www.insightscaremagazine.com






www.insightscaremagazine.com






Natalie May
Everydrugortreatmentthattodaysaveslivesfromcritical
diseaseswasoncedeemedimpossibleorevenmagical.Yetitis visionarieswhodaretochallengeconventionalboundariesand pursuetheseeminglyunattainablethathavemadethesebreakthroughsa reality.Thefieldofclinicalresearchcontinuestoadvancerapidly,driven bytechnologicaladvancementsthathavemadeinvestigations significantlyfaster,moreprecise,andmoreefficientthaneverbefore.
ThisspecialeditionofInsightsCareexaminesthecontributionsofthe leaderswhoareactivelyshapingthefutureoftheindustry Titled 'RedefiningtheFutureofClinicalResearch:Top5CROLeadersto Watchin2025,'itservesasarecognitionoftheiroutstanding achievements,strategicvision,andlastingimpactonglobaldrug developmentandpatientoutcomes.
Throughin-depthprofilesandexclusiveinsights,wehighlighthowthese distinguishedexecutivesareadvancingoperationalexcellence, integratinglatesttechnologiessuchasartificialintelligenceand decentralizedtrialmodels,enhancingpatientdiversityandengagement, andnavigatingincreasinglycomplexregulatoryenvironments.Their leadershipisacceleratingthedeliveryofinnovativetherapieswhile upholdingthehigheststandardsofquality,ethics,andscientificrigor
Asthefieldofclinicalresearchentersaneweraofpossibility,these innovatorsdemonstratethattransformativeprogressisachievedthrough boldthinking,collaborativeinnovation,andarelentlesscommitmentto improvinghumanhealth.
Turn the page for an insightful and inspiring read!
Gary Zammit The Visionary Steering Innovation and Impact in CNS Trials




Featuring Person
Costa Panagos CEO
Gary Zammit President & CEO
Lloyd Presco� CEO
Samira Moran CEO
Teena Pisarev CEO Organiza�on
Syneos Health syneoshealth.com
Clinilabs clinilabs.com
Southern Star Research southernstarresearch.com
Ark Clinical Research arkclinicalresearch.com
Nucleus Network nucleusnetwork.com
Brief
Costa specializes in driving growth and innova�on in biopharmaceu�cal services by leveraging technology to improve clinical trial opera�ons, customer delivery, and pa�ent outcomes globally
Gary is an expert in neuropsychiatric and sleep disorder drug development, with over 230 publica�ons, pioneering therapeu�cs for psychiatric and neurological condi�ons, and a prominent academic and industry leader.
Lloyd works in the clinical research industry, leading innova�ve trials, ensuring regulatory compliance, and u�lizing advanced technology to bring new medical products to market efficiently.
Samira leads clinical research programs focusing on drug development, clinical trial management, and regulatory affairs to advance innova�ve therapies and pa�ent care.
Teena directs strategic planning and business development in clinical services, driving efficient delivery of early-phase trials to advance medicine and improve pa�ent outcomes.


Wherethepastmetmethod,thefuturenowmeets
intelligence—clinicalresearchisenteringanew erawherescienceandsmarttechnologycome together Astreatmentsbecomemoreadvancedandpatient groupsmorevaried,contractresearchorganizations(CROs) playamoreimportantrolethanever Theydon’tjusthandle theday-to-dayworkofclinicaltrials,theyalsoactaskey partnersindevelopingnewmedicinesandtherapeutic devices.Inthiscomplexfield,afewleadersarepushingthe limitsofwhatCROscando,focusingoninnovation, expertise,andpatientoutcomes.Oneofthoseleadersis GaryZammit,whosecareerbringstogetherstrongscientific knowledge,adrivetobuildsomethingnew,andaclear focusonpeople.
GaryZammitisthePresidentandChiefExecutive Officerof ,agloballyrecognizedCROthat Clinilabs specializesexclusivelyinthedevelopmentoftreatmentsfor centralnervoussystem(CNS)disorders.Hispathinto clinicalresearchwasbornnotoutofconvenience,butof necessityanddeepconviction.Havingservedasaprincipal investigatoratahospital-basedclinic,Zammitexperienced firsthandthepitfallsofanindustrythatoftenlacked therapeuticdepth.Herecounts,“BeforeIstartedthe company,Iworkedasaprincipalinvestigator,often workingwithCROstoconductCNStrialsinmyoutpatient clinic.IfeltthatmanyCROsatthattimelackedthe therapeuticareaexpertiseneededtounderstandthescience andrunclinicaltrialsefficiently.”
Itwasduringtheseearlyyearsthattheideaofadedicated CNS-focusedCROtookshape.Theroadto entrepreneurship,however,wasnotwithoutpersonal sacrifice.“Afteroneparticularlydisappointingtrial experience,Zammitdecidedtoresignfromasecurejob whilehiswifewassixmonthspregnantwiththeirfirst child,takingaleapthatwouldlaterreshapeCNSresearch. Zammitrecalls,“theideaofleavingmyjobandstartingthe businessdidnotgoovertoowellathomeatfirst,butmy wifeeventuallyencouragedmydecisionandbecamemy greatestcheerleader.”Whatbeganasaboldpersonal missionhassinceevolvedintoagloballyrespected organizationknownforbothitsscientificacumenand patient-centeredphilosophy
Let’s take a closer look at how Zammit is leading Clinilabs toward new breakthroughs in clinical research!
ZammitviewsleadershipintheCROspaceas fundamentallyservice-driven.“CROsareservice organizations.Itisallaboutthepeople,processes,and systemstheyapplyinthecourseoftheirwork,”he explains.Thistrinityofpeople,process,andtechnology formsthecornerstoneofClinilabs’operationalexcellence. HebelievesthatthemostsuccessfulCROscombinetop-tier talentwithworld-classprocessesandcutting-edgedigital tools.


Beyondoperationalefficiency,leadershipalsomeans instillingpurposeacrosseverylayeroftheorganization.For Clinilabs,thispurposeisembodiedinitsinternalcredo:the “CultureofQuality.”Zammitelaborates,“Leadershipmust keepeveryonefocusedonthecompany’smission,vision, andculture.AtClinilabs,wecoinedthephrase‘Cultureof Quality’todefineus,andourcorevaluesarerepresentedin theacronymASPIRE,whichstandsforAccountability, Science,Partnership,Integrity,Responsibility,and Excellence.I’madamantthatwedon’tsimplypostourcore valuesonourwebsite–welivebythem.”
Inanindustrypopulatedwithlarge,generalistCROs, Clinilabs’exclusivefocusonCNSgivesitaformidable competitiveedge.“CNStrialsarecomplex,andtheyfail moreoftenthantrialsinothertherapeuticareas,so expertiseisattheheartofsuccess,”Zammitemphasizes. Indeed,CNSdisorderssuchasdepression,schizophrenia, epilepsy,Alzheimer’s,andPTSDdemandmorethanroutine execution;theyrequireadeepunderstandingof neurobiology,patientassessment,regulatorypathways,and statisticalinterpretation.
ThissingularfocusenablesClinilabstoofferatruly comprehensivesuiteofservices—spanningfirst-in-human studies,proof-of-concepttrials,andpivotalphase3 programs.Fromthedesignofclinicalprotocolstopatient recruitmentandsiteselection,everyelementistailoredto theuniquedemandsofCNSresearch.“Wearetheonly globalCROthatisfocusedexclusivelyonthedevelopment
ofCNStherapeutics,bothdrugsanddevices.AsCNS experts,weknowthepatientpopulations,studydesigns, assessmenttools,investigatorsites,andregulatory strategiesthatsponsorsneedtoachievesuccess,”hesays.
Importantly,Clinilabsisstructuredtoprioritizeclientswho maybesidelinedbylargerCROs.“Asamid-sized organization,weprioritizesponsorsandprojectsthatlarge CROsoftencannot,andourseniormanagementteamis fullyengagedineveryprojectwedo.Ourvalueproposition isentirelydifferentthanthatoflargeCROs.”
Zammitiskeenlyawarethattechnology,whenproperly harnessed,canenhanceboththeaccuracyandspeedof clinicaltrials.Clinilabshasmadeconsiderableinvestments initsdigitalinfrastructure,fromconventionalsystemslike eCTMS,eTMF,andePROtoproprietaryinnovationssuch asClinicalInSite2.0.“Wecontinuouslystrivetobeatthe forefrontoftechnologyadvances,applyingdigitalsolutions tooptimizeworkflowsandimprovedataquality,”henotes.
ClinicalInSite2.0isparticularlynoteworthyforitsability tointerfacewithmedicaldevicesandwearables,facilitating real-timedatatransfer.Thisimmediacyallowsresearchers tomakeinformeddecisionsquicklyandenhancesthe integrityofthedatacollected.“Thesystemsweuseare tailoredtothespecificneedsofeachprotocol,”hesays, emphasizingthecustomizednatureoftheirtechnological integrations.
AccordingtoZammit,oneofthegreatestchallengesin early-phaseCNStrialsisthetemptationtoover-engineer Hewarnsthatexcessivecomplexitycanunderminestudy success.“Inphase1and2trials,itisimportanttokeep studydesignssimple,clearlydefinepatientpopulationsand outcomes,andlimitnon-coreelements.Toomuch complexitycancrushastudy,”heexplains.
Beyonddesignsimplicity,thereareadditionalnuancesto considerinCNSresearch,suchasplaceboeffects,diversity amongpatientcohorts,andtheselectionofpsychometric instruments.“InCNS,wemustcarefullyconsiderthings likeratertraining,theuseofcentralizedratings,and placeboresponsemitigation.Itisnotacceptabletolook backatthelastsuccessfuldevelopmentprogramandtryto copyit.Everyprojectisunique.”
Inasectorwheremanyengagementsareproject-based, Clinilabshasmanagedtobuildenduringpartnerships. Zammitattributesthissuccesstoconsistentdeliveryandan unwaveringfocusonsponsorneeds.“Long-term relationshipsarebuiltonperformance.Ifwedoourjobs well,actinthebestinterestsofthesponsor,andcollaborate aspartners,sponsorswillreturnagainandagain,”hesays.
Thisphilosophyhasledtoextraordinarycontinuity.“Some ofourlargepharmasponsorshaveplacedmorethan50 projectswithusover25years,”Zammitreveals.Inan industrywherelong-termloyaltyisrare,thesenumbers speaktothedeeptrustClinilabshasearned.
Talentacquisitionandretentionareparamountinafield wherehumancapitaldefinessuccess.Zammithasa nuancedviewonwhatattractsandholdstop-tier professionals.“Theseareimportantquestions,somuchso thatIdedicatedabouthalfofmybook,‘Beyondthe Science,’tothedevelopmentofhighperformingteams,”he shares.
Forhim,trueA-playersarenotmerelyenticedonlyby compensationpackages.“Interestingly,I’vefoundthat thesepeoplearenotprimarilydrivenbycompensation. Theyaredrivenbytheabilitytoachievetheirpersonalbest, tobepartofateamthatreachesloftygoals,andtowork withinanorganizationthatbothchallengesandsupports themmorethananyother.”
Maintainingcomplianceandsafeguardingpatientwellbeingarenon-negotiables.AtClinilabs,theseprioritiesare deeplyembeddedintoeverydaypractices.“Thefoundation isourSOPs.WeareanSOPdrivenorganization,andwe
relyonrobustprocessesforqualitycontrol,quality assurance,andcontinuousqualityimprovement,”says Zammit.
Butheisquicktostressthatqualityisnotthedomainofa singledepartment.“Qualityisnotsomethingthatisdone onlybytheQAdepartment–everyoneisresponsiblefor quality,andweholdeachotheraccountable!”Thisculture ofsharedresponsibilityfostersanenvironmentwhere excellenceisacollectivegoal.
Zammit’svisionforthefutureisoneofoptimism,propelled bytechnologicaladvancementanddeepenedscientific understanding.“Ibelieveweareonthevergeofsignificant breakthroughsinneurotherapeutics;onesthatwillresultin remarkableinnovationsinthewaypatientsaretreated,”he states.
HepointstocurrentresearchintopsychedelicsforPTSD anddepression,disease-modifyingagentsforAlzheimer’s, andevenvaccinesforsubstanceusedisorders.“Wearenow lookingatdiseasemodifyingtreatmentsforcognitive disorders,includingAlzheimer’sdisease,andwe’reeven workingonvaccinesforsubstanceusedisordersandother CNSconditions.”Thisiscoupledwiththeemergenceof AI-baseddiagnostics,brain-computerinterfaces,and mobileapplicationsthataretransformingpatientcare.
Hisforecastisambitiousbutgrounded:“Wewill accomplishmoreinthenext10yearsthanwehaveinthe past50.”
Tothosedeterminedtoleadthenextgenerationofclinical research,Zammitofferscounselforgedthroughpersonal experience.“Myadviceistoidentifythegoalsyouwantto achieveandstaylasertargetedonthosegoals.Besingularly focused.Everystepyoutakeshouldbeinthedirectionof fulfillingyoursetgoals.Don’tletanyoneoranythingdeter you,anddon’tbediscouragedbysetbacksbecause,if you’relikeme,youwillhavemany!”
Zammit’sjourneyfromafrustratedinvestigatorto pioneeringCEOillustrateswhatispossiblewhendeep scientificknowledge,boldentrepreneurship,andheartfelt purposeconverge.Underhisguidance,Clinilabshas becomemorethanjustaCRO;itisasymbolofwhat focusedspecializationandvalues-drivenleadershipcan accomplishinanever-evolvinghealthcaresector


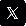





www.insightscaremagazine.com
Medicineisenteringanewerashapedby
biotechnology,digitalinnovation,andadvanced engineering.Thesedevelopmentsarechanging howresearchersfind,design,andtestnewtreatments.What usedtobesoexpensiveandtime-consumingisnow accomplishedinamuchshorterperiodoftimeandmore accurately.Drugdiscoveryandtestinghavebeenmade easier,morepersonalized,andencouraging,providinghope fordiseasesthatwerethoughttohavenocure.
This article explains how biotechnology and digital innovation are changing modern medicine, including how microfluidics and 3D bioprinting are revolutionizing drug discovery and testing.
Newdigitalsystemsnowdrivemuchoftoday's pharmaceuticalresearch.Computermodelscansimulate intricatebiologicalprocessesthatwouldhaverequired yearsoflabtimeyearsago.Theyareusefulinforecasting thebehaviorofvariouscompoundswiththetargetproteins, whichwouldsavetimeandcostintheearlystagesofthe developmentprocess.
ToolssuchasDeepVariantandAlphaFoldhavehelped scientistsbetterunderstandhowproteinsfold,akeypartof creatingtreatmentsfordiseasessuchasAlzheimer's,cancer andgeneticdisorders.Othercomputermodels,likeGenie,

areevenabletodesignnovelproteinswithcertainmedical applications.
Virtual"insilico"trialscannowpredicthowthehuman bodymightreacttonewdrugsbeforetestingbegins.These computer-basedtrialsreducetheneedforearly-stage animalorhumantesting,lowerrisks,andmakethepathto clinicaltrialsmuchfaster.
Microfluidictechnologyistransformingthewayscientists conducttrialsonpossiblemedicines.Thesesystems involvetheuseofsmallchip-basedsystemsthattransport smallvolumesofliquid,formingpreciselyregulated environmentsinwhichcellscanbestudied.
Incontrasttotheprevioustestingtechnologiesthat intermixtheoutputofalargenumberofcells, microfluidicsallowsscientiststostudyindividualcellsto determinehoweachcellrespondstoadrug.Thisisoneof thereasonswhycertainpatientsrespondwelltotreatment, unlikeothers.

Whenusedwithbioactivity-guided screening,whichtrackshowcells respondinrealtime,microfluidics helpsidentifyeffectivecompounds morequickly.Combinedwithorganoid andtissuemodels,thismethodmakes drugdiscoveryandtestingmore accurateandclosertorealhuman biology
Conventional2Dcellculturesand animalmodelscannotdemonstratethe actualhumanorganresponsetodrugs.
3Dbioprintingisasolution,asit produceslivingtissuesandsmall organ-likestructures,knownas organoidswhichrespondanalogously torealorgans.
Thesebioprintedmodelsarenowkey toolsfortestingnewdrugssafely As anexample,liverandheartorganoids canbeusedtopredictpotentialside effectsbeforethehumantrial.
Bygatheringthecellsofapatient,thetestingcanalsobe tailoredtohisorhergeneticmakeup,whichisamajor accomplishmenttowardspersonalizedmedicine.
Inthefuture,doctorsmaytesttreatmentsonlab-growntissue madefromapatient'scellsbeforeprescribingthem,ensuring thesafestandmosteffectiveresults.
Anewgenerationofbiotechnologiesistransforminghow medicinesaredeveloped.RNAinterference(RNAi)can silenceharmfulgenes,whilecircularRNAsserveasearly warningsignsfordiseasesandguidetreatmentchoices.
Proteinengineeringalsohasthepotentialtohelpscientists designthetailor-madeenzymesandantibodiesthatwould fightthediseasesthatwereonceregardedasuntreatable. Regenerativemedicineusesstemcellsandtissuerepairto healdamagedorgansandtreatlong-termillnesses.
Asbiotechnologygrows,italsoraisesnewethicalandsafety questions.Geneeditingisassociatedwiththeissuesof privacy,consent,andthelong-termoutcomes.Theregulators havetoguaranteethatthenewcompoundsthathavebeen developedusingdigitalsystemsaresafeandeffective.
Astheuseofpatient-derivedtissuesinresearchincreases, therehavetobeexplicitregulationstoensurethatdatais secureandthatthereisequitableaccessibility.Responsible developmentofthebiotechnologysphereshouldbeputat theforefront,withconsiderationofthesafetyandtrustofthe population.
Thefutureofanymedicineisshiftingitsdirectiontowards speed,accuracy,andindividualization.Theresearcherscan nowdealwithdiseasesthatusedtobeconsideredincurable, andthetoolsavailable,suchasCRISPR,mRNA,anddigital modeling,allowthemtodesignandtesttreatmentsinamore efficientway
Whensuchtechnologiesareontheincrease,oneshould balancebetweeninnovation,safety,andethics.Withcareful regulationandresponsibleuse,biotechnologycanmake healthcaremoreeffectiveandaccessibleforpeoplearound theworld.
- Natalie May


FertilitycareinIndiahaschangedremarkablyoverthe last thirty years. Thirty years ago handful of specialized clinics were available in major metropolitan cities, but now assisted reproductive technologies(ART)suchasIVF(InVitroFertilization),ICSI (IntracytoplasmicSpermInjection),andfertilitypreservation arenowwidelyavailableacrosstier1,tier2cities,andsemiurban areas. This expansion has given hope to millions of couplesstrugglingwithinfertility
However,thetraditionaldefinitionofsuccessinfertilitycare has often changed mainly around clinical outcomes, pregnancy rates, live birth rates, and the sophistication of laboratorytechniques.Whiletheseremainessential,theyare only one side of the story. For couples and individuals navigating infertility, the journey is not only about biology alone; it is equally, if not more, about emotional tolerance, socialsupport,andmentalwell-being.
Today,thereisaneedfortimeforfertilitycareinIndiamust evolvefrombeingpurelyclinicallydriventoaholisticmodel that integrates psychological, emotional, and social dimensions.Sustainability,compassion,andpatient-centered reproductive healthcare are the factors that this shift will address,andthismakesitnecessarilysignificantandnotjust desirable.
TheEvolvingLandscapeofFertilityinIndia
• RisingIncidenceofInfertility
According to the Indian Society of Assisted Reproduction, 10-15% of Indian couples of reproductive age are affected by infertility. Delayed marriages, late childbearing, lifestyle disorders like obesity and diabetes, PCOS, environmental pollution, andstressarethecontributingfactorstothis.
Male infertility, once a less-discussed issue, is now recognizedascontributingtonearly40–50%ofcases.
• EasierAccesstoART
TherapidriseofIVFcentersinTierIandTierIIcities
has improved access. India is a global hub for fertility treatments due to cost-effectiveness and clinical expertise. New technologies like time-lapse embryo imaging, pre-implantation genetic testing, and fertility preservation for cancer patients have raised clinical successrates.
• TheRegulatoryboundary
The Assisted Reproductive Technology (Regulation) Act, 2021, and Surrogacy (Regulation) Act, 2021, brought essential governance. They promote ethical practices, standardize care, and protect patients. However, regulation alone cannot address infertility's emotionalandsocialchallenges.
EmotionalBurdenofInfertility
• Psychologicalburden
Infertility is often described as a “silent struggle.” For manycouples,theinabilitytoconceivenaturallybrings profound grief, guilt, and a sense of inadequacy. Women, in particular, face disproportionate emotional pressure, often bearing the brunt of blame in a patriarchalsocialmilieu.
Commonpsychologicaleffects,anxietyanddepression, loss of self-esteem, strained marital relationships, sexualdysfunction,andsocialwithdrawal,increasethis burden.
• StigmaandSocialPressure
In the Indian scenario, usually any marriage is linked with obvious expectations of parenthood. Irrespective oftheagecoupleofyearsofmarriageistakenascriteria for this social expectation. Due to this expectation in lawscreateorpassthepressureofthesameexpectation tothecouple.Injointfamily,thissituationcreatesstress on couples especially the woman faces more stressful situation and conversation.The criteria of a successful marriage are taken as being parents of a child. Peer pressureisaffectingallagegroupsandgenerations.The peer pressures on in-laws and parents makes intense expectationsforparenthoodfromdaughtersand
Dr. Vinoad Bharrati is a leading expert in gynaecology and obstetrics, serving as Director and Consultant Gynaecologist at Rising Medicare Hospital and Elite Momz in Pune. His patientcentered approach, combined with cutting-edge medical solutions, ensures comprehensive care for women at every stage of life. With DGO and DNB certification along with a Diploma in Endoscopy from Germany, Dr. Bharrati’s expertise spans a wide range of services, including advanced IVF treatments and VBAC (vaginal birth after cesarean). Backed by a multidimensional experience spread over more than two decades, his leadership promotes the use of modern technologies and minimally invasive surgeries, reducing recovery times and improving patient outcomes.
daughterinlaws.Thepeerpressureofcomparisonmakesthe situationworseinacouple'slife.
Theotheraspect,whichistheinabilitytoconceivepregnancy, stillattractssocialstigmainmostpartsofIndia.Thesituation is not different in rural and urban communities. The older generations take pride in having grandchildren, and couples considerparenthoodasaunitofcompletionformarriedlife.
When any couple is going through painful journey of infertilitytheyusuallyfacethefollowing:
• Isolation
• Unsolicitednon-professionaladvice
• Blame(especiallytowardswomen)
• Comparisonswithothercoupleshavingchild
• Pressuretojustrelaxortohavefaith
Fertility treatments are often expensive and not widely coveredbyinsurance.TheaveragecostofonecycleofIVFin Indiaisaround1.5lakhsto3lakhs.MultiplecyclesofIVFcan drain families emotionally and financially This financial burden amplifies the psychological stress, especially when successisuncertain.
Usually,successrateshavebeentheelementofadvertisement forfertilityclinics.Althoughapositivepregnancytestisnot equaltotheoverallwell-beingoftheindividual.
Truesuccessshouldinclude:
1. Emotional Stability – Helping couples cope with the highsandlowsoftreatment.
2. Marital Harmony – Supporting partners to strengthen theirrelationship.
3. Social Integration – Enabling individuals to navigate societalstigmawithconfidence.
4. Long-Term Wellness – Promoting reproductive and mentalhealthbeyondtheimmediatetreatmentcycle.
IntegrativeCounselling
Patients would learn to cope easily with the inclusion of professional counselling as an imperative part of fertility treatment.Reproductivementalhealthpsychologistscanhelp in normalizing emotions, reducing anxiety, and enhancing treatmentadherence.
Infertility patients can attain stress reduction and improvementinemotionalbalancethroughyoga,meditation, and mindfulness, showing promising benefits for the same. Integration of such practices has begun in several fertility centersinIndia.
Connecting with people undergoing similar struggles and expressing your experiences provide hope and reduces the feelingofisolation.Thispeersupportcanbetransformativein the whole process. Unsolicited advice remains a negative aspectofsuchgroups.
Appropriate family members and spouses must also be engagedinfertilitycare.Developingasenseofempathyand shared responsibility by educating partners about the emotionalburdenisacrucialpartofIndia.
HealthcareProvider’sresponsibility
Clinicals play a role in the core of this shift from clinical successtoemotionalwellness.
• Empathy in Communication: Doctors who listen activelyandaddressemotionalconcernsfostertrust.
• Setting Realistic Expectations: Transparent conversationsaboutsuccessrates,potentialfailures,and alternativespreventpatientsfromunrealistichopes.
• EthicalResponsibility:Recommendingunnecessaryor repeated cycles without considering the patient’s emotionalstateriskslong-termharm.
Nurses, embryologists, and administrative staff also play a role in shaping the emotional experience of patients. A compassionate, patient-friendly environment can significantlyreducetreatment-relatedstress.
Oneofthebarrierstoemotionalwellnessisfinancialburden. Fertility treatments in India are largely out-of-pocket expenses Government policies that mandate insurance coverage for ART would ease this burden. Additionally, funding support for counselling services within fertility clinicsshouldbeconsideredpartofcomprehensivecare.
Based on above information emerging best practices would be:
1. HolisticFertilityCenters–offeringintegratedprograms thatincludecounselling,yoga,andnutritionalguidance alongwithART.
2. Digital Health Platforms – Mobile apps providing mental health support, treatment tracking, and communityforumsforfertilitypatients.
3. Corporate Wellness Programs – offering fertility benefits, including IVF coverage and counselling, as partofemployeewellnessinitiatives.
Lookingahead,theredefinitionoffertilitycarewouldinvolve threekeyshifts:
1. From Biomedical to Biopsychosocial Model –Rediscovering and realising that infertility is both a medicalandemotionalcondition.
2. FromPatienttoPerson-CenteredCare–Seeingpatients as wholeindividualswithaspirations,fears, andsocial realitiesbeyondtheirdiagnosis.
3. From Stigma to Support – Normalizing infertility, validationofemotionalstrugglesandopendialogueon thesubjectcanbeattainedtobuildingacommunity narrative.
Artificial intelligence, genetic testing, and advanced lab techniques will continue to improve clinical success rates . Although, the real sign of progress will be when emotional, socialandfinancialsupportisfeltbyeverycoupleundergoing fertilitytreatmentinIndia.
Fertility care in India has reached a point of paradigm shift where clinical excellence is no longer enough. As success rates climb and technologies advance, the human side of infertility must take center stage. By integrating emotional wellnessintofertilitytreatment,Indiahastheopportunityto redefine reproductive healthcare in a way that is holistic, compassionate,andsustainable.
Thismeanslottocouples,notjustthehopeofparenthoodbut alsodignity,resilience,andhealingthroughouttheirjourney. For society, it indicates next level maturity an acknowledgmentthatparenthoodisdeeplypersonalandthat supportingemotionalwell-beingisasimportantasscientific.
The future of fertility care in India, therefore, lies in ART laboratories as well as in the hearts, minds, and lives of the peopleitseekstoserve.

Modernclinicalresearchstandsatacrucialpoint
wherescientificprogressmustalignwiththe responsibilitytoprotecthumandignity Withthe developmentofmedicine,whichunitesdata-driven approaches,genetics,andcollaborationacrosstheworld,it isincreasinglyquestionedhowtobecomeinnovative withoutgoingtoofar.Balancingprogresswithprotection remainsthecoreofmodernclinicalresearchinnovation.
The article examines the evolving ethical challenges in modern clinical research innovation, focusing on how science can advance responsibly while safeguarding human dignity and trust.
Theethicsofclinicalresearchcontinuetoshiftas technologyandglobalizationreshapethefield.Thedigital healthtools,telemedicine,andremotetrialshaveenabled individualsinnearlyanylocationtoparticipateinresearch. Thisenhancesaccessibilityandthepaceofdiscoveries,but alsobringsinroughquestions:Aretheparticipantsawareof whattheyaregettingthemselvesinto?Areacademics upholdingidenticalethicsinternationally?
By2025,moretrialswillbeconductedinlowandmiddleincomecountries.Theselocationsofferlowercostsand diverseparticipants,butmostlyfaceweakeroversight.
Researchersmusthandledifferencesinculture,education, andlocalhealthsystemscarefully.Ethicalconcernsabout consentandfairnessgrowwhenparticipantsdonotfully understandwhatatrialinvolvesorwhenglobalsponsors operateunderlessstrictrules.
Socialminorities,includingchildren,theelderly demographic,inmates,andlow-incomecitizens,shouldbe additionallyguardedsincetheirparticipationshouldalready bevoluntary Theethicalregulationsshouldkeepchanging inlinewiththeglobalandtechnologicalshiftsthatdefine ClinicalResearchInnovation.
Informedconsentiscentraltoethicalresearch,butithas becomehardertoensure.Inonlineandremotetrials, participantsoftenreadconsentformsonscreensinsteadof talkingdirectlywithresearchers.Thiscanturnconsentinto abox-tickingstepratherthanaclearunderstandingof rights,risks,andbenefits.
Modernmedicaldevicesnowtrackhealthdata continuously Whilethisimprovessafetyandprecision,it alsoblursthelinebetweencareandprivacy.Many participantsdonotfullyknowhowtheirdatawillbestored, shared,orusedinternationally,creatingnewethical concernsaboutprivacyandtrust.
Theethicaldilemmasthatplaguedmodernresearchfora longtimearenotlimitedtotechnology:
• InformedConsent:Realconsentisnotmerelysigning apaper.Thisisusuallycomplicatedbylanguage barriers,complicatedstudydesignsanddigitalsystems.
• UseofPlacebos:Placebotrialsareessentialtogood evidence,butusingthemwhereeffectivetreatments existcanbeethicallytroubling,especiallyinserious diseases.
• Risk-BenefitBalance:Theresearchersshouldconsider theadvantagesanddangersofresearch,especiallyat thebeginningofthetrials,andbeopenwiththe participants.
• EqualOpportunityduringRecruitment:Equality involvesvariedinvolvement.Butwomen,theminority, andindividualswithotherhealthissuestendtobe underrepresented.
• DataPrivacy:Thediscoveryofbighealthdatasetsis fast,butthechancesofdataleaksormisusearehigh,a factthatwillharmthetrustofthepopulationin ClinicalResearchInnovation.
• ConflictsofInterest:Financialorinstitutional affiliationsmaycompromiseresearchoutcomesand closesupervisionanddisclosurearecrucial.
Oversightsystemshavebecomemorecomplextoaddress thesechallenges.TheethicscommitteesandIRBs, accordingtotheDeclarationofHelsinki,assistinthesafety oftheparticipants.Internationalregulators(FDA,EMAand WHO)strivetobringuniformity,yetthelocalregulations andculturalnormsstilldifferalot.
Manyexpertsbelievethatstrict,one-size-fits-alloversight nolongerworks.Instead,adaptivesystemsthatmatchthe levelofreviewtothelevelofriskaregainingground.The futureofClinicalResearchInnovationdependsonflexible andresponsiblegovernancethatsupportsbothprogressand protection.
Historyknowstheriskofprioritizingprogressover morality.TheTuskegeeSyphilisStudyandothercasesof consentbreachgaverisetothestringentregulationsinthe presenttime.Theselessonsremindresearchersthat discoverywithoutethicsleadstoharm,notadvancement.
Still,toomuchcautioncanslowusefulresearch.Overly strictrulesmightstopwillingparticipantsfromaccessing promisingtreatmentsorcontributingtoprogress.The challengenowistoprotectpeoplewhilerespectingtheir freedomtochoose.
ClinicalResearchInnovationshouldcenteronethical innovationtopreservetrust.Researchers,regulators,and communitiesmustworktogethertocreateflexible frameworksthatkeepupwithsciencewithoutsacrificing integrity.
Consentprocessesshouldbesimple,transparent,and people-centered,evenindigitalsettings.Datasystemsmust protectprivacywhileallowingresponsiblesharingfor publicbenefit.Globalpartnershipsmustvaluemoral responsibilityasmuchaslegalcompliance.Thefutureof medicinedependsonkeepingthesevaluesinharmony. Onlywhenethicsandinnovationmoveforwardtogether canresearchtrulyservehumanity -Natalie May




www.insightscaremagazine.com