Aseptic Non-Touch Technique (ANTT) Standard Operating Procedure
Ratified by: Infection Prevention and Control Group
Date ratified: 17/07/24
Job Title of author: Specialist Infection Prevention Nurse, Infection Prevention and Control Team
Reviewed by Committee or Expert Group Infection Prevention and Control Group
Related procedural documents

IPPOL17-Aseptic Non-touch Technique Policy
CPOL25 - Policy for the administration of IV drugs in the community
IPPOL21 – Standard Principles for Infection Prevention
IPPOL03 - Hand Hygiene Policy and Procedure
IPPOL09 - Decontamination of Medical Equipment Policy and Procedure
MMPOL30 - Medicines Management Policy
IPPOL18 - Management and Safety of Sharps Policy
Review date: 17/07/27

It is the responsibility of users to ensure that you are using the most up to date document template – i.e. obtained via the intranet.

In developing/reviewing this procedure Provide Community has had regard to the principles of the NHS Constitution.
Version Control Sheet
Version Date
21/6/2024
V1

Author
Lucy Ellis
Status
Comment
New SOP New SOP devised to replace ANTT policy, with reference to Asepsis within overarching IPC policy IPPOL21.


1. Introduction
Aseptic Non-Touch Technique (ANTT) is a standard for safe and effective aseptic practice using a theoretical framework which has been developed using research-based evidence. It that can be applied to all aseptic procedures such as intravenous therapy, wound care and urinary catheterisation. A core component of ANTT is the protection of key parts. These are those parts that if contaminated by infectious material increase the risk of infection. In IV therapy, key parts are usually those which come into direct contact with the liquid infusion e.g. needles, syringe tips, exposed central line lumens, etc
The procedures outlined in this standard operating procedure section refer and s been devised from the Royal Marsden online manual that Provide CIC adheres to.
Step by step clinical ANTT guidelines for cannulation, venepuncture, IV administration of medication, wound care, urinary catheterisation and central and peripheral line management are found in Appendix to this SOP.
2. Purpose
The purpose of this SOP is to establish ANTT as the safe and effective technique for all aseptic procedures.
3. Definitions
Aseptic Free from pathogenic organisms (in sufficient numbers to cause infection)
Clean Free from visible marks and stains
Sterile Free from (all) microorganisms
Sterile Technique A historical term used interchangeably for aseptic technique. (NB: ANTT does not recognize or use this term because due to the ever presence of microorganisms in air, it is virtually impossible to achieve in even the most specialist health care environment). By using a non-touch technique this promotes best practice. The commonly used term, ‘sterile technique' is therefore inaccurate, as practitioners are not actually achieving their stated objective and therefore have failed prior to procedure. When termed sterile technique these can only be referred to as those undertaken in controlled air flow environments.
Aseptic Technique For a venous-access-device related infection to occur it must be contaminated by a sufficient number of virulent, pathogenic organisms. Therefore, a technique that prevents such a level of pathogenic organisms from entering the patient’s blood stream is a safe technique. Such a technique is most accurately termed an ‘aseptic technique', as the word asepsis means, ‘freedom from infection or infectious (pathogenic) material'. An aseptic technique is the ‘effort to keep a patient as free from micro-organisms as possible’. Therefore, unlike sterile techniques, aseptic techniques are those described when a sterile technique cannot be achieved as the environment of airflow cannot be controlled. These techniques are those carried out in typical ward and home settings with the healthcare worker competent in.
Aseptic NonTouch Technique (ANTT)
This term is both accurate and achievable in normal clinical or non-clinical settings such as on hospital wards or a patient’s home. Pathogenic organisms cannot always be removed by effective hand washing. Additionally, hand washing is not always effective. Therefore, a non-touch technique (i.e. being able to identify the ‘key-parts’ and not touching them either directly or indirectly) is perhaps the single most important component of achieving asepsis.

‘Clean’ Technique
Historically interpretation of this term has created doubt over the aim of the technique. In particular, a clean technique does not have an aim of asepsis and has been associated with the notion that asepsis isn’t achievable in certain settings such as the community (Haslett 2007). ANTT is based on accurate and achievable terminology and practice. ANTT does not recognize or use the term clean technique to describe or define aseptic technique. The aim of practice is always to achieve asepsis. This is achieved by using the ANTT framework for managing a micro aseptic field either through surgical or standard ANTT.
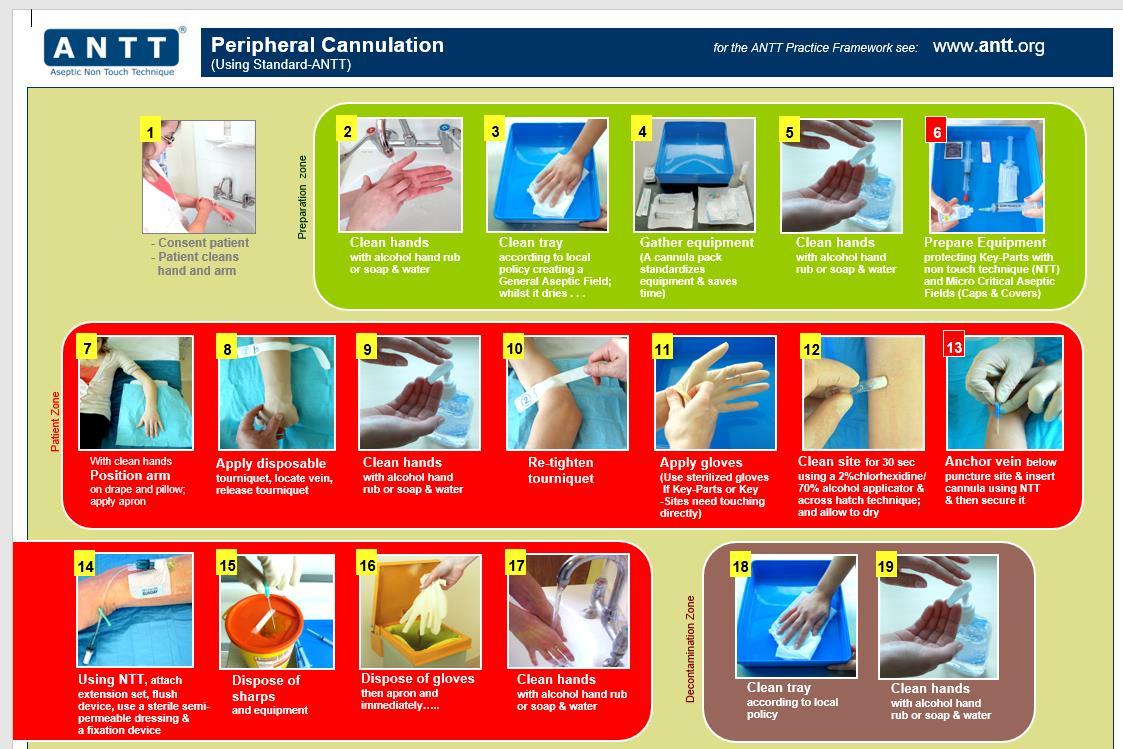
Standard ANTT Used for technically uncomplicated ANTT invasive procedures that involve minimal Key-Parts and small Key-Sites.
Some examples are:
• Simple wound dressings
• Reconstituting and administering certain IV medication
• Cannulation
• Venepuncture
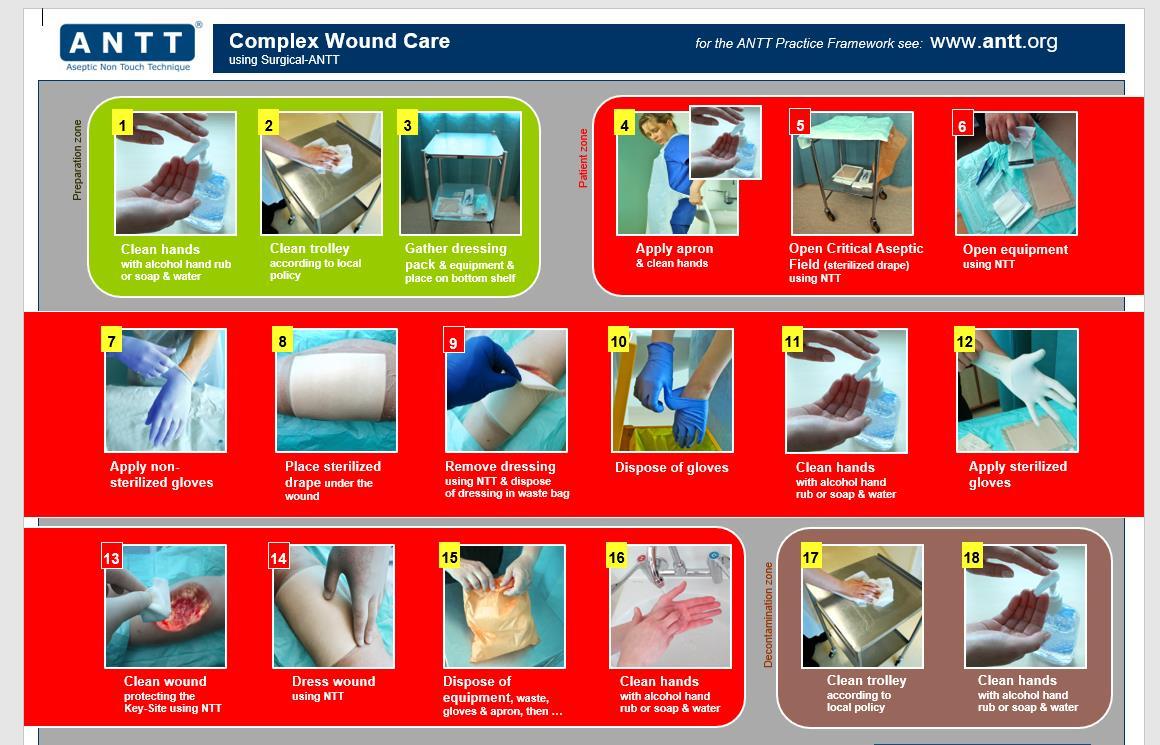
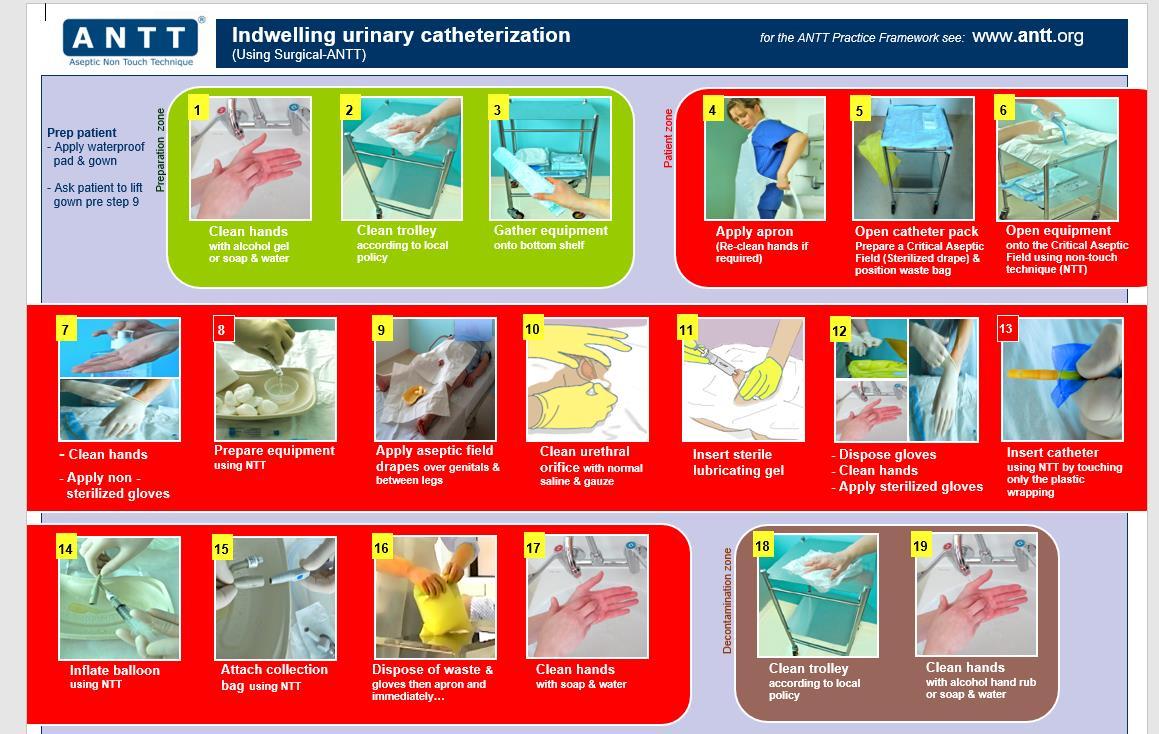
Surgical ANTT Used for technically complicated ANTT invasive Procedures that involve large or numerous Key-Parts or Key-Sites and would be considered procedure longer in duration >20mins.
Some examples are:
• Surgical wound dressings
• Any procedure involving incisions through skin
• Placement of central venous line
• Urinary catheterisation
Healthcare workers should always risk assess correctly between Standard and Surgical-ANTT, using the definitions. In practice, the main difference between Standard and Surgical-ANTT is the type and management of aseptic field(s) depending on the number of Key-Parts and Key-Sites that require protection.
Key-Part Active Key-Parts are the critical parts of the procedure/equipment that come into contact with Key-Sites, such as IV fluids connected to a cannula (puncture site). If contaminated during a procedure, Key-Parts provide a route for the transmission of pathogens onto or into the patient and present a significant infection risk.
Key-Site Open wounds and insertion and puncture sites for invasive medical devices.
Key-Part/Site Protection
The protection of Key-Parts and/or Key-Sites from pathogenic microorganisms. During clinical procedures this is achieved by a range of methods including non-touch technique, aseptic field management, standard infection prevention

precautions such as hand washing, and appropriate glove usage etc. as defined in ANTT. In between clinical procedures, wounds and medical devices may have Sustained Key-Part Protection from medical equipment or supplies. E.g. a wound care dressing, a passive IV hub cap protector.
4. Duties
Group Chief Executive Officer
• To ensure that infection prevention and control is a core part of clinical governance and patient safety programme
• Promote compliance with infection prevention policies in order to prevent Healthcare Associated Infections (HCAI)
• Awareness of legal responsibilities to identify, assess and control the risk of infection
Chief Executive Officer Health and Chief Nurse /Director of Infection Prevention and Control (DIPC)
• Oversee infection prevention policies and their implementation
• Reports directly to the Chief Executive and Board members
Infection Prevention and Control Team
• Review and update ANTT guidelines/SOP in line with national guidance.
• Monitor compliance as part of healthcare associated infection surveillance and incidents reported and relating to infection from medical devices.
• Provide advice regarding of ANTT when required to support staff.
• Include ANTT in education programme as part of Induction training for all new starters
Infection Prevention and control Link Practitioners & Associate Practitioners
• Audit standards of compliance with ANTT practice as part of High Impact interventions audits on digital app Tendable in clinical areas/departments.
Clinical Practice Facilitators
• Provide ANTT training to all new clinical staff as required and will ensure that their training, policies and guidelines are ANTT compliant
• Audit ANTT compliance through ANTT competency assessment for each clinical practice of IV administration, wound care, Urinary catheterisation, venepuncture, cannulation, venepuncture.

Assistant Directors
• Establish a positive culture across all services and promote compliance with ANTT as part of infection control practice within their teams
• Support managers by providing resources for implementation of ANTT in all clinical areas
• Support managers and infection prevention link practitioners in monitoring levels of ANTT compliance
Line Managers
• Identify staff requiring ANTT training
• Ensure that all staff able to undertake invasive techniques have undertaken training and assessment of competence in ANTT procedures
• Promote good practice and challenge poor compliance
All Clinical/healthcare Staff
• All staff must ensure they are trained and competent in ANTT procedures as required within their clinical areas and role.
• Apply ANTT principles to all procedures requiring aseptic technique
• Promote good practice and challenge poor compliance
• Must be familiar with and adhere to the relevant infection prevention and control policies to reduce the risk of cross infection of patients including ANTT
5. Consultation and Communication
This SOP has been distributed to members of the Infection prevention and control group for comment and approval as part of the organisation ratification process of procedural documents. This SOP has been developed from consulting the Department of Health, Health and Social Care Act 2008 code of practice on the prevention and control of infections.
6. Monitoring
• Managers will ensure that all staff who perform invasive techniques are trained and competent in Aseptic Non-Touch Technique (ANTT)
• New staff joining Provide, who have received ANTT training from a previous employer, and deemed competent, must provide documentary evidence of ANTT competence to their line manager prior to undertaking any invasive procedure within their role.
• Compliance of ANTT competence will be reviewed at local level. (Appendix 1)
7. General Steps for ANTT compliance
Step 1 ANTT risk assessment use of Standard or Surgical ANTT
Step 2 Decontaminate Hands
Step 3
Step 4

Clean trolley/plastic blue tray with disinfection/detergent wipe -work top down on trolley and inside first of tray to the outside. Using Clinell universal wipe use a S motion.
Gather equipment- if procedure is performed at the bedside ensure no cleaning or bedmaking is ongoing or been undertaken for 30 mins. Ensure windows shut and fans are not in operation.
Ensure patient is in suitable position for access to required procedure.
Step 5 Decontaminate hands
Step 6 Put on disposable plastic apron
Step 7 Open and prepare equipment – if required open sterile dressing pack or drape keep all equipment in their packaging or place on the open sterile pack using a non-touch technique as part of protection of key parts.
Step 8 Decontaminate hands
Step 9 Apply non-sterile or sterile gloves as required as part of risk assessment
Step 10 Perform ANTT procedure as either standard or surgical ANTT for risk assessment
Step 11 Remove gloves and apron
Step 12 Dispose of waste
Step 13 Decontaminate hands
Step 14 Clean trolley/tray environment surface
Step 15 Decontaminate hands
8. References
Dougherty L & Lister S (2008). The Royal Marsden Hospital Manual of Clinical Nursing Procedures. 10th Edition Chichester Wiley Blackwell
Epic 3: H P Loveday, J A Wilson, R J Pratt, M Golsorski, A Tingle, A Bak, J Browne, J Prieto, M Wilcox (2014) National Evidence-based Guidelines for Preventing Healthcare Associated Infections in NHS Hospitals in England: Journal of Hospital Infection: 8651, S1-S70
Maki, D.G., Goldman, D.A., Rhame, F.S. (1973) Infection Control in IV therapy. Annals of Internal Medicine 79(6) p867-887
National Institute for Health and Care Excellence (2012) Infection Control: Prevention of healthcare-associated infection in primary and community care (Updated 2017)
Rowley, S and Clare S, (2009) Improving standards of aseptic practice through an ANTT trust-wide implementation process: a matter of prioritisation and care Journal of Infection Prevention, vol. 10, 1_suppl: pp. S18-S23.

Clare, S. and Rowley, S. (2017) Implementing the Aseptic Non-Touch Technique clinical practice framework for aseptic technique: a pragmatic evaluation using a mixed methods approach in two London hospitals. Journal of Infection Prevention Vol. 19(10), pp. 3-4.
Royal Marsden Manual online Home - Royal Marsden Manual (rmmonline.co.uk)
9. Appendix 1: Competency Assessment for ANTT

Standard-ANTT® -Afterbasicprecautionsandappropriatepersonalprotectiveequipment are applied such as hand cleaning and glove use, all the Key-Parts are protected individually, by non-touch technique and individual Micro Critical Aseptic Fields
Surname: Forename:
Job Title: Ward / Department:
An Observational Assessment or a Simulation of Practice
- Only assessors with evidence of ANTT® competence can assess staff - healthcare worker (HCW)
- The assessor must include the theory and practice questions before or during the procedure
- This tool allows for assessment of three clinical procedures – if required
Competency Assessment (mark all components : ✓ X or n/a)
Date: Initial: Date: Initial: Date: Initial: Procedure Type (abbreviations)
Venepuncture – V; Cannulation – C; Blood cultures – BC; Simple wound Intravenous drug admin./ flush – IV; Other Procedures & abbreviations:
Urinary catheterisation – UC care – SW; Complex Wound Care – CW Other – O
Type: Type: Type: ANTT® principles & practice terms
Pre-Procedure
State the three main ways that equipment can be contaminated during aseptic technique
State a short definition of the terms: a) Sterile b) Aseptic c) Clean
State the practice aim of ANTT®
State the type of invasive procedures ANTT® is suitable for
State the fundamental practice concept that ANTT® is based upon
Name the two types of ANTT®
Explain the type of ANTT you are going to use and why you selected it
State some practice variables you’ve considered when determining the type of ANTT
Then state the ANTT Risk Assessment question that selects the type of ANTT
Preparation

Procedure
Inter-Procedure
Ask the practitioner to identify all the procedure Key-Parts of the procedure
State the definition of a Key-Part
State the definition of a Key-Site
State the Key-Part / Key-Site Rule
State the three types of aseptic field used in ANTT. Point them out in the procedure
Did the HCW clean their hands prior to equipment preparation?
If a cleanable procedure tray is used was it disinfected effectively?
Was all equipment gathered before cleaning hands and initiating equipment assembly?
Was the personal protective equipment (PPE) appropriate for the procedure?
Did the HCW clean their hands at the start of the procedure?
Was the choice of glove type appropriate for this Standard-ANTT® procedure?
Were gloves applied at the appropriate stage for the procedure?
When assembled was the equipment placed into a main General Aseptic Field (e.g. plastic or cardboard tray as per local policy)?
Was only necessary equipment in the tray and kept organised throughout?
Did the HCW avoid touching Key-Parts (i.e. non-touch technique)?
Were all Key-Parts protected by Micro Critical Aseptic Fields when not in use (e.g. aseptic caps, covers or inside of packaging)?
Were Key-Parts were not touched by any objects or surfaces
Did the HCW remove or avoid any obvious environmental risks – such as bed making or dusting taking place near the procedure area?
Did the HCW clean their hands and apply appropriate PPE before starting the procedure?
Were inactive Key-Parts disinfected before use (e.g. IV injection hub –scrubbed for 15 sec with an alcoholic /CHG wipe & allowed to dry)?
Were all Key-Parts individually protected at all times when not in use during the procedure by Micro Critical Aseptic Fields (Caps, covers, inside of packaging)?
Was the asepsis of Key-Parts promoted by organised management of the General Aseptic Field throughout the procedure?
Did the HCW avoid touching all Key-Parts with their gloved hands?
Did any Key-Part touch any objects or surfaces such as the procedure tray or non-Key-Parts.
Did the patients’ Key-Site(s) touch any non-Key-Parts or gloved hands?
Decontamination

Assessors
Did the HCW safely dispose of all sharps, equipment and waste according to local policies?
Did the HCW clean their hands IMMEDIATELY after glove removal?
Did the HCW decontaminate and disinfect the General Aseptic Field (e.g. plastic tray), and allow it to dry before storage?