

GMAN Fall Virtual Meeting Latest Updates in the Use of Dexamethasone & Activities on Myeloma Action Month
25 September 2025
8:00AM PST / 11:00AM EST / 12:00PM BRT / 5:00PM CET / 1:00AM DST



Thank You to Our Sponsors!

Meeting Agenda

5:00pm - 5:10pm CET Welcome & Introductions (Martine Elias & Serdar Erdogan)
5:10pm - 5:30pm CET Updates from IMS Meeting (Dr. Joseph Mikhael, IMF)
5:30pm - 5:45pm CET Q&A
5:45pm - 6:05pm CET Global Strategy of Myeloma Action Month 2026 (Peter Anton, IMF)
6:05pm - 6:20pm CET Q&A
6:20pm - 6:30pm CET Meeting Close (Martine Elias & Serdar Erdogan)

Welcome & Meeting Kickoff
Martine Elias
Chief Executive Officer, Myeloma Canada
Serdar Erdoğan Director, Global Myeloma Action Network and European & Middle Eastern Patient Programs


Updates from IMS Meeting
Joseph Mikhael, MD
IMF Chief Medical Officer




Highlights from IMS 2025
GMAN Quarterly Meeting & IMF Staff Meeting

Joseph Mikhael, MD, MEd, FRCPC Chief Medical Officer, International Myeloma Foundation Professor, Translational Genomics Research Institute (TGen) City of Hope Cancer Center

IMS 2025
A fantastic meeting in my motherland of Canada – downtown Toronto

Over 3000 attendees
Over 70 countries represented
Four full days of meetings:
Core Sessions, Abstracts, Industry Symposia and Special Sessions





• Linvo in SMM - plenary
• MajesTEC 5 Study
• Iberdomide-Dara-Dex



LINKER-SMM1 (NCT05955508) Phase 2 study design
• LINKER-SMM1 is an open-label study of linvoseltamab monotherapy in adults with HR-SMM, defined as SMM diagnosis within 5 years of study entry and high-risk features by “2-20-20”1 and/or PETHEMA criteria*2
Inclusion criteria
• Adults with HR-SMM diagnosis within 5 years of enrollment and no prior treatment
• HR-SMM based on the following markers:
‒ At least 2 of the following:
Serum M-protein >20 g/L or 2 g/dL; FLC ratio >20; BMPCs >20%
AND/OR
‒ ≥95% aberrant/clonal BMPCs and immunoparesis
• ECOG PS ≤1
Safety run-in

• Adequate organ function Study portions Part 1
(n=6)
Linvoseltamab treatment schedule (Parts 1/2) (28-day cycles) Part 2
• Expansion (n=34)
Up to Week 1–3 Week 4–
28 days (step-up period) (Cycle 1)
Week 8–23 (Cycles 2–5)
Week 24+ (Cycles 6–24)¶
Follow-up up to 5 years after last dose
Post-treatment follow-up** Long-term follow-up
Primary endpoints
• Part 1: Frequency of AESIs, including Grade ≥2 CRS and ICANS, and frequency and severity of TEAEs
• Part 2: CR rate per IMWG criteria, and MRD negativity at 12 and 24 months
Key secondary endpoints Parts 1/2
• ORR, MRD negativity rate, DOR
• Treatment began with a 3-week step-up schedule of 1 mg (Week 1), 4 mg (Week 2), and 25 mg (Week 3), followed by linvoseltamab 200 mg in 28-day cycles; premedication was required during the step-up period and at Cycle 1 D1
• First MRD assessment was conducted at ≥VGPR
*“2-20-20” criteria1 was defined as ≥2 of the following: serum M-protein >2 g/dL, serum involved/uninvolved FLC ratio >20, BMPCs >20%; PETHEMA criteria:2 ≥95% aberrant/clinical BMPCs and immunoparesis defined as reduction of ≥1 uninvolved Ig isotype of ≥25% below the lower normal limit; †Safety run-in period was 35 days (5 weeks), beginning D1 of step-up dosing; ‡Inpatient monitoring was required for the first 24 hours after each step-up dose; §Weekly administration of linvoseltamab occurred during the first cycle of treatment before patients transitioned to Q2W for the next four cycles. At the start of Cycle 6, the dosing frequency was decreased to Q4W. Treatment was continued for up to 2 years; ¶Until completion of 24 cycles or fulfillment of a protocol-defined criterion for study drug discontinuation; **Until biochemical progression, start of non-protocol cancer therapy, or death; ††For up to 5 years after last dose of study drug, unless death or study termination by the sponsor occurs earlier. AESI, adverse event of special interest; BMPC, bone marrow plasma cell; CR, complete response; CRS, cytokine release syndrome; D, day; DOR, duration of response; ECOG PS, Eastern Cooperative Oncology Group performance status; FLC, free light chain; HRSMM, high-risk smoldering multiple myeloma; ICANS, immune effector cell-associated neurotoxicity syndrome; IV, intravenous; MRD, minimal residual disease; ORR, objective response rate; PETHEMA, Programa de Estudio y Tratamiento de las Hemopatíás Malignas (The Spanish Society of Hematology); QW, weekly; Q2W, every 2 weeks; Q4W, every 4 weeks; TEAE, treatment-emergent adverse event. 1. Lakshman A, et al. Blood Cancer J 2018;8(6):59; 2. Pérez-Persona E, et al. Blood 2007;110(7):2586–2592.
Preliminary efficacy: ORR
• Among efficacy-evaluable patients who received ≥1 cycle of full-dose linvoseltamab (N=19)† , investigator-assessed ≥CR per IMWG criteria was 37% (ORR 100%; ≥VGPR 74%)
Data cut-off date: May 28, 2025.
GMMG-HD10/DSMM-XX/MajesTEC-5:
Study Design
Key eligibility criteria:
• TE NDMM
• ECOG PS score of 0-2
• Aged 18-70 years
Induction (6 × 28-day cycles)a
Arm A (n=10):
Tec (1.5 mg/kg QW)-DR
Arm A1 (n=20):
Tec (3 mg/kg Q4W)-DR
Arm B (n=19):
Tec (3 mg/kg Q4W)-DVR
Maintenanceb,c (× 18 cycles)
Primary endpoints:
• AEs, SAEs
Select secondary endpoints:
• MRD negativity (10–5 and 10–6)
• ORR
• ≥CR
• ≥VGPR
• Stem cell yield

• Tec (Cycle 1): Tec step-up dosing (0.06 and 0.3 mg/kg on Days 2 and 4) + 1.5 mg/kg on Days 8 and 15e
− Tec (Cycles 2-6): 1.5 mg/kg QW on Day 1 (Arm A); 3 mg/kg Q4W on Day 1 (Arm A1 and B)
• D: 1800 mg SC per label (QW for Cycles 1-2; Q2W for Cycles 3-6)
• V: 1.3 mg/m2 SC QW
• R: 25 mg PO daily starting in Cycle 2 (Days 1-21)
• d: 20 mg (PO or IV) in Cycles 1-4 (Arm A) or Cycles 1-2 (Arm A1/B) only
aStem cell collection was planned after 3 cycles of induction. bFollowing maintenance therapy, patients could receive additional SoC maintenance treatment per institutional standard and local investigator decision. cMaintenance treatment can be discontinued when 12 months of sustained MRD negativity (10–5) have been observed, beginning in induction. dPlanned maintenance treatment in Arm A was Tec-DR. A protocol amendment permitted patients initially assigned to TecDR maintenance to receive Tec-D maintenance per investigator’s choice (patients who started Tec-DR may have discontinued R to receive Tec-D per investigator’s choice). ePatients in Arm A received an additional dose of Tec 1.5 mg/kg on Day 22. AE, adverse event; ASCT, autologous stem cell transplant; CR, complete response; D, daratumumab; d, dexamethasone; DSMM, Deutsche Studiengruppe Multiples Myelom; ECOG PS, Eastern Cooperative Oncology Group performance status; GMMG, German-speaking Myeloma Multicenter Group; HDT, high-dose therapy; MRD, minimal residual disease; NDMM, newly diagnosed multiple myeloma; NGF, next-generation flow cytometry; NGS, next-generation sequencing; ORR, overall
lenalidomide; SAE, serious adverse event; SoC, standard of care; TE, transplant-eligible; Tec, teclistamab; V, bortezomib; VGPR, very good partial response.

GMMG-HD10/DSMM-XX/MajesTEC-5: Response Rates

GMMG-HD10/DSMM-XX/MajesTEC-5:
Conclusions
• Tec-Dara–based immunotherapy induction was well managed, with no discontinuations of all study drugs due to TEAEs, confirming its combinability
• Infections are common (grade 3/4, 36.7%); however, no infections led to discontinuation of all study drugs, and no grade 5 infections were reported
– Infection prophylaxis, including Ig replacement, was adopted and is strongly recommended
• 96% of patients were able to complete successful stem cell mobilization with Tec-D(V)R,a with a median total stem cell yield surpassing minimum protocol requirements
• Unprecedented levels of 100% ORR and MRD negativity at Cycle 3 (10–5) and maintained through Cycle 6 (10–5 and 10–6)b seen with Tec-Dara–based immunotherapy induction
• Results build confidence in Tec-Dara–based regimens as we await results of the first phase 3 study with this immunotherapy doublet in the setting of 1-3 prior LOT (MajesTEC-3)1
immunotherapy induction is manageable with unprecedented MRD negativity in TE NDMM

Iber-Dara-Dex
Key eligibility criteria Objectives Treatmentsc
• Adults (≥ 18 years of age) with NDMM
• Previously untreated symptomatic MMa
• No ASCT planned for initial therapy or ASCT ineligibleb
• Measurable disease
DARA: 1800 mg SC, QW C1–2, Q2W C3-6, Q4W C ≥ 7 DEX: 40 mg (20 mg if > 75 years of age) QW
• Evaluation of the preliminary efficacy and safety of IberDd in TNE NDMM
• PK assessment, MRD evaluation, biomarkers
• MRD evaluation during treatment for patients with ≥ VGPR
aRadiotherapy, bisphosphonates, or a single short course of steroids were permitted; bDue to age (≥ 65 years) or severe comorbidities; cVenous thromboembolism prophylaxis was administered from C1D1 until end of treatment; dIBER was given orally on D1–21; eDARA was given SC at 1800 mg on D1, 8, 15, and 22 during C1–2, D1 and 15 during C3–6, and D1 on C≥ 7; fDEX was given orally at a dose of 40 mg on D1, 8, 15, and 22, or a dose of 20 mg in patients > 75 years of age. C, cycle; MRD, minimal residual disease; PK, pharmacokinetics; QW, once a week; Q2W, every 2 weeks; Q4W, every 4 weeks; SC, subcutaneous; VGPR, very good partial response.

Response and MRD negativity
Sureda Balari A, et al. IMS 2025. Presentation OA-50

High Risk Myeloma
• GMMG analysis
• Functional High Risk – Core Session
• Approach to High Risk – Core Session



CONCEPT Study Design and Background

HRMM criteria: ISS stage II or III PLUS ≥1 of: del(17p), t(4;14), t(14;16) and/or ≥3 copies 1q21
Primary objective: MRD negativity after consolidation (NGF, 10-5)
Secondary objective: PFS; Selected tertiary objectives: ORR, OS
ASCT, autologous stem-cell transplant; d, dexamethasone; HDT, high-dose therapy; HRMM, high-risk multiple myeloma; Isa, isatuximab; ISS, International Staging System; K, carfilzomib; MRD, minimal residual disease; ND, newly-diagnosed; NGF, next-generation flow; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; R, lenalidomide; TE, transplant-eligible; TNE, transplant-ineligible. Following a protocol amendment in 2021, carfilzomib application was switched to once weekly 56 mg/m2 .
LB, Tichy D, Besemer B, et al. Isatuximab, Carfilzomib, Lenalidomide, and Dexamethasone for the Treatment of High-Risk Newly Diagnosed Multiple Myeloma. J Clin Oncol. 2024;42(1):26-37.
Leypoldt L et al., EHA 2025 Abstract S209

Leypoldt

Conclusions
The full GMMG-CONCEPT cohort represents the largest prospective trial cohort of purely HR NDMM patients, including uHR patients, reported so far.
These CONCEPT data underline the high potency of Isa-KRd to induce high rates of MRD negativity and the importance of multi-agent maintenance to sustain MRD-negative remissions across different cytogenetic subgroups in HR NDMM patients.
An unmet need still remains for patients with double-hit HRCA including del(17p) whereas e.g. patients with ≥3 copies 1q21 seem to be adequately addressed by this isatuximab-containing regimen.
D, dexamethasone; HR, high-risk; Isa, isatuximab; K, carfilzomib; MRD, minimal residual disease; NDMM, newly diagnosed multiple myeloma; R, lenalidomide.

Universitäres Cancer Center Hamburg (UCC Hamburg)

International Myeloma Society Meeting 2025, Toronto
Functional high-risk patients at relapse
Katja Weisel,
MD
University Cancer Center Hamburg Hamburg, Germany

Universitätsklinikum Hamburg-Eppendorf
Early Relapse is a predictor of impaired outcome

1 y 3 y
• French experience, 2,474 patients
• Primary refractory excluded
• Relapse within 18 months of start of Tx
• app. 16% patients with Early Relapse
Corre J, et al., Haematologica. 2020;105(9):E480-E483; Mai E, et al. ASH 2024
ER<18: NO
ER<18: YES
Number at risk (censored):
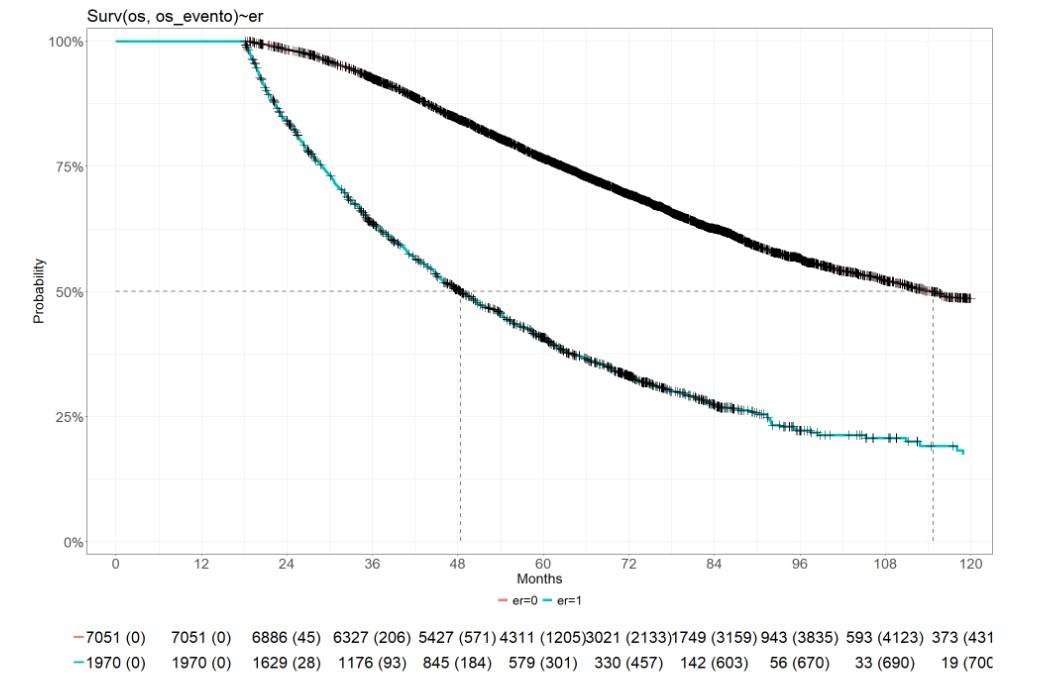
Time (months)
• EMN/Harmony Alliance, 10,843 patients
• Primary refractory excluded
• Relapse within 18 months of start of Tx
• app. 25% patients with Early Relapse

Universitätsklinikum Hamburg-Eppendorf
How to upfront identify FHR – Properly Defining High-Risk Myeloma
If measuring only t(4;14) and del(17p)
67% ER without risk marker
If measuring t(4;14), del(17p) + gain(1q)
30% ER without risk marker
If measuring t(4;14), del(17p) + gain(1q), del(1p) 24% ER without risk marker
IMS consensus on genomic definition of high risk myeloma
in more than 20% of sorted plasma cells
Association of 2 among t(4;14) or t(14;16) or t(14;20)
1q
β2-microglobulin ≥ 5.5 mg/dL in case of normal renal function (serum creatinine < 1.2 mg/dL)
The new IMS Consensus defines 20% of myeloma patients with the worst prognostic outcome – please use the novel definition of high-risk!


Optimal treatment for high-risk MM
Martin Kaiser, MD, FRCP, FRCPath Professor of Haematology
The Royal Marsden Hospital & The Institute of Cancer Research
London, United Kingdom
How to treat HRMM differently in 2025?

Prospective HRMM trial evidence in 2025
Maintain intensity and tolerability
2+ HRCA, GEP-HR, PCL

GMMG-CONCEPT
Cyclo+Quad x4
Dara-CVRD
Dara-VR(d) VASCT
Quad x18
ISS 2/3 AND del(17p), amp(1q), t(4;14), t(14;16); TE and NTE
Quad x6 Isa-KRD
Quad x4 Isa-KRD ASCT
Doublet maintenance until PD with Dara-Len
Triplet maintenance until PD with Isa-KR
del(17p), t(4;14), t(14;16)
Quad x6 Isa-KRD
Quad x4 Isa-KRD ASCT
IFM2018-04 ASCT
Doublet maintenance for 2 years with Isa-Len
HRMM treatment in 2025



1 HRCA



2 HRCA or GEP-HR 3+ HRCA or PCL Quad + (Doublet) maintenance (PERSEUS/(GMMGHD7?))

Presented by: Martin Kaiser, MD, FRCP, FRCPath
@MyMKaiser
Content of this presentation is property of the author. Permission required for use
Clinical Trials ?
T Cell Fitness
Optimal treatment approaches






Role of target/tumor as mechanism ofresistance to T-cell based therapies
Paola Neri, MD, PhD
Associate Professor, Arnie Charbonneau Cancer Research Institute, University of Calgary

Conclusion
• Antigen escape due to BCMA and GPRC5D loss/mutations represents the major cause of acquired resistance to T-cell therapies in MM.
• Distinct genomic alterations mediate BCMA and GPRC5D antigen escape in 60% of RRMM.
OA-06 Inactivation of TRAF3 and CYLD Drive Resistance to T-Cell
Based Therapies in Multiple Myeloma by Promoting Inflammation and an Immunosuppressive Microenvironment that Impair T Cell Function
Jung D et al Sep 18 2025 at 4:20 PM
Clinical Implications
Baseline screening for BCMA or GPRC5D expression/mutations using genomic-based methods
Surveillance for emerging BCMA or GPRC5D mutants (during TCE therapies)
Consider limited-duration of TCE therapy
Evaluate dual targeting or sequencing approaches
Develop TCE with high avidity and binding to multiple target epitopes







Optimal Dose, Schedule and Timing of Immunotherapy in Multiple Myeloma
Surbhi Sidana, MD
Associate Professor
Stanford University
Sept 18, 2025
Cilta-cel: Peak CAR-T expansion associated with delayed neurotoxicity
Longitudinal ALC expansion

CITADEL: Multicenter observational study of pre-emptively suppressing CAR-T expansion




Bridging and Timing of CAR-T Infusion
Non-response to bridging – Increased immune-mediated toxicity (Parkinsonism) – High non-relapse mortality – Decreased CAR-T efficacy

Bridging Can Mitigate Risk of Toxicity

Summary
CAR-T dose, phenotype and expansion can impact efficacy and toxicity with CAR-T
High, rapid cilta-cel expansion Increased risk of Parkinsonism
Timing of CAR-T infusion: After effective bridging (PR or better) to reduce severe toxicity
BsAb: After achieving response, reducing frequency is associated with ongoing responses, but reduced risk of toxicity
Novel Therapies and Approaches
• Dual Targeting CAR T
• Bispecific



September 18, 2025
Concurrent Administration of BCMA and GPRC5D Chimeric Antigen Receptor (CAR) T Cells for the Treatment of Relapsed or Refractory Multiple Myeloma: Results from the Phase I TANDEMM
Clinical Trial

TANDEMM: Study Design and Baseline Characteristics
Study Baseline Characteristics
Key eligibility criteria:
- 3 or more lines of therapy
- Prior PI, ImiD, CD38 antibody based therapy
- Prior BCMA and CART allowed
- Non-secretory myeloma allowed
- Prior allogeneic SCT allowed
Primary endpoint: Safety of concurrent infusion
Secondary endpoints: Efficacy, MCARH125 and MCARH109 expansion and persistence

Lines of Therapy, median (range)
Summary
- Co-infusion of two CAR products concurrently is feasible and not associated with excess toxicities
- In a heavily treated patient population, co-infusion was associated with high and durable responses
- Relapses after co-infusion can be associated with down regulation of one or both antigens
- Baseline T cell factors are associated with refractoriness and early relapse
- Optimal strategy for targeting BCMA and GPRC5D is unknown: co-infusion vs. single CAR targeting both antigens vs. bi- or tri-specific antibodies
Adam D. Cohen, MD

Abramson Cancer Center, University of Pennsylvania
22nd International Myeloma Society Annual Meeting
September 18, 2025

Division of Hematology/Oncology
Sequential T-Cell Engagement for Myeloma (“STEM”):
Phase 2 study of cevostamab consolidation following BCMA CAR T cell therapy
Hypotheses
1. Consolidation of BCMA CAR T cell therapy with cevostamab may:
a) Re-invigorate persisting CAR+ T cells against residual BCMA+ MM cells
b) Eliminate residual BCMA low/negative MM cells by redirecting endogenous T cells against FcRH5
c) Improve rates of sustained MRD-negativity and duration of response
2. MRD-directed, fixed duration cevostamab therapy may limit risk of infections and other complications

Primary endpoint: MRD-negative* CR rate at 12 months post-CAR T cells
n=up to 30 to have 26 evaluable opened July 2023

Preliminary efficacy

Conclusions
‣ Fixed-duration cevostamab consolidation following BCMA CAR T cells appears feasible and well-tolerated in heavily-pretreated MM patients
• 15% CRS, no ICANS or HLH, 15% G3/4 infections
• Primarily hematologic AE’s – G3/4 lymphopenia (74%), neutropenia (44%), thrombocytopenia (22%)
• Unusual immune toxicities in 4/27 (15%)
‣ To date, over 90% of evaluable patients sustaining MRD-negative CR at 1 year post-CART
‣ Enrollment complete; treatment and follow-up ongoing
‣ Correlative studies underway
• CAR T cell expansion/persistence
• CAR+ and CAR- T cell phenotyping
• Soluble BCMA and FcRH5 concentrations
• Interrogation of bone marrow samples pre- and post-CART and cevostamab

Dr. Joe’s Take Home Messages
1. Bispecific antibodies and CelMods are likely going to be used earlier in MM including smoldering and frontline therapy and optimal maintenance strategies are yet to be determined

2. High risk MM is now better defined, but remains sub-optimally managed, but strategies with combination approaches achieving MRD negativity hold the greatest promise
3. Use of immunotherapies continue to evolve as we better understand resistance and T cell fitness – future approaches will likely incorporate more testing for antigen resistance, limited duration bispecific therapy and effective bridging
4. The future is bright with CelMods, other oral agents, combinations with CAR T and bispecifics and novel CAR Ts....


The Evolution of Myeloma Therapy
New
Isa-KRD
SCT +/- More induction
Lenalidomide
Bortezomib
Ixazomib
Bortezomib
Lenalidomide
Carfilzomib
Pomalidomide
Selinexor
Panobinostat
Daratumumab
Ixazomib
Elotuzumab
Isatuximab
Idecabtagene autoleucel
Ciltacabtagene autoleucel
Lenalidomide + PI
Carfilzomib

Teclistamab Talquetamab
Elranatamab Linvoseltamab
Dara + Lenalidomide
Belantamab or Bispecifics?
CAR T or Bispecifics?
Iberdomide, Belanatamab or Bispecifics?

Novel CAR T Cell Therapies
Bispecific/Trispecific Antibodies
Iberdomide and Mezigdomide
Venetoclax?
Belantamab soon?
Multiple small molecules ++++++++

ASCT, autologous stem cell transplant; CAR, chimeric antigen receptor; Cy, cyclophosphamide; d- daratumumab; D/dex, dexamethasone; isa, isatuximab; K, carfilzomib; M, melphalan; PD-L1, programmed death ligand-1; PI, proteasome inhibitor; Rev, lenalidomide; V, bortezomib. Speaker’s own opinions.
Joseph Mikhael, MD, MEd, FRCPC

Professor,


Global Strategy on Myeloma Action Month
Peter Anton Marketing Vice President, IMF



MAM 2026 UPDATE & APPROACH

Thursday September 25th, 2025
Presented by, Peter Anton VP Marketing, IMF
2026 Campaign Overview
Approach & Last Mtg Recap
AGENDA

Exciting Updates & PR
Schedule & Asset Creation
Next Steps & Discussion


Myeloma Action Month is a global social awareness campaign that takes place every March to raise awareness of multiple myeloma. We urge the community to champion Myeloma Action Month to help make a positive impact on those suffering from this blood cancer.
Will you take action for the myeloma community?

MAM 2026 RECAP & CAMPAIGN OVERVIEW
MAM 2026 + GMAN: Campaign Overview
Myeloma Action Month 2026: #MoreThanMyeloma
Theme: "Light the World Red"
• Global Landmarks will be lit in red anytime during March 2026!
• Oncology centers, partners & community invited to illuminate campuses, offices, and anyone can participate by lighting their own homes in red
• Storytelling & Awareness: Campaign amplification through personal stories + facts & stats shared via graphics, videos, across all social channels
• Digital Wall of Action: Global story collection featured on myelomaactionmonth.org
• GMAN Engagement: Localized campaigns aligned with MAM Logo and hashtags and assets highlighting strength, resilience & disparities globally
• Facebook Live Q&As: Dr. Joe & a couple select healthcare professionals
• Call to Action: Join the global movement of unity and resilience – light the world red and show the world we are #MoreThanMyeloma !!
MAM 2026 + GMAN: Campaign Approach / Recap
Country by Country Campaign Participation
• All will use the MAM Logo and can choose to align company branding colors to personalize––for those countries that need help aligning the MAM logo with their brand, IMF MarCom can help
• All can take part in the "Light the World Red" element of the campaign
Campaign Tracking & Assets:
• To effectively track the campaign, ALL will use the hashtag #MyelomaActionMonth and secondary #MoreThanMyeloma
• To effectively communicate the campaign, ALL will use Myeloma Action Month in any of their associated text in posting and communicating the campaign i.e. NOT Myeloma Awareness Month
• IMF will share all assets for the campaign from their DAM (Digital Asset Management) System, sortable by type and language
• IMF will share social posting schedule to help others devise their campaign
• IMF will update www.myelomaactionmonth.org website and will be the main hub
MAM 2026: Partner Examples Recap


MAM 2026: "Light the World Red!" Recap
Each territory should begin planning which key point of interests in their territory to light up in red supporting MAM for the month of March. For example, from left to right: Eifel Tower, Niagara Falls, Golden Gate Bridge, London Bridge, and the Empire State building.










MAM 2026: Exciting Updates!
Light the World in Red
2 Confirmed Lighting Opportunities
• All Mayo Campuses in US, March 26th
o Rochester, Minnesota (exampled here)
o Phoenix, Arizona
o Jacksonville, Florida
o Mayo Clinic Health System sites across Wisconsin and Minnesota
• Nissan Stadium in Nashville TN, March
16th
NYC Times Square Nasdaq Tower (BHM)
• Nasdaq Tower display, runs 18 hrs daily
• IMF will have 4x :15 second spots each hour the whole month of march
• 4x :15 second spots an hour = 18 mins every day
• 9.3 hours of video for the month of March
PR Update
• RLM out of NYC –– IMFs PR agency of record







CAMPAIGN MILESTONES/SCHEDULE
AND ASSET CREATION
MAM 2026: Campaign Timeline/Key Milestones
MAM 2026: Timeline
September 25: Present roadmap at Global Myeloma Action Network (GMAN) meeting.
• Utilize simple campaign letter for landmark lighting requests (IMF can provide)
• Identify organizations that can self-edit templates vs those needing design support.
October 1–November 1, 2025:
• Partners begin outreach to local landmarks and institutions Partners who require graphical assistance provide IMF with:
• Local/regional myeloma facts and statistics and key messaging for localization.
• Brand guidelines and color palettes.
• List of local landmarks contacted including any early confirmations.
November 1–15: IMF Design Lead defines the overall look and feel of MAM 2026 graphics and creates standardized English templates.
November 15–December 20:
• EU graphic designer adapts for partners, applying localized branding and messaging.
• IMF monitors which partners require design support, and which can self-manage.
January 1–31:
• Toolkit for campaign delivered.
• Begin community messaging encouraging households and local businesses to participate.
MAM 2026: Timeline
By January 15–February 15:
• Partners provide IMF with final confirmations of landmarks, including name, city/country, and illumination date(s).
• IMF prepares and verifies data for the interactive illumination map on MAM website
By February 15:
• Launch Myeloma Action Month website with partner toolkit and global illumination map.
• Partners highlight early community engagement stories to build momentum.
• Campaign soft launch: Pre campaign messaging.
March 1–31: Global Activation. Campaign Official Start.
• Landmarks illuminated in red according to confirmed schedules.
• Partners capture and share photos/videos of illuminated landmarks on social media
• Partners promote “Light Your Home in Red” activities in their communities.
March 28: World Myeloma Day.
• Coordinated global push highlighting red-lit landmarks and community participation worldwide.
• Social media updates global illumination in real time with new images and reports on the date of illumination.
April 2026: Wrap Reporting
• IMF compiles and publishes a Global Impact Report (reach, impressions, activations, media) for GMAN and partners.
MAM 2026: Asset Creation
Key Notes & Guidelines for Partners
• Logo: Must remain in English; color may be adjusted per brand palette.
• Copy & Graphics: Partner orgs must provide copy, color palettes, and brand guidelines if requesting custom graphics.
• Templates: Designed for ease of localization to ensure timely delivery.
• Reporting: Social media & engagement to be tracked across platforms for global reporting.
Must always include English language hashtag and English language logo for tracking.
Schedule for Asset Creation:
• Have all MAM assets ready to share on IMF DAM by January 21st, 2025, including:
o Program Overview MAM country by country branded logos (for those needing support)
o Stats & Facts infographics (customize for SA & CA, any others?)
o Generic graphics
o Video ‘reels’ for social
o Updated web site www.myelomaactionmonth.org
o FB Lives scheduled (2-3)

NEXT STEPS & OPEN DISCUSSION
• IMF MarCom Team will share a DAM (digital asset management) link to MAM logo & style guide, landmark letter, and campaign overview.
• IMF MarCom Team will provide support where needed to align branding i.e., if a country does not have a resource to update MAM Logo in their company colors.
• For any questions, please email: o panton@myeloma.org & jlondon@myeloma.org
• Next Check-in ––

Meeting Close
Martine Elias & Serdar Erdogan


Thank You to Our Sponsors!




