THE
EMERGING ROLE OF CIRCULATING TUMOR DNA (CTDNA) AND OVARIAN CANCER CARE

June Y. Hou, MD. MBA
Associate Professor, Gynecologic Oncology
Columbia University Irvings Medical Center / NY-Presbyterian Hospital



June Y. Hou, MD. MBA
Associate Professor, Gynecologic Oncology
Columbia University Irvings Medical Center / NY-Presbyterian Hospital

•I was the PI and lead author in a clinical trial sponsored by Natera
•I am a voting member of the Developmental Therapeutics committee at NRG

•What is it: ctDNA
•How is it useful in oncology care
•Examples of ctDNA assays in oncology
Tumor agnostic: Multi-cancer early detection assay
Tumor informed: Molecular residual disease assay
•Hopes, limitations and takeaway
•Q&A



Selecting the right treatment at the right time
Improve clinical outcome
Minimize use of ineffective treatment

Selecting the right treatment at the right time
Improve clinical outcome
Minimize use of ineffective treatment
Ideal biomarker: minimally invasive; real-time info; sensitive
Tumor mass shedding into bloodstream

Blood sample
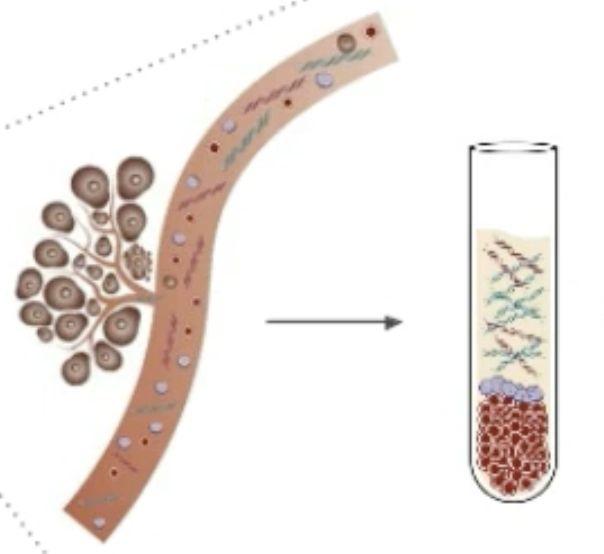
ctDNA extraction for analysis

Dying tumors constantly shed DNAinto the bloodstream.
Free floating tumor DNAfragments = circulating tumor DNA(ctDNA)
Liquid biopsies: Blood samples from which ctDNAis extracted and analyzed for molecular abnormalities
Results can be used to guide cancer care
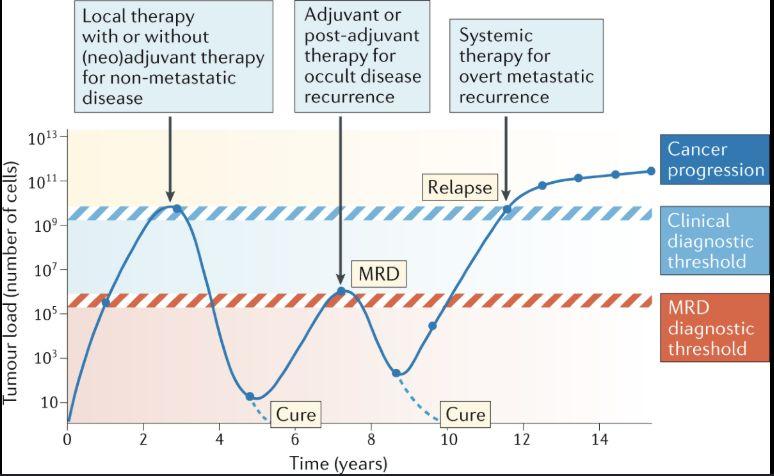
Pantel K. et al. Nat Rev Clin Oncol 2025


• ctDNA assay detects cancer after treatment that cannot be detected using imaging modalities
• Increased adoption in solid cancer treatments to more accurately monitor tumor burden (better than imaging)
• Considerations: not all tumor types shed ctDNA at the same level
• Different assays have different balance of sensitivity and specificity

Early cancer detection
•Multi-cancer early detection test
Treatment decision
•Molecular sequencing for targeted therapy; Intensifying or de-escalating treatments
Treatment monitoring Detecting relapse
•Molecular residual disease assay




Sequencing of tumor tissue to identify a unique signature Development of customized assay Serial tracking of ctDNA in blood

Identify a genomic and epigenomic signature common in specific cancers Serial tracking for presence of ctDNA

Tumor-informed: Looking for specific mutation, paired with tumor
Tumor-agnostic Detecting common mutations in blood

Characteristics
Tumor Informed
Molecular Residual Disease (MRD)
• Need tumor biopsy
• Use NGS to create a ‘mutational map’of the individual patient / tumor
• Longer turnaround: 3-4 weeks initially => 7-10 days serially
• Downside: does not account for new or acquired mutations
Tumor Agnostic
Multi-cancer Early DetectionTests (MCED)
• No biopsy needed
• Use NGS to identify a ‘common footprint’of cancer(s) of interest
• Fast results. (7-10 days)
• Downside: Lower sensitivity
Purpose
• Disease surveillance; treatment response tracking
• Population cancer screening

• When added to SOC cancer screening by USPSTFA/B: MCED test detected 7X cancers (cervical, CRC, prostate, breast, lung) compared to guidelines alone
• Approx 50% of cancers detected (Pancreatic, liver, ovary) were early stage
• 92% cancer localization
accuracy
• Prospective multi-centered NorthAmerica trial
• Multicancer Early Detection (MCED) test added to standard of care cancer screening in low-risk adults 50yo or older
• 2021-2025: 35,878 adults enrolled. (Data from first 25,578 participant with 12 mo follow up)
USPSTF cancer screening test characteristics:

99%sensitivity
99% specificity
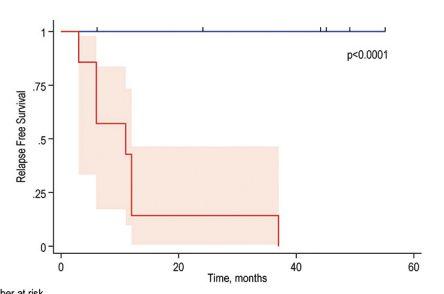
• ctDNAdetection preceded radiological imaging finding by 10 months

• ctDNA, not CA125 abnormality, predicts relapse with almost 100% sensitivity and specificity.
• ctDNApost-surgery is associated with reduced recurrence-free survival
• ctDNAis prognostic of disease recurrence and informs treatment decisions across multiple tumor types: Colon, Breast, Lung and Bladder



• Lack of inter-assay standardization

• Lack of standardization for usage
• Lack of large-scale clinical trial validation for each decision point in ovarian cancer
• ctDNA can offer a more personalized approach to monitor disease compared to imaging or other standard methods
• A simple blood test can now detect cancer activity earlier than scans or CA-125
• ctDNA is promising – but not ready for routine usage for everyone yet




•Limitations of CA-125 for treatment response monitoring:
•CA-125 progression do NOTcorrelate with radiologic progression across large clinical trials ( concordance rate < 50% in PARPinhibitor trials)
•Difficulty tracking immunotherapy response
• Radiology imaging lacks sensitivity and specificity to distinguish pseudo-progression from true progression
•10% rate of pseudo-progression
•ctDNAclearance is associated with super-responders to IO