

Minerals in the Soil Environment
■ Brenda S. Tubana
Louisiana State University Agricultural Center, School of Plant, Environmental and Soil Sciences
■ Wooiklee S. Paye
New Mexico State University, Department of Plant and Environmental Sciences
Introduction
Seventeen elements are essential for plant growth and reproduction. A plant may not be able to complete its life cycle if one of these essential elements is absent. Other elements that promote growth of certain plants are classified as beneficial nutrients. The plant-essential nutrients are grouped into macronutrients and micronutrients. Macronutrients are taken up and utilized by plants in the greatest quantities. Micronutrients, while equally essential, are utilized by plants in much smaller quantities. Plants obtain three of the macronutrients, carbon, hydrogen, and oxygen, from air and water. The others are absorbed from the soil, also known as mineral essential nutrients, in ionized forms bearing either positive (cations) or negative (anions) charge, which interact with the soil, often in complex ways. The availability of these nutrients is governed by mechanical, chemical, and biological transformation processes in the soil and its components—organic fraction, clays, and minerals (Figure 1.1). The nutrients removed from the soil solution via plant uptake are replenished by mineralization, desorption, dissolution, and weathering processes. Fertilization readily increases available nutrients in the soil
and is a routine management practice in crop production. Nutrients in soil solution are rendered unavailable for plant uptake through immobilization, adsorption, and precipitation. Nutrients, especially the mobile ones, are lost from the soil/plant system via leaching, runoff, erosion, and through volatilization for some nutrients. The purpose of this chapter is to elucidate the processes in soil that affect the plant availability of essential elements.
Macronutrients
Carbon, Oxygen, and Hydrogen
A. von Thaer in Germany, Sir Humphry Davy in England, and J. J. Berzelius in Sweden were among the respected scientists in the early 1800s who considered that plants absorbed carbon (C) through their roots largely from soil organic matter, i.e., humus (Russell 1961). Today it is known that plants obtain C through their leaves from atmospheric carbon dioxide (CO2).
Oxygen (O), on the other hand, can be absorbed by both leaves and roots, but always in the gaseous
Adapted from Mineral Nutrition and Plant Disease (Datnoff, Elmer, and Huber 2007), Chapter 1, by Samira H. Daroub and George H. Snyder, University of Florida, Institute of Food and Agricultural Sciences, Everglades Research and Education Center, Belle Glade.
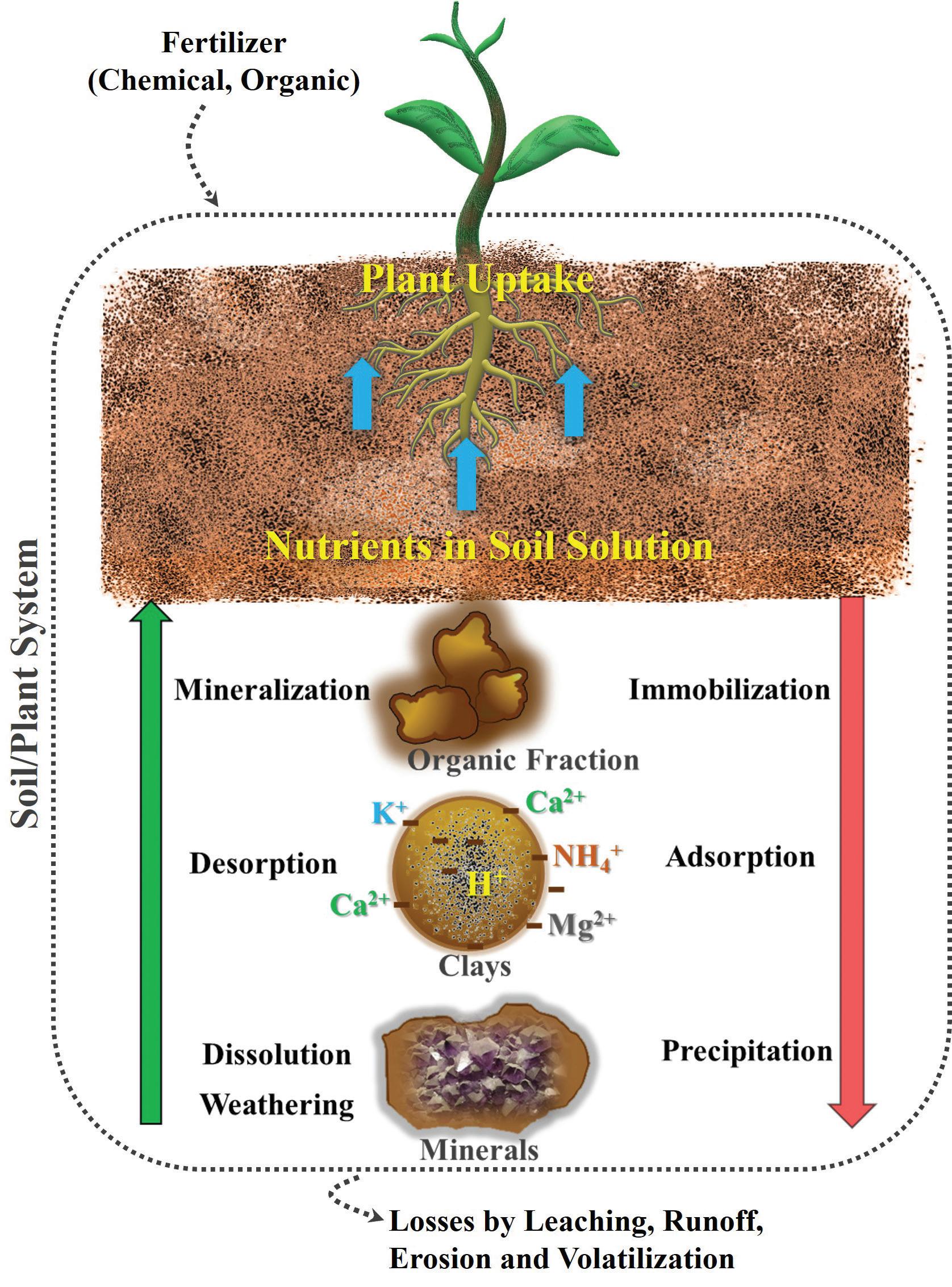
FIGURE 1.1. Processes in the soil and its components affecting the concentration of nutrients in the soil solution. Blue arrows represent nutrient uptake, and green and red arrows represent processes in the soil system that increase and reduce the concentration of nutrients in the soil solution, respectively. Fertilization readily increases available nutrients in the soil whereas leaching, runoff, erosion, and volatilization are lost pathways of nutrients out of the soil-plant system. (Courtesy B. S. Tubana and W. S. Paye—© APS)
diatomic form, O2. Oxygen in soil is generally present at much lower concentrations than in the atmosphere because of consumption by soil microorganisms. For this reason, plant root activity can be limited by the availability of O2. Oxygen is especially limited when the pore spaces between soil particles are filled with water since
O2 diffusion in water is very low, only 1 × 10–4 times that of diffusion in air. Under low-O2 conditions, terrestrial plant roots may have difficulty absorbing nutrients and water. Aquatic plants, on the other hand, contain parenchyma tissue that transmits O2 from the leaves to the roots, so the roots can function even in flooded soils.
Hydrogen (H) enters plants as a component of water (H2O). Water is considered the most limiting factor in a plant’s normal growth and development. As long as sufficient water is present to maintain plant turgor, sufficient H is available for metabolic needs.
Nitrogen
Most nitrogen (N) in soils is present in the organic form as a component of humus. As such, N is not available for plant absorption (uptake). Plant roots absorb N in its ionic forms: ammonium (NH4+), nitrite (NO2–), and nitrate (NO3–). The NO2– form is quite transitory in soils and is toxic to plants so there is very little uptake. Ammonium is produced from the ammonification process or decomposition of organic N-rich microbial, plant, and animal biomass by soil microorganisms. The rate of decomposition is regulated by factors that affect microbial activity, such as soil temperature, moisture, O2, and pH. For this reason, the supply of N for plant uptake can be limited when these factors are low or too high for optimal microbial activity. In well-aerated soils, nitrification proceeds wherein NH4+ is rapidly converted to NO2– and then NO3–. Thus, except in O2-depleted or flooded soil, most N absorbed by plants is in the NO3– form. Hydrogen ions (H+) are generated in the conversion of NH4+ to NO3– thus can subsequently acidify soils. Because NO3– is rapidly absorbed by plants and is also readily leached, soils generally contain little NO3– in the root zone. Continual replenishment of N is required to maintain rapid plant growth. Apart from leaching and soil surface runoff, N is lost from the soil in gaseous forms via denitrification. This is microorganism-mediated conversion of NO3– to nitric oxide (NO), nitrous oxide (N2O), and dinitrogen (N2) in O2-limited conditions as in very wet soils. On the other hand, N can be added to soils through fertilization and varying quantities can come from acid (diluted nitric acid) rain, dust, atmospheric deposition of reactive N from the ocean, and atmospheric N (N2) fixation by lightning. Also, certain microorganisms are capable of converting N2 to plant-available forms of N. The process is termed biological N2 fixation (BNF) and largely contributes N to soil at an estimated amount of 128 teragram (Tg, 1 × 109 kg) N yr–1 (Galloway et al. 2004). Some BNF is accomplished by free-living microorganisms, but most BNF occurs as the result of symbiotic interaction of bacteria and legumes. Biological N fixation is reduced in low pH soils due to restricted survival, growth, and reproduction of N2-fixing microorganisms, and excessive (e.g., manganese, iron) or deficient (e.g., calcium, molybdenum, and phosphorus) levels of nutrients (Rice et al. 1977; Hernandez et al. 2009). Movement of H2O and O2 is restricted in
soils with poor porosity thus microbial activities that facilitate BNF are reduced (Stott et al. 1990; Breland and Hansen 1996; Franzluebbers et al. 1996).
Phosphorus
Phosphorus (P) is one of the most limiting nutrients in crop production mainly due to its very reactive nature. Phosphorus is present in both organic and inorganic forms in soils. While the percentages of each can vary widely, substantial amounts of both forms generally are present. Phosphorus is absorbed by plant roots mainly as phosphate anions (H2PO4– and HPO42–). The concentration of P in the soil solution is very low generally ranging from 0.001 mg L–1 in very infertile soils to 1 mg L–1 in heavily fertilized soils (Brady and Weil 2002). For uptake to occur, P in soil organic matter must be converted to orthophosphate anions by soil microorganisms through a process known as mineralization. The release of P from organic matter, like that of N, depends on soil temperature, moisture, O2, and pH. The amount of plant-available P in soils is generally low because phosphate anions are quite chemically active. In acid soils in which cations of metals such as aluminum (Al), iron, and manganese are abundant, insoluble complexes are formed with P. In alkaline soils, P forms relatively insoluble compounds with calcium. Phosphate ions are strongly adsorbed by silicates in soils of near neutral pH. Consequently, while soils may contain considerable quantities of P, most of it is present in forms that are not found in the soil solution and available for plant uptake (Figure 1.2). Fertilizers contain P generally in the form of highly soluble monocalcium phosphate, Ca(H2PO4)2. Upon addition to soil, substantial amounts of the highly reactive H2PO4– form insoluble compounds with Al, iron, or calcium, depending on soil pH. Some plant-available P is eventually released by the slow dissolution of these compounds.
The concentration of P in soil solution is often too low to meet the plant’s requirement in most agricultural soils. Plants obtain additional P from the soil solid phase through a series of P transformation involving biotic and abiotic processes. The biotic process that governs the release of P into soil is through microbial biomass degradation and organic P mineralization (Frossard et al. 2000; Havlin et al. 2005). Soil microorganisms decompose plant and animal residues, resulting in the release of phosphate containing organic compounds that are later transformed into inorganic P. Abiotic P transformation involves adsorption and desorption, weathering of primary and secondary P minerals, precipitation, and dissolution reactions. Adsorption occurs on the surfaces of clay particles and on iron and Al oxides. When P concentration in soil solution is low, it encourages

FIGURE 1.2. Effect of soil pH on the relative abundance of various chemical forms of phosphorus. Phosphates available to plants constitute relatively little of the total phosphorus in the soil and are most abundant at pH 6–7. (From Brady, Nyle C.; Weil, Ray R., The Nature and Properties of Soils, 13th Edition, © 2002. Reprinted by permission of Pearson Education, Inc. Upper Saddle River, NJ.)
the release of P from clay surfaces and/or dissolution of P-containing minerals back into soil solution. A high concentration of P in soil solution promotes a reaction that precipitates P by Al and iron (mostly in acidic soil) as well as calcium (mostly in calcareous soils).
Potassium
Potassium (K) is one of the primary macronutrients and is considered the third most limiting nutrient in crop production. The concentration of K in most mineral soils is usually higher than N and P (Reitemeier 1951). On average, the K concentration in mineral soils ranges between 0.04 and 3% and the total K content within the top 0.2 m of the soil profile can be anywhere between 3,000 and 10,000 kg ha–1. Unlike N and P, almost all K in soils is present in inorganic forms and no gaseous phase, with only 1–2% in soil solution and exchangeable phases (Figure 1.3) (Schroeder 1979; Bertsch and Thomas 1985). Plants take up K in large quantity second only to N and the removal of K from soils through harvested crops is far greater than the removal rate of N (Kayser and Isselstein 2005). Sandy soils, being composed primarily of quartz (SiO2), frequently contain little K, but many soils contain very large amounts of it. However, most of the K in these soils is present as relatively insoluble minerals (90–98%) such as feldspars and micas, or is “fixed” in the mineral lattice of certain
clays (1–10%) such as hydrous micas also known as illite (Figure 1.3). Consequently, K deficiency can be observed in plants growing in soils containing considerable amounts of total K.
Potassium exists in soils in four different forms as solution, exchangeable, fixed, and nonexchangeable K (Figure 1.3). Potassium is absorbed by plants in the cationic (K+) form directly from the soil solution. This is termed solution K. However, K concentration in the soil solution is usually low ranging only between 2 and 5 mg L–1 in normal and unfertilized agricultural soils of humid regions; and may be higher in arid regions (Haby et al. 1990; Sparks 2000). Soil humus and soil clays generally are negatively charged so K+ is adsorbed and bounded as an outer-sphere complex on the surfaces of these materials. Such K is termed exchangeable because plants exchange secreted hydronium ions (H3O+) for adsorbed K+. Exchangeable K and solution K are both readily available for plant uptake but constitute only 1–2% of the total soil K (Hinsinger 2006; Brady and Weil 2017). Once present in the soil solution as K+, it forms few insoluble compounds. However, hydrous mica clays can reduce soil solution K by entrapping it in their mineral lattice. The term nonexchangeable or fixed K refers to the form of K not found in crystalline minerals but is rather tightly held between adjacent layers of clay minerals such as mica and vermiculite. When the binding force between K+ ions and the clay

surfaces is greater than the hydration forces between individual K+ ions, it gives rise to K fixation. This is because the partial collapse of the crystal structures of the clay mineral traps the K ions, only releasing them through a slow diffusion process (Sparks 1987). Hence, when levels of solution and exchangeable K are diminished by leaching, runoff, crop removal, or microbial use, the release of some nonexchangeable K may occur (Sparks 1980; Sparks 2000). The K in fertilizers is generally found in the form of soluble K salts such as potassium chloride, potassium sulfate, or potassium magnesium sulfate.
Plant K availability is influenced by clay content and type, soil organic matter, pH, and cation exchange capacity (CEC). The negative charges on the large surface area of clay particles and organic matter (humus) increase soil K retention. At high pH, H+ ions are dislodged from the soil exchange sites, allowing other
cations such as K to bind. The soil exchange sites serve as storage of readily available forms of K, which increases plant-available K and minimizes loss potential via the leaching process.
Calcium and Magnesium
Calcium (Ca) and magnesium (Mg) are needed by crops in large amounts but are less likely to limit crop yield unlike the primary macronutrients N, P, and K. Thus, both Ca and Mg are classified as secondary macronutrients absorbed by plants in the divalent cationic form found in the soil solution and on exchange sites. Like K, most Ca and Mg exist in soil in mineral forms. However, the predominant forms in alkaline soils are the carbonates, CaCO3 and MgCO3. In comparison to N and P, relatively little plant-available Ca and Mg originates from soil humus.
In addition to carbonates, minerals such as anorthite, augite, biotite, gypsum, hornblende, mica, and olivine supply soil Ca and Mg as they weather. Highly acid soils may be quite low in plant-available Ca and Mg. Clays, being negatively charged, adsorb Ca2+ and Mg2+, often preferentially to other cations. Therefore, a substantial quantity of the Ca and Mg in soils is in the readily available exchangeable form. Low solution Ca and Mg are then replenished by those exchangeable forms bonded to the soil exchange sites by means of desorption or through dissolution of Ca and Mg containing minerals. Excess Ca and Mg in soil solution can either precipitate to form secondary minerals or can be adsorbed to clay particles. Weathering can also release Ca and Mg, which can be adsorbed by clay minerals. However, Ca and Mg are less likely to be affected by clay fixation like K.
Calcium reacts with P to form relatively insoluble compounds, but the reaction generally has more consequence for limiting P availability than for Ca. Also, the divalent Ca2+ causes strong electrostatic attraction with negatively charged clay particles and soil organic matter. This promotes soil aggregation, which is a process known as flocculation. Calcium and sometimes Mg fertilization is often accomplished by adding lime or dolomite to soils to increase the soil pH. Thus, in cultivated acidic soils with good liming programs, Ca and Mg deficiencies seldom limit crop productivity. When an increase in pH is not desired, Ca and Mg may be supplied in the form of sulfates.
Deficiencies of Ca and Mg likely occur in sandy, highly leached acidic soils. The occurrence of Mg deficiency is more common than Ca deficiency because most liming materials contain Ca, and Mg poorly competes against other cations for both plant absorption and soil exchange sites. Plant uptake of Mg can be strongly affected by the presence and abundance of other cations such as Ca2+, K+, and NH4+ (Diem and Godbold 1993; Fageria 2001; Römheld and Kirkby 2007). Unlike Ca, K, and NH4, which are strongly attracted by soil particles, Mg is relatively mobile in soils due to its unique chemical property. Magnesium has a relatively smaller ionic radius compared to Ca2+, K+, or Na+, but a significantly larger hydrated radius (Shaul 2002; Maguire and Cowan 2002; Gardner 2003). This causes a weaker bonding of Mg to the soil CEC, relative to the other cations, making it highly mobile in soils and prone to leaching.
Sulfur
Sulfur (S) is also a secondary macronutrient but unlike Ca and Mg, the cases of S deficiency in crops has become a major concern in recent years. The decline in soil S can be attributed to several factors such as: use of chemical fertilizers with a high degree of purity, reduction in S
dioxide (SO2) emission from burning of coal and fossil fuel, use of high-yielding crop varieties, intensive cropping systems, and decreases in use of organic fertilizers (Scherer 2001; Grant et al. 2012). Sulfur is taken up in similar quantity as P but to a lesser extent when compared to N and K (Havlin et al. 1999). Sulfur is present in soils in both organic and inorganic forms. In temperate humid regions, over 90% of the S in soils is present in soil organic matter. However, S is absorbed by plants as the sulfate anion, SO42–. Consequently, organically bound S must be released by microbial decomposition to be available for plant uptake so the factors that affect microbial activity (e.g., temperature, moisture, O2, and pH) affect S availability. Sulfur mineralization releases 1 to 3% of organic S per year, which is equivalent to 4–14.5 kg SO42– ha–1 (Havlin et al. 1999).
The transformation of S to its oxidized and reduced forms is governed by biological and chemical reactions. In soils with very poor aeration, S is present in reduced forms such as elemental S (S0) or as sulfides (S2–). The rotten-egg smell of hydrogen sulfide (H2S) is a classic indicator of very poor soil aeration. Mineral sulfides include the well-known compound fool’s gold (iron pyrite). In well-aerated soil, S0 readily oxidizes producing SO42– and H+ ions. Thus, the oxidation of S0 increases the acidity of the soil. Most bacteria function poorly under highly acidic conditions, but those that oxidize S continue to function in spite of the acidity that results from the production of SO42–. Sulfates can be adsorbed on variable-charged clay surfaces, especially in acid soils high in iron and Al oxides and by the clay mineral kaolinite, but adsorbed SO42– represents a small component of the total S in most agricultural soils. Other than forming gypsum with Ca in arid soils, S undergoes few inorganic reactions that limit its availability to plants. In industrial areas, considerable S may be added to soils by way of airborne contaminants such as those produced during the combustion of fossil fuels. Sulfur dioxide diffusion into the stomata delivers low concentrations of S to the crop. Depending on the location, between 3 to 12 kg ha–1 yr–1 can be added to cropland through atmospheric deposition (Foth and Ellis 1997). When S fertilization is required, S0 or various SO42– salts such as gypsum or magnesium sulfate may be used.
Micronutrients
Micronutrients include iron, zinc, copper, manganese, boron, molybdenum, chlorine, and nickel. The availability of micronutrients depends on their solubility in soils, which is often determined by factors other than concentration. Deficiency usually occurs due to
insufficient amounts of available (soluble) forms to the plant. The primary factors include soil pH and organic matter content. Like the macronutrients, the dynamics and availability of micronutrients in the soil is also influenced by redox potential. Both soil pH and redox potential can be used to establish the domains that characterize nutrient availability and risk areas for deficiency and toxicity (Figure 1.4) (Husson 2013). In soils with optimal pH level and organic matter content, the small quantities of micronutrients required by plants are often sufficient thus, overall micronutrient deficiency is not common. Micronutrient deficiency can be induced by the presence of other nutrients in excessive amounts.
Iron
Iron (Fe) is the fourth most abundant element in the lithosphere. It is geochemically unique in its ability to form numerous stable compounds both with S
and with O plus silicon. Most of the Fe in soil occurs in primary minerals, clays, oxides, and hydroxides. The primary minerals in which Fe is present include ferromagnesian silicates, such as augite, biotite, hornblende, and olivine (Mengel and Kirkby 2001). Weathering of these minerals produces secondary FeIII minerals such as hematite (α-Fe2O3) and geothite (α-FeOOH). The solubility of the FeIII oxides is extremely low at high pH. Iron deficiency is most often observed in high-pH and calcareous soils of arid regions, but it also occurs in acidic soils that are very low in total Fe. An increase in rhizosphere pH results in Fe deficiency in soils that receive repeated applications of NO3–-N fertilizer (Marschner et al. 1986). Deficiency of Fe can be induced in the presence of excessive P in soils (Sánchez-Rodríguez et al. 2014). The reduction-oxidation (redox) potential of soils also affects Fe solubility. Compounds of Fe become more soluble as FeIII oxides are reduced to Fe2+ (dissolved in

FIGURE 1.4. Eh-pH domains that characterize favorable conditions for nutrient availability and risk areas of deficiency and toxicity. (Adapted from Husson 2013. Courtesy B. S. Tubana—© APS.)
the soil solution) when the O2 supply in soils is low, as in wet soils containing decomposable organic matter. The concentration of soluble Fe in soils is extremely low in comparison with the total Fe concentration. Iron in the soil solution is found as Fe2+, Fe (OH)2+, Fe(OH)2+, and Fe3+, with Fe3+ being dominant with Fe2+. Both Fe2+ and Fe3+ are forms taken up by plants but Fe2+ is more preferred than Fe3+. Iron solubility is largely controlled by the solubility of hydrous FeIII oxides. These give rise to Fe3+ and its hydrolysis species (Lindsay 1979):
+ 3OH– ↔ Fe(OH)3 (solid)
For every unit increase in pH, the Fe3+ concentration decreases 1,000-fold. Over the normal pH range in soils, soil solution Fe is not sufficient to meet plant requirements even in acidic soils. Numerous natural organic compounds in soil are able to complex or chelate Fe3+ and other micronutrients and therefore increase the availability of these micronutrients in the soil solution. Chelates are soluble organic compounds that bond with metals such as copper, Fe, manganese, and zinc, increasing the solubility of these metals and their supply to plant roots. The Fe chelates (siderophores) are highly soluble and are stable over a wide range of pH. Natural organic chelates in soils are products of microbial activity and degradation of soil organic matter and plant residues. Most animal wastes contain small quantities of plant-available Fe. Synthetic chelates of copper, Fe, manganese, and zinc are often applied to soils as fertilizers. Common natural and synthetic chelates are listed in Table 1.1. Chelation increases the solubility and transport of micronutrients. The stability of chelates is
greatly affected by pH (Norvell 1972) (Figure 1.5). The Fe-EDDHA (ethylenediaminedi-o-hydroxyphenylacetic acid) is stable over the pH range of 4 to 9, while chelates like Fe-EDTA (ethylenediaminetetraacetic acid) and Fe-DTPA (diethylenetriaminepentaacetic acid) are not stable at high pH. Abundant Ca in high-pH soils will easily replace the Fe on EDTA or DTPA and the Fe will precipitate as an oxide.
Manganese
Manganese (Mn) is found in various primary rocks, particularly ferromagnesian (Fe-Mg) materials. The weathering of primary rocks releases Mn, which forms
Ethylenediaminetetraacetic acid
Diethylenetriaminepentaacetic acid
Ethylenediaminedi-ohydroxyphenyl-acetic acid
Citric acid
Oxalic acid
Pyrophosphoric acid
C10H16O8N2 EDTA
C14H23O10N3 DTPA
C18H20O6N2 EDDHA
C6H8O7 CIT
C2H2O4 OX
H4P2O7 P2O7
a From Norvell 1972—Reproduced, by permission, of Soil Science Society of America and American Society of Agronomy.
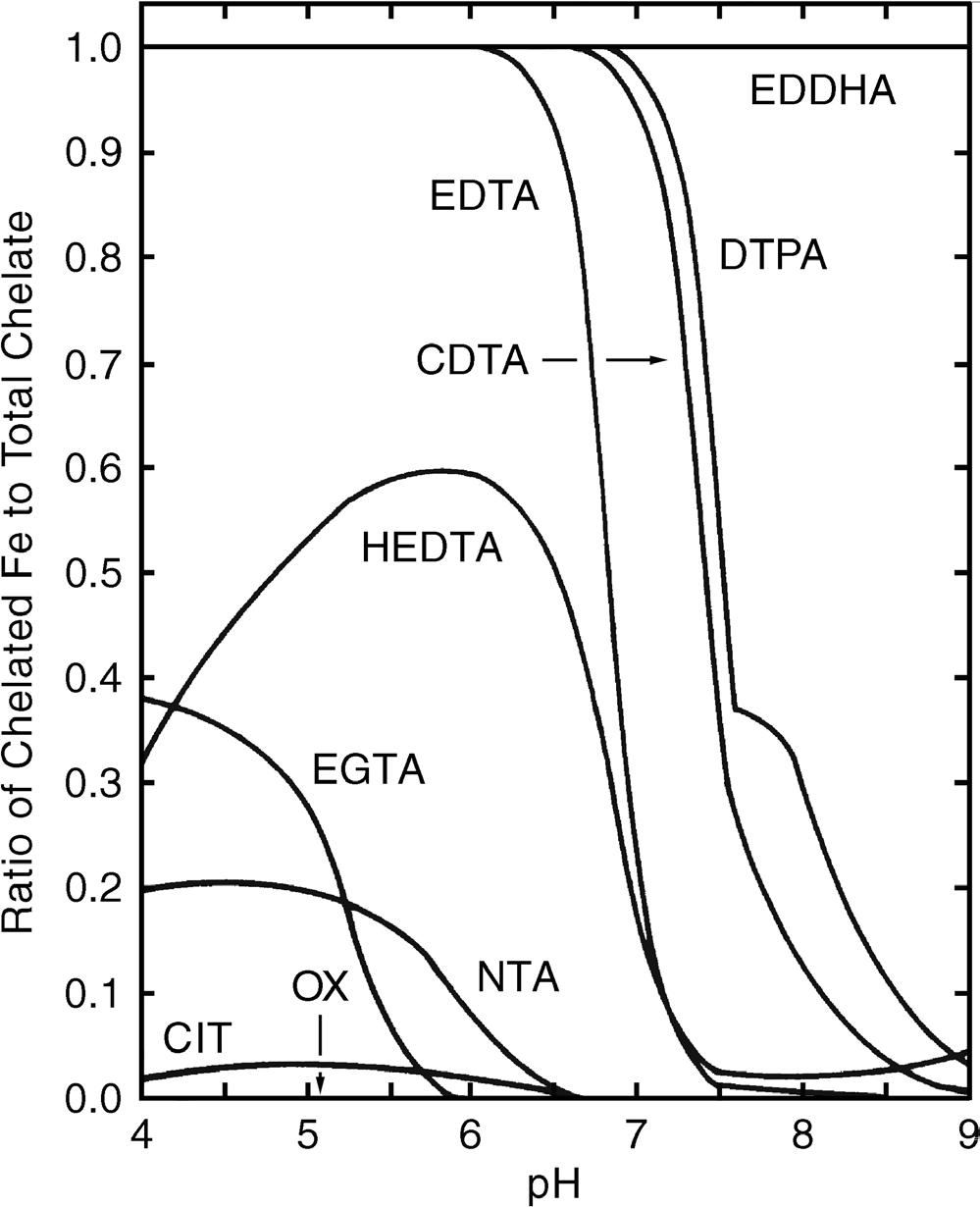
FIGURE 1.5. Stability of iron chelates in the soil solution. Equilibrium between H+, Ca2+, Mg2+, Al3+, and Fe3+ is assumed, and the concentration of each chelating agent is assumed to be 10 –4 M. CDTA = cyclohexanediaminetetraacetic acid. CIT = citric acid. DTPA = diethylenetriaminepentaacetic acid. EDDHA = ethylenediaminedi- o -hydroxyphenylacetic acid. EDTA = ethylenediaminetetraacetic acid. EGTA = ethyleneglycol-bis(2-aminoethylether)-tetraacetic acid. HEDTA = hydroxyethylethylenediaminetriacetic acid. NTA = nitrilotriacetic acid. OX = oxalic acid. (From Norvell 1972—Reproduced, by permission, of Soil Science Society of America and American Society of Agronomy)
