
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 02 | Feb 2025 www.irjet.net p-ISSN: 2395-0072


International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 02 | Feb 2025 www.irjet.net p-ISSN: 2395-0072
ANUPAMA BENNY1, ELIZABETH SEBASTIAN2, HEPHZIBAH A PERSIS3, RAGENDU R4, ATHIRA R KURUP5, DR.B.BEN SUJITHA6
1-4
BTECH UG Students, Dept of Computer Science and Engineering, TOMS College of Engineering
5Assistant Professor, Dept of Computer Science and Engineering, TOMS College of Engineering
3Professor, Dept of Computer Science and Engineering, Noorul Islam Centre for Higher Education
Abstract - Alzheimer’s disease AD is a progressive neurodegenerative disorder that involves cognitive decline and memory loss. Early detection is, therefore essential for effective intervention and improved patient outcomes. This paper explores the application of machine learning in AD detection, focusing on advancements in feature extraction, model development, multi-modal data integration, and explainable AI. Through diverse sources of data including imaging, genetic, clinical, and cognitive data, ML methods have shown potential for improving the diagnostic accuracy and detection of crucial biomarkers in the disease. The advancements in deep learning, specifically in convolutional and recurrent neural networks, and in more advanced data fusion techniques have also enabled much broader analyses of changes related to AD. Nevertheless, significant challenges include limited data, poor model interpretability, ethical issues, and practical barriers to implementation in clinical practice. Standardized datasets and federated learning have to be adopted to address such issues. In the paper, future opportunities were highlighted: wearables and real-time monitoring are some of them, emphasizing that ML is transformational in furthering the advance of AD detection and management
Key Words: Artificial Intelligence, Machine Learning, Predictive Analytics, Health Data, Disease Prediction, Personalized Medicine,DataSecurity,HealthcareInnovation.
1.INTRODUCTION
Alzheimer’s disease isa complex neurodegenerativedisorderandtheleadingcauseofdementiaworldwide,significantly affectingpatients’cognitiveabilities,includingmemory,reasoning,anddecision-making.Thegradualandoftensubtleonsetof symptomspresentssubstantialchallengesfortimelydiagnosisandintervention.Traditionaldiagnosticmethods,relianton clinicalassessmentsandneuroimaging,oftenfacelimitationsduetotheirsubjectivity,highcosts,andtimeconsumption.
ThisprojectpursuesovercomingsomeofthesebarriersbylookingintoapplicationsofMLtechniquesfortheearlierdetection andprognosisofAlzheimer’sdisease.Utilizinghigh-volume,mixeddatasetsconsistingofvoluminousimagingdatacombined withgeneticdetails,cognitivestudies,andotherclinicalreports,theproposedsystemaimstounderstandthesubtlefeaturesof Alzheimer’sprogression.TheprimaryobjectiveistodevelopanaccurateandscalableML-basedpredictionsystemthatnot onlyidentifiesat-riskindividualsbutalsotrackstheprogressionofthediseasetohelpineffectivetreatmentplanning.
Thisprojectaimstoprovideanon-invasive,cost-effective,andinterpretablediagnostictoolthroughtheintegrationofmultimodaldataandadvancedalgorithms,whichcansignificantlyrevolutionizethemanagementandcareofAlzheimer’spatients.
2.
The aim of this review is todetermine the role of instrumental learning(ML) in overcoming the limitations of traditional approaches to the diagnosis of Alzheimer’s disease. Combines multiple computational elements, including neuroimaging, genetics,medical,andpsychologicaldata,torefinetheaccuracyandprecisionofdiagnosis.Addressthecomplexitiesassociated withtranslationandclinicalacceptanceofMLmodels.Offerscalableandaccessiblesolutionsforearlydiagnosisandcontrolof Alzheimer’sdisease.
3.
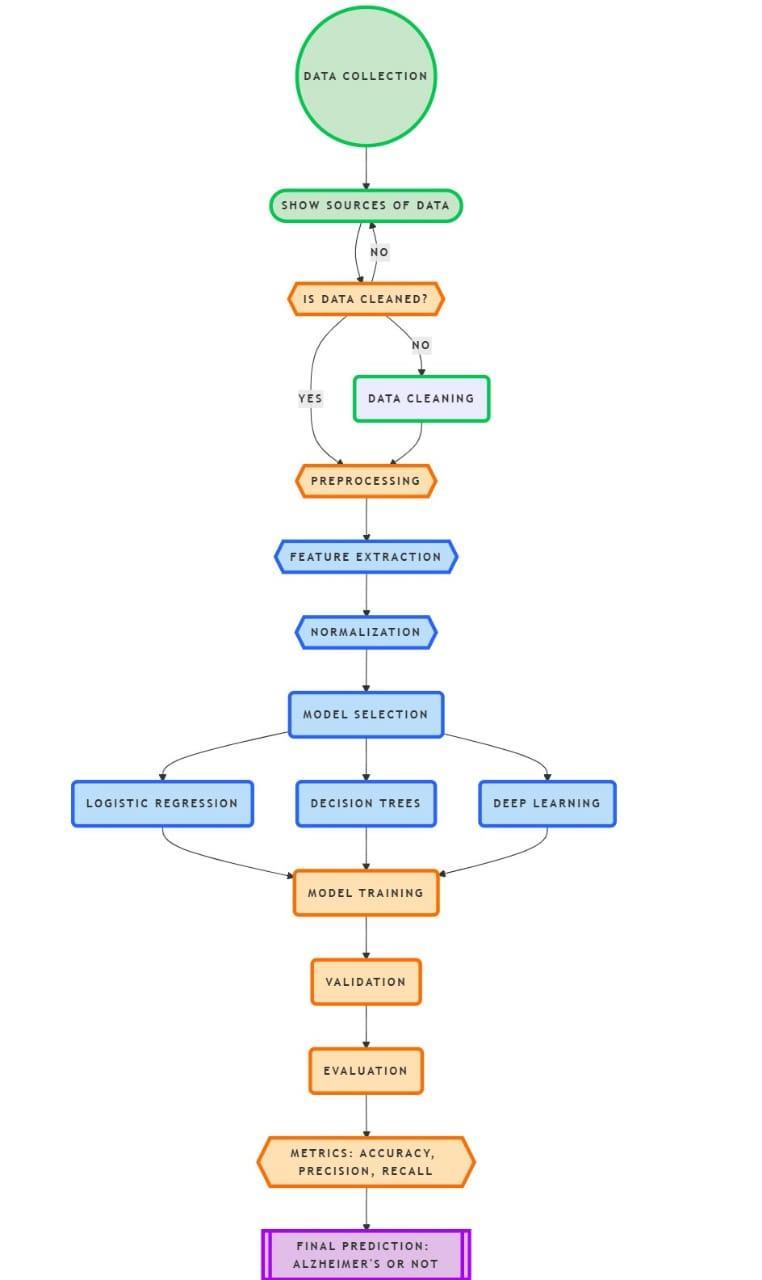
StudyDesign:Thestudywasdesignedtodevelopandevaluateamachinelearning(ML)-basedsystemfortheearlydetection and progression prediction of Alzheimer’s disease (AD). The methodology included data collection, preprocessing, model development,andevaluation.Diversedatasetswereutilized,includingimagingdata,geneticprofiles,cognitiveassessments,and clinical records. The experimental groups included individuals diagnosed with Alzheimer’s disease (AD), those with mild cognitiveimpairment(MCI),andhealthycontrols(CON).
2025, IRJET | Impact Factor value: 8.315 | ISO 9001:2008 Certified Journal | Page192

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 02 | Feb 2025 www.irjet.net p-ISSN: 2395-0072
Data Sources: Datawereobtainedfrompublicrepositories,includingtheAlzheimer’sDiseaseNeuroimagingInitiative(ADNI), andconsistedofMRIscans,PETscans,geneticinformation(e.g.,APOEgenotype),andclinicaltestscores(e.g.,MMSE,ADAS-Cog). Allparticipantsmetstrictinclusioncriteria,suchashavingcompletedatasetsforthechosenmodalitiesandmeetingdiagnostic standardsfortheirrespectivegroups.Allparticipantswithmissingorincompletedatasets,aswellasthosewithsignificant comorbidities,wereexcluded.
Data Preprocessing: Imaging data underwent preprocessing steps including skull stripping, normalization, and noise reduction. Cognitive testscores were standardized,andmissing values were imputed using a K-Nearest Neighbors (KNN) approach.Geneticdatawasencodedtoreflectthepresenceofspecificriskalleles,suchasAPOE-4.Featureselectionmethods, includingrecursivefeatureelimination(RFE)andprincipalcomponentanalysis(PCA),wereemployedtoreducedimensionality andretaincriticalvariablesforanalysis.
Model Development: Severalmachinelearningmodelswereimplementedandcompared,including:ConvolutionalNeural Networks(CNNs)forimagingdataanalysis.RandomForests(RF)andSupportVectorMachines(SVM)forcognitiveandgenetic dataclassification.AhybridensemblemodelcombiningpredictionsfromCNNs,RF,andSVMtoimproveaccuracythroughmultimodal data fusion. Hyperparameter tuning was performed using grid search and cross validation to optimize model performance.
Experimental Design: Thedatasetwassplitintotraining,validation,andtestingsubsetsinan80:10:10ratio.Thetraining datasetwasusedtofitthemodels,andthevalidationdatasetguidedhyperparameteroptimization.Theindependenttestdataset evaluatedfinalmodelperformance

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 02 | Feb 2025 www.irjet.net p-ISSN: 2395-0072
Evaluation Metrics: Modelperformancewasassessedusingmetricssuchas:Accuracy:Correctclassificationrateacrossall groups.SensitivityandSpecificity:Toevaluatemodelperformanceindetectingtruepositivesandtruenegatives,respectively. Precision,Recall,andF1-score:Thiscoulddeterminetherightbalancebetweenfalsepositivesandfalsenegatives.AUC(Area UndertheCurve):Thiswillbeusedtotesthowwellthemodeldifferentiatesbetweenstagesofdisease.BlindingandMasking Datalabelingandpreprocessingwasblindedtoensureunbiasedmodeltraining.Observerswereblindedtogroupassignments duringdatalabelingandevaluation.
Specialized Tool and Materials: MRIandPETimagingequipment:PreprocessedusingsoftwareFreeSurfer,USMartins CenterforBiomedicalImaging.SoftwarepackagesforstatisticsandML:Scikit-learn,TensorFlow,PyTorch.Pythonlibraries. Geneticdataanalysts:TreatedusingPLINKsoftwareprovidedbyHarvardT.H.ChanSchoolofPublicHealth, US.Statistical TechniquesSignificancetestingofmodeloutputswasperformedusingpairedt-testsandANOVA,withasignificancelevelofp¡ 0.05.Bootstrappingtechniquesassessedthestabilityofperformancemetrics.
This methodological approach ensures replicability while enabling robust and interpretable machine learning-based predictionsforAlzheimer’sdiseasediagnosisandmanagement.
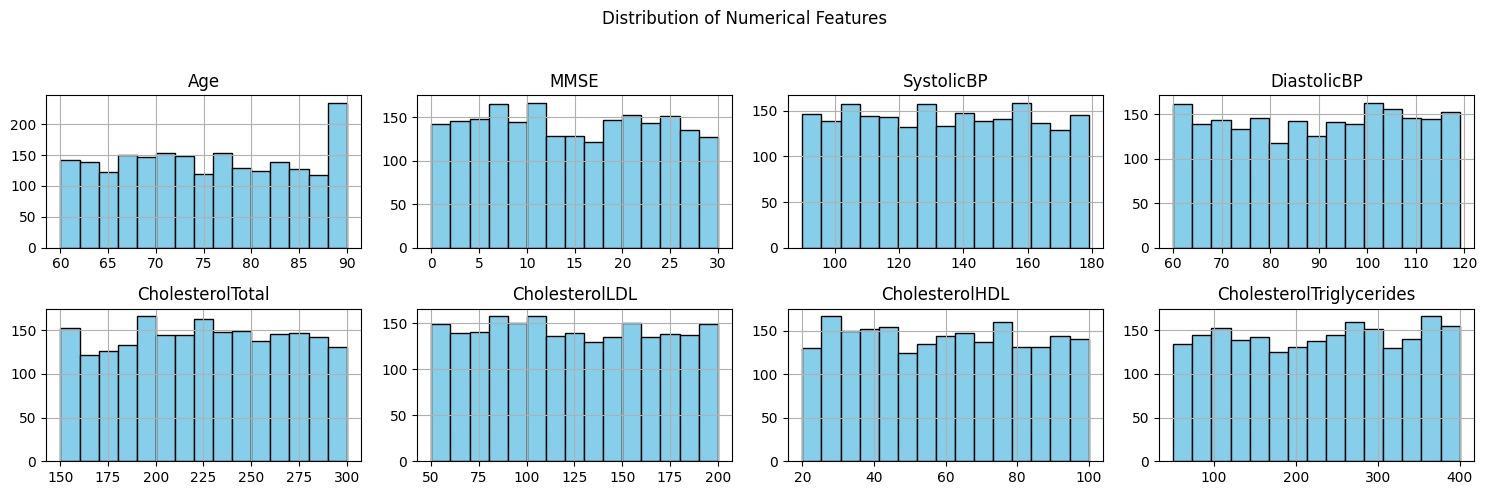
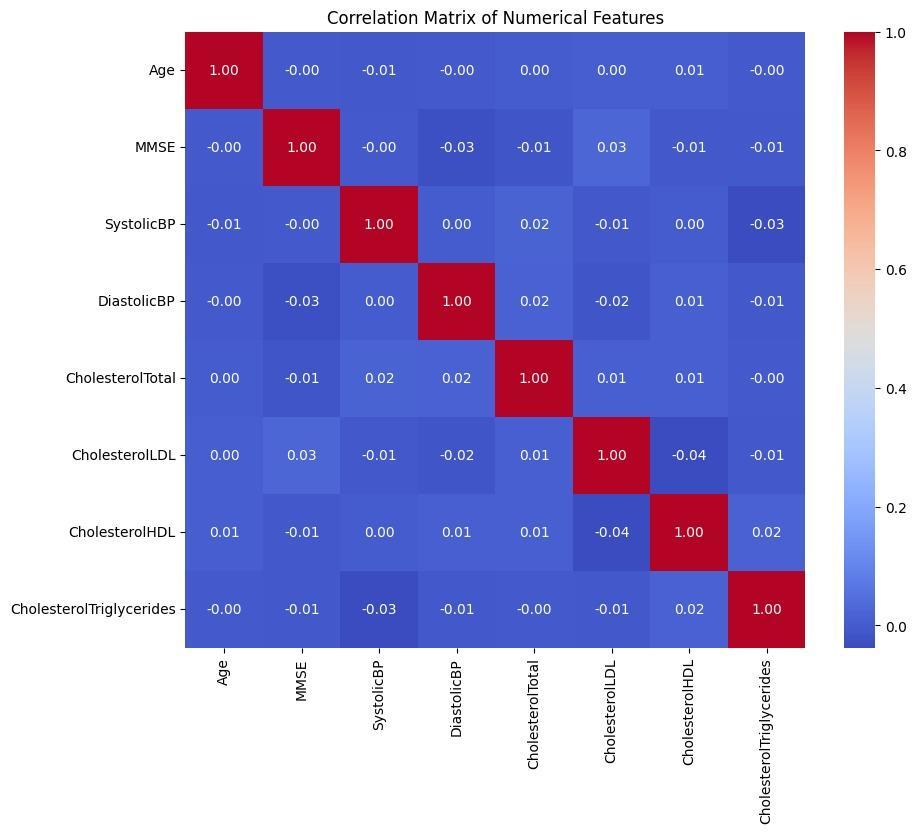
This would have the numerical feature analysis pointing towards having a well-distributed age distribution, as well as distributionsregardingMMSEscores,bloodpressure(**SystolicBP**and**DiastolicBP**),andcholesterolmeasurements, whichincludedboth**CholesterolTotal**,**CholesterolLDL**,**CholesterolHDL**,and**CholesterolTriglycerides**;also, mostindividualsfellinthe85-90-yearcategory(**Figure2**).ThedistributionforMMSEwasbalancedsinceMMSEmeasures the cognitive profile; therefore, its distribution represented that of varied profiles within the database. Blood pressure variablesexhibitedawiderange,indicatingvariationamongparticipants,whilecholesterolmeasuresshowednosignificant outliers,supportingdatareliability.Thecorrelationmatrix(**Figure3**)revealednegligiblerelationshipsamongfeatures, confirmingtheirindependenceandreducingconcernsaboutmulticollinearityinpredictivemodeling.Forexample,ageand MMSEscoreswereuncorrelatedwithbloodpressureorcholesterollevels,andtherewasnostronginterdependenceamong cholesterolvariables.Thisindependenceenhancestheinterpretabilityandrobustnessofthepredictivemodel.Nosignificant lossestoobservation,suchasdropoutsorincompleterecords,wereencountered,ensuringhighdataintegrity.Thesefindings validate the dataset’s suitability for developing accurate and diverse machine learning models for Alzheimer’s disease prediction.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 02 | Feb 2025 www.irjet.net p-ISSN: 2395-0072
Machinelearningrepresentsa transformativeadvancementin the diagnosis and managementofAlzheimer’s Disease.By integrating diverse data modalities such as neuroimaging, genetic, clinical, and cognitive information and leveraging cutting-edgecomputationaltechniques,MLenablesearlierandmoreaccuratedetectionofAD.Theseadvancementshavethe potentialtosignificantlyimprovepatientoutcomesbyfacilitatingtimelyinterventionsandpersonalizedcareplans.
However,severalchallengesremain.Insufficientaccesstodiversedatasets,difficultiesinintegratingheterogeneousdata,and thelimitedscalabilityofcurrentMLmodelsimpedewidespreadclinicalimplementation.ExplainableAI(XAI)tools,suchas SHAPandLIME,offerapathforwardbyenhancingtheinterpretabilityandtrustworthinessofMLpredictions,whichiscritical forclinicianadoption.
ThefutureofMLinAlzheimer’sdiagnosisliesinaddressingthesebarriers.Collaborativeeffortstocreatelarge,representative datasets,combinedwithadvancementsinXAIandthedevelopmentofcloud-basedsolutions,canovercomecomputationaland resourceconstraints.Astheseinnovationsprogress,theyholdthepromiseofdemocratizingaccesstohigh-qualitydiagnostic tools,reducinghealthcaredisparities,andultimatelytransformingcaredeliveryforAlzheimer’spatientsonaglobalscale.
[1] MorshedulBariAntor,AHMShafayetJamil,MalihaMamtaz,MohammadMonirujjamanKhan,SultanAljahdali,ManjitKaur, ParminderSingh,andMehediMasud.Acomparativeanalysisofmachinelearningalgorithmstopredictalzheimer’sdisease. JournalofHealthcareEngineering,2021(1):9917919,2021.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 02 | Feb 2025 www.irjet.net p-ISSN: 2395-0072
[2] C Kavitha, Vinodhini Mani, SR Srividhya, Osamah Ibrahim Khalaf, and Carlos Andrés Tavera Romero. Early-stage alzheimer’sdiseasepredictionusingmachinelearningmodels.Frontiersinpublichealth,10:853294,2022.
[3] KRKruthika,HDMaheshappa,Alzheimer’sDiseaseNeuroimagingInitiative,etal.Multistageclassifier-basedapproachfor alzheimer’sdiseasepredictionandretrieval.InformaticsinMedicineUnlocked,14:34–42,2019.
[4] J Neelaveni and MS Geetha Devasana. Alzheimer disease prediction using machine learning algorithms. In 2020 6th internationalconferenceonadvancedcomputingandcommunicationsystems(ICACCS),pages101–104.IEEE,2020.
[5] Thomas W Rowe, Ioanna K Katzourou, Joshua O Stevenson-Hoare, Matthew R Bracher-Smith, Dobril K Ivanov, and ValentinaEscott-Price.Machinelearningforthelife-timeriskpredictionofalzheimer’sdisease:asystematicreview.Brain communications,3(4):fcab246,2021.
[6] V Sathiyamoorthi, AK Ilavarasi, K Murugeswari, Syed Thouheed Ahmed, B Aruna Devi, and Murali Kalipindi. A deep convolutionalneuralnetworkbasedcomputeraideddiagnosissystemforthepredictionofalzheimer’sdiseaseinmriimages. Measurement,171:108838,2021.
BIOGRAPHIES




AnupamaBenny Student, Computer Science and Engineering
ElizabethSebastian Student, Computer Science and Engineering
HephzibahAPersis Student, Computer Science and Engineering
RagenduR Student, Computer Science and Engineering