

Welcome!

Robin Tuohy,
IMF Vice President,
Patient Support

25-Year Care Partner | Advocate | Support Group Leader

Thank You to Our Sponsors!


Thank You to our Donors!


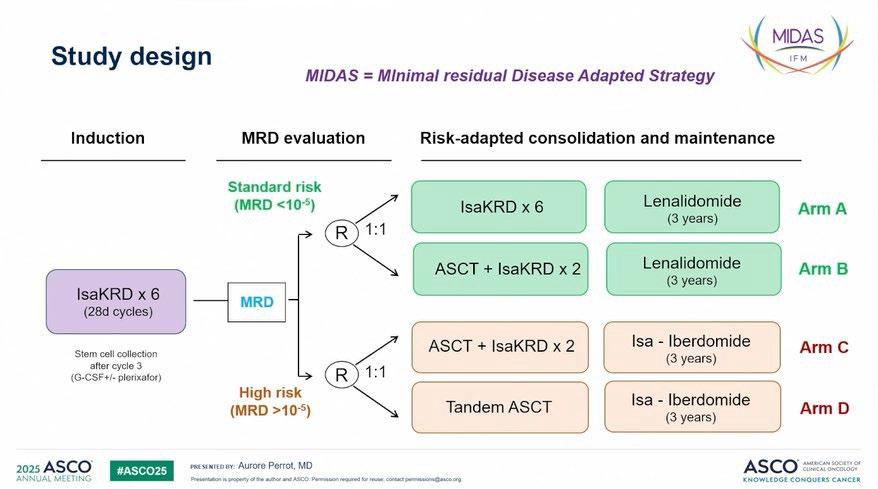
IN-PERSON PROGRAM | JULY 17 – 20, 2025 Omni Viking Lakes - Minneapolis, MN
Welcome First Time and Returning Leaders!

There are 108 Support Group Leaders in attendance representing 81 Support Groups.
We are thrilled to welcome 21 first-time attendees and welcome back 87 returning leaders!

Meeting Room Wi-fi Information Network:

Omni Select Guest

Please remember to enroll in the
Omni Select Guest Program

Free WiFi in your room

Two bottles of water replenished daily

Please
use this QR code for Friday's

Evaluation!



Friday Morning Agenda

Key Questions in Myeloma Research:
How the IMF is Addressing Them

Joseph Mikhael, MD, MEd, FRCPC, FACP, FASCO
IMF Chief Medical Officer, SAB & IMWG

WE ARE LIVING IN THE GOLDEN AGE OF MYELOMA WITH IMPROVING QUANTITY AND QUALITY OF LIFE!

BUT WE ARE ALSO LIVING IN A DAY OF UNPRECEDENTED
DISPARITIES IN MYELOMA WORLDWIDE...
RESEARCH IS ESSENTIALLY BASED ON
ANSWERING IMPORTANT QUESTIONS
I will describe the “state” of myeloma research by proposing 10 critical unanswered questions in myeloma

Black Swan Research Initiative
• Black Swan events refers to unpredictable events that have significant impact which are often criticized before and then post hoc rationalized as something that was predictable.
• When we started BSRI over ten years ago, the three interrelated “black swan” ideas we put out were:
• Myeloma was curable if treated in the early asymptomatic stage
• Myeloma was curable if we can get to sustained MRD negative status with treatment.
• Sustained MRD negative status was an indicator of cure

Black Swan Research Initiative
To accomplish the goals of BSRI we needed:
• A cure trial targeting high risk SMM (ASCENT)
• Widely available MRD test development (NGF, Peripheral blood MRD)
• MRD surrogacy studies (I2TEAMM)
• Screening and Early detection studies

Black Swan Research Initiative
Current and Future BSRI goals (for funding, for grants, IMWG guidelines and research projects)
1. MRD as a surrogate endpoint for full regulatory approval in myeloma trials
2. Improved MRD testing (deeper; peripheral blood, etc)
3. Successor cure trials (ASCENT 2, and more)
4. Early Detection


1. SHOULD WE BE SCREENING FOR MYELOMA?


Iceland Screens, Treats, or Prevents Multiple Myeloma
Control arm
Inclusion criteria:
-Born ≤1975
-Icelandic resident

Exclusion criteria:
-Previous lymphoproliferative disease
Only arm 2 and 3:
-Previously known MGUS (excluded for this analysis)
Psychiatric health: Assessed at registration, after MGUS notification, and annually
Intervention arm
Rögnvaldsson S et al. (2021) Blood Cancer Journal; 11, 94

Dr. Joe’s Take on Screening
This is a MASSIVE and critical study to educate us about the feasibility and usefulness of screening
We have to remember Iceland does not represent the whole planet but is an important place to start (especially due to genetic information there)
All screening programs are focused by age, gender, family history, etc and we should expect the same in MM – especially as it is twice as common in patients of African descent
The ISTOPMM trial will likely answer the key question of whether or not screening saves lives in the next 2 years...
IMF Role – We are involved in iSTOPMM and the CHAAMP study in Charlotte – this is part of the Black Swan Research Initiative

2. SHOULD WE TREAT SMOLDERING MYELOMA?
AQUILA: Study Design
AQUILA
Screening
Key eligibility criteria:
• ≥18 years of age
• Confirmed SMM diagnosis (per IMWG criteria) for ≤5 years
• ECOG PS score of 0 or 1
• Clonal BMPCs ≥10% and ≥1 of the following risk factors:
- Serum M-protein ≥30 g/L
- IgA SMM
- Immunoparesis with reduction of 2 uninvolved Ig isotypes
- Serum involved:uninvolved FLC ratio ≥8 and <100
- Clonal BMPCs >50% to <60%
All patients were required to have CT/PET-CT and MRI imaging during screening
enrollment period: December 2017 to May 2019 at 124 sites in 23 countries
Treatment/active monitoring phase Follow-up phase
DARA monotherapy
1800 mg SCb QW Cycles 1-2, Q2W Cycles 3-6, Q4W thereafter in 28-day cycles until 39 cycles/36 months*
Active monitoring
No disease-specific treatment, with AE monitoring up to 36 months*
• Efficacy follow-up until progression by SLiM-CRAB
• Survival follow-up every 6 months until end of study
Primary endpoint:
• PFS by IRC per IMWG SLiM-CRAB criteriac
Key secondary endpoints:
• ORR
• Time to first-line treatment for MM
• PFS on first-line treatment for MM
• Overall survival
*Or confirmed disease progression (whichever occurred first).
Stratified by number of risk factorsa for progression to MM (<3 vs ≥3)
Disease evaluation schedule
• Laboratory efficacy – Every 12 weeks by central lab until disease progression
• Imaging (CT/PET-CT, MRI) – Yearly (central review)
• Bone marrow – At least every 2 years
IMWG, International Myeloma Working Group; ECOG PS, Eastern Cooperative Oncology Group performance status; BMPC, bone marrow plasma cell; FLC, free light chain; CT, computed tomography; MRI, magnetic resonance imaging; QW, weekly; Q2W, every 2 weeks; Q4W, every 4 weeks; AE, adverse event; IRC, independent review committee; ORR, overall response rate. aRisk factors included involved:uninvolved FLC ratio ≥8 (yes vs no), serum Mprotein ≥30 g/L (yes vs no), IgA SMM (yes vs no), immunoparesis (reduction of 2 uninvolved immunoglobulins vs other), or clonal BMPCs (>50% to <60% vs ≤50%). bDARA SC (1800 mg co-formulated with recombinant human hyaluronidase PH20 [rHuPH20; 2,000 U/mL; ENHANZE® drug delivery technology; Halozyme, Inc.]). cPFS was defined as duration from randomization to initial documented progression to active MM or death due to any cause, whichever occurred first. 22

AQUILA: Progression to MM by IMWG SLiM-CRAB Criteria (IRC Assessment)

AQUILA: Overall Survival
*Deaths due to an event occurring after the AE reporting window (ie, events that happened after patient started subsequent therapy or >30 days after last dose) or deaths with unknown reason.
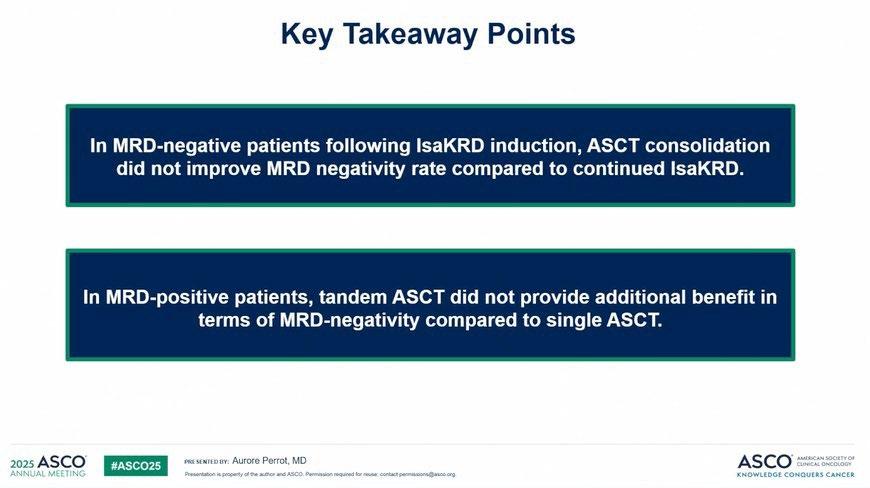
Early intervention with fixed duration DARA extended overall survival versus active monitoring

AQUILA: Safety Overview

Dr. Joe’s Take on Smoldering Myeloma
• Wow this is a VERY important study and may well change the way we think about and treat high risk smoldering myeloma
• The study was critical to really prove we can delay the progression to active myeloma and even improve survival with 3 years of daratumumab
• It underscores the important of a DISCUSSION with the health care team as many options can be offered to patients with high risk smoldering MM
• There will me MANY more trials coming in this area, with even more intense therapies like combinations and even CAR T Cell therapy...
• IMF Role – We have conducted the ASCENT and CESAR trials and are planning the ASCENT 2 trial – intensely treating high risk SMM

3. IS IT QUADRUPLETS FOR ALL IN
FRONTLINE MYELOMA?
PERSEUS: Study Design

Induction

• Transplanteligible NDMM
• Age 18-70 years
• ECOG PS ≤2

V: 1.3 mg/m2 SC
Days 1, 4, 8, 11
R: 25 mg PO Days 1-21
d: 40 mg PO/IV Days 1-4, 9-12

: 1,800 mg SCb
Cycles 1-2 Q2W Cycles 3-4
VRd administered as in the VRd group

Consolidation

V: 1.3 mg/m2 SC Days 1, 4, 8, 11
: 25 mg PO Days 1-21

DARA: 1,800 mg SCb Q2W
VRd administered as in the VRd group

Maintenance
DARA: 1,800 mg SCb Q4W R: 10 mg PO Days 1-28



Primary endpoint: PFSc
Key secondary endpoints: Overall ≥CR rate,c overall MRD-negativity rate,d OS
Discontinue DARA therapy only

Discontinue DARA therapy only after ≥ 24 months of D-R maintenance for patients with ≥CR and 12 months of sustained MRD negativity
Restart DARA therapy upon confirmed loss of CR without PD or recurrence of MRD
ECOG PS, Eastern Cooperative Oncology Group performance status; V, bortezomib; SC, subcutaneous; PO, oral; d, dexamethasone; IV, intravenous; QW, weekly; Q2W, every 2 weeks; PD, progressive disease; Q4W, every 4 weeks; MRD, minimal residual disease; CR, complete response; OS, overall survival; ISS, International Staging System; rHuPH20, recombinant human hyaluronidase PH20; IMWG, International Myeloma Working Group; VGPR, very good partial response. aStratified by ISS stage and cytogenetic risk. bDARA 1,800 mg co-formulated with rHuPH20 (2,000 U/mL; ENHANZE drug delivery technology, Halozyme, Inc., San Diego, CA, USA). cResponse and disease progression were assessed using a computerized algorithm based on IMWG response criteria. dMRD was assessed using the clonoSEQ assay (v.2.0; Adaptive Biotechnologies, Seattle, WA, USA) in patients with ≥VGPR post consolidation and at the time of suspected ≥CR. Overall MRD-negativity rate was defined as the proportion of patients who achieved both MRD negativity (10–5 threshold) and ≥CR at any time.

Isa-VRD vs VRD: Study design – Part 1
Stratification for randomization prior to:
1. Induction: R-ISS stage (I/II versus III versus not classified)
Treatment for 3 years or until PD Cycles 4+ Isa: 10 mg/kg IV Cycle 1 Cycles 2-3
1
2-3 R: 10/15/25 mg PO; Days 1-14 and 22-35
V: 1.3 mg/m2 SC; Days 1, 4, 8, 11, 22, 25, 29, and 32
d: 20 mg POb; Days 1-2, 4-5, 8-9, 11-12, 15, 22-23, 25-26, 29-30, and 32-33
R: 10 mg (up to 15 mg, if tolerated) PO; continuously
d: 20 mg PO; Days 1, 8, 15, and 22
2. Maintenance: R-ISS stage at study entry (I/II versus III versus not classified) and MRD– after last HDM (no versus yes versus unknown) Induction (3 x 6-week cycles) Maintenance (4-week cycles) Days 1, 8, 15, and 22 Days 1 and 15 Day 1 Days 1, 8, 15, 22, and 29 Days 1, 15, 29
Primary end pointsc: Post-induction MRD– (NGF, 10–5); PFS after second randomization
Key secondary end points: PFS (whole study); OS (whole study and from second randomization); post-induction CR; CR and MRD– after HDM and during and after maintenance therapy
Selected secondary end point: PFS after first randomization

Study design: Isa-VRd vs VRd in transplant-ineligible NDMM
Treatment until PD, unacceptable toxicities, patient withdrawal

Primary endpoint: PFS
Key secondary endpoints: CR rate, MRD– CR (NGS, 10-5) rate, ≥VGPR rate, OS
*Patients considered Ti due to age or comorbidities.
†In the continuous phase, patients randomized to the VRd arm who experience PD may cross over to receive Isa-Rd.
‡10 mg/day if eGFR 30–<60 mL/min/1.73 m2.
§If aged ≥75 years, d was administered on days 1, 4, 8, 11, 15, 22, 25, 29, and 32.
BY: Thierry Facon, MD
C, cycle; d, dexamethasone; Isa, isatuximab; R, lenalidomide; SC, subcutaneous; V, bortezomib. Orlowski RZ, et al. ASCO 2018.
CEPHEUS: Phase 3 Study of DARA SC-VRd Versus VRd
in TIE or Transplant-deferred Patients With NDMM
Key eligibility criteria:
• NDMM (TIE or transplant deferred)
• ECOG PS score of 0-2
• Frailty score of 0-1
V: 1.3 mg/m2 SC Days 1, 4, 8, 11
: 25 mg PO Days 1-14
: 20 mg PO Days 1, 2, 4, 5, 8, 9, 11, 12
DARA: 1,800 mg SC QW Cycles 1-2, Q3W Cycles 3-8 VRd: schedule as above
21-day cycles
8 cycles of bortezomib treatment
Cycle 9+ R: 25 mg PO Days 1-21
: 40 mg PO Days 1, 8, 15, 22
Primary endpoint:
• Overall MRD (≥CR) negativity
DARA: 1,800 mg SC Q4W Rd: schedule as above 28-day cycles until disease progression or unacceptable toxicity
Key secondary endpoints:
• PFS
• Sustained MRD (≥CR) negativity (≥12 months)
• ≥CR rate
• OS
SC, daratumumab and recombinant human hyaluronidase for subcutaneous injection; ECOG PS, Eastern Cooperative Oncology Group performance status; V, bortezomib; SC, subcutaneous; R, lenalidomide; PO, oral; d,

DARA
dexamethasone; DARA, daratumumab; QW, weekly; Q3W, every 3 weeks; Q4W, every 4 weeks; CR, complete response.
Dr. Joe’s Take on Quadruplets
• It is very clear that combining more mechanisms of action controls the disease better
• With these newer agents, it is feasible to give quadruplets to most patients with newly diagnosed myeloma
• There is an art in dosing these agents to maximize the synergy and minimize the toxicity
• dose of lenalidomide
• dose and frequency of bortezomib
• dosing and tapering of dexamethasone
• We are still working on the ideal duration of agents in frontline and how to de-escalate or even discontinue therapy
• IMF Role – we continue to advocate for the PATIENT’S voice in the design of these trials, especially in de-escalation

4. WHAT IS THE OPTIMAL MAINTENANCE STRATEGY
IN MYELOMA?
PERSEUS: Study Design
Maintenance
• Transplanteligible NDMM
• Age 18‒70 years
• ECOG PS ≤2
4 cycles of 28 days
28-day cycles
Continue DR until PD Stop Dara and Restart Dara continue R per criteria
• MRD-negativityc rate was defined as the proportion of patients achieving MRD negativity and ≥CR in the ITT population
– Patients who were not evaluable or had indeterminate results were considered MRD positive
– MRD was evaluated post consolidationd at the time of suspected CR/sCR; at 12, 18, 24, 30, and 36 months after cycle 1 day 1; and yearly thereaftere
aStratified by ISS stage and cytogenetic risk. bDara 1800 mg co-formulated with rHuPH20 (2000 U/mL; ENHANZE ® drug delivery technology, Halozyme, Inc., San Diego, CA, USA); VRd administered as in the VRd group. cMRD was assessed using the clonoSEQ assay (v.2.0; Adaptive Biotechnologies, Seattle, WA, USA) in patients with ≥VGPR post consolidation and at the time of suspected ≥CR. dIn patients with ≥VGPR. eIn patients who achieved CR/sCR and remained on study.
CR, complete response; Dara, daratumumab; DR, daratumumab and lenalidomide; DVRd, daratumumab, bortezomib, lenalidomide, and dexamethasone; ECOG PS, Eastern Cooperative Oncology Group performance status; ISS, International Staging System; ITT, intent-to-treat; MRD, minimal residual disease; NDMM, newly diagnosed multiple myeloma; PD, progressive disease; rHuPH20, recombinant human hyaluronidase PH20; R, lenalidomide; sCR, stringent

PERSEUS: Sustained
MRD-Negativity (10‒5) ≥CR Rates
by P Moreau at the American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2025; Chicago, IL, USA & Virtual

Dr. Joe’s Take on Maintenance
• Historically we know that lenalidomide maintenance prolongs PFS and even improves survival
• Other approaches have added agents to lenalidomide to improve outcomes, especially in higher risk patients
• Due to its convenience and emerging data, there has been a trend to use daratumumab in maintenance
• The recent results from the PERSEUS is quite convincing for its use for up to 2 years
• Several ongoing studies are evaluating the potential to STOP maintenance primarily based on MRD status
• IMF Role – we continue to study MRD and its importance in decision making in MM

5. CAN MRD STATUS GUIDE THERAPY?
The Evolution of Response Assessment in Myeloma

Courtesy of Rafael Fonseca
Dr. Joe’s Take on MRD
• MRD is an incredibly powerful biomarker
• MRD is diagnostic and prognostic currently and will be more ”therapeutic” very soon
• It may help reduce the need of certain more intense therapies like stem cell transplant
• It is a particularly important goal in high risk myeloma
• It is now an accepted endpoint in clinical trials
• I believe it will become standard practice in myeloma to guide escalation and de-escalation of therapy....
• IMF Role – we continue to study MRD and its importance in decision making in MM

6. HOW DO WE OVERCOME HIGH RISK MM?
GMMG-CONCEPT Trial design
Stem cell mobilization after cycle 3
Isa: 10 mg/kg D1,8,15,22 in C1; D1,15 in C2+; K: 20 mg/m² D1,2 of C1; 36 mg/m² D8,9,15,16 of C1 and D1,2,8,9,15,16 in C2+; from 2021 onwards: 56 mg/m² on D1,8,15 and 70 mg/m² on D1,15 in maintenance; R: 25 mg D1-21 all Cycles; d: 40 mg D1,8,15,22 all Cycles (20 mg age >75).
Arm A: app. 15-18 months after inclusion
Arm B: app. 12 months after inclusion
HRMM criteria: ISS stage II or III PLUS ≥1 of: del(17p), t(4;14), t(14;16) and/or ≥3 copies 1q21 (amp1q21)
Primary objective: MRD negativity after consolidation (NGF, 10-5)
Secondary objective: PFS; Selected tertiary objectives: ORR, OS
ASCT, autologous stem-cell transplant; d, dexamethasone; HDT, high-dose therapy; HRMM, high-risk multiple myeloma; Isa, isatuximab; ISS, International Staging System; K, carfilzomib; MRD, minimal residual disease; ND, newly-diagnosed; NGF, next-generation flow; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; R, lenalidomide; TE, transplant-eligible; TNE, transplant-ineligible. Following a protocol amendment in 2021, carfilzomib application was switched to once weekly 56 mg/m2.
Progression-free and Overall Survival
• Median follow-up of 43 months (0-90.2 months)
1st cohort: 69 months (0-90.2 months)
2nd cohort: 33 months (5.5-43.3 months)
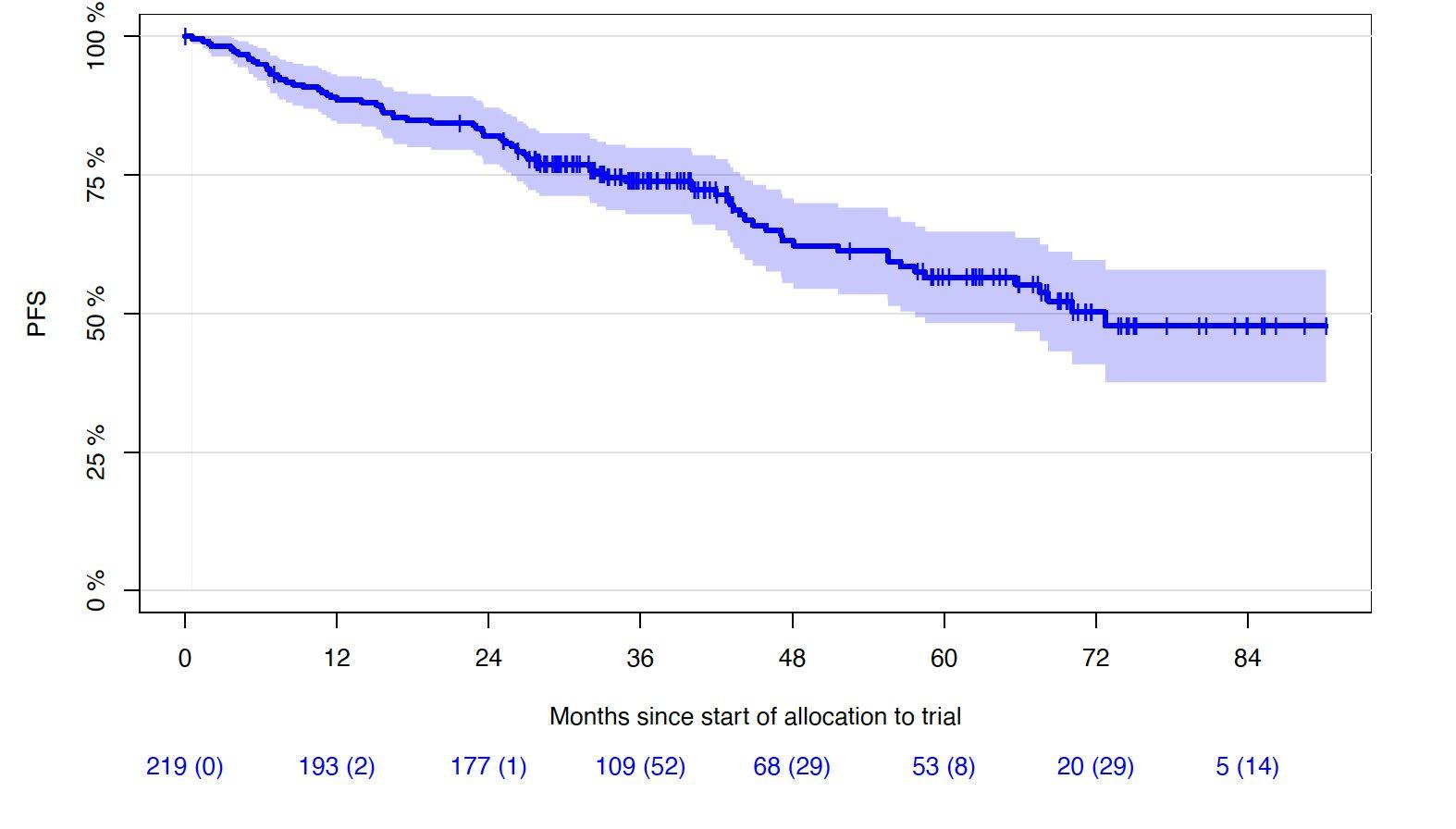
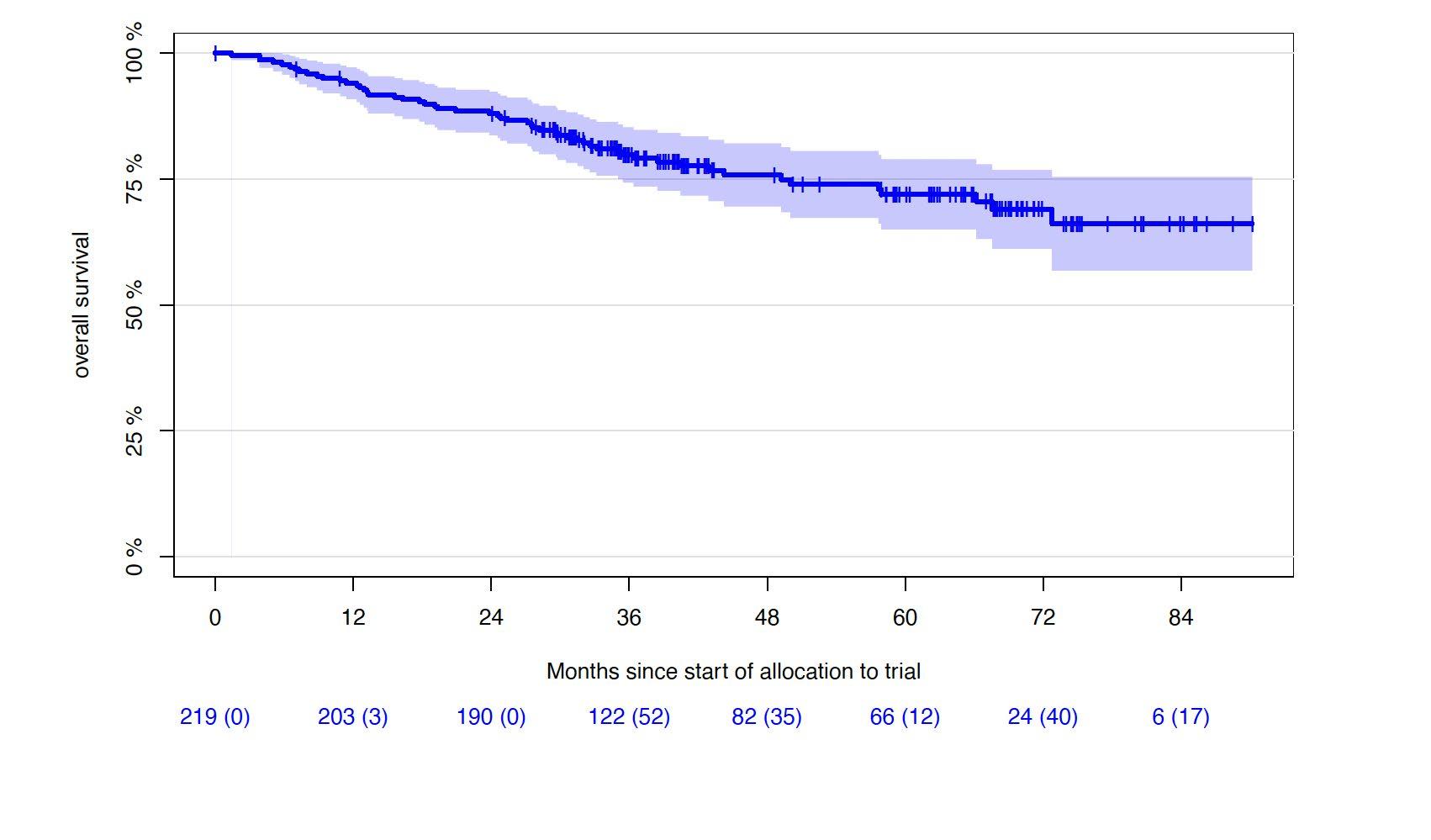
5Y-OS-rate: 72% (65.0-78.9%)
6Y-OS-rate: 69% (61.2-76.8%)
Lisa B. Leypoldt, MD
Results
Sustaining MRD-negativity was associated with increased PFS
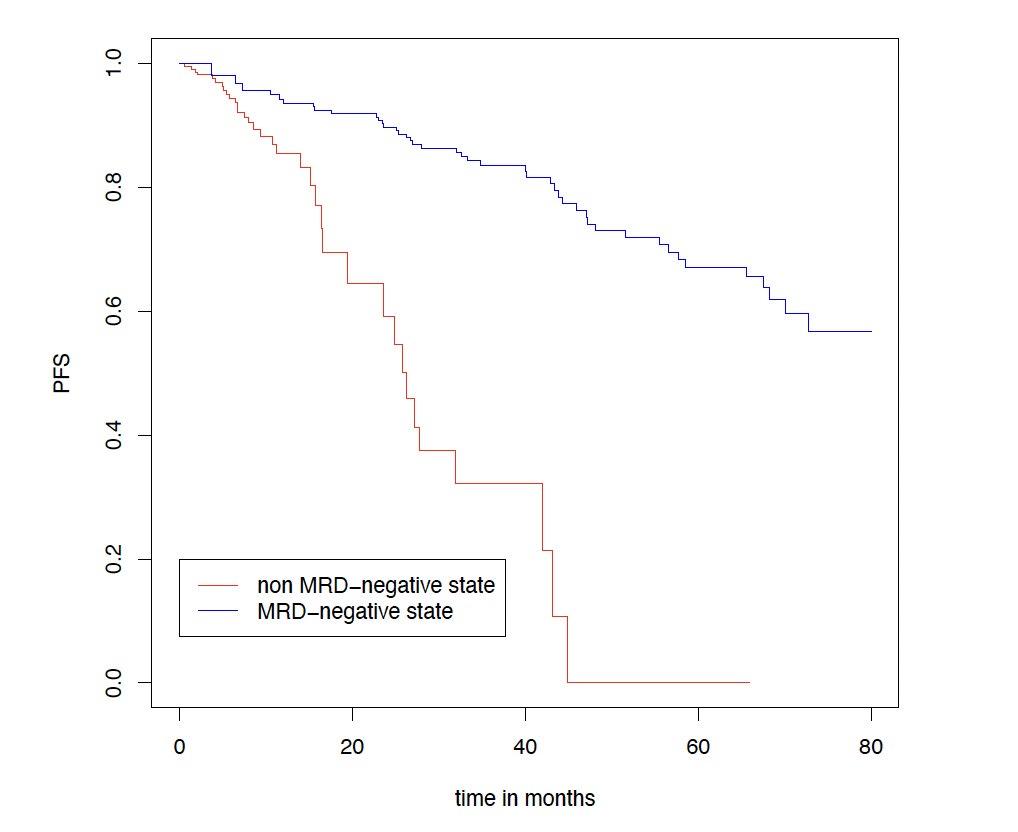
CI, confidence interval; HR: hazard ratio; MRD, minimal residual disease. Clinical data cut-off: April 28, 2025.
Multivariable time-dependent Coxregression analysis showed a prognostic PFS benefit for remaining in MRD negativity versus non–MRD negativity with a hazard ratio of 0.15 (95% CI, 0.07-0.29, p<0.0001)
Results for TE patients. Figure shows a Simon-Makuch plot illustrating the estimated survival while staying in the state of MRD-negativity compared to the estimated survival while staying in the state of MRD-positivity. Patients may switch between the two states under therapy.
PRESENTED BY:
Lisa B. Leypoldt, MD
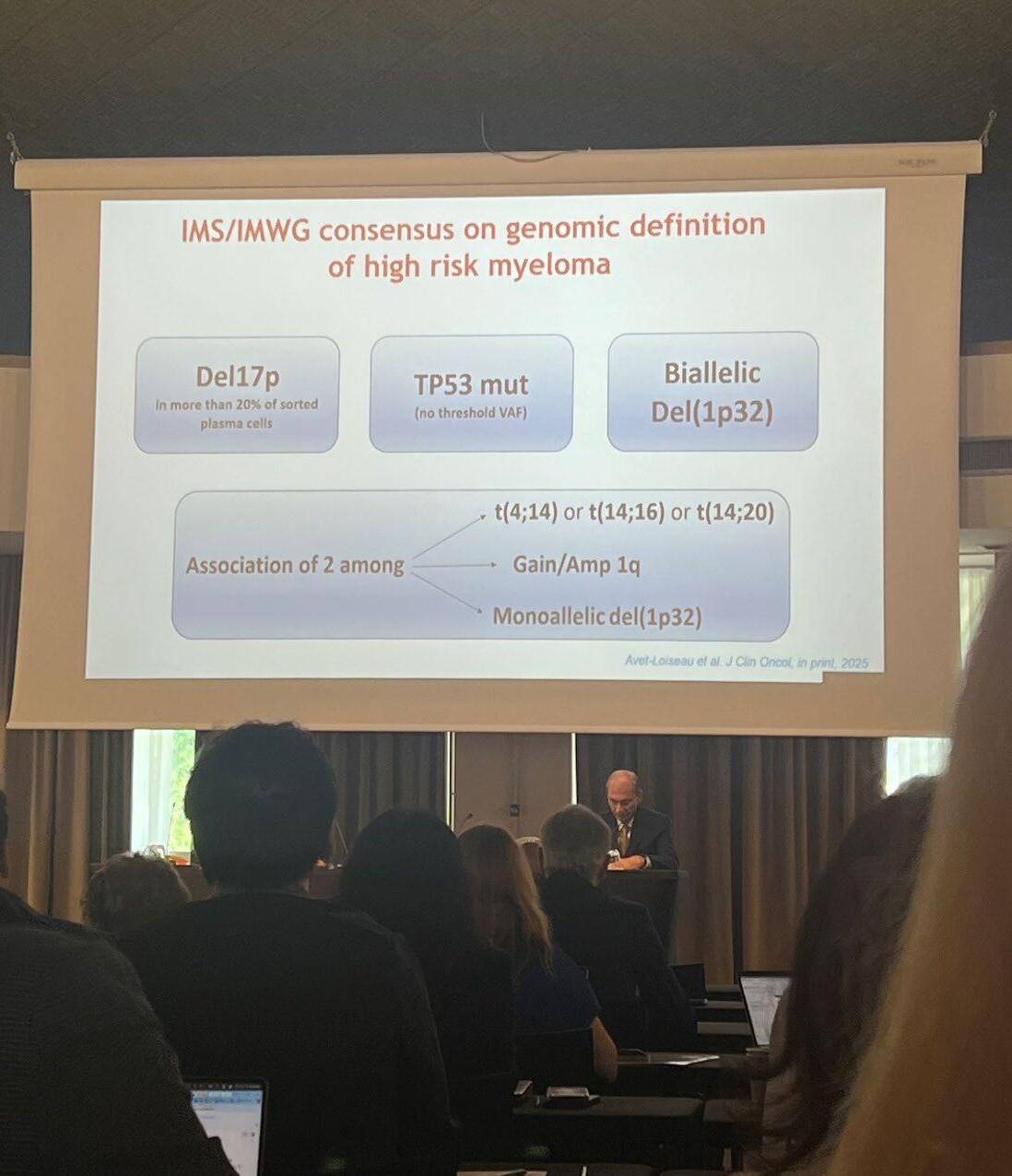
IMS/IMWG consensus on high risk myeloma definition

Del17p in more than 20% of sorted plasma cells
2 among t(4;14) or t(14;16) or t(14;20)
Dr. Joe’s Take on High Risk Myeloma
• A NEW definition is was JUST released to truly identify those patients at highest risk
• It is genuinely an unmet need in MM, as sadly some still succumb to their disease in the first year of diagnosis
• Patients require combination therapies, given continuously and with MRD negativity as the goal
• It is possible that CAR T Cell therapy may change this approach as even high risk patients can have deep and durable responses to CAR T
• IMF Role – in conjunction with the IMS (International Myeloma Society) we convened the IMWG to write the new definition of high risk MM

7. CAN WE MORE EFFECTIVELY, SAFELY AND CONVENIENTLY GIVE BISPECIFIC ANTIBODIES?
Cytokine Release Syndrome (CRS)
Severity for BsAbs and CAR T Ranges From Mild to Life-Threatening

CAR T: Need to Manufacture
Monitoring for CRS, Neurotoxicity
• Vital signs (eg, temperature, O2 saturation)
• Review of systems and physical exam
– Focus on cardiovascular, pulmonary, and neurologic systems
• Rule out infection
• Laboratory monitoring
– CRP
– Cytokines
– Ferritin
– LDH
• Mental status scoring

RESPIRATORY
Hypoxia
Dyspnea
Capillary leak syndrome
HEPATIC
Transaminitis
↑ ALP
Hyperbilirubinemia
RENAL
↑ Serum creatinine
Renal insufficiency
TLS
HEMATOLOGIC
Anemia
Thrombocytopenia
Neutropenia
CONSTITUTIONAL
Fever
Fatigue, malaise
Headache

NEUROLOGIC
Delirium
Somnolence
Dysphagia
CARDIOVASCULAR
Sinus tachycardia
Hypotension
Arrhythmias
GASTROINTESTINAL
Nausea
Vomiting
Diarrhea
MUSCULOSKELETAL
↑ CPK
Myalgia
Weakness
Neurotoxicity in Immunotherapy

Monitoring for Immune Effector CellAssociated Neurotoxicity Syndrome (ICANS)1,2
ICE screening tool
Review of systems and physical examination
– Focus on neurologic systems
Rule out infection
If ICANS suspected – Neuroimaging (ideally MRI)
– Diagnostic lumbar puncture for opening pressure and infection tests
Corticosteroids are typically indicated for ICANS ≥ grade 2
Patient and care partner information
Abbreviations: AE, adverse event; ICE, immune effector cell encephalopathy. 1. Brudno JN, Kochenderfer JN. Blood. 2016;127:3321-3330. 2. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625-638.
Neurotoxicity
Ataxia
Apraxia
Facial nerve palsy
Tremors
Seizures
Hallucinations

ICE Screening Tool for Neurologic Assessment
Dr. Joe’s Take on Managing Immunotherapies
• Every treatment in myeloma goes through an evolution after approval to increase efficacy and reduce toxicity
• We are in the midst of that evolution now
• working towards outpatient management
• understanding side effects
• developing strategies to prevent and treat them rapidly
• sharing information between experts to speed up the process
• The IMF Immune Therapy Registry was designed to do this!
• IMWG guidelines for bispecific antibodies and CAR T Cell therapy
• now with a “living” component by keeping them up to date online
• IMF Role – we are hosting the living guideline with help from our NLB (Nurse Leadership Board)

8. IS THERE AN IDEAL SEQUENCING APPROACH IN
RELAPSED MYELOMA?
Don’t Save the Best for Last!

In every new LOT, ~15-35% of patients are lost
Figure adapted from: Yong, K et al. Br J Haematol 2016;175(2):252-264
Shared Decision-Making Strategies
Patients and providers should make individualized decisions together regarding treatment
SHARE approach
Seek patient participation
Help the patient explore and compare treatment options
Assess patient’s values and preferences
Reach a decision with the patient
Evaluate the patient’s decision


Noonan K, et al. J Adv Pract Oncol. 2022;13:15-21; Faiman B, et al. Clin J Oncol Nurs. 2019;23:540-42;
International Myeloma Foundation. https://www.myeloma.org/resource-library/tip-card-myeloma-treatment-discussion-tool.
Dr. Joe’s Take on Sequencing
• There really is no IDEAL sequence that we know of in myeloma
Key Principles include:
• Use the most effective therapies as early as possible in the disease course
• in eligible patients sequence CAR T prior to other immunotherapies
• The BCMA target can be used more than once
• Shared decision making (SDM) is critical to matching the right therapy to the patient
• IMF Role – The IMF’s NLB essentially defined SDM and we continue to support the importance of patient preference

9. HOW CAN WE ADDRESS THE GLOBAL
DISPARITIES IN MYELOMA?




Reference:

Liu, X., Zhuang, H., Li, F. et al. Trends and projections of the global and regional burden of multiple myeloma in adults aged 40 and over, 1990–2044. Sci Rep 15, 13595 (2025). https://doi.org/10.1038/s41598-025-96981-w

M-Power = Myeloma Power
Thefactsaresobering:
• MyelomaistwiceascommoninAfrican Americans
• Survival in African Americans in HALFthat of White Americans
• Thedisparityismultifactorial
The core vision of this initiative is to improve the short- and long-term outcomes of African American and Latino patients with myeloma. We want to empower patients and communities to change the course of myeloma…
Enhance access to optimal care by educating myeloma providers about the disparity and how to reduce it
Engage the community to increase awareness and provide support
Shorten the time to diagnosis by educating primary care providers to recognize the disease and order the right tests

Juneteenth 2025 - Brooklyn



Dr. Joe’s Take on Disparities
• There is no greater problem in myeloma worldwide!
• It is a combination of multiple factors
• Social determinant of Health
• Delayed Diagnosis
• Reduced Access to Novel therapies
• It is not an easily solved problem but critical advances can be made
• The IMF M-Power program is an example of a strategy that can be applied more broadly with short and long term goals
• IMF Role – we are truly a leader in reducing health disparities in medicine through M-Power and GMAN (Global Myeloma Action Network)

10. ARE WE CURING MYELOMA?
Survival in multiple myeloma has improved over time
Overall survival by decade of MM diagnosis1

CI, confidence interval; HR, hazard ratio; MM, multiple myeloma; OS, overall survival.

1. Puertas B, et al. Cancers (Basel). 2023;15(5):1558
Long-Term (≥5 Year) Remission and Survival After Treatment With Ciltacabtagene Autoleucel in CARTITUDE-1
Patients With Relapsed/Refractory Multiple Myeloma
Peter M Voorhees1, Thomas G Martin2, Yi Lin3, Adam D Cohen4, Noopur Raje5, Myo Htut6, Abhinav Deol7, Mounzer Agha8, Jesus G Berdeja9, Binod Dhakal10, Andrzej J Jakubowiak11, Samir Parekh12, Hui Li13, Rocio Montes de Oca14, Huabin Sun15, Nikoletta Lendvai15, Deepu Madduri15, Mythili Koneru16, Nitin Patel16, Sundar Jagannath12
1Atrium Health/Levine Cancer Institute, Wake Forest University School of Medicine, Charlotte, NC, USA; 2University of California, San Francisco, CA, USA; 3Mayo Clinic, Rochester, MN, USA; 4Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; 5Massachusetts General Hospital Cancer Center, Boston, MA, USA; 6City of Hope Comprehensive Cancer Center, Duarte, CA, USA; 7Karmanos Cancer Institute, Wayne State University, Detroit, MI, USA; 8UPMC Hillman Cancer Center, Pittsburgh, PA, USA; 9Tennessee Oncology, Nashville, TN, USA; 10Medical College of Wisconsin, Milwaukee, WI, USA; 11University of Chicago, Chicago, IL, USA; 12Icahn School of Medicine at Mount Sinai, New York, NY, USA; 13Johnson & Johnson, Shanghai, China; 14Johnson & Johnson, Spring House, PA, USA; 15Johnson & Johnson, Raritan, NJ, USA; 16Legend Biotech USA Inc., Somerset, NJ, USA
https://www.congresshub.com/Oncology/ AM2025/Cilta-cel/Voorhees
Copies of this presentation obtained through Quick Response (QR) Codes are for personal use only and may not be reproduced without permission from ASCO® or the author of this presentation.

Presented by PM Voorhees at the American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2025; Chicago, IL, USA & Virtual
CARTITUDE-1 Long-Term Remission:
One-Third of Patients Were Progression-Free for ≥5 Years
Overall population (N=97); median follow-up: 61.3 months
Progression-free survival
32 of 97 (33%) patients were treatment- and progression-free at ≥5 years

cilta-cel, ciltacabtagene autoleucel.
Dr. Joe’s Take on Cure
• I like to define cure in the way someone on the street would likely define it... someone is given limited therapy then no longer has to think about the disease
• We have always had a very small fraction of patients who with limited therapy have particularly long remissions
• I believe that fraction continues to grow with the novel approaches we are providing
• It will not happen overnight – but will be the result of a global coalition against myeloma bringing together the best science, clinical trials, experts and motivated patients...
• IMF Role – we are leading the effort to “define” cure for scientific purposes and conduct “cure” trials...

Bonus Topic – Down with Dex!


Dr. Joe’s Approach to Dex
• Dexamethasone Dose De-escalation Protocol

1. Assess 40 mg versus 20 mg weekly starting dose
2. Limit starting dose to 2 to 3 months
3. Then taper dose each month over the course of 3 to 4 months
4. Plan to stop dexamethasone at 6 months
5. Regular assessments for toxicity and de-escalate sooner if clinically indicated Join the DOWN with

FFinal Thoughts...

• There are still MANY more unanswered questions! • But the future for myeloma patients is genuinely bright • We must
all be a part of the solution!
Turning Personal Stories into Powerful Advocacy:
How to Get Involved and Make a Difference

Michael Riotto
IMF Grassroots Advocacy Consultant, Support Group Leader (Philadelphia,
PA)

Turning Personal Stories into Powerful Advocacy: Our Advocate Panel




Kathy Missel Support Group Leader (Sebring, FL)
Diane Hunter Support Group Leader (Montgomery, AL) Support Group Leader (Staten Island, NY)
Elissa Anticev

1. Ensure Access to Care: We advocate to ensure all myeloma patients have equitable, comprehensive, patient-centered care without insurance barriers that limit options or delay treatment initiation.
2. Eliminate Financial Barriers: We advocate for policies that allow myeloma patients access to treatments and supportive care interventions without facing financial hardships.
3. Advance Myeloma Research: We advocate for annual appropriations funding for myeloma research and the advancement of clinical trial eligibility and research protocols that ensure representation from diverse populations.

The IMF Grassroots Advocacy Program is multi-faceted and growing
• Advocacy Training & Leadership Development
• Policy and Legislative Education
• Grassroots Campaign Planning
• Health Policy Forums & Roundtables
• Advocacy Resource Development
• Storytelling and Personal Narratives



Stretch & Morning Break


Serdar Erdogan Myeloma has no Borders
IMF Director of GMAN
EU/ME Patient Programs

Global Myeloma Action Network (GMAN) | Uniting a Global Patient Community

GMAN is a global consortium of 50 myeloma patient organizations representing 46 countries.
GMAN works at the intersection of research, advocacy and member support in collaboration with patients, care partners, physicians/KOLs, policymakers, the pharmaceutical industry and other global stakeholders.
Research
Increase awareness of and participation in clinical trials worldwide.
Access
Influence policymaking for improved access to timely diagnosis and critical therapies.
Member Support
Education, awareness and capacity building to strengthen GMAN’s National Member Organizations.


GMAN’s National Member Organizations | Global Reach

GMAN brings together 50 National Member Organizations from 46 countries across 5 continents
EUROPE & MIDDLE EAST (29)
MYMU BELGIUM
MIJELOM CROATIA
CZECH MYELOMA GROUP

NA - IMF USA

KPMM CZECH REPUBLIC
ARMENIAN HEMATOLOGY ASSOCIATION
MYELOMA UND LYMPHOMHILFE ÖSTERREICH

LATAM - COLOMBIA
FUNLEUCEMIALIMFOMA

CHILE, PARAGUAY & URUGUAY
FUNCA PARAGUAY
AUMM URUGUAY
FUNDACION MYELOMA CHILE


LATAM – BRAZIL
IMF LATIN AMERICA

MULTIPLES MYELOM SELBSTHILFE ÖSTERREICH
DANSK MYELOMATOSE FORENING
SYOPAPOTILAAT FINLAND
AF3M FRANCE
LHRM GERMANY
AMEN ISRAEL
AIL ITALY
AFRICA
KENYAAMPATH
ASIA PACIFIC

ICELANDIC MYELOMA ASSOCIATION
BLODKREFTFORENINGEN NORWAY
CARITA POLAND
POLISH ASSOCIATION FOR MYELOMA PATIENTS
FUNDACJA CENTRUM LECZENIA SZPICZAKA POLAND
APLL PORTUGAL & APCL PORTUGAL
MYELOMA EURONET ROMANIA
MYELOMA MULTIPLEX CSOPORT HUNGARY


ASSOC. OF MYELOMA PATIENTS OF SERBIA
SLOVAK MYELOMA SOCIETY
ASSOC. OF PATIENTS WITH BLOOD DISEASES SLOVENIA
SLOVENSKO ZDRUZENJE BOLNIKOV
CEMMP SPAIN

KOREA BLOOD CANCER ASSOCIATION
ASIA PACIFIC (8)
ASIAN MYELOMA NETWORK (6)
MYELOMA FRIENDS INDIA
MYELOMA AUSTRALIA NA - MYELOMA CANADA

LATAM - ARGENTINA
FUNDACIÓN ARGENTINA DE MIELOMA
BLODCANCERFORBUNDET SWEDEN
STIFTUNG ZUR FÖRDERUNG DER
KNOCHENMARKTRANSPLANTATION SWISS
MYELOMLA YASAM TURKEY
ASSOCIATION FOR PATIENTS WITH CHRONIC LYMPHOPROLIFERATIVE DISEASES - UKRAINE
KANSER SAVASCILARI DERNEĞI TURKEY
MYELOMA UK
AUSTRALIA

NEW ZEALAND
MYELOMA NEW ZEALAND

GMAN: One voice globally, collaboration regionally, and independent at national level GMAN’s National Member Organizations | Global Reach

Umbrella Organizations
Finland. Kenya, Paraguay, Turkey
Myeloma Groups
Hungary, Philippines, Thailand
Blood Cancer Organizations
Armenia, Colombia, Italy, Norway, Portugal(2), South Korea, Sweden, Ukraine
Well Structured Organizations
Australia, Brazil, Canada, Denmark, France, Italy, United Kingdom. United States
GMAN’s National Member Organizations


Denmark, France & Italy: Strong regional structure, volunteers operate the organization

Czechia: Strong clinical trial integration with support groups

Germany: Focused regional support groups

Hong Kong, Malaysia, Philippines, Singapore, Thailand, Taiwan, South Korea:
Patient organizations are growing under the roof of AMN

Argentina, Brazil, Chile, Colombia, Uruguay:
Patient organizations are growing under the roof of LAMN




GMAN Annual Summit | 2025


Topics Included:
• Barriers to accessing both standard of care & novel therapies
• HTA and regulations in EU
• Capacity building

June 2025 | Milan, Italy
• 2 days
• 30 country patient leaders attending
• 4 national educational grants awarded
• Scientific updates, panel discussions, policy and awareness


GMAN Annual Summit | 2025

Mercy Oduor receiving grant on behalf of AMPATH:
Expanding Early Detection, Access and Support for Myeloma Patients in Kenya

Susie Novis Durie
Grants:
2025 | Grant Recipients
• Armenia
• Kenya
• Poland
• Serbia
Each project is awarded with $8,000 USD
Armenian Hematology Association
“Myeloma Armenia 2025/26 – National Educational Seminars on MM”
AMPATH - The Academic Model Providing Access to Healthcare
Expanding Early Detection, Access, and Support for MM Patients in Kenya
CARITA Foundation
“In The Blood” – A Quarterly Magazine for Myeloma Patients & Carers
Association of Myeloma Patients Serbia
Knowledge Unites the CoMMunity

PROJECT FROM CROATIA:
A MEAN DISEASE WITH ORDINARY SYMPTOMS

Case Study: Mijelom CRO
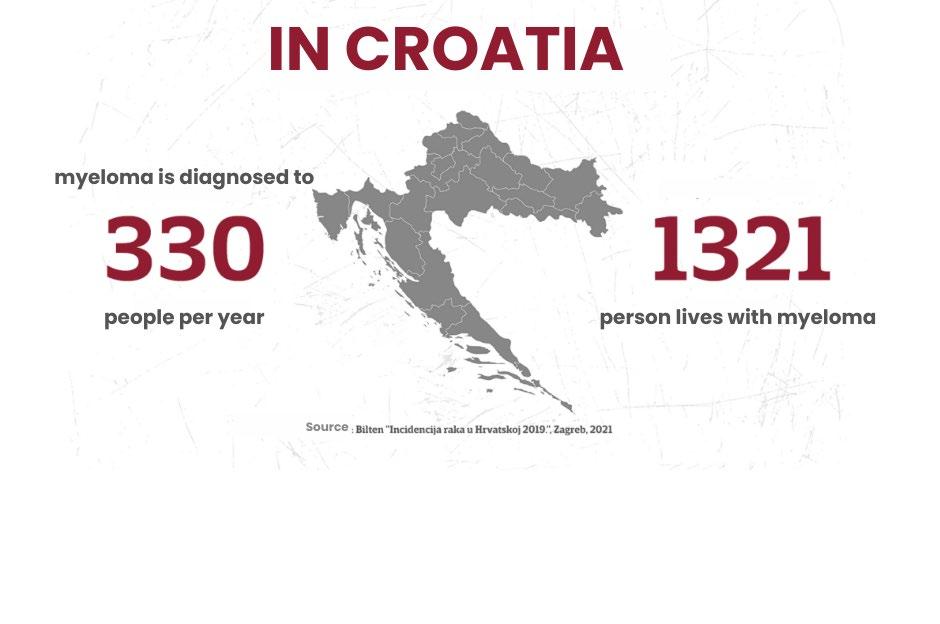

Mijelom CRO: A Mean Disease with Ordinary Symptoms


Objectives:

Educate and remind general medical practitioners about myeloma and amyloidosis.
Raise local awareness about multiple myeloma.
Connect people who are affected by these diseases, living in smaller towns within Zagreb Municipality and provide printed materials.
Mijelom CRO: A Mean Disease with Ordinary Symptoms (Results)

Samobor’s event attracted GPs and nurses
Dugo Selo’s event welcomed mainly patients and families (connection to existing patient).
Participants valued sharing experiences and networking.
Future efforts should focus more on GPs and physiotherapists to enhance impact.

Printed materials provided free to participants and distributed amongst 13 hospitals in Croatia
Raising awareness especially among GPs, nurses, and physiotherapists
Patient handbook - Physical Activity and Sport for People with Myeloma

PROJECT FROM DENMARK: LIVING WITH MYELOMA
Danish Project: Identifying Challenges in Living With Myeloma
Dansk Myelomatose Forening
• Celebrating 25 years of support for myeloma patients.
• Currently has 1,200 members in Denmark.
• Represents approximately 34% of myeloma patients in the country.
• Focus on raising awareness and providing resources.
• Aims to improve the quality of life for patients and families.



Danish Project: Identifying Challenges in Living With Myeloma

Both patients and relatives shared experiences and insights on the theme of cognitive challenges.
Responses highlighted difficulties in coping with everyday
Cognitive challenges were a prominent concern for care partners
Terms like “chemo brain” and “mental fog” were frequently mentioned.
Danish Project: Identifying Challenges in Living With Myeloma
SMART Training, a Danish-developed program that combines cognitive and motor exercises was implemented.

25.5% indicated experiencing at least one area of severe impairment, and 65.6% reported challenges rated 3 or higher on a 0–5 scale.



International Bill of Rights


2026 MAM + GMAN Campaign Idea
Each country can work on having a key point of interest in their territory light up in red supporting MAM for the month of March. It would be great if….Paris, Rio, Niagara Falls, Toronto, San Francisco, New York could have a landmark lit in red!!!




















Any Questions?


Fireside Chat #1:
Screening, Smoldering Myeloma & Frontline Therapy

Joseph Mikhael, MD
Shaji Kumar, MD Mayo Clinic, Rochester, MN
IMF SAB & IMWG



Robin Tuohy Morning Wrap-Up


Share a Memory



Headshots available by Jason London from 12
- 1 PM






Reminder to wear your name badge that includes your dinner tickets throughout the program




Lunchtime 12:00 - 1:00 PM





Welcome Back!


Fireside Chat #2:
Maintenance & Relapsed Therapy


Joseph Mikhael, MD
Kumar, MD

Shaji
Support Group Leader Guide:
Making Sense of Lab & Diagnostic Tests in Multiple Myeloma

Beth Faiman, PhD, MSN,
APN-BC, AOCN, BMTCN, FAAN,
FAPO
Cleveland Clinic Taussig Cancer Institute
IMF Nurse Leadership Board

Overview

• Describe common tests in myeloma and how to interpret
• Review the concepts of biomarkers and genomics
• Identify important findings of pathology and radiology reports

Biomarkers and Genomics are Important in Myeloma
Biomarker: A distinctive biological characteristic that can be objectively measured to diagnose disease, determine response to medications, or estimate prognosis
Numerous biomarkers in Myeloma
Genomics: A discipline concerned with mapping the genes (“gen-”) and chromosomes (“-ome”) of an organism as well as the identification of dysregulated or misprogrammed genes
Genomics as a science has gained popularity with the human genome project (HGP), an international, collaborative research program completed in 2003 which “mapped” the human genome and developed a “blueprint” of human genetic material
The HGP revealed there are approximately 20,500 human genes in which 99% of humans share
Changes in genes arise from a series of mutations, alterations to normal cells
What is Multiple Myeloma (MM)?
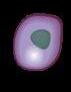












Cancer of the bone marrow

plasma cells that make excess proteins in the blood, urine
Understanding Your Test Results, International Myeloma Foundation 2025.

Not everyone with an abnormal protein has multiple myeloma -
Biomarkersandgeneticscan helpdiagnose

Spike on SPEP/UPEP
Abnormal Freelite Test
Bone Marrow < 10% PCs
Low Risk Risk of Progression: ≈ 1% per year
High Risk: Likely to Progress to Active MM Within 2 Years MGRS*
M-spike ≥ 2 g/dL
Freelite Ratio ≥ 20%
Bone Marrow ≥ 20%

CRAB Criteria
Calcium Elevation
Renal Dysfunction
Anemia
Bone Lesions
MDEs
Bone Marrow ≥ 60% PC
Freelite Ratio ≥ 100
CCR < 40 mL/min
MRI ≥ 1 focal lesion ≥5mm

CCR = creatinine clearance rate; MDE = myeloma-defining event; MGUS = monoclonal gammopathy of undetermined significance; MM = multiple myeloma; MRI = magnetic resonance imaging; PC = plasma clone; SMM = smoldering multiple myeloma; SPEP = serum protein electrophoresis; UPEP = urine protein electrophoresis. aMonoclonal gammopathy of renal significance (MGRS) does not meet criteria for myeloma but has kidney manifestation; kidney biopsy is the gold standard for diagnosis. Rajkumar SV, et al. Lancet Oncol. 2014;15(12):e538-48. Bridoux F, et al. Kidney Int. 2015;87(4):698-711. Terpos E, et al. Lancet Oncol. 2021;22(3):e119-e130. Hillengass J, et al. Lancet Oncol. 2019;20(6):e302-e312.
What kind of tests can one expect?

There are several tests you can expect:
Blood
Urine
Bone Imaging
Bone Marrow Biopsy
Ask your provider how often these need to be done (Monthly, every 1, 3, 6 months or annually)

Know who is responsible for ordering and monitoring these tests (community or academic center)
Interpreting the Blood Tests: “Monoclonal Protein” Work Up
Organ function: complete blood count, blood chemistry panel
Serum protein electrophoresis – “MSPIKE”
How much monoclonal is present? Low(0.1gms) or high (10.00gms)?
Serum immunofixation – “IgG Kappa, or IgA lambda”, etc…..
What type of monoclonal
Serum free light chains “Kappa or Lambda”
Does one have a high serum free Kappa or Lambda?
Serum immunoglobulins – total values
IgG, IgA, IgM
Urine studies, protein electrophoresis and immunofixation, light chains 24-hr is the best, especially if there is proteinuria on recent UA
In a recent study, the age of first chromosomal gain to age of sample collection 30-40 yrs
MGUS to MM
Gene translocations can be present many yrs before genetic changes

Alberge, J.-B., Dutta, A. K., Poletti, A., Coorens, T. H. H., Lightbody, E. D., Toenges, R., Loinaz, X., Wallin, S., Dunford, A., Priebe, O., Dagan, J., Boehner, C. J., Horowitz, E., Su, N. K., Barr, H., Hevenor, L., Towle, K., Beesam, R., Beckwith, J. B., . . . Ghobrial, I. M. (2025). Genomic landscape of multiple myeloma and its precursor conditions. Nature Genetics, 57(6), 1493-1503. https://doi.org/10.1038/s41588-025-02196-0
Testing Needed To Define The Diagnosis
Lab tests – Blood and Urine
– CBC + differential, complete metabolic panel (albumin, creatinine, calcium), Beta-2 Microglobulin
– Serum protein electrophoresis (SPEP), Free light chain assay with kappa/lambda ratio, serum immunofixation
– Urine immunofixation & protein electrophoresis (UPEP)
Bone marrow biopsy & aspirate
– Plasma cell concentration (%)
– Congo Red staining if concern for AL-Amyloid
– Cytogenetics, “BRAINS” of the cells with “FISH”, for genetic information on prognosis
Imaging:
– Skeletal survey
– WB-LD CT ± PET (Whole body low dose CT)
– MRI ± PET scan

When
do you Treat Patients for Multiple Myeloma
The current goal is to find patients at

Clonalbonemarrow≥10%,orplasmacytomaANDany one or more Myeloma Defining Events(MDE)

Common findings in Myeloma: Anemia
• A reflection of the bone marrow’s ability to produce cells that are obtained through peripheral blood draws

Lab values vary slightly amongst institutions and laboratories
• Anemia is a functional inability of the blood to supply the tissue with adequate oxygen for proper metabolic function
• It is not a disease, but rather the expression of an underlying disorder or disease
• Morphologic approach: MCV
CBC (Complete Blood Count): A window into your body’s ability to make healthy
red cells, white cells and platelets

WhiteCells fight infection
Hemoglobincarries Oxygen Platelets Clot your blood
• The “differential” –numbers at the bottomare a break down of the healthy cells
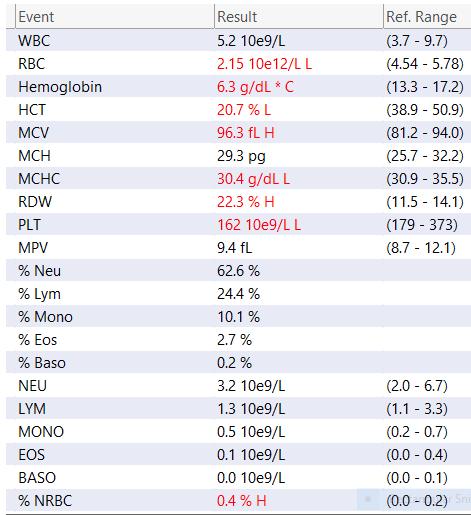
Tip: Make sure to look at the reference range, AND look for your past results (high,)low? Stable?
This shows findings of anemia and low blood platelets.

Complete Metabolic Panel (Blood test)
Indicators of myeloma-related organ dysfunction on CMP
• BUN
• Creatinine
• Calcium
• ALB
• Alkaline phosphatase
Metabolic Panel: Shows organ function (kidney,liver,bone)

Glucose: Diabetes?
ALT, AST, (liver) ALK
PHOS: Bone or liver
Total Protein, Albumin: low in AL amyloidosis, Active myeloma due to high total Protein levels
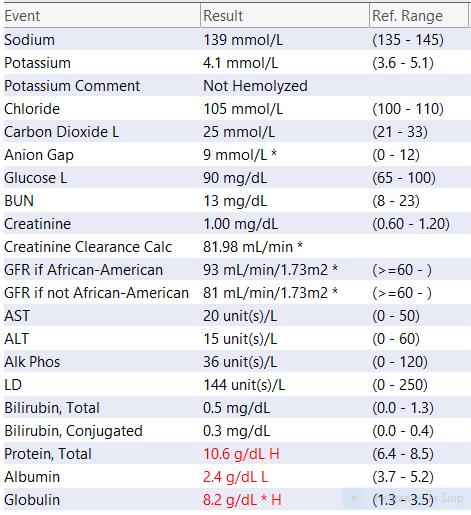
Many of these are important Electrolytes to keep our bodies in balance
Creatinine/ BUN: Kidney function
LD (LDH): tumor burden, prognosis
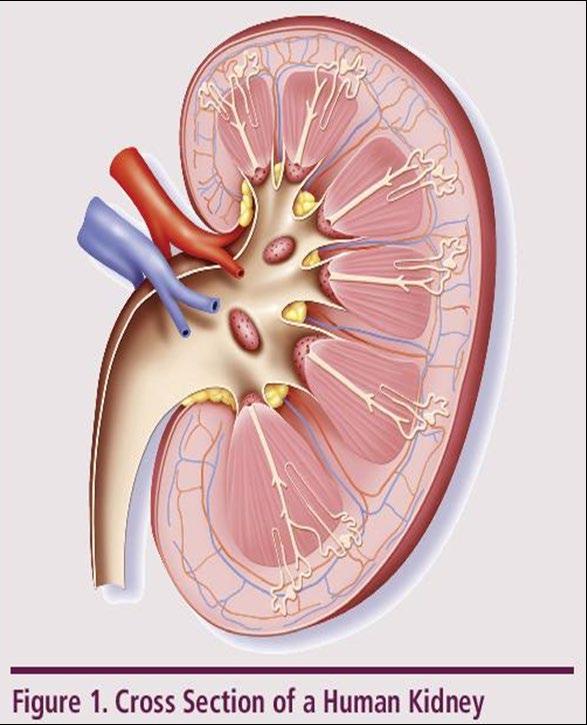
Understanding Changes to Kidney Function
►Risk Factors
– Active multiple myeloma (light chains, high calcium)
– Other medical issues (ex: Diabetes, dehydration, infection)
– Medications (MM treatment, antibiotics, contrast dye)
Prevention
– Drink, Drink, Drink
– Avoid certain medications, when possible
Treatment
– Treatment for myeloma – Hydration – Dialysis


Myeloma Cells Can Cause Damage To Bones
Most
myeloma patients will experience
bone involvement at some point; it is important to protect your bone health
► Approximately 85% of myeloma patients develop bone disease
► Protecting bone health
– Nutrition
– Weight-bearing activity
– Medications
• Vitamin D

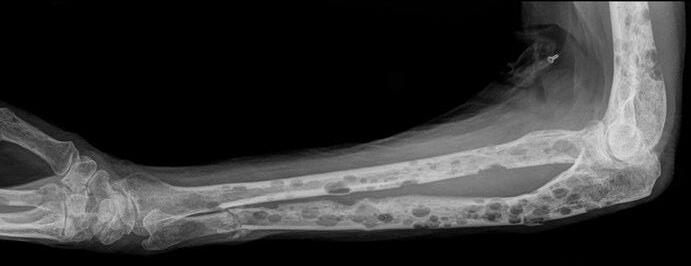
• Calcium (if approved by doctor)
• Bone strengthening agents: Zometa® (zoledronic acid), Aredia (pamidronate), or Xgeva® (denousamab)
► Report new pain to your health care provider
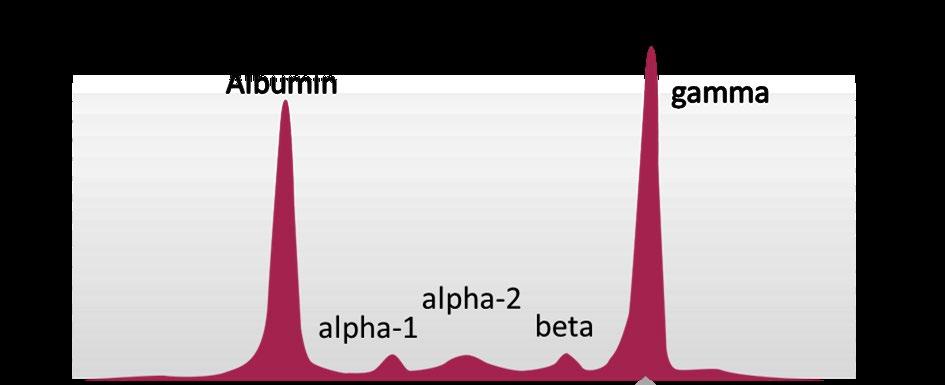
SPEP and UPEP – looks at “M-SPIKE”

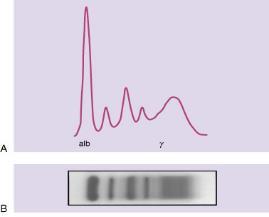
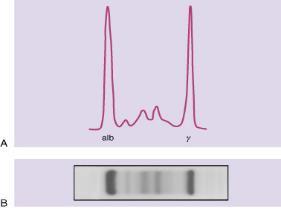
Monoclonal Proteins and Immunoglobulins

Immunoglobulin structure
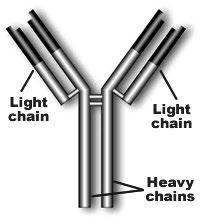
Monoclonal Proteins and Immunoglobulins

Hypogammaglobulinemia:
Serum (Blood) Free Light Chains
Normal Abnormal
Kappa light chain: 3.3–19.4 mg/L
– Normal ratio for free lightchain kappa: Lambda is 0.26–1.65
Elevation of kappa: Lambda ratio suggests kappa light chain M gammopathy is present
Increasing trend of kappa sFLC in a patient with MGUS that transforms to myeloma

Lambda light chain: 5.7–26.3 mg/L
– Kappa: Lambda free lightchain ratio < 0.26 typically defined as having M lambda free light chain
Decreased in Kappa: Lambda ratio suggests lambda light chain M gammopathy is present/dominant clone
(H)
(H)
(H)
(H)
24-Hour Urine for Protein and Immunofixation

Why is my provider asking for a 24-hr urine evaluation if a secondary analysis says it doesn’t change response?
- Still very accurate to detect a monoclonal protein for diagnosis of MM, amyloidosis
- Measures kidney health, bone loss
- Study procedures will still require for some time

Lab values vary slightly amongst institutions and laboratories
Bone Imaging Tests

Whole Body Low -Dose CT (WBLDCT)
Best for early screening for bone disease

Response assessment: active residual disease
MRI
WB or spine + pelvis
Gold standard to assess bone
marrow involvement
CT = computed tomography
DEXA = dual-energy x-ray absorptiometry
MRI = magnetic resonance imaging; PET = positron emission tomography
WB = whole body
WBLDCT = whole-body lowdose computed tomography
NOTE: Bone scan (DEXA) for bone density is useful in some situations
Brigle K, et al. JAdv PractOncol . 2022;13(Suppl 4):7-14. Hillengass J, et al. LancetOncol . 2019;20(6):e302-e312. Rome SI, et al. ClinJ OncolNurs . 2017;21(5 suppl):47-59. Faiman B. ClinLymphomaMyelomaLeuk . 2014;14:436-440. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-1556.

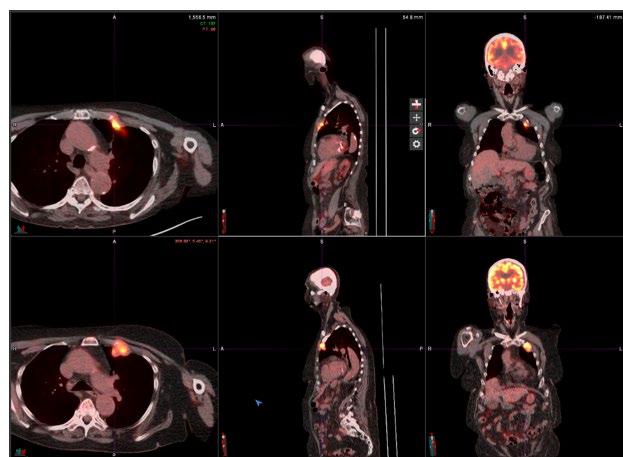
PET Scan Example

What is DNA?

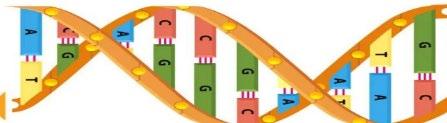
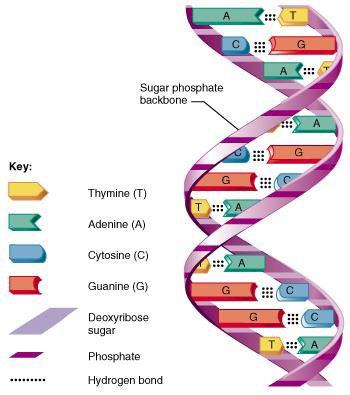
• Integral to the identification of the human genome was an understanding of the coiled double helix structure known as deoxyribonucleic acid (DNA), covered with proteins called histones
• Genetic information is contained in cells, which forms a sequence
• A DNA consists of four nucleotide base pairs including: adenine (A), thymine (T), guanine (G) and cytosine (C)
• Each base pair develops a sequence which can be followed and replicated
• For example, the purine adenine always pairs with the pyrimidine thymine (“A with T”), while the pyrimidine cytosine always pairs with the purine guanine (“C with G”)
• All individuals are born with DNA that express genes and represents opportunities for variability from one human to the next
Detection of Genetic Alterations in Myeloma



• Cytogenetics
• Large fragments of DNA, easily visible with a microscope

• FISH (Fluorescence In Situ Hybridization)
• Much smaller fragments of DNA (closer to the size of individual genes/gene regions) – requires tagging with a fluorescent substance, visible under a special fluorescent microscope
• Molecular / Next Generation Sequencing (NGS)
• Submicroscopic alterations in the nucleotides (single or clusters of few nucleotides) that make up the sequence specific for each gene
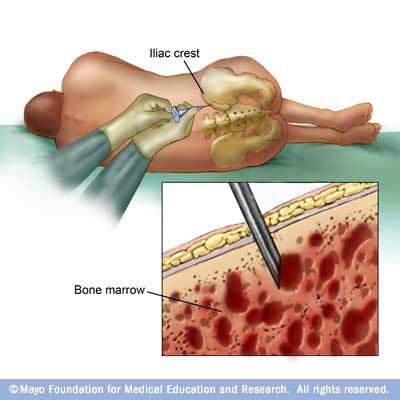
Bone Marrow Aspirate and Biopsy: A way to obtain DNA
Adult red marrow has a large reserve capacity for cell production

Only found in the upper ends of the femur and humerus; flat bones of the sternum, ribs, cranium, pelvis, and vertebrae
Important to characterize plasma cell percentage, FISH, and cytogenetics
Bone Marrow Biopsy with a Needle

In humans, each cell normally contains 23 pairs of chromosomes –total of 46. Twenty-two of these pairs, called autosomes, look the same in both males and females.
The 23rd pair, the sex chromosomes, differ between males and females.
Chromosomal Analysis

Trisomies can be seen in chromosomes 1, 5, 9, 11, 15, 19, a tetrasomy can be seen in chromosome 7 and deletions of chromosome can be seen in chromosomes 14, 16, 17 and 21.
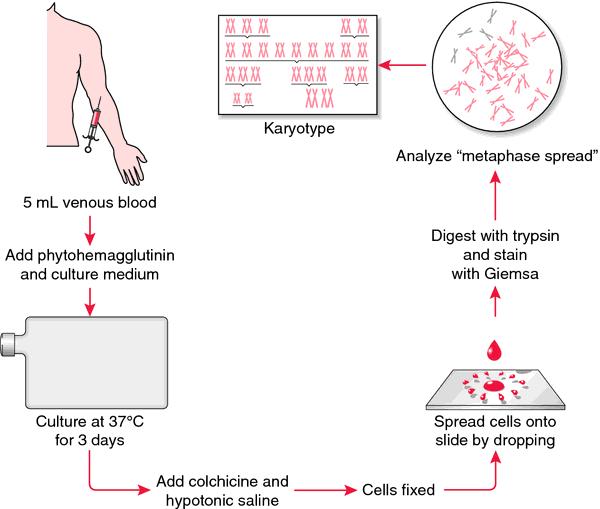
Process for Cytogenetics (Karyotyping)

Require presence of live cells or intact nuclei Can be analyzed on bone marrow, peripheral blood, body fluids, and solid tissue
Cells exposed to mitotic inhibitors, hypotonic solution, and fixation followed by banding methods
Can identify related and distinct clonal populations Allows for stratification into favorable, intermediate, and adverse-risk
Fluorescent In-Situ Hybridization (FISH)
More sensitive to detect specific translocations

Performed on same sample submitted for chromosome analysis
Blood, bone marrow, body fluids
Detection of gene amplification
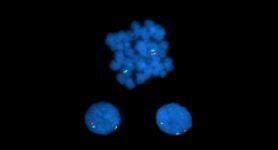
Confirmation of rearrangements detected on conventional cytogenetics
Detection of early relapse based on lineage of neoplastic cells
FISH image showing an abnormal cell with one red signal and two green signals, indicating the long arm of one copy of chromosome 7 is deleted
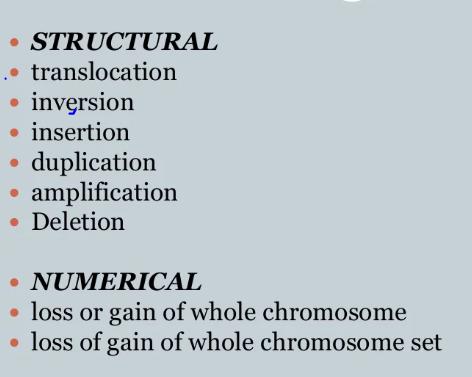
Recap: Detection of Genetic Alterations in Myeloma
Cytogenetics
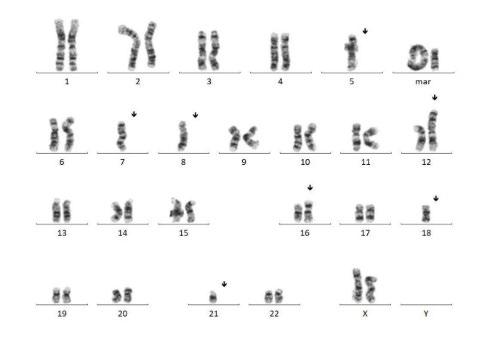

Can detect (Screening):
- Any translocations
- deletions
- additional material (large fragments)
FISH
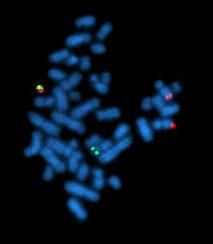
Can detect (specific to the genes interrogated):
- Translocations
- Deletions
- Additional copies of genes (amplification)
Nucleotides
Can detect:
- DNA
- Sequencing – mutations (single bases)
- PCR: Gene rearrangements
- RNA (RT-PCR)
- Specific translocations
What does Risk With Multiple Myeloma Mean?
Prognosis Findings from Bone Marrow Biopsy
Itisimportanttoknowthenumberofbonemarrowplasmacellsandprognosisinformation

No abnormalities detected
OR
Abnormalities that are not defined as high-risk
• Trisomies
• t (11;14)
• t (6;14)

Identified by FISH
• t (4;14)a
• t (14;16)
• t (14;20)
• Del (17p)
• gain (1q)a
Double hit: any 2 high-risk factors
Triple hit: any 3 high-risk factors

CKS1B = cyclin-dependent kinases regulatory subunit 1 gene; FISH = fluorescence in situ hybridization; TP53 = tumor protein 53. aHigh risk if detected in SMM; intermediate risk in MM according to Rajkumar SV 2022. Rajkumar SV. AmJofHematology.2022;97(8):1086-1107.

New categories for high risk: What does this mean?
Consider 17P high risk even if not ISS 3
Do you have to check TP53 status if 17P is present? Yes
– since sometimes 17 p without TP53 mutational status present
TP53: Biallelic TP53 alterations can involve a mutation on one allele and a deletion on the other, or two distinct mutations on each allele
Bialleic del (1p32), 10% of NDMM; in 20% of these cases, if bialleic then poor prognosis
Example: Biallelic deletion of 1p32:This means both copies of the chromosome region 1p32 are deleted, resulting in a loss of both allele
t(4;14) high risk if assess w/ another intermediate lesion
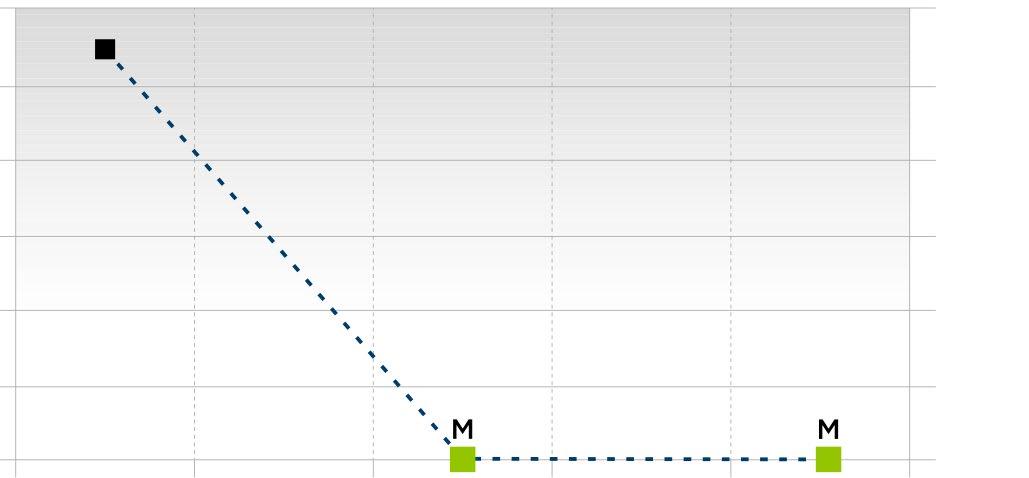
Prognosis: Minimal Residual Disease (MRD)-Negative


ASCT = autologous stem cell transplant; CR = complete response; FDA = US Food and Drug Administration; MRD = minimal residual disease; VGPR = very good partial response. Munshi N, et al. Blood Adv. 2020;4(23):5988-5999. ClonoSEQ website. Accessed March 15, 20222. https://www.clonoseq.com/. ClonoSEQ® [technical summary]. Seattle, WA. Adaptive Biotechnologies; 2020. Accessed March 23, 2023. https://www.clonoseq.com/technical-summary/.
• Predict outcomes
• Support treatment decisions?
• VGPR or better
• Often prior to transplant or cellular therapy
• Often retest at set intervals (eg, every 6-months)
• ClonoSEQ—FDA cleared for diagnostic use
• Within a clinical trial protocol
• Nice to know vs. need-to-know information

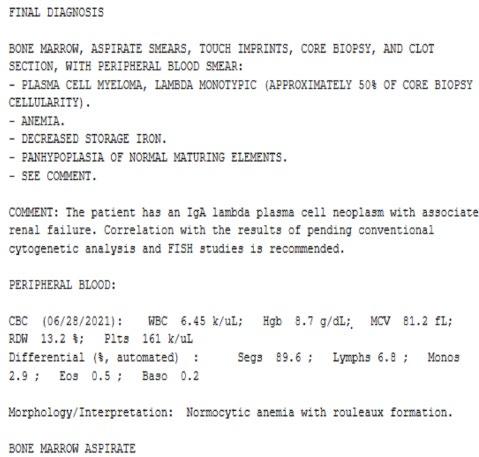
Bone marrow biopsy report

Complex Cytogenetics, FISH






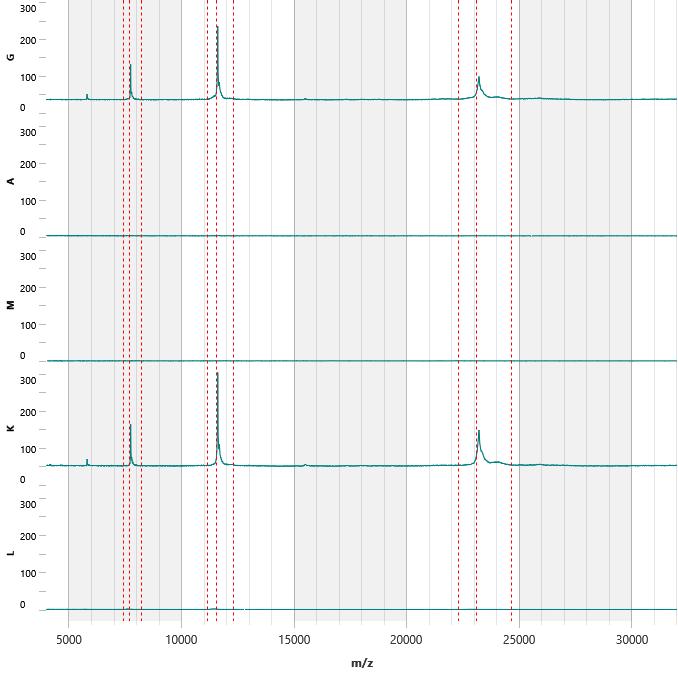
IgG, IgA , IgM, κ, λ Spectra
Mass Spectrometry
Peak Analyzer Isotyper Interpreter Analyzer System
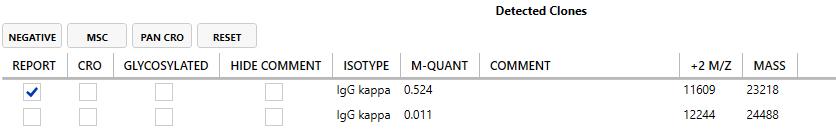
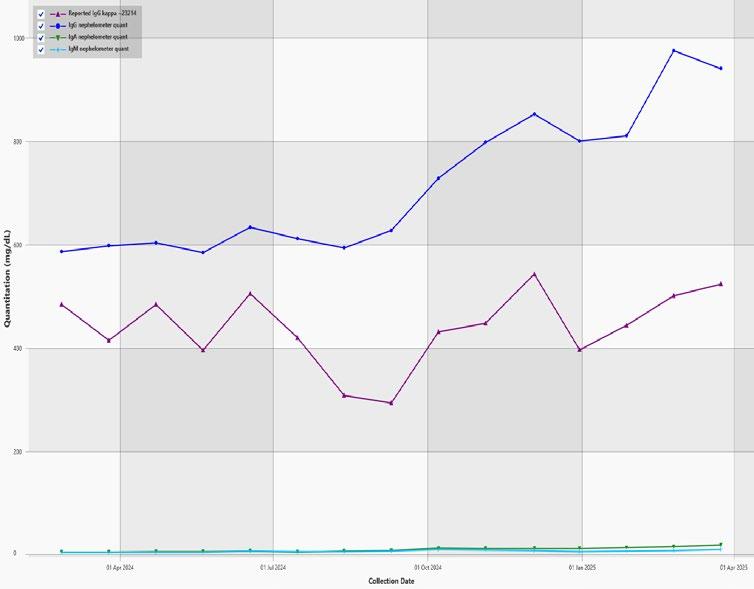
Current Detected M -Proteins M -Protein, FLC and Ig Quant History
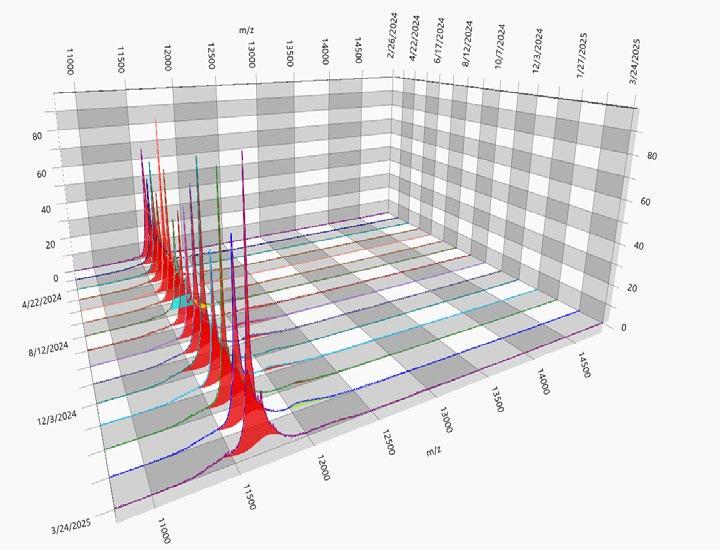
3D Spectra History
2D Spectra with Peaks highlighted


Lab Tech and Lab Director Review Manual regating if necessary Release
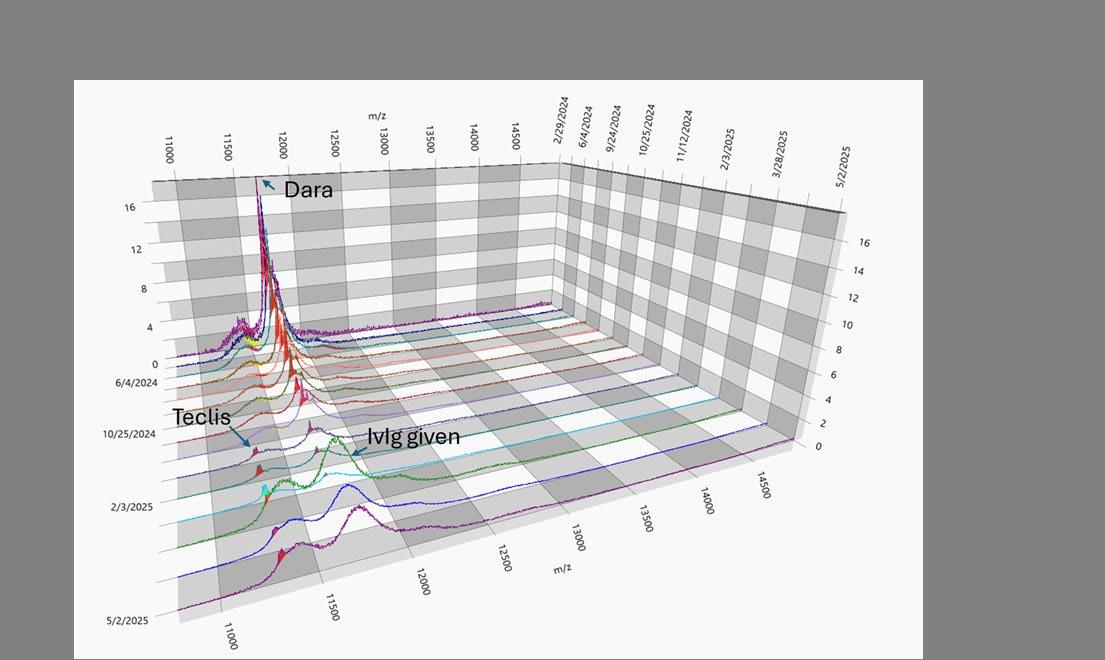
Timeline of mass-fix

Initial development of MALDI-TOF method
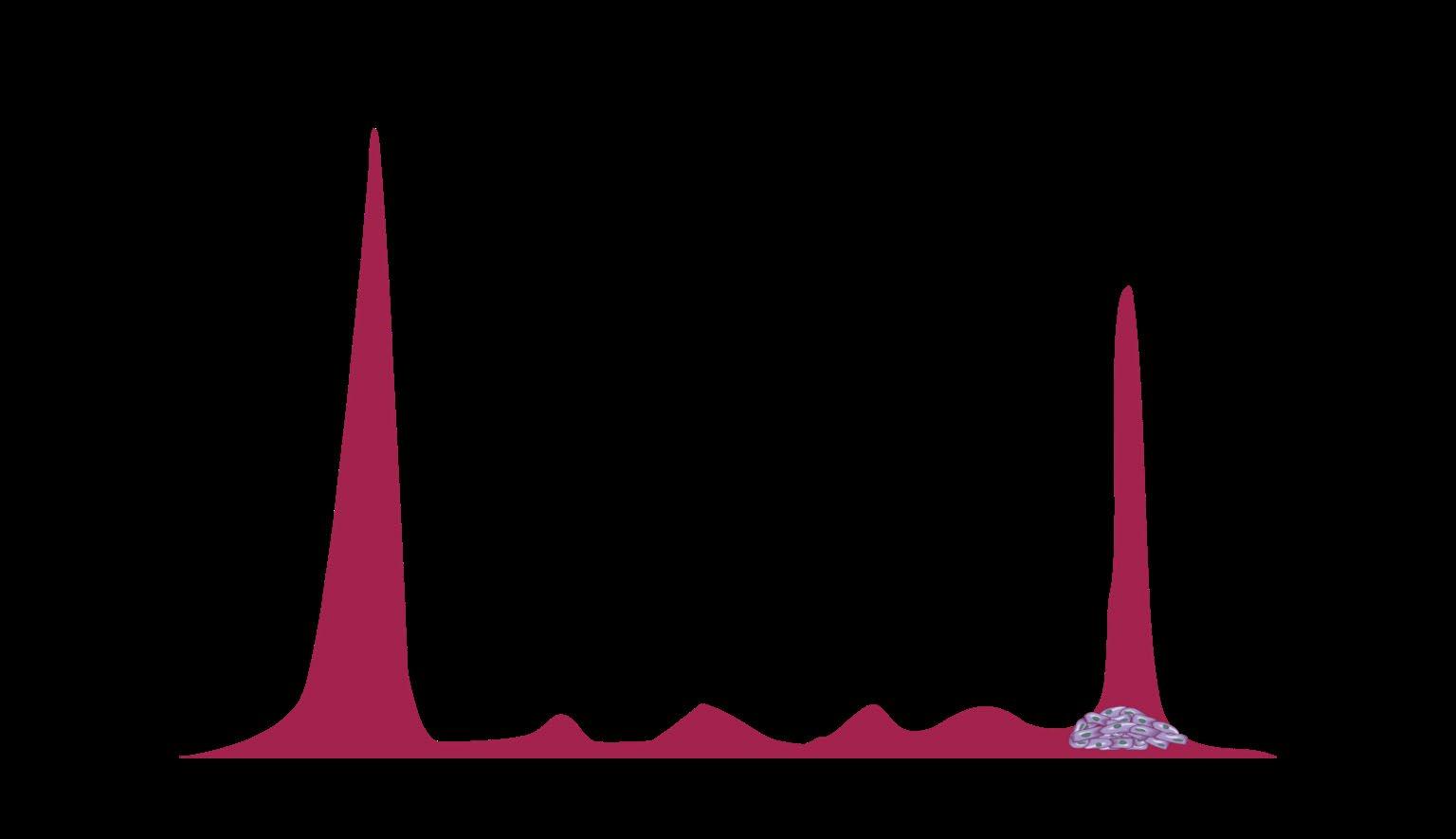
EXAMPLE OF DYNAMIC RANGE IN AN IGG LAMBDA MM
PATIENT
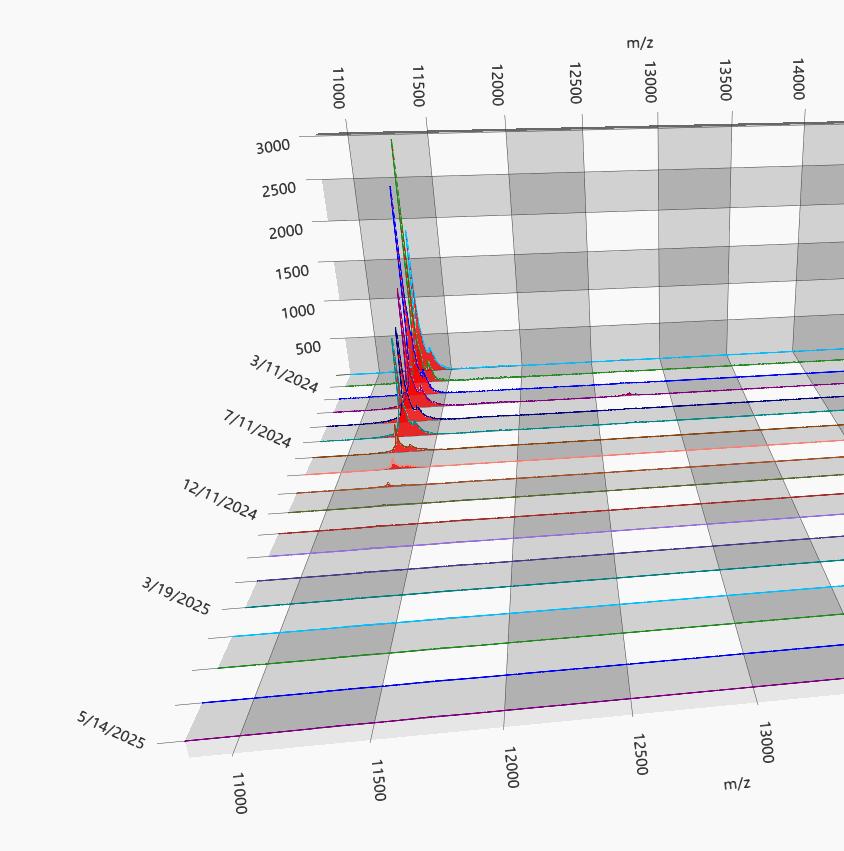
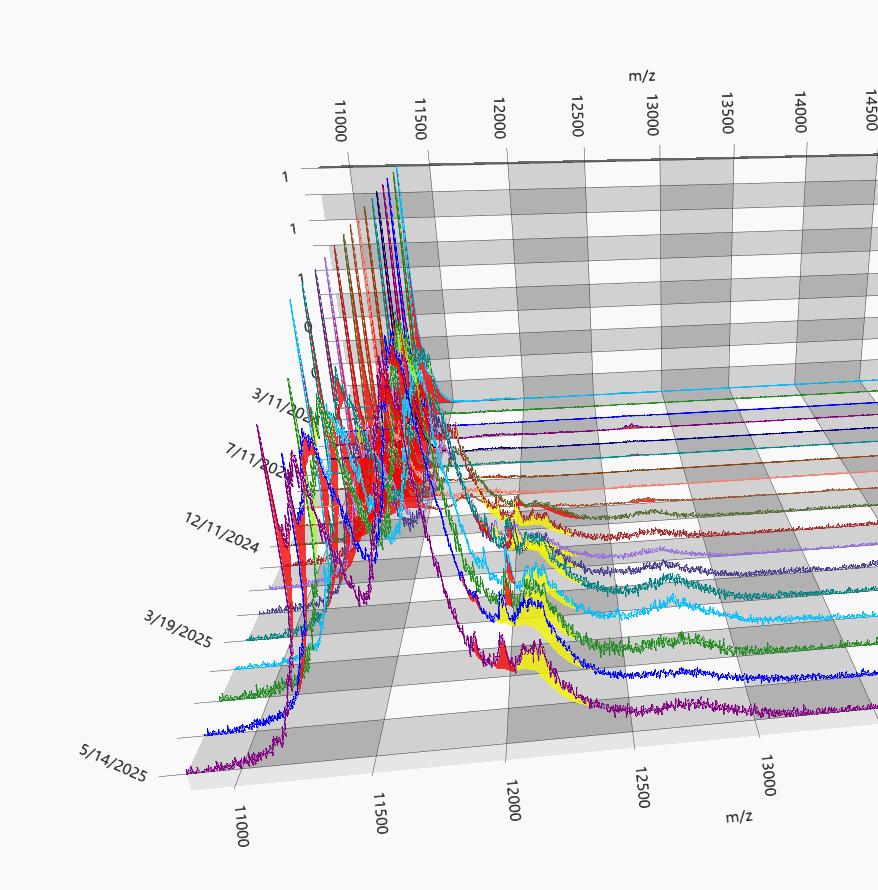
80 g/L Max Mode 0.01 g/L 80 g/L
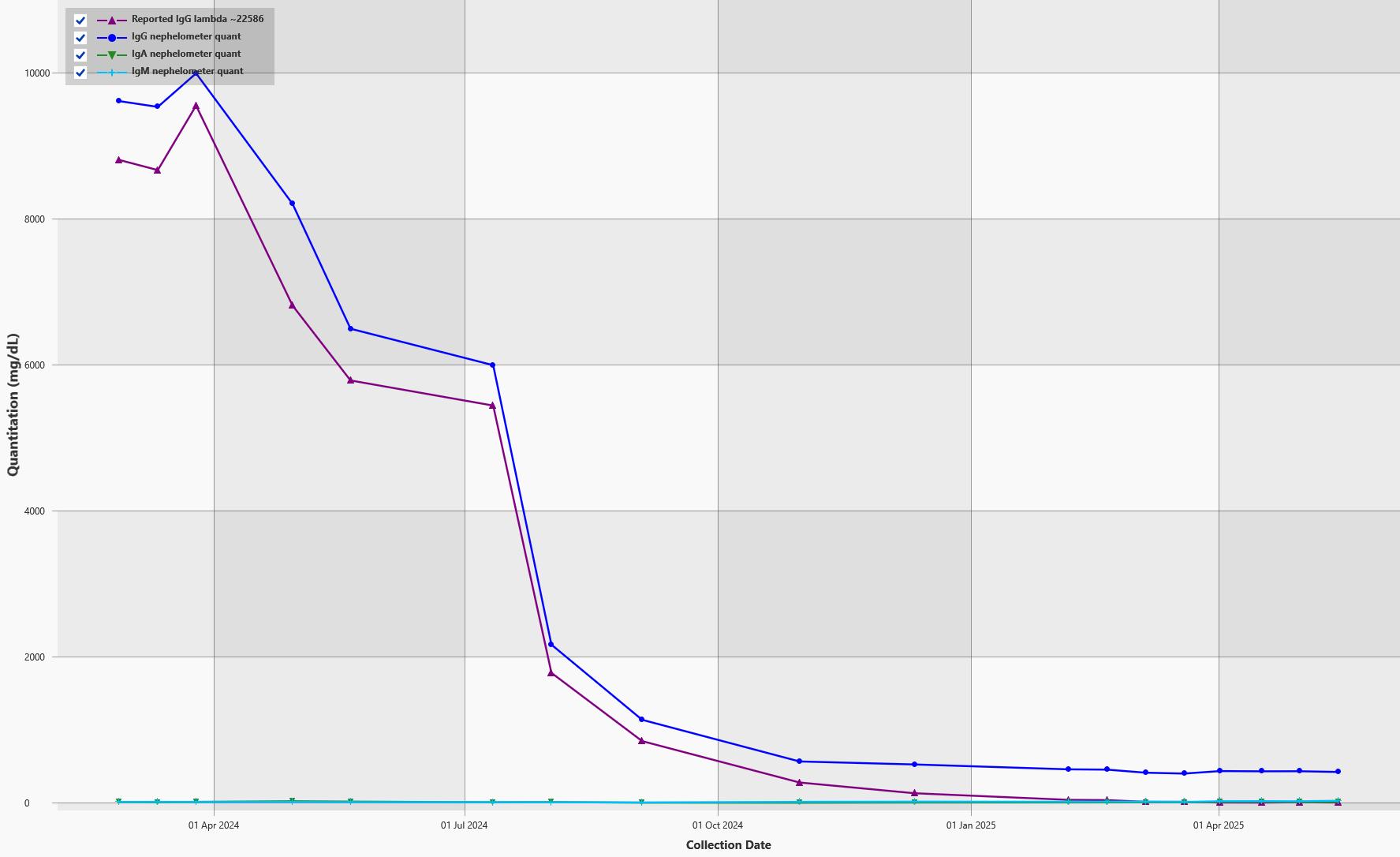
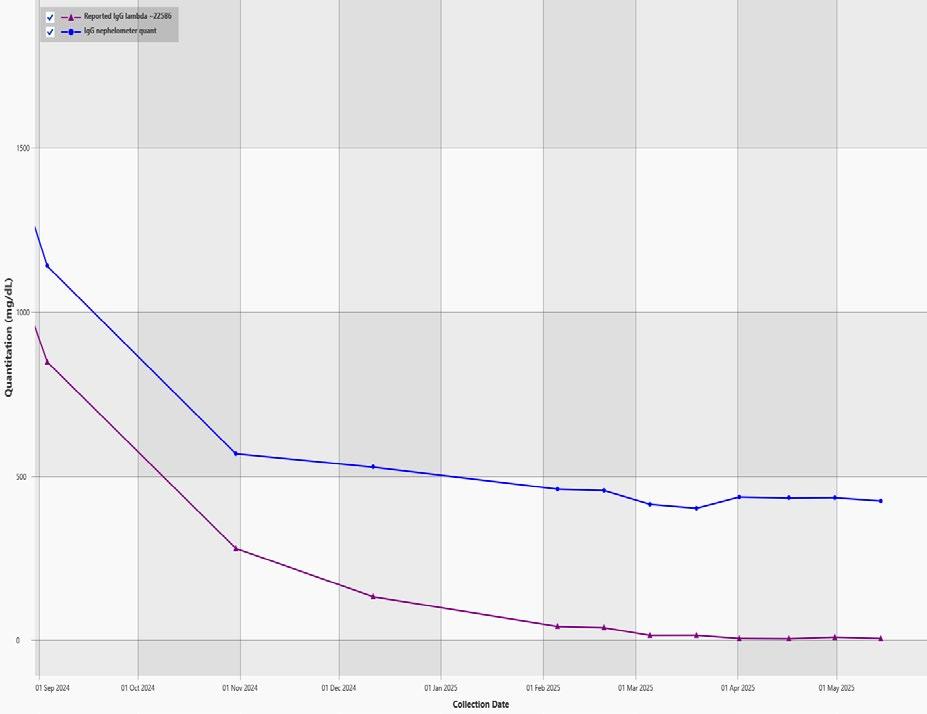
M-protein slowly decreasing
1+ year course of MM treatment

IMPLICATIONS OF LOWER LOQ FOR M-PROTEIN QUANTITATION
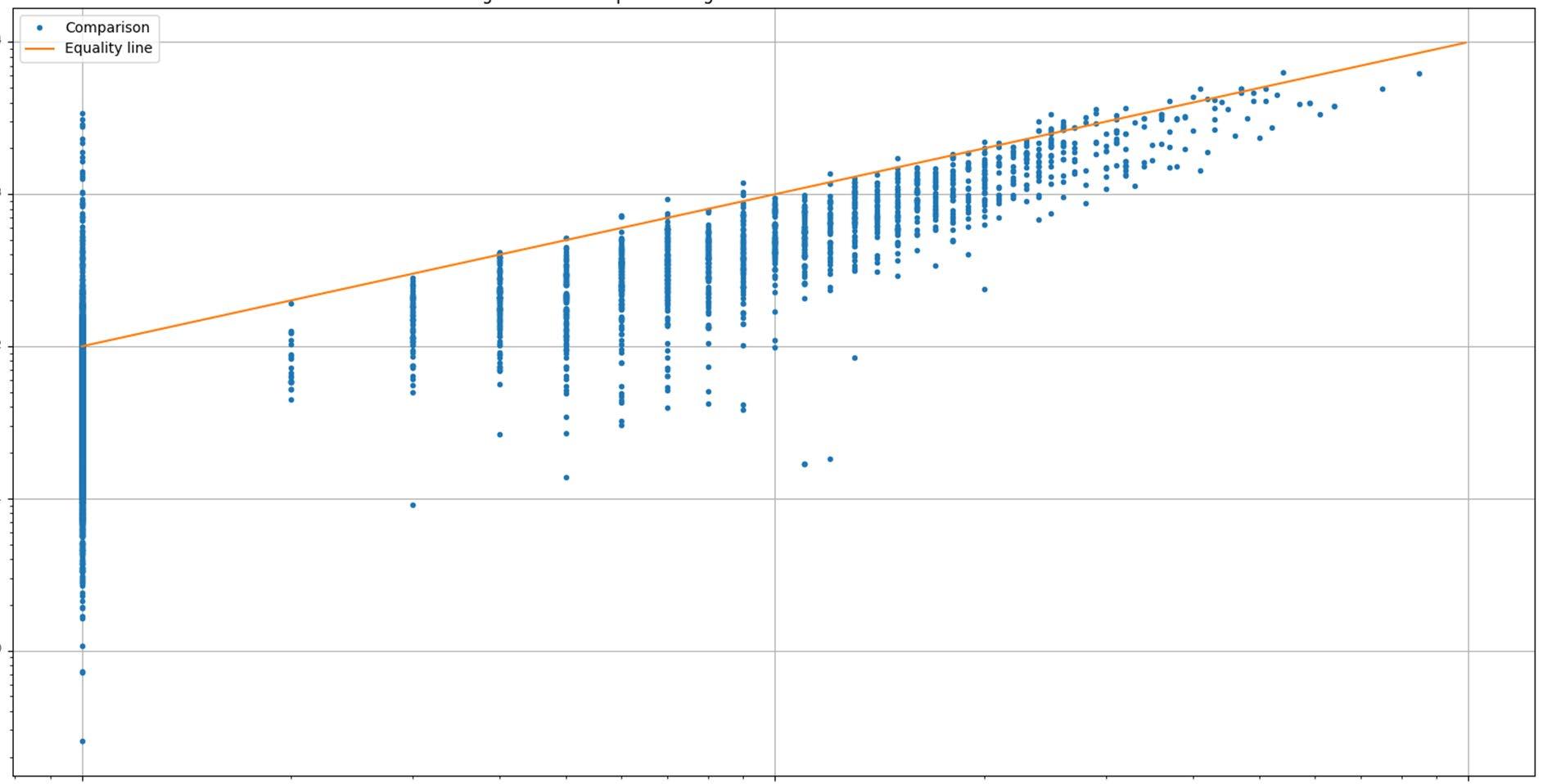
SPEP and IFE
• 65% of M-protein positive samples were below the LOQ of SPEP
• The range of IFE positive samples below the SPEP LOQ was 0.05 g/L to 40 g/dL by Mass-Fix
Mass-Fix
• 0.03% of M-protein samples had values below LOQ of Mass-Fix

Mass
Special Considerations With Antibody Therapy
Interference with IFE laboratory tests
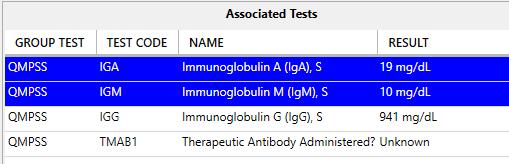
Daratumumab, isatuximab, and elotuzumab are IgG antibodies that co-migrate with IgG M-protein
IFE cannot distinguish between therapeutic IgG antibodies and IgG Mprotein; Lowers apparent CR rates
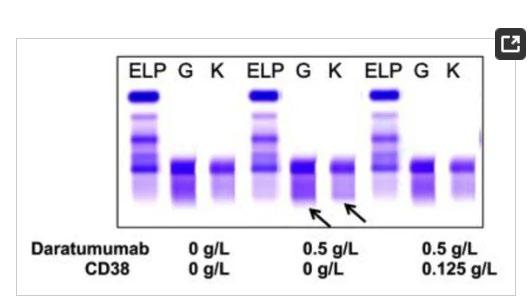
Mass spectrometry (available in some centers) can resolve antibody interference
• Patients with MM can be by MS replacing SPEP and IFE
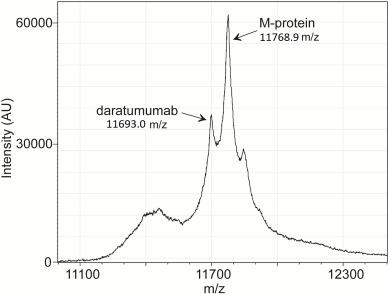
• MS also has a role in amyloidosis (eg, it has improved accuracy for typing amyloid plaques and has led to the discovery of new types of amyloid)

CR = complete response; IFE = immunofixation electrophoresis; IgG = immunoglobulin G; M-protein = monoclonal protein; SPEP = serum protein electrophoresis.
Mills JR, Murray DL. J Appl Lab Med. 2017;1(4):421-431. Murray DL, Dasari S. Clin Lab Med. 2021;41(2):203-219. Deulofeu M, et al. ACS Chem Neurosci. 2023;14(2):300-311. Moore LM, et al. Clin Chim Acta. 2019;492:91-94.
How Well Treatment Is Working: IMWG Myeloma Response
and Relapse Criteria Assessment

CR Complete
Remission

CR: myeloma protein undetectable in serum or urine (negative immunofixation); no more than 5% plasma cells in bone marrow; no new lytic lesions; plasmacytomas resolved
VGPR Very Good
Partial
Remission 90% reduction in myeloma protein
PR
Partial
Remission At least 50% reduction in myeloma protein
PD Progressive Disease Increase of > 25% from lowest response value in myeloma protein or other assessment
Further categorization of CR: sCR, MRD-negative

Know: Order labs regularly
Know who is monitoring (local or referral center)
Monitor for relapse
– CRAB symptoms OR increase of 25% in M protein from the lowest point
How Patients With Myeloma Relapse

Myeloma protein

No symptoms, “Biochemical” Relapse
• Sequentially rising myeloma protein or free light chain (>25% increase from low point)
• No other symptoms
• Decisions: if, when, how to treat
New symptoms, “Symptomatic”
New, worsening bone pain
Increasing fatigue, anemia
Next step: relapse workup; many therapy choices
Knowing Your Myeloma Numbers

Immunoglobulin: (Ig)___: _______

FLC: kappa _____ lambda _____
G A M E D
Ratio (K/L) ______ M-spike _______ Bone Marrow Plasma Cells: _____% Urine Protein: _____mg/24hours M-spike _____mg/24hours
What questions should I ask about treatment?

• It is OK to ask your provider about lab results & possible treatments
• Consider trade offs
• Consider burden versus benefit
• If clinical trials are not offered, please ask if one is right for you!

SHARED DECISION-MAKING
Be empowered to be part of the treatment decision-making, come prepared with questions
• Ask for time to consider options (if needed/appropriate)
• Understand options; consider priorities
• Use reliable sources of information
• Use caution considering stories of personal experiences
• Consider your goals/values/preferences
• Express your goals/values/preferences; create a dialog
• My top priority is [goal/value]; additional [preferences] are also important.
• I think [treatment] may be a good choice given my priorities… What do you think?
• Arrive at a treatment decision together

TREATMENT DECISION
Preference
Philippe Moreau. ASH 2015.
THE MEMBERS OF YOUR TEAM
Understand their different roles Myeloma specialist: for myeloma care
Primary care: for health screening, general check ups, vaccinations

Others: for specific needs (Kidney, Bone)
Keep a contact list of your providers
Come prepared to office visits:
Bring a list of current medications, prescribed and over the counter
Write down your questions and concerns. Prioritize them including financial issues
Have there been any medical or life changes since last visit?
Primary Care
(PCP)

Subspecialists



You and Your Caregiver

Current symptoms - how have they changed (improved, worsened, stable)? Keep a symptom diary. Bring it along
Communicate effectively: your health care team can’t help if they don’t know

Family/Support Network

Allied Health Staff


• Blood, urine, bone and bone marrow testing can provide important information into the state of your myeloma
• Regular monitoring is important for all patients with an abnormal protein
• Don’t be afraid to ask questions about your labs, or treatments


Robin Tuohy Day 1 Wrap-Up

Headshots available by Jason London from


4:30 - 5:30 PM




Leaders who are Health Care Providers, please choose either option to attend

Day 1 Wrap Up
3:30PM – 60-minute session
Please attend the option you selected:
Care Partners (Gale)
Patients (Valhalla)

4:30PM – Open Options
Walk around Viking Lake
Indoor Pool (2nd Floor)
Lawn Games (Valhalla Terrace)
Rest & Relax
Resilience Gallery

Sponsors, we kindly ask you to allow leaders privacy during these sessions


6:00PM – Networking & Evening Reception (Valhalla Terrace & Prefunction North)

7:00PM – Dinner (Valhalla Ballroom)
Tomorrow: 6:30 – 7:45AM – Breakfast (Kyndred Hearth)
Please use this QR code for Friday's Evaluation!





Thank You to Our Sponsors!


Thank You to our Donors!

