BE AHEAD OF THE TIMES STAY HUNGRY FOR THE UNKNOWN AND WRITE YOUR OWN HISTORY. THAT’S WHAT WE DO.
Great people | Great instruments




BE AHEAD OF THE TIMES STAY HUNGRY FOR THE UNKNOWN AND WRITE YOUR OWN HISTORY. THAT’S WHAT WE DO.
Great people | Great instruments


A world where people with cancer live better and longer lives. That’s Pfizer’s vision. We are accelerating breakthrough cancer medicines that bring new hope to patients everywhere.
Pfizer is tackling one of the most challenging health crises of our time. Nothing is more urgent, or more personal, than our goal to outpace, outsmart, and outmaneuver cancer at every turn.





4 MINISTER’S FOREWORD
Minister Peter Burke underlines the Government’s commitment to enhancing competitiveness and discusses the development of Ireland’s National Life Sciences Strategy.
6 SECTORAL OVERVIEW
Sinead Keogh, Director, BioPharmaChem Ireland, reflects on a tumultuous year for Ireland’s BioPharmaChem sector, which displayed tremendous resilience in the face of global challenges.
12 NATIONAL LIFE SCIENCES STRATEGY
30 BIODIVERSITY
How the Lower Harbour Biodiversity Forum is creating a shared vision for Cork Harbour and restoring nature across Cork’s pharma cluster, by Michael Kilkelly, Senior Executive, EHS, BioPharmaChem Ireland.
33 BIOPHARMACHEM SKILLNET
2025 was a very successful year for BioPharmaChem Skillnet, including new programmes, exciting collaborations and respected award wins.
36 NIBRT
Lisa Goodman, Senior Public Affairs Executive, BioPharmaChem Ireland, provides the backdrop to the development of Ireland’s first-ever National Life Sciences Strategy and argues that to deliver targeted outcomes, the Strategy must be governed by a dedicated national Office of Life Sciences.
14 DIGITAL HEALTH
Ireland is in a unique position globally to lead the development of Digital Health, explains Sinéad Keogh, Director, BioPharmaChem Ireland and Head of Sectors, Ibec.
16 SUSTAINABILITY
The transition to Green Chemistry presents a business opportunity for Ireland’s API sector, writes Michael Kilkelly, Senior Executive, EHS, BioPharmaChem Ireland.
20 SUPPLY CHAIN
Karina Cassidy, Senior Executive, Regulatory Affairs, BioPharmaChem Ireland, stresses the importance of ensuring an agile supply chain for Ireland’s Pharmaceutical industry, in the face of a volatile trade environment.
22 BIOPHARMACHEM IMPACT 2026
BioPharmaChem Impact 2026 is a much-attend event for senior leaders in the industry. Sinéad Keogh, Director of BPCI, provides a sneak preview of what to expect.
24 MANUFACTURING
Sharon Higgins, Executive Director, Membership and Sectors, Ibec, reflects on Ibec’s fifth Annual Manufacturing Report, which presents a mandate for Ireland’s manufacturing future based on securing competitiveness by controlling costs and mastering AI.
27 ARTIFICIAL INTELLIGENCE
Adopting Generative AI is huge challenge, but one Ireland’s Life Sciences sector must embrace if it is to continue its international success, argues Sinead Keogh, Director BioPharmaChem Ireland and Ibec Head of Sectors. 4 33 24 6
Navigating disruption: opportunities and challenges in a year of transition, by Killian O’Driscoll, Chief Commercial Officer, NIBRT.
42 EDUCATION
A partnership between a Waterford school and BioPharma company Sanofi points the way to how we can advance education for sustainable development through school/industry partnerships.
44 PHARMACEUTICAL MANUFACTURING TECHNOLOGY CENTRE
PMTC brings together multinationals, SMEs, and academic institutions to tackle realworld challenges in Pharmaceutical and BioPharmaceutical manufacturing.
46 SSPC
SSPC’s recent impact report highlights the centre’s incredible economic contribution to Ireland since 2008.
47 LEAP COMPLIANCE
Leap Compliance work with Pharmaceutical and medical device manufacturers to build skills, strengthen capability, and create high performing teams.
48 ASTELLAS
Astellas recently announced a multi-site investment of €129 million over the next three years.
50 IDA IRELAND
Rory Mullen, Global Head of BioPharma, IDA Ireland, reflects on a buoyant year in 2025, despite challenging global headwinds and geopolitical conditions.
52 BIOLOGICS TESTING & MICROBIAL QC SOLUTIONS
Charles River provide world class biologics testing, alongside regulatory guidance and consultative expertise, helping their clients towards regulatory approval.

56 CHEMICAL SOLUTIONS: CSG
CSG, including Clemifloc and GI Chemicals, provide reliable chemical solutions for Ireland’s Life Sciences and utilities sectors.
58 LEADERSHIP IN LIFE SCIENCES
The upcoming National Life Sciences Strategy is a huge opportunity for Ireland to cement its competitive advantage in global Life Sciences, write Niall Best and Conor Cunningham, McCann FitzGerald’s Life Sciences Group.
60 KELSIUS
CoolCheck by Kelsius is an automated, wireless monitoring system that delivers efficiency without compromising compliance.
62 HUB PACKAGING
Hub Packaging can be your trusted partner in industrial packaging, with a broad range of packaging solutions available.
64 MICRO-BIO
Ireland’s only Chlor-alkali manufacturer, Cork’s Micro-Bio are pioneers in Membrane Cell electrolysis.


65 HOVIONE
Hovione are a science-based, integrated CDMO, with over 60 years of experience in Pharmaceutical development and manufacturing operations.
66 PHARMACEUTICAL TESTING
Anton Paar’s series of FTIR spectrometers and software solutions are ideal for the Pharmaceutical industry, where they are used to confirm the identity of raw materials, verify the composition of intermediates, and assess the quality of finished products.
70 RENEWABLE ENERGY: GREENVOLT NEXT
Greenvolt Next have developed a 4 MW solar PV plant for global BioPharmaceutical company Sanofi in Waterford.
72 EDUCATION: ATLANTIC TECHNOLOGICAL UNIVERSITY
Atlantic Technological University’s new School of Life Sciences offers an innovative range of programmes and research for the Pharma, BioPharma and wider Life Sciences industries.
74 GE HEALTHCARE
GE HealthCare announced a massive $138 million investment in their Cork campus in 2025, with work progressing well on-site.
76 BIO PHARMA TECHNICAL CONSULTING
Chemistry, Manufacturing and Controls (CME) should be the backbone of any drug development programme and Bio Pharma Technical Consulting can help you to accelerate success.
78 ENDRESS+HAUSER
Endress+Hauser’s dedicated Flow Calibration Lab in Cork has achieved ISO 17025 accreditation, providing superior service for customers.
80 NATIONAL CHEMICAL COMPANY
Alan Looney, Managing Director of NCC, explores how the company’s strategic approach delivers a competitive advantage in chemical sourcing.
82 HEALTHCARE LOGISTICS: KUEHNE+NAGEL
Seamus Keane, VP Pharma and Healthcare Europe, Kuehne+Nagel, advises on optimising your Pharma and BioPharma logistics network.
84 MASON TECHNOLOGY
Mason Technology have built a reputation as a trusted partner to Ireland’s Pharmaceutical, BioPharmaceutical, and Medical Device industries.
85 AUTOMATION: BONNER
Bonner’s automation specialists work together with clients to review existing systems, create tailored upgrade strategies, and deliver solutions with minimal disruption.
86 ANALYTICAL SOLUTIONS: ALMAC
With more than 55 years of experience across five global locations, Almac can add real value to drug development and commercialisation programmes.
88 WASTE SOLUTIONS: INOPSYS
InOpSys are committed to providing circular waste or side stream solutions for the chemical and Pharmaceutical industry.
90 SOLTEC
Soltec enjoyed a great 2025, and were recently rewarded with two major sustainability awards.
Managing Director: Patrick Aylward
Editor:
Advertising

Currently it is a time of many challenges for procurement professionals. Reliably sourcing and securing supply of vital chemicals. It seems there’s ever greater pressure, fewer certainties, more hurdles.
But some of the world’s best-known companies have a hidden advantage. They don’t spend too much time problem solving If they need something, it’s sourced and supplied. Quietly and efficiently. They don’t need the headaches of multiple vendors and different standards, it’s streamlined. Compliance, paperwork, it’s taken care of. They have an advantage.
They have Camida.
Minister Peter Burke , underlines the Government’s commitment to enhancing competitiveness and discusses the development of Ireland’s National Life Sciences Strategy.
As Minister for Enterprise, Tourism and Employment, I am honoured to contribute to this year’s Irish PharmaChem Yearbook. I had the opportunity this summer to contribute to the summer edition of the Yearbook and I set out the strengths of this sector in Ireland, its agility and its resilience in the face of increasing uncertainties.
The current Programme for Government called for the preparation of an Action Plan on Competitiveness and Productivity which was published in September. Our commitment to the Life Sciences is underpinned in each theme of the Action Plan.
• Under Productivity, we’re embracing research, innovation and skills, particularly through R&D, talent development, and collaboration between academia and industry.
• We are Internationally Focused, we are aiming to boosting FDI and exports by using Ireland’s role as a global hub for Pharma/Medtech with strong EU-US trade links.
• We’re creating and scaling more SMEs, leaning into partnerships between multinationals and SMEs, and creating opportunities for indigenous enterprises.

• On Competitiveness, we’re regulating for growth and controlling costs so Ireland can uphold its reputation as a pro-enterprise nation and remain a destination of choice for global BioPharma investment.
• Increasing the State’s capacity to deliver Infrastructure is key to our ability to attract investment and enable regional development.
• And the Action Plan’s final theme, Sustainability, is about growing sustainable Irish businesses and boosting regional development, with an emphasis on decarbonisation, renewable energy integration and green innovation.
The Action Plan highlights the importance of the development of a National Life Sciences Strategy. Engagement with stakeholders will be key in developing an ambitious strategy, clearly aligned with the needs of the industry.
The public consultation on the development of the National Life Sciences Strategy was opened late last year to seek your views on key themes including scope, objectives, areas for opportunities and alignment with the European Life Sciences Strategy. With the input of key stakeholders like you, we can develop an ambitious, coherent strategy that will deliver real-world impact.
I am confident that in 2026 the Life Sciences sector will continue to be a cornerstone of Ireland’s economic success, and the development of the strategy will further harness this.
I wish the Irish PharmaChem Yearbook, and everyone involved, every success in 2026.
Peter Burke TD, Minister for Enterprise, Tourism and Employment



Sinéad Keogh, Director, BioPharmaChem Ireland, reflects on a tumultuous year for Ireland’s BioPharmaChem sector, which displayed tremendous resilience in the face of global challenges.
The past year has been defined by complexity, challenge, and, most importantly, strategic resilience and tangible delivery. The BioPharmaChem sector in Ireland, a sector that directly and indirectly employs over 80,000 people, has achieved unprecedented success despite headwinds.
As an industry, we have navigated shifting global supply chains, absorbed fundamental international tax reforms, and managed the acute pressures of talent acquisition, all while continuing to drive record exports and therapeutic innovation. Crucially, this period has been profoundly marked by geopolitical risk and the chilling effect of trade uncertainties. The sector has faced mounting risks from factors like the introduction of US tariffs and increasing global competition, which has directly impacted forward-looking sentiment and investment decisions across our membership.

It is precisely within this dynamic and pressurised environment that BioPharmaChem Ireland (BPCI) has reinforced its role as the essential strategic partner for our members and for the Government. The period under review marks a pivotal moment where the collective advocacy and strategic influence of our organisation translated directly into quantifiable, pro-investment policy outcomes, securing the sector’s competitive edge for the decade ahead.
Our focus has been uncompromising: to ensure Ireland remains the globally recognised centre of excellence for the sustainable manufacture and supply of pharmaceuticals, biopharmaceuticals, and chemicals.
This success is measured against our core framework, the Five Strategic Pillars which guide our advocacy, resource allocation, and value proposition to every member company: Quarterly Trends and Risk Analysis, Talent
& Workforce Development, Sustainability & Responsible Care, Digitalisation & AI, and Ecosystem Development.
Pillar 1. The Strategic Imperative: Quarterly Trends, Opportunities, and Risk Analysis
The first and most foundational strategic pillar is ensuring our organisations have the foresight and analysis necessary to navigate a volatile global environment.
The BioPharmaChem sector, contributing approximately €116 billion in annual exports, operates at the sharp end of the knowledge economy, and its stability is a national economic priority.
The new theme: uncertainty and cost pressures
Our internal analysis, powerfully reflected in our BioPharmaChem Manufacturing Report,

December 2025, identifies a pervasive theme: Uncertainty. The assessment of the wider manufacturing environment is significantly less positive than this time last year.
This sentiment is directly tied to mounting cost pressures and geopolitical risks. The findings register higher expectations of increased costs for wages (86%), and for both raw materials and energy (71%) in the months ahead. This cost inflation, coupled with market uncertainty, is dampening forward-looking metrics, with reduced numbers expecting profitability to increase (down from 33% to just 14%), and lower levels of expected increased capital investment (54% to 39%).
The most prominent challenge is explicitly external: trade uncertainties (68%), a figure that has tripled since 2024 (21%). Moreover, three-quarters of participating businesses expect to be impacted by US tariffs, with the majority anticipating a negative effect. This pervasive risk is even leading to concerns over Ireland’s fundamental appeal, as the nation's reputation as a manufacturing hub poses a major or minor challenge to half of Pharma firms (54%).
The policy wins of the past year were designed precisely to combat these headwinds and preserve Ireland's competitive edge:
• R&D Tax Credit Increase: Our most significant policy victory, working with Ibec, was securing a major enhancement to the national R&D Tax Credit, increasing the rate from the previous 30% to a globally competitive 35%. This is an immediate, quantifiable benefit that directly lowers the effective unit cost of innovation, targeting capital investment and acting as a crucial defence against international competitive pressures. Our influence is built on direct, strategic engagement with Government leaders; the BPCI Board met with then Finance Minister Paschal Donohoe TD to discuss core priorities, including incentives for innovation.
• National Life Sciences Strategy Commitment: Following years of sustained lobbying, we successfully secured a formal Government
commitment to develop a National Life Sciences Strategy for Ireland. Our influence in this process means the strategy is being designed by industry, for industry, creating a predictable, competitive landscape for the decade ahead. We hosted a roundtable meeting with Irish Medtech and subsequently submitted a cross-sectoral White Paper to the Government, and the public consultation phase is now officially underway.
In line with this global focus, BPCI actively engaged with EU and international partners throughout the year:
• Brussels Delegation and EU Policy Influence: In March, BPCI led a delegation to Ibec's Annual St Patrick’s Day event at the European Parliament, engaging with key figures including Commissioner Michael McGrath, Séan Kelly MEP, Billy Kelleher MEP, Nina Carberry MEP, and Cynthia Ní Mhurchú MEP. This advocacy work directly influences the debates on key policy files, such as the EU Biotech Act, which


BPCI responded to the EU Commission consultation in collaboration with members, as well as the General Pharmaceutical Legislation, the Critical Medicines Act, and the Urban Waste Water Treatment Directive. We also met with Minister of State for European Affairs and Defence, Thomas Byrne TD, in October to discuss priorities for the upcoming Irish Presidency of the Council of the EU.
• Presence at BIO International: BPCI maintained a vital presence on the global stage, with our attendance at the annual BIO International Convention in Boston in May. This included attending the annual ICBA meeting and engaging with EU Health Commissioner Olivér Várhelyi. This engagement is critical for maintaining dialogue with international counterparts and shaping global trade and regulatory environments. The ICBA continues to meet monthly.
The engine of the BioPharmaChem industry is its talent. Our association’s focus is on securing and continuously upgrading the skills pipeline for roles across the entire value chain.
With Ibec, our lobbying efforts for increased public sector research funding resulted in a major success: the substantial €4.55 billion investment into the Department of Further and Higher Education, Research, Innovation and Science (DFHERIS) under the National Development Plan. We advocated for this investment to close the funding gap in the higher education system and ensure a pipeline of high-calibre researchers.
A key highlight of our strategic influence this year was the dedicated member roundtable with James Lawless TD, Minister for Further and Higher Education, Research, Innovation and Science, in July and a meeting with Secretary General (DFHERIS) Colm O‘Reardon in June. This direct engagement allowed BPCI to shape the Government's focus on innovation, talent, and skills, ensuring our specific needs are recognised at state level.
To champion the critical role of these research centres, we produced essential thought leadership, including impactful podcasts with the SSPC (Solid State Pharmaceutical Cluster) – the SFI Research Ireland Centre for Pharmaceuticals - and the National Institute for Bioprocessing Research and Training (NIBRT).
We are deeply involved in governance and strategy: BPCI continues to work closely with the Expert Group on Future Skills Needs (EGFSN) Biopharma Skills Implementation
Group, and we secured a commitment to take on the sectoral lead role for this group from February 2026 to November 2027; the group held its inaugural Skills Forum in Cork on November 6, 2025.
Driving industry/school engagement and skills development
We recognise the necessity of engaging early to build the pipeline: in November, BPCI, in collaboration with the Department of Education, unveiled the Inspiring Futures: Mapping Industry-School Engagement in Ireland Report 2025. We engaged directly with Education Minister Helen McEntee TD in October to discuss the findings.
Furthermore, our practical work ensures the sector offers diverse and compelling career pathways, achieving a 20% increase in the numbers on the Lab Apprenticeships programme, and launching a new Bio Process/Chemical Process Technician Apprenticeship, along with extensive training and development run through our award winning BioPharmaChem Skillnet.
These focused initiatives provide a crucial, immediate supply line for skilled technicians and combat the acute pressures of talent acquisition. This is supported by specific working groups, including the HR Forum and the inaugural Learning and Development (L&D) Forum, ensuring continuous, peerled professional development across our member companies.
The industry’s commitment to responsible growth is articulated through our work on Sustainability and Responsible Care. This commitment is a strategic necessity, ensuring both environmental stewardship and operational licence.
A key highlight was the publication of the BPCI Sustainability Strategy and Responsible Care Report 2025 in March, tracking industry progress in energy, emissions, data management, and culture. The knowledge and data gathered here have helped members benchmark themselves against other companies in the sector. Our commitment to this pillar includes the oversight of the BPCI Sustainability Steering Group and the launch of the BPCI Biodiversity Network in lower Cork Harbour, with ambitions to establish a second network soon.
As a testament to the impact of this collective work, BPCI was honoured to receive the highly prestigious National Association Prize at Cefic’s annual Responsible Care Awards in Brussels in December, recognising the measurable impact we are making across this strategic theme.
Beyond policy, we focused on practical engagement and best practice sharing across the industry. This included hosting the Water Stewardship Workshop in Waterford in May, directly addressing the critical issue of

resource management, and the BPCI Energy Forum in October, which included a site tour at Takeda, Grange Castle, focusing on achieving energy efficiency and stability in the face of rising costs. These best practice guides derived from these key regional sites are immediately transferable to operations nationwide, providing a national blueprint for compliance and efficiency.
We are proactively shaping environmental policy: we secured the establishment of an Interdepartmental Group on Urban Waste Water Treatment Directive (UWWTD) through extensive lobbying with other partners, including the Irish Pharmaceutical Healthcare Association (IPHA) and Medicines For Ireland (MFI), which is crucial for industry input on national policy implementation. We maintain continued bi-annual formal engagement with the EPA, OEE, ECHA, and HSA on crucial issues such as EPA licencing and auditing, ensuring we support members with compliance through multi-annual engagements.
The rapid integration of technology is foundational to the future competitiveness of our sector, making Digitalisation and AI a core strategic pillar. Our BioPharma Report validates this focus, showing an uplift in those planning AI initiatives in the next one to two years from 75% to 86%, with the primary objective being to improve efficiencies. Digitalisation remains the second most widely mentioned business priority (43%).
A major output was the joint release of the Generative AI for Life Sciences Playbook


2025 in September, with Connected Health Skillnet and lead authors Brightbeam. This Playbook serves as a strategic, GxPcompliant roadmap for adoption and scaling of GenAI. The playbook identified 100+ use cases across five value streams in manufacturing, and was developed over the course of 10 months. The playbook was launched at our GenAI Forum in September and we’ve established a new Digitalisation & AI Steering Group (Board-led) in 2025 to provide strategic oversight.
Furthermore, we continue to collaborate

with ISPE on the joint Lab 5.0 objective, hosting ‘Smarter Labs, Stronger Science' in November to share best practice on the digitalisation transformation in the QC Lab. These forums are crucial for embedding new technologies.
Collaborating on cross-sector digital health strategy
Our work in this area is intrinsically collaborative, exemplified by the crosssectoral campaign 'Where Digital Health Thrives', which we drive alongside the Irish Medtech Association and Technology Ireland. The Digital Health Report, co-launched in July, provided critical research underpinning our national vision.
This pillar is dedicated to building the depth, resilience, and diversity of the entire BioPharmaChem ecosystem in Ireland. We launched the first-ever BioPharma Business Services (BBS) Strategy 2025 in May. This positions Ireland as the global location of choice for BioPharma Business Services, a segment that represents approximately 20% of total employment in the sector.
An interesting facet is the resilience and growth of the API (Small Molecule) sector; contrary to some previous predictions, it is currently growing in Ireland, with over 140 APIs being manufactured here and increasing. This is work we will continue into 2026.
Furthermore, work has commenced on a BioPharma Indigenous Strategy. This strategy, launching in Q2 2026, is being designed specifically to address the scaling needs of

our indigenous members, focusing on access to capital, supplier networks, and peer-topeer technical support. To date, we have already mapped 130 indigenous companies across the ecosystem.
Community, events, and member value
Our success is amplified by the platform we provide for networking, learning, and collaboration. Our flagship events are essential for senior-level connection: The BioPharmaChem Impact Conference and Awards in May was an outstanding success, bringing together over 300 delegates, and this was complemented by the crucial Annual BPCI Leaders Forum and Dinner for site leaders in October.
Conclusion and acknowledgements
The past year has been a period of highimpact delivery and unprecedented collaboration. While the challenging sentiment in our new BioPharma Report
signals caution, it underscores the strategic importance of BPCI's core mandate: to secure a competitive, predictable, and de-risked operating environment for every member.
This work is possible only through the dedication of our leadership and the commitment of our network. I offer my sincere gratitude to our Chair, Joyce Fitzharris, President, Small Molecule, SK Pharmteco, and our outgoing Vice Chair, Vice President, Dana Danescharavi, Johnson & Johnson Innovative Medicine, for their extraordinary guidance and tireless commitment over the past year. We also warmly welcome our incoming Vice Chair, Tim Shanahan, Vice President, Ipsen.
A profound thank you is due to the entire BioPharmaChem Ireland Board for their strategic foresight. We extend our sincere appreciation to all our Working Group Chairs and every participant who contributes their knowledge and time. Their voluntary time is a strategic asset. This peer-led engagement prevents costly regulatory missteps, de-risks new technology
adoption, and identifies operational efficiencies. Your operational insights are the engine of BPCI's best practice development.
Finally, we thank the many external stakeholders and partners who collaborated with us throughout 2025. This includes government departments and agencies such as the HSA, EPA, Skillnet, SOLAS, Enterprise Ireland, IDA Ireland, DFHERIS, D. Health, D. Enviro, Uisce Éireann, CRU, HPRA, SEAI, and the Department of Education, alongside our European and global partners, including EuropaBio, Cefic, and BIO. Your willingness to engage with the BPCI voice is fundamental to shaping a fit-for-purpose regulatory and legislative environment.
By maintaining our unified focus across the Five Strategic Pillars, we ensure that the Irish BioPharmaChem sector is wellpositioned, well-supported, and ready to meet the global opportunities that lie ahead, securing our status as a global centre of excellence for decades to come.


We are driven by a mission to change what it means to live with a rare disease. Built on a legacy of turning pioneering science into trans formative treatment s , we lis ten to and par tner with the rare disease ecos ys tem to help improve outcomes for more people impac ted by rare diseases across the globe. alexion.com
Lisa Goodman , Senior Public Affairs Executive, BioPharmaChem Ireland, provides the backdrop to the development of Ireland’s first-ever National Life Sciences Strategy and argues that to deliver targeted outcomes, the Strategy must be governed by a dedicated National Office of Life Sciences.
The close of 2025 marks a year defined by profound progress and pivotal change for Irish BioPharma. Our sector, a vital pillar of the Irish economy and fundamental to providing patients worldwide with essential medicines, has leveraged our ecosystem strengths to remain a resilient, globally competitive hub. Yet, we stand at a critical juncture. The rapidly shifting global landscape is now defined by intensifying global competition for investment, geopolitical uncertainty and disruptive technological leaps. In this new era, standing still is not an option.
This backdrop is what makes one of the key achievements of 2025 so monumental. After years of sustained, strategic advocacy by BioPharmaChem Ireland (BPCI) and Irish Medtech, we have successfully secured a landmark commitment from Government: the development of Ireland’s first-ever National Life Sciences Strategy (NLSS), under the Department of Enterprise, Tourism and Employment.
This is more than just a policy win; it is a game-changer. For the first time, our nation will have a single, cohesive roadmap to guide the next phase of growth for our interconnected ecosystem.
Securing this commitment was a multiyear endeavour, the culmination of BPCI’s persistent calls in our manifestos, from
‘Electing for Business’ to ‘BPCI - Global Impact’, and our annual budget submissions. Securing Government’s commitment to developing the strategy in the Programme for Government in January 2025 was the critical first victory. But this victory immediately presented a new, more profound challenge: ensuring the strategy is not just a theoretical blueprint, but a living charter designed to drive tangible action, create accountability and delivery measurable change for the next decade.
With the commitment for National Life Sciences Strategy secured, we faced a critical question: what strategic priorities must be included and how can they be effectively delivered? BPCI's leadership in 2025 was defined by our work to answer that very question, not in isolation, but by working with our colleagues in Irish Medtech and stakeholders from across the ecosystem.
Convening the ecosystem: building a unified voice
The true strength of Ireland’s Life Sciences sector is its unique convergence of BioPharma, Medtech and digital health. Therefore, our first and most crucial task was to bring these powerful, interconnected voices to the same table.
We began developing an understanding of the necessary scope and deliverables for

the NLSS in consultation and collaboration with our members since we first identified the need for such a strategy four years ago. In 2025, as part of our ongoing advocacy on the NLLS, BPCI and our partners at Irish Medtech jointly hosted the ‘Shaping our Future’ workshop. We gathered 40 cross-disciplinary and cross-sectoral leaders from across our member companies and the wider ecosystem for a single purpose: to identify and prioritise the core considerations that must underpin the National Life Sciences Strategy, ultimately ensuring the strategy addresses industry needs and incorporates strategic imperatives required for future global competitiveness.
This was not a time for incremental thinking. The clear consensus was that in the face of global challenges, the NLSS must be ambitious and must set a bold, long-term vision for Ireland.
Our industry's vision, forged in this workshop and subsequent member validation, was then detailed in a White Paper for Government, to set the context for a strategy that delivers clear, transformative outcomes. We have called for a prioritised set of actions designed to leverage and enhance Ireland's unique and collective capabilities, ultimately positioning Ireland as a globally recognised leader in innovation and healthcare excellence.

BioPharmaChem Ireland and Irish Medtech jointly hosted the ‘Shaping our Future’ workshop to identify and prioritise the core considerations that must underpin the National Life Sciences Strategy.

Sinéad Keogh (standing, centre), Director, BioPharmaChem Ireland, and Eoghan Ó Faoláin, Director, Irish Medtech, at the ‘Shaping our Future’ workshop.
This means the strategy must rapidly address barriers, establish Ireland as a global R&D leader, and future-proof our national talent pipeline. Crucially, it must align the efforts of government departments and agencies to ensure a cohesive, whole-ofgovernment approach.
The headline ask: a new engine for execution
Our White Paper contains many critical recommendations, but it is built upon one central, non-negotiable pillar: governance.
The challenges facing our sector, from developing a future-proof talent pipeline to enhancing our R&D infrastructure and ensuring regulatory agility, are complex. They transcend the remit of any single department. A siloed approach, where different parts of government work without coordination, will lead to a siloed and ineffective strategy.
Therefore, BPCI's headline recommendation is the establishment of a dedicated, empowered and well-resourced National Office of Life Sciences.
This Office would not be another layer of bureaucracy. It would be the ‘engine room’ of the NLSS, responsible for its implementation, oversight, and adaptation. To be effective, this cross-departmental and cross-agency
body must have real authority and be comprised of senior officials from all critical departments, including the Departments of the Taoiseach, Health and Further and Higher Education, Research, Innovation and Science.
Furthermore, it must embed all key implementing agencies, from IDA Ireland and Enterprise Ireland to the HPRA, HSA and EPA, to ensure policy is grounded in operational reality.
Most importantly, our submission insists that industry must have a formal seat at the table. We have called for national industry associations, research centres and patient representatives to be embedded in this governance structure. This is the only way to ensure the strategy is practical and remains agile enough to respond to the real-world needs of the sector. A strong governance structure is the fundamental prerequisite for the strategy's long-term success.
The establishment of this Office is the how, but the why is even more compelling.
The NLSS is our vehicle to secure Ireland's competitiveness for the next decade.
This strategy must be a lever to move up the value chain. Ireland is already a global leader in BioPharma manufacturing. The NLSS
A dedicated, empowered and well-resourced national Office of Life Sciences would be the ‘engine room’ of the NLSS, responsible for its implementation, oversight, and adaptation.
is our chance to build on that excellence and become a world leader in high-value innovation and R&D. It must create the conditions for more investment in clinical trials, advanced therapeutics and nextgeneration drug discovery.
It must also be the catalyst for building our indigenous ecosystem. The strategy must provide targeted funding and growth supports for indigenous start-ups and scale-ups, fostering the next generation of Irish-owned Life Science champions that can compete on the global stage.
We have an opportunity that is truly unique to Ireland. Our unprecedented levels of interconnectedness between globally leading BioPharma, Medtech and technology companies create a convergence that other nations can only dream of. The NLSS must be the plan to harness this unique advantage, fostering collaboration and enabling new entrants to reinvent traditional operating models.
The work of 2025 centred around building the blueprint for success. The mandate for 2026 and beyond will be to ensure Government’s development and execution of the NLSS is robustly ambitious, fully resourced, and effectively delivered.
We have a rare window of opportunity to deliver something truly transformative. But in the face of rife global competition for investment, this is more than an opportunity; it is an imperative. The National Life Sciences Strategy is not a ‘nice to have’. It is the ‘musthave roadmap’ that will secure our sector’s success, drive innovation, and cement Ireland’s position as a global Life Sciences leader for the next decade. BPCI is proud to have led the charge to this point, and we are ready to partner with Government to make this vision a reality.

Ireland is in a unique position globally to lead the development of Digital Health, explains Sinéad Keogh, Director, BioPharmaChem Ireland and Head of Sectors, Ibec.
The transformation of healthcare is no longer a distant vision; it is a present and accelerating reality. Digital Health, broadly defined as the intersection of healthcare and technology, involves the innovative use of software, hardware, and data. Its goal is to deliver better, more efficient patient care, empower individuals in their own self-management, and provide clinicians with powerful tools to support decision-making.
The scale of this global shift is immense. The Digital Health market is projected to reach over $946 billion by 2030, expanding at a compound annual growth rate of over 22% (Grand View Research, 2025).
Here in Ireland, we are in a globally unique and enviable position to lead this convergence. This is a cross-sectoral
endeavour, driven by the powerful collaboration of Ibec's Irish Medtech, BioPharmaChem Ireland, and Technology Ireland associations. This ‘triple helix’ of expertise has built a global powerhouse ecosystem, with over 700 Life Sciences and health technology companies employing 90,000 people directly. The presence in Ireland of nine of the top 10 global medtech firms, all 10 of the top 10 Pharma companies, which includes over 200 Digital Health businesses, makes Ireland the place ‘Where Digital Health Thrives’.
Core business imperative
This is not just a theory; it is a core business imperative. A recent Ibec Digital Health Sector Report, launched at a dedicated Digital
Health Forum in Ibec's Dublin head office in July 2025, confirmed this momentum. An overwhelming 83% of Digital Health business leaders identified digitalisation as ‘very important’ to their operations, with 82% actively planning to invest in Digital Health activities. These are not abstract concepts; they are tangible solutions, with 48% of companies already engaged in connected medical devices and remote patient monitoring, and others focused on personalised healthcare (30%) and digital therapeutics (26%).
This planned investment is sharply focused. The top priorities for Irish operations are Artificial Intelligence (65%) and Cybersecurity (61%). This reflects a mature understanding that to deliver connected
The
Ibec’s ‘Where Digital Health Thrives’ is a crosssectoral campaign with BioPharmaChem Ireland, the Irish Medtech Association and Technology Ireland, to enable Ireland to become a recognised global hub for Digital Health.
top priorities for Irish operations are Artificial Intelligence (65%) and Cybersecurity (61%). This reflects a mature understanding that to deliver connected devices and personalised therapeutics, the systems must be intelligent, predictive, and secure.
devices and personalised therapeutics, the systems must be intelligent, predictive, and secure. This investment is about building the fundamental infrastructure for the next generation of healthcare.
This push for innovation is happening within a complex new regulatory landscape. The Ibec report highlights that industry is preparing for significant impacts from new European frameworks, with 61% of leaders expecting the EU AI Act and the European Health Data Space (EHDS) to affect their business.
This new reality is creating an urgent need for new capabilities. Business leaders identified significant gaps in access to essential skills. Looking to the future, the top skills needs are in Data Science and AI (identified by 70% of leaders) and, critically, in Regulation (61%), a direct response to the new compliance demands of the AI Act and EHDS. The report reveals companies are planning a dual strategy to fill these roles, balancing external recruitment with upskilling existing staff.

This clear focus on skills, combined with the defined investment priorities, demonstrates a sector that is moving in unison. The data is unequivocal: Irish industry is not just investing in Digital Health, it is defining what it means in
practice and strategically building the expert talent pool required to deliver its future.
For more information visit about Ibec’s ‘Where Digital Health Thrives’ campaign, visit: www.ibec.ie/digitalhealth
Ibec’s ‘Where Digital Health Thrives’ is a crosssectoral campaign with BioPharmaChem Ireland, the Irish Medtech Association and Technology Ireland, to enable Ireland to become a recognised global hub for Digital Health.


The transition to Green Chemistry presents a business opportunity for Ireland’s API sector, writes Michael Kilkelly, Senior Executive, EHS, BioPharmaChem Ireland.
The goals of Irelands Climate Action Plan 2024 1 and the EU’s Chemical Strategy for Sustainability 2 are key drivers of sustainability and environmental policy in Ireland. They share an ambition to protect public and environmental health, and they both align on strategies to do so: setting 2050 as the target year for net zero 3 climate neutrality and supporting innovation in the production and use of safe, sustainable chemicals.
In terms of net zero targets 3, the biggest challenge for the BioPharmaChem industry is decarbonisation in the supply chain. Typically, Scope 3 emissions ⁴ account for about 80% of greenhouse gas GHG emissions for the sector. Bundled under the category of Purchased Goods and Services, the largest share of Scope 3 emissions (60%) comes from the procurement of ingredients, chemicals, solvents and packaging. Chemical processes are a major part of this category, as the manufacture of active pharmaceutical ingredients (API’s) and intermediates involve energy-intensive chemical synthesis and raw material extraction.
The big challenge is that for many of the

current first-generation API processes, change is restricted by competing regulatory pressures. Changes to processes for API’s require costly development of new chemistries, rigorous application of change control, multiple risk-assessments, extensive validation activities, increased inspections and regulatory approval across multiple jurisdictions to fulfil strict GMP and EHS requirements. To employ these secondgeneration processes takes years to complete and requires significant investment and risk.
However, the medium-term sustainability challenges of API production and substitution of restricted chemicals could present long term business opportunities.
The estimated cumulative investment required for the global transition to sustainable chemical feedstocks is between US$440 billion and US$1.5 trillion by 2040 and potentially reaching between US$1.5 trillion and US$3.3 trillion by 2050 ⁵. This represents a huge potential for Europe to revitalise its chemical industry through innovative technologies such as carbon capture, green hydrogen, biomass utilisation, etc.
Even though Ireland’s role in chemical manufacture is limited, there is great
potential to position itself as a leader in green chemistry for API’s in small molecules. The global API market itself is projected to be worth US$330 billion by 2034 ⁶.
However, most API manufacturing sites in Ireland are not directly involved in the development of first-generation API processes. These processes generally come from discovery chemists based outside Ireland.
Discovery chemists use lab grade, lab-scale reagents, catalysts, synthetic pathways that


2050 is the target year for net zero climate neutrality and supporting innovation in the production and use of safe, sustainable chemicals.
provide small amounts of pure materials for pre-clinical trials. Focus is on high throughput screening.
In the past, little if any consideration was given to the environmental impact of the synthetic routes. Once successful in clinical trials, the synthesis is often locked in. It is almost always the case that efficiency and environmental impacts are considered only post-approval. Manufacturing plants and reactor configurations are then built around these resource-heavy processes, essentially scaling up these inefficiencies. Then validation locks this in for years.
A second-generation process (focusing on efficiency, cost, lower environmental impact) needs to go through a lengthy and expensive approval process from a quality point of view. The time gap from first generation synthesis scale-up to validation of an efficient second-generation process represents lost opportunity.
The question for the Irish small molecule API sector is this: do we focus our decarbonisation efforts on second generation process development and leverage on the existing process development experience and skills in the sector?
In the shorter term, this makes sense, given that most, if not all, of the firstgeneration discovery chemistry is carried out elsewhere. However, the more strategic option would be for Ireland to start focusing more on drug discovery and to develop an end-to-end life cycle approach from discovery to market. This presents an opportunity to develop the indigenous industry and to embed Sustainability by

Design (SbD) at the earliest stage.
An end-to-end approach would also facilitate the overlay of a single data source from discovery to market using AI to collate and analyse all aspects of the life cycle and remove data silos. Localising discovery chemistry coupled with green chemistry principles would reduce dependence on volatile supply chains.
How do we, as the broader Irish BioPharmaChem sector, influence and embed green chemistry principles as early as possible in small molecule drug development trajectory? The first step is to align it with a broader strategy in developing the Life Science Sector in Ireland through policy.
An aligned approach through a dedicated Life Science Office would help co-ordinate a National Life Sciences Strategy ⁷. A significant part of this is to aggressively develop and support indigenous drug discovery in Ireland. The recent increase of the R&D tax credit from 30 to 35% and expansion of its remit are positive developments but we need to go further. Successful drug discovery can then be the starting point of embedding green chemistry across the drug life cycle.
BPCI will launch its Indigenous strategy at BioPharmaChem Impact 2026, which will focus on supporting the ecosystem around this strategic growth area. BPCI is also working on mapping the broader small molecule API sector in Ireland, which will be published early in 2026. It is significant to note that there are over 140 small molecule API’s currently manufactured in Ireland and so the opportunity to deploy green chemistry is immense.
We need to build upon the current skills and talent in chemistry and engineering to focus on green chemistry. Curricula need to be updated to include not just green chemistry technologies but also life cycle analysis (LCA).
Embedding green chemistry principles as early as possible into the chemistry, pharmacy and chemical engineering programmess is essential. Sustainability by Design is as much of a mindset as it is a technical challenge.
A great initiative in this area is the partnership of Research Ireland with My Green Lab to promote certification of laboratories in third level institutions.
BioPharmaChem Ireland are also working with My Green Lab to promote the uptake of this same standard with the BioPharmaChem sector in Ireland. The aim here is to make this accreditation a standard across industry and education. We also need to develop expertise in hard technologies.
‘ Hard’ technologies
There are several technologies that can be deployed in reducing the environmental burden of API processes.
Bio-catalysis:
Several companies now employ enzymes such as lipases to effect very selective chemical transformations and the possibilities are expanded beyond nature's transformations through advances in protein engineering and enzyme immobilisation techniques. This removes toxic, precious metal catalysts from API synthesis.
Solvent free processes:
Small molecule API manufacture often uses large amounts of solvents, which present a significant scale of hazard. Complete removal of solvents through mechano-chemical processes or flow chemistry is an area worth exploring. Where solvents can’t be removed, then it is worth considering replacing toxic solvents with natural or biodegradable solvents and also looking at renewable raw materials, shifting from petroleum-based substances to bio-based feedstocks.
Catalyst technology:
Heavy metal catalysts such as palladium, platinum, and rhodium are widely used as catalysts due to their efficiency, but they pose challenges including supply volatility, high cost and the need for rigorous removal to meet regulatory limits on metal impurities in drugs and the environment. In the medium term, we can look at Closed Loop (advanced recovery and recycling). In the longer term, replacing heavy metals with different modalities such as nanoparticle catalysts may be the way forward.

Photochemistry:
Light-driven reactions often occur at room temperature and avoid the need for high thermal energy. Photochemical processes can offer direct access to target molecules, avoiding lengthy multi-step procedures and improving atom economy.
Electrochemistry:
This can often replace traditional oxidation or reduction methods that involve hazardous chemicals and is starting to be used more broadly
Continuous processing:
Continuous processing and in particular the use of flow chemistry provides techniques to perform energetic chemistry at scale, to reduce solvent and energy use, to have higher yields and more selective chemistries, and overall can be safer and more environmentally friendly. It does require a unique blend of chemistry, engineering and process control to design and scale-out. Several manufacturing sites in Ireland have already implemented flow chemistry and interesting process research in this area is carried out by SSPC, APC and Pfizer’s Process Development Centre in Cork. There is an opportunity to build in modules on continuous processing into courses and leverage the current experience in industry to help deliver these modules.
In silico methods:
Drug design using AI is a powerful screening tool that can be used for selecting drug candidates and is often cited as a game changer. In addition, design of experiments (DoE), when properly employed, can significantly reduce the number of experiments in process development, thus reducing environmental impact. Computational techniques such as Dynochem pioneered by Dr Joe Hannon in Ireland have helped development chemists use modelling to optimise and scale up processes faster, thus saving significant resources.

How do we, as the broader Irish BioPharmaChem sector, influence and embed green chemistry principles as early as possible in small molecule drug development trajectory? The first step is to align it with a broader strategy in developing the Life Science Sector in Ireland through policy.
Future advances will leverage precision automation and advanced process analytical techniques (PAT) with CMC teams to accelerate smarter more efficient processes. The recent partnership between Metler-Toledo International and indigenous company APC is a great example of these partnerships. These types of combinations will be further enhanced by deploying edge LLM’s to analyse field data in real time.
Summary
There is a significant opportunity for the BioPharmaChem sector in Ireland to become a world leader in green chemistry. This is not just a business opportunity but also a way to de-risk our exposure to complex supply chains and significantly reduce our carbon footprint. We have the technology; we have the talent; we have the ideas. What we now need is the funding and the policy to bring all the disparate groups together to build the infrastructure. The future is bright and the future is green.
Sources
1. https://www.gov.ie/en/department-of-climate-energyand-the-environment/publications/climate-actionplan-2025/
2. https://echa.europa.eu/el/hot-topics/chemicals-strategyfor-sustainability
3. https://www.climatecouncil.ie/media/CBWG%20 Report%20TIMES-Ireland%20Model.pdf
4. https://ghgprotocol.org/corporate-value-chain-scope-3standard
5. https://www.futuremarketsinc.com/the-globalmarket-for-sustainable-chemical-feedstocks-20252035/#:~:text=The%20transition%20to%20sustainable%20 chemical%20feedstocks%20represents,US$1.5%20 trillion%20to%20US$3.3%20trillion%20by%202050.
6. https://www.biospace.com/press-releases/smallmolecule-api-market-size-to-reach-usd-331-56-bn-by-2034
7. https://enterprise.gov.ie/en/consultations/publicconsultation-on-national-life-science-strategy.html


DELIVERING COMPLIANT, HIGH-PERFORMANCE


FACILITY SERVICES THROUGH ENGINEERING AND TECHNICAL EXPERTISE

35+ years supporting world-leading pharmaceutical manufact urers.
Expertise in commissioning, calibration, and o perations & maintenance.
Co-d esign partnersh ip mod el ensuring compliance, relia bility, and e fficiency.
Operations across EMEA and the USA.


Karina Cassidy, Senior Executive, Regulatory Affairs, BioPharmaChem Ireland, writes on the importance of ensuring an agile supply chain for Ireland’s Pharmaceutical industry, in the face of a volatile trade environment.
In today’s complex and dynamic global environment, supply chain agility is no longer a competitive advantage for Ireland's Pharmaceutical industry; it is a business necessity. The mission to reliably deliver essential medicines to patients worldwide is challenged by persistent volatility, regulatory complexity, and global disruptions.
As a key global manufacturing hub, Ireland’s Pharmaceutical sector is deeply integrated into global supply networks. This brings great strength but also inherent vulnerability. Key challenges for our members include significant global disruptions; a heavy reliance on Active Pharmaceutical Ingredients (APIs) from third countries, for example, exposes the sector to geopolitical tensions and international trade disruptions, such as the recent US tariff measures. This is not just a global trend; BPCI’s Manufacturing Report 2025 found that 'trade uncertainties' were the single greatest major challenge cited by members (68%).
Additionally, members face notoriously unpredictable demand volatility, which is influenced by factors from patent expirations to epidemics. With forecast errors averaging up to 40% and lengthy manufacturing lead times that can vary from two to 12 months, flexibility is constrained. Finally, there is the resource-intensive challenge of regulatory and handling complexity. Adhering to stringent global standards like Good Manufacturing Practices (GMP) and Good Distribution Practices (GDP) is demanding, and the growth of biologics and specialty drugs requires sophisticated, temperature-controlled cold chain and specialised handling capabilities to maintain product integrity.
Collaboration and insight through BPCI BPCI plays a key role in addressing these challenges by strengthening the entire value chain. This work is a direct expression of our 2025 strategic theme, 'Ecosystem development'. The BPCI Supply Chain
Working Group is the primary way we bring this strategy to life. It acts as the central hub, connecting our members with each other and with expert solution providers to build a more resilient and collaborative sector. This group provides the vital platform for members to share best practices and navigate this complex landscape together.
The value for members is generated through this group's dual focus. First, it drives strategy by focusing on members' highest priorities. This focus is a direct response to member feedback, as our 2025 research highlighted 'managing supply chains/ suppliers' and 'introducing digitalisation/ advanced manufacturing initiatives' as top business priorities.
The group's work on risk mitigation, transparency, and sustainability directly addresses these stated needs. Future work is already targeted at supplier diversification, emerging risks, and compliance to ensure the continuity of supply and protect patient outcomes.
Second, this strategy is brought to life by fostering direct, peer-to-peer collaboration through sessions where members share best practices and develop actionable solutions. Recent forums, such as those hosted by members Camida and McArdle Skeath, have proven invaluable for this essential, practical engagement.
In this environment, third-party logistic (3PL) providers have evolved from simple vendors into essential strategic partners. They provide the specialised expertise, scalable infrastructure, and advanced technologies necessary to manage the sector's unique demands. These partners are critical in managing the compliance burden of GMP and GDP standards, offering the scalability to manage demand volatility, and providing the sophisticated cold chain logistics vital for biologics.
Crucially, they are also drivers of innovation, increasingly employing digital tools like AI and advanced analytics to improve forecasting, enhance inventory management, and provide the real-time supply chain visibility that is critical for proactive risk mitigation.
As the sector moves toward more complex therapies, 3PLs manage the growing complexity of specialty and biologic drugs, which often require precise handling and direct-to-patient distribution models.
As John Halpin, Senior Manager Business Development, Life Sciences & Healthcare Ireland at DHL Health Logistics, notes: “We are expanding rapidly to meet the increasing demand for best-in-class 3PL services supporting the BioPharma sector across Ireland. Anchored by our state-of-the-art

HPRA approved facilities, Quantum, and Horizon, we offer robust, GxP-compliant solutions that span the full spectrum of cold chain management, including chill and deepfrozen regimes, as well as extensive controlled ambient materials handling.
“Our close collaboration with industry ensures our services are precisely aligned with the evolving needs of the sector, particularly in supporting in-bound logistics to manufacturing and seamless export to global markets. This reinforces our commitment to delivering agile, scalable, and regulatoryready supply chain solutions that enable our customers to thrive in a highly competitive and quality-driven environment.”
Building a resilient ecosystem
The path forward requires integrating supply chain agility into the core of our business strategies. This means fostering deep, transparent collaboration with partners, aligned on quality standards and data-sharing.
As the global landscape continues to revolutionise, Ireland’s industry leaders are redefining supply chain resilience. Through the collaborative platform BPCI provides, our members are building strategic alliances, progressing new levels of agility, and driving resilience. This is the strategic imperative for sustaining our global impact and, most importantly, delivering lifesaving medicines to the patients who need them most.

BioPharmaChem Impact 2026 is a much-attend event for senior leaders in the industry. Sinéad Keogh, Director of BPCI, provides a sneak preview of what to expect.

BioPharmaChem Ireland are proud to announce the return of our flagship event, BioPharmaChem Impact 2026. Set for May 6, 2026, at The InterContinental Hotel in Dublin, this third annual event is the must-attend gathering for senior leaders in our sector. We will once again bring together over 400 of our most influential executives, policymakers, and academic partners for a day of high-level dialogue.
This year's conference comes at a pivotal moment. With Ireland assuming the Presidency of the Council of the European Union in July 2026, we have a unique and powerful opportunity to shape the future of European policy.
Ireland’s leadership role during EU presidency
The day's agenda is structured around 10 dynamic sessions, bookended by two pivotal plenaries. We will open by exploring Ireland’s leadership role during the EU Presidency and its impact on European competitiveness, before closing with a strategic call to action for building our Indigenous BioPharmaChem Ecosystem.
Throughout the day, parallel tracks will offer deep dives into our industry's most critical challenges.
Strategic sessions will cover the future of medicine development, the practical implementation of AI and cybersecurity, and the

2025.
urgent need to close the talent and skills gap.
Our technical tracks will feature C-suite leaders from global CDMOs discussing the future of external manufacturing, expert case studies on green chemistry and sustainability, and essential analysis on future-proofing QA/ RA and compliance. Finally, we will tackle supply chain resilience from every angle, from high-level geopolitical risk to on-the-ground technical logistics.
Of course, the conference is not just about the speakers; it's about the connections. The day is filled with networking opportunities and concludes with our prestigious Annual Awards Dinner. This is our chance to celebrate the outstanding individuals and organisations that make the BioPharmaChem community here a global success.
Last year's attendees called the awards ceremony a "very enjoyable way to celebrate our successes in the industry", and we look forward to building on that tradition.
With 94% of last year's delegates rating the conference either very good or excellent, this is an event that truly delivers.
On behalf of the BPCI team, I look forward to welcoming you on May 6, 2026. This is our moment to connect, share, and lead the future of our industry, together.
To book your place at BioPharmaChem Impact 2026, visit bpcimpact.ie

Sharon Higgins , Executive Director, Membership and Sectors, Ibec, reflects on Ibec’s fifth Annual Manufacturing Report, which presents a mandate for Ireland’s manufacturing future based on securing competitiveness by controlling costs and mastering AI.

Ireland's manufacturing sector is the backbone of our economy. The fifth Annual Manufacturing Report 2025, launched on November 18, 2025, in Ibec's new Galway offices, confirms that this success, though robust, is no longer guaranteed. Our industry, which employs over 240,104 individuals in 2024 and generates over €10 billion in corporation tax, is revealing a deep resilience. However, the report shows this resilience is under intense pressure from global volatility and escalating domestic costs. This analysis provides a critical roadmap for controlling the controllables and securing the future of this essential industry.
The new global backdrop: unpredictability and the margin squeeze
The most significant finding of the 2025 survey is the dramatic escalation of external risk. Trade uncertainties have surged to become the number one challenge for 59% of leaders (up from 30% in 2024). This is not abstract risk; it is a clear and present threat to our export-driven model, validated by the fact that seven in 10 manufacturers are affected by US tariffs. This geopolitical friction, coupled with the expected challenge from Weaker Global Growth (42% challenge), is placing exceptional pressure on investment decisions.

This volatility has forced a shift in mindset: the overall positive outlook ('Very Good' / 'Good' sentiment) has dropped sharply from 69% to 51%. This is not pessimism, but a proactive 'Cautious Resilience’. Our leaders are strategically managing expectations, with the majority of sentiment now concentrated in the 'Average' category (43% concentration).
The cost structure underpinning this caution is noted:
• Wage growth is expected to increase for 81% of respondents.
• Expectations for the cost of energy have doubled to 64%.
• The result is a severe margin squeeze: expected profitability increases dropped sharply to 18% (down from 37% in 2024), with around 30% now expecting a decrease.
This dynamic - increasing costs against flat sales expectations - explains the moderation of general investment, with capital investment dipping to 34% and R&D expenditure dropping to 19%. The need for defensive cost management is paramount. Members should utilise the Ibec Pay and Resourcing Forecast for authoritative benchmarking to manage intense labour cost pressures and prepare strategically for compliance demands.
The AI imperative: the sector's strategic response
The most encouraging data confirms that manufacturers are strategically investing their way out of this uncertainty by controlling the controllables and embracing technology. AI is now a priority for 52% of businesses (up from 39% in 2024), and the motivation is crystal clear: 97% plan AI adoption primarily 'to improve efficiency/ productivity'.
This singular focus confirms that AI is the core resilience investment, immune to the general investment downturn, as our members recognise that mastering AI and digitalisation is the only viable path to defending margins against high labour costs and securing future global competitiveness.
Given that AI adoption is a core competitiveness strategy, members must utilise the Ibec AI Hub for practical guidance on adoption, toolkits for responsible implementation, and EU AI Act compliance. Furthermore, the launch of Ibec's Tariff Hub provides
vital, expert-driven intelligence and tools to assess and navigate the complex risks associated with trade uncertainties and tariffs.
While the sector is proactively investing in AI, structural deficits in the national infrastructure are compounding competitive threats.
The challenges surrounding our talent pipeline and housing remain acute, as the availability of housing for employees remains a major challenge for 48% of leaders. Furthermore, the cost of energy (64% concern) is so acute it is eclipsing the administrative focus on sustainability measures (down to 18% concern), meaning high costs are making long-term green capital investments unaffordable.
Finally, regulatory and planning friction is rising, with Irish planning regulations now a challenge for 25% of leaders, slowing critical infrastructure delivery and undermining our reputation for operational certainty.
Policy mandate: partnership for future growth
Ibec strongly commends the Government’s proactive response in Budget 2026, which delivered on key long-term enablers: the vital increase of the R&D Tax Credit to 35% is a welcome signal to unlock innovation, and the €2 billion commitment to infrastructure is crucial for future capacity.
However, the task now is to build on that momentum and address the immediate operational cost and skills gaps that remain critical threats. Our policy priorities for the next Programme for Government are clear and targeted:
1. Champion an Ambitious EU Trade Policy:
To counter global trade fragmentation, Ibec urges the Government to renew its commitment to openness and advance an ambitious EU trade policy, safeguarding Irish competitiveness in collaboration with key trading partners such as the US and the UK. This includes leveraging the EU mandate to deliver on developing the Single Market.
2. Mitigate Rising Business Costs:
This is our immediate ask. We would like the Government to introduce a PRSI rebate for exposed companies and accelerate the rollout of low-cost renewable generation to reduce Ireland’s high electricity costs. The Government must also formally assess the impact of all new labour market policy measures on employers using an enhanced SME Test.

The fifth Annual Manufacturing Report 2025 provides a critical roadmap for securing the future of this essential industry and is available to download from www.ibec.ie/manufacturinginireland.
3. Invest in AI Skills Development:
To bridge the critical gap between AI ambition (52% priority) and execution capability, we urge the Government to urgently increase matched funding for Skillnet Business Networks, unlocking private capital for essential AI upskilling through the National Training Fund (NTF).
4. Accelerate Climate Action and Digital Strategy:
The Government must secure a competitive net-zero economy by adopting an interventionist role. This requires implementing an all-of-government masterplan to overcome policy gridlock and accelerating infrastructure delivery by streamlining the permitting process.
5. Optimise the R&D Tax Credit to Unlock Innovation:
Building on the 35% rate, we urge the Government to use the forthcoming R&D Compass to expand the qualifying expenditure definitions and reform outsourcing rules (for connected and unconnected parties) to reward innovation and IP creation, where the value created benefits for Ireland. Clarity is critical for claimant companies.
Ibec in action: inspiring the future
Ibec ensures our members are prepared for these shifts through targeted support initiatives:
• Benchmarking and Compliance: The Ibec Pay and Resourcing Forecast provides indispensable data for managing the intense labour cost pressures (58% challenge) and setting competitive pay rates.
• Talent Pipeline: The launch of Ibec’s National Manufacturing Day Ireland (MDI) saw over 60 manufacturers open their doors, providing firsthand access and immersive experiences to students and jobseekers. This initiative is vital for tackling the long-term talent attraction challenges and showcasing modern manufacturing as a high-tech career path.
• Advocacy Success: We continue to demonstrate Influence at the highest levels, campaigning effectively for the policy changes needed, as demonstrated by the enhanced R&D Tax Credit and our ongoing fight for vital cost mitigation measures.
Manufacturing remains a central pillar of the Irish economy. By addressing these critical cost, talent, and infrastructure barriers, the Government can secure the foundation upon which the sector's inherent resilience and strategic AI investments can deliver the sustained, top-tier growth Ireland relies upon. We look forward to working with all stakeholders to champion these priorities.

























Adopting Generative AI is huge challenge, but one Ireland’s Life Sciences sector must embrace if it is to continue its international success, argues Sinead Keogh, Director BioPharmaChem Ireland, and Ibec Head of Sectors.
Generative AI presents a profound challenge for Ireland's €116 billion Life Sciences sector. It is, at once, the greatest opportunity for competitive advantage and a significant new frontier for regulatory compliance. Successfully navigating this landscape is the defining leadership challenge of our time.
The global prize is transformative.
McKinsey estimates GenAI could create $60 billion to $110 billion in annual value for the global Life Science industries. This value will come from radically accelerating R&D, a process that traditionally takes 10-15 years and costs over $1 billion per drug. But for Ireland, AI is also a critical competitiveness shield. Faced with what our 2025 Manufacturing Report calls escalating geopolitical risk and trade uncertainties, adopting AI to drive productivity is not just an option, it is a core defensive strategy to protect our export-led model.
Our members are moving decisively. Our latest BioPharmaChem Manufacturing Report 2025 reveals that AI is now a top priority for 64% of businesses, up from 54% last year. The survey also shows that 86% of businesses are now actively planning AI initiatives, a significant increase from 75% in 2024. The driver is clear: 100% of those investing are doing so to improve efficiency/productivity, with 75% aiming to improve innovation.
The playbook for GXP-scaled AI
Our industry is focused on the specific, highstakes task of GxP-scaled AI. This is why BPCI, with our partners, lead authors Brightbeam and Connected Health Skillnet, developed the 'GenAI in Life Sciences Manufacturing Playbook.' This is not just a report; it is a roadmap for our entire ecosystem to collaborate, build a culture of GenAI adoption with foundational applications, and ultimately scale into the complex, regulated GxP environments that define our sector.
Developed through collaboration with our members over a 10-month work programme, the playbook contains over 100 practical use cases across five value streams, from foundational applications in process development and supply chain management to critical GxP tasks like accelerating deviations management. Our aim is to help the ecosystem collectively build a culture of
AI, with a plan to scale these use cases into more complex GXP environments next year.
As Brian Hanly, CEO of Brightbeam, noted at the launch: "Generative AI is a generational opportunity but many companies are stuck in 'proof-of-concept purgatory'. They are running experiments that don't always scale into real value. This collaborative playbook, on which we were the lead authors, is the antidote. It provides a clear, GxP-compliant roadmap to move from ambition to adoption."
This collaborative model is Ireland's unique advantage. As highlighted in our BioPharma Business Services Strategy 2025, AI will be transformative across the entire business ecosystem, bridging the gap between advanced manufacturing and global services. Today, nearly 9,500 professionals, representing close to 20% of our total employment, drive a dynamic services sector that is transforming from
back-office support into a strategic engine for global corporations. Crucially, AI now presents a new opportunity for these hubs to recapture and secure high-value transactional activity. By leveraging AI to automate and optimise complex, high-volume tasks, Ireland can now be globally competitive in these areas. The EU AI Act positions our GBS hubs as the logical "compliance nerve centre" to deploy trusted, GxP-compliant AI globally.
To win, three critical enablers are required: culture, skills, and regulation.
The critical enablers
First, we must establish the right culture, which starts with leadership. This must be treated as a strategic leadership programme, not an IT project. The greatest challenge in our sector is integrating this technology within stringent GxP-regulated environments. Therefore, the non-negotiable foundation of this culture is a robust "Human-in-theLoop" (HITL) governance model. This ensures a qualified subject matter expert reviews,


approves, and remains accountable for all AIassisted outputs, thereby maintaining patient safety, GxP compliance, and traceability.
Second, we must industrialise our talent pipeline. We cannot scale technology without scaling our talent. Ibec and BPCI are actively lobbying for this increased investment, calling on the Government to scale AI skills development by increasing matched funding for agile, industry-led platforms like Skillnet Business Networks and Springboard+. These are the proven mechanisms to deliver the AI fluency our workforce needs.
Finally, we must implement enabling regulatory frameworks. We must de-risk adoption to accelerate it. We strongly advocate for sectoral AI regulatory sandboxes. This would allow companies to test and validate new AI solutions in a controlled environment, in partnership with regulators
like the HPRA. The recent designation of the HPRA as an AI competent authority and the new National AI Office are welcome steps. We must ensure the BioPharma sector is central to the design of these sandboxes, a key priority for us in the new National Life Sciences Strategy consultation.
EU: The global standard on AI
The European Union is already setting the global standard for ethical, trustworthy AI. By executing on these pillars, we can build on this strong foundation of trust and accountability. This is not about a guaranteed win, but the necessity of mastering GxPscaled AI to ensure Ireland remains a leading global hub. Amidst rife global competition, we must embrace this challenge or risk forfeiting our hard-won leadership.

Our 'GenAI Playbook' identifies over 100 use cases. Here are five examples of AI in action:
AI accelerates GxP compliance and quality oversight. By analysing unstructured text from historical Quality Management System (QMS) reports, the AI can instantly identify patterns to suggest probable root causes, accelerating Root Cause Analysis (RCA). This acts as a "knowledge agent" for the QA team, recommending actions based on past incidents.
Compiling mandatory GxP reports like Annual Product Reviews (APRs) is a manual process that consumes thousands of person-hours. AI solves this by automatically aggregating data from siloed systems (MES, LIMS, QMS), drafting the report, and reducing the risk of human error from manual reconciliation. This frees up expert time, which can be as much as 70% of their focus on certain tasks.
GenAI strengthens supply chain security by analysing global data streams, from geopolitical shifts to weather patterns, to predict disruptions before they happen. It enables rapid 'what-if' scenario planning, allowing teams to proactively adjust sourcing and logistics to protect delivery timelines.
Meeting our climate goals requires precise data. GenAI automates the tracking of Scope 3 emissions by extracting and standardising data from thousands of diverse supplier invoices and reports. This identifies carbon hotspots instantly, moving sustainability from manual reporting to real-time strategic management.
This key low-risk application builds AI culture. AI can summarise complex SOPs and vendor manuals into concise training modules, providing a virtual assistant for operators, and reducing dependency on senior Subject Matter Experts (SMEs). Crucially, it helps retain and transition knowledge of experienced personnel, which is vital for business continuity.

With sites across Ireland, the U.S., and South Korea, SK pharmteco provides supply chain resiliency you can count on.
From development to commercialization, our teams deliver the expertise, reliability, and quality to advance your molecules.
Small molecule APIs • Advanced intermediates • Peptides • ADC payloads & linkers
Energetic & Specialty Chemistry • High Potency • Particle Engineering
Continuous Flow • Chromatography • Catalyst Design • Analytical Services

How the Lower Harbour Biodiversity Forum is creating a shared vision for Cork Harbour and restoring nature across Cork’s pharma cluster, by Michael Kilkelly, Senior Executive – EHS, BioPharmaChem Ireland.
Cork’s Lower Harbour is one of the country’s great industrial success stories, a thriving hub for Pharmaceutical and BioPharmaceutical manufacturing that supports thousands of skilled jobs and global exports. Yet it is also a place of remarkable natural beauty, bordered by coastal wetlands, grasslands, and the local communities of Ringaskiddy, Monkstown, Carrigaline, and Crosshaven.
Recognising the unique setting in which they operate, companies across the Harbour, including Pfizer, J&J, Sterling, Thermofisher, Recordati, Biomarin, Hovione, DePuy Synthes, Moog, and Carbon Chemicals, have come together with local representatives, educational institutes, IDA Ireland, Cork County Council with BioPharmaChem Ireland (BPCI) to form the Lower Harbour Biodiversity Hub, a collaborative network working to protect, connect, and celebrate nature across the industrial landscape.
This model is part of the BPCI Sustainability Strategy 2025 and Ringaskiddy was selected by BPCI Sustainability Steering Committee as a pilot case. It is planned that this hub will serve as a template for rolling out more hubs around the country that have natural clusters of member companies.
This isn’t just another sustainability committee; it is a new way of thinking about how manufacturing, community, and biodiversity can coexist and thrive.
The Hub grew out of a simple idea: that while every company in the Lower Harbour has its own biodiversity initiatives, greater impact could be achieved by working together, sharing lessons, and showcasing progress collectively.
Following an initial meeting hosted at Sterling Pharma Solutions in September 2025, industry representatives from BioPharmaChem Ireland (BPCI), and Cork County Council agreed to begin the first steps to collaborate under a shared banner.
The goals were clear:
1. Co-ordinate biodiversity actions across sites to avoid duplication and identify regional opportunities.
2. Engage the local community and schools to create a shared sense of ownership of the landscape.
3. Communicate successes through a biannual newsletter, openhouse events, and an online biodiversity map.
At its first meeting, members shared biodiversity case studies from their respective sites, from wildflower meadows and pollinator trails to coastal clean-ups and employee allotments. It was immediately clear that, although each company’s journey was unique, there were strong common threads: native planting, pollinator protection, and local engagement.
Some of the highlights include:
• Pollinator-friendly planting and meadow management across several sites, reducing mowing frequency and encouraging native flora.
• On-site apiaries and bee hotels, managed by staff volunteers and
local beekeepers, helping to support pollinator populations, while engaging employees in hands-on conservation.
• Habitat protection for local wildlife, including coastal birds and small mammals such as the Arctic hare, whose presence is now being recorded around green buffers and rewilded zones.
• Employee allotments that combine wellbeing, biodiversity, and local food production.
• Community clean-ups and coastal litter collections, often organised in partnership with Clean Coasts Ireland and local Tidy Towns groups.
By sharing these efforts, the Forum hopes to build a picture of biodiversity stewardship across the entire Lower Harbour, showing that industrial zones can also be ecological corridors.
A platform for collaboration
The group is now developing several tools to formalise collaboration:
• Shared biodiversity information initiatives and programmes to gather consistent data on habitats, species, and site activities.
• A shared digital platform for project updates, photo libraries, and event calendars.
• A biannual newsletter, coordinated initially by Colt Schafer (J&J), to showcase local success stories and raise visibility of the Harbour’s nature-positive actions.
• Plans for an Open House event in early 2026 at Ringaskiddy Community Centre, inviting local residents and students to see what biodiversity looks like within a world-class manufacturing region.
Connecting industry and community
One of the Forum’s strengths is its emphasis on community connection. Members are reaching out to local schools, Tidy Towns committees and sports clubs to co-create small, high-impact initiatives such as tree-planting, biodiversity talks, and joint clean-ups.
This engagement builds trust and demonstrates that the Pharma industry can be a positive environmental neighbour, a message that resonates strongly with both employees and the public.
Why biodiversity matters to industry
While environmental stewardship is the heart of the initiative, the benefits extend far beyond compliance or CSR. Biodiversity programmes help:
• Enhance site resilience, providing natural storm-water management, soil health, and aesthetic value.
• Support employee wellbeing, giving staff access to nature during the workday.
• Strengthen sustainability reporting, contributing to ESG metrics and Nature-Related Disclosures (TNFD).
• Show leadership within Ireland’s industrial landscape, where collaboration and transparency are increasingly expected by regulators and investors.
As one member put it at the September meeting: “If we can show that Pharma manufacturing in the Lower Harbour actively supports biodiversity, we set the tone for what sustainable industry in Ireland looks like.”
The Lower Harbour Biodiversity Hub represents something new in Ireland’s industrial sustainability landscape: collaborative biodiversity governance. Rather than working in isolation, companies are sharing ideas, resources, and communications to achieve regional impact.

The hope is that this model can be replicated in other Irish industrial clusters, like those at Damastown, Waterford, Grangecastle, and Athlone, where multiple BPCI member companies operate sideby-side in environmentally sensitive areas.
The Forum also complements Ireland’s National Biodiversity Action Plan (2023-2030) and aligns with corporate sustainability strategies that increasingly require site-level biodiversity reporting and community engagement.
As the Forum grows, participants are exploring ways to deepen scientific collaboration, such as co-ordinated biodiversity audits, shared habitat mapping, and ecological monitoring that transcends site boundaries.
By the time of its first newsletter, the group aims to publish its first Lower Harbour Biodiversity Map, showcasing the collective impact of local industry and community partners.
Ultimately, the message is simple: we can be good community partners, while giving life back to the landscape around us.
In Cork’s Lower Harbour, industry and nature are learning to thrive together and the Biodiversity Forum is proof that collaboration is the key to both.

Cork’s Lower Harbour Biodiversity Hub sees members working together, sharing lessons, and showcasing progress collectively.







2025 was a very successful year for BioPharmaChem Skillnet , including new programmes, exciting collaborations and respected award wins.
As Ireland’s Life Sciences sector continues to evolve at pace, BioPharmaChem Skillnet remains at the forefront of workforce development, supporting businesses and professionals to upskill, adapt, and thrive. Through industry-led collaboration, tailored training, and forward-thinking initiatives, the Network continues to address the sector’s most pressing skills needs across biopharmaceuticals, pharmaceuticals, and chemicals.
In 2025, BioPharmaChem Skillnet continued to expand its portfolio of specialised programmes, ensuring Ireland’s BioPharma and chemical sectors are equipped with the talent required to sustain growth and innovation. With the industry facing increased digitalisation, regulatory complexity, and global competition, the Network’s focus remained on enabling enterprise-led learning and supporting talent mobility within the sector.
Pilot programmes delivering impact Among the year’s key highlights were two major pilot programmes. The Special Purpose Certificate in Essentials for Pharmaceutical Manufacturing – Level 5, in collaboration with Innopharma Education, was developed and piloted to meet the growing demand for entry-level skills in manufacturing. This QQI-accredited programme provides learners with a foundation in production processes,

quality systems, and regulatory compliance, blending theoretical knowledge with practical application. It has already gained strong industry interest from companies seeking to build new talent pipelines.
The Inclusive Leadership Programme Level 8, in collaboration with PDI and University College Cork, had two successful cohorts this year, and has also become a cornerstone of leadership development across the sector. Designed to help managers and senior leaders foster inclusive, highperforming teams, the programme encourages empathy, communication, and self-awareness as vital skills for the modern workplace.

Dr. Kevin O'Donnell, Market Compliance Manager, HPRA; Iva Peradenic, Office Administrator; Marie O’Brien, Accounts Assistant.
therapies, and emerging skills
BioPharmaChem Skillnet’s commitment to progressive and inclusive learning extended through several other key initiatives. The Neurodiversity in the Workplace Level 8, in collaboration with UCC, continued to gain significant traction, with strong engagement from businesses eager to create more inclusive environments for neurodivergent employees. This commitment to inclusion was recognised nationally when the programme won the Inclusion in Action award at the 2025 Learning & Development Institute (L&DI) Awards.
The Network also continued to collaborate with University College Cork (UCC) on the Certificate in CPD: Introduction to Biopharmaceuticals and Advanced Personalised Therapies Level 8, which provides a deep dive into the rapidly advancing world of personalised medicine.
Additionally, BioPharmaChem Skillnet, in collaboration with Viral Lab TU Dublin, explored the use of Virtual Reality (VR) as an innovative training tool, demonstrating its commitment to adopting new technologies that enhance learning engagement and technical skills development in training like health and safety and powder handling.


Collaboration remains at the heart of BioPharmaChem Skillnet’s success. A notable example in 2025 was the webinar on Advertising Compliance and Regulatory Compliance Inspections for Marketing Authorisation Holders (MAHs), run in partnership with the Health Products Regulatory Authority (HPRA). This webinar provided practical guidance to member businesses on regulatory expectations for promotional activity and advertising, helping organisations to navigate compliance risks and strengthen their internal governance. The HPRA event illustrated the practical benefit of regulator–industry engagement and reinforced the Network’s role as a bridge between regulatory guidance and day-to-day business practice.
Another highlight of the year was theTechBrek: Leading the Future – AI, Change Management & the New Workplace Experience, hosted jointly with Technology Ireland ICT Skillnet and Tech Industry Alliance Skillnet. The event brought together professionals from across the Life Sciences, technology, and manufacturing sectors to

explore innovation and digital transformation trends shaping the future of work. This cross-sector collaboration reflected BioPharmaChem Skillnet’s ongoing commitment to fostering learning beyond traditional industry boundaries and encouraging dialogue around technology adoption and workforce readiness.
The Network also works closely with a wide range of training providers and industry experts to deliver Lunch & Learn sessions, offering short, focused insights on emerging topics relevant to the sector. These sessions have become a valuable platform for knowledge exchange, fostering dialogue across businesses on key issues from sustainability to Artificial Intelligence in the sector.

• Women in Pharma (Small) — Pharma Industry Awards (nomination)
These accolades reflect the collective effort of the Network’s team, training providers, and industry partners in delivering impactful, industry-aligned learning that supports Ireland’s life sciences ambitions.
Beyond its scheduled programmes, BioPharmaChem Skillnet also partners directly with member businesses to design and deliver customised training solutions, tailored to their unique workforce needs. Delivered onsite or online, these bespoke programmes cover a diverse range of topics, from digital literacy and project management to technical writing and supply chain management, reflecting the Network’s enterprise-led and responsive approach to industry training.
2025 has been a strong year of recognition for BioPharmaChem Skillnet and its partners UCC and TU Dublin. Highlights include:
• Winner: Learning & Development Institute (L&DI) Award - Inclusion in Action (Neurodiversity in the Workplace Programme).
• Finalist: Pharma Industry Awards - Health & Safety Award for the Network’s VR programme on health and safety.
• Finalist: Pharma Industry Awards - Pharma Education & Training Award for the Neurodiversity in Workplace course.
• Finalist: Life Science Industry AwardsExcellence in Health & Safety for the Network’s VR programme on health and safety.
In addition to these network honours, Susan Costello, Network Director, received multiple individual nominations in recognition of her leadership in skills development and sector engagement, including:
• Best Education Leadership — Women in Pharma Awards (nomination)
• Women in Life Science — Life Sciences Industry Awards (nomination)
As the BioPharma and chemical industries continue to grow, BioPharmaChem Skillnet remains committed to supporting that momentum by fostering talent, inclusion, and innovation across all levels of the workforce. Through collaboration with industry, academia, and government, the Network will continue to evolve its offerings to ensure Ireland remains a global leader in the BioPharmaceutical and chemical sector.



Amneal Ireland Ltd is part of Amneal Pharmaceuticals, Inc. (NYSE:AMRX), a global biopharmaceuticals company making healthy possible through the development, manufacturing, and distribution of a diverse portfolio of over 280 generic and specialty pharmaceutical products

Amneal Ireland is located in Cashel, County Tipperary, where our state of the art facility is dedicated to the development and production of inhalation products.
We are Amneal and we make healthy possible.
That’s a powerful reason to come to work.
If you’re excited about joining a company with a purpose that believes in, encourages and invests in the growth of its people, let’s talk!

To share your interest in joining our Amneal Cashel team, please reference the appropriate area of interest and email CV to careers.cashel@amneal.com
NIBRT’s focus on research and innovation ensures that Ireland remain ahead of emerging technologies and industry needs, helping to drive the future of Biopharmaceutical manufacturing, writes Killian O’Driscoll , Chief Commercial Officer, NIBRT.
“In the middle of every difficulty lies opportunity.” - Albert
The Irish BioPharma sector has long been recognised for its global success, consistently ranking among the top pharmaceutical exporters worldwide, often cited as the third largest. Over the decades, the industry has demonstrated remarkable resilience in the face of major challenges, from patent cliffs, the transition to biologics, to the unprecedented disruption caused by the Covid-19 pandemic. Now, amid a new wave of global economic disruption, the sector is once again being called upon to showcase its adaptability, innovation, and strength.
In 2025, a series of economic policy announcements from the Trump administration introduced significant uncertainty and disruption to the global BioPharma landscape. These developments have coincided with a surge in BioPharmaceutical manufacturing investment announcements within the United States. While there are still many unknowns and the situation changes frequently, there is a requirement for strategic reassessments across all stakeholders.
At the same time, the pace of innovation in BioPharmaceuticals is accelerating. Beyond the well-established classes of protein-based therapies, a new generation of therapeutic modalities is emerging, including highly personalised medicines. Advanced data science and artificial intelligence are beginning to reshape how medicines are developed, manufactured, and commercialised. Integrated, patientspecific solutions are no longer a distant vision; they are rapidly becoming a reality.
Globally, more regions are prioritising Life Sciences and deploying innovative incentives to attract multinational investment. Notably, China has made significant strides as a BioPharma research hub, with China-based companies now accounting for 20% of drugs in development worldwide—compared to 40% for the US and 11% for the EU (Source: Global Data’s Drug Database).

NIBRT’s core mission is to support the growth and development of the BioPharmaceutical manufacturing sector in Ireland and globally by delivering world-class training and research solutions. In response to the evolving global landscape, the Institute is focusing on six strategic priorities to ensure Ireland remains well-positioned to meet future challenges and seize emerging opportunities:
• Reinforce Ireland as a stable, resilient and adaptable global manufacturing hub for BioPharma production.
• Support a national step-change in research activity, impact and funding with a strong with a strong emphasis on manufacturing innovation.
• Establish NIBRT as the global leader in in workforce development for the BioPharma industry.
• Drive leadership in advanced manufacturing, leveraging digital technologies and artificial intelligence to transform production processes.
• Position Ireland at the forefront of research, development, and manufacturing of advanced therapeutics and next-generation biologics.
• Foster a vibrant indigenous Biotech ecosystem, synergistic with the multinational BioPharma manufacturing sector.
Between 2020 and 2025, NIBRT’s research programme experienced sustained growth across all key performance indicators, including:
• 22.5% of publications appeared in the top 10% of journals globally.
• 73.4% of publications featured at least one international collaborator, with 23% of publications included industry partners, underscoring NIBRT’s commitment to translational research.
• Prestigious Research Professorship awards to Professor Mark Smales, who securted a Taighde Éireann Award, and Professor Sakis Mantalaris, who received a €4.8 million SFI Research Professorship award.
• €4.2 million in funding from SFI's Research Infrastructure programme to enable the development of CONCEPT, as a core facility for early-stage biotherapy development.
A cornerstone of NIBRT’s strategy is to build on this research capability by fostering impactful, large-scale collaborations at both national and international levels. In 2025, NIBRT played a pivotal role in several high-profile research centre proposals currently under final review by Research Ireland, including:
• The IMPACT Centre (led by Trinity College Dublin): Aims to transform the development of personalised advanced cellular therapeutics, ensuring Irish patients have access to innovative, effective, and affordable treatments.
• The INSPIRE Centre (led by the University of Limerick): Dedicated to advancing Pharmaceutical and BioPharmaceutical manufacturing technologies to improve efficiency and sustainability.
As therapeutic pipelines diversify, CONCEPT, a cutting-edge facility within NIBRT’s Advanced Therapies unit, has emerged as a key stakeholder for early-stage Biotherapeutic development. Supporting research across biologics, cell, gene, and RNA therapies, CONCEPT offers academic and industry partners access to state-of-the-art instrumentation and expert guidance, accelerating the development of next-generation treatments.
In 2025, the CONCEPT team were delighted to be part of the Can-Vas Consortium which received €10.7 million funding to advance a new class of cell therapy treatment to clinic for early brain injury.
and belfast:
While Ireland has built a globally respected BioPharma sector largely through foreign direct investment in manufacturing, the growth of a robust indigenous industry has been more gradual. In contrast, Catalonia has emerged as one of Europe’s most dynamic Life Sciences ecosystems, offering a compelling case study in the power of strategic investment, scientific excellence, and entrepreneurial culture (Source: Catalonia Health and Life Sciences Sector 2024 BioRegion Report)
Key highlights include:
• Scale: The Life Sciences sector contributes 7.6% of Catalonia’s GDP, encompassing over 1,500 companies and 93 research institutions. Small and medium-sized enterprises (SMEs) make up 90% of the sector, driving innovation and job creation.
• Research Excellence: Catalonia ranks first in Europe for Highly Cited Papers and fifth in Life Sciences publications, reflecting its strong academic and research output.
• Start-up Ecosystem: In 2024, a record €347 million was invested in start-ups and scale-ups, with 75% of funding sourced internationally. Over the past five years, 108 new international venture capital firms have entered the BioRegion. Barcelona now hosts over 470 start-ups and scaleups, supported by world-class universities, research centres, and science parks.
• Clinical Impact: The region boasts a robust pipeline of 75 molecules and therapies in clinical development. It is a European leader in CAR-T cell therapy, with Hospital Clínic pioneering two EMA-recognised treatments.
An excellent example of regional leadership in strategic research investment is Queen’s University Belfast, which is spearheading the development of iREACH Health, a £64 million world-class integrated clinical research innovation centre. iREACH Health is dedicated to making Northern Ireland a region of choice for the set-up and delivery of clinical trials. The new purposebuilt innovation centre will streamline clinical trials, equip healthcare professionals with specialised training, and leverage digital technologies to enhance recruitment and boost remote participation in clinical trials – including up to a dedicated 100 hospital beds for clinical trial activities.
The Irish BioPharma sector employs more than 50,000 people directly in Ireland (and multiples of that indirectly) with a 2024 report from the Expert Group on Future Skills Needs predicting that number to grow by more than 21,000 by 2027. Throughout 2025, NIBRT has been delighted to participate in an Implementation Group to drive the comprehensive recommendations in the report.
A particularly significant initiative is the launch of NIBRT Global Qualifications (NGQs) in April 2025 (www.nibrt.ie/ngqs). NGQs provide internationally recognised training qualifications, setting the benchmark for BioPharmaceutical education and workforce development. Developed by NIBRT and our educational partners, NGQs deliver standardised, high-quality learning qualifications for professionals across the industry. Combining theoretical knowledge with hands-on practical training, NGQs equip professionals with the expertise needed for success in the BioPharma sector. The NGQs provide certainty to industry that their trainees have been accredited to world class standards in both practical and theoretical core BioPharma operations.

James Lawless TD, Minister for Further and Higher Education, Research, Innovation and Science of Ireland meeting members of the NIBRT Research team (for more, visit www.nibrt.ie/9499-2).
NIBRT the global leader in BioPharma workforce development There are numerous reports highlighting the continued growth of the demand for skilled workforce in BioPharma manufacturing. In April 2025, US Congress: National Security Commission on Emerging Biotechnology published a report on Charting the Future of Biotechnology concluded “The need for talented biotechnology workers has never been more urgent (Source: US Congress: National Security Commission on Emerging Biotechnology Charting the Future of Biotechnology).”
The NIBRT Global Partners Programme (GPP) is a NIBRT strategic initiative designed to address the growing global shortage of skilled professionals in BioPharmaceutical manufacturing. As the industry continues to expand, the need for high-quality, standardised training remains critical.
The GPP enables selected international partners to license and deliver NIBRT’s world-class curriculum, methodologies, and operational models, thereby building regional capabilities and ensuring global consistency in workforce development.
Since its inception in 2018, the programme has evolved into a robust global alliance, with active partners in the USA, Canada, South Korea, and Senegal. These partners operate under a licensing model that includes facility design guidance, curriculum access, train-thetrainer programmes, and participation in NIBRT’s Global Qualifications (NGQ) framework.
NIBRT is also in the final stages of assessment to be designated as a WHO Biomanufacturing Regional Training Hub (RTH). The RTH supports Lower Middle Income Countries (LMICs), to develop the workforce with relevant skills for Biomanufacturing. A partnership with the Department of Foreign Affairs is a key component of this initiative.

In an interview with former NIBRT Chairman, Brendan O’Callaghan, Executive Vice President of Manufacturing and Supply at Sanofi detailed the benefits Sanofi are now accruing from their strategic investment in digital and AI capabilities (Source: ISPE, Sanofi's Digitalization Road Trip Shifts into High Gear).
• Proprietary AI-powered system that uses telemetry from sensors across our bioreactors and production lines. It tracks thousands of data points in real time, which allows us to optimise yield and performance batch by batch. We’ve seen gains of 5% to 10% in production yield.
• We've seen remarkable returns from putting sensors on production lines to monitor performance parameters like vibration, temperature, and electrical pull. This telemetry approach, inspired by our McLaren Racing partnership, has improved overall equipment effectiveness (OEE) by 20-25% in some of our lines. Even a modest sensor deployment can yield significant insights without requiring a complete factory rebuild.
• McLaren brought us a mindset of continuous optimisation and real-time telemetry that has transformed our operations. Their engineers helped us improve changeover times on our production lines by up to 40% through real-time AI video analysis and achieved 10-times productivity gains on a series of manufacturing processes.

x e n g i n e e r i n g p r o b l e m s .
W e d e l i v e r w o r l d - c l a s s , i n n o v a t i v e s o l u t i o n s a c r o s s v a r i o u s s e c t o r s , e s p e c i a l l y P h a r m a c e u t i c a l s ,
D a t a C e n t e r s a n d L i f e S c i e n c e s . W e c o n t i n u e t o i n v e s t i n c u t t i n g - e d g e , i m m e r s i v e t e c h n o l o g i e s t o
m a n a g e a l l o u r p r o j e c t s . W e u t i l i z e A I , o f f s i t e m a n u f a c t u r i n g , a n d s t a n d a r d i z a t i o n t o f i n d n e w
w a y s t o i m p r o v e t h e b u i l d i n g p r o c e s s a n d o v e r a l l p e r f o r m a n c e .
Can Europe be the most attractive place in the world for life sciences by 2030?
In July 2025, the European Commission launched a new strategy to make Europe the most attractive place in the world for Life Sciences by 2030. The strategy focuses on three core areas of optimising the research and innovation ecosystem, enabling rapid market access for Life Science innovations and boosting trust, uptake and use of innovation – all areas while Ireland can make a significant contribution. NIBRT is part of these conversations through our membership of strategic groupings in the EU (IBISBA, EIT Health, EuropaBio).
On July 1, 2026, Ireland will take on the Presidency of the Council of the European
Union, which provides a unique opportunity, in particular as this will align with the publication of the pivotal EU Biotech Act.
From an Irish perspective, NIBRT warmly welcome the Government’s commitment to developing a National Life Sciences Strategy, which provides a wonderful chance for all stakeholders to address barriers and avail of the numerous opportunities to ensure the next 10 years are as successful as the last decade.
2025 has been a transformative year for the global BioPharma sector, marked by rapid scientific innovation, shifting geopolitical dynamics, and an urgent demand for skilled
talent. Ireland continues to play a central role in shaping this evolving landscape.
At the heart of NIBRT’s approach is a deliberate focus on research and innovation, ensuring that we remain ahead of emerging technologies and industry needs. Through world-class research centres, Global Qualifications, international partnerships, and cutting-edge facilities like CONCEPT, NIBRT is not simply responding to global disruption, it is helping to drive the future of BioPharmaceutical manufacturing.
By integrating research with training and industry collaborations, we are building the scientific and skills foundation that will define the next generation of advanced manufacturing.





A partnership between a Waterford school and BioPharma company Sanofi points the way to how we can advance education for sustainable development through school/industry partnerships.
In October 2025, BioPharmaChem Ireland and the Irish Schools
Sustainability Network (ISSN) invited industry partners and other stakeholders to Ardscoil na Mara (ASNM) in Tramore, Co. Waterford, for a Partners in Change event. The gathering explored how a dynamic school/industry partnership between Ardscoil na Mara and Sanofi has driven transformative changes in sustainability practices across the campus, the curriculum, and the wider community.
Sanofi funded the ISSN’s Environmental Leadership Development Programme, enabling ASNM to build a sustainability team within the school and empower student groups to lead meaningful environmental initiatives.
During the event, the ISSN highlighted the low levels of ecological and climate literacy in Irish schools and the persistent barriers to advancing Education for Sustainable Development (ESD). These barriers include limited knowledge and confidence among staff, insufficient training, competing priorities, and a lack of time and funding.
TY programme
Ardscoil na Mara has demonstrated how these challenges can be overcome through partnership. Each year, approximately 25 Transition Year (TY) students deliver around 170 one-hour workshops in the school’s outdoor classroom for all students in first, second, and third year.
The programme covers various environmental modules, and each module explores a theme, such as the importance of biodiversity and practical ways to protect it, and combines short discussions with handson activities like propagating plants, building bird boxes, and preparing and tasting food from the vegetables they have grown in school.
“We feel so lucky to have this programme and opportunity, but we shouldn’t feel lucky,” explained Amelia Dunphy, a 6th -year student ambassador. “This should be standard in all schools.”
Beyond the classroom, students lead a variety of sustainability initiatives, including an Active Travel Programme that has reduced

The partnership between Sanofi and Ardscoil na Mara extends far beyond financial support, with Sanofi hosting TY students for work experience, welcoming student leaders to present at company events, and collaborating with the school to run a career speed-networking event.
on-site traffic by an average of 40%, a composting initiative, and a treeplanting campaign encouraging families to plant trees in their gardens, which has resulted in over 2,200 trees planted in the past four years.
Patrick Kirwan, who drives the initiative within the school, explained: “Schools have a crucial role to play in sparking social change and showing young people what’s possible. All teachers who come here for place-based training are inspired and energised by what they see. Schools need the right level of support, and Sanofi have proven that industry has an important role to play.”
The partnership between Sanofi and Ardscoil na Mara extends far beyond financial support. Sanofi hosts TY students for work experience, welcomes student leaders to present at company events, and collaborates with the school to run a career speed-networking event involving 120 TY students. Each year, Sanofi also hosts an awards ceremony at its site to celebrate the Environmental Leadership Development Programme, presenting Bronze, Silver, and Gold Awards to recognise student achievement and leadership.
We feel so lucky to have this programme and opportunity, but we shouldn’t feel lucky. This should be standard in all schools.

The success of this partnership exemplifies how collaboration between education and industry can advance sustainability literacy, empower young leaders, and foster lasting cultural change within schools and across communities.
Developing leadership skills in sustainability
“The TY sustainability leaders programme is unique in that it is very structured and puts the emphasis on developing leadership skills for students in sustainability,” notes Michael Kilkelly, Senior Executive - EHS, BioPharmaChem Ireland. “The passion, confidence and communication skills of the students who have been through this programme is amazing. This programme is building future leaders for our society and we hope that more of our members will engage with this innovative programme.”
Jennifer Murphy, Head of Environment at Sanofi, summed up the success of the programme: “Through a meaningful partnership, Sanofi have supported Ardscoil na Mara to create a mutually beneficial programme in environmental stewardship. Students gain knowledge, purpose, and a voice in environmental awareness to create tomorrow’s environmental leaders. This programme doesn't just teach sustainability; it lives it, proving that the greatest impact emerges when we work together and learning meets action in service of our shared planet.”
If you would like to support a school to run this Environmental Leadership Development Programme, contact Codie Preston, ISSN Programme Manager: codie@issn.ie

Each year, approximately 25 Transition Year students deliver one-hour workshops in the school’s outdoor classroom.
The Pharmaceutical Manufacturing Technology Centre (PMTC) brings together multinationals, SMEs, and academic institutions to tackle real-world challenges in Pharmaceutical and BioPharmaceutical manufacturing, delivering solutions that optimise processes, lower technologyadoption risks, speed innovation transfer, and offer an environment to test, validate, and scale innovations before full deployment.
Ireland’s Pharmaceutical and BioPharmaceutical industry continues to play a central role in the global Life Sciences sector. Maintaining that competitive edge amid rising complexity, evolving technologies, and growing economic and regulatory pressures, requires continuous innovation. At the heart of this transformation is the Pharmaceutical Manufacturing Technology Centre (PMTC).
Established in 2014 and funded by Enterprise Ireland with the support of IDA Ireland, PMTC has become a cornerstone of applied research and technological innovation for the Irish Pharmaceutical and BioPharmaceutical manufacturing sector. Its industry-led model brings together a vibrant community of multinational (MNCs) and SME members, and academic institutions to deliver impactful research and ensure that Irish Pharmaceutical and BioPharmaceutical Manufacturing remain efficient, resilient, and globally competitive.
A unique industry-led research model What sets PMTC apart in the Irish RDI (Research, Development & Innovation) landscape is its industry-informed research. The centre focuses on real-world challenges facing Pharmaceutical and BioPharmaceutical companies, delivering solutions that focus on process intensification and improvement, pharmaceutical plant

Damon Warnock, Centre Director, PMTC.
cleaning, the development and integration of novel technologies, data analytics, and digitalisation. This is a real need for the sector in these difficult times.
For instance, improving capacity through better cleaning processes, reducing waste by improving yields and using greener solvents and processes etc. will help keep production and jobs in Ireland, as well as fostering innovation mindsets and relevant skills in the Irish plants’ personnel, which is critical for future continued success.
Acting as a strategic connector between industry and academia, PMTC enables:
• Collaborative research projects between academia and industry, targeting highpriority industry needs;
• Knowledge and technology transfer, ensuring research is translated into practical, on-site improvements;
• SME/MNC member engagement, opening doors for small and medium enterprises to access and contribute to global supply chains. By fostering an open, academia/industry collaborative environment, PMTC drives innovation across every tier of the Irish Pharma and BioPharma ecosystem.
One of PMTC’s key contributions is in enabling SMEs members to engage directly with large MNCs. This is critical to building a stronger, more resilient Pharma and BioPharma value chain within Ireland. PMTC acts as a test-bed
and showcase for new technologies, giving SMEs the opportunity to:
De-risk their innovations/technologies through lab and pilot-scale demonstration;
Validate performance in realistic settings at the pilot-scale;
• Gain visibility and credibility among major global players operating in Ireland.
Moreover, the centre is playing a vital role in de-risking the implementation and use of new technologies by MNCs, enabling Pharma and BioPharma companies to ‘test before invest’ and ensuring seamless industry readiness in a safe testing environment. This access to expertise and collaboration is invaluable. It helps indigenous firms build the capability and confidence needed to scale, diversify, and become strategic partners in an industry traditionally dominated by large multinationals. This collaborative dynamic not only boosts the performance of individual companies but also enhances the overall robustness and agility of Ireland’s Pharmaceutical and BioPharmaceutical value chain.
As the industry becomes more advanced, it must also invest in future-ready skills and talent development. PMTC recognises this and is helping to shape Ireland’s talent pipeline through:
Engagement with higher education institutions and globally renowned academics and subject matter-experts;
• Industry-led training, workshops, and communities of practices in industry relevant topics;
• Collaborative projects that offer hands-on learning for researchers and Manufacturing staff.
By supporting the development of a highly skilled workforce equipped for next-generation manufacturing, PMTC is ensuring that Ireland remains an attractive location for high-value Pharma and BioPharma investment.
PMTC's Phase 3 research programme has been co-created with PMTC industry members, agencies, and academic partners to ensure maximum alignment with sectoral needs and emerging global trends. It translates Ireland’s Pharma and BioPharma industry challenges into practical, mission-driven research programmes that are directly relevant to both MNCs and SMEs.
The research programme is structured around six core themes:
1. Pharmaceutical & BioPharmaceutical Plant Cleaning & Enhancing Asset Utilisation
2. Advanced Manufacturing of Drug Substance & Drug Product
3. Process Intensification & Improvement
4. Digitalisation & Advanced Data Analytics
5. Sustainability in Pharmaceutical & BioPharmaceutical Manufacturing
6. Pharmaceutical & BioPharmaceutical Regulatory & Compliance
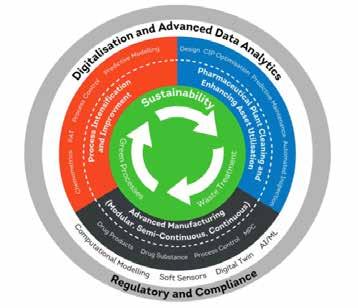
These themes operate not as standalone research themes but enablers designed to support and accelerate adoption across the entire research programme:
• Digitalisation & Advanced Data Analytics – Embedding AI/ML, hybrid modelling, predictive soft sensors, and digital twins to optimise processes, enable RTRT, and accelerate decision-making.
• Sustainability – Integrating decarbonisation, solvent reduction, and waste minimisation into all missions with measurable ESG metrics.
• Regulatory & Compliance – Co-developing frameworks with regulators to ensure rapid adoption of new technologies in GMP environments.
• Resource Development (Talent & Infrastructure) – Developing a future-ready workforce through the ML-BioPharma Hub, while establishing the National BioPharma Testbed and Lab 5.0 as “test-before-invest” environments for scaling and digitalising operations.
The Pharmaceutical and BioPharmaceutical sector is facing growing complexity in drug processing, digitalisation, and regulatory demands. To address this, PMTC is scaling its Research, Development & Innovation (RDI) operations with a strategic focus on:
• Long-term research missions, ensuring sustained Research, Development, and Innovation (RDI) momentum and fostering innovative interconnectivity.
• Advanced Pharma and BioPharma drug manufacturing technologies, aimed at accelerating the operationalisation of late-stage Technology Readiness Level (TRL) to industrial-scale production.
• Enhanced collaborations (inter-institutional and cross-centres), maximising cumulative value through leveraged matrixed capabilities and synergistic expertise.
• Development of tailored talent pipelines, delivering industryready professionals in cutting-edge Pharma and BioPharma manufacturing, equipped with the required future skills.

By aligning these initiatives with industry needs and objectives, PMTC will drive transformative change, ensuring Ireland remains at the forefront of high-value Pharma and BioPharma manufacturing.
While PMTC has already delivered impressive outcomes, it will continue to deliver on the successful impacts from the current phase of operation, via process development and innovation; industrialacademic collaborations, knowledge, and skill development; building infrastructure and capabilities; and showcasing industrially relevant research.
By remaining aligned with national priorities and international best practices, PMTC is uniquely equipped to help maintain existing investments and attract new ones, ensuring Ireland stays at the forefront of Pharmaceutical and BioPharmaceutical innovation.
For more information:
If you would like to know more about PMTC or are interested in becoming a member of our growing pharmaceutical community, please contact catriona.hassett@ul.ie or adam.barry@ul.ie
SSPC’s recent impact report highlights the centre’s incredible economic contribution to Ireland since 2008.
SSPC, Research Ireland Centre for Pharmaceuticals, which is hosted at University of Limerick, has revealed extraordinary national contributions in its latest impact report. The study highlights over €1.3 billion in economic impact since 2008 and a 26-fold return on core investment, safeguarding the nation’s position as a global leader in the BioPharmaceutical sector. The Centre has generated €5.27 for the Irish economy for every €1 of public and industry funding received.
As a cornerstone of Ireland’s innovation ecosystem, SSPC has been instrumental in attracting €3.7 billion in Foreign Direct Investment (FDI), while simultaneously cultivating the nation’s indigenous enterprise base. This dual impact, which protects and creates high-value jobs, is highlighted by the success of APC/VLE Ltd. An indigenous company co-founded in 2011 by SSPC alumnus Dr Mark Barrett and SSPC cofounder, Professor Brian Glennon. APC/ VLE has grown from a two-person start-up into a global leader and is now investing €100 million in a new ‘Medicine Accelerator’, creating 300 new roles.
The Centre has supported over 397 alumni, with 65% of its PhD graduates from its first decade transitioning directly into industry roles. Crucially, 57% of international alumni have chosen to remain in Ireland, a powerful ‘brain gain’ that directly strengthens the national skills base and addresses critical talent shortages in the sector.
Securing this pipeline for the long term, SSPC’s Education and Public Engagement (EPE) programme has reached over 5.6 million people worldwide, promoting greater awareness, scientific literacy and understanding of medicines. SSPC researchers actively contribute to international education research, shaping policy, and ensuring that scientific discovery continues to deliver meaningful benefits to society.
Magnet for global investment
“At SSPC, world-class research is the engine of our impact,” said Professor Damien Thompson, SSPC Scientific Director. “This

Professor Shane Kilcommins,
Institute; and Dr Sinéad
report showcases our proven success in building a research ecosystem that acts as a magnet for global investment and cultivates a pipeline of exceptional scientific talent. We are committed to developing highly-skilled future leaders who will drive Ireland’s national imperative to move decisively up the value chain, co-locating R&D with our world-class manufacturing. The fact that two-thirds of our international alumni choose to build their careers here is a powerful ‘brain gain’ directly fuelled by our research environment. Through this strategic focus, SSPC is strengthening Ireland’s position in global BioPharmaceutical innovation and securing our competitive edge for the decade to come.”
Dr Diarmuid O’Brien, Chief Executive Officer, Research Ireland, added: “Ireland’s future as a location for global pharmaceutical companies is dependent on our ability to demonstrate leadership in research and innovation and to invest in developing the talent which can drive the future ambition of industry. The SSPC Research Ireland Centre for Pharmaceuticals exemplifies this mission by advancing cutting-edge science, forging strategic partnerships and producing the next generation of pharmaceutical scientists and process engineers. Ireland has attracted over €3.7 billion in investment linked to pharmaceuticals and an important factor of this success is the pipeline of PhD research talent which SSPC produces for the country.”
UL Vice President Research and innovation
Professor Kevin Ryan said: “As host of SSPC, University of Limerick is proud to see such a resounding success for Irish research and the national economy. The SSPC report demonstrates the valuable work being caried out at the centre and the significant impact it is having on Ireland’s economy. Added to this, the SSPC’s strong network of international collaborations is greatly enhancing Ireland’s reputation in international research. Investment in research infrastructure is key to the sustainable and ongoing development of our economy with SSPC delivering exceptional return on investment.”
SSPC is a dynamic ecosystem of interdisciplinary research excellence, comprising 81 scientific leaders across 10 of Ireland’s leading research-performing organisations. The Centre connects these Irish researchers with collaborators in 54 countries, spanning six continents, driving innovation on a global scale. With a collaborative network of more than 50 industry partners, including global leaders as well as Irish SMEs, SSPC has successfully delivered over 201 collaborative research projects with industry.
Leap Compliance is an Irish-owned QA/RA training company based in Galway. They work with pharmaceutical and medical device manufacturers across Ireland and globally to build skills, strengthen capability and create high performing teams. Quality culture is one of their flagship programmes, reflecting a growing industry priority and an area where meaningful change is needed.
With increased focus from the FDA and the introduction of a dedicated ISO standard, culture can no longer be taken for granted.

It must be understood, shaped and sustained. Leap Compliance’s Quality Culture Course provides practical support through structured workshops, which are tailored to audience levels and needs.
Quality as a mindset
Leap Compliance believe quality is not a function; it is a shared way of thinking and behaving. When that mindset is clear, it influences decisions, communication and everyday actions across the organisation.

Galway-based Leap Compliance work with pharmaceutical and medical device manufacturers across Ireland and globally to build skills, strengthen capability and create high performing teams.

Astellas recently announced a multi-site investment of €129 million over the next three years.
Astellas, one of Japan’s largest Life Sciences companies and a leading developer and manufacturer of pharmaceutical products globally, recently announced a series of significant investments across their Irish operations. These investments, amounting to a total of €129 million over the next three years, reinforce the company’s long-term commitment to Ireland as a strategic hub for its global manufacturing, research, and development activities.
“This commitment by Astellas highlights Ireland’s role in the global life sciences sector,” noted Peter Burke TD, Minister for Enterprise, Tourism and Employment. “Our ongoing investment in education, infrastructure, and innovation continues to create the optimum environment for companies to thrive. Astellas’ decision to grow its footprint in both Kerry and Dublin demonstrates the strength of Ireland’s offering and our commitment to supporting enterprise development across all regions.”
With a heritage in Ireland spanning more than 35 years, Astellas’ operations have a significant local impact and global reach. The company currently employs over 650 people in Ireland across two facilities in Killorglin and Mulhuddart, supplying medicines to 133 countries worldwide.
The multi-site investments, focusing on development, sustainability, and Research Development & Innovation (RD&I), aim to enhance Astellas’ operations across the country, further embedding their Irish footprint as a key pillar in the company’s global network.
The investments at Tralee, Co. Kerry, include:
• Talent Development Plan: Astellas will undertake a comprehensive plan to equip employees with skills to address strategic challenges, focusing on leadership development, digital literacy, environmental sustainability, innovation, and compliance.
• RD&I Project: This project will enhance the production of antibody-drug conjugates and monoclonal antibodies, aiming to establish a high-efficiency, low-waste, multi-product biologics facility.

• Green Capital Project: A significant initiative designed to reduce energy use and lower CO₂ emissions. Planned upgrades include Heating, Ventilation, and Air Conditioning (HVAC) systems, a woodchip boiler, solar panels, and an onsite wastewater treatment plant.
These investments in Tralee follow on from the company’s announcement in 2023 that it will build a new Biologics Sterile FillFinish Facility, which when fully operational in 2028 will create 120 new jobs. Additionally, more than 500 workers will be involved in the construction phase. The new facility will accelerate the expansion of Astellas’ in-house production capabilities and ensure a stable supply of high-quality medicines to patients around the world. The new facility will also be fully digitally enabled and will adhere to high energy and environmental standards.
The Killorglin, Co. Kerry, investment includes Green Projects. Astellas Killorglin will implement several initiatives to significantly reduce energy consumption and CO₂ emissions. These measures include wind turbines, woodchip boiler economisers, chiller upgrades, BioLPF/Hydrotreated Vegetable Oil (HVO) tanks, water heat pumps, and energy metering systems.
At Damastown, Co. Dublin, Astellas will invest in sustainability enhancements. Astellas Damastown is embarking on a project to improve environmental sustainability through the installation of solar panels and heat pumps across multiple facilities. This transition to electricity for heating will substantially decrease carbon emissions.
E xpanding capacity in ireland
Lisa Murphy, General Manager of Astellas Pharma Co. Ltd, said: “We’re delighted to see great progress across the various projects ongoing across the Astellas operational footprint here in Ireland. The continued investment by Astellas in Ireland will expand our capacity and capabilities for aseptic drug products, reinforce stable production for global supply, and accelerate the development and commercialisation of innovative antibody drugs and other new products.
“Astellas’ commitment to Ireland is a testament to the excellent record, past and present, of Astellas employees that have delivered for over 30 years across our Irish operations and the great relationship that Astellas has with the local community and local officials.”
Michael Lohan, CEO of IDA Ireland, described the multi-site investment by Astellas as “a strong endorsement of Ireland’s reputation as a global centre of excellence in Life Sciences”. He said: “It exemplifies IDA Ireland’s strategic objectives to strengthen long term investment, scale cutting edge innovation, drive sustainable change and maximise regional opportunities. With its well-established talent base, robust infrastructure, and pro-business environment, Ireland continues to attract strategic investments from world-leading companies. Astellas’ long-standing presence here and its ongoing commitment to both Dublin and Kerry is a testament to the strength of our partnership and the value Ireland offers as a key location for innovation and growth.”



































































Rory Mullen, Global Head of BioPharma, IDA Ireland, reflects on a buoyant year in 2025, despite challenging global headwinds and geopolitical conditions.
Despite the challenging trade and geopolitical conditions that have developed during the year, employment in Ireland’s BioPharmaceutical sector remained buoyant during 2025 as the investments announced in recent years continue to be built out. The industry now employs over 50,000 and exports over €100 billion of medicines every year.
The range of therapeutic modalities that are manufactured in Ireland continues to grow as Antibody Drug conjugates (ADCs), mRNA vaccines and Gene Therapies are added to the small molecule and biologics facilities already operating in the country. We are also seeing more new indigenous companies spinning out from the world class research being carried out in our universities and research centres.
Against this very positive backdrop for Ireland as a global leader, the world of BioPharma manufacturing is now encountering a period of significant disruption on a number of fronts. Global supply chains are being challenged by the altering geopolitical picture and economic realities, including recent changes to global trade policy. The pace of innovation in BioPharmaceuticals is also accelerating, and a range of new

therapeutic modalities are being developed and launched, including advanced individualised medicines, to add to the wellestablished classes of small molecule and protein-based biopharmaceuticals.
Advanced data science and artificial intelligence are also beginning to transform the development, manufacture and commercialisation of medicines and integrated, as comprehensive, patient-specific solutions are becoming a reality.
Competition for mobile investment projects is also intensifying, with increasing numbers of countries in Europe, the Middle East, Far East and beyond prioritising Life Sciences FDI and using incentives and other innovative instruments to attract multinational investment.
Multi-million investments in ireland
The year started with an announcement from MSD in January that they were acquiring Wuxi’s vaccine site in Dundalk to add to their manufacturing portfolio in Ireland. In Munster, GE Healthcare announced an investment to expand the capacity of their contrast media manufacturing operation in Cork. During the summer, Astellas announced significant continuing investments in Dublin and Kerry, and Aenova announced the expansion of their CDMO drug delivery operations in Killorglin. In September, Merck KGaA opened their new €150 million climate neutral investment in Blarney, Co. Cork, with the creation of 200 jobs.
Within this fast-changing global landscape, Ireland needs to ensure that it remains


Michael Lohan, CEO, IDA Ireland; Kevin O’Neill, President & CEO, GE HealthCare’s Pharmaceutical Diagnostics; An Taoiseach Micheál Martin TD; and Eugene Barrett, Managing Director, GE HealthCare Ireland, at the turning of the sod at GE HealthCare in Carrigtohill, Co. Cork.
competitive by proactively adapting to these emerging trends. Ireland stands out as a reliable hub for BioPharma production, especially for firms seeking secure, regionally resilient supply chains in a volatile world. Ireland needs to continue to leverage its geopolitical stability, regulatory track record, and highly skilled workforce to be an ever-reliable, responsive manufacturing base, and especially where firms are looking for secure, regionally resilient supply chains in a volatile world.

Minister for Education Normal Foley TD, and IDA Chief Executive Michael Lohan were amongst the guests at the official announcement by Aenova in a significant expansion at their Temmler Ireland Ltd facility in Killorglin, Co. Kerry.
Ireland can be the pioneer in effective, regulator-compliant use of digital technologies and AI in BioPharma through testbeds, attracting world tech leaders, and developing digital and data competence within the manufacturing value chain.
The current Irish Government has started on this journey by committing to develop a holistic Life Sciences strategy for the country. Responsibility for the development of this strategy has been given to the Department of Enterprise, Tourism and Employment, who have now established a dedicated team and have started to engage with all stakeholders, including Government, industry and academia, in the development of a comprehensive strategy.
Today’s investment climate for BioPharmaceuticals in Ireland remains highly favourable. Ireland’s success in both small molecule and biologics manufacturing has solidified its position as a global leader in the Pharmaceutical industry. The sector remains dynamic, with significant ongoing expansions and new facilities being established, which have been instrumental in growing employment in the BioPharmaceutical sector from 30,000 in 2016 to over 50,000 today.
Ireland can be the pioneer in effective, regulator-compliant use of digital technologies and AI in BioPharma through testbeds, attracting world tech leaders, and developing digital and data competence within the manufacturing value chain.
Charles River provide world class biologics testing, alongside regulatory guidance and consultative expertise, helping their clients towards regulatory approval.
Budget, capacity, and strict regulatory requirements are just some of the hurdles that can prevent the next big breakthrough therapy from commercialisation. Less than 1% of all drug candidates entering clinical trials reach the market, which means you need a partner with a proven track record of success.
Bringing a biologic therapy to market requires more than testing - it requires a trusted partner who understands the challenges of regulatory submission. Charles River work as an extension of their clients' teams, providing consultative expertise and regulatory guidance. With a proven track record in biologics testing, they help clients reduce uncertainty, optimise timelines, and move toward regulatory approval with confidence.
Their global network of facilities and scientific expertise can help accelerate the delivery of biologic therapies with confidence and ensure quality, compliance, and the safety of those who need them most.
Navigating regulatory hurdles
Every decision in biologics testing influences downstream success. By working alongside clients as trusted partners, Charles River help them navigate regulatory hurdles, optimise testing strategies, and ensure a smooth path to commercialisation.
Their facility in Ballina, Co. Mayo, is purpose-built to offer a comprehensive package of GMP services in support of recombinant biologics, vaccines, cell and gene therapies, biosimilars, and medical devices. With a >95% on-time performance and same-day sample delivery (for Ireland and UK), clients can outsource to Ballina with ease and confidence.
GMP biologics services that are available at Ballina include method development, method transfer and phase-appropriate validation to process/cleaning validation, impurities testing, stability storage and sample analysis.
Charles River also provide GMP quality control, routine testing, and release testing services for bulk drug substances and clinical and marketed drug products for the European Union (EU), United States (US), and other regulatory-distinct markets, and can act as a single site for your global release testing needs. The combined expertise of their Ballina and Dublin teams offers analytical, biosafety, in vivo bioassay, and microbiology services. Microbiology testing covers compendial and validated alternative sterility testing, water analysis, bioburden, LAL, identification, monocyte activation test (MAT) & more.
Stability studies are an essential part of product development and are conducted throughout a product's life cycle. They are required by a number of regulatory agencies. Charles River’s Ballina site offers a comprehensive suite of storage conditions and the necessary testing required to meet regulatory requirements. Their walk-in and reach-in chambers are monitored and controlled 24/7, and each programme is defined by a customised stability study protocol based on clients' needs.
They also offer vaccine development services, including in vivo disease models for challenge studies, in vivo rodent research models,
adjuvant selection studies, and stability assessment of product formulations. The Ballina facility can perform these services under BSL-2 and BSL-3 standards. The added barriers and constraints will allow them to support GMP-compliant testing of high-risk pathogens like SARS-CoV-2.
Charles River’s scientists have also supported vaccines including, and not limited to, JEV, Pseudomonas aeruginosa, tetanus toxoid, Equine flu, Diphtheria anti-toxin, Influenza, and Meningitis B, as well as influenza (attenuation, immunogenicity, and anti-sera).
For more information, visit criver.com

Charles River’s global network of facilities and scientific expertise can help accelerate the delivery of biologic therapies with confidence.



WuXi Biologics Ireland: Advancing Biologics
Manufacturing, Innovation, and Global Health
ISPE Facility of the Year
Award Winner
Large-scale single-use bioreactor capacity 54,000L
3 Manufacturing Facilities fully GMP Certified


CSG , including Clemifloc and GI Chemicals , provide reliable chemical solutions for Ireland’s Life Sciences and utilities sectors.
CSG partner with a range of customers in the potable water, wastewater, environment and hygiene sectors to deliver clean water, safe food and some of life’s essential products. Through their brands, Chemifloc and GI Chemicals, they combine 40 years of specialist chemistry expertise, a guaranteed supply of vital chemicals, and exceptional customer service.
CSG provides a wide portfolio for water and wastewater treatment and broader industrial applications: high-performance coagulants and flocculants, pH/alkalinity correction, anti-scalants and corrosion inhibitors, absorbents, disinfectants and detergents, and speciality chemicals. Customers can also access equipment, telemetry, laboratory analysis, and on-site technical services; an end-to-end model that reduces risk, simplifies audits and supports continuous improvement.
Chemifloc
Established over 40 years ago, Chemifloc, a CSG company, offers a guaranteed supply of vital chemicals to optimise all stages of the water treatment process. From primary coagulants to advanced, they help operators improve clarification, tackle colour and turbidity, and maintain stable operations in challenging conditions.
Chemifloc also work closely with their customers to manufacture bespoke products to satisfy their unique processes. All backed by a robust supply chain, expert logistics, and specialist services, including laboratory testing and telemetry.
GI chemicals
For nearly 10 years, GI Chemicals have been a trusted specialist in the supply of vital chemicals to industry. They maintain a robust supply chain, extensive stocks and run a logistics operation delivering across Ireland and the UK, using their own fleet of licensed tankers.
GI Chemicals offer a responsive, flexible service, with rapid delivery of consistent, high-quality chemicals. Their product range includes hydrochloric acid, potassium hydroxide, sodium hydroxide and sulphuric acid.
Why CSG:
• Logistics & Distribution:
Experienced team ensuring uninterrupted supply of essential chemicals via a robust global network and a fleet of 75+ ADRcompliant trucks, delivering to 1,600+ sites. Services include just-in-time delivery, flexible pack sizes, emergency response, and vendor-managed inventory supported by advanced telemetry.
• Technical Services:
Deep application expertise to solve site-specific challenges and boost efficiency. They advise on off-the-shelf or bespoke solutions and provide comprehensive lab service, from testing and dose optimisation to refining treatment plans.
• Specialist Equipment:
Flexible rental options for safe storage and accurate dosing, including ISO tankers, dosing rigs, mobile bunds, and tank telemetry, all helping optimise handling, compliance, and chemical performance.
CSG’s headquarters are in Smithtown, Shannon, Co. Clare, with operations in Foynes, Co. Limerick, and Immingham, UK.

From improving clarification with dual-action coagulants to optimising sludge conditioning and scaling control, CSG’s teams focus on outcomes that reduce downtime, protect assets and support ESG targets.
If you need validated products, secure supply and hands-on technical support, CSG are ready to help. For more information, email info@csg-corporate.com or visit www.csg-corporate.com









The upcoming National Life Sciences Strategy is a huge opportunity for Ireland to cement its competitive advantage in global Life Sciences, write Niall Best and Conor Cunningham , Partners and Co-chairs of McCann FitzGerald’s Life Sciences Group.
Ireland’s Life Sciences sector is preparing for the landmark opportunity that the upcoming National Life Sciences Strategy will present. A Government priority for delivery in 2026, having featured in both the Programme for Government and the Action Plan on Competitiveness and Productivity, the plans for a National Strategy demonstrate Ireland’s clear ambition to position itself as the global jurisdiction of choice for Life Sciences market participants by building on its existing competitive advantage in the areas of BioPharmaceuticals, Pharmaceuticals and medical technology.
Ireland’s Life Sciences industry is already a global leader, employing approximately 100,000 people and generating close to €100 billion in exports. The IDA’s recent ‘White Paper: The future of biopharma in Ireland’, confirmed that Ireland has attracted more than €15 billion in BioPharma foreign direct investment in the last decade. The White Paper also highlighted Ireland’s stable geopolitical environment, skilled workforce and rigorous regulatory standards as key advantages against the backdrop of evolving global supply chains and inhibitors to cross-border trade, the pace of innovation, the transformative nature of advanced data science and AI, and the increasing investment in Life Sciences across other jurisdictions.
Life Sciences continue to feature prominently on the EU agenda too, contributing close to €1.5 trillion to the EU economy and supporting 29 million jobs. The European Commission launched its EU Life Sciences Strategy in July 2025 with a clear ambition to make the EU the world’s most attractive location for Life Sciences by 2030.
The EU strategy is pragmatic about the challenges facing the industry, including fragmented research ecosystems, regulatory complexity, and the slow translation of innovation into market-ready products.
The volume of deliverables under the EU Strategy is ambitious and continues at pace: the
EU proposed the Critical Medicines Act in March 2025, which will complement the sweeping reforms to the EU’s 20-year-old pharmaceutical rules that were agreed in December 2025.
As Ireland prepares to assume the Presidency of the Council of the EU in July 2026, a National Strategy that consolidates Ireland’s position while aligning with the EU agenda is key. To maximise Ireland’s influence while holding the EU Council Presidency, and to continue to attract FDI into the Life Sciences sector by highlighting its track record as a stable, innovation-friendly jurisdiction, it is key that the National Strategy is published before the end of June 2026.
The first consultation on the National Strategy published by the Department of Enterprise, Tourism and Employment (DETE) in November 2025 asked the key question: is the Life Sciences sector broader than pharmaceuticals and biopharmaceuticals, medical technology, agriculture, fisheries and food production?
Biotechnologies, digitisation and AI (all components of the EU Strategy) should form part of the National Strategy – this would clearly demonstrate that the data- driven innovation and research that is critical for the Life Sciences sector in the longer term is a priority.
The core strategic objectives proposed by the DETE in that consultation (innovation, global competitiveness, patient outcomes, talent and sustainability) are key, and Ireland’s record in educating and training in STEM subjects will position its workforce well to meet the challenges ahead: the need for regulatory simplification, policy certainty, faster approvals and speed-to-market.
The objective of the Action Plan on Competitiveness and Productivity: ensuring that Ireland can control its resistance to global economic shocks, and the priority given to planned actions such as increasing investment in research, development and innovation; positioning Ireland as a global intellectual property hub; and updating the national digital and AI strategies, will be key in supporting the roll-out of the National Strategy.
To maximise Ireland’s influence while holding the EU Council Presidency, and to continue to attract FDI into the Life Sciences sector, it is key that the National Strategy is published before the end of June 2026.

Some law firms react to regulatory shifts. Others anticipate them.
The best combine both – understanding of today’s legal complexity whilst seeing tomorrow’s growth opportunity.
In a transforming Life Science landscape, that’s how we see the whole picture. And that’s how you go further.

Go Further
CoolCheck by Kelsius is an automated, wireless monitoring system that delivers efficiency without compromising compliance.
The ongoing shift toward digital solutions has transformed how we maintain quality and compliance across the Pharmaceutical and Life Sciences industries. While early waves of digital transformation focused on connectivity and mobility, the most meaningful evolution today is found in how automation supports precision and accountability.
The pandemic accelerated this change, highlighting the importance of robust, remote-ready monitoring systems capable of sustaining production and research in even the most challenging conditions.
In the years since, our sector has faced an increasingly complex landscape. Supply chain fragility, rising costs, and heightened sustainability demands are redefining the standards of operational excellence. For quality professionals, this has translated into the need for more reliable data, faster decision-making, and systems that can withstand scrutiny from auditors, regulators, and customers alike.
At the same time, the industry continues to balance regulatory pressures, data security expectations, and workforce challengesall while maintaining absolute integrity in research and production environments.
To maintain the highest quality standards, many organisations in Ireland are embracing automated, wireless monitoring systems that deliver efficiency without compromising compliance.

CoolCheck by Kelsius is one such innovation - a fully automated monitoring platform designed to safeguard samples, reagents and finished products. Using a network of wireless sensors, CoolCheck continuously records temperature, humidity, oxygen, and CO₂ levels across laboratories, cleanrooms and storage facilities. Data is captured and displayed in real time through a secure digital portal, eliminating manual logging and reducing the risk of human error.
Through the Kelsius App, users receive instant notifications via mobile push alerts, email or voice call whenever conditions fall outside defined limits. Each alert can be traced to corrective actions, detailing who responded, when, and what action was taken. The process ensures total traceability for audits and regulatory inspections. The system supports monitoring of fridges, freezers, incubators,
Real-time push notifications on the Kelsius app alert staff in real-time when deviations are detected.
water baths, and cryogenic storage, among many other controlled environments.
Secure, compliant, and sustainable
CoolCheck operates within a fully GDPRcompliant infrastructure, featuring multifactor authentication, user permissions, and advanced access control. Incremental data backups occur every five minutes, allowing full restoration to any point in time. Historical records remain accessible for over 30 years, providing an unrivalled audit trail for regulators and internal reviews alike.
Sustainability is also a key driver. By digitising temperature records and eliminating paper-based logs, Kelsius solutions help laboratories and production sites reduce waste, lower energy use, and contribute to corporate ESG objectives.

The Kelsius thermocouple sensor provides high-accuracy temperature monitoring, particularly effective in extreme high and low temperature conditions.
As a Guaranteed Irish company, Kelsius partners with leading pharmaceutical and research organisations across Ireland and beyond. Kelsius systems protect the integrity of medicines, materials, and research outcomes - and ultimately, the trust that patients place in suppliers of their healthcare.
For more information, call (074) 9162982, email sales@kelsius.com or visit www.kelsius.com


Hub Packaging can be your trusted partner in industrial packaging, with a broad range of packaging solutions to suit your needs.
Having the right packaging solutions is critical to running an efficient, reliable and safe business. Hub Packaging are a leading provider of industrial and transit packaging solutions, working with businesses across all sectors to protect their products and goods. They are the go-to partner for businesses looking for optimal high-performance packaging solutions.
Protection to meet your every need
Hub Packaging offer one of the most extensive ranges of packaging solutions and stock over 3,500 lines, including:
• Off the shelf Cardboard Boxes: Over 70, single and double wall boxes.
• Pallet Protection Specialists: Pallet Goods/Covers, sheets and edge boards.
• Premium Tape & Sealing Products: Quality tapes, glues and sealants.
• Professional Wrapping Solutions: Pallet wrap and stretch films designed for securing large shipments and pallets.
• End of Line Automation: High-quality machinery for pallet wrapping, strapping, and case taping, streamlining packaging operations for improved efficiency and consistency.
• Polythene & Plastic Packaging: Bags and covers for moisture and dust protection.

Specialists in industrial packaging
Whether you need robust packaging for heavy machinery parts, tamper-proof solutions for pharmaceuticals, or transit packaging for the food & beverage industry, Hub Packaging have a solution. Indeed, Hub Packaging have partnered with businesses across most sectors, including:
• Manufacturing & Engineering
• Logistics & Distribution
• Construction & Builders Merchants
• E-commerce & Retail
• Food & Beverage
• Pharmaceuticals & Healthcare

Hub Packaging offer one of the most extensive ranges of packaging solutions, with more than 3,500 lines.
Unmatched industry expertise & customer support
“With years of experience in industrial packaging, we understand the challenges businesses face regarding product protection, compliance, and cost efficiency,” explains Greg Brennan, Head of Sales at Hub Packaging. “Our team is dedicated to helping businesses find the perfect packaging solution tailored to their needs.”
Hub Packaging offer:
• Expert Consultation: Their specialists work closely with you to identify the most effective packaging solutions.
• Fast & Reliable Nationwide Delivery: Ensuring your packaging materials arrive on time, every time.
• Competitive Pricing: Cost-effective solutions without compromising on quality.
• Sustainable Options: Eco-friendly packaging alternatives that support your environmental commitments.
Partner with Hub Packaging
Contact Hub Packaging today for a full packaging audit. “We can help you reduce damages, reduce your carbon footprint and save you money,” Greg concludes.
For more information, visit www.hub-packaging.com or call them on (01) 866 0136 & +44 28 4175 4977 to speak with one of their packaging specialists.











Manufactured in Fermoy, Co. Cork, using a renewable-energy powered process, Micro-Bio is Ireland’s only Chlor-alkali manufacturer and a trusted partner to the country’s leading Biopharma, Pharmaceutical, dairy and water-treatment companies.
A pioneer in Membrane Cell electrolysis, Micro-Bio produces Sodium Hydroxide, Hydrochloric Acid, Sodium Hypochlorite, Sodium Chloride and Purified Water Solutions, all manufactured to GMP and multi-compendial standards (USP/EP/JP/BP/NF).
As Ireland’s only domestic producer of these essential raw materials, Micro-Bio provides security of supply, shorter lead times and direct access to decision-makers, ensuring unmatched responsiveness for its customers.
From raw materials to cleanroom filling, every stage of production is tightly controlled to ensure purity, consistency and full traceability, critical for applications in highly regulated industries.
Your sustainable partner
Recent investment in Generation 6 Electrolysis Cells has enhanced safety and efficiency, reducing carbon emissions by 90 percent and achieving EcoVadis Silver Medal. This investment shows their commitment to the market and gives them solace in security of supply.

With all production, testing and filling completed on site in Fermoy, Micro-Bio offers complete supply chain integrity and product traceability.
Micro-Bio continues to expand internationally while maintaining its strong community focus through local employment, education and sponsorship initiatives.

“We’re proud to demonstrate that an Irish-owned SME can deliver world-class manufacturing and sustainability standards,” says Peter McNamara, Managing Director.
www.micro-bio.ie | (025) 31388 | Fermoy Industrial Estate, Co. Cork.



Hovione is a science-based, integrated CDMO (Contract Development and Manufacturing Organisation) with over 60 years of experience in Pharmaceutical development and manufacturing operations. With FDAinspected sites in the US, Portugal, Ireland, and Macau, and development labs in Lisbon and New Jersey, Hovione delivers integrated services across drug substances, drug product intermediates, and drug products. The company supports Pharmaceutical

and biotech companies throughout the entire drug lifecycle, creating distinctive value through integrated technology platforms, combining expert teams, proprietary technology and engineering, specialised assets, and digitalised systems. These platforms are built not just for capacity, but for scientific depth, process excellence, and long-term value, helping solve the industry’s most complex development and scale-up challenges.
A global leader in spray drying, Hovione operates the largest commercial capacity in the industry. With over 25 years of expertise in amorphous solid dispersion (ASD), the company is a trusted partner in overcoming solubility and bioavailability challenges from early development through to commercial supply. Hovione is also at the forefront of

continuous tableting, combining sciencebased manufacturing and real-time process control to accelerate scale-up and ensure consistency. In the inhalation area, the company provides a full-service platform, from API development and formulation to capsule filling and device integration.
Trusted by 19 of the top 20 Pharmaceutical companies, Hovione has a flawless regulatory track record and contributes to up to 10% of new FDA drug approvals each year, with treatments reaching an estimated 80 million patients annually. This impact reflects Hovione´s commitment to improving patients’ lives, a purpose at the heart of everything the company stands for: “In it for life.”

Hovione is a global CDMO dedicated to helping pharmaceutical partners bring new and o -patent drugs to market.
With FDA-inspected sites in Ireland, the US, Portugal and China, we combine scientific excellence, large capacity and world-class capabilities across drug substance, drug product intermediates and drug product.
We are committed to mastering what is di cultand delivering it exceptionally well.

Anton Paar’s series of FTIR spectrometers and software solutions are ideal for the pharmaceutical industry where they are used to confirm the identity of raw materials, verify the composition of intermediates, and assess the quality of finished products.
Quality in pharmaceuticals is more than a requirement. It is a promise that every product reaching a patient is safe, effective, and authentic. For laboratories faced with increasing regulatory expectations, maintaining that promise depends not only on scientific accuracy but also on the ability to document and prove every analytical step.
Among the many analytical tools that ensure this trust, infrared spectroscopy stands out for its ease of use and ability to handle a wide range of samples. It offers the accuracy demanded by pharmacopoeia standards and the speed required by modern production schedules. Yet, as compliance frameworks evolve, pharmaceutical laboratories are asking for more than measurement precision; they need workflows that are digital, traceable, and simple to manage.
Infrared spectroscopy as a global standard for material authenticity
Fourier transform infrared (FTIR) spectroscopy works by passing infrared light through a sample and measuring how much of that light is absorbed at different wavelengths. Each molecule interacts with infrared radiation in a specific way, depending on its chemical bonds and structure. The result
is a unique absorption pattern – a molecular fingerprint – that allows clear identification of a compound or mixture.
This capability makes FTIR particularly valuable for the pharmaceutical industry, where it is used to confirm the identity of raw materials, verify the composition of intermediates, and assess the quality of finished products. Because most pharmaceutical substances contain functional groups that absorb strongly in the infrared region, FTIR can reveal subtle differences between chemically similar materials. It is a rapid, non-destructive technique that requires little or no sample preparation, making it ideal for incoming goods inspection and final product release.
In addition to identification, FTIR also supports material integrity monitoring. It can detect contamination, monitor polymorphic changes, and verify that excipients or active ingredients remain stable during production. For solid, liquid, and semi-solid samples alike, FTIR offers a direct and reliable method to ensure material authenticity and consistent product quality.
Its importance is also reflected in international regulation. The European Pharmacopoeia general chapter 2.2.24 on infrared

absorption spectrophotometry is referenced in over 1,200 monographs, while comparable procedures appear in USP <854>, JP 2.25, and ChP 0402. Together, these chapters form a global framework that establishes FTIR as a recognised standard for material identification.
FTIR made simple: Lyza series While FTIR is well-established, many laboratories still face challenges related to training, method setup, and data management. These are the challenges Anton Paar addressed with the Lyza series of FTIR spectrometers and connected software solutions, designed specifically for the needs of regulated industries.
The Lyza series combines robust optical engineering with intuitive operation. Each instrument is built for long-term stability and comes with a 15-year warranty on key optical components. For laboratories operating under GMP, longevity is more than a convenience; it is part of compliance assurance. Instruments that maintain alignment and performance over decades minimise recalibration risks and support continuous, verifiable data integrity throughout their lifecycle.
Spectroscopy suite: data integrity by design
In response to the strict data integrity principles pharmaceutical laboratories must operate under, Anton Paar developed Spectroscopy Suite Premium, a software environment built to combine analytical precision with full regulatory compliance. Spectroscopy Suite provides an intuitive interface that guides analysts through the complete measurement process. Each method, library, and dataset is stored in a secure SQL database, and all actions are automatically logged in a detailed audit trail that fulfils 21 CFR Part 11 and EU GMP Annex 11 requirements. Role-based permissions and configurable electronic signatures ensure that only authorised personnel can modify or approve results, while version-controlled methods maintain a complete history of changes.
For the user, the workflow remains simple and efficient. Automatic pass or fail evaluation, transparent validation steps, and predefined checks eliminate uncertainty and minimise manual input. By enforcing consistency and traceability, Spectroscopy Suite ensures that every FTIR result can stand up to regulatory review.
AISQ+: Qualification and lifecycle assurance
To support full compliance over time, Anton Paar provides the AISQ+ qualification package with every Lyza instrument. Covering design, installation, operational, and performance qualification (DQ, IQ, OQ, PQ), this
documentation aligns with 21 CFR Part 11 and EU Annex 11 and ensures that every instrument is demonstrably fit for its intended use.
AISQ+ simplifies the qualification process, providing ready-to-use templates and auditready documentation. In addition, scheduled requalification and preventive maintenance ensure that performance remains consistent over many years of operation.
While instrument-level integrity is essential, true compliance in modern laboratories requires data control across the entire analytical workflow. This is the role of AP Connect Pharma Edition, Anton Paar’s secure laboratory integration platform.
AP Connect acts as a central data hub, automatically collecting FTIR results from Spectroscopy Suite and storing them in a protected database. Every dataset, method, and signature is version-tracked, ensuring that information remains traceable and reviewable at any time. The system integrates with Active Directory, supporting centralised user authentication and policy management. Even existing laboratory instruments from other vendors can be connected through adapters, helping facilities move toward
complete digitalisation without replacing legacy equipment.
By linking FTIR analysis directly to LIMS or ELN systems, AP Connect eliminates manual data transfer, eliminating transcription errors and ensuring results remain consistent from measurement to final approval. This creates a closed, transparent digital workflow that supports both operational efficiency and regulatory confidence.
Confidence in every spectrum
As pharmaceutical laboratories continue to evolve, FTIR spectroscopy remains a trusted method for delivering clarity where it matters most – in understanding materials, verifying identity, and ensuring that every product fulfils its promise. With the Lyza series, Spectroscopy Suite, AISQ+, and AP Connect, Anton Paar provides a cohesive framework built around one goal: helping laboratories transform compliance into efficient, reliable workflows.
From the first spectrum to the final archived record, every result is traceable, verifiable, and secure, giving laboratories not only regulatory assurance, but lasting confidence in every measurement.
For more information please visit: https://www.anton-paar.com/ie-en/
The Lyza series of FTIR spectrometers and connected software solutions were designed specifically for the needs of regulated industries, combining robust optical engineering with intuitive operation.
We are a global pharmaceutical group, listed on the Italian Stock Exchange, with over 4,450 employees. We are a group of like-minded, passionate individuals who go to extraordinary lengths for our partners, customers, investors, and the people across the globe we serve.
We develop and commercialise medicines to serve people living with common diseases, as well as those living with some of the rarest.
Our site in Ringaskiddy, Cork is dedicated to the manufacturing of Lercanidipine HCl with dedicated Production and Quality units ensuring compliance with the highest specifications.

Greenvolt Next have developed a 4 MW solar PV plant for global BioPharmaceutical company Sanofi in Waterford.
Greenvolt Next, part of Greenvolt Group, a leading specialist in renewable energy solutions for the commercial and industrial sector, have completed the development of a 4 MW solar PV plant for Sanofi, one of the leading global BioPharmaceutical companies.
Now in operation at Sanofi’s manufacturing campus in Waterford, the project was structured under a Power Purchase Agreement (PPA), requiring no upfront investment from the pharma company and allowing them to access renewable electricity at a stable and predictable price, while benefiting from longterm operational certainty.
The project was formally inaugurated in a ceremony attended by the Minister of State, Mary Butler TD, underscoring the national relevance of investments that accelerate Ireland’s clean-energy transition. Sanofi, established in Ireland more than 20 years ago, develop and manufacture healthcare solutions across therapeutic areas such as cardiovascular disease, diabetes, multiple sclerosis and vaccines, with the Waterford site playing a central role in their operations.
Greenvolt Next designed and delivered the 4 MW solar PV plant, comprising more than 5,700 panels and 10 inverters. The installation will generate approximately 3.2 GWh of
renewable electricity annually, covering around 20% of the site’s energy needs and enabling the avoidance of 950 tonnes of CO₂ each year. This reduction in carbon emissions directly supports Sanofi’s global environmental strategy, while demonstrating the Waterford site’s leadership in climate action within the Irish Pharmaceutical sector.
Its delivery was completed within a four-month schedule, mobilising a multidisciplinary team of more than 100 professionals across design, engineering and installation. Greenvolt Next will operate and maintain the facility for 20 years, ensuring performance, safety and reliability throughout its lifecycle. The project strengthens the site’s long-term competitiveness, ensuring it can continue to grow, innovate and manufacture high-quality medicines sustainably.
“This is an impressive initiative that represents a significant step forward for clean energy and sustainable infrastructure in Waterford,” noted Mary Butler TD, Minister of State at the Department of the Taoiseach and the Department of Health. “It is so important that we strive to continue developing smart energy solutions at community level. Such local investments in renewable energy are particularly important in the context of our national commitments and climate targets. I was particularly struck by the team leading out on this initiative in Sanofi, the energy,
the enthusiasm is so encouraging. It sets a precedent for others to follow, having one of the largest renewable installations of this kind in the heart of our city.”
João Manso Neto, CEO of Greenvolt Group, said: “This project reflects the trust placed in our expertise and execution capabilities by a global company of Sanofi’s scale, reinforcing our leadership in the commercial and industrial renewable energy sector, both in Ireland and across Europe. We have built a strong track record across multiple industries, including the pharma cluster, which given its substantial energy needs, has increasingly adopted on-site generation as a strategic pillar for decarbonisation and energy resilience.”
Cian O’Brien, Site Lead, Sanofi Ireland, said: “Generating our own renewable energy on-site is vital in helping us meet our sustainability targets, aiming for net zero greenhouse gas emissions by 2045 across all scopes, with a trajectory towards carbon neutrality by 2030. With Greenvolt Next Ireland’s expertise in renewable energy installations for large businesses like us, we will be able to significantly reduce our reliance on the grid, generating 20% of our energy on-site each year.”

Atlantic Technological University’s new School of Life Sciences offers an innovative range of programmes and research for the Pharma, BioPharma and wider Life Sciences industries.
Atlantic Technological University (ATU), with campuses in Sligo, Donegal, Galway and Mayo, ranks among Ireland’s largest multi-campus universities, serving around 30,000 students across more than 600 undergraduate and doctoral programmes. ATU’s commitment to academic excellence empowers students to become global citizens, critical thinkers, and lifelong learners.
ATU is deeply rooted in industry engagement. Their new School of Life Sciences offers an innovative portfolio of programmes and research in Pharmaceutical, BioPharmaceutical, MedTech and wider Life Sciences, across their campuses at Sligo, Letterkenny and Galway. They prepare graduates for success in a rapidly evolving global landscape, equipped with the skills and perspectives needed to thrive and contribute meaningfully to Ireland’s leading industries.
Educating future-ready pharmacists
In September 2025, at the state-of-the-art Sligo campus, ATU launched its new Master of Pharmacy programme. Accredited by the Pharmaceutical Society of Ireland, the programme will, for the first time ever, educate pharmacists in the Northwest. Graduates of ATU’s MPharm programme also meet the educational requirements to work as QPs in industry.

The MPharm programme is modern and future-focused, integrating evidence-based clinical pharmacy practice with cutting-edge healthcare and pharmaceutical sciences research ongoing across ATU’s new Department of Pharmacy & Pharmaceutical Sciences, in areas such as Pharmaceutical and MedTech innovation, translational sciences and clinical therapeutics, medicines optimisation and future health. Undertaking work placements in community, hospital and industrial pharmacy, graduates will be future-ready pharmacists, with the clinical, digital health and pharmaceutical skills for a changing world. See: www.atu. ie/courses/master-of-pharmacy for more information
ATU is ambitious in expanding the range of programmes on offer in the Northwest. In 2025, in addition to the new Pharmacy programme, ATU also launched a new physiotherapy programme at its Letterkenny Campus. In September 2026, ATU will launch a new veterinary medicine programme at its Letterkenny Campus and a new Dietetics programme at its Sligo Campus. See www.atu.ie/courses for more information.
ATU’s Sligo campus also offers an extensive

ATU research is delivering real-world impact for patients, practitioners, communities and industry across the west and north-west of Ireland, and beyond.
range of undergraduate and postgraduate programmes in Pharmaceutical and BioPharmaceutical sciences, including their flagship programmes: MSc in Industrial Pharmaceutical Science (QP), as well as the MSc in BioPharmaceutical Sciences, a suite of ATMP programmes, and BioIndustry 4.0 Masters programmes, offered jointly with NIBRT. See: www.atu.ie/faculties/sciencehealth/life-sciences
At ATU’s Galway campus, the Department of Analytical, BioPharmaceutical and Medical Sciences delivers a range of undergraduate and postgraduate programmes in the area of chemical and Pharmaceutical, BioPharmaceutical and medical sciences. See: www.atu.ie/ faculties/science-health/analyticalbiopharmaceutical-medical-sciences
Many of these programmes are supported through Springboard+ and others such as the Irish Medtech Skillnet.
From optimising BioPharmaceutical production to developing Artificial Intelligence platforms for novel diagnostics, and from advanced manufacturing of medical devices to digital therapeutics that enhance wellbeing among vulnerable populations, ATU research is delivering real-world impact for patients, practitioners, communities and industry across the west and north-west of Ireland, and beyond.
ATU has grown extensive expertise in health-tech research and innovation, building on the University’s interdisciplinary research, innovation and collaborative across ATU’s faculties and Research Centres, while reinforcing Ireland’s position as a global leader in health innovation.
Through alignment with Ireland’s National Smart Specialisation Strategy (S3) for Innovation 2022–2027, ATU contributes to sectoral strengths and potential opportunities in Pharmaceutical and Life Sciences, MedTech, and medical devices, while also driving emerging opportunities in Advanced Manufacturing, Engineering, ICT, and Digital Services, reinforcing its role as a key innovation partner.


GE HealthCare announced a massive $138 million investment in their Cork campus in 2025, with work progressing well on-site.
In January 2025, GE HealthCare announced a $138 million (approximately €118 million) investment in their Cork campus to help meet growing global demand for their contrast media products, driven by ageing populations and the increasing global prevalence of chronic disorders.
An Taoiseach, Micheal Martin, Kevin O’Neill, President & CEO of GE HealthCare’s Pharmaceutical Diagnostics (PDx) segment, and CEO of IDA, Michael Lohan, visited the Cork campus for the sod turning to mark the announcement.
Contrast media are injectable diagnostic imaging agents used in Computed Tomography, X-ray and interventional imaging, to enhance visualisation of organs, blood vessels and tissues, to support clinical diagnosis.
In 2024, the Cork site, along with GE HealthCare’s other contrast media fill and finish production sites in Shanghai, China, and Oslo, Norway, supplied over 100 million
patient doses of contrast media around the world (Source: GE HealthCare data on file –Pharmaceutical Diagnostics Contrast Media Capacity and Investment, 2025).
The new 3000 square metre facility, which will support both established and pipeline products, will include solution preparation vessels, multi-functional powder handling systems, a new filling line and autoclaves, with advanced automation systems underpinning production.
By the end of 2027, the additional capacity will enable the production of 25 million additional patient doses per year. It will help to address growing global demand, while offering increased flexibility and resiliency across GE HealthCare’s contrast media production network for security of supply.
There has been considerable work on the site, since the investment was announced. Enabling work has progressed, including changes at the site to re-align the site entrance, car parks, drains, water main, and electricity lines. The
main manufacturing steel frame is now in place, with cladding being added.
The project is being led by Edward Kelly, Project Manager, who has long service who has long service with GE HealthCare and experience across the site operations. He is being supported by a team of highly talented colleagues, across a range of disciplines.
At the earliest stages of design for the new facilities at the site, environmental considerations and impact were taken into account. When finished, the project will help deliver significant environmental improvement in GE HealthCare’s carbon emissions, as well as larger use of electrification.
The Cork site has been producing vital pharmaceuticals for over 30 years. The investment in the site, and its expansion, cements the Cork campus as a key contrast media fill and finish facility – and one which will continue to support customers and their patients, across the world, for many years to come.



On 31st of January 2025, GE HealthCare, Cork announced a €132 million investment to expand their fill and finish production site. Global demand for contrast media, injectable pharmaceuticals used every day around the world to enhance and enable medical imaging procedures, is expected to double over the next ten years.
This investment will allow the Cork site to produce an additional 25 million patient doses of contrast media annually. An Taoiseach Micheál Martin, Kevin O’Neill, President & CEO, Pharmaceutical diagnostics (PDx) and Michael Lohan, Chief Executive Officer, IDA, helped turn the sod ahead of the new facility construction.
Eugene Barrett, Site Leader and Managing Director, GE HealthCare Ireland, said: “This expansion strengthens our longstanding presence in Cork, where we have a highly skilled team, access to leading talent in the pharmaceutical industry, strong distribution links around the world and a great partnership with IDA Ireland. First doses from our new facility are expected by the end of 2027 and we are proud of the impact our site will continue to make for patients around the world.”
Chemistry, Manufacturing and Controls (CMC) should be the backbone of any drug development programme and Bio Pharma Technical Consulting (BPTC) can help you to accelerate success, writes Tiffany D. Rau, PhD, co-owner and Principal Consultant, BPTC.
In biotech, scientific innovation is only the starting point. The real challenge lies in translating discovery into a safe, scalable, and compliant product. This is where Chemistry, Manufacturing, and Controls (CMC) becomes a strategic enabler, not just a regulatory checkbox.
Why CMC matters
Chemistry, Manufacturing and Controls (CMC) is the backbone of any successful drug development programme. It encompasses the processes, analytical methods, and quality systems that ensure your product is safe, effective, and reproducible. While science drives innovation, CMC ensures that innovation can be scaled, regulated, and delivered to patients.
Starting CMC early delivers measurable benefits:
• Regulatory Alignment: Agencies expect robust CMC data at every milestone.
• Risk Mitigation: Early process characterisation prevents expensive rework during scale-up.
• Investor Confidence: A strong CMC strategy signals readiness and de-risked execution.
• Accelerated Time-to-Market: Streamlined tech transfer and GMP readiness reduce bottlenecks.
Why process and manufacturing readiness are critical
Process readiness ensures your development work translates seamlessly into commercialscale production. It involves defining critical process parameters, validating analytical methods, and establishing control strategies early. Without this, scale-up can introduce variability, jeopardising product quality and regulatory compliance.
Facility design and start-up must align with your product’s requirements from day one. Delays in GMP readiness can stall clinical supply and jeopardise launch timelines. Early planning ensures:
• Fit-for-purpose facility design that supports scalability and compliance.
• Start-up strategies integrating equipment qualification, utilities, and workflows.
• Manufacturing initiatives embedding quality systems and digital tools for efficiency and audit readiness.
Current CMC landscape
Between 2020 and 2024, 74% of FDA Complete Response Letters (CRLs) were driven by quality and manufacturing deficiencies, not clinical data. CMC-related delays can add 6-24 months to development timelines for biologics and advanced therapies and some never make it to the market.
Early engagement with regulators and proactive CMC readiness is essential for success under expedited timelines.

Tiffany D. Rau, PhD, co-owner and Principal Consultant of Bio Pharma Technical Consulting.
The bottom line is that CMC isn’t just a regulatory checkbox; it’s a strategic enabler. Starting early reduces risk, saves money, and accelerates your path to patients. In developing medicines, timing is everything, and CMC done right, done early, is the difference between success and setback. Delaying CMC planning is one of the most common, and costly, mistakes in biotech development.
How BPTC can help
BPTC partner with biotech innovators to make CMC, process readiness, and manufacturing readiness a competitive advantage. Our services include:
• Process & Analytical Development: Building robust, scalable processes and validated methods.
• Scale-Up & Tech Transfer: Seamless transition from lab to GMP manufacturing.
Facility Design & Start-Up: Advising on layout, utilities, and workflows to meet global GMP standards.
• Manufacturing Strategy & Readiness: Implementing initiatives that ensure operational excellence and inspection success.
• GMP Compliance: Preparing documentation, training, and systems for regulatory approval.
For Cell and Gene Therapy, we address unique challenges - vector production, comparability studies, and specialised facility requirements - so you can move confidently through clinical phases.
Your science deserves a clear path to patients. Let BPTC help you pave it.


At BPTC, we help biotech and pharmaceutical innovators accelerate development and commercialisation by making Chemistry, Manufacturing & Controls (CMC), and manufacturing readiness a competitive advantage. From early-stage development to post-commercial GMP operations, our team delivers strategic solutions that reduce risk, save time, and ensure compliance.
Our Services Include:
• CMC Strategy & Process Development.

• Scale Up, Technology Transfer & Manufacturing
• Facility Design & Start-Up
• GMP Compliance & Quality Systems
• Biologics and Advanced Therapies Expertise
Your science deserves a clear path to patients. Let BPTC help you pave it - starting today.
www.bptc.ie or contact@bptc.ie

Endress+Hauser’s dedicated Flow Calibration Lab in Cork has achieved ISO 17025 accreditation, providing superior service for customers.
Endress+Hauser Ireland recently announced that their dedicated Flow Calibration Laboratory in Carrigtwohill, Co. Cork, has achieved ISO 17025 accreditation for flow calibrations. The accreditation was awarded by the Irish National Accreditation Board (INAB) in June 2025. This milestone marks a significant expansion of the company’s calibration capabilities and reinforces their commitment to delivering high-quality, traceable calibration services to customers across Ireland.
The newly accredited lab offers flow calibrations traceable to ISO 17025 standards for both Endress+Hauser and third-party

Endress+Hauser Ireland’s Flow Calibration Laboratory in Carrigtwohill, Co. Cork, offers flow calibrations traceable to ISO 17025 standards for both Endress+Hauser and thirdparty instrumentation.
instrumentation. With this enhanced capability, customers can benefit from shorter lead times, faster turnaround, and minimal disruption to operations, ultimately reducing costs and improving process efficiency.
E xpanding flow calibration capabilities
Endress+Hauser Ireland offer accredited calibration, helping customers stay in compliance, while reducing costs and increasing uptime. The new calibration rig expands their local calibration capabilities to include DN08 to DN80, with flow rates ranging from 0.1 m³/h to 60 m³/h. The lab provides accredited calibration services for Coriolis, Electromagnetic, and Vortex flowmeters, performed by certified and experienced service engineers based locally.
“Our investment in this dedicated Calibration Lab allows us to better support the installed base of flowmeters nationwide,” said Finbarr O’Neill, Service Manager at Endress+Hauser Ireland. “It’s about helping our customers ensure measurement accuracy, maintain compliance, and enhance process reliability.”
Providing added value to customers
Endress+Hauser Ireland’s calibration services are designed with customer needs in mind. They offer a standard five-day turnaround time, with a two-day express service available upon request. Additional services
such as cleaning and passivation are also offered, along with full documentation and certification traceable to national and international standards.
Endress+Hauser’s calibration services are designed to support your compliance and enhance efficiency. Endress+Hauser continue to invest in technology which brings them closer to the customers and the industries they serve.
For more information about Endress+Hauser Ireland’s Calibration Services, please visit www.endress.ie/flowcal
Endress+Hauser are leading global suppliers of meters and automated technology for process and laboratory use. The family-owned company is headquartered in Reinach, Switzerland, with approximately 17,000 employees worldwide. Endress+Hauser was founded in 1953 by Georg H. Endress and Ludwig Hauser. Since then, the company has been driving the development and deployment of innovative technologies and today is helping to shape the digital transformation of industry. Intellectual property is protected by 8,900 patents and patent applications. Endress+Hauser have sales companies in more than 50 countries, as well as representatives in another 70 countries, ensuring a global support network.

Process improvement is like climbing. With a strong partner, you can overcome any obstacle.
Just as athletes rely on their teammates, we know that partnering with our customers brings the same level of support and dependability in the area of manufacturing productivity. Together, we can overcome challenges and achieve a shared goal, optimizing processes with regards to economic efficiency, safety, and environmental protection. Let’s improve together.
Alan Looney, Managing Director of NCC, explores how the company’s strategic approach delivers a competitive advantage in chemical sourcing.
At National Chemical Company (NCC), we understand that sourcing chemicals for the Pharmaceutical industry is not merely about cost and compliance; it’s about creating strategic value. As regulatory demands, supply chain risks, and sustainability goals evolve, Pharmaceutical companies need sourcing partners who can deliver reliability, technical expertise, and access to global markets across East Asia, India, Europe, and North America.
For over five decades, NCC have built a reputation as a trusted chemical sourcing partner to leading Pharmaceutical manufacturers, helping them move from compliance to competitive advantage.
Compliance as the foundation
In the Pharmaceutical sector, compliance isn’t optional; it’s the cornerstone of every successful operation. NCC’s sourcing process is built around traceability, transparency, and regulatory assurance. For every product we source, we conduct full manufacturer authentication, regulatory verification, and due diligence.
Our in-house experts ensure that all materials comply with applicable regulatory requirements and quality standards. This includes NCC conducting REACH compliance checks and assisting with audit management, setting a pathway to secure compliance.
This meticulous approach gives Pharmaceutical companies peace of mind, knowing their raw materials are sourced from audited and approved suppliers, and that every product is supported by the necessary regulatory documentation and analytical data. At NCC, compliance is where we start, but it’s not where we stop
NCC’s sourcing services extend well beyond traditional procurement. We work collaboratively with Pharmaceutical clients to identify and authenticate global manufacturers and secure supply of required materials. Our assessment includes evaluating intellectual property, root of synthesis, upstream products, back integration and allied chemistries based on the root of synthesis
This strategic approach helps our clients:
• Access high-purity fine chemicals, intermediates, process solvents, and excipients.
• Accelerate R&D and scale-up through to commercial production.

Alan Looney, Managing Director, NCC.

By integrating sourcing expertise with technical and regulatory support, NCC enables Pharmaceutical companies to focus on what they do best, discovering and delivering life-changing medicines.
Supply chain resilience has never been more vital. NCC’s global network of vetted suppliers ensures our Pharmaceutical partners are not reliant on a single source or region.
NCC’s global footprint includes offices in Ireland, the UK and a new office in the United States. Our membership of the PlusChem network extends our reach across Europe, Asia, and North America.
This presence allows us to provide seamless sourcing support, faster logistics, and localised customer service, giving Pharmaceutical clients direct access to our global chemical supply capabilities and regulatory expertise. For global partners, this footprint supports reliability, responsiveness, and risk mitigation across every stage of the supply chain, even during disruption.
At NCC, we believe responsible sourcing is fundamental to longterm competitiveness. We work with manufacturers who share our commitment to environmental stewardship, ethical operations, and sustainable practices.
By embedding ESG principles into our sourcing strategy, we help Pharmaceutical clients align with growing expectations from regulators, investors, and patients for transparency and sustainability, without compromising performance.
When Pharmaceutical companies partner with NCC, they gain more than a supplier; they gain a strategic ally. Our approach transforms chemical sourcing from a necessary function into a driver of quality, innovation, and resilience.
With NCC, Pharma clients benefit from:
• Support achieving regulatory compliance.
• Faster innovation, assisting speed to market.
• Operational continuity, multi-region sourcing and secure logistics.
• Global service, support from Europe and the United States, backed by decades of experience.
For more information, call (01) 6131400, email hello@ncc.ie or visit ncc.ie




Pharmaceutical companies working with NCC don’t just source materials, they gain a strategic partner. Our smart sourcing and compliance expertise help accelerate innovation and keep supply chains resilient across regions.
Backed by decades of global experience, NCC delivers the reliability, speed, and expertise that move pharma forward.
NCC. Where smarter sourcing gives you the edge. Learn more about

As VP Pharma and Healthcare Europe, Kuehne+Nagel, Seamus Keane is well positioned to report on volatility within the global healthcare logistics industry, and to advise Pharmaceutical and BioPharmaceutical companies on how to build resilience into their supply chains.
What are the big issues facing the healthcare logistics market, worldwide and in Ireland?
“The healthcare logistics network remains volatile, with geopolitical unrest and natural disasters contributing to disruptions. Building resiliency in your supply chains with a key trusted partner is more critical than ever. Kuehne+Nagel support our customers with a quality led approach, ensuring we deliver as promised for our customers and their patients.”
How does a logistics service provider like Kuehne+Nagel help healthcare organisations with supply chain challenges?
“Healthcare logistics is about more than just supply chain efficiency; it is about improving the outcomes of the people at the end of healthcare supply chains waiting for their life-saving, life-changing, life-enhancing healthcare products. As regulatory pressures intensify and global supply chains become more intricate, quality plays an ever bigger role.
“A well-optimised and quality-driven logistics network is the key to making sure that the right products reach the right place at the right time and in the right condition.
The pandemic exposed the vulnerabilities in global healthcare supply chains, from shortages of critical medical supplies to disruptions in the distribution of healthcare products. As a result, quality is now being recognised as an essential pillar of healthcare logistics, demanding more sophisticated, data-driven, and resilient strategies.”
What difference can Kuehne+Nagel make over the entire product lifecycle, from R&D to clinical trials and through to commercialisation and even when a product comes off patent?
“Kuehne+Nagels’s HealthChain-certified network ensures consistent GxP quality at every product lifecycle stage, from early R&D to post patent expiry distribution. We manage clinical trials, commercial launch, and post market logistics, leveraging QuickSTAT for expedited transport during trials, then transitioning to full-scale distribution at launch. Our network comprises 270+ GxP-certified sites spanning 60+ countries, staffed by 4,000+ specialists, ensuring truly global coverage for over 95% of the world’s population. Kuehne+Nagel and our fully owned QuickSTAT entity offer a real end to end logistics solution to our key strategic partners in healthcare.”
What are the benefits for a pharma/ biopharma supplier in partnering with Kuehne+Nagel?
“Partnering with Kuehne + Nagel means
securing a comprehensive, quality-controlled, end-to-end logistics ecosystem built for sensitive healthcare products, backed by global coverage, proactive risk intelligence, real-time monitoring, regulatory rigor, and digital innovation. We deliver for when it matters the most.”

Logistics

For all your shipping needs:
No matter where along a product’s lifecycle you operate—from product discovery to patent expiry—choose a partner you can rely on to consistently apply the highest GxP compliant quality standards. Contact our Healthcare Logistics experts to learn more: ireland.sales@kuehne-nagel.com
■ Personalised medicines
■ Medical devices
■ Pharmaceuticals
■ Vaccines
■ Animal health products
■ Consumer healthcare products
Founded in 1780, Mason Technology have built a reputation as a trusted partner to Ireland’s Pharmaceutical, BioPharmaceutical, and Medical Device industries. With more than two centuries of experience, the company delivers high-quality scientific equipment and technical solutions that enable customers to achieve operational excellence, ensure compliance, and drive innovation in regulated environments.
Comprehensive scientific solutions
Partnering with over 50 global manufacturers, including Mettler Toledo, Edwards Vacuum, BUCHI, Huber, Evident, Shimadzu, and Buchiglasuster,

Mason Technology are a reliable partner for organisations seeking scientific excellence, dependable technical support, and a proactive approach to compliance.
Mason Technology provide a broad portfolio spanning analytical instrumentation, precision weighing, microscopy, spectroscopy, bioprocessing technologies, laboratory equipment, and data management software.
The company continues to expand its portfolio with innovative technologies such as the UVD Robot, an autonomous UV-C disinfection system that enhances contamination control and operational efficiency in controlled environments.
E xpert support for optimal equipment performance
A dedicated team of service engineers delivers end-to-end technical support, from installation and commissioning to INAB-accredited calibration and Validation Services (IQ, OQ, PQ, RQ). Every service is carried out in full compliance with manufacturer standards and regulatory requirements, ensuring reliable system performance and peace of mind throughout the equipment lifecycle.
Specialised software support
Mason Technology’s software specialists provide expert consultation and Computer System Validation (CSV) services to help customers maintain compliance with Data Integrity and 21
New Generation of UV-C Germicidal Efficacy and Efficiency

Mason Technology have been around for more than 200 years.
CFR Part 11 requirements in cGxP environments. This ensures digital systems remain secure, compliant, and audit-ready.
A trusted partner for quality and compliance
With continuous ISO certification since 1994, Mason Technology remain a reliable partner for organisations seeking scientific excellence, dependable technical support, and a proactive approach to compliance. The company continues to evolve, supporting Ireland’s Life Sciences sector with innovative solutions and expert services that uphold the highest standards of quality and performance.
Key Features: masontechnology.ie
3 minutes of staff involvement per room
Disinfects an area of 50m² in less than 15 minutes
Eliminates more than 99.99% of multiple organisms
100% autonomous UV-C radiation
Key Benefits:
Production capacity increase >50%
Full traceability and 21 CFR compliance
100% chemical free disinfection
Return on investment in less than a year

Ireland’s Life Sciences sector is under growing pressure to deliver efficiency, maintain strict regulatory compliance, and accelerate digital transformation. One of the most important enablers of this progress is modern industrial automation.
Bonner focus on upgrading legacy control systems such as SCADA, PLC, and DCS. By helping Pharmaceutical and biotech manufacturers modernise without halting production, they ensure facilities remain compliant, while realising the benefits of advanced technology. These systems are the backbone of reliable, validated operations, and updating them delivers both immediate and long-term improvements.
But efficiency is only the beginning. Advanced automation provides real-time visibility through data capture, monitoring, and reporting, empowering managers with deeper insight and supporting proactive, risk-based decision-making.
Integrated systems also strengthen batch traceability, enable predictive maintenance, and support energy efficiency, all vital to minimising downtime and protecting consistent, compliant product quality.
Perhaps the most powerful benefit is seamless data integration. Today’s control platforms connect directly with enterprise systems such as MES, ERP, and cloud analytics, creating the foundation for digital transformation. The result is a more connected, agile, and scalable manufacturing environment that’s ready for the future.
Bonner’s automation specialists work together with clients to review existing systems, create tailored upgrade strategies, and deliver solutions with minimal disruption.
Bonner_Ad_180x131_Automation_03.pdf 1 17/09/2025 11:59
With decades of experience supporting Ireland’s regulated industries, Bonner are your trusted partner for smarter, compliant, and more flexible Life Sciences manufacturing.
Automation isn’t just an improvement; it’s a strategic advantage.
Visit www.bonner.ie or email contact @bonner.ie to begin your automation journey.


With more than 55 years of experience across five global locations, Almac can add real value to drug development and commercialisation programmes.
Almac employs over 700 highly skilled analytical scientists across five global locations working in regulatory-approved GMP laboratories.
With 55+ years of experience, their expert teams develop 1,000+ analytical methods and validate 250+ methods annually. Drawing upon an extensive range of analytical technology, combined with a wealth of analytical knowledge, Almac can add real value to your drug development and commercialisation programmes.
Within Almac’s specialised testing laboratories, they have significant experience in a wide range of analytical techniques delivered by highly skilled analytical scientists, ensuring all testing is conducted with the highest level of accuracy and reliability.
Almac support drug substance and drug product analytics across all clinical phases, from early pre-clinical/clinical development to commercial release:
Test Articles Services
Raw materials
Drug substance / API
Small molecule
Peptides
Biologics
Drug product
Small molecule
Peptides
Biologics
Over encapsulated drug
• Medical devices
• Conjugates
• Highly potent
• Controlled substances
Method development
Method validation
Method transfer
Stability Study programmes
Release analysis: all lifecycle stages
Spectroscopy services (inc. structural elucidation and impurity identification)
• Biodistribution Studies by ICP-MS
• Investigational analysis
• Reference standard management
• Microbiological testing
• Physical sciences
• Analytical support for clinical trial
supplies
Almac offers a diverse suite of analytical methods with applications across the biologic development space, with extensive experience in the analysis of drug substance, drug product and reference material, from pre-clinical phase through to commercial manufacture for monoclonal antibodies (mAb) and mAb-like molecules, antibody drug conjugates, biosimilars and biobetters, recombinant proteins, cell/gene therapies and long peptides.
Almac look deeper so you don’t have to:
Specialised instrumentation and techniques: most recently they have invested in state-of-the-art chromatography, spectroscopy, and

biologics testing equipment, including the Raman TRS100 system, which allows faster assay of tablets, capsules, and other dosage forms.
Digitisation upgrade: a multi-million-euro digitisation upgrade, including enhancements to their existing laboratory information management system (LIMS), promotes seamless information sharing across multiple teams, departments and facilities, offering clients a reliable, optimised and streamlined solution.
People: Almac’s expert teams conduct continuous training and collaboration with local academia fosters talent, offering clients the very best partnership possible now, and for the future.
Ongoing investment: guaranteeing best-in-class services, Almac continue to invest in expanding their analytical labs, technology and people. Most recently, their new lab space at their global headquarters site has enabled Almac to increase capacity, with additional upgrades featuring across all sites.
Highly experienced and dedicated teams: their teams bring a wealth of experience and dedication, ensuring expert solutions for every project.
Industry leading project management: Almac excel in project management, setting high standards for efficiency and effectiveness.
Flexibility to meet your growing needs: their flexible approach allows Almac to adapt and meet your evolving requirements seamlessly by leveraging their five global sites.
Reputation for quality: Almac is a quality-driven organisation and makes the commitment to clients, large and small, to deliver highquality results every time.
Results-driven: their focus is on achieving tangible, impactful results that drive the success of your product.
Collaborative partnership: Almac believe in building strong, collaborative partnerships to achieve the mutual goal of drug development and commercial success.
Continual investment in their people, facilities, capabilities, and technology: they continuously invest in their team, infrastructure and technology to stay at the forefront of innovation and capability.
For more information, visit www.almacgroup.com
Purifying water and recovering materials with mobile & modular installations
InOpSys is committed to providing circular waste or side stream solutions for the chemical and pharmaceutical industry, by building and operating mobile & modular purification installations on the customer site. Using a train of selective technology combinations, InOpSys efficiently closes water and material loops and thus helps the industry reach their sustainability goals by reducing waste and CO2 emissions.
for linear destruction
The costs associated with waste and emissions can thus be reduced and value can be created by the recovery of materials (e.g. precious metals like Pd, Pt). InOpSys wants to do better than linear destruction (e.g. incineration) of hazardous side or waste streams in times of water and material scarcity.
Award-winning concept for api removal & metal recovery
InOpSys offers solutions to treat water and solvent streams, for:
• Active Pharmaceutical Ingredient (API) removal & recovery
• Metal removal & recovery (e.g. precious metals)
• Micropollutant removal
Their award wins include:
• Imagine Chemistry Challenge AkzoNobel (2017)
• Belgian Business awards for the Environment (2018): first prize
• Solar impulse foundation (2021): efficient solution label
Selective and very high rate of removal
InOpSys uses a decentralised model, creating installations on-site, close to the side stream source. This allows working with unmixed and well defined side streams, and avoids transport as a plus.
InOpSys is a one-stop shop, because they do not focus on one single technology, but on a hybrid combination of different technologies. Thanks to this “relay team” of technologies, very high removal rates

can be achieved in a more efficient way compared to monotechnology solutions. This also allows InOpSys to remove pollutants in a selective way, leaving easily biodegradable components untouched.
The customers are unburdened as InOpSys takes ownership from start to finish, and
offers its service in a CAPEX-free way. InOpSys finances the installation via a pay-per-use model, which spares customers an investment which is not interesting enough according to their internal return on investment guidelines.
Contact details
www.inopsys.eu • info@inopsys.eu
+32 (0)495 653 821

circular chemistry can save the world.

we believe in circles. advantages.
we believe in circles. advantages.
SELECTIVE REMOVAL


circular chemistry can save the
Soltec recently celebrated 30 years in business by winning two prestigious environmental awards.
As the Pharmaceutical sector intensifies its focus on circularity and carbon reduction, Soltec (Ireland) Ltd ended 2025 with a sweep of national honours that underscore their growing influence in sustainable waste management. The company has been awarded the Ernst and Young Sustainability Entrepeneur of the Year Award 2025 and the Excellence in Business Waste Management Solutions Award 2025, recognising their leadership in developing innovative, lowcarbon recycling pathways for hazardous and non-hazardous waste.
Soltec CEO David Corcoran was named Ernst & Young Sustainability Entrepreneur of the Year 2025 at an awards ceremony at the Powerscourt Hotel, a testament to the company’s long-standing commitment to innovation and the transformative impact of their model on high-waste, high-regulation industries, including the Pharmaceutical sector.
At the forefront of hazardous waste recycling
For more than three decades, Soltec have been at the forefront of hazardous waste recycling in Ireland, converting materials traditionally destined for incineration into valuable, reusable products that re-enter the market. With clients across Pharma, education, manufacturing and more, the

company now recovers and recycles 91% of the waste it manages, delivering over 6,000 tonnes in carbon savings annually.
That capability expanded significantly in 2024, when Soltec completed a €6.8 million investment in a new EPA-licensed treatment facility, boosting hazardous waste processing capacity by 300%, from 5,000 to 20,000 tonnes per year.
The expansion followed a year of national recognition: Soltec secured both the Overall Repak Award and the Waste Recycling

and Recovery Facility of the Year title in 2024, reinforcing their reputation as a sector leader in Ireland’s circular economy.
Today, Soltec accept more than 450 waste streams, including solvents, laboratory chemicals, corrosives, aerosols, PPE, glycols, cutting fluids and contaminated packaging. These materials are particularly prevalent in Pharmaceutical research, production and quality-control environments. Their on-site distillation technology enables the creation of recycled solvent products and industrial fuels, including low-carbon alternatives used within the cement industry.
The company’s sustainability strategy is reinforced by ISO9001, ISO14001 and ISO45001 accreditations, as well as EPA Licence Ref. P1093-01. Their operational footprint is further decarbonised by a substantial solar array that now generates 55% of the electricity required for treatment and recovery processes.
For Pharmaceutical manufacturers under increasing pressure to decarbonise supply chains, reduce waste exports, and meet stringent regulatory obligations, Soltec’s model offers a practical and scalable pathway. By keeping materials in use, eliminating unnecessary disposal, and maximising resource recovery, Soltec are helping move Ireland’s high-tech industries toward a more resilient, regenerative future.
Providing sustainable solutions through recycling hazardous waste.
Securing cost-effective treatment options for our industry partners.
Reducing Ireland’s carbon footprint.
30 YEARS EXPERIENCE IN TREATMENT AND RECYCLING




IRELAND’S ONLY PROVIDER OF SOLVENT DISTILLATION SERVICES



A pioneer in the plasma industry, Grifols manufactures lifesaving plasma-derived medicines to treat chronic and rare conditions, as well as infectious diseases. Learn more about
at www.grifols.com

Corcoran Chemicals Limited
ACIDS & ALKALIS
AQS Environmental Solutions
Associated Chemicals Ltd
Brenntag Ireland
Camida Ltd
Carbon Group
Chemco Ireland Ltd
Chemifloc Ltd
Corcoran Chemicals Ltd
Fisher Scientific Ireland Ltd
GI Chemicals
Greenfield Global
Heterochem Dist Ltd
Micro-Bio Ireland Ltd
National Chemical Company
Q1 Scientific
Solv-Echem Ireland Ltd
Univar
Associated Chemicals Ltd
Brenntag Ireland
Chemco Ireland Ltd
Fisher Scientific Ireland Ltd
National Chemical Company
Univar
ACTIVE PHARMACEUTICAL INGREDIENTS
AbsorboPak
Barentz
Corcoran Chemicals Ltd
Mallinckrodt Pharmaceuticals
SK Pharmteco
BIOCHEMICALS
Alexion
Arran Chemical Company
Associated Chemicals Ltd
Camida Ltd
Carbon Group
Corcoran Chemicals Ltd
Fisher Scientific Ireland Ltd
GI Chemicals
Pharmalex
Univar
BIOCIDES
Associated Chemicals Ltd
Brenntag Ireland
Camida Ltd
Carbon Group
Chemco Ireland Ltd
Corcoran Chemicals Ltd
Fisher Scientific Ireland Ltd
Heterochem Dist Ltd
Mason Technology
Univar
CATALYSTS
Arran Chemical Company
Associated Chemicals Ltd
Camida Ltd
Fisher Scientific Ireland Ltd
Ortec Inc.
National Chemical Company
SK Pharmteco
CHIRAL COMPOUNDS
Arran Chemical Company
Associated Chemicals Ltd
Camida Ltd
Fisher Scientific Ireland Ltd
Hovione Ltd
National Chemical Company
SK Pharmteco
EXCIPIENTS
A&C Your Global GMP Partner
Associated Chemicals Ltd
Azelis
Barentz
Brenntag Ireland
Camida Ltd
Chemco Ireland Ltd
Greenfield Global
Heterochem Dist Ltd
Micro-Bio Ireland Ltd
National Chemical Company
Ortec Inc.
Mason Technology
Univar
FINE CHEMICALS
Actylis
AbsorboPak
Arran Chemical Company
Associated Chemicals Ltd Azelis
Brenntag Ireland
Camida Ltd
Carbon Group
Chemco Ireland Ltd
Corcoran Chemicals Limited
Fisher Scientific Ireland Ltd
Greenfield Global
Heterochem Dist Ltd
IDA Ireland
Micro-Bio Ireland Ltd
National Chemical Company
Ortec Inc.
SK Pharmteco
Solv-Echem Ireland Ltd
GASES
National Chemical Company
HETEROCYCLICS
Arran Chemical Company
Associated Chemicals Ltd
Camida Ltd
Corcoran Chemicals Ltd
National Chemical Company
INDUSTRIAL CHEMICALS
Corcoran Chemicals Ltd
GI Chemicals
Mason Technology
INORGANIC CHEMICALS
Associated Chemicals Ltd
Brenntag Ireland
Camida Ltd
Carbon Group
Chemco Ireland Ltd
Chemifloc Ltd
Corcoran Chemicals Limited
Fisher Scientific Ireland Ltd
GI Chemicals
Heterochem Dist Ltd
Micro-Bio Ireland Ltd
National Chemical Company
Ortec Inc.
Solv-Echem Ireland Ltd
Univar
LABORATORY REAGENTS
Associated Chemicals Ltd
Bonner
Corcoran Chemicals Ltd
Fisher Scientific Ireland Ltd
Heterochem Dist Ltd
IDA Ireland
Mason Technology
Solv-Echem Ireland Ltd
MISC. CHEMICALS
AbsorboPak
Arran Chemical Company
Associated Chemicals Ltd
Azelis
Brenntag Ireland
Camida Ltd
Carbon Group
Chemco Ireland Ltd
Chemifloc Ltd
Corcoran Chemicals Limited
Fisher Scientific Ireland Ltd
Greenfield Global
Heterochem Dist Ltd
Mason Technology
National Chemical Company
Solv-Echem Ireland Ltd
Univar
OILS, FATS AND WAXES
Associated Chemicals Ltd
Brenntag Ireland
Carbon Group
Corcoran Chemicals Limited
Mason Technology
Heterochem Dist Ltd
ORGANIC INTERMEDIATES
Arran Chemical Company
Associated Chemicals Ltd
Camida Ltd
Corcoran Chemicals Ltd
Fisher Scientific Ireland Ltd
Heterochem Dist Ltd
Hovione Ltd
IDA Ireland
National Chemical Company
Ortec Inc.
Mason Technology
SK Pharmteco Univar
ORGANOMETALLICS
Arran Chemical Company
Associated Chemicals Ltd
Camida Ltd
Mason Technology
National Chemical Company
REAGENTS
Associated Chemicals Ltd
Camida Ltd
Corcoran Chemicals Ltd
Fisher Scientific Ireland Ltd
Heterochem Dist Ltd
IDA Ireland
Mason Technology
National Chemical Company
Source BioScience
SILANES
Arran Chemical Company
Associated Chemicals Ltd
Brenntag Ireland
Camida Ltd
Fisher Scientific Ireland Ltd
Heterochem Dist Ltd
Ortec Inc.
Univar
SOLVENTS
Brenntag Ireland
Camida Ltd
Carbon Group
Chemco Ireland Ltd
Corcoran Chemicals Limited
Fisher Scientific Ireland Ltd
Greenfield Global
Heterochem Dist Ltd
National Chemical Company
Univar
SURFACTANTS
Associated Chemicals Ltd
Brenntag Ireland
Camida Ltd
Carbon Group
Corcoran Chemicals Limited
Fisher Scientific Ireland Ltd
Greenfield Global
Heterochem Dist Ltd
TREATMENT CHEMICALS
Corcoran Chemicals Ltd
GI Chemicals
Micro-Bio
Ireland Ltd

AGITATORS
CPI Technology Ltd
Quitmann O’Neill
AIR / ROAD / OCEAN FREIGHT
AbsorboPak
Dawsongroup | TCS Ireland
Hazchem Training Ltd
Kuehne + Nagel
Portakabin Ireland
UPS Healthcare
AIR FILTRATION / MONITORING / INGREDIENTS
CMS Chemstore Engineering Ltd
ALUMINIUM PRODUCTS
Foltech Engineering Ltd
Quitmann O’Neill
ANALYSIS SERVICES
Almac Sciences Ltd
ATG Scientific Ltd
Charles River Laboratories Ireland Ltd
Charles River Microbial Solutions
Corcoran Products Ltd
Eurofins Biopharma Product Testing
GI Chemicals
IDA Ireland
Particular Sciences Ltd
Reading Scientific Services Ltd
SK Pharmteco
ANALYTICAL EQUIPMENT
ATG Scientific Ltd
Endress & Hauser (Ireland) Ltd
Fisher Scientific Ireland Ltd
LABPLAN
Mason Technology
Particular Sciences Ltd
Scientific Instruments Ireland
ASSOCIATIONS
GS1 Ireland
AUTOCLAVES
Fisher Scientific Ireland Ltd
Scientific Instruments Ireland
Mason Technology
AUTOMATION
Bonner
Endress & Hauser (Ireland) Ltd
LABPLAN
O’Flynn Medical Ltd
Portakabin Ireland
ProSys Containment and Sampling Technology
Tekpak Automation Ltd
BALANCES
Bonner
Fisher Scientific Ireland Ltd
Mason Technology
Scientific Instruments Ireland
BARCODE VERIFICATION
GS1 Ireland
Holfeld Graphics
Horan Automation
Tekpak Automation Ltd
BARCODING / LABELLING / TRACEABILITY
Corcoran Products Ltd
GS1 Ireland
Holfeld Graphics
Horan Automation
Tekpak Automation Ltd
BIOINTERACTION ANALYSIS
ATG Scientific Ltd
BIOLOGICS
MSD Ireland
BIOPHARMACEUTICALS
AbsorboPak
Alexion
Alkermes Pharma Ireland Limited
Almac Sciences Ltd
Barentz
BS&B Safety Systems Ltd
Charles River Laboratories Ireland Ltd
Charles River Microbial Solutions
Eurofins Biopharma Product Testing
Horan Automation
IDA Ireland
Janssen
Mason Technology
Micro-Bio Ireland Ltd
MSD Ireland
Pharmalex
Portakabin Ireland
Reading Scientific Services Ltd
BIOPHARMA / BIOTECH SUPPLIERS
BS&B Safety Systems Ltd
BIOPHARMA / BIOTECH SUPPORTS
Flexachem Manufacturing Ltd
BIOTECHNOLOGY
Almac Sciences Ltd
Charles River Microbial Solutions
DHL Supply Chain
IDA Ireland
Labplan
Mallinckrodt Pharmaceuticals
Mason Technology
Micro-Bio Ireland Ltd
MSD Ireland
Particular Sciences Ltd
Pharmalex
BLENDERS
Fisher Scientific Ireland Ltd
Ortec Inc.
BLISTERING / DE-BLISTERING
Holfeld Graphics
Horan Automation
BPRV
BS&B Safety Systems Ltd
CPI Technology Ltd
BURSTING/RUPTURE DISCS
Horan Automation
CABINETS
Dawsongroup | TCS Ireland
Fisher Scientific Ireland Ltd
Foltech Engineering Ltd
Horan Automation
Source BioScience
CAD
Horan Automation
CALIBRATION
Ballinlough Pharma Solutions
Bonner
Dawsongroup | TCS Ireland
Labplan
LotusWorks
Mason Technology
CARTONING SYSTEMS
Tekpak Automation Ltd
CASE ERECTING / PACKING
Horan Automation
Tekpak Automation Ltd
CASE SEALING
Horan Automation
CENTRIFUGES
AQS Environmental Solutions
CPI Technology Ltd
Fisher Scientific Ireland Ltd
Labplan
Mason Technology
CHEMICAL CONSULTANTS
Corcoran Chemicals Limited
Hazchem Training Ltd
CHILLED WATER
Astatine
Cross Technical Solutions
CHROMATOGRAPHY
Charles River Microbial Solutions
Fisher Scientific Ireland Ltd
Labplan
Mason Technology
SK Pharmteco
CLEANROOMS
Charles River Microbial Solutions
Cross Technical Solutions
Dawsongroup | TCS Ireland
Fisher Scientific Ireland Ltd
Horan Automation
KUKA Robotics Ireland Ltd
Mason Technology
Ortec Inc.
Portakabin Ireland
CLEANING SERVICES / EQUIPMENT
AQS Environmental Solutions
Portakabin Ireland
CLINICAL RESEARCH
ORGANISATION
Charles River Microbial Solutions
COLD CHAIN CONSULTATION
DHL Supply Chain
COLD CHAIN PACKAGING
Alexion
CRS Pharma Solutions
DHL Supply Chain
Horan Automation
Kuehne + Nagel
Quitmann O’Neill
UPS Healthcare
COMPRESSED AIR / COMPRESSORS
Ballinlough Pharma Solutions
COMPUTER SYSTEMS
Charles River Microbial Solutions
Chemishield
Westbourne IT Global Services
CONDENSORS
Ballinlough Pharma Solutions
CPI Technology Ltd
Cross Technical Solutions
Dawsongroup | TCS Ireland
Flexachem Manufacturing Ltd
CONDITION MONITORING
AbsorboPak
Bonner
Charles River Microbial Solutions
Flexachem Manufacturing Ltd
Foltech Engineering Ltd
LotusWorks
Q1 Scientific
CONSTRUCTION MANAGEMENT
Astatine
Dawsongroup | TCS Ireland
Portakabin Ireland
Tandem Project Management Ltd.
CONSULTANCY
Astatine
Dawsongroup | TCS Ireland
Hazchem Training Ltd
Horan Automation
Reading Scientific Services Ltd
Tandem Project Management Ltd
CONTENT STRATEGY
TWi
CONTRACT DEVELOPMENT & MANUFACTURING
Hovione
Ortec Inc.
SK Pharmteco
CONTRACT DEVELOPMENT & MARKETING
Charles River Microbial Solutions
Hovione
CONTRACT PHARMA SERVICES
Alkermes Pharma Ireland Limited
Charles River Laboratories Ireland Ltd
Charles River Microbial Solutions
Eurofins Biopharma Product Testing
Hovione
Ortec Inc.
Pharmalex
Reading Scientific Services Ltd
CONTROL SUBSTANCES
CONVEYORS
Horan Automation
Tekpak Automation Ltd
COOLING SYSTEMS
Astatine
Ballinlough Pharma Solutions
CPI Technology Ltd
Cross Technical Solutions
CRS Pharma Solutions
Dawsongroup | TCS Ireland
DATA ACQUISITION
Bonner
Charles River Microbial Solutions
IDA Ireland
Labplan
DEHUMIDIFIERS
Cross Technical Solutions
CRS Pharma Solutions
Dawsongroup | TCS Ireland
DESIGN
Ballinlough Pharma Solutions
Horan Automation
Nelipak Healthcare Packaging
Pharmalex
Quitmann O’Neill
Tandem Project Management ltd
Tekpak Automation Ltd
DISPERSERS
Fisher Scientific Ireland Ltd
Horan Automation
DISTILLATION
Fisher Scientific Ireland Ltd
Labplan
DOCUMENTATION SOLUTIONS
Charles River Microbial Solutions
Kuehne + Nagel
TWi
DRAINS
AQS Environmental Solutions
Foltech Engineering Ltd
DRUMS / CONTAINERS
AbsorboPak
CMS Chemstore Engineering Ltd
Complas Packaging Ltd
Corcoran Products Ltd
Interpac
National Chemical Company
Quitmann O’Neill
ECONOMIC DEVELOPMENT AGENCY
IDA Ireland
EDUCATION & TRAINING
Charles River Microbial Solutions
GS1 Ireland
Pharmalex
Reading Scientific Services Ltd
Science Foundation Ireland (SFI)
TWi
Westbourne IT Global Services
EFFLUENT MONITORING / TREATMENT
Carbon Group
ELECTRICAL
Portakabin Ireland
Tandem Project Management ltd.
ELECTRONIC COMPONENTS
Horan Automation
ENERGY EFFICIENCY / MANAGEMENT
Astatine
Horan Automation
Sustainable Energy Authority of Ireland
ENGINEERING SERVICES
Astatine
Bonner
Charles River Microbial Solutions
Chemishield
Dawsongroup | TCS Ireland
Endress & Hauser (Ireland) Ltd
Horan Automation
LotusWorks
Tandem Project Management ltd
Tekpak Automation Ltd
ENVIRONMENTAL CONSULTING
Charles River Microbial Solutions
CMS Chemstore Engineering Ltd
Quitmann O’Neill
ENVIRONMENTAL MONITORING
Bonner
Charles River Microbial Solutions
ENVIRONMENTAL SERVICES / EQUIPMENT
AQS Environmental Solutions
Charles River Microbial Solutions
CMS Chemstore Engineering Ltd
GI Chemicals
Mason Technology
O’Flynn Medical Ltd
Portakabin Ireland
Quitmann O’Neill
Source BioScience
EVAPORATORS
Cross Technical Solutions
Dawsongroup | TCS Ireland
Fisher Scientific Ireland Ltd
Flexachem Manufacturing Ltd
Labplan
Mason Technology
EXPLOSION PROOFING
CMS Chemstore Engineering Ltd
Henley Forklift Group Limited
EXPLOSION PROTECTION / PANELS
BS&B Safety Systems Ltd
CMS Chemstore Engineering Ltd
Henley Forklift Group Limited
EXTRUDERS
BS&B Safety Systems Ltd
FACILITY DESIGN
ATG Scientific Ltd
Dawsongroup | TCS Ireland
Pharmalex
Tekpak Automation Ltd
FACILITIES MANAGEMENT
AbsorboPak
AQS Environmental Solutions
Foltech Engineering Ltd
O’Flynn Medical Ltd
Quitmann O’Neill
FILLING EQUIPMENT
Horan Automation
Ortec Inc.
Quitmann O’Neill
Tekpak Automation Ltd
FILTERS
Fisher Scientific Ireland Ltd
Flexachem Manufacturing Ltd
Mason Technology
FILTRATION
Associated Chemicals Ltd
Fisher Scientific Ireland Ltd
Mason Technology
FIRE DETECTION / PREVENTION / PROTECTION
BS&B Safety Systems Ltd
FLAME ARRESTERS
BS&B Safety Systems Ltd
Flexachem Manufacturing Ltd
FLEXCO PLATES
Holfeld Graphics
FLOW CONTROL
Bonner
Endress & Hauser (Ireland) Ltd
Flexachem Manufacturing Ltd
Horan Automation
FLUID HANDLING
Horan Automation
Interpac
Labplan
ProSys Containment and Sampling Technology
Quitmann O’Neill
Fisher Scientific Ireland Ltd
Foltech Engineering Ltd
FURNACES
Fisher Scientific Ireland Ltd
Scientific Instruments Ireland
GAS DETECTION
Bonner
CMS Chemstore Engineering Ltd
Fisher Scientific Ireland Ltd
GAS SUPPLY
Corcoran Products Ltd
Scientific Instruments Ireland
GAUGES
BS&B Safety Systems Ltd
Fisher Scientific Ireland Ltd
GENERATORS
Dawsongroup | TCS Ireland
Scientific Instruments Ireland
GLASSWARE
Associated Chemicals Ltd
Fisher Scientific Ireland Ltd
Quitmann O’Neill
GRINDING
Fisher Scientific Ireland Ltd
HAZARDOUS WASTE DISPOSAL INGREDIENTS
Chemishield
Indaver Ireland
Interpac
Quitmann O’Neill
HEALTHCARE LOGISTICS
Kuehne + Nagel
HEALTH & SAFETY / FIRST AID
Charles River Microbial Solutions
Hazchem Training Ltd
Tandem Project Management ltd.
HEAT EXCHANGERS
BS&B Safety Systems Ltd
Cross Technical Solutions
Flexachem Manufacturing Ltd
Labplan
HEATERS
Ballinlough Pharma Solutions
CMS Chemstore Engineering Ltd
HOMOGENISERS
Fisher Scientific Ireland Ltd
Flexachem Manufacturing Ltd
HUMIDITY / HUMIDIFIERS
AbsorboPak
Ballinlough Pharma Solutions
Cross Technical Solutions
Dawsongroup | TCS Ireland
Interpac
Source BioScience
HYGIENIC DIAPHRAGM VALVES
Flexachem Manufacturing Ltd
INCUBATORS
Ballinlough Pharma Solutions
Charles River Microbial Solutions
Cross Technical Solutions
Dawsongroup | TCS Ireland
Fisher Scientific Ireland Ltd
ProSys Containment and Sampling Technology
INJECTION MOULDING
Horan Automation
Quitmann O’Neill
INSPECTION EQUIPMENT
Holfeld Graphics
Horan Automation
Mason Technology
O’Flynn Medical Ltd
Tekpak Automation Ltd
INSTRUMENTATION
ATG Scientific Ltd
BS&B Safety Systems Ltd
Charles River Microbial Solutions
Endress & Hauser (Ireland) Ltd
Horan Automation
Labplan
LotusWorks
Mason Technology
Scientific Instruments Ireland
IT
Charles River Microbial Solutions
Westbourne Global IT Services
INVESTMENT
IDA Ireland
INVESTMENT PROMOTION AGENCY
IDA Ireland
ISOLATORS
Foltech Engineering Ltd
ProSys Containment and Sampling Technology
LABELLING
CMS Chemstore Engineering Ltd
DHL Supply Chain
Horan Automation
Tekpak Automation Ltd
LAB IT
Westbourne Global IT ServicesS UPPLIERS
LABORATORY EQUIPMENT / SUPPLIERS
ATG Scientific Ltd
Charles River Microbial Solutions
Chemishield
CMS Chemstore Engineering Ltd
Fisher Scientific Ireland Ltd
Labplan
Mason Technology
Micro-Bio Ireland Ltd
O’Flynn Medical Ltd
Particular Sciences Ltd
Portakabin Ireland
ProSys Containment and Sampling Technology
Scientific Instruments Ireland
Solv-Echem Ireland Ltd
LABORATORY MOBILE BENCHES
ATG Scientific Ltd
LABORATORY SERVICES
Charles River Laboratories Ireland Ltd
CMS Chemstore Engineering Ltd
Eurofins Biopharma Product Testing
GI Chemicals
Mason Technology
Nelipak Healthcare Packaging
Reading Scientific Services Ltd
Westbourne Global IT Services
LIFT TABLES
Horan Automation
ATG Scientific Ltd
Horan Automation
Tekpak Automation Ltd
LOGISTICS
AbsorboPak
AQS Environmental Solutions
Ballinlough Pharma Solutions
Charles River Laboratories Ireland Ltd
Chemco Ireland Ltd
Dawsongroup | TCS Ireland
DHL Supply Chain
Kuehne + Nagel
Portakabin Ireland
Quitmann O’Neill
Solv-Echem Ireland Ltd
UPS Healthcare
MACHINE TOOLS
Horan Automation
Lister Machine Tools Ltd
MAINTENANCE
AQS Environmental Solutions
Bonner
Cross Technical Solutions
Endress & Hauser (Ireland) Ltd
Horan Automation
LotusWorks
Mason Technology
Particular Sciences Ltd
Source BioScience
MANUFACTURERS
Abbvie
Amneal
BS&B Safety Systems Ltd
Horan Automation
Hovione
IDA Ireland
Micro-Bio Ireland Ltd
Portakabin Ireland Ltd
Quitmann O’Neill
Source BioScience
Tekpak Automation Ltd
MATERIALS HANDLING / FORKLFTS / PALLET TRUCKS
AbsorboPak
Ballinlough Pharma Solutions
Henley Forklift Group Limited
Horan Automation
Interpac
Portakabin Ireland
Toyota Material Handling Ireland Ltd
Foltech Engineering Ltd
LotusWorks
Portakabin Ireland
Tandem Project Management ltd
Tekpak Automation Ltd
MECHANICAL & PROCESS ENGINEERING
Endress & Hauser (Ireland) Ltd
Foltech Engineering Ltd
Horan Automation
LotusWorks
Tandem Project Management ltd.
MEDICAL DEVICE MANUFACTURE
B. Braun Medical
Charles River Microbial Solutions
Horan Automation
LotusWorks
Mallinckrodt Pharmaceuticals
Ortec Inc.
Tekpak Automation Ltd
MEMBRANE FILTRATION SYSTEMS
Microfiltration: Fisher Scientific Ireland Ltd
Nanofiltration: Fisher Scientific Ireland Ltd
Ultrafiltration: Fisher Scientific Ireland Ltd
Reverse osmosis: Fisher Scientific Ireland Ltd
METERS
Fisher Scientific Ireland Ltd
MICRO QC
Charles River Microbial Solutions
Reading Scientific Services Ltd
MICROSCOPES
Fisher Scientific Ireland Ltd
Mason Technology
Particular Sciences Ltd
MICROWAVE TECHNOLOGY
Scientific Instruments Ireland
MILLING
Fisher Scientific Ireland Ltd
SK Pharmteco
MIXERS
Fisher Scientific Ireland Ltd
Flexachem Manufacturing Ltd
NOISE / ODOUR CONTROL
AbsorboPak
Associated Chemicals Ltd
ATG Scientific Ltd
Flexachem Manufacturing Ltd
NON-HAZARDOUS WASTE DISPOSAL/RECOVERY
Indaver Ireland
NON-HAZARDOUS WASTE MANAGEMENT
Indaver Ireland
NON-HAZARDOUS WASTE TREATMENT
Indaver Ireland
OEM MANUFACTURING
B. Braun Medical
Foltech Engineering Ltd
IDA Ireland
PACKAGING / BLENDING UNDER GMP
National Chemical Company
PACKAGING / DESIGN
Charles Tennant & Co Ltd
Complas Packaging Ltd
Holfeld Graphics
IDA Ireland
Interpac
Kuehne + Nagel
Limerick Packaging
Nelipak Healthcare Packaging
Quitmann O’Neill
Tekpak Automation Ltd
UPS Healthcare
PACKAGING / MACHINERY
AbsorboPak
Horan Automation
IDA Ireland
Interpac
NPP Group Ltd
Limerick Packaging
Nelipak Healthcare Packaging
Quitmann O’Neill
Smurfit Kappa Ireland
Tekpak Automation Ltd
PALLETS
Ballinlough Pharma Solutions
Interpac
Quitmann O’Neill
Tekpak Automation Ltd
PALLETISING / DEPALLETISING
Tekpak Automation Ltd
PARTICLE SIZING SURFACE AREA
Particular Sciences Ltd
Reading Scientific Services Ltd
PHARMACEUTICAL FABRICATION
Charles River Microbial Solutions
IDA Ireland
ProSys Containment and Sampling Technology
PHARMACEUTICAL MANUFACTURE
Abbvie
Amneal
LotusWorks
Mallinckrodt Pharmaceuticals
SK Pharmteco
PICK & PLACE MACHINES
Tekpak Automation Ltd
PIPES / CORES
Quitmann O’Neill
Smurfit Kappa Ireland
PLASTIC CONTAINERS
Interpac
Nelipak Healthcare Packaging
PrimePac Ltd
Quitmann O’Neill
PLASTIC CORES / TUBES
Quitmann O’Neill
Smurfit Kappa Ireland
PNEUMATICS
Flexachem Manufacturing Ltd
Horan Automation
POWDER HANDLING
AQS Environmental Solutions
Dawsongroup | TCS Ireland
Foltech Engineering Ltd
Interpac
Mason Technology
ProSys Containment and Sampling Technology
Quitmann O’Neill
PRESSURE MEASUREMENT / SWITCHES / VESSELS
BS&B Safety Systems Ltd
Flexachem Manufacturing Ltd
Horan Automation
PRESSURE RELIEF
BS&B Safety Systems Ltd
Flexachem Manufacturing Ltd
PRINT PACKAGING
Horan Automation
Limerick Packaging
Quitmann O’Neill
PROCESS CONTROL
Bonner
BS&B Safety Systems Ltd
Charles River Microbial Solutions
Dawsongroup | TCS Ireland
Horan Automation
Mason Technology
O’Flynn Medical Ltd
Scientific Instruments Ireland
PROCESS DESIGN
Bonner
BS&B Safety Systems Ltd
Pharmalex
Tandem Project Management ltd.
PROCESS & MECHANICAL ENGINEERING CONTRACTORS
Bonner
PROJECT MANAGEMENT
Astatine
Chemishield
Horan Automation
Pharmalex
Reading Scientific Services Ltd
Tandem Project Management Ltd
PROTECTIVE CLOTHING / APPARATUS
O’Flynn Medical Ltd
ProSys Containment and Sampling Technology
PUMPS
Fisher Scientific Ireland Ltd
Flexachem Manufacturing Ltd
Horan Automation
Quitmann O’Neill
QC RELEASE TESTING
Charles River Laboratories Ireland Ltd
QUALITY & COMPLIANCE
AbsorboPak
Charles River Microbial Solutions
Chemifloc Ltd
LotusWorks
Mason Technology
Pharmalex
ProSys Containment and Sampling Technology
Reading Scientific Services Ltd
SK Pharmteco
Westbourne Global IT Services
R&D
Almac Sciences Ltd
ATG Scientific Ltd
Charles River Microbial Solutions
Chemifloc Ltd
IDA Ireland
Pharmaceutical Manufacturing Technology Centre (PMTC)
ProSys Containment and Sampling Technology
Reading Scientific Services Ltd
SK Pharmteco
Synthesis and Solid State Pharmaceutical Centre
REACTORS
Flexachem Manufacturing Ltd
RECRUITMENT
Chemishield
ICDS Recruitment Consultants
Tandem Project Management ltd.
REFRIGERATION / FREEZING
Ballinlough Pharma Solutions
Cross Technical Solutions
CRS Pharma Solutions
Dawsongroup | TCS Ireland
Fisher Scientific Ireland Ltd
Mason Technology
Q1 Scientific
Source BioScience
RENEWABLE ENERGY
Astatine
Dawsongroup | TCS Ireland
Sustainable Energy Authority of Ireland
RESEARCH FUNDING
Pharmaceutical Manufacturing Technology Centre (PMTC)
Science Foundation Ireland (SFI)
RESPIRATORY PHARMACEUTICALS
Reading Scientific Services Ltd
Teva Pharmaceuticals Ireland
ROBOTICS
Charles River Microbial Solutions
KUKA Robotics Ireland Ltd
Labplan
Tekpak Automation Ltd
SANITARY TUBING
Flexachem Manufacturing Ltd
SCADA /DCS / MIS
Bonner
Horan Automation
ProSys Containment and Sampling Technology
Tekpak Automation Ltd
SCREENS
Horan Automation
SCRUBBERS
Dawsongroup | TCS Ireland
Flexachem Manufacturing Ltd
SEALS & GASKETS
Flexachem Manufacturing Lt
SIEVING
Fisher Scientific Ireland Ltd
Particular Sciences Ltd
SLEEVE / STRETCH WRAPPING
Horan Automation
SOFTWARE
Ballinlough Pharma Solutions
Charles River Microbial Solutions
Chemishield
CMS Chemstore Engineering Ltd
Mason Technology
Westbourne Global IT Services
SOLVENT RECOVERY / SERVICES
Carbon Group
STABILITY STORAGE
Almac Sciences Ltd
ATG Scientific Ltd
Ballinlough Pharma Solutions
Charles River Laboratories Ireland Ltd
Charles River Microbial Solutions
CMS Chemstore Engineering Ltd
Corcoran Products Ltd
Cross Technical Solutions
CRS Pharma Solutions
Dawsongroup | TCS Ireland
Eurofins Biopharma Product Testing
Kuehne + Nagel
Mason Technology
Q1 Scientific
Reading Scientific Services Ltd
SK Pharmteco
Source BioScience
STABILITY TESTS
Charles River Laboratories Ireland Ltd
Eurofins Biopharma Product Testing
Ortec Inc.
Particular Sciences Ltd
Reading Scientific Services Ltd
SK Pharmteco
STAINLESS STEEL / FITTINGS / PRODUCTS
CMS Chemstore Engineering Ltd
Flexachem Manufacturing Ltd
Foltech Engineering Ltd
Henley Forklift Group Limited
Interpac
ProSys Containment and Sampling Technology
Quitmann O’Neill
STEAM EQUIPMENT
BS&B Safety Systems Ltd
Flexachem Manufacturing Ltd
Foltech Engineering Ltd
STERILITY TESTING
Charles River Laboratories Ireland Ltd
Charles River Microbial Solutions
Eurofins Biopharma Product Testing
Reading Scientific Services Ltd
Source BioScience
STORAGE / BUNDING
Ballinlough Pharma Solutions
CMS Chemstore Engineering Ltd
Charles River Microbial Solutions
CRS Pharma Solutions
Dawsongroup | TCS Ireland
Interpac
Kuehne + Nagel
Quitmann O’Neill
STORAGE OF HAZARDOUS MATERIALS
CMS Chemstore Engineering Ltd
Corcoran Products Ltd
CRS Pharma Solutions
Dawsongroup | TCS Ireland
Hazchem Training Ltd
Indaver Ireland
Kuehne + Nagel
STRAPPING EQUIPMENT
AbsorboPak
Horan Automation
SUPPLY CHAIN MANAGEMENT
AbsorboPak
Alexion
Ballinlough Pharma Solutions
Chemco Ireland Ltd
DHL Supply Chain
GS1 Ireland
IDA Ireland
Kuehne + Nagel
KWE (Ireland) Ltd
Limerick Packaging
National Chemical Company
Portakabin Ireland
Quitmann O’Neill
Solv-Echem Ireland Ltd
SURFACE PLASOMON RESONANCE
ATG Scientific Ltd
TABLETING EQUIPMENT
ATG Scientific Ltd
Flexachem Manufacturing Ltd
Horan Automation
TANKS
Complas Packaging Ltd
Flexachem Manufacturing Ltd
Quitmann O’Neill
TEMPERATURE CONTROL
Ballinlough Pharma Solutions
Bonner
CMS Chemstore Engineering Ltd
Corcoran Products Ltd
Cross Technical Solutions
CRS Pharma Solutions
Dawsongroup | TCS Ireland
DHL Supply Chain
Fisher Scientific Ireland Ltd
Flexachem Manufacturing Ltd
KWE (Ireland) Ltd
Labplan
LotusWorks
Mason Technology
Q1 Scientific
Source BioScience
TESTING SERVICES
Almac Sciences Ltd
ATG Scientific Ltd
Bonner
Charles River Laboratories Ireland Ltd
Charles River Microbial Solutions
Chemifloc Ltd
CMS Chemstore Engineering Ltd
Eurofins Biopharma Product Testing
Reading Scientific Services Ltd
SK Pharmteco
THERMAL IMAGING /THERMOGRAPHY
Ballinlough Pharma Solutions
Reading Scientific Services Ltd
TOOLS
Charles River Microbial Solutions
TRAINING
Hazchem Training Ltd
Reading Scientific Services Ltd
TWi
TRANSPORT & LOGISTICS
AbsorboPak
AQS Environmental Solutions
Ballinlough Pharma Solutions
CRS Pharma Solutions
Dachser Ireland Ltd
Dawsongroup | TCS Ireland
DHL Supply Chain
Hazchem Training Ltd
Kuehne + Nagel
KWE (Ireland) Ltd
Quitmann O’Neill
Skycell AG
UPS Healthcare
TRAY HEAT SEALER
Nelipak Healthcare Packaging
TUBE SETS / DISPOSABLES
Quitmann O’Neill
VACCINES
Ballinlough Pharma Solutions
MSD Ireland
VACUUM SYSTEMS
BS&B Safety Systems Ltd
Fisher Scientific Ireland Ltd
Flexachem Manufacturing Ltd
Horan Automation
Particular Sciences Ltd
VALVES
Bonner
BS&B Safety Systems Ltd
Flexachem Manufacturing Ltd
Foltech Engineering Ltd
Horan Automation
VALIDATION
Almac Sciences Ltd
Bonner
Charles River Microbial Solutions
Chemishield
CRS Pharma Solutions
Dawsongroup | TCS Ireland
Horan Automation
LotusWorks
Mason Technology
Pharmalex
Reading Scientific Services Ltd
SK Pharmteco
Source BioScience
Tandem Project Management ltd
Tekpak Automation Ltd
Westbourne IT Global Services
VENTILATION
Cross Technical Solutions
VISION SYSTEMS
Bonner
Horan Automation
KUKA Robotics Ireland Ltd
Particular Sciences Ltd
Tekpak Automation Ltd
WAREHOUSE MANAGEMENT
CMS Chemstore Engineering Ltd
Complas Packaging Ltd
DHL Supply Chain
Horan Automation
Henley Forklift Group Limited
Kuehne + Nagel
Portakabin Ireland
UPS Healthcare
WASTE MANAGEMENT / BALERS / RECYCLING
AQS Environmental Solutions
Indaver Ireland
Quitmann O’Neill
WATER FOR INJECTION
National Chemical Company
WATER TREATMENT
BS&B Safety Systems Ltd
Charles River Microbial Solutions
Chemco Ireland Ltd
Chemifloc Ltd
Flexachem Manufacturing Ltd
GI Chemicals
InOpSys
Portakabin Ireland
WEIGHING
Bonner
Fisher Scientific Ireland Ltd
Horan Automation
Mason Technology
Tekpak Automation Ltd
WORKWEAR
O’Flynn Medical Ltd
WORKWEAR MANAGEMENT D
O’Flynn Medical Ltd

Address: ABBVIE Sligo – Manorhamilton Road.
Tel: (071) 913 6600
Address: 14 Riverwalk, Citywest Business Campus, D24 XN32.
Tel: (01) 428 7900
Business: Pharmaceutical.
Address: 6 Main Street, Howth, Dublin, D13P2C1, Ireland.
Tel: +353 (1) 6978733
Email: info@absorbopak.com
Web: www.absorbopak.ie
Business: With our range of desiccants, we help clients all over the world prevent moisture damage.
Contact: Sé O'Leary, Sales / Technical Support


Address: Grange Business Park, Skule Hill, Fedamore, Co. Limerick
Tel: (061) 574 404
Email: biopharma@actylis.com
Web: www.actylis.com
Business: Actylis is a global GMP manufacturer of Excipients, APIs and PIs, Process and Cleaning Solutions, Buffers, Cell Culture Ingredients, Amino Acids & Cell and Gene Therapy Ingredients.
Contact: biopharma@actylis.com
Address: College Business & Technology Park, Blanchardstown, Dublin 15, D15 R925.
Tel: (01) 254 6400
Email: julie.carney@alexion.com
Web: www.alexion.com
Business: Pharmaceutical.
Contact: Site Operations Coordinator: Julie Carney

Address: IDA Technology & Business Park, Garrycastle, Athlone, Ireland, N37 X061.
Tel: +353 (0)90 646 0200
Email: almacanalytical@almacgroup.com
Web: www.almacgroup.com
Business: Analytical services provider working in GMP environments across UK, Europe and US with significant experience in the analysis of small and large molecules. Our state-of-the-art labs support drug substance (API) and drug product (finished product) analytics across all phases of clinical development and into commercial release.
Contact: Business Development: Anna Cousens
Address: West Building, Carrigaline Industrial Park, Carrigaline, Co. Cork, Ireland, P43 HK76.
Tel: +353 (0)21 242 8800
Email: info@technicallywriteit.com
Web: www.technicallywriteit.com
Business: TWi is a team of content strategists and content creators supporting leading technology and life sciences companies to streamline their content processes.
Contact: Business Development Director: Emmet Kearney

Address: Cashel Road, Cashel, Co. Tipperary.
Tel: +353 (0) 62 27000
Email: careers.cashel@amneal.com
Web: www.amneal.com/cashel
Business: Pharmaceutical Manufacture.
Contact: VP Operations, Ireland: Niall Prendergast
Address: Block 20A, Beckett Way, Park West Business Park, Dublin 12, D12 P8R2.
Tel: (01) 270 7973
Email: info.ireland@amtivo.com
Web: www.certificationeurope.com
Business: ISO Management Standards Certification body for ISO 9001, 14001, 45001, 50001, 27001, 22301 and 20000-1.
Contact: Holly Fitzpatrick, Sales and Marketing Manager

Address: Unit 21, Grattan Business Park Clonshaugh Business and Technology Park Dublin D17 H526
Tel: +353 (1) 2479820
Email: info.ie@anton-paar.com
Business type: Laboratory instruments and process measuring systems
Address: Kiloran, Moyne, Thurles Co. Tipperary.
Tel: +353 (0)504 57800
Email: info@aqssolutions.ie
Web: www.aqssolutions.ie
Business: Provider of drainage, industrial cleaning, waste management and processing services.
Contact: Commercial Director: Stan O’Reilly

Address: Monksland Industrial Estate, Athlone, Co. Roscommon, N37 DN24, Ireland.
Tel: +353 (0) 90 644 5700
Email: info@arranchemical.ie
Web: www.arranchemical.ie
Business: Fine chemical company specialising in the manufacture of products for Pharma, Healthcare, Flavour/ Fragrance & other specialised chemicals & industrial applications.
Contact: Business Development Manager: Gareth Maguire.
Address: Riverstown 5 Complex, Tramore, Republic of Ireland.
Tel: +353 (0) 51 338435
Web: www.astoriom.com
Address: Oxford Centre for Innovation, New Road, Oxford, OX1 1BY, United Kingdom.
Tel: +44 (0) 1865 261423
Email: enquiries@atgscientific.co.uk
Web: www.atgscientific.co.uk
Business: Supply of Laboratory Products and Equipment Solutions.
Contact: Andrew Graham

Address: 2E Purcellsinch Industrial Estate, Dublin Road, Kilkenny, R95 D882.
Tel: +353 56 7771771
Email: enquiries@asgardcleanrooms.com
Web: www.asgardcleanrooms.com
Business: Cleanroom Design & Construction.
Contact: Group VP / Director Business Development & Marketing: Jimmy Blaney
Address: 16D Euro Business Park, Little Island, Co. Cork, Ireland.
Tel: +353 (0) 21 4351014
Email: info@acl.ie
Web: www.associatedchemical.wixsite.com
Business: Chemical Suppliers.
Contact: Managing Director: Sylvester Cotter
Address: Suite 301, Guinness Enterprise Centre, Dublin 8, D08 T1WY.
Tel: (01) 525 3006
Web: www.astatine.ie
Main Products & Services: Solar PV and High Temperature Heat Pump Installation.
Address: Unit 23, Sandyford Office Park, Blackthorn Avenue, Sandyford Industrial Estate, Foxrock, Dublin D18 X9X7.
Tel: (01) 295 6977
Fax: (01) 295 8338
Email: graeme.locke@azelis.com
Business: Chemical Distributor.
Contact: Managing Director: Graeme Locke
Address: Block S, Grants View, Greenogue Business Park, Rathcoole, Co. Dublin.
Tel: +353 1 4039518
Email: pharmaireland@barentz.com
Web: www.barentz.com
Business: Distributor of excipients, amino acids and APIs to the Pharmaceutical, Nutraceutical & Biopharma industries in Ireland.
Contact: Sales Manager: Storme Delaney
Address: 3 Watergold, Douglas, Cork. International
Offices: Singapore, London & Dubai.
Tel: (021) 428 9600
Email: info@Berkley-group.com
Web: www.berkeley-group.com
INSTRUMENTATION, CALIBRATION AND AUTOMATION SOLUTIONS
Address: 35 Western Parkway
Business Centre, Ballymount Drive, Ballymount, Dublin 12.
Tel: (01) 450 5050
Fax: (01) 450 5183
Email: contact@bonner.ie
Web: www.bonner.ie

Address: Clondalkin, Dublin, Annacarton Bridge, Co. Cork & Ballinlough, Co. Roscommon.
Tel: Dublin (01) 460 0322 Cork (021) 488 2077 Roscommon (094) 964 0045
Email: info@brltd.ie
Web: www.brltd.ie
Business: Suppliers of Thermo King Pharma Transport Refrigeration. Sales - Service - Parts
Contact: Joe Malone
Business: Instrumentation, Calibration and Automation Solutions including calibration, maintenance, temperature mapping and automation systems including SCADA, DCS and MES. Instrumentation product sales from international manufacturers.
Contact: Managing Director: Patrick M Bonner
Service Manager: Roddy Jefferson
Automation Solutions: Darren Roche

Address: 3 Naas Road
Industrial Park, Dublin 12.
Tel: (01) 709 1800
Email: robert.bannon@bbraun.com
Web: www.bbraun.ie
Business: OEM manufacturing.
Contact: Sales Consultant: Susan Cleary

Address: Unit 405, Greenogue Business Park, Rathcoole, Dublin 24.
Tel: +353 (0) 1 401 3500
Web: www.brenntag.ie
Business: Chemical Suppliers & Distributors.
Contact: Evelyn O'Connor: evelyn.o'connor@brenntag.ie Cole Carroll: colman.carroll@brenntag.ie

Address: Little Island Industrial Estate, County Cork.
Tel: (021) 4520500
Email: mkirk@carapartners.ie
Business: Pharmaceuticals.
Contact: Site Head: Michael Kirk

Address: Charles Tennant & Co. (Eire) Ltd. Unit J, Aerodrome Business Park, Rathcoole, Dublin, D24 FP89.
Tel: (01) 451 4099
Email: info@ctennant.ie
Web: www.charlestennant.com
Business: Your Trusted Chemical Distributor Across the Aerospace, Building, Food Ingredients, Packaging, Agriculture, Chemical, Surface Coatings & Pharmaceutical Sectors
Contact: sales@ctennant.ie
Address: Raheen Business Park, Limerick, Ireland V94 N4V2.
Tel: (061) 484 700
Fax: (061) 304 774
Email: sales@bsb.ie
Web: www.bsbsystems.com
Business: Design, manufacture and supply of Pressure Relief Devices such as rupture disks, safety heads, custom engineered products, explosion vents, flame arresters, breather valves, safety valves and more.
Contact: Regional Sales Manager: Patrick Murphy
Address: Ringaskiddy, Co. Cork, P43 R772.
Tel: +353 (0)21 437 8988
Mobile: +353 (0) 86 2612 485
Fax: +353 (0)21 437 8950
Email: cork@carbon.ie dublin@carbon.ie
Web: www.carbon.ie
Business: Pharma/biopharma chemicals.
Contact: Area Sales Manager: Carol Deegan
Address: Unit 21, Primeside Park, Kilshane Way, Ballycoolin, Dublin15.
Tel: (01) 861 2326
Email: info@centralpumpsupplies.com
Web: www.centralpumpsupplies.com
Business: Appliances, Electrical, and Electronics Manufacturing.
Contact: Sales/Digital Marketing Assistant: Beatriz Baldiviezo

Address: Unit 2, Stadium Business Park, Ballycoolin, Cappagh, Dublin 11, D11 X205.
Tel: +353 (0)1 829 3600
Email: info@chemco.ie
Web: www.chemco.ie

Address: Carrentrila, Ballina, Co. Mayo, F26 D786, Ireland.

Address: New Quay, Clonmel, Co. Tipperary, E91 YV66, Ireland.
Tel: +353 52 6125455
Email: info@camida.com
Web: www.camida.com
Contact: Company Secretary: Deirdre McGrath
Ingredients Sales Manager: Donal O’Neill
Mob: 087 7060774
Email: donal.oneill@camida.com
Email: askcharlesriver@crl.com
Web: criver.com/biologics
Business: Biologics Testing Solutions.

Address: 49, Greenogue Business Park, Jordanstown Ave, Jordanstown, Rathcoole, Co. Dublin, D24 NF21, Ireland.
Email: askcharlesriver@crl.com
Web: criver.com/microbial
Business: Micro QC.



Address: Smithstown Industrial Estate, Shannon, Co. Clare, V14 VY67.
UK Address: GI Chemical Solutions UK Ltd., 110-114 Duke Street, Liverpool, L1 5AG, United Kingdom
Tel: +353 (0)61 708 699
Email: info@csg-corporate.ie
Web: www.csg-corporate.ie
Business: Chemifloc: Water Treatment, Chemilabs: Technical Services GI Chemicals: Industrial Chemicals
Contact: David Bourke, Chief Revenue Officer
Address: Leeson House, Forty-one, Leeson Street Lower, Dublin 2
Tel: +353 (0)51 576 025
Email: sales@chemishield.com
Web: www.chemishield.com
Business: Software & Life Sciences Consultancy.
Contact: Commerical Director: Kevin Walsh
Address: Clondrinagh Industrial Estate, Ennis Road, Limerick, V94 XT27.
Tel: (061) 327 792
Email: sales@chemstore.ie
Web: www.chemstore.ie
Business: Solutions provider for the safe storage and management of Hazardous Materials.
Address: Southern Link Business Park, Naas, Co. Kildare.
Tel: (045) 874 088/9
Email: sales@complas.ie
Web: www.complas.ie

Address: 17 Parkgate Street, D08 NRP2.
Tel: (01) 633 0400
Fax: (01) 679 3521
Email: info@corcoran-group.com
Web: www.corcoran-group.com
Business: Distributors of raw materials for the food, pharmaceutical, polymer & chemical industry.
Contact: Sales

Address: Unit 12, Northern Cross Business Park, Finglas, D11 DC67.
Tel: (01) 864 4422
Email: info@corcoran-group.com
Web: www.corcoran-group.com
Business: Suppliers of packaging to the food, pharmaceutical and chemical industry.
Contact: Derek Lennon
Address: 1-5 Eastgate Drive, Eastgate Retail Park, Little Island, Co. Cork, T45 A433.
Tel: (021) 435 4690
Email: info@cpitechnology.com
Web: www.cpitechnology.com
Business: Providers of Process Equipment. Contact: Managing Director: Adrian Giltinan

Address: Groun d Floor, One Haddington Buildings, Haddington Road, Dublin 4, D04 X4C9.
Tel: (01) 614 6000
Email: hello@talentevolutiongroup.com
Web: www.cpl.com/ie
Business: Specialised talent and recruitment solutions company.

Address: 9/10 Broomhill Road, Tallaght, Dublin 24.
Tel: (01) 405 6777
Fax: (01) 413 6932
Email: sales@ctsolutions.org
Web: www.crosstechnicalsolutions.ie
Business: Refrigeration.
Contact: Technical Director: Jonathan McGrath General Manager: Jason Keating
Address: Ireland HQ: Summerhill Enterprise Centre, Summerhill, Co. Meath, A83 XE40. UK HQ: Unit 3, Thornton Park, North Road, Ellesmere Port, CH65 1AB.
Tel: Ireland: +353 (0) 46 943 5000 UK: 0800 085 2298
Email: info@crspharmasolutions.ie
Web: www.crspharmasolutions.ie
Business: Climate controlled storage specialists. Internal coldroom and freezer installations with temperatures ranging from -65°C to +60°C. Close control temperature and humidity stores, stability chambers, incubators, Atex cold stores, dual redundant cooling systems with integral back-up generators, blast freezers, freezers for fast freezing of phase change materials (Eutetic plates and gel packs).
Contact: Technical Director: Patrick Tyrrell
Address: Blackchurch Business Park, Rathcoole, Co. Dublin, D24 C796. Tel: +353 1 401 3333
Email: dachser.dublin@dachser.com
Web: www.dachser.ie
Business: Founded in 1930, DACHSER is a global market leader in logistic services with a revenue of €7.1 billion. With our comprehensive European road transport network of system and charter service, as well as a homogeneous structure of branches, subsidiaries and partner companies, we will support you in fulfilling your logistics requirements reliably, cost-effectively and on time. We transport your groupage, your full or partial loads and manage your procurement and distribution, both Europe-wide and national, to the highest level.

Address: Momentum Logistics Park, Unit JB, Beech Ave, Newhall, Naas, Co. Kildare, W91 A090
Tel: (045) 448 810
Email: info@ie.dawsongrouptcs.com
Web: www.dawsongrouptcs.com
Business: Temperature Controlled Solutions. Clean rooms, Stability chambers -70c to +60c, ATEX Modular Solutions, Humidity control, Test Chambers, Blast Freezers (gel packs / Eutectic plates), Hybrid Generators.
Contact: Head of Sales: Christian Visser

Address: Unit 1 Quantum Distribution Park, Kilshane Cross, Dublin, D11 KV1W, Ireland.
& Unit B1 Horizon Logistics Park, Harristown, Co. Dublin, K67 N5C3, Ireland.
Tel: 086 0665865
Email: john.halpin@dhl.com
Web: http://www.dhl.com/globalen/home/our-divisions/ supply-chain/sectors-overview/ life-sciences-and-healthcare.html
Business: Supply Chain & Logistics.
Contact: Business Development Manager, Life Sciences: John Halpin

Address: 10 Eastgate Ave, Eastgate, Little Island, Cork, T45 PC63.
Tel: 00353 212 330 900
Email: alyson.murphy@dornangroup.com
Web: www.dornan.ie
Business: Dornan Group is a wellestablished mechanical, electrical and HVAC engineering and construction company, with major project experience across a wide range of sectors in Europe.
Contact: Business Development Coordinator: Alyson Murphy

Address: Eirgen Pharma, Unit 507, IDA Waterford Business Park, Waterford, X91 H72T
Tel: 051 591 944
Email: bd@eirgen.com
Web: www.lilly.co/ie
Business: Eirgen Pharma is a leading Contract Development & Manufacturing Organization headquartered in Waterford, Ireland. We specialize in the development, co-development, and manufacturing of highpotency oral solid dose (OSD) products and advanced softgel formulations, serving global pharma partners with flexible, innovative solutions for human and companion animal health.

Address: Dunderrow, Kinsale, Co. Cork
Tel: 085 878 3827
Email: ezara.ahern@network.lilly.com
Web: www.lilly.co/ie
Business: Pharmaceutical.
Contact: External Communications: Ezara Ahern

Address: Ellab Ireland, Whitegate Cross, Virginia Co Cavan, A82 TFF9
Tel: 0818 250 250
Email: ireland@ellab.com
Web: www.ellab.com
Business: Complete solutions provider to the Biotech and Pharma sectors offering validation & calibration services, monitoring solutions & product sales.
Contact: Director of Business Development: Kevin Davis

Address: Exchequer House, Embassy Office Park, Kill, Co. Kildare.
Tel: (045) 989 200
Email: info.ie@endress.com
Web: www.ie.endress.com
Business: Leading supplier of products, services and solutions for industrial process measurement and automation industry.
Contact: Sales Manager: Brian O’Connell

Address: Clogherane, Dungarvan, Co. Waterford, X35 T628, Ireland.
Tel: +353 (0) 58 48300
Email: EurofinsBPT-IE@eurofins.ie
Web: www.eurofins.ie/biopharma-services

Address: Blanchardstown Corporate Park, Ballycoolen, Dublin 15.
Tel: (01) 885 5854
Email: fsie.sales@thermofisher.com
Web: www.ie.fishersci.com
Business: Laboratory supplies, Chemicals, Consumables, Reagents, Equipment & Instruments.
Contact: Portfolio Manager: Gerry Fitzmaurice

Address: Donnybrook Commercial Centre, Douglas, Cork, T12 X68Y.
Tel: (021) 461 7200
Email: sales@flexachem.com
Web: www.flexachem.com
Business: Mechanical Process Equipment Supplier - Pumps, Seals, Valves, Process Equipment, Biotech and Pharma Equipment and Technical Support.
Contact: Commercial Manager: Michael Bradley
Address: The Store House, Charleston Maltings, Midleton, Co. Cork.
Tel: (021) 463 9592
Email: info@foltech.ie
Web: www.foltech.ie
Business: Mechanical Engineering.
Contact: Director: John Foley
Address: Friars Industrial Estate, Bradford Road, Idle, Bradford, BD10 8SX, UK.
Tel: (0044) 1274 617021
Email: post@graham-hart.com
Web: www.graham-hart.com
Address: IDA Business & Technology Park, Mountrath Road, Portlaoise, Co. Laois.
Tel: +353 (0) 57 8671400
Email: Portlaoise.info@greenfield.com
Web: www.greenfield.com
Business: Excipients, Buffer Solutions and CIP Solutions producer.
Contact: Business Development Manager: Johnny Geraghty
Address: Second Floor, The Merrion Centre, Nutley Lane, Donnybrook, Dublin 4.
Tel: (01) 208 0660
Email: healthcare@gs1ie.org
Web: www.gs1ie.org/healthcare
Business: Global Supply Chain Standards Body.
Address: Clogherane, Knockbrack, Dungarvan, Co. Waterford
Tel: 086 4081878
Email: gillian.m.power@haleon.com
Web: www.haleon.com
Business: Healthcare manufacturer.
Contact: Business Manager/Comms & Engagement Lead: Gillian Power

Address: G10, Maynooth Business Campus, Maynooth, Co. Kildare.
Tel: (01) 629 1800
Email: info@hazchem.ie
Web: www.hazchem.ie
Business: Training and Consultancy Services.
Contact: Director: Michelle Cleere
Address: Henley Industrial Park, Killeen Road, Dublin 10.
Tel: (01) 620 9200
Web: www.henley.ie/pharma
Business: Forklift and warehousing equipment, sales, service, hire, parts, driver training, thorough examinations.
&
HORAN AUTOMATION AND ROBOTICS LIMITED
Address: Drangan, Thurles, Co Tipperary, E41 DA36.
Tel: (052) 915 2208
Email: sales@horan.ie
Web: www.horan.ie
Business: Automation, Integration, Service, Packing, Palletising, Filling machinery, Robotics, Consulting, Design, Prototyping, Medical device assembly, Sub Component assembly.
Contact: Sales & Marketing Director: Gary Monks
Address: Loughbeg, Ringaskiddy, Co. Cork, Ireland. P43 DY23
Tel: +353 21 451 2856
Email: hello@hovione.com
Web: www.hovione.com
Contact: General Manager: Angela Moriarty

Address: Block 2, Newtown & Business Enterprise Park, Newtownmountkennedy, Co. Wicklow.
Tel: +353 89 700 3476
Email: niall.otoole@hubbcat.com
Business: Telecommunications.
Contact: COO: Niall O'Toole

Address: 1 Newry Road Rathfriland Newry BT34 5AL
Tel: +3531 866 0136
Email: sales@hub-packaging.com
Web: https://www.hub-packaging.com
Business: Industrial & Transit Packaging Solutions
Contact: Management: Ben Crozier, Andrew Ward, Julian Coldrick, Russell Brennan
Address: Connacht House, 24 Upper Fitzwilliam Street, Dublin 2.
Tel: +353 1 632 1200
Email: info@icds.ie
Web: www.icds.ie
Business: Specialist Recruitment Consultants to the Pharmaceutical, Life Science, Chemical, Medical Device, Food and Technology sectors.

Address: Three Park Place, Hatch Street Upper, Dublin 2.
Tel: (01) 603 4000
Email: idaireland@ida.ie
Web: www.idaireland.com
Business: Investment Promotion & Development Agency.

Address: The Highline, 1st Floor, Bakers Point, Pottery Road, Dun Laoghaire, Co. Dublin, A96 KW29.
Tel: (01) 697 2900
Email: info@indaver.ie
Web: www.indaver.ie
Business: Hazardous & nonhazardous waste disposal and recovery ensuring full compliance.

Address: Maanstraat 9B, 2800 Mechelen, Belgium.
Tel: +32495653821
Email: thomas.windels@inopsys.eu
Web: www.inopsys.eu
Business: Providing circular side stream solutions. Purifying water and recovering materials with mobile & modular installations.
Contact: Sales Manager: Thomas Windels

Address: 67E Heather Road, Sandyford Industrial Estate, Sandyford, Dublin D18 NV90.
Tel: (01) 294 0600
Email: taylor@interpac.ie
Web: www.interpac.ie
Business: Interpac provides a complete range of UN-approved packaging solutions from steel drums, fibreboard boxes, IBCs and FIBCs, to labels, placards, and packaging materials, sustainable options available & Our services support businesses with compliance and safety, offering expert packing & repacking, dangerous goods consultancy & audits, and tailored training courses covering ADR, IATA, IMDG and lithium battery regulations
Contact: Director: Taylor Sutton
Address: Bedford Square, Bedford Street, Belfast, BT2 7ES.
Tel: (0044) 800 181 4422
Web: www.investni.com
Business: Economic development agency.

Address: Ballyconnell Industrial Estate, Falcarragh, Co. Donegal
Tel: (074) 916 2982
Email: sales@kelsius.com
Web: www.kelsius.com
Business: Wireless Temperature Monitoring
Contact: Sales Manager: Ciaran Gallagher

Address: Unit D2 Horizon Logistics Park, Harristown, Swords, Co. Dublin, K67 A5W6.
Tel: (01) 823 9777
Email: Seamus.keane@kuehne-nagel.com
Web: ie.kuehne-nagel.com
Business: Global Freight Forwarding & Supply Chain Management.
Contact: Seamus Keane, VPPharma&Healthcare - Europe Mob: +353 85 8666646

Address: Unit 16, Brewery Business Park, Ardee Rd, Cambrickville, Dundalk, Co. Louth, A91 ATX4, Ireland.
Tel: (042) 939 5034
Email: sales.ie@kuka.com
Web: www.kuka.com
Business: Industrial robotics & automation.
Contact: Managing Director KUKA IE: Brian Cooney Your trusted Global Logistics Par tner

GDP Wholesale Licence Holder
Dublin Head Office & Temperature Controlled
Warehouse Facility
Address: Horizon Logistics Park, Harristown, Swords, Co. Dublin.
Tel: (01) 823 9600
Email: kwedub@kwe.com
Web: www.kwe.com
Cork Regional Office & Warehouse Facility
Address: South Ring West Business Park, Tramore Road, Cork.
Tel: (021) 497 5722
Email: kweork@kwe.com
Address: Allenwood Enterprise Park, Allenwood, Naas, Co Kildare.
Tel: (045) 870 560
Email: info@labplan.ie
Web: www.labplan.ie
Business: Lab supplier of analytical instrumentation, services, technical support.
Address: John F. Kennedy Drive, Naas Road, Dublin, D12FP79.
Tel: (01) 460 7600
Email: customerservice@lennox.ie
Web: www.lennox.ie
Contact: Padraig Callan, Head of Business Development
Address: Eastlink Business Park, Ballysimon Rd., Limerick.
Tel: (061) 400 035
Email: info@lmkpkg.ie
Web: www.limerickpackaging.ie
Business: Packaging manufacturers, Distributors, Designers and Auditors.
Contact: Sales Director: John Blessing
Address: PO Box 838, Bluebell Industrial Estate, Dublin 12.
Tel: (01) 450 8866
Email: sales@listermachinetools.com
Web: www.listermachinetools.com
Business: Supply of machine tools and ancilliary equipment, service and support.
Contact: Sales: Ryan McGrath

Address: Building 3, Finisklin Business Park, Sligo, F91 KAP2.
Tel: (071) 916 9783
Email: contactus@lotusworks.com
Web: www.lotusworks.com
Business: Engineering & Technical Solutions Provider.
Address: Unit 10, 4075 Kingswood Road, Citywest Business Campus, D24
Tel: (01) 676 3465
Email: mail@maclachlan.ie
Web: www.maclachlan.ie
Business: Intellectual Property Attorneys.
Address: Red Oak North, South County Business Park, Leopardstown, Dublin 18.
Tel: +353 (0)1 299 8700
Email: info@msd.ie
Web: www.msd.ie
Address: 228 South Circular Road, Dublin, D08 DX8P
Tel: (01) 453 4422
Email: info@masontec.ie
Web: www.masontechnology.ie
Business: Provider of scientific and laboratory equipment, and technical services including installation and calibration.
Contact: Strategic Marketing Manager: Eilish Sutton

Address: Industrial Estate, Fermoy, Co. Cork
Tel: (025) 31388
Fax: (025) 32458
Email: info@micro-bio.ie
Web: www.micro-bio.ie
Business: Chemical Manufacturer.
Contact: Sales Director: Tom Tobin
Business: MSD Ireland is one of the country’s leading healthcare companies, having first established here over 50 years ago. We have a dynamic and diverse team of over 2,800 employees currently across six sites in Ballydine, Co Tipperary, Brinny, Co Cork, Dunboyne, Co Meath, Carlow and Dublin, and, in addition, operate substantial Human Health and Animal Health businesses. At MSD Ireland, we work at the forefront of science and technology to advance manufacturing excellence and R&D across our Irish sites and global company network. With a long-standing footprint in Ireland, our Irish sites manufacture approximately half of MSD’s top twenty products, helping save and enhance lives in over sixty countries around the world.
Contact: Mairead McCaul
MSD Ireland (Human Health) LTD

Address: NCC House, 42 Lower Leeson St., Dublin 2, D02 FX39.
Tel: (01) 613 1400
Fax: (01) 634 0132
Email: sales@ncc.ie
Web: www.ncc.ie
Business: Supply Chain PartnerLife Sciences Industry.
Contact: Sales Director: Christy Smith

Address: Galway: Unit 6D, Mervue Business Park, Mervue, Galway, H91 C9D0, Ireland.
Tel: (091) 757 152
Address: Offaly: Kilbeggan Road, Clara, Co. Offaly, R35 F583, Ireland.
Tel: (057) 933 1888
Address: Derry:
1 Acorn Road, Campsie Industrial Estate, Derry, BT47 3GQ, Northern Ireland.
Tel: 0044 28 7181 4000
Email: info@nelipak.com
Web: www.nelipak.com
Business: Innovative packaging solutions and complementary products and services for the healthcare market serving the medical and pharmaceutical sectors.
Contact: Director of Global Marketing: Sean Egan
Address: Unit 2, Vantage Business Park, Coldwinters, Dublin 11.
Tel: (01) 880 9299
Email: sales@npp.ie bmcmahon@npp.ie
Web: www.npp.ie
Business: Flexible packaging suppliers & distributors.
Address: Unit 3, M.F.T. Business Park, Doughcloyne Ind. Estate, Sarsfield Road, Co. Cork.
Tel: (021) 431 8555
Email: info@oconchemicals.com
Web: www.oconchemicals.ie

Address: Newcastle West Business Park, Station Road, Newcastle West, Limerick, V42 E765.
Tel: +353 87 7752914
Email: jgeraghty@ortecinc.com
Web: www.ortecinc.com
Business: Contract Manufacturing.
Contact: Business Development Manager: John Geraghty

(PMTC)
Address: Bernal Institute, University of Limerick, Co. Limerick, V94 T9PX.
Tel: 061202293
Email: pmtc@ul.ie
Web: pmtc.ie
Business: Research and development.
Contact: Centre Director: Damon Warnock

Address: Unit D1, North Dublin Corporate Park, Swords, Co. Dublin
Tel: +353 1 846 4742
Web: www.pharmalex.com/ireland
Business: An award winning EU and US Quality, Technical & Compliance consultancy to the life science industry.
Address: Fox & Geese House, Naas Road, Dublin 22.
Tel: (01) 450 8759
Email: sales@thepackagingcentre.ie
Web: www.thepackagingcentre.ie
Contact: Managing Director: Ivan Powell

Address: 2 Birch House, Rosemount Business Park, Ballycoolin Road, Finglas, Dublin D11 T327.
Tel: (01) 820 5395
Fax: (01) 822 8813
Email: info@particular.ie
Web: www.particular.ie
Business: Supply, service and support of laboratory equipment.
Contact: Business Development Manager: Donnchadha Quilty
O’FLYNN
Address: Macroom Environmental Park, Bowl Road, Macroom Co. Cork, P12 YD92.
Tel: (029) 21 799
Email: info@oflynnmedical.com
Web: www.oflynnmedical.com
Business: Distributor of OSL “Automated Protective Clothing Distributor”.
Contact: Conor O’Flynn
Address: 4D, Western Business Park, Shannon, Co. Clare, V14 RW92
Tel: 061704740
Email: bronagh@pbcbiomed.ie
Business: Medical device consultancy
Contact: Ms Bronagh O'Doherty
Contact: Country Manager: Jane Lyons
Address: Roseville Business Park, Turvey Avenue, Donabate, Co. Dublin.
Tel: (01) 960 9482
Web: www.portakabin.ie
Business: Portakabin is the pioneer of modular construction in Europe, delivering interim and permanent bespoke buildings, of any size and to fulfil almost any application, site and design. Portakabin has provided award-winning off-site built environments of outstanding quality, with unrivalled on time and on budget performance, for over 50 years.
Address: Building C, Athlone Business & Technology Park, Garrycastle, Athlone, Co. Westmeath.
Tel: (0906) 460 300
Web: www.ppd.com
Business: Contract Research Organisation.

Address: IDA Business Park, Carrigtohill, Co. Cork, Ireland, T45 AP82.
Tel: +353 21 4853900
Email: info@prosysgroup.com
Web: www.prosysgroup.com
Contact: Head of Global Sales: Michael Hennessy
Address: 87 Westside Business Park, Co. Waterford.
Tel: (051) 355977
Email: info.cqw@cambrex.com
Web: www.q1scientific.com
Business: Stability Storage (ICH/GMP).
Contact: Stephen Delaney, MD
Address: St. Brendan’s Road, Portumna, Co. Galway, H53 HX51.
Tel: (090) 9741148
Email: sales@quitmannoneill.com
Web: www.quitmannoneill.com
Business: Packaging Disributor and Stockist.
Contact: David O’Neill

Address: Watery Lane, Swords, Co. Dublin.
Tel: (01) 813 9000
Email: skpharmteco@sk.com
Web: www.skpharmteco.com
Business: Contract Development Manufacturing Organisation.
Contact: Director Business Development & Marketing: Brian Fairley

Address: Bahnhofplatz 6300, Zug, Switzerland.
Tel: 00 4144 533 2300
Email: info@skycell.ch
Web: skycell.ch
Business: Pharmaceutical Logistics & Supply Chain Solutions.
Contact: Sales Department: sales@skycell.ch
Address: The Reading Science Centre, Whiteknights Campus, Pepper Lane, Reading, Berkshire, RG6 6LA, UK.
Tel: +44 (0) 118 918 4076
Web: www.rssl.com
Business: Analytical Testing Laboratory.
Address: Sobo Works, 2 Windmill Lane, Sir John Rogerson's Quay, Dublin Docklands, Dublin 2, D02 K156.
Tel: (01) 661 4420
Web: www.red-eng.com
Business: Engineering & Project Management Company.
Contact: Senior Director: Killian O'Neill
Address: Ballymount Road, Walkinstown, Dublin 12.
Tel: (01) 409 0000
Email: info@smurfitkappa.ie
Web: www.smurfitkappa.ie
Business: Packaging.

Address: Zone C, Clonmore, Mullingar Business Park, Mullingar, Co. Westmeath.
Tel: (044) 933 5133
Email: info@soltec.ie
Web: www.soltec.ie
Business: Solvent Recovery and Hazardous Waste Management
Contact: Managing Director: Michael Corcoran
Address: Great Island Industrial Park, Ballincollig, Co. Cork.
Tel: (021) 487 7066
Email: info@solvechem.com
Web: www.solvechem.com
Business: Chemical distribution, chemicals, solvents, water treatment, warehousing.

Address: Bernal Institute, University of Limerick, Limerick, Ireland.
Web: www.sspc.ie
Business: Pharma Research Centre.
Contact: Chief Operating Officer, Dr. Sarah Hayes
Address: 3 Park Place, Hatch Street Upper, Dublin 2.
Tel: (01) 808 2100
Email: info@seai.ie
Web: www.seai.ie
Business: Energy advice & information.


Address: 11 Nessan House, Mahon Industrial Estate, Blackrock, Cork, T12 XN4V.
Tel: (021) 2038130
Email: info@tandempm.ie
Web: www.tandempm.ie
Business: Tandem is an expert project management and engineering design consultancy successfully delivering to life science clients in Ireland, the UK and Europe. Tandem’s results orientated services include project management, project controls, multi-disciplined engineering design, construction management, energy & carbon reduction and CQV.
Tandem also provide client representative teams, operational readiness consultancy and talent solutions.
Contact: Customer Relations Manager: Linda Nugent 0876897995 linda.nugent@tandempm.ie

Address: Whitemill Industrial Estate, Wexford, Y35 A620, Ireland.
Tel: +353 (0)53 9163033
Email: info@tekpak.ie
Web: www.tekpakautomation.com
Business: End-of-Line Automated Packaging Line Design, Manufacture, and Integration for High-Speed Retail Packaged Products and Pharmaceuticals. Tekpak Automation is Focused on the Life Sciences Industry range of pharmaceutical, biomedical, medical device, biotechnology, and cosmeceutical products. With Clients across Ireland, the UK, Europe, North America, Asia and the Middle East, Tekpak Automation can produce customised packaging machinery for product manufactures at any size or scale. Automated Applications include Robotic Pick and Place Systems, Vertical and Horizontal Cartoning, Brite Stock Loading/Unloading from Trays, Stacking, Nesting and Collating, Feeding Product to a Flow Wrapper Infeed, Labelling (TE, Print and Apply, or Thermal Transfer), Batch Data Printing with Camera Verification, Carton and Case Erectors/Closers, Case Packing and Palletising, Machine Linking Conveyors with Signal Exchange, Integration of Serialisation and Aggregation Systems, 21 CFR Part 11 Ready Machines, GAMP 5 Project Management, GMP Design Features and Full Validation Documentation. Tekpak also offers a complete range of after-sales support and service packages to reduce machine downtime and disruptions to maximise the return on investment. Contact us today to assist with your next end-of-line automated
Contact: Managing Director: John Kehoe

Address: Currabinny, Carrigaline, Co Cork, P43 AY66.
Tel: (021) 437 8800
Email: contactcork@thermofisher.com
Web: thermofisher.com/patheon
Business: Active Pharmaceutical Ingredient Contract Development and Manufacturing.

Address: 536 Grants Crescent, Greenogue Business Park, Rathcoole, Co. Dublin.
Tel: (01) 401 9800
Email: irelandsalesoffice@univarsolutions.com
Web: www.univarsolutions.com/pharma
Business: Chemical Distribution.
Contact: Key Account Manager: Carla Byrne

Address: Unit 3, Mygan Park, Jamestown Road, Finglas, Dublin 11.
Tel: +44 7979 704423
Email: ukiehealthcare@ups.com
Web: www.ups.com/ie/en/healthcare/Home.page
Business: Transport & Logsitics.
Contact: Business Development Manager: Cathal Murtagh
Address: Unit 3, Innovation Park, Carrigaline Ind. Estate, Kilnagleary, Co. Cork.
Tel: (021) 483 2644
Email: mwren@wrentech.ie
Web: www.wrentech.ie

Address: WuXi Biologics, Dundalk Science and Technology Park, Haynestown, Dundalk, Co. Louth, A91X56F
Tel: (021) 483 2644
Email: Dundalk_Ireland@ wuxibiologics.com
Web: www.wuxibiologics.com
Business: CRDMO (Contract Research Development Manufacturing Organisation)

Address: The Rubicon Centre, MTU, Bishopstown, Co. Cork.
Tel: (021) 431 4310
Email: dennis.blanck@westbourneit.com
Web: www.westbourneit.com
Business: General IT and Laboratory IT Service Provider to the pharmaceutical industry in Ireland.
Contact: CEO: John O'Sullivan

ACADEMY OF CLINICAL
SCIENCE AND LABORATORY MEDICINE
Tel: (01) 905 9730
E-mail: mail@acslm.ie
Web: www.acslm.ie
AN BORD PLEANALA
Tel: (01) 858 8100
E-mail: bord@pleanala.ie Web: www.pleanala.ie
BIOPHARMACHEM
IRELAND
Tel: (01) 605 1500
E-mail: info@ibec.ie
Web: www.biopharmachemireland.ie
CHAMBERS IRELAND
Tel: (01) 400 4300
E-mail: info@chambers.ie
Web: www.chambers.ie
COMPANIES
REGISTRATION OFFICE
Tel: (01) 804 5200
LoCall: 0818 452 000
E-mail: cro.info@enterprise.gov.ie Web: www.cro.ie
DEPARTMENT OF THE ENVIRONMENT, CLIMATE ACTION AND COMMUNICATIONS
Tel: (01) 678 2000
E-mail: customer.service@decc.gov.ie Web: gov.ie/decc
DEPARTMENT OF ENTERPRISE, TRADE AND EMPLOYMENT
Tel: (01) 631 2121
LoCall: 0818 302 121
E-mail: info@enterprise.gov.ie Web: enterprise.gov.ie
DEPARTMENT OF HOUSING, LOCAL GOVERNMENT AND HERITAGE
Tel: (01) 888 2000
E-mail: qcsofficer@housing.gov.ie
Web: www.housing.gov.ie
ELECTRICITY SUPPLY
BOARD
Tel: (01) 676 5831
E-mail: esbnetworks@esb.ie
Web: www.esb.ie
ENTERPRISE
IRELAND
Tel: (01) 727 2000
Web: www.enterprise-ireland.com
ENVIRONMENTAL HEALTH ASSOCIATION OF IRELAND
Tel: (01) 276 1211
Web: www.ehai.ie
ENVIRONMENTAL PROTECTION AGENCY
Tel: (01) 268 0100
Web: www.epa.ie
HEALTH AND SAFETY AUTHORITY
Tel: (01) 614 7000
LoCall: 0818 289 389
E-mail: contactus@hsa.ie Web: www.hsa.ie
HEALTH PRODUCTS REGULATORY AUTHORITY
Tel: (01) 676 4971
E-mail: info@hpra.ie Web: www.hpra.ie
HEALTH RESEARCH BOARD
Tel: (01) 234 5000
E-mail: hrb@hrb.ie Web: www.hrb.ie
HIGHER EDUCATION AUTHORITY
Tel: (01) 231 7100
E-mail: info@hea.ie Web: www.hea.ie
IDA - INDUSTRIAL DEVELOPMENT AGENCY
Tel: (01) 603 4000
E-mail: idaireland@ida.ie Web: www.idaireland.com
INSTITUTE OF CHEMISTRY OF IRELAND
E-mail: secretary@instituteofchemistry.org Web: chemistryireland.org
INTELLECTUAL PROPERTY OFFICE OF IRELAND
Tel: (056) 772 0111
E-mail: ipinfo@ipoi.gov.ie Web: https://www.ipoi.gov.ie/en/
INTERNATIONAL SOCIETY FOR PHARMACEUTICAL ENGINERING (ISPE)
Tel: +1 (813) 960 2105
E-mail: ask@ispe.org
Web: www.ispe.org
INVEST NORTHERN IRELAND
Tel: 0800 181 4422
Web: www.investni.com
IRISH BUSINESS & EMPLOYERS CONFEDERATION (IBEC)
Tel: (01) 605 1500
E-mail: info@ibec.ie Web: www.ibec.ie
IRISH CLEANROOM SOCIETY
Web: www.cleanrooms-ireland.ie
IRISH COSMETICS, DETERGENT & ALLIED PRODUCTS ASSOCIATION
Tel: (01) 605 1500
E-mail: kevin.maher@ibec.ie Web: www.icda.ie
IRISH EXPORTERS ASSOCIATION
Tel: (01) 661 2182
E-mail: contact@irishexporters.ie Web: www.irishexporters.ie
IRISH MEDTECH ASSOCIATION
Tel: (01) 605 1500
E-mail: info@irishmedtechassoc.ie
Web: www.irishmedtechassoc.ie
IRISH NATIONAL ACCREDITATION BOARD
Tel: (01) 614 7182
E-mail: info@inab.ie
Web: www.inab.ie
IRISH PHARMACY UNION
Tel: (01) 493 6401
E-mail: info@ipu.ie
Web: www.ipu.ie
IRISH VENTURE CAPITAL ASSOCIATION
Tel: (01) 276 4647
Web: www.ivca.ie
Email: ciara@ivca.ie
MANDATE TRADE UNION
Tel: (01) 874 6321
E-mail: mandate@mandate.ie
Web: www.mandate.ie
NATIONAL INSTITUTE FOR TRANSPORT & LOGISTICS (NITL)
Tel: (01) 402 3898
Web: www.nitl.ie
PARENTERAL DRUG ASSOCIATION (PDA)
Tel: +1 (301) 656 5900
Web: www.pda.org
PHARMACEUTICAL SOCIETY OF IRELAND
Tel: (01) 218 4000
E-mail: info@psi.ie
Web: www.thepsi.ie
REPAK
Tel: (01) 467 0190
E-mail: info@repak.ie
Web: www.repak.ie
SCIENCE FOUNDATION IRELAND
Tel: (01) 607 3200
E-mail: info@sfi.ie
Web: www.sfi.ie
UCD SCHOOL OF CHEMICAL & BIOPROCESS ENGINEERING
Tel: (01) 716 1825
E-mail: SCBE.enquiries@ucd.ie
Web: www.ucd.ie/chembioeng

A leaf from Ginkgo Biloba , believed to be the oldest living species of tree. The seeds and leaves of Ginkgo Biloba have been used in Chinese traditional medicine for over 800 years
Cara Partners manufactures a quantified extract of Ginkgo Biloba for the EU and Chinese market Ginkgo Biloba extract products are used to treat a variety of conditions associated with blood circulation disorders


Keenova Therapeutics is a leading global developer and manufacturer of branded therapeutics that strives to help patients with rare or unaddressed conditions live happier and healthier lives.
Globally headquartered in Dublin, Ireland, we deliver medicines grounded in science and guided by our commitment to responsible practices.