
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 02 | Feb 2025 www.irjet.net p-ISSN: 2395-0072


International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 02 | Feb 2025 www.irjet.net p-ISSN: 2395-0072
Uday A.Satpute1 , Manik P.Satwadhar2 , Shifa Sayyad3 , Shubham Mhaske4
1,2,3Student, Pravara Rural College of Pharmacy, Pravaranagar, Maharashtra, India,413736 4Assistant Professor, Department of Quality Assurance, Pravara Rural College of Pharmacy, Pravaranagar, Maharashtra, India,413736 ***
Abstract - Pharmaceutical development has been completely transformed by Quality by Design (QbD), which integrates quality into the product from the very beginning rather than depending solely on end-product testing. The main ideas of Quality-Based Design (QbD), such as design space, Critical Process Parameters (CPPs), and Critical Quality Attributes (CQAs), are discussed in this study along with the advantages and disadvantages of using QbD to pharmaceutical production. The analysis also looks at how QbD lowers variability, increases overall product quality, and improves regulatory compliance. Widespread adoption is hampered by early costs, regulatory obstacles, and the complexity of data administration, despite its many benefits. This study emphasizes the significance of innovation, risk management, and continuous improvement within the QbD framework as pharmaceutical businesses move more and more toward QbD.
Key Words: Quality by Design (QbD), Pharmaceutical Development, Critical Quality Attributes (CQAs), Critical Process Parameters (CPPs), Design Space, Risk Management, Process Analytical Technology (PAT), Regulatory Compliance, Pharmaceutical Quality System, ICH Guidelines, Quality Risk Management
Pharmaceutical quality control has historically depended on a number of end-product testing techniques to make sure drugsfulfilspecifiedrequirementsforsafety,identification,potency,andpurity.Insteadofanticipatingproblemsearlyon inthedevelopmentprocessandproactivelyaddressingthem,thismethodfrequentlyconcentratesonreactive measures, such as reviewing final goods. However, a move toward more comprehensive quality assurance systems has become necessaryduetothecomplexityofcontemporarydrugresearch,growingregulatoryscrutiny,andtheneedfor increased efficiency.Inordertomakesurethatqualityisingrainedintheprocessfromthebeginningratherthanbeingtestedatthe conclusion, Quality by Design, or QbD, was presented as a proactive framework during the product design and developmentphases.
In the current pharmaceutical landscape, where there is an increasing desire for safer, more effective medications, the adoptionofQbDisparticularlycrucial.QbDlowersvariability,promotesinnovation,andimprovesoutcomepredictability by placing a high priority on a methodical understanding of the product and its production process. It promotes the incorporationofrisk-basedandscientificmethodologiesintodrugdevelopment,whicheventuallyresultsinmoreeffective medicinal products and efficient regulatory procedures. With the market shifting toward complicated biologics and personalized medicine, QbD provides a strong foundation for handling the inherent uncertainties of these cutting-edge treatments. This paper aims to provide an overview of Quality by Design, clarify its advantages and disadvantages, and investigatepotentialfutureapplicationsforthepharmaceuticalsector.Thisreviewseekstogiveinsightsforresearchers, practitioners, and regulatory agencies looking to improve pharmaceutical quality and innovation by looking at how QbD canbeadoptedsuccessfullyandidentifyingpotentialroadblocks.
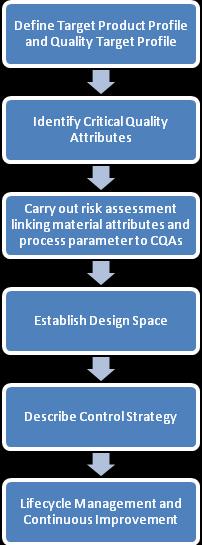
The Quality by Design (QbD) methodology is a methodical approach to pharmaceutical development that prioritizes the designofqualityintoproductsattheoutset,asopposedtodependingsolelyonend-producttesting.Toguaranteeconstant quality, the basic concept is to comprehend the relationship between the raw materials, production procedures, and the finished product. Defining quality attributes, determining essential parameters, and using risk management to reduce variabilityareallpartofQbD.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 02 | Feb 2025 www.irjet.net p-ISSN: 2395-0072
2. Improved Product Quality: QbD is centered on identifying and managing the variables that affect product quality. Manufacturersmayguaranteethatthefinishedproductregularlysatisfiespredeterminedqualitycriteriabyincluding qualityintothedesignphase.
3. Decreased Risk and Variability: QbD assists in identifying possible problems early in the development process by means of thorough risk assessment and management. In manufacturing, this proactive strategy improves predictabilityandlowersvariability.
4. Regulatory Compliance: The application of QbD concepts is promoted by regulatory organizations like the FDA. Applying QbD shows a dedication to quality and dependability, which helps expedite regulatory evaluations and clearances.
5. Enhanced Product Lifecycle Efficiency and Cost Savings: Quality-Based Development (QbD) can result in reduced waste,fewermanufacturingdisruptions,andoverallcostsavingsbydetectingandcorrectingpossibleissuesearlyon.
6. Better Process Understanding: Quality by Design (QbD) encourages a more thorough comprehension of the manufacturingandformulationprocesses.Innovationsandadvancementsinmanufacturingandproductdevelopment processesmayresultfromthisinformation.
7. Patient-CentricFocus:Theultimategoal of QbDistoimprovedrugsafetyandtherapeutic efficacy.Assuringuniform qualityhelpsimprovepatientoutcomesandboostsconsumerconfidenceinpharmaceuticals.
1) Critical Quality Attributes (CQAs):
The physical, chemical, biological, or microbiological qualities or traits that have to be kept within specified bounds in ordertoguaranteetheintendedlevelofproductqualityareknownascriticalqualityattributes,orCQAs. Asanexample:
Potency:Theabilityofamedicationtoproducethedesiredtherapeuticoutcome.
Purity:Theabsenceofunwantedsubstancesorbreakdownproductsthatcouldcompromiseaproduct'seffectiveness orsafety.
DissolutionRate:Thespeedatwhichamedicationbreaksdowninaparticularmedium,affectinghowbioavailableit is.
Stability: A medicinal product's capacity to hold onto its intended molecular, physical, and microbiological characteristicsoveranextendedperiodoftime.
2) Critical Process Parameters (CPPs):
The primary factors influencing CQAs during the manufacturing process are known as Critical Process Parameters, or CPPs.Controllingthesevariablesiscrucialtoguaranteeingthatthefinalproductfulfilsitsqualityrequirements. EffectontheQualityoftheProduct:
Temperature:Changesintemperatureduringgranulationormixingcanhaveanimpactonthedistributionofparticle sizes,whichinturnaffectstherateofdissolutionandbioavailability.
pHLevels:Anactiveingredient'ssolubilitycanbeimpactedbyaformulation'spH,whichcanthushaveanimpacton theingredient'spotencyandstability.
Mixing Time: An uneven formulation might affect the product's consistency and efficacy. An excessive or insufficient mixingcancausethis.
3) Design Space:
Ithasbeenshownthatthe designspace,whichisa multidimensional rangeofinputvariables(suchasmaterial qualities and process parameters), assures quality. Variations are possible in this area without sacrificing the calibre of the final result.
RegulatoryImplications:
ManufacturingFlexibility:Manufacturerscanmakemodificationswithout priorclearanceaslongastheystay within thespecifieddesignspace,whichisfrequentlyacceptedbyregulatorybodies.
Simplified Approval Process: Because it shows a thorough comprehension of the relationship between inputs and outputsinthemanufacturingprocess,aclearlydefineddesignspacecanspeeduptheregulatoryreviewprocedure.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 02 | Feb 2025 www.irjet.net p-ISSN: 2395-0072
Acontrolstrategyisaprearrangedsetofcontrolsthatguaranteeprocessperformanceandproductquality.Itisdeveloped from a methodical assessment of the manufacturing process. DevelopmentandApplications:
Combining CPPs and CQAs: management strategies combine monitoring and management of important quality attributesandcriticalprocessparameterstomaintainquality.
Real-time Monitoring: By putting in place in-process controls (such online measurements), it is possible to quickly spotdeviationsfromtheintendedcondition.
Corrective Actions: The control strategy ought to specify what should be done in the event that deviations arise in ordertomaintaintheproduct'sacceptablelevelofquality.
5) Risk Management: ToolandTechnique:
Failure Mode and Effects Analysis, or FMEA, is a methodical technique for locating possible process failure modes, evaluatingtheirimplications,andrankinghazardsaccordingtotheirseriousness,frequency,anddetectability.Making educateddecisionsaboutwhichhazardstohandleisaidedbythis.
Risk-basedDecision-Making:Thepossiblehazardsconnectedtochangesinthesefactorsaretakenintoconsideration whenmakingdecisionsonCQAs,CPPs,andcontrolmeasures.Thisguaranteesthatresourcesareallocatedtotheareas thathavethemosteffectsonthequalityoftheproduct.
Importance in QbD: Risk management aids in the prioritization of activities and resources within the framework, guaranteeing that possible problems are dealt with beforehand and, in the end, improving the quality and safety of products.
Methods validated as a check-box tool as defined in International Conference on Harmonization (ICH) Q2 guidance, Validation of Analytical Procedure: Text and Methodology.
Effectofvariationinmethodparametersonperformanceof methodislessunderstood.
Methodtransferseenasseparateexercisefromvalidation
Suitability of a method demonstrated against an analytical target profile, which defines the specific characteristics and criteria required by the process controlstrategy.
A science based structured approach for identifying and exploring method variables and their effect (method designandqualificationstages)
Method-transferactivitiesseenascomponentsofthelife cycleapproachand considered change control exercises; appropriate method installation and verification actions determined by assessment (method performance verificationstage)
The terms e.g; method verification, method, method validation and revalidation are confusing in traditional approach
Method validation used to performed onetime event performedoncompletionofmethoddevelopment.
In lifecycle-approach more clear terms aligned with process validation and equipment qualification terminologyareused.
Method lifecycle validation used to performed all activities that ensure a method produces fit-for-purpose data during the whole lifecycle (i.e; from development through to on-going routine operating environment and includesknowledgetransferfromasendingunit)

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 02 | Feb 2025 www.irjet.net p-ISSN: 2395-0072
Improving Product Quality-Embedding quality considerations into the design and development phases leads to consistentproductquality.Manufacturerscancreaterobustprocessesthatminimizevariabilitybyidentifyingcritical qualityattributes(CQAs)andunderstandingtheirrelationshipwithcriticalprocessparameters(CPPs).Thisproactive approach ensures that products consistently meet predefined specifications, leading to higher patient safety and satisfaction.
Flexibility in Regulations-QbD principles is endorsed by regulatory bodies such as the FDA, EMA, and ICH because they acknowledge that a thoroughly characterized product development process can result in improved quality outcomes. Manufacturers can work within a specified range of conditions without requiring extensive regulatory reevaluation for changes made within this "design space." This flexibility can streamline product development and enablefasterresponsestomarketdemands.
Minimizing Product Failures-Whenqualityisintegratedintothedesignstage,QbDdecreasesthechancesofout-ofspecificationoutcomes.Recognizingtheconnectionbetweenprocessvariablesandproductqualityaidsinpinpointing possiblefailureareasatthestartofthedevelopmentprocess.Thispredictiveabilityenablesmanufacturerstotackle problemsbeforetheyarise,leadingtofewerrecallsandreducedrework,ultimatelyfosteringgreaterconfidenceinthe product.
Efficiency and Cost Savings-Long-termQbDbenefitscanbeverysubstantialeconomically.Reducingthefrequencyof productrecallsandfailurescansavebusinessesmoneyonreputationaldamageandcleanupexpenses.Furthermore, shorterproductionrunsandlowerproductioncostsarefrequentlytheresultofincreasedprocessefficiency.Allthings considered,applyingQbDprinciplescanimproveproductqualityandregulatorycomplianceandresultinsignificant costsavings.
Initial Outlay of Funds and Resources-A substantial initial time and money commitment is frequently needed to implement QbD. It may be necessary to conduct significant research and development in order to gain a thorough grasp of critical quality attributes (CQAs) and critical process parameters (CPPs). This could entail investing in new technologies, developing laboratory capacity, and educating personnel. The adoption of a QbD framework may be impededbythelargeinitialexpensesinvolved,whichmaybeparticularlydifficultforsmallerbusinesses.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 02 | Feb 2025 www.irjet.net p-ISSN: 2395-0072
Data-Intensive Approach-Because QbD mostly relies on data analysis and collecting, it requires strong data management systems. To properly comprehend the links between CQAs and CPPs, companies must collect, analyse, and interpret massive volumes of data. Advanced analytical tools may need to be adopted in order to meet this requirement,whichmayputaburdenoncurrentdatamanagementsystems.Furthermore,maintainingdataintegrity and adhering to regulatory requirements raises complexity and necessitates infrastructure and specialist training expendituresfromenterprises.
Regulatory Difficulties-Despitethegrowingregulatorylandscape'stendencytoembraceQbDconcepts,therearestill obstaclestoovercome.RegionalregulationsandagencyinterpretationsofQbDrecommendationscandiffer.Examples of these agencies are the FDA and EMA. For businesses that are not familiar with QbD, navigating these regulatory frameworks can be challenging because they demand a deep understanding of compliance standards. Additionally, producersmaybecomehesitantduringthefirstdeploymentphaseduetothepossibilityofregulatoryinspection.
Developing Design Space's Complexity-AcrucialstepinQbDisdefiningthedesignspace,whichcanbedifficultand time-consuming. Finding the best operating ranges for CPPs while maintaining constant product quality requires a great deal of experimentation. Because this process is iterative, businesses may find it difficult to strike a balance betweenthoroughnessandspeedwhiledevelopingnewproducts.Furthermore,teamsmayfinditchallengingtoagree onacceptableboundsiftheyrequireagreatdealofstatisticalanalysistovalidatethedesignspace.
1. Progress in Digital Technology-The application of QbD is being revolutionized by digital technologies like automation,AI,andmachinelearning.Theseinstrumentsenable:
EnhancedDataAnalysis:ArtificialIntelligenceandmachinelearninghavethecapacitytoanalyselargevolumesof data andmore effectivelyfindpatternsand linksbetweencritical processparameters(CPPs)andcritical quality attributes(CQAs).Thismakesitpossibletoanticipateandoptimizedevelopmentprocesseswithgreateraccuracy.
Processautomation:Automatedsystemscanreducehumanerrorandincreasethedependabilityofoutcomesby streamlining data collecting and analysis. Additionally, automation speeds up process modifications and experimentation,enablingmorerapiditerationsthroughoutthedevelopmentcycle.
Real-Time Monitoring: Advanced sensors and IoT technologies enable real-time monitoring of manufacturing processes.Thisdatacanbefedintomachinelearningalgorithmstomakeinstantadjustments,enhancingproduct qualityandconsistency.
2. New Developments in QbD-ThefutureofQbDisbeingshapedbyseveraltrends:
PersonalizedMedicine: QbD principlescanbemodified tomeet eachpatient'sspecificneedsasthe medical field moves toward personalized care. This emphasizes the significance of comprehending CQAs in the context of diversepatientpopulationsandcallsforanadaptivedesignspacethatcanrespondtoarangeofpatientprofiles.
ContinuousManufacturing:QbDconceptsarewell-alignedwiththeshiftfrombatchmanufacturingtocontinuous manufacturingprocesses.Ongoingmonitoringandcontrolaremadepossiblebycontinuousprocesses,whichalso enable quick decisions based on data collected in real time. This can result in less waste and time to market as wellasbetterproductqualityandefficiency.
3. Constant Observation and Modification-It is impossible to exaggerate the significance of using data to inform decisionswhenitcomestoQbD.Organizationsthatcontinuouslymonitortheirproceduresandoutputareableto:
Adapt Strategies: Organizations can improve their QbD strategies by modifying the design space and CPPs to maximize efficiency and quality as new data becomes available. This flexibility is essential for responding to shiftingmarketdemands,legalrestrictions,andtechnologybreakthroughs.
Establish feedback loopstoensurethatfuturedevelopmentandprocessimprovementsareinformedbyinsights gathered from continuous manufacturing and post-market surveillance. As a result, a culture of continuous improvementisestablished,andQbDproceduresmethodicallyincorporatethelessonslearnt.
8. CONCLUSIONS:
Throughout the development process, the quality of pharmaceutical goods may be guaranteed according to a revolutionary framework called Quality by Design (QbD). Manufacturers may dramatically lower variability, improve efficiency, and more closely conform to regulatory standards by integrating quality issues from the beginning. The longterm benefits such as less product failures and improved patient safety far surpass the early expenditures associated with training, resources, and data infrastructure. The production of safe and effective pharmaceuticals will depend

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 02 | Feb 2025 www.irjet.net p-ISSN: 2395-0072
increasingly on QbD as the pharmaceutical industry develops, especially with advances in automation, artificial intelligence, and continuous manufacturing. The necessity for flexible QbD solutions that can accommodate a range of patientdemographicsisunderscoredbyemergingconcepts,suchascustomizedmedicine.ProspectsforQbDinthefuture include integrating digital technologies even more to improve data analysis and expedite procedures. Still needing more study and development are issues like improving data management systems, developing standardized procedures for defining and verifying design space, and improving regulatory frameworks to better incorporate QbD principles. In the end,resolvingtheseissueswillresultinmorerobustandresilientdrugdevelopmentprocessesbyoptimizingthepotential ofQbDinthepharmaceuticalindustry.
1. U.S.FoodandDrugAdministration(FDA).(2006).Pharmaceuticalqualityforthe21stcentury:Arisk-basedapproach. U.S.DepartmentofHealthandHumanServices.https://www.fda.gov/media/77391/download
2. International Conference on Harmonisation (ICH). (2009). Q8(R2) pharmaceutical development. https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf
3. InternationalConferenceonHarmonisation(ICH).(2012).Q9:Qualityriskmanagement. https://database.ich.org/sites/default/files/Q9_Guideline.pdf
4. International Conference on Harmonisation (ICH). (2008). Q10: Pharmaceutical quality system. https://database.ich.org/sites/default/files/Q10_Guideline.pdf
5. Patil,A.S.,&Pethe,A.M.(2013).Qualitybydesign(QbD):Anewconceptfordevelopmentofqualitypharmaceuticals. International Journal of Pharmaceutical Quality Assurance, 4(2), 13-19. https://www.researchgate.net/publication/269107872_Quality_by_Design_QbD_A_New_Concept_for_Development_of _Quality_Pharmaceuticals
6. Montgomery, D. C. (2017). Design and analysis of experiments (9th ed.). Wiley. https://www.wiley.com/enus/Design+and+Analysis+of+Experiments%2C+9th+Edition-p-9781119113478
7. Yu, L. X., Amidon, G., Khan, M. A., & Hoag, S. W. (2014). Understanding pharmaceutical quality by design. European Journal of Pharmaceutics and Biopharmaceutics, 91, 1-13. https://www.sciencedirect.com/science/article/pii/S0939641114001861
8. U.S. Food and Drug Administration (FDA). (2011). Process validation: General principles and practices. U.S. DepartmentofHealthandHumanServices.https://www.fda.gov/media/71021/download
9. Kourti,T.(2010).Processanalyticaltechnologyandqualitybydesign.AnalyticalandBioanalyticalChemistry,398(1), 53-58.https://link.springer.com/article/10.1007/s00216-010-3699-1
10.Lionberger,R.A.,Lee,S.L.,Lee,L.,&Raw,A.(2008).Qualitybydesign:ConceptsforANDAs.TheAAPSJournal,10(2), 268-276.https://pubmed.ncbi.nlm.nih.gov/18800218/
11.Basu, P. K., & Walker, S. (2012). Implementing Quality by Design (QbD) in generic pharmaceutical product development.JournalofPharmaceuticalInnovation,7(1),22-31.https://doi.org/10.1007/s12247-012-9125-1
12.Rathore, A. S., & Winkle, H. (2009). Quality by Design for biopharmaceuticals. Nature Biotechnology, 27(1), 26-34. https://doi.org/10.1038/nbt0109-26
13.Iyer,R.,&Singhal,D.(2015).Roleofqualitybydesign(QbD)inpharmaceuticalproductdevelopment.Pharmaceutical RegulatoryAffairs:OpenAccess,4(2),1-5.https://doi.org/10.4172/2167-7689.1000140
14.Chang, S. Y., Morrison, K. A., & Parekh, H. S. (2013). Pharmaceutical Quality by Design: A practical perspective on implementation. International Journal of Pharmaceutics, 451(1-2), 1-8. https://doi.org/10.1016/j.ijpharm.2013.05.012
15.Nasr, M. M. (2007). Risk-based CMC review paradigm. The AAPS Journal, 9(1), E121-E126. https://doi.org/10.1208/aapsj0901012