When patients build medicine
MEDTECH Why Pediatric MedTech Breaks the Standard Playbook P30 FEBRUARY
CLINICAL TRIALS
Christine Nimalasiri on Clinical Trial Regret P60

FROM INNOVATION TO IMPACT How EvoMed is Rethinking Alignment in Healthcare P48



MEDTECH Why Pediatric MedTech Breaks the Standard Playbook P30 FEBRUARY
CLINICAL TRIALS
Christine Nimalasiri on Clinical Trial Regret P60

FROM INNOVATION TO IMPACT How EvoMed is Rethinking Alignment in Healthcare P48


The 2026 calendar year is underway, and there’s no better place to begin than Dubai - an emirate at the forefront of change and innovation. A concerted effort has been made to take our efforts to a global stage - and this year, MedTech World will host summits across the Middle East, West Palm Beach (USA), Hong Kong (Asia), and Malta. For the first time, four special editions of MedTech World magazine will also launch in tandem, each supporting its respective event. In many ways it feels like a milestone that marks our progress.
I hope you’ll find this issue as worthwhile as a read as it was to make. We’ve tried to include a little bit of everything. From the next big thing to inspirational tales, there’s plenty to unpack. Expect regional insights alongside deeply personal stories that showcase both triumphs and tribulations, with the spotlight firmly on the individuals leading the charge for change in medicine and healthcare worldwide.
For Shavini Fernando, Founder and CEO of OxiWear, it was profoundly personal. Faced with the choice between letting her condition define her life or finding a way to live safely on her own terms, Fernando chose a third path: she decided to build what did not yet exist. Her journey features as this edition’s cover story - follow it in full from page 38.
This spirit of personal agency sits at the heart of the edition. On page 32, Edwin Lindsay opens up about watching his newborn grandson undergo emergency heart surgery - a deeply moving moment that reshaped his perspective. As he explains, the stakes “became intensely personal.”
With all that said, there’s just enough space for thank yous. No list would be complete without mentioning Dr. Dylan Attard. His reputation precedes him. From a single first event in Malta five years ago to four quarterly global summits, he has quietly nurtured a vision that has fostered a powerful sense of trust, collaboration and engagement within the MedTech community. Backing this vision is sharp design and masterful storytelling, which have come together to create something quite special.
In short, healthcare innovation is having a moment and we’re here for it.
Katy Micallef, Editor MedTech World Magazine

















































Faced with the choice between letting her condition define her life or finding a way to live safely on her own terms, SHAVINI FERNANDO, Founder and CEO of OxiWear chose a third path. She decided to build what did not yet exist. 36

10
DR. NARGES
SHEIKHANSARI highlights the growing need to strengthen governance and better translate evidence into coordinated, preventive action.

:Newgevity
THORSTEN WALOSCHEK advocates for a life-course approach to health that treats pregnancy, birth and early childhood as the foundation of long-term resilience. 18

26
Beyond Capital: Redefining the Role of the Healthcare Investor

30

52
Women’s Healthcare from the Ground Up
Building technologies that genuinely improve human health requires more than funding rounds and financial projections.
RACHNA DAYAL shares her approach to investing.
Why Pediatric MedTech Breaks the Standard Playbook
Success in pediatric MedTech depends on designing with children in mind from day one, building flexible regulatory and clinical strategies, and using technology to support – but not replace – the critical decisions at the bedside, says EDWIN LINDSAY
SOPHIE SMITH on countering the challenges in women’s healthcare with a hybrid model that integrates technology directly into clinical pathways, rather than layering digital tools on top of existing care.

As a surgeon, I experienced the deep satisfaction of caring for one patient at a time. Over the years, however, a question stayed with me: what if that impact could extend far beyond a single operation room. The wish to improve healthcare on a broader, global scale is what ultimately gave life to MedTech World.
Six years ago, we held our first summit in Malta. It was modest in size but ambitious in vision. What began as a single gathering has evolved into a global movement, bringing together innovators, investors, hospitals, insurers, and providers across continents. Today, our events span from Dubai to Singapore, Hong Kong, Houston, San Jose, and cities throughout Europe. We have become known for attracting the minds that genuinely move medical technology forward.
Dubai holds a special place in our journey. It was among our earliest events, and it remains one of our favourites.
The Middle East embodies precisely what MedTech World stands for. The region's openness to new ideas, its genuine interest in nurturing investment, and its genuine welcome of international talent have impressed us profoundly. UAE demonstrated that appetite for meaningful collaboration and innovation that we seek to foster across all our summits.
MedTech World exists as more than a series of conferences: we have built a global connector that brings together innovation, funding, expertise, and the practical adoption of solutions, creating partnerships that turn ideas into real-world impact.
In 2026, we will host four quarterly summits, each anchored in a region of genuine strategic significance: Dubai in the Middle East, West Palm Beach in the USA, Hong Kong in Asia, and Malta, our home base in Europe. Throughout the year, these events will underscore our commitment to being a true global connector of ideas, partnerships, and opportunities for those seeking to shape the future of healthcare innovation.
I remain grateful to our community, partners, and attendees for their steadfast support. It is an honour to nurture this trust and to bring together minds committed to advancing healthcare. I invite readers of this magazine to join us, to participate in what we are building, and to become part of a movement dedicated to creating meaningful change in medicine and healthcare globally.
Dr. Dylan Attard MD, MRCSI, MEnt. CEO AND CO-FOUNDER, MEDTECH WORLD



Modern healthcare systems are under unprecedented strain. The global rise of chronic diseases, mental health challenges, aging populations, and climate-related health risks have exposed the limits of infrastructures designed primarily for acute, episodic care. According to DR. NARGES SHEIKHANSARI, an executive advisor and public health expert, “healthcare systems are confronting complex, long-term health risks using frameworks built for short-term medical intervention,” highlighting the growing need to strengthen governance and better translate abundant evidence into coordinated, preventive action across sectors.
The need for alignment becomes particularly evident in how longevity is defined. For decades, increased life expectancy has been the major measure of success, often overlooking the quality of life in those added years.
Dr. Sheikhansari calls for a more meaningful reframing, one that moves beyond survival to sustained wellbeing. “Longevity should be redefined not as the extension of life alone, but as the extension
Healthy choices are only possible when systems make them accessible and sustainable.
of healthy, functional, and meaningful years of life.”
Reframing longevity around healthspan places physical function, cognitive resilience, and social participation at the center of public health strategy. It also shifts the policy lens upstream, recognizing that healthy aging is shaped across the life course through early investment in mental wellbeing, metabolic health, supportive environments, and social connection.
This broader perspective helps explain why population health has become central to sustainable healthcare. Poor population health is no longer solely a clinical concern; it carries significant economic and social implications. Rising non-communicable diseases reduce workforce participation, increase long-term care needs, and place sustained pressure on public finances. “Healthcare systems cannot compensate indefinitely for poor population health,” Dr. Sheikhansari explains. “When governments invest in population health, they reduce long-term healthcare expenditure, strengthen workforce resilience, and support social stability.”
Despite growing consensus around prevention, structural constraints continue to shape how health systems operate. Financing mechanisms still prioritize treatment volume over outcomes, and performance is often assessed by services delivered rather than disease prevented. Prevention, by contrast, delivers its benefits over longer time horizons and across multiple sectors, requiring coordination that extends well beyond the healthcare system. “Prevention demands shared accountability,” she emphasizes. “Without aligned incentives and cross-sector governance, systems remain reactive; treating consequences rather than shaping conditions for health.”
The challenge is particularly visible in the global rise of non-communicable diseases. Conditions such as obesity, diabetes, and cardiovascular disease are influenced by food systems, urban design, work patterns, stress, and socioeconomic factors; determinants that sit largely outside the healthcare settings. While individual behaviors and choices play a significant role, the living and working environments also matter. “Healthy choices are only possible when systems make them accessible and sustainable,” Dr. Sheikhansari notes. “Public health succeeds when responsibility is shared between individuals, institutions, markets, and governments.” These dynamics also help explain why reactive models of care are increasingly being reconsidered. Systems designed primarily to respond after illness occurs face growing pressure
as populations age and multimorbidity becomes more common. Dr. Sheikhansari asserts that “addressing these realities requires a stronger emphasis on predictive analysis, early intervention, and policy-led prevention.”
Healthcare systems are confronting complex, long-term health risks using frameworks built for short-term medical intervention.
Crucially, decisions made today have consequences far beyond the short-term health outcomes. Policies on nutrition, mental health, education, urban planning, and women’s health establish the foundations into which the next generation is born. “Public health is a legacy function,” Dr. Sheikhansari reflects. “Every policy decision either compounds risk or builds resilience over time.” Redefining public health in this way requires more than technical reform. It demands long-term vision, coordinated governance, and a societal shift that values prevention and healthy choices. The transition from reactive care to population-level prevention is not optional; it is essential to building resilient societies capable of thriving in the decades ahead.
In medical device innovation, failure sometimes happens because the technology does not work. More often, however, it happens much later, when a product that is clinically sound collides with the complexity of real healthcare systems. Few people understand this gap better than SHAI POLICKER, Managing Partner of Edge Medical Ventures, a US-Israeli medical-device focused venture capital fund and venture studio, which invests in category-defining early stage medical device innovation working in collaboration with the Israeli innovation authority and the New-Jersey economic development authority. Policker has spent more than 25 years working across Israel, Europe and the United States, helping MedTech companies move from development to commercialization.
Policker’s perspective is shaped by contrast. Israel is one of the world’s most prolific hubs for medical device innovation, known for its engineering talent, speed, and problem-solving mindset. But the US healthcare market – the destination for many of these startups – operates under very different rules. “When you are based in Israel and occasionally visit the US, you only get limited exposure to this complexity,” he explains. Living and working in the US, and experiencing the system as a patient as well as a professional, reveals a reality that is difficult to grasp from afar.
That reality is a healthcare ecosystem in general and medical devices specifically where the user, buyer, payer, and decision-maker are rarely the same. Physicians, hospitals, insurers, distributors, and regulators all play distinct roles, and any one of them can become a blocking point. In Policker’s words, “it only takes one of them to become an obstacle for your product to fail.” Understanding this ecosystem is not an option, it’s the very foundation for your product to succeed.
This is where many earlystage MedTech companies struggle. Founders often invest enormous effort into clinical validation and regulatory approval, assuming that commercialization will naturally follow. In practice, it rarely does. Reimbursement, in particular, is consistently underestimated. It feels distant
when a company is still in R&D, and there is often a quiet hope that a strategic acquisition will happen before real market entry is required. “Once you reach commercialization,”
Policker notes, “Even when proper ground work has been done, securing orders with hospitals and clinicians and getting payers to cover and pay is without exception a very bumpy road, full of surprises – mostly unpleasant ones. When the original analysis and preparation of the commercial channels is superficial and naive then startups end up spending a lot of money and time figuring out the right path while running out of cash with no revenues and skeptical investors”
Edge Medical Ventures was created to address this exact gap. Positioned as a bridge between Israeli MedTech startups and the US market, Edge provides hands-on support across the most fragile stages of commercialization: Alignment of R&D with early commercial strategy, reimbursement and sales channel exploration and preparation, local hiring, logistics, distribution, and contracting. The goal is to provide the medtech startups value beyond funding and an unfair advantage - Capital is expensive at this stage, but time is even more so. Figuring out the optimal regulatory pathway, the optimal market entry point and the optimal commercial structure at this point can mean the difference between survival and collapse.
Policker is particularly blunt about how seemingly “simple” decisions can become fatal if mishandled. Incorporating in the US, and hiring a local clinical and commercial team can quickly drain resources . Here the secret is sometimes resource sharing between companies when possible and growing overhead cautiously. On the product side, overengineering is another common trap. Designing for the minimal viable product that is still attractive to customers addressing a real market need – rather than packing in every possible feature – can also help companies reach the market earlier and avoid unnecessary regulatory and reimbursement barriers.
Despite these challenges, Policker remains deeply optimistic about Israeli MedTech. The country’s strength
Even when proper ground work has been done, securing orders with hospitals and clinicians and getting payers to cover and pay is without exception a very bumpy road, full of surprises – mostly unpleasant ones.

When the original analysis and preparation of the commercial channels is superficial and naive then startups end up spending a lot of money and time figuring out the right path while running out of cash with no revenues and skeptical investors.
lies in its multidisciplinary thinking and its ability to deliver results under tough conditions.
Looking ahead, the problem that increasingly captures his attention is not a single disease or device category, but a systemic constraint: the global shortage of healthcare professionals. Technologies that meaningfully reduce clinician workload, extend capacity, or compensate for workforce gaps are likely to define the next wave of impactful MedTech innovation.
What we can learn from Policker’s experience is that in healthcare, success is not decided by how impressive a technology looks in isolation, but by how well it navigates a complex, human, and highly constrained system. Innovation may begin in the lab, but it is tested, often brutally, in the market. And it’s this broader picture that professionals should always be looking at.
For decades, longevity has been treated as a problem to solve later in life. We invest in managing chronic disease, slowing decline, and extending lifespan once damage is already visible. THORSTEN WALOSCHEK, a MedTech executive and CEO of NeoPredics, with more than two decades of experience in maternal and neonatal healthcare, argues that this framing is fundamentally backwards. Longevity, he says, does not begin in midlife or old age. It begins at the very start of life.

Waloschek calls this shift Newgevity: a life-course approach to health that treats pregnancy, birth and early childhood as the foundation of long-term resilience. “Longterm health is not something that begins in midlife or old age,” he explains. “It is established from the very beginning of life, when biology is most adaptable.” In that window, small changes can shape health trajectories for decades.
His conviction comes from experience. Across leadership roles in newborn care, including
at Draeger Medical and NATUS Newborn Care, Waloschek saw the same pattern repeat Healthcare had become extraordinarily good at reacting to emergencies, yet far weaker at anticipating them. “We had incredible technology to manage the crisis,” he recalls, “but we weren’t doing enough to predict and prevent problems before they became critical. Every adverse outcome felt like a missed opportunity.”
That insight led him to NeoPredics, where the focus is not on automation for its own
Technology only has value in healthcare if it earns the trust of clinicians and fits naturally into clinical decision making.

sake, but on clinical decision support that helps clinicians act earlier and more precisely.
NeoPredics develops predictive algorithms for maternal and neonatal care, built around real clinical decision points and designed to fit into existing workflows. The goal is simple: deliver the right insight at the right time, in a way clinicians can trust and use..
Trust is not a slogan, it is a design requirement.
“Technology only has value in healthcare if it earns the trust of clinicians and fits naturally into clinical decision making,” Waloschek says. Rather than building black-box models, NeoPredics prioritizes interpretability, workflow fit and real-world validation. In practice, that means tools that translate data into clear guidance, earlier monitoring when needed, preventative treatment when appropriate, earlier discharge if safe and timely escalation when risk is rising.
NeoPredics’ work is grounded in clear clinical use cases. Its first solution, BiliPredics, support newborn jaundice management by forecasting bilirubin trajectories, helping care teams intervene earlier and reduce avoidable and costly readmissions.The company has also developed PreFree, a prediction tool that flags risk of severe adverse outcomes related to preeclampsia, early enough to support timely intervention. These are exactly the moments Newgevity is about, moving from rescue
care to prevention. At the same time enabling reduction in length of stay whenever possible.
Waloschek is blunt about the limits of prediction without action. “Prediction without action is just anxiety,” he notes. A risk score alone does not improve outcomes. What matters is whether the insight arrives early enough, is understandable in seconds, and clearly guides what to do next.
Despite the stakes involved, maternal and pediatric health remain structurally underfunded. Waloschek points out that only around 2-3% of global MedTech investment flows into this space, even though it touches every human being at their most vulnerable stage. The reason, he argues, is not lack of impact, but misaligned incentives. Returns from early intervention unfold over years, while investment cycles tend to chase short-term wins. “It’s not just inequitable,” he says. “It’s economically shortsighted.”
The consequences are generational. Poor outcomes in pregnancy and infancy increase lifetime disease risk, and shape the health of the next generation. Conversely, earlier optimization creates a compounding effect, where resilience carries forward.
If Newgevity were adopted as a guiding principle across healthcare systems, it would represent a paradigm shift. Early life would be treated as strategic infrastructure, not a niche specialty. Prevention would become proactive and data-driven, guided by individualized risk rather than population averages. Care would be longitudinal, with insights from pregnancy and childhood informing decisions later in life.
“The technology largely exists today,” Waloschek emphasizes. “What we lack is systemic commitment.”
Newgevity is not about abandoning older people, everyone deserves care at every stage. It is about building stronger foundations from day one, so more families get the start they deserve.
Newgevity: the

Innovation in healthcare is often celebrated for its breakthroughs, yet the real challenge lies in turning promising ideas into everyday practice. Around the world, new solutions can struggle to move from concept to clinic, not because they lack potential, but because health systems are rarely designed to support seamless adoption and sustained impact.
In Dubai, the establishment of Dubai Health, the emirate’s first integrated academic health system, brought care delivery, medical education, research, and philanthropy together within one structure. As YACINE HADJIAT, Head of Dubai Health Innovations at Dubai Health and Faculty at MBRU College of Medicine, explains, “Following COVID-19, Dubai fundamentally rethought how public healthcare should be organised... The idea was to move away from silos and build a model where clinicians, researchers, educators, and philanthropies are aligned around a shared promise to put the patient first.”
This connected model has important implications for innovation. Implementation is often the point at which healthcare innovations face the most difficulty, when ideas encounter clinical realities, operational pressures, or fragmented governance. Dubai Health Innovations was designed to operate inside this healthcare system, translating real clinical needs into solutions that can be tested, adopted, and scaled across everyday care settings. As Hadjiat notes, “Innovation often fails not because the idea isn’t good, but because implementation is hard.”
That perspective shapes how innovation is defined operationally. At Dubai Health, innovation is not measured by novelty, but by value. “Innovation is defined in very concrete terms: it’s about adding value,” Hadjiat says. “What matters is not just the idea, but the
Innovation often fails not because the idea isn’t good, but because implementation is hard.
ability to implement it, adopt it, and scale it within the health system. Innovation is therefore not only invention, but true, adopted change.” This framing deliberately shifts attention away from invention and toward execution, the point where many healthcare innovations struggle.
One of the reasons Dubai Health can take this approach is its integrated model, which embeds research and education directly within clinical environments. Academia and research are not peripheral actors but active contributors to patient care, ensuring that insights move continuously between practice and development. “They help build a sustainable innovation ecosystem by grounding research and education in real clinical needs.
A portfolio of more than 20 active innovation projects address care-pathway, operational challenges, and integration barriers common to large healthcare systems. Being an integrated academic health system, shortens feedback loops, aligns stakeholders, and makes it easier to test solutions where care actually happens. Integration does not eliminate complexity, but it makes complexity manageable.
Interdisciplinary collaboration is another defining feature.
Under Dubai Health Innovations, clinicians, researchers, technologists, designers, and industry partners work together. “The value of this

model is simple,” Hadjiat notes. “It brings all the capabilities needed to move from problem to solution into one place.”
Clinicians define real problems, researchers bring evidence, technologists build and test, and partners support scaling.
International collaborations further reinforce this strength. Dubai Health connects local clinical priorities with global expertise, ensuring that solutions benefit from international best practice
while remaining practical and relevant within the local system.
Ultimately, what sets Dubai Health’s approach apart is the commitment to embedding innovation across the entire system. By aligning care, learning, discovery, and giving, Dubai Health ensures that innovation translates into a future ready workforce, better patient experiences, improved health outcomes, and elevated standard of care for patients and families.
In healthcare and MedTech, capital is necessary – but rarely sufficient. Building technologies that genuinely improve human health requires more than funding rounds and financial projections. It demands patience, technical understanding, and most importantly a willingness to stay close to the realities of medicine long after the check is written. This belief sits at the core of how RACHNA DAYAL, Founder and Managing Partner at Sugati Ventures, approaches investing.
With an engineering background and professional experience spanning three continents, Dayal brings a perspective that is both global and deeply technical. Today, she focuses on medical devices and AI-enabled platforms. Not because healthcare is fashionable, but because it is one of the few sectors where impact is unquestionable: “There was no doubt in my mind that healthcare was one of these areas where I would not question impact: every medical device, pharma drug or AI platform that I work with either saves lives or makes people healthier and happier. That is where I draw my inspiration from”, she says.
Dayal’s global experience strongly shapes how she evaluates innovation.
Opportunity size, in healthcare, cannot be confined to a single geography. Diseases do not respect borders, and neither should solutions. “If a medical device or platform cannot create impact globally, it becomes a limited vision,” she explains. At the same time, global thinking is not about assuming uniformity: ““True innovation comes from diverse perspectives. How we lead our lives, how we deal with situations, our culture, our climate, it all shapes our perspective and thinking process.” True scale requires understanding both similarities and differences, a balance she sees many companies underestimate.
Technical depth is also fundamental in healthcare investing. Being able to quickly evaluate the strengths and weaknesses of machines, software, and AI architectures allows her to go beyond surface-level narratives.
What most clearly differentiates Dayal, however, is her view of what it means to be an investor in healthcare. “I believe a VC’s job begins with writing the check and not ends with it,” she says. In MedTech and HealthTech, startups face a uniquely complex set of hurdles: regulatory approval, human safety, clinical trials, usability, clinician adoption, reimbursement, and payer alignment – often all at once. Capital alone does not solve these problems.

As a hands-on investor, Dayal is offering operational guidance, strategic input, and access to a global network of clinicians, partners, and co-investors. This advisory role reflects a broader shift she believes is necessary in regulated sectors. Investors must continue learning, stay close to evolving technologies, and recognize the limits of their own expertise. No one can master every dimension of healthcare, which is why Dayal relies on a collective model, drawing on expert advisors who bring complementary skills to the table.
Of course, nowadays many healthcare investors are focused on AI. But Dayal is wary of hype. She avoids startups that are little more than “wrappers” around foundational models, or those unable to work with unstructured data, which is a critical limitation in a field where clinical notes, images, voice data, and fragmented records are the norm. If a system cannot handle that complexity, she questions whether it can truly function in healthcare at all.
More fundamentally, Dayal believes AI should not be treated as a standalone feature. Instead, she envisions it as an operating system embedded across healthcare environments. Hospitals, she notes, are already overwhelmed by disconnected point solutions that do not communicate with one another. What they need are integrated AI systems working quietly in the background, processing data, automating tasks, supporting reimbursement workflows, and optimizing clinician scheduling – all without adding friction or compromising safety.
This philosophy is reflected in the projects Dayal feels most connected to within her portfolio. One is Neurava, a company addressing the challenge of sudden death in epilepsy. By combining medical devices with AI, the team monitors seizure-induced cardiorespiratory collapse and alerts caregivers and clinicians in real time.
Another is Perceive Now, a company developing an agentic AI operating system designed to function across messy, incomplete, and unstructured data environments. Built with multiple layers of validation and enterprise-grade AI swarms, the platform aims to deliver explainable, auditready decisions by default – a critical requirement in healthcare and other regulated industries.
There was no doubt in my mind that healthcare was one of these areas where I would not question impact: every medical device, pharma drug or ai platform that I work with either saves lives or makes people healthier and happier.
Behind these roles, however, there is also a deeply personal dimension. This past year marked the loss of Dayal’s father, an experience that shaped her reflections on purpose and legacy. He instilled in her a sense of confidence and freedom, encouraging her to think globally and pursue unconventional paths. In an industry often reduced to capital flows and technology stacks, this is a reminder for everyone of us that behind every ambition and achievement, there is a human story.
And this is what we can learn from Dayal’s story and approach to healthcare: healthcare innovation is ultimately built by people, for people. And the role of the investor, when done well, is not to stand apart from that reality –but to engage with it from start to end, and even beyond.


EDWIN LINDSAY is a seasoned expert in Regulatory, Quality, and Clinical for early-stage MedTech, currently Co-Founder and Director at SURE, as well as Principal Consultant and Managing Director at Compliance Solutions (Life Sciences). Lindsay has spent decades guiding startups through the complexities of bringing medical devices to market, yet it was a deeply personal experience that reshaped his perspective very recently, in the closing moments of 2025: watching his newborn grandson undergo emergency heart surgery.
Every step done right early on can save time, reduce rework, and most importantly, change a child’s outcome.
usually doesn’t work. Children grow, anatomy changes, physiology is different. That means more scientific and design complexity, more validation work, and often more SKUs – all for a market that can look less attractive on a spreadsheet or balance sheet.”
“Before my grandson’s emergency surgery, I genuinely believed the work I do in Regulatory, Quality, and Clinical mattered—but in a way that was still professional and sometimes a bit abstract,” Lindsay recalls. “Sitting through those night shifts at his bedside flipped that completely. Now, I don’t just see ‘a clinical protocol’ or ‘a regulatory strategy’ as a deliverable –I see it as something that can directly shape whether a child gets access to a lifesaving intervention in time, whether care teams have better tools, and whether families get a different or better outcome. The stakes became intensely personal.”
For Lindsay, the pediatric MedTech space exposes a unique set of challenges that go far beyond what founders might expect. “Paediatric innovation is still underserved not because people don’t care – but because the system isn’t built to reward it,” he explains. “Patient populations are often smaller, the needs are more specialised, and ‘just shrinking the adult version’
This complexity translates into regulatory and clinical hurdles that can trip up even the most passionate innovators. According to Lindsay, “Earlystage pediatric MedTech doesn’t fail because founders don’t care. It struggles because the regulatory and clinical path is simply harder and less forgiving than most people expect. Adult data often won’t translate cleanly, and ‘it’s the same device, just smaller’ usually doesn’t hold up once you factor in anatomy, physiology, and growth. Patient numbers can be small, the right specialist sites are limited, and recruitment can be slow – often happening in highstress, high-stakes moments for families. Endpoints can be messy too, because outcomes look different across development stages, ages, and weights, and follow-up may need to account for durability over time as kids grow.”
For startups entering this space, the risk of missteps is real, but still avoidable with disciplined early planning. Lindsay advises: “The most practical thing founders can do early is be disciplined about intended use and claims from day one, because a few words about age group, user, setting,
or ‘diagnose vs support’ can completely change the regulatory pathway and the clinical evidence burden. And before anyone writes a ‘perfect’ clinical protocol, engage real paediatric clinicians early – get KOL/site feedback on who the realworld patients are, how the pathway actually works, what endpoints are meaningful, and whether recruitment and follow-up are feasible at all.”
Despite the challenges, Lindsay remains inspired by early-stage innovations that are already making an impact. He cites Seluna, a Scottish startup developing software to help clinicians rapidly identify pediatric sleep disorders: “What makes this so meaningful is that in pediatrics, the biggest harm is often time delays to diagnosis (or misdiagnosis), which can quietly compound into missed development, worsening symptoms, and huge stress for families. Tools that improve triage don’t just ‘speed up admin’- they help scarce specialist capacity reach the right kids sooner. Work like this is a reminder that earlystage innovation can change outcomes long before a child ever reaches a hospital bed.”
Looking forward, Lindsay sees emerging technologies like AI as powerful enablers, but only if applied responsibly. “AI is going to be a powerful tool in regulatory and clinical development, but it’s not a strategy on its own – and it’s definitely not automatically ‘the answer.’ If used well, it can remove friction: improving documentation consistency, supporting signal detection, and helping teams structure evidence, trend analysis, and post-market monitoring in a more systematic way. The risk is when AI gets bolted on because it’s fashionable or investors want an ‘AI story’ – because then it adds complexity, validation burden, cybersecurity, explainability challenges, and ongoing change-control headaches without improving outcomes.”
Ultimately, Lindsay’s reflections highlight a clear truth: success in pediatric MedTech depends on designing with children in mind from day one, building flexible regulatory and clinical strategies, and using technology to support – but not replace – the critical decisions at the bedside. “Every step done right early on can save time, reduce rework, and most importantly, change a child’s outcome. That’s what this work is really about.”














































Photos by Dhanush De Costa

OxiWear did not begin as a startup idea, a research grant, or a market opportunity. It began as a survival strategy.
For SHAVINI FERNANDO, the founder and CEO of OxiWear, medicine was not an abstract system or a professional domain. It was something she depended on, something that had already failed her in critical ways. Living with pulmonary hypertension, her oxygen levels could drop dangerously low without warning. There were no symptoms to rely on, no signals her body could offer before it was too late. The consequences were devastating: four cardiac arrests and three strokes, often occurring before anyone, including Fernando herself, realized something was wrong.
Doctors advised her to avoid living alone, climbing stairs, exerting herself, or doing many of the things that defined her independence. The implicit message was clear: safety required limitation. For Fernando, that was not an acceptable trade-off.
“OxiWear was born directly from my experience living with silent hypoxia,” she explains. “My oxygen levels could drop dangerously low without any warning symptoms. Because of that, I suffered four cardiac arrests and three strokes – events that often happened before anyone, including me, realized something was wrong.”
Faced with the choice between letting her condition define her life or finding a way to live safely on her own terms, Fernando chose a third path. She decided to build what did not yet exist.
“I didn’t want false reassurance or a lifestyle gadget,” she says. “I wanted agency.”
That word – agency – sits at the center of OxiWear’s story. From day one, OxiWear was designed to give patients real-time visibility into silent, life-threatening oxygen drops during everyday activity, so they could make informed decisions in the moments that mattered most.

This patient-first origin is what fundamentally differentiates OxiWear from much of the wearable technology market. Fernando did not approach the problem as a clinician, researcher, or engineer trying to optimize an existing category. She approached it as someone living inside the gaps of the healthcare system, gaps that are often invisible from the outside.
“The biggest lesson is that patients live in the gaps of the healthcare system every day,” she says. “Those gaps are often invisible to clinicians, researchers, and companies unless they’re willing to listen deeply. As a patient, I wasn’t thinking in terms of publications
From the very beginning, many people told us it couldn’t be done,” she recalls.
“We were told repeatedly that we would fail. Instead of discouraging us, that skepticism became fuel.”
We don’t smooth over data to make it look reassuring – we preserve clinically meaningful signals, even when they’re uncomfortable.
or market categories – I was thinking about survival, quality of life, and peace of mind.”
Those gaps are not theoretical. They exist between clinic visits, outside controlled environments, and during movement, precisely when many traditional monitoring tools fail. Most pulse oximeters and oxygen-monitoring systems are designed and cleared for patients at rest. But for many cardiopulmonary conditions, the most dangerous oxygen drops happen during activity.
That insight shaped every technical and strategic decision behind OxiWear. It also led Fernando into unfamiliar and unforgiving territory: regulated medical hardware.
Her background spans software, design, and immersive technologies, but entering med-tech meant starting over. Electrical engineering, sensor design, biomedical signal processing, algorithm development, much of it she studied herself, determined to understand the system deeply enough to challenge it when necessary. Over time, she assembled a team of specialists who shared the
same commitment to rigor and purpose, not just execution.
What surprised her most was not the complexity of the technology, but the rigidity of the regulatory framework.
“When we entered the FDA process, we quickly learned that many regulatory testing requirements are applied almost uniformly – whether you’re building a CT scanner or a wearable device and protocols for testing are not inclusive of skin tones” Fernando says. “The framework hasn’t fully evolved to reflect the nuances of modern, body-worn technology.”
Rather than accepting those constraints at face value, OxiWear engaged directly with regulators, explaining not just how the technology worked, but why it mattered. Why motion mattered. Why the ear – long dismissed as a viable measurement site – could provide more reliable physiological data during activity. Why testing across all skin tones for accuracy was essential, And why certain legacy tests, while appropriate for stationary equipment, were not meaningful for a wearable designed to function in real life.
At the same time, the company faced another structural challenge: funding. Hardware is notoriously difficult to finance. Regulated medical hardware even more so. And for Fernando, who was neither a U.S. citizen nor a permanent resident at the time, everything depended on external investment. Many potential investors expressed interest, but on one condition: change the mission.
“That was never an option,” she says. “Compromising patient safety or impact was never on the table.”
Instead, OxiWear took a deliberate and often overlooked path. When the first version of the device launched in 2022, it was positioned closer to the wellness space, not because the vision was smaller, but because survival demanded proof. The company needed to demonstrate that accurate oxygen measurement during movement was possible, that people genuinely needed it, and that the technology worked outside the lab.
What followed validated the original premise. Patients for whom oxygen monitoring was a safety requirement, and not a nice-to-have, actively sought out the device. That realworld adoption generated meaningful usage data, refined the product, and demonstrated impact across multiple verticals, from chronic disease management and post-acute care to aerospace, military, and high-risk occupational settings.
Crucially, it also enabled OxiWear to secure the funding necessary to complete the FDA process. In August 2024, OxiWear received FDA clearance, becoming the only FDAcleared wearable capable of measuring blood oxygen levels during physical activity with proven accuracy across all skin tones. For Fernando, the milestone was deeply personal.
“From the very beginning, many people told us it couldn’t be done,” she recalls. “We were told repeatedly that we would fail. Instead of discouraging us, that skepticism became fuel.”
The team worked through years of uncertainty, often for minimal compensation, driven by belief rather than guarantees. FDA clearance was not just validation of the technology, it was recognition that a patient-driven vision could meet the highest medical standards.
“As a patient, FDA clearance felt like validation that taking control of my own safety – and refusing to let my condition define my limits – was the right decision,” Fernando says. “What began as a way for me to protect myself had grown into a medical device held to the highest standards of safety and effectiveness.”
Professionally, clearance transformed OxiWear’s role in the healthcare ecosystem. Clinicians began to see it not as an experimental or supplemental device, but as a legitimate medical
tool. Partners saw credibility and long-term viability. And patients saw something even more important: trust.
That trust rests on a fundamental distinction between OxiWear and most consumer wearables. The difference is not form factor or user interface, but the very intent of the device.
“Consumer wearables are designed for trends, averages, and lifestyle insights,” Fernando explains. “OxiWear is designed for clinical decision-making, prevention, and safety. We don’t smooth over data to make it look reassuring –we preserve clinically meaningful signals, even when they’re uncomfortable.”
Today, OxiWear is being used across a wide range of clinical settings, from pulmonary hypertension and COPD to post-surgical recovery, oncology, and emergency care. It has proven especially valuable for patients who are difficult to assess with traditional finger-based sensors, including those with poor peripheral perfusion or autoimmune conditions. Beyond healthcare, adoption is growing in environments where realtime physiological insight can prevent catastrophe rather than respond to it.
Looking ahead, Fernando’s vision extends beyond oxygen alone. The next-generation OxiWear device aims to deliver deeper, real-time cardiovascular insight, capturing how the heart and lungs respond together during everyday life. The goal is to shift care from reactive to proactive, giving clinicians and patients earlier signals that something is changing, before symptoms escalate.
At its core, however, the philosophy remains unchanged. OxiWear exists because one patient refused to accept the limits imposed by a system not designed for real life. It is a reminder that some of the most meaningful medical innovation does not come from optimizing what already exists, but from listening to those who live with its failures.
OxiWear

Beyond the Pilot:

Standfirst: As the MENA region transitions from healthcare pilots to national infrastructure, "Enterprise Readiness", defined by rigorous regulation, proactive cybersecurity, and seamless interoperability, has become the mandatory currency for scaling MedTech innovation.
WORDS BY WARA SAMAR
For the last five years, the Middle East and North Africa (MENA) region, spearheaded by the digital-first ambitions of Saudi Arabia and the UAE, has served as a global laboratory for health innovation. From AI-driven diagnostics in Riyadh to the integration of genomics in Dubai, the region has successfully leapfrogged legacy infrastructure. However, as we move through 2026, a quiet but significant friction point has emerged. While the region is awash in medical innovation, it is struggling with implementation.
This is the MedTech Procurement Paradox: we have some of the world’s most ambitious healthcare buyers and visionary sovereign wealth funds, yet we have a shrinking pool of vendors who are truly "Enterprise Ready." In this new era, clinical efficacy is no longer the sole ticket to entry. To succeed today, a MedTech firm must be as proficient in cybersecurity and meeting regulatory compliance as it is in medicine.
The "wild west" of digital health pilots is officially over. Regulatory bodies, most notably the Saudi Food and Drug Authority (SFDA) and the Emirates Drug Establishment (EDE), have matured into world-class entities. They have shifted toward a "Reliance Model," which aligns regional approvals with global gold standards like the EU Medical Device Regulation (MDR).
For a MedTech startup or an international firm looking to enter the MENA market, this means that innovation is no longer simply about a sleek user interface or a promising algorithm. It is about a rigorous Technical File and a Quality Management System (QMS) that can withstand 2026-level scrutiny. Procurement teams at national providers are now looking for regulatory sustainability, the assurance that a product won't be pulled from the shelf because its certification was built on a shortcut.
In 2026, a medical device is more than a tool; it is a network node. As healthcare data accounts for an increasingly massive share of the world’s digital footprint, the MENA region has become a prime target for sophisticated cyber threats. Consequently, cybersecurity has shifted from an IT checkbox to a core clinical requirement.
Modern procurement reality dictates that if a device cannot demonstrate resilience against ransomware or provide a clear Software Bill of Materials (SBOM), it is a liability, not an asset. Compliance with the UAE’s Health Data Protection Law and Saudi Arabia’s National Cybersecurity Authority (NCA) standards is now non-negotiable. Hospitals are no longer just asking, "Does this save lives?" They are asking, "Does this compromise my entire network?" Cybersecurity is now the new clinical vital sign.
The final hurdle is "New Procurement Reality." Hospital CFOs and government health ministries have moved away from purchasing isolated tools toward investing in ValueBased Care ecosystems.
The greatest barrier to scaling in MENA today is a lack of Interoperability by Design. If an AI diagnostic tool cannot feed data seamlessly into
national Electronic Health Records (EHR) or unified platforms like Malaffi or Seha Virtual Hospital, it becomes a "data island." At a time when data liquidity is the goal, data islands are being systematically decommissioned. True enterprise readiness means a product is built to talk to other systems from day one, using global standards like FHIR and HL7.
The Path Forward
To bridge the gap between innovation and implementation, we need a two-pronged approach:
1. Regulators must continue the push toward GCC Regulatory Convergence. Harmonizing standards across borders will reduce the "compliance tax" that often prevents the most innovative, smaller players from scaling across the region.
2. Innovators must stop viewing regulation and cybersecurity as "red tape." In 2026, these are your most powerful sales features. A startup that leads with its security architecture and regulatory roadmap will win over a competitor that leads only with a clinical pilot.
The MENA region is no longer just a promising market; it is a global leader in healthcare transformation. But to maintain this momentum, our innovators must stop building for the laboratory and start building for the Enterprise. The future belongs to those who are not just clinically brilliant, but infrastructure-secure.


In the MedTech industry, innovation is often framed as a technical challenge: better algorithms, more data, smarter systems. But for AMEL HAVKIC, Founder & CEO at EvoMed Consulting, that framing misses the point. As a practicing physician turned strategic advisor, his work sits at the uncomfortable intersection where innovation meets the realities of clinical practice – and where many promising solutions quietly fail.
Havkic’s background in intensive care, respiratory medicine, and private practice fundamentally shapes how he approaches strategy today. In clinical environments, decisions are rarely made under ideal conditions. Time pressure, cognitive overload, imperfect workflows, and human factors are part of everyday reality. “Healthcare is not IT,” he says. Technology may be involved, but lives are on the line, and the margin for error is thin.
This perspective led Havkic to found EvoMed Consulting, a firm positioned not as a traditional consulting provider, but as a strategic partner deeply embedded in the logic of healthcare systems. His work focuses on one recurring problem: innovation that looks strong on paper but collapses at the point of adoption. Not because the
idea is flawed, but because it was never aligned with how healthcare actually functions in real life practice.
Over the years, working with MedTech startups, established companies, hospitals, and increasingly public institutions, Havkic began to notice the same pattern repeating itself. Teams would optimize one dimension – be it product features, clinical validation, reimbursement strategy, or marketing – while neglecting others. The result was fragmentation. Solutions that were technically sound failed to integrate into clinical workflows. Products with clinical value struggled to gain trust. Projects stalled despite clear demand.
With unlimited resources, you could generate the best outcome for every patient. In the real world, the challenge is achieving the best possible outcomes for the largest number of patients, while remaining sustainable.
EvoMed
The root cause, Havkic argues, is systemic misalignment. Healthcare is a complex ecosystem involving clinicians, nurses, administrators, IT departments, payers, regulators, and patients. Even seemingly simple changes can ripple across multiple stakeholders.
“Healthcare is a microcosm of its own,” he says.
Clinicians, in particular, sit at the center of this tension. While patients ultimately benefit from innovation, clinicians are often the gatekeepers. Yet their reality is frequently misunderstood. They are not looking for innovation in the abstract. They are looking for tools that reduce friction, support decisions, and fit seamlessly into already overloaded workflows.
This insight has direct implications for how EvoMed approaches strategy. Rather than starting with market positioning or growth narratives, Havkic starts with the clinical environment. Does a solution remove friction from existing processes? Does it help clinicians make better decisions without interrupting their workflow? Does it introduce new risks or demand behavior change without clear payoff? If the answer to these questions is unclear, adoption risk is already high.
Healthcare is a microcosm of its own.
At the same time, the economic realities faced by founders and managers must be taken into account as well. Unlike clinicians, they must ensure that processes are scalable, financially viable, and attractive to investors. The tension between outcome-driven clinical thinking and resource-driven business thinking is unavoidable.
“With unlimited resources, you could generate the best outcome for every patient,” Havkic notes. “In the real world, the challenge is achieving the best possible outcomes for the largest number of patients, while remaining sustainable.”
His role, as he defines it, is to bridge these two worlds – not by compromising one in favor of the other, but by aligning them. This can become more complex when working with public healthcare systems. In the private sector, decisions are often driven by speed, return on investment, and competitive advantage. In public institutions, decisionmaking is shaped by political accountability, long procurement cycles, and risk avoidance.
It was this recurring exposure to misalignment that led Havkic to develop StarMap, a framework now at the core of EvoMed’s work. StarMap was created to address a simple but persistent problem: the lack of a structured way to assess and align the multiple factors that determine success in healthcare innovation.
Rather than focusing on a single dimension, StarMap evaluates elements such as workflow integration, patient safety, implementation barriers, trust, and economic viability – and, most importantly, how these factors influence one another. It functions as a diagnostic tool, helping teams identify where adoption risk originates and which gaps matter most. “Instead of saying ‘we need better marketing,’” Havkic explains, “it might show that workflow integration is weak, value is not measurable, and clinicians don’t trust the tool yet. That makes action possible.”
What makes StarMap particularly relevant is its adaptability. Whether applied to a MedTech startup, a hospital, or a public healthcare system, the underlying success conditions remain similar. Human behavior and system dynamics do not change simply because the organizational context does.
The goal is not to “fix” healthcare – which is a trivial concept – but rather to help it function more coherently. By aligning innovation with clinical reality, operational constraints, and economic logic, EvoMed aims to move projects from promising ideas to real-world impact.
This serves as a reminder that progress in healthcare is rarely about moving faster. More often, it is about aligning better – and designing innovation that can survive the pressure of real life.
Women’s healthcare is often described as “underserved,” but that term oversimplifies a complex problem. The issue is not a lack of technology or interest; it is that traditional healthcare systems remain reactive, episodic, and misaligned with women’s real lives. Symptoms are often normalized, care is fragmented, and prevention is rarely integrated into mainstream delivery.

SOPHIE SMITH, founder and CEO of Nabta Health, has approached this challenge by grounding innovation in large-scale clinical insight. During the company’s R&D phase, her team engaged with approximately 25 million women across the Middle East and Africa. What became clear was that women were “bad at identifying symptoms as symptoms,” particularly when fatigue, pain, or hormonal changes were normalized in daily life or overshadowed by stigma and societal expectations. “Even when women identify symptoms as symptoms, because of the stigma and shame attached to women’s bodies at every age and stage, they are slow to act on them,” she notes. This delay often results in late diagnosis and worsened outcomes.
Traditional healthcare systems amplify these challenges. They are structured around acute interventions rather than prevention, assuming patients have the time, mobility, and health literacy to navigate care. As Smith observes, “Traditional healthcare systems are built around episodic (symptom-led), acute care and reimbursement structures that reward intervention rather than prevention. They also assume time, mobility, money, and health literacy that many women simply don’t have.”
Nabta Health’s solution is a hybrid model that integrates technology directly into clinical pathways, rather than layering digital tools
on top of existing care. The company emphasizes “high tech, high touch,” where licensed medical professionals remain central, supported – but not replaced – by technology. AI and automation handle documentation, risk stratification, and monitoring, freeing clinicians to focus entirely on patient care. “A key aspect of our hybrid model is the understanding that human connection and in-person interactions continue, at least for now, to be a critical aspect of care delivery,” Smith explains. Technology is used only where it meaningfully reduces friction and enhances clinical outcomes, creating augmented intelligence rather than artificial intelligence in isolation.
This grounding in clinical rigor also defines how NABTA differentiates itself from wellness-focused platforms. Insights and lifestyle recommendations, however sophisticated, are insufficient unless they influence real clinical decisions, treatment pathways, or long-term outcomes. Smith emphasizes, “Every model is constrained by clinical oversight, explainability, and real-world validation. If AI doesn’t save clinician time, improve the quality of clinician-patient interactions, or improve health outcomes in the short and long term, we won't deploy it.”
Prevention remains a central challenge. Smith argues that it is not a question of desire but of structure. Insurance models reward intervention today, while the benefits of prevention accrue over years. “In order for prevention to become widely adopted, there needs to be a change in the government-mandated payor setup. As long as we pursue exclusively insurance-based reimbursement models, prevention will remain structurally underfunded because benefits accrue later while costs are immediate,” she says.
Cultural context further shapes NABTA’s approach. In multigenerational households, which are common in the Middle East, interventions targeting individuals often fail. NABTA adapts screening and care at the household level, aligning recommendations with how women actually live and make decisions, ensuring care is accessible, safe, and practical. But building a clinically rigorous, culturally aware, and technology-supported model has required tradeoffs. Growth has been slower than many venture-backed peers, with a heavier burden of proof and lower funding.
However, that doesn’t change the fact that the future of women’s healthcare will not be defined by standalone apps or superficial innovation. It will be shaped by clinically validated platforms that integrate diagnostics, AI, and longitudinal care, while respecting cultural context, and while treating prevention as a structural necessity rather than a premium add-on. Progress in this space may be slower and less glamorous than headlines imply – but far more durable, as Nabta Health is indeed proving.


In conversations about healthcare innovation, MedTech is often framed as a race to build better technology. Yet one of the sector’s most persistent challenges is not innovation itself, but integration: how to introduce new tools into public health systems that are complex, highly regulated, and accountable to citizens. This is the space where KENNETH BRINCAT, CEO of the Malta Digital Innovation Authority (MDIA), has focused his work, treating regulation not as a constraint, but as a foundational enabler of sustainable innovation.
Photo by Bernard Polidano
Rather than competing on scale, Malta has chosen to compete on structure. Its approach is built on the premise that innovation in sensitive sectors like healthcare cannot be detached from governance, trust, and institutional readiness. Under Brincat’s leadership, the MDIA operates at the intersection of these forces, combining regulatory oversight with hands-on support for startups, research institutions, and public bodies. The goal, as he describes it, is to create an ecosystem “where technology can thrive responsibly,”
without forcing innovators to choose between speed and safety.
A central pillar of this strategy is DiHubMT, Malta’s European Digital Innovation Hub. Designed around a “test before you invest” philosophy, the hub provides startups and SMEs with access to advanced technologies, regulatory guidance, and validation environments before they face the pressures of market deployment. High-performance computing infrastructure plays a key role, transforming data into actionable insight for research
and product development. Programs such as the Startup Launchpad further reinforce this approach by helping early-stage teams validate problem-solution fit before incorporation, reducing both technical and regulatory risk. Artificial intelligence is where this model is being tested most visibly. In healthcare, AI directly influences clinical decisions, patient safety, and professional accountability. With the introduction of the EU AI Act, Malta has positioned itself at the forefront of responsible AI implementation, designating the MDIA as the Market Surveillance Authority. Brincat emphasizes that compliance alone is not enough. What matters is creating pathways that allow innovators to experiment safely while aligning with legal and ethical standards from the outset.
This is where the MDIA’s regulatory sandbox becomes critical. By offering a controlled environment for testing AI systems, including those used in MedTech, the sandbox enables developers to validate solutions before largescale deployment. “Access to guidance and advisory support is just as important as access to advanced technologies,” Brincat notes, highlighting the Authority’s role in aligning innovation with international best practices such as the OECD and UNESCO AI ethics frameworks. Complementary tools, including the AI Helpdesk and self-assessment guides under the EU AI Act, are designed to reduce uncertainty and help companies navigate complex regulatory terrain.
Crucially, Malta’s approach treats regulation as infrastructure. In a landscape where AIpowered medical devices are classified as high-risk systems, clarity and predictability become strategic advantages. The MDIA’s close coordination with the Medicines Authority ensures alignment between the AI Act, the Medical Device Regulation (MDR), and the In Vitro Diagnostic Regulation (IVDR), simplifying compliance without diluting standards. This integrated oversight model reflects a broader shift, from regulation as a gatekeeper to regulation as a system that enables safe scale.
Trust remains the underlying challenge. Even the most robust regulatory frameworks can falter if patients, clinicians, and the public lack
understanding or confidence in new technologies. Brincat is explicit about this risk, pointing to transparency and communication as essential tools for addressing ethical concerns and public resistance. Equally complex is stakeholder coordination, which means aligning regulators, innovators, healthcare providers, and policymakers who often operate on different timelines and incentives.
Looking ahead, Malta’s ambition is not to become a closed innovation hub, but a connective one. Initiatives such as CALYPSO, the AI Factory Antenna linked to Greece’s PHAROS supercomputer, reinforce Malta’s role within the wider European AI ecosystem. Healthcare is one of the priority sectors, enabling access to infrastructure, datasets, and expertise that support the development of trustworthy, scalable MedTech solutions.
Ultimately, the MDIA’s contribution under Brincat’s leadership lies in cementing Malta’s position as a centre for excellence by making complex technological innovation workable and secure for the wider market. At a time when technology is advancing faster than institutions can adapt, Malta offers a different perspective: in healthcare, progress depends less on disruption and more on building the regulatory and ethical foundations that allow innovation to endure.

By CHRISTINE NIMALASIRI, Clinical Director, Ascend CRO
In MedTech, innovation naturally gets most of the attention. New technologies, novel delivery mechanisms, and breakthrough concepts dominate conversations. But when it comes to clinical trials, it is the data — not the device — that ultimately determines whether a product progresses, stalls, or is forced back to the drawing board.
At Ascend CRO, almost a quarter of our work last year involved helping sponsors repeat their First-in-Human (FIH) clinical trial. Ouch. We work with MedTech teams across all stages of development, from early feasibility through to post-market studies. While the technologies themselves vary widely, the data challenges we see are remarkably consistent. Too often, promising devices run into trouble not because they lack potential, but because the data generated fails to meet regulatory expectations.
The good news is that this kind of clinical trial regret is avoidable when you choose the right partners.
Below are the five most common data pitfalls we encounter in clinical trials — and how sponsors can avoid the costly mistake of having to do it all again.
One of the most frequent mistakes is designing a trial around what is interesting, familiar, or operationally convenient, rather than what regulators actually need to see.
This approach can produce data that looks robust on the surface but does not adequately support safety or performance claims. Without clear alignment to a regulatory pathway, sponsors may complete a study only to discover it cannot be used in a key market submission.
A stronger approach is to define the regulatory objective first. This means clarifying the intended use, target claims, and jurisdictional pathway before the protocol is finalised. When the end goal is clear, the data requirements become far more focused, efficient, and defensible.
In an effort to reduce risk, many trials collect far more data than is actually required. While this may feel conservative, it often creates new problems.
Large datasets increase the burden on investigators and sites, raise the likelihood of missing or inconsistent data, and complicate analysis. They also increase the level of scrutiny during monitoring and audits.
Regulators are not impressed by volume. They are looking for relevance, consistency, and clarity. A lean dataset that clearly supports defined endpoints is almost always more persuasive than an expansive one with gaps and inconsistencies.
Poor pre-clinical data is one of the highest hidden risks going into a First-in-Human medical device trial. It doesn’t just weaken the science; it undermines the entire clinical, regulatory, ethical, and operational foundation of the study.
FIH trials are approved on trust in the pre-clinical package. Weak or poorly designed data breaks that trust immediately. When gaps emerge, sponsors are often forced into protocol amendments, trial pauses, or additional non-clinical testing.
Each of these outcomes extends timelines, increases burn rate, and erodes confidence. FIH rework is among the most expensive fixes in medical device development.

Too often, promising devices run into trouble not because they lack potential, but because the data generated fails to meet regulatory expectations.
Another common pitfall is treating data quality as something to address at the end of the trial. By that point, options to correct issues are limited.
Regulators expect data quality to be actively managed throughout the study, not retrospectively cleaned. Ongoing data review allows teams to identify trends, inconsistencies, or protocol deviations early, when corrective action is still possible.
Proactive quality oversight protects both timelines and credibility, and significantly reduces the risk of data being questioned later.
Traceability is one of the most critical and most underestimated aspects of clinical trial data.
Regulators need to clearly understand how reported results link back to source data. Without strong documentation, validated systems, and reliable audit trails, even accurate data can be challenged.
Traceability is not administrative overhead. It is how trust in the data is established and maintained, particularly during audits and regulatory review.
For sponsors, avoiding these pitfalls is not just about compliance — it is about protecting time, capital, and credibility.
Ascend’s role is to help sponsors make the right decisions early, before they become expensive or irreversible. We work closely with sponsors to translate regulatory expectations into practical trial design, ensuring data is not only collected correctly, but is usable across the full development pathway. This includes stress-testing protocols, simplifying data strategies, and building quality and traceability into trials from day one.
The result is confidence. Confidence that the data will stand up to scrutiny, support future submissions, and reduce the need for rework. Because nothing defines clinical trial regret quite like having to run your First-in-Human study for a second time.
Surgery is probably one of the most fast-paced and high-stakes areas of healthcare. Every second counts, and every inefficiency carries a cost. For THOMAS KNOX, co-founder of VitVio, a startup leveraging AI to transform surgical workflows, the challenge is clear: healthcare is complex, high-risk, and resistant to shortcuts. Knox’s vision is not to replace clinicians but to empower them, using AI as a tool to reduce the noise, friction, and administrative burden that too often distract from patient care.
by Thomas Knox

This real-time observation extends far beyond efficiency in a single procedure. Knox explains, “Hospitals do not fail because people do not care. They fail because they operate without real visibility. By understanding what is happening in the operating room in real time – case progress, delays, instrument usage, and readiness states – AI transforms the OR from a black box into a live system.” With this visibility, downstream teams can prepare proactively, bottlenecks are addressed before they cascade, and overall hospital operations become smoother. The result is a better workflow that brings benefits for everyone, clinicians and patients alike.
VitVio is also pioneering the concept of a connected operating room, where disparate systems – from surgical robots to anesthesia machines to monitoring devices – communicate and generate a unified understanding of the surgical environment. “VitVio’s role is to unify those clinical and operational signals into a shared understanding of what is actually happening during a case,” Knox says. “That real-time awareness improves surgery first – but its impact extends far beyond the OR… making the entire hospital operate more predictively.” In this vision, the operating room becomes the central hub for hospital efficiency, informing staffing, bed flow, and downstream care with unprecedented accuracy.
Looking to the future, Knox sees AI’s most significant impact in areas that are often overlooked: operations, coordination, and cognitive offloading. “No clinician or supporting staff went into healthcare because they like doing admin work,” he observes. The potential is enormous, but he also emphasizes caution: black-box decision-making, over-automation, and systems that erode clinical judgment are real dangers. AI can recommend, surface, and prepare – but it’s still up to humans to make decisions, maintaining accountability and safety at every step.
VitVio’s work is a study in balancing speed, credibility, and rigor. By embedding AI seamlessly into clinical reality, the company helps hospitals operate more efficiently while preserving the human judgment that is central to patient care. Knox’s philosophy is clear: technology should be a tool, not a replacement; a lens on reality, not a filter of it. In doing so, VitVio is redefining what operational excellence looks like in modern surgery, creating a model where AI, humans, and complex systems work in harmony.
That real-time awareness improves surgery first –but its impact extends far beyond the OR... making the entire hospital operate more predictively.









Malta: A Life Sciences Powerhouse
Malta offers a thriving pharmaceutical and medical device ecosystem supported by top hospitals, skilled talent, and the Life Sciences Park.
With the Malta Medicines Authority at its core, and the support of Malta Enterprise, the country’s regulatory and business framework ensures safety, quality, and innovation for global life sciences.


From medical cannabis research to biotech, med-tech, and drug development, Malta’s start-up friendly environment creates fertile ground for pioneering ventures.
Embracing AI, personalised care, and clinical trials, Malta is investing in tomorrow’s breakthroughs, making it an ideal destination for innovators and investors alike.




































































































































































































































































42globalventures.com
Anna is a seasoned expert in HealthTech innovation and founder of 42 Global Ventures. She leads the HealthTech & AgeTech Chapter at Monaco-based White Castle Partners and is Venture Partner at Aegis Fund. She helps investors identify top opportunities and supports startups that have collectively raised over €1 billion in the past decade.
Anna Badurska CEO & Founder, 42 Global Ventures


us.allotex.com
Allotex pioneers tissue-addition refractive surgery, restoring near vision by adding natural human collagen to the cornea—preserving adaptability and any future options for presbyopic patients seeking youthful, glasses-free vision.
Michael Mrochen CEO, Allotex


alalphahealth.com/ar
Hamzeh is a digital health investor with a decade of experience at Debiopharm Innovation Fund and Hikma Ventures, involved in closing more than 20 global deals. He served on multiple boards, held finance roles at Hikma, PMI, and Capital Investments, graduated from GWU, and co-founded initiatives in Jordan and Spain.
Hamzeh Abdul Hadi Investor, Angelini Ventures


alvarezandmarsal.com
Health informatics futurist, quantum physicist, startup mentor and coach, inspiring speaker, value based transformation researcher, and Web3 ambassador who loves anything about blockchain, Metaverse, NFT, Nanotechnology, Big Data and Human Transformation. Has worked in healthcare innovation and digital disruption for over 25 years.
Mazin Gadir Director of Healthcare Life Sciences, Alvarez and Marsal


alliancecaretech.com
Michele Tarnow is Founder and CEO of Alliance Care Technologies, driving digital health innovation to advance equitable, value-based care globally. With leadership spanning healthcare technology, venture startups, and social impact, she pioneers solutions that integrate AI and connected care to transform patient outcomes and strengthen health systems worldwide.
Michele Tarnow CEO, Alliance Care Technologies


ascendcro.com
Ascend is Australasia’s premier med tech clinical research partner for FIH, pivotal and post market studies offering premium access to animal labs, sites and KOL outreach/development, ethics submissions, governance, monitoring and more. Recognised by FDA, CE as a Center of Excellence, Australia delivers Tier 1 quality data affordably.
Joseph Panetta Chief Marketing Officer, Ascend Clinical Research Organization


atlasmedical.ai
Has 28+ years of experience, digital health & AI, medical devices and consumables, built collaborative teams, worked on commercial operation and launching, innovative products, managed team size of 45 sales, and managed 25+ partners, including Philips, Hillrom, Varian, Masimo, Vocera etc, Turnkey Projects, PPP Projects, and Establish New Business.
Suresh Babu
Chief Business Officer , Atlas Medical LLC part of Sirius Holding Investment C ompany


cslifesciences.com
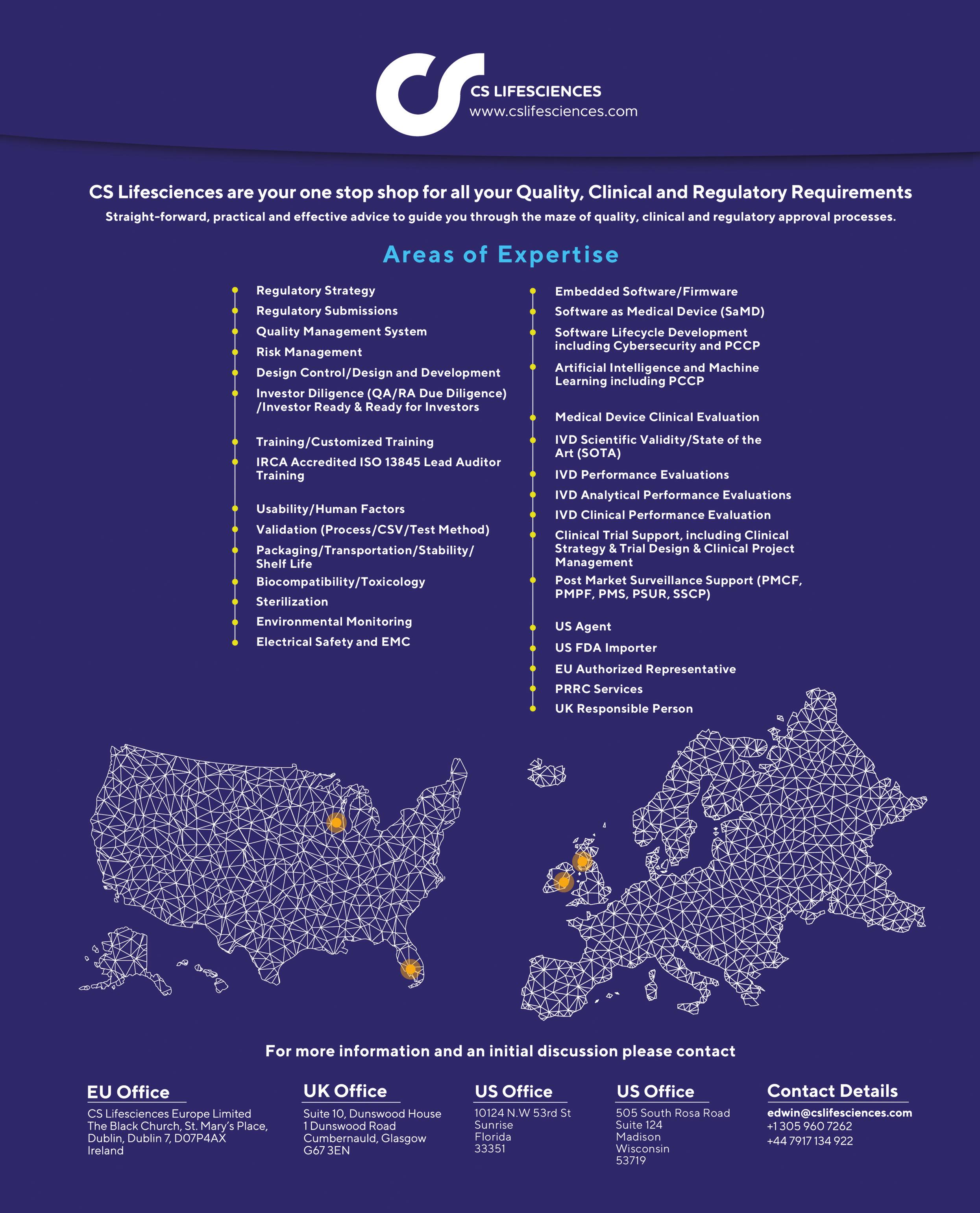
CS Lifesciences accelerates MedTech success with end-to-end regulatory, quality, and market access expertise. We guide devices and diagnostics through MDR, IVDR, FDA, and ISO 13485 compliance, build robust QMS, support clinical evidence, and drive commercialization—delivering faster approvals, sustainable compliance, and scalable growth for innovators worldwide.
Edwin Lindsay Managing Director & Principal Consultant, CS Lifesciences


bluegoatcyber.com
Blue Goat Cyber is a medical device cybersecurity firm that helps manufacturers accelerate regulatory readiness and deliver safer, connected products. With 200+ successful FDA and global submissions, we provide fixed-fee, turnkey support across the total product lifecycle—including penetration testing, SBOM management, and postmarket monitoring—backed by a submissionready deliverables guarantee.
Christian Espinosa CEO, Blue Goat Cyber


Calla Lily Clinical Care is a British women’s health medtech developing Callavid®, a transformative intravaginal drug-delivery platform. It aims to be the world’s first drug-device to prevent threatened miscarriage and support IVF, with future expansion into oncology, menopause, infectious diseases, and live biotherapeutics to reduce antibiotic use and antimicrobial resistance.
Thang Vo-Ta
CEO & Co-founder, Calla Lily Clinical Care


deerfield.com
Dr. Mussaad has over 20 years of healthcare management and private-equity experience. He is a former Chief Investment and Business Development Officer at Kuwait Life Sciences Company and board member of multiple regional pharmaceutical firms. He holds an MBA in Healthcare Management and Finance from Columbia Business School.
Dr. Mussaad Al-Razouki, MD MBA Operating Partner, Deerfield Management

doceree.com
Dr. Ruchi Dass is a globally recognised clinician-executive and digital health strategist, renowned for her pioneering work at the intersection of healthcare, artificial intelligence, and technology-driven transformation. With a career spanning clinical leadership, AI innovation, and health policy, she has consistently driven meaningful change across global healthcare systems.
Dr. Ruchi Dass
SVP Strategy (CEO Office), Doceree Inc.



dha.gov.ae/en
Dr. Khulood Alsayegh is an internationally recognised healthcare leader with over 20 years of clinical and regulatory expertise. Trained at RCSI and Harvard, she leads clinical standards at Dubai Health Authority, pioneers AI ethics in healthcare, shapes global policy, and has received prestigious national honors for transformative impact.
Dr. Khulood Alsayegh
Head of Clinical Standards & Guidelines, Dubai Health Authority


electroducer.io
Electroducer is a medical device company developing the Electroducer Sleeve, designed to make temporary cardiac pacing safer, simpler, and faster. The Sleeve integrates the Direct Wire Pacing (DWP) technique while preserving standard operator workflows, avoiding any change in clinical habits. In parallel, the Electroducer Sleeve is under clinical investigation as a non-invasive tool to optimize renal denervation procedures.
Lucien Goffart CEO, Electroducer


evomed-consulting.eu
Amel Havkic, is a lung and critical care specialist, clinical lead, and founder of EvoMed Consulting. He’s helped leading innovators secure millions in funding and launch clinician-approved solutions globally. His vision is safer, smarter healthcare, delivered through strategies that scale innovation, win markets, and change lives worldwide.
Dr. Amel Havkic
Founder & CEO, EvoMed Consulting

frost.com
Reenita is a Partner and Senior Vice President at Frost & Sullivan, and its first female Partner. She leads global healthcare futures research, forecasting operations, rethinking consumer models, advancing Femtech, and advising companies on innovation and growth in digital health and wellness.
Reenita Das
Partner & Senior Vice President, Healthcare & Lifesciences, Frost & Sullivan


ehs.gov.ae/en/home
Dr. Kareemah Alraesi is the Director of Primary Health Care at Emirates Health Services (EHS), with over 19 years of medical experience and seven years in health management and innovation. Dr. Kareemah leads strategic oversight of 63 health centers across the UAE, pioneering urgent care and family medicine telemedicine, AI-enabled decision support, cancer screening, premarital genetic testing national program and zero-bureaucracy services.
Kareemah Alraeesi Director of Primary Healthcare, Emirates Health Services


huma.com
Dan Vahdat is Founder and CEO of Huma, a $1B+ healthtech company advancing digital healthcare and research. Huma’s AI-powered platform supports 1,000+ clinical trials, is used in 4,500+ hospitals, and reaches 50M+ people globally. Dan is a two-time Prix Galien winner, 2023 HealthTech Leader of the Year, and a World Economic Forum member.
Dan Vahdat CEO & Founder, HUMA Technologies




linkedin.com/company/ ib-ventures/about/
Dr. Yazeed Alsufyani is the CEO & General Partner of IB Ventures, the first biotech-focused VC, incubator, and accelerator in Saudi Arabia. He leads institutional-grade investments and ecosystem development aligned with Vision 2030 and the National Biotechnology Strategy, partnering with global platforms and public stakeholders to scale MedTech, AI-driven healthcare, and advanced biotech commercialisation.
Dr. Yazeed Alsufyani
CEO & General Partner, IB Ventures


modivc.com
Sahir Ali is the founder of Modi Ventures, a VC firm focused on the convergence of technology and biology, with expertise spanning AI, medical imaging, cancer research, and cloud computing. In addition, Sahir is a successful entrepreneur and investor, advises global organisations on the integration of advanced technologies and speaks regularly on relevant topics.
Sahir Ali
Founder & General Partner, Modi Ventures


leucadiatx.com
Leucadia Therapeutics restores a critical brain clearance mechanism that deteriorates with age, causing neurological disorders including Alzheimer’s. Arethusta is a small implanted device that rejuvenates this pathway to age-25 levels. Clinical trials begin June 2026, aiming to reverse early-stage Alzheimer’s within months of treatment.
Doug Ethell, Phd Chief Executive Officer, Leucadia Therapeutics

mediclinic.ae
Dr. Ramzy Ross is Director of Shared Clinical Services at Mediclinic Middle East, leading a portfolio including diagnostics, precision medicine also involving AI-enabled transformation. His work includes a focus on integrated diagnostics, data-driven care models, and scaling personalised healthcare through advancing imaging, laboratory, genomics, and digital capabilities.
Dr. Ramzy Ross Director Shared Clinical Services, Mediclinic Middle East


moh.gov.sa
Dr. Kheder is a healthcare quality consultant. He advises the Vice Minister of Health, chairs the Quality in Health Care Association, serves on professional boards, teaches master’s students, and supports national hospital standards development within the Saudi healthcare system nationally.
Dr. Khalid Kheder Advisor to H.E. Vice Minister for Planning and Development, Ministry of Health Saudi Arabia and the Board Chairman of the Quality in Health Care Association, MOH KSA


neom.com/en-us
Eng. Muhammad Mudassar leads Data & AI at NEOM Health & Wellbeing, driving digital-first, preventive healthcare. With 20+ years’ global experience, he bridges technology and clinical innovation. An IEEE senior member, certified CPHIMS, he holds engineering, MBA, MIT and Cornell credentials and authored “Humanizing Healthcare AI.”
Eng. Muhammad Mudassar Head of Healthcare AI NEOM




As Abu Dhabi’s designated Life Sciences hub, Masdar City brings together research, pre-clinical development, clinical trials, regulation, manufacturing support, and commercialization within a single, connected ecosystem. Companies benefit from rapid approvals, strong regulatory alignment, and direct access to shared labs, biobank, genomics programmes, and AI-driven health platforms. masdarcity ae


neopredics.com/en
NeoPredics is a healthcare technology company specializing in predictive analytics for maternal and neonatal care. Its flagship solution, PreFree™, predicts preeclampsia risk before symptoms, enabling earlier intervention and personalized care. The platform integrates into clinical workflows, supporting scalable deployment and improving outcomes for mothers and newborns.
Thorsten Waloschek CEO, NeoPredics


un.org
Dr. Nabhit Kapur is a mental health advocate and pioneer of wellbeing diplomacy. He serves as UN Ambassador for the Pan African Intergovernmental Agency for Water and Sanitation. Founder of Peacefulmind Foundation and World Leaders for Mental Health, he advances psychosocial wellbeing, peacebuilding, and global policy integration.
Ambassador Nabhit Kapur Ambassador and Permanent Representative to United Nations, PAN African Intergovernmental Agency


plugandplaytechcenter.com
Michael Maylahn is a 3x founder and investor. He previously scaled a medical technology company across three continents as Founder and CEO. He currently serves as a Director for Plug and Play, building innovation programs that connect global startups with regional partners and invests in early-stage companies developing scalable, high-impact technologies.
Michael Maylahn Director, Plug and Play


purelandgroup.co
Chris is a Partner at Pureland Venture, looking at early-stage healthtech value creation opportunities in the Asia Pacific and beyond. In addition, Chris serves various roles around the regional ecosystem, such as healthcare policy advising on UHC matters, executive hospital leadership education, and regulatory and reimbursement pathway design. Mostly, Chris is excited about bringing safe, affordable medical innovations to the masses in need.
Dr. Chris L. Hardesty Partner, Pureland Venture


Philippe Gerwill is a global Digital Health Futurist and Innovation Advisor active in many countries. A former Novartis executive, he now champions humancentric AI, digital transformation, and healthcare innovation. He is also an Adjunct Professor at the Rome Business School and a sought-after international keynote speaker.
Philippe Gerwill
Founder and Managing Director, PGEA Limited


purposetech.vc
Professor Shafi Ahmed is a worldrenowned, multi-award-winning surgeon, teacher, futurist, innovator, entrepreneur, humanitarian, and international keynote speaker.
Prof. Shafi Ahmed
Senior Partner, Purpose Tech Capital




quadriacapital.com
Dr. Amit is Co-founder and Managing Partner of Quadria Capital and Co-sponsor of HealthQuad. With 33 years’ experience across the USA and Asia, he is a critical care physician passionate about advancing healthcare. He has held leadership roles at Manipal, Narayana Hrudayalaya, Fortis, and Religare Healthcare.
Dr. Amit Varma
Managing Partner & Co-Founder, Quadria Capital


sagana.com
Aina Gaur is an Associate Partner at Sagana, co-leading its global investment team overseeing USD 800M+ across 60 companies and 20 funds worldwide. With 15+ years investing in healthcare and technology, she advises family offices, foundations and founders, and champions women’s health and science, and brings deep cross-market board experience globally and strategically
Aina Gaur
Associate Partner, Sagana Gmbh


sinapticatx.com
Sinaptica delivers a personalized, non-invasive 20-minute weekly therapy with successful Phase 2 trials meeting all endpoints, earning FDA Breakthrough status. Its real-time TMSEEG innovation is patent-protected with new CPT codes. Pivotal-ready, it targets a major market by 2029.
Ken Mariash CEO, Sinaptica Therapeutics

berger-invest.com
Dr. Sascha Berger is an experienced biotech and medtech investor with nearly 20 years of international track record. Based in Saudi Arabia, he serves as Investment Director at NEOM and advises several national stakeholders on shaping the country’s innovation and investment landscape in healthcare and biotechnology.
Sascha Berger Investor, Saudi Arabia


subqbionics.com
Sub-Q was founded to give breastcancer survivors their mobility and confidence back. Lymphedema remains one of the most overlooked complications of treatment - daily compression, recurring infections, constant fatigue. Our team, in partnership with the Weizmann Institute of Science, has built the world’s first fully implantable bionic lymphatic-drainage system, designed to manage the condition automatically so women can return to normal life.
Jordan Pollack CEO, Sub-Q Bionics Inc.


sugativentures.com
Rachna Dayal is an outcomes-driven executive with global experience across Philips, Johnson & Johnson, startups, and venture capital. She is Managing Partner of Sugati Ventures, a healthcare VC investing in early-stage MedTech and HealthTech, and co-founder of the AI Enabled Health think tank.
Rachna Dayal
Managing Partner, Sugati Ventures
















surgeomniqx.ai
SurgeOmniQ-X Robotics develops autonomous technologies for minimally invasive surgery, enhancing ergonomics, efficiency, and patient outcomes. Focused on autonomous pneumosufflation and port entry, it integrates AI and patent-pending optical systems to provide surgeons with superior precision and innovative solutions for complex procedures.
Dr Siddharthan Rajagopal CEO, SurgeOmniQ-X Robotics


Joseph is co-founder and Managing Partner of Verge HealthTech Fund, investing in early-stage healthcare technology with global impact. Formerly a cancer researcher, medical device entrepreneur, and Oliver Wyman consultant, he holds a PhD from the University of Toronto and an MBA from Ivey. He has 19 publications, 3 patents, and 46 healthcare investments.
Joseph Mocanu Partner, Verge HealthTech Fund


Steve Moore is CEO and Co-Founder of Welcome Health Ventures, scaling healthcare innovation across MENA. An Honorary Executive in Residence at Brunel and PhD candidate at UCL, he has 20 years’ experience leading health transformation in the UK, Qatar and Saudi Arabia, advising ministries on policy, digital health and system redesign.
Steve Moore CEO and Co-Founder, Welcome Health Ventures

Dr. Narges Sheikhansari is a public health executive with over a decade of experience in various global health affairs. While her main area of focus is developing policies and implementing strategies for healthcare transformation, her expertise spans population health and longevity, research, health behavior change and digital health and innovation. Dr. Sheikhansari has successfully led significant public health programs, influencing policy at national and international levels.
Dr. Narges Sheikhansari Executive Advisor and Public Health Expert


ArabMedicare.com is a comprehensive digital platform dedicated to healthcare professionals in the Middle East and North Africa (MENA) region. Established over 25 years ago, it offers medical news, job listings, country-specific healthcare profiles, and resources on medical devices, health insurance, and environmental health.
arabmedicare.com

Bovia is a Brussels-based health innovation cluster formed by the merger of three Belgian organisations. It supports start-ups and established firms in life sciences, providing infrastructure, business advice and collaborative networks to enhance growth and develop disruptive health technologies.
biovia.be

CIOCoverage bridges the gap between rapidly advancing technology and technologists striving to keep up. It offers insights into the latest tech trends, like AI, Big Data, and Cloud, aimed at helping entrepreneurs and industry leaders, including CEOs, CXOs, and CIOs, stay ahead of innovations and tackle emerging challenges in enterprise technology.
ciocoverage.com

BioPartner UK is an independent trade organisation promoting international partnerships, trade, and investment with UK life science companies. Collaborating with public and private sectors, it organises events, projects, and missions, leveraging technology partners to connect global stakeholders and efficiently support the UK life science sector with innovative solutions.
biopartner.co.uk

CIO Applications Europe delivers peer-driven insights, offering professionals comprehensive coverage of technology trends across the continent. As a leading Pan-European publication, it guides decision-makers through the fastevolving tech landscape while providing services and solutions that help businesses address challenges, innovate, and stay ahead in the dynamic technology industry.
cioapplicationseurope.com

DigitalHealth.London connects NHS staff, digital health companies, and academics to improve NHS and social care through technology. Delivered by the Health Innovation Network, it provides mentoring, education, networking, and partnership opportunities. Supported by government and partners, it empowers digital health leaders to tackle challenges and drive innovation across London’s healthcare system.
digitalhealth.london

The Distretto Biomedicale Mirandolese in northern Modena, Italy, is Europe’s leading biomedical cluster and third largest globally. Hosting over 300 companies, it specializes in medical devices and disposables. Renowned for innovation, export strength, and advanced manufacturing, the district plays a pivotal role in healthcare production and supports the global medical technology industry.
distrettobiomedicale.it

EIT Health promotes entrepreneurship and innovation in healthy living and active ageing across Europe. By providing resources, programs, and opportunities, it enables citizens to lead healthier and more productive lives. Delivering products, services, and concepts that improve quality of life, EIT Health contributes to sustainable healthcare and advances innovation across the continent.
eithealth.eu

FirstWord HealthTech delivers fast, reliable news and analysis for digital health professionals worldwide. Offering daily updates, executive interviews, physician polls, and premium tracking of products, companies, and regulations, it provides deeper insights than competitors. Trusted by clients, the platform ensures users stay ahead in the rapidly evolving digital health industry.
firstwordhealthtech.com

Doctorpreneurs is a non-profit global community empowering doctors, medical students, and healthcare professionals passionate about transforming healthcare through innovation and entrepreneurship. It provides resources, networking, and opportunities to inspire, connect, and accelerate medical entrepreneurs, fostering collaboration and supporting initiatives that drive meaningful, innovative, and sustainable improvements in healthcare worldwide.
doctorpreneurs.com

Esimatic is a global eSIM provider offering prepaid mobile data plans for travelers in over 200 countries. Through its user-friendly app, users can access high-speed 4G/ LTE and 5G networks without physical SIM cards, enjoying flexible data packages ranging from 1GB to unlimited options. Ideal for tourists, remote workers, and digital nomads, Esimatic provides instant activation, no ID verification, and eco-friendly connectivity solutions. esimatic.co

Flying Health is a leading ecosystem connecting startups, corporates, and stakeholders to advance nextgeneration healthcare. By combining consulting expertise with trend research, we empower industry leaders and entrepreneurs to innovate. Together with partners, we develop strategies, business models, and networks that shape the future of healthcare and technology.
flyinghealth.com






































Good MedTech News delivers the latest positive updates from the medtech sector, highlighting innovations that save lives. Covering CE markets, venture capital, cardiovascular advancements, robotics, and emerging technologies, it also publishes MedTech Opinion Leader, a quarterly magazine featuring in-depth insights, interviews, and leadership perspectives shaping the future of healthcare.
medtechlead.eu

HealthTech Magazines is an award-winning Wilmingtonbased publication providing healthcare technology professionals a platform to share insights on industrydriving innovations. Offering neutral, in-depth analysis of companies and solutions, it helps decision-makers tackle complex challenges, make informed technology choices, and advance organizational efficiency, innovation, and patient care across the healthcare sector.


Healthy.mt is a trusted health and industry news platform delivering evidence-based information on medical research, healthcare innovation, and pharmaceuticals. Providing expert insights, practical advice, and updates, it empowers patients, professionals, and policymakers to stay informed, make confident decisions, and focus on progress, prevention, and the future of health.
healthy.mt

HealthcareWorld.com is a global healthcare platform offering news, insights, events, and consultancy services that connect healthcare professionals, organisations, and policymakers. It features a leading international magazine, high-level forums, webinars, and strategic support to help partners expand, network, and influence healthcare markets worldwide.
healthcareworld.com

HealthTechX shares the latest industry insights, trends and ideas through a series of editorials, reports and thoughtleadership summits. Powered by IBIS Capital, we connect the global health technology community to accelerate the positive impact that technology can have across the sector.
impactx2050.com/healthtechx

Impulse Podcast is a healthcare technology podcast founded in 2022 by biomedical engineer Mathieu Chaffard. It features in-depth conversations with innovators, entrepreneurs, and experts shaping the future of medical technology, from surgical robotics to AI in care delivery, offering insights into breakthrough ideas and the people driving change in health innovation.
impulsepodcast.com

International Business Magazine (INTLBM), based in the UAE, is a digital publication serving over 50,000 subscribers, including investors, C-suite executives, and policymakers. It delivers timely news and insights across banking, finance, technology, and corporate sectors. The platform also hosts annual awards to recognise industry excellence. intlbm.com

Medarabia is the Middle East’s largest healthcare network, founded to empower the industry by enabling users to share experiences and post reviews about doctors, clinics, hospitals, and other service providers. It bridges the communication gap between patients and practitioners, helping users make informed healthcare decisions. m.edarabia.com

MD101 helps medical device companies accelerate time-to-market while ensuring regulatory compliance. Leveraging a vast expert network, we provide guidance in data strategy, quality assurance, regulatory and clinical affairs, reimbursement, and international business development. Our healthcare-focused expertise ensures manufacturers overcome challenges and achieve sustainable business success.
md101.io

Medgate Today is a leading international MedTech and healthcare magazine headquartered in India. It connects innovators with the medical community, offering in-depth reporting through its monthly print and digital editions. The publication also hosts the prestigious MT India Healthcare Awards to recognise excellence in the sector. medgatetoday.com

Medical Buyer is a leading monthly magazine and web portal in India, connecting medical equipment buyers and vendors. It provides reliable information for purchase decisions, vendor promotion, and customer lead generation. Published by ADI Media, it focuses on unbiased reporting, ensuring relevant business information for healthcare professionals. medicalbuyer.co.in
Medical Device Developments is the leading source for medical device manufacturing professionals. Through expert journalism, research, and analysis, it provides key insights into market trends and technology innovations. Aimed at senior executives, it supports critical business decisions and serves as the definitive, authoritative platform across print and digital channels worldwide. medicaldevice-developments.com

Medicaspace is a dedicated medical networking and marketing platform connecting professionals, companies, and organizations across the healthcare sector. Targeting doctors, biomedical engineers, consultants, manufacturers, and educational centers, it enables efficient C2C networking and B2B/B2C marketing, delivering accurate, affordable, and specialised solutions to reach the medical market quickly and effectively.
medicaspace.com

MedTech Europe promotes innovative medical technology across Europe, supporting sustainable healthcare and valuebased solutions. Formed in 2012 from EDMA and Eucomed, it engages policymakers, drives research, and organizes events to highlight medical technology’s impact. By fostering collaboration and innovation, MedTech Europe ensures healthcare systems meet Europe’s evolving needs effectively.
medtecheurope.org

MEDtube is a global eLearning platform for healthcare professionals, offering 35,000+ peer-reviewed videos, cases, images, and publications in five languages. Founded by transplant surgeons in 2010, it enables knowledge sharing, CME-accredited programs, digital learning, and networking, supporting clinicians worldwide to enhance skills, teach peers, and solve clinical challenges.
medtube.net

Mediworld Middle East is a dynamic platform offering in-depth coverage of healthcare developments across the region. Through its magazine, website, and active social media presence, it provides timely news, expert interviews, and event coverage. The platform keeps professionals informed on the latest trends, innovations, and industry discussions.
mediworldme.com

MedTech Spectrum is an online media platform dedicated to medical technology and healthcare innovation. It publishes news, analysis, interviews, events and job listings covering medical devices, diagnostics, digital health and industry trends, keeping professionals and enthusiasts informed about breakthroughs, regulatory updates and the evolving MedTech landscape.
medtechspectrum.com

Mid-East.info is an online portal offering business and financial news for the Middle East. It publishes company news and press releases free of charge and is building a comprehensive business directory for the region. The platform provides timely insights, helping businesses stay informed and connected across various industries.
mid-east.info
NXGN is a platform dedicated to championing the rising leaders in healthcare, offering support and inspiration for those shaping the future of healthtech and biotech. It provides a space for emerging professionals to be heard, fostering a community committed to innovation and leadership in the evolving healthcare industry. nxgn.health

Plug and Play is a global innovation platform connecting entrepreneurs, corporations, and investors. With 50+ locations worldwide, it has supported 35,000+ startups and 500+ corporations across 20+ industries, facilitating investment, partnerships, and meaningful introductions. plugandplaytechcenter.com

Sigma Squared is a global, invite-only community of founders under 26, spanning 35 countries. Fellows connect, collaborate, and scale ventures, raising over $2.5 billion collectively. Supported by top partners like Sequoia and Redalpine, the society empowers young entrepreneurs to tackle world challenges, innovate, and shape the future. sigma-squared.org

One HealthTech is a global community promoting diversity and inclusion in health innovation. Supporting women and underrepresented groups, OHT provides mentorship, education, and networking across digital health. With hubs in the UK, Europe, and Australia, it champions leadership, collaboration, and participation in healthtech events and initiatives.
onehealthtech.com

SEHTA (Science and Engineering Health Technologies Alliance) is a UK-based, not-for-profit health technology network founded in 2005 that supports innovators, SMEs, clinicians, care providers and academics. It accelerates health tech innovation from concept to commercialisation with business support, training, events and networking, including the International MedTech Expo & Conference. SEHTA’s mission is to improve healthcare outcomes and grow economic impact through collaboration and expert guidance. sehta.co.uk

Startups Magazine, launched in April 2018, is a bi-monthly platform championing tech startups. It offers expert content, digital issues, event access, and startup features, while connecting a passionate community of founders, investors, and innovators. It also curates industry events, podcasts, and practical guidance for entrepreneurial success.
startupsmagazine.co.uk
The Arab Hospital Magazine addresses the growing need for knowledge in the healthcare sector across the Arab world. It provides reliable, professional journalism on public and private healthcare developments, new medical technologies, and career opportunities. With regional correspondents, it presents timely news to enlighten the public and healthcare professionals.
thearabhospital.com

Zapyrus is a machine learning-powered sales research assistant for MedTech leaders. It provides real-time industry insights on medical devices, IVD/diagnostics, and SaMD, ensuring businesses never miss key opportunities. Trusted globally, Zapyrus helps companies make informed decisions and take immediate action, transforming the way MedTech professionals approach sales research. welcome.zapyrus.com

UAE Vartha is a news platform focused on providing the latest updates, insights, and information on events and developments in the UAE. It covers various sectors, including business, politics, culture, and lifestyle. If you need specific details, I recommend checking out their website directly!
uaevartha.com

ZEX PR WIRE® is an automated press release distribution platform, syndicating content to over 500 global media outlets. Designed for PR teams and agencies, it allows users to conduct and manage campaigns with zero mail trails and mediators. The platform delivers press releases within 72 hours, ensuring fast, efficient service.
zexprwire.com





Dr. Dylan Attard
CEO MedTech World

Yulia Yurevich
Head of Client Relations & Partnerships

Dr. Miguel Fenech Business Development
Manager

Sara De Luca Business Development Manager



Dylan strongly believes in collaborative efforts to enhance the healthcare system. He is dedicated to making MedTech World the epicenter of investment and progress. He aims to create a platform for meaningful discussions, fostering innovation and connections for entrepreneurs to scale their solutions.
As an entrepreneurial enthusiast in the MedTech industry, Yulia specialises in connecting global innovators, investors, and healthcare professionals to accelerate healthcare transformation also creating strategic opportunities for emerging ventures in the MedTech ecosystem.
Miguel is a medical doctor with extensive clinical experience across London and the UK. He has a keen interest in the safety, regulation, and ethical integration of AI-driven healthcare solutions in patient care and is a certified Clinical Safety Officer with NHS England.
With 8+ years in MedTech and HealthTech, and a background in biomedical engineering, product management, and business development, Sara combines technical insight with business strategy to support ecosystems where innovation scales and relationships last.

Miguel Musu Sales & Customer Success Manager

Shara Layton Head of Marketing

Emma Giannatiempo Junior Events Manager

Amisha
Patidar Junior Media Operations


Miguel is a physiotherapist with a prior finance background, combining clinical and business insight. He is focused on healthcare innovation, digital health and rehabilitation. Miguel also researches exergames for older adult balance.
Shara believes working in the MedTech industry is not just a profession but a purpose. She finds it incredibly fulfilling to contribute to groundbreaking innovations that are transforming global healthcare and improving lives around the world.
With a background in tourism and hospitality, She’s passionate about bringing people together, connecting industry leaders, and creating meaningful conversations across the MedTech industry.
An Economics and Business graduate with hands-on experience in marketing, media relations and digital strategy. Amisha helps build impactful partnerships in the healthcare technology sector. Ready to connect with driven individuals who share a passion for innovation and growth.