

2025 IMF REGIONAL COMMUNITY WORKSHOP
WALTHAM, MA

SEPTEMBER 27, 2025
Thank you to our speakers & our sponsors!









Welcome & Introductions
Robin Tuohy
Vice President, Patient Support
International Myeloma Foundation
Understanding Myeloma Basics
Shonali Midha, MD
Dana-Farber Cancer Institute, Boston, MA
IMF REGIONAL COMMUNITY WORKSHOP
WALTHAM MORNING AGENDA

(Video) Closing the Gap: Health Disparities in Myeloma
Joseph Mikhael, MD, MEd, FRCPC, FACP, FASCO,
Chief Medical Officer, International Myeloma Foundation
Advancing Treatment Options Through Clinical Trials
Andrew Yee, MD
Massachusetts General Hospital, Boston, MA
Q&A with Panel
Coffee Break
Breakout: Frontline or Relapsed Treatment Approaches
-NDMM: Getting Started with Myeloma Management
Shonali Midha, MD
-RRMM: Continuing the Myeloma Treatment Journey
Andrew Yee, MD
Housekeeping Items
Presentation Slides: Are available by scanning the QR code, Instructions are on the QR code handout on each table.
Program Evaluations: evaluations at the end of today.

Restrooms: Outside the meeting hall, take a left and then proceed towards the hotel reception. Bathrooms are on the right just before reception desk.
Badge Holders: Please return your badge holders and we can recycle them.
We greatly appreciate your time and feedback!




Scientific Advisory Board


S. Vincent Rajkumar, MD IMF Board Chair


Thomas Martin, MD
UCSF, Helen Diller Family Comprehensive Cancer Center


Wee Joo Chng, MD
National University of Singapore


María-Victoria Mateos, MD, PhD University of Salamanca


Vania Hungria, MD, PhD Santa Casa de São Paulo


Joseph Mikhael, MD, MEd, FRCPC, FACP IMF Chief Medical Officer


Sigurður Yngvi Kristinsson, MD, PhD University of Iceland


Philippe Moreau, MD University Hospital of Nantes


Shaji Kumar, MD Mayo Clinic


NIkhil Munshi, MD Dana-Farber Cancer Institute


Jesús San Miguel, MD, PhD University of Navarra


Sagar Lonial, MD, FACP
Winship Cancer Institute, Emory University


Saad Zafar Usmani, MD, MBA, FACP, FASCO
Memorial Sloan Kettering Cancer Center
The IMF Support Group Team is Here
For You!
Shared Experiences Help to Better Understand the Myeloma Journey
• Support Groups empower patients & care partners with information, insight & hope
• The IMF provides educational support to a network of over 150 myeloma specific groups
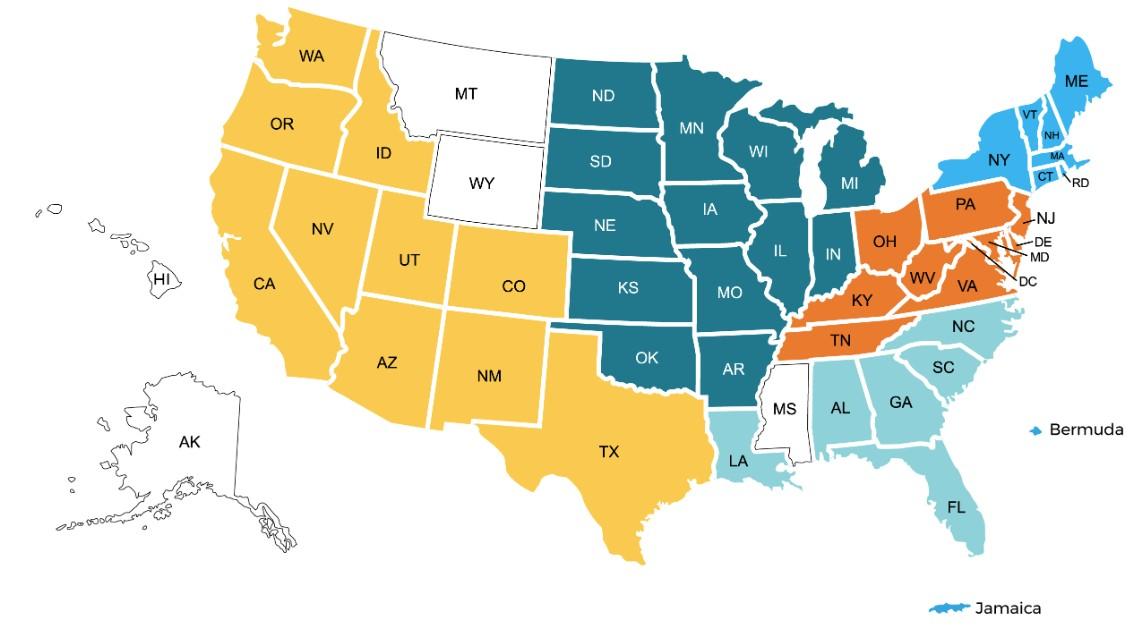
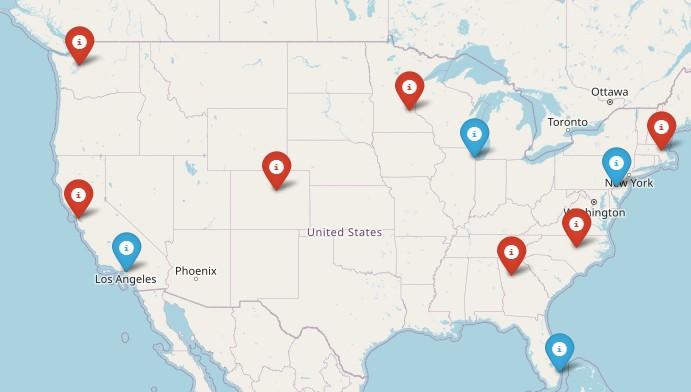
150+ US Support Groups
Over 200 Support Group Visits/year


Boston-Dana Farber, MA
Meets virtually on the 2nd Wednesday of each month at 5:30 PM
Prospect, CT
Meets virtually, with quarterly in-person on the 2nd Tuesday of each month at 6 PM
Greater Rhode Island
Meets hybrid on the 1st Wednesday of each month at 6 PM
Manchester, NH
Meets virtually on the 3rd Wednesday of each month at 6 PM
Cape Cod, MA
Meets in-person on the 2nd Tuesday of each month at 3 PM
Central MA
Meets hybrid on the 2nd Monday of each month at 6 PM
UConn (Farmington), CT
Meets virtual on the 1st Thursday of each month at 3:30 PM


Fairfield, CT
Meets virtually on the 1st Tuesday of each month at 7 PM
Portland, ME
Meets virtually on the 4th Wednesday of each month at 6 PM
Burlington, VT
Meets virtually on the 3rd Tuesday of each month at 5 PM
Special Interest Groups
Special interest groups are designed as a supplemental support for specific populations of patients, in addition to their local Support Groups
MM Families
Founded in 2021
For patients & care partners with young children
Las Voces de Mieloma
Founded in 2022
For Spanish speaking patients & care partners
Living Solo & Strong
Founded in 2022
For patients without a care partner

Click here for more inf
Smolder Bolder
Founded in 2023
For smoldering myeloma patients & care partners

Veterans SIG
Founded in 2025
For those who served our country
High Risk Multiple Myeloma
Founded in 2023
For high-risk myeloma patients & care partners
Care Partners Only
Founded in 2024
For myeloma care partners only
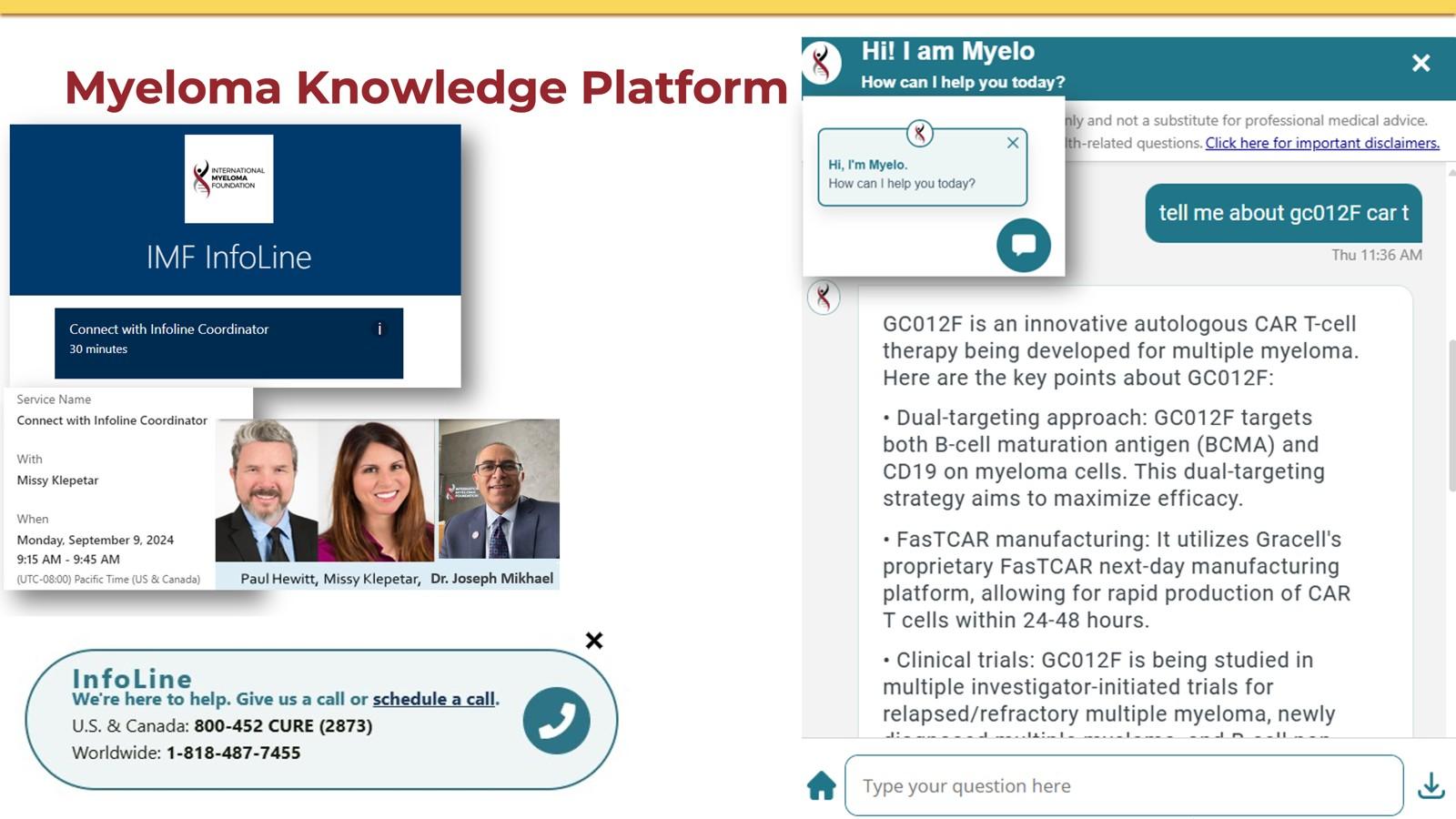
CLINICAL TRIALS MATCHING ENGINE
Seamlessly matching patients to the latest clinical trials.
• Discover clinical trials tailored to your myeloma journey with the IMF.
• Filter by diagnosis, treatment, and location to find the best fit for you.
Clinical Trials Matching Engine Usage











Clinicaltrials.gov https://clinicaltrials.gov/



ncreasing-diversity-in-cancer-clinical-research


WRITTEN EDUCATION





































Live Patient Education



& Family Seminars
• Boca Raton, FL – March 14 – 15
• Philadelphia, PA – May 2 – 3
• Los Angeles, CA – August 15 – 16
• Chicago, IL – October 3 – 4 Myeloma Community Workshops

Virtual-March 4-replay on myeloma.org • San Mateo, CA - March 29 • Atlanta, GA - April 5 • Edina, MN - April 26 • Denver, CO - June 21 • Virtual – July 29 – replay on myeloma.org
Seattle, WA - August 9
Waltham, MA - September 27
Raleigh-Durham, NC - November 15
Virtual – November 18

1. Ensure Access to Care: We advocate to ensure all myeloma patients have equitable, comprehensive, patient-centered care without insurance barriers that limit options or delay treatment initiation.
2. Eliminate Financial Barriers: We advocate for policies that allow myeloma patients access to treatments and supportive care interventions without facing financial hardships.
3. Advance Myeloma Research: We advocate for annual appropriations funding for myeloma research and the advancement of clinical trial eligibility and research protocols that ensure representation from diverse populations.

The IMF Grassroots Advocacy Program is multi-faceted and growing
• Advocacy Training & Leadership Development
• Policy and Legislative Education
• Grassroots Campaign Planning
• Health Policy Forums & Roundtables
• Advocacy Resource Development
• Storytelling and Personal Narratives
Scan for Upcoming Programs and Events!






Understanding Myeloma Basics
Shonali Midha, MD
Dana-Farber Cancer Institute, Boston, MA
Understanding Myeloma Basics

Shonali Midha, MD
Dana-Farber Cancer Institute ,Boston, MA

Review the basics of blood and cancer
OBJECTIVES
Define multiple myeloma and its key features
Discuss the staging and classification of myeloma
Outline the approach to therapy of myeloma



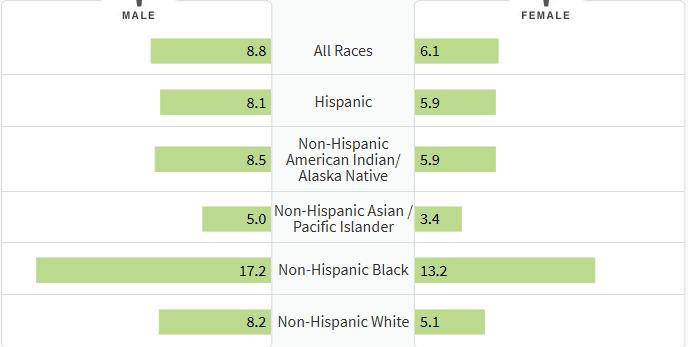
How common is Myeloma in the US?


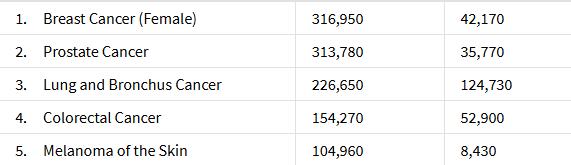



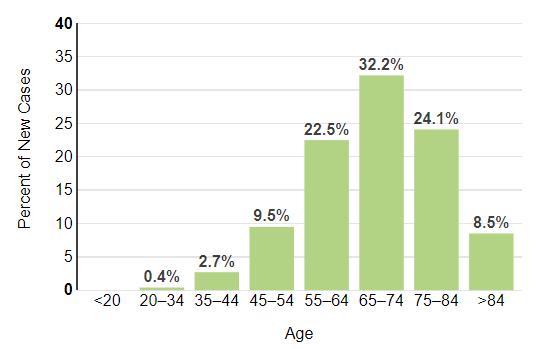



What Causes Myeloma? How/Why Did
I Get This?
Environmental Factors:
• Exposure to some chemicals
• Radiation exposure
Examples:
Agent Orange
Burn pits
Pesticides, Herbicides
Firefighter/First Responder exposures
Individual Factors:
• Age
• Family History of related disorders
• Personal History of MGUS or SMM
• Obesity
VA Study Documents Health Risks for Burn Pit Exposu
res
Leukemia and Multiple Myeloma Set to Be Added to List of Conditions Linked to Burn Pits
In most cases, the honest truth
WE DON’T KNOW



Multiple Myeloma Diagnosis Can Be Challenging




Kyle RA. Mayo Clin Proc. 2003;78:21-33.



What is the Connection Between Bone Marrow & Myeloma ?
Hematopoietic stem cell
Red Blood Cells Carry Oxygen White Blood cell Fight Infection Platelets Prevent Bleeding



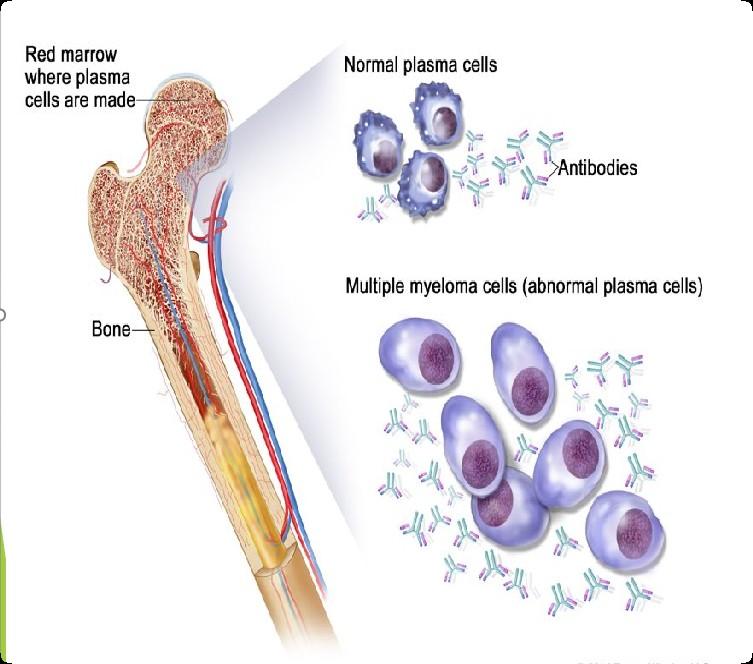



Photo Credit
Understanding (Mono)clonal Plasma Cells

Heavy Chain: G, A, M, D, E


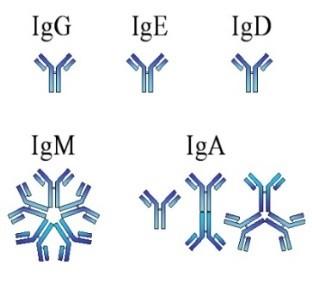


Chain = M-Spike

65% IgG – most common
20% IgA – associated with AL Amyloid
5%
Less common: IgD, IgE, IgM



• AL-Amyloid
Is Myeloma the Only Protein Disorder?
• POEMS
• Light or Heavy Chain Deposition Disease
• MGCS = Clinical
• MGRS = Renal
• MGNS = Neuro
Condition
MGUS1-4
(Monoclonal Gammopathy of Undetermined Significance)
SMM1-5,8 (Smoldering Multiple Myeloma) Active Multiple Myeloma6-8
Clonal plasma cells in bone marrow
Presence of Myeloma Defining Events
Likelihood

* In clinical trial



Multiple Myeloma and Myeloma Defining Events


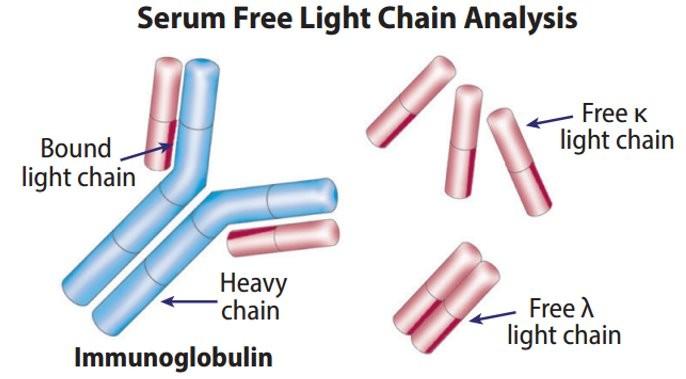
Testing For Myeloma: Blood & Urine
Test Name What it means
CBC + differential
Complete metabolic panel

Beta-2 Microglobulin (B2M)
Hemoglobin, WBC, Platelets
Creatinine, Calcium, Albumin, Liver function
Lactate Dehydrogenase (LDH) Part of staging and risk stratification
Serum Immunofixation and Protein electrophoresis (SPEP+IFE)
Immunoglobulins (G, A, M, D, E)
Free light chain assay with kappa/lambda ratio
Urine immunofixation & protein electrophoresis (UPEP+IFE)
Measures the level of normal and clonal protein Identifies the type of clonal protein

SV, et al. Lancet Oncol. 2014;15:e538-3548. Ghobrial IM, et al. Blood. 2014;124:3380-3388; mSMART.org; NCCN.org
Measures the level of normal and clonal protein Identifies the type of clonal protein




This Photo by Unknown Author is licensed under CC BY-SA-NC

Testing For Myeloma: Imaging
Imaging:
– Skeletal survey: Series of X-rays; less sensitive than other techniques
– Whole body low dose (CTWB-LD CT )
– Positron Emission Tomography (PET/CT)
– Magnetic Resonance Imaging (MRI)

Healthy bone versus myeloma bone disease

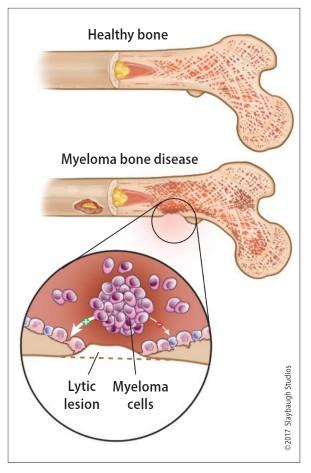
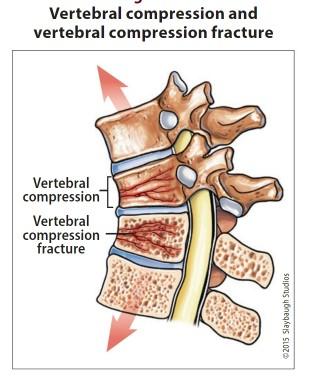


This Photo by Unknown Author is licensed under CC BY-NC-ND

Testing For Myeloma: Bone Marrow
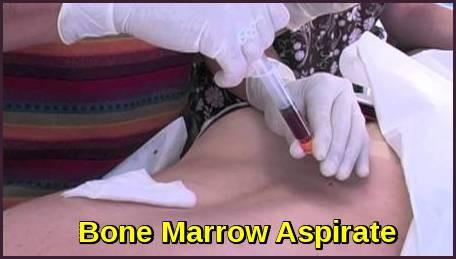
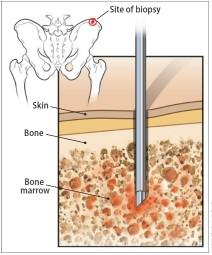
Bone marrow genetics
• Cytogenetics
• Fluorescence in situ hybridization (FISH)
• Next generation sequencing (NGS)





What is (the importance of) Myeloma Staging & Risk Stratification?
Putting It Together

• Updated as new information becomes available
• Helps to guide therapy and measure response to treatment
• Provides some prognostic value
• Standardizes terminology in medical practice








What is the Myeloma Treatment Landscape?
Initial Therapy (a.k.a. Frontline, Induction)
Quad Therapy (ex. CD38+ MoAb + VRd)
HD-Melphalan + Stem Cell
Transplant (ASCT)
Treatment for Relapse
Supportive Care and Living Well




Drug Class Overview

(thalidomide)
(lenalidomide)
(pomalidomide)


Drug Class Overview
Peptide Drug Conjugate*
BCMA Targeted Antibody Drug
Conjugate (ADC)*
CAR T Cell therapy
Bispecific Antibodies


Pipeline
Pepaxto (Melphalan Flufenamide)
Blenrep (belantamab mafodotin-blmf) Bela, Belamaf, or B
Abecma (idecabtagene vicleucel) Ide-cel
Carvykti (ciltacabtagene vicleucel)
Tecvayli (teclistimab)
Talvey (Talquetamab)
Elrexfio (Elranatamab)
Lynozyfic (Linvoseltamab)
Cilta-cel
Tec Talq Elra Linvo
Cevostamab, Iberdomide, Mezigdomide, Venetoclax. Sonrotoclax, anitocel, arlo-cel, Etentamig (ABBV-383), ISB2001, JNJ-79635322 ………MUCH
MORE TO COME! * These agents are currently off the market but available through special programs


Measuring Disease Response: IMWG Response Criteria


















Negative by next generation flow (NGF) or next generation sequencing (NGS) (minimum sensitivity 1 in 10-5 nucleated cells or higher)*
mCR AND normal Free Light Chain ratio, Bone Marrow negative by flow, 2 measures
CR AND negative PCR
Complete Response: Negative immunofixation (IFE); no more than 5% plasma cells in BM; 2 measures
Very Good Partial Response: 90% reduction in myeloma protein
Partial Response: at least 50% reduction in myeloma protein
Minimal Response
Stable Disease: Not meeting above criteria
Progressive Disease: At least 25% increase in identified myeloma protein from lowest level
MRD = Minimal Residual Disease
sCR = Stringent Complete Response; BM = Bone Marrow



When Do I Need A New Treatment?
• Not every relapse requires immediate therapy
• Each case is different
Asymptomatic high-risk disease or rapid doubling time or extensive marrow involvement
Symptomatic or extramedullary disease
Initiate Treatment
Asymptomatic biochemical relapse on 2 consecutive assessments
Consider Observation Monitor Carefully Consider Treatment
Patient-/Disease-Specific Monitor Carefully



Targets on the Myeloma Cell Surface and Therapeutic Antibodies

Bi-Specific Antibodies
Talvey (Talquetamab) CAR-T




Antibody Drug
Empliciti (Elotuzumab)
Bi-Specific Antibodies
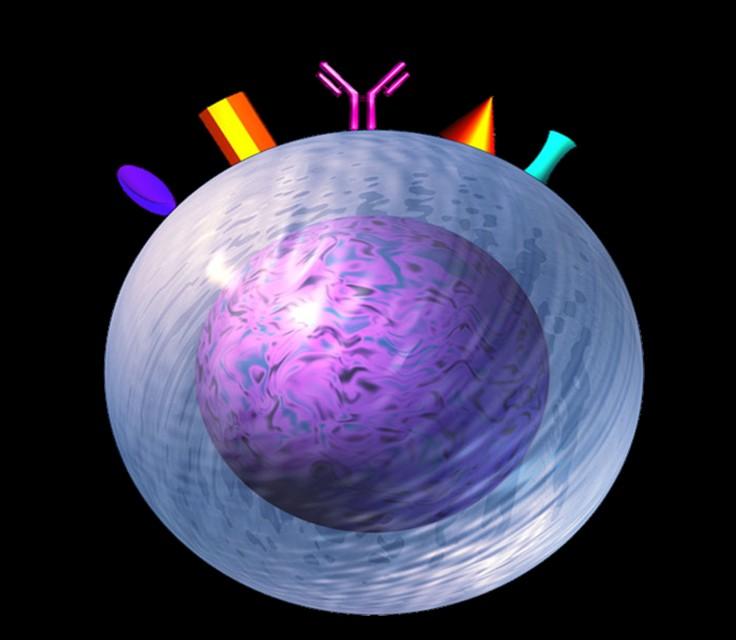

Bi-Specific Antibodies CAR-T


Monoclonal Antibodies
Daratumumab and Darzalex Faspro
Sarclisa (Isatuximab)
TAK-079 MOR202
Immune Therapies
Abecma (Ide-cel CAR-T)
Carvykti (Cilta-cel CAR-T)
Tecvayli (Teclistamab)
Elrexfio (Elranatamab)


Other CAR-Ts
Other Bi-Specific Antibodies



Antibody Drug Conjugates

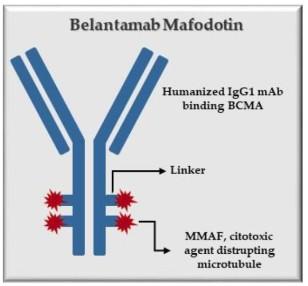

How it works:
An antibody directed at a target (BCMA) combined with a cytotoxic agent (chemotherapy)
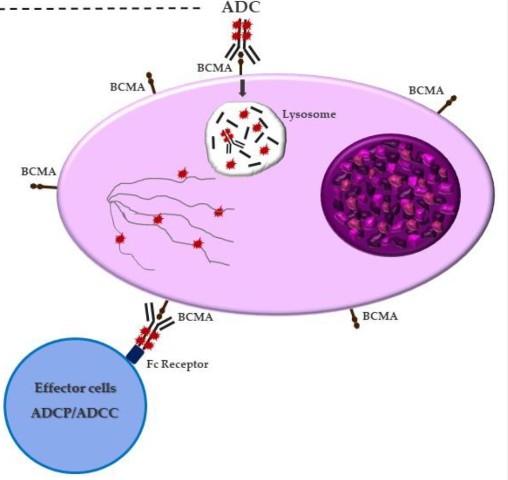
ADC = Antibody-Drug Conjugate
BCMA = B-Cell Maturation Antigen
ADCP/ADCC = Antibody-Dependent Cellular Cytotoxicity & Phagocytosis
Image Credit: https://creativecommons.org/licenses/by-nc/3.0/



Bispecific Antibodies: Mechanism of Action
• Incorporates 2 antibody fragments to target and bind both tumor cells and T cells
• Brings target-expressing MM cells and T cells into close proximity, enabling T cells to induce tumor-cell death
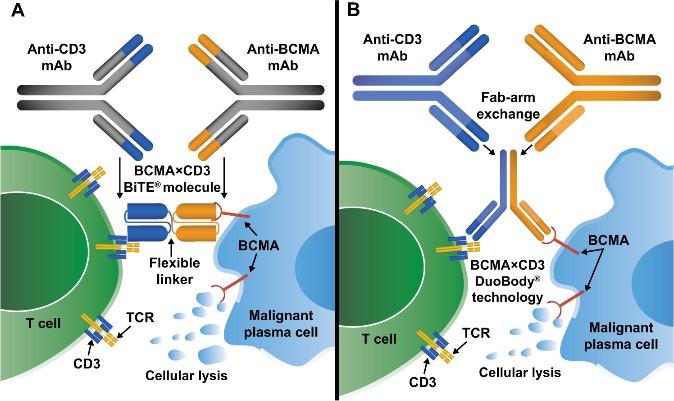

Targets of Bispecific Molecule Vary
“Off the Shelf” Advantage
• No manufacturing process, unlike CAR T-cell therapy (but like ADC/belantamab therapy)
• Thus, no delay between decision to treat and administration of drug
ADC = Antibody-Drug Conjugate; BCMA = B-Cell Maturation Antigen; CD3 = Cluster of Differentiation 3; FcRH5 = Fc receptor-homolog 5; GPRC5D = G-protein coupled receptor family C group 5 member D



The Process of CAR T Cell Therapy






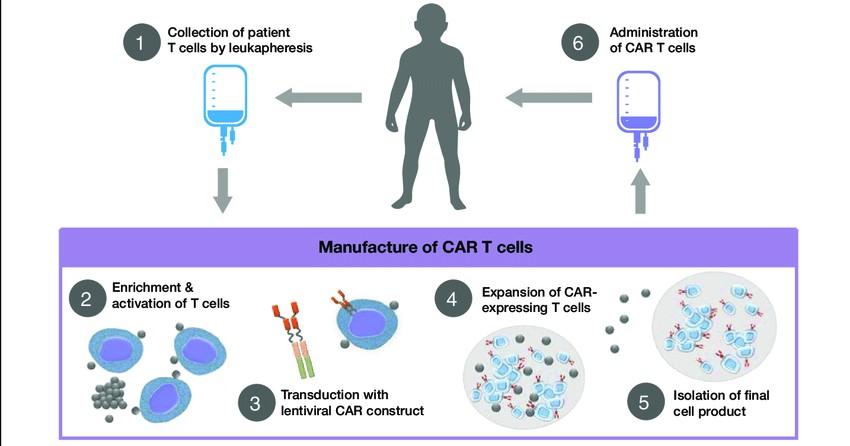






The Evolution of Myeloma Therapy
VD
Rev/Dex
CyBorD
VTD
VRD
KRD
D-VMP
DRD
ASCT (?)
Tandem ASCT (?)
Nothing
Thalidomide?
Bortezomib
Ixazomib
Lenalidomide
Combinations
Bortezomib
Lenalidomide
Carfilzomib
Pomalidomide
Selinexor
Panobinostat
Daratumumab
Ixazomib
Elotuzumab
Isatuximab
Belantamab mafodotin*
Melphalan flufenamide*
Idecabtagene autoleucel
Ciltacabtagene autoleucel
Teclistamab, Talquetamab
Elranatamab, Linvoseltamab
D-VRD
Isa-VRD
D-KRD
Isa-VRD “More” induction?
Daratumumab?
Carfilzomib?
Lenalidomide + PI
ASCT, autologous stem cell transplant; CAR, chimeric antigen receptor; Cy, cyclophosphamide; d- daratumumab; D/dex, dexamethasone; isa, isatuximab; K, carfilzomib; M, melphalan; PD-L1, programmed death ligand-1; PI, proteasome inhibitor; Rev, lenalidomide; V, bortezomib.
Speaker’s own opinions.
CAR T Cell Therapy
Bispecific/Tri-specific
Antibodies
Cell Modifying Agents
Venetoclax
PD/PDL-1 Inhibition?
Small Molecules
* These agents are currently off the market but available through special programs
Anito-cel
Cevostomab
Iberdomide, Mezigdomide
Sonrotoclax



Second/Expert Opinion
• You have the right to get a second opinion. Insurance providers may require second opinions.
• A second opinion can help you:
– Confirm your diagnosis
– Give you more information about options
– Talk to other experts
– Introduce you to clinical trials
– Help you learn

which health care team you’d like to work with, and which facility



















Closing the Gap: Health Disparities in Myeloma (Video)
Joseph Mikhael, MD, MEd, FRCPC, FACP, FASCO Chief Medical Officer, International Myeloma Foundation
Advancing Treatment Options Through Clinical Trials

Massachusetts General Hospital, Boston, MA
Andrew Yee, MD
Clinical Trials

Andrew J. Yee, MD
Clinical Director, Center for Multiple Myeloma
Massachusetts General Hospital Cancer Center
Assistant Professor of Medicine
Harvard Medical School
Waltham, MA
September 2025


Clinical Trials - Overview

Some Of The Important Principles Of Clinical Trials:
The drive of research has brought us to where we are
No one is expected to be a “guinea pig” with no potential benefit to them
Research is under very tight supervision and standards
Open, clear communication between the physician and the patient is fundamental Driving research forward!





MYTH: If I participate in a clinical trial, I might get a placebo, not active treatment
MYTH: If I participate in a clinical trial, I can’t change my mind
Clinical Trials: Myths
• Phase 1 and 2, everyone gets active treatment
• Phase 3 standard of care vs new regimen: often standard regimen with/without additional agent in MM trials
• Patients can withdraw their consent for clinical trial participation at any time

MYTH: Clinical trials are dangerous because they have new medicines and practices
• Some risk is involved with every treatment, but medicines are used in clinical trials with people only after they have gone through testing to indicate that the drug is likely to be safe and effective for human use
MYTH: Clinical trials are expensive and not covered by insurance
• Research costs are typically covered by the sponsoring company
• Standard patient care costs are typically covered by insurance
• Check with clinical trial team/insurers; costs such as transportation, hotel, etc. may not be reimbursed and are paid by patient
PhRMA website. Accessed March 25, 2024. https://phrma.org/-/media/Project/PhRMA/PhRMA-Org/PhRMA-Org/PDF/A-C/CLINICAL-TRIALS-MYTH-FACT-PRINT.pdf?hsCtaTracking=f6689b95-1626-40d9-8c87-c6b 8d31600a4%7C35221aa8-d487-4db3-9416-b9c3c35e3bac
.



Overview of New Drug Development
Identify a target for therapy in the laboratory
Confirm the anticancer activity in laboratory and animal studies
Clinical trials (human studies) to determine safety, dosing and effectiveness
The whole process costs millions of dollars and years of effort!



Preclinical
PHASE 1
Clinical Trial Phases
PRECLINICAL STUDIES: Examine safety and potential for efficacy in the laboratory, in animal models
FIRST INTRODUCTION OF AN INVESTIGATIONAL DRUG INTO HUMANS
• Determine metabolism and pharmacokinetic, pharmacodynamics, maximum tolerate dose, and dose limiting toxicities, identify adverse events
• Gain early evidence of efficacy, studied in many conditions; determine best dose (recommended phase 2 dose) to use in larger studies; typically, 20 to 80 patients
PHASE 2
PHASE 3
• Everyone gets the investigational drug
EVALUATION OF EFFECTIVENESS IN A CERTAIN TUMOR TYPE
• Determine efficacy at a given dose; everyone gets the investigational drug
• Determine short-term adverse events and risks
• Includes up to 100 patients, typically
COMPARED TO STANDARD OF CARE
• Standard of care is not a placebo in oncology!
• Hundreds to several thousand patients
• Often multiple institutions
PHASE 4
• Sometimes treatment is blinded
APPROVED AGENTS IN NEW POPULATIONS OR NEW DOSE FORMS



Clinical Trials – Why Me??
Benefits of trials are numerous and include:
Early access to “new” therapy
Contribution to myeloma world – present and future
Closer connection with your myeloma team
Must be balanced with potential risks
“Toxicity” of side effects
Possibility of lack of efficacy
More testing to establish efficacy and monitoring
Treatments and monitoring all done (generally) at site of clinical trial



Why Do So Few Cancer Patients Participate in Trials?
Patients may:
• Treating team may not be participating in clinical trials
• Be unaware of clinical trials
• Have practical and logistical barriers
• Face insurance or cost problems
• Fear, distrust, or be suspicious of research




Importance of Clinical Trial Participation by Diverse Populations
[P]eople from racial and ethnic minorities and other diverse groups are underrepresented in clinical research. This is a concern because people of different ages, races, and ethnicities may react differently to certain medical products.
– FDA

Leadership and commitment
Community engagement practices
Investigator hiring, training, and mentoring practices


Patient engagement practices

US Cancer Centers of Excellence: Strategies for Increased Inclusion of Racial and Ethnic Minorities in Clinical Trials
FDA = US Food and Drug Administration. Regnante JM, et al. J Oncol Pract. 2019;15(4):e289-e299. FDA website. Clinical Trial Diversity. Accessed March 27, 2024. https://www.fda.gov/consumers/minority-health-and-health-equity/clinical-trial-diversity.



Is A Clinical Trial Right For Me?
Discuss with your physician if you are eligible for a clinical trial
Clinical trials have eligibility criteria that generally require “measurable disease,” adequate blood counts, kidney function, etc.
Work with your physician to determine the best trial for you
Meet with the clinical research nurse or trials coordinator to discuss the trial
Carefully review the provided “Informed Consent”
Describes the study and any potential safety concerns related to the experimental medication




Commonly Asked Questions
How does the study work? How often will I need to see my doctor or visit the cancer center?

Will I need to undergo additional tests?
What is currently known about the new drug or combination?


What benefits can I expect?
What side effects should I expect? Who should I notify if I have side effects?


Can I take my vitamins or other medications?

Can I get the treatment with my local doctor?



Will my insurance pay for my participation in the clinical trial?



Clinicaltrials.gov https://clinicaltrials.gov/




ncreasing-diversity-in-cancer-clinical-research









Q&A WITH PANEL
Housekeeping Items
Presentation Slides: Are available by scanning the QR code, Instructions are on the QR code handout on each table.
Program Evaluations: evaluations at the end of today.
Restrooms: Outside the meeting hall, take a left and then proceed towards the hotel reception. Bathrooms are on the right just before reception desk.

Badge Holders: Please return your badge holders and we can recycle them.
We greatly appreciate your time and feedback!


BREAK
WHEN YOU RETURN FROM BREAK
PLEASE HEAD TO YOUR SELECTED BREAKOUT SESSION:

BREAKOUT A: NDMM - GETTING STARTED WITH MYELOMA
MANAGEMENT
Dr. Shonali Midha, MD
Please move to Hastings II Room
BREAKOUT B: RRMM - CONTINUING THE MYELOMA TREATMENT JOURNEY
Dr. Andrew Yee, MD
Please remain in this room
Thank you to our speakers & our sponsors!











Frontline Therapy

Shonali Midha, MD
Dana-Farber Cancer Institute ,Boston, MA

Objectives
Review the importance of DEPTH of response in early treatment of myeloma and the increasing use of MRD testing
Discuss emerging approaches in transplant eligible patients, including quadruplet therapy and stem cell transplantation
Outline the approach to a patient not going to transplant and how to optimize therapy

Goals of Therapy: The Iceberg Model of Myeloma

Treatment
>1 Billion
>1 Trillion D i s e a s e B u r d e n ( # o f m y e l o m a c e l l s )
>10 Million
1 myeloma cell in 100K to 1 million normal cells

Symptomatic Myeloma

At diagnosis
Partial response

50% reduction in M protein
Very good partial response
90% reduction in M protein immunofixation positive only

Complete remission No M-protein immunofixation negative

Minimal Residual Dis Flow Cytometry

Minimal Residual Dis
Next Generation Molecular testing


Depth of response matters!
MRD refers to the persistence of residual tumor cells after treatment and is responsible for relapse1
Current techniques can detect MRD with a sensitivity of 10-6 for MM cells2
DEPTH
MR→PR→ VGPR→CR →sCR
1.
minimal response; neg, negative; pos, positive; R, relapse


Adapted from Hauwel M, Matthes T. Swiss Med Wkly 2014:144:w13907 2. Biran N, et al. Curr Hematol Malig Rep 2014;9:368–78
MRD is Prognostic – Both for PFS and OS
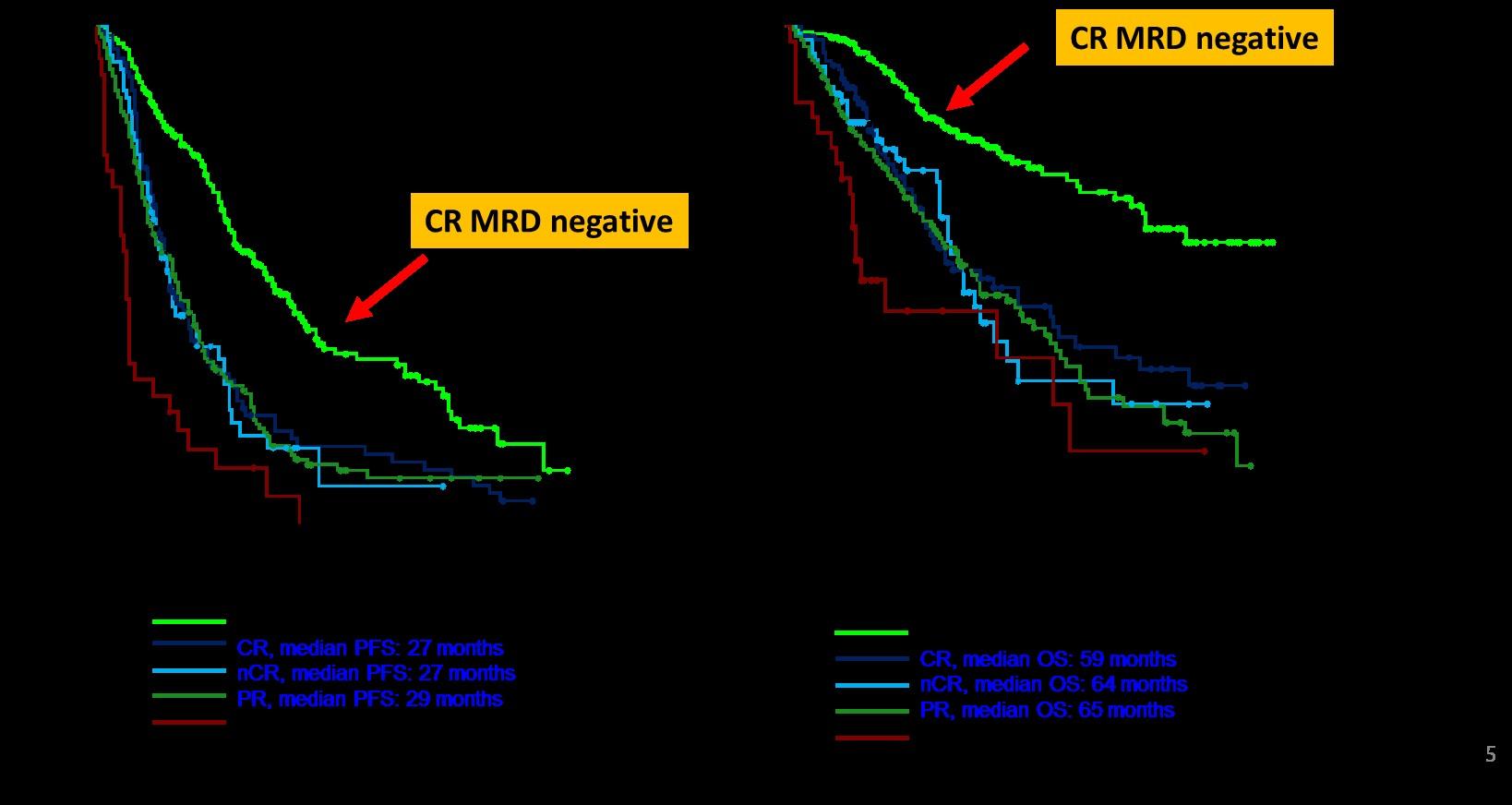
JJ,
B, et al. J Clin Oncol. 2017; 35(25): 2900–2910

Lahuerta
Paiva

SMOLDERING MULTIPLE MYELOMA
What about Smoldering Myeloma?
• Recall that all patients prior to having active myeloma, have MGUS (monoclonal gammopathy of undetermined significance) then SMM (smoldering myeloma)
• Historically we have not treated smoldering myeloma, but redefined MM to include “ultra-high risk smoldering myeloma”
• But now clinical trials are being conducted in patients with smoldering myeloma – mostly “high risk”
• High risk SMM is defined in different ways, often including at least 2 out of 3 of the following factors:
• M spike 2 or more
• Light chain ratio (involved/uninvolved) 20 or over
• Bone Marrow Plasmacytosis of 20% or higher

Phase 3 Randomized Study of Daratumumab Monotherapy Versus Active Monitoring in Patients With High-risk Smoldering Multiple Myeloma: Primary Results of the AQUILA Study
Meletios A Dimopoulos1, Peter M Voorhees2, Fredrik Schjesvold3, Yael C Cohen4, Vania Hungria5, Irwindeep Sandhu6 ,
Jindriska Lindsay7, Ross I Baker8, Kenshi Suzuki9, Hiroshi Kosugi10, Mark-David Levin11, Meral Beksac12 , Keith Stockerl-Goldstein13, Albert Oriol14, Gabor Mikala15, Gonzalo Garate16, Koen Theunissen17, Ivan Spicka18 ,
Anne K Mylin19, Sara Bringhen20, Katarina Uttervall21, Bartosz Pula22, Eva Medvedova23, Andrew J Cowan24 , Philippe Moreau25, Maria-Victoria Mateos26, Hartmut Goldschmidt27, Tahamtan Ahmadi28, Linlin Sha29, Els Rousseau30 , Liang Li29, Robyn M Dennis31, Robin Carson32, S Vincent Rajkumar33
1National and Kapodistrian University of Athens, Alexandra General Hospital, Athens, Greece; 2Levine Cancer Institute, Atrium Health Wake Forest University School of Medicine, Charlotte, NC, USA; 3Oslo Myeloma Center, Department of Hematology, Oslo University Hospital, Oslo, Norway; 4Tel-Aviv Sourasky (Ichilov) Medical Center and Tel Aviv University, Tel Aviv, Israel; 5Clínica Medica São Germano, São Paulo, Brazil; 6Cross Cancer Institute, University of Alberta, Edmonton, AB, Canada; 7Kent and Canterbury Hospital, Kent, UK; 8Perth Blood Institute, Murdoch University, Perth, Australia; 9Japanese Red Cross Medical Center, Tokyo, Japan; 10Ogaki Municipal Hospital, Ogaki City, Japan; 11Albert Schweitzer Hospital, Dordrecht, The Netherlands; 12Ankara University, Ankara, Turkey; 13Washington University School of Medicine, St. Louis, MO, USA; 14Institut Català d'Oncologia and Institut Josep Carreras, Hospital Germans Trias I Pujol, Barcelona, Spain; 15South-Pest Central Hospital, National Institute for Hematology and Infectious Diseases, Budapest, Hungary; 16Hospital Alemán, Buenos Aires, Argentina; 17Jessa Hospital, Hasselt, Belgium; 18Charles University and General Hospital, Prague, Czech Republic; 19Rigshospitalet, University of Copenhagen, Copenhagen, Denmark; 20SSD Clinical Trials in Oncol-ematologia e Mieloma Multiplo, AOU Città della Salute e della Scienza di Torino, Torino, Italy; 21Medical Unit Hematology, Karolinska University Hospital, Stockholm, Sweden; 22Institute of Hematology and Transfusion Medicine, Warszawa, Poland; 23Knight Cancer Institute, Oregon Health & Science University, Portland, OR, USA; 24University of Washington and Fred Hutchinson Cancer Center, Seattle, WA, USA; 25University Hospital Hôtel-Dieu, Nantes, France; 26University Hospital of Salamanca/IBSAL/Cancer Research Center-IBMCC (USAL-CSIC), Salamanca, Spain; 27GMMG Study Group at University Hospital Heidelberg, Internal Medicine V, Heidelberg, Germany; 28Genmab US Inc., Plainsboro, NJ, USA; 29Janssen Research & Development, LLC, Shanghai, China; 30Janssen Research & Development, Beerse, Belgium; 31Janssen Research & Development, LLC, Raritan, NJ, USA; 32Janssen Research & Development, LLC, Spring House, PA, USA; 33Mayo Clinic, Rochester, MN, USA.
Presented by MA Dimopoulos at the 66th American Society of Hematology (ASH) Annual Meeting & Exposition; December 7-10, 2024; San Diego, CA, USA
https://www.congresshub.com/ASH2024/ Oncology/Daratumumab/Dimopoulos
The QR code is intended to provide scientific information for individual reference, and the information should not be altered or reproduced in any way.

AQUILA: Study Design
AQUILA enrollment period: December 2017 to May 2019 at 124 sites in 23 countries
Screening
Key eligibility criteria:
• ≥18 years of age
• Confirmed SMM diagnosis (per IMWG criteria) for ≤5 years
• ECOG PS score of 0 or 1
• Clonal BMPCs ≥10% and ≥1 of the following risk factors:
- Serum M-protein ≥30 g/L
- IgA SMM
- Immunoparesis with reduction of 2 uninvolved Ig isotypes
- Serum involved:uninvolved FLC ratio ≥8 and <100
- Clonal BMPCs >50% to <60%
All patients were required to have CT/PET-CT and MRI imaging during screening
Treatment/active monitoring phase Follow-up phase
DARA monotherapy
1800 mg SCb QW Cycles 1-2, Q2W Cycles 3-6, Q4W thereafter in 28-day cycles until 39 cycles/36 months*
Active monitoring
No disease-specific treatment, with AE monitoring up to 36 months*
• Efficacy follow-up until progression by SLiM-CRAB
• Survival follow-up every 6 months until end of study
Primary endpoint:
• PFS by IRC per IMWG SLiM-CRAB criteriac Key secondary endpoints:
• ORR
• Time to first-line treatment for MM
• PFS on first-line treatment for MM
• Overall survival
*Or confirmed disease progression (whichever occurred first).
Stratified by number of risk factorsa for progression to MM (<3 vs ≥3)
Disease evaluation schedule
• Laboratory efficacy – Every 12 weeks by central lab until disease progression
• Imaging (CT/PET-CT, MRI) – Yearly (central review)
• Bone marrow – At least every 2 years
IMWG, International Myeloma Working Group; ECOG PS, Eastern Cooperative Oncology Group performance status; BMPC, bone marrow plasma cell; FLC, free light chain; CT, computed tomography; MRI, magnetic resonance imaging; QW, weekly; Q2W, every 2 weeks; Q4W, every 4 weeks; AE, adverse event; IRC, independent review committee; ORR, overall response rate. aRisk factors included involved:uninvolved FLC ratio
30 g/L (yes vs no), IgA SMM (yes vs no), immunoparesis (reduction of 2 uninvolved immunoglobulins vs other), or clonal BMPCs (>50% to <60% vs 50%). bDARA SC (1800 mg co-formulated with recombinant human hyaluronidase PH20 [rHuPH20; 2,000 U/mL; ENHANZE® drug delivery technology; Halozyme, Inc.]). cPFS was defined as duration from randomization to

AQUILA: Progression to MM by IMWG SLiM-CRAB Criteria (IRC Assessment)
follow-up: 65.2 months

AQUILA: Overall Survival
*Deaths due to an event occurring after the AE reporting window (ie, events that happened after patient started subsequent therapy or >30 days after last dose) or deaths with unknown reason.
Early intervention with fixed duration DARA extended overall survival versus active monitoring

AQUILA: Safety Overview

Points for Smoldering Myeloma
• Wow this is a VERY important study and may well change the way we think about and treat high risk smoldering myeloma
• The study was critical to really prove we can delay the progression to active myeloma and even improve survival with 3 years of daratumumab
• It underscores the importance of a DISCUSSION with the healthcare team as many options can be offered to patients with high-risk smoldering MM
• There will be MANY more trials coming in this area, with even more intense therapies like combinations and even CAR T Cell therapy...



NEWLY DIAGNOSED MULTIPLE MYELOMA
Newly Diagnosed MM and Risk
Stratified
Factors to be considered for ASCT
Age, performance status (PS), comorbidities (R-MCI score, HCT-Cl) and organ function

General Principles of Initial Therapy
1. Most patients will be given a combination of drugs to control the disease quickly, usually a QUADRUPLET
2. We don’t “save the best for last” because early therapies have a long term effect on survival
3. We seek a DEEP and DURABLE response
4. We mix and match from the 3 major classes of drugs and add steroids:
Proteasome Inhibitors – most often botezomib (Velcade)
Immunomodulatory Drugs – lenalidomide (Revlimid)
Monoclonal Antibodies – daratumumab (Darzalex) and Isatuximab (Sarclisa)
5. We decide early on whether or not someone will have a stem cell

THIS JUST IN!!
QUADRUPLET therapies are becoming the standard of care for MOST (but not all) patients with newly diagnosed MM
DVRD and Isa-VRD combinations below now FDA approved
Transplant Eligible
Darzalex-Velcade-Revlimid-Dexamethasone (PERSEUS)
Transplant Ineligible
Sarclisa-Velcade-Revlimid-Dexamethasone (IMROZ)
Sarclisa-Velcade-Revlimid-Dexamethasone (BENEFIT)
Darzalex-Velcade-Revlimid-Dexamethasone (CEPHEUS)


PERSEUS: Study Design
Induction
V: 1.3 mg/m2 SC Days 1, 4, 8, 11
R: 25 mg PO Days 1-21
d: 40 mg PO/IV Days 1-4, 9-12
Consolidation
Maintenance
VRd administered as in the VRd group
Primary endpoint: PFSc Key secondary endpoints: Overall CR rate,c overall MRD-negativity rate,d
D-R until PD Discontinue DARA therapy only
Discontinue DARA therapy only after 24 months of D-R maintenance for patients with CR and 12 months of sustained MRD negativity
Restart DARA therapy upon confirmed loss of CR without PD or recurrence of MRD
ECOG PS, Eastern Cooperative Oncology Group performance status; V, bortezomib; SC, subcutaneous; PO, oral; d, dexamethasone; IV, intravenous; QW, weekly; Q2W, every 2 weeks; PD, progressive disease; Q4W, every 4 weeks; MRD, minimal residual disease; CR, complete response; OS, overall survival; ISS, International Staging System; rHuPH20, recombinant human hyaluronidase PH20; IMWG, International Myeloma Working Group; VGPR, very good partial response. aStratified by ISS stage and cytogenetic risk. bDARA 1,800 mg co-formulated with rHuPH20 (2,000 U/mL; ENHANZE drug delivery technology, Halozyme, Inc., San Diego, CA, USA). cResponse and disease progression were assessed using a computerized algorithm based on IMWG response criteria. dMRD was assessed using the clonoSEQ assay (v.2.0; Adaptive Biotechnologies, Seattle, WA, USA) in patients with VGPR post consolidation and at the time of suspected CR. Overall MRD-negativity rate was defined as the proportion of patients who achieved both MRD negativity (10 –5 threshold) and CR at any time.
PERSEUS: Progression-free Survival
PERSEUS: Overall CR Rates
Subgroup no. of patients with ≥CR/total no. (%)
143/205 (69.8) 105/149 (70.5)
186/267 (69.7) 62/87 (71.3)
226/323 (70.0) 22/31 (71.0)
129/178 (72.5)
84/125 (67.2) 34/50 (68.0)
122/185 (65.9) 73/96 (76.0)
Cytogenetic
182/266 (68.4) 59/78 (75.6) 7/10 (70.0)
160/230 (69.6) 88/124 (71.0)
185/211 (87.7) 127/144 (88.2)
(90.0) 77/94 (81.9)
(87.6)
(92.0)
(89.8)
(88.6)
(80.0)
(87.3) 72/78 (92.3)
234/264 (88.6) 63/76 (82.9) 15/15 (100)
195/221 (88.2) 117/134 (87.3)
3.34 (1.87-5.95) 3.79 (1.91-7.54) 1.88 (0.77-4.58)
3.54 (2.12-5.90) 3.78 (1.45-9.83)

Phase 3 Study Results of Isatuximab, Bortezomib, Lenalidomide, and Dexamethasone (Isa-VRd) Versus VRd for
Transplant-Ineligible Patients With Newly Diagnosed Multiple Myeloma (IMROZ)
Thierry Facon,1 Meletios-Athanasios Dimopoulos,2 Xavier Leleu,3 Meral Beksac,4,5 Ludek Pour,6
Roman Hajek,7 Zhuogang Liu,8 Jiri Minarik,9 Philippe Moreau,10 Joanna Romejko-Jarosinska,11 Ivan Spicka,12
Vladimir Vorobyev,13 Michele Cavo,14 Hartmut Goldschmidt,15 Thomas Martin,16 Salomon Manier,17
Marie-France Brégeault,18 Sandrine Macé,18 Christelle Berthou,18 Robert Z. Orlowski19
1Department of Haematology, University of Lille, and French Academy of Medicine, Paris, France; 2Department of Clinical Therapeutics, National and Kapodistrian University of Athens, Greece; 3Service d'Hématologie et Thérapie Cellulaire, CHU and CIC Inserm 1402, Poitiers Cedex, France; 4Department of Hematology, Ankara University, Ankara, Turkey; 5Istinye University Ankara Liv Hospital, Ankara, Turkey; 6Department of Internal Medicine, Hematology and Oncology, University Hospital Brno, Brno, Czech Republic; 7Department of Hemato-Oncology, University Hospital Ostrava and Faculty of Medicine, University of Ostrava, Ostrava, Czech Republic; 8Shengjing Hospital of China Medical University (Huaxiang Br), Shenyang, China; 9Department of HematoOncology, University Hospital Olomouc and Faculty of Medicine and Dentistry, Palacký University Olomouc, Olomouc, Czech Republic; 10Department of Hematology, University Hospital HôtelDieu, Nantes, France; 11Department of Lymphoid Malignancies, Marie Sklowdoska-Curie National Research Institute of Oncology, Warszawa, Poland; 12Charles University and General Hospital in Prague, Prague, Czech Republic; 13SP Botkin Moscow City Clinical Hospital, Moscow, Russia; 14IRCCS Azienda Ospedaliero-Universitaria di Bologna, Istituto di Ematologia "Seràgnoli," Università di Bologna, Bologna, Italy; 15Department of Internal Medicine V, University of Heidelberg, Heidelberg, Germany; 16Department of Hematology, University of California at San Francisco, San Francisco, California, USA; 17Department of Hematology, University Hospital Center of Lille, Lille, France; 18Sanofi, R&D, Vitry-sur-Seine, France; 19Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.

ID #OA-49

Study design: Isa-VRd vs VRd in transplant-ineligible NDMM
Initiation phase (4 x 6-week
Treatment until PD, unacceptable toxicities, patient withdrawal
Primary endpoint: PFS
Key secondary endpoints: CR rate, MRD– CR (NGS, 10-5) rate, ≥VGPR rate, OS
(bone marrow aspirate)
In case of CR or VGPR
*Patients considered Ti due to age or comorbidities.
†In the maintenance phase, patients randomized to the VRd arm who experience PD may cross over to receive Isa-Rd.
‡10 mg/day if eGFR 30 to <60 mL/min/1.73 m2 .
§If aged ≥75 years, d was administered on days 1, 4, 8, 11, 15, 22, 25, 29, and 32.
C, cycle; CR, complete response; d, dexamethasone; eGFR, estimated glomerular filtration rate; Isa, isatuximab; MRD, minimal residual disease; NDMM, newly diagnosed multiple myeloma; NGS, next-generation sequencing; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PO, orally; R, lenalidomide; SC, subcutaneous; Ti, treatment-ineligible; V, bortezomib; VGPR, very good partial response. Orlowski RZ, et al. ASCO 2018.

Baseline characteristics
Patient characteristics were balanced in both arms
*One patient in the Isa-VRd arm had an ECOG PS of 3. †High risk defined as the presence of del(17p) and/or t(4;14) and/or t(14;16), with cutoffs defined in footnote ‡ . ‡Abnormality defined as present in at least 30% of abnormal bone marrow plasma cells for t(4;14) and t(14;16) and 1q21+ (at least 3 copies), and at least 50% of abnormal plasma cells for del(17p). Only one patient had 2 high-risk cytogenetic abnormalities: del(17p) and t(4;14). §1q21+ defined as at least 3 copies of 1q21. Amplification 1q21 defined as at least 4 copies of 1q21. ¶In addition, there were 67 (25.3%; Isa-VRd) and 49 (27.1%; VRd) patients with paramedullary disease and 1 patient in each group with both
Primary endpoint met: Interim PFS analysis–IRC assessment in ITT population

60-mo PFS rate: 63.2%
mPFS: NR
60-mo
mPFS: 54.34 months (95% CI, 45.207 to NR)
*Cutoff date for PFS analysis: September 26, 2023 (median follow-up, ~5 years). †Nominal one-sided P value. CI, confidence interval; HR, hazard ratio; Isa, isatuximab; ITT, intent-to-treat; mPFS, median PFS; NR, not reached; PFS, progression-free survival; Rd, lenalidomide and dexamethasone; VRd, bortezomib, lenalidomide and dexamethasone. Facon T, Dimopoulos MA, Leleu X, et al. Isatuximab, Bortezomib, Lenalidomide and Dexamethasone for Multiple Myeloma.
BENEFIT Study design: Isa-VRd vs Isa-Rd in Ti NDMM
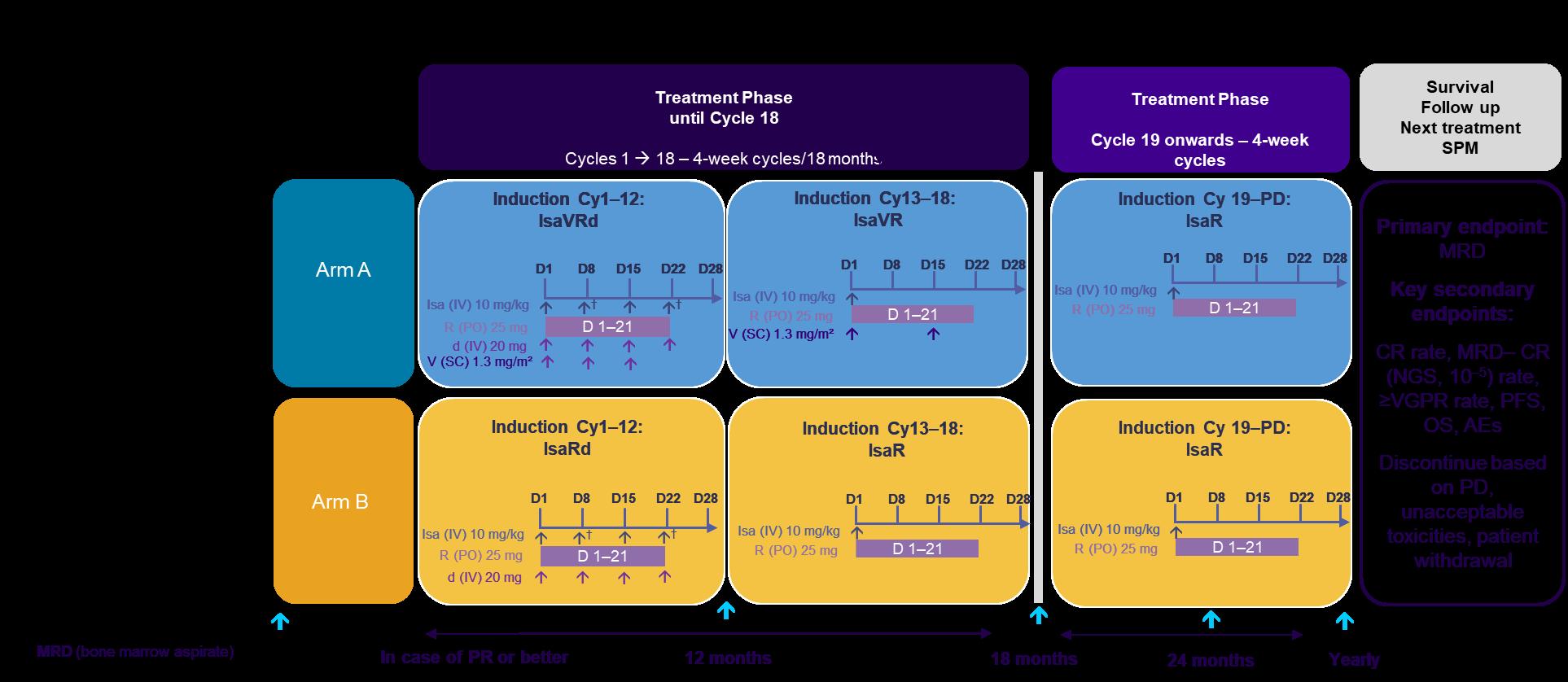
†Cycle 1 only. CR, complete response; Cy, cycle; d, dexamethasone;
PRESENTED BY: Xavier Leleu, MD, PhD
D, day; Isa, isatuximab; M, month; MRD, minimal residual disease; NDMM, newly diagnosed multiple myeloma; NGS, next generation sequencing; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; R, lenalidomide; SPM, second primary malignancy; Ti, transplant-ineligible; V, bortezomib; VGPR, very good partial response.
Quadruplet Frontline Summary
Quadruplets are indeed better than triplets in patients going to transplant
They also seem to be better in transplant ineligible patients but with some caveats we have less evidence in patients over 80 dosing of drugs is CRITICAL to ensure tolerability
Revlimid – we don’t need 25mg in most patients
Velcade – should be given weekly – still unclear as to length of time
Dexamethasone – DOWN with DEX! – we can taper and discontinue in the first 4-6 months
Darzalex-VRD for transplant eligible and Sarclisa-VRD for transplant ineligible now FDA approved!

#DownWithDex
HISTORICAL TREND TOWARD OPTIMAL DEXAMETHASONE DOSING
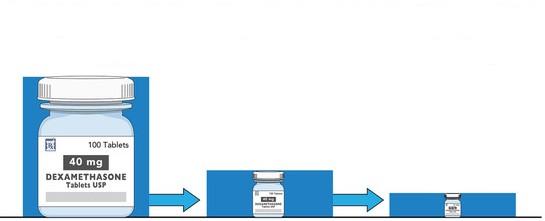
Dose
Dose
Dose
40 mg x 12 days per month x 6 months
40 mg x 4 days per month x 6 months
Harding and Mikhael, Blood Editorial “Down with dex!” 2025
Optimal dose yet to be defined


DO WE REALLY STILL NEED TRANSPLANT??
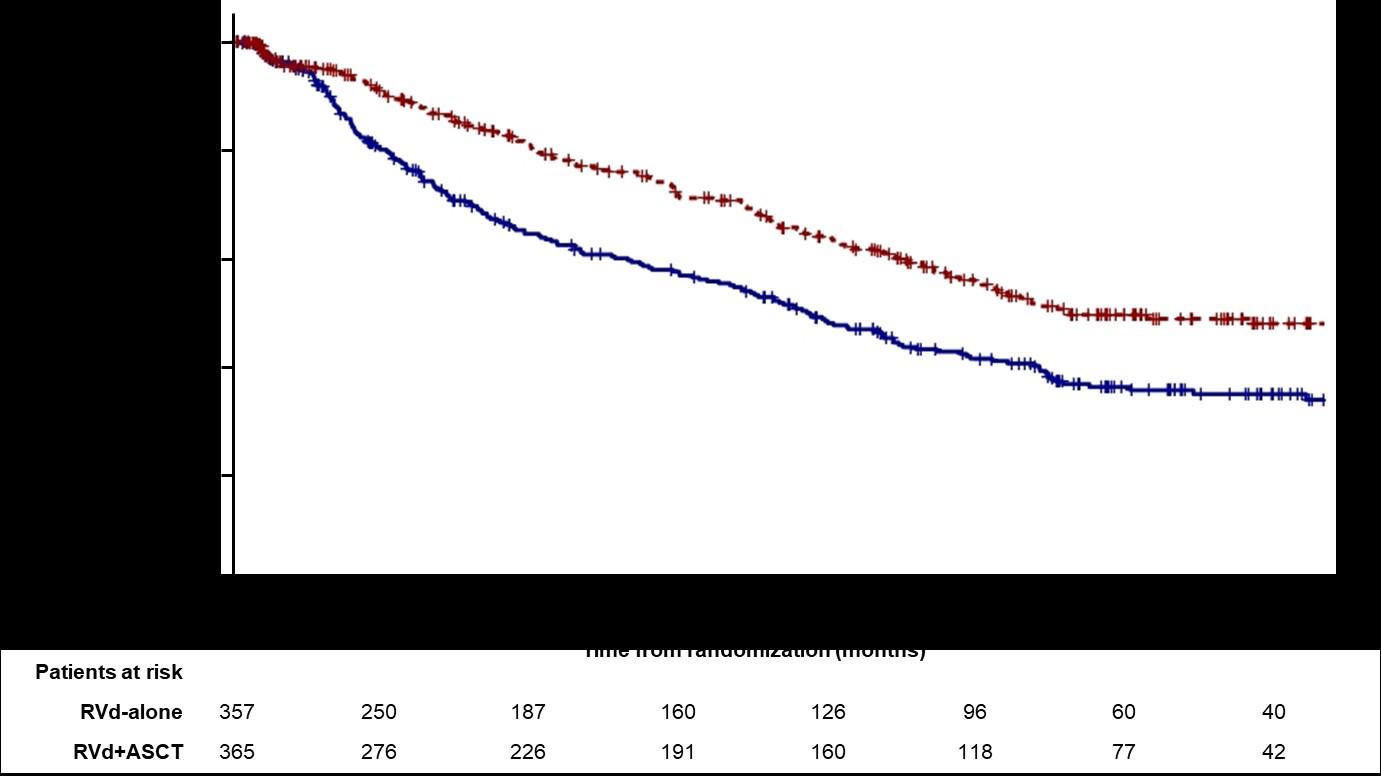
RVD +Stem Cell Transplant vs. RVD without Transplant
DETERMINATION Trial of Newly Diagnosed MM: DESIGN
-Patients aged 18-65 yrs with symptomatic newly diagnosed MM following 1 cycle of RVD -56 sites within the United States from 2010 to 2018
End Points of Study and Follow-up
Melphalan 200 mg/m2 + Stem Cell Support (n = 310)
• Primary end point: progression-free survival (time to next relapse)
• Secondary end points included:
• Response rates, overall survival, quality of life, and adverse events
• Follow-up on participant status : median of 6 years

Primary endpoint: Progression-free survival (PFS)


DETERMINATION Trial of Newly Diagnosed MM Quality of Life
Global Health Status/QoL, Physical Functioning

DETERMINATION Discussion
ASCT remains very relevant and important in prolonging PFS in younger and eligible patients
BUT it may not be mandatory in all eligible patients upfront
As with other agents, we INDIVIDUALIZE the sequencing patterns
ASCT does carry genuine toxicity, short term and long term
We may become callous to these toxicities
Maintenance therapy remains an important part of myeloma therapy
Joseph Mikhael – ASCO Plenary Discussant DETERMINATION

ASCO Guidelines: What criteria are used to assess eligibility for autologous stem cell transplant (SCT)?
Recommendation
Patients should be referred to a transplant center to determine transplant eligibility
Evidence Rating
Type: Evidence based
Evidence quality: Intermediate, benefit outweighs harm
Strength of recommendation: Moderate
Chronologic age and renal function should not be the sole criteria used to determine eligibility for SCT.
Type: Evidence based
Evidence quality: Intermediate, benefit outweighs harm
Strength of recommendation: Moderate
Mikhael J, et al. J Clin Oncol. April 1, 2019. DOI:10.1200/JCO.18.02096.

What about patients not eligible for transplant?
As noted before, quadruplets are now becoming the standard of care for most patients
However, some patients may not be “quad eligible” In these patients, triplets, or even doublets may be considered the most commonly used regimen is DRD – darzalex, revlimid and dex this is based on the MAIA trial of DRD vs RD

MAIA Study Design – DRD vs RD
‒ Patients were enrolled in MAIA from March 2015 through January 2017
D: 16 mg/kg IV
QW Cycles 1-2, Q2W Cycles 3-6, then Q4W thereafter until PD
Key eligibility criteria
• TIE NDMM
• ECOG PS
score 0-2
• CrCl
≥30 mL/min
R: 25 mg PO Days 1-21 until PD
da: 40 mgb PO or IV Days 1, 8, 15, 22 until PD
Primary endpoint

R: 25 mg PO Days 1-21 until PD
d: 40 mg PO Days 1, 8, 15, 22 until PD
Cycles: 28 days Rd
End-oftreatment visit (30 days after last dose) Longterm follow-up
• PFS Key secondary endpoints
• OS
• PFS2
• ORR
• CR/sCR rate
• MRD (NGS; 10–5) 1
Updated PFS

Overall Survival

Key Take-Aways
Although historically we have not treated smoldering myeloma, new evidence suggests we can treat patients with high risk SMM with daratumumab monotherapy for 3 years
D-VRD is the new standard of care in Transplant Eligible Patients
Isa-VRD is a new standard of care in Transplant Ineligible Patients
DRD or Sarclisa+Rd may still be considered in some patients
Although ASCT remains the standard of care, use is likely to decline in patients who are 65-75 or with significant comorbidities
Continuous therapy has resulted in better outcomes
The balance of toxicity and efficacy is particularly important in this population
ESPECIALLY with dexamethasone
Ongoing studies will help us decide if CAR T cell therapy should move upfront and possibly even replace transplant
What we do frontline has an impact in the long term...



Breakout B
RRMM: Continuing the Myeloma Treatment Journey
Dr. Andrew Yee, MD


Relapsed Therapy

Andrew J. Yee, MD
Clinical Director, Center for Multiple Myeloma
Massachusetts General Hospital Cancer Center
Assistant Professor of Medicine
Harvard Medical School
Waltham, MA
September 2025

Discuss an approach to treating relapsed myeloma based on patient, disease, and treatment characteristics
Outline the key results from recent trials in relapsed disease
OBJECTIVES
Discuss the approach to relapse in later lines and the use of novel therapies such as CAR T-cells and bispecific antibodies


Before 2020s: three core drug classes


thalidomide
lenalidomide

cereblon

Immunomodulatory drugs
E3 ubiquitin ligase complex

melphalan cyclophosphamide bendamustine melflufen*

pomalidomide 19S cap
bortezomib
carfilzomib

core


selinexor

Proteasome inhibitors




Anti-CD38 monoclonal antibodies





venetoclax dexamethasone

glucocorticoid receptor


*Withdrawal from US market October 2021
†Withdrawn from US market November 2021
After 2020s: BCMA therapies


thalidomide
lenalidomide

E3 ubiquitin ligase complex

pomalidomide
venetoclax







isatuximab
ciltacabtagene autoleucel Feb 2022 cereblon selinexor





glucocorticoid receptor panobinostat†






teclistamab Approved Oct. 2022
elranatamab Approved Aug 2023
linvoseltamab Approved July 2025


bispecific antibodies CAR T-cell therapies

idecabtagene vicleucel March 2021




*Withdrawal from US market October 2021
†Withdrawn from US market November 2021

talquetamab Aug 2023

bispecific antibody
‡Withdrawn from US market November 2022 after accelerated approval August 2020 but may return based on recent DREAMM-7 and DREAMM-8 studies; awaiting FDA announcement October 2025

An Approach to Relapsed MM
• It is not a simple algorithm of treatment #1 then #2 then #3…
• Leverage the benefit of multiple mechanisms of action in combination therapy
Categories:
• 1-3 prior lines
• ≥4 prior lines
• Triple class refractory = refractory to proteasome inhibitor (e.g. bortezomib), immunomodulatory drug (e.g. lenalidomide) and anti-CD38 monoclonal antibody (e.g. daratumumab or isatuximab)



Definitions:
What is relapsed/refractory disease and a line of therapy?
• Relapsed: recurrence (reappearance of disease, progression) after a response to therapy
• Refractory: progression despite ongoing therapy
• Biochemical progression: increase in M protein or free light chain values above a threshold
• Clinical progression: symptoms of disease such as new bone lesions, low blood counts; new imaging findings
• New line of therapy: change in treatment due to either progression of disease or unmanageable side effects
• Note: initial (or induction) therapy + auto stem cell transplant + consolidation/ maintenance therapy = 1 line of therapy



Multiple myeloma is not one disease: multiple journeys
Followed for MGUS
Routine surveillance shows anemia, bone lesions on imaging
Both patients have relapsed disease but treatment considerations will be different
Presents to emergency department with fatigue and back pain and found to have bone lesions 2nd line
line
Better response than initial therapy


Approach to relapsed multiple myeloma
Disease factors
Biochemical (based on laboratory studies) vs clinical relapse (anemia, new bone lesions, etc.)
Patient factors
Functional status
Comorbidities
Treatment history
Patient preferences
Eligibility and interest in a clinical trial
Earlier vs later relapse in disease course
Response to previous therapy
Tempo of relapse (gradual vs rapid rise in monoclonal protein, light chain)
Clinical features (new bone lesions, cytopenias, renal dysfunction, extramedullary disease)
Biology of disease (high-risk FISH)
Actionable genetic findings: t(11;14), BRAF mutation
Choice of therapy
Treatments available now may not have been available before Overcoming drug resistance; trying therapies with new mechanisms of action
Therapy factors
Route of administration
Schedule
Monitoring and availability of caregiver
Side effect profile
Availability of therapy locally v. at a major medical center
Cost of therapy (e.g. oral medications)


Naked antibodies“Naked” monoclonal
antibody
Daratumumab (Darzalex) — recognizes CD38
Isatuximab (Sarclisa) — recognizes CD38
Elotuzumab (Empliciti) — recognizes SLAMF7
Multiple myeloma cell



Antibody drug conjugate (ADC)
linker toxin

Example: Belantamab mafodotin (Blenrep) targets BCMA
Toxin mafodotin enhances efficacy
Mafodotin inhibits microtubule polymerization
Induces immunogenic cell death

Mafodotin associated with visual side effects (transient and reversible)



Accelerated approval August 2020 but withdrawn from US market November 2022.
May return based on recent DREAMM-7 and DREAMM-8 studies; FDA announcement expected October 2025


Myeloma cell

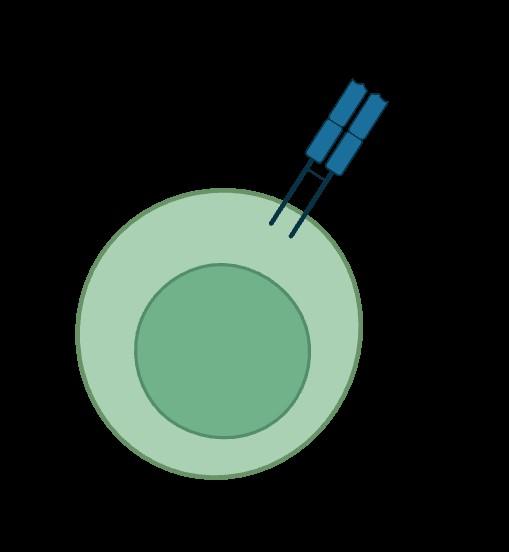
Bispecific antibodies
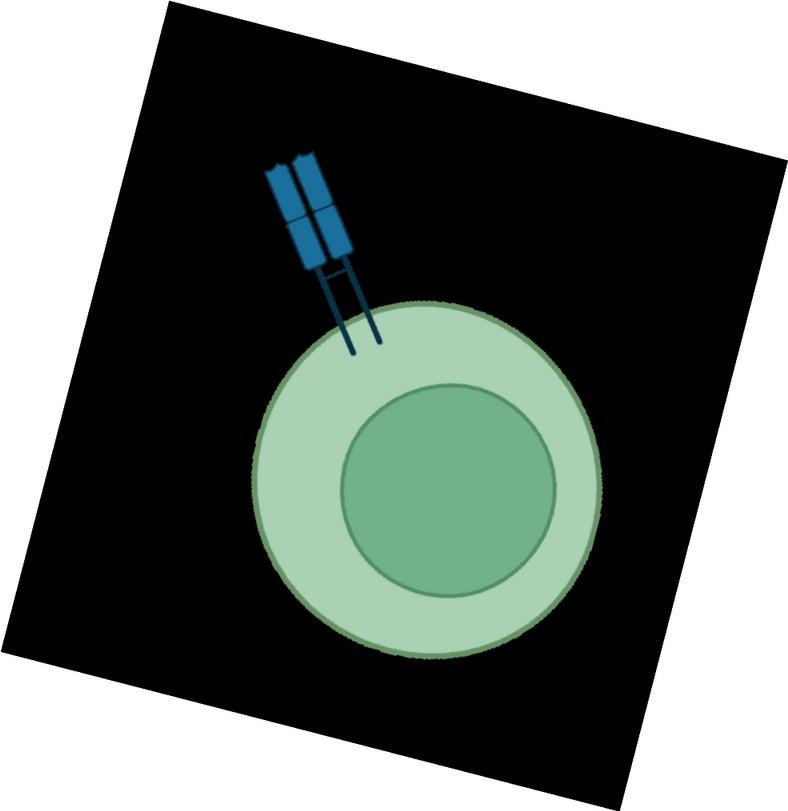
Four bispecific antibodies for two different targets on myeloma cells: BCMA and GPRC5D
Anti-BCMA
Teclistamab (2022, Tecvayli)
Elranatamab (2023, Elrexfio)
Linvoseltamab (2025, Lynozyfic)
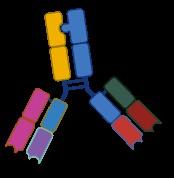
Bispecific antibodies bind to both CD3 on T-cells and a target on myeloma cells


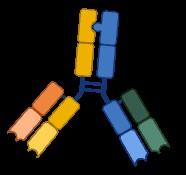
Anti-GPRC5D
Talquetamab (2023,
Cytokine release
T-cell activation
Perforin, granzymes
Multiple myeloma cell death


Talvey)
T-cells
T-cells
CD3
CD3
chimeric antigen receptor

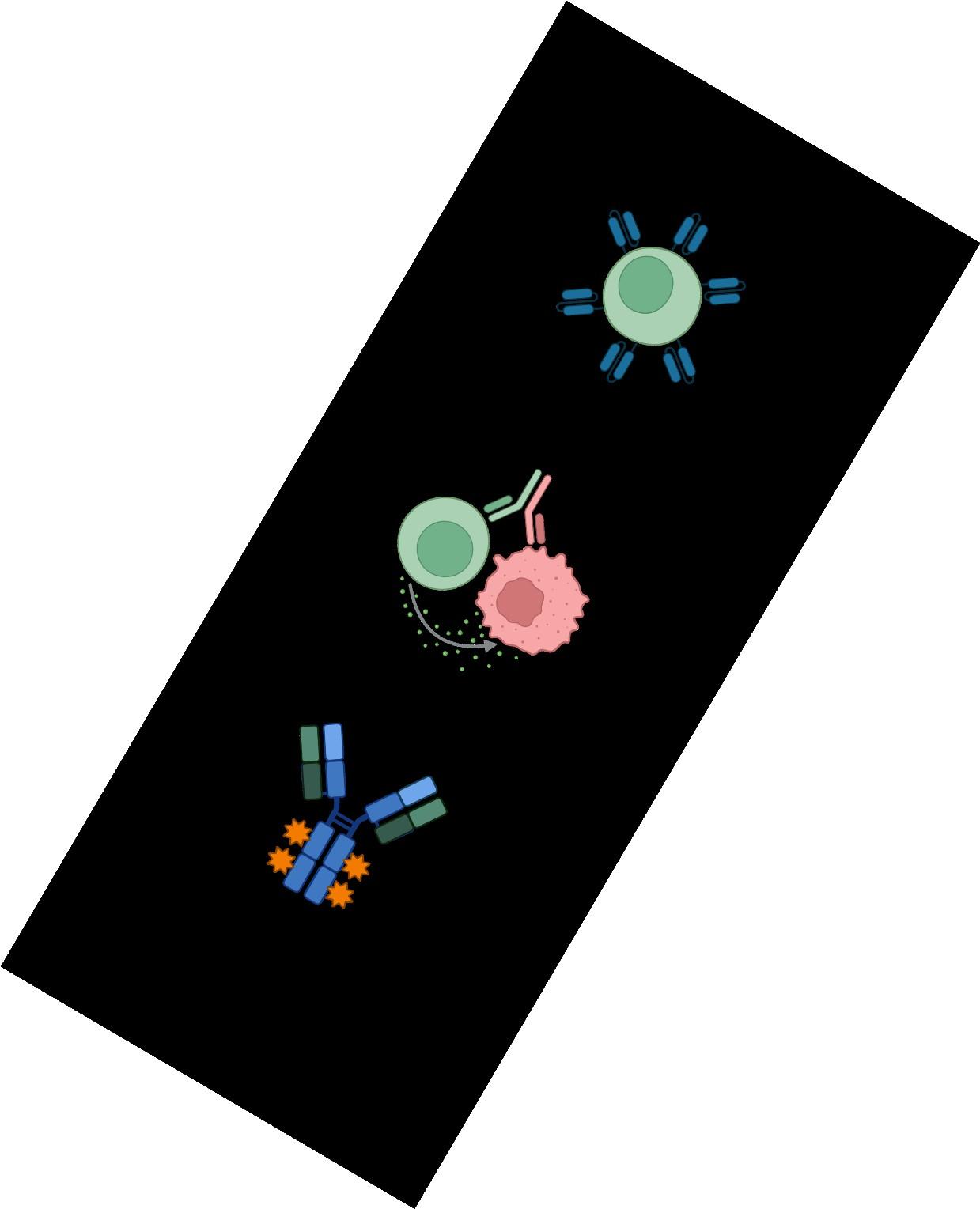
Idecabtagene vicleucel (Abecma, 2021)
Ciltacabtagene autoleucel (Carvykti, 2022)




CAR T-cell

General principles
For first relapse
Consider mechanisms of action not previously used
Triplets have more efficacy over doublets
In practice, for patients receiving VRd (bortezomib, lenalidomide, dex) like regimens and who have not had an anti-CD38 monoclonal antibody, multiple options for 1st relapse:
Daratumumab + pomalidomide + dex (APOLLO)
Isatuximab + pomalidomide + dex (ICARIA)
Daratumumab + carfilzomib + dex (CANDOR)
Isatuximab + carfilzomib + dex (IKEMA)
Selinexor + bortezomib + dex (BOSTON) or selinexor + carfilzomib + dex
Important Update (2024) – CAR T cell therapies can be used as early as first relapse!



First or Second Relapse (Options after Considering Clinical Trials)
(Modified from mSMART With New CAR T-cell Approvals)
Not refractory to anti-CD38 Less common with upfront use of anti-CD38
Not refractory to lenalidomide and standard risk or long 1st remission
Refractory to anti-CD38 and len
DRd
Refractory to lenalidomide
Standard risk and >2-3 years remission <2 years of remission and/or HRCA > 2-3 year remission and standard risk < 2 year remission or HRCA
DPd or IsaPd Or DVd CAR-T
Cilta-cel CAR T or anti-CD38 + Kd
KPd, KCd, PVd PCd, EloPd Or CAR T (Cilta-cel if 1+ prior LOT, Ide-cel if 2+ LOT)
Cilta-cel (1+ prior LOT) Or Ide-cel (2+ prior LOT)
Others to the left if not candidate for CAR-T
C = cyclophosphamide
D = daratumumab
d = dexamethasone
Elo = elotuzumumab
Isa = isatuximab
K = carfilzomib (Kyprolis)
P = pomalidomide
R = lenalidomide (Revlimid)
V = bortezomib (Velcade)
HRCA = high risk chromosomal abnormalities e.g. del17p, t(4;14), t(14;16) t(11;14) present venetoclax-based combinations
Dingli D, et al. Mayo Clin Proc. 2017;92(4):578-59. Updated 2024.



CAR T-cell therapy: from experimental to now standard of care

December 2012

UPenn (class of 2027)


CAR T-cell therapy: from experimental to now standard of care

December 2012

Axicabtagene
ciloleucel approved in October 2017 for diffuse large B-cell lymphoma
Tisagenlecleucel approved in August 2017 for acute lymphoblastic leukemia
June 5, 2025
Ciltacabtagene
autoleucel (Carvykti) approved in 2022 for multiple myeloma

UPenn (class of 2027)

Ide-cel CAR T (Abecma) vs standard of care:
2-4 prior lines of therapy, triple class exposed
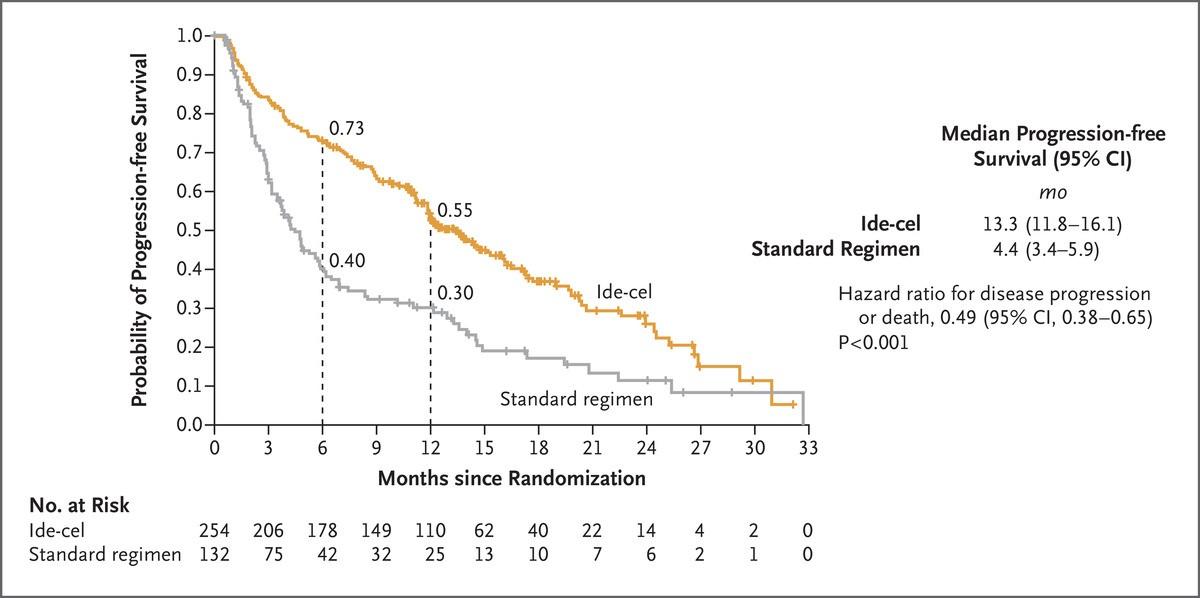
SOC = DPd, Kd, elo Pd, ixa Rd, DVd
P et al., N Engl J Med


Rodriguez-Otero

Cilta-cel
of therapy, lenalidomide refractory
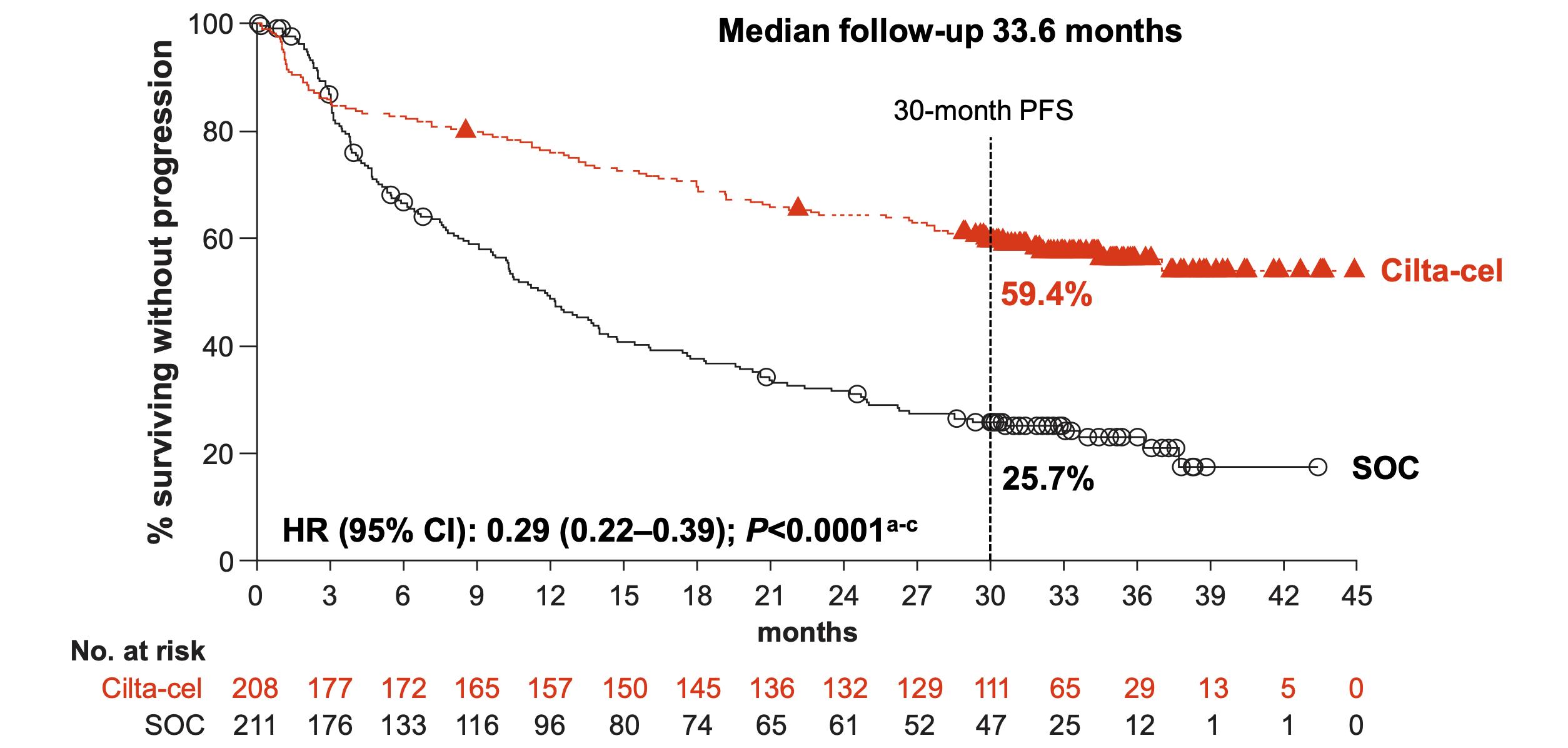


Mateos
OVERALL RESPONSE RATE AND PFS OF RECENTLY APPROVED THERAPIES IN RRMM
≥4 LOT* and triple refractory
Carfilzomib-dex
Richardson Blood 2014; 123:1826-32
Siegel Blood 2012; 120:2817-25
Lonial Lancet 2016; 387:1551-60
Rasche EHA 2024, P915
*Ide-cel approved after 2 prior lines
*Cilta-cel approved after 1 prior line
Van de Donk ASCO 2023; abs 8011
Lesokhin Nat Med 2023; 29:2259-67
Munshi NEJM 2021; 384:705-16
Munshi EHA 2023; S202
Huang ASCO 2024; 7511


CAR T-cell therapy in multiple myeloma available in earlier lines of therapy
Idecabtagene vicleucel (Abecma)
Approved in 4 prior lines, triple class exposed (March 2021)
Ciltacabtagene autoleucel (Carvykti)
Approved in 2 prior lines, triple class exposed (April 2024)
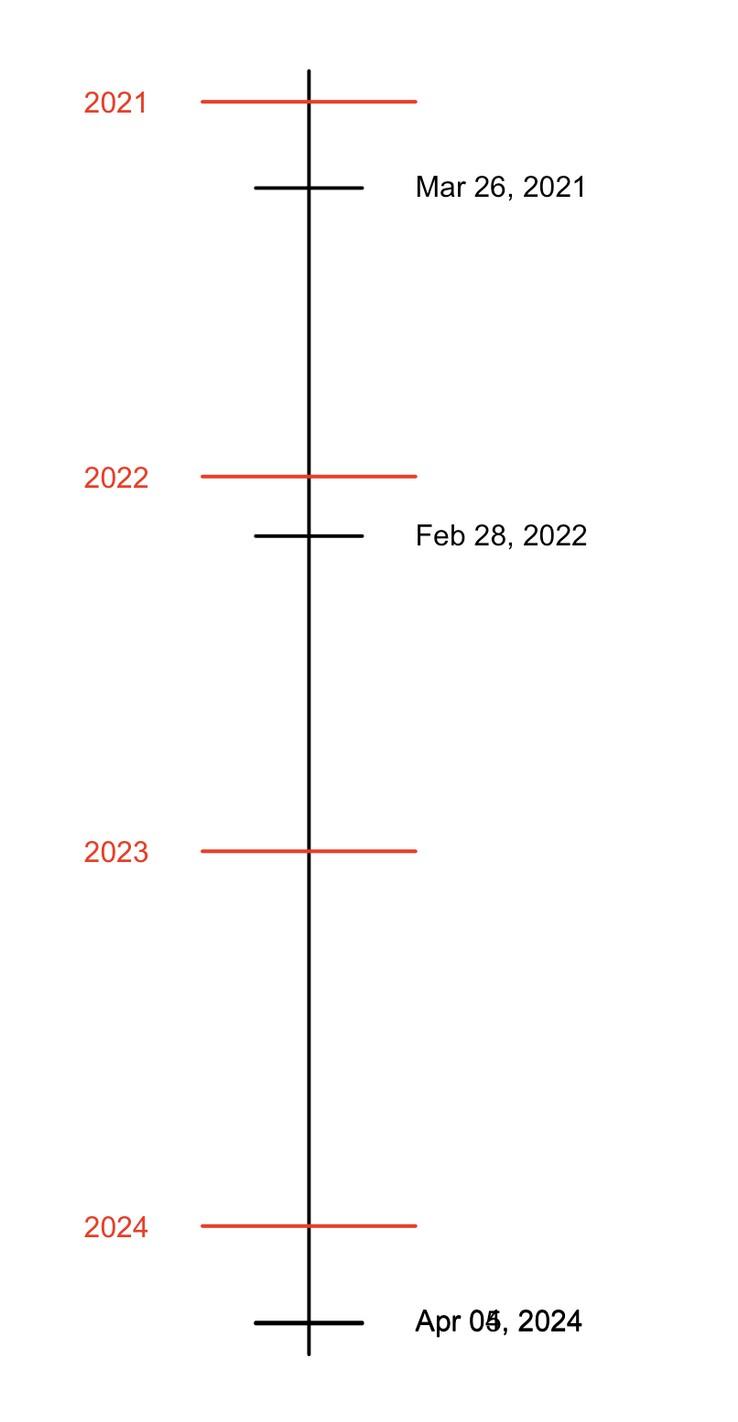
Approved in 4 prior lines, triple class exposed (February 2022)
What’s new in 2025
CAR T is more accessible as more sites become active
No more waiting lists
More patients will be eligible for CAR T-cell therapy as indications move to earlier lines of therapy
Approved in 1 prior line, lenalidomiderefractory (April 2024)
Ongoing trials in newly diagnosed multiple myeloma


CAR T-Cell Therapy Patient Journey

Immune cells from the patient are collected

Standard of care therapy (bridging) until CAR T-cells are ready for infusion

Fludarabine and cyclophosphamide are used to create “immunologic space” to CAR T cells to expand


Infusion of CAR T-cells may occur in the hospital or may occur as an outpatient
Monitoring for side effects may occur in the hospital (~1 week) and as an outpatient in proximity of the hospital



CAR T: Expected side effects in first 2 weeks

Cytokine release syndrome Neurotoxicity (ICANS)


Cytopenias Infections
CRS ICANS
Onset 1 9 days after CAR T-cell infusion 2 9 days after CAR T-cell infusion
Duration 5 11 days 3 17 days
Symptoms
• Fever
• Difficulty breathing
• Dizziness
• Nausea
• Headache
• Rapid heartbeat
• Low blood pressure
Management
• Tocilizumab
• Corticosteroids
• Supportive care
• Headache
• Confusion
• Language disturbance
• Seizures
• Delirium
• Cerebral edema
• Corticosteroids
• Anti-seizure medications
CRS: fever is common; uncommon low blood pressure, need for ICU is ICANS: some patients may have transient confusion
After two weeks, CRS and ICANS have generally resolved Monitoring for above generally involves hospitalization (about 1 week)



CAR T-cell therapy: other side effects in first month
• Blood counts can take take time to recover
• With cilta-cel: small risk of parkinsonism (~5% in initial trials, ~0.5% in more current trials)
• Most cases are reversible with supportive care, chemotherapy
• With cilta-cel: Bell’s palsy ~7% , transient
• Small risk of second blood cancer like leukemia
While neurological side effects above (mainly with cilta-cel) can occur, strategies at managing these are improving.
Beyond a month, patients are feeling well off therapy.


Bispecific antibodies: practical considerations
• Cytokine release syndrome (CRS) (less than CAR T-cells)
• First 2-3 doses involve step up dosing to minimize risk of CRS
• Monitoring for CRS during step up dosing
• Hospitalization per label, but increasingly this is being done as outpatient
• More and more sites have the capability to administer bispecific antibodies
• Minimize risk of infections with IVIG (with anti-BCMA)
• Dosing can be spaced out since deep responses happen early
• Some side effects are target dependent. With GPRC5D, change in taste, weight loss, rash, nail changes


Approved product
Anti-BCMA therapies
Anti-BCMA bispecific antibody Anti-BCMA CAR T-cell
Teclistamab
Elranatamab
Linvoseltamab
Efficacy +++
Idecabtagene vicleucel
Ciltacabtagene autoleucel
Combinations under investigation ++++
How given Weekly to biweekly to monthly until progression
“One-and-done”
Trials evaluating addition of maintenance therapy and consolidation
Notable adverse events Infection, hypogammaglobulinemia CRS and neurotoxicity
Late neurotoxicity with movement disorders (cilta-cel)
Immune effector cell diarrhea (cilta-cel)
Cytokine release syndrome ++ +++
Neurotoxicity Rare +
Availability Off-the-shelf; hospitalization for initial step-up dosing (but outpatient initiation increasing)
Limited to major medical centers
Wait time for manufacturing; hospitalization for monitoring
Ongoing randomized studies evaluating above therapies in earlier lines of treatment, including in newly diagnosed patients

Emerging Therapies for Relapsed/Refractory Multiple Myeloma
Bispecific antibodies and trispecific antibodies
• Etentamig, another anti-BCMA bispecific antibody with a favorable dosing schedule
• Cevostamab targeting a new cell surface protein, FcRH5
• Combinations of bispecific antibodies with e.g. daratumumab, being evaluated in earlier lines of therapy, including newly-diagnosed patients
• Trispecific antibodies: an antibody that can bind to three proteins all at once (e.g. BCMA,GPRC5D, and CD3 on T-cells)
CAR T-cell therapies in development
• Anito-cel (Arcellx), an anti-BCMA CAR T-cell in clinical trials, may be approved in 2026, favorable profile
• GC012F (AstraZeneca), targets both BCMA and CD19
• Anti-GPRC5D CAR T-cells
Small molecule inhibitors
Cereblon E3 ligase modulators (CELMoDs)
• Iberdomide, mezigdomide, cemsidomide
• More potent inhibition of cereblon (compared to lenalidomide and pomalidomide)
• Enhances tumoricidal and immune-stimulatory effects compared with immunomodulatory agents
• Sonrotoclax (similar to venetoclax), in t(11;14); targets
Bcl-2
• p300/CBP inhibitors (inobrodib, OPN-6602)
With recently approved therapies and therapies on the horizon, patients are living longer and better, and we are getting closer to a cure.







LUNCH
Please remain in this room
Walk Through the IMF Website
Robin Tuohy
Vice President, Support Groups, International Myeloma
Foundation
IMF REGIONAL COMMUNITY WORKSHOP
WALTHAM
AFTERNOON AGENDA

Seasons of Myeloma: Managing Side Effects and Living Well
Kimberly Noonan, DNP, ANP-BC, AOCN, FAAN
VNA Care
Living the Myeloma Life: Local Patient & Care Partner
Jill Zitzewitz (Patient) and Osman Bilsel (Care Partner)
Beyond Myeloma Therapy: Unlocking Emotional Freedom Technique (EFT) and Stress Management
Rebecca Rooney, PhD
Retired Lt. Col. U.S. Army, Clinical Psychologist
Q&A with Panel
Closing Remarks
Robin Tuohy
Vice President, Support Groups, International Myeloma
Foundation
Coffee/Network

Walk Through the IMF Website
Robin Tuohy, Vice President, Support Groups
International Myeloma Foundation

Seasons of Myeloma: Managing Side Effects and Living Well
Kimberly Noonan, DNP, ANP-BC, AOCN, FAAN
VNA Care
Seasons of Multiple Myeloma

Kimberly Noonan, DNP, ANP-BC, AOCN,
IMF
Nurse Leadership Board member
VNA Care

Wintery Mix of Treatment Options Spring into Managing Side Effects
Summer of Success






Wintery Mix of Treatment Options

Diverse and Complex Treatment Combinations









Myeloma Treatment Common Combinations
Velcade® (bortezomib)
Lenalidomide
DVRd, VRd, Vd
DVRd, VRd, Rd
Kyprolis® (carfilzomib) KRd, Kd, DKd, Isa-Kd
Pomalyst® (pomalidomide) Pd, DPd, EPd, PCd, Isa-Pd
Darzalex® (daratumumab) DVRd, DRd, DVd, DPd, DVMP, DKd
Ninlaro®(ixazomib) IRd
Empliciti® (elotuzumab) ERd, EPd
Xpovio® (Selinexor) XVd, XPd, XKd
Sarclisa® (Isatuximab) Isa-Kd, Isa-Pd
Blenrep® (Belantamab mafodotin) Bela-d
Abecma® (Idecabtagene Vicleucel) --
Carvykti® (ciltacabtagene autoleucel)
Elrexfio® (elranatamab)

Lynozyfic™ (linvoseltamab)
Tecvayli® (teclistamab) --
Talvey® (talquetamab) --
Venclexta® (venetoclax) Vd + ven
New agents or regimens in clinical trials are possible options
ASCT = autologous stem cell transplant; Bela = belantamab; C = cyclophosphamide; D = daratumumab; d = dexamethasone; E = elotuzumab; Isa = isatuximab; I = ixazomib; K = carfilzomib; M = melphalan; P = pomalidomide; R = lenalidomide; V = bortezomib; ven = venetoclax.


Stem Cell Transplant
ELIGIBILITY
Measuring Treatment Response
Determining Transplant Eligibility
Insurance Authorization Collecting Stem Cells



TRANSPLANT
High Dose Chemotherapy
Stem Cell Infusion
Supportive Care Engraftment
Duration: Approximately 2 weeks
Location: Transplant Center
Duration: Approximately 3-4 weeks
Location: Transplant Center

POST-TRANSPLANT
P H A S E 1 P H A S E 2 P H A S E 3

Restrengthening
Appetite recovery
“Day 100” assessment
Begin maintenance therapy
Duration: Approximately 10-12 weeks
Location: HOME





CAR T: Another Treatment Approach
Ask for a referral to CAR Tcell center as soon as it is possible as next treatment option (ie, before relapse)
Manufacturing takes
≈ 4 to 6 weeks
Bridging therapy may be needed
T-Cell Collection

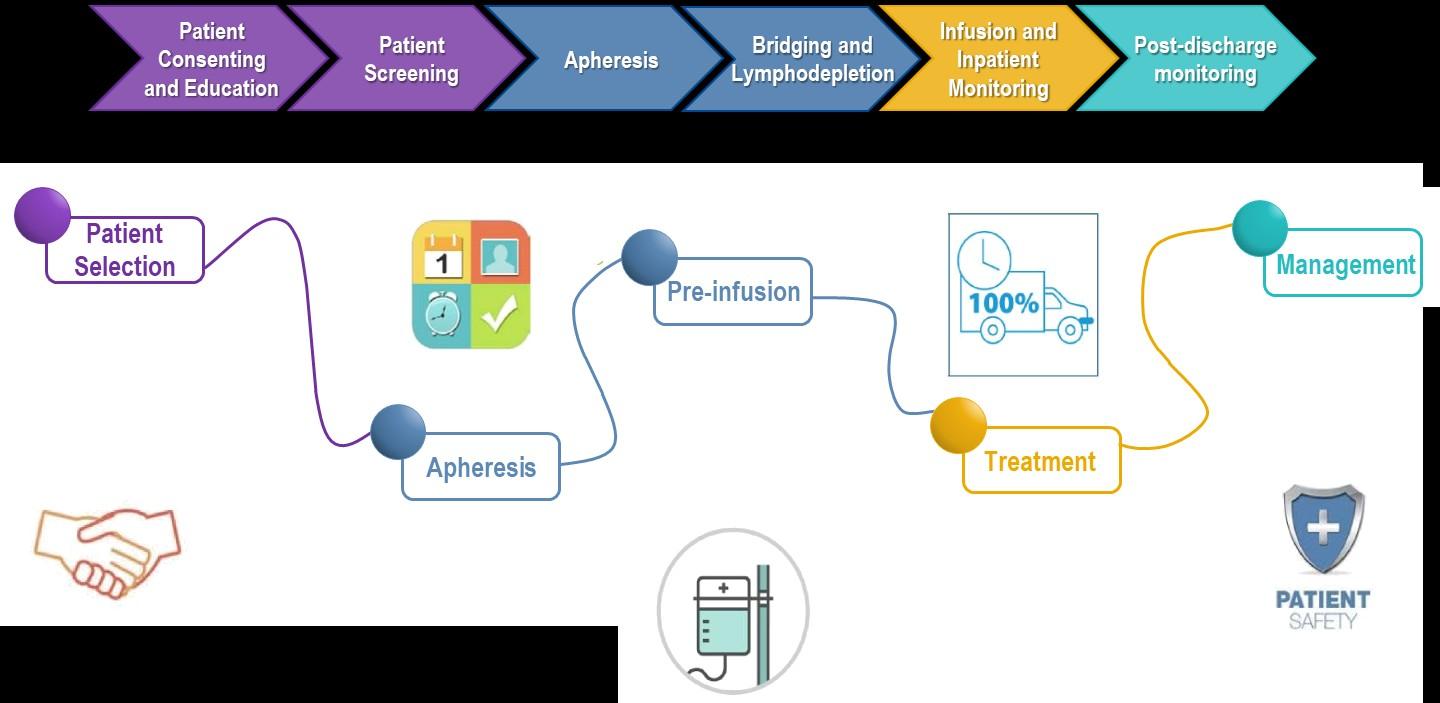
No driving for 8 weeks
“One & Done” with continued monitoring
• Away from home
• Often some hospital stay
• Care Partner needed
• Side effect management
• CRS, ICANS
• Low blood counts
• Fatigue and fever
• Some patients need ongoing transfusion support


Bispecific Antibodies
• Different bispecific antibodies have differences in efficacy, side effects
– Available after 4 prior lines of therapy (or clinical trial)
– About 7 in 10 patients respond
– Off-the-shelf treatment; no waiting for engineering cells
– CRS and neurotoxicity
– Risk of infection
• BCMA target: greater potential for infection
– Tecvayli® (teclistamab)
– Elrexfio ® (elranatamab)
– Lynozyfic™ (linvoseltamab)
BISPECIFIC ANTIBODIES

• GPRC5D target: potential for skin and nail side effects, GI issues of taste change, anorexia and weight loss
– Talvey ® (talquetamab)





CAR T and Bispecific Antibodies: Unique Side Effects





CRS is a common but often mild & manageable side effect



CAR = chimeric antigen receptor; CRS = cytokine release syndrome. Oluwole OO, Davila ML. J Leukoc Biol. 2016;100:1265-1272. June CH, et al. Science. 2018;359:1361-1365. Brudno JN, Kochenderfer JN. Blood. 2016;127(26):3321-3330. Brudno JN, Kochenderfer JN. Blood Rev. 2019:34:45-55. Shimabukuro-Vornhagen, et al. J Immunother Cancer. 2018;6:56. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625-638.




CAR T and Bispecific Antibodies: Unique Side Effects











Spring Into Managing Side Effects




The Early Bird Gets the Worm: Communicate Proactively with Your Healthcare Team
Your team may be able to help, but only if they know how you feel.
Unmanaged Myeloma can cause:
• Calcium elevation
• Renal dysfunction
• Low blood counts
• Infection Risk
• Blood clots
• Bone pain
• Neuropathy
• Fatigue

Side Effects of Treatment can
cause:
• GI symptoms
• Renal dysfunction
• Low blood counts
• Infection Risk



Tip: proactively discuss common side effects and what to do if they occur





How You Feel





• Blood clots
• Neuropathy
• Fatigue











Tip: Keep a Symptom Diary and bring it to appointments









Are Steroids Messing With Your Sunny Disposition?


Steroids enhance the effectiveness of other myeloma therapies
Your provider may adjust your dose. Do not stop or alter your dose of steroids without discussing it with your provider
Steroid Side Effects
• Irritability, mood swings, depression
Managing Steroid Side Effects
• Consistent schedule (AM vs. PM)
• Take with food
• Stomach discomfort: Over-the-counter or prescription medications
• Medications to prevent shingles, thrush, or other infections

• Difficulty sleeping (insomnia), fatigue
• Blurred vision, cataracts
• Flushing/sweating
• Increased risk of infections, heart disease

• Muscle weakness, cramping
• Increased blood pressure, water retention
• Stomach bloating, hiccups, heartburn, ulcers, or gas
• Weight gain, hair thinning/loss, skin rashes
• Increased blood sugar levels, diabetes


Infection Can Be Serious for People With Myeloma
Preventing infections is paramount.
Infection remains the leading cause of death in patients with multiple myeloma. Several factors account for this infection risk, including the overall state of immunosuppression from multiple myeloma, treatment, age, and comorbidities (e.g., renal failure and frailty).
IMWG Consensus guidelines and recommendations for infection prevention in multiple myeloma; Lancet Haematol.2022;9(2):143–161.
Infection Prevention Tips
Good personal hygiene (skin, oral)



Report fever of more than 100.4°F, shaking chills even without fever, dizziness, shortness of breath, low blood pressure to HCP as directed.
Environmental control (avoid crowds and sick people; use a high-quality mask when close contact is unavoidable)
As recommended by your healthcare team:
Immunizations:
Flu, COVID, RSV & and pneumococcal vaccinations; avoid live vaccines

Preventative and/or supportive medications (next slide)


Medications Can Reduce Infection Risk
Type of Infection Risk
Viral: Herpes Simplex (HSV/VZV); CMV
Medication Recommendation(s) for Healthcare Team Consideration
Acyclovir prophylaxis
Bacterial: blood, pneumonia, and urinary tract infection Consider prophylaxis with levofloxacin
PJP (P. jirovecii pneumonia)
Fungal infections
COVID-19 and Influenza
IgG < 400 mg/dL (general infection risk)
ANC < 1000 cells/μL (general infection risk)


Consider prophylaxis with trimethoprim-sulfamethoxazole
Consider prophylaxis with fluconazole
Antiviral therapy if exposed or positive for covid per institution recommendations
IVIg recommended
Consider GCSF 2 or 3 times/wk (or as frequently as needed) to maintain
ANC > 1000 cells/μL and maintain treatment dose intensity



GI Symptoms: Prevention & Management
Fluid intake can help with both diarrhea and constipation and helps kidney function
Constipation is more common in the induction phase
• Opioid pain relievers, antidepressants, heart or blood pressure medications (check with provider, pharmacist)
• Supplements: Calcium, Iron, vitamin D (rarely), vitamin B-12 deficiency Increase fiber
• Stay well hydrated
• Fruits, vegetables, high fiber whole grain foods
• Fiber binding agents – Metamucil® , Citrucel®, Benefiber®


Anorexia, the inability to eat, is common during transplant and resolves with time.
• Hydration is most important
• Small, frequent meals with a focus on protein intake
• You will work closely with a dietician to help monitor your calorie intake
Diarrhea is common during transplant and long-term maintenance therapy. Other medications and supplements
• Hydration is very important
• Electrolyte replacement is common
• Good skin care will help prevent irritation
• Stool exam may be needed to rule-out infection
• If no infection, anti-diarrheal medication may be prescribed


Discuss GI issues with healthcare providers to identify causes and make adjustments to medications and supplements


Management of Oral Side Effects

OTC dry mouth rinse, gel, spray are recommended. Advise patients to avoid hot beverages. Initiate anti-fungal therapy for oral thrush
Dexamethasone oral solutions “swish and spit” have been tried but with no proven benefit yet. Sour citrus or candies before meals are also recommended. Taste Changes


Dietary modifications with small bites, eating upright, and sips with food can help manage symptoms.
Some medications lead to weight gain, others to weight loss.
Dry mouth leads to taste changes which can lead to anorexia. Meet

Dry Mouth
Dysphagia
Catamero D, Purcell K, Ray C, et al. Presented at the 20th
Myeloma Society (IMS) Annual Meeting Nurse
September 27–30, 2023; Athens, Greece.

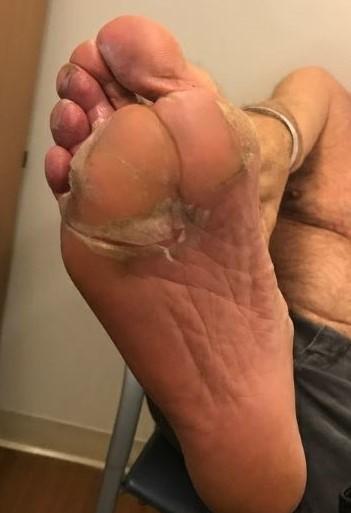
Skin and Nail Side Effects
Possible side effect to some treatments and supportive care medications
Skin Rash:
• Prevent dry skin; apply lotion
• Report changes to your care team
• Medication interruption or alternative, as needed
• Steroids:
– Topical for grades 1-2,
– Systemic and topical for Grade 3

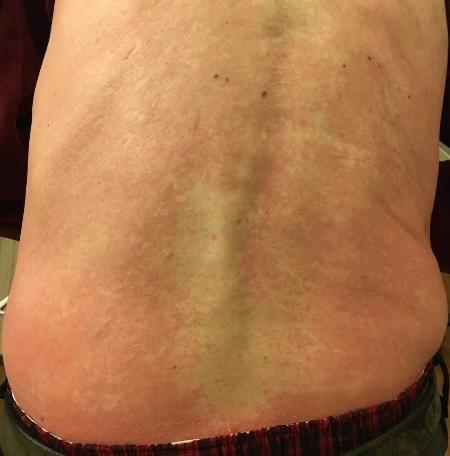
• Antihistamines, as needed
Nail Changes:
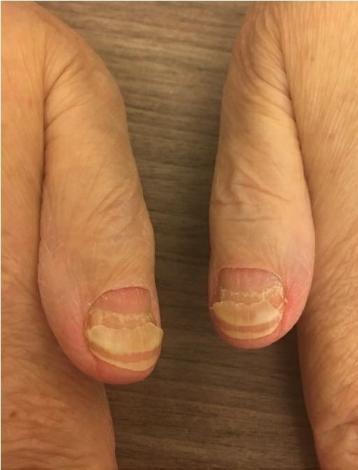
• Keep your nails short and clean. Watch for “catching and tearing”

• Apply a heavy moisturizer like Vaseline or salve. Wear cotton hand coverings to bed
• A nail hardener may help with thinning
• Tell the team if you have signs of a fungal infection, like thickened or discolored nails

Photos: Mount Sinai Hospital, NY, NY

Feel Like a Spring Chicken: Prevent and Manage Pain
Pain can significantly compromise quality of life
Sources of pain include bone disease, neuropathy and medical procedures
• Management
– Prevent pain when possible
• Bone strengtheners to decrease fracture risk
• Antiviral to prevent shingles
• Sedation before procedures
– Interventions depend on source of pain
• May include medications, activity, surgical intervention, radiation therapy, etc
• Complementary therapies (Mind-body, medication, yoga, supplements, acupuncture, etc)
• Scrambler therapy for neuropathy




Tell your healthcare provider about any new bone or chronic pain that is not adequately controlled


Peripheral Neuropathy Management
Peripheral neuropathy happens when there is damage to nerves in the extremities (hands, feet, limbs). Damage can be the result of myeloma, treatment or unrelated conditions (i.e. diabetes).
Symptoms:
• Numbness
• Tingling
• Prickling sensations
• Sensitivity to touch
• Burning and/or cold sensation
• Muscle weakness
Prevention / management:
• Bortezomib once-weekly and/or subcutaneous administration
• Massage area with cocoa butter regularly
• Neuroprotective Supplements:
– B-complex vitamins (B1, B6, B12)
– Green tea
• Safe environment: rugs, furnishings, shoes

If neuropathy worsens, your provider may:
• Adjust your treatment plan
• Prescribe oral or topical pain medication
• Suggest physical therapy
Report symptoms of peripheral neuropathy early to your healthcare provider; nerve damage from neuropathy can be permanent if unaddressed


Understanding Changes to Kidney Function
• Risk Factors
– Active multiple myeloma (light chains, high calcium)
– Other medical issues (ex: Diabetes, dehydration, infection)
– Medications (MM treatment, antibiotics, contrast dye)
– Poor Nutrition
• Prevention
– Stay hydrated – drink water
– Avoid certain medications when possible (eg, NSAIDs), dose adjust as needed
• Treatment
– Treatment for myeloma
– Hydration
– Dialysis




Many myeloma patients will experience kidney issues at some point; protecting your kidney function early and over time is important


Additional Supportive Care


Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56. Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36.
Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.


Summer of Success

Let the Sun Shine In
Fatigue is the most reported symptom. Sources include anemia, pain, reduced activity, insomnia, treatment toxicity, bone marrow suppression 98.8%
Often, people do not share these symptoms with their providers. Talk to your provider about symptoms that are not well controlled or if you have thoughts of self-harm.


>35% of patients
≈25% of patients




Bee an Empowered Patient
Ask
questions
– What are my treatment options?
– What are the pros and cons of the different options?
– How will we know if treatment is working?
– What do the different labs mean?
– Who will be monitoring my labs?
– How can I access my test results (eg, patient portal)?
– What will we do if my treatment doesn’t work or quits working?
Participate in decisions
– Share your priorities and preferences
– Include care partner(s) in your discussion



Speak up if something seems different or unusual
– Normally 4 vials of blood but only drawing 3?
– Normally specialty pharmacy confirms delivery but haven’t heard from them this month?

Live in the sunshine, swim the sea, drink the wild air.
– When is my next appointment?
Communicate with your healthcare team
– Understand the roles of each team member
– Who to contact for your needs (eg, side effects, insurance issues, other)
Develop a support network
– Learn from others: IMF has many support groups or you can start one (IMF’s can help)


– Ralph Waldo Emerson

Care Partners Are Vital for Success


If you want to go fast, go alone, if you want to go far, go together
• Care partners may help with medical appointments, managing medication, daily living, physical assistance, emotional support, myeloma knowledge, healthy lifestyle, patient advocacy, financial decisions
• Care partners can be a spouse, close relative, a network of people (family, friends, neighbors, church members, etc)





African Proverb
• Caring for the Care Partner
– Recognize that caregiving is difficult/stressful
– Encourage care partners to maintain their health, interests, and friendships
– The IMF has information and resources to help care partners




Cultivate A Care Network



If you want to go fast, go alone, if you want to go far, go together
• Multiple studies demonstrate that strong social ties are associated with longevity, improved adherence to medical treatment and overall improved health outcomes
• Care partners can be a spouse, close relative, a network of people (family, friends, neighbors, church members, etc)
• Care partners may help with medical appointments, managing medication, daily living, physical assistance, emotional support, myeloma knowledge, healthy lifestyle, patient advocacy, financial decisions


• Caring for the Care Partner
– Recognize that caregiving is difficult/stressful
– Encourage care partners to maintain their health, interests, and friendships
– The IMF has information and resources to help care partners







Martino J, et al. Am J of Lifestyle Med. 2015;11(6):466-475. Yang YC, et al. Proc Natl Acad 2016;113(3):578-583. Pinquart M and Duberstein PR. Crit Rev Oncol Hematol. 2010; 75(2):122–137.
African Proverb
IMF Care Giver Tip Cards

Enjoy Life’s Bounty

Harvest Good Health
Have a Primary Care Provider & Have Recommended Health Screenings
• Blood pressure
• Cholesterol
• Cardiovascular disease
• Diabetes
• Colonoscopy
• Women specific: mammography, pap smear
• Men specific: prostate
• Vision
• Hearing
• Dermatologic evaluation
• Dental checkups & cleaning

Develop & maintain healthy behaviors
• Good nutrition
• Regular activity
• Quit tobacco use
• Sufficient Sleep (next slide)
An ounce of prevention is worth a pound of cure. Benjamin
Franklin

Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56. Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.



































Living the Myeloma Life: Local Patient & Care Partner
&
Jill Zitzewitz (Patient Advocate)
Osman Bilsel (Care Partner)

LIVING THE MYELOMA LIFE: LESSONS LEARNED

Jill Zitzewitz (patient) & Osman Bilsel (care partner)
AGENDA

Share a Bit of Our Story
Share Lessons We Learned Along Our Myeloma Journey
Hear Your Stories & Lessons Learned
Goal: Build Community & Learn From Each Other

A LITTLE BIT ABOUT US AND LESSONS WE LEARNED ALONG THE WAY
Lesson
1: Your Life Journey Matters & Prepares You For What’s
Ahead
• We just celebrated 29 years of marriage, and we’re the parents of 4 beautiful young adult children.
• We are scientists & have worked together for most of our career.

• We are very involved in our faith, town, and work






Lesson 2: You’re Not Alone – Seek Out Support
















Lesson 2b: Join a Myeloma Support Group
• Other myeloma patients and care partners understand in ways that your extended family, friends, and community members just don’t because they haven’t experienced what you are experiencing.
• Myeloma support groups provide communities of support, educational information, inspiration, and often lead to new friendships.




Lesson 3: Knowledge is Power – Use Reliable Sources
• Be your own best advocate, ask your myeloma specialist questions, and seek out reliable sources of information about your myeloma.
• International Myeloma Foundation website is amazing!
• Support group finder, nurse’s hotline, clinical trial finder, Gen AI toolMyelo!

Don’t consult Dr. Google! – Instead, ask Myelo!





Lesson 4: Take Care of Yourself
• Make time for things that bring you joy and fill you up!
• Ensure that care partners also have time for themselves.
• Don’t be afraid to ask for help or to help others when you






Lesson 5: Never Lose Hope!
• There are people who care about you and want to help you on your journey.
• Education and research is leading to earlier diagnosis and better outcomes with less suffering for patients and their families.
• Basic science has led to many new therapeutic options for myeloma patients so they can live longerand better lives.





WHAT LESSONS HAVE YOU LEARNED?


Beyond Myeloma Therapy: Unlocking Emotional Freedom Technique (EFT) and Stress Management
Rebecca Rooney, PhD
Retired Lt. Col. U.S. Army, Clinical Psychologist
Emotional Freedom Techniques: EFT
Presentation for IMF Regional Community Workshop
Rebecca Rooney, PhD, Lieutenant Colonel, US Army (Retired)
Goals for the Day
Learn EFT
Understand the theory and history of EFT
Understand when and why to use EFT
Understand the versatility of EFT
Grow and have fun
YOUR GOALS?
First Assignment
Write down some issues that cause you stress or distress
Rate the level of distress from 0 (no distress) to 10 (the WORST you could feel)
Pick one issue that you want to resolve today
Highlight that issue
Tap on that issue at some point today
Background
What is EFT?
Energy Psychology : “acupressure for the emotions”
Energy Meridians, Acupuncture, Acupressure
Emotional and Physical Challenges
Blocked Energy
Tapping
Change
Healing
THIS IS EFT.
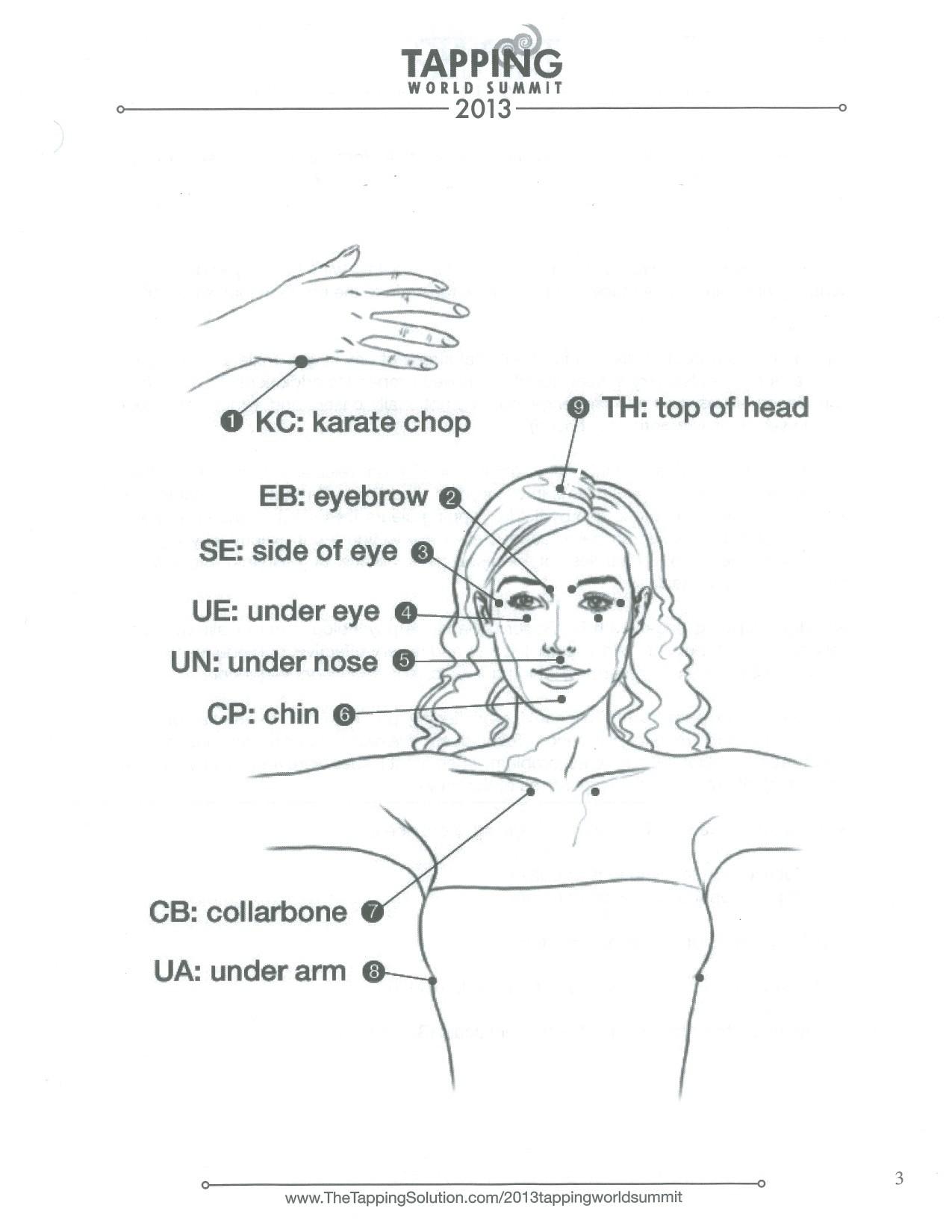
EFT Tapping Points
The Basic EFT Protocol
1. Choose a problem or issue (this craving for chocolate; this anxiety, etc)
2. Rate the level of disturbance (SUD) from zero (0) to ten (10).
3. Set up Phrase: “Even though I have this-------------, I deeply and completely accept myself.” (3 times)
4. TAP about 5-7 times on each point, saying “this ------”.
5. Breathe. Notice thoughts and feelings. Check SUD. If not zero,
6. Repeat #3 and #4 saying: “Even though I still have this ---” and “this remaining ---------”.
EFT LET’S JUST DO IT!
Energy Psychology
EINSTEIN: e = mc2 Matter = Energy
Therapies that work directly on and in the body to produce psychological change
Visualization and Verbalization
SPECIFIC Routines: tapping, touching, breathing, massage
Energy Psychology
Recall + Physical Stimulation = Relief
Focus + Physical Stimulation = Change
Methods:
Thought Field Therapy TFT : Roger Callahan, PhD
Meridian Tapping Techniques
Tapas Acupressure Techniques
Emotional Freedom Techniques: Gary Craig
When to use EFT
Stressors
Anxiety, fear, disappointment, uncertainty, anger
Pain: Headaches, backaches, bunions
Finances
Self-sabotage
Weight Loss
Self Care
Identity Issues
Saying NO
Fear of Change
Relationships
Sports Performance
Whenever !
The Choices Method
Patricia Carrington, PhD
“Accept myself” ????
Use CHOICE phrase instead “Even though
I have this ------, I CHOOSE to have --------.”
CHOICES TRIO:
Negative Phrase
Positive Phrase
Alternate Phrases
CHOICES DEFAULT PHRASES
I choose to be calm and confident.
I choose to feel comfortable and at ease.
I choose to be in harmony with (name the person with whom you are experiencing some difficulty).
I choose to enjoy myself and be happy.
I choose to be loving, kind and compassionate.
I choose --------
EFT JUST DO IT!
Other Energy and EFT Options and Methods
Something for Everyone. JUST Do It.
Touch and Breathe
Touch each point gently with fingertip(s)
Take an easy, deep breath at each point and let the breath out slowly
Repeat your tapping phrase still touching the spot with your fingertip(s)
Use to go to sleep, in public places, or to create a quiet, meditative mood
The SORE Spot
Two locations, one on each side of the chest
Painful to press on
Use in a crisis or when full tapping is not possible
Rub or palpitate the spot
Breathe
Release
CALM will come (most of the time)
Discreet Tapping
Fingertip Tapping Use thumb to tap on ends of fingers
Touch, rub or hold any of the energy points Simple Energy Techniques (SET)
Simple Energy Techniques (SET)
Tap lightly on the Energy Points while focusing on a problem
Focus on bodily feelings, anxiety, or thoughts
Regularly check emotional intensity
Continue and be aware of other issues, aspects, thoughts, events, memories
PERSIST and recognize many possible ASPECTS
Tap daily for ENERGY TONING
EFT
with Power Memories
Positive Personal Resources
Effective Coping Experience
Personal Memory
Borrowed Memory
Collect Power Memories DAILY
Regularly TAP on Power Memories Use them when you
really need to ……and when you think you don’t!
REMEMBER:
When you can’t do EFT in public, or if you don’t want to, you can almost always go to the restroom and tap.
EFT JUST DO IT!
Tapping with Children
Tap on YOURSELF first
Match the tapping to the child
Match the child’s language
Tap at the child’s pace
Use books and pictures to help
Introduce tapping as a game: Dance with your fingers
On special points on your body
Help you feel good
Children’s Book
“The Wizard’s Wish: Or, How He Made the Yuckies Go Away
– A Story about the Magic in You” by Brad Yates
A bad storm disrupts a village. The Village Wizard discovers a way to make himself and the people in his village feel better. They simply need to use their finger tips to tap on some magic points to get rid of the yuckies. They end up feeling even happier.
Tell the Story Technique
Bodily Sensation Based
Think of an experience that disturbs you
Rate the disturbance; locate it in your body
Tell the Story as you remember it
Check often for clearing or blocks
Tap through the feelings when apparent
Borrowing Benefits
Go back to the issue you started with this morning
Rate the level of intensity of the disturbance now
Review some of the other issues you wrote about
Just tapping with another person or other people can help reduce and heal our own issues because we are still changing the energy in the body.
Personal Peace Procedure
Write down every specific troublesome event
Give each event a specific Title
Rate the events starting with the worst
Tap on one event at a time to a SUD of zero
Clear one to three events DAILY
Pay attention to your body, your emotions and your relationships for over time
Check for the EFT Generalization Effect
EFT Humanitarian Projects
The Tapping Solution for Newtown: Stress and Trauma Relief Project - Nick Ortner
Iraq Veterans Stress Project - Gary Craig, Marilyn McWilliams, Dawson Church, Gary Feinstein
World Trade Center
Rwandan Genocide project
EFT: Making a Difference
“We must not, in trying to think about how we can make a big difference, ignore the small daily differences we can make which, over time, add up to big differences that we often cannot foresee.”
Marion Wright Edelman
EFT Research
Veterans: Five Studies
Rwandan Genocide Survivors (single session)
Refugees and Immigrants to the US
Peruvian Male Adolescents (single session)
EFT Research
Journal of Nervous & Mental Disease
February 2013 - Volume 201 - Issue 2 - p 153–160
doi: 10.1097/NMD.0b013e31827f6351
Original Articles
Psychological Trauma Symptom Improvement in Veterans Using Emotional Freedom Techniques: A Randomized Controlled Trial
Church, Dawson PhD*; Hawk, Crystal MEd†; Brooks, Audrey J. PhD‡; Toukolehto, Olli MD§; Wren, Maria LCSW∥; Dinter,, Ingrid¶; Stein, Phyllis PhD#
Abstract
Abstract: This study examined the effect of Emotional Freedom Techniques (EFT), a brief exposure therapy combining cognitive and somatic elements, on posttraumatic stress disorder (PTSD) and psychological distress symptoms in veterans receiving mental health services. Veterans meeting the clinical criteria for PTSD were randomized to EFT (n = 30) or standard of care wait list (SOC/WL; n = 29). The EFT intervention consisted of 6-hour–long EFT coaching sessions concurrent with standard care. The SOC/WL and EFT groups were compared before and after the intervention (at 1 month for the SOC/WL group and after six sessions for the EFT group). The EFT subjects had significantly reduced psychological distress (p < 0.0012) and PTSD symptom levels (p < 0.0001) after the test. In addition, 90% of the EFT group no longer met PTSD clinical criteria, compared with 4% in the SOC/WL group. After the wait period, the SOC/WL subjects received EFT. In a within-subjects longitudinal analysis, 60% no longer met the PTSD clinical criteria after three sessions. This increased to 86% after six sessions for the 49 subjects who ultimately received EFT and remained at 86% at 3 months and at 80% at 6 months. The results are consistent with that of other published reports showing EFT’s efficacy in treating PTSD and co morbid symptoms and its long-term effects
EFT Testimony before
Congress
Innovative Treatments for PTSD and TBI
House Veterans Affairs Committee July 20, 2010
Iraq Veterans Stress Project
David Feinstein, PhD, Dawson Church, PhD, and Wayne Miller
Research, Evidence Based, Rapid Results
Request for VA and DOD Implementation
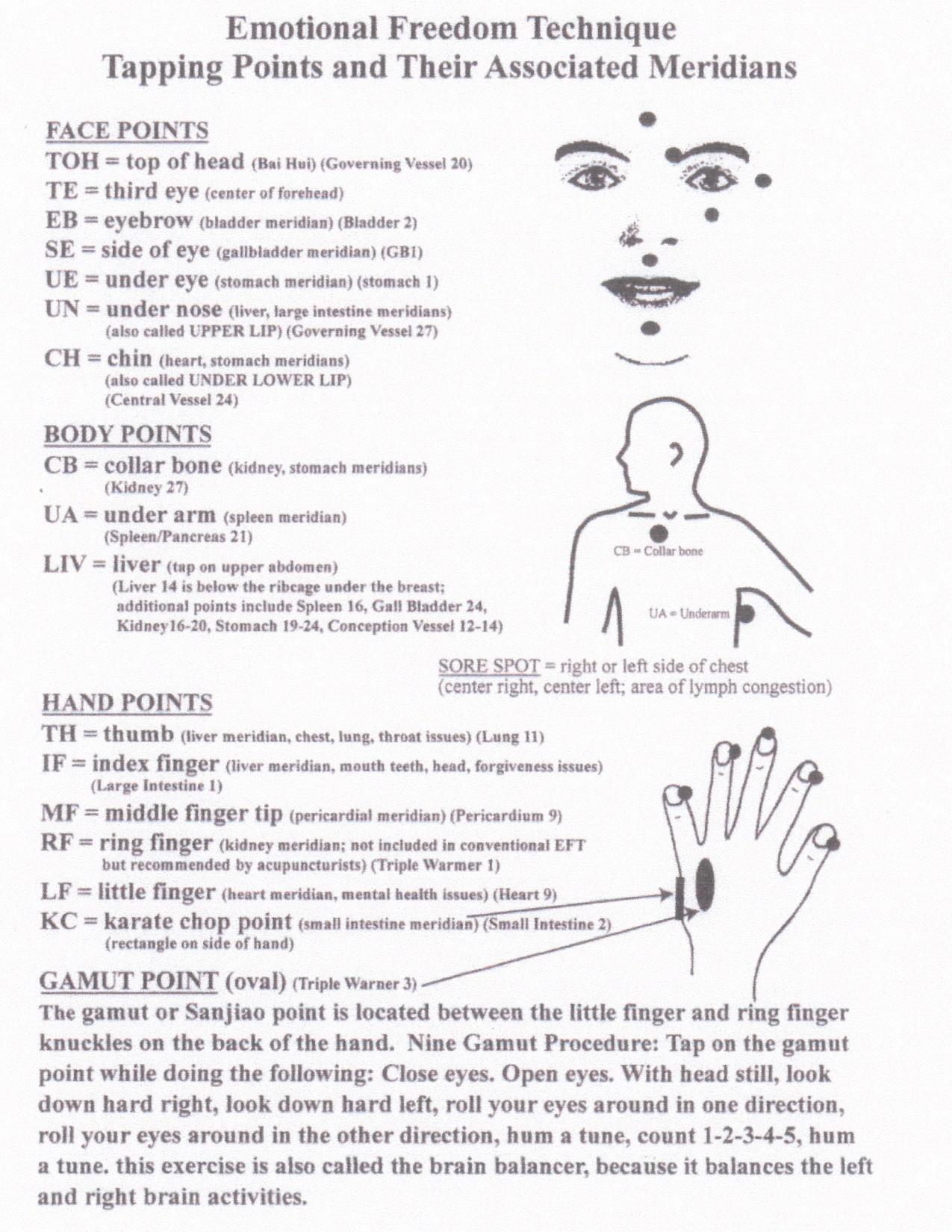
EFT JUST DO IT!

Q&A WITH PANEL

Closing Remarks
Robin Tuohy, Vice President, Patient Support International Myeloma
Foundation
Thank you to our speakers & our sponsors!









Housekeeping Items
Presentation Slides: Are available by scanning the QR code, Instructions are on the QR code handout on each table.
Program Evaluations: evaluations at the end of today.
Parking: All day parking is complimentary and there are no vouchers or tickets required.
Badge Holders: Please return your badge holders and we can recycle them.

We greatly appreciate your time and feedback!


OUR VISION:
A world where every myeloma patient can live life to the fullest, unburdened by the disease.
OUR MISSION:
Improving the quality of life of myeloma patients while working toward prevention and a cure.

IMF Core Values:
These are the core values we bring to accomplishing our mission each day.
Patient Centric
The patient experience is the focus of everything we do. Every interaction is an opportunity to establish a personal connection built on care and compassion which is the basis for continued support.
Respect All
As a team, we value honesty and transparency while creating a culture of mutual respect. We foster a myeloma community built on sincerity, authenticity, and kindness.
Excellence and Innovation
We value accountability, personal responsibility, and a steadfast commitment to excellence. We respect the legacy and reputation of our organization while seeking new solutions and advancements to improve outcomes, quality of life, and access to the best available resources for everyone impacted by myeloma.
Honor differences
We recognize each team member's skills and talents through collaboration and cooperation. Our programs aim to celebrate and support the diversity of our patients and their communities.





