was identified in ocelots in Texas and a select number of samples dating from 1985 to 2012 (2/9, 22.2%) [A. Reeves et al, unpublished data, in prep.]. Leishmania spp. (2/22; 9%) was identified in ocelot populations in Texas, however, it was not identified prior to 2021 [A. Reeves, East Foundation, unpublished data]. For some of the listed species, continuing to screen for emergence within the United States could provide information about emerging infectious pathogens. For others, the clinical significance remains unknown as these were one time captures and a snapshot in time of the individual’s health. It is important to note the pathogens that released individuals may be exposed to and screen for negative impacts to their health.
Pathogen Genus and Species
E. canis
Countries of Detection
Genus Ehrlichia
Canada, USA, Brazil, Portugal
E. chaffeensis USA, Brazil
E. ewingii USA
Ehrlichia spp. Italy, USA, Kenia, France
Genus Anaplasma
Vector
The brown dog tick (Rhipicephalus sanguineus), the lone star tick (Amblyomma americanum), and the blacklegged tick (Ixodes scapularis)
A. phagocytophilum USA, Sweden, Finland, Poland, Switzerland, Germany, Italy, Spain Ixodes ricinus
A. platys USA Brazil Rhipicephalus sanguineus (suspected)
A. platys-like Italy
A. bovis Japan
Genus Rickettsia
R. rickettsii USA
Dermacentor andersoni (W) D. variabilis (MW and E)
R. conorii Spain Rhipicephalus sanguineus
R. massiliae Spain
Rickettsia spp. Italy
Hepatozoon spp. India, South Africa, Nigeria, USA, Brazil, Israel, Spain, France, Portugal, Italy,
Rhipicephalus sanguineus (brown dog tick) suspected; but unknown in felines
Table 14: Rickettsial and Vector-Borne Pathogens, Countries of Detection in Cats, and Known Vectors
Turkey, Cape Verde archipelago, Cyprus, Switzerland, Austria
Sample Type and Testing [220- 222]
Both Anaplasma and Ehrlichia spp. give rise to cytoplasmic inclusion bodies: small elementary bodies (0.2-0.4 μm diameter) and larger reticulate bodies and morulae (up to 2-6 μm). These inclusion bodies are mostly found in neutrophils. Antibodies to rickettsial infections can be detected by IFA and ELISA; however, cross-reaction between other species can occur, antibody detection may not be possible early in infection, and some cats may not have yet seroconverted. Blood PCR analysis is a sensitive and specific method for confirming the diagnosis at the onset of acute clinical disease before starting therapy; however, this early infection will likely result in a negative antibody test. The use of genus-inclusive primers is suggested, followed by sequencing of any resulting PCR products to determine the infecting species. The diagnosis of hepatozoonosis in cats can be made by observation of the parasite gamonts in blood smears within the neutrophils and monocytes, meronts in muscles by histopathology and detection of parasite DNA in blood and tissue by PCR.
Quarantine
Any ocelots entering the Ocelot Conservation Facility from an AZA or similar institution or from the wild will have a pre-entry health examination, a quarantine period, and a post-quarantine examination prior to any future transfer and/or contact with other ocelots in the Ocelot Conservation Facility or in the wild. When possible, ocelots should be tested for disease prior to transfer to the Ocelot Conservation Facility. Disease testing may not be possible for transfer of ocelots originating from the wild, and supervising veterinarians at the Ocelot Conservation Facility will have discretion on whether to accept an ocelot to the Ocelot Conservation Facility based on its origin and available disease testing results. Upon an individual’s arrival to the Ocelot Conservation Facility, the minimum components of the quarantine examination (arrival exam) should include a physical examination under anesthesia, weight, blood collection (for use in CBC, blood chemistry, disease, and genetic testing), confirmation or insertion of a permanent identification applied (PIT tag), and fecal exam. Additionally, recommended components include a urinalysis, thoracic and abdominal radiographs, and ultrasound exam. After the arrival exam, quarantine is important to protect resident animals from contracting disease from outside sources. Quarantine at the Ocelot Conservation Facility must occur in an area separate from other ocelots. There must be proper sanitation plus footbaths and removable clothing for staff to use prior to working at quarantine facilities. Quarantine length will be determined based on individual circumstance and the results of health testing. The minimum length of time separated from the main population is 30 days, and quarantine may occur for up to 45 days, or longer. At the end of the quarantine period, the same required components of the prearrival exam should be repeated, with the exception of sample collection for genetic testing and placement of a PIT tag.
References
[1] American Association for Clinical Chemistry. Lab Tests Online: Complete Blood Count (CBC). 2015. Available at: https://labtestsonline.org/tests/complete-blood-count-cbc. Accessed April 1, 2021.
[2] Canadian Cancer Society: Complete blood count (CBC). 2021. Available at: https://www.cancer.ca/en/cancernformation/diagnosis-and-treatment/tests-and-procedures/complete-blood-count-cbc/?region=on. Accessed April 1, 2021.
[3] American Association for Clinical Chemistry. Lab Tests Online: Chemistry Panels. 2014. Available at: https://labtestsonline.org/tests/chemistry-panels. Accessed April 1, 2021.
[4] Canadian Cancer Society: Blood chemistry tests. 2021. Available at: https://www.cancer.ca/en/cancer-information/diagnosis-andtreatment/tests-and-procedures/blood-chemistry-tests/?region=on. Accessed April 1, 2021.
[5] Widmer CE, Matushima ER, de Azevedo FCC. 2016. Clinical Evaluation, Hematology, and Serum Chemistry of Ocelots (Leopardus pardalis) in the Atlantic Forest of Brazil. Journal of Wildlife Diseases. 52(4): 916-621.
[6] ISIS (International Species Information System). 2002. Ocelot (Leopardus pardalis) physiological reference ranges (both sexes, all ages). ISIS, Apple Valley, Minnesota.
[7] English AW, Lepherd EE. 1981. The haematology and serum biochemistry of wild fallow deer (Dama) in New South Wales. Journal of Wildlife Diseases 17(2): 289-95.
[8] Huber D, Kusak J, Zvorc Z, Rafaj RB. 1997. Effects of sex, age, capturing method, and season on serum chemistry values of brown bears in Croatia. Journal of Wildlife Diseases 33(4): 790-94.
[9] Broughton H, Govender D, Shikwambana P, Chappell P, Jolles AE. 2017. Bridging gaps between zoo and wildlife medicine: establishing normal reference ranges for free-ranging African lions (Panthera leo). Journal of Zoo Wildlife Medicine 48(2): 298-311.
[10] Moen R, Rasmussen JM, Burdett CL, Pelican KM. 2010. Hematology, serum chemistry, and body mass of free-ranging and captive Canada lynx in Minnesota. Journal of Wildlife Diseases 46(1): 13-22.
[11] Constable P, Hinchcliff K, Demma N, Callahan M, Dale B, Fox K, Adams L, Wack R, Kramer L. 1998. Serum biochemistry of captive and free-ranging gray wolves (Canis lupus). Journal of Zoo Wildlife Medicine 29(4): 435-440. PMID: 10065853.
[12] Couch CE, Movius MA, Jolles AE, Gorman ME, Rigas JD, Beechler BR, Serrano Ferron E. 2017. Serum biochemistry panels in African buffalo: Defining reference intervals and assessing variability across season, age and sex. PLos ONE. 12 (5).
[13] Borjesson DL, Christopher MM, Boyce WM. 2009. Biochemical and hematologic reference intervals for free-ranging desert bighorn sheep. Journal of Wildlife Diseases 36(2): 294-300.
[14] Bush M, Smith EE, Custer RS. 1981. Hematology and serum chemistry values for captive dorcas gazelles: variations with sex, age and health status. Journal of Wildlife Diseases 17(1): 135-43.
[15] Doornenbal H, Tong AK, Murray NL. 1988. Reference values of blood parameters in beef cattle of different ages and stages of lactation. Canadian Journal of Veterinary Research 52(1): 99.
[16] Marler RJ. 1975. Some hematologic and blood chemistry values in two herds of American bison in Kansas. Journal of Wildlife Diseases 11(1): 97-100.
[17] Dodham J. 2020. Anesthetic monitoring: blood pressure (direct pressure). Felis ISSN 2398-2950. Vetstream Ltd https://www.vetstream.com/treat/felis/freeform/anesthetic-monitoring-blood-pressure-(direct-pressure).
[18] Robertson SA, Gogolski SM, Pascoe P, Shafford HL, Sager J, Griffenhagen GM. 2018. AAFP Feline Anesthesia Guidelines. Journal of Feline Medicine and Surgery 20: 602-634.
[19] Barnette C. 2020. Veterinary Anesthesia Monitoring: Cheat Sheet and FAQs. Dispomed.
[20] Marti I, Ryser-Degiorgis MP. (2018) A tooth wear scoring scheme for age estimation of the Eurasian lynx (Lynx lynx) under field conditions. European Journal of Wildlife Research 64: 37.
[21] Aiello SE, Moses MA, editors. 2012a. The Merck veterinary manual online. Hematologic reference ranges. Merck, Sharp, and Dohme Corp. New York, New York.
http://www.merckvetmanual.com/mvm/appendixes/reference_guides/hematologic_reference_ranges.html. Accessed December 2021.
[22] Aiello SE, Moses MA, editors. 2012b. The Merck veterinary manual online. Serum biochemical reference ranges. Merck, Sharp, and Dohme Corp. New York, New York. http://www.merckvetmanual.com/mvm/appendixes/reference_guides/serum_biochemical_reference_ranges.html. Accessed December 2021
[23] DeNicola DB, Cowell RL, Frye M. 2014. Urine Sediment Guide. IDEXX Laboratories, Inc. 09-80958-00. https://www.idexx.com/files/urine-sediment-guide.pdf
[24] ECLINPATH. Cornell University College of Veterinary Medicine. 2020. https://eclinpath.com/urinalysis/.
[25] Chung PH. 2020. Urinalysis and Urine Culture in Merck Manual Consumer Version. https://www.merckmanuals.com/home/kidney-and-urinary-tract-disorders/diagnosis-of-kidney-and-urinary-tract-disorders/urinalysisand-urine-culture
[26] Callealta I, Ganswindt A, Lueders I. 2020. Reproductive cycle stage assessment using vaginal cytology evaluation in African lions (Panthera leo). Animal Reproduction Science 213: 106260
[27] Raju K. 2016. Evolution of Pap Stain. Biomedpress 3(2): 490-500.
[28] Kutzler, MA, Yeager, AE, Mohammed, HO and Meyers-Wallen, VN. 2003. Accuracy of canine parturition date prediction using fetal measurements obtained by ultrasonography. Theriogenology 60: 1309-1317.
[29] Nyland, TG and Mattoon, JS. 1995. Veterinary diagnostic ultrasound. W.B. Saunders Company, Philadelphia.
[30] O’Brien, R and Barr, F (Eds) 2009. BSAVA Manual of canine and feline abdominal imaging. British Small Animal Veterinary Association, Gloucester.
[31] Mondolfi E. 1986. Notes on the biology and status of the small wild cats in Venezuela. In: Miller SD, Everett DD (Eds.). Cats of the world: biology, conservation and management. Washington, D.C.: National Wildlife Federation. pp. 85-124.
[32] Goodrowe KL, Howard JG, Schmidt PM, Wildt DE. 1989. Reproductive biology of the domestic cat with special reference to endocrinology, sperm function and in-vitro fertilization. Journal of Reproduction and Fertility Supplement 39: 73-90. PMID: 2695644
[33] Wildt DE, Chan SYW, Seager SWJ, Chakrabory PK. 1981. Ovarian activity, circulating hormones, and sexual behavior in the cat. I. Relationships during the coitus-induced luteal phase and the estrous period without mating. Biology of Reproduction 25: 15–28.
[34] Schmidt AM, Nadal LA, Schmidt MJ, Beamer NB. 1979. Serum concentrations of oestradiol and progesterone during the normal oestrous cycle and early pregnancy in the lion (Panthera leo). Journal of Reproduction and Fertility 57: 267-72.
[35] Schmidt PM, Chakraborty PK, Wildt DE. 1983. Ovarian activity, circulating hormones and sexual behavior in the cat. II. Relationships during pregnancy, parturition, lactation and the postpartum estrus. Biology of Reproduction 28(3): 657–71.
[36] Schmidt AM, Hess DL, Schmidt MJ, Lewis CR. 1993. Serum concentrations of oestradiol and progesterone and frequency of sexual behavious during the normal oestrous cycle in the snow leopard (Panthera uncia). Journal of Reproduction and Fertility 98: 91-5.
[37] Bonney RC, Moore HDM, Jones D. 1981. Plasma concentrations of oestradiol-17β and progesterone, and laparoscopic observations of the ovary in the puma (Felis concolor) during oestrus, pseudopregnancy and pregnancy. Journal of Reproduction and Fertility. 63: 523-31.
[38] Seal US, Plotka ED, Smith JD, Wright FH, Reindl N, Taylor RS, Seal MF. 1985. Immunoreactive luteinizing hormone, estradiol, progesterone, testosterone, and androstenedione levels during the breeding season and anestrus in Siberian tigers. Biology of Reproduction 32: 361-8.
[39] Schramm RD, Briggs MB, Reeves JJ. 1995. Spontaneous and induced ovulation in the lion (Panthera leo). Zoo Biology 13: 3017.
[40] Brown JL. 2006. Comparative endocrinology of domestic and nondomestic felids. Theriogenology. 66:25-36.
[41] Lasley BL, Kirkpatrick JF. 1991. Monitoring ovarian function in captive and free-ranging wildlife by means of urinary and fecal steroids. JJournal of Zoo and Wildlife Medicine. 22: 23-31.
[42] Shille VM, Wing AE, Lasley BL, Banks JA. 1984. Excretion of radiolabeled estradiol in the cat (Felis catus, L): a preliminary report. Zoo Biology 3: 201-209.
[43] Shille VM, Haggerty MA, Shackleton C, Lasley BL. 1990. Metabolites of estradiol in serum, bile, intestine and feces of the domestic cat (Felis catus). Theriogenology. 34: 77994.
[44] Brown JL, Wasser SK, Wildt DE, Graham LH. 1994. Comparative aspects of steroid hormone metabolism and ovarian activity in felids, measured noninvasively in feces. Biology of Reproduction 51(4): 776–86.
[45] Moreira N, Monteiro-Filho ELA, Moraes W, Swanson WF, Graham LH, Pasquali OL, Gomes MLF, Morais RN, Wildt DE, Brown JL. 2001. Reproductive steroid hormones and ovarian activity in felids of the Leopardus genus. Zoo Biology 20: 103-16.
[46] Braun BC, Frank A, Dehnhard M, Voigt CC, Vargas A, Goritz F, Jewkenow K. 2009. Pregnancy diagnosis in urine of Iberian lynx (Lynx pardinus). Theriogenology 71:754-761.
[47] Brown JL, Graham LH, Wu J, Collins D, Swanson WF. 2002. Reproductive endocrine responses to photoperiod and exogenous gonadotropins in the Pallas’ cat (Otocolobus manul). Zoo Biology 21: 347-64.
[48] Brown JL, Wildt DE, Graham LH, Byers AP, Barrett S, Howard JG. 1995. Comparison of natural versus chorionic gonadotropininduced ovarian responses in the clouded leopard (Neofelis nebulosa) assessed by fecal steroids. Biology of Reproduction 53: 93-102.
[49] Brown JL, Wildt DE, Wielebnowski N, Goodrowe KL, Graham LH, Wells S, Howard JG. 1996. Reproductive activity in captive female cheetahs (Acinonyx jubatus) assessed by faecal steroids. Journal of Reproduction and Fertility 106: 337-46.
[50] Johnston SD, Root Kustritz MV, Olson PNS. 2001. Vaginal Cytology. In: Johnston SD, Root Kustritz MV, Olson PNS (Eds.), Canine and Feline Theriogenology, first ed. Saunders, Philadelphia. 32-40.
[51] Ammersbach M, Bienzle D. 2011. Methods for assessing feline immunodeficiency virus infection, infectivity and purification. Veterinary Immunology and Immunopathology 143(3): 202–214.
[52] Little S, Levy J, Hartmann K, Hofmann-Lehmann R, Hosie M, Olah G, Denis K. 2020. AAFP Feline Retrovirus Testing and Management Guidelines. Journal of Feline Medicine and Surgery 22(1): 5–30.
[53] Sacristán I, Acuña F, Aguilar E, García S, López MJ, Cabello J, Hildalgo-Hermoso E, Sanderson J, Terio KA, Barrs V, Beatty J, Johnson WE, Millán J, Poulin E, Napolitano C 2021. Cross-species transmission of retroviruses among domestic and wild felids in human-occupied landscapes in Chile. Evolutionary Applications 14: 1070–1082.
[54] Franklin SP, Kays RW, Moreno R, TerWee JA, Troyer JL, VandeWoude S. 2008. Ocelots on Barro Colorado Island Are Infected with Feline Immunodeficiency Virus but Not Other Common Feline and Canine Viruses.Journal of Wildlife Diseases 44(3): 760–765.
[55] Gomerčić T, Perharić M, Kusak J, Slijepčević V, Starešina V, Stevanović V, Mojcec V, Toplicanec I, Sindičić M. 2021. A retroviral survey of endangered Eurasian lynx (Lynx lynx) from Croatia. Veterinarski arhiv. 91(1): 65-71.
[56] Filoni C, Adania CH, Durigon EL, Catão-Dias JL. 2003. Serosurvey for feline leukemia virus and lentiviruses in captive small neotropic felids in São Paulo state, Brazil. Journal of Zoo and Wildlife Medicine 34(1): 65-68.
[57] Zhang Z, Gu Q, Marino D, Lee KL, Kong IK, Häussinger D, Münk C. 2018. Feline APOBEC3s, Barriers to Cross-Species Transmission of FIV? Viruses 10(4): 186.
[58] Troyer JL, VandeWoude S, Pecon-Slattery J, McIntosh C, Franklin S, Antunes A, Johnson W, O’Brien SJ. 2008. FIV crossspecies transmission: an evolutionary prospective. Veterinary Immunology and Immunopathology 123(1-2):159-166.
[59] VandeWoude S, Troyer J, Poss M. 2010. Restrictions to cross-species transmission of lentiviral infection gleaned from studies of FIV. Veterinary Immunology and Immunopathology 134, 25–32.
[60] Tandon R, Cattori V, Willi B, Lutz H, Hofmann-Lehmann R. 2008. Quantification of endogenous and exogenous feline leukemia virus sequences by real-time PCR assays. Veterinary Immunology and Immunopathology. 123(1-2): 129–133.
[61] Hartmann K. 2012. Clinical aspects of feline retroviruses: a review. Viruses. 4(11): 2684-710.
[62] Burling AN, Levy JK, Scott HM, Crandall MM, Tucker SJ, Wood EG, Foster JD. 2017. Seroprevalences of feline leukemia virus and feline immunodeficiency virus infection in cats in the United States and Canada and risk factors for seropositivity. Journal of the American Veterinary Medical Association 251: 187–194.
[63] Cunningham MW, Brown MA, Shindle DB, Terrell SP, Hayes KA, Ferree BC, McBride RT, Blankenship EL, Jansen D, Citino SB, Roelke ME, Kiltie RA, Troyer JL, O’Brien SJ. 2008. Epizootiology and management of feline leukemia virus in the Florida puma. J. Wildl. Dis. 44: 537-552.
[64] Kennedy-Stoskopf S. 1999. Emerging viral infections in large cats. In: Zoo and Wild Animal Medicine (Fowler, M. E., R. E. Miller, Eds.), W. B. Saunders, Philadelphia. 401-410
[65] Leutenegger CM, Hofmann-Lehmann R, Riols C, Liberek M, Worel G, Lups P, Fehr D, Hartmann M, Weilenmann P, Lutz H. 1999. Viral infections in free-living populations of the European wildcat. J. Wildl. Dis. 35: 678–686.
[66] Brown MA, Cunningham MW, Roca AL, Troyer JL, Johnson WE, O’Brien SJ. 2008. Genetic characterization of feline leukemia virus from Florida panthers. Emerging Infectious Diseases. 14 (2). Gale Academic OneFile. link.gale.com/apps/doc/A175285235/AONE?u=googlescholar&sid=bookmark-AONE&xid=42d74442.
[67] Chiu ES, Kraberger S, Cunningham M, Cusak L, ROelke M, VandeWoude S. 2019. Multiple Introductions of Docestic cat feline leukemia virus in endangered Florida panthers. Emerging Infectious Diseases 25 (1) 92-101.
[68] Meli ML, Cattori V, Martínez F, López G, Vargas A, Palomares F, López-Bao JV, Hofmann-Lehmann R, Lutz Hans. 2010. Feline leukemia virus infection: a threat for the survival of the critically endangered Iberian lynx (Lynx pardinus). Veterinary Immunology and Immunopathology 134(1-2):61-67.
[69] Lamberski N. 2015. Felidae. Fowler's Zoo and Wild Animal Medicine. 8: 467-476.
[70] Levy JK, Crawford PC, Tucker SJ. 2017. Performance of 4 Point-of-Care Screening Tests for Feline Leukemia Virus and Feline Immunodeficiency Virus. Journal of Veterinary Internal Medicine 31: 521-526.
[71] Tandon R, Cattori V, Gomes-Keller GH, Meli ML, Golder MC, Lutz H, Hoffmann-Lehmann R. 2005. Quantitation of feline leukemia virus viral and proviral loads by TaqMan real-time polymerase chain reaction. Journal of Virological Methods 130: 124-132.
[72] Hoffmann-Lehmann R, Huder JB, Gruber S, Boretti F, Sigrist B, Lutz H. 2001. Feline leukaemia provirus load during the course of experimental infection and in naturally infected cats. Journal of General Virology 82: 1589-1596.
[73] Attias M, Teixeira D, Benchimol M, Vommaro R, Crepaldi P, De Souza W. 2020. The life-cycle of Toxoplasma gondii reviewed using animations. Parasites & Vectors 13(1): 588–588.
[74] Robert-Gangneux F, Darde M. 2012. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clinical Microbiology Reviews 25(2): 264–296.
[75] Hill D, Dubey J. 2002. Toxoplasma gondii: transmission, diagnosis, and prevention. Clinical Microbiology and Infection 8(10): 634–640.
[76] Al-Malki ES. 2021. Toxoplasmosis: stages of the protozoan life cycle and risk assessment in humans and animals for an enhanced awareness and an improved socio-economic status. Saudi Journal of Biological Science. 28(1): 962–969.
[77] Brister J, Shell L. 2019. Vincyclopedia of Diseases: Toxoplasmosis. Vincyclopedia of Diseases https://www.vin.com/Members/Associate/Associate.plx?from=GetDzInfo&DiseaseId=814&pid=607.
[78] Charlesworth D, Willis JH. 2009. The genetics of inbreeding depression. Nature Reviews: Genetics 10(11): 783–796.
[79] Spielman D, Brook BW, Briscoe DA, Frankham R. 2004. Does Inbreeding and Loss of Genetic Diversity Decrease Disease Resistance? Conservation Genetics 5(4): 439–448.
[80] Dubey J, Quinn W, Weinandy D. 1987. Fatal neonatal toxoplasmosis in a bobcat (Lynx rufus). Journal of Wildlife Diseases 23(2): 324–327.
[81] Desmonts G, Couvreur J. 1974. Congenital toxoplasmosis. A prospective study of 378 pregnancies. The New England Journal of Medicine 90(20): 1110–1116.
[82] Dunn D, Wallon M, Peyron F, Petersen E, Peckham C, Gilbert R. 1999. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet (London, England). 353(9167): 1829–1833.
[83] Delair E, Latkany P, Noble AG, Rabiah P, McLeod R, Brézin A. 2011. Clinical manifestations of ocular toxoplasmosis. Ocular Immunology and Inflammation. 19(2): 91–102.
[84] Remington JS, McLeod R, Thulliez P, and Desmonts G. 2001. Toxoplasmosis. In: Remington JS and Klein J (ed), Infectious diseases of the fetus and newborn infant, 5th ed. WB Saunders, Philadelphia, PA. 205–346.
[85] Roberts F, Mets MB, Ferguson DJ, O'Grady R, O'Grady C, Thulliez P, Brézin AP, McLeod R 2001. Histopathological features of ocular toxoplasmosis in the fetus and infant. Archives of ophthalmology. (Chicago, Ill. : 1960). 119(1): 51–58. PMID: 11146726
[86] Verma SK, Minicucci L, Murphy D, Carstensen M, Humpal C, Wolf P, Calero-Bernal R, Cerqueira-Cézar CK, Kwok OC, Su C, Hill D, Dubey JP. 2016. Antibody Detection and Molecular Characterization of Toxoplasma gondii from Bobcats (Lynx rufus), Domestic Cats (Felis catus), and Wildlife from Minnesota, USA. The Journal of Eukaryotic Microbiology 63(5): 567–571.
[87] Verma SK, Sweeny AR, Lovallo MJ, Calero-Bernal R, Kwok OC, Jiang T, Su C, Grigg ME, Dubey JP. 2017. Seroprevalence, isolation and co-infection of multiple Toxoplasma gondii strains in individual bobcats (Lynx rufus) from Mississippi, USA. International Journal for Parasitology 47(5): 297–303.
[88] Humphreys JG. 2006. Seroprevalence of Antibodies to Toxoplasma gondii in the Pennsylvania Bobcat (Lynx rufus rufus). Journal of Wildlife Diseases 42(1): 188–191.
[89] Rendón-Franco E, Caso-Aguilar A, Jiménez-Sánchez NG, Hernandez-Jauregui DMB, Sandoval-Sánchez AL, Zepeda-López HM. 2012. Prevalence of anti-Toxoplasma gondii antibody in free-ranging ocelots (Leopardus pardalis) from Tamaulipas, Mexico. Journal of Wildlife Diseases 48(3): 829–831.
[90] Desmonts G, Remington JS. 1980. Direct agglutination test for diagnosis of Toxoplasma infection: Method for increasing sensitivity and specificity. Journal of Clinical Microbiology 11: 562-568.
[91] Dubey JP, Desmonts G. 1987. Serological responses of equids fed Toxoplasma gondii oocysts. Equine Veterinary Journal 19:337-339.
[92] Jansen A M, Roque ALR. 2010. Domestic and wild mammalian reservoir. In: Telleria J, Tibayrenc M, editors. American Trypanosomiasis Chagas Disease-one hundred years of research. London: Elsevier. 249-276.
[93] Curtis-Robles R, Hamer SA, Lane S, Levy MZ, Hamer GL. 2018. Bionomics and spatial distribution of triatomine vectors of Trypanosoma cruzi in Texas and other southern states. USA. American Journal of Tropical Medicine and Hygiene 98: 113-121.
[94] Gurtler RE, Cecere MC, Lauricella MA, Cardinal MV, Kitron U, Cohen JE. 2007. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwest Argentina. Parasitology 134: 69-82.
[95] Enriquez GF, Cardinal MV, Orozco MM, Wirth S, Schijman AG, Gurtler RE. 2013. Detection of Trypanosoma cruzi infection in naturally infected dogs and cats using serological, parasitological, and molecular methods. Acta Tropica 126(2): 11-217.
[96] Enriquez GF, Bua J, Orozco MM, Wirth S, Schijman AG, Gurtler RE, Cardinal MV. 2014. High levels of Trypanosoma cruzi DNA determined by qPCR and infectiousness to Triatoma infestans support dogs and cats are major sources of parasites from domestic transmission. Infectection, Genetics and Evolution 25: 36-43.
[97] Jiminez-Coello M, Acosta-Viana KY, Guzman-Marin E, Gomez-Rios A, Ortega-Pacheco A. 2012. Epidemiological survey of Trypanosoma cruzi infection in domestic owned cats from the tropical southeast of Mexico. Zoonoses and Public Health 59: 102-109.
[98] Zecca IB, Hodo CL, Slack S, Auckland L, Rodgers S, Killets KC, Saunders AB, Hamer SA. 2020. Prevalence of Trypanosoma cruzi infection and associated histologic findings in domestic cats (Felis catus). Veterinary Parasitology 278: 109014.
[99] Rocha FL, Roque ALR, Saab de Lima J, Cheida CC, Lemos FG, Cavalcanti de Azevedo F, Arrais RC, Bilac D, Herrera HM, Mourao G, Jansen AM. 2013a. Trypanosoma cruzi infection in neotropical wild carnivores (mammalia : carnivora): at the top of the T. cruzi transmission chain. PLOS ONE 8(7): e67463.
[100] Rocha FL, Roque AL, Arrais RC, Santos JP, Lima VD, Chagas Xavier SC, Cordeir-Estrela P, D’Andrea PS, Jansen AM. 2013b. Trypanosoma cruzi TcI and TcII transmission among wild carnivores, small mammals and dogs in a conservation unit and surrounding areas, Brazil. Parasitology 140: 160-170.
[101] Herrera HM, Rocha FL, Lisboa CV, Rademaker V, Mourao GM, Jansen AM. 2011. Food web connections and the transmission cycles of Trypanosoma cruzi and Trypanosoma evansi (Kinetoplastida, Trypanosomatidae) in the Pantanal region, Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene 105: 380-387.
[102] Zecca IB, Hodo CL, Swarts HM, DeMaar TW, Snowden KF, Prestridge HL, Light JE, Hamer SA. 2021. Trypanosoma cruzi and incidental Sarcocystis spp. in endangered ocelots (Leopardus pardalis) of south Texas, USA. Journal of Wildlife Diseases. 57(3): 667671.
[103] Andre MR, Adania CH, Machado RZ, Allegretti SM, Felippe PAN, Silva KF, Nakaghi ACH, Dagnone AS. 2009. Molecular Detection of Cytauxzoon Spp. in Asymptomatic Brazilian Wild Captive Felids. Journal of Wildlife Diseases 45(1): 234–237
[104] Lewis K. 2011. Cytauxzoon felis: An emerging feline pathogen and potential therapy. University of Missouri- Columbia, Ann Arbor.
[105] Meinkoth J, Kocan AA, Whitworth L, Murphy G, Fox JC, Woods JP. 2000. Cats Surviving Natural Infection with Cytauxzoon Felis: 18 Cases (1997–1998). Journal of Veterinary Internal Medicine 14(5): 521–525.
[106] Gallusová M, Jirsová D, Mihalca AD, Gherman CM, D'Amico G, Qablan MA, Modrý D. 2016. Cytauxzoon Infections in Wild Felids from Carpathian-Danubian-Pontic Space: Further Evidence for a Different Cytauxzoon Species in European Felids. The Journal of Parasitology 102(3): 377–380.
[107] Drechsler Y, Alcaraz A, Bossong FJ, Collisson EW, Diniz PPVP. 2011. Feline coronavirus in multicat environments. Veterinary Clinics of North America: Small Animal Practice 41(6): 1133-69.
[108] Pedersen NC. 2009. A review of feline infectious peritonitis virus infection: 1963–2008. Journal of Feline Medicine and Surgery 11(4):2 25–258
[109] Pedersen NC. 1976. Serologic studies of naturally occurring feline infectious peritonitis. American Journal of Veterinary Research 37(12): 1449–1453. PMID: 793459
[110] Cave TA, Golder MC, Simpson J. 2004. Risk factors for feline coronavirus seropositivity in cats relinquished to a UK rescue charity. Journal of Feline Medicine and Surgery 6(2): 53–58.
[111] Luria BJ, Levy JK, Lappin MR. 2004. Prevalence of infectious diseases in feral cats in Northern Florida. Journal of Feline Medicine and Surgery 6(5): 287–296.
[112] Pedersen NC, Allen CE, Lyons LA. 2008. Pathogenesis of feline enteric coronavirus infection. Journal of Feline Medicine and Surgery 10(6): 529–541.
[113] Addie DD, Jarrett O. 2001. Use of a reverse-transcriptase polymerase chain reaction for monitoring the shedding of feline coronavirus by healthy cats. Veterinary Record 148(21): 649–653.
[114] Addie DD, Paltrinieri S, Pedersen NC. 2004. Recommendations from workshops of the second international feline coronavirus/feline infectious peritonitis symposium. Journal of Feline Medicine and Surgery 6(2): 125–130.
[115] Han JI, Kang SY, Yoon KJ, Na KJ. 2014. Nucleic acid-based differential diagnostic assays for feline coronavirus. Journal of Virological Methods. 208: 21-25.
[116] Pedersen NC, Black JW, Boyle JF 1984a. Pathogenic differences between various feline coronavirus isolates. Advances in Experimental Medicine and Biology 173: 365–380.
[117] Pedersen NC, Evermann JF, McKeirnan AJ. 1984b. Pathogenicity studies of feline coronavirus isolates 79-1146 and 791683. American Journal of Veterinary Research 45(12): 2580–2585. PMID: 6084432
[118] Pedersen NC. 1987. Virologic and immunologic aspects of feline infectious peritonitis virus infection. Adv Exp Med Biol. 218:529–550.
[119] Chang HW, de Groot RJ, Egberink HF. 2010. Feline infectious peritonitis: insights into feline coronavirus pathobiogenesis and epidemiology based on genetic analysis of the viral 3c gene. J Gen Virol. 91(Pt 2): 415–420.
[120] Poland AM, Vennema H, Foley JE 1996. Two related strains of feline infectious peritonitis virus isolated from immunocompromised cats infected with a feline enteric coronavirus. J Clin Microbiol. 34(12): 3180–3184.
[121] Vennema H, Poland A, Foley J. 1998. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virolog. 243(1): 150–157.
[122] Pedersen NC, Floyd K. 1985. Experimental studies with three new strains of feline infectious peritonitis virus FIPV-UCD2, FIPV-UCD3, and FIPV-UCD4. Compendium Continuing Education Practicing Veterinarians. 7:1001–1011.
[123] Sharif S, Arshad SS, Hair-Bejo M, Omar AR, Zeenathul NA, Hafidz MA. 2009. Prevalence of feline coronavirus in two cat populations in Malaysia. Journal of Feline Medicine and Surgery, 11 (12): 1031-1034.
[124] Addie DD, Toth S, Murray GD. 1995. Risk of feline infectious peritonitis in cats naturally infected with feline coronavirus. American Journal of Veterinary Research 56(4): 429–434. PMID: 7785816
[125] Foley JE, Poland A, Carlson J. 1997. Risk factors for feline infectious peritonitis among cats in multiple-cat environments with endemic feline enteric coronavirus. Journal of the American Veterinary Medical Association 210(9): 1313–1318. PMID: 9143536
[126] Addie DD, Jarrett O. 1992. A study of naturally occurring feline coronavirus infections in kittens. Veterinary Record 130(7): 133–137.
[127] Herrewegh AA, Mahler M, Hedrich HJ. 1997. Persistence and evolution of feline coronavirus in a closed cat-breeding colony. Virology 234(2): 349–363.
[128] Pesteanu-Somogyi LD, Radzai C, Pressler BM. 2006. Prevalence of feline infectious peritonitis in specific cat breeds. Journal of Feline Medicine and Surgery 8(1): 1–5.
[129] Foley JE, Pedersen NC. 1996. The inheritance of susceptibility to feline infectious peritonitis in purebreed catteries. Veterinary Practitioner 24(1): 14-22.
[130] Pedersen NC. 2009. A review of feline infectious peritonitis virus infection: 1963–2008. Journal of Feline Medicine and Surgery 11(4): 225–258.
[131] Rohrbach BW, Legendre AM, Baldwin CA. 2001. Epidemiology of feline infectious peritonitis among cats examined at veterinary medical teaching hospitals. Journal of the American Veterinary Medical Association 218(7): 1111–1115.
[132] Hardy WD, Jr. 1982. Immunopathology induced by the feline leukemia virus. Seminars in Immunopathology 5(1): 75–106.
[133] Cotter SM, Hardy WD, Jr, Essex M. 1975. Association of feline leukemia virus with lymphosarcoma and other disorders in the cat. Journal of the American Veterinary Medical Association 166(5): 449–454. PMID: 163223
[134] Addie D, Belak S, Boucraut-Baralon C. 2009. Feline infectious peritonitis: ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery 11(7): 594–604.
[135] Hartmann K. 2005. Feline infectious peritonitis. Veterinary Clinics of North America: Small Animal Practice 35(1): 39-79
[136] Kipar A, May H, Menger S. 2005. Morphologic features and development of granulomatous vasculitis in feline infectious peritonitis. Veterinary Pathology 42(3): 321–330.
[137] Andrew SE. 2000. Feline infectious peritonitis. Veterinary Clinics of North America: Small Animal Practice 30(5): 987–1000.
[138] Kennedy M, Citino S, McNabb AH, Moffatt AS, Gertz K, Kania S. 2002. Detection of Feline Coronavirus in Captive Felidae in the USA. Journal of Veterinary Diagnostic Investigation 14 (6): 520-2.
[139] Stout AE, André NM, Whittaker GR. 2021. Feline coronavirus and feline infectious peritonitis in nondomestic felid species. Journal of Zoo and Wildlife Medicine 52(1): 14-27.
[140] Juan-Sallés C, Domingo M, Herráez P, Fernandez A, Segalés J, Fernández J. 1998. Feline infectious peritonitis in servals (Felis serval). Veterinary Record.
[141] Stephenson N, Swift P, Moeller R, Worth S, Foley J. 2013. Feline Infectious Peritonitis in a Mountain Lion (Puma concolor), California, USA. Journal of Wildlife Diseases 49(2): 408-12.
[142] Kennedy M, Kania S, Stylianides E, Bertschinger H, Keet D, van Vuuren M. 2003. Detection of feline coronavirus infection in southern African nondomestic felids. Journal of Wildlife Diseasess 39(3): 529-35.
[143] Van Rensburg IB, Silkstone M. 1984. Concomitant feline infectious peritonitis and toxoplasmosis in a cheetah (Acinonyx jubatus). Journal of the South African Veterinary Association 55(4): 205-207
[144] Evermann J, Heeney J, Roelke M, McKeirnan A, O’Brien S. 1988. Biological and pathological consequences of feline infectious peritonitis virus infection in the cheetah. Archives of Virology 102(3-4): 155-171.
[145] Horhogea C, Floristean V, Lazăr M, Creţu C, Solcan C. 2017. Immunohistochemical Methods to Diagnose Atraumatic Spleen Rupture in Feline Infectious Peritonitis of Tiger (Panthera tigris). Revisita de Chimie-Bucharest-Original Edition 68(5): 1055.
[146] Mwase M, Shimada K, Mumba C, Yabe J, Squarre D, Madarame H. 2015. Positive immunolabelling for feline infectious peritonitis in an African lion (Panthera leo) with bilateral panuveitis. Journal of Comparative Pathology 152 (2): 265-268.
[147] McOrist S, Boid R, Jones T, Easterbee N, Hubbard A, Jarrett O. 1991. Some viral and protozool diseases in the European wildcat (Felis silvestris). Journal of Wildlife Diseases 27(4): 693-6.
[148] Watt N, Macintyre N, McOrist S. 1993. An extended outbreak of infectious peritonitis in a closed colony of European wildcats (Felis silvestris). Journal of Comparative Pathology 108(1): 73-9.
[149] Schmitt AC, Reishak D, Clavac CL, Monforte CHL, Couto FT, Almeida BPF, Santos DGG, Souza L, Alves C, Vecchi K. 2003. Infection by feline leukemia virus and feline infectious peritonitis in a wild and captive felid from the pantanal region of mato grosso. Acta Scientiae Veterinariae 31(3): 185–188.
[150] Filoni C, Catão-Dias JL, Bay G, Durigon EL, Jorge RSP, Lutz H, Hoffman-Lehmann R. 2006. First evidence of feline Herpesvirus, Calicivirus, Parvovirus, and Ehrlichia exposure in Brazilian free-ranging felids. Journal of Wildlife Diseases 42(2): 470477.
[151] Cornell University: Baker Institute for Animal Health. 2016. “Feline Calicivirus” https://www.vet.cornell.edu/departmentscenters-and-institutes/baker-institute/our-research/feline-calicivirus
[152] Radford AD, Sommerville L, Ryvar R, Cox MB, Johnson DR, Dawson S, Gaskell RM. 2001. Endemic infection of a cat colony with a feline calicivirus closely related to an isolate used in live attenuated vaccines. Vaccine 19(31): 4358-4362.
[153] Radford AD, Coyne KP, Dawson S, Porter CJ, Gaskell RM. 2007. Feline calicivirus. Veterinary Research 38(2): 319-335. [154] Henzel A, Lovato LT, Weiblen R. 2015. Epidemiological status of felid herpesvirus type-1 and feline calicivirus infections in Brazil. Cienca. Rural: Microbiology 45(6).
[155] Ellis TM. 1981. Jaundice in a Siamese cat with in utero feline calicivirus infection. Australian Veterinary Journal 57 (8): 383385
[156] Radford AD, Addie D, Belák S, Boucraut-Baralon C, Egberink H, Frymus T, Gruffydd-Jones T, Hartmann K, Hosie MJ, Lloret A, Lutz H, Marsilio F, Pennisi MG, Thiry E, Truyen U, Horzinek MC. 2009. Feline calicivirus infection ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery 11(7): 556-564.
[157] Henzel A, Brum MCS, Lovato LT, Widblen R. 2013. Serological Survey of Feline Calicivirus and Felid herpesvirus in Rio Grande do Sul, Brazil. Acta Scientiae Veterinariae 41:1-6.
[158] Ostrowski S, Vuuren MV, Menain DM, Durand A. 2003. A serologic survey of wild felids from central west Saudi Arabia. Journal of Wildlife Diseases 39: 696–701.
[159] Thalwitzer S, Wachter B, Robert N, Wibbelt G, Müller T, Lonzer J, Meli ML, Bay G, Hofer H, Lutz H. 2010. Seroprevalences to viral pathogens in free-ranging and captive cheetahs (Acinonyx jubatus) on Namibian farmland. Clinical and Vaccine Immunology 17:232–238.
[160] Hoffman-Lehmann R, Fehr D, Grob M, Elgizoli M, Packer C, Martenson JS, O’Brien SJ, Lutz H. 1996. Prevalence of antibodies to feline parvovirus, calicivirus, herpesvirus, coronavirus, and immunodeficiency virus and of feline leukemia virus antigen and the interrelationship of these viral infection in free-ranging lions in east Africa. Clinical and Diagnostic Lab Immunology 3(5): 554-562.
[161] Fiorello CV, Noss AJ, Deem SL, Maffei L, Dubovi EJ. 2007. Serosurvey of small carnivores in the Bolivian Chaco. Journal of Wildlife Diseases 43(3): 551-557.
[162] Furtado MM, de Ramos Filho JD, Scheffer KC, Coelho CJ, Cruz PS, Ikuta CY, de Almeida Jácoma AT, de Oliviera Porfírio GE, Silveira L, Sollmann R, Tôrres NM, Neto JSF. 2013. Serosurvey for selected viral infections in free-ranging jaguars (Panthera onca) and domestic carnivores in Brazilian cerrado, pantanal and Amazon. Journal of Wildlife Diseases 49: 510–521.
[163] Gaskell R, Dawson S, Radford A, Thiry E. 2007. Feline herpesvirus. Veterinary Research 38: 337-354.
[164] ABCD Feline Herpesvirus infection. 2017. Journal of Feline Medicine and Surgery 17: 570-582
[165] Gaskell R, Dawson S, Radford A. 2006. Feline respiratory disease. Greene CE, ed. Infectious diseases of the dog and cat. Missouri: WB Saunders, 145-154.
[166] Gaskell RM, Povey RC. 1982. Transmission of feline viral rhinotracheitis. Veterinary Record 111: 359-362.
[167] Westermeyer HD, Thomasy SM, Kado-Fong H, Maggs DJ. 2009. Assessment of viremia associated with experimental primary feline herpesvirus infection or presumed herpetic recrudescence in cats. American Journal of Veterinary Research 70: 99-104.
[168] Johnson RP, Povey RC. 1985. Vaccination against feline viral rhinotracheitis in kittens with maternally derived feline viral rhinotracheitis antibodies. Journal of the American Veterinary Medical Association 186:149-152. PMID: 2982774.
[169] Dawson S, Willoughby K, Gaskell RM, Woog G, Chalmers WCK. 2001. A field trial to assess the effect of vaccination against feline herpesvirus, feline calicivirus and feline panleukopenia virus in 6-week-old kittens. Journal of Feline Medicine and Surgergy 3: 17-22.
[170] Gaskell RM, Povey RC. 1979. The dose response of cats to experimental infection with feline viral rhinotracheitis virus Journal of Comparative Pathology 89: 179-191.
[171] Jas D, Aeberle C, Lacombe V, Guiot AL, Poulet H. 2009. Onset of immunity in kittens after vaccination with a non-adjuvanted vaccine against feline panleukopenia, feline calicivirus and feline herpesvirus. The Veterinary Journal 182: 86–93.
[172] Munson L, Marker L, Dubovi E, Spencer JA, Evermann JF, O'Brien SJ. 2004. Serosurvey of viral infections in free-ranging Namibian cheetahs (Acinonyx jubatus). Journal of Wildlife Diseases 40(1): 23–31.
[173] Thalwitzer S, Watcher B, Robert N, Wibbelt G, Müller T, Lonzer J, Meli ML, Bay G, Hofer H, Lutz H. 2010. Seroprevalences to viral pathogens in free-ranging and captive cheetahs (Acinonyx jubatus) on Namibian farmland. Clinical Vaccine Immunology 17(2): 232–238
[174] Van Vuuren M, Goosen T, Rogers P. 1999. Feline herpesvirus infection in a group of semi-captive cheetahs: case report. Journal of the South African Veterinary Association 70(3): 123–134.
[175] Munson L, Wack R, Duncan M, Montali RJ, Boon D, Stalis I, Crawshaw GJ, Cameron KN, Mortenson J, Citino S, Zuba J, Junge RE. 2004. Chronic eosinophilic dermatitis associated with persistent feline herpes virus infection in cheetahs (Acinonyx jubatus). Veterinary Pathology. 41(2): 170–176.
[176] Junge RE, Miller E, Boever WJ, Scherba G, Sundberg J. 1991. Persistent cutaneous ulcers associated with feline herpesvirus type 1 infection in a cheetah. Journal of the American Veterinary Medical Association 198(6): 1057–1058. PMID: 1851739
[177] Flacke GL, Schmidt-Kuntzel A, Marker L. 2015. Treatment of chronic herpesviral dermatitis in a captive cheetah (Acinonyx jubatus) in Namibia. Journal of Zoo and Wildlife Medicine 46(3): 641–646.
[178] Hara M, Fukuyama M, Suzuki Y, Kisikawa S, Ikeda T, Kiuchi A, Tabuchi K. 1996. Detection of feline herpes virus 1 DNA by the nested polymerase chain reaction. Veterinary Microbiology 48: 345-352.
[179] Nasisse MP, Weigler BJ. 1997. The diagnosis of ocular herpes virus infection. Veterinary and Comparative Ophthalmology 7: 44-51.
[180] Stiles J, McDermott M, Bigsby D, Willis M, Martin C, Roberts W, Greene C. 1997a. Use of nested polymerase chain reaction to identify feline herpesvirus in ocular tissue from clinically normal cats and cats with corneal sequestra or conjunctivitis. American Journal of Veterinary Research 58: 338-342. PMID: 9099374
[181] Stiles J, McDermott M, Willis M, Roberts W, Green C. 1997b. Comparison of nested polymerase chain reaction, virus isolation, and fluorescent antibody testing for identifying feline herpesvirus in cats with conjunctivitis. American Journal of Veterinary Research 58: 804-847. PMID: 9256959
[182] Weigler BJ, Babineau A, Sherry B, Nasisse MP. 1997. High sensitivity polymerase chain reaction assay for active and latent feline herpesvirus-1 infections in domestic cats. Veterinary Record 140: 335-338.
[183] Maggs DJ, Lappin MR, Nasisse MP. 1999. Detection of feline herpesvirus-specific antibodies and DNA in aqueous humor from cats with and without uveitis. American Journal of Veterinary Research 60: 932-936. PMID: 10451199
[184] Sykes JE, Allen JL, Studdert VP, Browning GF. 2001. Detection of feline calicivirus, feline herpesvirus 1 and Chlamydia psittaci mucosal swabs by multiplex RT-PCR/PCR. Veterinary Microbiology 81: 95-108.
[185] Vögtlin A, Fraefel C, Albini S, Leutenegger CM, Schraner E, Spiess B, Lutz H, Ackermann M. 2002. Quantification of feline herpesvirus 1 DNA in ocular fluid samples of clinically diseased cats by real-time TaqMan PCR. Journal of Clinical Microbiology 40: 519-523.
[186] Helps C, Reeves N, Egan K, Howard P, Harbour D. 2003. Detection of Chlamydophila felis and feline herpesvirus by multiplex real-time PCR analysis. Journal of Clinical Microbiology 41: 2734-2736.
[187] Marsilio F, Di Martino B, Aguzzi I, Meridiani I. 2004. Duplex polymerase chain reaction assay to screen for feline herpesvirus1 and Chlamydophila spp. in mucosal swabs from cats. Veterinary Research Communications 28: 295-298.
[188] Maggs DJ, Clarke HE. 2005. Relative sensitivity of polymerase chain reaction assays used for detection of feline herpesvirus type 1 DNA in clinical samples and commercial vaccines. American Journal of Veterinary Research 66: 1550-1555.
[189] Creevy KE. (2020) Canine Distemper Overview in Merck Manual: Veterinary Manual. https://www.merckvetmanual.com/generalized-conditions/canine-distemper/canine-distemper-overview
[190] Appel MJG, Yates RA, Foley GL, Bernstein JJ, Santinelli S, Spelman LH, Miller LD, Arp LH, Anderson M, Barr M, PearceKelling S, Summers BA. 1994. Canine distemper epizootic in lions, tigers, and leopards in North America. Journal of Veterinary Diagnostic Investigation 6(3): 277-288.
[191] Blythe LL, Schmitz JA, Roelke M, Skinner S. 1983. Chronic encephalomyelitis caused by canine distemper virus in a Bengal tiger. Journal of the American Veterinary Medical Association 183(11): 1159-1162. PMID: 6685717
[192] Deem SL, Spelman LH, Yates RA, Montali RJ. 2000. Canine distemper in terrestrial carnivores: a review. Journal of Zoo and Wildlife Medicine 31 (4): 441-451.
[193] Martinez-Gutierrez M, Ruiz-Saenz J. 2016. Diversity of susceptible hosts in canine distemper virus infection: a systematic review and data synthesis. BMC Veterinary Research 12(1): 78.
[194] Gilbert M, Miquelle DG, Goodrich JM, Reeve R, Cleaveland S, Matthews L, Joly DO. 2014. Estimating the potential impact of canine distemper virus on the Amur tiger population (Panthera tigris altaica) in Russia. PLoS One 9(10): e110811.
[195] Gilbert M, Soutyrina SV, Seryodkin IV, Sulikhan N, Uphyrkina OV, Goncharuk M, Matthews L, Cleaveland S, Miquelle DG. 2015. Canine distemper virus as a threat to wild tigers in Russia and across their range. Integrative Zoology 10(4): 329-343.
[196] Goodrich J, Lynam A, Miquelle D, Wibisono H, Kawanishi K, Pattanyibool A, Htun S, Tempa T, Karki J, Jhala Y, Karanth K. 2015. Panthera tigris. In: The IUCN red list of threatened species. Available from www.iucnredlist.org
[197] Montali RJ, Tell L, Bush M, Cambre RC, Kenny D, Sutherland-Smith M, Appel MJG. 1994. Vaccination against canine distemper in exotic carnivores: successes and failures. In: Proc Am Assoc Zoo Vet Ann Conf: Pittsburgh, PA; 340–344.
[198] Association of Zoos and Aquariums. Association of Zoos and Aquariums tiger species survival plan® 2016. tiger care manual. Silver Spring (MD): Association of Zoos and Aquariums; 2016.
[199] Bronson E, Deem SL, Sanchez C, Murray S. 2007. Serologic response to a canarypox-vectored canine distemper virus vaccine in the giant panda (Ailuropoda melanoleuca). Journal of Zoo and Wildlife Medicine 38(2): 363-366.
[200] Connolly M, Thomas P, Woodroffe R, Raphael BL. 2013. Comparison of oral and intramuscular recombinant canine distemper vaccination in African wild dogs (Lycaon pictus). JJournal of Zoo and Wildlife Medicine 44(4): 882-888.
[201] Vickers TW, Garcelon DK, Fritcher DL, Christianson M. 2004. Evaluation of an oral and food-based canarypox-vectored canine distemper vaccine in endangered Channel Island foxes (Urocyon littoralis). In: Proceedings of the American Association of Zoo Veterinarians and the American Association of Wildlife Veterinarians, Wildlife Disease Association Joint Conference 87-88.
[202] McEntire M, Ramsay EC, Kania S, Prestia P, Anis A, Cushing AC, Wilkes RP. 2020. Tiger (Panthera tigris) and domestic cat (Felis catus) immune responses to canarypox-vectored canine distemper vaccination. Journal of Zoo and Wildlife Medicine 50(4): 798-802.
[203] Sadler RA, Ramsay EC, McAloose D, Wilkes RP. 2016. Evaluation of two canine distemper vaccines in captive tigers (Panthera tigris). JJournal of Zoo and Wildlife Medicine. 47(2): 558-563.
[204] Nava AFD, Cullen Jr L, Sana DA, Nardi MS, Filho JDR, Lima TF, Abreu KC, Ferreira F. 2008. First evidence of canine distemper in Brazilian free-ranging felids. EcoHealth 5: 512-518.
[205] Avendano R, Barrueta F, Soto-Fournier S, Chavarria M, Monge O, Gutierrez-Espeleta GA, Chaves A. 2016. Canine distemper virus in wild felids in Costa Rica. Journal of Wildlife Diseases 52(2): 373-377.
[206] Ikeda Y, Nakamura K, Miyazawa T, Chen M, Kuo T, Lin JA, Mikami T, Kai C, Takahaski E. 2001. Seroprevalence of canine distemper virus in cats. Clinical & Diagnostic Laboratory Immunology 8: 641–644.
[207] Gollakner R, Kimmerlein A. Coronavirus Disease (COVID-19). VCA Animal Hospitals. https://vcahospitals.com/know-yourpet/coronavirus-disease-covid19
[208] Hammer AS, Lauge Quaade M, Bruun Rasmussen T, Fonager J, Rasmussen M, Mundbjer K, LohseL, Strandbygaard B, Svaerke Jorgensen C, Alfaro-Nuñes A, Rosenstierne MW, Boklund A, Halasa T, Fomsgaard A, Belsham GJ, Botner A. (2021). SARS-CoV-2 transmission between mink (Neovison vison) and humans, denmark. Emerging Infectious Diseases 27: 547–551.
[209] Oude Munnink BB, Sikkema RS, Nieuwenhuijse DF, Molenaar RJ, Munger E, Molenkamp R, van der Spek A, Tolsma P, Rietveld A, Brouwer M, Bouwmeester-Vincken N, Harders F, Hakze-van der Honing R, Wegdam-Blans MCA, Bouwstra RJ, GeurtsvanKessel C, van der Eijk AA, Velkers FC, Smit LAM, Stegeman A, van der Poel WHM, Koopmans MPG. (2021). Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 371: 172–177. [210] CDC Centers for Disease Control and Prevention. Animal Testing Guidance. 2021. https://www.cdc.gov/coronavirus/2019ncov/animals/animal-testing.html#content-3
[211] CDC Centers for Disease Control and Prevention. Animals and COVID-19. 2022. https://www.cdc.gov/coronavirus/2019ncov/daily-life-coping/animals.html
[212] Terio KA, Bronson E, McAloose D. 2021. Felid TAG guidance for working around non-domestic felid species during the SARS CoV 2 pandemic. 1-2. https://cdn.ymaws.com/www.aazv.org/resource/resmgr/docs/Guidance_for_working_around_.pdf [213] AVMA. American Veterinary Medical Association. Feline panleukopenia. https://www.avma.org/resources-tools/petowners/petcare/feline-panleukopenia
[214] Lane EP, Brettschneider H, Caldwell P, Oosthuizen A, Dalton DL, du Plessis L. 2016. Feline panleukopenia virus in captive non-domestic felid in South Africa. Onderstepoort Journal of Veterinary Research 83(1): a1099.
[215] Nakamura K, Sakamoto M, Ikeda Y, Sato E, Kawakami K, Miyazawa T, Tohya Y, Takahashi E, Mikami T, Mochizuki M. 2001. Pathogenic potential of canine parvovirus types 2a and 2c in domestic cats. Clinical and Diagnostic Laboratory Immunology 8(3): 663-668.
[216] Squires RA. 2020. Merck Manual Veterinary Manual. Feline Panleukopenia. https://www.merckvetmanual.com/generalizedconditions/feline-panleukopenia/feline-panleukopenia
[217] van Vuuren M, Steinel A, Goosen T, Lane E, van der Lugt J, Pearson J, Truyen U. 2000. Feline panleukopenia virus revisited: Molecular characteristics and pathological lesions associated with three recent isolates. Journal of the South African Veterinary Association 71(3): 140-143.
[218] Wasieri J, Schmiedeknecht G, Forster C, Konig M, Reinacher M. 2009. Parvovirus infection in a Eurasian lynx (Lynx lynx) and in a European wildcat (Felis sylvestris silvestris). Journal of Comparative Pathology 140(2-3): 203-207.
[219] Chandran NDJ, Saravanabava K, Albert A, Venkatesan RA. 1993. Occurrence of parvoviral infection in lions. Indian Journal of Animal Science 63(7): 727-728.
[220] Pennisi MG, Radford A, Tasker S, Belák S, Addie DD, Boucraut-Baralon C, Egberink H, Frymus T, Gruffydd-Jones T, Hartmann K, Horzinek MC, Hosie MJ, Lloret A, Lutz H, Marsilio F, Thiry E, Truyen U, Möstl K. 2017. Anaplasma, Ehrlichia, Rickettsia Infections. Journal of Feline Medicine and Surgery 19(5): 542-548.
[221] Allison RW, Little SE. 2013. Diagnosis of rickettsial diseases in dogs and cats. Veterinary Clinical Pathology 42: 127-144.
[222] Lloret A. 2020. Hepatozoonosis in European Advisory Board on Cat Diseases (ABCD). http://www.abcdcatsvets.org/hepatozoonosis/
[223] Foley JE, Leutenegger CM, Dumler JS, Pedersen NC, Madigan JE. 2003. Evidence for modulated immune response to Anaplasma phagocytophila sensu lato in cats with FIV-induced immunosuppression. Comparative Immunology, Microbiology & Infectious Diseases 26: 103-113.
[224] Lappin MR, Chandrashekar R, Stillman B, Liu J, Mather TN. 2015. Evidence of Anaplasma phagocytophilum and Borrelia burgdorferi infection in cats after exposure to wild-caught adult Ixodes scapularis. Journal of Veterinary Diagnostic Investigation 27: 522-525.
[225] Bjöersdorff A, Svendenius L, Owens JH, Massung RF. 1999. Feline granulocytic ehrlichiosis – a report of a new clinical entity and characterization of the infectious agent. Journal of Small Animal Practice 40: 20-24.
[226] Lappin MR, Breitschwerdt EB, Jensen WA, Dunnigan B, Rha JY, Williams CR, Brewer M, Fall M. 2004. Molecular and serological evidence of Anaplasma phagocytophilum infection in cats in North America. Journal of the American Veterinary Medical Association 225: 893-896.
[227] Schaarschmidt-Kiener D, Graf F, von Loewenich FD, Müller W. 2009. Anaplasma phagocytophilum infection in a cat in Switzerland. Schweiz Arch Tierheilkd 151: 336-341.
[228] Heikkilä HM, Bondarenko A, Mihalkov A, Pfister K, Spillmann T. 2010. Anaplasma phagocytophilum infection in a domestic cat in Finland: case report. Acta Veterinaria Scandinavica 52: 62.
[229] Adaszek L, Górna M, Skrzypczak M, Buczek K, Balicki I, Winiarczyk S. 2013. Three clinical cases of Anaplasma phagocytophilum infection in cats in Poland. Journal of Feline Medicine and Surgery 15: 333-337.
[230] Savidge C, Ewing P, Andrews J, Aucoin D, Lappin MR, Moroff S. 2015. Anaplasma phagocytophilum infection of domestic cats: 16 cases from northeastern USA. Journal of Feline Medicine and Surgery Feb 13. pii: 1098612X15571148.
[231] Tarello W. 2005. Microscopic and clinical evidence for Anaplasma (Ehrlichia) phagocytophilum infection in Italian cats. Veterinary Record 156: 772-774.
[232] Baneth G, Sheiner A, Eyal O, Hahn S, Beaufils JP, Anug Y, et al (2013): Redescription of Hepatozoon felis (Apicomplexa: Hepatozoidae) based on phylogenetic analysis, tissue and blood from morphology, and possibly transplacental transmission. Parasite Vectors 6: 102.
[233] Klopfer U, Nobel TA, Neumann F 1973. Hepatozoon-like parasite (schizonts) in the myocardium of the domestic cat. Veterinary Pathology 10(3): 185-190.
[234] Beaufils JP, Martin-Granel J, Jumelle P 1998 Hepatozoon spp. parasitemia and feline leukemia virus infection in two cats. Feline Pract 26: 10–13. ISSN : 1057-6614
[235] Basso W, Görner D, Globokar M, Keidel A, Pantchev N 2019. First autochthonous case of clinical Hepatozoon felis infection in a domestic cat in Central Europe. Parasitology International 72: 101945.
[236] Scott FW, Geissinger CM. (1999) Long-term immunity in cats vaccinated with an activated trivalent vaccine. American Journal of Veterinary Research 60: 652-658.
[237] Mouzin DE, Lorenzen MJ, Haworth JD, King VL. (2004) Duration of serologic response to three viral antigens in cats. Journal of the American Veterinary Medical Association 224: 61-66
[238] Bronson E, Terio K. (2018) 2018 Felid Taxon Advisory Group Preventative Medicine Recommendations. http://www.felidtag.org/Husbandry
[239] Rivas AE, Langan JN, Colegrove KM, Terio K, Adkesson MJ. (2015) Herpesvirus and calicivirus infection in a black-footed cat (Felid nigripes) family group following vaccination. Journal of Zoo and Wildlife Medicine 46: 141-145.
[240]Schmidt-Posthaus H, Breitenmoser-Wörsten C, Posthaus H, Bacciarini L, Breitenmoser U. (2002). Causes of mortality in reintroduced Eurasian lynx in Switzerland. Journal of Wildlife Diseases 38: 84–92.
[241] Wassmer DA, Guenther D, Layne JN. (1988). Ecology of the bobcat in South-Central Florida. Bulletin of the Florida Museum of Natural History 33: 176–178.
[242] López G, López-Parra M, Garrote G, Fernández L, del Rey-Wamba T, Arenas-Rojas R, García-Tardío M, Ruiz G, Zorrilla I, Moral M, Simón MA (2014) Evaluating mortality rates and causalities in a critically endangered felid across its whole distribution range. European Journal of Wildlife Research 60: 359–366.
[243] Nájera F, Grande-Gómez R, Peña J, Vázquez A, Palacios MJ, Rueda C, Corona-Bravo AI, Zorrilla I, Revuelta L, Gil-Molino M, Jiménez J. (2021). Disease surveillance during the reintroduction of the Iberian Lynx (Lynx pardinus) in southwestern Spain. Animals 11(2): 547.
[244] Nakamura J, Iwasa T. 1942. On the fowl-pest infection in cat. Japanese Journal of Veterinary Science 4: 511–523.
[245] Kuiken T, Rimmelzwaan G, van Riel D, van Amerongen G, Baars M, Fouchier R, Osterhaus A. 2004. Avian H5N1 influenza in cats. Science 306: 241.
[246] Rimmelzwaan GF, van Riel D, Baars M, Bestebroer TM, van Amerongen G, Fouchier RAM, Osterhaus ADME, Kuiken T. 2006. Influenza A virus (H5N1) infection in cats causes systemic disease with potential novel routes of virus spread within and between hosts. American Journal of Pathology 168: 176–183.
[247] Leschnik M, Weikel J, Möstl K, Revilla-Fernández S, Wodak E, Bagó Z, Vanek E, Benetka V, Hess M, Thalhammer JG. 2007. Subclinical infection with avian influenza A (H5N1) virus in cats. Emerging Infectious Diseases 13: 243–247.
[248] Vahlenkamp TW, Harder TC, Giese M, Lin F, Teifke JP, Klopfleisch R, Hoffmann R, Tarpey I, Beer M, Mettenleiter TC. 2008. Protection of cats against lethal influenza H5N1 challenge infection. Journal of General Virology 89: 968–974.
[249] Vahlenkamp TW, Teifke JP, Harder TC, Beer M, Mettenleiter TC. 2010. Systemic influenza virus H5N1 infection in cats after gastrointestinal exposure. Influenza and Other Respiratory Viruses 4: 379–386.
[250] Ayyalasomayajula S, DeLaurentis DA, Moore GE, Glickman LT. 2008. A network model of H5N1 avian influenza transmission dynamics in domestic cats. Zoonoses and Public Health 55: 497–506.
[251] Lee CT, Slavinski S, Schiff C, Merlino M, Daskalakis D, Liu D, Rakeman JL, Misener M, Thompson C, Leung YL,Varma JK, Fry A, Havers F, Davis T, Newbury S, Layton M, Influence A(H7N2) Response team. 2017. Outbreak of influenza A(H7N2) among cats in an animal shelter with cat-to-human transmission New York City, 2016. Clinical Infectious Diseases 65: 1927–1929.
[252] Newbury SP, Cigel F, Killian ML, Leutenegger CM, Seguin MA, Crossley B, Brennen R, Suarez DL, Torchetti M, TooheyKurth K. 2017. First detection of avian lineage H7N2 in Felis catus. Genome Announc 5: e00457–17. doi:10.1128/genomeA.00457-17
[253] Wasik BR, Voorhees IEH, Parrish CR. 2021. Canine and Feline Influenza. Cold Spring Harbor Perspecies in Medicine. Cold Spring Harbor Laboratory Press.
[254] Hatta M, Zhong G, Gao Y, Nakajima N, Fan S, Chiba S, Deering KM, Ito M, Imai M, Kiso M, Naktsu S, Lopes TJ, Thompson AJ, McBride R, Suarez DL, Macken CA, Sugita S, Neumann G, Hasegawa H, Paulson JC, Tooher-Kurth KL, Kawaoka Y. 2018. Characterization of a feline influenza A(H7N2) virus. Emerging Infectious Diseases 24: 75–86.
[255] Keawcharoen J, Oraveerakul K, Kuiken T, Fouchier RAM, Amonsin A, Payungporn S, Noppornpanth S, Wattanodorn S, Theambooniers A, Tantilertcharoen R, Pattanarangsa R, Arya N, Ratanakorn P, Osterhaus DME, Poovorawan Y. 2004. Avian influenza H5N1 in tigers and leopards. Emerging Infectious Diseases 10: 2189–2191.
[256] Thanawongnuwech R, Amonsin A, Tantilertcharoen R, Damrongwatanapokin S, Theamboonlers A, Payungporn S, Nanthapornphiphat K, Ratanamungklanon S, Tunak E, Songserm T, Vivatthanavanich V, Lekdumrongsak T, Kesdangsakonwut S, Tunhikorn S, Poovarawan Y. 2005. Probable tiger-to-tiger transmission of avian influenza H5N1. Emerging Infectious Diseases 11: 699–701.
[257] Bouvier NM, Lowen AC. 2010. Animal models for influenza virus pathogenesis and transmission. Viruses 2: 1530– 1563.
[258] Belser JA, Barclay W, Barr I, Fouchier RAM, Matsuyama R, Nishiura H, Peiris M, Russell CJ, Subbarao K, Zhu H, Yen, H-L. 2018. Ferrets as models for influenza virus transmission studies and pandemic risk assessments. Emerging Infectious Diseases 24: 965–971.
[259] Berg M, Englund L, Abusugra IA, Klingeborn B, Linné T. 1990. Close relationship between mink influenza (H10N4) and concomitantly circulating avian influenza viruses. Archives of Virology 13: 61–71.
[260] Yoon K-J, Schwartz K, Sun D, Zhang J, Hildebrandt H. 2012. Naturally occurring influenza A virus subtype H1N2 infection in a Midwest United States mink (Mustela vison) ranch. Journal of Veterinary Diagnostic Investigation 24: 388–391.
[261] Jiang W, Wang S, Zhang C, Li J, Hou G, Peng C, Chen J, Shan H. 2017. Characterization of H5N1 highly pathogenic mink influenza viruses in eastern China. Veterinary Microbiology 201: 225–230.
[262] Geraci JR, St Aubin DJ, Barker IK, Webster RG, Hinshaw VS, Bean WJ, Ruhnke HL, Prescott JH, Early G, Baker AS, Madoff S, Schooley RT. 1982. Mass mortality of harbor seals: pneumonia associated with influenza A virus. Science 215: 1129–1131.
[263] Anthony SJ, St Leger JA, Pugliares K, Ip HS, Chan JM, Carpenter ZW, Navarrete-Macias I, Sanchez-Leon M, Saliki JT, Pedersen J, et al. 2012. Emergence of fatal avian influenza in New England harbor seals. mBio 3: e00166– 00112.
[264] Bodewes R, Bestebroer TM, van der Vries E, Verhagen JH, Herfst S, Koopmans MP, Fouchier RAM, Pfankuche VM, Wohlsein P, Siebert U, et al. 2015. Avian influenza A (H10N7) virus–associated mass deaths among harbor seals. Emerging Infectious Diseases 21: 720–722.
[265] Elsmo EJ, Wünschmann A, Beckmen KB, Broughton-Neiswanger LB, Buckles EL, Ellis J, Fitzgerald SD, Gerlach R, Hawkins S, Ip HS, Lankton JS, Lemley EM, Lenoch JB, Killian ML, Lantz K, Long L, Maes R, Mainenti M, Melotti J, Moriarty ME, Nakagun S, Ruden RM, Shearn-Bochsler V, Thompson D, Torchetti MK, Van Wettere AJ, Wise AG, Lim AL. 2023. Pathology of natural infection with highly pathogenic avian influenza virus (H5N1) clade 2.3.4.4b in wild terrestrial mammals in the United States in 2022. bioRxiv.
[266] Savage HM, Burkhalter KL, Godsey Jr MS, Panella NA, Ashley DC, Nicholson WL, Lambert AJ. (2017). Bourbon virus in field-collected ticks, Missouri, USA. Emerging Infectious Diseases 23(12): 2017-2022
Wilding Enclosures
Enclosure Size and Materials
The wilding enclosures are located within the Ocelot Conservation Facility. There will be at least four 0.25-acre (~1,012 m2) enclosures that will provide a quasi-natural environment for development of wild behaviors in captive-bred ocelots. As needed, each enclosure can be connected to adjacent enclosures to provide larger spaces or subdivided to provide more individual enclosures. Enclosures should be approximately three meters in height and though not top-covered, should contain positive cantilevers to avoid ocelot escape through the top of the enclosures. Enclosures should also have a series of electric fencing to prevent ocelot escape at the ground level and to prevent other animals from entering. To prevent excessive escape of prey items from inside the enclosures, smooth surface barriers will be placed along the bottom of the enclosure fencing. Other designs options may also be considered to decrease the chances of prey escape.
To promote development of social awareness and behavior, ocelots in the wilding enclosures should be allowed to live with their dam and siblings and to make visual - but not physical - contact with other unrelated ocelots in nearby enclosures. There must be physical distance or actual physical barriers between adjacent enclosures to completely prevent any direct physical contact between unrelated ocelots. Holding unrelated ocelots in the same enclosure (except for breeding pairs or surrogate dam/kitten groups) is not recommended in order to avoid ocelot-ocelot conflict.
General Environment/Habitat of Enclosures
The wilding enclosures should be environmentally rich to support ocelots’ ability to develop wild behaviors [56] The environment should include free-ranging live native prey and a vegetation community that will mimic the community present at the reintroduction site: Tamaulipan thornscrub. Raising ocelots in a similar environment to that they will experience in the reintroduction site will support their ability to prepare for life at the reintroduction site. Additionally, the presence of a variety of natural materials (including plant structures, logs, rocks, shelves, etc.) will allow ocelots to experience a three-dimensional space and ultimately develop behavioral and psychomotor skills necessary for successful movements in the wild.
Tamaulipan thornscrub habitat is characterized by diverse woody and herbaceous thorny shrub communities with canopy cover that ranges from open to dense and can be 3 to 10 meters high. Shorter woody vegetation may form an understory below an upper story of Texas ebony (Ebenopsis ebano), mesquite (Prosopis glandulosa), or live oak (Quercus virginiana) trees. Woody and shrub understory species may include whitebrush (Aloysia gratissima), lime prickly ash (Zanthoxlyum fagara), cat-claw acacia (Acacia greggii), blackbrush (Acacia rigidula), and a variety of cacti [7-8]. Dense, medium-height native and invasive grasses often occur interspersed between woody communities and can provide suitable cover for ocelots’ exploratory and hunting movements [9-10]. Existing vegetation already present at the wilding enclosures can be maintained there during facility construction and other species may be planted and grown as necessary to make the environment more similar to the reintroduction site.
The density of vegetation in the wilding enclosures must be balanced with monitoring needs. Monitoring is needed to evaluate behavioral development of ocelots to determine whether they are prepared for release to the wild. While ocelots may use highly dense vegetation in the wild, especially for daytime resting sites, vegetation throughout the enclosure cannot be so dense that video monitoring of ocelots is impossible; there must be some openings in vegetation for monitoring.
Environmental Enrichment
Complex environmental enrichment, beyond vegetation and free ranging live prey, is necessary to mimic the habitat at the reintroduction site, avoid ocelot habituation to captivity, and create an overall stimulating environment for ocelot’s behavioral development. There should be standing environmental enrichment always present in the enclosures, while introducing new and variable environmental enrichment will be important in cases where ocelots show signs of abnormal or repetitive stress behaviors and thus a need for increased stimulation [11-12]. For any enrichment added to an enclosure, the desired outcome of its use should be clear, and personnel should evaluate whether the environmental enrichment will contribute to the development of a specific natural wild ocelot behavior and if the enrichment is appropriate for a given individual based on age, experience, and personality.
Standing environmental enrichment should include:
● Free-ranging live native prey species (small mammals) that ocelots can catch and consume. See sections below for further information on prey.
● Natural objects to touch such as sand, water, leaves, grass, stones, etc. and logs to scratch or use for marking of territory.
● Areas such as wildlife trails, open areas, and refuge spaces (under branches, on ledges, etc.) that provide places for hiding, eating, resting, and sheltering from weather conditions.
○ Refuge spaces should be created from natural, not human-made, materials except for a nest box or den for dams with young kittens. A human-made but natural-like nest box or den can be provided for dams with kittens, and it can be removed or remotely closed once kittens have aged to encourage the dam and kittens to seek more natural refuge spaces. The nest box can be replaced or re-opened as needed.
● Objects to climb on (to explore, see the habitat from above, develop motor skills, and experience the enclosure three-dimensionally) such as trees (fallen and upright), series of connected logs, slopes and valleys/ditches, and rocks.
○ Any climbable objects should not allow ocelots to escape from the enclosure.
● Other ocelots
○ Siblings and the dam should be held in the same enclosure to allow natural social behaviors, though the dam must be captured and removed from the enclosure to separate her from the kittens after they have matured and developed sufficiently (see more in sections below)
■ Cross fostering of kittens to other ocelot dams may be warranted due to a negligent or aggressive dam or other issues (e.g., maternal health concerns, lactational failure, etc.).
Fostered kittens are typically within 2-3 weeks of age of and similar in size to the neonatal kittens of the foster dam. A dam ocelot with similarly aged kittens may not be available for
cross fostering. The Ocelot Conservation Facility should coordinate with partner zoological institutions during breeding to assess the availability of possible surrogate feline species for cross foster opportunities. If no cross-fostering matches are available, use of a domestic cat and kittens to aid in raising ocelot kittens and developing their behavior may be possible Supplement feeding may be necessary for ocelots raised by domestic cats, that may not produce enough milk for ocelot kittens. See Kitten Rearing section for more information. Any surrogate felines used for cross fostering must be screened for disease so they do not introduce any health risks to ocelots.
○ Otherwise, ocelots are typically not held with unrelated individuals in the same enclosure unless they are a male-female pair being housed together for breeding or surrogate dam/kitten groups
○ Ocelots in nearby enclosures are physically separated from each other, though individuals should be able to see, smell, and hear one another. Removing visual access between enclosures or reducing visual access to small viewsheds by using a shade cloth may be necessary to obscure views into neighboring enclosures if viewing of other ocelots is causing individuals to pace or exhibit other stereotypy or abnormal behavior.
Additional environmental enrichment may be added on a case-by-case basis to the wilding enclosures and may include:
● Movement of ocelots to different enclosures
○ Ocelots can be moved to different wilding enclosures to provide novel enrichment via new environmental interactions. Moving ocelots to different enclosures may also be needed to relocate ocelots who are causing stress to others or to resolve abnormal behavior in an ocelot.
■ For example, ocelots can be transferred into recently unoccupied enclosures so they can experience the scents, pawprints, etc. of the ocelot who was there before.
● Olfactory stimuli from potential prey or other ocelots
○ All scents must be free of any health risks.
○ Feces or urine of other unknown ocelots may be placed in the enclosure.
■ Scents may be captured from the wild or elsewhere in the Ocelot Conservation Facility and placed in an enclosure.
■ Some feces or urine of ocelots who previously lived in the enclosure can be left behind when new ocelots enter the enclosure.
○ Prey scat, urine, or parts (such as feathers, tails, ears, etc.)
■ Can be collected from the wild or the prey colony.
○ Plant parts collected from the reintroduction site.
○ Bobcat, coyote, or puma (potential predator/competitor) urine or feces
■ Smells from these carnivores should only be used minimally and for short periods to prevent ocelots from becoming habituated to other carnivores’ smells absent of any actual interaction with the other species. Scent objects used will be removed following brief introduction to ensure its does not linger in the environment resulting in habituation to the smell.
● Auditory stimuli (sound recordings) from potential prey, from other ocelots, and from other carnivores
○ As for scents, noises should only be played minimally to avoid habituation or excessive stress to an ocelot.
● Novel human objects for interaction
○ Any human object used as enrichment must support development of a specific behavior, such as exploring the environment or learning to use livestock or wildlife water tanks that will be available in the reintroduction site. The purpose/goal of the enrichment must be established prior to its use and there should be defined criteria for evaluation of the effectiveness of human objects as enrichment. Additionally, prior to the use of any human-made objects for enrichment, a defined process and schedule for presenting the objects must be established.
○ Positive enrichment items, such as hanging balls or bags with smells or meat inside can be used to stimulate ocelots as well as help them to practice motor skills. Objects such as these should be provisioned at random times, moved around the enclosure, and eventually removed completely so that any human objects remain novel, and ocelots do not become habituated to them.
○ Commercially available pet toys such as balls should not be used.
● Negative enrichment for behavioral training
○ Negative enrichment can be used to teach ocelots about dangerous objects. A dangerous item, for example, a human-made object, a domestic animal, or a predator/competitor species could be presented safely but with a negative reinforcer (e.g., loud noise or other harassment) to discourage possible interactions with that dangerous object in the future [13].
Abnormal Behavior
Abnormal behavior, including stereotypy, could be a sign of behavioral estrus in females or simply the ocelot patrolling its home range. However, it could be a sign of a behavioral issue for an ocelot. Should an abnormal behavior such as stereotypy occur in an ocelot in the wilding facility, the ocelot should first be further monitored and evaluated by personnel trained in behavioral studies. Personnel should assess the details of the ocelot's behavior, the ocelot’s emotional state, the location of the behavior, the potential cause of the behavior (i.e., keepers, noises, ocelots in neighboring enclosures, etc.), and any impacts of the behavior to other ocelots. Multiple complete observations of an abnormal behavior should be evaluated by necessary personnel to determine an individualized behavioral intervention, which may include the introduction of additional environmental enrichment presented above.
Water
Multiple water sources will be present in every wilding enclosure to assure access to clean drinking water. Small, natural-like water pools underneath vegetative cover should be constructed, if possible, to provide water to ocelots and prey animals. Water at these sources will be non-flowing and will be monitored for algal growth and mosquito larvae. Additionally, each enclosure should have one or more tanks that are plumbed for running water. These tanks will mimic existing cattle/wildlife water tanks present on ranches in southern Texas that could be used by ocelots upon release to the wild. If necessary, water tanks can be placed close to the perimeter of the enclosure to ensure the ability to refill from the outside of the enclosure.
Cleaning
Given that enclosures are large and should be wild-like, cleaning needs will be minimal. Cleaning of enclosures may occur as necessary but must be balanced with the need to limit human interaction with ocelots in the wilding enclosures. Removing food remains, waste, or other biological materials and cleaning of water pools and/or tanks may occur, when necessary, at the discretion of veterinarian and keepers. The best opportunity for cleaning is likely after an ocelot has been removed from the enclosure and is being held elsewhere.
Humans at the Wilding Enclosures
Human intervention in the wilding enclosures should be minimized as much as possible so that ocelots can be allowed to cope with natural elements and the various challenges of living in a natural environment without assistance. Human-ocelot contact must be minimized at the wilding enclosures. Enclosures should only be visited by authorized personnel on official business; interested persons cannot observe ocelots in the wilding enclosures in-person (although they can watch live video surveillance obtained from cameras in the wilding enclosures). Enclosure size (just over 1,000 m2) and the presence of natural cover will reduce the likelihood of a keeper encountering an ocelot when entering an enclosure for feeding, watering, maintenance, or other activities. However, if keepers do encounter an ocelot while working in or around an enclosure, they should scare the animal away and introduce a negative stimulus by yelling, throwing water, or throwing other objects near the ocelot, for example. Keepers may also periodically walk the perimeter of an enclosure to test whether any ocelot approaches and should scare away any ocelots that do approach people or fail to flee. An ocelot that shows signs of a positive or neutral response to human presence, even with negative stimuli, may ultimately be considered unsuitable for release to the wild.
Feeding
Prey Species and Sources
Provisioning a variety of live prey to ocelots in the wilding enclosures is essential for allowing ocelots to learn to catch and consume different types of prey, as they must do upon release to the wild. Ocelots being trained for release to the wild should be provided live prey as much as possible, and hunting and feeding behaviors should be monitored with video surveillance to assess hunting and feeding proficiency. Feeding ocelots with chunks of meat that are not associated with a whole live prey animal should be avoided where possible. If meat must be fed to ocelots, it is recommended to use meat from native prey animals found in Texas. Prey species native to southern Texas will be utilized for feeding in the wilding enclosures to avoid the possibility of introducing nonnative species to the environment. Prey escape from the wilding enclosures will be minimized by placing surface barriers at the bottom of enclosures.
In Texas, ocelots are known to have a generalist diet that includes birds (roadrunners and passerines), small mammals (shrew, rabbit, mouse, gopher, rat), meso mammals (white-tailed deer fawns), and reptiles (lizards) [14]. The wilding enclosures should be managed to retain as much naturally occurring live wild prey inside to way to mimic conditions in the natural environment. Additional prey animals may be placed inside the wilding enclosures and will be sourced from a prey colony at the Ocelot Conservation Facility. The colony will raise native species of mice, rats, and rabbits. Prey can also be trapped from wild sources and brought to the wilding
facility. All food must be appropriately checked by a veterinarian or other staff to verify safety and cleanliness before providing the prey to ocelots. To the best of their ability, keepers should record every source of prey and its estimated weight or age category (e.g., adult, juvenile, or neonate prey) that is provided to ocelots. The results of feeding (fully eaten, mostly eaten, partially eaten, or not eaten) should be recorded, if observed, after feeding.
Food Delivery
Live, free-ranging prey animals should be living in the wilding enclosures prior to the placement of any ocelots there. The method for delivery of additional live prey into the enclosure can be through a tunnel system or other remote system. In such systems, ocelots will not associate humans or human activities with the provisioning of food. In a tunnel system, prey is placed into the top of the tunnel outside of the enclosure, moved through the enclosure, and are ultimately released inside. Multiple tunnels are recommended for each enclosure to increase the complexity of food provisioning and to avoid any habituation; different tunnels should be utilized randomly and at diverse times so that ocelots do not habituate to a single location or time for prey provisioning. Additionally, to avoid ocelots becoming habituated to noise in tunnels leading to the appearance of a prey item, routine use of tunnels and noisemaking without the provisioning of prey is recommended. Prey provisioning can be scheduled to occur mostly when ocelots are active, likely after 4 PM, and at different times to avoid habituation. Video monitoring should occur at the bottom of each tunnel to capture hunting behavior in cases where the prey does not move far from the end of the tunnel.
Types of Food
Four possible types of food for ocelots - meat, dead prey, easy prey, and difficult prey - are summarized in Table 1. The four options represent an increasing level of difficulty for ocelots, who should be encouraged to hunt and eat difficult live prey. The progression from easier to more difficult food sources will be an important part of behavioral development in ocelots. It is recommended to provide as much difficult live prey to ocelots as possible so there are always ample opportunities to practice hunting behaviors. Ocelots who have problems feeding and develop poor health or body condition should be transitioned to easier food types as appropriate.
Meat
In the wilding enclosures, meat should only be provided as a necessary health intervention to support ocelots who are unable to eat whole prey. It is preferable to source meat from native live prey animals, though meat from other sources could be used as necessary. In the event meat alone is provided for substantial periods of time (greater than a few days), supplementation of minerals and vitamins may be recommended. At that time, personnel will consult with nutritionists and zoo-based feeding systems.
Dead Whole Prey
Dead whole prey can be provided as a health intervention for ocelots that prove unable to catch live prey as well as to kittens that should be beginning to eat meat and play with prey (beginning at approximately 8 weeks age) but whose dams are unable to kill prey and bring it to the kittens. When dead prey must be provided, unskinned carcasses are recommended, as this will require ocelots to learn to remove the skin. The exception is for kittens
needing to start eating meat but whose dams will not skin dead prey; unskinned carcasses may be provided instead.
Dead prey can be added to the enclosure through a tunnel at random times and locations, it may be placed by a keeper in hidden or difficult-to-access places as appropriate, or it could be thrown into an enclosure Providing prey in diverse locations will reduce the likelihood of ocelot habituation to any location, including to the bottom of tunnels. Placing dead prey (for sub adult or adult ocelots, not kittens) in difficult places will require ocelots to explore and search for food to find it and then exert energy to get it. Dead prey can be placed high up or hung to require some climbing or jumping to get the prey. Program managers must consider a dam’s ability to find dead prey when providing it for the benefit of kittens. Case-by-case management of the feeding of dam and young kittens, including where and when to place dead prey and whether it should be skinned, is expected to be necessary.
Easy Live Prey
Easy live prey should be provided to young ocelots that are beginning to learn to hunt or to ocelots that are having challenges with hunting and need easier hunting opportunities. Easy prey may include young, domesticated, or more habituated animals that do not fear predators or have the skills to escape from them efficiently. Additionally, small prey of a consistent variety may be easier for ocelots to catch. Easy live prey can be added to the enclosure through the tunnel system. The prey will be naive to the enclosure upon placement there, potentially making the prey animals easier to catch. If an ocelot is unable to catch easy prey that is freeranging, program managers may consider placing prey in confinement so that ocelots can easily catch the prey and develop hunting skills. Small, open-top cages that an ocelot can climb or jump into, but the prey cannot escape from, can be used, though care should be taken to minimize disruption to ocelots while placing this prey and to avoid any habituation to prey at the cage. Habituation can be avoided by changing the location of the cage or the timing of prey placement.
Difficult Live Prey
Ocelots who are at higher stages of behavioral development should be able to sustain themselves with only difficult prey, including free-ranging prey already living in the enclosure and additional prey animals added through the tunnel system or other remote systems. The most difficult prey to catch in the enclosure will be wild, live, free-ranging prey that has been established in the enclosure prior to ocelots’ placement there. This prey may already be adapted to the enclosure and may prove the most difficult for ocelots to find and kill. Such prey should always be present in the enclosures to provide enrichment for ocelots through constant opportunity to catch and eat prey. Surface barriers will be placed along the bottom of the enclosure fencing to reduce prey escape. Difficult live prey can also be added to the enclosure through the tunnel system. To make this prey difficult to catch, the prey animals should be healthy adult animals. A variety of native wild – not domesticprey species, including larger ones, should be provided. Wild-trapped prey may prove more difficult to catch than prey sourced from the prey colony.
Table 1. Categories of food for ocelots at the wilding facility, with meat being the easiest source of food for ocelots that do not hunt and difficult live prey the most challenging food for ocelots being prepared for life in the wild.
Food Category Meat Dead Prey “Easy Live Prey” “Difficult Live Prey”
Types
Chunks of meat, preferably from native prey animals
Uses Only in cases of health concern where ocelots will not eat other food sources.
Unskinned, dead whole animals
Skinned (for nursing or young, but no longer nursing, kittens)
For ocelots learning to eat whole prey but not yet hunting and whose dams do not bring them dead prey
Young, small, domestic breed, confined to cage, naive to enclosure, same species always
For ocelots beginning to learn to hunt, needing easy opportunities to successfully kill prey, and not yet ready for more difficult prey
Adult, larger, healthy, wild breed, freeranging, adapted to enclosure, variety of species, wild trapped.
For individuals showing success in hunting easy prey
Placement Tunnels
Placed by keeper in hidden or difficult-toaccess location
Tunnels
Placed by keeper in hidden or difficult-toaccess location
Tunnels
Placed by keeper in confinement, if necessary
Treatment of Female Breeding Ocelots at the Wilding Facilities
Tunnels
Free-ranging in enclosure before ocelots are placed there.
The ability to hold adult females in wilding enclosures before they breed or give birth will depend on the availability of open wilding enclosures. If possible, adult female ocelots, especially captive-born individuals, should be exposed to the wilding enclosures prior to giving birth to kittens. Spending time in the wilding enclosures will support practice of natural behaviors - such as hiding, exploring, and hunting - that the females can practice and then teach to their offspring (though it is possible that kittens may be able to learn to hunt even if the dam does not hunt). Females who have experienced the wilding facility and/or show better inclination to hunt and search for food may be preferred for breeding.
Adult breeding females can be held alone in wilding enclosures before they breed to become familiar with the more natural environment present there. This pre-exposure to wilding enclosures will give them experience utilizing the enclosures and it may reduce stress following relocation to the enclosures with kittens. Additionally, live breeding events with males can occur in the wilding enclosures. This may work well for consistently breeding pairs or for wild-sourced individuals who may prefer to breed in the wilding enclosures versus in the breeding enclosures.
Program managers and veterinarians will determine, based on individual behavior and space needs, when it is appropriate for a breeding female to be in a wilding enclosure, and if she should breed and give birth in the large wilding enclosures or the smaller breeding enclosures. It is not recommended to move ocelots to different enclosures during pregnancy due to possible stress. First time, inexperienced, or previously unsuccessful dams should give birth in the breeding enclosures, which are easier to monitor, while proven dams that have already raised one or more successful litters may give birth in wilding enclosures if program personnel are comfortable with this. While in the wilding enclosures, females should be encouraged to catch and eat live wild prey animals. Live, free-ranging prey animals (i.e., difficult prey) should be consistently available in the wilding enclosure when a female is present so that hunting and feeding skills are practiced.
An individualized feeding plan based on health and hunting abilities will be developed for each female, who will be monitored through video surveillance within enclosures. Food for females can range from the dead whole prey to difficult live prey. If an adult female is initially unable to successfully sustain herself by hunting live prey, easy prey will be provided to encourage development of hunting and feeding skills. The “difficulty” of live prey should be increased over time as hunting and feeding proficiency increases and body condition is maintained. Additionally, the location and timing of prey provisioning can also become increasingly random as the female develops hunting and eating behaviors. Fasting periods (1-3) days can be used to stimulate hunger and hunting behavior. However, if a female is unable to hunt and shows signs of declining body condition, easier prey should be provided to maintain the ocelot’s health. As described previously, both the types of prey provided to ocelots and the degree of ocelots’ consumption should be recorded so that managers and veterinarians can evaluate the efficacy of the feeding plan and make adjustments as needed.
Timeline for Management of Releasable Ocelot Offspring
The following is a suggested timeline for management of releasable ocelots in the breeding and wilding enclosures (summarized in Table 2). The timelines presented here are general and are based on what is known of ocelot biology (including anticipated age of motor skill development, hunting skill development, and dispersal from the dam, for example) by field ecologists and zoological managers. However, all individual management of ocelots should ultimately be dictated not by the exact timelines here but by the actual behavior and development of the individuals. For example, the exact timing of separating a dam from the kittens should be determined based on the offspring’s ability to hunt and its degree of independence from the dam, including time spent away from her. The determination of when exactly to remove an ocelot from the wilding facility for transfer to the reintroduction site will depend on its “readiness,” which includes its success in hunting and consuming prey, exploring habitat, and avoiding humans. When the program is implemented, managers will gain experience in identifying the development of key behaviors or other signals that indicate it is an appropriate time to transition management. Creating a behavioral development timeline for each dam and her kittens will establish baseline information used to inform subsequent management.
The dam and offspring should be kept together in the breeding and wilding enclosures so the offspring can learn from the dam and socialize together normally. Throughout their time in the enclosures, siblings should have the opportunity to play, compete for space or food, stay close to each other, move farther apart from each other, or even avoid each other. The social interactions between kittens and dam should be monitored to evaluate the growing independence of kittens as they age. Only when offspring reach approximately 10-12 months old and
show proper independence and hunting ability should the dams be separated from the offspring. Decisions will be made based on behavioral criteria evaluated by program managers.
Table 2. General suggested timelines for management of captive-bred ocelots from birth to release. Ocelots are born in a breeding or wilding enclosure, live in the wilding enclosure with their dam until they have developed necessary behavior, and are separated from their dam for several months to practice skills without the dam, and are finally transferred to the reintroduction site.
Offspring approximate age
Birth until 6-12 weeks (kitten)
6-12 weeks up to 10-12 months (kitten)
From 10-12 months up to 1218 months (subadult)
From 12-18 months (subadult)
Location Breeding enclosures or wilding enclosures, depending on circumstances Wilding enclosures Wilding enclosures Reintroduction site (soft-release facility or hard release)
Offspring behavior
Kittens nursing and staying in den with siblings and dam
Kittens, with dam and siblings, transferred to wilding enclosures (if not already there) to develop exploratory, hunting, and social behaviors
Once behaviorally ready to be separated from the dam, offspring are separated from their dam to practice hunting on their own
Birth Until 6-12 Weeks: Breeding or Wilding Enclosures with Dam
Soft or hard release of ready subadult ocelots
Kittens may be born in breeding enclosures or in the wilding enclosures based on veterinarian and facility manager evaluation of factors such as the dam’s behavior, veterinary needs, and the availability of enclosures. For ocelot kittens who are intended to be released to the wild, preparation for life in the wild will begin at birth. Even in the first weeks of life when kittens are blind and moving minimally, kittens should be exposed to natural sounds, smells, and surfaces so that their senses can begin developing naturally. For example, once kittens begin to stand and walk, they should be able to touch various surfaces (such as sand, pebbles, vegetation) on the floor/ground so they can gain sensory knowledge of different surfaces and develop their motor skills. Further, kittens should not be allowed to habituate to human presence, as this could cause a lack of fear of humans. Human contact (including touching, sounds, or smell) with kittens and their dam should be limited to necessary veterinary care. Around 8-10 weeks, kittens should receive a full physical exam, blood
workup, and vaccinations. This process is likely aversive for the kittens because human handling during the exam will be stressful. While kittens are in breeding enclosures and during any care, personnel must avoid any unnecessary touching or talking when in close proximity to kittens. Kittens that are intended to be released into the wild should not be raised to develop positive relationships with people. The number of caretakers in any area should be limited to three or fewer people as possible to limit the number of unique humans who may interact with ocelots. Ocelots may become accustomed to specific humans from their visual, auditory, and/or olfactory cues and may develop better with a smaller number of keepers. Additionally, limiting the number of humans that interact with an ocelot may improve wilding of captive reared ocelots.
Two or more nest boxes or den structures must be provided for the birth of the kittens and for shelter in their early weeks of life before they are moving. Providing multiple options for nests gives options to the dam. Nest boxes can be elevated approximately one foot off the ground where possible and around vegetative cover to allow ocelots to use the cover below the nest box. Each nest box/den structure should be well-monitored by a video camera, preferably with a wide-angle low light camera inside and with a camera outside to monitor activity directly outside the structure. If the dam is giving birth in the wilding enclosure, the nest box/den structure should be as natural as possible. Additionally, there should be a variety of substrates and climbing surfaces present near the nest box/den so that once kittens begin walking, they can experience different environments without moving far from the den. If kittens are born in the smaller indoor-outdoor breeding enclosures, there must be some natural elements added to the space, such as natural materials, sounds, smells, and different floor surfaces. The dam of newborn kittens can be given either meat, whole dead prey, easy live prey, or difficult live prey depending on the environment and its own abilities. Dams must be provided with sufficient food to avoid nutritional stress while nursing the kittens and caring for them.
Between 6-12 Weeks and 10-12 Months: Wilding Enclosures with Dam
Nursing Kittens
Kittens open their eyes around 3-4 weeks old. After 6 weeks, they may begin nursing less, gaining more interest in food, becoming more mobile, and showing greater interest in exploring their surroundings. At this point, the dam may allow the kittens more opportunity to leave the den. Once the dam is consistently allowing the kittens to leave the nest box to move around and once the kittens’ veterinary exam has been conducted, the kittens and their dam may be removed from the breeding enclosure and transferred to an available wilding enclosure (if kittens were not born there). The exact timing of a transition will be determined by program managers based on the health and behavior of the ocelot dam and kitten. When the kittens and dam are moved to a wilding enclosure, the nest box should also be moved there. It should be placed near different structures and surfaces that kittens can experience proximate to the nest box. Camera monitoring of the nest box should continue until dam and kittens no longer use the location. If there are no negative effects of the nest box, it may be left in the enclosure. The nest box may be equipped with a remote device to open and close it. This may assist in capturing dam and kittens and transferring them to appropriate locations.
When the dam and offspring are in the wilding enclosure, the dam should continue to have access to prey/food based on its hunting skill level and energetic needs. When kittens are still nursing, they should start to develop single elements of hunting behavior in their play, such as hiding and stalking. Since kittens may initially be
afraid of moving live prey, any delivery of live prey should occur away from the kittens. Though kittens will not yet accompany the dam on hunts, the dam should begin bringing her kittens dead prey or prey parts (tails, ears, etc.) so they can learn to play with prey parts. For dam who cannot do this, prey parts can be delivered to the kittens through remote systems, by tossing prey parts inside the enclosure, or by hand by keepers
Once kittens begin the process of being weaned from the dam (after 6-12 weeks) and can begin eating solid food, the proportion of wild meat in the kittens’ diet should be maximized. The dam should be provided additional food as necessary, including easier and smaller live prey if that helps her bring more to the kittens and encourage their curiosity about live prey. Program managers should determine on a case-by-case basis what prey should be provided for the dam, how that prey should be provided, and if food needs to be delivered more directly to the kittens to support interest in prey, meat eating, and playing with prey or prey parts. All data regarding provisioning and consumption of prey will be recorded to the degree possible. As kittens age, develop, play with prey, or prey parts, and eat more meat, they should also begin to show the first signs of the ability to perform sequences of hunting behavior while playing and moving around the enclosure.
Weaned Kittens Developing Wild Behaviors
When kittens no longer are receiving milk from the dam, they will begin learning to hunt by playing with prey brought to them by their dam and then by following their dam and participating in hunts. Once kittens begin to participate in hunts, dead prey or meat should no longer be provided, unless this is necessary to rescue declining body condition. If dead prey or meat must be fed, it should be hidden in different areas around the enclosure, delivered at random times, and/or placed in difficult-to-access locations so that the kittens must search for the prey and expend energy to get it.
When kittens are first participating in hunts with the dam, a large amount of easy live prey should be provisioned in the enclosure so that kittens have ample opportunities to learn. Initially, kittens should learn how to catch prey, though they may have challenges with properly killing and eating it. As kittens develop skills in hunting and feeding themselves, prey provisioning should transition to more difficult prey items. Management of this transition must be dictated by the kittens’ hunting success and body conditions. As kittens improve in hunting ability, they should begin hunting and successfully catching prey independently. Managers may explore temporarily separating the dam from the kittens for short periods to encourage the kittens to hunt on their own and to evaluate their ability to hunt independently. This would require capture and removal of the dam or holding of the dam in parts of the enclosure where doors can be remotely closed.
In all cases, it is important for any provisioning of prey through the tunnel system to occur at random times and locations around the enclosure so that kittens learn to search for prey and do not become habituated. Additionally, periods of 1-3 days with no provisioning of additional prey can be introduced to stimulate hunger, hunting behavior, and attempts to catch the free-ranging prey in the enclosure. Finally, since hunting failures may stimulate hunger and further hunting behaviors, failures should not automatically result in the feeding of easier prey, including dead prey or meat. Evaluation of both individual behavior and body condition is necessary for making management decisions on feeding.
From 10-12 Months Until Release: Wilding Enclosures Without Dam
Eventually, subadult ocelots and their siblings should be permanently separated from their dam to further their independence, test the offspring’s ability to support itself, and allow the dam to breed again. The exact timing of separation will be dictated by the subadults’ “readiness” for separation and is expected to occur sometime after 10-12 months of age. Once offspring are separated from the dam, they should not be reunited with the dam, though siblings may stay together. As such, offspring and the dam should not be separated until the offspring shows proper development of behavior and readiness for independence.
Readiness for separation should be determined by several factors. First, subadults must be observed spending an increasing amount of time away from their dam, including during both active and resting periods. This signals advancing degrees of independence. When subadults are spending time with the dam, they should be at a farther distance apart from her than they were at younger ages and they should engage in fewer positive social interactions (licking, grooming, etc.) than they did as younger kittens. Negative interactions during close contact may also increase as the dam and offspring are becoming ready to be separated. Separation should not occur if dam and offspring are still closely tied socially. Second, subadults should be spending more time exploring the enclosure without the accompaniment from the dam. They should be observed using a larger extent of available space in the enclosure than they did as younger kittens. While exploring, ocelot subadults may also begin performing marking behaviors (e.g., urine spray, scratching). Finally, subadults must be able to consistently catch, kill, and eat “difficult” live prey and maintain their body condition independent from the dam’s help or human intervention.
When a subadult is ready to be separated, the dam can be removed from the wilding enclosure and placed back into the breeding enclosures. Siblings may stay together after the dam has been removed if both show the ability to hunt and feed themselves. The siblings may continue to interact with each other and hunt together or by themselves. In cases where there is concern that one sibling hunts and the other only steals food from its sibling, siblings can be separated to test the hunting abilities of all siblings independently. Finally, in a case where one subadult is ready and other sibling is not, the ready subadult could be separated from the sibling and dam while the sibling who is unable to hunt can stay with the dam Once separated from the dam, the ocelot subadults should be fitted with a Global Positioning System (GPS) collar with an accelerometer for monitoring purposes
Upon separation from the dam, subadults should be held in the wilding enclosures for approximately two additional months, or more, without their dam to continue developing skills and to ensure that they are ready for release. Subadults in the wilding enclosures without their dam should be provisioned with only live, difficult prey for the remainder of their time in the wilding enclosures. Subadults should not be transitioned to easier prey unless their body conditions decline, and easier prey is needed to maintain health. Once offspring have been separated from the dam, they should continue to practice their natural behaviors in the wilding enclosures up until they are ready to transfer to the reintroduction site. Ocelots’ health, exploratory behavior, hunting skills, reaction to humans, and other factors as necessary will be evaluated to determine if they are ready for release to the wild.
Selection of Ocelots for Release
Only healthy ocelots who clearly display proper wild behaviors are suitable for transfer to the reintroduction site and release to the wild. Wild behaviors will be monitored using cameras, radio collars or other methods in the wilding enclosures. Health may be observed remotely or after capture and full assessment, under anesthesia, of the ocelot. If there are concerns about the readiness of an ocelot for release, regardless of its age, it should not be transferred out of the wilding facility. An ocelot should be kept in the wilding facility to continue its behavioral development with individualized management until it is ready for release. Animals that never reach levels of behavior that are clearly suitable for release could be maintained at the facility for breeding or could be transferred to cooperating zoological institutions.
Characteristics of releasable ocelots include:
● Have no health/physiological problems currently being treated or otherwise undesirable for reintroduction to the wild (disease, injury, body condition, defect, etc.)
○ Ocelots should be sedated for a full health check and disease screening prior to release to the wild
● Ideal body condition score of 2.5-3/5 or 4-5/9 that, along with its estimated weight, is maintained by the ocelot’s own hunting efforts.
● Proper hunting behaviors
○ Consistent successful hunting behavior, including searching, stalking, catching, killing, and eating multiple types of difficult live prey.
● Proper exploratory behaviors
○ Use of a large extent of the enclosure to explore, look for prey, etc.
○ Full command of different forms of locomotion (walking, running, climbing, stalking, pouncing, etc.)
● Fear of humans
○ Never approaches humans.
○ Flees or hides from humans if it sees them.
Monitoring
It is vital to monitor ocelots’ health and behavior in the wilding enclosure to inform management practices, including feeding, use of enrichment, and transition of ocelots to new settings, for example.
Camera monitoring
Since the enclosures will include Texas-Tamaulipan thornscrub communities, direct visual observation of ocelots in the enclosures will be difficult. Direct observation may also be disruptive to ocelots. Instead, remotely accessible (cellular signal-equipped) cameras should be placed around the enclosure to provide video monitoring of ocelot behavior in all areas of the enclosure. A grid system of cameras should be used to cover the entire enclosure. Additional cameras should be placed at high-use areas, such as watering points, the bottoms of feeding tunnels, the inside of nest boxes/dens, entrances of nest boxes/dens, and the area
surrounding nest boxes/dens. Prior to an ocelot’s placement in an enclosure, the vegetation there should be managed as necessary to ensure that the enclosure is not so densely vegetated that monitoring with camera traps is impossible (there must be some openings in vegetation to allow ocelots to be monitored by camera trap).
Feeding locations
The wilding facilities may also have locations where ocelots are fed above a hidden scale that is monitored by camera. Attracting an ocelot to food where it will have to step onto the scale and into live video monitoring will allow remote assessment of health. This area may also include doors that can be closed remotely to capture the individual for veterinary intervention.
Scat analysis
Ocelot scat may be collected from the wilding enclosures for analysis of diet and identification of prey species consumed. Scat may also be used for other health monitoring procedures.
Radio collar monitoring
Ocelots in the wilding enclosures can also be collared with expandable collars that automatically adjust with individual growth Ocelots must be large enough in size (typically collar weight cannot exceed 3% of individual body weight) to be monitored via collar; accordingly, kittens would not be collared. Collar monitoring of dams with kittens will provide additional activity data to supplement monitoring via camera. Though kittens will initially be too small to wear collars, collars could be placed once they become larger. Further, their collars could be used only for a short period of time and designed to drop off or expand in circumference to respond to growth. Subadults separated from their dams and appearing ready for transfer to the reintroduction site could be collared to allow more detailed monitoring of their behavior and suitability for release to the wild. Prior to transfer to the reintroduction site, all individuals should receive a new radio collar with full battery life. Collar monitoring of ocelots in the wilding enclosures can also be useful for collecting baseline activity data (resting, walking, jumping, hunting, etc.) that can inform analysis of wild-activity data obtained from ocelots who are collared and released to the wild.
Behaviors to monitor
Video monitoring (and collar monitoring where possible) should allow daily monitoring of ocelots’ behavior in the wilding enclosures. For each ocelot, a journal that contains a checklist of behaviors with additional detailed notes on the behaviors will allow for tracking of behavioral changes of individuals over time, assessment of behavioral development, and comparison between individuals. A professional animal behaviorist from a zoological or academic background, with the assistance of graduate student(s) and technicians as necessary, will be engaged to provide metrics for behavioral monitoring and to evaluate monitoring data based on these metrics. All monitoring will be used to inform management of ocelots in the facility and ultimately determine whether ocelots are suitable for release to the wild.
Behaviors to monitor in the wilding enclosures should include (though are not limited to) the following. The frequency, location, and time length of all behaviors should be recorded. A time budget for each individual
should be constructed regularly to record percent of time spent performing various behaviors, identity of conspecifics interacted with during the behaviors or proximity to other ocelots when behaviors were performed, and where the behavior occurred (three-dimensionally) in the enclosure.
Health
● Body condition score (see veterinary section)
● Signs of injury or illness
● Drinking water
○ Location
○ Source (tank versus natural pool)
● Vocalizations
○ Type
○ Social context
● Defecation and urination, potentially as marking.
Feeding
● Nursing
● Eating meat
● Eating dead or live prey
○ How prey was delivered to enclosure
○ Type of live prey (easy or difficult)
○ Where prey was obtained
○ How prey was obtained (who caught the prey)
● Feeding results
○ Fully, mostly, or partially ate item
○ Did not eat item
● Searching for food
● Hunting (stalk, rush, kill, consume), see below
● Stealing food from sibling or dam
● Etc.
Socializing
● Social activities and whether each is positive, negative, or neutral
○ Social playing with other ocelot (younger age)
○ Solitary play with objects (older age)
○ Social grooming
○ Self grooming
○ Threats (hissing, growling)
○ Fighting
○ Urine marking
○ Flehmen
● Participants
○ Who initiated social action
○ Who received interaction
○ Who stopped the interaction
● Time spent together versus apart
○ By activity type
● Distance between offspring and dam during different activities
Hunting behaviors
Play hunting
● Playing with prey or prey parts
● Fear of live prey
● Performance of single elements of hunting behavior, including stalk, rush, kill, or consume
● Performance of full series of hunting, from stalk to rush to kill to consume
Accompanying the dam who is hunting
● Watching the hunt
● Participating in the hunt
● Characterization of participation
● Catching prey
● Killing prey
● Playing with prey
● Etc.
Independent hunting without the dam
● With sibling or not
● Type of prey pursued
● Use of and success of elements of hunting behavior
● Searching, Waiting/watching, Stalking, Chasing, Pouncing, Catching, Killing, Eating
● Moving dead prey to a new location to eat
● Sharing prey with sibling or dam
● Etc.
● Play hunting with siblings/dam (stalking, jumping, etc.)
● Etc.
Resting or sleeping
• Time spent resting versus active
• Location of resting or sleeping
• In nest box/den
• Other location if not in nest box/den
• Distance between sleeping offspring and dam
Movements – measures of dexterity
● Use of different geographic locations in enclosure
● Climbing
● Jumping
● Etc.
Interactions with objects
● Scratching (potentially as marking) for claw sharpening
● Smelling objects
● Manipulating items with paws, nose, mouth
● Urine marking
Human interactions or other dangers
Interacting with man-made items
• Enrichment items
• Nest box
• Cameras
• Water tank
• Fence lines
• Etc.
Contact with people or dangerous objects
• Positive (approaching people), neutral, or negative (fleeing or hiding) response to people or any dangerous objects presented (actual or simulated predators, competitors, human objects, etc.)
• How ocelots were scared away from dangerous objects or people
References
Behavior of person scaring ocelots
• Yelling
• Throwing objects
• Etc.
[1] Vargas A, Breitenmoser C, Vreitenmoser U. eds. 2009. Iberian Lynx Ex situ Conservation: An interdisciplinary approach. Fundación Biodiversidad in collaboration with IUCN Cat Specialist Group.
[2] Rozhnov VV, Yachmennikova AA, Dronova NA, Pkhitikov AB, Magomedov M-RD, Chestin IE, Mnatsekanov RA, Blidchenko EY, Voshchanova IP, Alshinetski MV, Alibekov AB 2020. The restoration of persian leopard in the Caucasus (scientific approach). Moscow. KMK Scientific Press Ltd. 219 p.
[3] Tamboni T, Solís G, Peña J, Ríos Noya M, Di Martino S, Carro N, Paviolo A, De Angelo C, Di Bitetti M, Quiroga Ve, Donadio, E 2018. Proyecto de reintroducción del yaguareté (Panthera onca) en el Parque Iberá, Corrientes, Argentina. The Conservation Land Trust.
[4] Solís G, Peña J, Spørring K, Boixader J, Jiménez I. 2014 Programa de funcionamiento del centro experimental de cría de yaguaretés en la Reserve Iberá. Version 3.0. The Conservation Land Trust.
[5] McDougall PT, Réale D, Sol D, Reader SM. 2006. Wildlife conservation and animal temperament: causes and consequences of evolutionary change for captive, reintroduced, and wild populations. Animal Conservation 9: 39-48.
[6] Tetzlaff SJ, Sperry JH, DeGregori BA. 2019. Effects of antipredator training, environmental enrichment, and soft release on wildlife translocations: A review and meta-analysis. Biological Conservation 236: 324-331.
[7] French, JT, Silvy, NF, Campbell, TA Tomeček, JM. 2022. Divergent predator activity muddies the dynamic landscape of fear. Ecosphere 13: e3927.
[8] Leivers S, Campbell T, Bodenchuk M, Tomeček J. 2023. Behavior of wild pigs toward conspecific carcasses: implications for disease transmission in a hot, semiarid climate. Transboundary and Emerging Diseases, 4195199.
[9] Sergeyev M, Holbrook JD, Lombardi JV, Tewes ME, Campbell TA. 2022. Behaviorally mediated coexistence of ocelots, bobcats, and coyotes using hidden Markov models. Oikos e09480.
[10] Lombardi JV, Sergeyev M, Tewes ME, Schofield LR, Wilkins RN. 2022. Spatial capture-recapture and LiDAR-derived vegetation metrics reveal high densities of ocelots on Texas ranchlands. Frontiers of Conservation Science 3: 1003044.
[11] Santymire R. 2019. Saving the Black-Footed Ferret from Extinction: In Theory and Practice. In A. Kaufman, M. Bashaw, & T. Maple (Eds.), Scientific Foundations of Zoos and Aquariums: Their Role in Conservation and Research (pp. 440-474). Cambridge: Cambridge University Press.
[12] Swaisgood R, Shepherdson D. 2006. Environmental enrichment as a strategy for mitigating stereotypies in zoo animals: a literature review and meta-analysis. In Stereotypical Animal Behavior: Fundamentals and Applications to Welfare, 2nd edition. eds. Mason, G., & Rushen, J.
[13] Booth-Binczik, SD, Bradley RD, Thompson CW, Bender LC, Huntley JW, Harvey JA, Mays JL. 2013. Food habits of ocelots and potential for competition with bobcats in southern Texas. Southwestern Naturalist 58: 403–410.