
SEATTLE, WA

AUGUST 9, 2025
Thank you to our sponsors!










Welcome & Introductions
Robin Tuohy
Vice President, Patient Support
International Myeloma Foundation
Understanding Myeloma Basics
Anupama Kumar, MD
University of California San Francisco, CA


SEATTLE, WA

AUGUST 9, 2025
Thank you to our sponsors!










Welcome & Introductions
Robin Tuohy
Vice President, Patient Support
International Myeloma Foundation
Understanding Myeloma Basics
Anupama Kumar, MD
University of California San Francisco, CA

(Video) Closing the Gap: Health Disparities in Myeloma
Joseph Mikhael, MD, MEd, FRCPC, FACP, FASCO, Chief Medical Officer, International Myeloma Foundation
Advancing Treatment Options Through Clinical Trials
Andrew Cowan, MD
Fred Hutchinson Cancer Center, Seattle, WA
Q&A with Panel
Coffee Break
Breakout: Frontline or Relapsed Treatment Approaches
-NDMM: Getting Started with Myeloma Management
Anupama Kumar, MD
-RRMM: Continuing the Myeloma Treatment Journey
Andrew Cowan, MD
Presentation Slides: Are available by scanning the QR code, Instructions are on the QR code handout on each table.
Program Evaluations: evaluations at the end of today.
Restrooms: Bathrooms are located upstairs on the Mezzanine level, and a code is required.
Men: 4550
Check in with IMF staff at welcome desk for the code as needed.
Parking: If you have parked at the hotel today, please check in with an IMF team member at the registration desk, our team will provide a voucher to provide to the valet team.

Badge Holders: Please return your badge holders and we can recycle them.
We greatly appreciate your time and feedback!






S. Vincent Rajkumar, MD IMF Board Chair


Thomas Martin, MD
UCSF, Helen Diller Family Comprehensive Cancer Center


Wee Joo Chng, MD
National University of Singapore


María-Victoria Mateos, MD, PhD University of Salamanca


Vania Hungria, MD, PhD Santa Casa de São Paulo


Joseph Mikhael, MD, MEd, FRCPC, FACP IMF Chief Medical Officer


Sigurður Yngvi Kristinsson, MD, PhD University of Iceland


Philippe Moreau, MD University Hospital of Nantes


Shaji Kumar, MD Mayo Clinic


NIkhil Munshi, MD Dana-Farber Cancer Institute


Jesús San Miguel, MD, PhD University of Navarra


Sagar Lonial, MD, FACP
Winship Cancer Institute, Emory University


Saad Zafar Usmani, MD, MBA, FACP, FASCO
Memorial Sloan Kettering Cancer Center
Shared Experiences Help to Better Understand the Myeloma Journey
• Support Groups empower patients & care partners with information, insight & hope
• The IMF provides educational support to a network of over 150 myeloma specific groups

150+ US Support Groups
Over 200 Support Group Visits/year







Meets mostly virtually on the 4th Saturday of each month at 10:00AM Pacific Time
Northwest MM Fighters Care Partners Support Group
Meets virtually on the 1st Saturday of each month at 10:00AM Mountain Time



Special interest groups are designed as a supplemental support for specific populations of patients, in addition to their local Support Groups
MM Families
Founded in 2021
For patients & care partners with young children
Las Voces de Mieloma
Founded in 2022
For Spanish speaking patients & care partners
Living Solo & Strong
Founded in 2022
For patients without a care partner

Click here for more inf
Smolder Bolder
Founded in 2023
For smoldering myeloma patients & care partners

Veterans SIG
Founded in 2025
For those who served our country
High Risk Multiple Myeloma
Founded in 2023
For high-risk myeloma patients & care partners
Care Partners Only
Founded in 2024
For myeloma care partners only

Seamlessly matching patients to the latest clinical trials.
• Discover clinical trials tailored to your myeloma journey with the IMF.
• Filter by diagnosis, treatment, and location to find the best fit for you.
Clinical Trials Matching Engine Usage


















































• Boca Raton, FL – March 14 – 15
• Philadelphia, PA – May 2 – 3
• Los Angeles, CA – August 15 – 16
• Chicago, IL – October 3 – 4

• Virtual - March 4
• San Mateo, CA - March 29
• Atlanta, GA - April 5
• Edina, MN - April 26
• Denver, CO - June 21
• Virtual – July 29
• Seattle, WA - August 9
• Waltham, MA - September 27
• Raleigh-Durham, NC - November 15
• Virtual – November 18

1. Ensure Access to Care: We advocate to ensure all myeloma patients have equitable, comprehensive, patient-centered care without insurance barriers that limit options or delay treatment initiation.
2. Eliminate Financial Barriers: We advocate for policies that allow myeloma patients access to treatments and supportive care interventions without facing financial hardships.
3. Advance Myeloma Research: We advocate for annual appropriations funding for myeloma research and the advancement of clinical trial eligibility and research protocols that ensure representation from diverse populations.

The IMF Grassroots Advocacy Program is multi-faceted and growing
• Advocacy Training & Leadership Development
• Policy and Legislative Education
• Grassroots Campaign Planning
• Health Policy Forums & Roundtables
• Advocacy Resource Development
• Storytelling and Personal Narratives






Anupama Kumar, MD
University of California San Francisco, CA



Anupama Kumar, MD
August 9, 2025


• You didn’t do anything wrong!
• Error in DNA of plasma cell leads to abnormal plasma cell
• Abnormal plasma cells can multiply until they reach a “critical threshold” and cause symptoms
• Generally NOT carried in families
• Age – median age is late 60s
• Usually there is no identifiable exposure
• Obesity
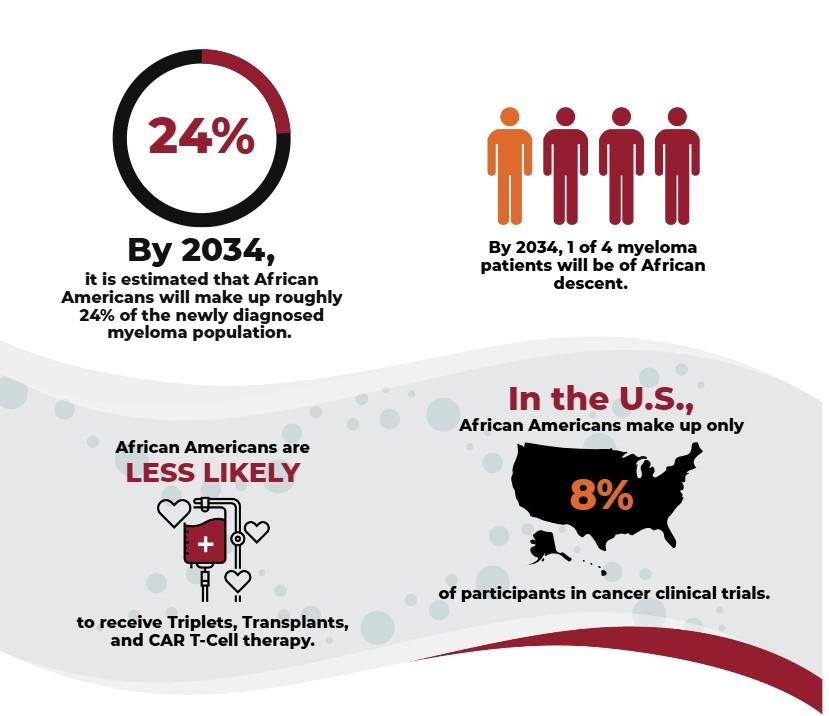
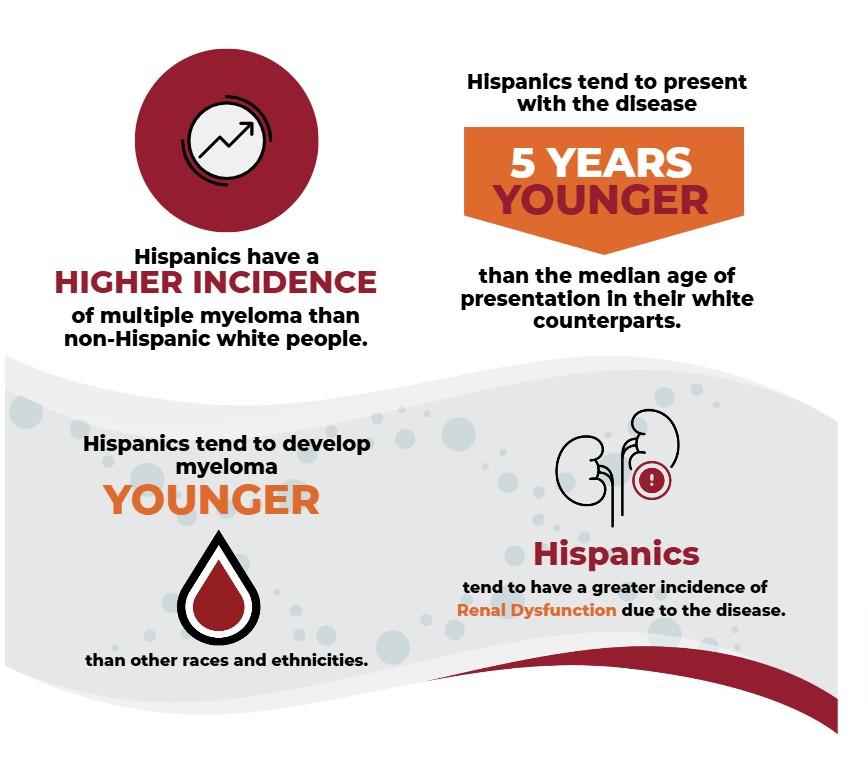
• Race/ethnicity
• 1 in 5 patients with myeloma are African American
• Higher incidence in Hispanic patients

MGUS has a 1% risk of progression per year
Smoldering myeloma has a 10% risk of progression per year
• Fatigue
• Weight loss
• Bone pain, fractures
• Cord compression – numbness, weakness
• Kidney failure – decreased urination, bubbly/frothy urine
• “Incidental” diagnosis – workup by hematologist, primary care, nephrologist, endocrinologist, neurologist
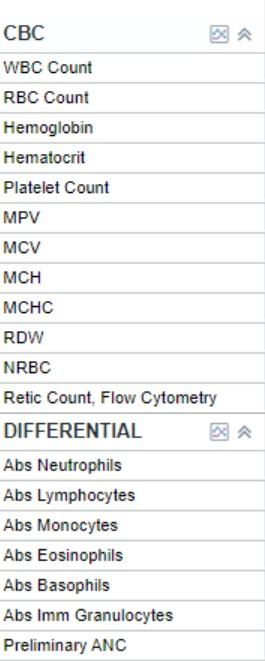
• Blood tests
• Complete blood count
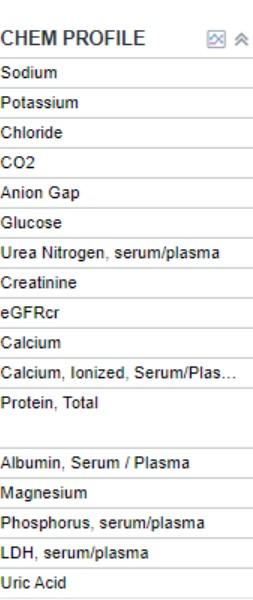
• Comprehensive metabolic panel
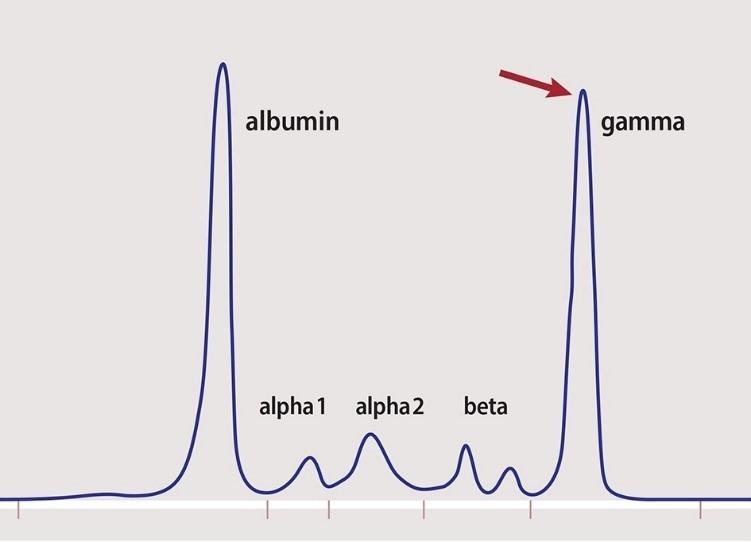
• SPEP
• Immunofixation
• Free light chains (kappa, lambda)
• Immunoglobulins (IgG, IgA, IgM)
• Beta 2 microglobulin, LDH, albumin
• Blood tests
• Complete blood count
• Comprehensive metabolic panel
• SPEP
• Immunofixation
• Free light chains (kappa, lambda)
• Immunoglobulins (IgG, IgA, IgM)
• Beta 2 microglobulin, LDH, albumin

• Blood tests
• Complete blood count
• Comprehensive metabolic panel
• SPEP
• Immunofixation
• Free light chains (kappa, lambda)
• Immunoglobulins (IgG, IgA, IgM)
• Beta 2 microglobulin, LDH, albumin

• Blood tests
• Complete blood count
• Comprehensive metabolic panel
• SPEP
• Immunofixation
• Free light chains (kappa, lambda)
• Immunoglobulins (IgG, IgA, IgM)
• Beta 2 microglobulin, LDH, albumin

• Blood tests
• Complete blood count
• Comprehensive metabolic panel
• SPEP
• Immunofixation
• Free light chains (kappa, lambda)
• Immunoglobulins (IgG, IgA, IgM)
• Beta 2 microglobulin, LDH, albumin
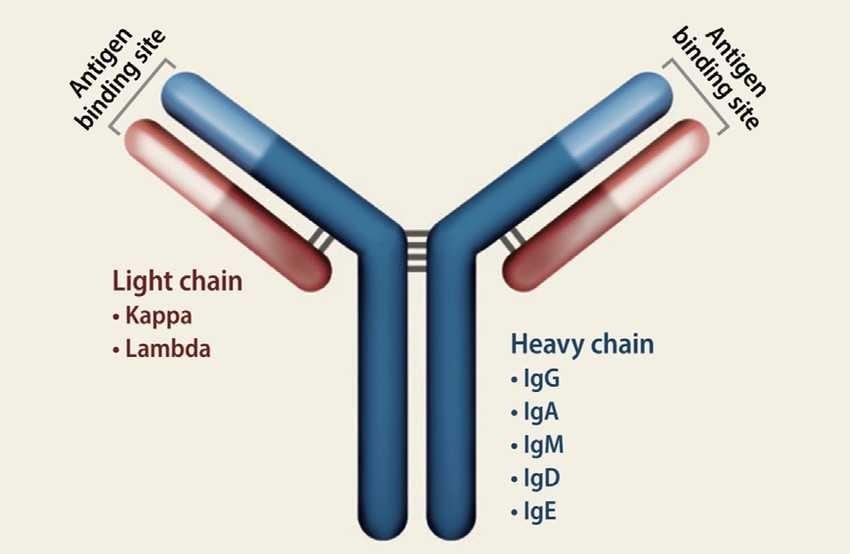
• Blood tests
• Complete blood count
• Comprehensive metabolic panel
• SPEP
• Immunofixation
• Free light chains (kappa, lambda)
• Immunoglobulins (IgG, IgA, IgM)
• Beta 2 microglobulin, LDH, albumin
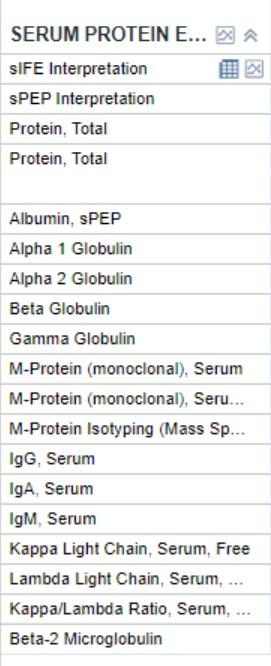

• SPEP/M-spike = 0.2
• IFE = IgG kappa
• This patient has IgD lambda MM
• How did this happen?

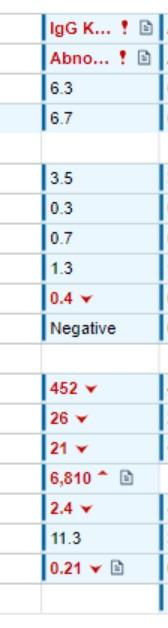
• SPEP/M-spike = 0.2
• IFE = IgG kappa
• This patient has IgD lambda MM
• The patient is on daratumumab


• Monoclonal antibodies (daratumumab/isatuximab) can cause a small M-spike, corresponding to IgG kappa
• Special tests, using mass spectrometry, can differentiate

• Urine tests – “random” or 24 hour
• Total protein
• UPEP
• UIFE
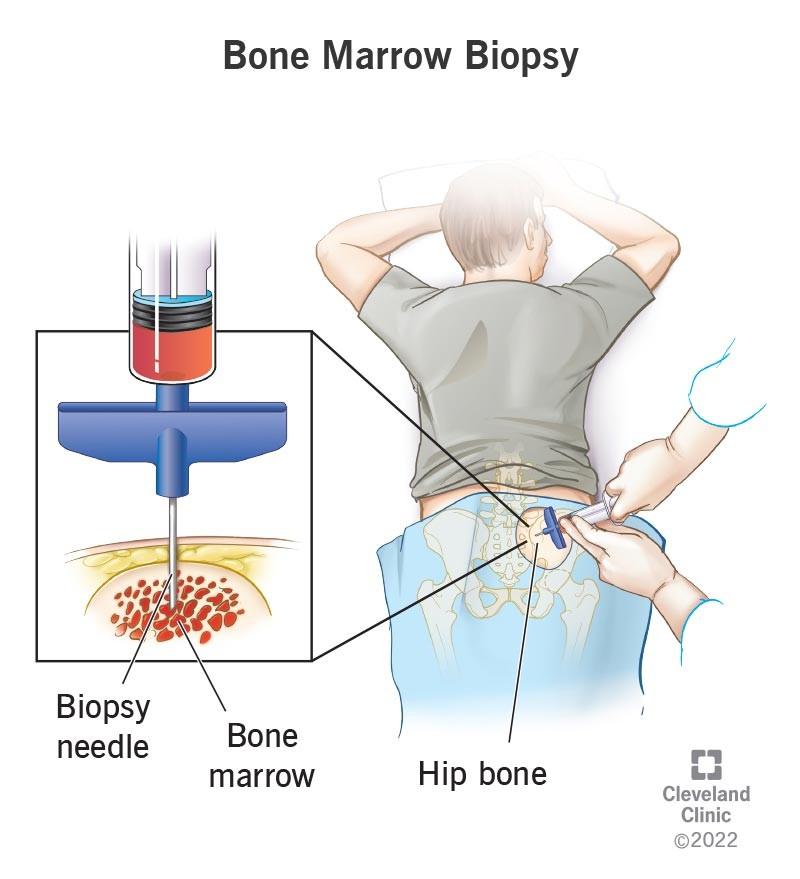
• Bone marrow biopsy
• Imaging
• X-rays
• CT skeletal survey
• PET/CT scan
• MRI
• Urine tests – “random” or 24 hour
• Total protein
• UPEP
• UIFE
• Bone marrow biopsy
• Imaging
• X-rays
• CT skeletal survey
• PET/CT scan
• MRI


• Urine tests
• Spot or 24 hr protein, UPEP, UIFE
• Bone marrow biopsy
• Imaging
• X-rays
• CT skeletal survey
• PET/CT scan
• MRI
Morphology


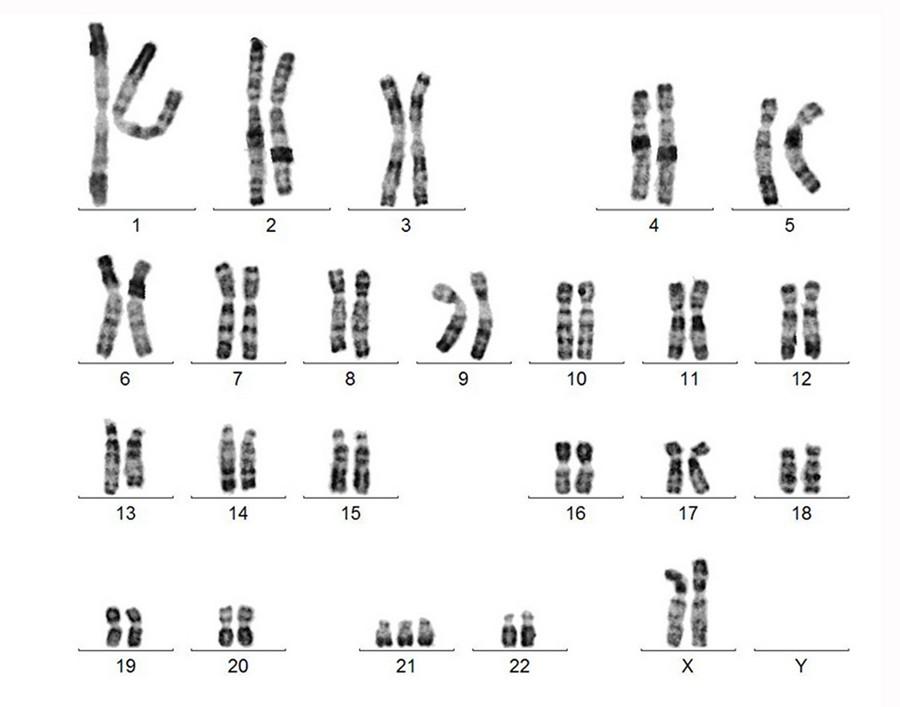
Karyotype FISH
Van De Donk et al. (2023), https://doi.org/10.1038/sj.leu.2402768
• MRD = minimal residual disease.
• MRD negative = no residual myeloma
• 2 methods:
• NGS (ClonoSEQ) – precise to 10-6, need original “ID” specimen

• Flow cytometry – precise to 10-5
• Urine tests
• Spot or 24 hr protein, UPEP, UIFE
• Bone marrow biopsy
• Imaging
• X-rays
• CT skeletal survey
• PET/CT scan
• MRI


PET/CT scan with diffuse (“salt and pepper”) involvement throughout bone marrow (A, B) and focal lesions at L humerus (C, D) and L sacrum (E, F)
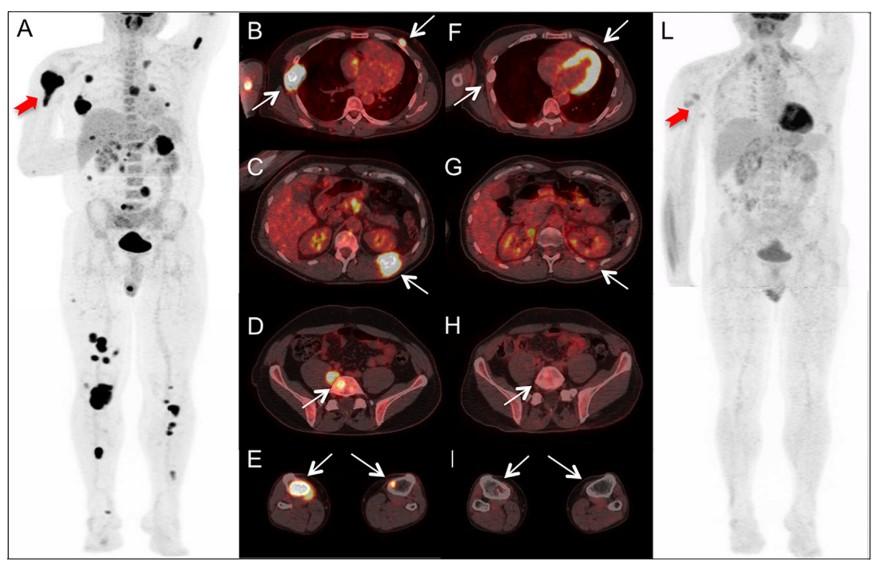
BEFORE treatment AFTER treatment
Rubini et al. (2014), https://doi.org/10.1016/j.critrevonc.2016.03.006
MGUS Smoldering myeloma Early Active Myeloma Multiple Myeloma
<10% myeloma cells on bone marrow
No SLiM-CRAB features 10-60% myeloma cells on bone marrow No SLiM-CRAB features
Presence of any “SLiM” features:
• ≥60% myeloma cells in bone marrow
• Involved/uninvoled light chain >100
• MRI with >1 lesion ≥5 mm
Presence of any “CRAB” features:
• Ca >11
• Cr>2 or CrCl<40
• Hgb <10
• ≥1 lytic lesion by skeletal survey, CT, or PET
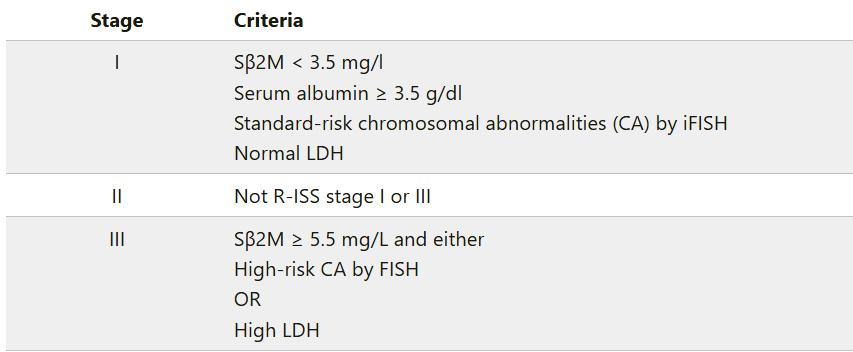
International Staging System (ISS)

Revised International Staging System (RISS)
Only del 17p, t(4;14) or t(14;16) included in this definition
• Translocation t(4;14), t(4;16), or t(14;20)
• Gain/amp of 1q
• Deletion of 1p32
• deletion 17p (>20%)
• TP53 mutation
• Biallelic deletion of 1p32,
• Beta-2 microglobulin >5.5 plus creatinine <1.2
• Plasma cell leukemia
• >5% plasma cells in blood
• High disease burden
• Functional high-risk

• Depth of response
• Partial response: 50% improvement in M-spike
• Very good partial response: 90% improvement in Mspike
• Complete response: M-spike and IFE negative, bone marrow with <5% myeloma cells
• Stringent complete response: CR + light chains normal
• MRD- stringent complete response
• Response generally deepens over the course of treatment
• The deeper the response, the longer the remission
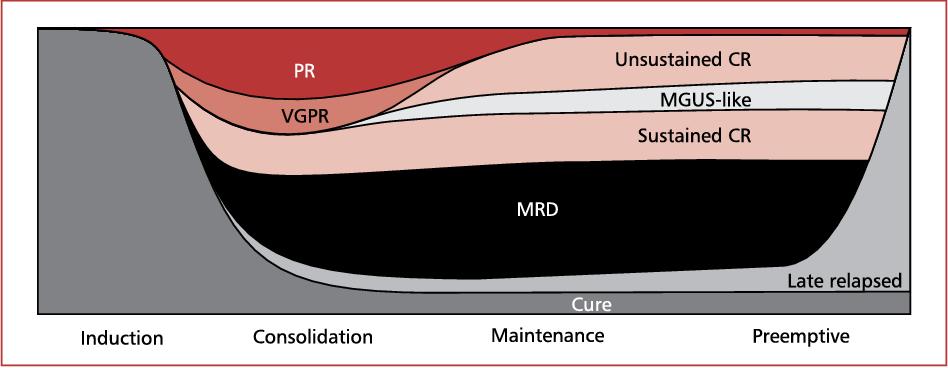
• PFS = progression free survival
• How long a patient stays in remission before having to switch to a different treatment
• OS = overall survival
A Kaplan-Meier curve
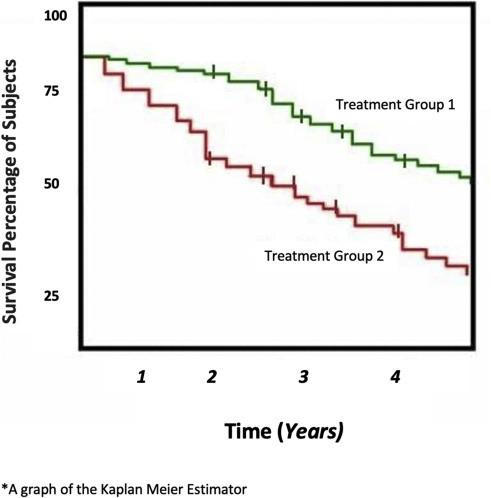
1. To prolong survival
2. To prevent permanent organ damage
3. To reduce symptoms (pain, nerve damage, etc.)
4. To cure patients

Puertas (2023), http://doi:10.3390/cancers15051558

Shah et al. (2021), http://doi:10.3390/cancers15051558
Induction – initial chemotherapy +/- transplant
Consolidation – similar to induction, given after transplant
Maintenance – less intensive phase of treatment
Proteosome Inhibitors IMiDs Monoclonal Antibodies Alkylators
Bortezomib (Velcade)
Ixazomib (Ninlaro)
Carfilzomib (Kyprolis)
Lenalidomide (Revlimid)
Pomalidomide (Pomalyst)
Daratumumab (Darzalex)
Isatuximab (Sarclisa)
Elotuzumab (Empliciti)
• Proteosome inhibitors. Proteosomes recycle protein. When proteosomes are inhibited > extra protein builds up > toxic to cell > cell dies
• IMiDs directly kill cancer cells and alter your immune system to better fight cancer
• Monoclonal antibodies target a specific marker, such as CD38 on plasma cells
• Alkylators alter the DNA by adding an alkyl group, inhibiting DNA/RNA replication and leading to cell death.
• Melphalan chemotherapy
• Reinfusion of your own stem cells (autologous)
• Typically done after initial “induction” chemotherapy

Target BCMA GPRC5D FcRH5
Bispecifics Teclistimab Elaranatamb Talquetamab In trials CAR-T Idecel Ciltacel In trials In trials


• Mental health is important and often overlooked!
• Support
• Friends/family
• Support groups
• Find a mentor
• Understand your medical team
• Doctors - Oncologist, radiation oncology, surgeon, nephrologist
• Nurse, transplant coordinator, MA
• Social work
• Nutrition
• Palliative care

• Patients, caregivers
• UCSF myeloma team – MDs, RNs, BMT coordinators, social work, nutrition, psychology

Joseph Mikhael, MD, MEd, FRCPC, FACP, FASCO Chief Medical Officer, International Myeloma Foundation


Andrew Cowan, MD
Associate Professor of Medicine
Research Director, Multiple Myeloma
Fred Hutch Cancer Center / UW Medicine



The drive of research has brought us to where we are
No one is expected to be a “guinea pig” with no potential benefit to them
Research is under very tight supervision and standards
Open, clear communication between the physician and the patient is fundamental Driving research forward!





MYTH: If I participate in a clinical trial, I might get a placebo, not active treatment
MYTH: If I participate in a clinical trial, I can’t change my mind
• Phase 1 and 2, everyone gets active treatment
• Phase 3 standard of care vs new regimen: often standard regimen with/without additional agent in MM trials
• Patients can withdraw their consent for clinical trial participation at any time

MYTH: Clinical trials are dangerous because they have new medicines and practices
• Some risk is involved with every treatment, but medicines are used in clinical trials with people only after they have gone through testing to indicate that the drug is likely to be safe and effective for human use



MYTH: Clinical trials are expensive and not covered by insurance
• Research costs are typically covered by the sponsoring company
• Standard patient care costs are typically covered by insurance
• Check with clinical trial team/insurers; costs such as transportation, hotel, etc may not be reimbursed and are paid by patient
PhRMA website. Accessed March 25, 2024. https://phrma.org/-/media/Project/PhRMA/PhRMA-Org/PhRMA-Org/PDF/A-C/CLINICAL-TRIALS-MYTH-FACT-PRINT.pdf?hsCtaTracking=f6689b95-1626-40d9-8c87-c6b 8d31600a4%7C35221aa8-d487-4db3-9416-b9c3c35e3bac
.




Identify a target for therapy in the laboratory
Confirm the anticancer activity in laboratory and animal studies
Clinical trials (human studies) to determine safety, dosing and effectiveness
The whole process costs millions of dollars and years of effort!


Preclinical
ANIMAL STUDIES: Examine safety and potential for efficacy
PHASE 1
PHASE 2
FIRST INTRODUCTION OF AN INVESTIGATIONAL DRUG INTO HUMANS
• Determine metabolism and PK/PD actions, MTD, and DLT
• Identify AEs
• Gain early evidence of efficacy, studied in many conditions; typically, 20 to 80 patients; everyone gets agent
• Determine short-term AEs and risks; closely monitored
• Includes up to 100 patients, typically
PHASE 3

PHASE 4
INFORMATION COMPARED TO STANDARD OF CARE
• Placebo may be involved if no standard of care exists; hundreds to several thousand patients
• Often multiple institutions; single or double blind; sometimes open label
APPROVED AGENTS IN NEW POPULATIONS OR NEW DOSE



Every patient is unique and must be viewed that way
Benefits of trials are numerous and include:
Early access to “new” therapy
Delay use of standard therapy
Contribution to myeloma world – present and future
Financial access to certain agents
Must be balanced with potential risks
“Toxicity” of side effects
Possibility of lack of efficacy



Patients may:
• Be unaware of clinical trials
• Lack access to trials
• Fear, distrust, or be suspicious of research
• Have practical or personal obstacles
• Face insurance or cost problems

• Be unwilling to go against their physicians’ wishes
• Not have physicians who offer them trials
• Have a disconnect with their healthcare team



[P]eople from racial and ethnic minorities and other diverse groups are underrepresented in clinical research. This is a concern because people of different ages, races, and ethnicities may react differently to certain medical products.
– FDA

Leadership and commitment


Community engagement practices
Investigator hiring, training, and mentoring practices



Patient engagement practices

US Cancer Centers of Excellence: Strategies for Increased Inclusion of Racial and Ethnic Minorities in Clinical Trials
FDA = US Food and Drug Administration. Regnante JM, et al. J Oncol Pract. 2019;15(4):e289-e299. FDA website. Clinical Trial Diversity. Accessed March 27, 2024. https://www.fda.gov/consumers/minority-health-and-health-equity/clinical-trial-diversity.



Discuss with your physician if you are eligible for a clinical trial
Work with your physician to determine the best trial for you
Meet with the clinical research nurse or trials coordinator to discuss the trial
Carefully review the provided “Informed Consent” Describes the study and any potential safety concerns related to the experimental medication




How does the study work? How often will I need to see my doctor or visit the cancer center?

Will I need to undergo additional tests?
What is currently known about the new drug or combination?


What benefits can I expect?
What side effects should I expect? Who should I notify if I have side effects?


Can I take my vitamins or other medications?

Can I get the treatment with my local doctor?



Will my insurance pay for my participation in the clinical trial?



Clinicaltrials.gov https://clinicaltrials.gov/




ncreasing-diversity-in-cancer-clinical-research




Presentation Slides: Are available by scanning the QR code, Instructions are on the QR code handout on each table.
Program Evaluations: evaluations at the end of today.
Restrooms: Restrooms are located upstairs across from the elevators.
Badge Holders: Please return your badge holders and we can recycle them.

We greatly appreciate your time and feedback!


PLEASE HEAD TO YOUR SELECTED BREAKOUT SESSION:

BREAKOUT A: NDMM - GETTING STARTED WITH MYELOMA
MANAGEMENT
Dr. Anupama Kumar, MD
Please move to Quimby Room
BREAKOUT B: RRMM - CONTINUING THE MYELOMA TREATMENT JOURNEY
Dr. Andrew Cowan, MD
Please remain in this room

Thank you to our speakers & our sponsors!












Anupama Kumar, MD
August 9, 2025
Proteosome Inhibitors IMiDs Monoclonal Antibodies Alkyalors
Bortezomib (Velcade)
Ixazomib (Ninlaro)
Carfilzomib (Kyprolis)
Lenalidomide (Revlimid)
Pomalidomide (Pomalyst)
Daratumumab (Darzalex)
Isatuximab (Sarclisa)
Elotuzumab (Empliciti)
• Proteosome inhibitors. Proteosomes recycle protein. When proteosomes are inhibited > extra protein builds up > toxic to cell > cell dies
• IMiDs directly kill cancer cells and alter your immune system to better fight cancer
• Monoclonal antibodies target a specific marker, such as CD38 on plasma cells
• Alkylators alter the DNA by adding an alkyl group, inhibiting DNA/RNA replication and leading to cell death.


• Landscape evolving with more use of quad therapy in MM
• Compared 4 drug combination to 3 drug combination
• Daratumumab + Revlimid + velcade + dexamethasone (DRVd)
• Revlimid + velcade + dexamethasone (RVd)
• Same drug regimen as the GRIFFIN trial, but in more patients (n=709) and international
• Primary endpoint: progression free survival
• Patient population
• Age 18-70 years
• Transplant eligible
• ECOG 0-2
Cycle Length = 28 days
D: 1800 mg SC weekly C1-2. Q2 weeks C3-6
V: 1.3 mg/m2 D1, 4, 8, 11
R: 25 mg PO D1 – 21
d: 40 mg PO/IV D1-4, 9-12
DRVd x 4 cycles
DRVd x 2 cycles
DR > R alone if MRD-
RVd x 4 cycles
RVd x 2 cycles
R until PD or AE
What is different from GRIFFIN?
• Cycle length 28 days (vs. 21 days)
• MRD-guided approach for DR duration (vs. fixed 2 years of dara)
• Dexamethasone pulses
• Those who had DRVd had deeper remission
• MRD rate got better over time
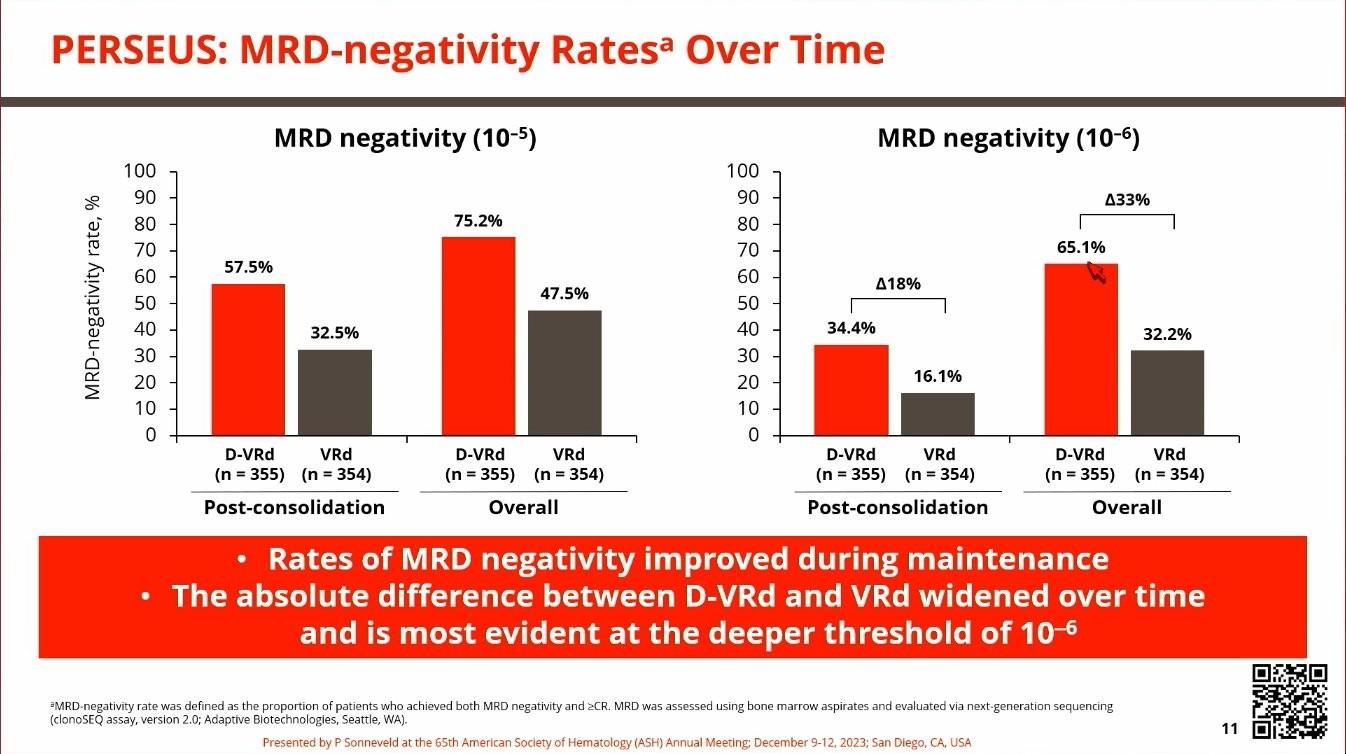
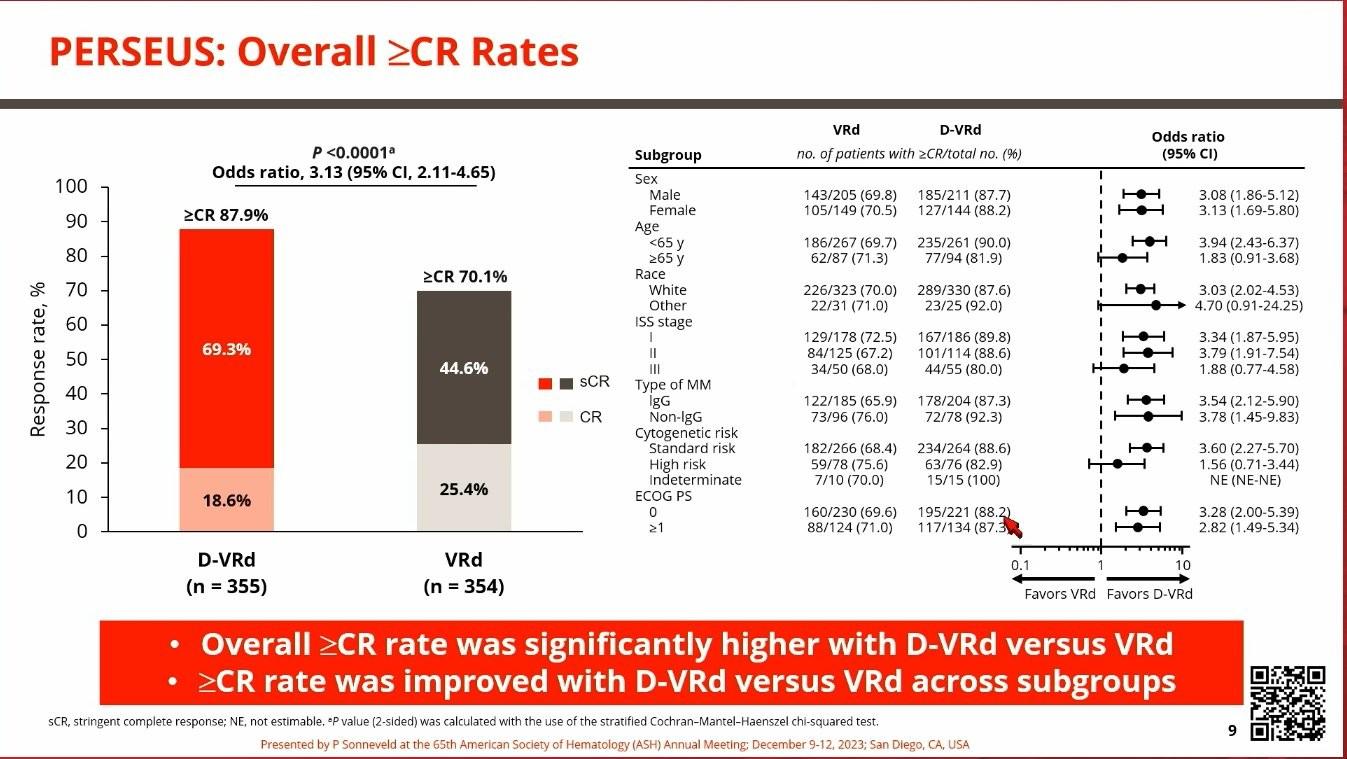


• After a median of 48 months, those who received DRVd had improved PFS
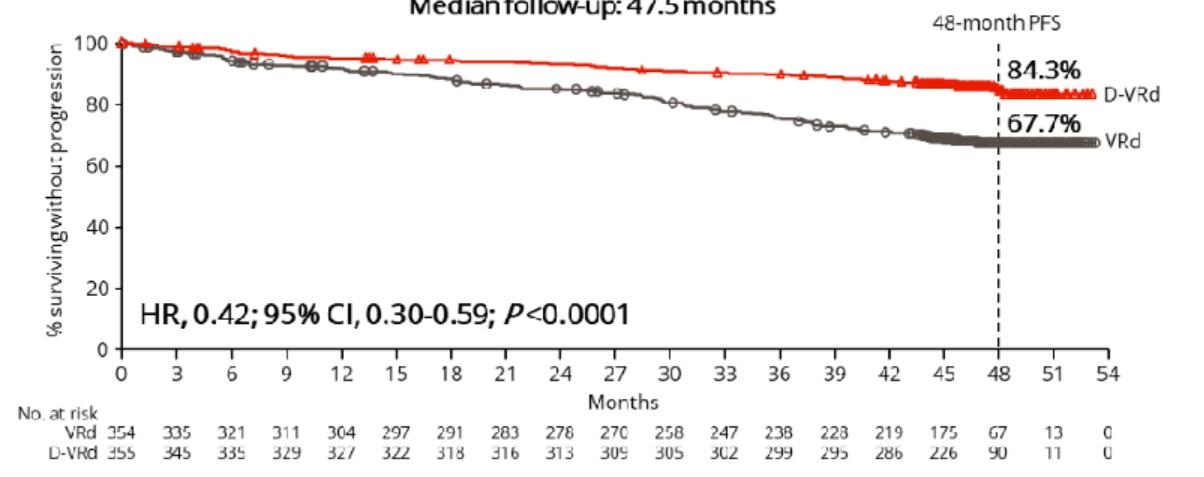
• It is too early to make any conclusion about survival

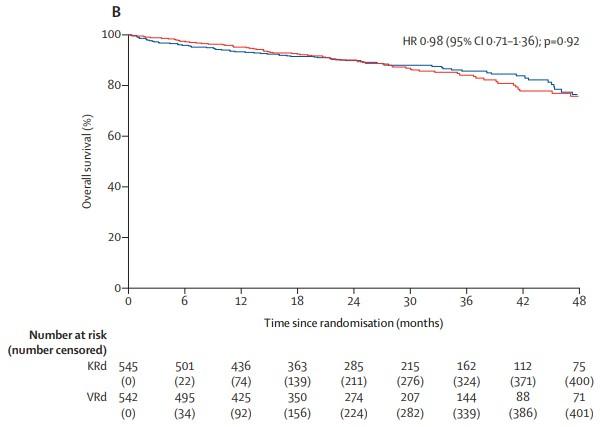
• Compared two different 3 drug regimens
• Kyprolis + revlimid + dexamethasone (KRd)
• Revlimid + velcade + dexamethasone (RVd)
• High-risk patients not included
• Carfilzomib associated with less neuropathy, but more shortness of breath, high blood pressure, rare heart failure

• Compared 4 drug regimen to 3 drug regimen
• Isatuximab + kyprolis + revlimid + dexamethasone (Isa+KRd)
• Kyprolis + revlimid + dexamethasone (KRd)
• Primary endpoint: MRD negativity rate
• Patient Population
• Age 18-70 years
• Transplant eligible
N= 151 N=151
INDUCTION
IsaKRd x 4 cycles
KRd x 4 cycles
Standard Induction/Consolidation, Cycle: 28 days
Isa: 10 mg/kg IV days 1, 8, 15, 22 in C1, 10 mg/kg IV days 1,15 in C2-4
K: 20/56 IV mg/m2 days 1, 8, 15
R: 25 mg PO days 1-21
d: 40 mg PO days 1, 8, 15, 22
IsaKRd x 4 cycles
Isa-KRd x 12 cycles
IsaKRd x 4 cycles
KRd x 12 cycles
Light consolidation, Cycle: 28 days
Isa: 10 mg/kg IV day 1
K: 56 IV mg/m2 days 1, 15
R: 10 mg PO days 1-21
d: 20 mg PO days 1, 8, 15, 22
• Median follow-up: 20 months (short!)
• MRD negativity at 10-6 improved over time
• 27% vs 14% after induction
• 52% vs 27% after transplant
• 67% vs 48% after consolidation
• PFS data not yet available
• High-risk myeloma accounted for 35% of patients
• DKRd transplant DKRd (4-8 cycles) lenalidomide
• Uses MRD guided approach (BMBx after each phase)
• If MRD- at 2 timepoints stopped further therapy
• 80% reached MRD- at 10-5
• 71% had 2 MRD and came off treatment
• PFS 87% at 2 years
• Should be a personalized decision
• Factors to consider
• Age
• Genetic features of your myeloma
• Overall fitness – Are you transplant candidate?
• Other medical problems
• Side effects
• If pre-existing neuropathy, be careful about velcade
• If pre-existing heart failure, be careful about kyprolis
• Convenience – route of administration (pill vs IV vs shot), # of infusion center visits required
• You don’t need to decide on day 1 of induction!
• We do a full assessment of your organ function to make sure you are healthy enough to get a transplant
• Transplant involves a ~2+ week stay in the hospital
• Transplant side effects – low energy, nausea, vomiting, diarrhea, mucositis, infection, low blood counts
• Need time off work and caregiver support
• 3 month recovery

• Transplant is considered standard of care in patients who are eligible after induction
• The DETERMINATION trial showed a significant PFS benefit
• In high-risk, PFS: 4.5 years in transplant vs 1.5 years in no-transplant

5.5 vs 3.8 yrs remission

• This should be a personalized decision
• Factors to consider
• Age
• Overall fitness
• Genetic features of your myeloma
• Other medical problems
• Organ function
• Tolerance to induction
• Response to induction
• If unsure, strongly consider banking your stem cells for later use
• IsaKRd induction x 6 cycles
• If MRD- at -105, randomized 1-to-1:
• Transplant + IsaKRd x 2 cycles
• IsaKRd x 6 cycles
• If MRD+ at -105, randomized 1-to-1:
• Transplant + IsaKRd x 2 cycles
• Tandem transplant)
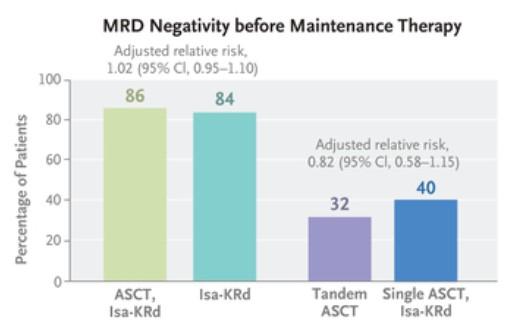
• In those who were MRD negative after induction, subsequent MRD negative rates after transplant vs additional IsaKRd consolidation were about the same
• We may be able to avoid transplant in MRD negative patients
and for how long?
• Maintenance revlimid prolongs survival, many years down the line
Revlimid vs placebo, IFM trial
PFS 3.5 years vs 2 years, HR=0.51

Revlimid vs placebo, CALGB 100104
Patients who got revlimid lived 3 years longer

• May be a preferred strategy in patients who are high-risk and/or MRD+
• Fixed-duration
• GRIFFIN gave revlimid indefinitely, + daratumumab for 2 years
• MRD-adapted
• Currently under investigation (PERSEUS, DRAMMATIC)
• Age – avoid if >70-75 yrs?
• Co-morbidities/organ function
• EF ≥40%, DLCOcorrected ≥40%, AST/ALT/ALK ≤3x ULN, TB≤2.0
• Tolerance of induction
• Performance status
How do we optimize care for those who are transplantineligible (TI?)

• RVd vs Rd without intent for immediate transplant
Both PFS and OS improved with VRd vs Rd, when adjusting for age
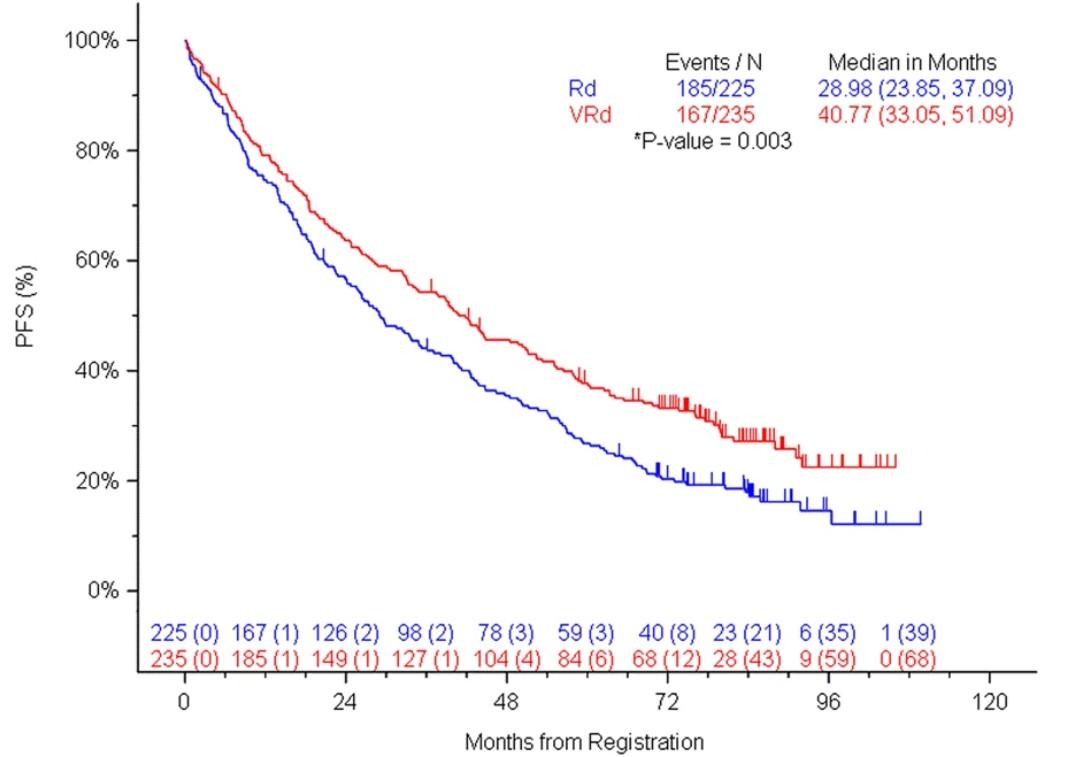

RVd, in pts age ≥ 65 yrs and/or transplant-ineligible
VRd (35d cycles) X 9 Rd (28d cycle) x 6
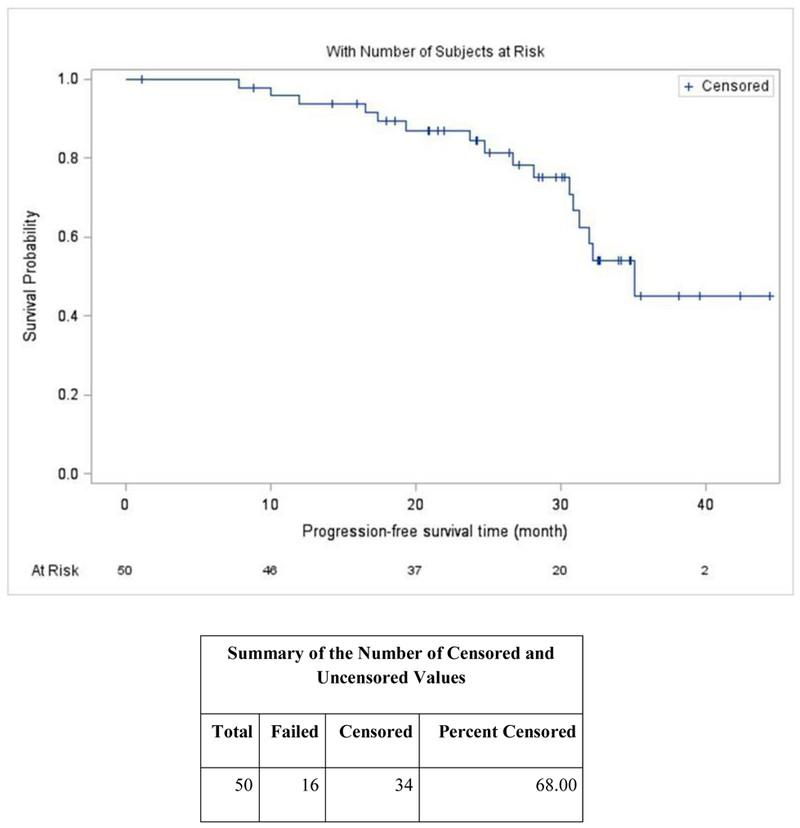


• Intended for older adults (65+) or those with other medical problems
• PFS 5 years (61.9 months) in DRd group
• PFS 3 years (34.4 months) in Rd group
• 32% MRD- in DRd group
• 11% MRD- in Rd group Weisel et al. (2023),
Great outcome!
Ph3, DRd vs Rd, in transplant-ineligible (age ≥ 65 yrs and/or co-morbidities)

Botzeomib-sparing regimen with lower rates of neuropathy!
DRd: 28% G1-2 neuropathy, 2% G3 neuropathy
Rd: 18% G1-2-neuropathy, <1% G3 neuropathy
Facon et al, 2020, Lancet Oncology ,
Ph3, IsaVRd vs VRd in transplant-ineligible
Induction
IsaRVd (6wk cycles) X 4 cycles vs
RVd (6wk cycles) x 4 cycles
• Primary endpoint:
• PFS
• Secondary endpoints:
• OS, PFS2, ORR, CR, safety, QOL, MRD
Ongoing Treatment
IsaRd (4wk cycles ) until PD vs Rd (4wk cycles) until PD, then crossover to IsaRd


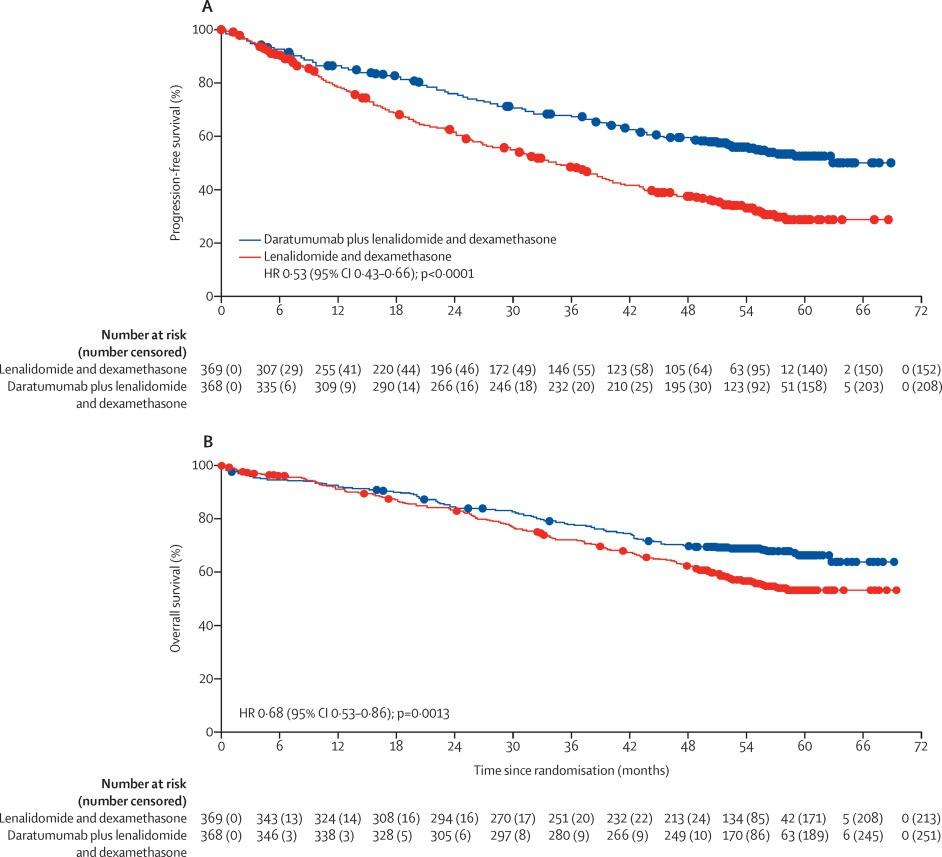
Ph3, IsaVRd vs VRd in transplant-ineligible

Estimated OS @ 60 months: 77.3% with IsaVRd vs 66.3% with VRd
Ph3, IsaVRd vs IsaRd in transplant-ineligible
IsaVRd (28d cycles) X 12 vs.
IsaRd (28d cycles) x 12
IsaVR (28d cycles) X 6 vs. IsaR (28d cycles) x 6
Primary endpoint: - MRD (at 10-5) @ 18 months
Secondary endpoints: - CR rate, MRD- CR rate, VGPR+ rate, PFS, OS, safety
until PD
Ph3, IsaVRd vs IsaRd in transplant-ineligible



At median of 23.5 months f/u, PFS and OS data still immature
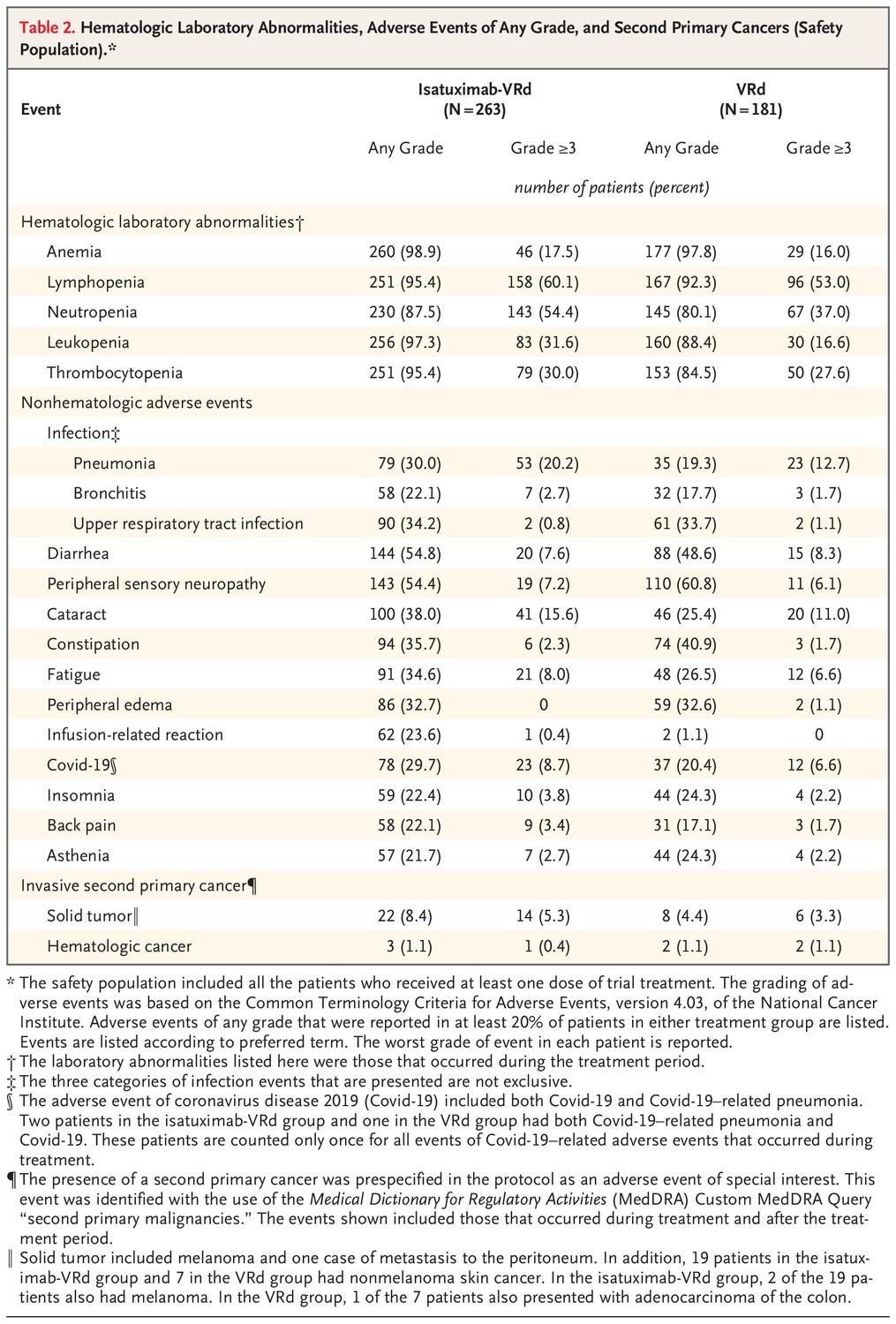
• Daratumumab/isatuximab – infusion reaction, decreased ability to make antibodies (infection)
• Lenalidomide – rash, diarrhea, cramping, low blood counts, blood clots, teratogenic, secondary cancer
• Bortezomib – neuropathy, GI toxicities, styes
• Carfilzomib – fever, shortness of breath, nausea, high blood pressure, heart failure
• Long-term dex associated with side effects, especially in elderly
• French trial compared revlimid + dexamethasone (Rd) to daratumumab + revlimid (DR)
• DR group had better response (ORR 89% vs 77%), quicker response (5 months vs 11 months to get to VGPR), and more MRD negativity (33% vs 18%)
Manier et al. (2022),
• Bone strengtheners
• Zoledronic acid
• Denosumab
• Calcium/vitamin D
• Infection prevention
• Acyclovir/valacyclovir – prevent VZV infections
• Trimethoprim sulfamethoxazole, atovaquone, dapsone – prevent certain types of pneumonia (PJP)
• IVIG – if IgG <400 + frequent infections
• Blood clot prevention
• Aspirin
• Apixaban, rivaroxaban, etc

• Patients, caregivers
• UCSF myeloma team – MDs, RNs, BMT coordinators, social work, nutrition, psychology


Andrew Cowan, MD
Associate Professor of Medicine
Research Director, Multiple Myeloma
Fred Hutch Cancer Center / UW Medicine

Discuss an approach to treating relapsed myeloma based on patient, disease and treatment characteristics
Review the important trend of using an aggressive approach in early treatment of myeloma
Outline the key results from recent trials in early relapse
Discuss the approach to late relapse and the use of novel therapies such as CAR T and bispecific antibodies




• It is not a simple algorithm of treatment #1 then 2 then 3…
• Leverage the benefit of multiple mechanisms of action in combination therapy
Categories:
• 1-3 prior lines
• Later Relapse
• Refractory to PI, IMiD and MoAb = Triple Class Refractory



What is relapsed/refractory disease and a line of therapy?
• Relapsed: recurrence (reappearance of disease) after a response to therapy
• Refractory: progression despite ongoing therapy
• Progression: change in M protein/light chain values
• Line of therapy: change in treatment due to either progression of disease or unmanageable side effects
• Note: initial (or induction) therapy + stem cell transplant + consolidation/ maintenance therapy = 1 line of





Disease-Related
• Nature of the relapse
– Biochemical vs symptomatic
• Risk stratification
– High-risk chromosomal abnormalities: del(17p), t(4;14), t(14;16)
• Disease burden
• Previous therapies
• Prior treatmentrelated adverse event
• Regimen-related toxicity
• Depth and duration of previous response
• Cost to patient
Patient-Related
• Renal insufficiency
• Hepatic impairment
• Comorbidities
• Preferences
• Social factors
– Support system
– Accessibility to treatment center
– Insurance coverage



RVD+ASCT+Lenalidomide Maintenance
Relapse 2
Relapse 1
Time: Years! D i s e a s e A c t i v i t y
Relapse 1
Relapse 2
Relapse 3
Relapse 1


1. Therapies change over time— clinical trials make this possible 2. Outcomes (e.g. survival) changes over time, as new therapies emerge
3. If one therapy did not work well, it doesn’t mean another won’t
4. Terms like “overall survival” and “progression free survival” typically refer to statistical probabilities for GROUPS of people and do not seal the fate of an INDIVIDUAL

5. At a given point in time, there may not be a known “right answer;” hence many opinions






Modified from: Shah A, Mailankody S. BMJ 2020; 370



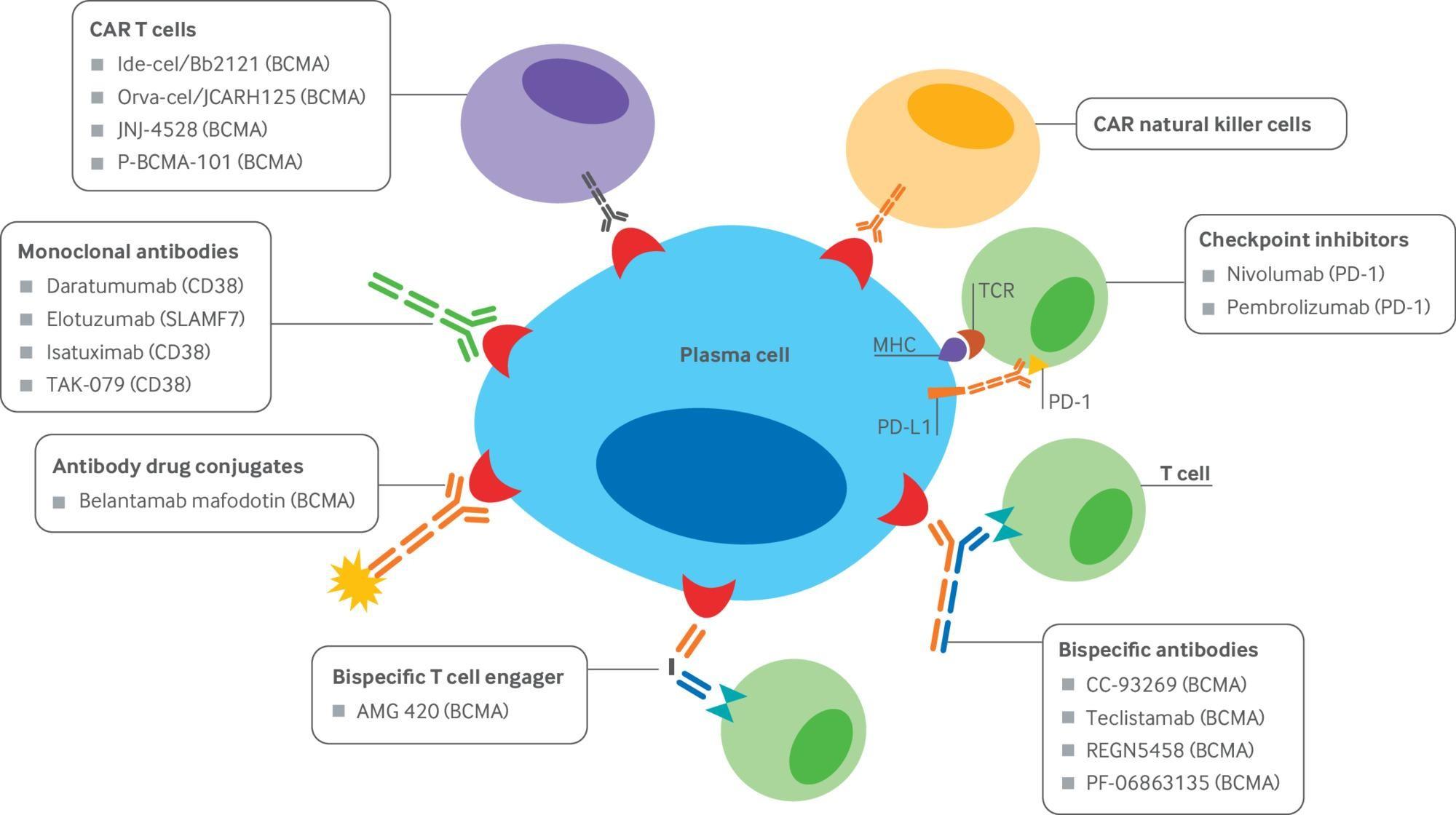
Examples:
Cancer cell




Examples:
1. Belantamab mafodotin (Blenrep) — recognizes BCMA





Progression free Survival Overall Survival
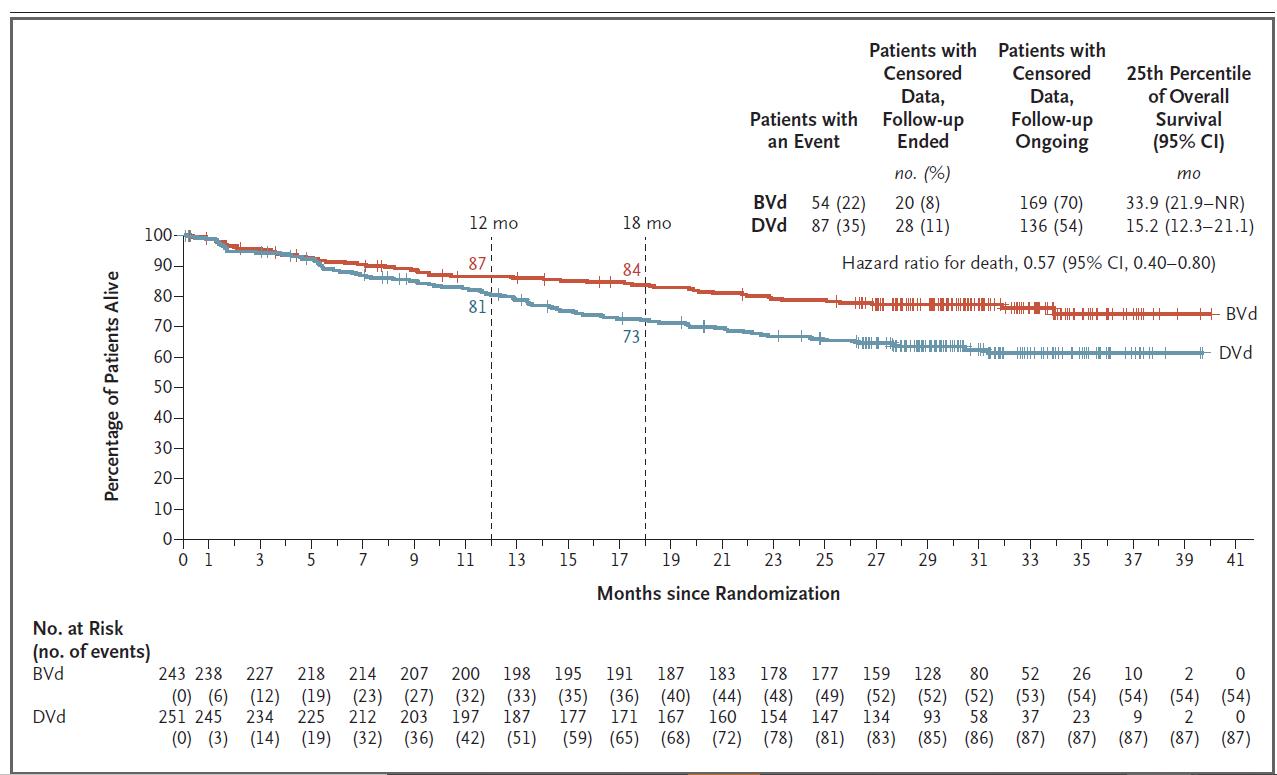
Hungria V. NEJM; 391(5): 393-407.

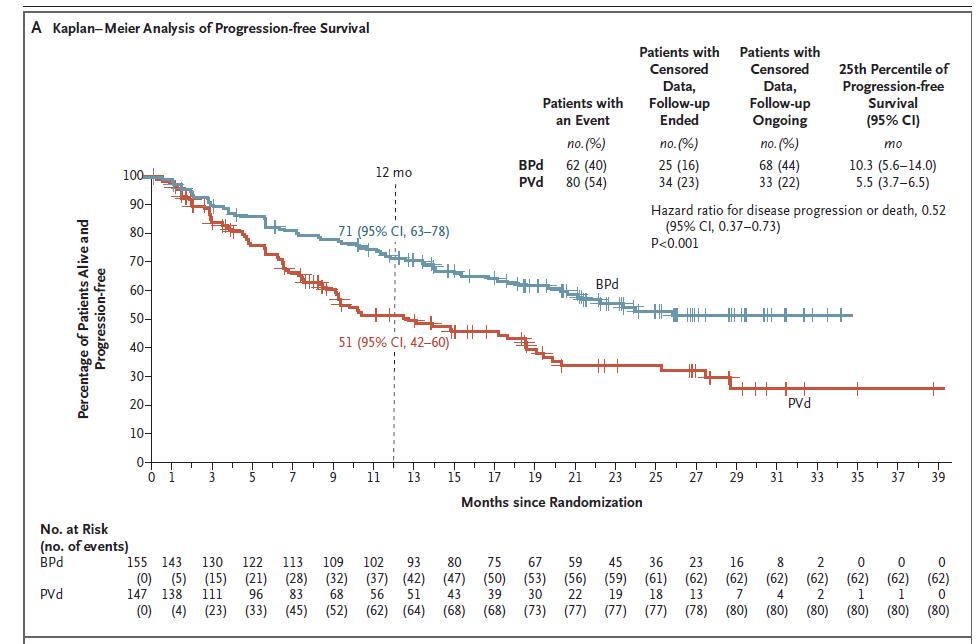
Progression free Survival
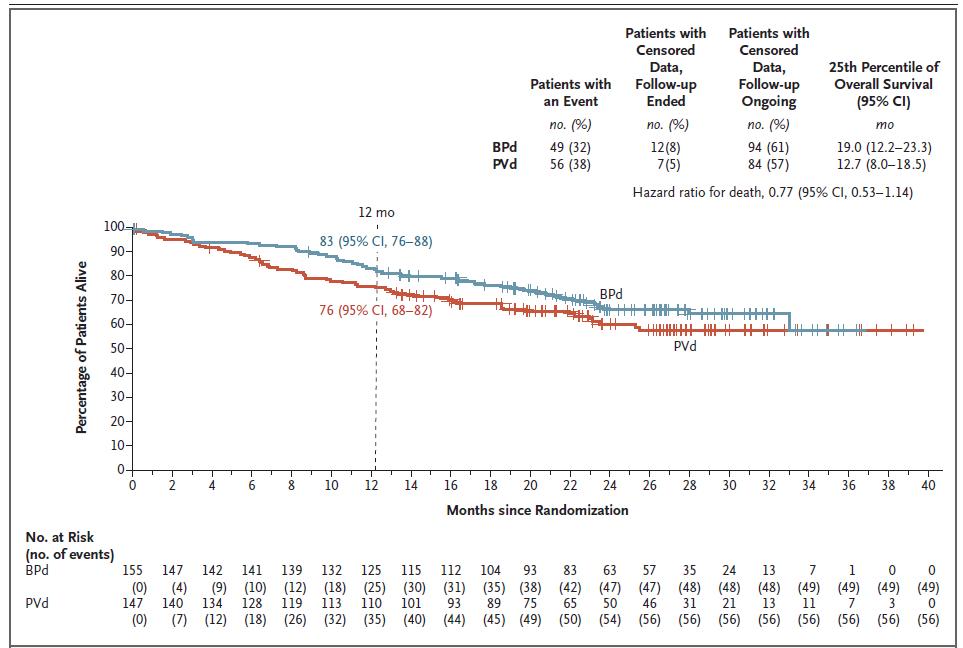
Dimopoulos MA. NEJM 2024; 391(5): 408-421.
Overall Survival

Bispecific MM target Brand name
Teclistamab BCMA Tecvayli
Talquetamab GPRC5D Talvey
Elranatamab BCMA Elrexfio
Cevostamab FcRH5
Livoseltamab BCMA
Bispecific antibody
Plasma cell aka myeloma cell T-cell • Cytokine release • T cell activation • Perforin/Granzymes
Fc domain



General Principles
Use mechanisms of action not previously used
Do not continue to use lenalidomide if progressing on len maintenance
Triplets are preferred over doublets
In real practice - most patients receiving VRD (Bortezomib-LenalidomideDex) like regimens, 1st relapse is typically
Daratumumab + Pomalidomide + Dex (APOLLO)
Isatuximab + Pomalidomide + Dex (ICARIA)
Daratumumab + Carfilzomib + Dex (CANDOR)
Isatuximab + Carfilzomib + Dex. (IKEMA)
Selinexor + Bortezomib + Dex (BOSTON)
Important Update – CAR T cell therapies can be used as early as first relapse!



Not refractory to Lenalidomide and standard risk or long 1st remission
Refractory to Lenalidomide
KPd or PVd or PCd or EloPd Or CAR-T (Cilta-cel If 1+ prior LOT, Ide-cel if 2+ LOT) DRd
Not Refractory to Anti-CD38 Refractory to Anti-CD38 and Len DPd or IsaPd Or DVd
Favor CAR-T Cilta-cel (1+ prior LOT) Or Ide-cel (2+ prior LOT)
Others to the left if not candidate for CAR-T
Standard Risk and >23 years remission <2 years of remission and/or HRCA > 2-3 year remission and standard risk < 2 year remission or HRCA Cilta-cel CAR T or Anti-CD38 + Kd
Dingli D, et al. Mayo Clin Proc. 2017;92(4):578-59. Updated 2024.



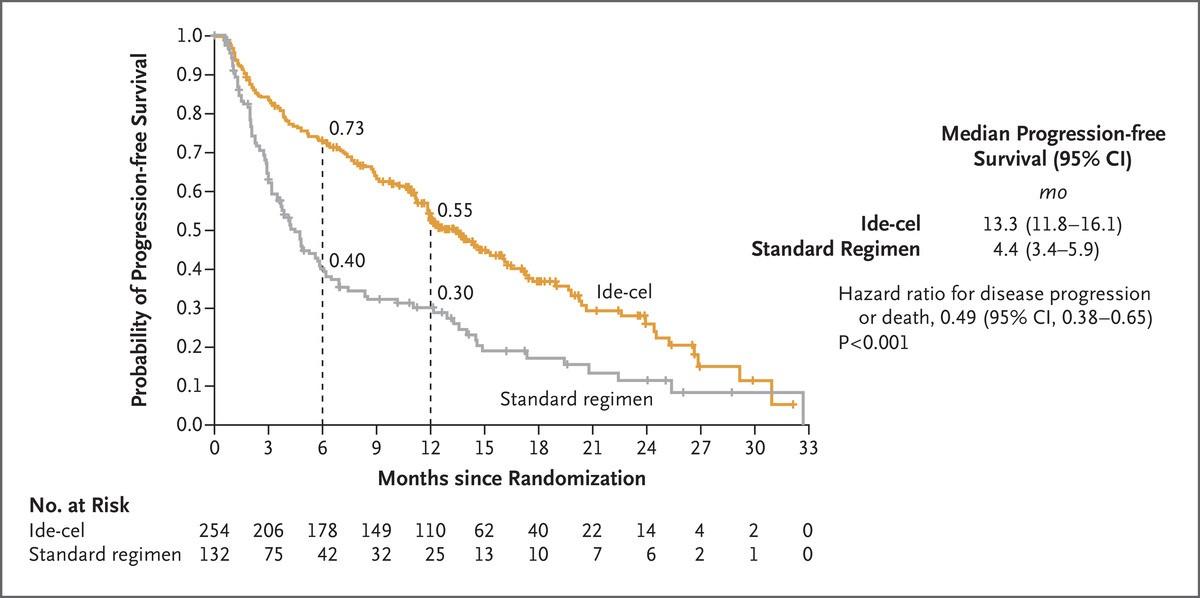



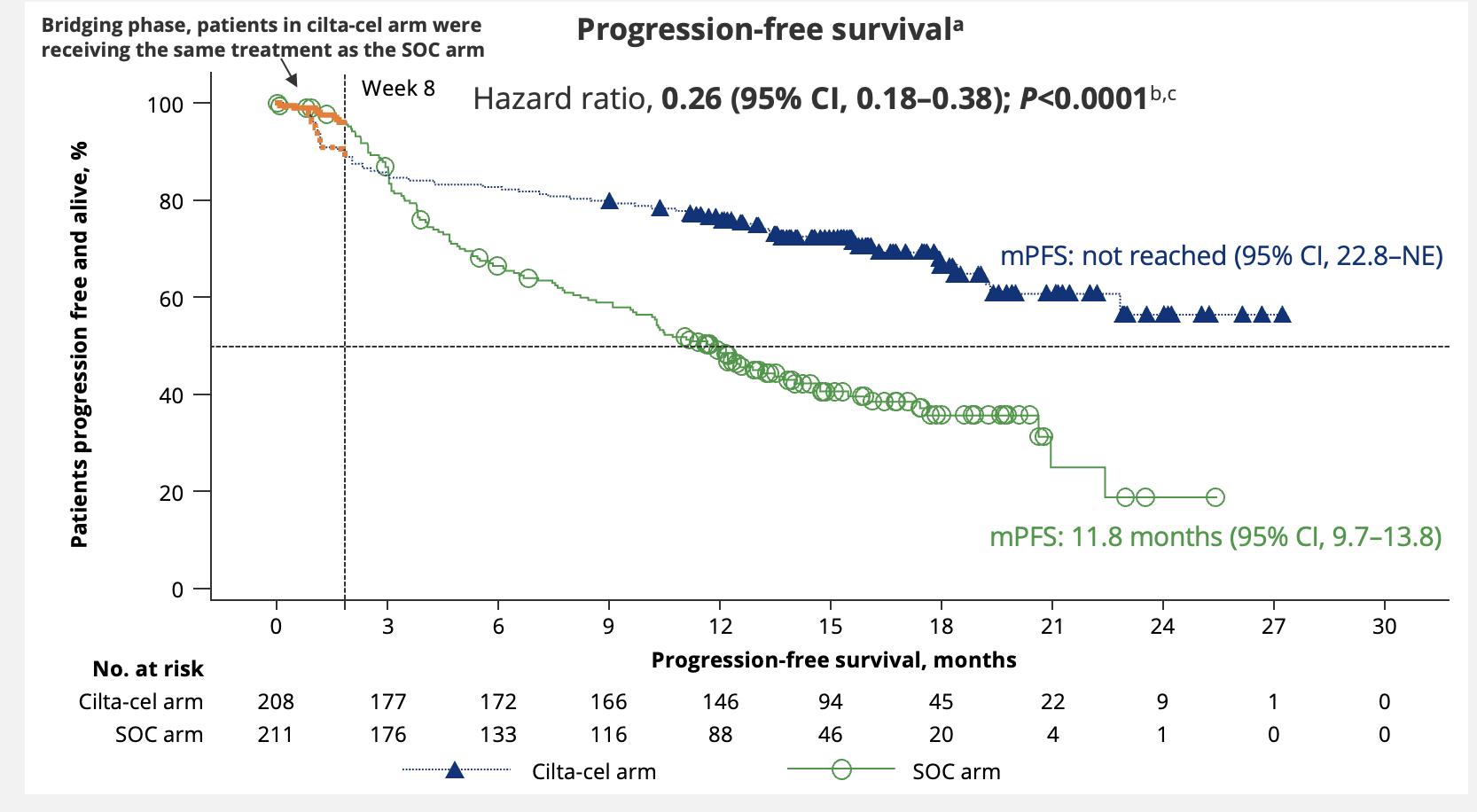


Richardson Blood 2014; 123:1826-32
Siegel Blood 2012; 120:2817-25
Lonial Lancet 2016; 387:1551-60
Rasche EHA 2024, P915
Van de Donk ASCO 2023; abs 8011
Lesohkin Nat Med 2023; 29:2259-67
Munshi NEJM 2021; 384:705-16
Munshi EHA 2023; S202
Huang ASCO 2024; 7511



Immune cells from the patient are collected

Standard of care therapy is permitted until CAR T cells are ready for infusion

Fludarabine and Cytoxan are used to create “immunologic space” to CAR T cells to expand







Cytokine release syndrome Neurotoxicity (ICANS)


Onset 1 9 days after CAR Tcell infusion 2 9 days after CAR Tcell infusion
Duration 511 days 317 days
Symptom
s
Managem
ent
• Fever
• Difficulty breathing
• Dizziness
• Nausea
• Headache
• Rapid heartbeat
• Low blood pressure
• Actemra (tocilizumab)
• Corticosteroids
• Supportive care
• Headache
• Confusion
• Language disturbance
• Seizures
• Delirium
• Cerebral edema
• Antiseizure medications
• Corticosteroids
*Based on the ASTCT consensus; †Based on vasopressor; ‡For adults and children >12 years; §For children ≤12 years; ‖Only when concurrent with CRS



There are currently 3 approved bispecific antibodies:
Teclistamab (Tecvayli)
Talquetamab (Talvey)
Elranatamab (Elrexfio)



Bispecific antibodies are also referred to as dual specific antibodies, bifunctional antibodies, or T-cell engaging antibodies
Bispecific antibodies can target two cell surface molecules at the same time (one on the myeloma cell and one on a T cell)
Many different bispecific antibodies are in clinical development; none are approved for use in myeloma
Availability is off-the-shelf, allowing for immediate treatment

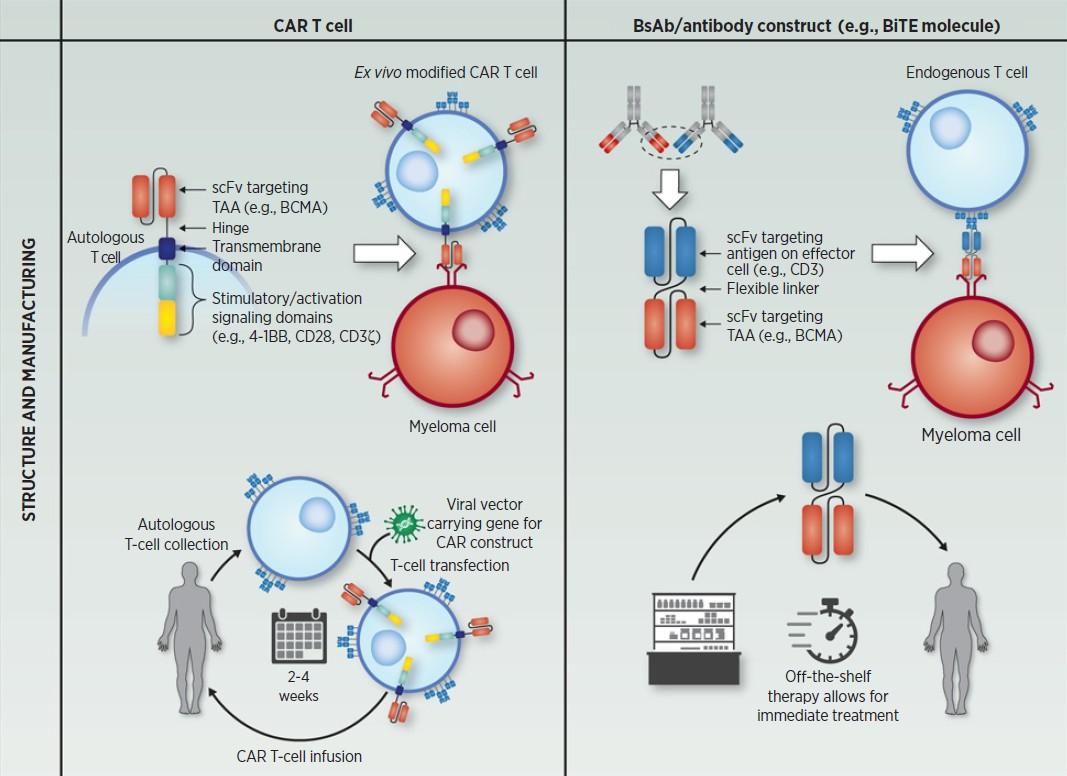

Cohen A et al. Clin Cancer Res. 2020;26:1541.


BCMA, GPRC5D, or FcRH5
Examples:
• Elranatamab
• Teclistamab
• TNB-303B (ABBV-383)
• REGN5458
• Cevostamab
• Talquetamab



• Cytokine release syndrome (CRS)
• Neurotoxicity (ICANS)
• Usually occurs within first 1–2 weeks
• Frequency (all grade and grade 3–5) higher with CAR T
• Cytopenias
• Target unique
• For example, rash, taste disturbance seen with GPRC5D, but not with BCMA
• Infections
• Incidence for bispecifics at RP2D not yet known
• Viruses: CMV, EBV
• PCP/PJP
• Ongoing discussions regarding prophylactic measures
IVIG
Anti-infectives



Approved product Abecma, Carvykti Tecvayli



CAR T and bispecific antibodies are very active even in heavily pretreated patients.
Side effects of CAR T cells and bispecific antibodies include cytokine release syndrome, confusion, and low blood counts, all of which are treatable.
Abecma and Carvykti are only the first-generation CAR T cells and target the same protein. Different CAR Ts and different targets are on the way.
Bispecific antibodies represent an “off-the-shelf” immunotherapy; Tecvayli was approved in October 2022, and now Talvey and Elrexfio in August 2023
Several additional bispecific antibodies are under clinical evaluation.



Bispecific antibodies
• Cevostamab, Alnuctamab, ABBV383, and others
• Target BCMA, GPRC5D, or FcRH5 on myeloma cells and CD3 on T cells
• Redirects T cells to myeloma cells
• Iberdomide
• Targets cereblon
• Enhances tumoricidal and immune-stimulatory effects compared with immunomodulatory agents
• Venetoclax
• Targets Bcl-2
• Induces multiple myeloma cell apoptosis



VD
Rev/Dex
CyBorD
VTD
VRD
KRD
D-VMP
DRD
ASCT
Tandem ASCT (?)
Nothing
Thalidomide?
Bortezomib
Ixazomib
Lenalidomide
Combinations
Bortezomib
Lenalidomide
Carfilzomib
Pomalidomide
Selinexor
Panobinostat
Daratumumab
Ixazomib
Elotuzumab
Isatuximab
Belantamab mafodotin*
Melphalan flufenamide*
Idecabtagene autoleucel
Ciltacabtagene autoleucel
Teclistamab, Talquetamab
Elranatamab
D-VRD
Isa-VRD
D-KRD
Isa-VRD “More” induction?
Daratumumab?
Carfilzomib?
Lenalidomide + PI
ASCT, autologous stem cell transplant; CAR, chimeric antigen receptor; Cy, cyclophosphamide; d- daratumumab; D/dex, dexamethasone; isa, isatuximab; K, carfilzomib; M, melphalan; PD-L1, programmed death ligand-1; PI, proteasome inhibitor; Rev, lenalidomide; V, bortezomib.
Speaker’s own opinions.
CAR T Cell Therapy
Bispecific/Tri-specific
Antibodies
Venetoclax
PD/PDL-1?
Small Molecules
* These agents are currently off the market but available through special programs
Anito-cel
Cevostomab
Linvoseltamab
Iberdomide, Mezigdomide
Sonrotoclax




Please remain in this room
Walk Through the IMF Website
Robin Tuohy
Vice President, Support Groups, International Myeloma
Foundation

Seasons of Myeloma: Managing Side Effects and Living Well
Daniel Verina, DNP, RN, ACNP-BC
Mount Sinai Hospital, New York City, NY
Living the Myeloma Life: Local Patient & Care Partner
Flora Ostrow (Patient) and Betsy Gilbert (Care Partner)
Beyond Myeloma Therapy: Palliative Care for the Life
You Are Still Living
Richa Thakur, MD
Fred Hutchinson Cancer Center, Seattle, WA
Q&A with Panel
Closing Remarks
Robin Tuohy
Vice President, Support Groups, International Myeloma
Foundation
Coffee/Network

Robin Tuohy, Vice President, Support Groups

Daniel Verina, DNP, RN, ACNP-BC
Mount Sinai Hospital, New York City, NY

Daniel Verina, DNP, MSN, RN, ACNP-BC
Mount Sinai Hospital - New York, New York & IMF Nurse Leadership Board member

















Myeloma Treatment Common Combinations
Velcade® (bortezomib)
Lenalidomide
DVRd, VRd, Vd
DVRd, VRd, Rd
Kyprolis® (carfilzomib) KRd, Kd, DKd, Isa-Kd
Pomalyst® (pomalidomide) Pd, DPd, EPd, PCd, Isa-Pd
Darzalex® (daratumumab) DVRd, DRd, DVd, DPd, DVMP, DKd
Ninlaro®(ixazomib) IRd
Empliciti® (elotuzumab) ERd, EPd
Xpovio® (Selinexor) XVd, XPd, XKd
Sarclisa® (Isatuximab) Isa-Kd, Isa-Pd
Blenrep® (Belantamab mafodotin) Bela-d
Abecma® (Idecabtagene Vicleucel) --
Carvykti® (ciltacabtagene autoleucel)
Elrexfio® (elranatamab)

Lynozyfic™ (linvoseltamab)
Tecvayli® (teclistamab) --
Talvey® (talquetamab) --
Venclexta® (venetoclax) Vd + ven
New agents or regimens in clinical trials are possible options
ASCT = autologous stem cell transplant; Bela = belantamab; C = cyclophosphamide; D = daratumumab; d = dexamethasone; E = elotuzumab; Isa = isatuximab; I = ixazomib; K = carfilzomib; M = melphalan; P = pomalidomide; R = lenalidomide; V = bortezomib; ven = venetoclax.


ELIGIBILITY
Measuring Treatment Response
Determining Transplant Eligibility
Insurance Authorization Collecting Stem Cells



High Dose Chemotherapy
Stem Cell Infusion
Supportive Care Engraftment
Duration: Approximately 2 weeks
Location: Transplant Center
Duration: Approximately 3-4 weeks
Location: Transplant Center

POST-TRANSPLANT
P H A S E 1 P H A S E 2 P H A S E 3

Restrengthening
Appetite recovery
“Day 100” assessment
Begin maintenance therapy
Duration: Approximately 10-12 weeks
Location: HOME





Ask for a referral to CAR Tcell center as soon as it is possible as next treatment option (ie, before relapse)
Manufacturing takes
≈ 4 to 6 weeks
Bridging therapy may be needed
T-Cell Collection


No driving for 8 weeks
“One
& Done” with continued monitoring
• Away from home
• Often some hospital stay
• Care Partner needed
• Side effect management
• CRS, ICANS
• Low blood counts
• Fatigue and fever
• Some patients need ongoing transfusion support


• Different bispecific antibodies have differences in efficacy, side effects
– Available after 4 prior lines of therapy (or clinical trial)
– About 7 in 10 patients respond
– Off-the-shelf treatment; no waiting for engineering cells
– CRS and neurotoxicity
– Risk of infection
• BCMA target: greater potential for infection
– Tecvayli® (teclistamab)
– Elrexfio ® (elranatamab)
– Lynozyfic™ (linvoseltamab)


• GPRC5D target: potential for skin and nail side effects, GI issues of taste change, anorexia and weight loss
– Talvey ® (talquetamab)






































Your team may be able to help, but only if they know how you feel.
Unmanaged Myeloma can cause:
• Calcium elevation
• Renal dysfunction
• Low blood counts
• Infection Risk
• Blood clots
• Bone pain
• Neuropathy
• Fatigue

cause:
• GI symptoms
• Renal dysfunction
• Low blood counts
• Infection Risk



Tip: proactively discuss common side effects and what to do if they occur










• Blood clots
• Neuropathy
• Fatigue











Tip: Keep a Symptom Diary and bring it to appointments











Steroids enhance the effectiveness of other myeloma therapies
Your provider may adjust your dose. Do not stop or alter your dose of steroids without discussing it with your provider

• Irritability, mood swings, depression
• Consistent schedule (AM vs. PM)
• Take with food
• Stomach discomfort: Over-the-counter or prescription medications
• Medications to prevent shingles, thrush, or other infections

• Difficulty sleeping (insomnia), fatigue
• Blurred vision, cataracts
• Flushing/sweating
• Increased risk of infections, heart disease

• Muscle weakness, cramping
• Increased blood pressure, water retention
• Stomach bloating, hiccups, heartburn, ulcers, or gas
• Weight gain, hair thinning/loss, skin rashes
• Increased blood sugar levels, diabetes


Infection remains the leading cause of death in patients with multiple myeloma. Several factors account for this infection risk, including the overall state of immunosuppression from multiple myeloma, treatment, age, and comorbidities (e.g., renal failure and frailty).
IMWG Consensus guidelines and recommendations for infection prevention in multiple myeloma; Lancet Haematol.2022;9(2):143–161.
Good personal hygiene (skin, oral)



Report fever of more than 100.4°F, shaking chills even without fever, dizziness, shortness of breath, low blood pressure to HCP as directed.
Environmental control (avoid crowds and sick people; use a high-quality mask when close contact is unavoidable)
As recommended by your healthcare team:
Immunizations:
Flu, COVID, RSV & and pneumococcal vaccinations; avoid live vaccines


Preventative and/or supportive medications (next slide)


Type of Infection Risk
Viral: Herpes Simplex (HSV/VZV); CMV
Medication Recommendation(s) for Healthcare Team Consideration
Acyclovir prophylaxis
Bacterial: blood, pneumonia, and urinary tract infection Consider prophylaxis with levofloxacin
PJP (P. jirovecii pneumonia)
Fungal infections
COVID-19 and Influenza
IgG < 400 mg/dL (general infection risk)
ANC < 1000 cells/μL (general infection risk)


Consider prophylaxis with trimethoprim-sulfamethoxazole
Consider prophylaxis with fluconazole
Antiviral therapy if exposed or positive for covid per institution recommendations
IVIg recommended
Consider GCSF 2 or 3 times/wk (or as frequently as needed) to maintain
ANC > 1000 cells/μL and maintain treatment dose intensity



Fluid intake can help with both diarrhea and constipation and helps kidney function
Constipation is more common in the induction phase
• Opioid pain relievers, antidepressants, heart or blood pressure medications (check with provider, pharmacist)
• Supplements: Calcium, Iron, vitamin D (rarely), vitamin B-12 deficiency Increase fiber
• Stay well hydrated
• Fruits, vegetables, high fiber whole grain foods
• Fiber binding agents – Metamucil® , Citrucel®, Benefiber®


Anorexia, the inability to eat, is common during transplant and resolves with time.
• Hydration is most important
• Small, frequent meals with a focus on protein intake
• You will work closely with a dietician to help monitor your calorie intake
Diarrhea is common during transplant and long-term maintenance therapy. Other medications and supplements
• Hydration is very important
• Electrolyte replacement is common
• Good skin care will help prevent irritation
• Stool exam may be needed to rule-out infection
• If no infection, anti-diarrheal medication may be prescribed





Sources of pain include bone disease, neuropathy and medical procedures
• Management
– Prevent pain when possible
• Bone strengtheners to decrease fracture risk
• Antiviral to prevent shingles
• Sedation before procedures
– Interventions depend on source of pain
• May include medications, activity, surgical intervention, radiation therapy, etc
• Complementary therapies (Mind-body, medication, yoga, supplements, acupuncture, etc)
• Scrambler therapy for neuropathy





Tell your healthcare provider about any new bone or chronic pain that is not adequately controlled


Peripheral neuropathy happens when there is damage to nerves in the extremities (hands, feet, limbs). Damage can be the result of myeloma, treatment or unrelated conditions (i.e. diabetes).
Symptoms:
• Numbness
• Tingling
• Prickling sensations
• Sensitivity to touch
• Burning and/or cold sensation
• Muscle weakness
Prevention / management:
• Bortezomib once-weekly and/or subcutaneous administration
• Massage area with cocoa butter regularly
• Neuroprotective Supplements:
– B-complex vitamins (B1, B6, B12)
– Green tea
• Safe environment: rugs, furnishings, shoes

If neuropathy worsens, your provider may:
• Adjust your treatment plan
• Prescribe oral or topical pain medication
• Suggest physical therapy
Report symptoms of peripheral neuropathy early to your healthcare provider; nerve damage from neuropathy can be permanent if unaddressed



• Risk Factors
– Active multiple myeloma (light chains, high calcium)
– Other medical issues (ex: Diabetes, dehydration, infection)
– Medications (MM treatment, antibiotics, contrast dye)
– Poor Nutrition
• Prevention
– Stay hydrated – drink water
– Avoid certain medications when possible (eg, NSAIDs), dose adjust as needed
• Treatment
– Treatment for myeloma
– Hydration
– Dialysis




Many myeloma patients will experience kidney issues at some point; protecting your kidney function early and over time is important







































CAR = chimeric antigen receptor; CRS = cytokine release syndrome. Oluwole OO, Davila ML. J Leukoc Biol. 2016;100:1265-1272. June CH, et al. Science. 2018;359:1361-1365. Brudno JN, Kochenderfer JN. Blood. 2016;127(26):3321-3330. Brudno JN, Kochenderfer JN. Blood Rev. 2019:34:45-55. Shimabukuro-Vornhagen, et al. J Immunother Cancer. 2018;6:56. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625-638.

















OTC dry mouth rinse, gel, spray are recommended. Advise patients to avoid hot beverages. Initiate anti-fungal therapy for oral thrush
Dexamethasone oral solutions “swish and spit” have been tried but with no proven benefit yet. Sour citrus or candies before meals are also recommended. Taste Changes


Dietary modifications with small bites, eating upright, and sips with food can help manage symptoms.
Some medications lead to weight gain, others to weight loss.
Dry mouth leads to taste changes which can lead to anorexia. Meet


Possible side effect to some treatments and supportive care medications

Skin Rash:
• Prevent dry skin; apply lotion
• Report changes to your care team
• Medication interruption or alternative, as needed
• Steroids:
– Topical for grades 1-2,
– Systemic and topical for Grade 3

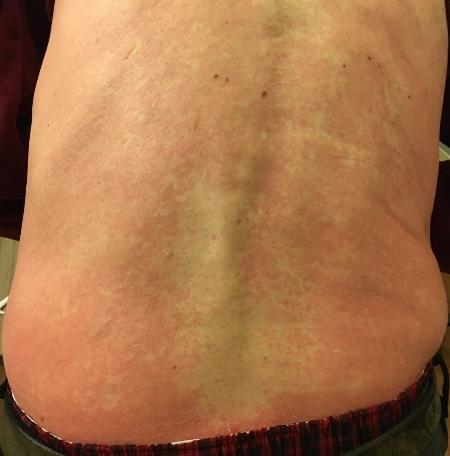
• Antihistamines, as needed
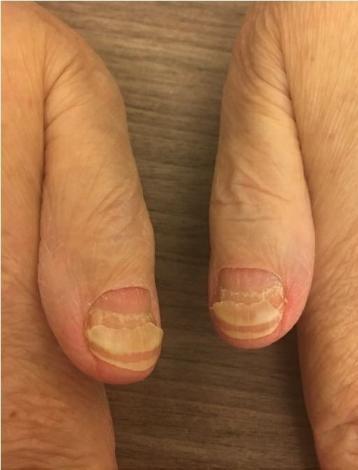
• Keep your nails short and clean. Watch for “catching and tearing”

• Apply a heavy moisturizer like Vaseline or salve. Wear cotton hand coverings to bed
• A nail hardener may help with thinning
• Tell the team if you have signs of a fungal infection, like thickened or discolored nails




Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56. Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36.
Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.



Fatigue is the most reported symptom. Sources include anemia, pain, reduced activity, insomnia, treatment toxicity, bone marrow suppression 98.8%
Often, people do not share these symptoms with their providers. Talk to your provider about symptoms that are not well controlled or if you have thoughts of self-harm.


>35% of patients
≈25% of patients




questions
– What are my treatment options?
– What are the pros and cons of the different options?
– How will we know if treatment is working?
– What do the different labs mean?
– Who will be monitoring my labs?
– How can I access my test results (eg, patient portal)?
– What will we do if my treatment doesn’t work or quits working?
Participate in decisions
– Share your priorities and preferences
– Include care partner(s) in your discussion



Speak up if something seems different or unusual
– Normally 4 vials of blood but only drawing 3?
– Normally specialty pharmacy confirms delivery but haven’t heard from them this month?

Live in the sunshine, swim the sea, drink the wild air.
– When is my next appointment?
Communicate with your healthcare team
– Understand the roles of each team member
– Who to contact for your needs (eg, side effects, insurance issues, other)
Develop a support network
– Learn from others: IMF has many support groups or you can start one (IMF’s can help)





If you want to go fast, go alone, if you want to go far, go together
• Care partners may help with medical appointments, managing medication, daily living, physical assistance, emotional support, myeloma knowledge, healthy lifestyle, patient advocacy, financial decisions
• Care partners can be a spouse, close relative, a network of people (family, friends, neighbors, church members, etc)





African Proverb
• Caring for the Care Partner
– Recognize that caregiving is difficult/stressful
– Encourage care partners to maintain their health, interests, and friendships
– The IMF has information and resources to help care partners







If you want to go fast, go alone, if you want to go far, go together
• Multiple studies demonstrate that strong social ties are associated with longevity, improved adherence to medical treatment and overall improved health outcomes
• Care partners can be a spouse, close relative, a network of people (family, friends, neighbors, church members, etc)
• Care partners may help with medical appointments, managing medication, daily living, physical assistance, emotional support, myeloma knowledge, healthy lifestyle, patient advocacy, financial decisions


• Caring for the Care Partner
– Recognize that caregiving is difficult/stressful
– Encourage care partners to maintain their health, interests, and friendships
– The IMF has information and resources to help care partners









Have a Primary Care Provider & Have Recommended Health Screenings
• Blood pressure
• Cholesterol
• Cardiovascular disease
• Diabetes
• Colonoscopy
• Women specific: mammography, pap smear
• Men specific: prostate
• Vision
• Hearing
• Dermatologic evaluation
• Dental checkups & cleaning

Develop & maintain healthy behaviors
• Good nutrition
• Regular activity
• Quit tobacco use
• Sufficient Sleep (next slide)
An ounce of prevention is worth a pound of cure. Benjamin
Franklin

Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56. Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.




































Richa Thakur,
MD
Fred Hutchinson Cancer Center, Seattle, WA
Richa Thakur, MD
Assistant
Professor in Hematology and Oncology
University of Washington/Fred Hutchinson Cancer Center
1. Palliative care is for any stage in myeloma - don’t wait to ask!
2. You can still get your cancer treatment
3. You stay in control - Palliative care helps you make informed decisions
4. Your symptoms are treatable
5. It supports you AND your whole family
The Interdisciplinary Team
Why it Matters for Patients with Myeloma
Take the First Step: Advance Care Planning
The Interdisciplinary Palliative Care Team
Why it Matters for Patients with Myeloma
Take the First Step: Advance Care Planning

What is Palliative Care?
The Interdisciplinary Palliative Care Team
Why it Matters for Patients with Myeloma
Take the First Step: Advance Care Planning
Palliative care is a crucial part of integrated, people-centred health services. Relieving serious health-related suffering, be it physical, psychological, social, or spiritual, is a global ethical responsibility. Thus, [regardless of] the cause of suffering … palliative care may be needed and has to be available at all levels of care.
The Interdisciplinary Team
Why it Matters for Patients with Myeloma
Take the First Step: Advance Care Planning
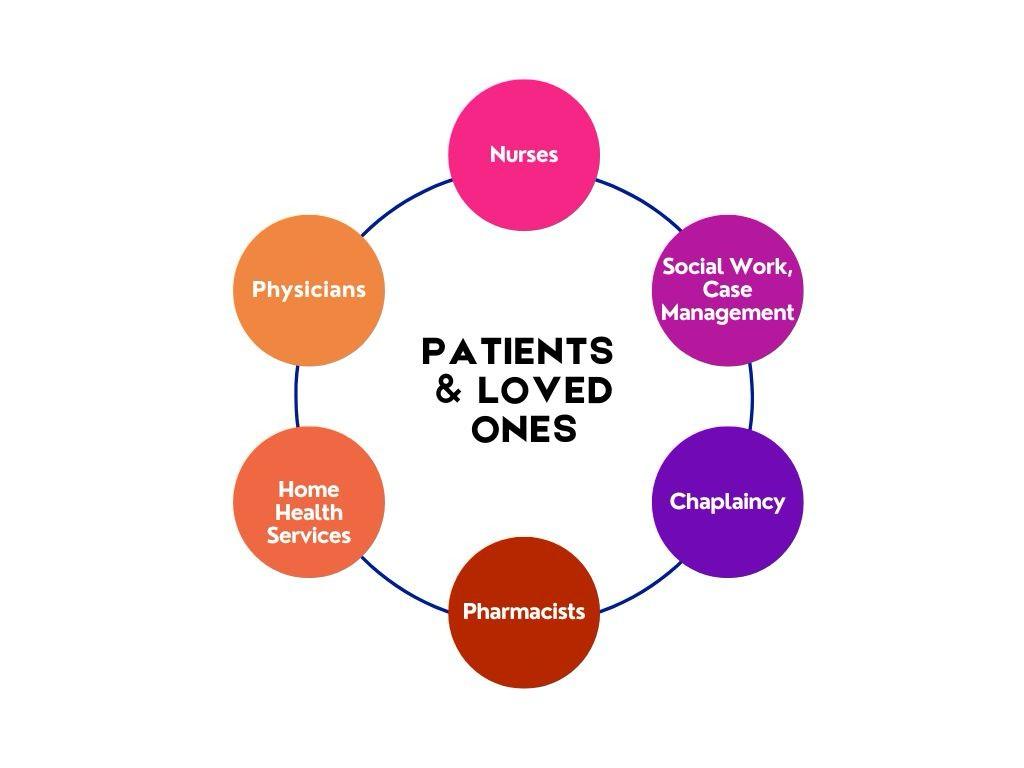
The Interdisciplinary Palliative Care Team
Why it Matters for Patients with Myeloma
Take the First Step: Advance Care Planning
● Multi-drug regimens can possibly increase side effect in some patients
● Limitations of hospice in patients with dialysis and transfusions
● Pain from both disease and treatment
Diagnosi
s
Advance Care
Planning
Goals of Care
Psychosocial distress
Goals of Care Family Meetings
I want to
- Continue chemotherapy?
- Do not have insurance?
- Want a clinical trial?
- I previously was on hospice and changed my mind?
- I do not live near a palliative care doctor?

What is Palliative Care?
The Interdisciplinary Palliative Care Team
Why it Matters for Patients with Myeloma
Take The First Step: Advance Care Planning
These are formal ways to outline your wishes and helps guide doctors when you cannot speak for yourself
Benefits of Advance Care Planning:
- Peace of mind for you and your family
- Reduces confusion or conflict during emergencies
- Ensures your care aligns with what Your wishes are
1. Guardian
2. Durable Medical Power of Attorney (not POA for finances)
3. Next of Kin (State Dependent)
a. Spouse or Domestic Partner
b. Adult Children (majority agreement)
c. Parents
d. Adult Siblings
e. Next closest relative…
But, what if I do not trust my decision maker?
Key Documents:
- Health Care Proxy
- Living Will
- Do Not Resuscitate/Do not Intubate Orders (Optional)
How to Get Started:
- Visit TheConversationProject.org
- Look at your state’s website for Advance Directives
- Talk to your doctor
1. Palliative care is for any stage in myeloma - don’t wait to ask!
2. You can still get your cancer treatment
3. You stay in control - Palliative care helps you make informed decisions
4. Your symptoms are treatable
5. It supports you AND your whole family
Bonus Tip: Advanced Care Planning is about protecting your wishes - not giving up.





Robin Tuohy, Vice President, Patient Support International Myeloma
Presentation Slides: Are available by scanning the QR code, Instructions are on the QR code handout on each table.
Program Evaluations: evaluations at the end of today.
Parking: If you have parked at the hotel today, please check in with an IMF team member at the registration desk, our team will provide a voucher to provide to the valet team.

Badge Holders: Please return your badge holders and we can recycle them.
We greatly appreciate your time and feedback!



Thank you to our speakers & our sponsors!









OUR VISION:
A world where every myeloma patient can live life to the fullest, unburdened by the disease.
OUR MISSION:
Improving the quality of life of myeloma patients while working toward prevention and a cure.

These are the core values we bring to accomplishing our mission each day.
The patient experience is the focus of everything we do. Every interaction is an opportunity to establish a personal connection built on care and compassion which is the basis for continued support.
As a team, we value honesty and transparency while creating a culture of mutual respect. We foster a myeloma community built on sincerity, authenticity, and kindness.
We value accountability, personal responsibility, and a steadfast commitment to excellence. We respect the legacy and reputation of our organization while seeking new solutions and advancements to improve outcomes, quality of life, and access to the best available resources for everyone impacted by myeloma.
We recognize each team member's skills and talents through collaboration and cooperation. Our programs aim to celebrate and support the diversity of our patients and their communities.




