

2025

RALEIGH, NC
NOVEMBER 15, 2025

Thank you to our speakers & our sponsors!









Welcome & Introductions
Robin Tuohy
Vice President, Patient Support
International Myeloma Foundation
Understanding Myeloma Basics
Cristina Gasparetto, MD
Duke Cancer Institute, Durham, NC
IMF REGIONAL COMMUNITY WORKSHOP
RALEIGH MORNING AGENDA

(Video) Closing the Gap: Health Disparities in Myeloma
Joseph Mikhael, MD, MEd, FRCPC, FACP, FASCO, Chief Medical Officer, International Myeloma Foundation
Advancing Treatment Options Through Clinical Trials
Attaya Suvannasankha, MD
Indiana University School of Medicine & Roudebush VAMC, Indianapolis, IN
Q&A with Panel
Coffee Break
Breakout: Frontline or Relapsed Treatment Approaches
-NDMM: Getting Started with Myeloma Management
Cristina Gasparetto, MD
-RRMM: Continuing the Myeloma Treatment Journey
Attaya Suvannasankha, MD
LUNCH
Walk Through the IMF Website
Robin Tuohy
Vice President, Support Groups, International Myeloma
Foundation
IMF REGIONAL COMMUNITY WORKSHOP
RALEIGH
AFTERNOON AGENDA

Seasons of Myeloma: Managing Side Effects and Living Well
Amy Pierre, MSN, RN, ANP-BC
Memorial Sloan Kettering Cancer Center & Flatiron Health
Living the Myeloma Life: Local Patient & Care Partner
Clark Murphy (Patient) & Janet Murphy (Care Partner)
Beyond Myeloma Therapy: Oral Health Survivorship
Katharine Ciarrocca, DMD, MSEd
Duke University Hospital, Durham, NC
Q&A with Panel
Closing Remarks
Robin Tuohy
Vice President, Support Groups, International Myeloma
Foundation
Housekeeping Items
Presentation Slides: Are available by scanning the QR code, Instructions are on the QR code handout on each table.
Program Evaluations: evaluations at the end of today.
Badge Holders: Please return your badge holders and we can recycle them.
Wifi: Network Name: Password: Sheraton2025

Restrooms: You are currently in Oak Ballroom –Noted with the RED STAR. Bathrooms are located outside Willow Oak –noted with the ORANGE X.
We greatly appreciate your time and feedback!










WELCOME BACK TO HEATHER COOPER ORTNER!
Incoming President & CEO International Myeloma Foundation


“I am humbled to serve alongside so many who are making a difference every day for patients and families affected by myeloma, and I look forward to building on the IMF’s legacy of impact”
INTERNATIONAL MYELOMA WORKING GROUP


• Under the guidance of the IMF Scientific Advisory Board, the IMWG identifies critical research needs, collaborations, and funding
• Primary Goal: To improve patient outcomes by identifying the most promising research to prevent and treat myeloma and ultimately, find a cure!
Clinical Trials
355 Doctors 43 Countries
70 Publications


RESEARCH – SCIENTIFIC ADVISORY BOARD




Joseph Mikhael, MD, MEd, FRCPC, FACP
IMF Chief Medical Officer


Sagar Lonial, MD, FACP
Winship Cancer Institute, Emory
University


Thomas Martin, MD
UCSF, Helen Diller
Family Comprehensive Cancer Center


Joo Chng, MD
National University of Singapore


María-Victoria Mateos, MD, PhD University of Salamanca




Sigurður Yngvi Kristinsson, MD, PhD
University of Iceland


Philippe Moreau, MD
University Hospital of Nantes


NIkhil Munshi, MD Dana-Farber Cancer Institute


Shaji Kumar, MD Mayo Clinic


Jesús San Miguel, MD, PhD University of Navarra


Saad Zafar Usmani, MD, MBA, FACP, FASCO Memorial Sloan Kettering Cancer Center

S. Vincent Rajkumar, MD IMF Board Chair
Wee
Vania Hungria, MD, PhD Santa Casa de São Paulo
Support Groups empower patients & care partners with information, insight & hope The IMF provides educational support to a network of over 155 myeloma specific groups
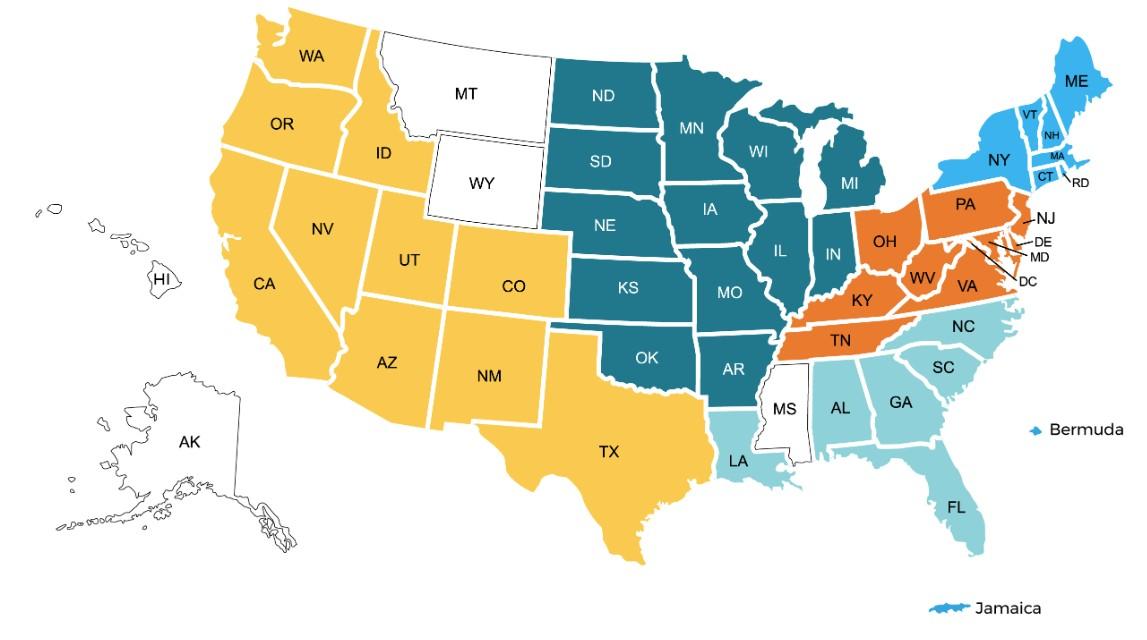





SUPPORT – LOCAL GROUPS
Western Wake
Meets hybrid on the 2nd Saturday of each month at 10:00 AM
Lake Norman
Meets in-person on the 3rd Thursday of each month at 6:30 PM
Asheville
Meets virtually on the 1st Tuesday of each month at 10:00 AM

Charlotte
Meets hybrid on the 3rd Saturday of each month at 10:00 AM
Triangle Area
Meets hybrid on the 4th Saturday of each month at 10:00 AM
Winston-Salem
Meets hybrid on the 4th Wednesday of each month at 11:30AM
Charlotte West
Meets virtually on the 3rd Saturday of each month at 11:00 AM

SPECIAL INTEREST GROUPS
Special interest groups are designed as a supplemental support for specific populations of patients, in addition to their local Support Groups
MM Families
Founded in 2021
For patients & care partners with young children
Las Voces de Mieloma
Founded in 2022
For Spanish speaking patients & care partners
Living Solo & Strong
Founded in 2022
For patients without a care partner


Veterans SIG
Founded in 2025
For those who served our country
Smolder Bolder
Founded in 2023
For smoldering myeloma patients & care partners
Living with High-Risk Multiple Myeloma
Founded in 2023
For high-risk myeloma patients & care partners
Care Partners Only
Founded in 2024
For myeloma care partners only

MYELOMA VOICES AT ASH



























MYELOMA INFOLINE
Promoting Knowledge, Empowerment, & Hope
Since 1990, InfoLine Coordinators, with medical oversight, connect patients around the world to needed resources and help answer their myeloma questions
Patient-Reported Needs
Include:
• Understanding lab results, terminology and disease state
• Preparing for medical visits
• Access to medical providers, specialty care (ASCT, CAR T)
• Access to specialty medications (Len, Dara, etc.)
• Financial resources





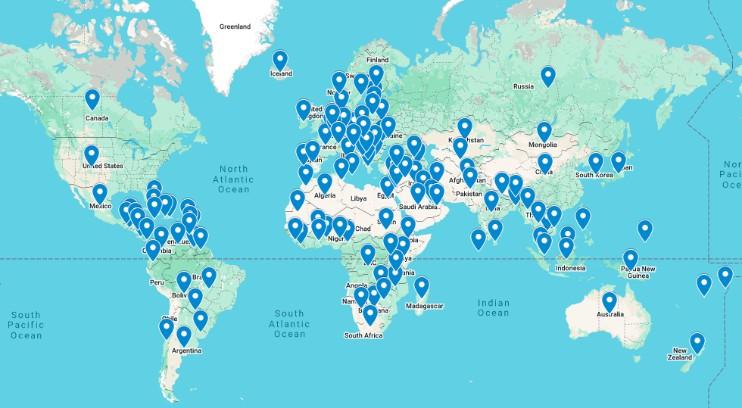






CLINICAL TRIALS MATCHING ENGINE
Seamlessly matching patients to the latest clinical trials.
• Discover clinical trials tailored to your myeloma journey with the IMF.
• Filter by diagnosis, treatment, and location to find the best fit for you.
Clinical Trials Matching Engine Usage











EDUCATION- WRITTEN







































EDUCATION – LIVE IN THE US




2025 LIVE PATIENT EDUCATION
Patient & Family Seminars
• Boca Raton, FL – March 14 – 15
• Philadelphia, PA – May 2 – 3
• Los Angeles, CA – August 15 – 16
• Chicago, IL – October 3 – 4

Myeloma Community Workshops
• Virtual - March 4 – focused on relapse
• San Mateo, CA - March 29
• Atlanta, GA - April 5
• Edina, MN - April 26
• Denver, CO - June 21
• Virtual – July 29 – focused on newly dx
• Seattle, WA - August 9
• Waltham, MA - September 27
• Raleigh-Durham, NC - November 15
• Virtual – November 17 – focused on Co ntroversies – Register here

WHAT DO WE ADVOCATE FOR?

The following policy principles are the foundation on which we prioritize our advocacy work.
1. Ensure Access to Care: We advocate for policies that ensure all myeloma patients have equitable, comprehensive, patientcentered care without insurance barriers that limit options or delay treatment initiation.
2. Eliminate Financial Barriers: We advocate for policies that allow myeloma patients access to treatments and supportive care interventions without facing financial hardships.
3. Advance Myeloma Research: We advocate for annual appropriations funding for myeloma research and the advancement of clinical trial eligibility and research protocols that ensure representation from diverse populations.










Understanding Myeloma Basics

Duke Cancer Institute, Durham, NC
Cristina Gasparetto, MD
The ‘Multiple Faces’ of Multiple Myeloma

November 15,2025
Cristina Gasparetto, MD
The Mysterious Illness of a Wealthy London Grocer
The story of this eponym begins with Thomas Alexander McBean, a 38-year-old London grocer, who, while on vacation in Scotland in 1844, suddenly developed pain in his chest after vaulting over an underground cavern.
McBean, a man of ‘temperate habits and exemplary conduct described the pain ‘as if something had snapped or given way within the chest’. McBean had also noted that ‘body linen was stiffened by his urine’.
Saturday,Nov1st,1845
DearDr.Jones,
Thetubecontainsurineofavery specificgravity.Whenboileditbecomes opaque.Ontheadditionofnitricacid, iteffervesces,assumesareddishhue, andbecomesquiteclear,butasitcools, assumestheconsistenceandappearance whichyousee.Heatreliquifiesit.
Whatisit?
“The History of Multiple Myeloma” R. Kyle
Dr.WilliamMacintyre

Dr. Henry Bence Jones
The Story of Sarah Newbury
She felt excruciating pain ‘just as if her thighs were being broken into thousand pieces’ while her husband was lifting her from the fireplace to carry her to bed. She was unable to walk thereafter.
Kyle R A , Rajkumar S V Blood 2008;111:2962-2972
Who gets Multiple Myeloma?
Approximately 30,000 new cases in the United States per year1
The second most common hematologic malignancy after lymphoma1
Slightly more common in men vs. women2
Incidence in African Americans is about twice that in Caucasians2
Median age at diagnosis is ~69 years2 1.
Incidence of MM by Age in the US
Myeloma Age-Specific SEER Incidence Rates, 2010-2014. https://seer.cancer.gov/csr/1975_2014/browse_csr.php?
sectionSEL=18&pageSEL=sect_18_table.07.html. Accessed June 14, 2017.
Incidence of MM by Race and Ethnicity
Stat Fact Sheets: Myeloma. Available at: https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed June 14, 2017
Etiology
Decline in the immune system
Genetic factors
Exposure to chemicals
Exposure to environmental toxins
Certain occupations
Exposure to radiation
Numerous viruses (HIV, HCV, HHV-8)
HIV = human immunodeficiency virus; HCV = hepatitis-C virus; HHV-8 = human herpes virus-8
Dispenzieri A, Lacy MQ, Greipp PR. Multiple myeloma. In: Greer JP, eds. Wintrobe’s Clinical Hematology. 12th ed. Philadelphia: Wolters Kluwer; 2009:2373-2374
Multiple Myeloma is a B-cell Malignancy
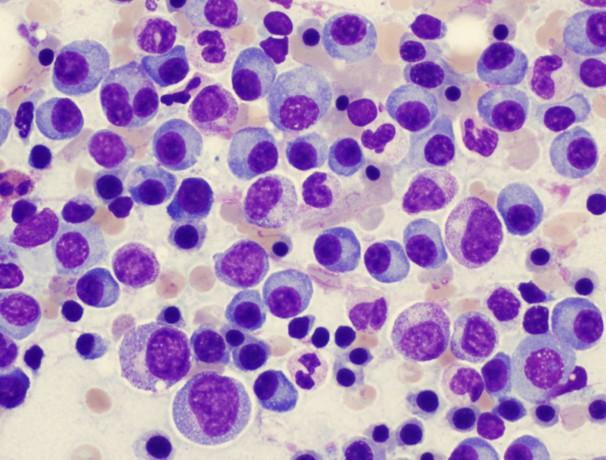
How we make the diagnosis of Multiple Myeloma

Blood Work


Bone marrow


Blood Work

• Beta 2 macroglobulin
• lactate dehydrogenase-LDH
• MM labs-SPEP-IFE, Free Light Chain
I I I I
> 10% Clonal Plasma cells

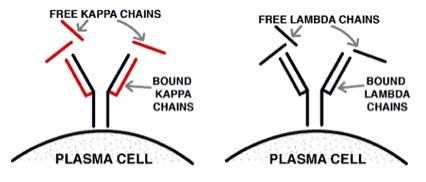

Type of monoclonal protein in 984 cases of multiple myeloma at the Mayo Clinic from 1982 to 1994.
Kyle R A The Oncologist 1996;1:315-323
24 Hours Urine Electrophoresis
A laboratory test that separates and identifies proteins in the urine
Purpose:
•To detect abnormal proteins that may indicate underlying medical conditions, such as:
•Kidney disease
•Multiple myeloma
•Amyloidosis
•Urinary tract infections
Procedure:
•A urine sample is collected.
•The sample is processed in a laboratory using electrophoresis, which separates the proteins
•based on their size and charge.
•The proteins are visualized and identified using staining techniques.
Results:
•Normal urine contains small amounts of albumin and other proteins.
•Abnormal results may show:
•Increased levels of certain proteins (e.g., albumin, globulins)
•Presence of monoclonal proteins (proteins produced by a single clone of cells)
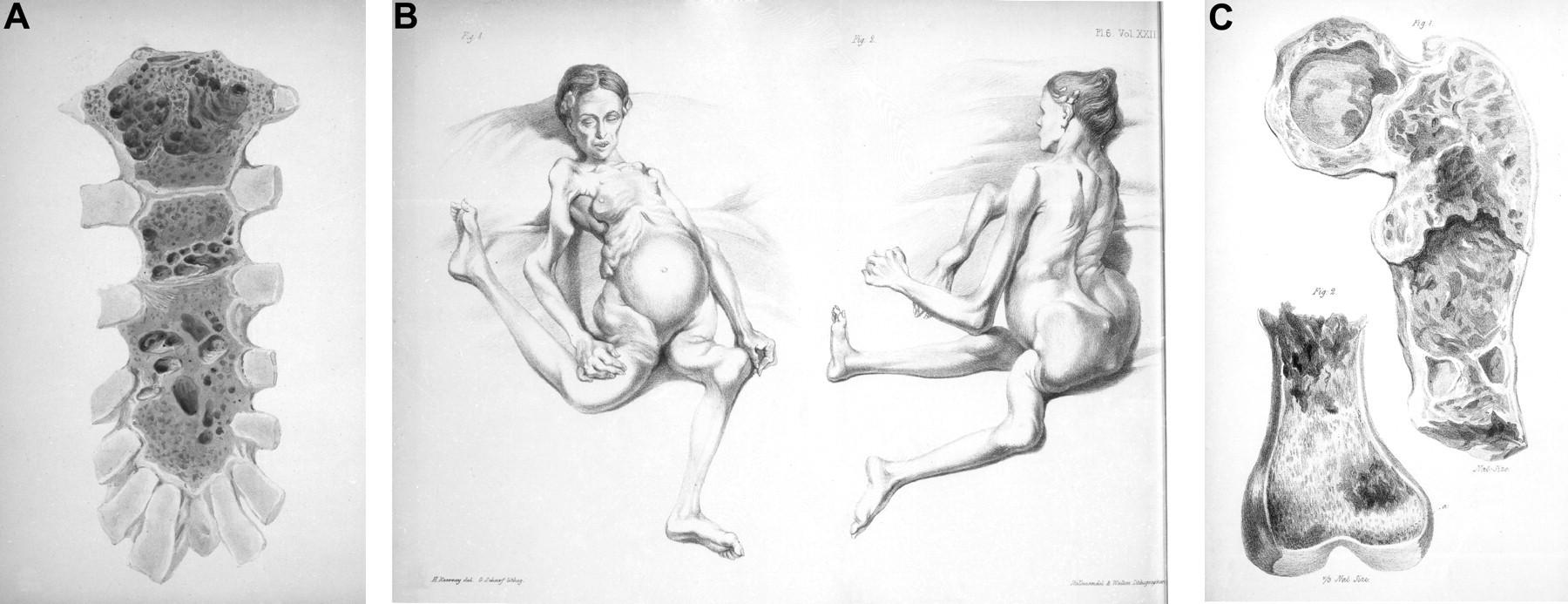
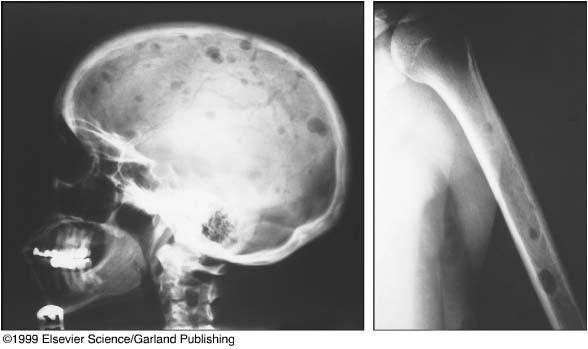
Bone Disease in Multiple Myeloma
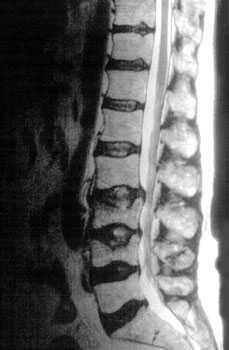
Kyle RA. Mayo Clin Proc. 1975;50:29.
• A burdensome and frequent complication in MM
• Present in up to 80% of patients at diagnosis
• Characterized by osteolytic bone lesions secondary to increased bone resorption and impaired bone formation
• Sequelae
• Pathological fractures
• Osteoporosis
• Hypercalcemia
• Bone pain
• Spinal cord compression
Skeletal CT scan
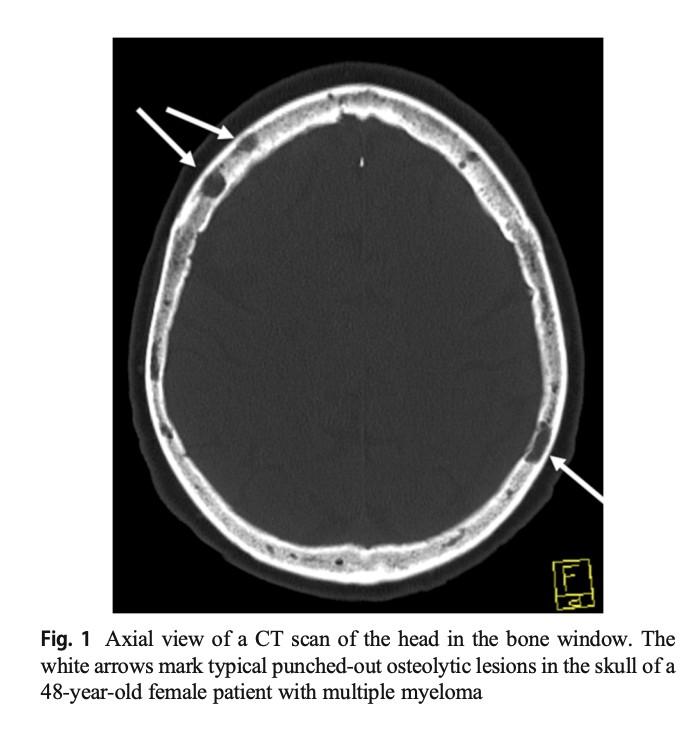
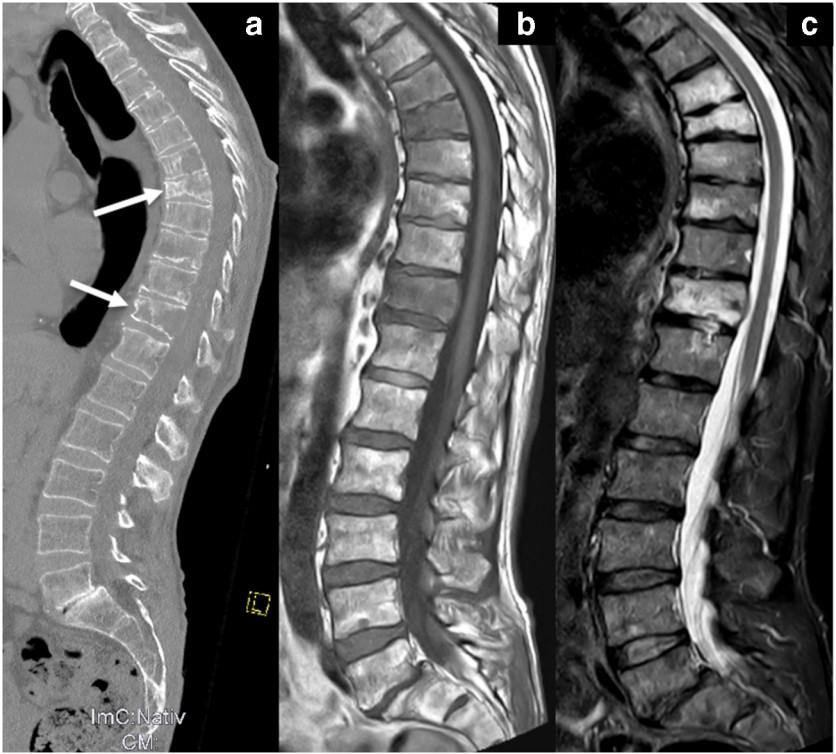
K. Treiti et al. Skeletal Radiology 2021
MRI Scan
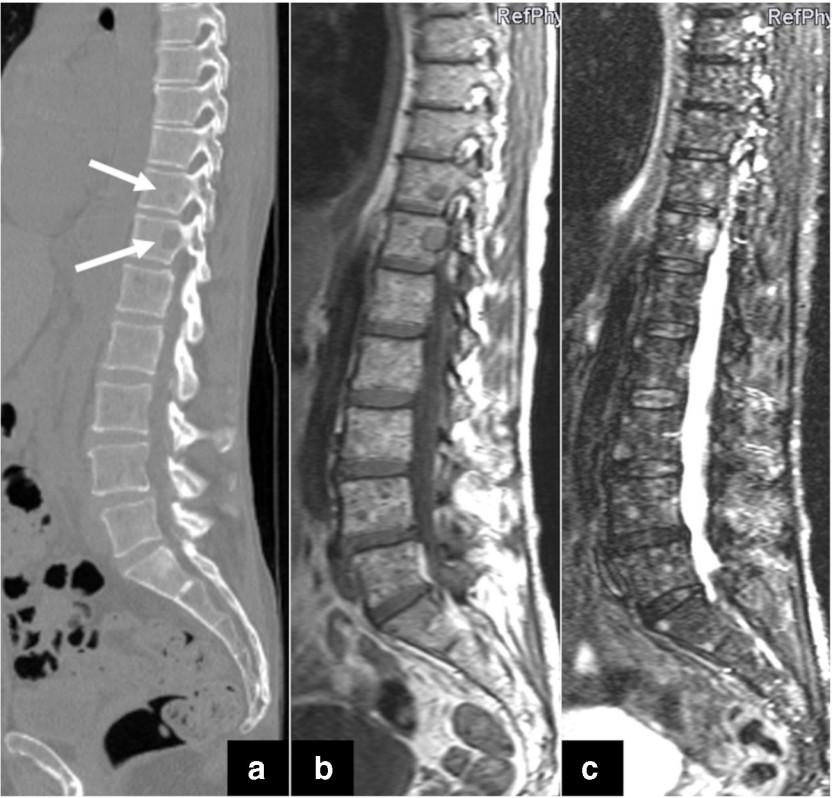
K. Treiti et al. Skeletal Radiology 2021
PET/CT Imaging in MM
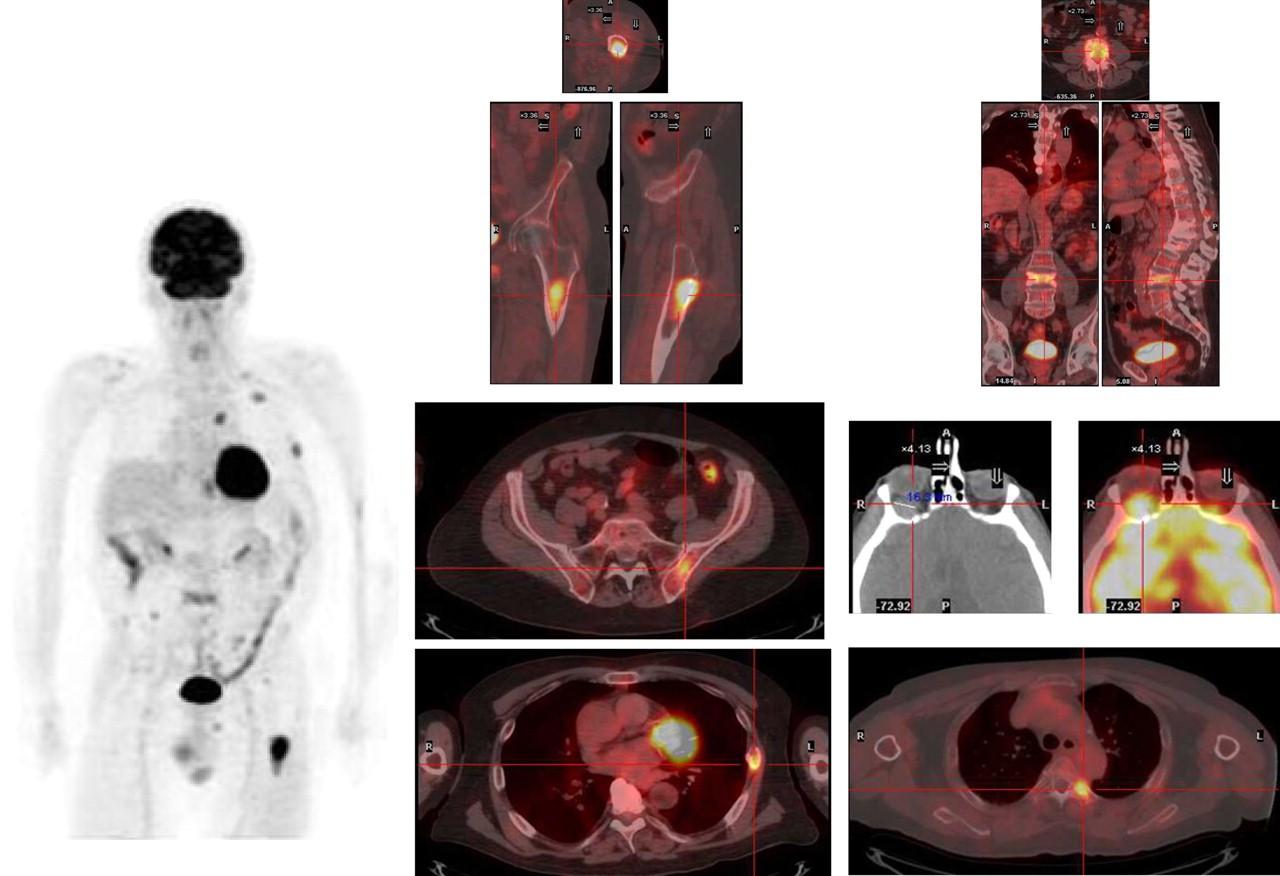
Stewart AK et al. Blood. 2009;114:5436. San Miguel JF et al. ASCO Educational Book. 2013;e313.
Bone Marrow Aspirate and
Biopsy
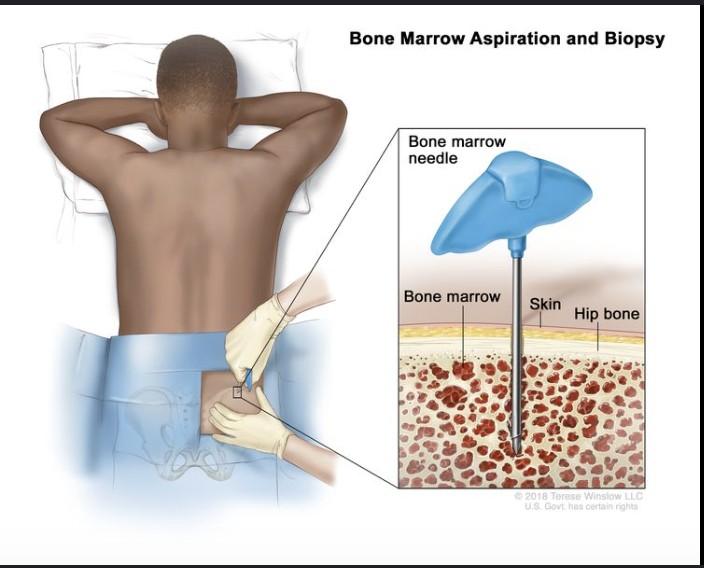
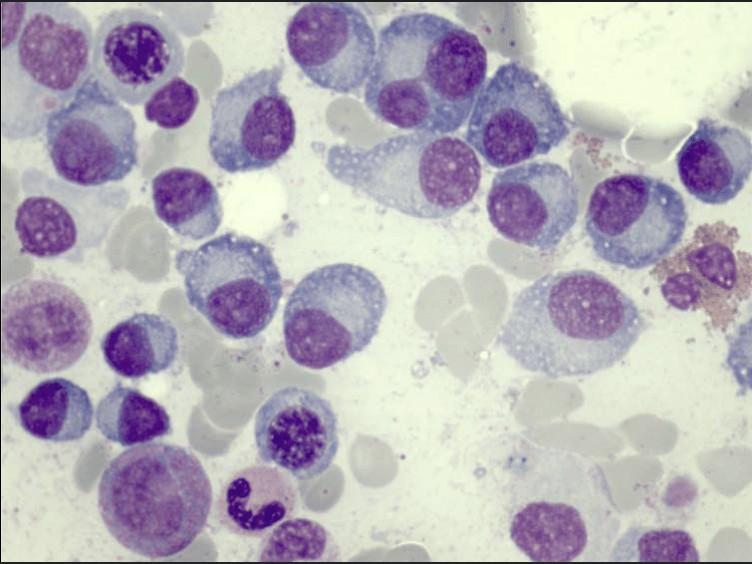
Staging and Risk Stratification

IMS consensus on genomic definitions of High-Risk Multiple Myeloma (HRMM)
Del17p
In >20% sorted PCs
TP53 mutation
Biallelic Del(1p32)
Association of 2 among:
T(4;14) or t(14;16) or t(14;20)
Gain/amp 1q
Monoallelic del(1p32)
B2m >5.5
Criteria for Diagnosis of Myeloma
MGUS
SMM
• <3 g M spike
• <10% plasma cells
• ≥3 g M spike
• ≥10% plasma cells
• > 500 mg Urine BJ proteinuris
Active
MM
• ≥10% plasma cells
• ++M spike
Kyle RA et al. Leukemia. 2009;23:3.
Kyle RA et al. N Engl J Med. 2002;346:564.
C - High calcium
R - Renal dysfunction
A - Anemia
B - Bone lesions
Hyperviscosity, amyloidosis, h/o recurrent infections
Algorithm
for reclassifying SMM and active MM
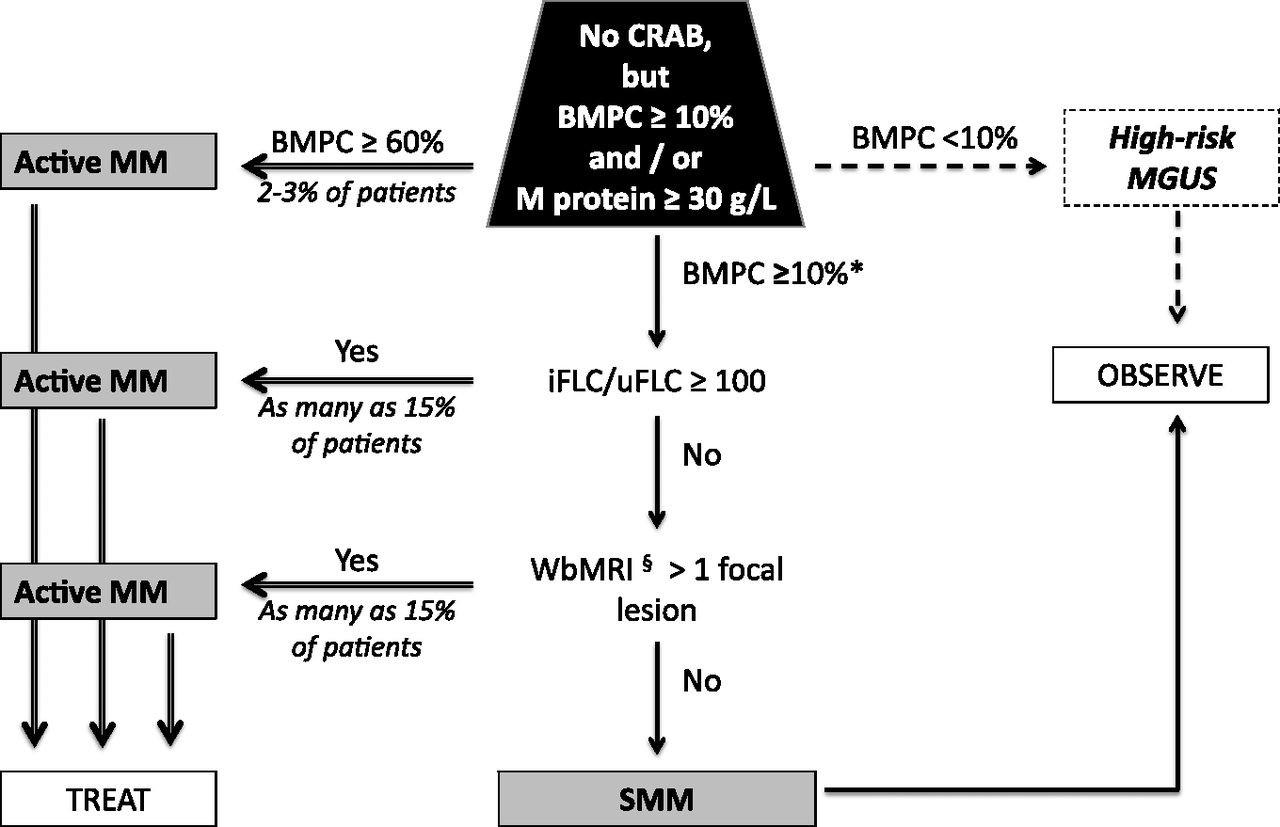
Dispenzieri A et al. Blood 2013;122:4172-4181



Provided by Dr. Robert Kyle
Survival from Onset of Treatment of Multiple Myeloma
Holland et al: Blood 27(3):335, 1966
Provided by Dr. Robert Kyle
Placebo
Targeted Therapies and Immunotherapy-2000-2025
• IMiDs
• Thalidomide
• Lenalidomide
• Pomalidomide
• PIs
• Bortezomib
• Carfilzomib
• Ixazomib
• Antibodies
• Daratumumab
• Isatuximab
• Elotuzumab
• XPO1- inhibitor
• Selinexor
• Immunotherapy
• Car-T cell
• Abecma
• Carvikty
• Bispecifics
• Teclistamab
• Talquetamab
• Elranatamab
• Linvoseltamab
• Antibody-Drug-Conjugated
• Belantamab
RELATIVE SURVIVAL HAS SIGNIFICANTLY INCREASED
OVER THE LAST 30 YEARS FOR PATIENTS WITH MYELOMA

• Relative survival for patients with multiple myeloma has increased due to more understanding of disease biology1,2
• The data points shown in this graph represent the 5-year relative survival obtained from the NCI SEER database from 1975 to 20141
*The difference in 5 year relative survival between 19751977 and 2007-2013 is statistically significant (p<.05).
1. National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER cancer statistics review 19752014. Available at www.seer.cancer.gov. Accessed June 15, 2017. 2. Kumar SK, et al. Leukemia. 2014;28(5):11221128.

Closing the Gap: Health Disparities in Myeloma (Video)
Joseph Mikhael, MD, MEd, FRCPC, FACP, FASCO Chief Medical Officer, International Myeloma Foundation

Advancing Treatment Options Through Clinical Trials
Attaya Suvannasankha, MD
Indiana
University School of Medicine & Roudebush VAMC, Indianapolis, IN
Advancing Treatment Options Through Clinical Trials
• Attaya Suvannasankha, MD
• Indiana University School of Medicine & Roudebush
VAMC, Indianapolis, IN


• Why clinical trials matter in myeloma
• How trials lead to new therapies
Overview
• Patient participation and impact
• How to find and join trials
The Progress in Myeloma
• Over the past 20 years: major advances
• Improved survival and quality of life
• Multiple classes of new drugs:
• - Proteasome inhibitors (e.g., bortezomib, carfilzomib)
• - Immunomodulatory agents (e.g., lenalidomide, pomalidomide)
• - Monoclonal antibodies (e.g., daratumumab, elotuzumab)
• - Bispecific antibodies and CAR-T therapies
• Research studies involving patient volunteers
What Are Clinical Trials?
• Evaluate new treatments, combinations, or sequences
• Determine safety, dosing, and effectiveness
• Essential for FDA approval and clinical use
Phase I Safety & dosage (20-80 patients)
Phases of Clinical Trials
Phase II Effectiveness & side effects (100-300 patients)
Phase III Compare to standard care (300-3000 patients)
Phase IV Long-term safety (Post-approval)
Examples of Myeloma
Trials That Changed Care

• Daratumumab: introduced a new class of antibody therapy
• CAR-T cell therapies: personalized immune therapy
• Bispecific antibodies: offthe-shelf immune redirection

• Maintenance studies: improved long-term control
Patient Benefits from Participation
• Access to promising new treatments
• Close monitoring by expert teams
• Contributing to future care improvements
• Often no additional cost for study drug
• 'Will I get a placebo?' – Rare; usually standard therapy vs. standard + new agent
Common Concerns
• 'Is it safe?' – Safety is top priority; trials are closely regulated
• 'Will it affect my care?' – Always coordinated with your myeloma specialist
• Discuss your goals and expectations
How Patients Can Prepare
• Ask about eligibility criteria
• Bring a list of your medications
• Have a family member or friend for support

• Smarter, more targeted approaches
• Combining immunotherapy with traditional drugs
• Personalized treatment based on genetic profiling
• Ongoing progress through patient partnership




Q&A WITH PANEL
Housekeeping Items
Presentation Slides: Are available by scanning the QR code, Instructions are on the QR code handout on each table.
Program Evaluations: evaluations at the end of today.
Badge Holders: Please return your badge holders and we can recycle them.
Wifi: Network Name: Password: Sheraton2025

Restrooms: You are currently in Oak Ballroom –Noted with the RED STAR. Bathrooms are located outside Willow Oak –noted with the ORANGE X.
We greatly appreciate your time and feedback!





BREAK
WHEN YOU RETURN FROM BREAK
PLEASE HEAD TO YOUR SELECTED BREAKOUT SESSION:

BREAKOUT A: NDMM - GETTING STARTED WITH MYELOMA MANAGEMENT
(For those currently picking their 1st therapy, or in the midst of their 1st therapy)
Dr. Cristina Gasparetto, MD
Please move to Willow Oak
BREAKOUT B: RRMM - CONTINUING THE MYELOMA TREATMENT JOURNEY
(For those currently past their 1st therapy, in a good remission, or looking to see next steps)
Dr. Attaya Suvannasankha, MD
Please remain in this room

Breakout A
NDMM: Getting Started with Myeloma Management
Dr. Cristina Gasparetto, MD
NDMM Getting Started with Myeloma Managment
International Myeloma Foundation Regional Community
Workshop Raleigh, NC
November 15, 2025
Cristina Gasparetto, MD
MM
is a heterogeneous
disease
with multiple patient factors to consider. Therefore, an individualized approach is required

Frailty and disability

Age
Multiple factors for consideration

Comorbidities and organ function

Performance status
Risk assessment

Refractory status

Patient preference and other psychosocial factors


Treatment history
The goal of treatment is to determine the most effective and safe regimen, which maintains quality of life
Laubach J, et al. Leukemia 2016;30:1005–17; Sanchez L, et al. Expert Rev Hematol 2020;13:943–58; Sonneveld P and Brojil A. Haematologica 2016;101:396–406; Goel U, et al. Am J Hematol 2022;97:S3–25
Newly Diagnosed MM Treatment Paradigm in 2025
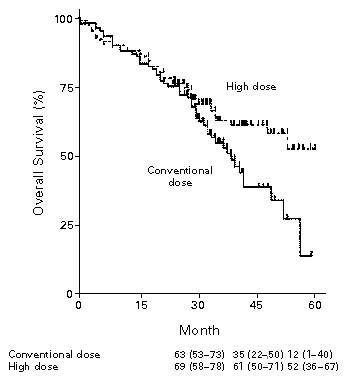
1996: Transplant Prolongs the Life of Patients With MM
Randomized phase III study of conventional chemotherapy ± autologous stem cell transplant (ASCT)

Median EFS: 27 months
Median EFS: 18 months
Median and 5-Year OS: Not reached and 52%
Median and 5-Year OS: 37.4 months and 12%
Why Transplant Work?
Induction 1

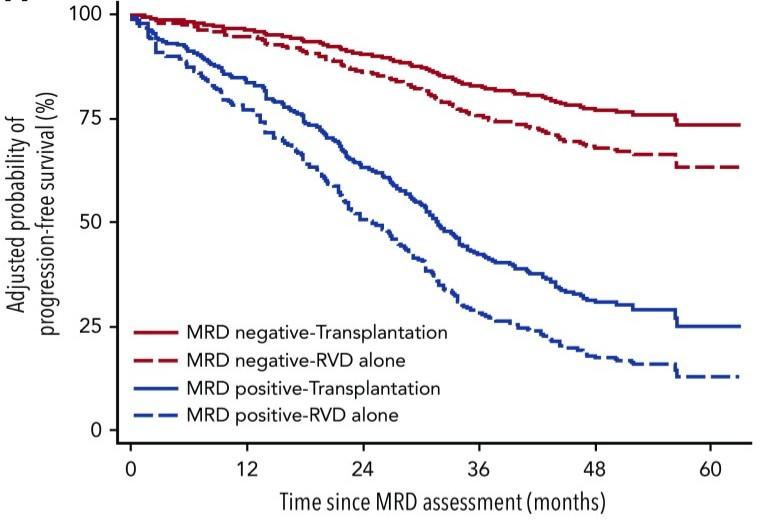
Large Meta-Analysis Established Role of MRD Negativity
in Long-Term Survival Outcomes in Patients With NDMM
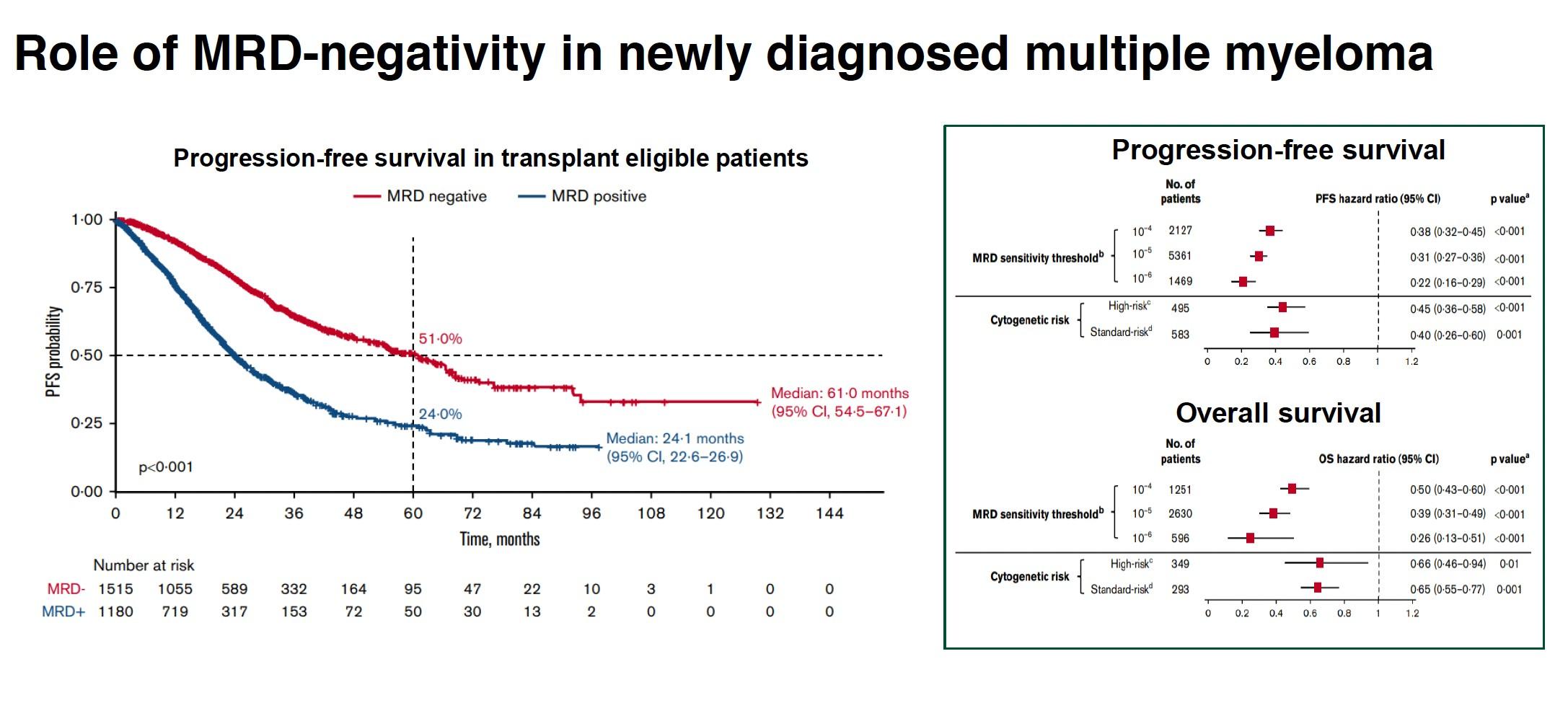
Munshi NC et al. Blood Advances. 2020;4(23):5988-5999.
2025: Transplant Prolongs Progression-Free Survival of Patients With MM
Major Failure of Transplant is ‘Relapse’ –
How Can We Improve Transplant? Induction Relaps

From Doublets to Triplets to Quadruplets
Bal S et al. Am J Hematol. 2021;96:367–78; Bazarbachi AH et al. Blood Cancer J. 2022;12:47; Costello C et al. Hematology Am Soc Hematol Educ Program. 2022:539–50.
C, cyclophosphamide; d, dexamethasone; D, daratumumab; Isa, isatuximab; K, carfilzomib; R, lenalidomide; T, thalidomide; V, bortezomib.
Trials Exploring Quadruplet Therapies in
Transplant-Eligible (Te) NDMM
Bela, belantamab mafodotin; d, dexamethasone; D, daratumumab; Isa, isatuximab; K, carfilzomib; R, lenalidomide; V, bortezomib.
1. Voorhees P et al. Lancet Haematol. 2023;10(10):e825-e837; 2. Moreau P et al. Lancet. 2019;394(10192):29-38; 3. Sonneveld P et al. N Engl J Med. 2024;390:301-13; 4. Goldschmidt H et al. Lancet Haematol. 2022;9:e810-21; 5. Gay F et al. ASH 2023; 6. Costa LJ et al. J Clin Oncol. 2022;40(25):2901-2912; 7. Leypoldt LB et al. J Clin Oncol. 2024;42:26-37; 8.
PERSEUS: Phase 3 Study of D-VRd
vs VRd

PERSEUS: Progression-Free Survival

Sonneveld P et al. N Engl J Med. 2024;390(4):301-313.
PERSEUS: MRD-Negativity Rates (10-5 and
• D-VRd + D-R doubled the rates of deeper MRD negativity at 10-6 versus VRd + R
• MRD negativity at 10-6 increased by approximately 30% during maintenance with D-R
GMMG-HD7: Phase 3 Study of Isa-VRd vs VRd
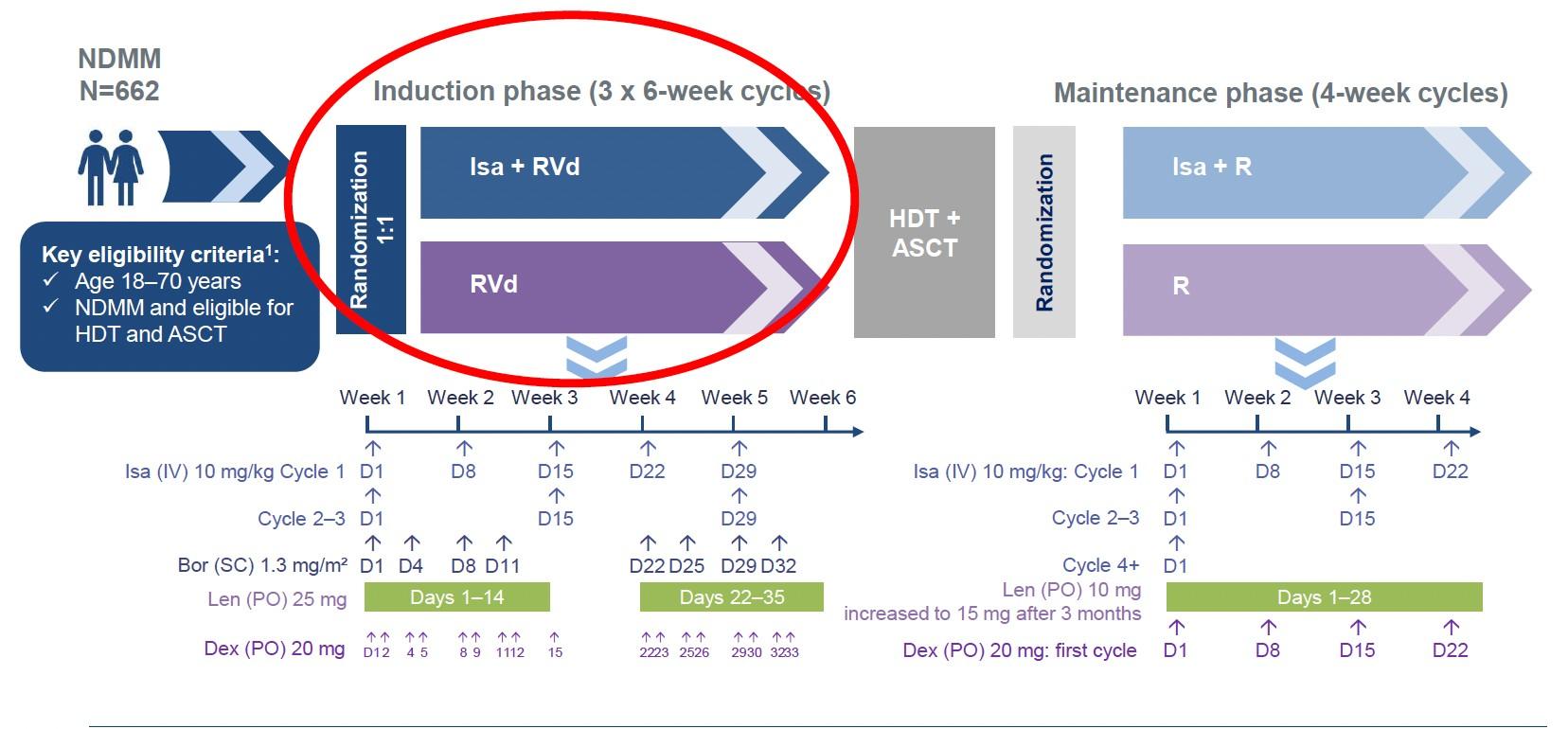
3 years or
GMMG-HD7: Results
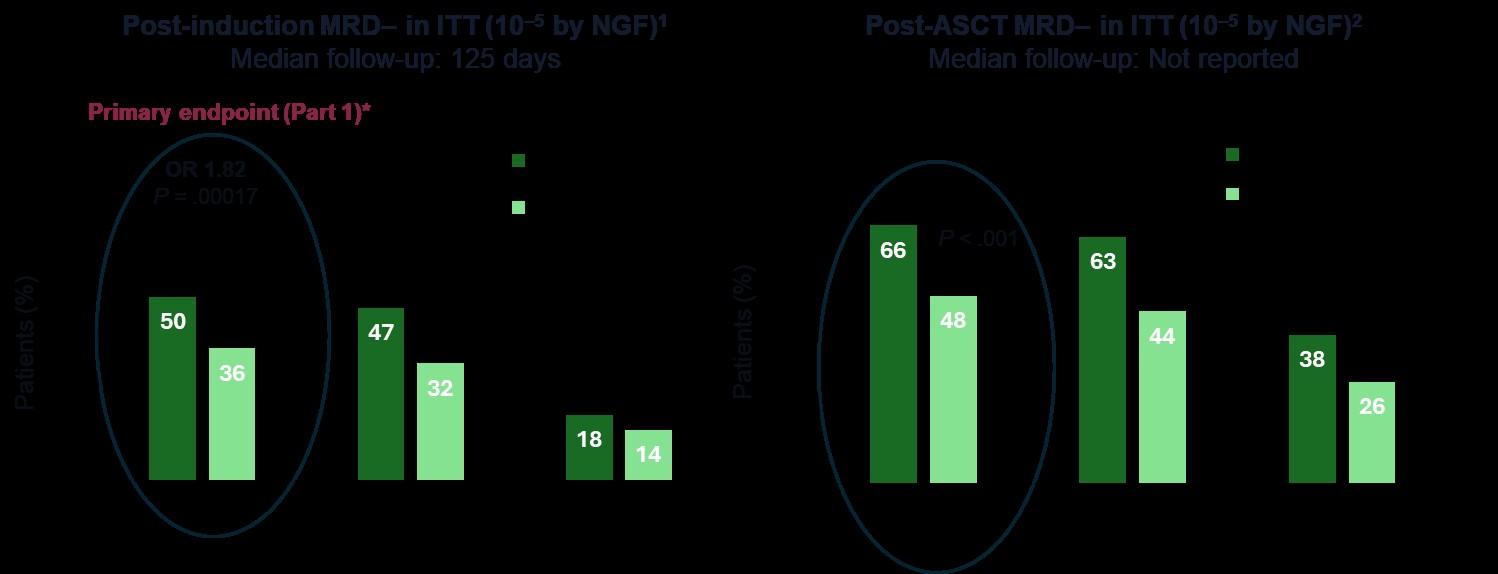
Goldschmidt H et al. Lancet Haematol. 2022;9(11):e810-e821; Raab M et al. EHA 2024.
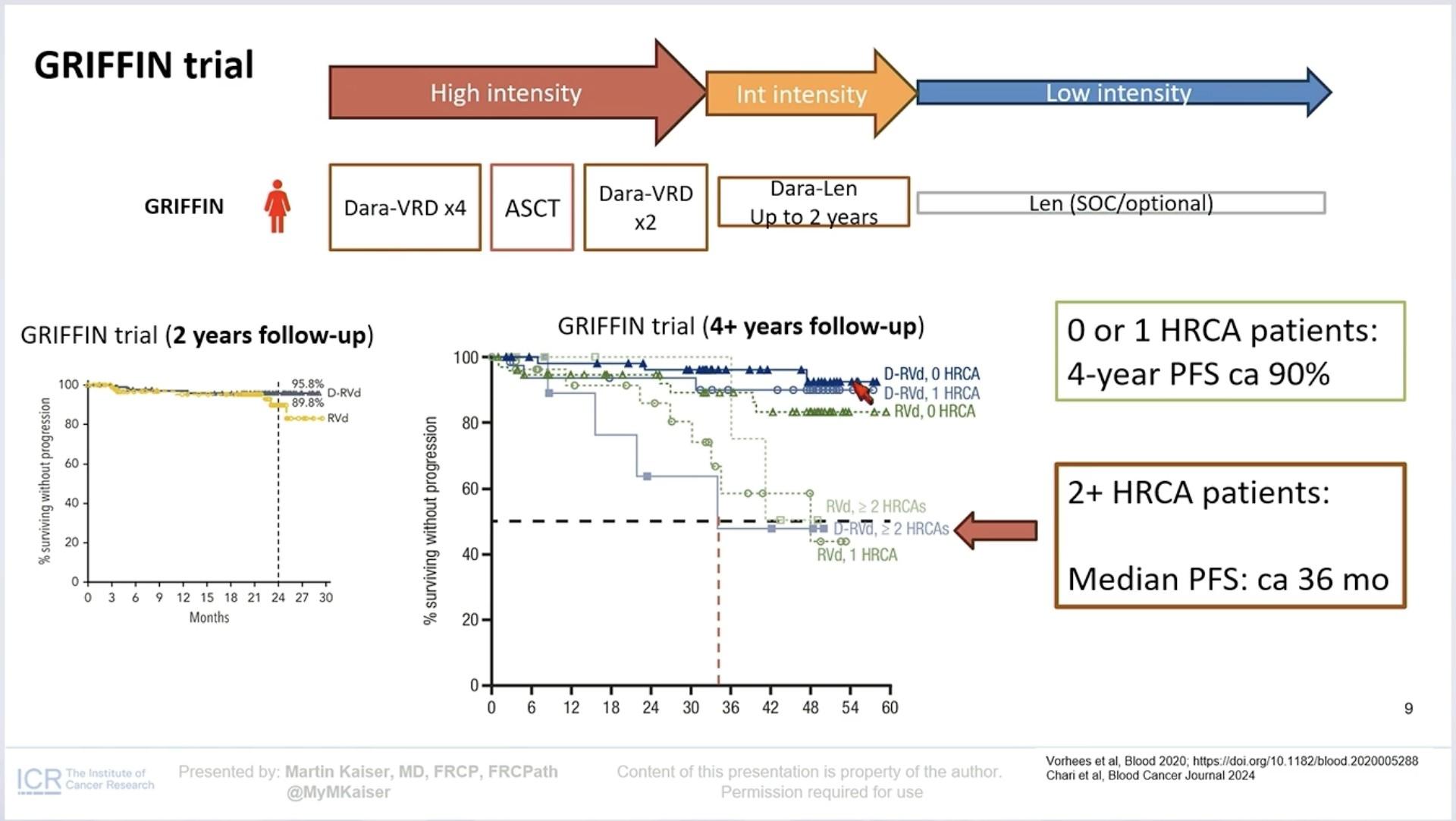

What Have we Learned?
• Diagnose High Risk MM Early
• Treat HRMM differently
• Maintain the intensity
Risk Stratification Based on Cytogenetic Abnormalities
IMS consensus on genomic definitions of High-Risk Multiple Myeloma (HRMM)
• Del17p
– In >20% sorted PCs
• TP53 mutation
• Biallelic Del(1p32)
• Association of 2 among:
– T(4;14) or t(14;16) or t(14;20)
– Gain/amp 1q
– Monoallelic del(1p32)
– B2m >5.5
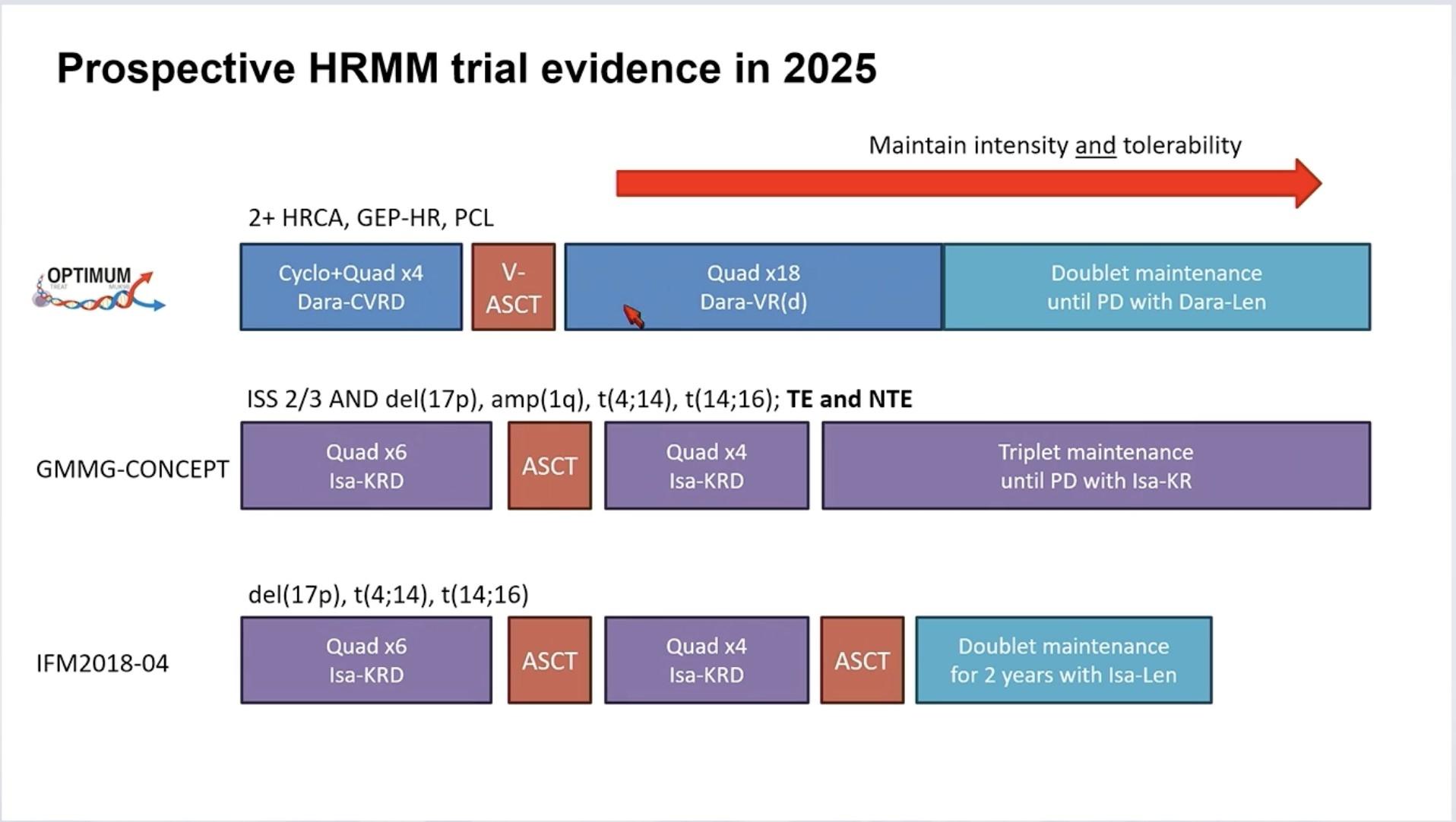
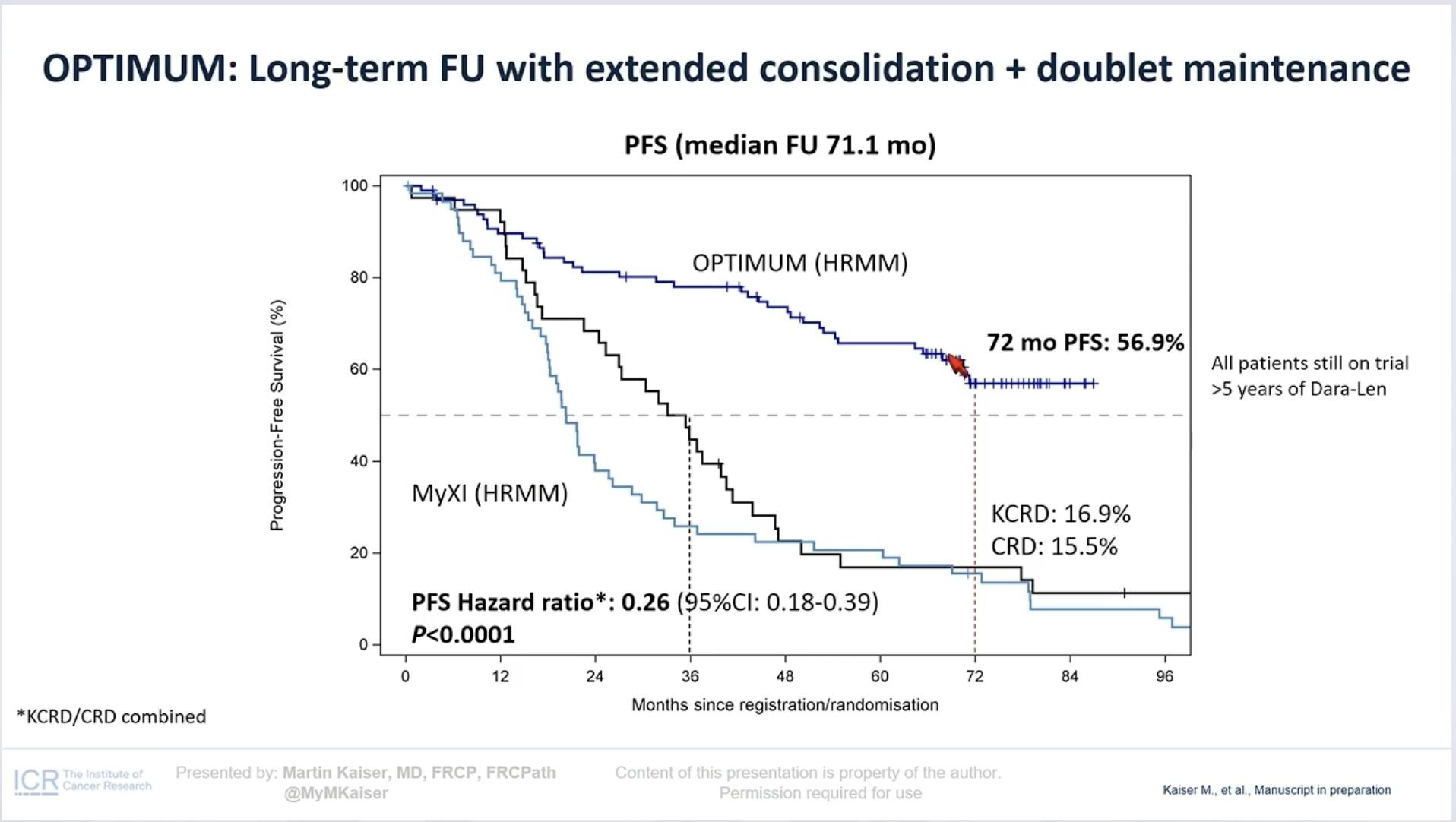
Transplant Eligible: Key Points
Quadruplet therapy is the new standard for patients with NDMM who are transplant eligible.
Continuous improvement of MRD rate is observed through induction, ASCT, consolidation, and maintenance treatment.
Combination maintenance therapy is clearly associated with sustained MRD negativity.
Achieving rapid and deep responses is crucial for patients with HR MM.
The Future: CAR-T cell, Bispecifics, ADC
Response Adapted therapy


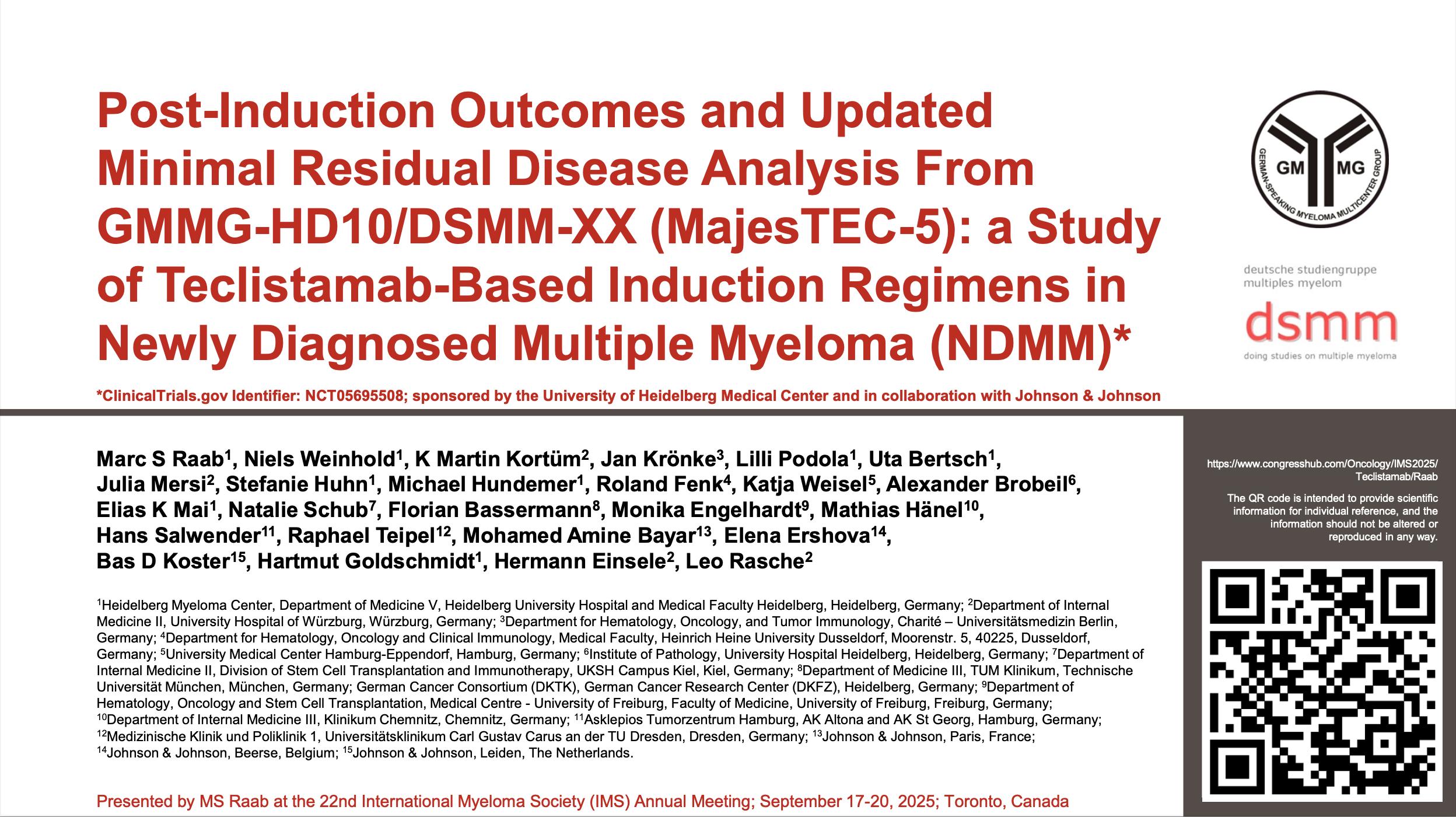

Cilta-Cel: Phase III Trials in NDMM
CARTITUDE-5: Transplant not intended/not eligible.
N = 650 planned. Patient enrollment began August 2021
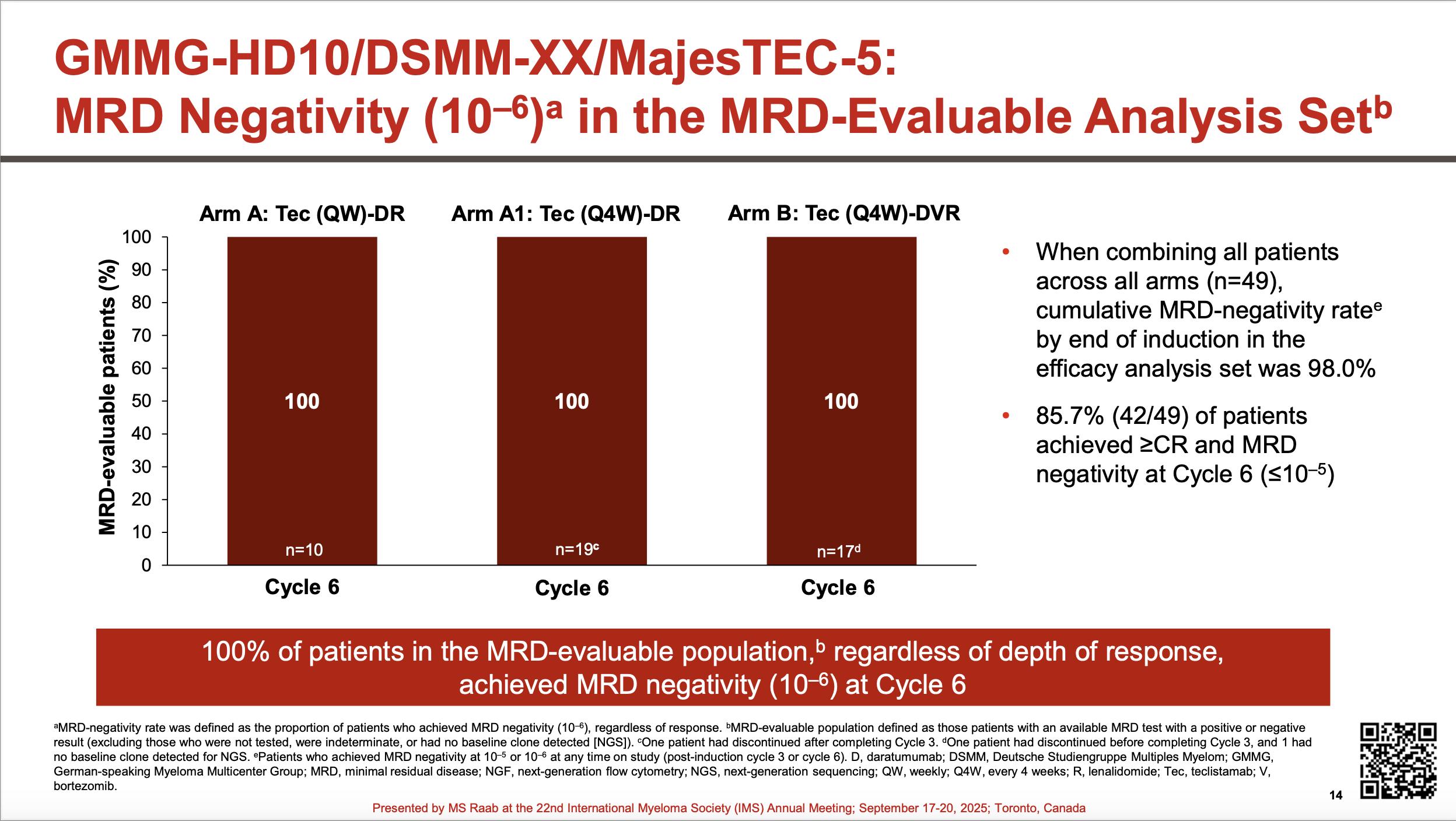
CARTITUDE-6: Transplant eligible.
N = 750 planned
MIDAS study : MInimal residual Disease
Adapted Strategy
Conclusions: Controversies and Gaps
• Induction Therapy will continue to evolve
• ADC
• Bispecifics
• HCT may go away, but cellular therapy (CART) is here to stay
• Maintenance therapy will be risk stratified and MRD directed
Transplant Ineligible (TIE) NDMM
The diversity of patient populations in Ti NDMM means flexibility is required to tailor treatment to patient needs

Frailty and disability

Age
Multiple factors for consideration

Comorbidities and organ function

Performance status


Risk assessment Refractory status

Patient preference and other psychosocial factors


Treatment history
Laubach J, et al. Leukemia 2016;30:1005–17; Sanchez L, et al. Expert Rev Hematol 2020;13:943–58; Sonneveld P and Brojil A. Haematologica 2016;101:396–406; Goel U, et al. Am J Hematol 2022;97:S3–25; Ramasamy K, et al. Blood Reviews 2021;49:100808 Ti, transplant ineligible
Treatment Attrition in Multiple Myeloma
Real-world assessment of lines of therapy and outcomes based on 3 US insurance claims databases
Put your best foot forward
Should we consider MRD in older patients?
Does Depth of Response Matter??
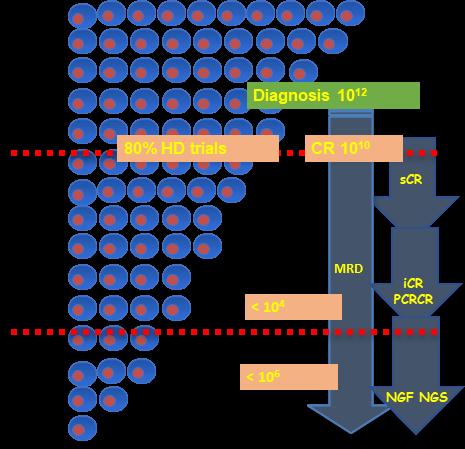

Paiva B et al. Blood. 2015;125(20):3059-68.
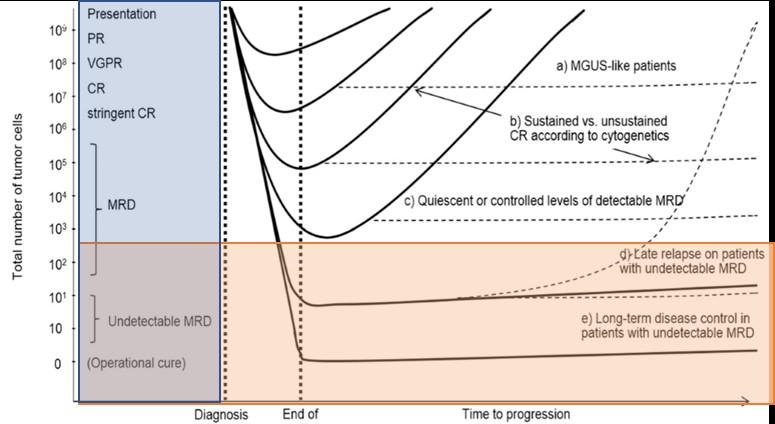
MRD Negative Patients Have Improved Outcomes
Irrespective of Therapy Used
Perrot A et al. Blood. 2018;132(23):2456-2464.
MAIA: Daratumumab-Lenalidomide-Dexamethasone for TIE NDMM
Median FU, 56.2 months
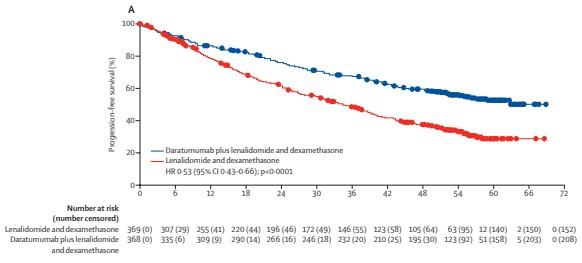
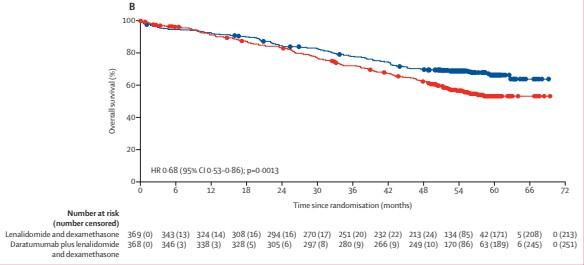
Facon T et al. Lancet Oncol. 2021;22(11):1582-96.
DaraRd
MRD-Negativity Rates and OS by MRD Status
• At a median follow-up of 64.5 months, the rates of MRD-negativity and sustained MRD negativity lasting ≥12 and ≥18 months were higher for D-Rd versus Rd
• At an extended median follow-up of 73.6 months, OS was improved in patients who achieved MRD negativity versus those who were MRD positive in both treatment arms; however, more patients in the D-Rd arm achieved MRD negativity
Can we Treat Fit Patients more Aggressively?
Quadruplet combinations?
Novel immunotherapies?
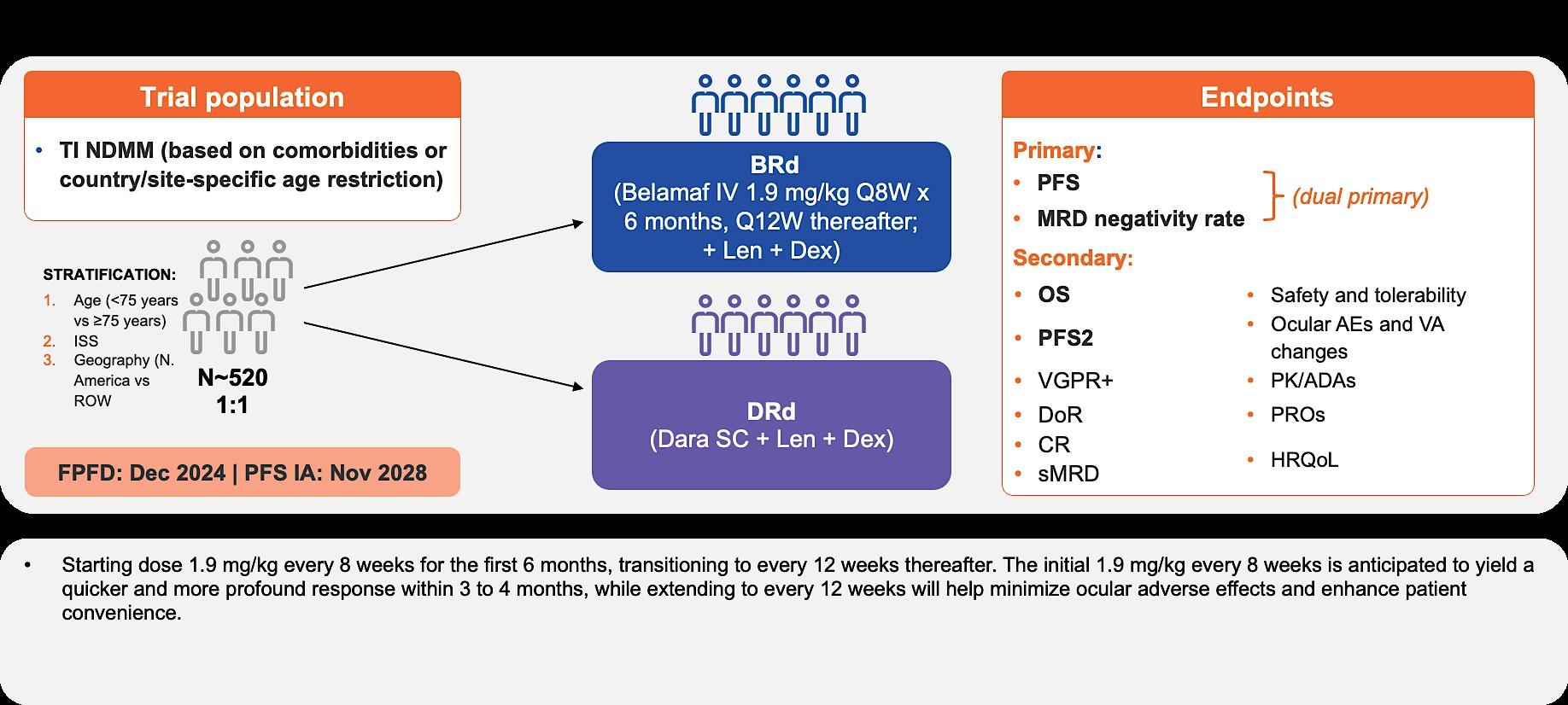
IMROZ (EFC12522) and CEPHEUS (MMY3019):
Study Designs
IMROZ1: NDMM patients ineligible for HDT-ASCT (N = 440)
Press Release Dec 7, 2023: Study met its primary endpoint
CEPHEUS2: phase 3 study of DARA SC-RVd vs RVd in transplantineligible FLMM (N = 360)
Primary endpoint:
• PFS (40 months vs 62.5 months)
Secondary endpoints:
• OS, PFS2
• ORR, CR
• Safety, QoL
• MRD
• PFS2 Long-term safety, subsequent therapy, PFS2, and survival followup Induction/Consolidation Maintenance
No cross-trial comparison is intended with this data. CR, complete response; d, dexamethasone; HDT-ASCT, high-dose therapy and autologous stem cell transplantation; ISA, isatuximab; MRD, minimal residual disease; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; QoL, quality of life; R, lenalidomide; SC subcutaneous; VGPR, very good partial response; V, bortezomib
Primary endpoint:
• MRD Secondary endpoints:
• PFS, OS
• Durable MRD
• ORR, VGPR, CR
1. IMROZ. NCT03319667. Updated April 8, 2024. Accessed April 8, 2024. https://clinicaltrials.gov/ct2/show/NCT03319667
2. CEPHEUS. NCT03652064. Updated March 27, 2024. Accessed April 8, 2024. https://clinicaltrials.gov/ct2/show/NCT03652064
IMROZ: Primary endpoint met: Interim PFS analysis–IRC assessment
Primary endpoint: PFS Median follow-up: 59.7 months
HR: 0.596 (95% CI: 0.406; 0.876); log-rank P=0.0005 Isa-VRd n=265: mPFS NR 60-month PFS: 63.2%
n=181:
0 6 12 18 24 30 36 42 48 54 60 66 72
OR: 1.73 (95% CI: 0.99; 3.01)
Response (ITT)
A 60-month PFS rate of 63.2% vs 45.2% with Isa-VRd and VRd, respectively, highlights a PFS benefit in patients with NDMM not intended for transplant OR: 1.79 (95% CI: 1.22; 2.63) OR: 1.80 (95% CI: 1.23; 2.65)
2.73 (95% CI: 1.80; 4.14)
At a median follow-up of 5 years, Isa-VRd followed by Isa-Rd resulted in a statistically significant reduction in the risk of progression or death by 40.4%, and consistent deep responses vs VRd followed by Rd.
confidence interval; CR, complete response; d, dexamethasone; HR, hazard ratio; Isa, isatuximab; ITT, intent to treat; m, median; MRD, minimal residual disease; NGS, next-generation sequencing; NR, not reached; PFS, progression-free survival; R, lenalidomide; SC, subcutaneous; Ti, transplant ineligible; V, bortezomib; VGPR, very good partial response
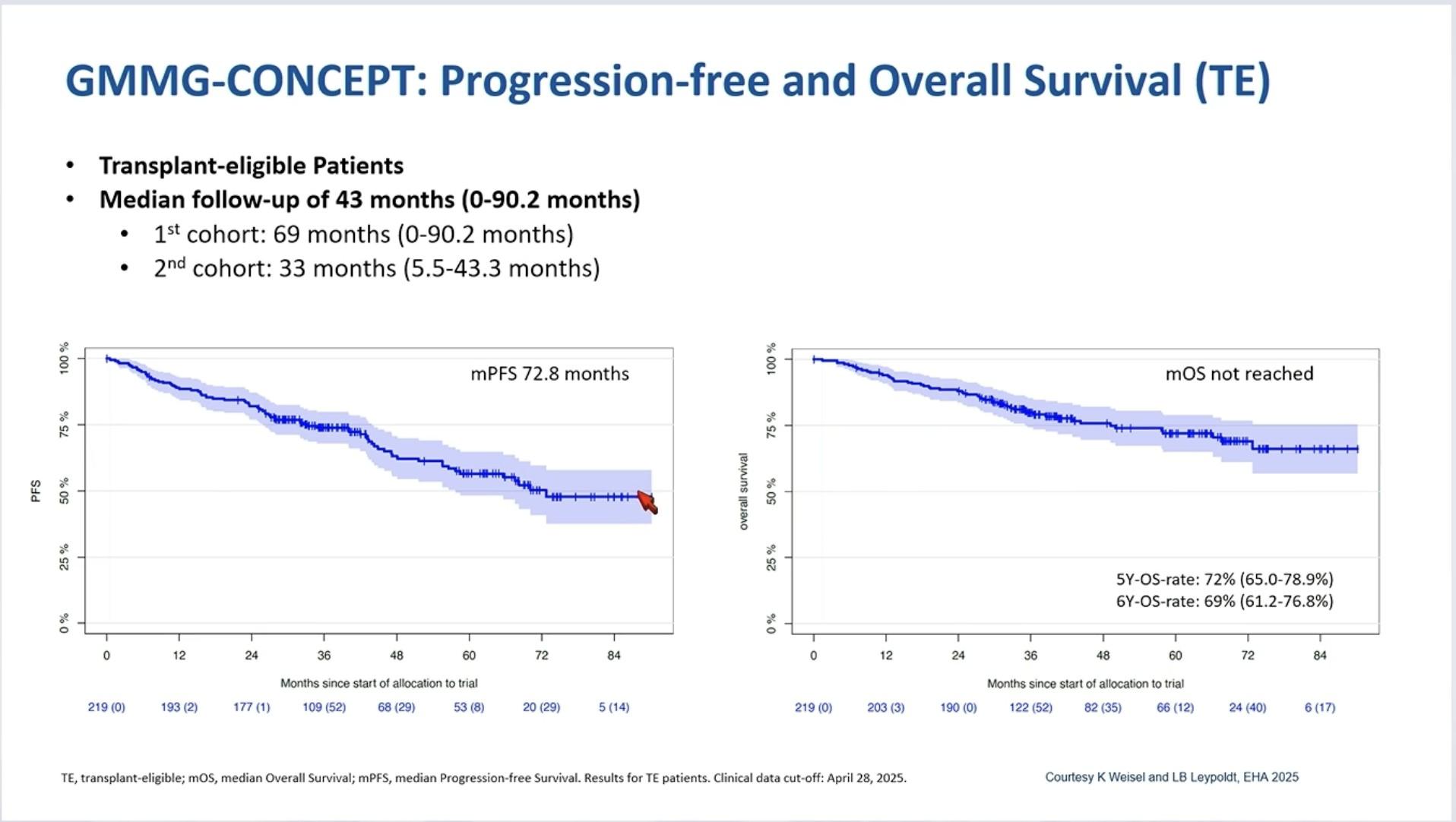
GMMG-CONCEPT: Response Rate and MRD
Negativity
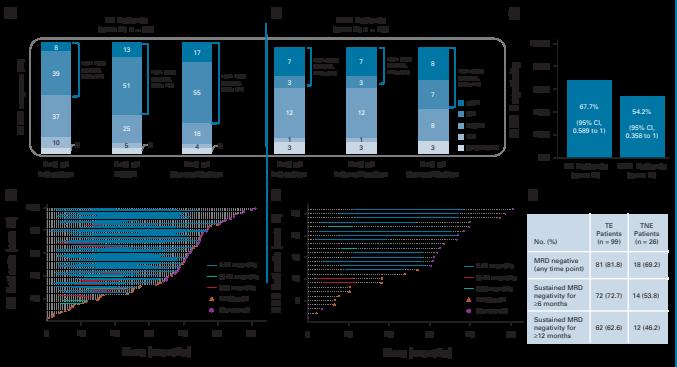
Primary endpoint met: 54% MRD negative at end of consolidation

Leypoldt LB et al. J Clin Oncol. 2024;42(1):26-37.

Leypoldt LB et al. J Clin Oncol. 2024;42(1):26-37.
BENEFIT: VRd using weekly bortezomib dosing in combination with Isatuximab turning monthly after 1year treatment

†Cycle 1 only. CR, complete response; Cy, cycle; d, dexamethasone; D, day; Isa, isatuximab; M, month; MRD, minimal residual disease; NDMM, newly diagnosed multiple myeloma; NGS, next generation sequencing; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; R, lenalidomide; SPM, second primary malignancy; Ti, transplant-ineligible; V, bortezomib; VGPR, very good partial response.
Leleu, X., Hulin, C., Lambert, J. et al. Isatuximab, lenalidomide, dexamethasone and bortezomib in transplant-ineligible multiple myeloma: the randomized phase 3 BENEFIT trial. Nat Med (2024). https://doi.org/10.1038/s41591-024-03050-2
BENEFIT:
MRD–*-CR rate at 18 months - ITT population
MRD–*-CR rate at 18 months - ITT population
Isa-VRd resulted in a significant improvement in the MRD– CR rate at 12 and 18 months, and at 10–5 and 10–6 in the ITT population OR (95% CI): 2.91 (1.64–5.16) P=0.0003
(95% CI): 3.86 (1.94–7.7) P=0.0001
(95% CI): 3.43 (1.53–7.65) P=0.003
(95% CI): 2.85 (1.49–5.45) P=0.002
How to improve and optimize treatment for TIE
Frail NDMM patients?
Dara-IRd in Transplant-Ineligible Patients


Yee A et al. NCT04009109. Updated February 5, 2024. Accessed May 28, 2024.
https://clinicaltrials.gov/study/NCT04009109
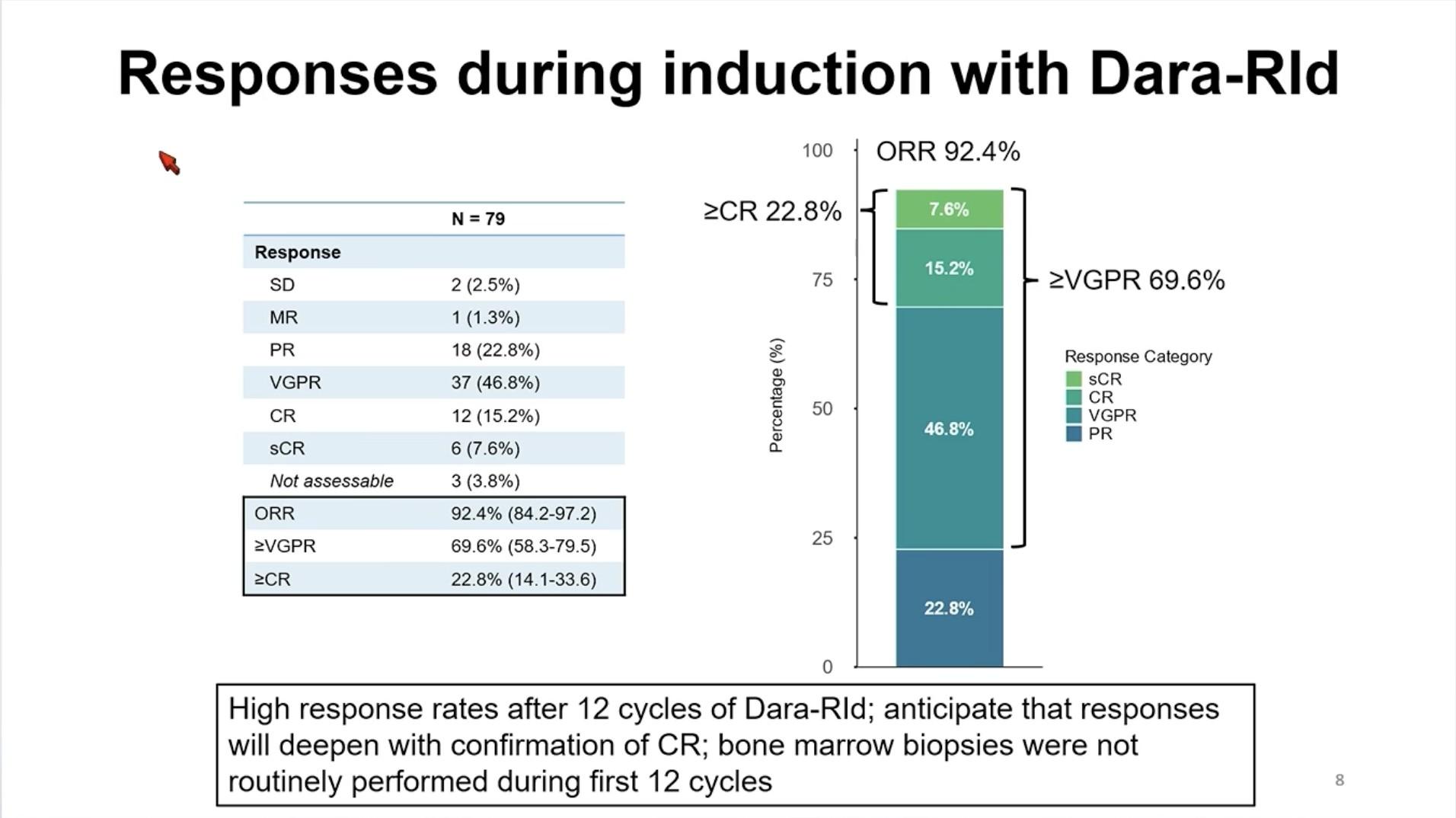
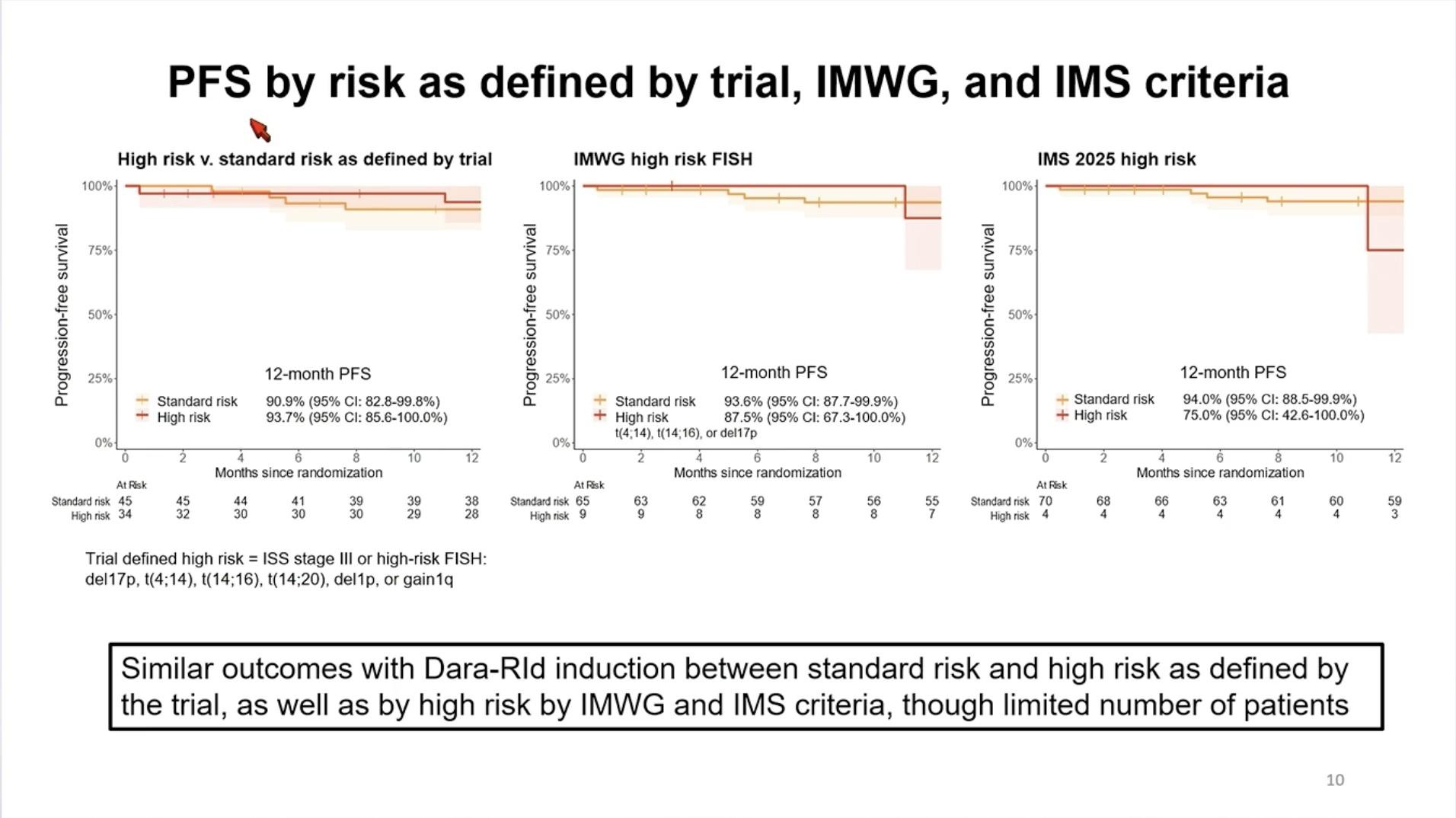
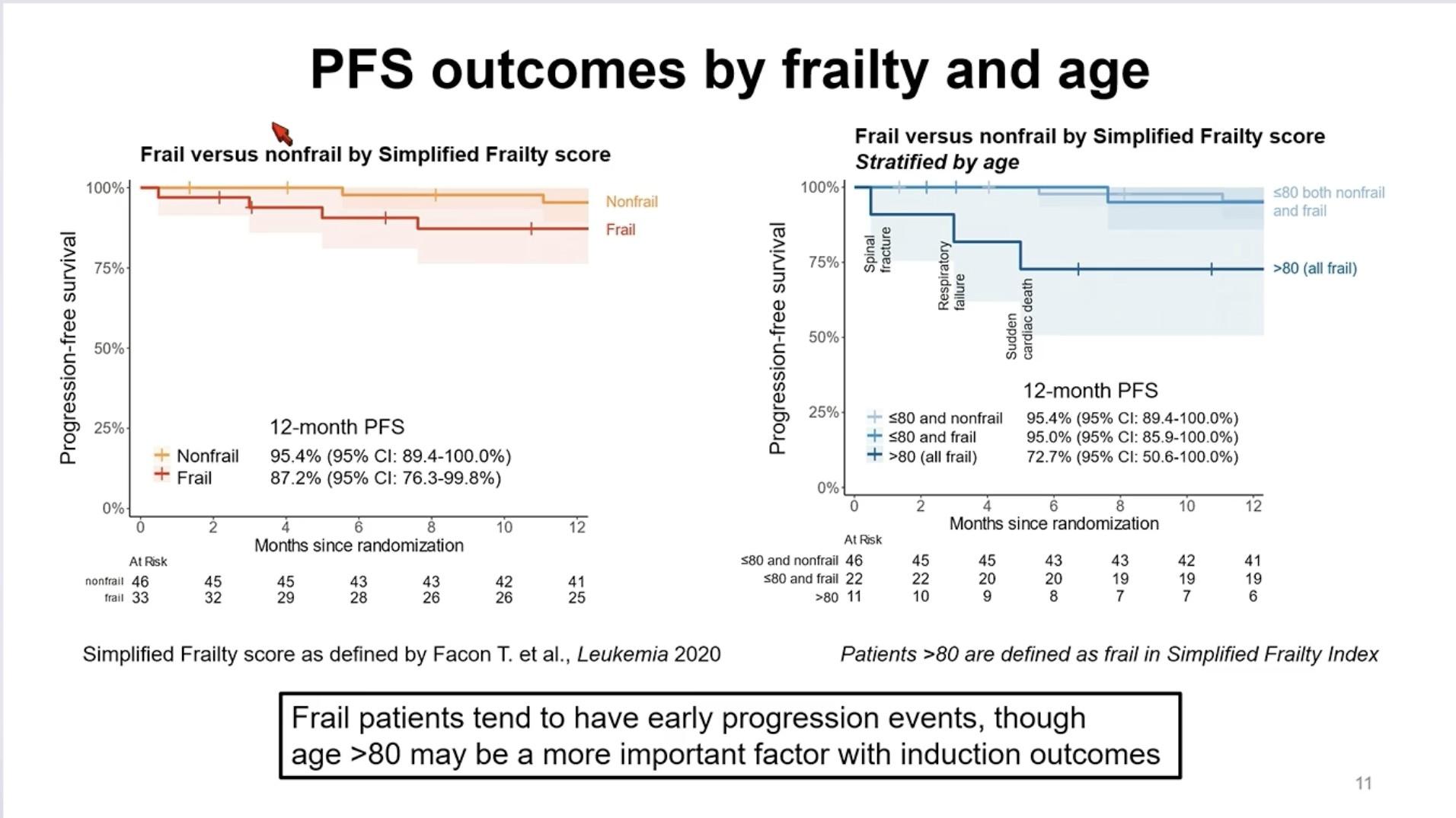
Conclusions – TI NDMM
• Outcomes continue to improve with triplet and now quadruplet treatment regimens
• Consider frailty status when treating older patients with MM
• Treatment endpoints and strategies should be different for fit and frail NTE patients
• Improving MRD negativity rates for fit patients to guide treatment and improve survival
• Limiting toxicity for frail patients not to worsen survival
• Future role of bispecific antibodies and CAR T-cell therapy???


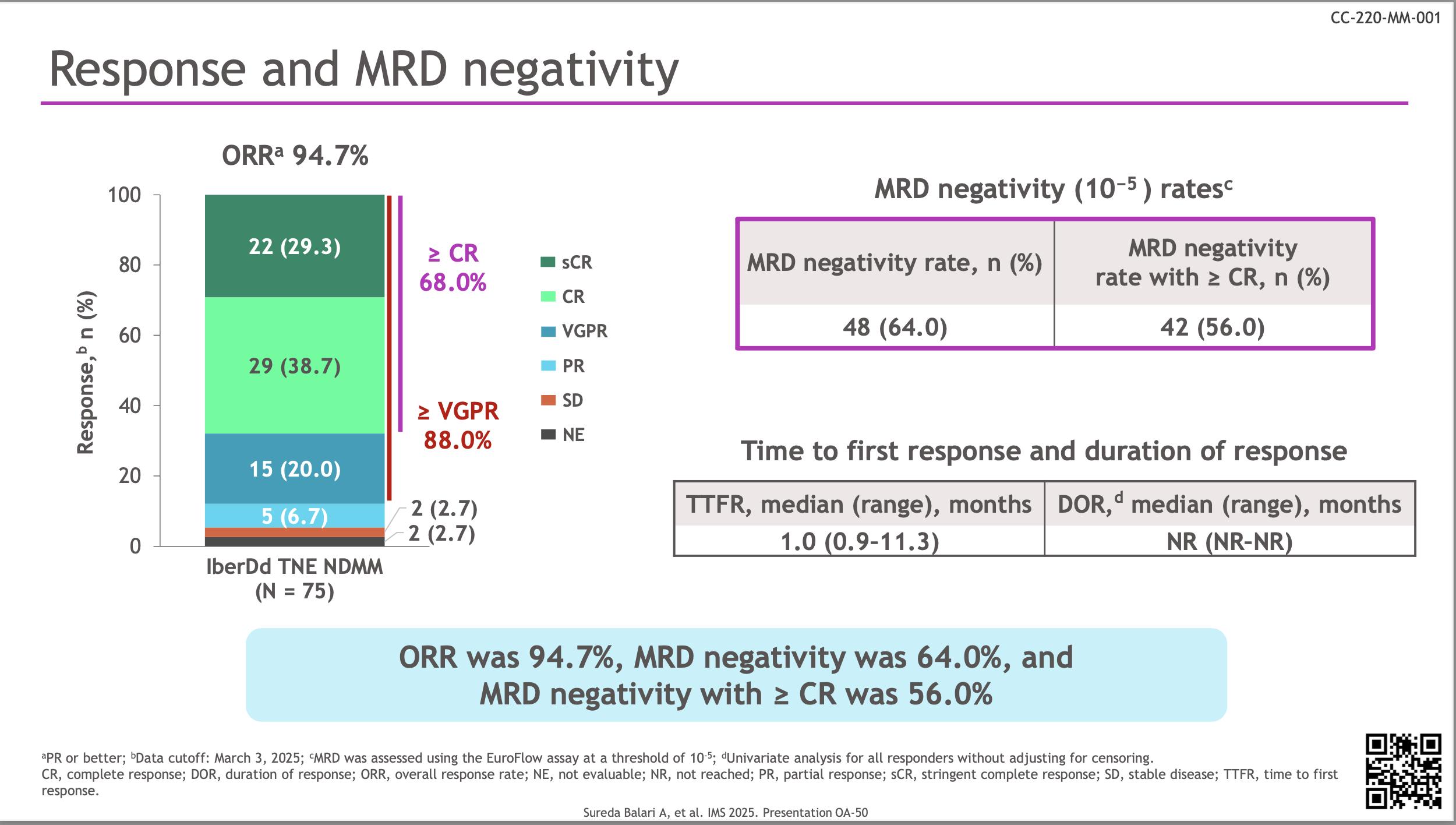
DREAMM-10 Belantamab Mafodotin
Frontline


Cilta-Cel: Phase III Trials in
Newly Diagnosed MM
CARTITUDE-5: Transplant not intended/not eligible.
N = 650 planned. Patient enrollment began August 2021

CARTITUDE-6: Transplant eligible.
N = 750 planned


Breakout B
RRMM: Continuing the Myeloma Treatment Journey
Dr. Attaya Suvannasankha, MD
Relapse Refractory Multiple Myeloma: Continuing the Myeloma Treatment Journey
A Patient-Focused Discussion on Modern Myeloma Care
Attaya Suvannasankha, MD
Professor of Clinical Medicine
Indiana
University School of Medicine

�� Myeloma biology or disease process
⚖ Decision-making and treatment choice
❤ Quality of life and emotional well-being
Visual Legend
�� Family or caregiver support
�� Treatments or clinical trials
�� Financial considerations
�� Hope and progress
�� Innovation and new research
�� Patient figures Patient vignettes / stories
Set the stage: continuing the myeloma journey.
Welcome & Overview
Session goals: understand relapse vs progression, treatment options, and practical steps.
Emphasis on shared decisionmaking: what matters to the patient and family. A patient-first, hopeful approach — science + quality of life.
Life with myeloma is a marathon
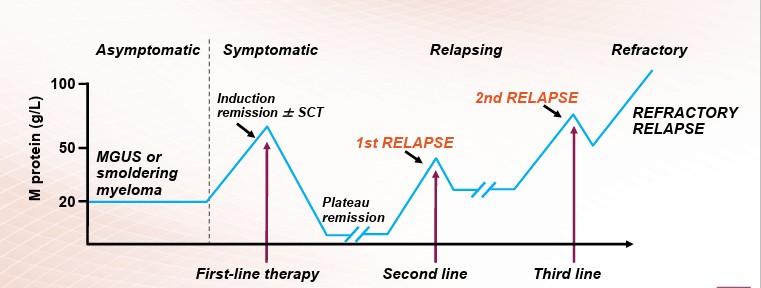
What is relapsed/refractory disease and a line of therapy?

Definitions:
• Relapsed:recurrence (reappearance of disease) after a response to therapy
• Refractory:progression despite ongoing therapy
• Progression: change in M protein/light chain values
• Line of therapy: change in treatment due to either progression of disease or unmanageable side effects–Note: initial (or induction) therapy + stem cell transplant + consolidation/maintenance therapy = 1 line of therapy
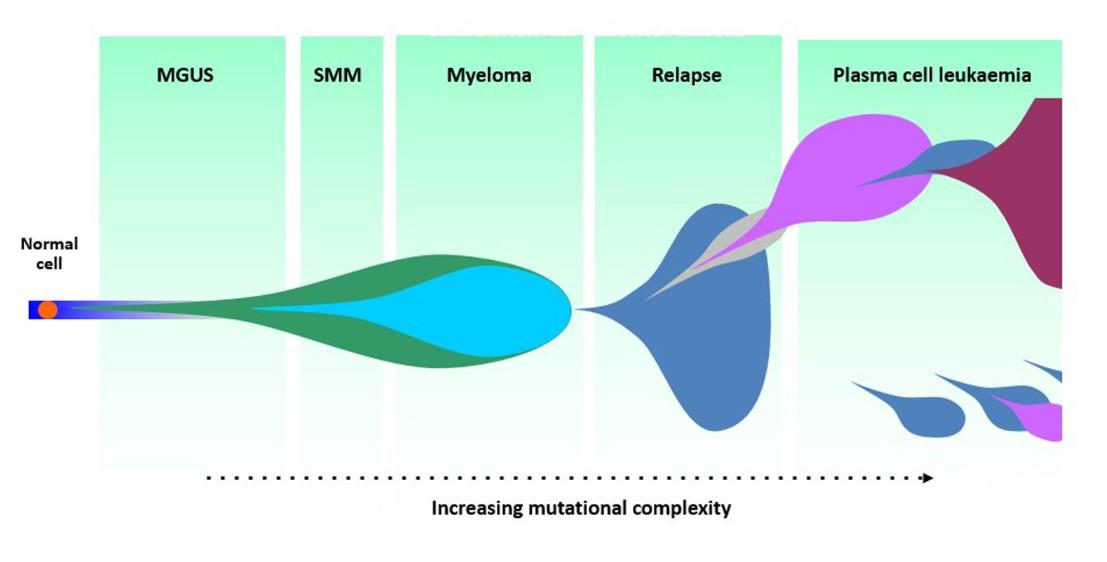
Why Myeloma Relapses
• Myeloma cells can adapt and change over time (clonal evolution).
• Some cells survive initial therapy and start growing again.
• Minimal Residual Disease (MRD): tiny cell groups left behind.
• Relapse ≠ failure — it’s part of the disease’s natural course.

Complete remission
Relapse vs Progressive Disease
Relapse: disease reappears after a period of control.
Progression: disease continues to worsen without a break.
Clinical decisionmaking differs for each situation.
Biochemical vs Clinical Relapse
Biochemical: lab values rising, but no new symptoms.
Clinical: new bone pain, anemia, kidney issues, or other signs.
Both indicate myeloma activity but guide timing differently.
Monitoring and Timing of Therapy
• Regular labs allow early detection.
• Some patients can safely observe mild biochemical changes.
• Treatment starts when progression affects organs or quality of life.
• Decisions are shared between patient, family, and care team.
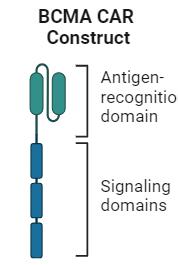
Novel Therapies in Multiple Myeloma

CD38 mAb

BCMA-directed ADC

BCMA-directed CAR T-cell therapy

BCMA-directed BsAb

GPRC5D-directed BsAb

Idecabtagene vicleucel (expanded approval 2024)


Ciltacabtagene autoleucel (expanded approval 2024)


Talquetamab

Daratumumab

Isatuximab
Belantamab mafodotin (withdrawn 11/2022)


Teclistamab Elranatamab
Ciltacabtagene autoleucel PI. Belantamab mafodotin PI. Daratumumab PI. Elranatamab PI. Isatuximab PI. Idecabtagene vicleucel PI. Teclistamab PI. Talquetamab PI.
Belantamab mafodotin
Choosing therapy


Myeloma care is individualized
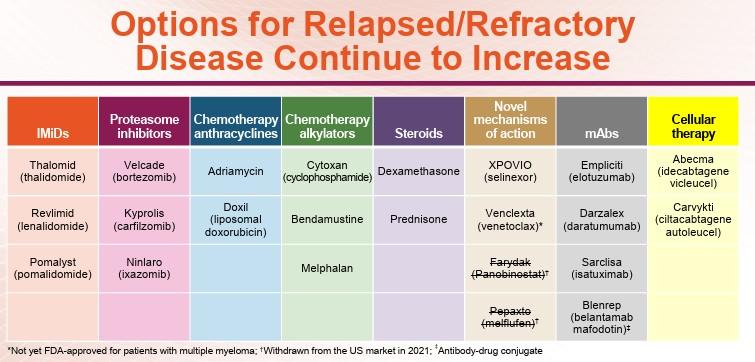

FDA Approved Autologous CAR T-Cell Therapies for
R/R MM
Idecabtagene Vicleucel Ciltacabtagene Autoleucel
CAR type BCMA/CD137 (4-1BB)/CD3ζ 2-BCMA binding domains/CD137 (4-1BB)/CD3ζ
Costimulatory domain 4-1BB
Vector Lentiviral
Lymphodepletion IV cyclophosphamide IV 300mg/m2 + fludarabine 30mg/m2 QD x 3 days
Pivotal trials KarMMa and KarMMa-3 CARTITUDE-1 and CARTITUDE-4
Median time from leukapheresis to delivery 35 days 32 days Ciltacabtagene autoleucel PI. Idecabtagene vicleucel PI. Abebe. Front Immunol. 2022;13:991092. Image created from BioRender.com.
Initial approvals: patients with R/R MM after ≥4 prior LoT, including an IMiD, PI, and a CD38 mAb.
Expanded indications granted (April 2024): ide-cel after ≥2 prior LoT including an IMiD, PI, and a CD38 mAb and cilta-cel after 1+ prior LoT including a PI and an IMiD and refractory to Len
Phase III Data With Approved CAR T-Cell Therapies in R/R Multiple Myeloma
Bridging Therapy
SoC ≥1 cycle (optional in KarMMa-3, up to 1 cycle)
Investigators’ Choice SoC
Cyclophosphamide + Fludarabine† Lymphodepletion
Enrollment criteria
R/R MM with 2-4 prior LOT, refractory to last regimen
1. Rodriguez-Otero. NEJM. 2023;388:1002. 2. Rodríguez-Otero. ASH 2023. Abstr 1028. 3. San-Miguel. NEJM. 2023;389:335.
CAR T-Cell Infusion (Day 0)
CAR T-Cell Therapy
PD, death, unacceptable toxicity (crossover allowed in KarMa-3)
R/R MM with 1-3 prior LOT, refractory to lenalidomide
KarMMa-3: Treatment Response and Final PFS Analysis
CARTITUDE-4: Outcomes
• Median follow-up of 15.9 mo
• MRD-negative: 60.6% for cilta-cel vs 15.6% for SoC
San-Miguel. NEJM. 2023;389:335.
Spectrum of Clinical Toxicities Associated With CAR T-Cell Therapy
Headache
Myalgia, arthralgia
Hypotension, tachycardia
Neurologic change
CAR T-Cell Therapy: Acute Toxicities
Acute Toxicities
Cytokine-release syndrome
Cytopenias
Immune effector cell–associated neurotoxicity syndrome
Immune effector cell associated HLH-like syndrome
Generally managed by treatment center
Management of Adverse Events
CRS Tocilizumab, corticosteroids, anakinra
Neurotoxicity (ie, ICANs)
Seizure prophylaxis, corticosteroids, anakinra, siltuximab, cyclophosphamide
Hines. Transplant Cell Ther. 2023;29:438.e1. Jain. Blood 2023;141:2430.
Maus. J Immunother Cancer. 2020;8:e001511. Tallantyre. J Neurol. 2021;268:1544.
IEC-HS
Anakinra, steroids, ruxolitinib, etoposide, emapalumab
CAR T-Cell Therapy: Delayed Toxicities
Delayed Toxicities
B-cell aplasia/hypogammaglobulinemia
Prolonged cytopenias
Late infections
Long-term neurologic events/ movement and neurocognitive treatment-emergent AEs
Transient cardiac toxicities
Secondary malignancies?
Management of Adverse Events
Prolonged cytopenias Growth factors (after CRS)
Immunosuppression
Prophylactic/preemptive IVIG for hypogammaglobulinemia
Infection (eg, HSV/VZV, encapsulated bacteria) Vaccines, acyclovir, sulfamethoxazole/ trimethoprim, other
Generally managed by primary oncologist (treatment center or community setting)
Chakraborty. Transplant Cell Ther. 2021;27:222. Hill. Blood. 2020;136:925. Munshi. NEJM. 2021;384:705. Berdeja. Lancet. 2021;398:10297. Cohen. Blood Cancer J. 2022;12:32.
Bispecific Therapy Options for Multiple Myeloma
• “Off the shelf” immunotherapy with multiple binding domains
– Target different tumor antigens like BCMA, GPRC5D, FcRH5
– Also binds to immune cell targets, including CD3 (T-cell)
Teclistamab*: CD3 x BCMA
Elranatamab*: CD3 x BCMA
Talquetamab*: CD3 x GPRC5D
Cevostamab: CD3 x FcRH5
Linvoseltamab: CD3 x BCMA
ABBV-383: CD3 x BCMA
• Variable administration: SC or IV options with required step-up dosing
*FDA approved for treating adults with R/R MM after ≥4 prior lines of therapy, including a PI, IMiD, and anti-CD38 mAb.
Elranatamab-bcmm PI. Teclistamab-cqyv PI. Talquetamab-tgvs PI. van de Donk. Lancet. 2023;402:142.
FDA-Approved Bispecific Antibody Therapies in R/R MM
Agent Type
Teclistamab
Elranatamab
BCMA x CD3
Bispecific antibody
BCMA x CD3
Bispecific antibody
Current Indication
≥4 prior LOT, including an IMiD, PI, and an anti-CD38 mAb
Talquetamab
GPRC5D x CD3
Bispecific antibody
≥4 prior LOT, including an IMiD, PI, and an anti-CD38 mAb
≥4 prior LOT, including an IMiD, PI, and an anti-CD38 mAb
Ciltacabtagene autoleucel PI. Elranatamab PI. Idecabtagene vicleucel PI. Talquetamab PI. Teclistamab PI.
Phase I/II MajesTEC-1: Teclistamab in
R/R MM
• R/R MM after ≥3 lines of therapy, including an IMiD, PI, and anti-CD38 mAb
– 26% high-risk cytogenetics
– Median 5 prior lines of therapy (range: 2-14)
– 78% triple-class refractory; 30% penta refractory
– 90% refractory to last therapy line
• Teclistamab: 1.5 mg/kg SC weekly, after step-up
ORR (N = 165)
MajesTEC-1:
PFS and DoR With Teclistamab in R/R MM
Patients
Patients
Phase II MagnetisMM-3:
Elranatamab for BCMA-Directed Therapy-Naive R/R MM (Cohort A)
Patients with MM refractory to ≥1, including an IMiD, PI, and anti-CD38 mAb
‒ 97% triple-class refractory
‒ Median 5 prior lines of therapy (range: 2-22)
‒ 25% with high-risk cytogenetics
‒ 32% with extramedullary disease
Elranatamab: 76 mg SC weekly with priming and/or premedication to reduce CRS
– If weekly dosing given for ≥6 cycles with achievement of ≥ PR for ≥2 mo, then dosing interval changed to every 2 wk
Primary endpoint: ORR
Secondary endpoint: DoR, OS, PFS, safety
Lesokhin. Nat Med. 2023;29:2259. Mohty. EHA 2024. P932.
follow-up: 14.7 mo
MagnetisMM-3: PFS and DoR
Overall
Patients
Median PFS, mo (95% CI)
Overall 17.2 (9.8-NE)
Patients
Patients
Median ODR, mo (95% CI)
Patients with OR NR (NE-NE)
Patients with ≥ CR NR (NE-NE)
Phase III MagnetisMM-5: Trial
of Elranatamab + Daratumumab in R/R Multiple Myeloma
• Part 1 enrolled 34 adults with R/R MM with ≥3 lines of therapy, including lenalidomide and a PI and no history of treatment with a BCMA-targeting agent, and no anti-CD38 mAb within 6 mo of first dose
– After step-up dosing, patients received 44 mg or 76 mg SC elranatamab + 1800 mg SC daratumumab
• Primary endpoint: DLT
• Secondary endpoints: ORR, CR, DoR, PFS, OS, MRD negativity, safety, and PK
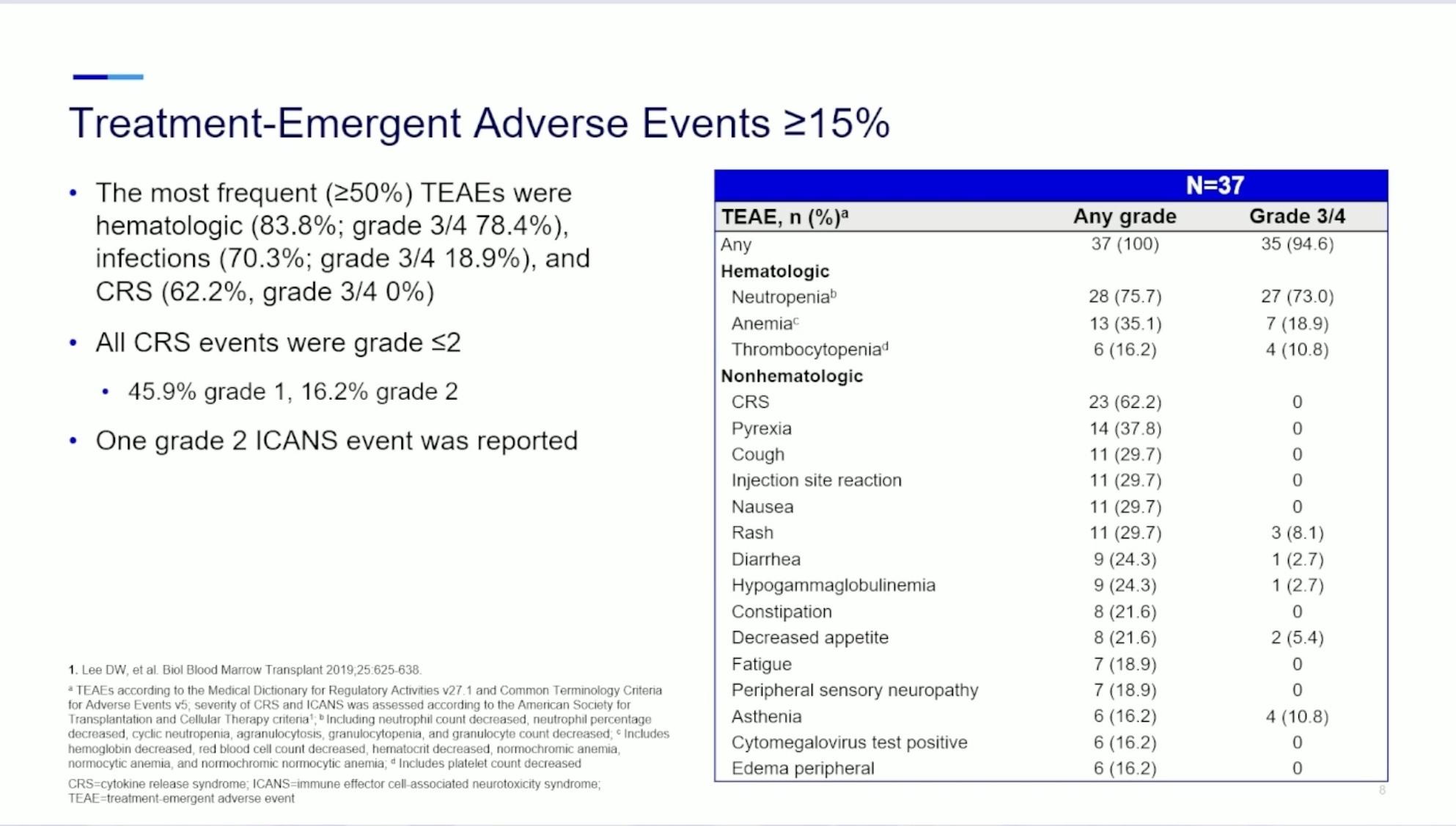
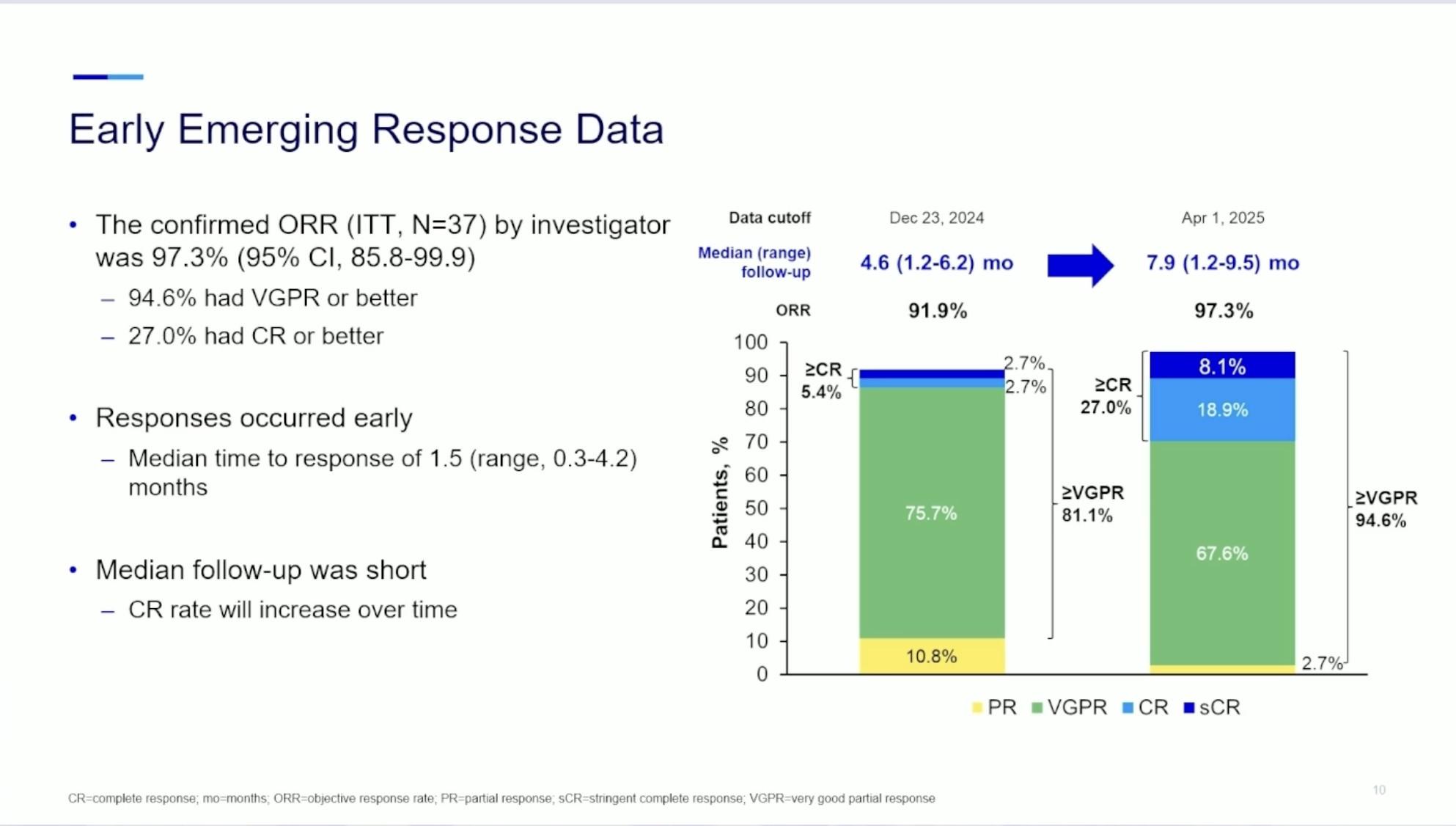
Safety Summary:
No DLTs were observed, and safety profile was tolerable
CRS: 47%, with no grade ≥3 CRS events
– There were no treatment discontinuations due to CRS
No patients experienced ICANS
Phase II MonumenTAL-1: Talquetamab in R/R MM
Patients with R/R MM after ≥3 lines of therapy, including an IMiD, PI, anti-CD38 mAb
‒ 69%-84% triple-class refractory
‒ Median 5-6 prior lines of therapy across all cohorts
‒ 27.1% with high-risk cytogenetics; 24.3% with EMD
Talquetamab: 0.4 mg/kg SC QW or 0.8 mg/kg SC Q2W with 2-3 step-up doses and/or premedication to reduce CRS
Primary endpoint: DLTs
Key secondary endpoint: ORR
Chari. NEJM. 2022;387:2232. Schinke. ASCO 2023. Abstr 8036. Rasche. EHA 2024. Abstr P915.
MonumenTAL-1: DoR and PFS Outcomes
DoR in 0.8 mg/kg Q2W Cohort
Phase Ib RedirectTT-1: Teclistamab Plus Talquetamab in
R/R Multiple Myeloma
• Open-label, phase Ib/II dose escalation and expansion trial of teclistamab plus talquetamab in patients with R/R MM with previous exposure to a PI, IMiD, and anti-CD38 mAb and refractory to last line of therapy
– Median prior LOT: 4 (1-11); extramedullary plasmacytomas: 37.6%
• Primary endpoints: safety, RP2R; secondary endpoints: ORR, PK, immunogenicity
ICANS
Occurrence
Safety of Approved Bispecific Antibodies for Multiple Myeloma
1/2: 3%
Neutropenia Gr 3/4: 56%
Infection Gr 3/4: 35%
0%
3/4: 35%
3/4: 17%
3/4: 51%
3/4: 31%
Skin/Nail Toxicity Not reported 62% Not reported
Oral Toxicity Not reported
80% (dysgeusia: 49%, dry mouth: 34%, dysphagia: 23%, ageusia: 18%) Not reported
Moreau. NEJM. 2022;387:495. Teclistamab-cqyv PI. van de Donk. ASCO 2023. Abstr 8011. Chari. NEJM. 2022;387:2232. Talquetamab-tgvs PI. Touzeau. EHA 2023. Abstr S191. Elranatamab-bcmm PI. Lesokhin. Nat Med. 2023;29:2259.
Ongoing Phase I/II Trials With Bispecific Antibodies in R/R Multiple Myeloma
Inclusion criteria
Triple-class refractory R/R MM, with prior BCMA-targeted ADC or CAR Tcell therapy, no BCMA-targeted bispecific*
R/R MM ≥3 prior LOT including PI, IMiD, and anti-CD38 mAb, no prior BCMA-targeted therapy
R/R MM ≥3 prior LOT including PI, IMiD, and anti-CD38 mAb
A1.
Belantamab Mafodotin: A BCMA-Targeted Antibody–Drug Conjugate
Belantamab mafodotin
• Humanized, afucosylated IgG1 anti-BCMA antibody
• Conjugated to a microtubule disrupting agent MMAF via a stable, protease resistant linker
MMAF (non-cell permeable, highly potent auristatin)

Managing Side Effects of Modern Therapies

Cytokine Release Syndrome (CRS): manageable with close monitoring.
Less severe with bispecific Ab

Infection prevention: hand hygiene, vaccinations, prompt reporting.

Fatigue and low counts: rest, nutrition, supportive medications.

Skin and oral side effects of Talquetamab requires preventive care

Eye care during BCMA ADC therapy: regular ophthalmology visits.
Report Report new symptoms early and clearly.
Practical Tips for Patients and Families
Stay Stay hydrated, eat well, and stay lightly active.
Lean on Lean on caregivers, family, and support groups.
Coordinate Coordinate schedules for appointments and rest.
Quality of Life and Emotional Health





EMOTIONAL
WELL-BEING IS AS VITAL AS LAB RESULTS.
ADDRESS ANXIETY, FATIGUE, AND UNCERTAINTY OPENLY.
USE MINDFULNESS , HOBBIES, AND SOCIAL CONNECTION FOR BALANCE.
SEEK COUNSELING OR SUPPORT WHEN NEEDED.
Living
with Hope

Myeloma treatment continues to evolve rapidly.
Each year brings new therapies and better outcomes.
Focus on resilience and teamwork with the care team.
Hope is grounded in science and compassion.

LUNCH
Please remain in this room
Walk Through the IMF Website
Robin Tuohy
Vice President, Support Groups, International Myeloma
Foundation
IMF REGIONAL COMMUNITY WORKSHOP
RALEIGH
AFTERNOON AGENDA

Seasons of Myeloma: Managing Side Effects and Living Well
Amy Pierre, MSN, RN, ANP-BC
Memorial Sloan Kettering Cancer Center & Flatiron Health
Living the Myeloma Life: Local Patient & Care Partner
Clark Murphy (Patient) & Janet Murphy (Care Partner)
Beyond Myeloma Therapy: Oral Health Survivorship
Katharine Ciarrocca, DMD, MSEd
Duke University Hospital, Durham, NC
Q&A with Panel
Closing Remarks
Robin Tuohy
Vice President, Support Groups, International Myeloma
Foundation

Walk Through the IMF Website
Robin Tuohy, Vice President, Support Groups
International Myeloma Foundation

Seasons of Myeloma: Managing Side Effects and Living Well
Amy Pierre, MSN, RN, ANP-BC
Memorial Sloan Kettering Cancer Center & Flatiron Health
Seasons of Multiple Myeloma

Amy E. Pierre MSN, RN, ANP-BC
Memorial Sloan Kettering Cancer Center, Flatiron Health & IMF Nurse Leadership Board member

Wintery Mix of Treatment Options Spring into Managing Side Effects
Summer of Success






Wintery Mix of Treatment Options

Diverse and Complex Treatment Combinations









Myeloma Treatment Common Combinations
Velcade® (bortezomib)
Lenalidomide
DVRd, VRd, Vd
DVRd, VRd, Rd
Kyprolis® (carfilzomib) KRd, Kd, DKd, Isa-Kd
Pomalyst® (pomalidomide) Pd, DPd, EPd, PCd, Isa-Pd
Darzalex® (daratumumab) DVRd, DRd, DVd, DPd, DVMP, DKd
Ninlaro®(ixazomib) IRd
Empliciti® (elotuzumab) ERd, EPd
Xpovio® (Selinexor) XVd, XPd, XKd
Sarclisa® (Isatuximab) Isa-Kd, Isa-Pd
Blenrep® (Belantamab mafodotin) Bela-d
Abecma® (Idecabtagene Vicleucel) --
Carvykti® (ciltacabtagene autoleucel)
Elrexfio® (elranatamab)

Lynozyfic™ (linvoseltamab)
Tecvayli® (teclistamab) --
Talvey® (talquetamab) --
Venclexta® (venetoclax) Vd + ven
New agents or regimens in clinical trials are possible options
ASCT = autologous stem cell transplant; Bela = belantamab; C = cyclophosphamide; D = daratumumab; d = dexamethasone; E = elotuzumab; Isa = isatuximab; I = ixazomib; K = carfilzomib; M = melphalan; P = pomalidomide; R = lenalidomide; V = bortezomib; ven = venetoclax.


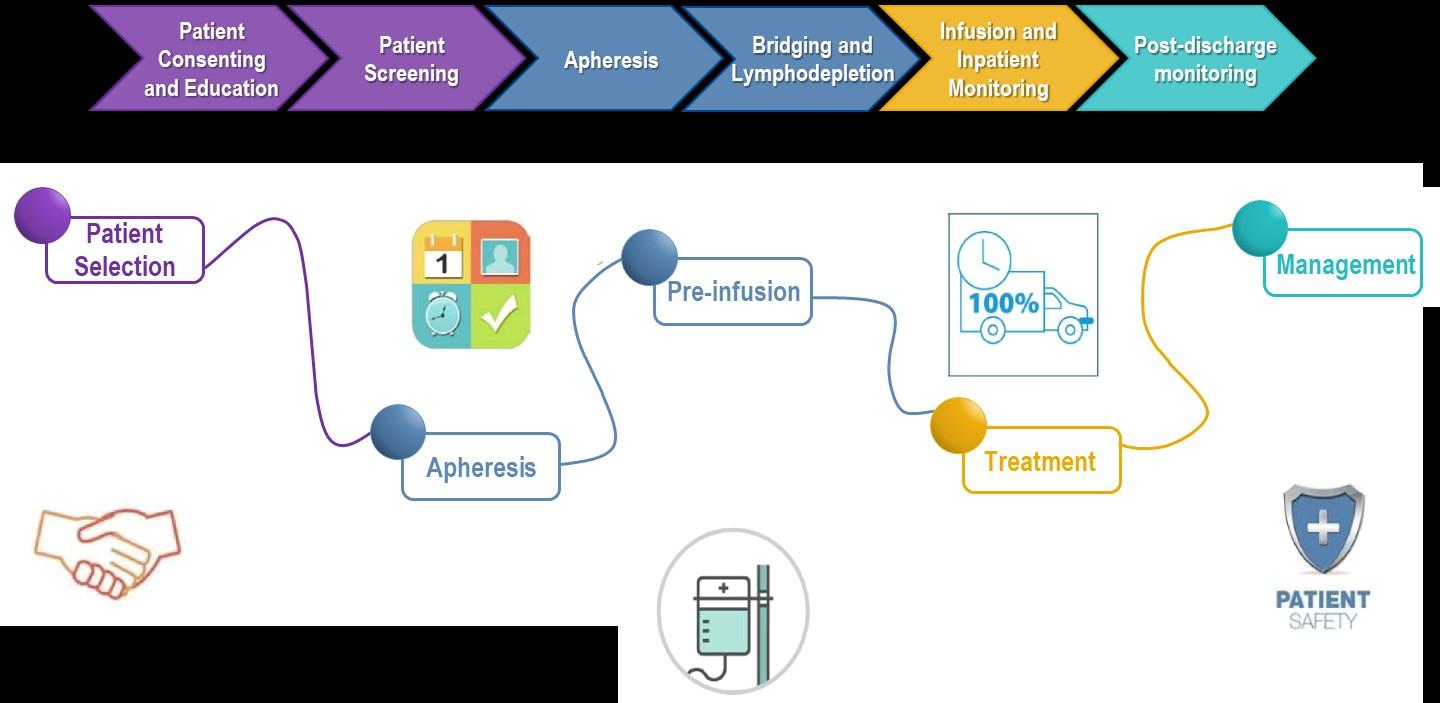
Stem Cell Transplant
ELIGIBILITY
Measuring Treatment Response
Determining Transplant Eligibility
Insurance Authorization Collecting Stem Cells



TRANSPLANT
High Dose Chemotherapy
Stem Cell Infusion
Supportive Care Engraftment
Duration: Approximately 2 weeks
Location: Transplant Center
Duration: Approximately 3-4 weeks
Location: Transplant Center

POST-TRANSPLANT
P H A S E 1 P H A S E 2 P H A S E 3

Restrengthening
Appetite recovery
“Day 100” assessment
Begin maintenance therapy
Duration: Approximately 10-12 weeks
Location: HOME





CAR T: Another Treatment Approach
Ask for a referral to CAR Tcell center as soon as it is possible as next treatment option (ie, before relapse)
Manufacturing takes
≈ 4 to 6 weeks
Bridging therapy may be needed
T-Cell Collection

No driving for 8 weeks
“One
& Done” with continued monitoring
• Away from home
• Often some hospital stay
• Care Partner needed
• Side effect management
• CRS, ICANS
• Low blood counts
• Fatigue and fever
• Some patients need ongoing transfusion support


Bispecific Antibodies
• Different bispecific antibodies have differences in efficacy, side effects
– Available after 4 prior lines of therapy (or clinical trial)
– About 7 in 10 patients respond
– Off-the-shelf treatment; no waiting for engineering cells
– CRS and neurotoxicity
– Risk of infection
• BCMA target: greater potential for infection
– Tecvayli® (teclistamab)
– Elrexfio ® (elranatamab)
– Lynozyfic™ (linvoseltamab)
BISPECIFIC ANTIBODIES

• GPRC5D target: potential for skin and nail side effects, GI issues of taste change, anorexia and weight loss
– Talvey ® (talquetamab)





CAR T and Bispecific Antibodies: Unique Side Effects





CRS is a common but often mild & manageable side effect



CAR = chimeric antigen receptor; CRS = cytokine release syndrome. Oluwole OO, Davila ML. J Leukoc Biol. 2016;100:1265-1272. June CH, et al. Science. 2018;359:1361-1365. Brudno JN, Kochenderfer JN. Blood. 2016;127(26):3321-3330. Brudno JN, Kochenderfer JN. Blood Rev. 2019:34:45-55. Shimabukuro-Vornhagen, et al. J Immunother Cancer. 2018;6:56. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625-638.




CAR T and Bispecific Antibodies: Unique Side Effects











Spring Into Managing Side Effects





The Early Bird Gets the Worm: Communicate Proactively with Your Healthcare Team
Your team may be able to help, but only if they know how you feel.
Unmanaged Myeloma can cause:
• Calcium elevation
• Renal dysfunction
• Low blood counts
• Infection Risk
• Blood clots
• Bone pain
• Neuropathy
• Fatigue

Side Effects of Treatment can
cause:
• GI symptoms
• Renal dysfunction
• Low blood counts
• Infection Risk



Tip: proactively discuss common side effects and what to do if they occur





How You Feel





• Blood clots
• Neuropathy
• Fatigue











Tip: Keep a Symptom Diary and bring it to appointments











Steroids enhance the effectiveness of other myeloma therapies
Your provider may adjust your dose. Do not stop or alter your dose of steroids without discussing it with your provider
Managing Steroid Side Effects
• Consistent schedule (AM vs. PM)
• Take with food
• Stomach discomfort: Over-the-counter or prescription medications
• Medications to prevent shingles, thrush, or other infections


Are Steroids Messing With Your Sunny Disposition?


Steroid Side Effects
• Irritability, mood swings, depression
• Difficulty sleeping (insomnia), fatigue
• Blurred vision, cataracts
• Flushing/sweating
• Increased risk of infections, heart disease
• Muscle weakness, cramping
• Increased blood pressure, water retention
• Stomach bloating, hiccups, heartburn, ulcers, or gas
• Weight gain, hair thinning/loss, skin rashes
• Increased blood sugar levels, diabetes


Infection Can Be Serious for People With Myeloma
Preventing infections is paramount.
Infection remains the leading cause of death in patients with multiple myeloma. Several factors account for this infection risk, including the overall state of immunosuppression from multiple myeloma, treatment, age, and comorbidities (e.g., renal failure and frailty).
IMWG Consensus guidelines and recommendations for infection prevention in multiple myeloma; Lancet Haematol.2022;9(2):143–161.
Infection Prevention Tips
Good personal hygiene (skin, oral)
Environmental control (avoid crowds and sick people; use a high-quality mask when close contact is unavoidable)



Report fever of more than 100.4°F, shaking chills even without fever, dizziness, shortness of breath, low blood pressure to HCP as directed.

As recommended by your healthcare team:
Immunizations:
Flu, COVID, RSV & and pneumococcal vaccinations; avoid live vaccines

Preventative and/or supportive medications (next slide)


Medications Can Reduce Infection Risk
Type of Infection Risk
Viral: Herpes Simplex (HSV/VZV); CMV

Medication Recommendation(s) for Healthcare Team Consideration
Acyclovir prophylaxis
Bacterial: blood, pneumonia, and urinary tract infection Consider prophylaxis with levofloxacin
PJP (P. jirovecii pneumonia)
Fungal infections
COVID-19 and Influenza
IgG < 400 mg/dL (general infection risk)
ANC < 1000 cells/μL (general infection risk)

Consider prophylaxis with trimethoprim-sulfamethoxazole
Consider prophylaxis with fluconazole
Antiviral therapy if exposed or positive for covid per institution recommendations
IVIg recommended
Consider GCSF 2 or 3 times/wk (or as frequently as needed) to maintain
ANC > 1000 cells/μL and maintain treatment dose intensity



GI Symptoms: Prevention & Management
Fluid intake can help with both diarrhea and constipation and helps kidney function
Constipation is more common in the induction phase
• Opioid pain relievers, antidepressants, heart or blood pressure medications (check with provider, pharmacist)
• Supplements: Calcium, Iron, vitamin D (rarely), vitamin B-12 deficiency Increase fiber
• Stay well hydrated
• Fruits, vegetables, high fiber whole grain foods
• Fiber binding agents – Metamucil® , Citrucel®, Benefiber®
Anorexia, the inability to eat, is common during transplant and resolves with time.
• Hydration is most important
• Small, frequent meals with a focus on protein intake
• You will work closely with a dietician to help monitor your calorie intake


Diarrhea is common during transplant and long-term maintenance therapy. Other medications and supplements
• Hydration is very important
• Electrolyte replacement is common
• Good skin care will help prevent irritation
• Stool exam may be needed to rule-out infection
• If no infection, anti-diarrheal medication may be prescribed



Discuss GI issues with healthcare providers to identify causes and make adjustments to medications and supplements


Management of Oral Side Effects
OTC dry mouth rinse, gel, spray are recommended. Advise patients to avoid hot beverages. Initiate anti-fungal therapy for oral thrush
Dexamethasone oral solutions “swish and spit” have been tried but with no proven benefit yet. Sour citrus or candies before meals are also recommended. Taste Changes


Dietary modifications with small bites, eating upright, and sips with food can help manage symptoms.
Some medications lead to weight gain, others to weight loss.
Dry mouth leads to taste changes which can lead to anorexia. Meet



Dry Mouth
Dysphagia
Catamero D, Purcell K, Ray C, et al. Presented at the 20th
Myeloma Society (IMS) Annual Meeting Nurse
September 27–30, 2023; Athens, Greece.

Skin and Nail Side Effects
Possible side effect to some treatments and supportive care medications

Skin Rash:
• Prevent dry skin; apply lotion
• Report changes to your care team
• Medication interruption or alternative, as needed
• Steroids:
– Topical for grades 1-2,
– Systemic and topical for Grade 3
• Antihistamines, as needed


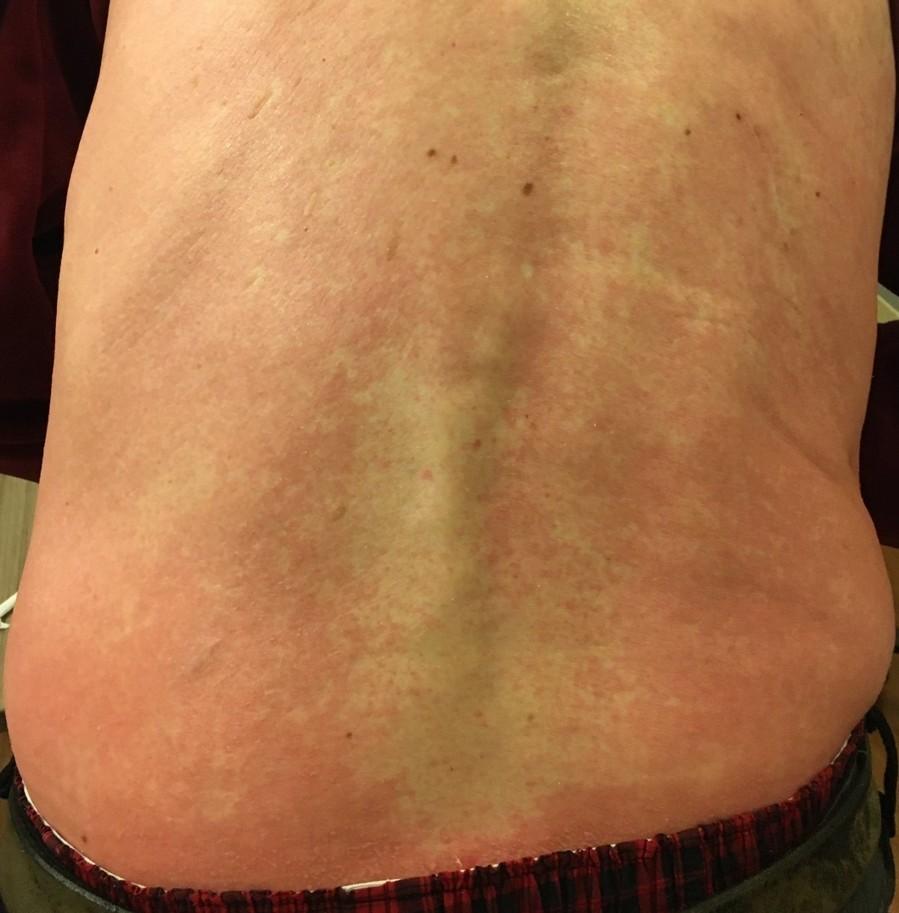


Nail Changes:

• Keep your nails short and clean. Watch for “catching and tearing”
• Apply a heavy moisturizer like Vaseline or salve. Wear cotton hand coverings to bed
• A nail hardener may help with thinning
• Tell the team if you have signs of a fungal infection, like thickened or discolored nails

Photos: Mount Sinai Hospital, NY, NY

Feel Like a Spring Chicken: Prevent and Manage Pain
Pain can significantly compromise quality of life
Sources of pain include bone disease, neuropathy and medical procedures
• Management
– Prevent pain when possible
• Bone strengtheners to decrease fracture risk
• Antiviral to prevent shingles
• Sedation before procedures
– Interventions depend on source of pain
• May include medications, activity, surgical intervention, radiation therapy, etc
• Complementary therapies (Mind-body, medication, yoga, supplements, acupuncture, etc)
• Scrambler therapy for neuropathy





Tell your healthcare provider about any new bone or chronic pain that is not adequately controlled


Peripheral Neuropathy Management
Peripheral neuropathy happens when there is damage to nerves in the extremities (hands, feet, limbs). Damage can be the result of myeloma, treatment or unrelated conditions (i.e. diabetes).
Symptoms:
• Numbness
• Tingling
• Prickling sensations
• Sensitivity to touch
• Burning and/or cold sensation
• Muscle weakness
Prevention / management:
• Bortezomib once-weekly and/or subcutaneous administration
• Massage area with cocoa butter regularly
• Neuroprotective Supplements:
– B-complex vitamins (B1, B6, B12)
– Green tea
• Safe environment: rugs, furnishings, shoes


If neuropathy worsens, your provider may:
• Adjust your treatment plan
• Prescribe oral or topical pain medication
• Suggest physical therapy
Report symptoms of peripheral neuropathy early to your healthcare provider; nerve damage from neuropathy can be permanent if unaddressed



Understanding Changes to Kidney Function
• Risk Factors
– Active multiple myeloma (light chains, high calcium)
– Other medical issues (ex: Diabetes, dehydration, infection)
– Medications (MM treatment, antibiotics, contrast dye)
– Poor Nutrition
• Prevention
– Stay hydrated – drink water
– Avoid certain medications when possible (eg, NSAIDs), dose adjust as needed
• Treatment
– Treatment for myeloma
– Hydration
– Dialysis




Many myeloma patients will experience kidney issues at some point; protecting your kidney function early and over time is important


Additional Supportive Care


Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56. Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36.
Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.


Summer of Success

Let the Sun Shine In
Fatigue
Fatigue is the most reported symptom.
Sources include anemia, pain, reduced activity, insomnia, treatment toxicity, bone marrow suppression 98.8%
Often, people do not share these symptoms with their providers. Talk to your provider about symptoms that are not well controlled or if you have thoughts of self-harm.


>35% of patients
of patients




Bee an Empowered Patient
Ask
questions
– What are my treatment options?
– What are the pros and cons of the different options?
– How will we know if treatment is working?
– What do the different labs mean?
– Who will be monitoring my labs?
– How can I access my test results (eg, patient portal)?
– What will we do if my treatment doesn’t work or quits working?
Participate in decisions
– Share your priorities and preferences
– Include care partner(s) in your discussion



Speak up if something seems different or unusual
– Normally 4 vials of blood but only drawing 3?
– Normally specialty pharmacy confirms delivery but haven’t heard from them this month?

Live in the sunshine, swim the sea, drink the wild air.
– When is my next appointment?
Communicate with your healthcare team
– Understand the roles of each team member
– Who to contact for your needs (eg, side effects, insurance issues, other)
Develop a support network
– Learn from others: IMF has many support groups or you can start one (IMF’s can help)


– Ralph Waldo Emerson

Care Partners Are Vital for Success


If you want to go fast, go alone, if you want to go far, go together
• Care partners may help with medical appointments, managing medication, daily living, physical assistance, emotional support, myeloma knowledge, healthy lifestyle, patient advocacy, financial decisions
• Care partners can be a spouse, close relative, a network of people (family, friends, neighbors, church members, etc)





African Proverb
• Caring for the Care Partner
– Recognize that caregiving is difficult/stressful
– Encourage care partners to maintain their health, interests, and friendships
– The IMF has information and resources to help care partners




Cultivate A Care Network



If you want to go fast, go alone, if you want to go far, go together
• Multiple studies demonstrate that strong social ties are associated with longevity, improved adherence to medical treatment and overall improved health outcomes
• Care partners can be a spouse, close relative, a network of people (family, friends, neighbors, church members, etc)
• Care partners may help with medical appointments, managing medication, daily living, physical assistance, emotional support, myeloma knowledge, healthy lifestyle, patient advocacy, financial decisions


• Caring for the Care Partner
– Recognize that caregiving is difficult/stressful
– Encourage care partners to maintain their health, interests, and friendships
– The IMF has information and resources to help care partners







Martino J, et al. Am J of Lifestyle Med. 2015;11(6):466-475. Yang YC, et al. Proc Natl Acad 2016;113(3):578-583. Pinquart M and Duberstein PR. Crit Rev Oncol Hematol. 2010; 75(2):122–137.
African Proverb
IMF Care Giver Tip Cards

Enjoy Life’s Bounty

Harvest Good Health
Have a Primary Care Provider & Have Recommended Health Screenings
• Blood pressure
• Cholesterol
• Cardiovascular disease
• Diabetes
• Colonoscopy
• Women specific: mammography, pap smear
• Men specific: prostate
• Vision
• Hearing
• Dermatologic evaluation
• Dental checkups & cleaning

Develop & maintain healthy behaviors
• Good nutrition
• Regular activity
• Quit tobacco use
• Sufficient Sleep (next slide)
An ounce of prevention is worth a pound of cure. Benjamin
Franklin

Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-56. Brigle K, et al. CJON. 2017;21(5)suppl:60-76. Faiman B, et al. CJON. 2017;21(5)suppl:19-36. Faiman B, et al. CJON. 2011;15suppl:66-76. Miceli TS, et al. CJON. 2011;15(4)suppl:9-23.






































Living the Myeloma Life: Local Patient & Care Partner
Clark Murphy (Patient) &
Janet Murphy (Care Partner)
Our Journey Navigating Multiple Myeloma Clark and Janet Murphy


The Discovery
What made us think something was going on?
• June 2018: Experienced back pain
• Physical Therapy
• Vacation: Calgary, Canada
• Referral for MRI: Cary


The Diagnosis
August 18, 2018
• MRI
• Phone Call
• Panic

What the Journey looked like…
Clark’s perspective
Janet’s perspective


Treatment
• Pills, pills, pills
• How do we manage it?
• The risk of overdose


Our system
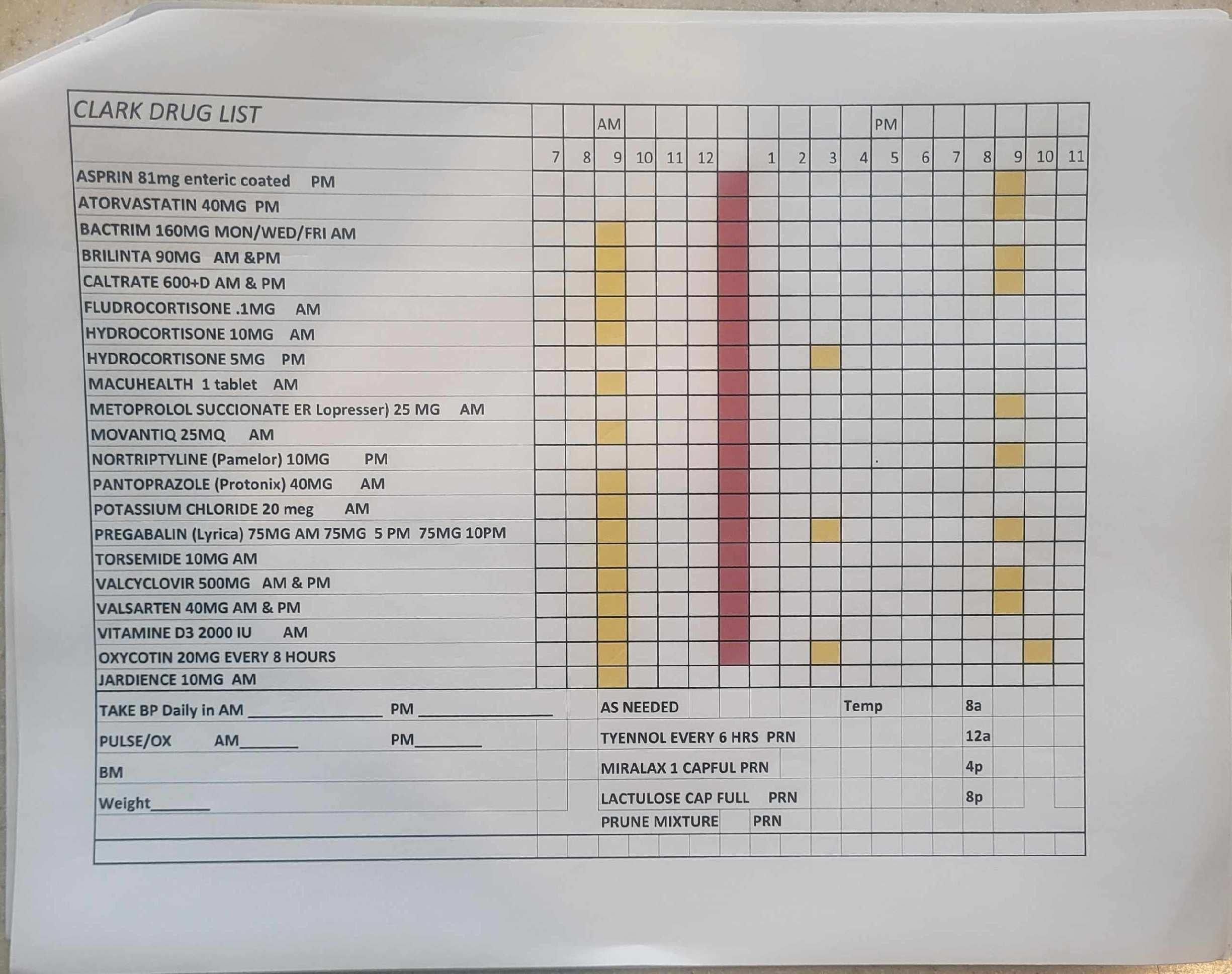
Our system
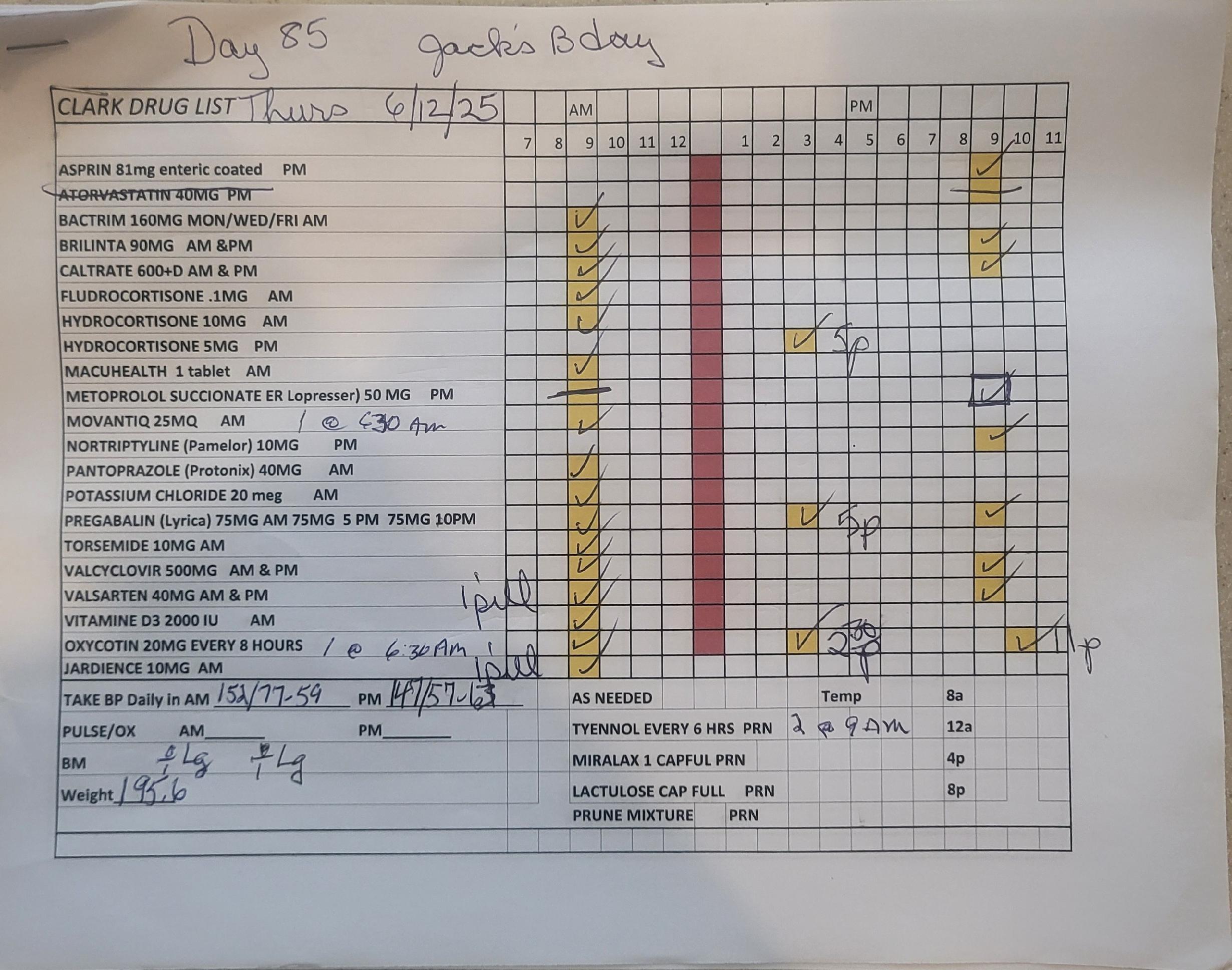
Appointments, Appointments


What’s happening now?




Beyond
Myeloma Therapy: Oral Health Survivorship
Katharine Ciarrocca, DMD, MSEd
Duke University Hospital, Durham, NC
Maintaining Oral Health During Cancer Treatment and Beyond
Maintaining Oral Health During Cancer Treatment and Beyond
Maintaining Oral Health During Cancer Treatment and Beyond

Beyond Myeloma Therapy: Oral Health Survivorship
Katharine (Kate) Ciarrocca, DMD, MSEd
Director, Oral Medicine, Duke University Hospital
Department of Surgery, Division of Plastic, Maxillofacial, and Oral Surgery
Department of Head and Neck Surgery & Communication Sciences
Diplomate and Secretary, American Board of Oral Medicine

Oral Medicine: Integrating Medicine & Dentistry

•Diagnosisandtreatmentofdiseasesin
orofacialcomplex

•Managementoforalcomplicationsof systemicdiseaseanditstreatment
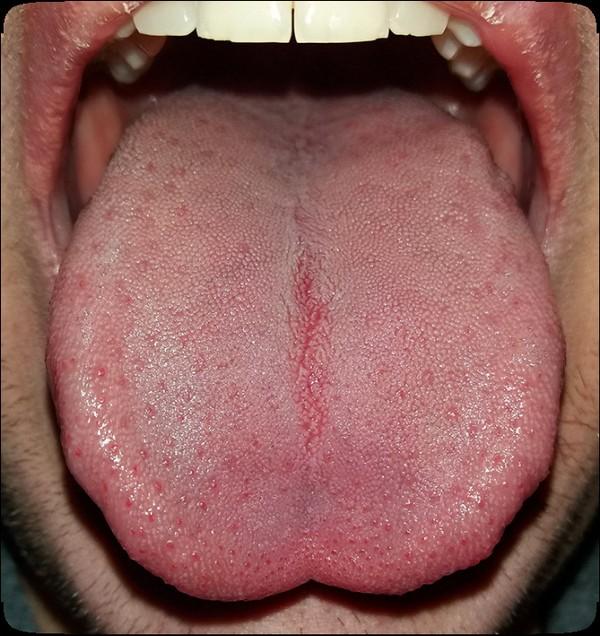

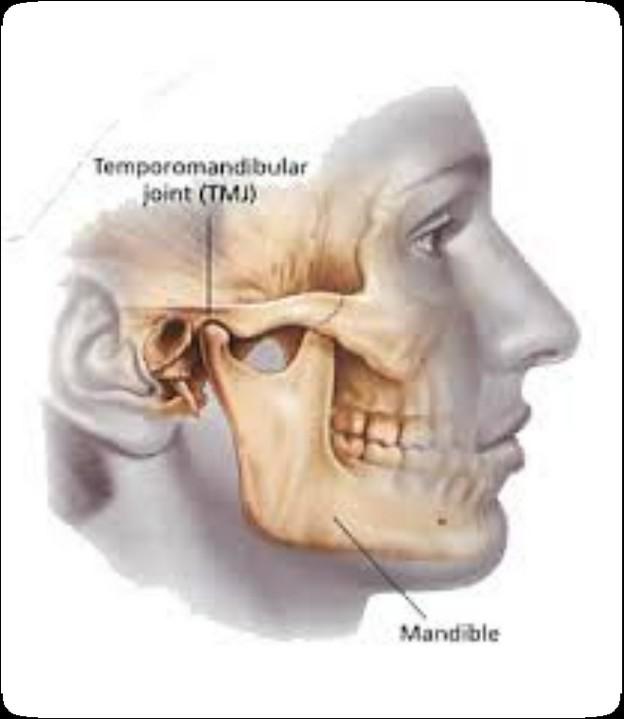
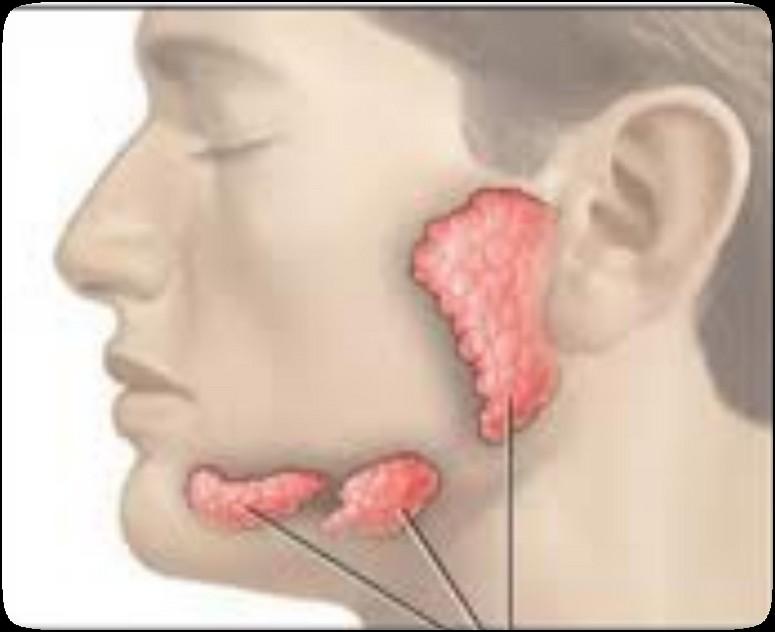
The Orofacial Complex
Dentition (teeth)
Periodontium (gums and bone)
Mucosa (soft tissue)
TMJ & Masticatory System (joint and chewing)
Salivary Glands & Saliva (spit)
Common Complications of Cancer Therapy
PRIMARY
• Anemia/Thrombocytopenia/Neutropenia*
• Dermatitis
• Infection*
• Irreversible damage to mucosa, vasculature, muscle and bone *
• Mucositis*
• Nausea/Vomiting*/Diarrhea/Constipation
• Neuropathy
• Salivary gland dysfunction *
• Trismus *

SECONDARY
• Dehydration*
• Dysgeusia *
• Malnutrition*
• Portal for septicemia *
• Xerostomia*
*related to or has effects on the oral cavity
Why is the mouth so susceptible?
• Rapid cell turnover
• Diverse and complex microflora
• Trauma to oral tissues during function
• Poor oral hygiene



Why the Mouth Matters
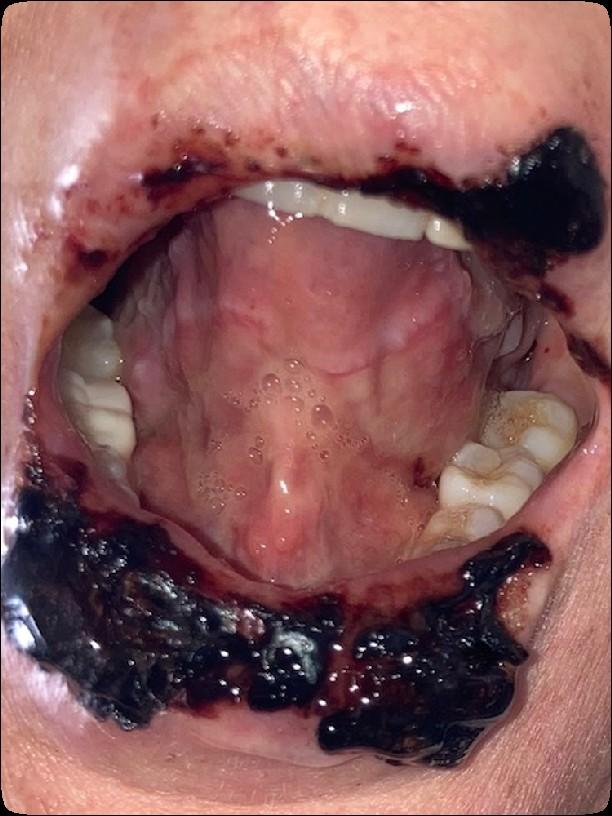
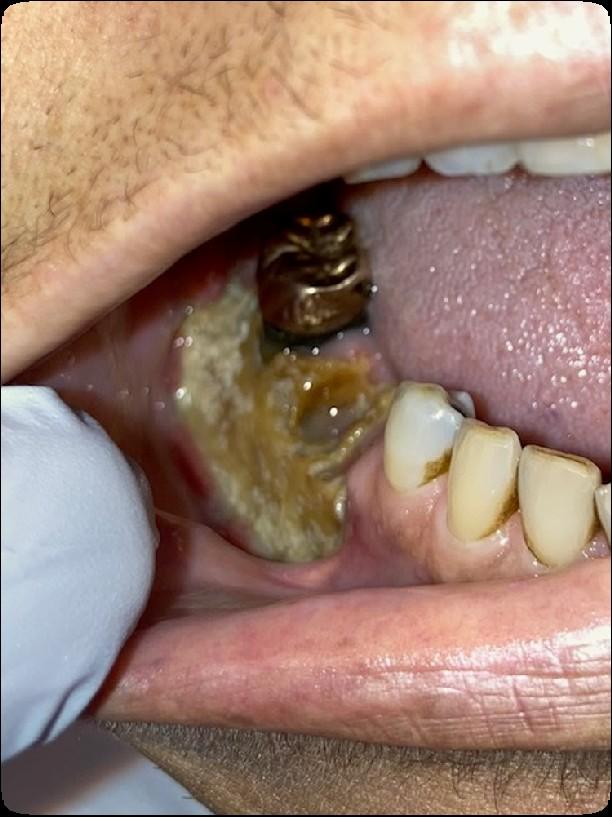
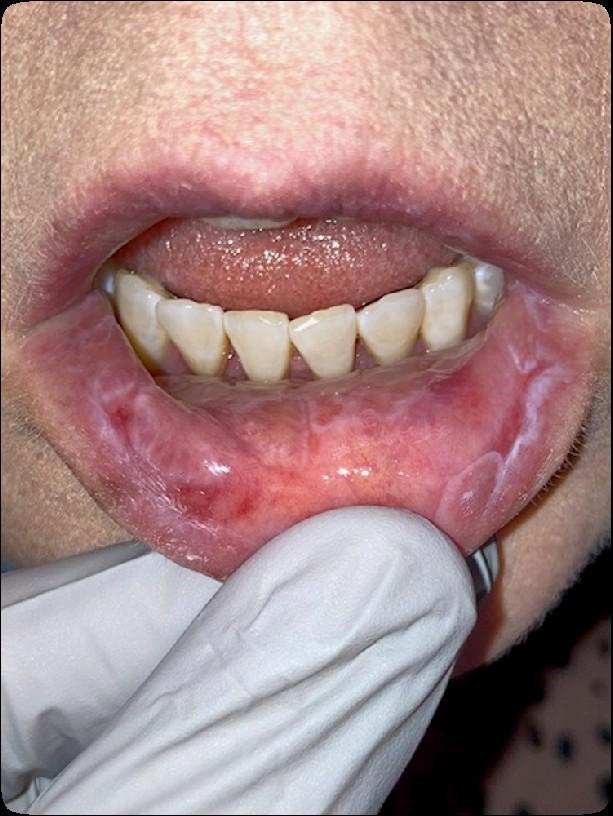
• Can compromise health and quality of life
• Can affect the ability to complete planned cancer treatment
• Can lead to serious systemic infections
• Can alter outcomes
Medically
necessary oral care before, during,
and
after
cancer treatment can prevent & reduce the incidence and severity of oral complications
ENHANCES SURVIVAL & IMPROVES QUALITY OF LIFE

Elad, Sharon, et al. "Basic oral care for hematology–oncology patients and hematopoietic stem cell transplantation recipients: a position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT)." Supportive Care in Cancer 23.1 (2015): 223-236.
Elad, Sharon, et al. "MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy." Cancer 126.19 (2020): 4423-4431.
Brennan, Michael T., et al. "Tooth failure post-radiotherapy in head and neck cancer: primary report of the clinical registry of dental outcomes in head and neck cancer patients (OraRad) study." International Journal of Radiation Oncology* Biology* Physics 113.2 (2022): 320-330.

Oral Evaluation Before Cancer Treatment
Prevent Infection
Maintain Nutrition
Maintain Quality of Life

Pre-Treatment Oral Evaluation
• Oral evaluation and management as early as possible prior to initiation of therapy by an Oral Medicine specialist

• GOAL: complete a comprehensive oral care plan that eliminates or stabilizes oral disease that could otherwise produce complications during cancer treatment

Oral Health During Cancer Treatment
Prevent Infection
Maintain Nutrition Maintain Quality of Life

Treatment Related Oral Complications
Due to both direct damage to oral tissues secondary to cancer treatment and indirect damage due to regional or systemic toxicity

• Mucositis (oral lesions) -> Managing Oral Pain
• Oral infections -> Prevention
• Poor oral hygiene -> Basic Oral Care
The Role of Basic Oral Care (BOC)
• Reduces the risk and severity of oral complications.
• Improves the likelihood that the patient will successfully complete planned cancer treatment.
• Prevents, eliminates, or reduces oral pain.
• Minimizes oral infections that could lead to potentially serious systemic infections.
• Prevents or minimizes complications that compromise nutrition.
• Improves the quality of life.

Elad S, Raber-Durlacher JE, Brennan MT, et al. Basic oral care for hematology-oncology patients and hematopoietic stem cell transplantation recipients: a position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT). Support Care Cancer. 2015 Jan;23(1):223-36.

Oral Care After Cancer Treatment: Managing Long Term Effects
Xerostomia (Dry Mouth)
Osteonecrosis of the Jaw
Oral GVHD
Surveillance for oral malignancies


Xerostomia (Dry Mouth)


Major functions of saliva
Protective Functions
•lubrication

•antimicrobial
•mucosal integrity
•lavage/cleansing
•buffering
•remineralization
Food & Speech Related Functions
•food preparation
•digestion
•taste
•speech
Causes of Dry Mouth

• Medications
• > 1100 medications
• Prescription or over the counter
• Taking more than one medication with dry mouth as a side effect makes it more likely to experience or increases the severity of dry mouth
• Head & Neck Radiation
• Chemotherapy
• Dehydration
• Mouth breathing
• Tobacco use
• Autoimmune Disease
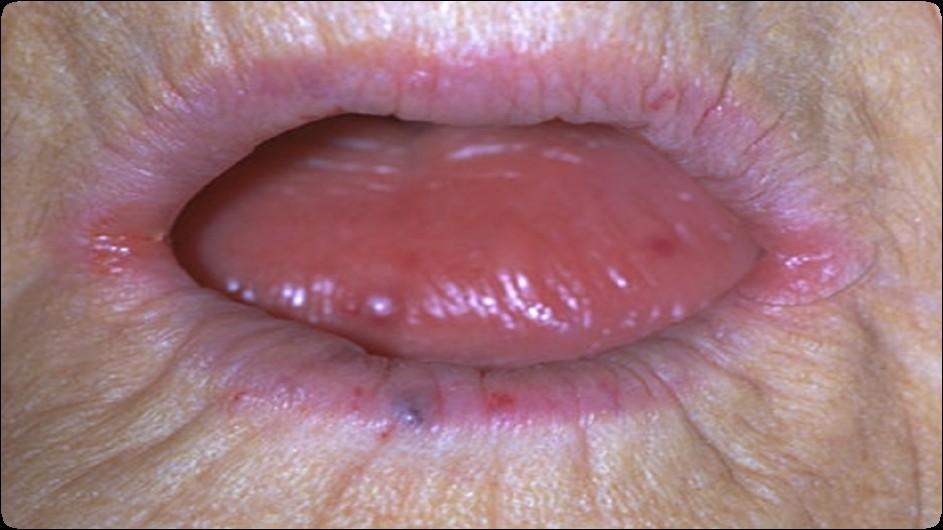
Complications of Dry Mouth
• Mucosal abnormalities
• Candidiasis
• Coated tongue
• Halitosis
• Difficulty swallowing
• Periodontal disease
• Caries
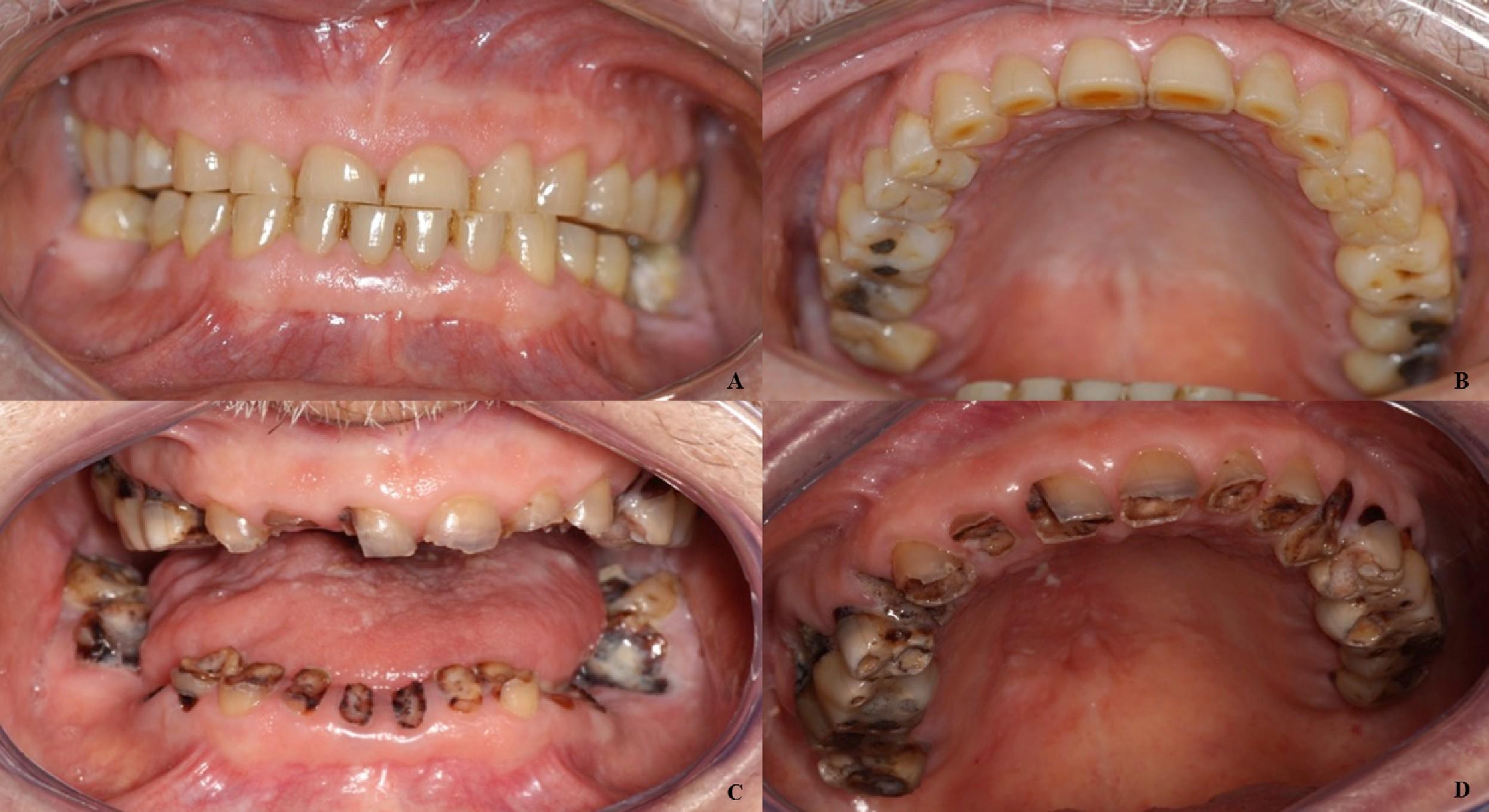




Dry Mouth Management
Relief of symptoms and prevention of oral complications

Behavior Modifications
• Limit alcoholic/caffeinated beverages
• Do not use mouthrinses with alcohol
• Frequently sip water; stay hydrated
• Ice chips
• Humidfier at night
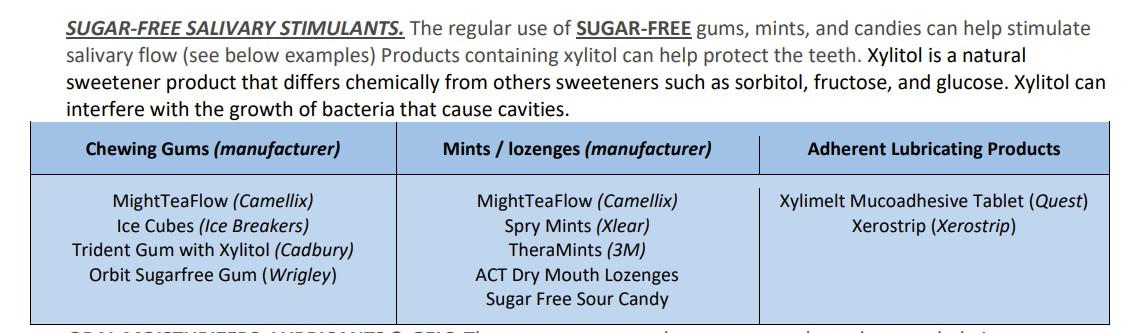
• Use sugarless salivary stimulants
• Sugarless chewing gum
• Sugarless hard candies, lozenges





Salivary Stimulants



Oral Moisturizers






Sialogogue Therapy
• Medications to increase salivary flow
• Two prescription drugs available:
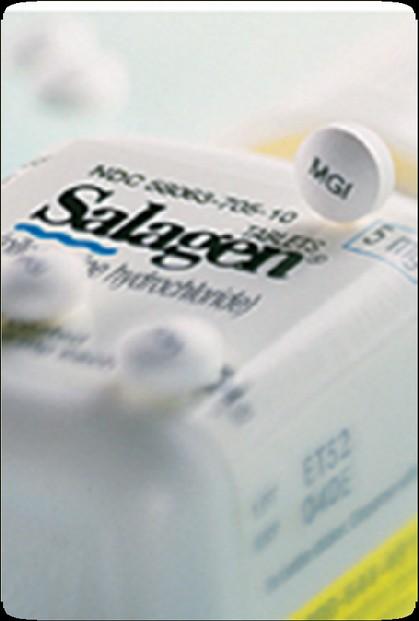
• pilocarpine (Salagen, MGI Pharma)
• cevimeline (Evoxac, Daiichi Sanko, Inc)
• GI, sweat, and cardiac side effects:
• Should be avoided in patients with significant heart disease, asthma, and narrow angle glaucoma
• Require functioning glands



Other Important Information
• See dentist regularly:
• may recommend you undergo more frequent check-ups, professional cleanings and in-office fluoride applications
• Perform oral hygiene as instructed:
• may recommend a prescription strength fluoride to be used in addition to your regular toothpaste
• consistently accomplishing daily oral hygiene measures is one of the most important steps in successfully managing the dental complications of oral dryness.
• Avoid sugary food and drinks:
• Decrease intake of sweet or sticky foods
• avoid the frequent intake of acidic beverages (such as most carbonated and sports replenishment drinks) and lemon products.

Osteonecrosis of the Jaw
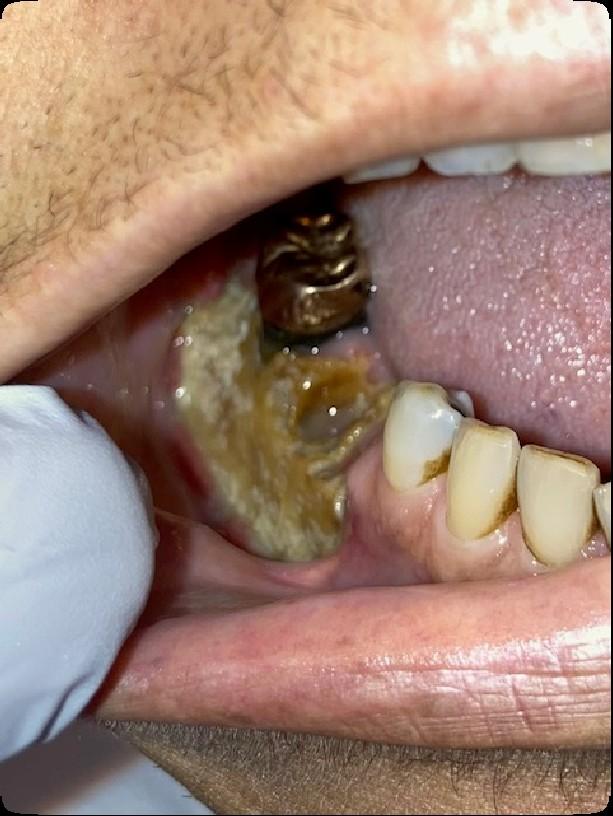
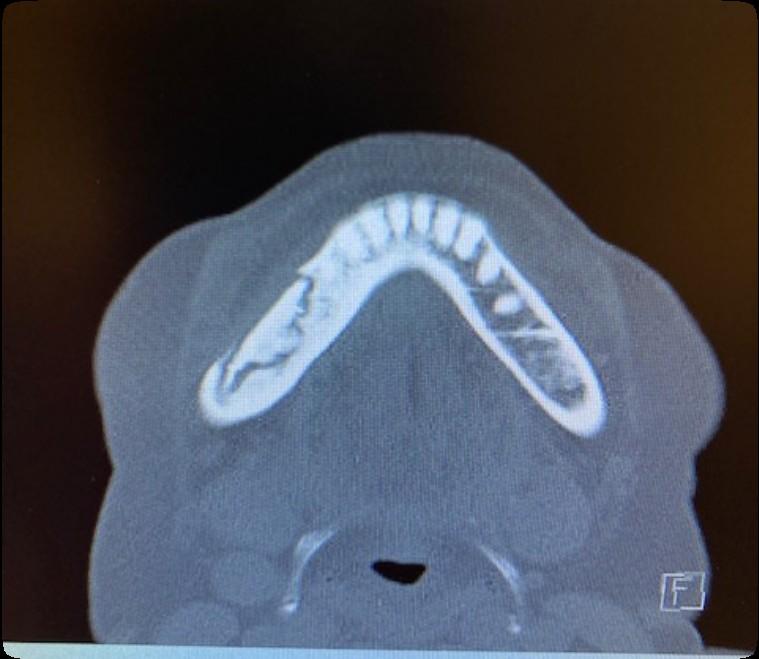

MRONJ: Medication Related Osteonecrosis of the Jaw
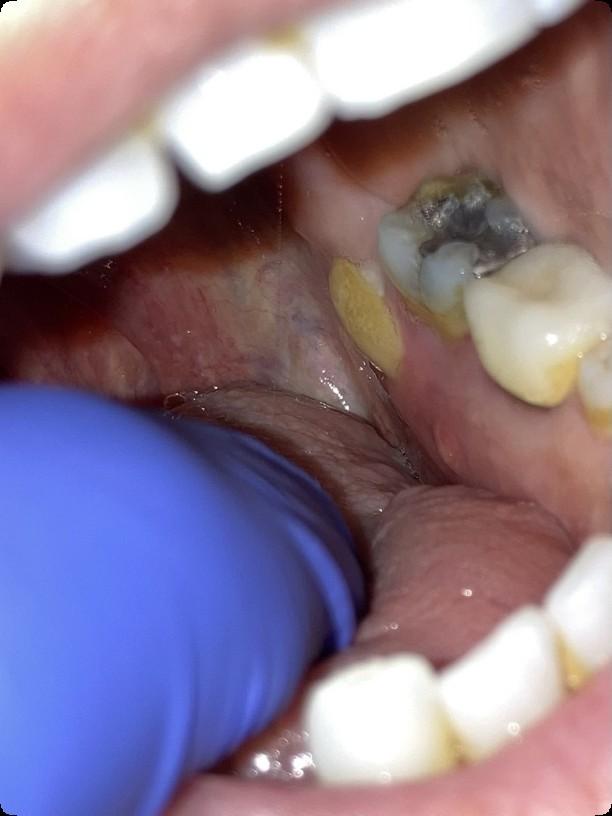
• Rare oral side effect called osteonecrosis of the jaw that is associated with certain medications, bone-modifying agents.
• Osteonecrosis of the jaw is a condition where small area(s) of the jawbone have difficulty healing and the bone starts to breakdown and die.

Bone Modifying Agents
• Medications used to prevent bone loss and demineralization (weakening/destruction)
• Some are given as pills; others must be given through an IV
• Examples of these medications include Actonel™, Zometa™, Fosamax™, and Boniva™, Xgeva™, and Prolia™
• Bone-modifying agents are approved for the treatment of:
• Osteoporosis: the loss of bone density often seen in postmenopausal females.
• Hypercalcemia of Malignancy: Increased calcium in the blood from bone breakdown.
• Metastatic disease to bone: Cancer spreading to bone tissue

How Common is MRONJ?
The risk is thought to be less than 1% for patients taking IV bonemodifying agents, and at least ten times less likely than that for patients taking the drugs by mouth.
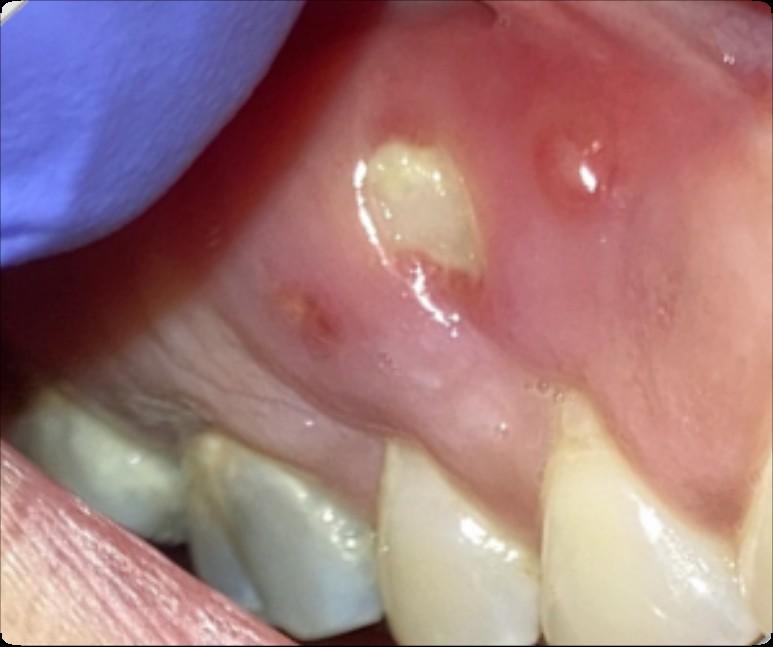

If I take these medications, am I automatically at risk?
• Anyone who takes these medications has a chance of developing ONJ.
• However, most reported cases occur after a type of oral “injury” – which could a tooth extraction, chronic periodontal (gum) disease, an oral surgical procedure, or simply a nonhealing traumatic ulceration.
• Tobacco use, treatment with corticosteroids, long-term use of bonemodifying agents, and diabetes also may increase the risk of this condition occurring.


What are the signs of MRONJ?
• The hallmarks of ONJ are exposed bone or gum/mucosa/tissue wounds that heal very slowly or do not heal at all for eight weeks or more after an injury to the mouth.
• Some patients report that this begins with a feeling of “roughness” on the gum tissue. If these open wounds become infected, there may be pus or swelling in the adjacent gum tissue.
• Many times, this condition is painless in the beginning, and patients only experience pain after the exposed bone becomes infected.
How is MRONJ treated?
• Education

• Careful oral hygiene
• Conservative interventions
• Avoid surgical intervention, if possible
• PATIENCE!

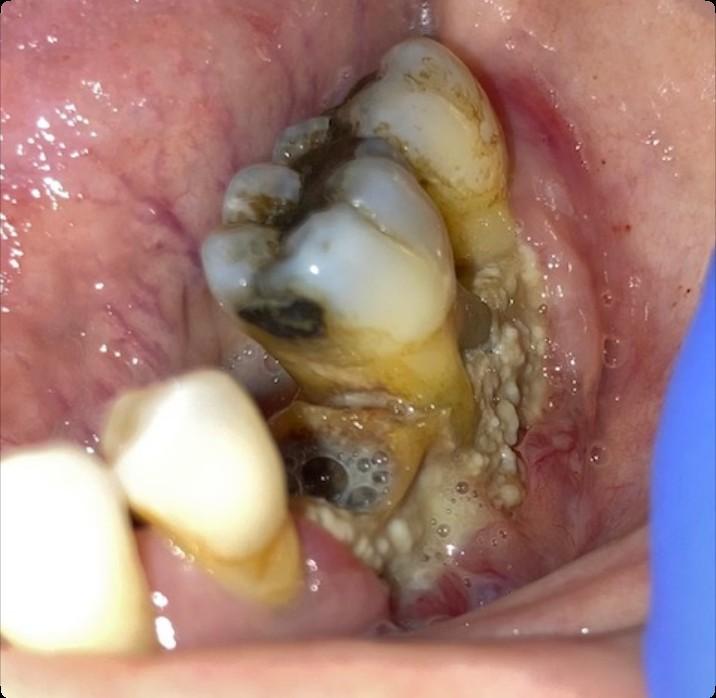



Oral GVHD
(Oral Graft vs. Host Disease)
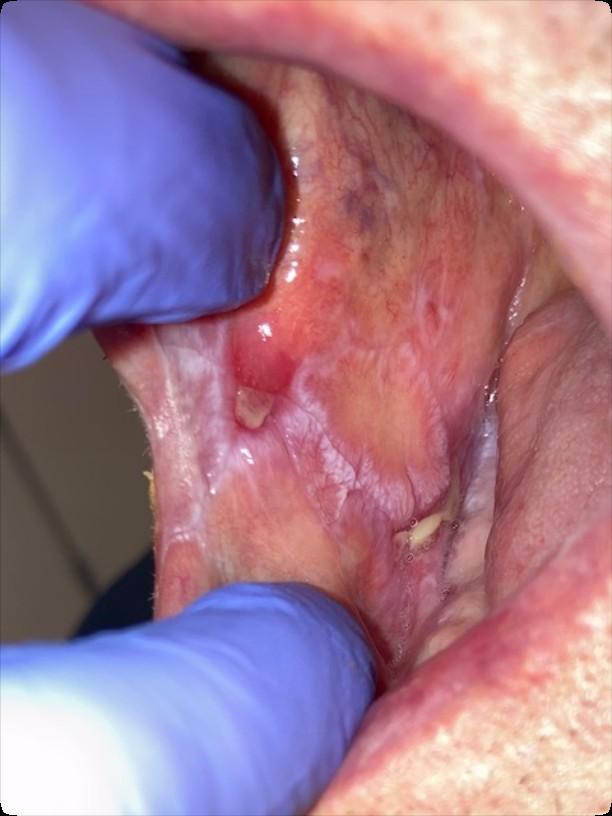

Oral GVHD
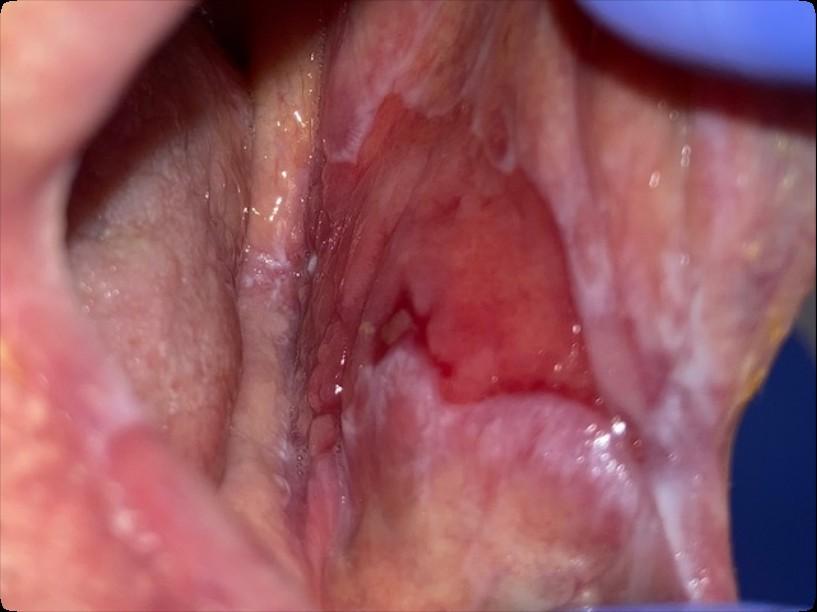
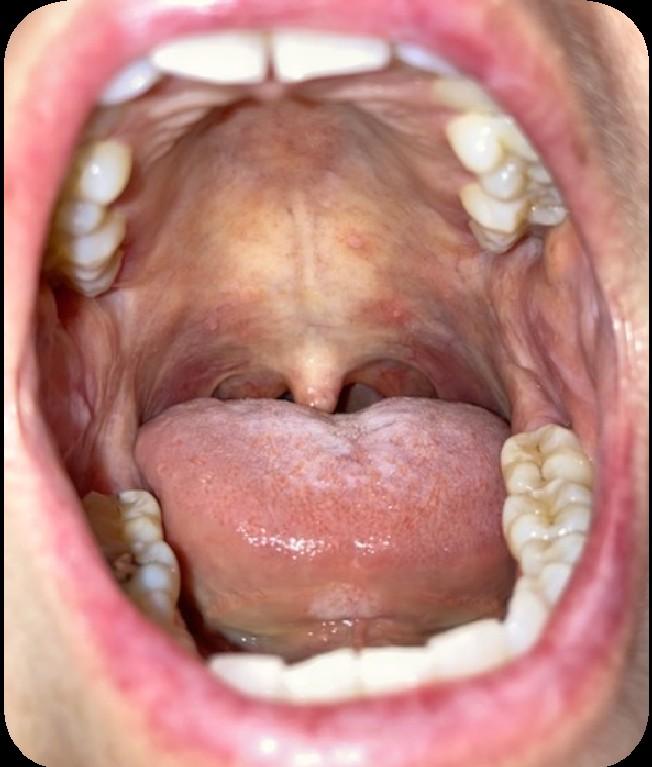

Oral Graft vs. Host Disease
• Complication following allogeneic HSCT which results in mouth sores, mouth pain and sensitivity to normally tolerated foods and drinks, and reduced mouth opening.
• May also affect the salivary glands, resulting in dry mouth and/or the development of small blisters (superficial mucoceles) on the inside of the lips and the palate.
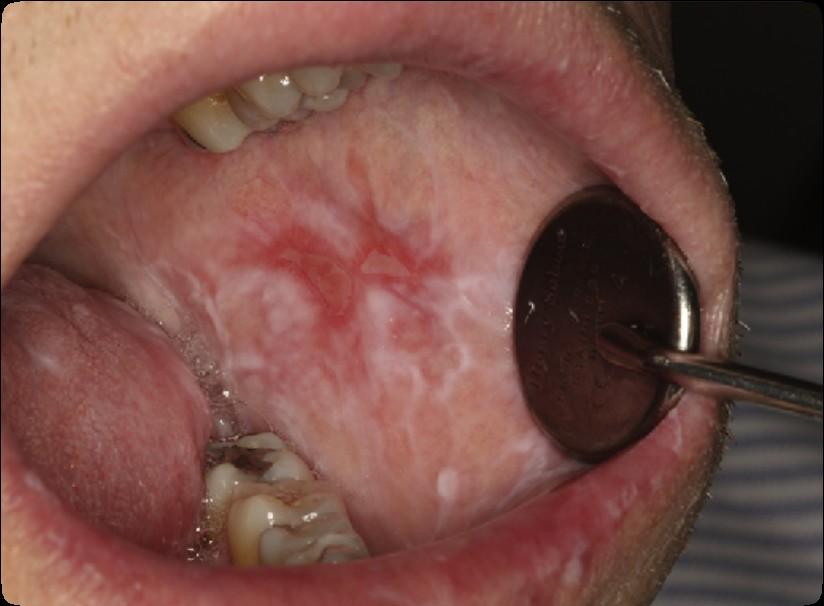
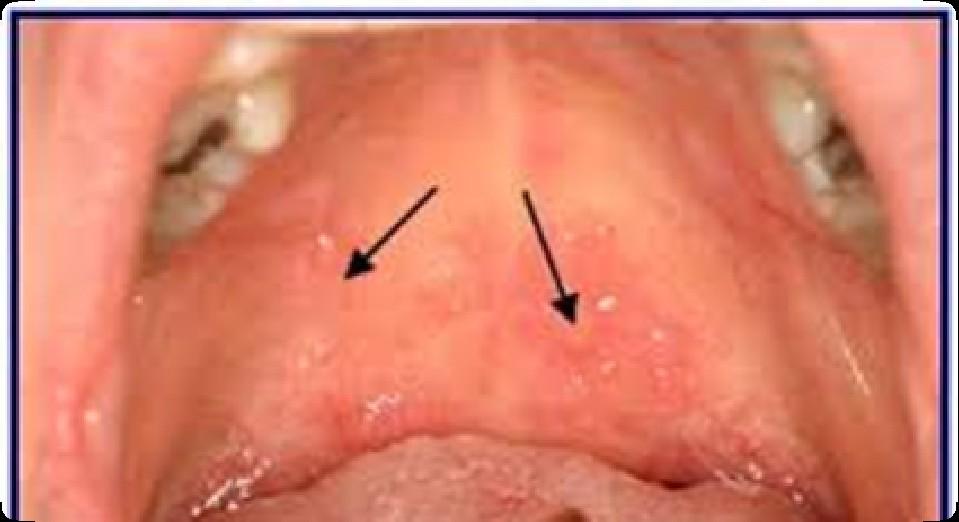
Oral Graft vs. Host Disease: Tips
• Avoid spicy and acidic foods
• there are no specific foods that actually make the condition get worse.
• Dry mouth may further worsen the condition
• For acute pain relief, topical anesthetic can be applied to the lesions.
• To reduce the inflammation, an antiinflammatory agent, such as a topical steroid can be used
• When using a steroid preparation, risk of developing an oral yeast infection is increased
• Oral cancer surveillance

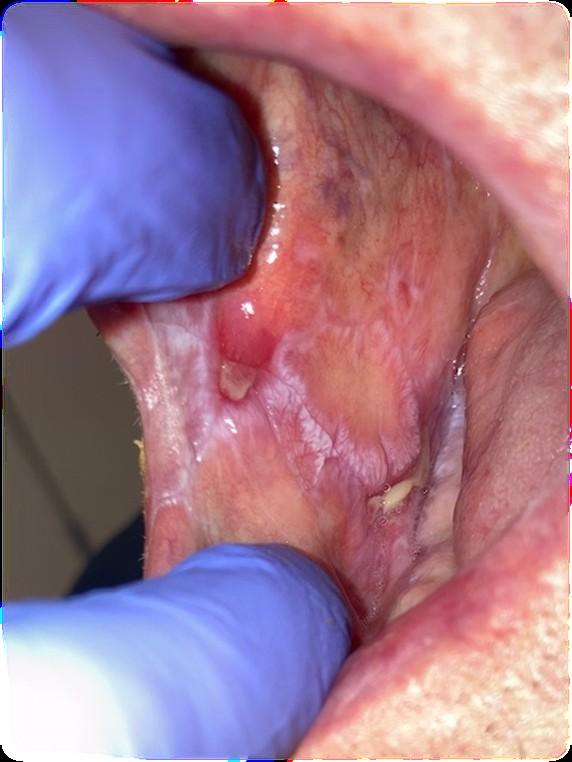
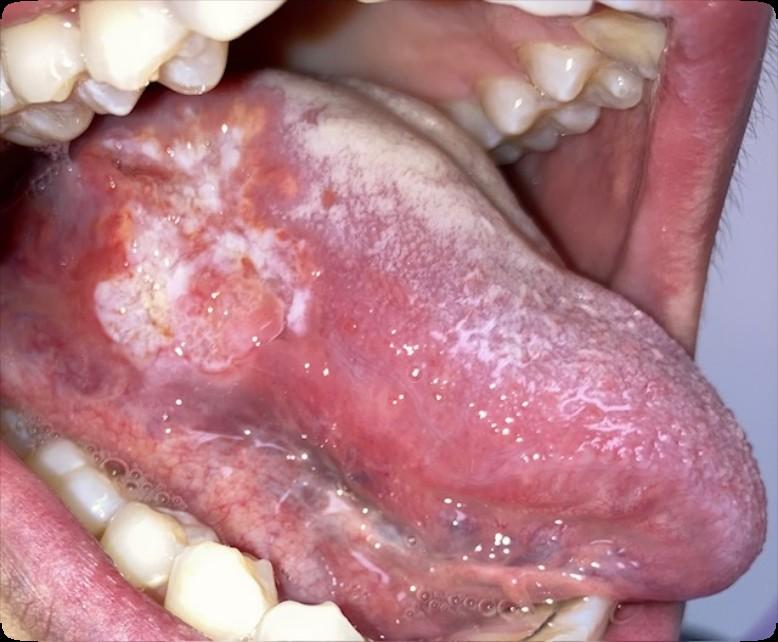

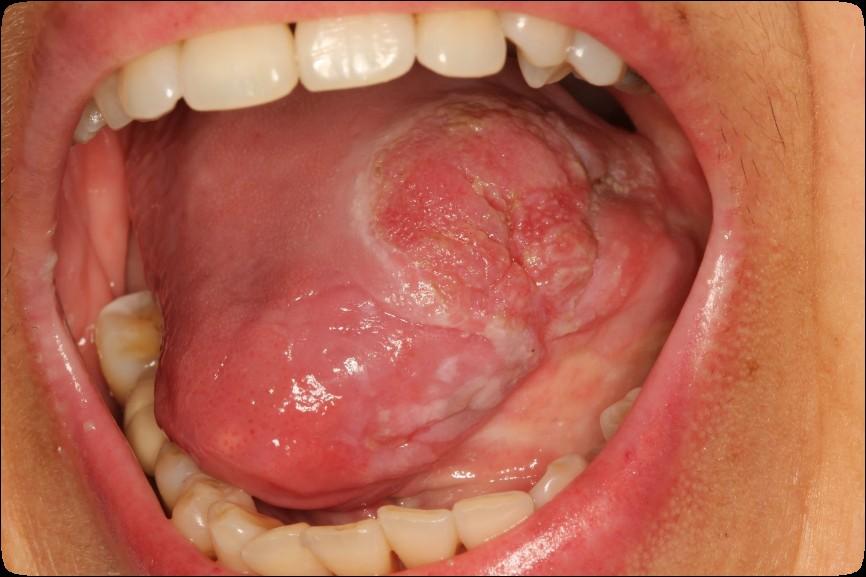
Cancer Surveillance





The Orofacial Complex

Dentition (teeth)
Periodontium (gums and bone)
Mucosa (soft tissue)
TMJ & Masticatory System (joint and chewing)
Salivary Glands & Saliva (spit)

Medically
necessary oral care before, during,
and
after
cancer treatment can prevent & reduce the incidence and severity of oral complications
ENHANCES SURVIVAL & IMPROVES QUALITY OF LIFE

Elad, Sharon, et al. "Basic oral care for hematology–oncology patients and hematopoietic stem cell transplantation recipients: a position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT)." Supportive Care in Cancer 23.1 (2015): 223-236.
Elad, Sharon, et al. "MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy." Cancer 126.19 (2020): 4423-4431.
Brennan, Michael T., et al. "Tooth failure post-radiotherapy in head and neck cancer: primary report of the clinical registry of dental outcomes in head and neck cancer patients (OraRad) study." International Journal of Radiation Oncology* Biology* Physics 113.2 (2022): 320-330.
QUESTIONS?



Q&A WITH PANEL

Closing Remarks
Robin Tuohy, Vice President, Patient Support International Myeloma
Foundation
Housekeeping Items
Presentation Slides: Are available by scanning the QR code, Instructions are on the QR code handout on each table.
Program Evaluations: evaluations at the end of today.
Badge Holders: Please return your badge holders and we can recycle them.
Wifi: Network Name: Password: Sheraton2025

Restrooms: You are currently in Oak Ballroom –Noted with the RED STAR. Bathrooms are located outside Willow Oak –noted with the ORANGE X.
We greatly appreciate your time and feedback!






Thank you to our speakers & our sponsors!









OUR VISION:
A world where every myeloma patient can live life to the fullest, unburdened by the disease.
OUR MISSION:
Improving the quality of life of myeloma patients while working toward prevention and a cure.

IMF Core Values:
These are the core values we bring to accomplishing our mission each day.
Patient Centric
The patient experience is the focus of everything we do. Every interaction is an opportunity to establish a personal connection built on care and compassion which is the basis for continued support.
Respect All
As a team, we value honesty and transparency while creating a culture of mutual respect. We foster a myeloma community built on sincerity, authenticity, and kindness.
Excellence and Innovation
We value accountability, personal responsibility, and a steadfast commitment to excellence. We respect the legacy and reputation of our organization while seeking new solutions and advancements to improve outcomes, quality of life, and access to the best available resources for everyone impacted by myeloma.
Honor differences
We recognize each team member's skills and talents through collaboration and cooperation. Our programs aim to celebrate and support the diversity of our patients and their communities.





