

Agenda through lunch




Agenda after lunch





5th Asian Myeloma Network Master Class
Meeting Chairs: Daryl Tan, MD
Raffles Cancer Center &
Chandramouli Nagarajan, MD
Singapore General Hospital & National Cancer Center
Singapore

Opening of the 5th AMN Master Class

Wee Joo Chng, MD
National University Cancer Institute, Singapore, National University Health System




Role of the Asian Myeloma Network

Dan Navid, JD VP, Global Affairs
International Myeloma Foundation
The IMF in Asia
The IMF is Dedicated to Improve the Quality of Life of Myeloma Patients While Working Towards Prevention and a Cure.
As Part of its Global Effort, IMF Devotes Increased Attention to Myeloma in Asia.
The Centerpiece of IMF's Asian Work is
– The Asian Myeloma Network

Tackle MM in Asia
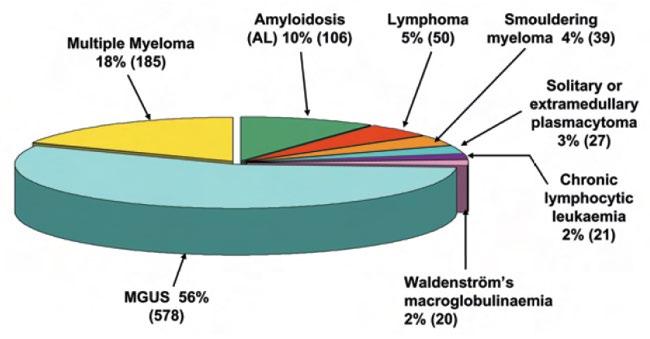
Epidemiology of MM (Am J Hem 2015)
Clinical Trials


AMN Focus of Work
1. Myeloma Database (led by Korea)
2. Clinical Trials and Treatment Guidelines (led by Singapore)
3. Physician Training (led by China)
4. Diagnosis and Smoldering Myeloma (led by Japan)

AMN Standing Committees
1. Executive Committee
2. International Advisory Committee
3. Program Committee
4. Clinical Trial Committee
5. Virtual Tissue Bank Committee
6. Award Committee

Growth of the AMN
1. Expanding membership
2. Outreach with other regions
3. Refining work arrangements

Annual AMN Summit



9th AMN

October 16-19, 2025
Nagoya, Japan
An Annual Expert Meeting to Review Latest Developments in Myeloma Research and to Determine Work Priorities.
AMN Master Class & Patient Forum


In addition to the AMN Summit, the AMN Master Class and AMN Patient Forum are also held annually.
5th AMN Master Class: July 25-27, 2025, Singapore
5th AMN Patient Forum: October 17, 2025, Nagoya, Japan

Current AMN Projects
AMN Clinical Trials
Tissue Bank
Virtual Master Class

Objectives
Early access to good treatment
Establish Asian Data
Study combinations that are relevant to Asia
Establish Asia as a partner for Industry in Cooperative group trials
Elevate standing of Asia in myeloma research

Current and Future Trials
in
Current AMN Projects: Virtual Tissue Bank


Current AMN Projects: Master Class

AMN Virtual Masterclass on Novel Immunotherapies – August 4, 2024
• Focused on novel immunotherapies, with consideration of patient selection, early success in this field, problems with toxicity and resistance, and finally future treatment directions
• Chaired by Dr. Wee Joo Chng (National University Hospital—Singapore)
Faculty: Dr. Thomas Martin (UC San Francisco—CA, USA); Dr. Shinsuke Iida (Nagoya City University Hospital—Japan); Dr. Juan Du (Chang Zheng Hospital China); Dr. Dok Hyun (Asan Medical Center S. Korea) and Dr. Chandramouli Nagarajan (Singapore General Hospital— Singapore)
• In person Masterclass planned for October 18, 2024, in Seoul, Korea
Possible New AMN Projects
Preceptorship Program
New Clinical Trials

AMN Executive Committee
Wen-Ming Chen (China)
Jian Hou (China)
James Chim (Hong Kong)
Masahiro Abe (Japan)
Hiroshi Handa (Japan)
Shinsuke Iida (Japan)
Gin Gin Gan (Malaysia)
Sen Mui Tan (Malaysia)
Priscilla B. Caguioa (Philippines)
Rosalio P. Torres (Philippines)
Wee Joo Chng (Singapore)
Chandramouli Nagarajan (Singapore)
Daryl Tan (Singapore)
Jin Seok Kim (South Korea)
Kihyun Kim (South Korea)
Jeffrey Huang (Taiwan)
Teeraya Puavilai (Thailand)

On behalf of the AMN Team,
IMF Executive Vice President of Research & Operations THANK YOU.
Wee Joo Chng
AMN Chairman
Dan Navid
IMF Senior Vice President, Global Affairs
Lisa Paik

Discussion




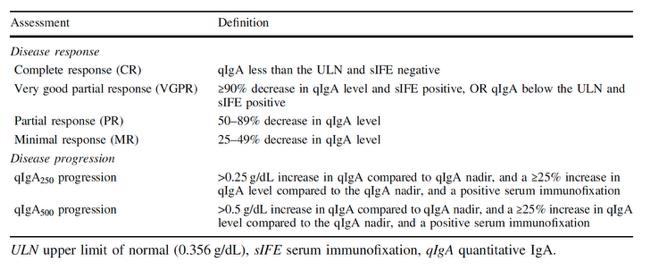
Global Multiple Myeloma Diagnostic Criteria,
Prognostic Factors, Risk Stratification

Wee Joo Chng, MD
National University Cancer Institute, Singapore, National University Health System

Multiple Myeloma: Diagnosis, prognosis and risk stratification

Prof Chng Wee Joo
Chairman, Asian Myeloma Network
Vice President (Biomedical Research) and Yong Loo Lin Professor in Medical Oncology, NUS
Restricted, Non-Sensitive
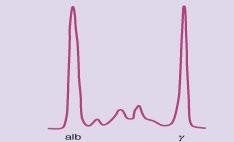
Monoclonal protein analysis
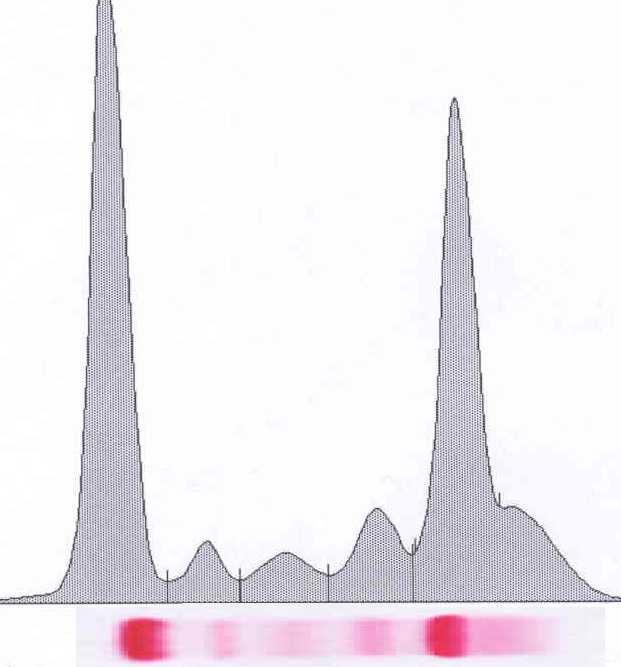
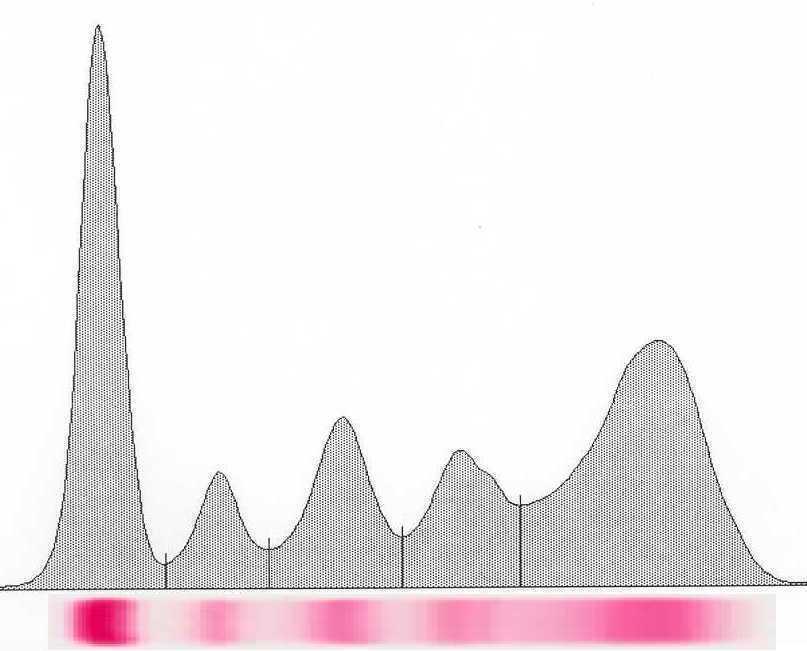
Picture courtesy of Drs. R. Kyle and J Katzmann, Mayo Clinic
Monoclonal protein Polyclonal protein “M spike” Polyclonal hump
Restricted, Non-Sensitive
Immunofixation
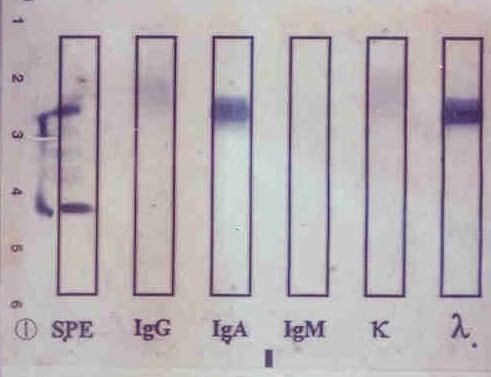
Restricted, Non-Sensitive
Special Situations
• Light Chain only
• Non-secretory MM
• Serum Free Light Chain assay are useful in monitoring these disease subtypes
• More sensitive than assessment of intact lg
• Normalization of kappa:lambda ratio an important goal
Light Chains – Biology
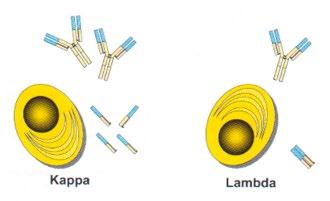

• Production of free light chains = 500 mg/d
• 40% excess free light chain produced over heavy chain
• κ is usually monomeric, λ usually dimeric
• Twice as many κ than λ producing plasma cells
Serum free light chain analysis. 2nd Ed. A.R. Bradwell

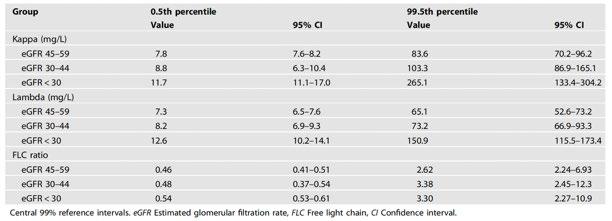
Blood Cancer J 2022
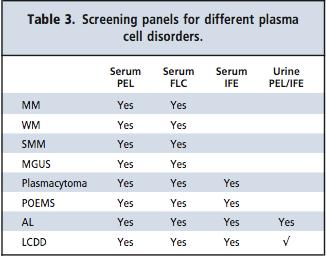
Myeloma Screening Panel
Restricted, Non-Sensitive
What about Serum lgs
• They do not distinguish monoclonal from polyclonal lgs
• However, they may be very useful for lgA MM

Leukemia 2021
Restricted, Non-Sensitive
Causes of monoclonal gammopathy
Restricted, Non-Sensitive
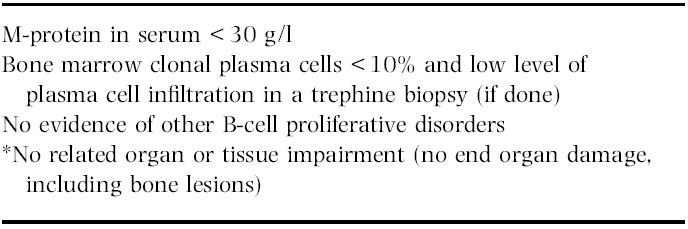
MGUS
Restricted, Non-Sensitive
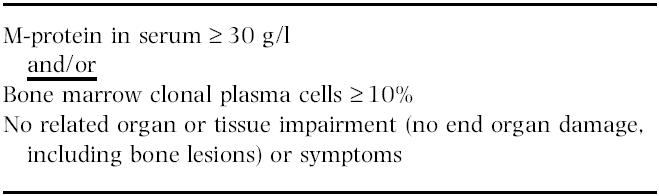
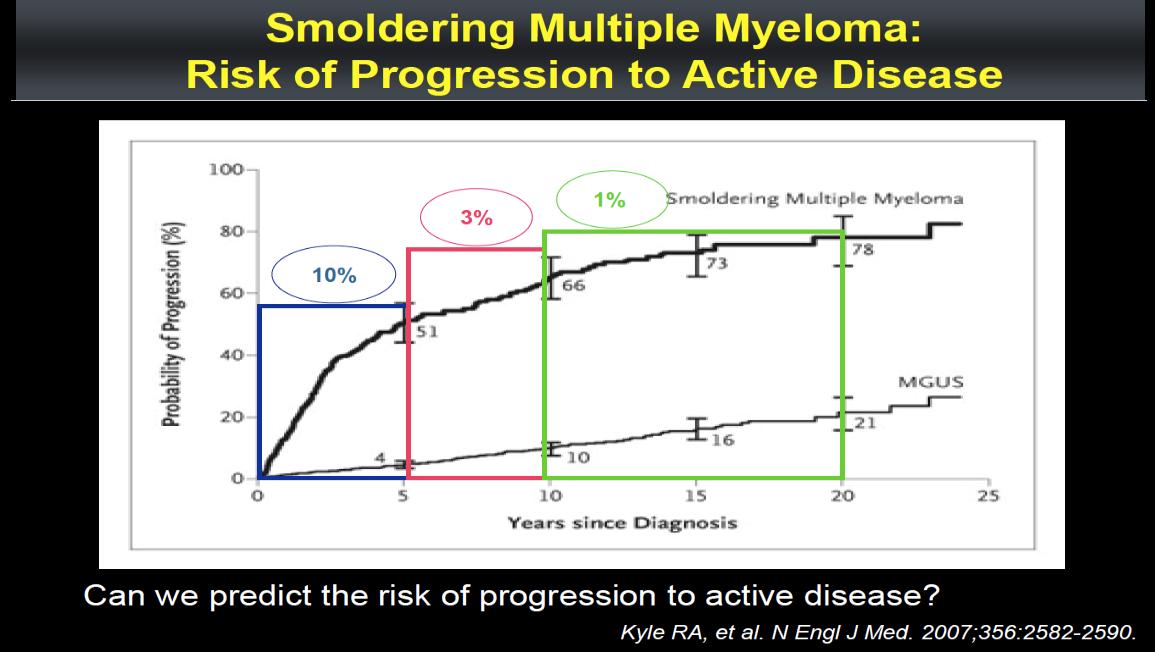
Asymptomatic / Smouldering Myeloma
Restricted, Non-Sensitive
Symptomatic Myeloma

Restricted, Non-Sensitive
Clinical Features
• Calcium elevation
• Renal disease
• Anemia
• Bone disease
• …and other constitutional

Smith. Br J Haematol. 2005;132:410.
Skeletal Survey
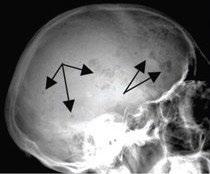
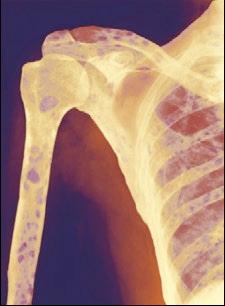
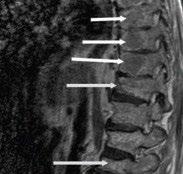
MRI Scan
PET/CT Scan
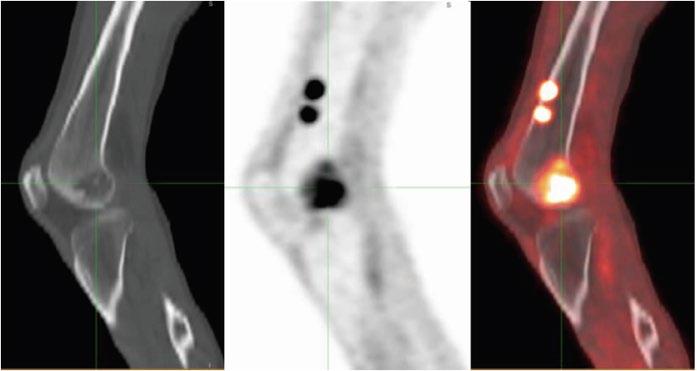
Restricted, Non-Sensitive
Clinical Application
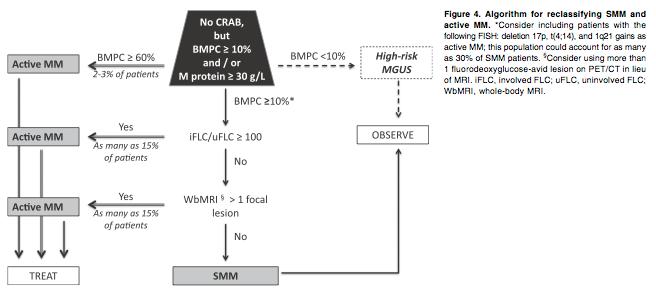
• ASH Review Article 2013
• Dispenzieri et al. Smoldering Multiple Myeloma requiring treatment: time for a new definition? Blood. 2013 122:4172-4181
Revised IMWG Criteria

Rajkumar. Lancet Oncol 2014; 15: e538-48
Investigations for Diagnosis
• Myeloma screening panel [SPEP, Serum IFE, sFLC]
• M -protein quantification (for response measurement)
• Bone Marrow Aspirate and Trephine
• FBC
• Ca Panel
• Renal Panel
• Skeletal Survey / LD CT Not Bone Scan
• MRI may be needed
Clonal PCs in BM (Flow Cytometry, IHC)
>10% Clonal PCs in BM (Flow Cytometry, IHC) ? MM Defining (FBC, CrCl, Ca, Lytic lesion, sFLC ratio >100)
Amyloidosis, IgM associated neuropathy, Light or heavy chain
Restricted, Non-Sensitive

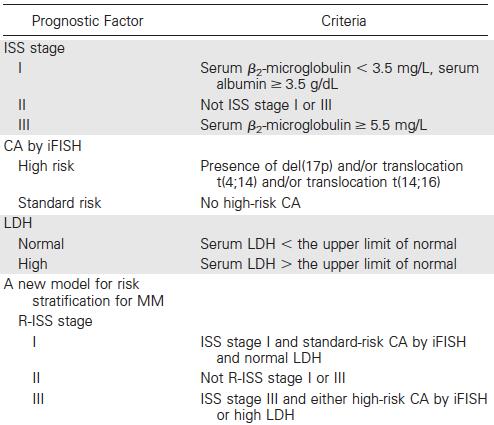
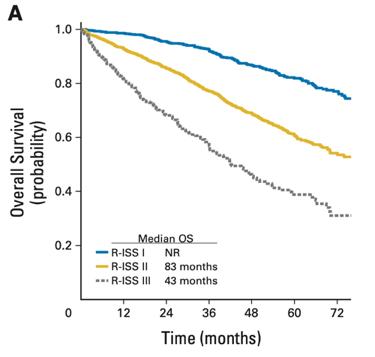
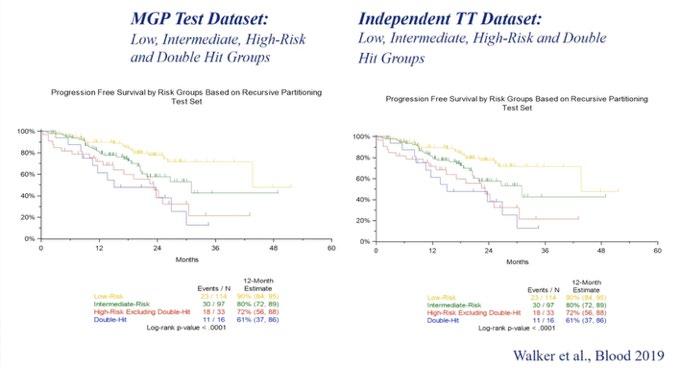
Prognosis
Revised ISS
Restricted, Non-Sensitive
Not all 17p Del are Bad
Clonal Fraction of 17p Del
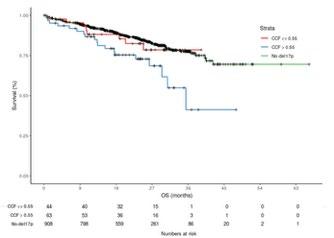
Bi-allelic loss of p53
Tharkurta et al. Blood 2019
Walker et al. Leukemia 2018
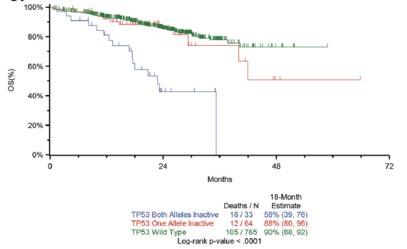
Outcomes are worse with more poor-risk genetic factors

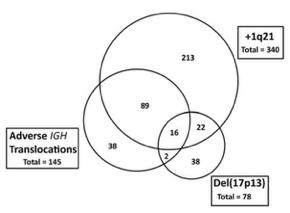
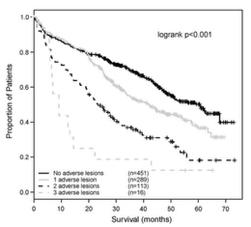
Double hit MM
Bi-allelic TP53 OR ISS3, +amp (1q)
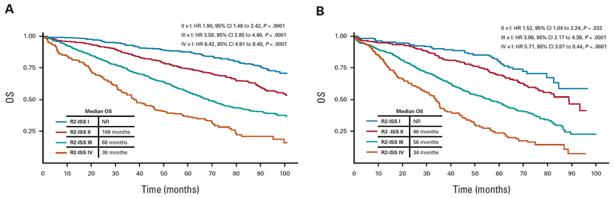
R2-ISS
Training Set (n=2226)
Validation Set (n=1214)
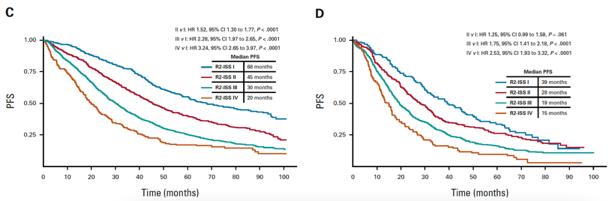
Functional HR
• Compass Dataset
• NDMM
• Suboptimal Response to Induction
• Progression within 12 Months of Induction
Soekojo C…Chng WJ. Blood Cancer J 2022
Summary of the New IMS-IMWG Consensus HRMM
Definition
Criteria for HRMM
Del(17p)a and/or TP53 mutationb
One of these translocations: t(4;14) or t(14;16) or t(14;20), cooccurring with +1q and/or del(1p32)
Monoallelic del(1p32) along with +1q, or biallelic del(1p32)
High β2M (>5.5 mg/dL) with normal creatinine (<1.2 mg/dL)
aCCF ≥20%, by analyses conducted on CD138-positive/purified cells.
bAssessed using an NGS-based method.
+1q, gain (3 copies) or amplification (≥4 copies) of the long arm of chromosome 1; CCF, cancer clonal fraction; HRMM, high-risk multiple myeloma; NGS, next-generation sequencing.

t(x;22) (light chains)
t(8;14)
t(4;14) breakpoint GEP
Chromothripsis
Hyper APOBEC
t(8;14)
t(x;22) (light chains)
GEP
Chromothripsis
t(4;14) breakpoint
Number and size of PET or MRI lesions at diagnosis Extra meduallary disease
IMS 2024
Hyper APOBEC
circulating DNA
circulating plasma cells
plasma cell leukemia
t(x;22) (light chains)
Chromothripsis
t(4;14) breakpoint
Number and size of PET or MRI lesions at diagnosis Extra meduallary disease
t(8;14)
t(x;22) (light chains)
Chromothripsis
t(4;14) breakpoint
IMS
Number and size of PET or MRI lesions at diagnosis Extra meduallary disease
Functional Risk Dynamic response to therapy - MRD
Hyper APOBEC
Microenvironment circulating DNA
Factors
Risk Stratification
1) Revised ISS 1
2) No CA
3) <55 years
1) IMS-IMWG HR
2) Functional HR
Median OS >10 years 8 years 2 years
Therapeutic
Questions ? Need CR or MRD? Need maintenance Aim to increase cure fraction Aim for MRDAim for maintenance
Need to test different strategy High risk procedure e.g. AlloSCT New therapeutics
These are patients that will benefit most from modern drugs and treatment paradigm and the potential group that we can aim for cure
Thank you.
Discussion




Break




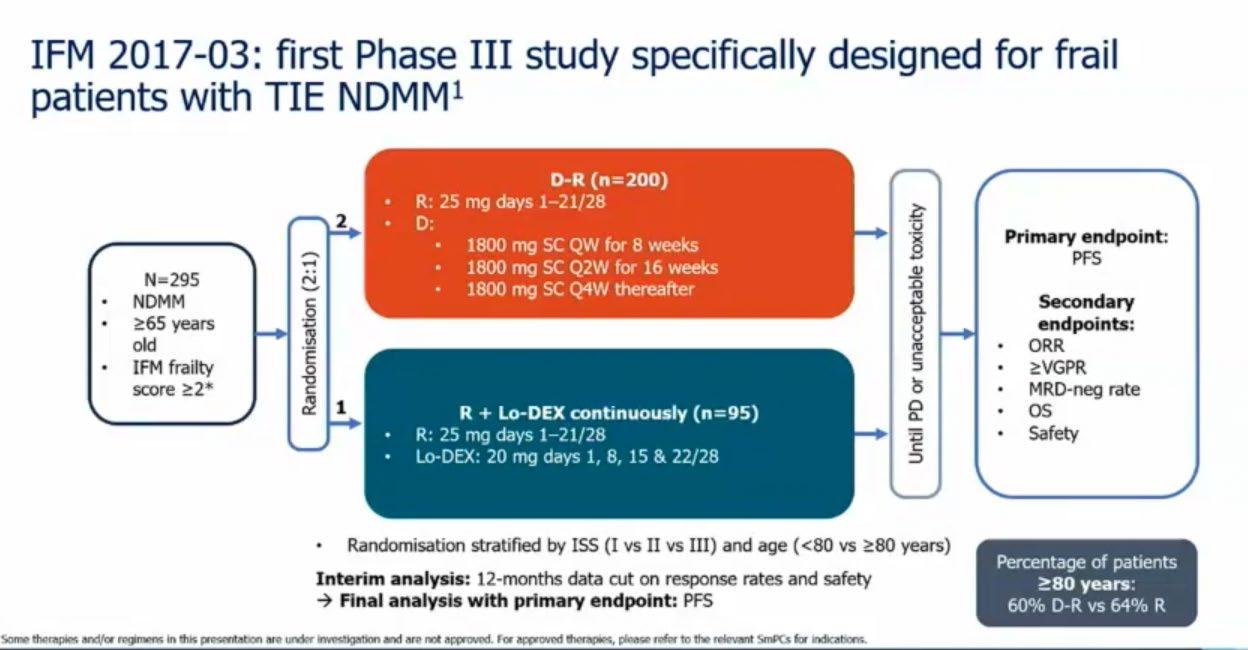
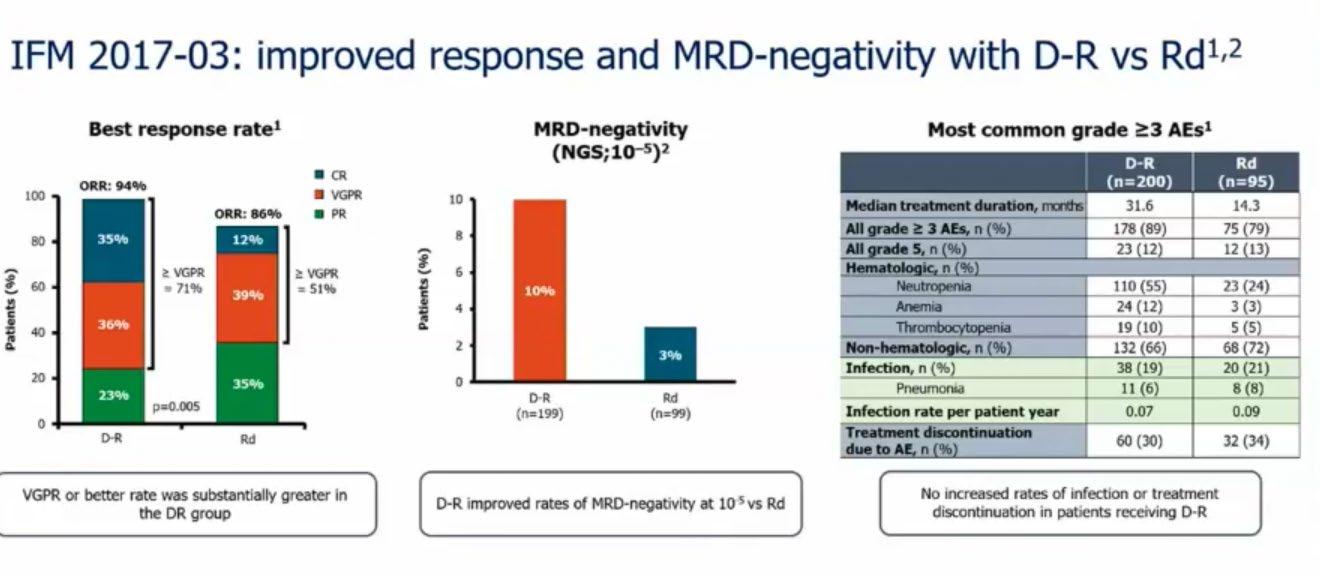
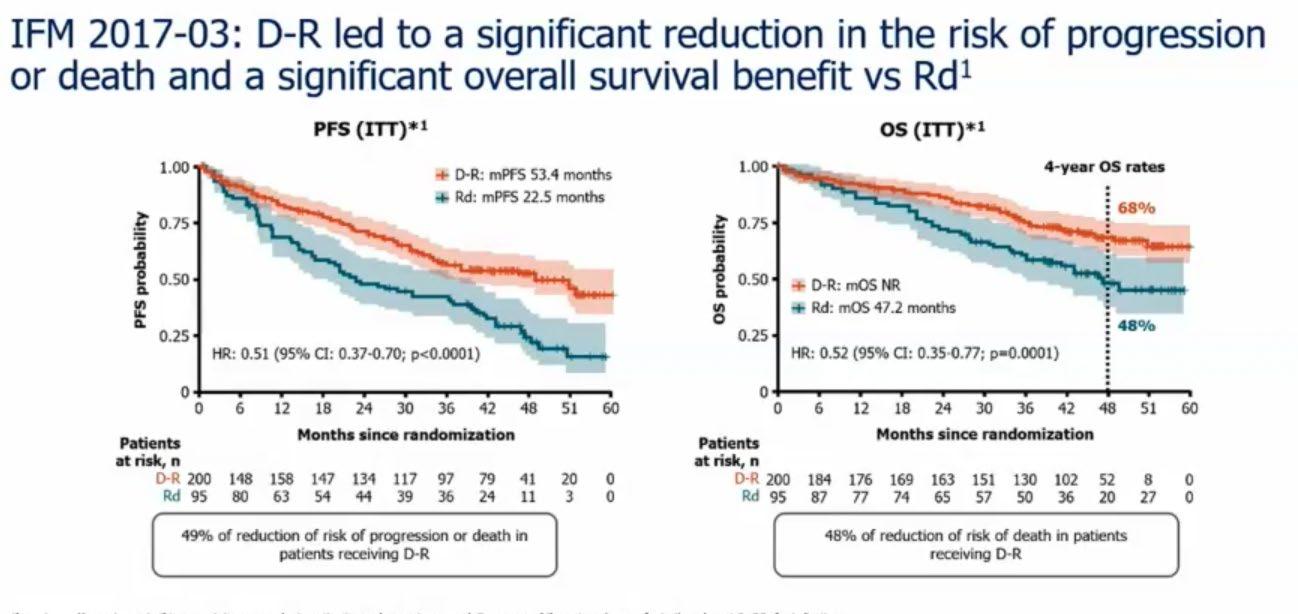
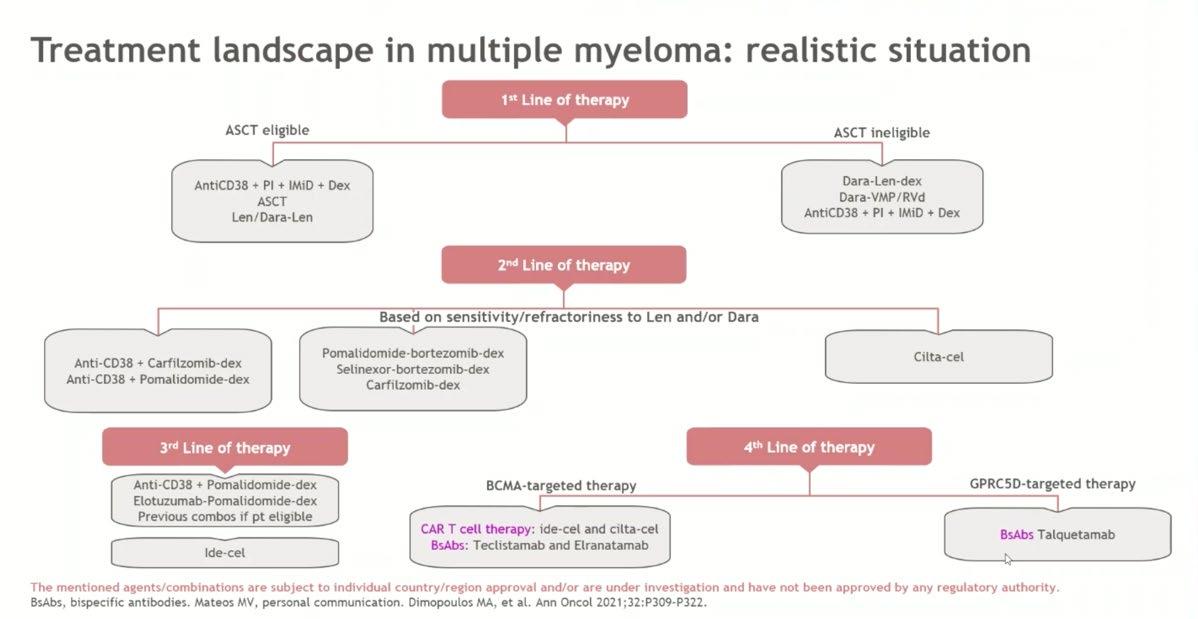
Treatment Options Asia - NDTE

Hiroshi
Handa, MD
Gunma University Graduate School of Medicine
Treatment option in Asia
Newly Diagnosed Transplanteligible Myeloma Patients
Hiroshi Handa Department of Hematology
Gunma University Graduate School of Medicine Japan
• Research Support
• Employee NA
• Consultant NA
• Major Stockholder NA
• Speakers Bureau NA
• Honoraria Janssen, Takeda, BMS, Ono
• Scientific Advisory Board Pfizer, Abbvie, Janssen, Takeda
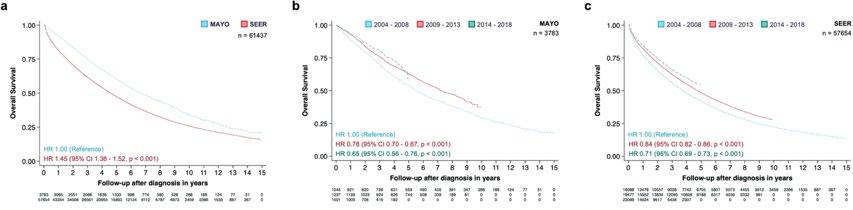
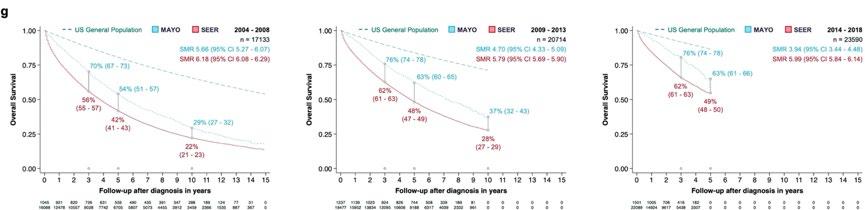
Myeloma survival continues to improve
(Mortality trends in multiple myeloma after the introduction of novel therapies in the United States)
Innovation of Treatment is contributing to the improvement




Primary analysis of a prospective cohort study of Japanese patients with plasma cell neoplasms
in the novel drug era (2016-2021)
Shibayama H, et al. Int J Hematol. 119:707-721.
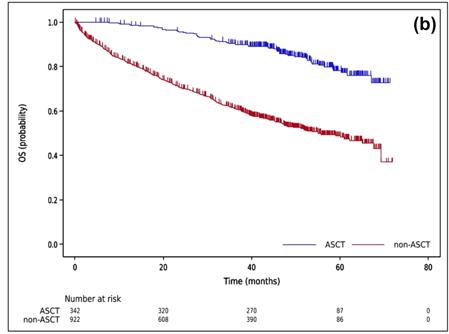
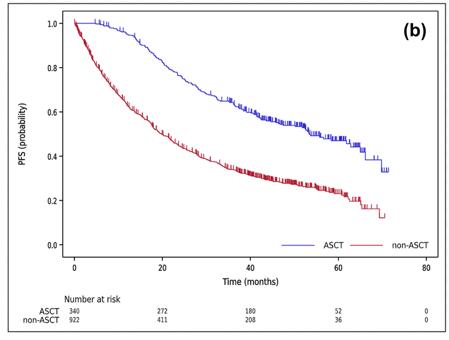
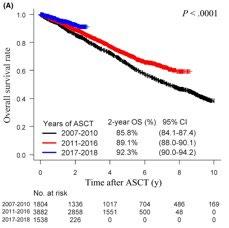
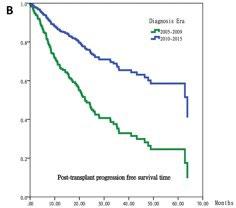
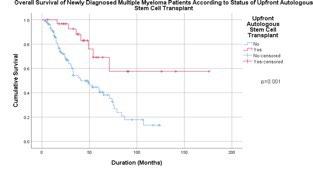
Autologous stem cell transplantation in Asian countries
Shimazu Y, et al. Cancer Sci. 112; 5034-5045. 2021
Huang TC, et al. J Formos Med Assoc. 118: 471-480 2019
Cheong CS, et al. Asian Pac J Cancer Prev;25(2):595-601. 2024
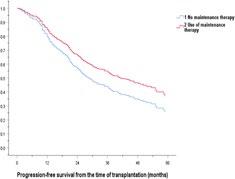
Kang KW, et al. (KMMWP) BMC Cancer.;25:204. 2025
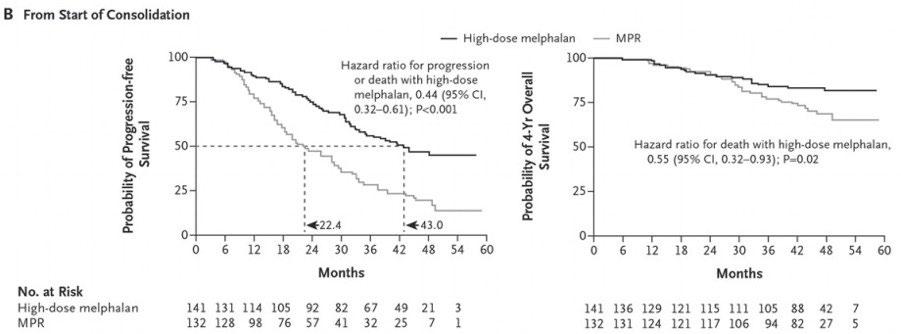
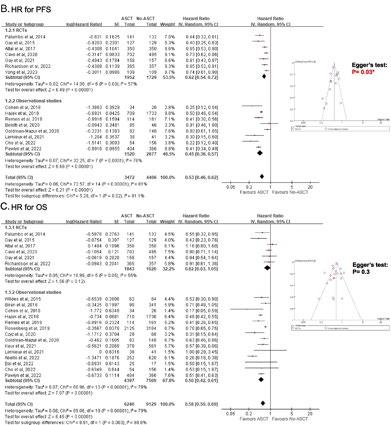
Treatment benefit of upfront ASCT for newly diagnosed multiple myeloma: a systematic review and meta-analysis
Lin CM, et al. BMC Cancer 23: 446 (2023)
Recent data do not convincingly demonstrate an OS advantage to HDM-ASCT
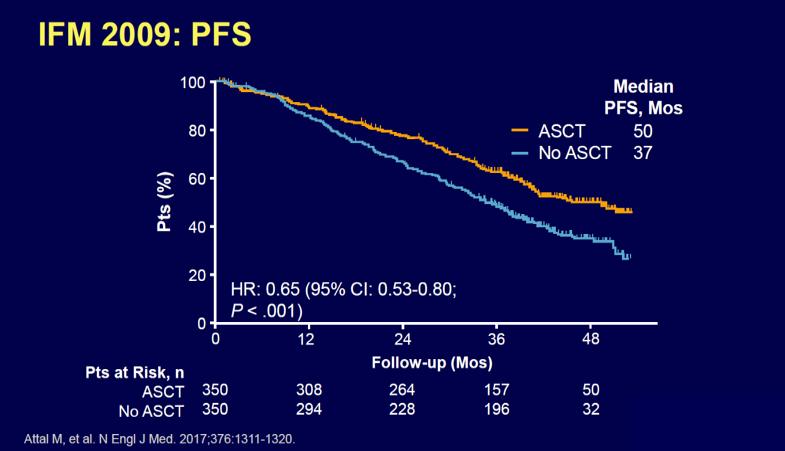
RVD+HDT+ASCT vs RVD alone Phase III IFM/DFCI 2009 TRIAL
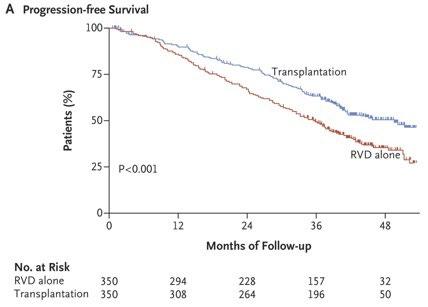
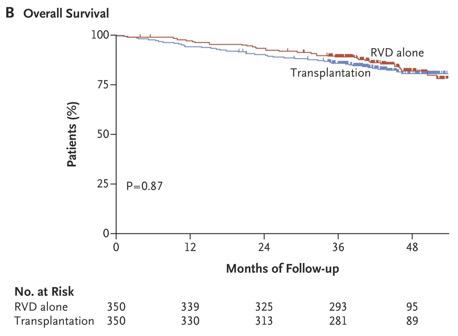
RVD plus ASCT was associated with significantly longer PFS than RVD therapy alone, but OS did not differ significantly
RVD+HDT+ASCT vs RVD alone Phase III DETERMINATION TRIAL
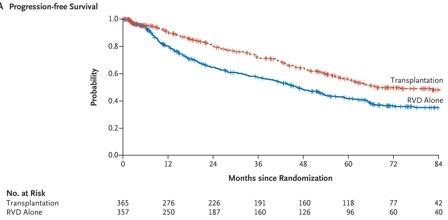
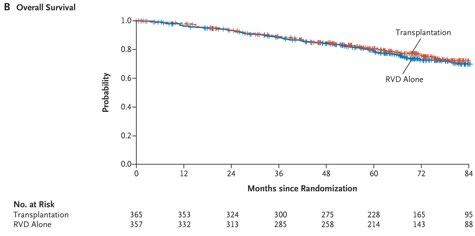
RVD+ASCT was associated with longer PFS than RVD alone, but no OS benefit was observed
So Question is,
• ASCT has long been considered to be the standard care.
• Do we need upfront ASCT for all NDMM-TE patients?
• If upfront ASCT did not show any OS difference, would deferring ASCT be a reasonable strategy?
• But some patients seem to need ASCT for longer OS How can we distinguish and construct effective strategy?
Depth of Response is important for OS
Ozaki et al. Japanese Society of Myeloma retrospective analysis
Measurable Residual Disease by Next-Generation Flow Cytometry
in Multiple Myeloma
PETHEMA/GEM2012MENOS65 clinical trial
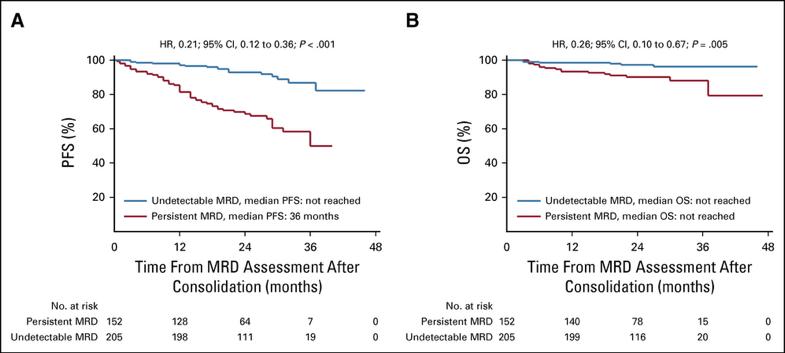
MRD negativity using NGS is major prognostic factor (IFM 2009 trial)
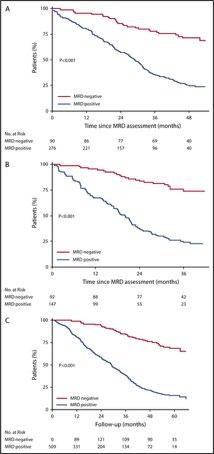
MRD negativity appears to be a stronger predictor of favorable outcome in both PFS and OS than ISS stage, cytogenetics
• MRD negativity is important and could be a surrogate endpoint of PFS/OS in the novel agent era.
• Could we construct MRD guided strategy for NDMM-TE patients?
• Before proceeding, let me talk about stronger induction/ consolidation/maintenance therapeutic regimen namely quadruplet regimen (anti-CD38 antibody+ PI+ IMiDs+ Dex), potentially have power deepening responses.
Clinical Trials with Quad Therapy including anti-CD38 antibody
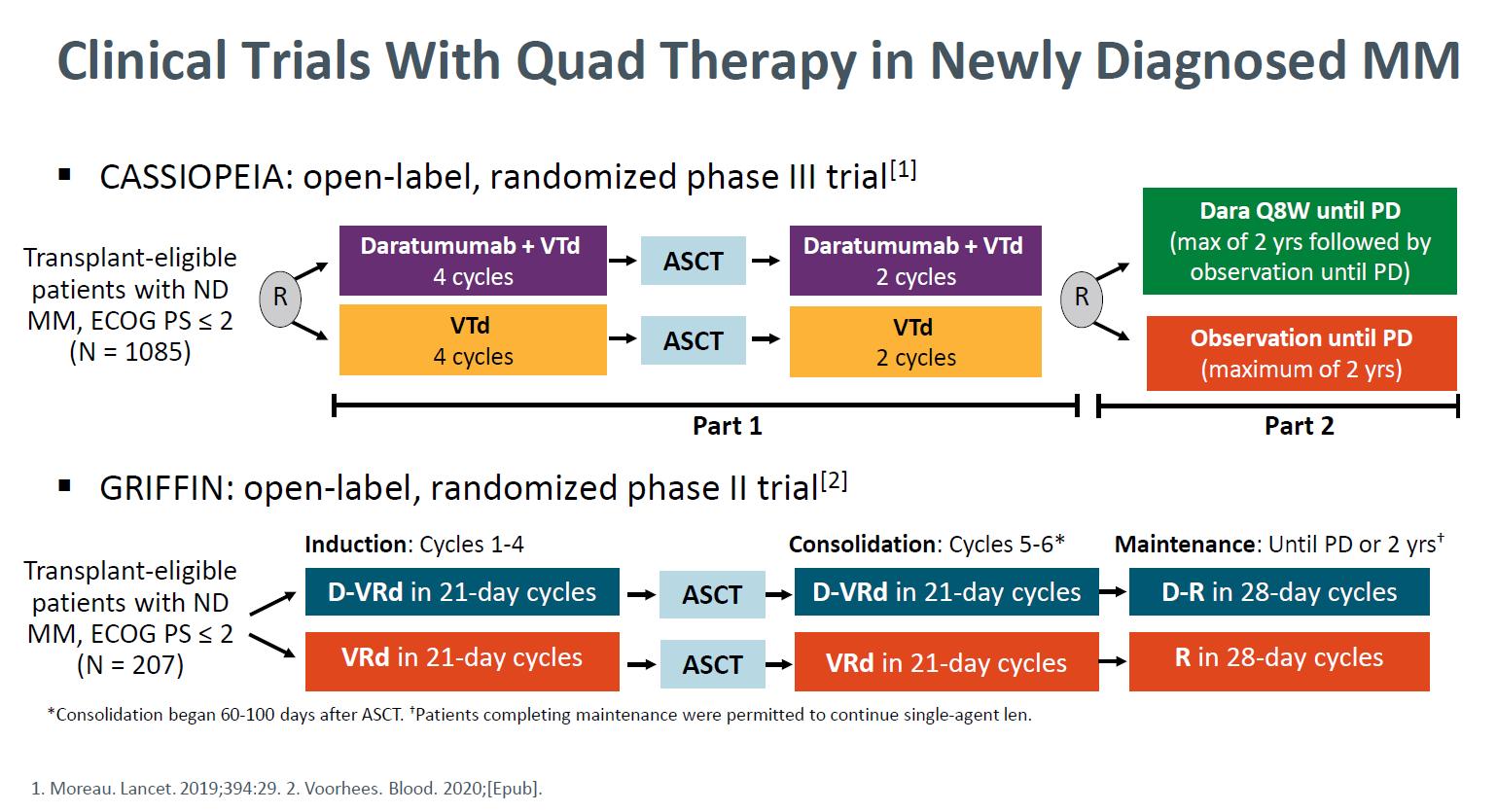
Daratumumab + Bortezomib + Thalidomide +
(D-VTD) vs VTD CASIOPEIA study
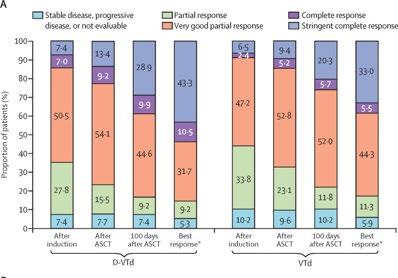
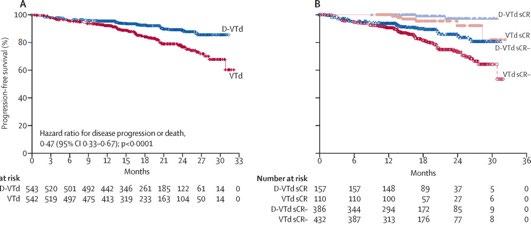
Moreau P. et al. Lancet. 394: 29-38. 2019
Daratumumab + RVd for NDMM GRIFFIN
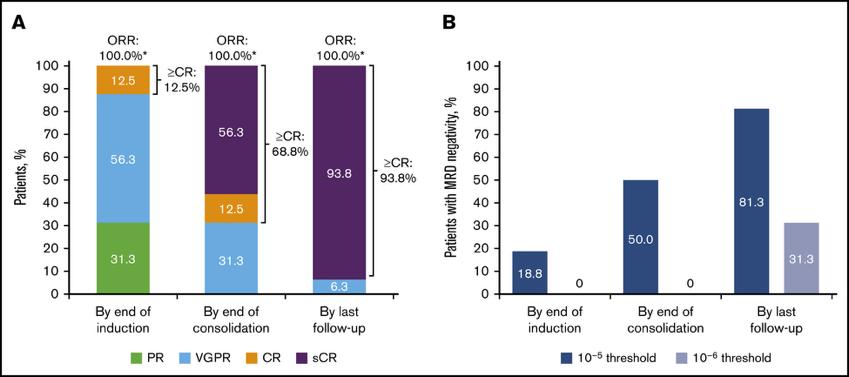
Voorhees PM, et al. Blood Adv. 5(4):1092-1096. 2021
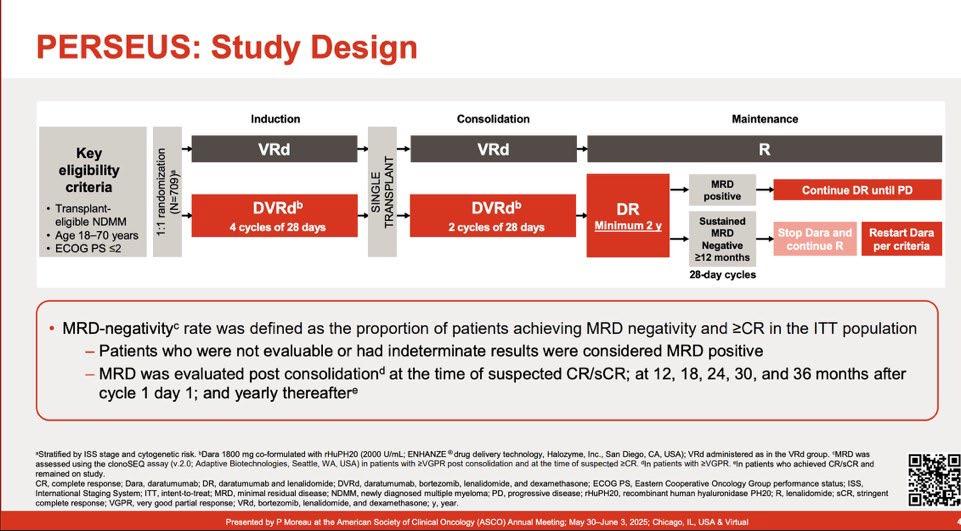
PFS of Daratumumab + RVd for TE-NDMM (PERSEUS)

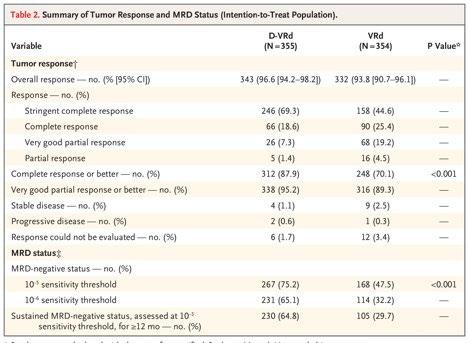
Advantage of achieving deep response following frontline daratumumab-VTd compared to VRd
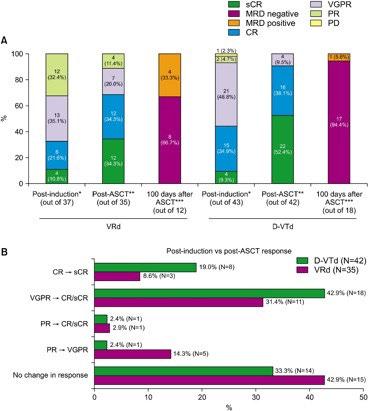
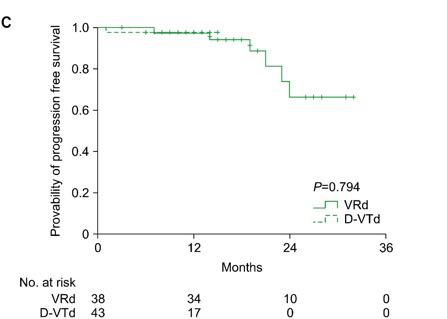
Byun JM, et al. Blood
GMMG-HD7: Isatuximab + VRd vs VRd

GMMG-HD7: MRD
negativity and response rate at end of induction
Main results of anti-CD38 monoclonal antibodies into ASCT program in newly diagnosed MM patients.
CASSIOPEIA [23,24] 3
GRIFFIN [25,26] 2
VTd vs. D-VTd induction (4 cycles) and consolidation (2 cycles), with D maintenance or observation Day 100 after ASCT
D-VRd induction (4 cycles) and consolidation (2 cycles) plus DR maintenance or VRd induction (4 cycles) and consolidation (2 cycles) plus R maintenance
(64% vs. 44%)
PERSEUS [27] 3
D-VRd induction (4 cycles) and consolidation (2 cycles) plus DR maintenance c or VRd induction (4 cycles) and consolidation (2 cycles) plus R maintenance
MASTER [28] 2 D-KRd induction (4 cycles) and consolidation (2 cycles), with R maintenance or observation e
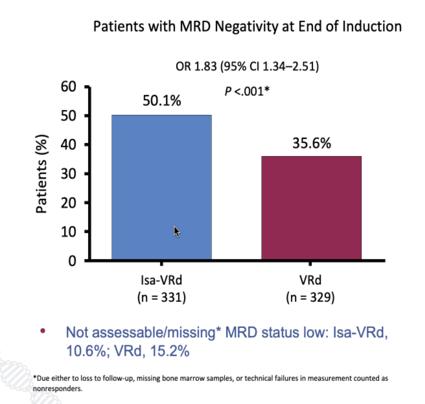
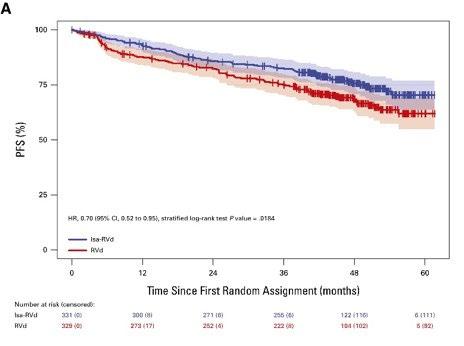
GMMG-HD7 [34] 3
GMMG-CONCEPT [35] 2
Isa-VRd vs. VRd induction (3 cycles) with maintenance with Isa-R or R After induction therapy (50% vs. 36%) Ongoing g
Isa-KRd induction (6 cycles) and consolidation (4 cycles) with Isa-KR maintenance h
(68%) i NR
Measurable
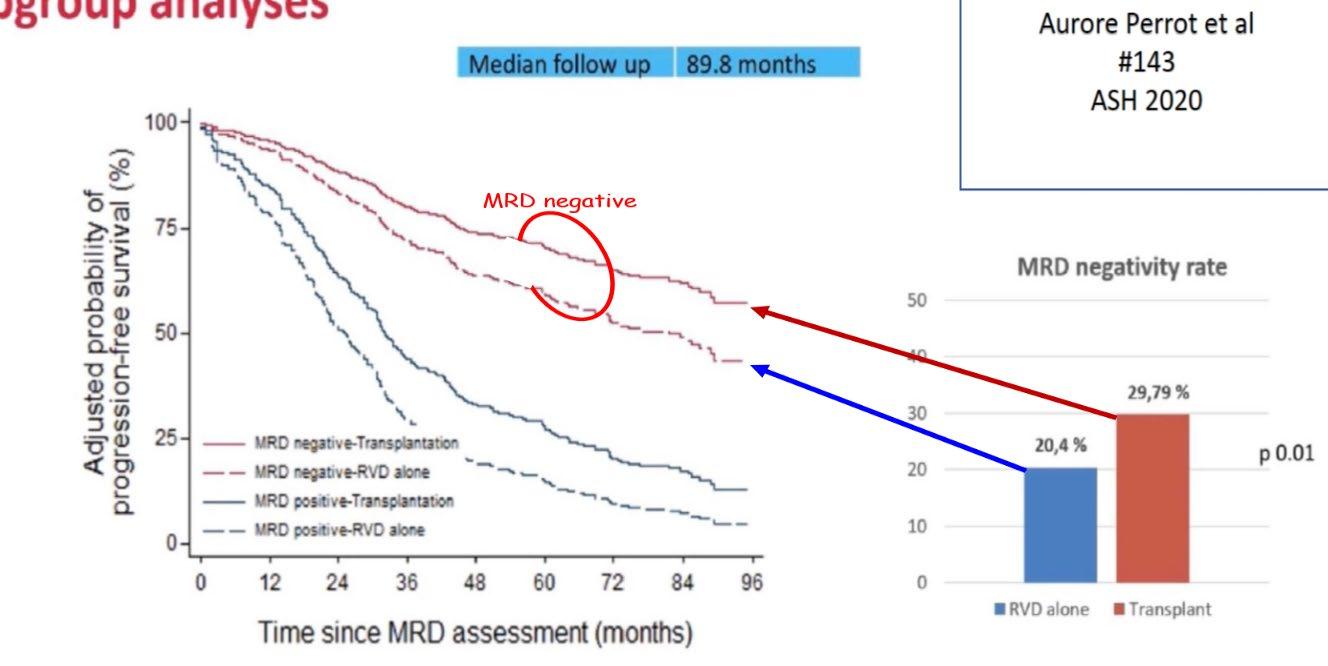
Residual Disease-Guided Therapy in Newly Diagnosed Myeloma (IFM2020-02 MIDAS study)
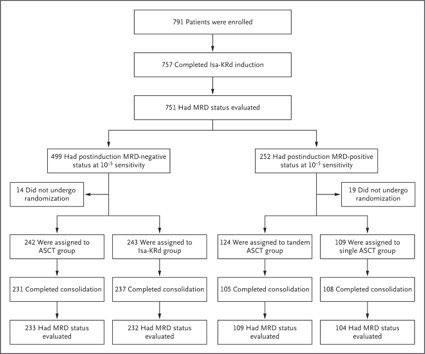
Among patients who were MRD-negative at 10-5 sensitivity after induction, the percentage with a premaintenance
MRD-negative status at 10-6 sensitivity was not significantly higher with ASCT than with Isa-KRd.
→If the patients get into MRD-negative by Isa-KRd, ASCT could not win Isa-KRd. Perrot A. et al.
Indirect comparisons of MRD-negativity rates after induction in ITT populations of studies focused on patients with transplant-eligible newly diagnosed MM
A quick break story
• Midas was a king of Phrygia, with whom many myths became associated.
• The most famous King Midas is popularly remembered in Greek mythology for his ability to turn everything he touched into pure gold and this came to be called the golden touch, or the Midas touch. [1]
• The Tragedy: Midas' daughter turns to a golden statue when he touches her
• Will the treatment scheme like MIDAS become the golden standard?

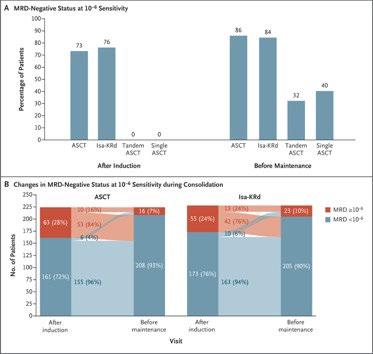
MRD oriented treatment for transplant eligible NDMM MASTER Trial
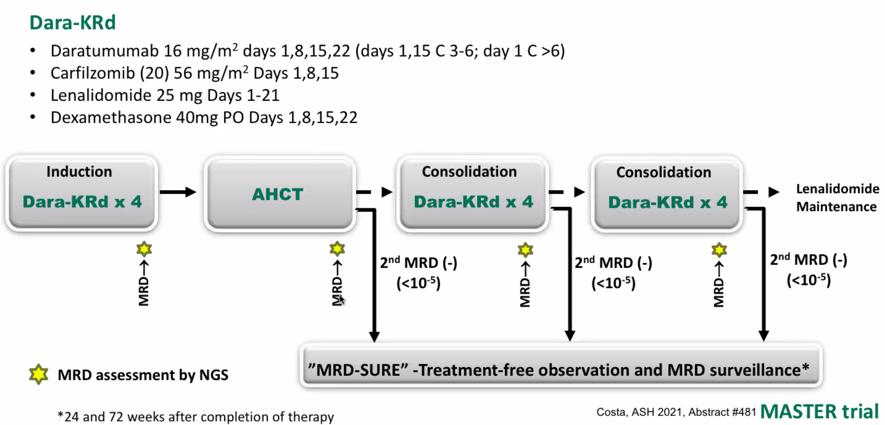
MRD kinetics differ according to cytogenetic risk: MASTER Trial
Around 40% achieved 10-5 MRD negative post induction, 60% post ASCT, 80% post consolidation.
And the almost same response was observed in the patients with high-risk cytogenetic abnormality (HRCA).
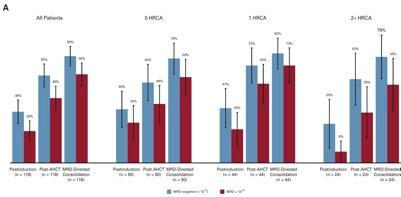
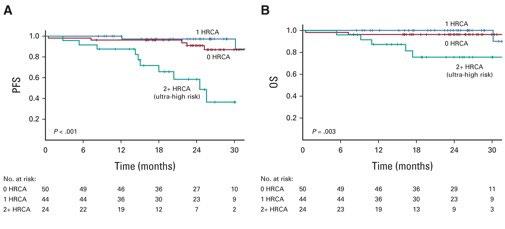
Caution !!
Even though 2+High risk cytogenetic abnormality (Ultra high risk) patients had deep, but the percentage of the patients with relapse after discontinuation was higher.
Costa LJ, et al. J Clin Oncol. 40: 2901-2912. 2022
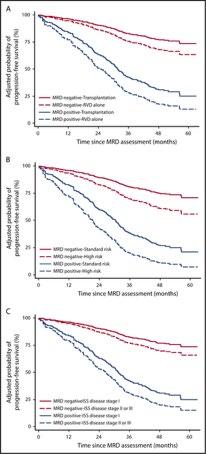
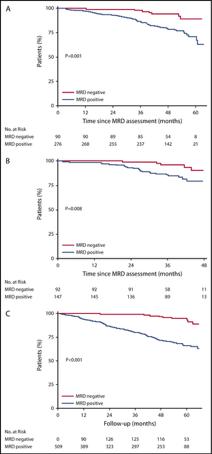
Cytogenetic risk stratification combined with minimal residual disease status influences the therapeutic outcome
and prognosis of multiple myelomas
The median PFS (C) and OS (D) in MM patients divided by the cytogenetic risk stratification combined with the MRD status.
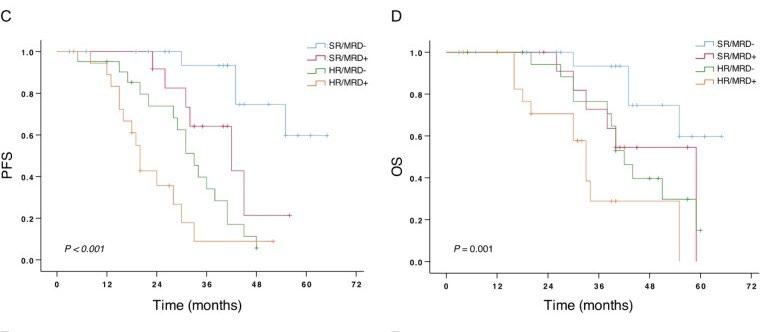
Mao J et al. Sci Rep. 2025 Apr 12;15(1):12545.
Next question
• How old should we or can we treat with ASCT?
• MM is predominantly a disease of older adults, with a median age at diagnosis of 69 years and over one-third of new cases occurring in patients aged 65 to 74 years.
How old is ASCT effective for ? ASCT for elderly patients (age>65)
Age no bar: A CIBMTR analysis of elderly patients undergoing autologous hematopoietic cell transplantation for multiple myeloma
MM is predominantly a disease of older adults, with a median age at diagnosis of 69 years and over one-third of new cases occurring in patients aged 65 to 74 years.
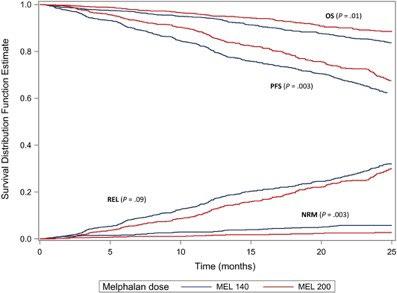
Among patients who received Mel at a dose of 200 mg/m2, there was no difference in OS noted by age group
Mel at a dose of 140 mg/m2 was associated with a worse NRM. both PFS and OS were worse among the patients aged ≥70 years who were treated with Mel at a dose of 140 mg/m2 Munshi PN et.al, Cancer. 127(20):3904. 2021
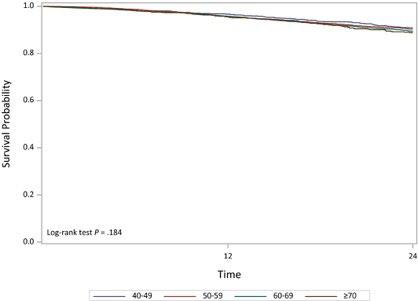
Autologous stem cell transplantation is safe and effective for fit, older myeloma patients: exploratory results from the Myeloma XI trial
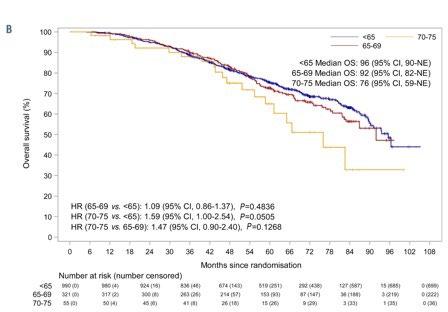
Pawlyn C. et al. Haematologica. 107: 231-242. 2022
Autologous stem cell transplantation is safe and effective for fit, older myeloma patients: exploratory results from the Myeloma XI trial
Pawlyn C. et al. Haematologica. 107: 231-242. 2022
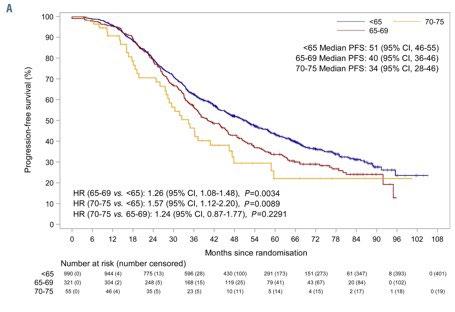
Daratumumab for newly diagnosed multiple myeloma: Pooled analysis of patients aged ≥65
years from GRIFFIN and
PERSEUS
MM is predominantly a disease of older adults, with a median age at diagnosis of 69 years and over one-third of new cases occurring in patients aged 65 to 74 years.
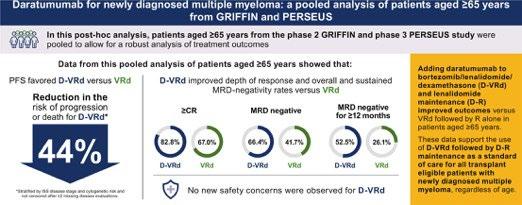
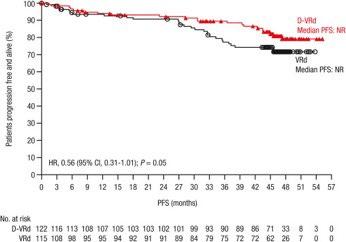
A trend in improved PFS was seen among patients aged ≥65 years favoring D-VRd
These data support use of D-VRd followed by D-R maintenance as standard of care for all transplant-eligible patients with NDMM, regardless of age up to 70 years.
Meaning of continuous/maintenance therapy
VRd followed by autologous stem cell transplant
Maintenance is important especially for High-risk cytogenetics
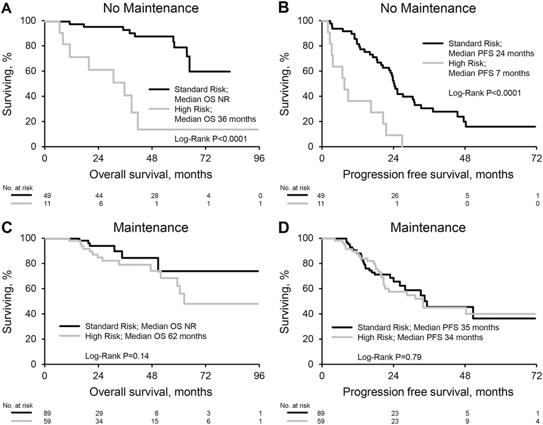
Sidiqi MH et al. Blood Cancer J. 2018;8(11):106.
Goldschmidt H et al, Leukemia.34: 3019-3027. 2020
Maintenance with daratumumab or observation following treatment with VTD with or without daratumumab and ASCT
in
patients
with NDMM (CASSIOPEIA)
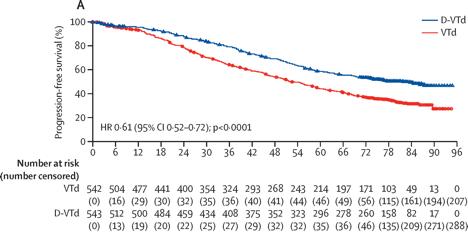
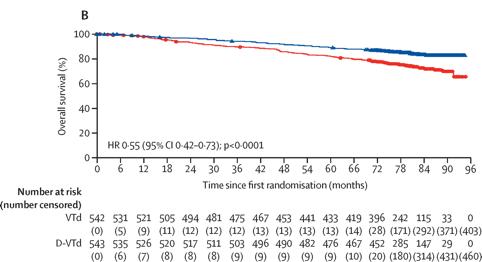
Moreau P et al. Lancet Oncol. 25:1003-1014. 2024
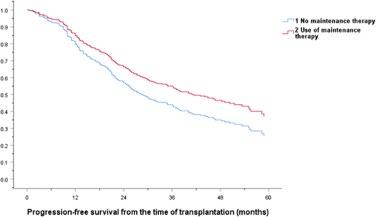
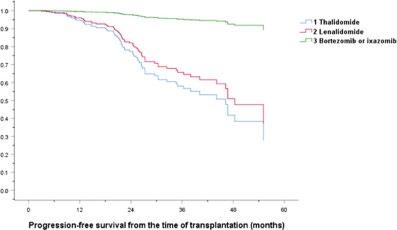
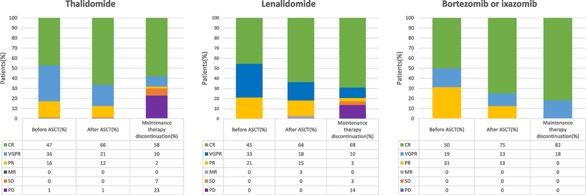
Kang KW, et al. (KMMWP) BMC Cancer.;25:204.
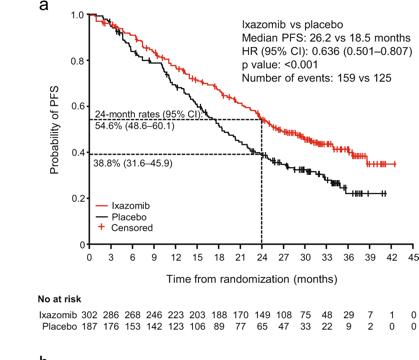
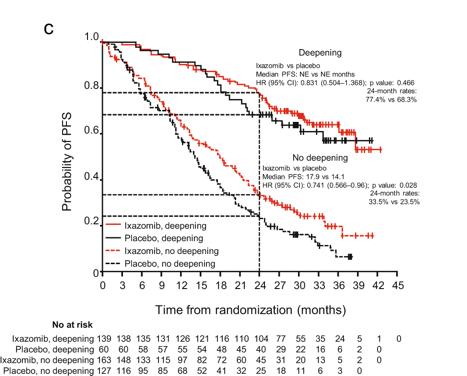
Lenalidomide remains the maintenance therapy of choice for patients with NDMM
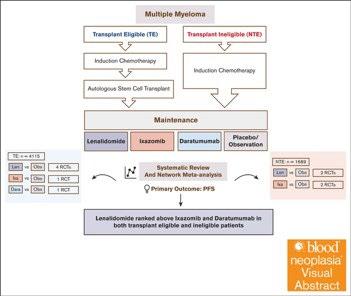
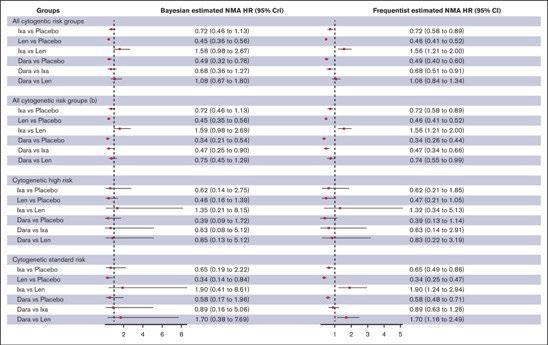
Lenalidomide and daratumumab but not ixazomib were associated with improved PFS compared with placebo in patients with TEMM None of the agents showed an OS benefit, and PFS benefits were not seen in patients with high-risk cytogenetics.
Lai E, et al. Blood Neoplasia (2024) 1 (4): 100042.
Stem Cell Mobilization and Harvesting
• A minimum of 2 × 106/kg CD34+ cells is required to perform ASCT, while the ideal target for 1 ASCT is >3 × 106/kg CD34+ cells, and that for 2 ASCT is >6 × 106/kg CD34+ cells.
• Stem cell mobilization can be performed with granulocyte colonystimulating factor (G-CSF), preceded or not by chemotherapy with Cyclophosphamide (Cy) at a variable dose of 1.5–4 g/m2.
• Plerixafor (scissor enzyme selective and reversible antagonist of CXCR4) has been approved, which is particularly effective in reducing the failure rate.
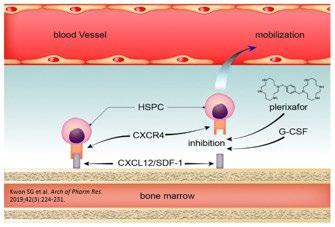
Stem Cell Mobilization and Harvesting
• After plerixafor approval, the chemotherapy-free protocol has been adopted by many centers because of the less adverse event rates, such as neutropenia or infections.
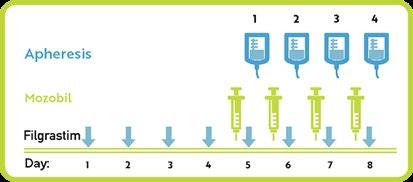
• Recent data seem to indicate that the introduction of anti-CD38 monoclonal antibodies in the induction therapy of NDMM is associated with a lower capacity to mobilize and collect HSC.
HDT-ASCT is a potentially toxic therapy
• Treatment related toxicity (TRM) <5%, <1% in expert centers
• Acute HDM-ASCT toxicity
Prolonged bone marrow suppression, infection, VOD (0.4%), Interstitial
Pneumonitis (0.5-8%), autologous GVHD (18%), graft failure (0.6%) (Fermand JP, Blood 92: 3131;1998)
• Decline of QoL
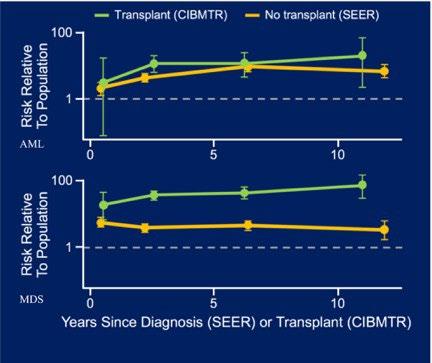
• Increased risk of secondary malignancies
AML/MDS risk is 11.5-fold higher in MM compared to general population.
AML/MDS risk is 50-fold and 100-fold higher respectively among patients treated with HDT-ASCT compared to the general population
The risk is approximately 10-fold higher than non-transplant patients.
Recent genomic study revealed that new mutational signature in previously exposed to high dose melphalan.
Relative risks of AML and MDS after autologous stem-cell transplant (CIBMTR data) or diagnoses (SEER data) for patients with MM
Patients receiving autologous transplant are at up to a 50-fold increased risk of developing AML compared to 10-fold and up to 100-fold risk of developing MDS compared to fivefold.
High dose melphalan induces mutation
EH, et al. Nat Commun. 11(1):1917. 2020
FZ et al. Mod Pathol. 36(6):100166.2023
Rustad
Jelloul
T cell-redirecting therapy in the front-line setting in transplant-eligible MM patients
KarMMa-4 NCT04196491 1
NDMM, TE bb2121 autologous CAR T cells + R as maintenance
NCT01352286
1/2
, TE b anti-CD3/anti-CD28-costimulated autologous T cells after ASCT c
Teclistamab + DRd ± V as induction and Teclistamab + DR as maintenance
Teclistamab + R vs Teclistamab vs R as maintenance after ASCT
+ Talquetamab + D
Positioning of ASCT
1. Recent advancement of induction treatment i.e. quadruplet regimen may change the treatment scheme in near future.
2. Including more potent drugs or antibody such as BCMA-bispecific antibody for the primary therapy will be likely pushed further in that direction
3. Using BCMA CAR-T instead of ASCT
4. But these T cell redirecting therapies are expensive, thus, despite its toxicity and limited ability to prolong OS, ASCT remains the standard treatment for NDMM-TE patients.
5. MRD driven and deferring ASCT may work.


Thank you !
Discussion




Close of Morning Session & Announcements

Dan Navid, JD VP, Global Affairs
International Myeloma Foundation
LUNCH







Treatment Options Asia - NDTI

Daryl Tan, MD
Mount Elizabeth Novena Hospital
Myeloma Treatment Options in Asia : Newly Diagnsoed Transplant-Ineligible Patients
Singapore
Daryl Tan
Mt Elizabeth Novena Hospital
Disclosures
Research Support/P.I. Novartis, Janssen
Employee . NA
Consultant . Janssen, Celgene
Major Stockholder . NA
Speakers Bureau NA NA
Honoraria
Scientific Advisory Board
Celgene, Janssen, Takeda, Amgen, Aztra Zeneca
Celgene, Janssen, Takeda, Novartis, Abbvie, Roche, Amgen, MSD
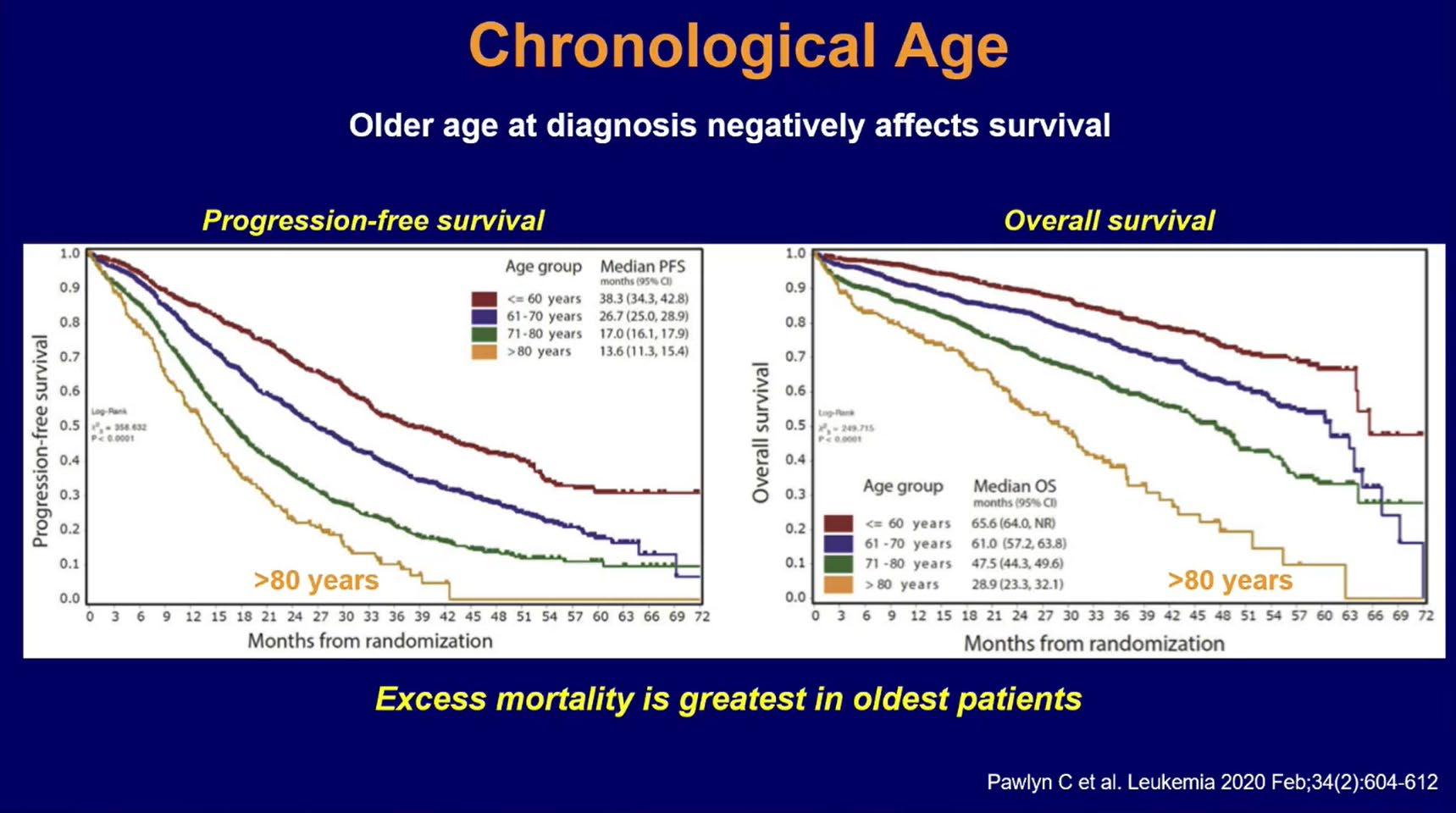
Chronological Age
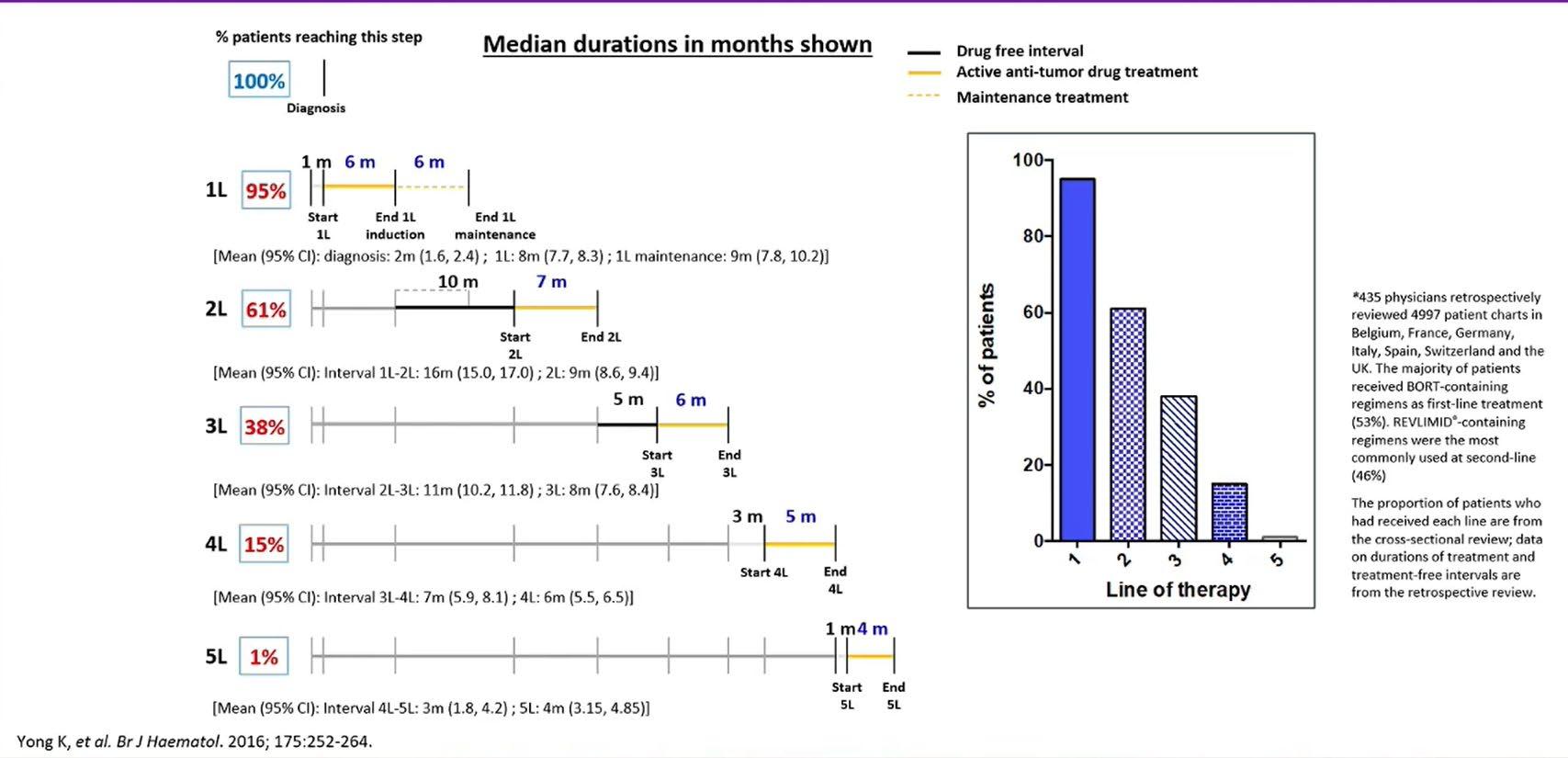
Treatment Durations and Treatment-free Intervals: A European Study
Myeloma Treatment Paradigm

































Treatment for Transplant-Eligible MM-Major RCTs
Goals
of Therapy in Elderly Patients
Symptom control
Quality of life
Deep response/MRD-
Long PFS
Long OS
Possible without ASCT ?
Toxicities
Tolerability
Frailty
Not all Elderly are Equal
Fit
Transplan
t- Eligible

Unfit / Intermediate
frail

Frail

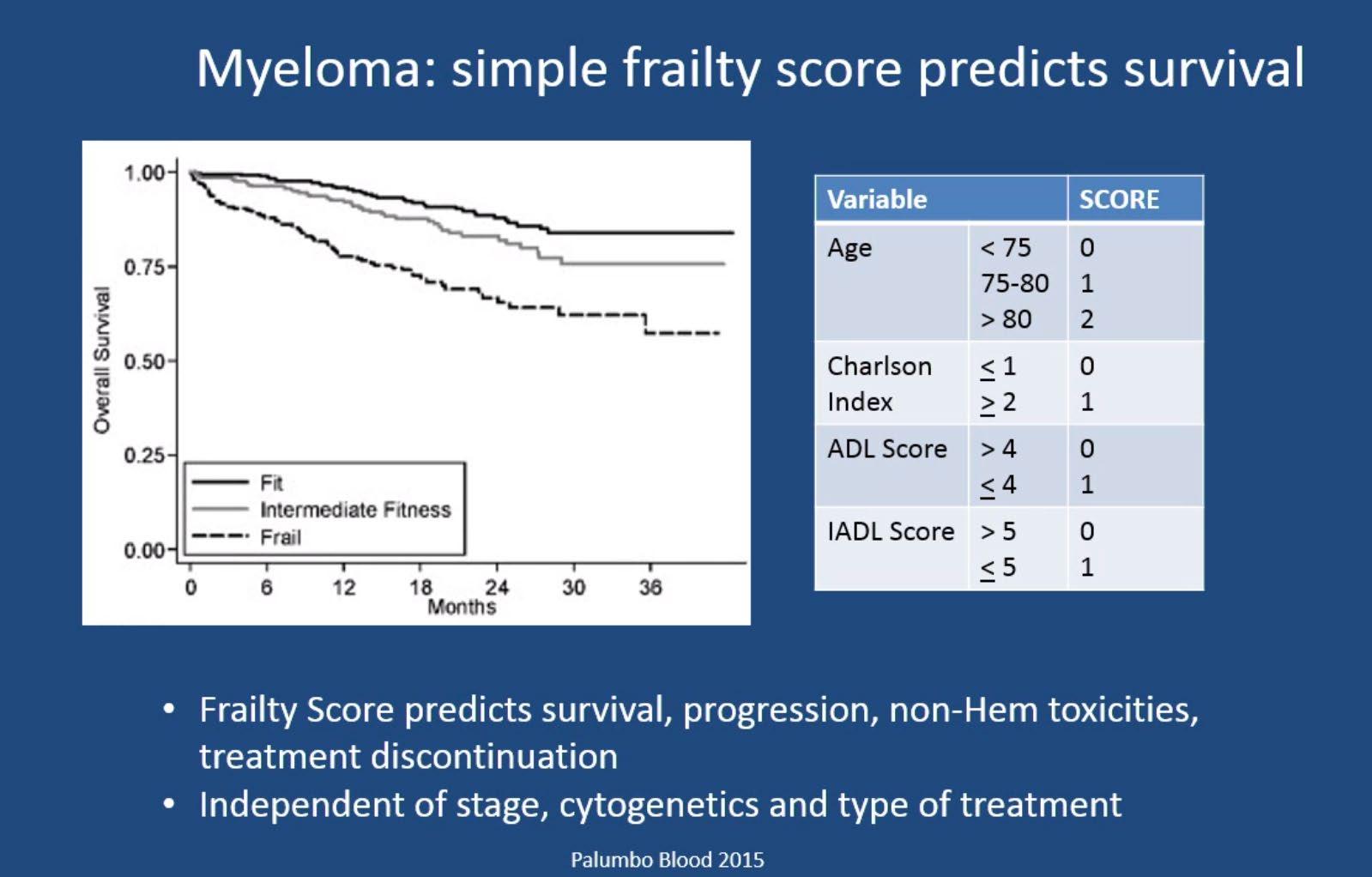
Overall Survival : Subgroup Analysis in All Patients
Report of EMN
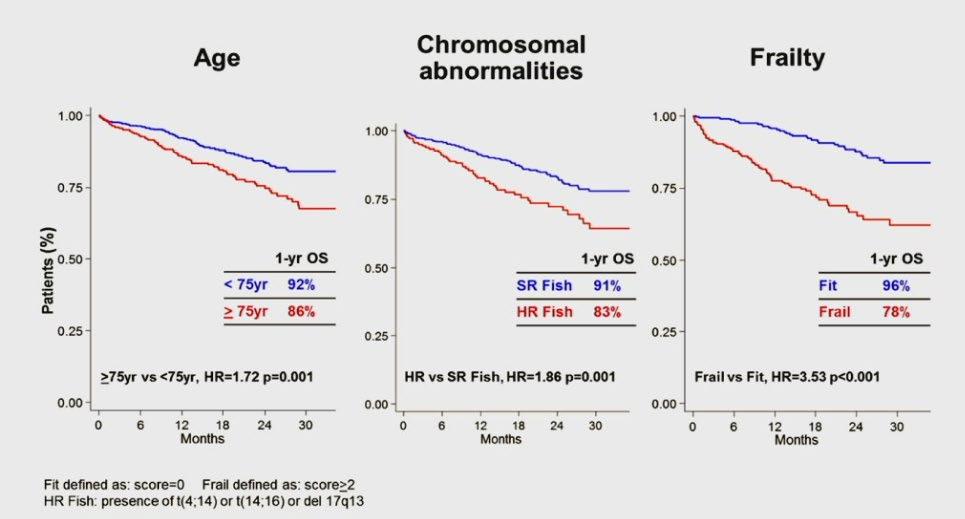
Palumbo A et al. Blood 2011; 125 2068-2074
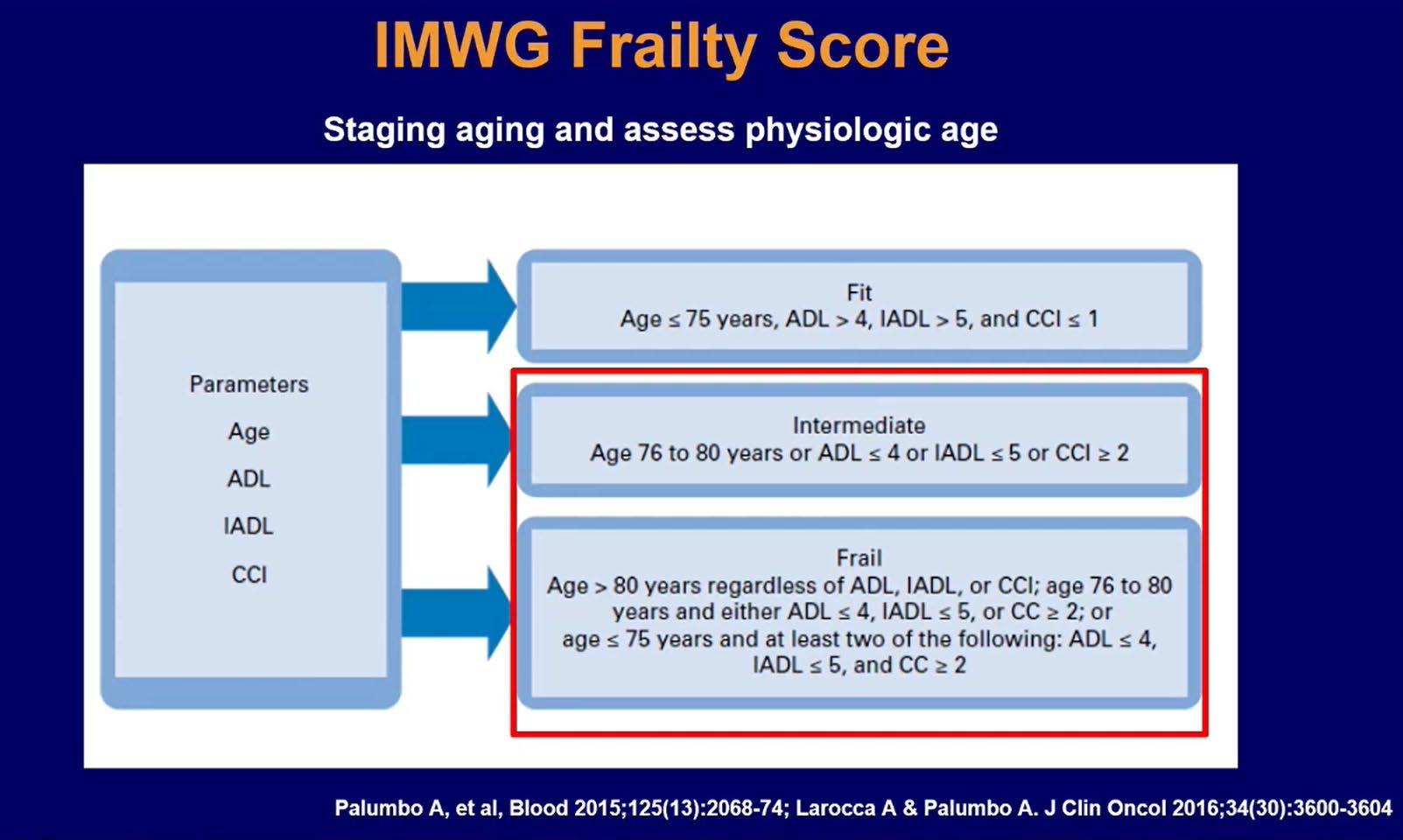
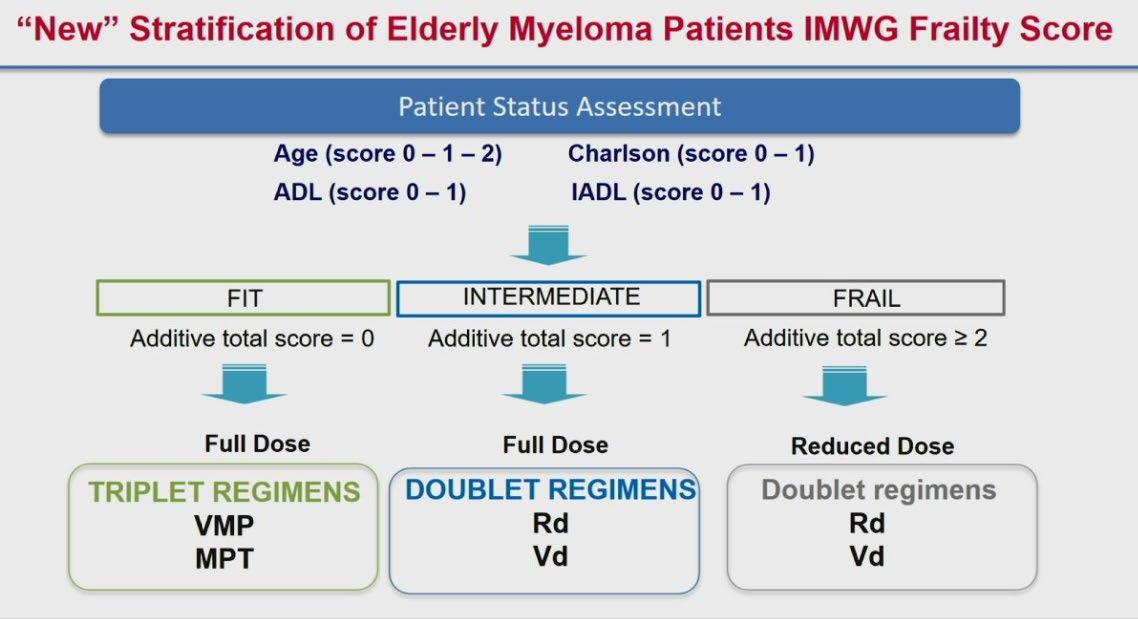
IMWG Frailty Score

IMWG Frailty Score Predicts Outcome and Toxicity
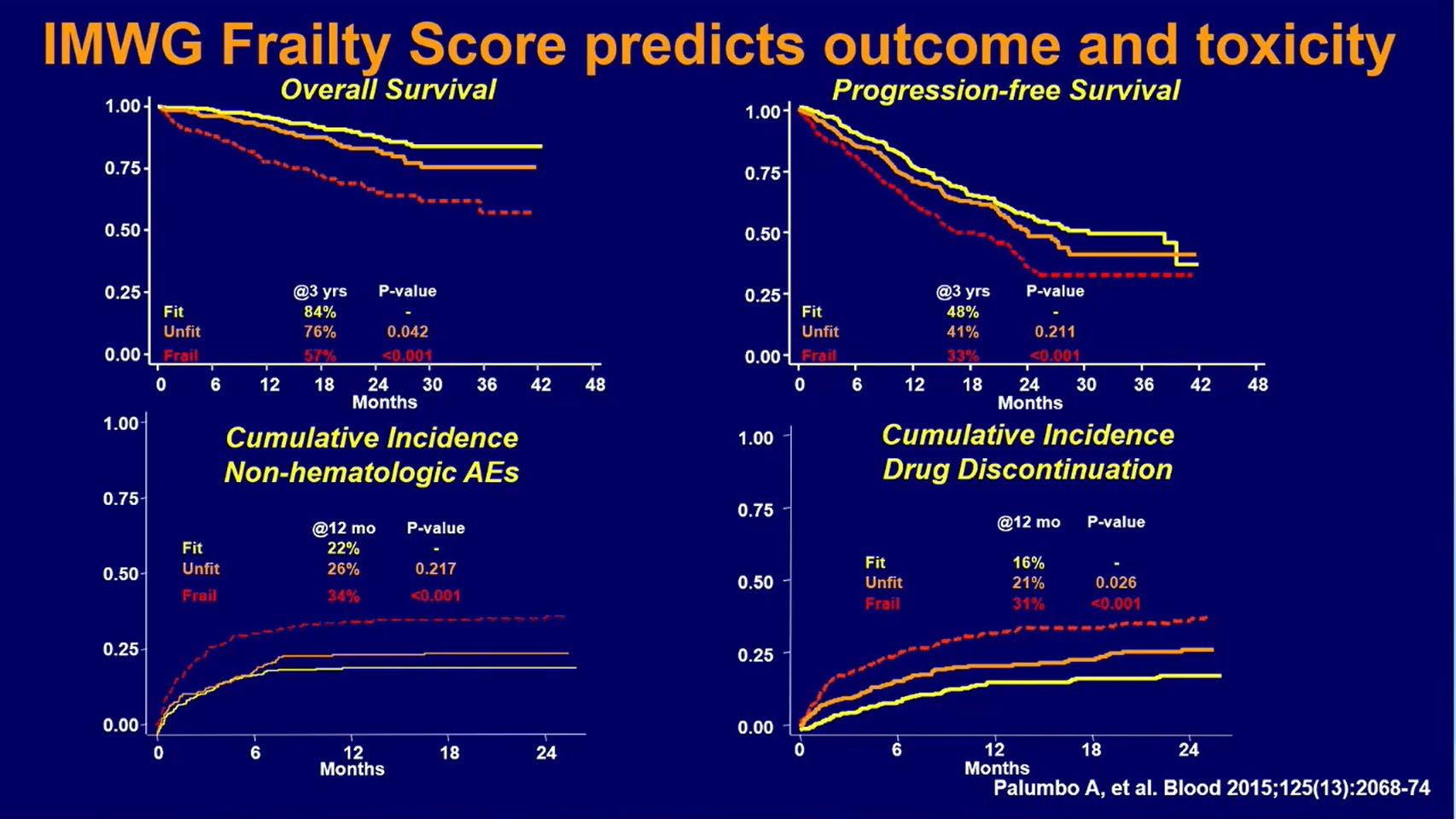
No matter how good a new drug is, it is useless if it cannot be tolerated by the patient
Evolution
of Treatment for Tpt-Ineligible MM
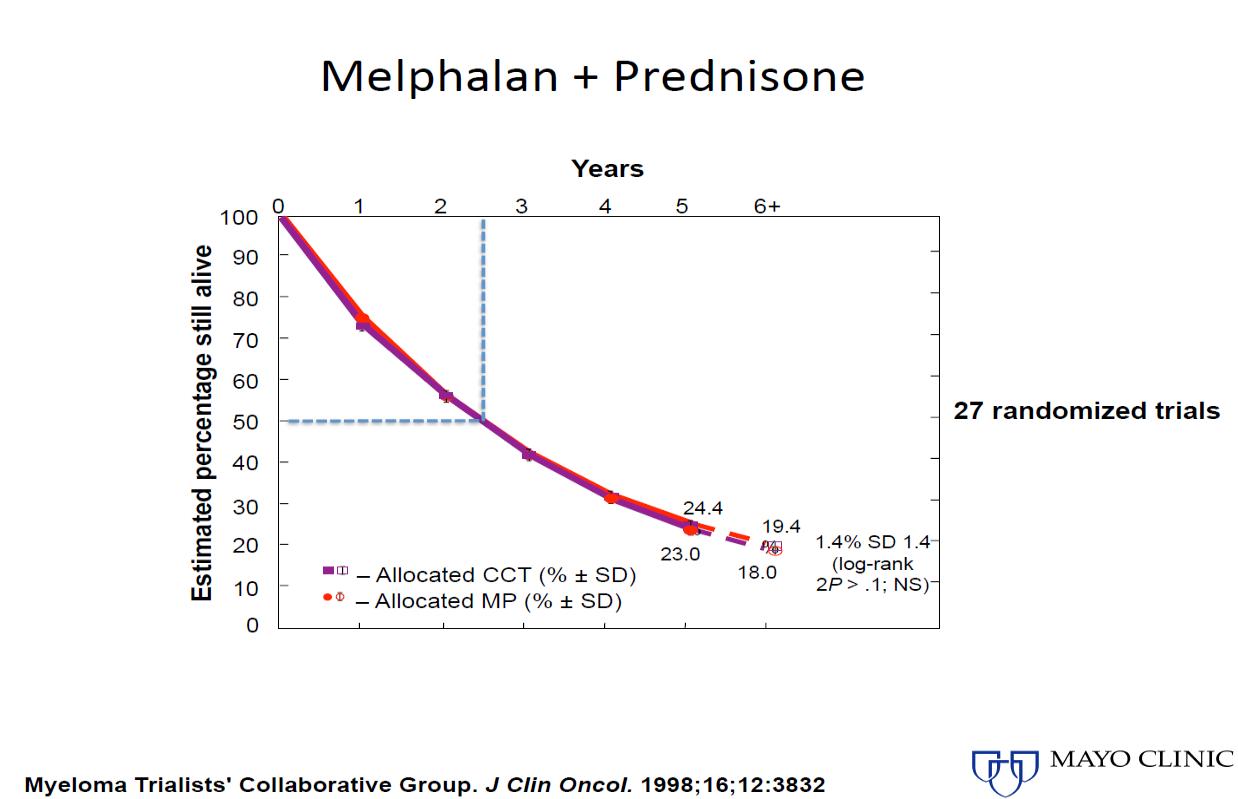
1980-90s 2000s
Evolution
of Treatment for Tpt-Ineligible MM
1980-90s
2000s
Fayers, et al. Blood 2011
Evolution of Treatment for Tpt-Ineligible MM
1980-90s
2000s
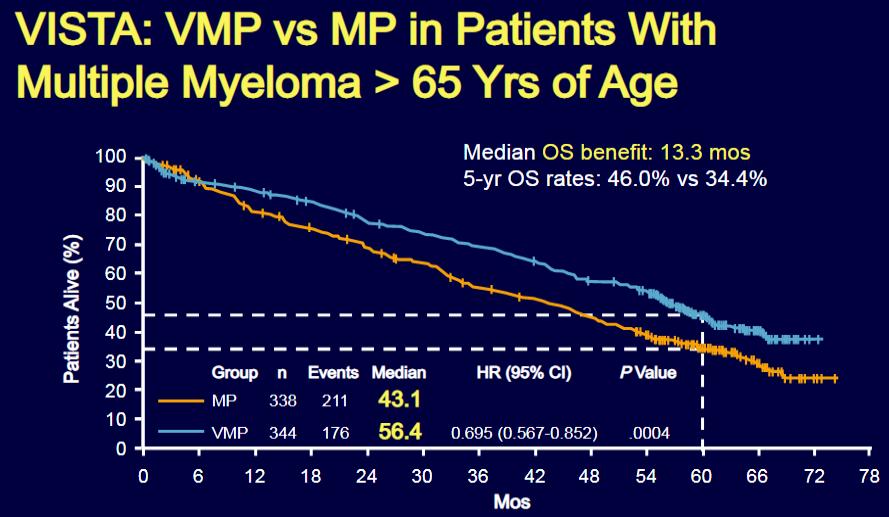
San Miguel J, et al. N Engl J Med. 2008: 359:906-917
Evolution of Treatment for Tpt-Ineligible MM
1980-90s
Evolution of Treatment for Tpt-Ineligible MM
1980-90s
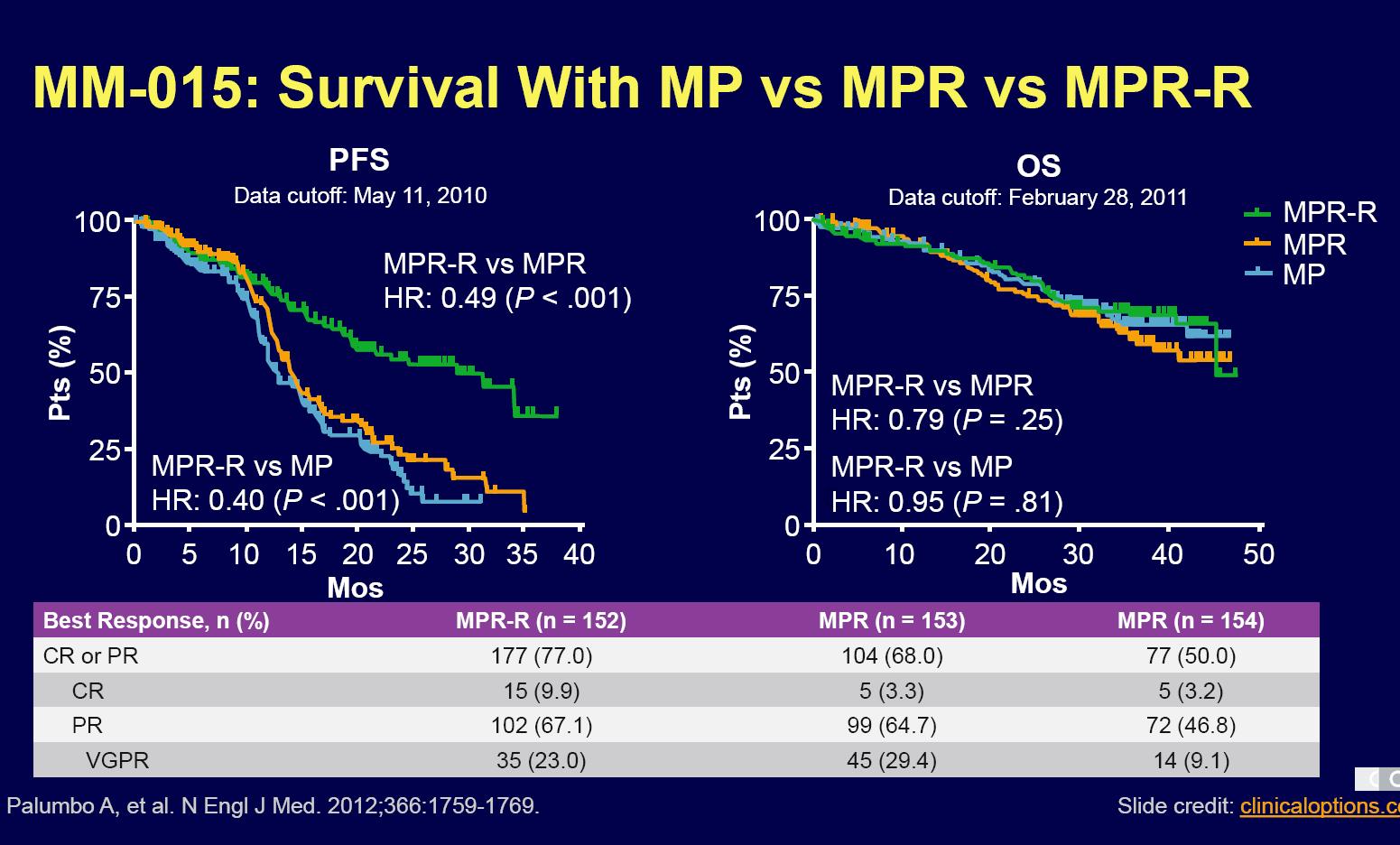
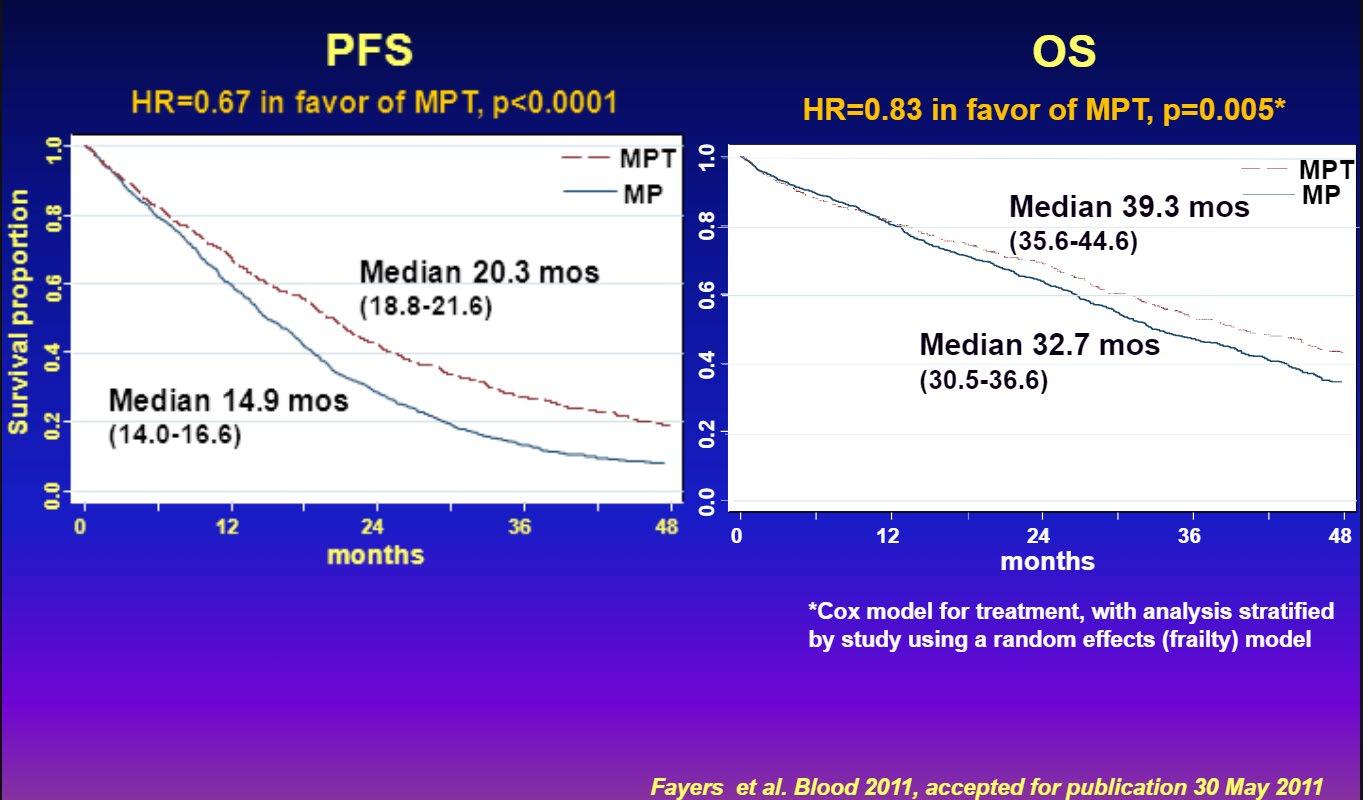
Continuous therapy is key if using IMiD combination
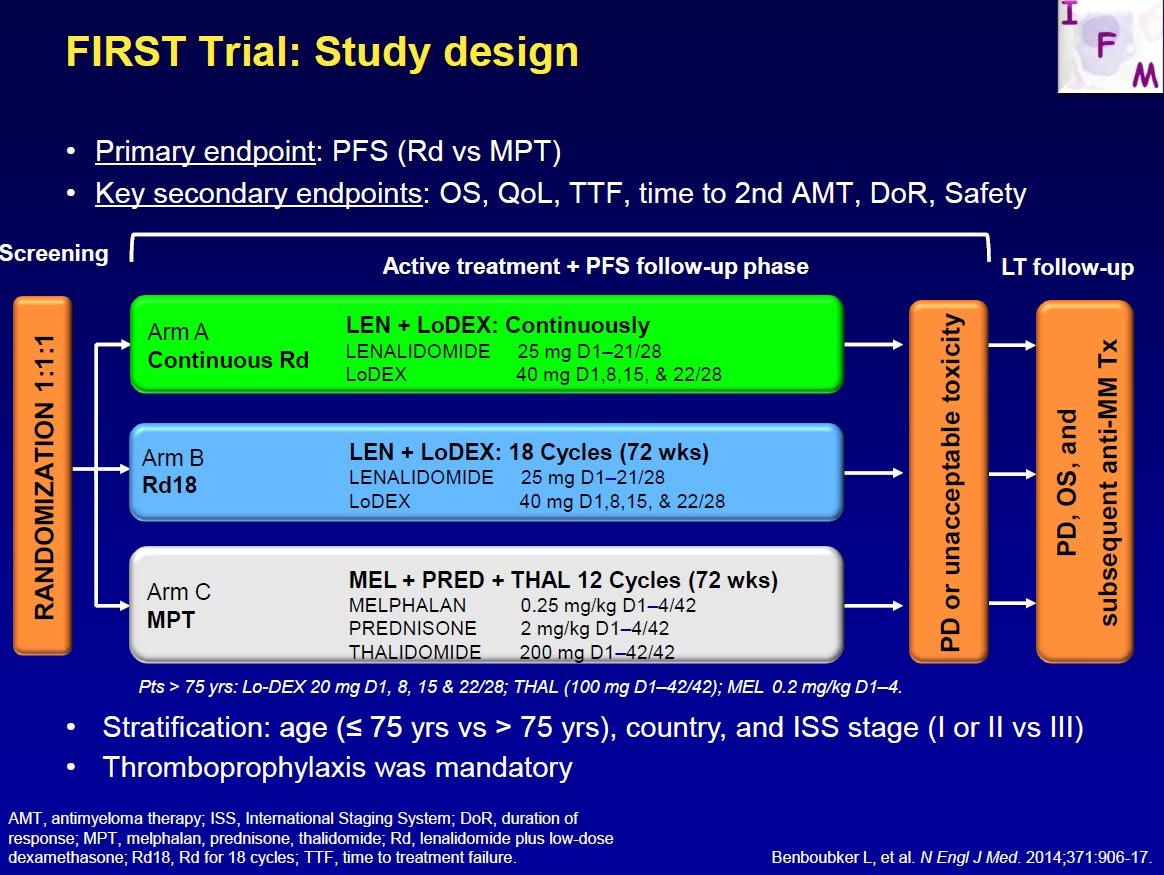
Final analysis of survival outcomes in the randomized phase 3 FIRST trial
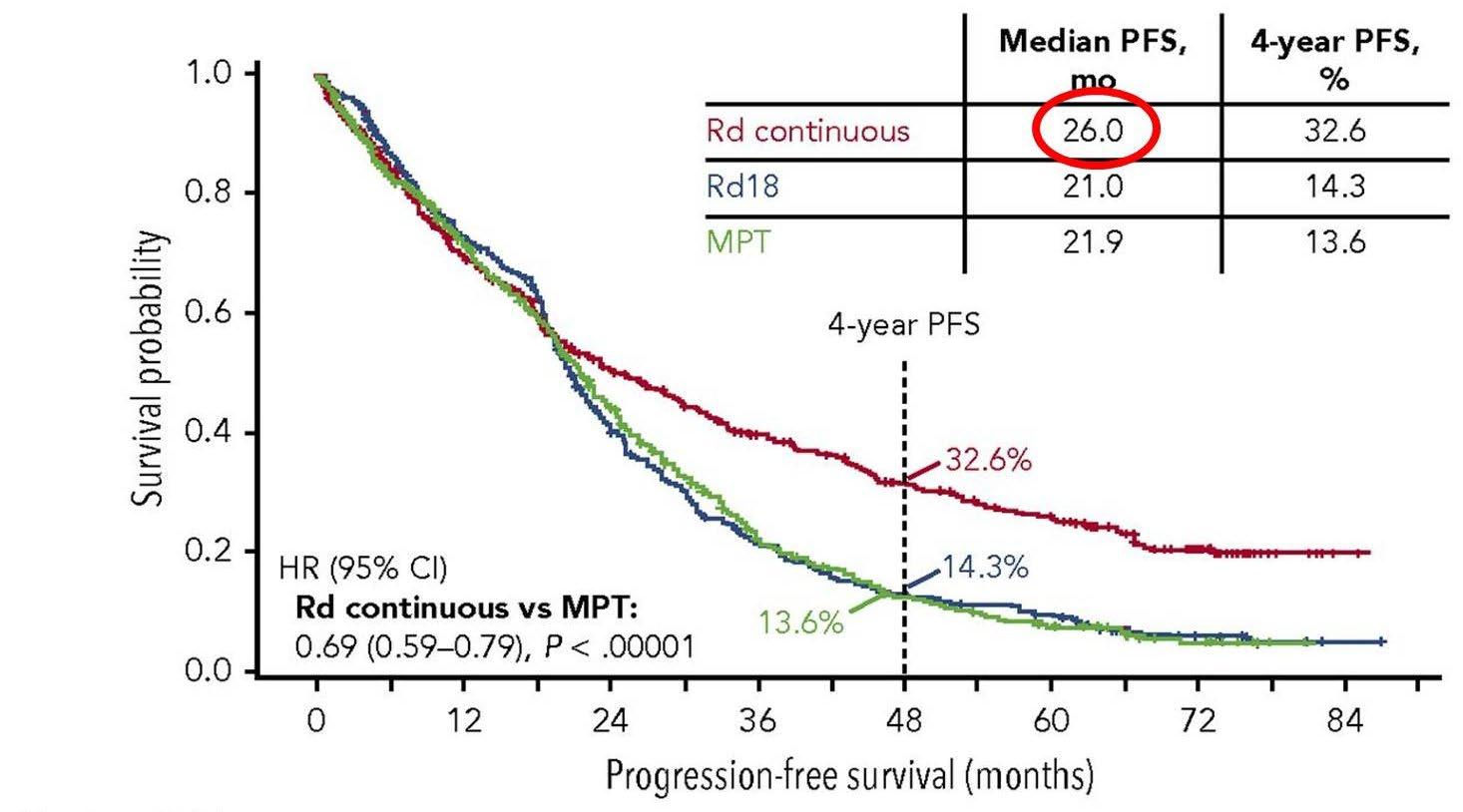
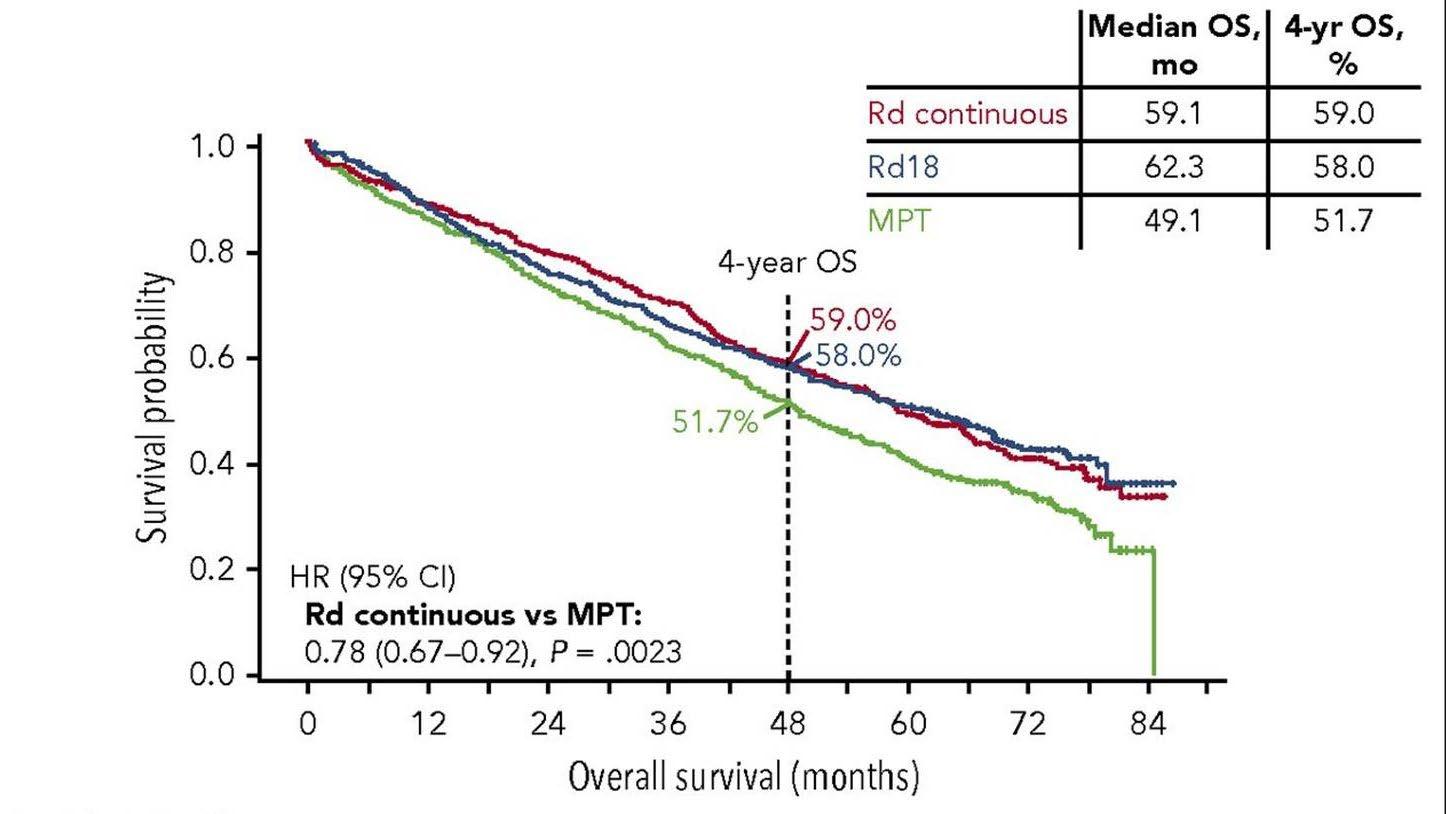
Rd continuous superior
Rd is superior to MPT
Facon T et al. Blood 2018
RD vs RD-R in Intermediate Fit Patients
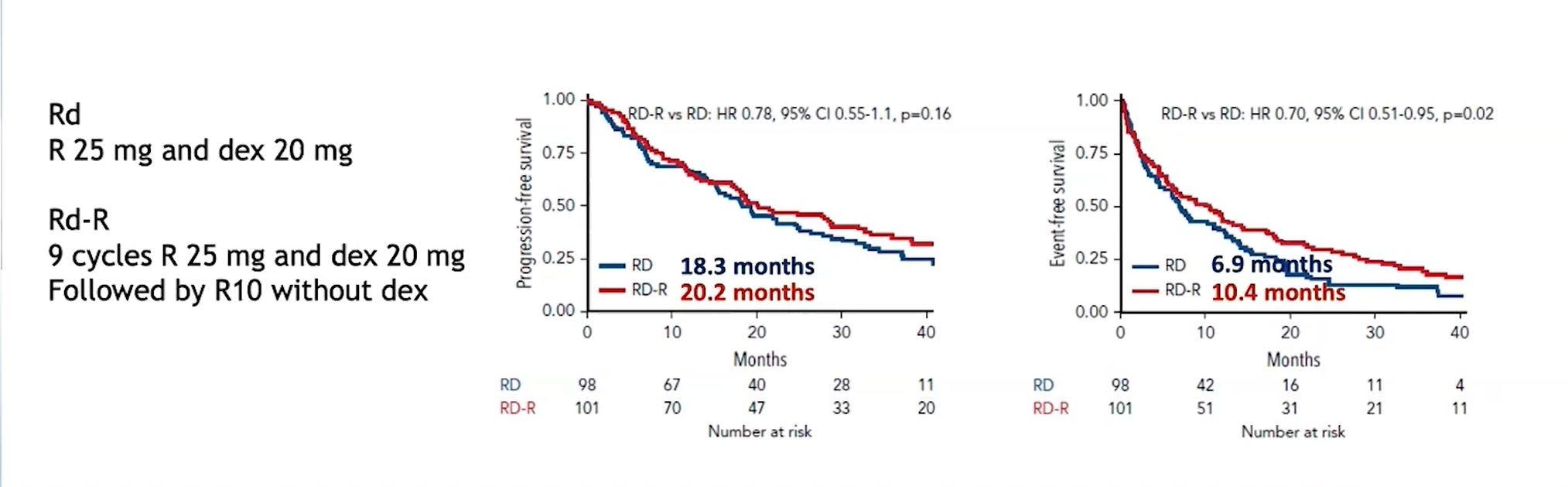
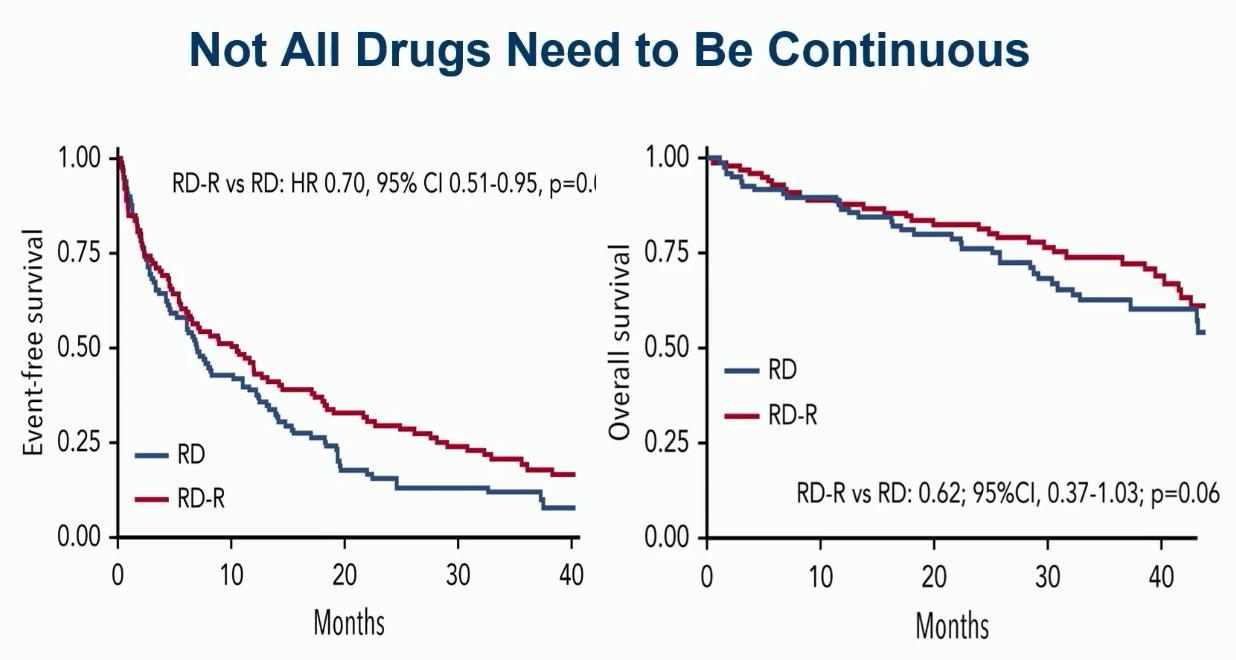
EFS: Death, Progression, Discontinuation of Len, Grade IV haem tox, Grade III/IV non-haem tox
Larocca A, et al. Blood 2021;137(22):3027-36
Evolution of Treatment for Tpt-Ineligible MM
1980-90s
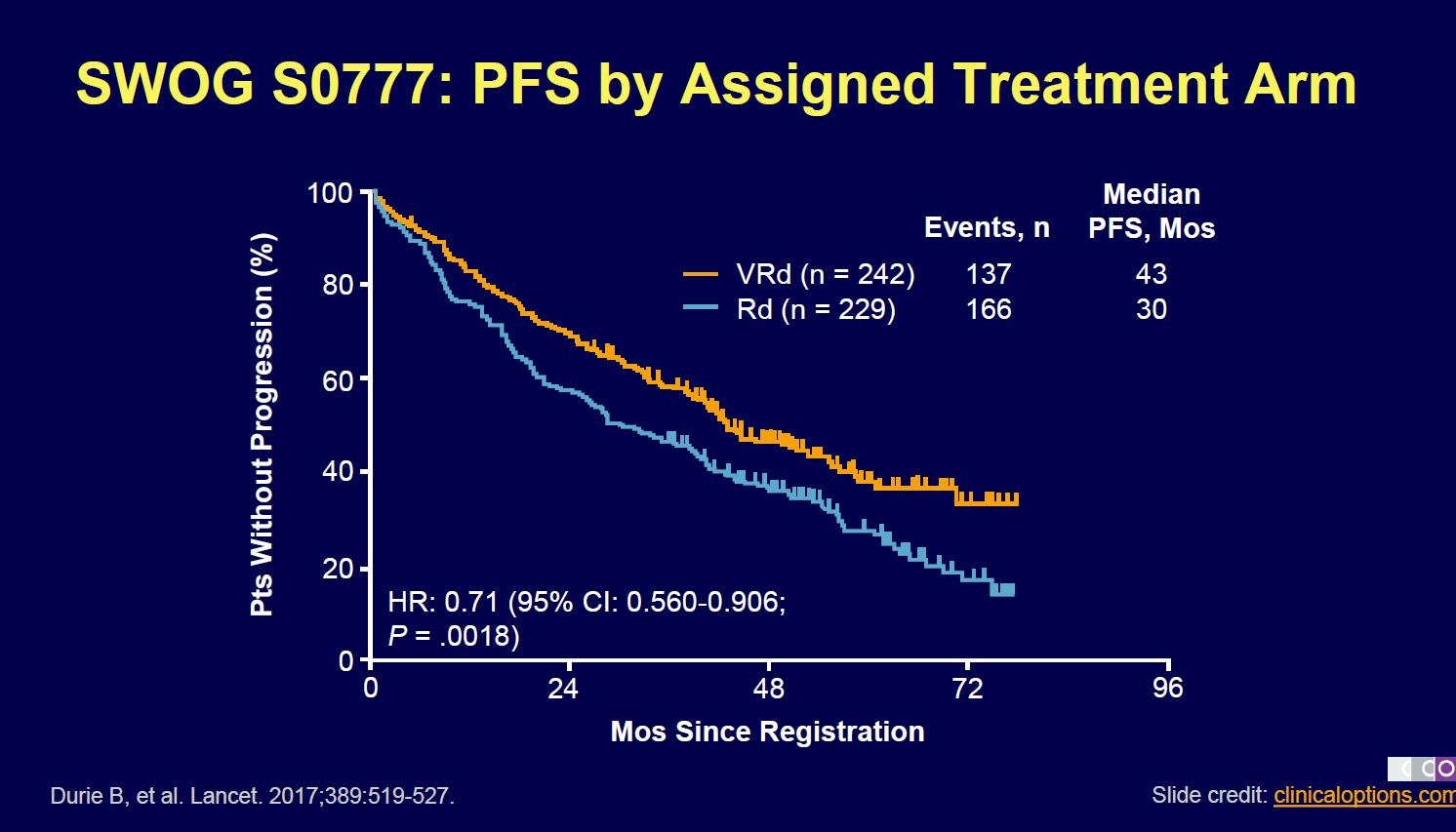
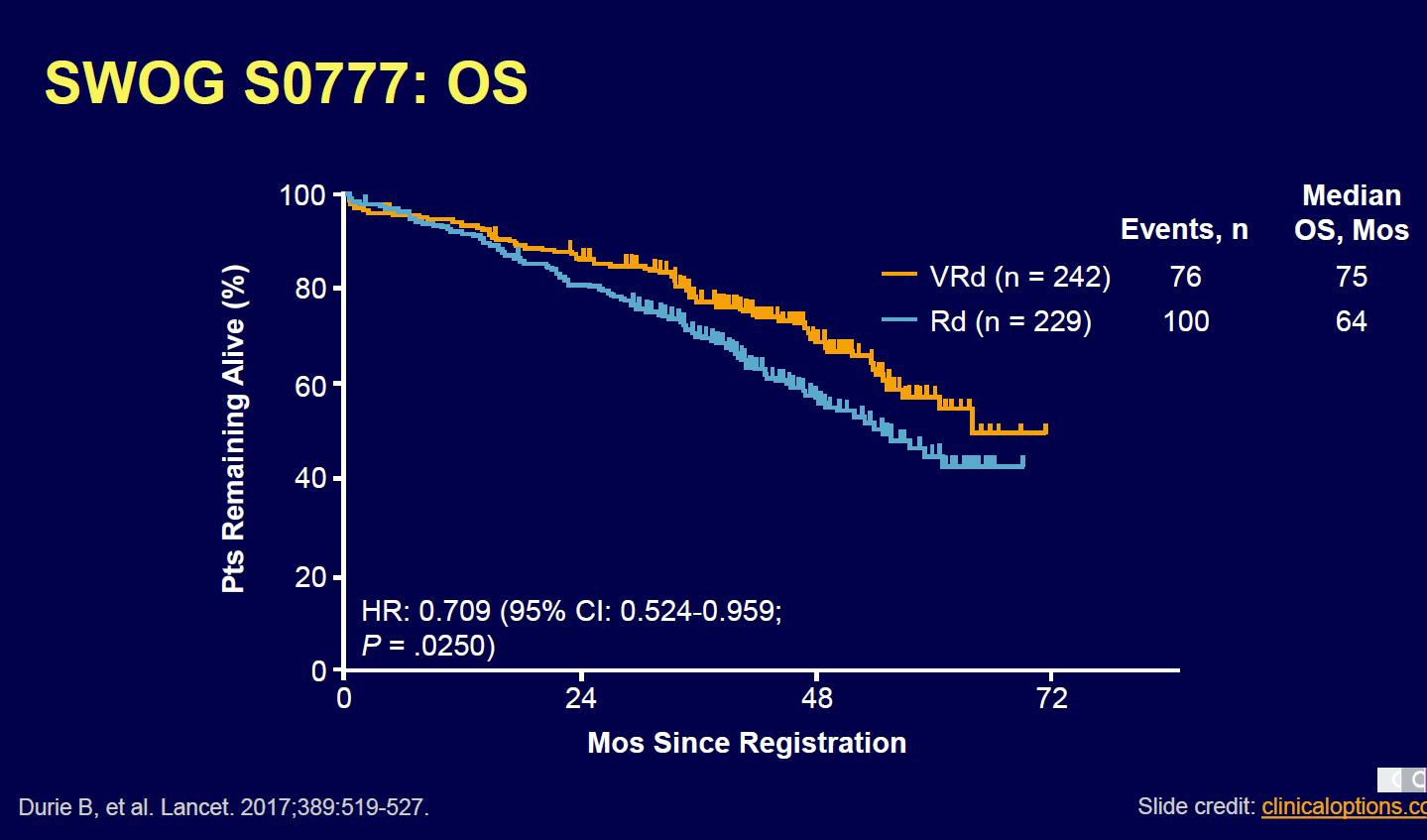
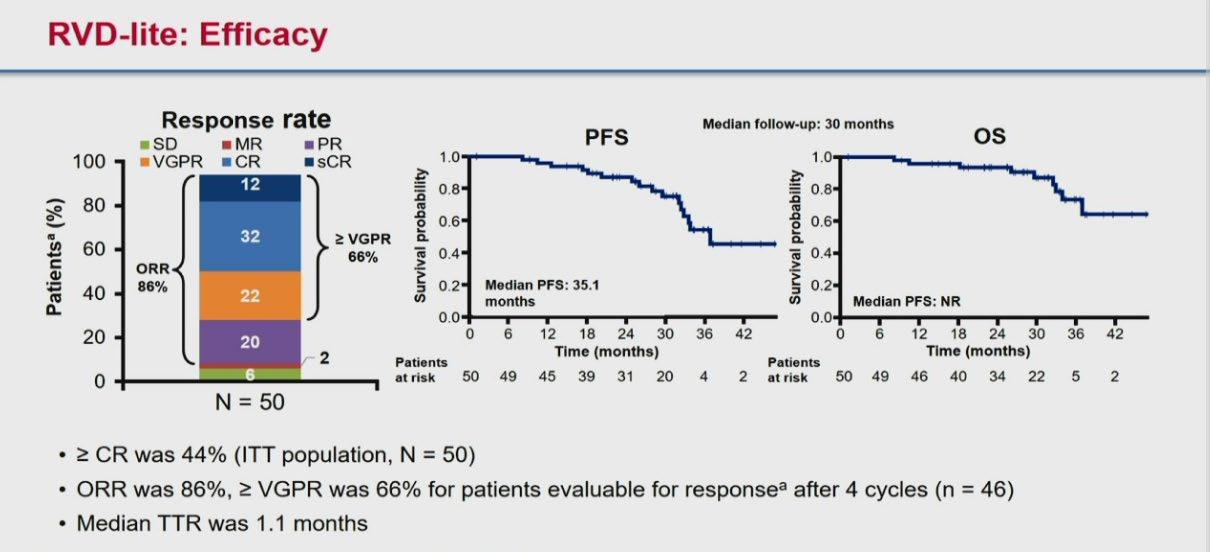
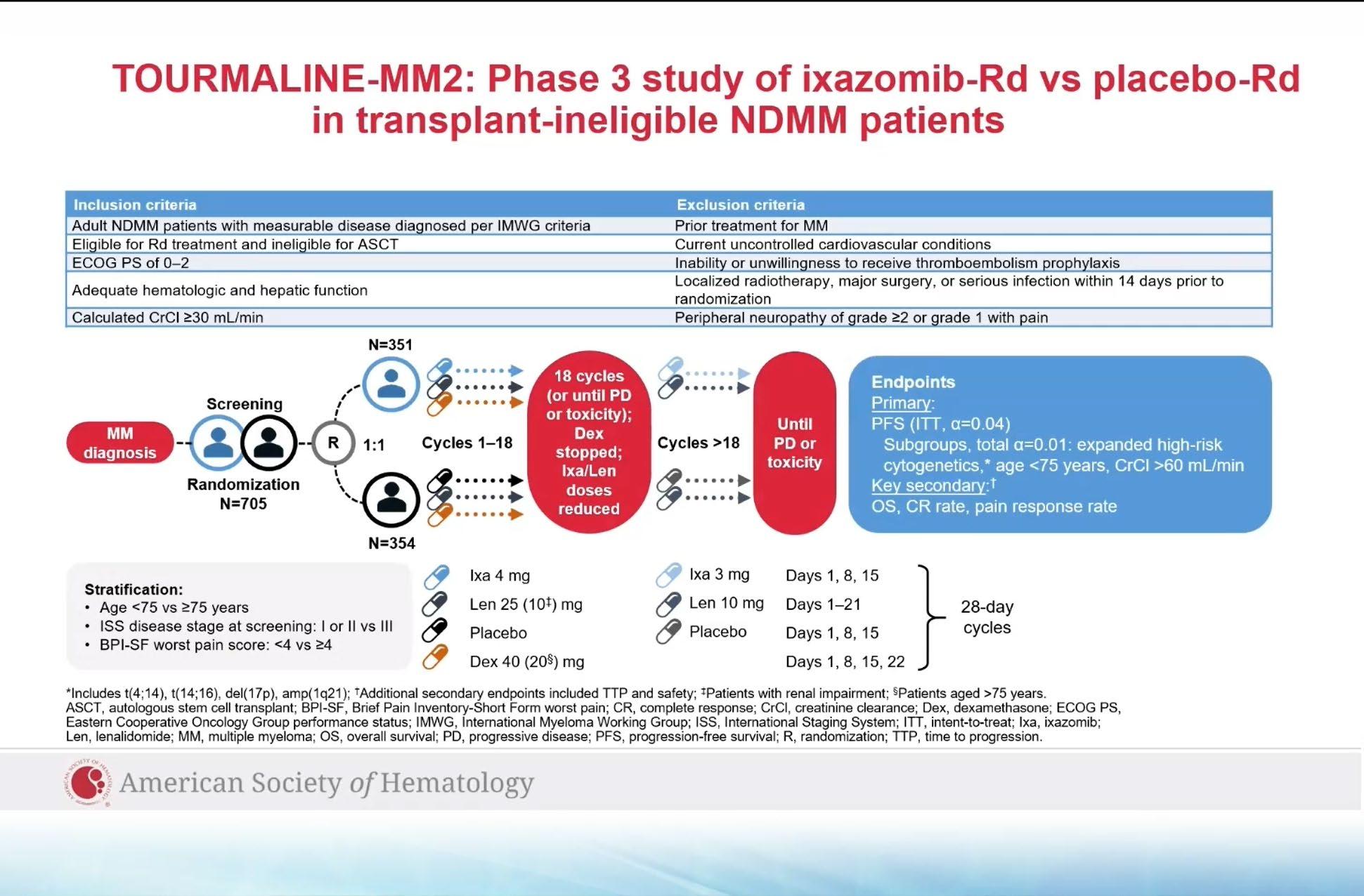
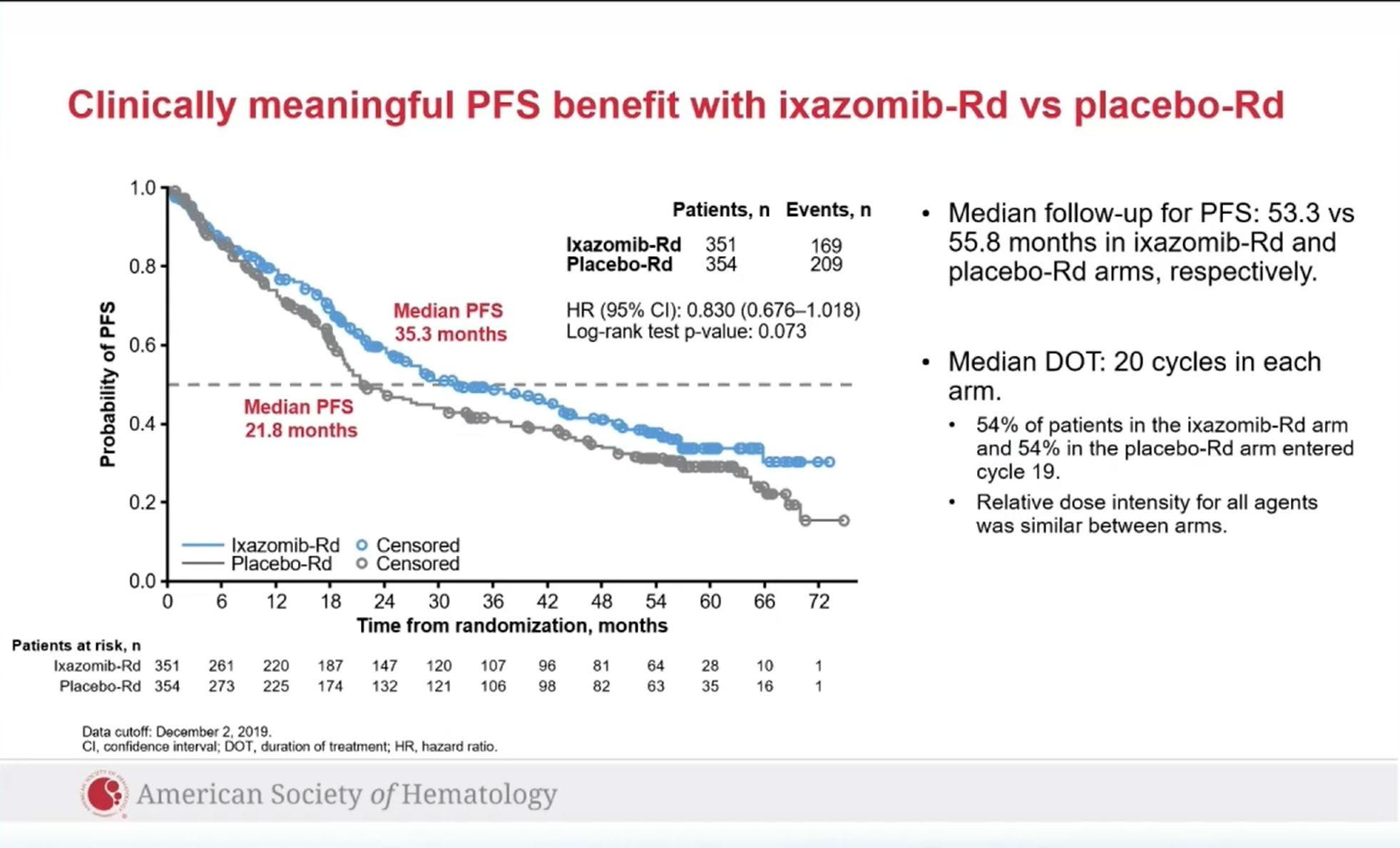
Evolution of Treatment for Transplant-Ineligible MM based on Major RCTs
How to improve upon VMP and VRD ?
1. Upgrade the PI >> Failed : KMP and KRD not superior
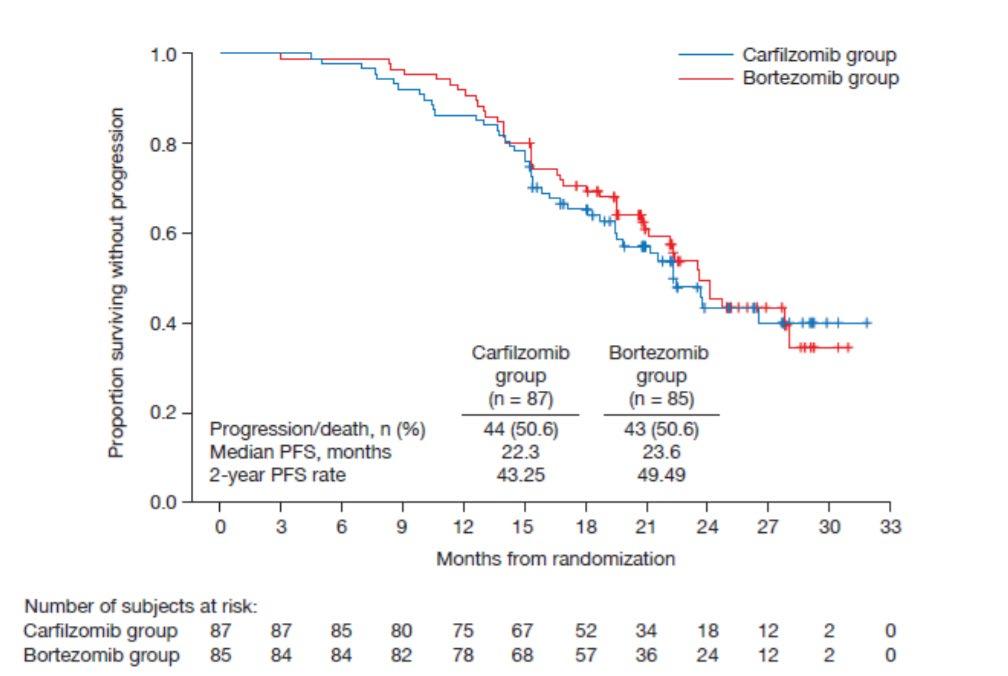
CLARION study
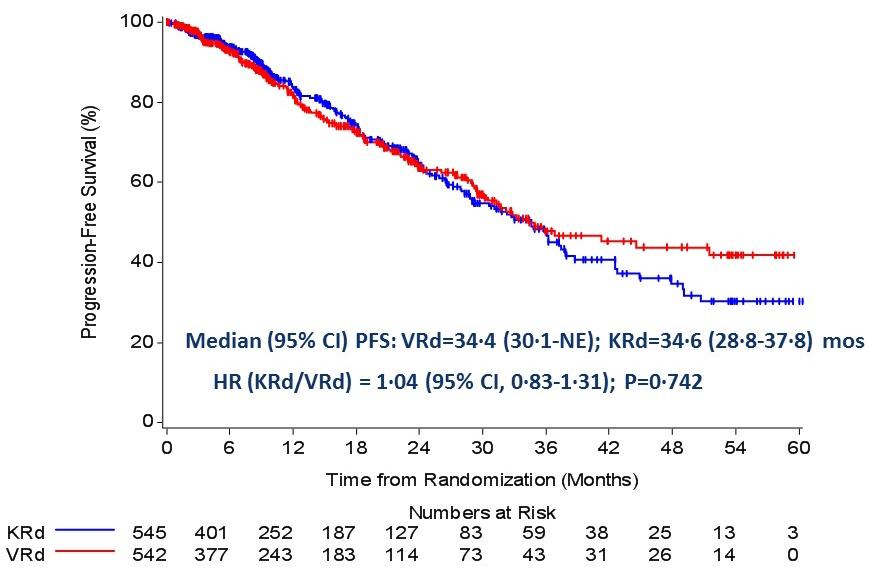
ENURANCE study
Evolution of Treatment for Transplant-Ineligible MM based on Major RCTs
1980-90s
SWOG-1211: Randomized Ph II VRD +/- Elotuzumab for High-risk MM

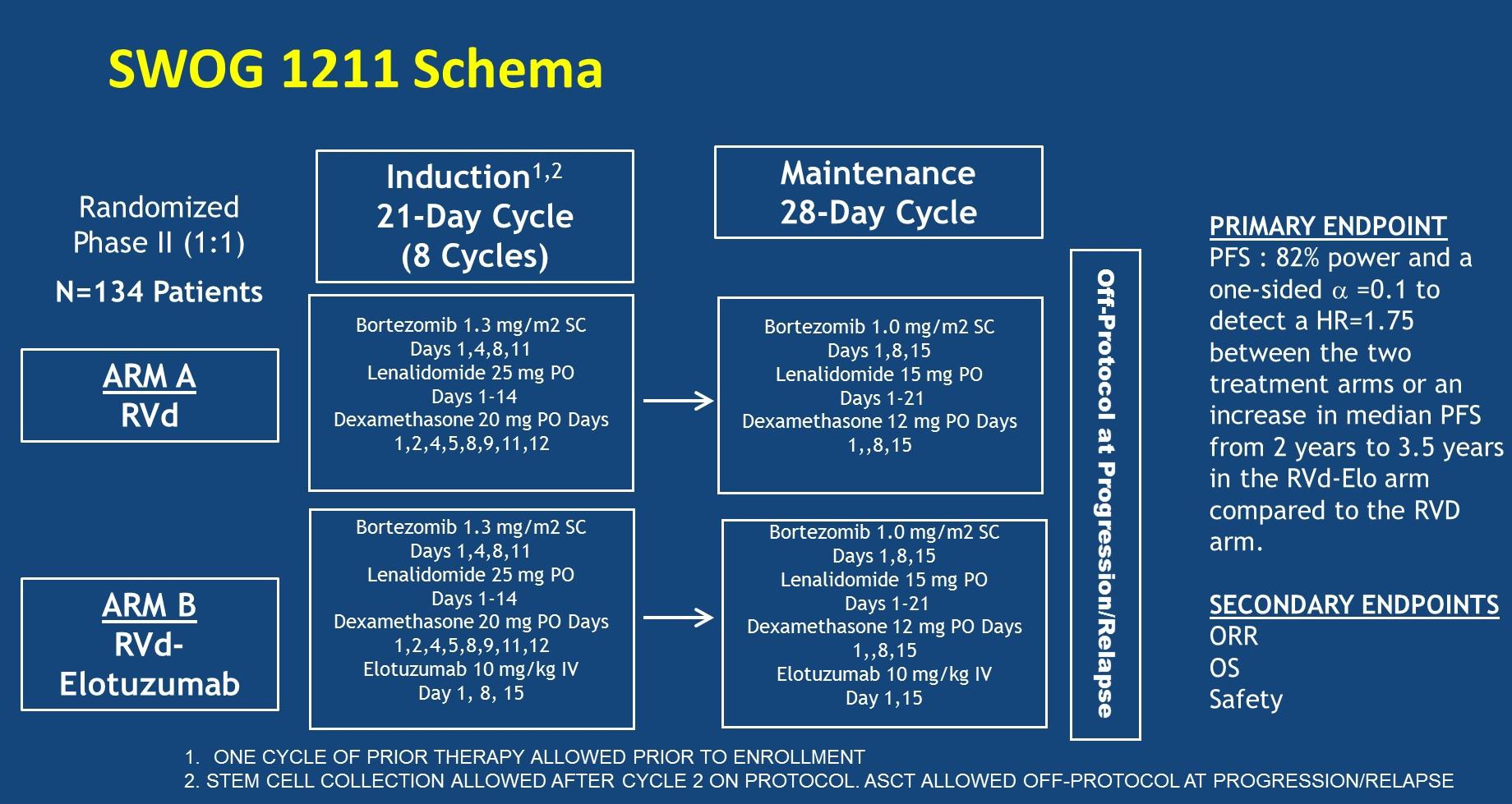
MM
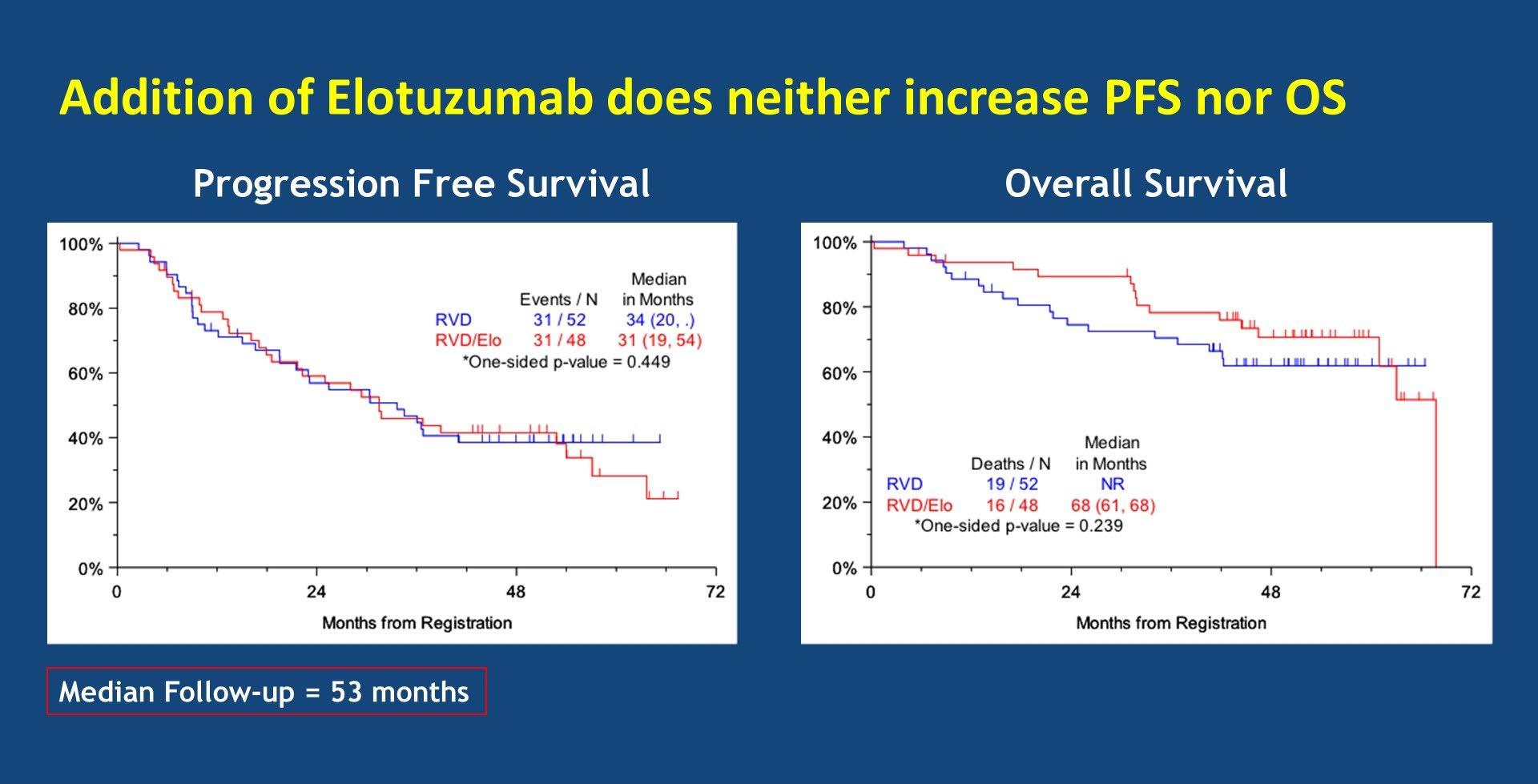
Evolution of Treatment for Transplant-Ineligible MM based on Major RCTs
1980-90s
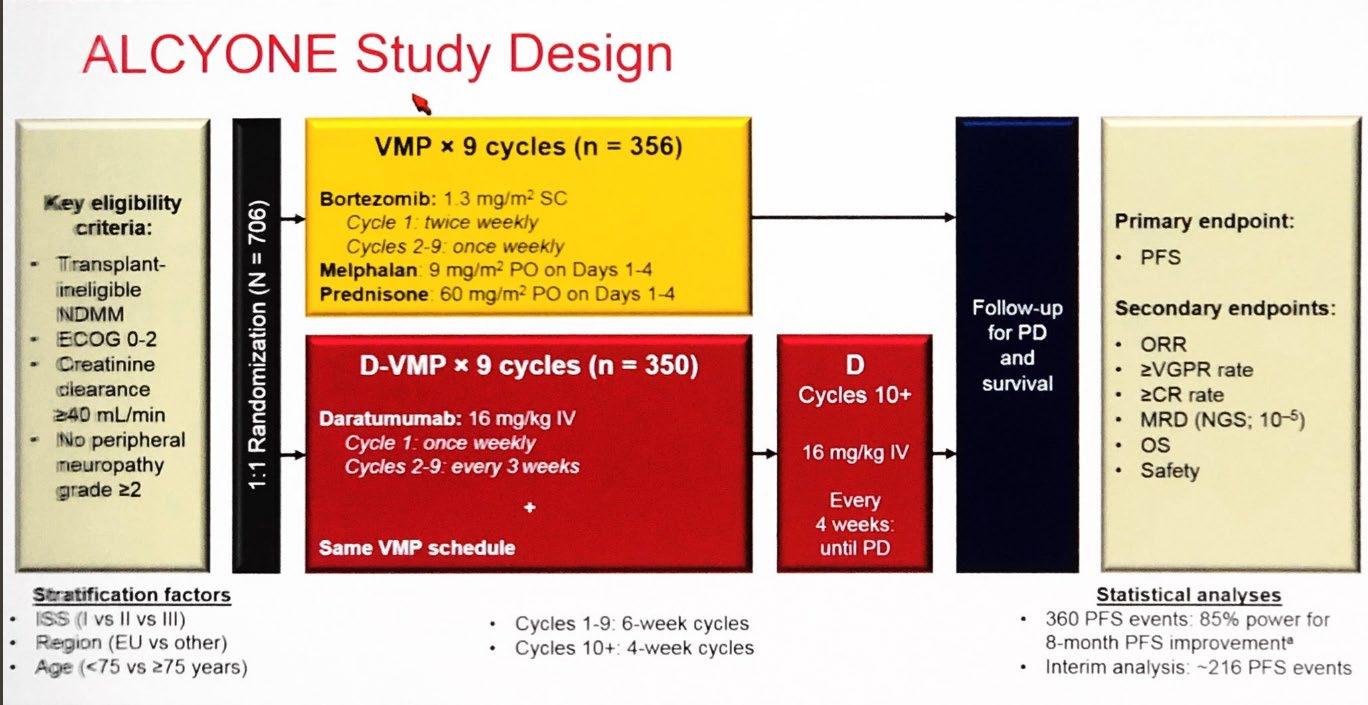
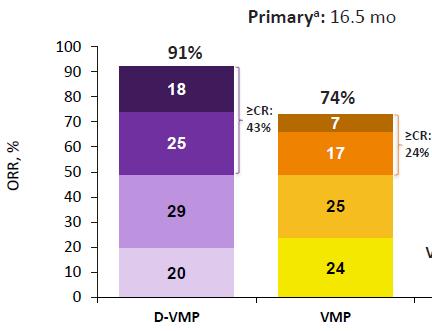
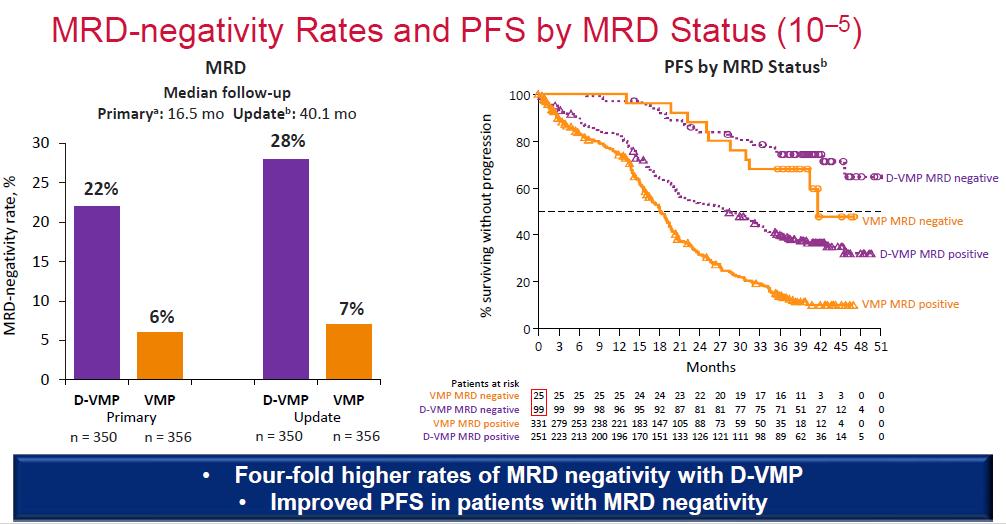
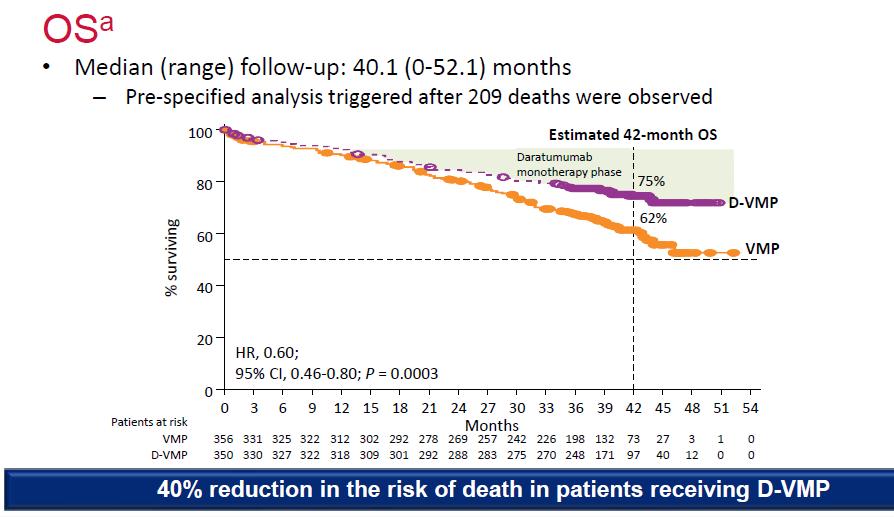
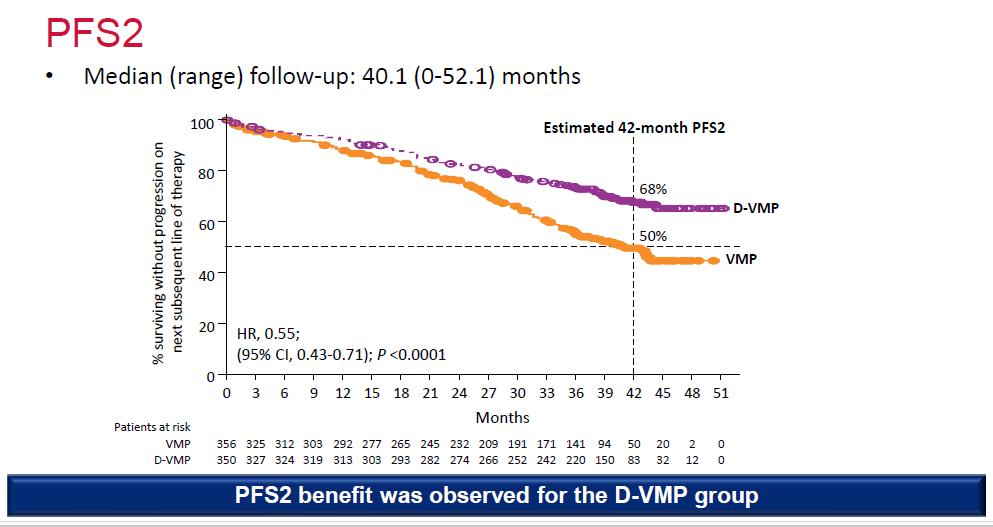
Median OS: 83 months
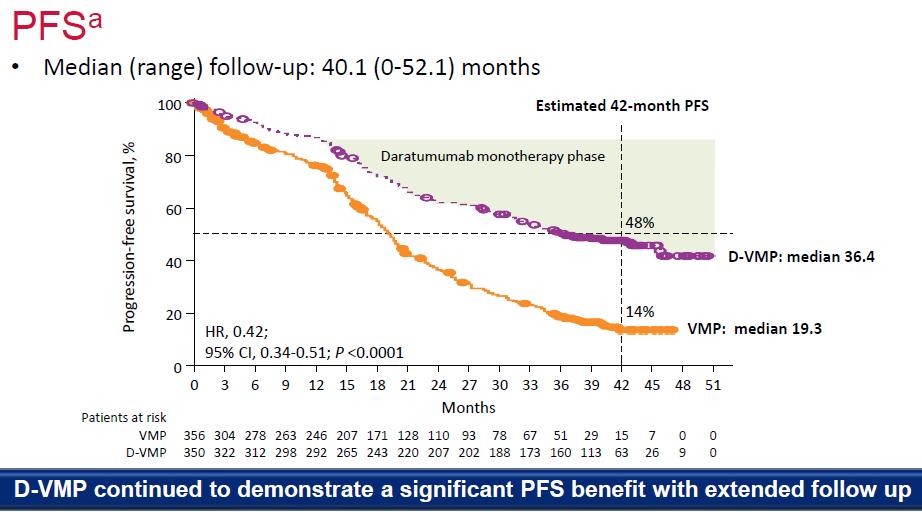
Evolution of Treatment for Transplant-Ineligible MM based on Major RCTs
1980-90s

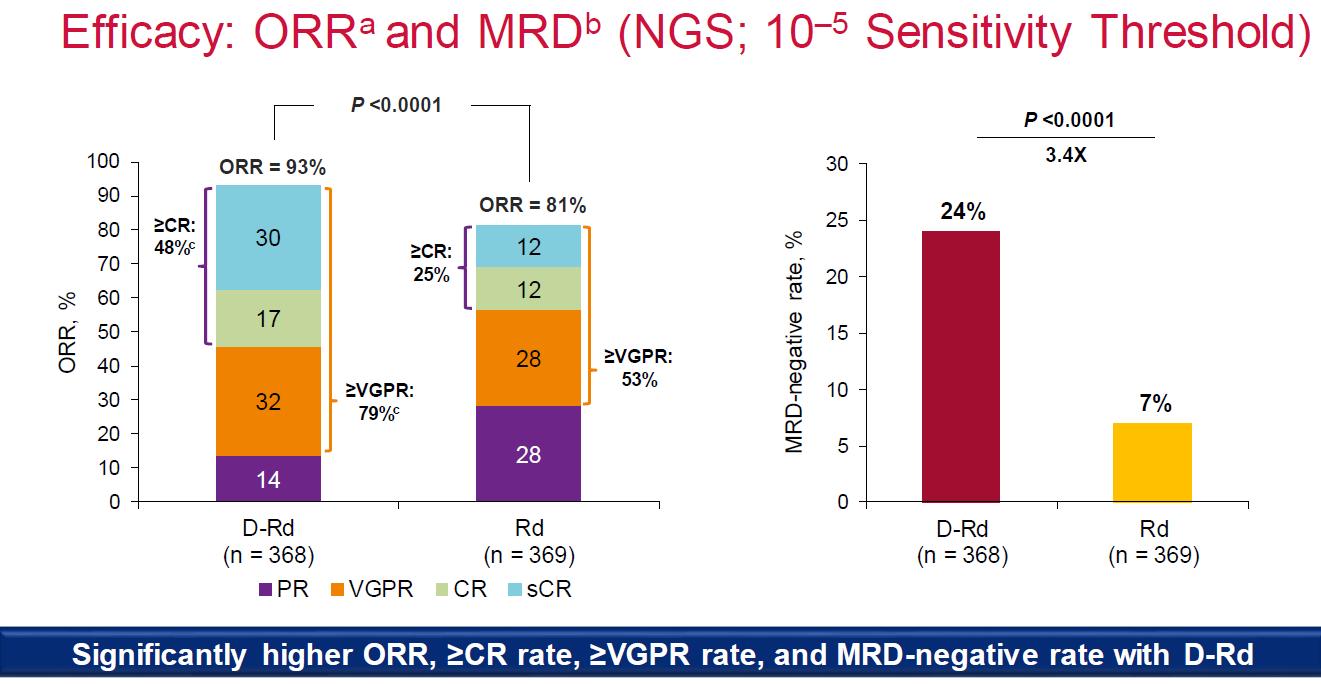
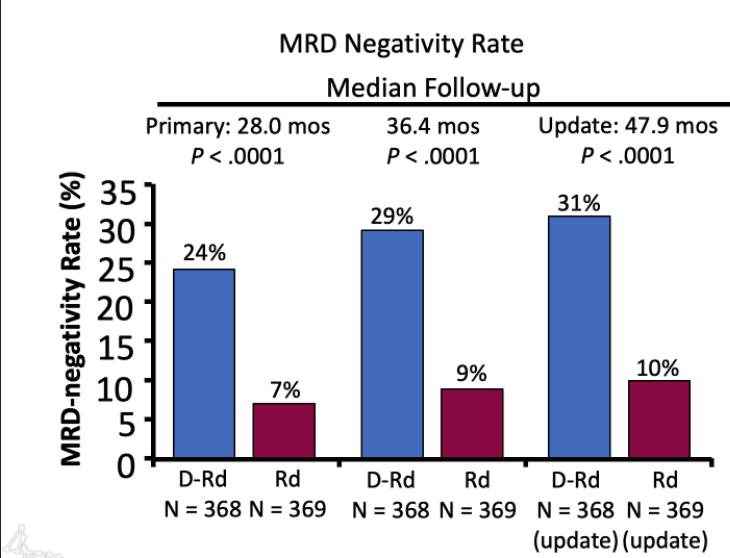
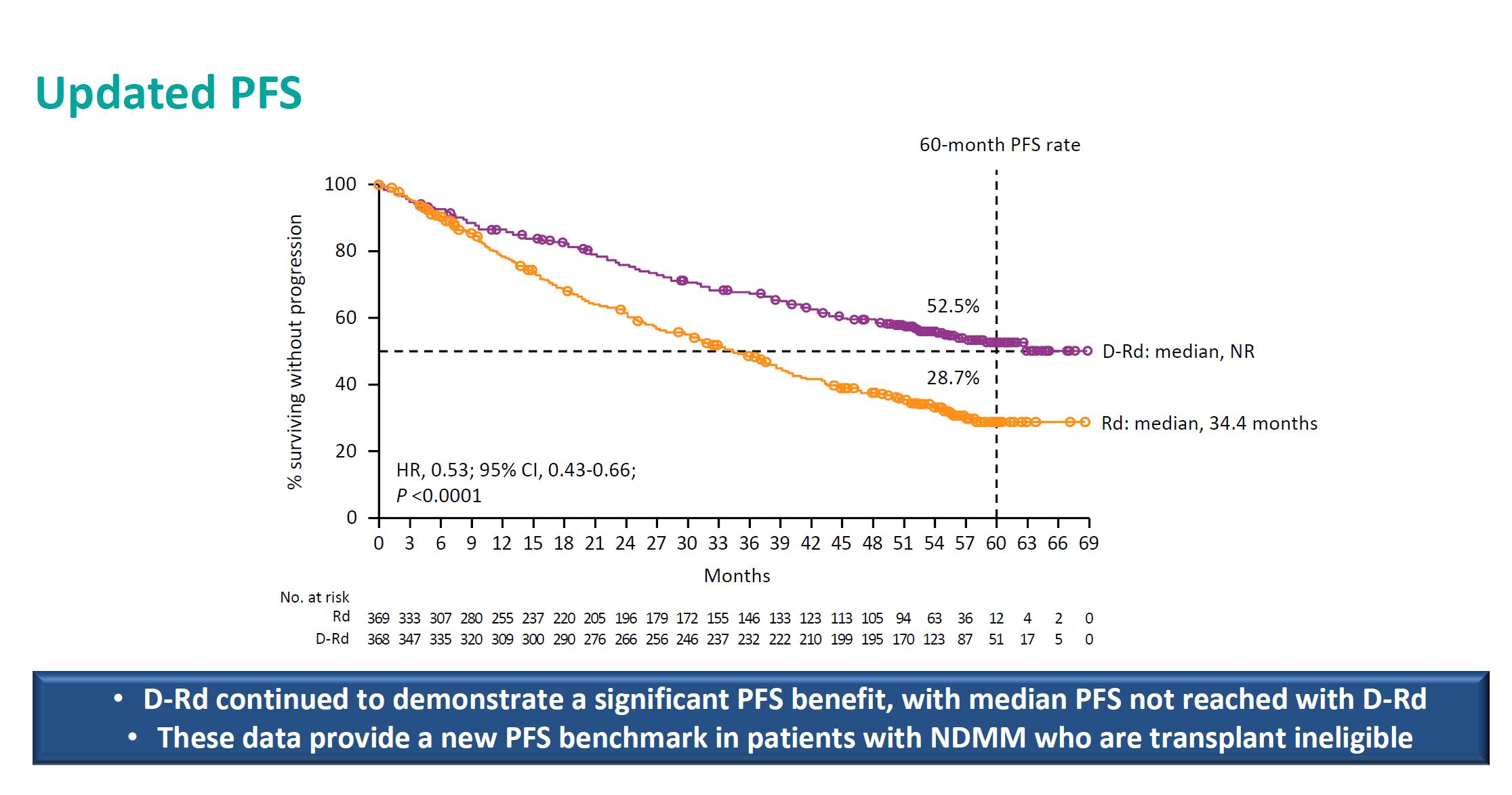
Facon T, et al.
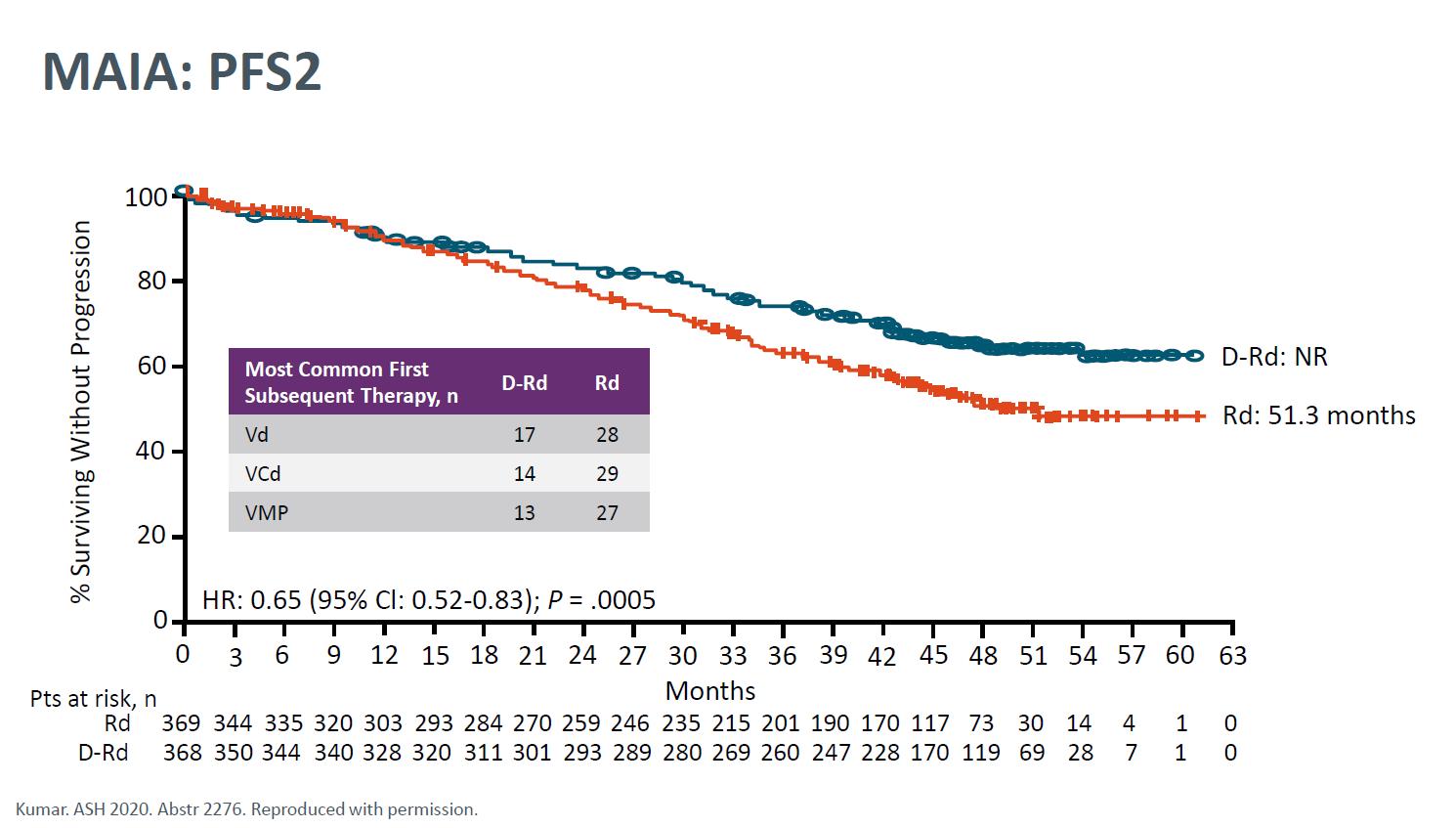
MAIA- Final Analysis
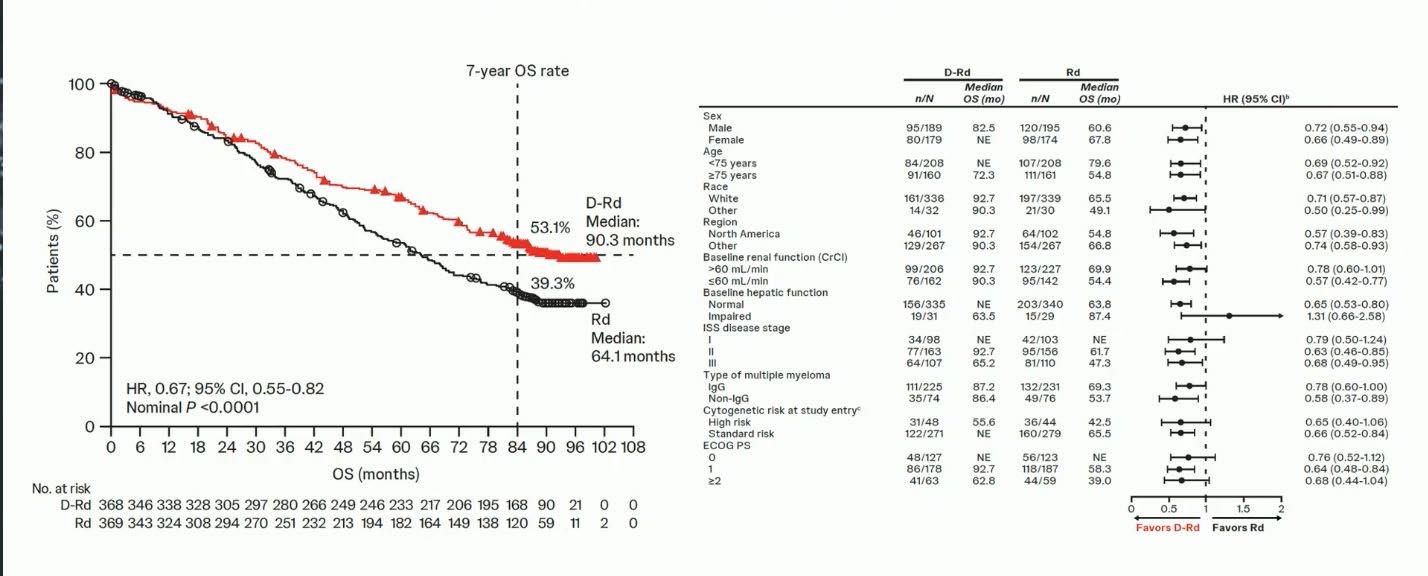
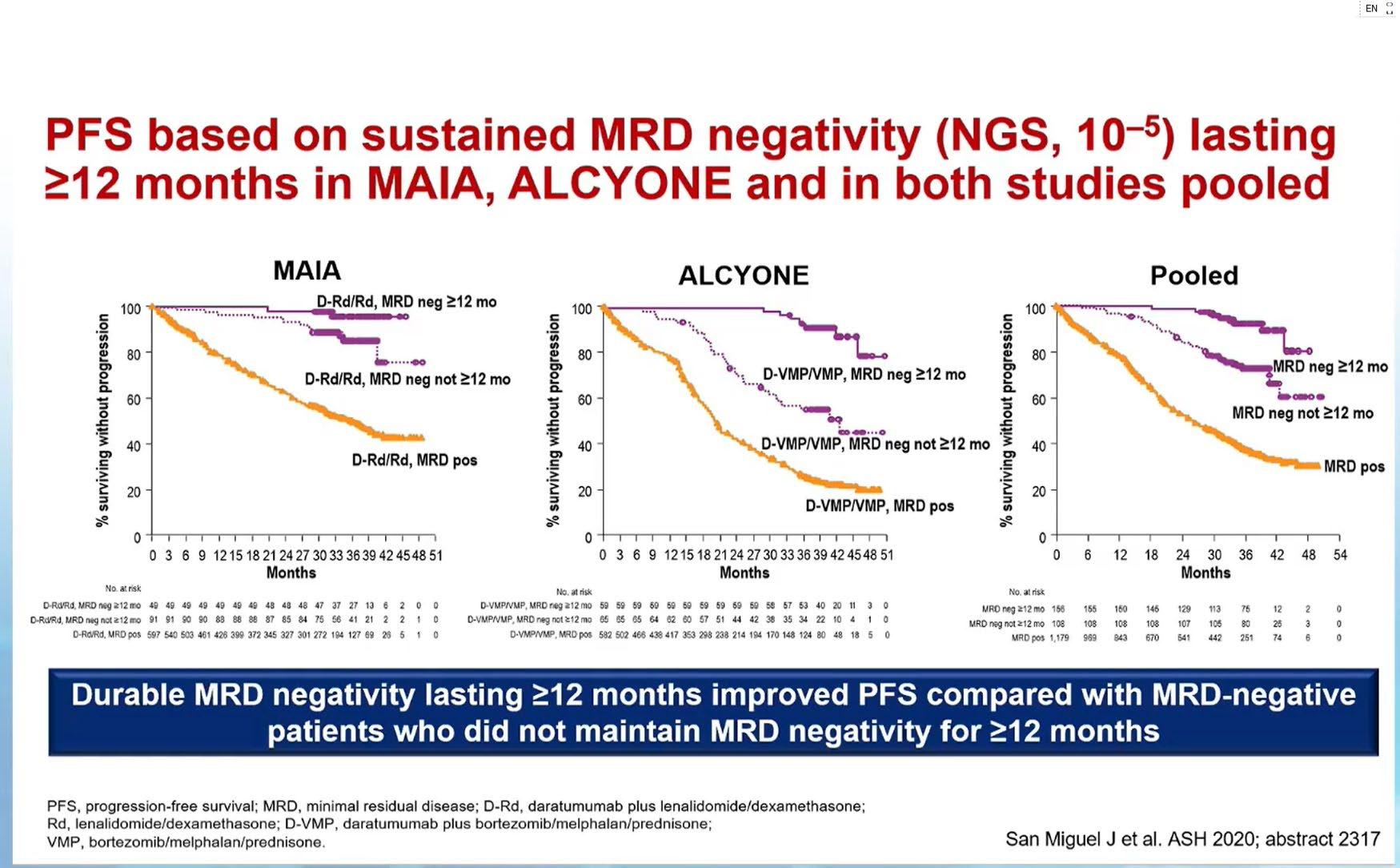
PFS based on Sustained MRD Negativity ≥ 12 months in MAIA and ALCYONE Studies
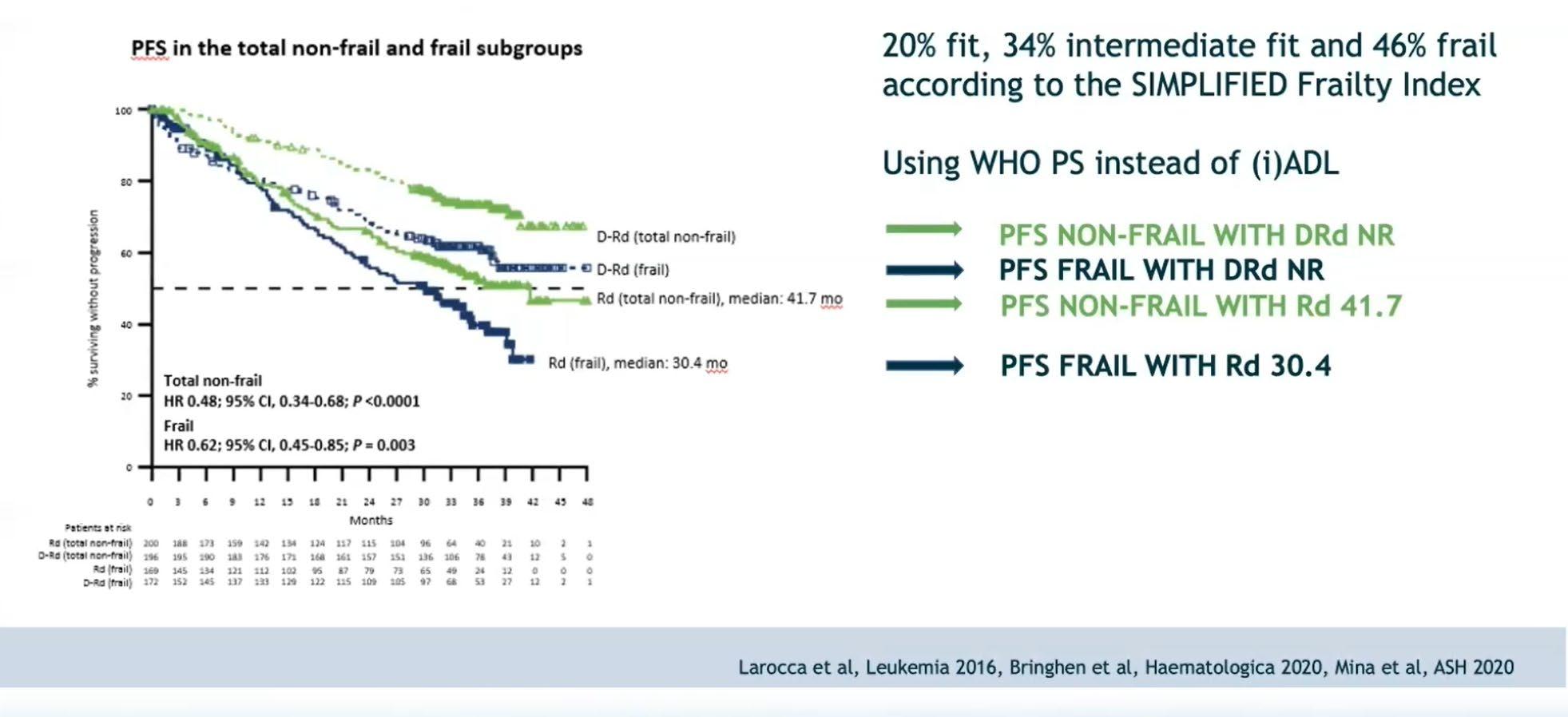
Sub-Analysis of the MAIA Trial Based on Frailty
Evolution of Treatment for Tpt-Ineligible MM
1980-90s
Evolution of Treatment for Tpt-Ineligible MM
Evolution
of Treatment for Tpt-Ineligible MM
1980-90s
Evolution
of Treatment for Tpt-Ineligible MM
1980-90s
Evolution of Treatment for Tpt-Ineligible MM
Evolution
of Treatment for Tpt-Ineligible MM

PFS of DRd Versus VRd in Transplant Ineligible Patients With Newly Diagnosed MM: TAURUS Chart Review Study
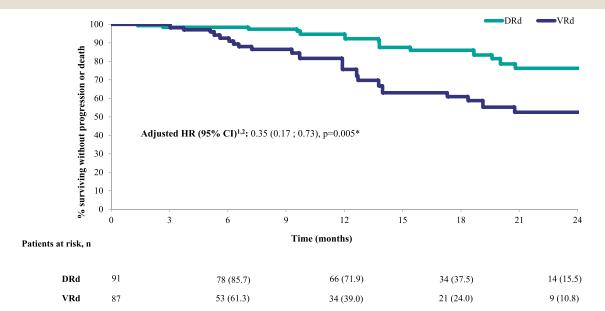
Gordon LN et al. Clin Lymphoma, Myeloma & Leukemia 2024.
Evolution of Treatment for Tpt-Ineligible MM
Evolution
of Treatment for Tpt-Ineligible MM
Evolution
of Treatment for Tpt-Ineligible MM
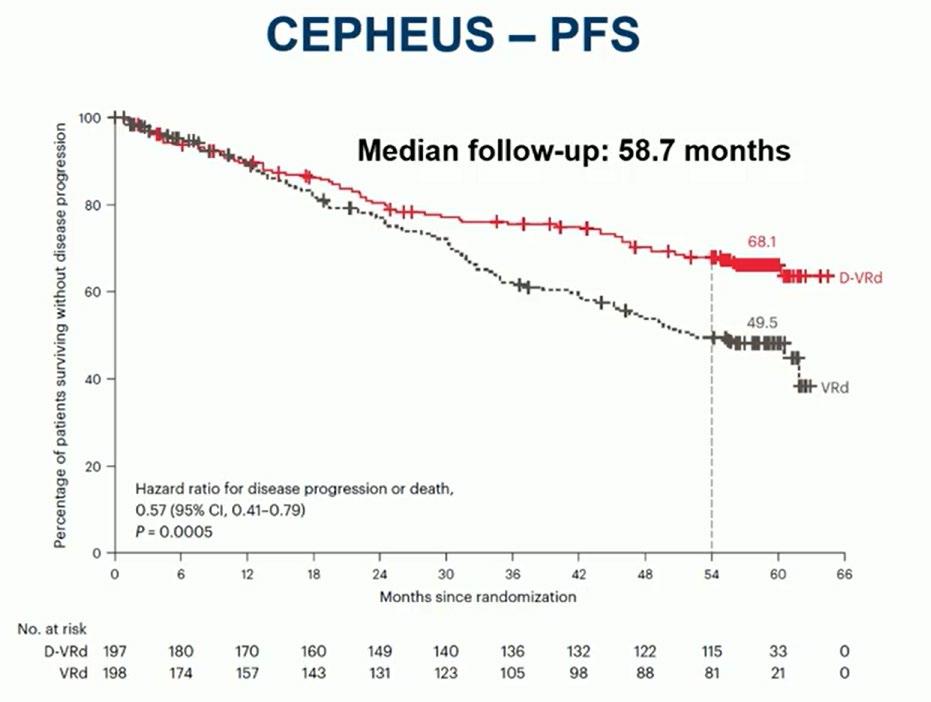
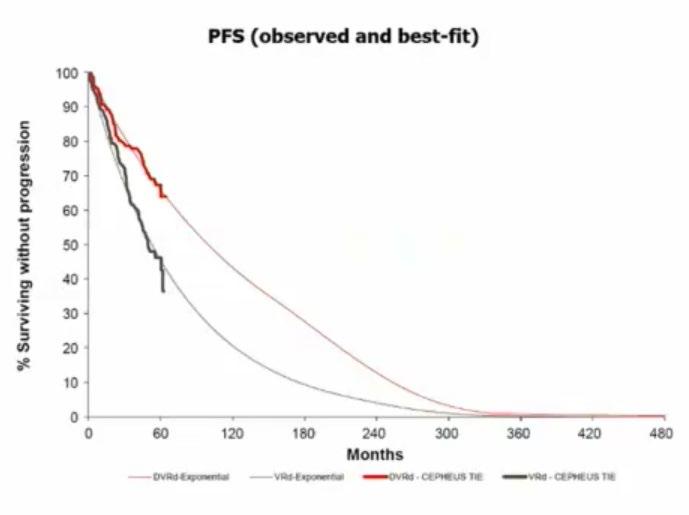
Evolution
of Treatment for Tpt-Ineligible MM
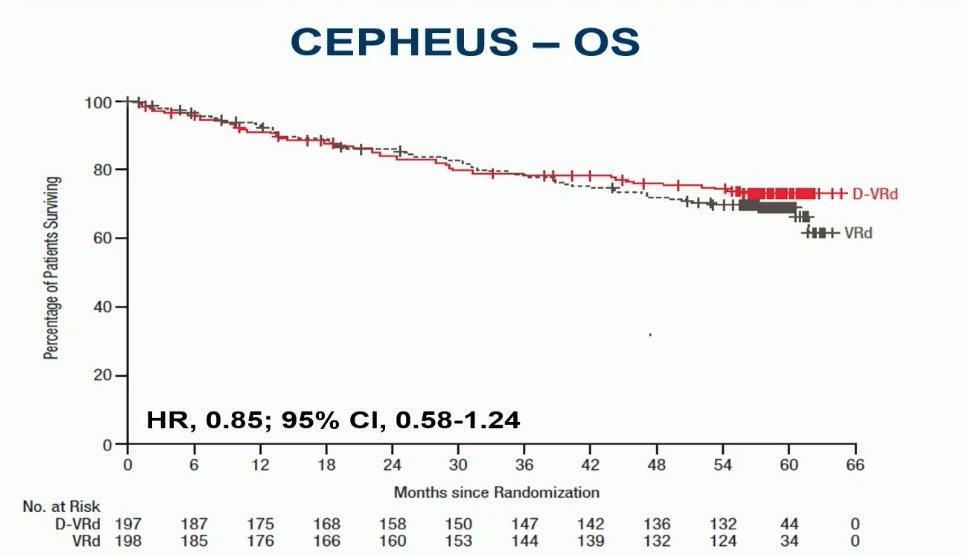
Evolution of Treatment for Tpt-Ineligible MM
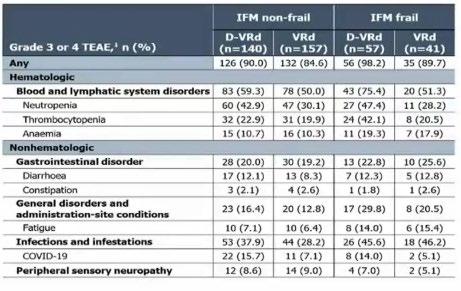
Evolution of Treatment for Tpt-Ineligible MM
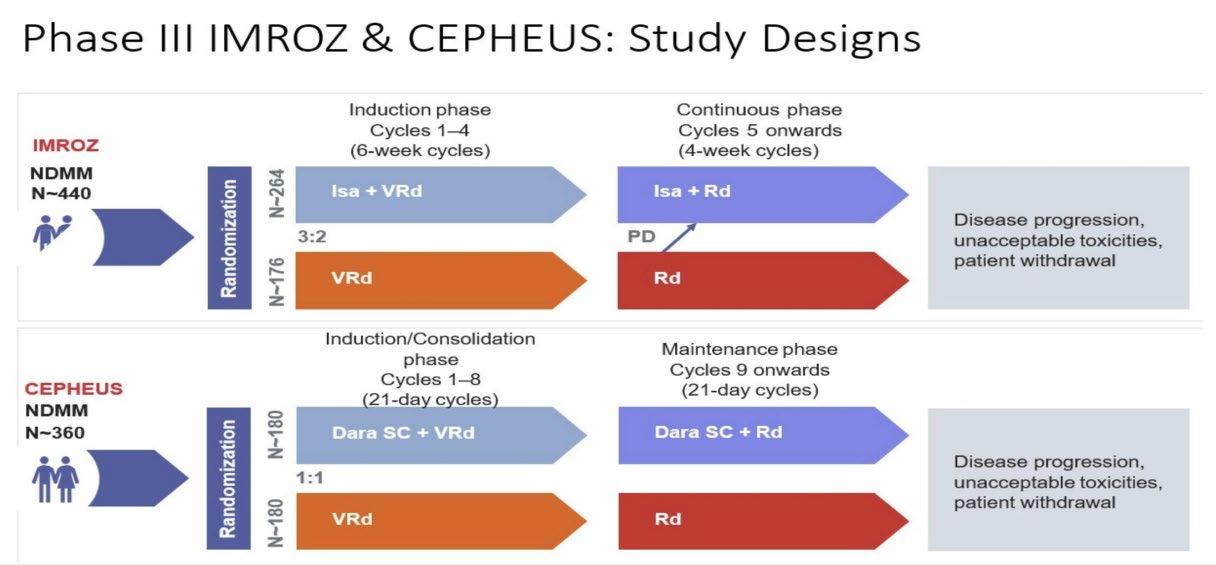
IMROZ: Isa-VRd vs VRd
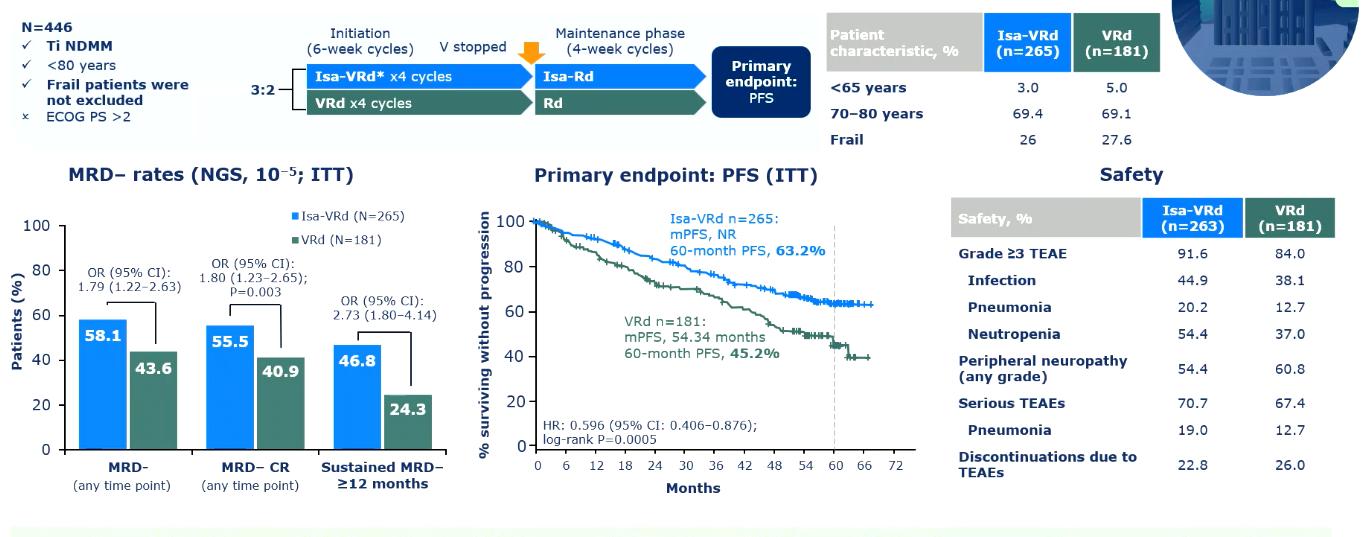
Evolution of Treatment for Tpt-Ineligible MM
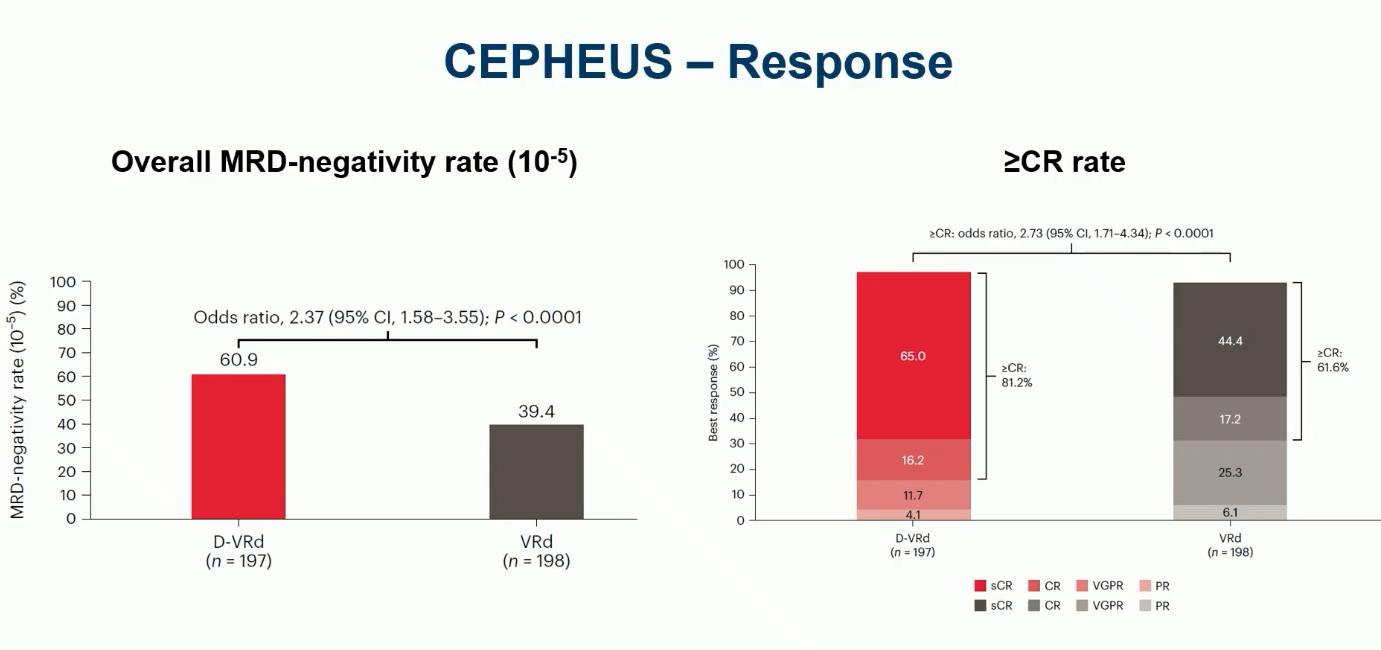
Leleu XP, et al. ASCO 2024. Abstract 7501
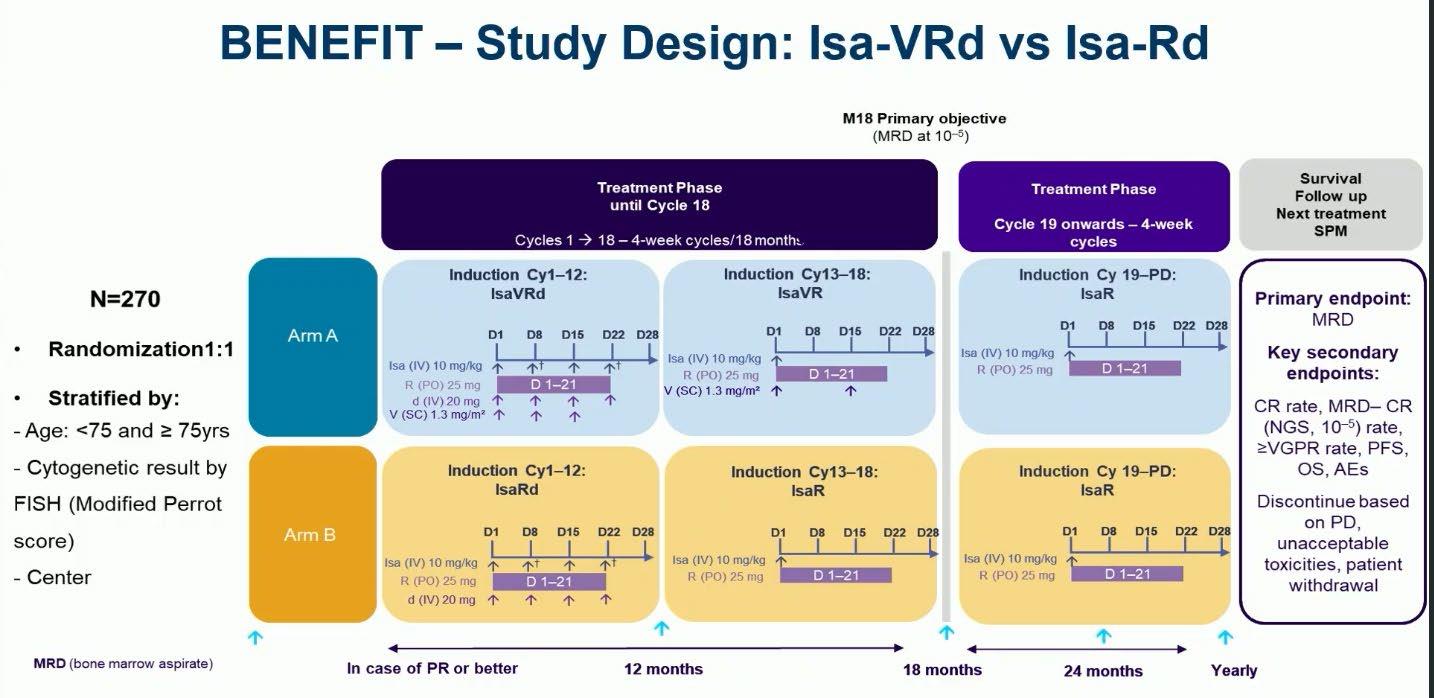
Evolution of Treatment for Tpt-Ineligible MM
Leleu XP, et al. ASCO 2024. Abstract 7501
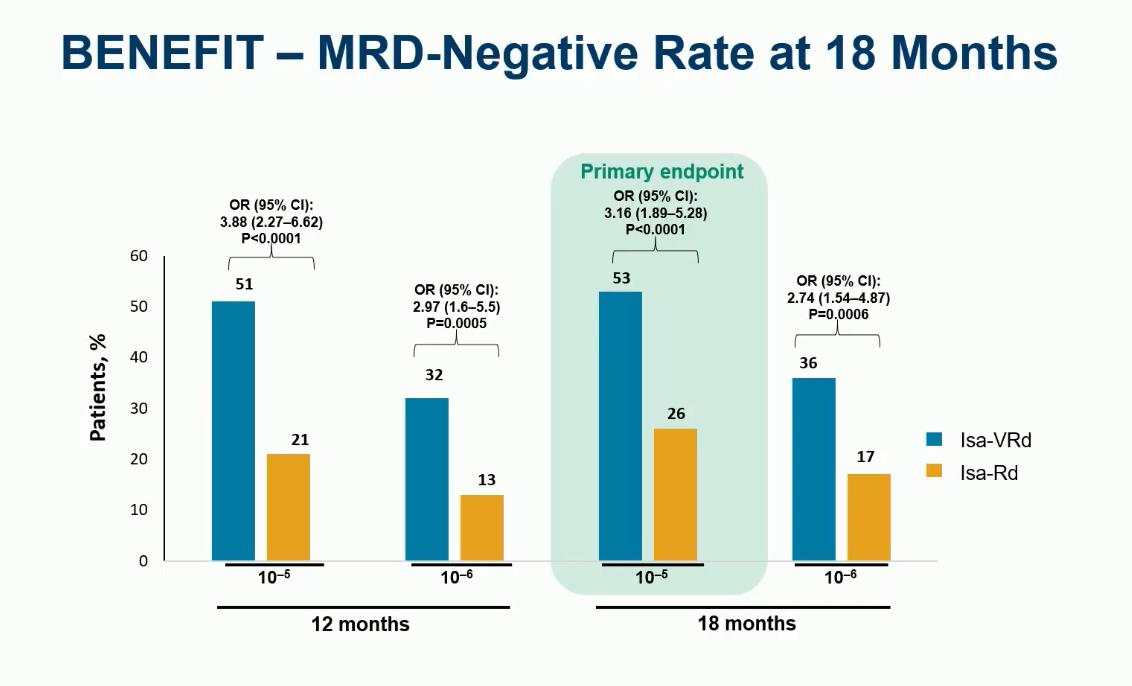
Evolution of Treatment for Tpt-Ineligible MM
Leleu XP, et al. ASCO 2024. Abstract 7501
Evolution
of Treatment for Tpt-Ineligible MM
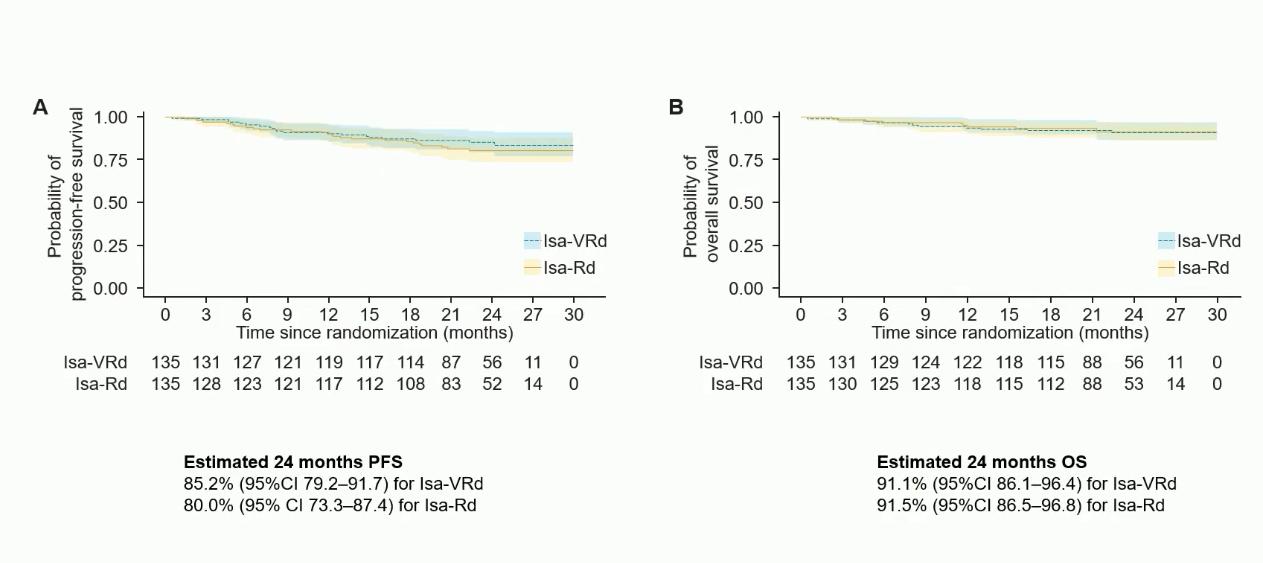
Evolution of Treatment for Tpt-Ineligible MM
Evolution of Treatment for Tpt-Ineligible MM
Evolution of Treatment for Tpt-Ineligible MM


Conclusions: Treatment of ND TIE MM
• Assessment of frailty should play a role in treatment decisions
• Continuous therapy and alkylator-free approach is new standard of care of elderly MM pts
• Targeting MRD in the elderly may be appropriate with addition of monoclonal antibodies
• OS and PFS benefits support frontline use of antiCD38 combinations

Thank You
Discussion




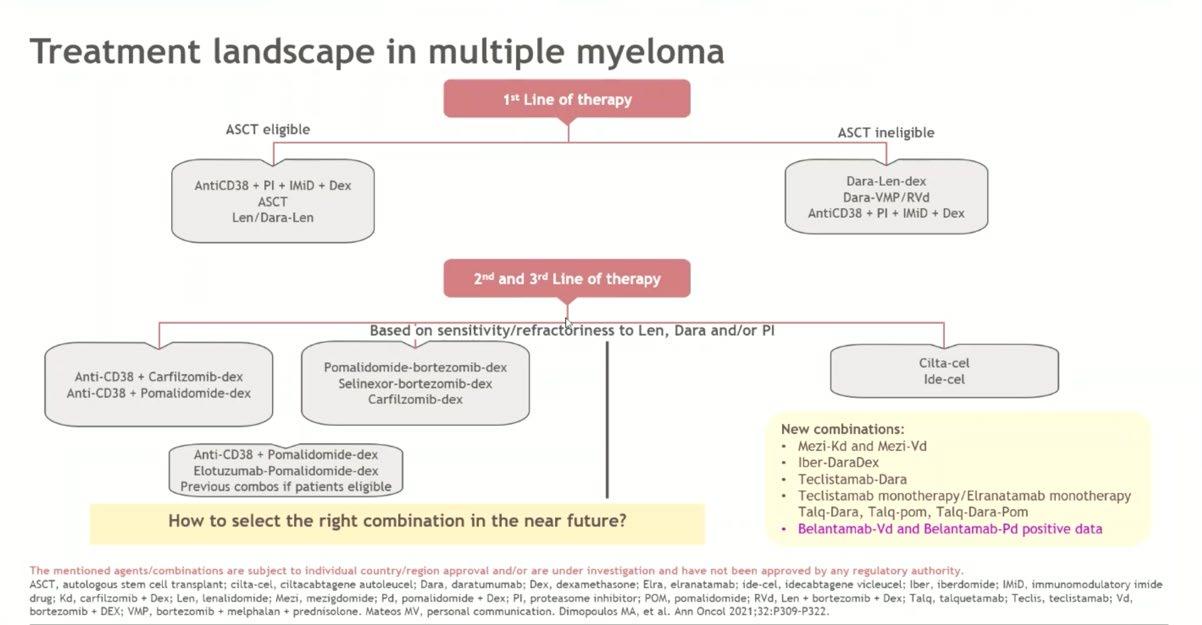
Treatment Options Asia – Relapsed & Refractory

Melissa Ooi, MD
National University Cancer Institute of Singapore

Treatment Options Asia - RRMM
AMN Masterclass 2025
Assistant Professor Melissa Ooi
Senior Consultant Department of Haematology
National University Cancer Institute, Singapore Department of Medicine
Disclosures 2024/5
Company Honoraria Advisory board
GSK, J&J X X
Novartis, Astellas, Amgen X
BMS, Antegene, X [Input
Definition of Relapsed MM: IMWG

Two relapse scenarios
Slow, asymptomatic relapse
• No symptoms
• Slowly progressing disease
• Low tumor burden
• Cytogenetic low risk
• Good performance status
Fast, symptomatic relapse
• Symptoms
• Radiply progressing disease
• High tumor burden
• Organ involvement
• Cytogenetic high risk
• Poor performance status
Prompt treatment initiation needed Observation
The challenge: Identify appropriate time to initiate treatment for situations ‘in between‘
How does the timing of relapse influence treatment decisions?
Early relapse: within < 1 year
• May indicate resistant disease
• Change from initial treatment
• Incorporate novel agent, if not previously used
Late relapse: after > 2 years
• Consider rechallenge with initial therapy
• If previous therapy was effective
• If previous therapy was given for a short, defined duration
• Consider addition of another agent
Factors affecting outcome at relapse
Patient
• Age
• Fit/Frail
• Co-morbidities
• Residual toxicities
• Social Support
Disease
• Risk Stratification
• Response to treatment
• Duration of response
• High risk cytogenetics
Treatment
• Type of treatment
• ASCT status
• Availability
• Financial consideration
Comorbidity(s) adjustment
Different treatment classes
TTNT was shorter with each progressive line of treatment
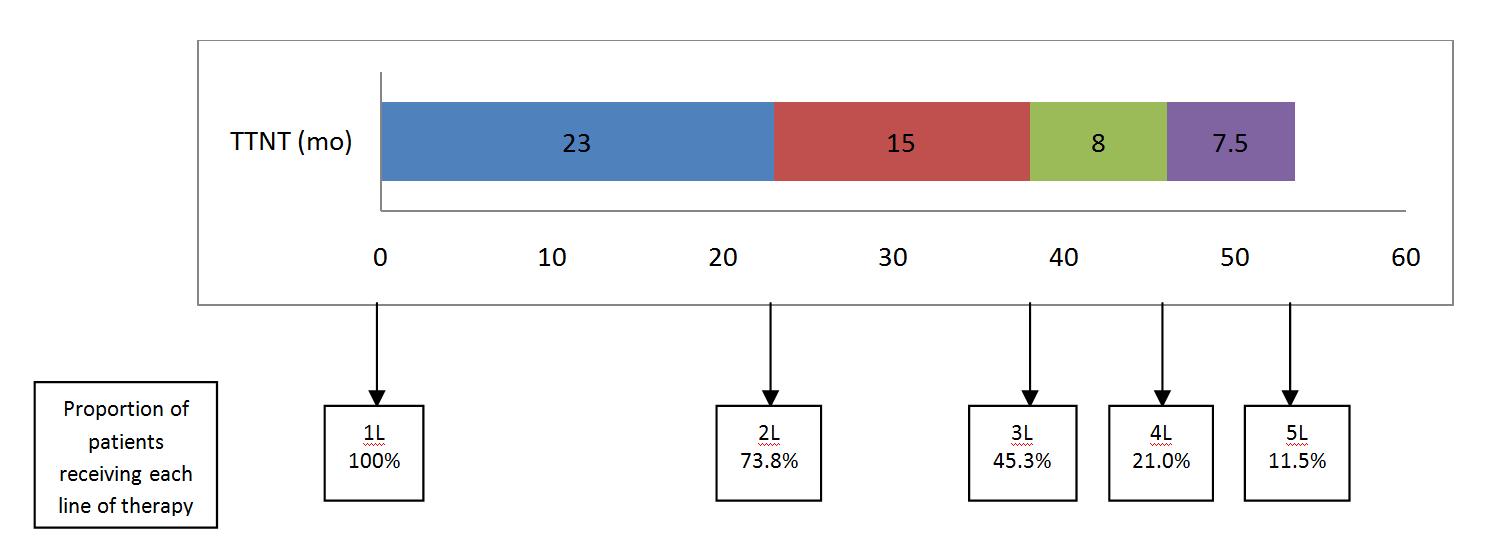
Efficacy was reduced with each progressive line of treatment
Best Response
VGPR or better PR
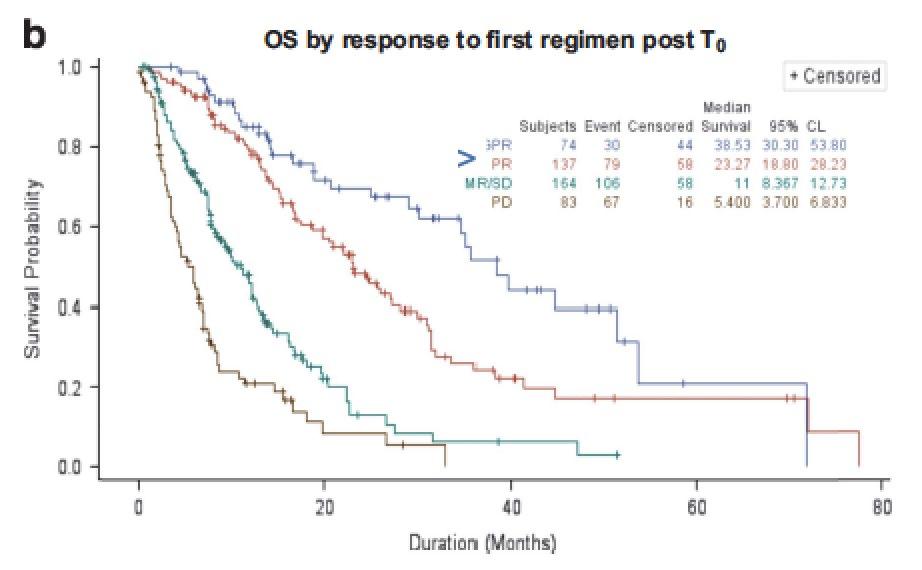
Depth of response of salvage affects outcome
EHA/EMN 2025 Guidelines for Second Line
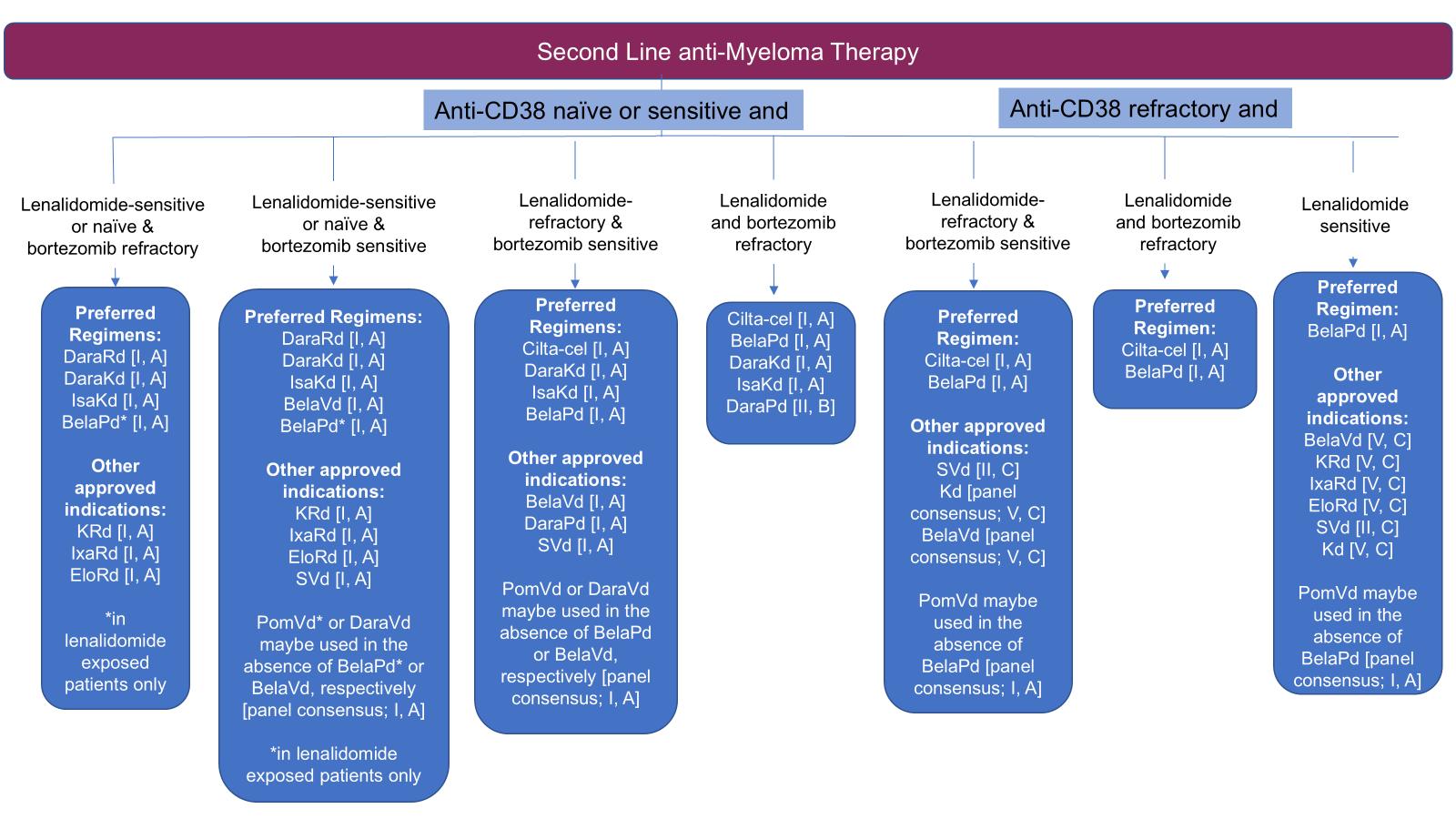

Dimopoulos MA, Terpos E,
HR
Dara naive, Len refractory trials in early RRMM
trial comparisons need to be interpreted with caution. References next slide
Dara Naïve, len refractory trials in early RRMM
References
1. Usmani 2022, Lancet Oncology
2. Dimopoulos 2021, Lancet Oncology
3. Mateos 2016, NEJM
4. Moreau 2021, Lancet
5. Attal 2019, Lancet
6. Bringhen 2021, Leuk Res
7. Martin 2023, Blood cancer J
Dara exposed, Len refractory trials in early RRMM
Dara exposed, Len refractory trials in early RRMM
References
8. Richardson 2019 Lancet Oncology
9. Grosicki 2020, Lancet
10. Song ASH 2023
11. Hungria 2024, NEJM
11a) Mateos, ASCO 2024
12. Dimoupoulos 2024, NEJM
12a) Bekset ASH 2024
AMN 003
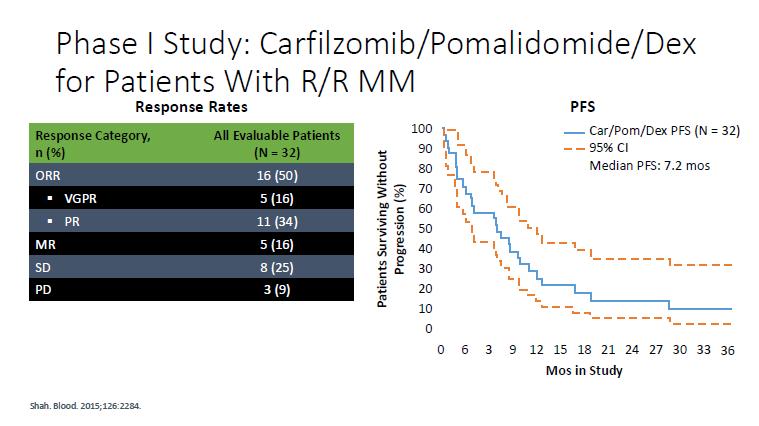
KPd – Phase 2
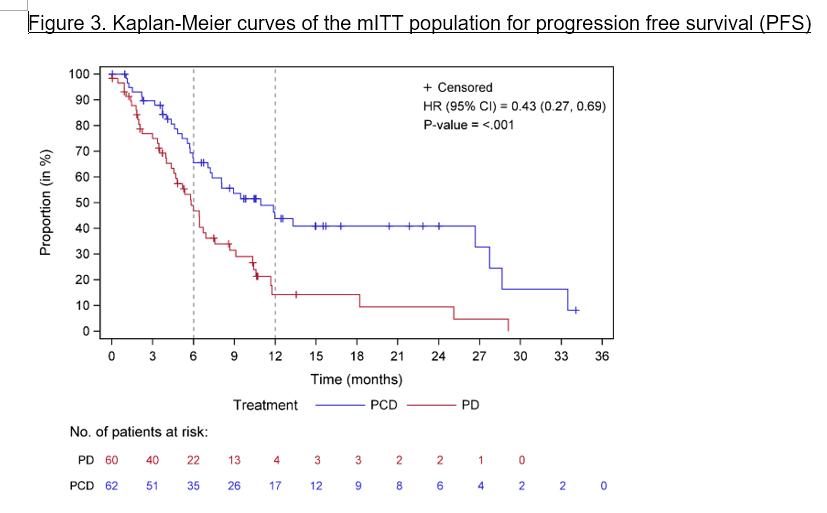
Regiment
Carfilzomib (20/36 mg/m2, days 1, 2, 8, 9, 15, 16) with pomalidomide (4 mg days 1–21) and dexamethasone (20 mg days 1, 2, 8, 9, 15, 16) (KPd) x 4-> ASCT (if never had) > KPd x 4.
Patients with stable disease or better were randomized to receive pomalidomide 4 mg alone (P) or with weekly dexamethasone (Pd) 40 mg in 21 of 28 days cycles continuously until progression or toxicity.
Results
Median OS 67 months
From randomisation, Median PFS 27 vs 18 mo in the pom-dex maintenance arm
Not lenalidomide refractory
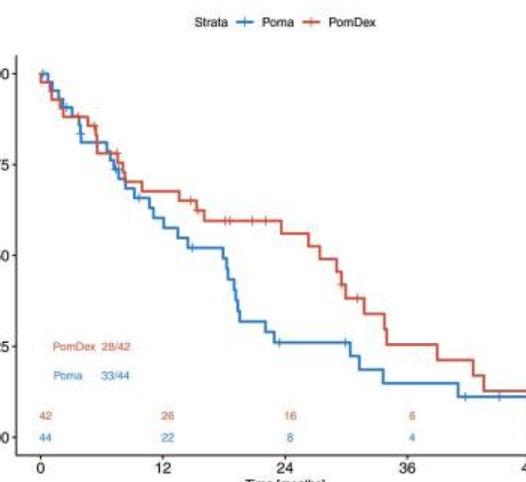
IMId based trials in early RRMM
Cross trial comparisons need to be interpreted with caution
Not Len refractory trials in early RRMM
References
8. Richardson 2019 Lancet Oncology
10. Stewart 2015, NEJM
11. Dimopoulos 2016, NEJM
12. Moreau 2016, NEJM
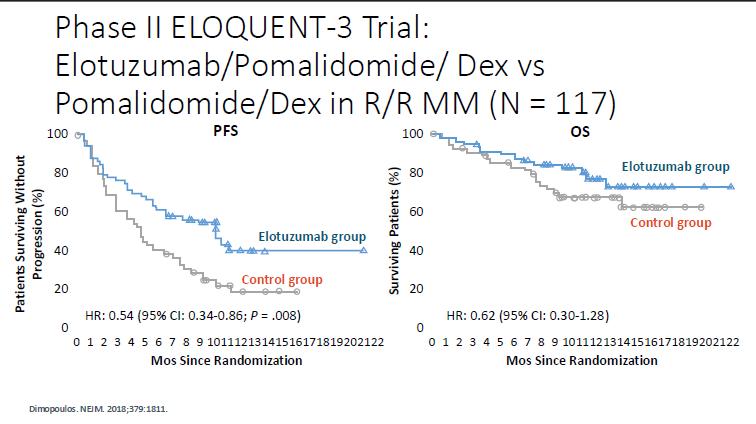
10.3 vs 4.7 mo
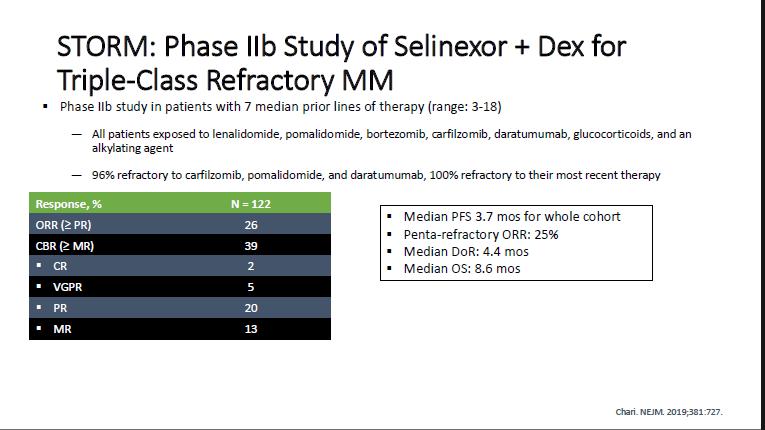

Newer Agents
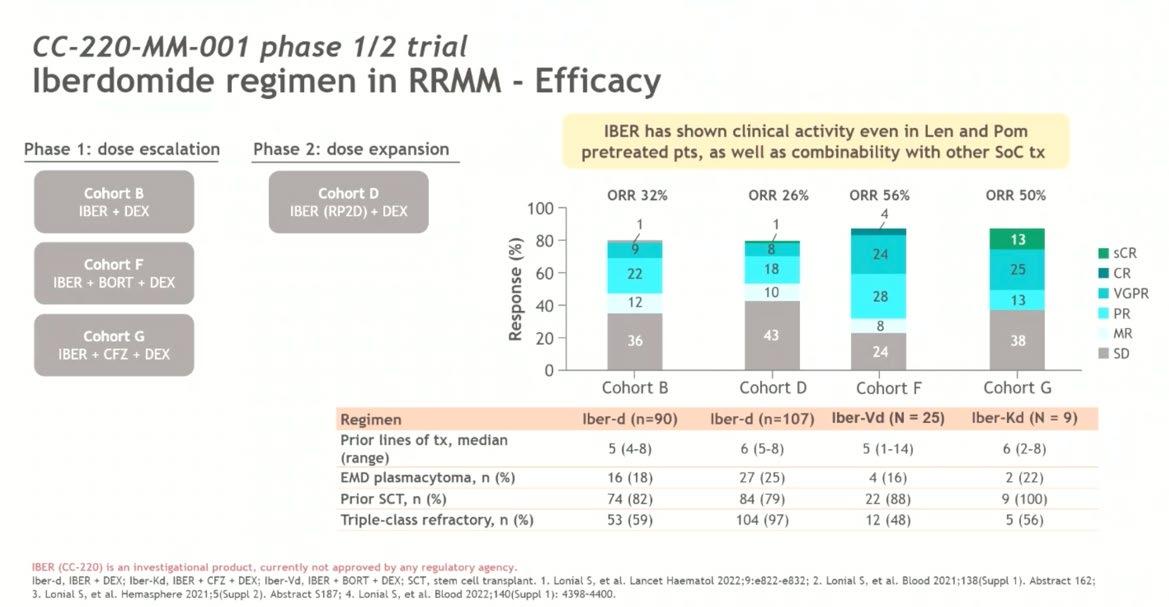
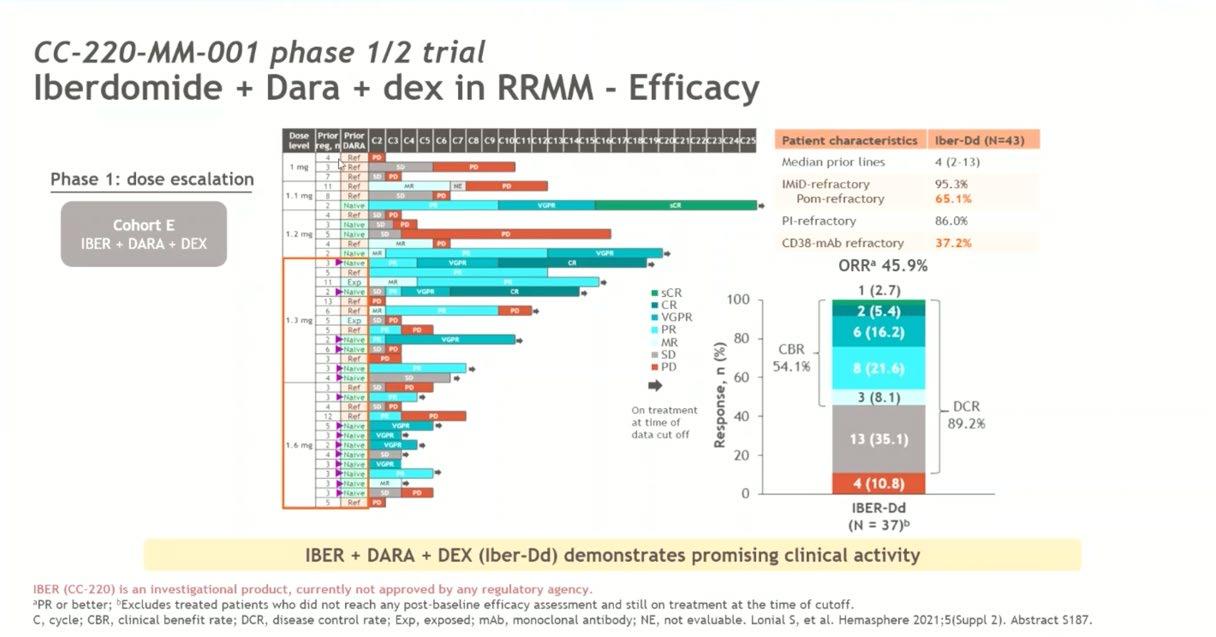
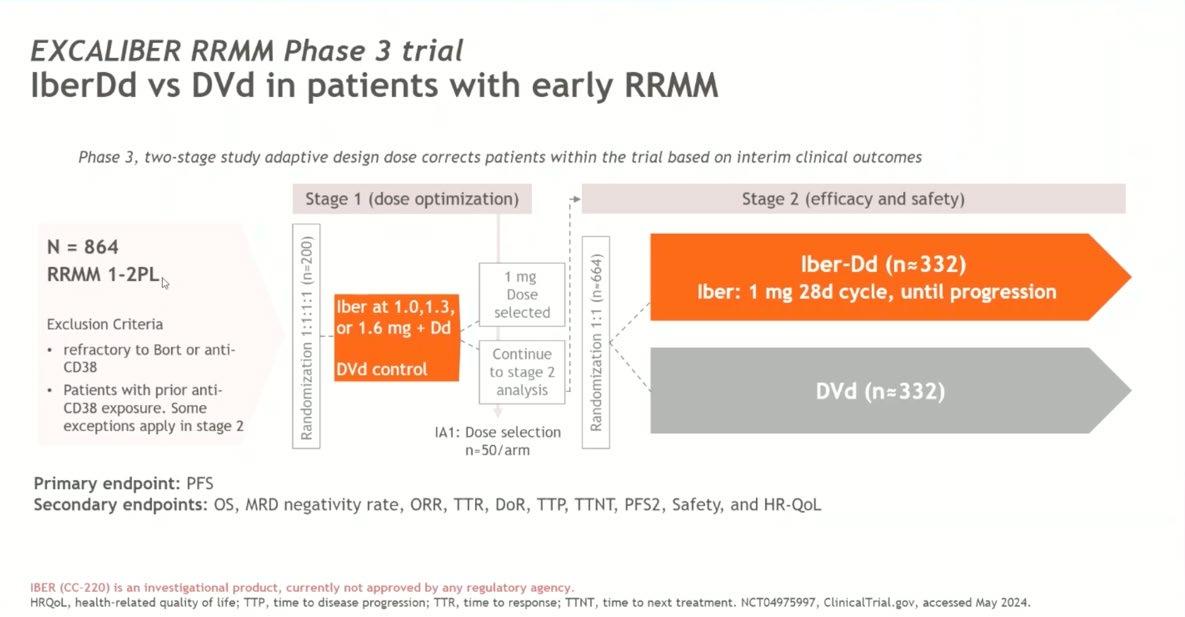
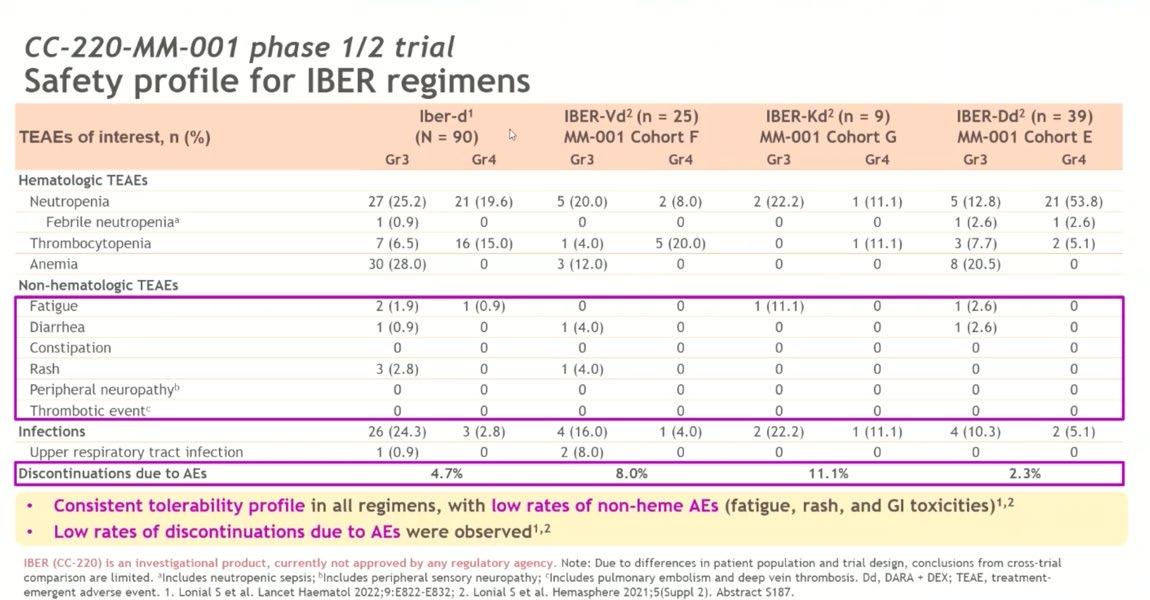
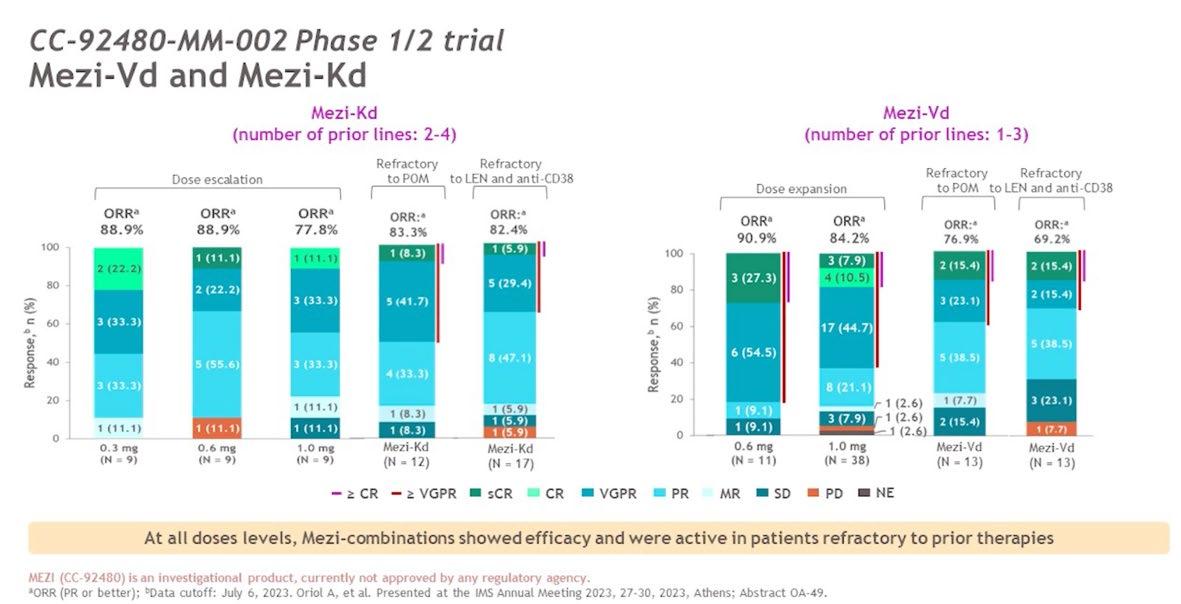
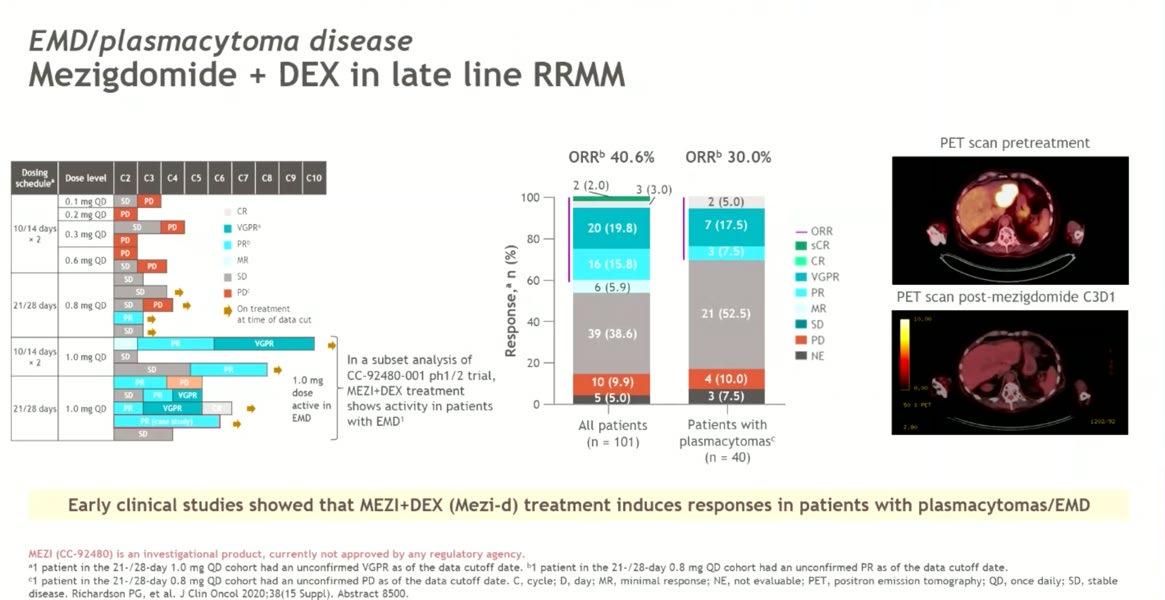

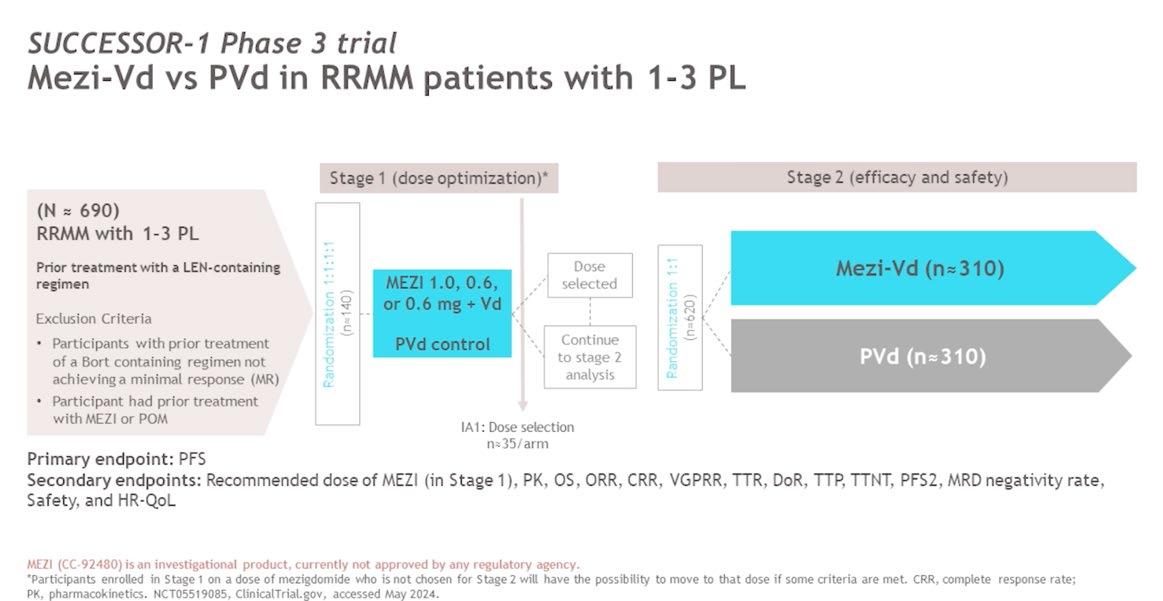
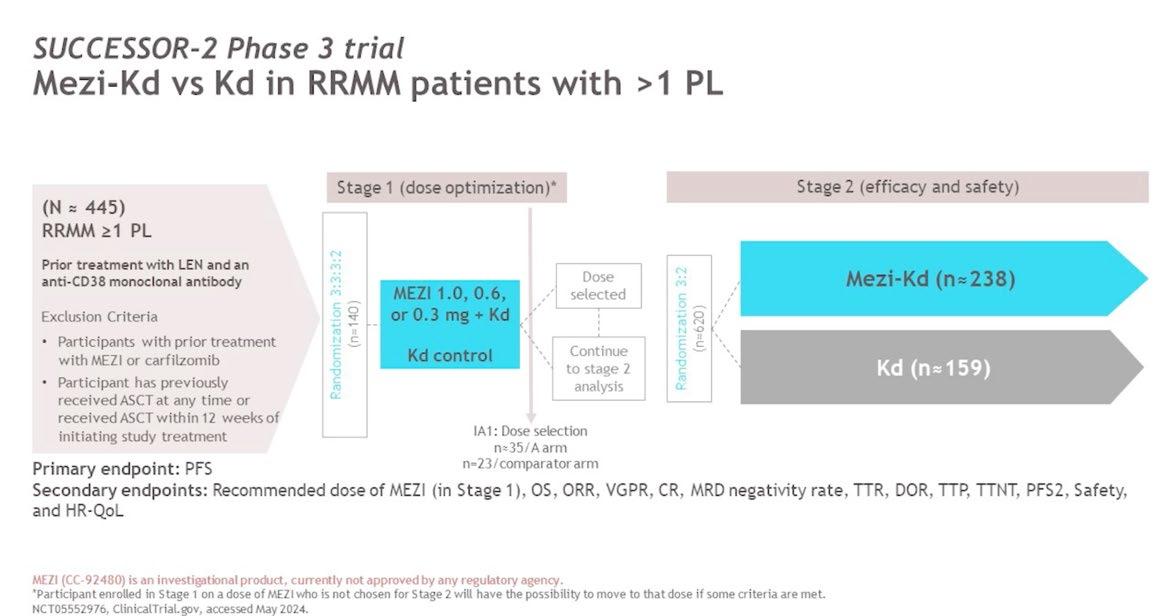
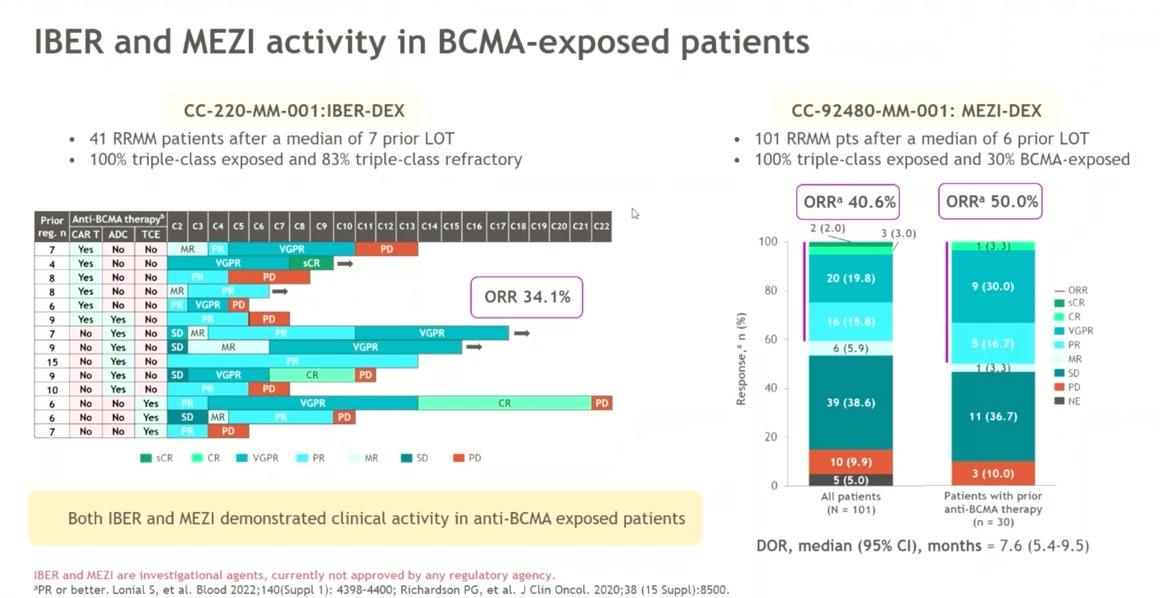
Conclusion
• Consideration for the next line of treatment – need to take in account patient, previous treatment and disease factors
• Need to also take account of local availability
• Many new options , so treatment need to be individualized
• CART and bispecifics should be considered earlier if available

Discussion




Break




CAR-T Cell Therapy and Other Immune Therapy Options

Chandramouli Nagarajan, MD
Singapore General Hospital and National Cancer Center Singapore


Cellular and other Immunotherapies in Multiple Myeloma

Dr. Chandramouli Nagarajan Consultant Haematologist, SGH / NCCS


changing treatment paradigm
Nothing
Thalidomide?
Bortezomib
Lenalidomide
Ixazomib combinations
Maintenance
Daratumumab + Carfilzomib
Pomalidomide
Ixazomib
Elotuzumab Combo Adding MAbs
Relapse
Post consolidation
Early relapse REFRACTORY

Late relapse
MaMMoth & LocoMMotion Studies
OS acc to refractoriness to
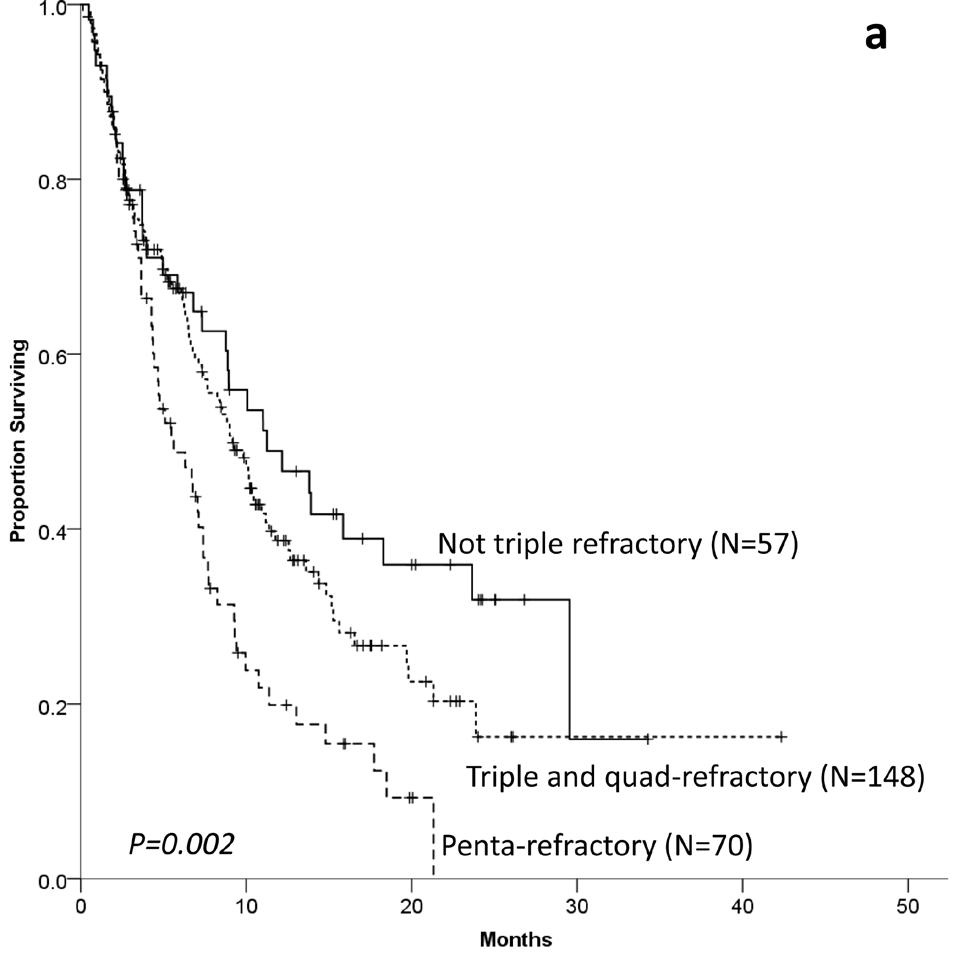




n = 275





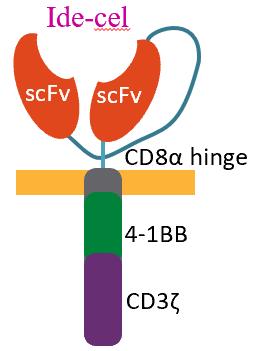
Idecabtagene Vicleucel (bb2121):
Second-generation
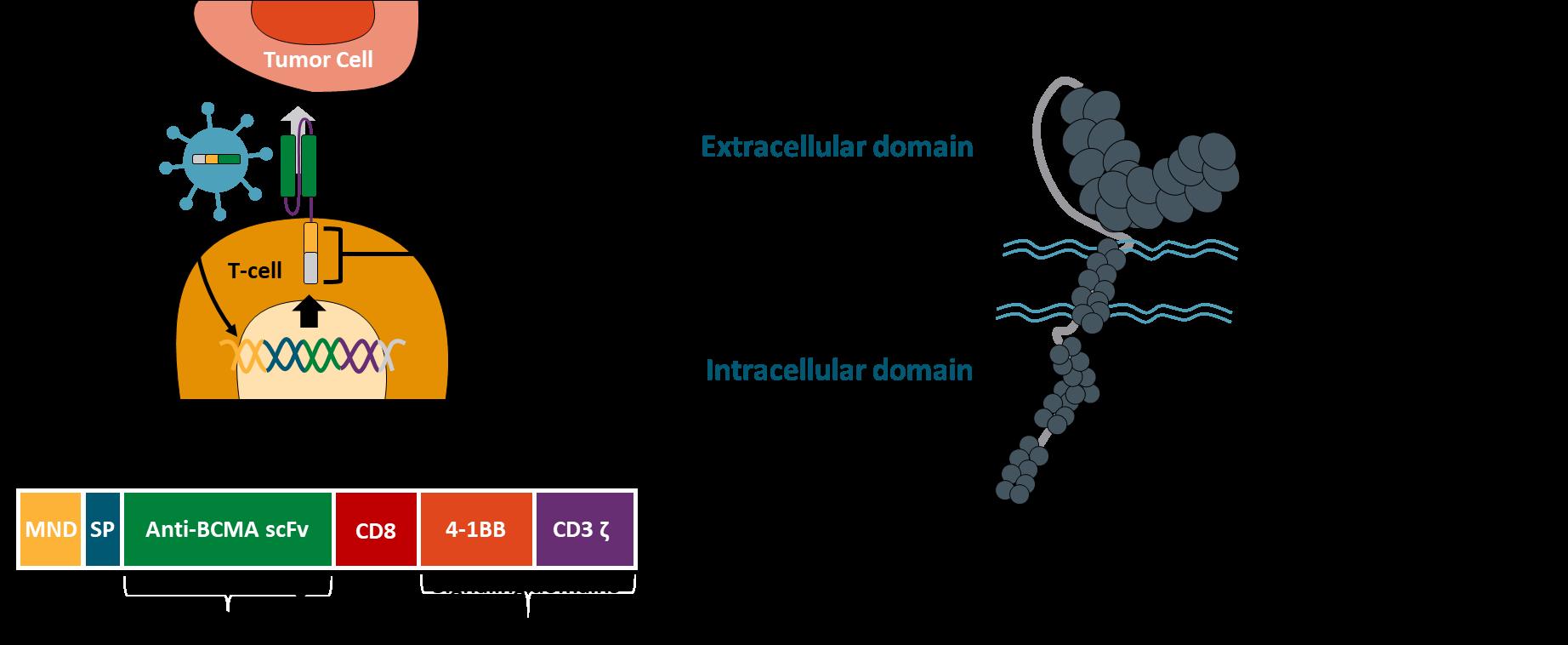


Ide-Cel – PhII Pivotal KarMMa Study
• RRMM
• ≥3 prior regimens with ≥2 consecutive cycles each (or best response of PD)
• Previously exposed to: – IMiD agent
– Proteasome inhibitor
– Anti-CD38 antibody
• Refractory to last prior therapy per IMWG*


Endpoints
Leukapheresis
ide-cel manufacturing (99% success rate)
1st Response Assessment (1 mo)
CAR T Infusion†
Bridging (≥14 before lymphodepletion)
Flu (30 mg/m2)
Cy (300 mg/m2)
Days -5,-4,-3 0
• Primary: ORR (null hypothesis ≤50%)
• Secondary: CRR (key secondary; null hypothesis ≤10%), time to response, Safety, DOR, PFS, OS, PK, MRD‡, immunogenicity
• Exploratory: Levels of cytokines and sBCMA, tumor BCMA expression
*Defined as documented disease progression during or within 60 d from last dose of prior antimyeloma regimen. ‡By next-generation sequencing.


Leukapheresed N=140
EudraCT: 2017-002245-29
Ide-Cel – Survival mfup -
63.6 months
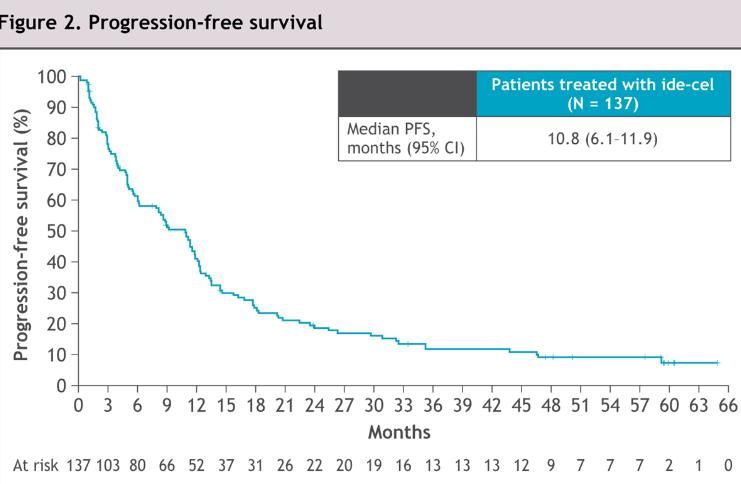

• First Car-T to receive US FDA approval for MM in 5th line
• Most relapsing patients had BCMA expression / sBCMA levels

Ide-Cel – RWE CIBMTR Data
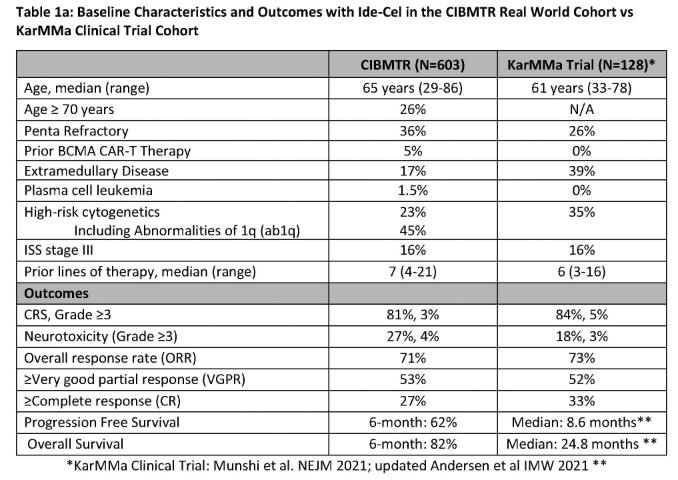

KarMMa-3 Ide-Cel Vs SOC in RRMM with 2-4 PLOT
• 2:1 RCT; Ide
• Bridging Therapy
• (n=386) Primary end point
• 44% HRMM; 24% EMD; 66% TCR; 95% CD38 Refractory
• ORR 72% Vs 42% MRD neg 22 Vs 1%
• mOS – Not significant (41mos Vs 37mos)
• Nil new safety signals



KarMMa-3 Ide-Cel
Vs SOC mFUP-30.9mo
CARTITUDE – 1 Cilta-Cel
Approved after 1 LOT
Berdeja, et al The Lancet. 2021

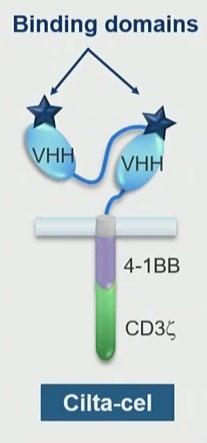
CARTITUDE – 1 Patient characteristics and final results
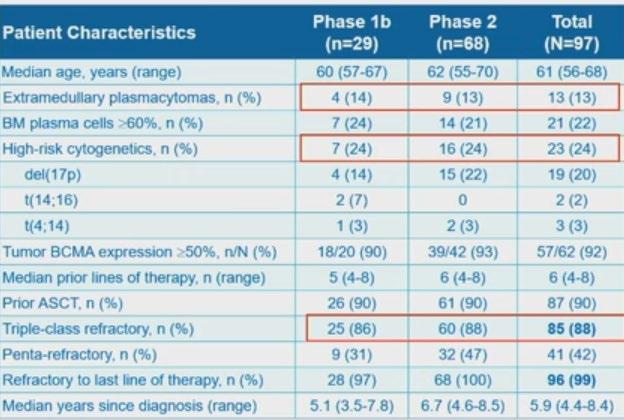


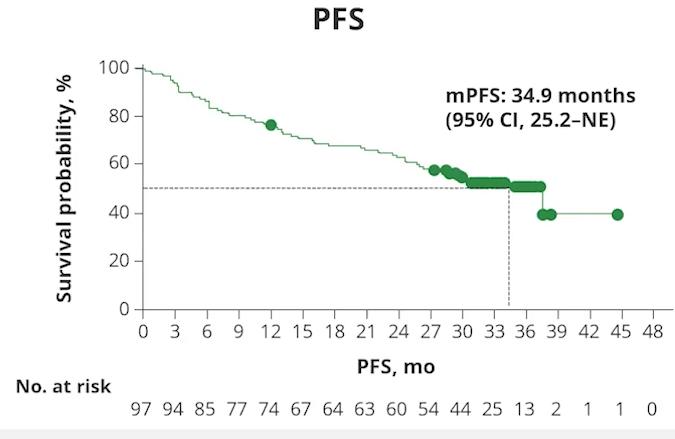
CARTITUDE – 1 Survival Outcomes 3-year fup


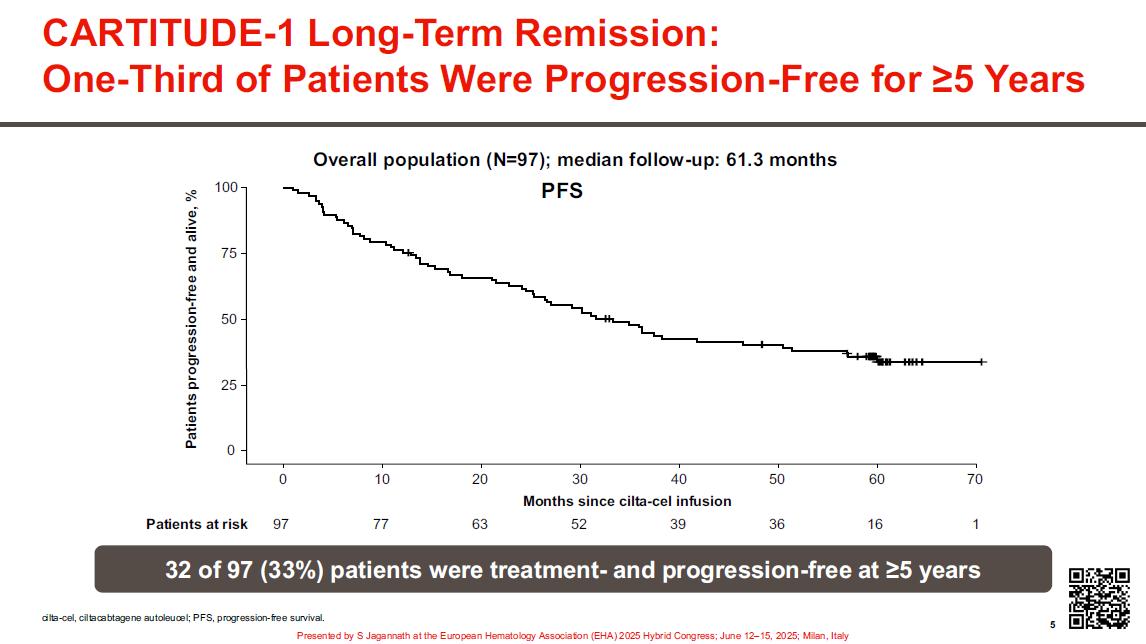
Extended >5-year fup –


CARTITUDE – 1 –
CRS / Neurotoxicity
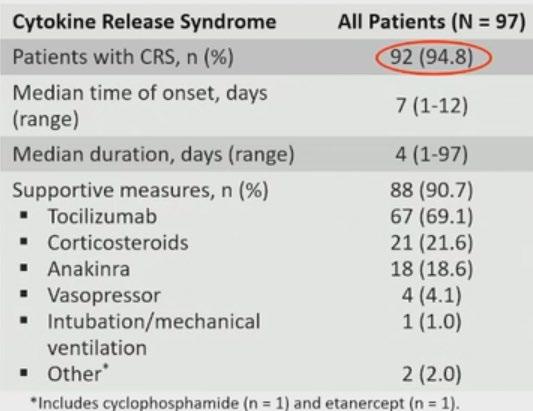
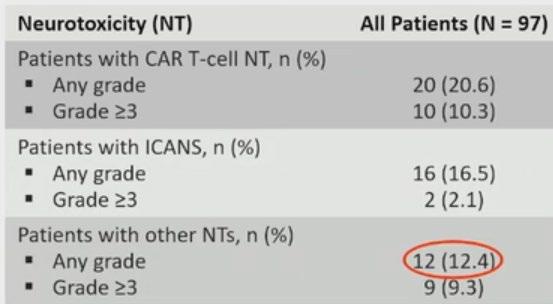
• Among 92 patients with CRS – 95% were Gd 1/ 2
• CRS resolved in 99% of pts
• Nil new safety signals at 3-year fup
• Cytopenias are the commonest Haematological Aes
Risk Factors Management strategies
≥ 2 factors such as:
• High Tumour Burden
• Prior Grade ≥ CRS
• Prior CNS events
• Higher CAR-T expansion and persistence
• Bridging to burden
• Screening & imaging (prior CNS events) + NT assessments
• Early and aggressive Rx of CRS and ICANS
• Extended monitoring >100d
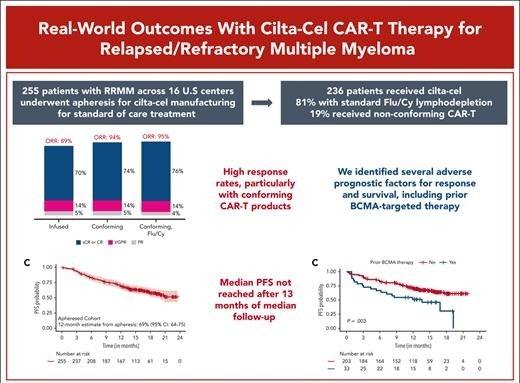

Sidana S, et al Blood . 2025;145(1):85-97

Cilta-Cel Vs SOC regimens CARTITUDE -

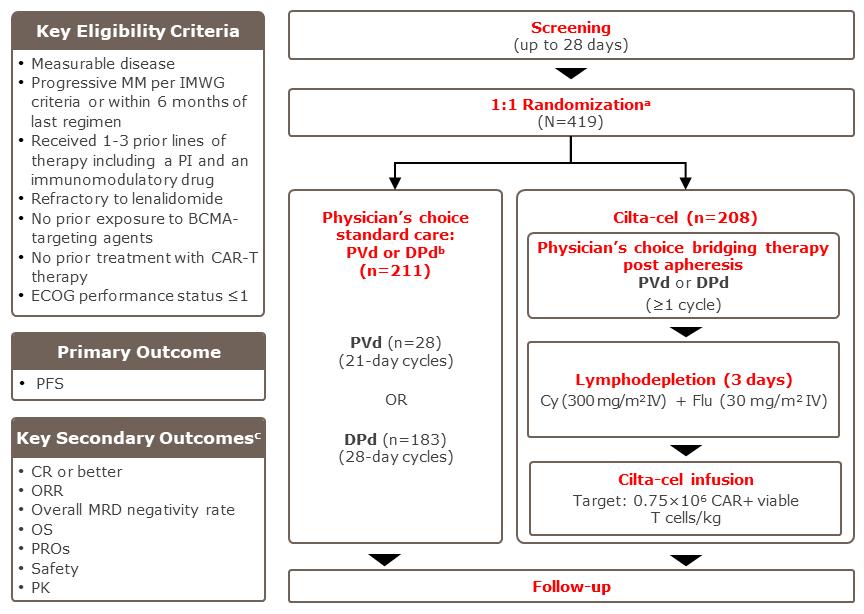

CARTITUDE 4 – PFS and

CARTITUDE 4 Vs 1


CAR T-Cell Therapy: Acute Toxicities
Cytokine-release syndrome
Cytopenias
Immune effector cell neurotoxicity syndrome
Immune effector cell associated
HLH-like syndrome
Generally managed by treatment center
Hines. Transplant Cell Ther. 2023;29:438.e1. Jain. Blood Maus. J Immunother Cancer. 2020;8:e001511. Tallantyre.

CAR T-Cell Therapy: Delayed Toxicities
B-cell aplasia/hypogammaglobulinemia
Prolonged cytopenias
Late infections
Long-term neurologic events/ movement and neurocognitive treatment
Transient cardiac toxicities
Secondary malignancies? Chakraborty. Transplant Cell Ther. 2021;27:222. Munshi. NEJM. 2021;384:705. Berdeja. Lancet. 2021;398:10297.
Generally managed by primary oncologist (treatment center or community setting)


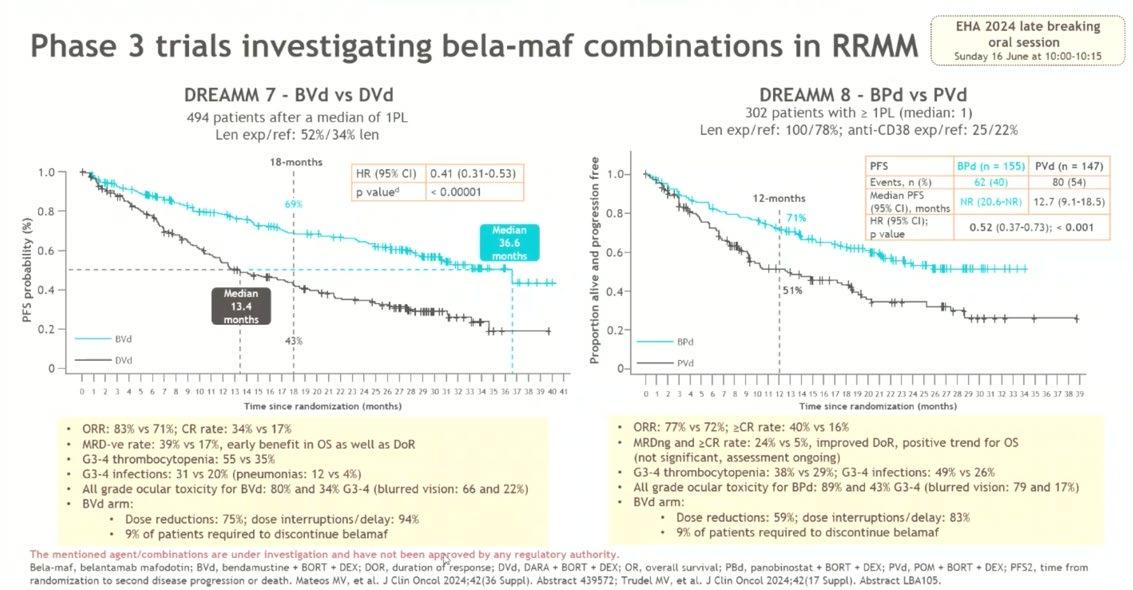
BCMA ADC

DREAMM-7 Ph 3 study Belantamab mafodotin +
(NCT04246047)
Recruitment period
~13-month from FPI (May 7, 2020) to LPI (June 28, 2021)
Eligibility criteria
• ≥1 prior line of MM therapy, and documented PD during or after their most recent therapy
• No prior treatment with anti-BCMA
• Not refractory or intolerant to daratumumab or bortezomib
Stratification:
• Prior lines of treatment (1 vs 2 or 3 vs ≥4)
• R-ISS (I vs II/III)
• Prior bortezomib (yes vs no)

Treatment period
Until end of study, withdrawal of consent, disease progression, death or unacceptable toxicity
Cycle 1-8
Bortezomib 1.3 mg/m2 SC on days 1,4,8, and 11 of cycle 1-8 (21-day cycle)
20 mga on the day of, and day after bortezomib for cycles 1-8
Daratumumab IV 16 mg/kg cycle 1-3; Q1W and cycle 4-8; Q3W
Bortezomib 1.3 mg/m2 SC on days 1,4,8, and 11 of cycle 1-8 (21-day cycle)
Dexamethasone 20 mga on the day of, and day after bortezomib for cycles 1-8
Follow-up period
Follow-up for PFS Q3W (for patients who discontinue due to reasons other than PD) Disease assessments Q3W End of
Disease assessment visits: Q3W from cycle 1 day 1 until disease progression
• Primary endpoint: PFS
• Key secondary endpoints: OS, DOR, MRD
Follow-up for OS Q12W (for patients who discontinue due to PD, or other reasons)
• Additional secondary endpoints: CRR, ORR, CBR, TTR, TTP, PFS2, AEs, Ocular findings, QOL


Bela Vd extended time to PD vs DVd, in RRMM

BVd PFS in subgroups, including in patients with len-refractory disease
All Subjects (Stratified)b
Number of Prior LOT (1 vs. 2/3 vs. ≥4)
1 2/3
≥4
Number of Prior LOT (1 vs. >1)
1 >1
Prior Bortezomib Yes No
Prior Lenalidomide
Yes No
Refractory to Lenalidomide Yes No
Revised ISS Staging at Screening I II/III
Age
<65 years
65-<75 years
≥75 years
Gender
Time to Relapse After Completion of 1L Treatment
≤12 months
>12 months
Cytogenetics Risk
High Riskc
Standard Riskd
Missing or Not Evaluable
Extramedullary Disease at Baseline
Yes No


BVd improved PFS regardless of
Mateos MVM et al. Presented at the ASCO Annual Meeting; May 31-June 4, 2024. Abstract 7503
BVd was associated with an early and sustained PFS benefit vs DVd in patients with Len-ref disease,
leading to a reduction in risk of progression or death in this group



PFS Benefit in Patients With High-Risk Cytogenetics1,2 High

1. Mateos MV, et al. ASCO 2024. Abstract 7503. 2. Mateos MV, et al. EHA 2024. Poster P938.

BVd (n=243) DVd (n=251)
BVd was associated with greater depth of response with a ≥CR rate that was double that with DVda
BVd (n=243) DVd (n=251)
MRD negativity rate (sensitivity of 10-5)b in patients treated with BVd was more than double that in patients treated with DVd (p-value <.00001)c Mateos MVM et al. Presented at the February American Society of Clinical Oncology Plenary Series. 2024 Abstract 439572.

DREAMM-7: Overall Survival
BVd Had an Early, Sustained, and Statistically Significant OS Benefit vs DVd
At 171 actual events (48.2% OS information fraction), OS was declared significant if the P value was <.00112. Simulation using an exponential distribution to predict median OS values in each a rm using the observed data at this interim analysis 2, with a 39.4-month median follow-up, to extrapolate time to death in ongoing censored patients. Predicted median OS values are subject to change as data mature.


DREAMM-8 Phase 3 study of
(NCT04484623)1,2
Recruitment period October 2020 to December 2022
Eligibility criteria
• ≥1 prior line of therapy including LEN
• Documented PD during or after their most recent therapy
• No prior treatment with anti-BCMA or pomalidomide; not refractory/intolerant to bortezomib
Stratificationb:
• Prior LOT (1 vs 2 or 3 vs ≥4)
• Prior bortezomib (yes vs no)
• Prior anti-CD38 therapy (yes vs no)

Treatment period
Until PD, death, unacceptable toxicity, end of study, or withdrawal of consent
2.5 mg/kg IV (cycle 1) then 1.9 mg/kg IV Q4W from cycle 2 onward
Pomalidomide 4 mg orally on days 1-21 (28-day cycles)
dexamethasone 40 mga on days 1, 8, 15, and 22 Bortezomib
1.3 mg/m2 SC on Day1,4,8,11 of cycles 1-8 then days 1 & 8 (21-day cycles)
Pomalidomide 4 mg PO days 1-14 (21-day cycles)
Dexamethasone 20 mga on the day and day after bortezomib
Primary endpoint: PFS (IRC assessed per IMWG)
Key secondary endpoints: OS, MRD negativity, and DOR
Additional secondary endpoints include: ORR, CRR, ≥VGPR, TTBR, TTR, TTP, PFS2, AEs, ocular findings, HRQOL, and PROs


Primary endpoint: PFS1,2

PFS outcomes in Lenalidomide refractory population
Reprinted from Dimopoulos MA, et al. N Engl J Med. 2024;391(5):408-421. Copyright © 2024 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Trudel S, et al. Presented at the International Myeloma Society Annual Meeting 2024. Abstract OA-62. Reprinted with permission by the author.

Median follow-up, 21.8 months (range, 0.03-39.23 months). Trudel S, et al. IMS 2024. Oral presentation OA-62.

Management of Ocular toxicity in D7 / D8
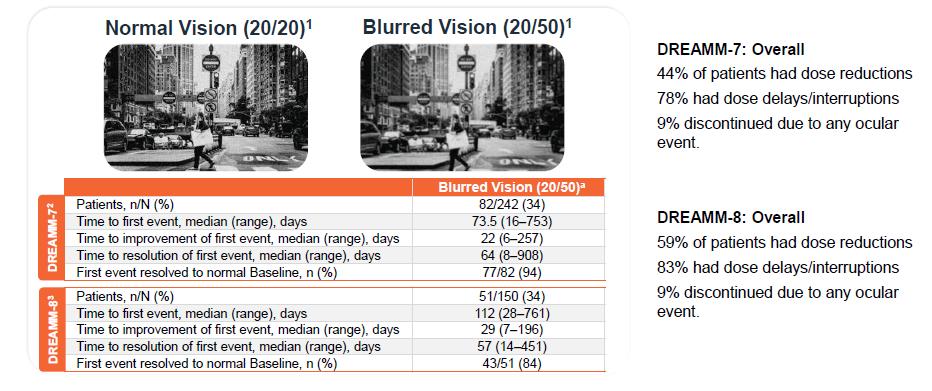


DREAMM-7: Median Dosing Interval, Response Rates & Ocular Events
High Response Rates Maintained Despite Longer Dosing Intervals With Low Incidence
of BCVA Worsening and Discontinuations Due to Ocular Events
• Median time between doses increased with treatment duration



• Responses (Best Response
≥ PR within each interval) remained high throughout treatment
• 23% patients experienced 20/ 50 or worse events in the first 3 months; prevalence decrea sed thereafter
• The rate of treatment discon tinuation due to ocular event s was low
Results from the primary analysis (data cutoff, October 2, 2023)
a Only the belantamab mafodotin treatment period was considered in these post hoc analyses. b Only patients with 20/25 or better in ≥1 eye at baseline are considered. c Mean of days between doses fo r each patient per interval is used. dGraph is truncated at 30 months because data beyond 30 months represented low number of patients on treatment (>30 to ≤33 months, n= 42; >33 to ≤36 months, n= 20; >36 to ≤39 months, n=8; >39 to ≤42 months, n=3).
Efficacy Overview of Key Studies in 2L+

1. Hungria V, et al. NEJM. 2024;391:393–407; 2. Mateos Manteca MV, et al. Presented at the European Haematology Association (EHA) 2024 Hybrid Congress; June 13–16; Madrid, Spain & virtual. 3. Mateos Manteca MV, et al. Presented at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting; May 31–June 4; Chicago, IL, USA & Virtual. 4. Dimopoulos MA, et al. NEJM. 2024;391:408–421; 5. Usmani SZ, et al. Lancet Oncol. 2022;23(1):65–76; 6. Landgren O, et al. Br J Haematol. 2022;198:988-993; 7. Usmani SZ, et al. . 2023;7(14):3739–3748; 8. Martin T et al. Blood Cancer J. 2023;13(1):1–9; 9. Moreau P, et al. Lancet 2021.397: 2361–71; 10. San-Miguel J, et al. NEJM. 2023;389:335–347

Study design and eligibility criteria differ across trials; therefore, data are not directly comparable.


EHA/EMN 2025 Guidelines for Second Line


Dimopoulos MA, Terpos E, et al. Nat Rev Clin Oncol 2025
Mechanism and prediction of responses to BiTEs


Pivotal study of Teclistamab


MAJESTEC-1 Results
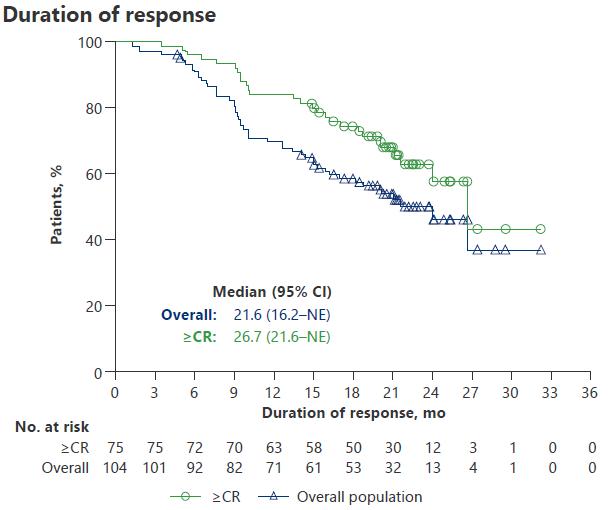
SAEs in MAJESTEC-1
Phase II MagnetisMM-3: Elranatamab for
Directed Therapy-Naive R/R MM (Cohort A)
Patients with MM refractory to ≥1, including an IMiD, PI, and anti
‒ 97% triple-class refractory
‒ Median 5 prior lines of therapy (range: 2
‒ 25% with high
‒ 32% with extramedullary disease
Elranatamab: 76 mg SC weekly with priming and/or premedication to reduce CRS
– If weekly dosing given for ≥6 cycles with achievement of ≥ PR for ≥2 mo, then dosing interval changed to every 2 wk
Primary endpoint: Secondary endpoint:

Lesokhin. Nat Med. 2023;29:2259. Mohty. EHA 2024. P932.


MagnetisMM-3: PFS and DoR

Phase II MonumenTAL-1: Talquetamab in
R/R MM
Patients with R/R MM after ≥3 lines of therapy, including an IMiD, PI, anti mAb
69%-84% triple
Median 5 cohorts
Key secondary endpoint:

Chari. NEJM. 2022;387:2232. Schinke. ASCO 2023. Abstr 8036. Rasche. EHA 2024. Abstr P915.


MonumenTAL-1: DoR and PFS Outcomes

ICANS
Safety of Approved BsAb for MM
3/4: 56%
3/4: 35%
3/4: 35%
3/4: 17%
3/4: 31% Skin/Nail
reported
reported Oral Toxicity Not reported 80% (dysgeusia: 49%, dry mouth: 34%, dysphagia: 23%, ageusia: 18%) Not reported
Moreau. NEJM. 2022;387:495. Teclistamab-cqyv PI. van de Donk. ASCO 2023. Abstr 8011. Chari. NEJM. 2022;387:2232. Talquetamab-tgvs PI. Touzeau.
EHA 2023. Abstr S191. Elranatamab-bcmm PI. Lesokhin. Nat Med. 2023;29:2259.
Phase Ib RedirectTT-1: Teclistamab Plus Talquetamab in R/R
Multiple Myeloma
Open-label, phase Ib/II dose escalation and expansion trial of teclistamab + talquetamab in patients with RRMM with prior exposure to a PI, IMiD, and anti-CD38 mAb and refractory to last line
‒ Median prior LOT: 4 (1-11); extramedullary plasmacytomas: 37.6%
Primary endpoints: safety, RP2R; secondary endpoints: ORR, PK, immunogenicity
Phase
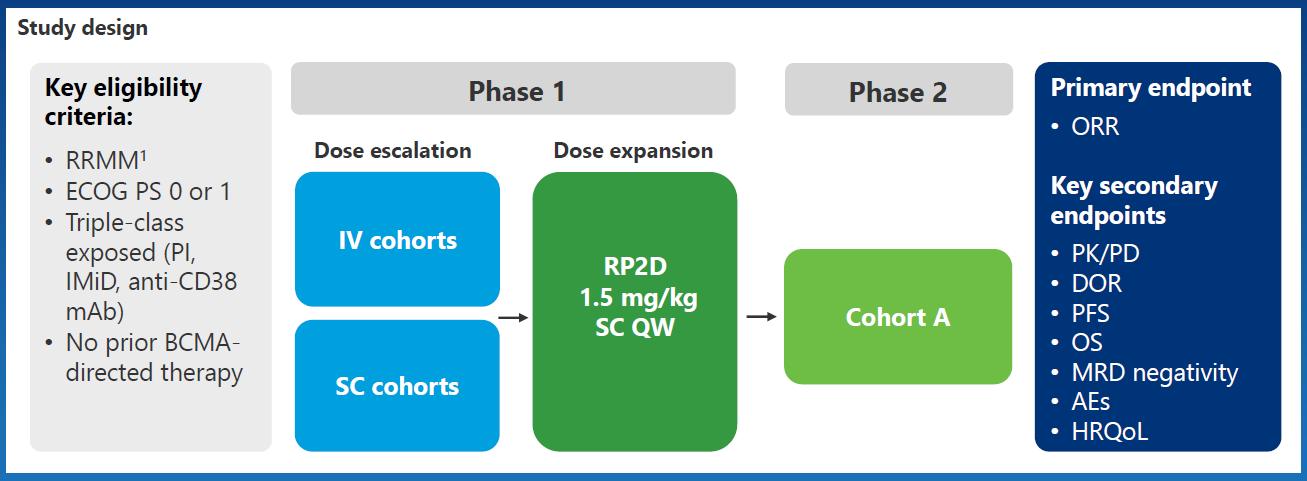
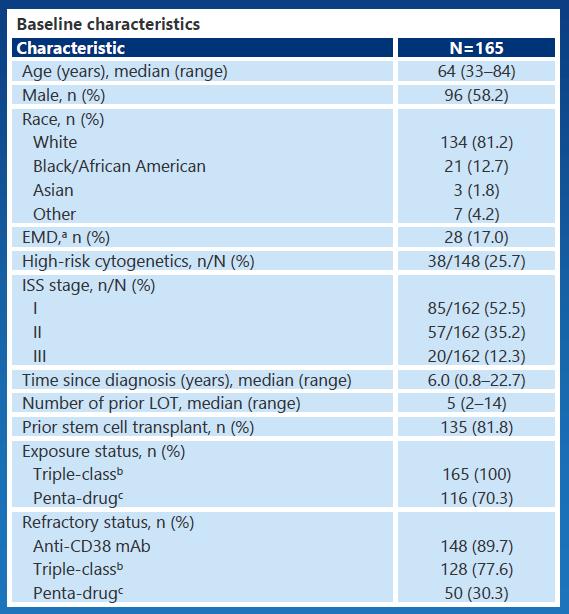
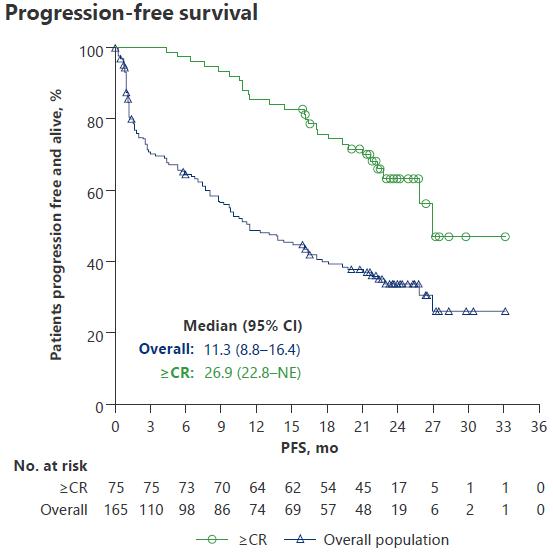
I/II Trial With BsAb in R/R Multiple Myeloma
Phase I/II CAMMA 2 Phase I NCT03933735 Phase I/II LINKER-MM1
Inclusion criteria Triple-class refractory R/R MM, with prior BCMA-targeted ADC or CAR T-cell therapy, no BCMAtargeted bispecific*
R/R MM ≥3 prior LOT including PI, IMiD, and antiCD38 mAb, no prior BCMAtargeted therapy
R/R MM ≥3 prior LOT including PI, IMiD, and antiCD38 mAb
Mechanism of Infections with BiTEs
Hypogammaglobulinaemia – Profound Plasma cell aplasia with BCMA targeting agents
Continuous BiTEs worsen it
Cytopenias – Neutropenia / Lymphopenia
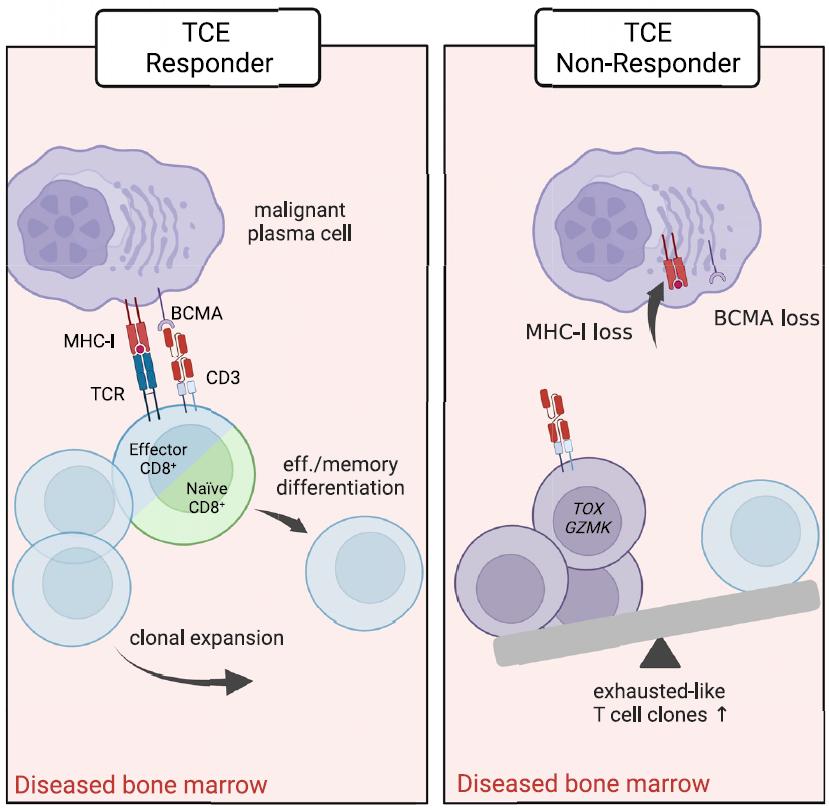
T-cell Exhaustion
Background
• BM plasma cell percentage > 50% - NDMM and at each relapse
• Inclusion criteria of Dara Exposure



Comparing BCMA Options: Advantages/Disadvantages
Antibody–
Off the shelf
Targeted cytotoxicity
Not dependent on T
No lymphodepletion
No corticosteroids
Available to any infusion center
Outpatient administration
Ocular toxicities requiring ophthalmology visits
Single-agent activity low in CD38-refractory patients
Requires continuous administration



Conclusions
Myeloma Survival has increased exponentially but cohorts of poor surviving patients remain
Early relapse management is crucial in improving OS and preventing devastating consequences
CAR-T & BsAbs are revolutionising early and late relapse management
Therapy related toxicities especially Infections and cost are challenges that needs solutions
Access in Sg / Asia is limited currently
Need to understand mechanisms underlying resistance

Dr. Chandramouli Nagarajan(Mouli) Chandramouli.Nagarajan@Singhealth.com.sg
Discussion




Infection and Side Effects

James CS Chim, MD, PhD
University of Hong Kong, Queen Mary Hospital
Infections & Adverse events of CAR-T & BiSP therapy of MM
James CS Chim
MBChB, MD, PhD, FRCP(London), FRCP(Edinburgh), FRCP(Glasgow), FRCPath(UK), FHKAM, FHKCP

Honorary Consultant, Hong Kong Sanatorium Hospital &
Honorary Clinical Professor, Department of Medicine, University of Hong Kong
Honorary Clinical Professor, Department of Medicine & Therapeutics, Chinese University of Hong Kong
Honorary Consultant, Hong Kong Sanatorium Hospital & Member, International Myeloma Working Group (IMWG)
Executive Council Member, Asian Myeloma Network (AMN)
Founding member, International Academy of Clinical Hematology (IACH)
Founder & Chairman, Hong Kong Society of Myeloma (HKSOM)
Infective complications of Immunotherapy for MM with BiSP & CAR-T
• Type (bacteria, viral, fungal)
• Site (resp, GI, GU, mucosal, soft tissue, etc)
• Impact (hospitalization, dose omission, discontinue, death)
• Onset in the course of treatment
Current issues about reporting calls for harmonization of reporting (infection/100 pts/month) :
1) Duration of FU not accounted for 2) Only the highest grade infection was reported
Bispecific Antibody Therapy for Multiple Myeloma
Antibodies with multiple binding domains
‒ Target different tumor antigens including BCMA, GPRC5D, FcRH5
‒ Also binds to immune cell targets including CD3 (T-cell)
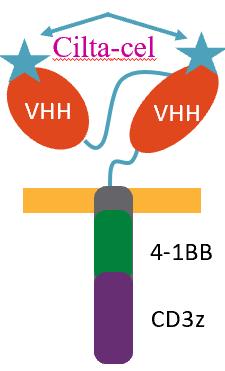
Teclistamab: CD3 x BCMA
Elranatamab: CD3 x BCMA
Talquetamab: CD3 x GPRC5D
Elranatamab PI. Talquetamab PI. Teclistamab PI. van de Donk. Lancet. 2023;402:142.
Bispecific antibodies (BsAbs) create an immune
synapse between T cells and malignant cells
Approximate toxicity timeline
CRS: mostly within first cycle onset, IV <24 hours, Subcut 24-48hr
Neurologic (ICANS): within first cycle
Infection: anytime during therapy
Cytopenias: during CRS, short duration
Slide credit: clinicaloptions.com

Infection risk
BsAb Therapy’s Infection Risk
Patient related
Heavily pre-treated
Plasma cell targeting (BCMA > nonBCMA) leads to significant hypogammaglobulinemia hence impaired ↓humoral immunity
Treatment MOA (T-cell redirection)
T-cell dysfunction/exhaustion leads to impaired cellular immunity
Myeloma related
immune paresis
Activation of immunosuppressive T-regulatory cell subtypes
CRS risk and related concomitant immunosuppressive supportive medications (corticosteroids, tocilizumab)
Treatment related
Image provided courtesy of Dr Lee. Koristka. J Immunol. 2012;188:1551.
Cumulative therapeutic exposure (long)
Continuous therapy by design in most protocols in already substantially pretreated patients
Significant DoR compared to historic conventional therapies used in R/R M → longer duration of therapy

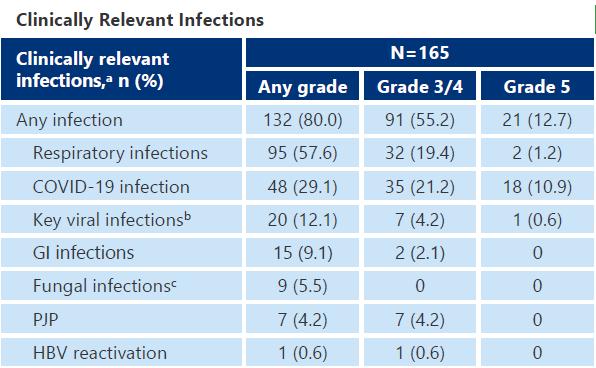
Infection: A Persistent Concern During Maintenance With Teclistamab
BCMA BsAbs deplete peripheral blood
B-cells & eliminate normal plasma cells
BCMA BsAbs ↓↓ polyclonal Ig levels & impair vaccination response The negative impact of BCMA BsAbs on humoral immunity can be partially reversed with IVIG supplementation

Infection Risk, incidence & mortality in Bispecific Antibody Trials in RRMM
Any grade infection occur in 76.4% in MajesTEC-1, 66.7% in MagnetisMM-3 studies, 57.3% in MonumenTAL-1 study
Despite no LD chemo as in CAR-T, G3/4 neutropenia still common
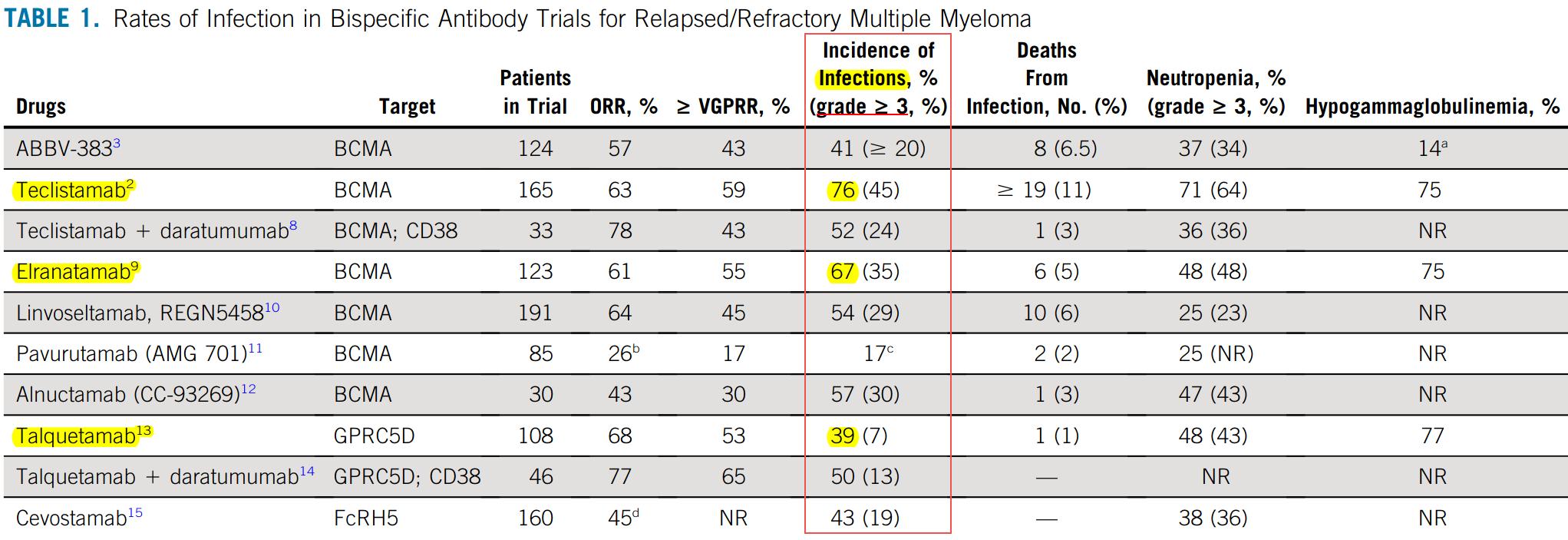
Non-BCMA
Similar rate of HGG
Less G3 neutropenia
Less infection mortality
Non-BCMA lowest
infection rate & mortality
But ~ rate of HGG
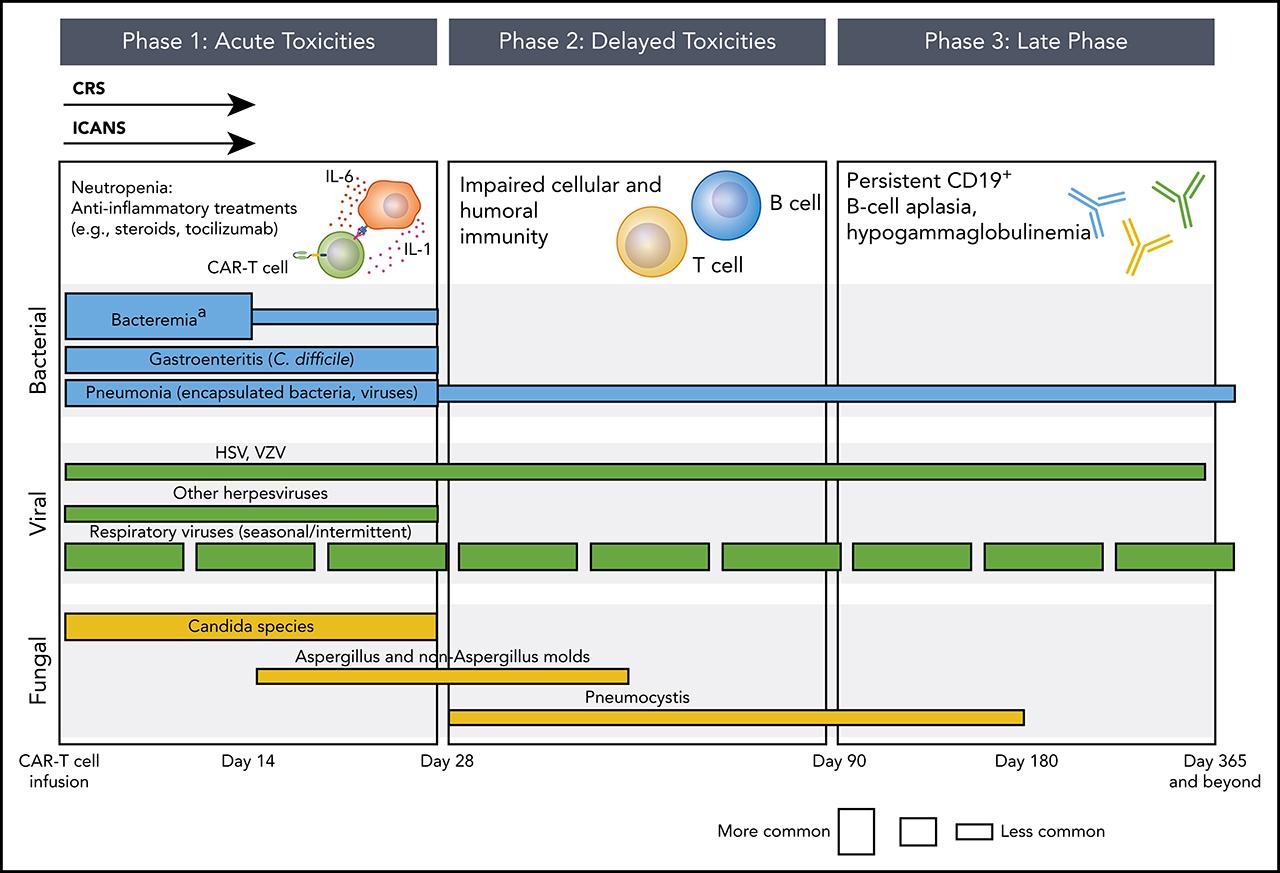
Infection is prevalent with BiSP therapy, especially with BCMA-targeted BiSP, with a notable mortality
This is due to the early neutropenia & then profound hypogammaglulinemia with prolonged BiSP treatment
Any grade infection occur in 76.4% in MajesTEC-1, 66.7% in MagnetisMM-3 studies, 57.3% in MonumenTAL-1 study
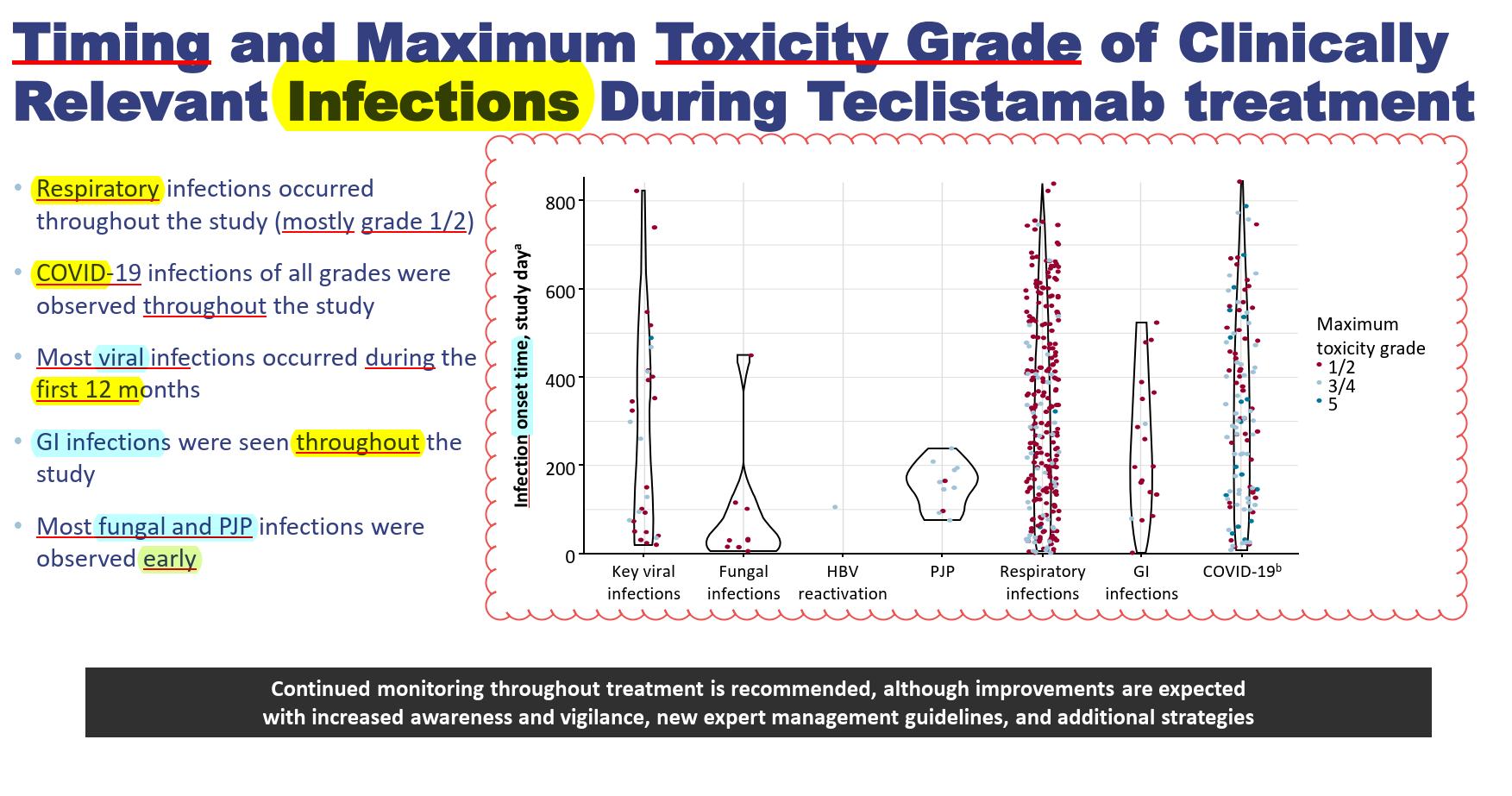
Viral, COVID, respiratory & GI infections occur throughout the year
Fungal & PJP mainly early on (neutropenia, CRS & steroid/Toci use)
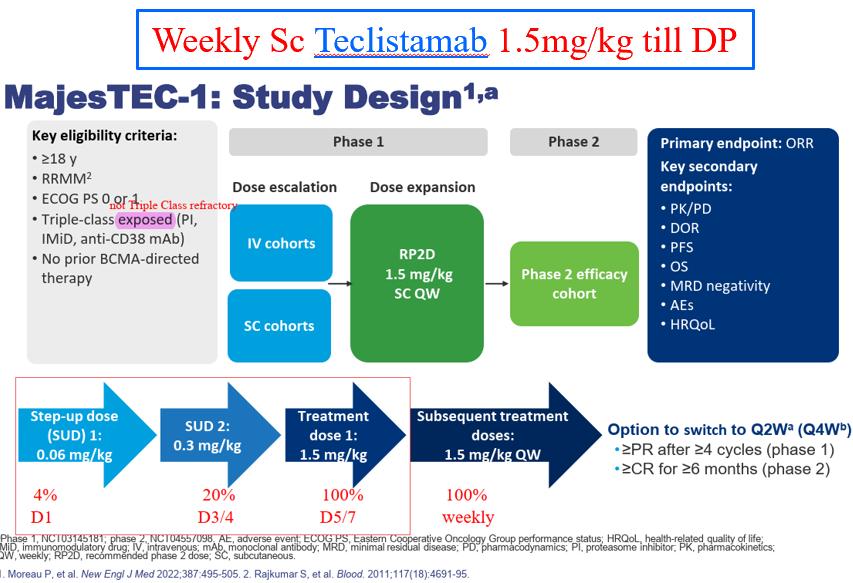
Sc Elranatamab 76mg Once weekly if PR at week 24 (6m) every 2 weeks
Any grade infection occur in 76.4% in MajesTEC-1, 66.7% in MagnetisMM-3 studies, 57.3% in MonumenTAL-1 study
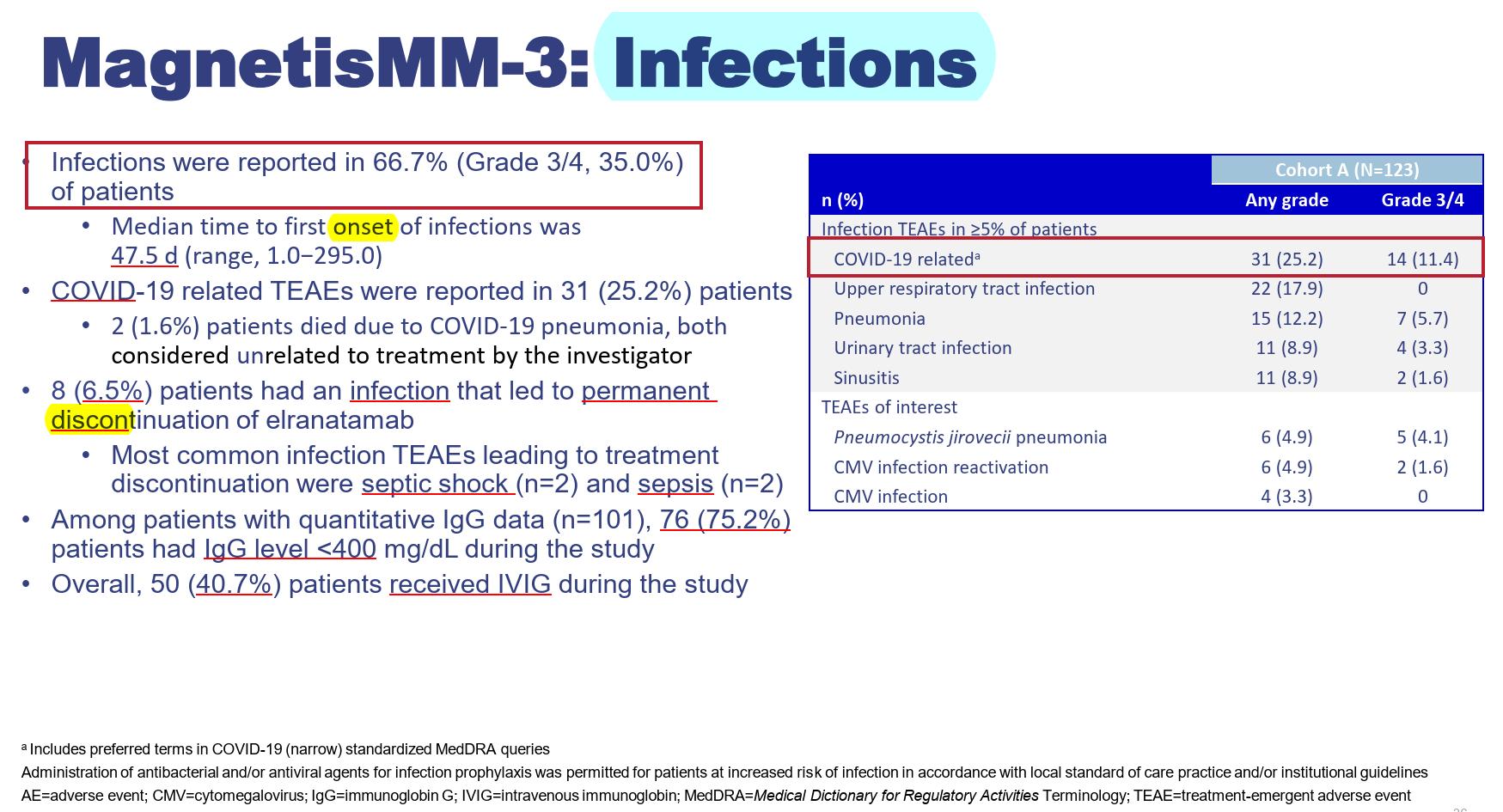
Resp (upper/lower), Sinus, UTI
CMV monitoring
What are the type & organisms of the infection in BiSP
?
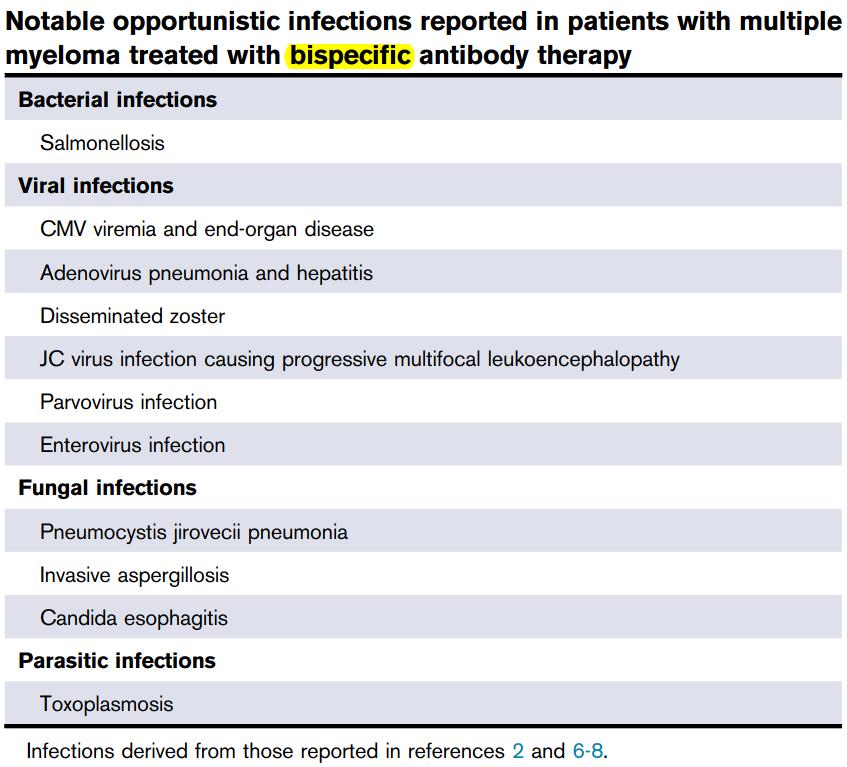
Type of Infection
• Most are viral & bacterial
• Samonella is common
• Viral: adeno-, entero-virus, dissem zoster, CMV viremia/infection
• Fungal: Pneumocystitis, Aspergillos
• Hence
• Prophylaxis for zoster & PJP
• How do we monitor CMV?
• By PCR or CMVpp65?
• When to start monitor?
Sarah P. Hammond, Edit, Blood Adv 2024
MajesTEC-1 :
Infection
Less infection with spacing out dosing
Patients With New
= 165)
to ≤6
= 113)
to ≤9
= 99)
to ≤12 (n = 184)
to ≤15 (n = 71)

to ≤18 (n = 66)
to ≤21 (n = 54)
to ≤24 (n = 44)
= 19)
Patients who switched to Q2W by 1 yr had fewer grade ≥3 treatment-emergent infections between 11.5 yr than those who remained on QW (15.6% vs 33.3%)

Consensus Recommendations : Infections in BsAb
Prevention is the key!
• Screen for HBV reactivation risk in all patients (level III)
• Use antiviral Prophylaxis against HSV and VZV in all patients (level III): acyclovir/valacyclovir
• Use PJP prophylaxis for all patients (level IIC): septrin, dapsone, atovaquone, neb pentamidine
• Administer monthly IVIG for duration of immunoparesis and in absence of life-threatening infectious manifestations (level IIC)
• Use G-CSF in persons with grade ≥3 neutropenia (level III)
• Follow CDC guidelines for vaccinations (eg, influenza, pneumococcus, RSV, COVID-19)
• No routine anti-fungal prophylaxis unless a history of fungal infection, prolonged neutropenia or steroid administration. Given that mould is the major culprit, azole (flucon-, itracon- or posaconazole) is used in case prophylaxis deemed worthy
• No routine anti-bacterial prophylaxis unless except in case of prolonged neutropaenia or history of recurrent bacterial infections
Devise achedule & methodology to monitor CMV
Omit BiSP dose if concurrent infection
Taper frequency of BiSP, e.g q1w q2w, when MM under control
• No routine CMV prophylaxis against as CMV “infection” uncommon BUT monitoring of CMV reactivation recommended Raje. Blood Cancer J. 2023;13:Art
Infections in CAR-T cell therapy
CAR T-Cell Treatment Schema
Patient stays within a certain radius of treating facility until approximately Day 30
Infections in CART
Late cytopenias
BCMA CARS
BCMA BsAbs
Infections
Lower with GPRC5D
Similar with Bispecifics
[Steroids/Toci for CRS] Neutropenia CRS - ICANs



Bacteria esp. encapsulated bacteria
Resp viruses
Respiratory infections
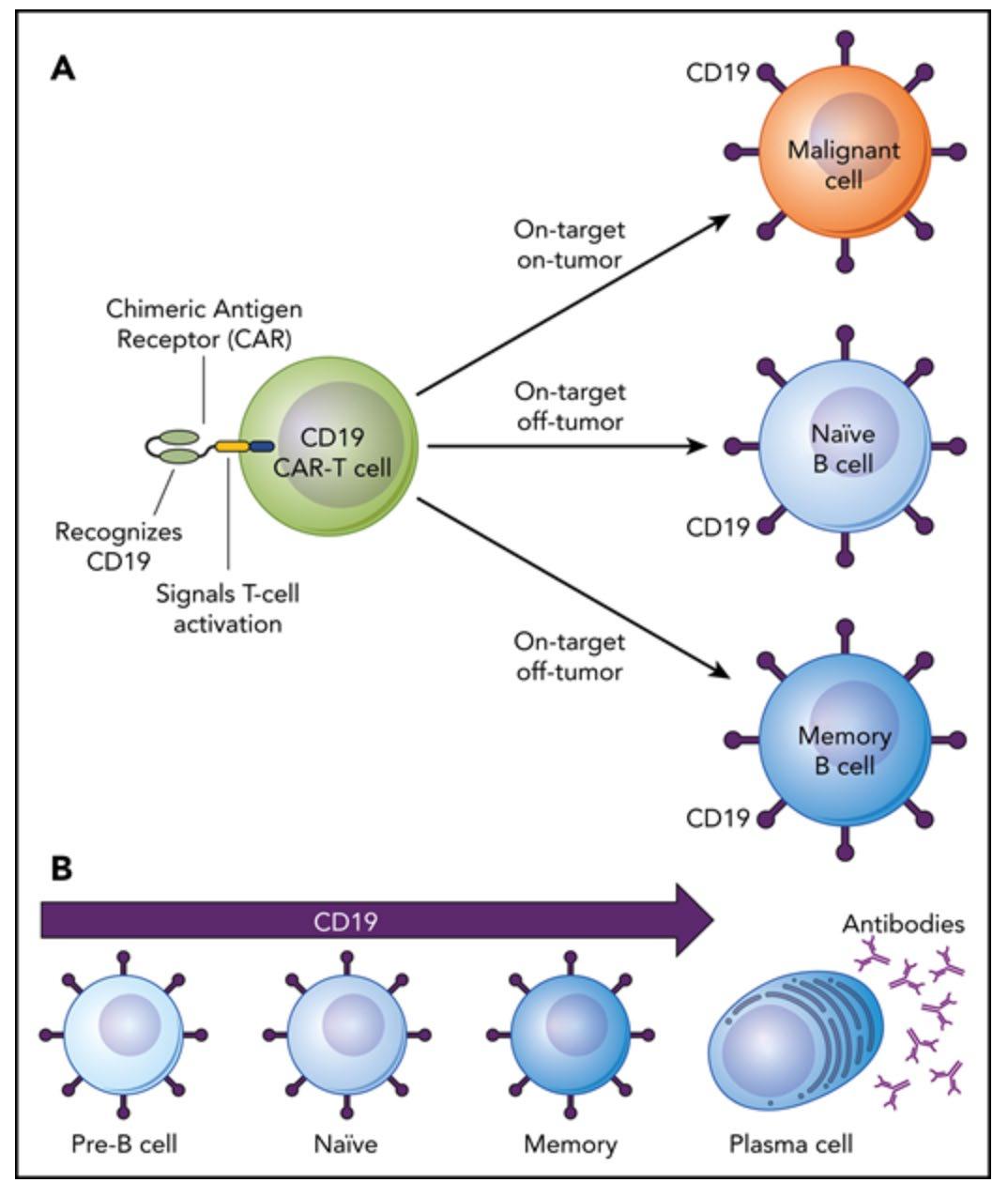
Suppression of B cells and T cell dysfunction
Joshua A. Hill, Susan K. Seo, Blood, 2020
Infectious complications in patients with RRMM after BCMA CAR T-cell therapy
Kambhampati Blood Adv 2022 single center retrospective study at UCSF
• RWD Infection in 55 MM BCMA CAR pts
• Prior to LD Therapy
• 1/3 (35%) severe hypogammaglobulinemia (HGG)
• 18% severe lymphopenia
• 68% received bridging chemotherapy (BC)
• In the 1st month post CAR-T
• 98% patients had grade 3-4 neutropenia.
• 1-year post CAR infusion HGG
• ¾ (76%) had HGG
• Infections
• 47 infectious events in 29 (53%) patients
• 40% bacterial
• 53% viral (CMV Pneumonia)
• 6% fungal (Aspergillus)
• 92% were mild-moderate
• 68% in the lower/upper respiratory tract system
BCMA - CAR
BCMA
Ide-cel NEJM 2021
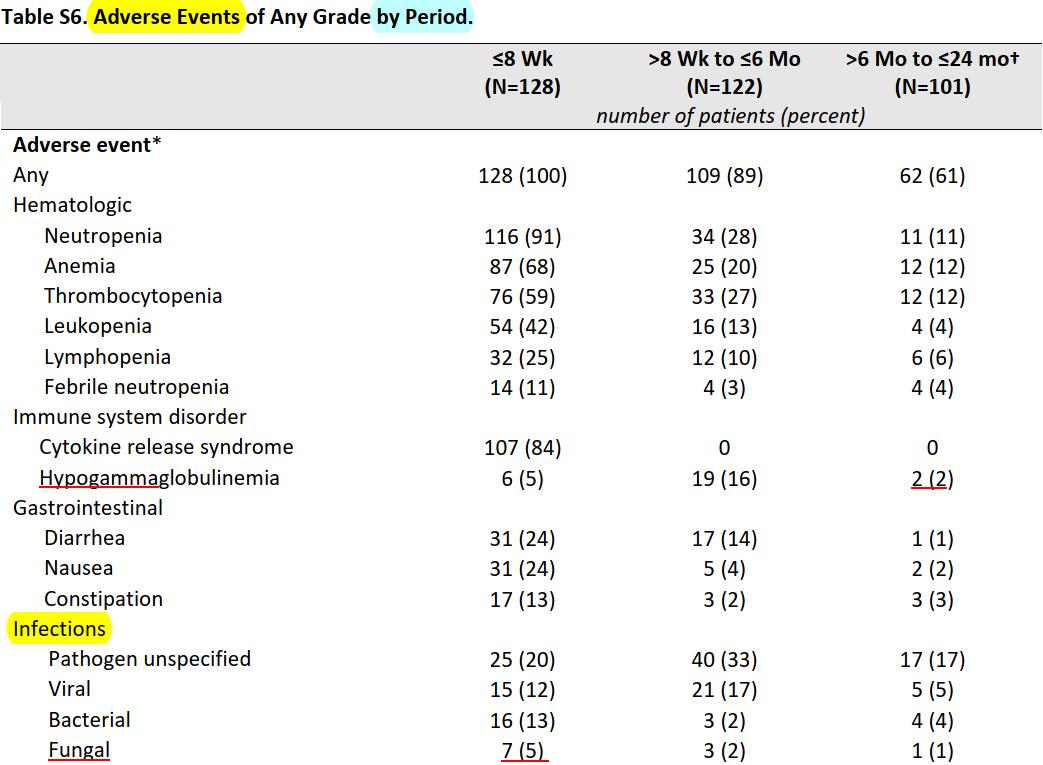
Viral prevalent in 1st 6 months
Bacteria & fungal mainly in 1st 2m when neutropenia prevails
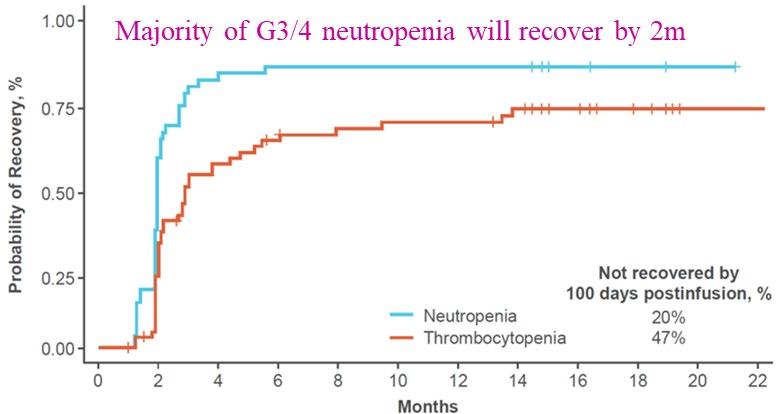
Cartitude-1 Deaths
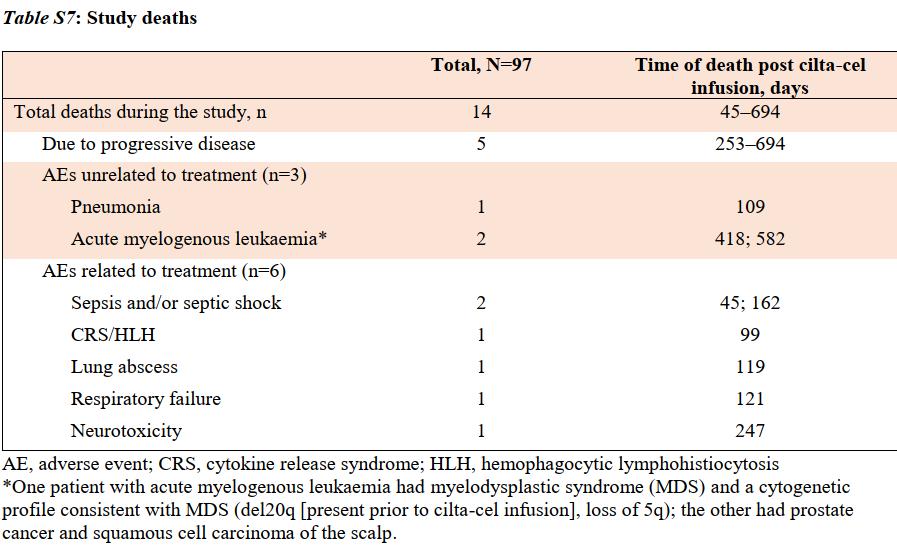
Lancet 2021suppl data
Causes of death:
1. DP: 5/14 (36%)
2. SPM (AML): 2/14 (14%)
3. TRM: 7/14 (50%)
a) infection : 4/7 (57%)
b) CRS/HLH : 1/7
c) ICANS: 1/7
d) resp failure: 1/7
Infection mortality: 4.1%
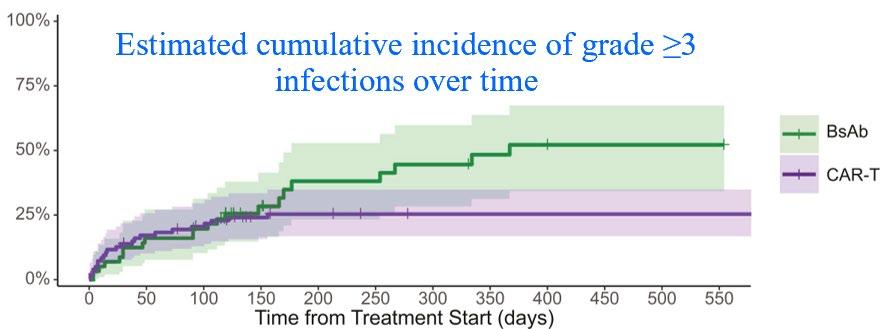

Table 1: Antimicrobial prophylaxis CAR-T same applies
to BiSP
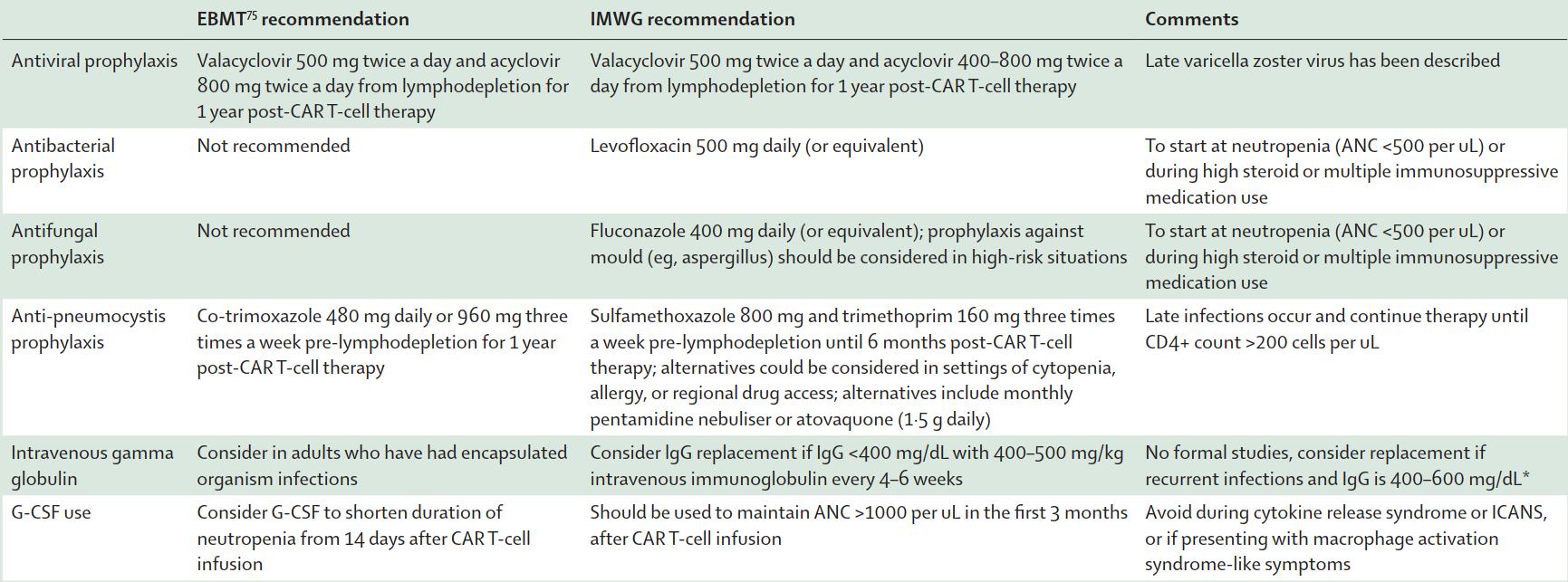
Unless high-risk
LiuYin, IMWG CAR-T,
HZV
PJP
Practical Guide
to
Minimizing
Infection Risk for BCMA CARTs and BCMA Bispecifics
• Seasonal vaccinations > 2 weeks prior to LD/CART or bispecific e.g. Flu, COVID, RSV
• Antibacterial/fungal: while ANC <500, levofloxacin and fluconazole
• Limited period (6 months) post BCMA CART vs indefinite on BCMA BiSP
• PJP PPX: trimethoprim/sulfamethaxazole tiw or alternative if cytopenic
• if Hep B-positive: entecavir
• IVIg replacement Q4W for 6 months
• VZV prophylaxis: acyclovir (for 1 year post CART)
• UCSF Post-CART Vaccination Schedule:
• Annual influenza @ 1+ months (Sep-Mar), 3+ months (Apr-Aug)
• 6 months: PCV20, Shingrix, RSV, COVID19 (3 doses, 3-4 weeks between dose 1-2, >4 weeks between dose 2-3)
• 9 months: Shingrix (if not rec’d previously)
• 12,18,24 months: HPV if <45 yo if not rec’d previously
UCSF guidelines, Ajai et al
CRS & ICANS in CAR-T & BiSP
Summary of Approved CAR T-Cell &Bispecific Antibodies in R/R Multiple Myeloma
** Table S10, Berdeja et al, Lancet 2021 Suppl

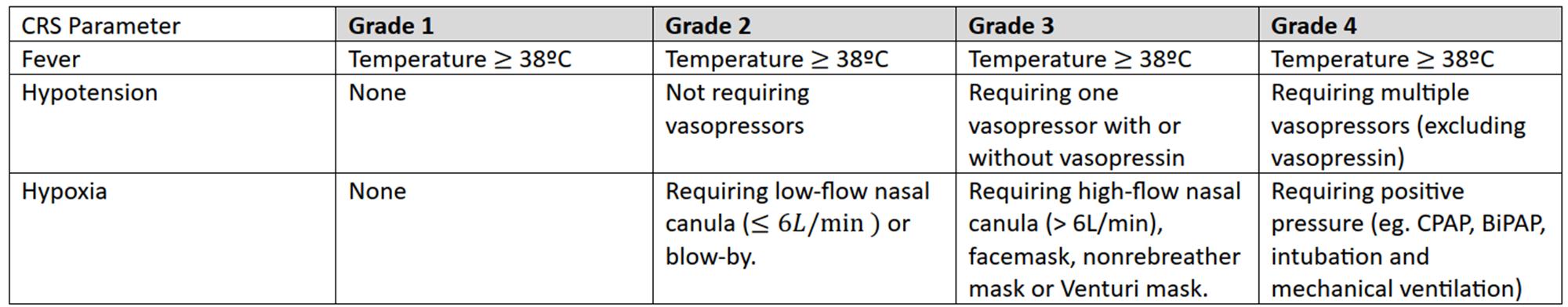
CRS Clinical Manifestations
Lee. Biol Blood Marrow Transplant. 2019;25:625.
Clinical Symptoms/Findings

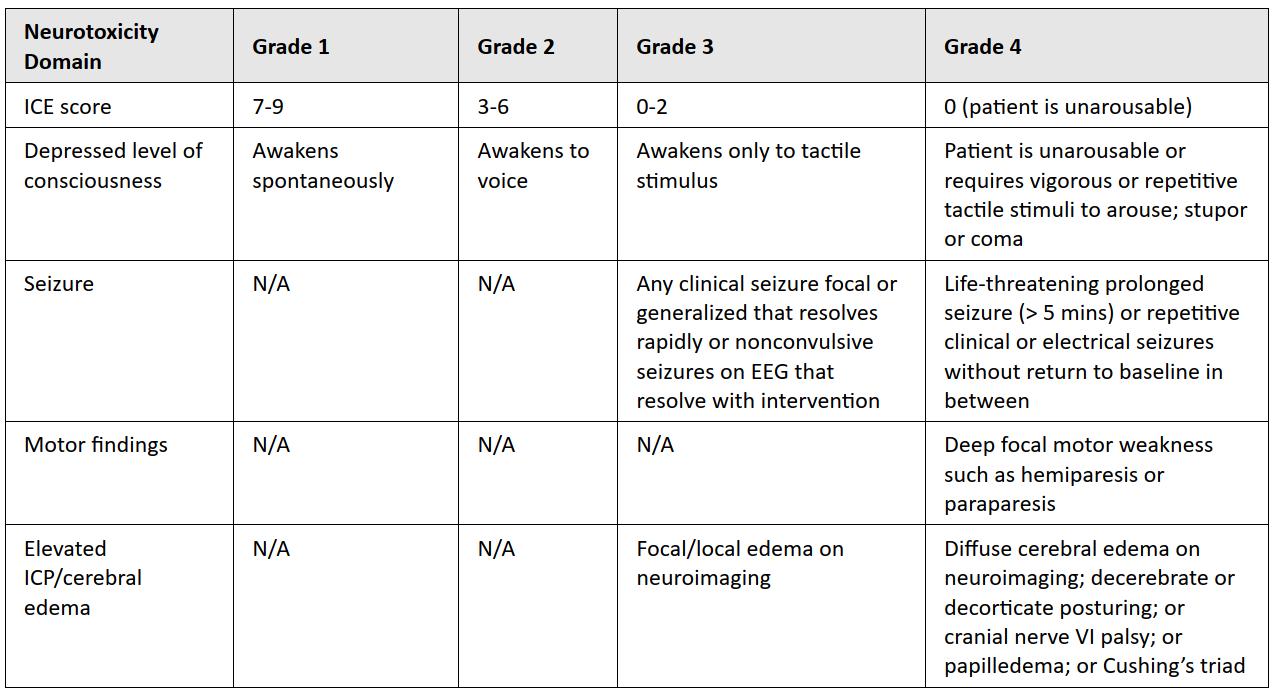
ICANS
Sx of Encephalopathy
headache, impaired consciousness, delirium, dysphasia, tremor, ataxia, myoclonus, seizure, ↑ICP, cerebral edema, and motor Sx
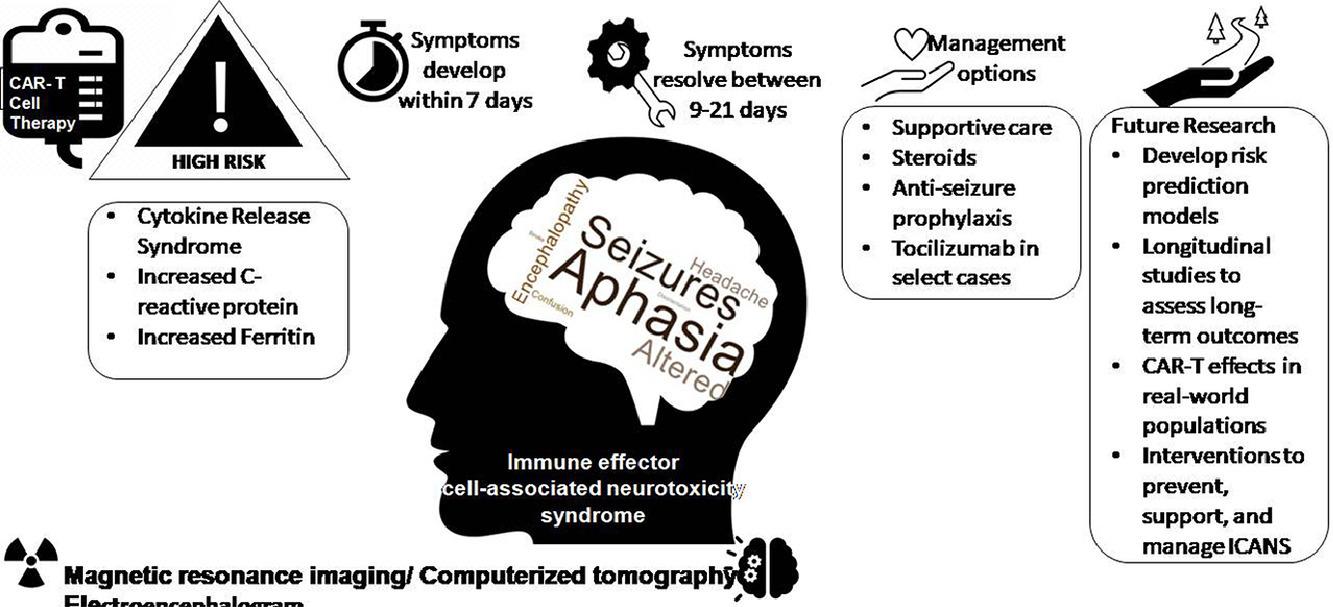
• ICANS can develop in 7 to 10 days and resolves within 21 days after chimeric antigen receptor T cell infusion.
• Common features of ICANS include aphasia, seizure, headache, and encephalopathy.
• Risk factors include cytokine release syndrome, C-reactive protein, and ferritin.
• Management includes supportive care, steroids, antiseizure prophylaxis, and tocilizumab
and Neurotoxicity: Time to Onset & Duration by CAR T-Cell or Bispecific Antibody
Bispecific antibodies with initial step-up dosing, followed by every 1–2-week dosing based on agent; median onset times are from last dose given

ICANS
ASTCT Grading for CRS & ICANS
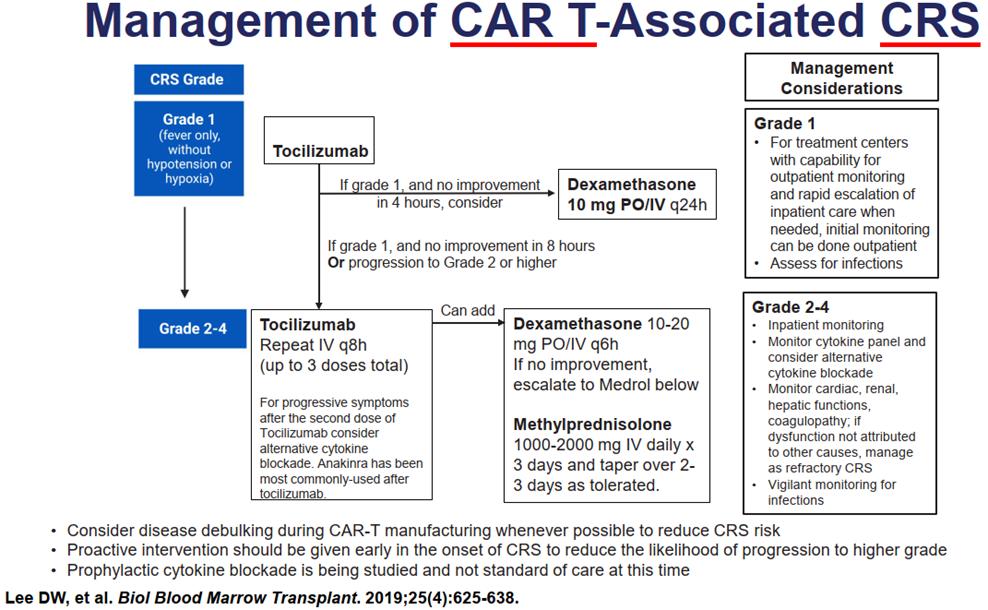
1) O2: Lo vs Hi-flow, CPAP, BiPAP, intubation
2) Hypotension: r/o sepsis, iv fluid, vasopressin, multiple vasopressors
3) ICU
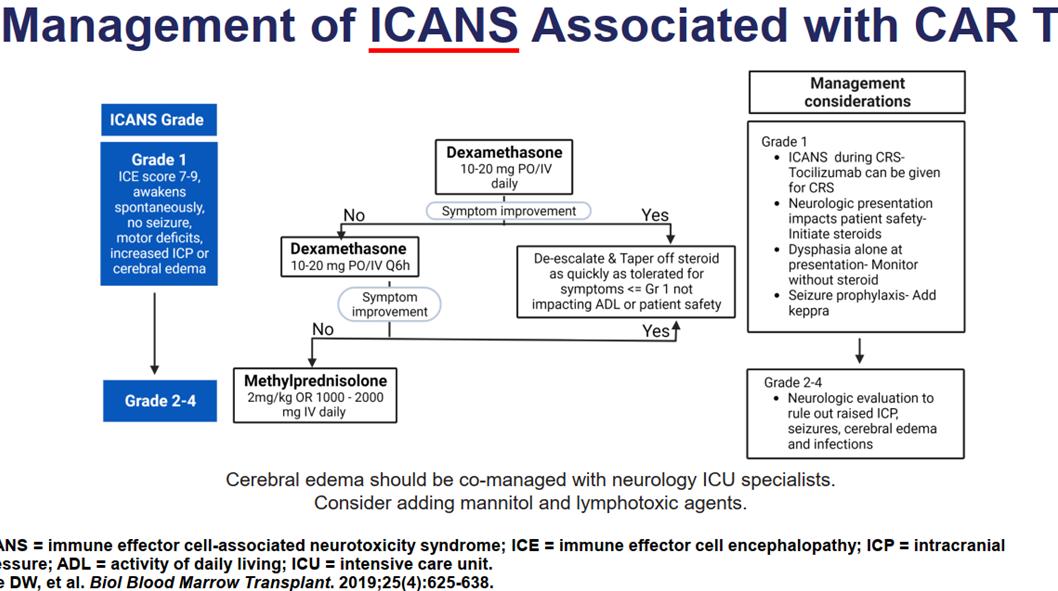
Consult neurology
Seizure prophylaxis
Brain imaging
EEG
Exclude sepsis

Discussion




Adjournment of Day One

Wee Joo Chng, MD
National University Cancer Institute, Singapore,
National University Health System




