A First-in-Class Cancer Immunotherapy

Fully Leverage the Immune System



By uniquely and specifically loading Dendritic Cells with both RNA and Proteins, we train the immune system to optimally attack cancer cells. Educate Activate Eliminate





By uniquely and specifically loading Dendritic Cells with both RNA and Proteins, we train the immune system to optimally attack cancer cells. Educate Activate Eliminate
Backed by decades of research and ongoing clinical trials, the Immunocine Dendritic Cell Treatment (IDCT) is a less invasive, more precise way to treat a large variety of cancers.


Our Dendritic Cell Treatment initiates a complete Immune Response against your cancer cells.
This top-down approach avoids the unspecific and ineffective targeting of other immunotherapies.

Our Dendritic Cell Treatment does not introduce any foreign compounds or genetically modified cells into the body.
By using your body’s own cells, our treatment significantly reduces the risk of side effects.

Our Dendritic Cell Treatment fully activates the Immune System and therefore continues after patients have received their last treatment.
This Immunological Memory can make it much harder for the cancer to return.

Scan to Watch Testimonials www.immunocine.com
In 2011, Dr. Matthew Halpert, PhD, joined a research team of immunologists studying and developing a revolutionary approach to cancer treatment at the prestigious Texas Medical Center. Leveraging the emerging science of immunotherapy, Dr. Halpert witnessed firsthand the powerful effects of this unique Dendritic Cell treatment both in the laboratory and in real-world case studies. This work has resulted in multiple, active clinical trials. While these trials represent significant potential for the future of cancer treatment, they don’t help patients who do not qualify for the clinical trials.
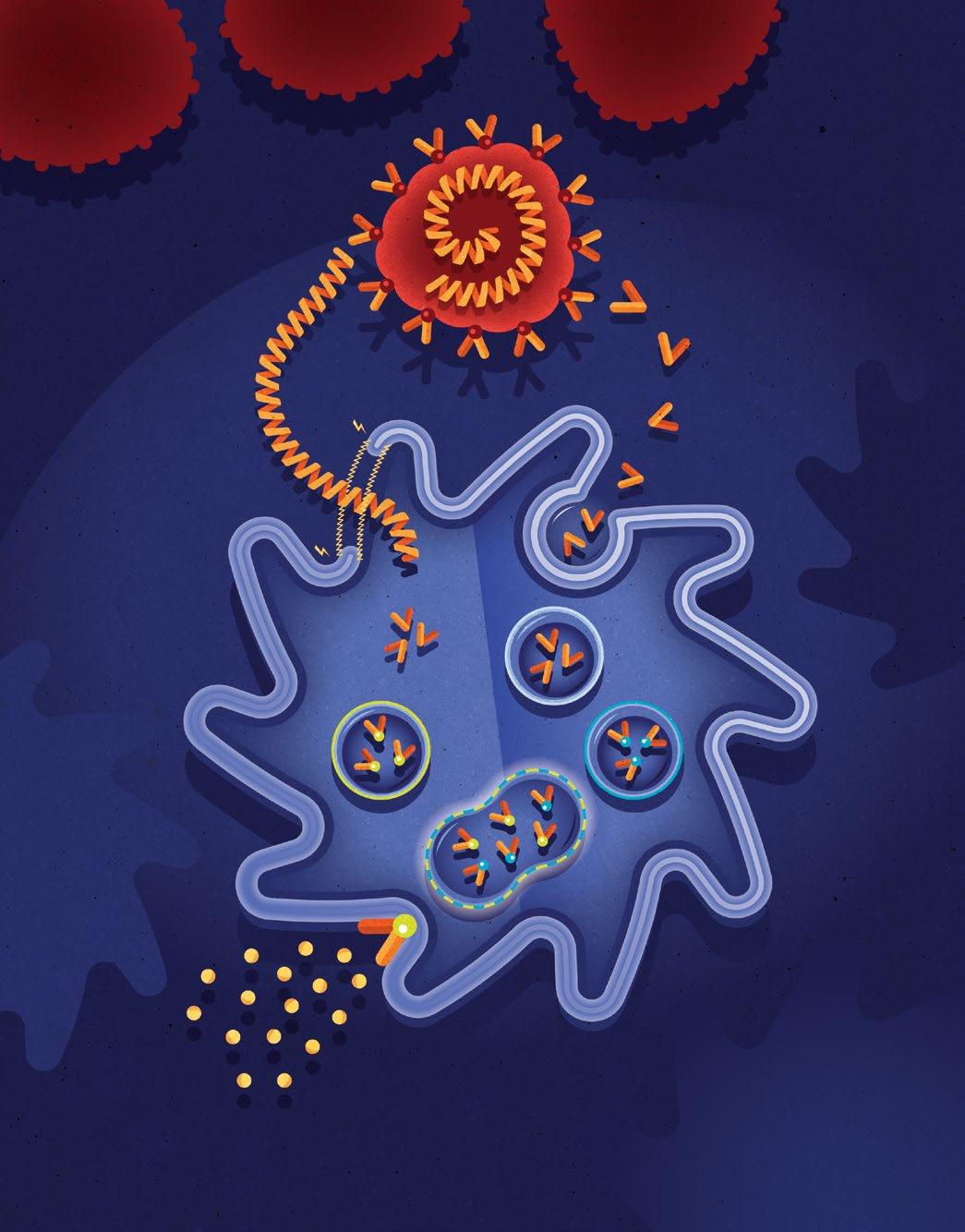
In our lab, Biopsied Cancer Cells are processed into specific mRNA and Proteins.
After the Cancer Cell processing, Cancer Antigens become free-floating outside of the Dendritic Cell.
2a Using an electrical current to open its membrane, the cancer mRNA is able to enter the Dendritic Cell, similar to a viral infection.
Free-floating cancer antigens are enclosed in a Dendritic Cell Endosome.
3a The Dendritic Cell processes the Cancer mRNA into specific Cancer Antigens. Cancer Antigens are enclosed in an Endosome and bound to MHC Class 1 Molecules, which detect foreign antigens within the cell.
Inside the Endosome, Cancer Antigens are bound to MHC Class 2 Molecules, which detect foreign antigens outside of the cell.
Dendritic Cell endosomes combine and confirm the MHC Class 1 and 2 molecules are bound to the same Cancer Antigens!
Dendritic Cell activates Immune Response by releasing antiviral cytokines and presents specific Cancer Antigens on its surface to educate Immune System.
Unlike other Dendritic Cell treatments, ours is the first to “Double Load” the patient’s Dendritic Cells with their extracted cancer cells. This simulates a viral-like infection and initiates specific T Cells to seek and destroy the cancer cells throughout the patient’s body. Using this completely new approach to Dendritic Cell Treatments, the Immunocine Dendritic Cell Treatment (IDCT) protocol is able to not only generate a natural immune response, but one that is comprehensive enough to attack a patient’s cancer throughout their body.
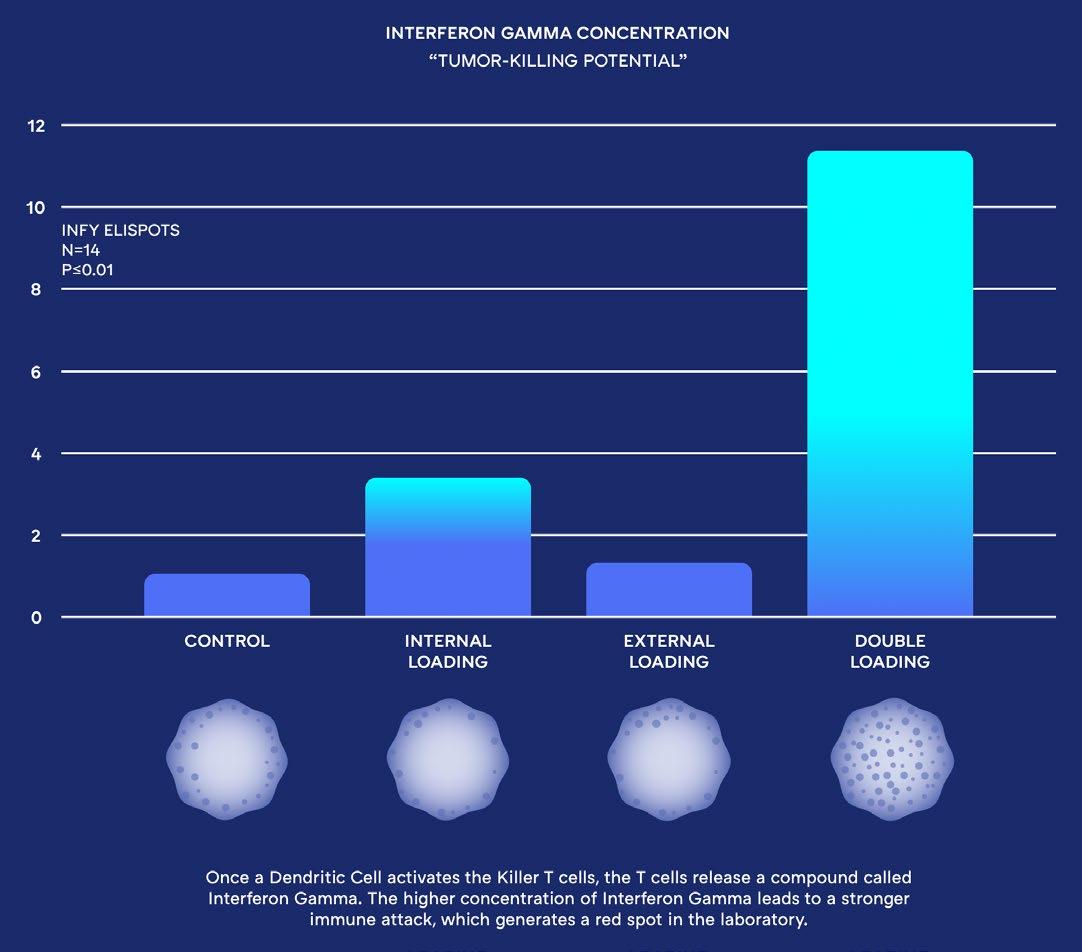
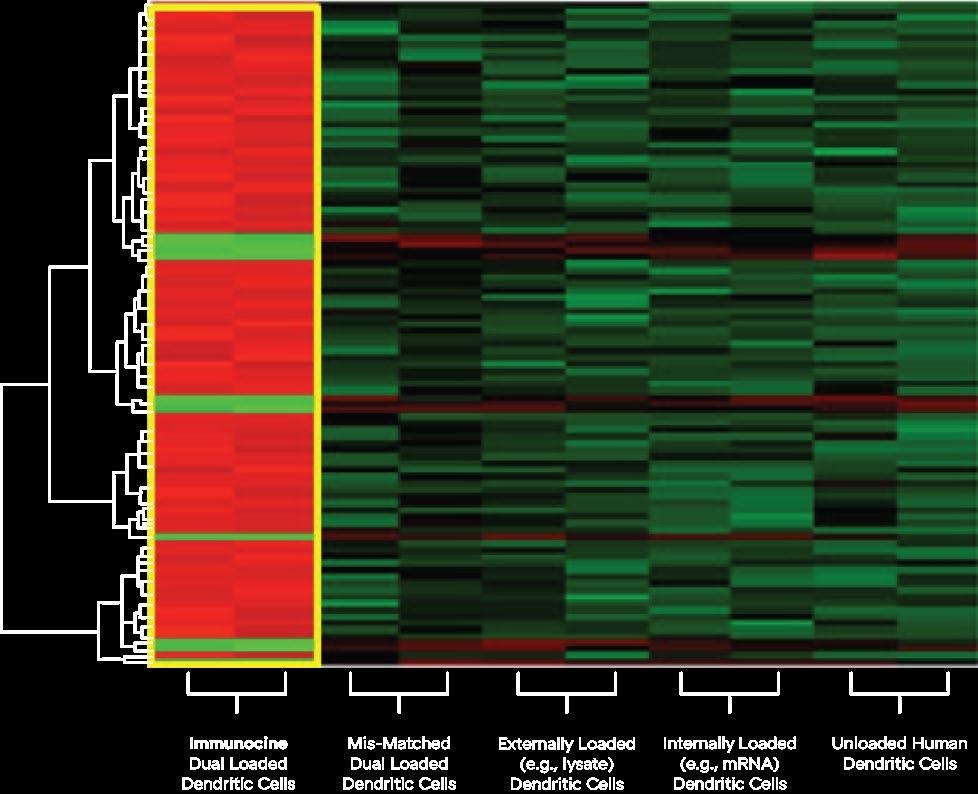

Dendritic Cells are quantitatively and qualitatively optimized for Th1 immune propagation only when both MHC Class I and II pathways are loaded with homologous antigens (a.k.a. Identical source material). This is represented by this heatmap of the top 100 most differentially expressed genes (1750 analyzed) of purified human Dendritic Cells loaded various ways, in duplicate, and equally matured.

Our cell therapy laboratory is one of the most advanced in Mexico. The lab equipment is equivalent to what is used at the Texas Medical Center for the clinical trials. And our state-of-the-art laboratory allows us to manufacture every personalized treatment to optimize patient safety and responses.


Since its discovery in 2006, the Immunocine Dendritic Cell Treatment (IDCT) Protocol has undergone continual research and clinical trials. It is a patented treatment and the first of its kind.

We focus our entire program on this specific Dendritic Cell immunotherapy protocol. This is the same protocol that has been studied extensively and seen significant successes in multiple research labs, oncological facilities, and clinical trials.
Across a wide range of tough-to-treat cancers, our patients have seen incredible compassion and results with our treatment despite often being previously deemed refractory or incurable by their physicians.

When Babette was diagnosed with metastatic growth near her heart, lungs and gall bladder, she faced an incredibly daunting battle for her life. After undergoing surgery to remove the tumors, the recommendation was to then do chemotherapy, which Babette had witnessed her best friend go through unsuccessfully just a year prior.
These are incredible real-world results backed by real science.

Brock’s cancer was spreading aggressively throughout his body. The debilitating side effects of his treatment and his progressing disease left him frail and with limited mobility. Upon completion of his treatment at Immunocine, Brock witnessed an astonishing improvement in his health. He regained his mobility, and most notably, his more than 10 tumors started disappearing.
When Al was diagnosed, his oncologist told him that the survival rate for this cancer is measured in months. He tried multiple types of chemotherapy and immunotherapy along with surgery to no measurable improvements. He then found the IDCT and upon completion of his treatment, quickly noticed an improvement in his overall health and his most recent CT scans show no signs of cancer.

This is the absolute best treatment in my research and experience.” “
She then learned about the Immunocine Dendritic Cell Treatment. She contacted Matt Halpert, Ph.D to learn more and then knew this was her path forward. Now, nearly five years following her terminal diagnosis, Babette is cancer free and credits the IDCT with defeating her cancer.

Relying on Lanreotide and diet changes, Kareema temporarily slowed her cancer’s progression. Unfortunately, her cancer progressed and her oncologist believed it was terminal. As a single mother, she refused to give up and found the IDCT. After treatment, she saw immediate success with her Chromagranin-A cancer markers falling from 7.6 nmol/L to 2.4nmol/L and a CT Scan detecting some of her tumors dissipating.

While also navigating the uncertainties of the pandemic, Justin searched for answers, leading him to undergo surgery. Despite what appeared to be a successful surgery, he had a recurrence, leading him on a journey that would challenge the limits of traditional cancer treatment. After receiving the IDCT, his cancer markers dropped drastically. He’s now thriving, having overcome a cancer with a five-year survival rate of less than 10%.
After a suspicious and steady rise in his Prostate-Specific Antigen (PSA) levels, Martin underwent a biopsy. The results were stunning with a Gleason Score of 9. As he researched his options, he was determined to find a non-pharmacological, non-surgical and non-radiation approach, leading him to Immunocine. Now, more than a year following treatment, his cancer is stable and confined.


Randy was diagnosed with Stage 4 colon cancer just two years after a clean colonoscopy. In January 2024, he underwent aggressive HIPEC surgery, which successfully removed visible disease, but odds remained steep. Hoping to prevent recurrence, he and his wife turned to Immunocine—after treatment, his follow-up scans have shown no evidence of cancer.

From flying through the air on dirt bikes to operating heavy machinery, Kevin has always thrived in high-risk environments. But in 2022, he faced his greatest challenge yet: Stage 2C Melanoma Cancer. Despite undergoing multiple surgeries and treatments in Toronto, Kevin’s condition stubbornly resurfaced, leaving him and his young family facing an uncertain future.
With everything on the line, Kevin and his wife discovered Immunocine and delved into the science behind the Immunocine Dendritic Cell Treatment (IDCT) before ultimately applying and being approved for treatment. In April 2023, Kevin embarked on the six-week treatment protocol at our center in Cancun. Just two months later, his oncologist in Toronto was astounded by the remarkable progress.
Today, Kevin is completely cancer-free, with multiple clean scans and biopsies to prove it. He credits the IDCT with not just saving his life, but also giving him a future to look forward to.
As a patient, you’re looking for the science behind it and proof that it works. Immunocine delivers that.” “
(6/12)
(4/4)
(3/3)
(2/3)
(1/1)
(13/18)
(9/16)
(5/12)
(2/5)
(3/4)
(3/3)
(2/3)
(1/1)
(8/11) (Avg: 19mo)
(8/8) (Avg: 20mo)
(5/8)
13mo)
(2/2) (Avg: 29mo)
(3/3) (Avg: 23mo)
(3/3)
14mo)
(2/2) (Avg: 22mo)
(1/1) (27mo)
(1/1)
(1/1)
(1/1)
(1/1)
(1/1)
(8/12)
(1/1)
(1/1)
(1/1) (12mo)
(1/1) (20mo)
(1/1) (12mo)
(1/1) (34mo)
Every Immunocine patient goes through three distinct phases of care, each tailored to their specific needs. From preparing your body, to activating your immune system with the IDCT, to supporting you after treatment, we design every step to maximize your potential to fight your cancer.
Most patients receive three separate treatments, each two weeks apart. During the treatment timeline, patients can either stay in Cancun for all six weeks or freely travel back and forth around their Dendritic Cell administrations.
Establish baseline metrics, develop a personalized treatment plan and optimize your immune system for the IDCT.
Assessment of current protocols and medical history
Baseline Measurements (e.g. scans, labs, immunological system)
Tissue procurement (e.g. biopsy) and white blood cell collection
(e.g. apheresis)
Personalized Dendritic Cell Vaccine
Manufacturing & Testing

The creation of your personalized treatment, followed by three injections, each two weeks apart. As well as, additional synergistic treatment modalities as determined beneficial.
Three Injections, Each Two Weeks Apart
Synergistic Treatment Modalities, If Applicable
Symptom Analysis & Management
Immunological Shift Measurements
We provide ongoing support as your immune system continues to fight and you return home.
Review of Scans and Blood Work
Telemedicine Follow Ups
Booster Treatments, if necessary and possible

While we are constantly expanding our capabilities, right now we only treat patients with solid tumors that can be biopsied. Blood malignancies, such as Leukemia, cannot be treated at this time.

For any immunotherapy to be successful, a patient needs a stable, functioning immune system. Patients with immune deficiencies and other conditions may not qualify for treatment until normal activity is restored.

Some medical conditions and other factors may prevent patients from traveling and our treatment is only administered at our facilities in Cancun. If you are unable to travel, you will not be able to receive treatment.

Our treatment protocol requires three separate treatments, each two weeks apart. Patients can either choose to stay in Cancun for six weeks or visit twice for two weeks at a time.

Impending organ failures would be a severe impediment to treatment response, thus proper functioning (e.g. liver, kidneys) is critical.

Due to safety protocols and equipment limitations, our lab is currently unable to process samples from individuals with active or recent infections of HIV, Hepatitis B, Hepatitis C, Syphilis, Chagas disease, Toxoplasmosis, or active Cytomegalovirus (CMV), or Epstein-Barr virus (EBV).
To qualify for treatment, patients must meet the requirements outlined here.


Until the IDCT receives regulatory approval, it is only available at our facilities in Mexico.

There are three hospitals located within close proximity to our lab. All three have state-of-the-art equipment, private rooms and are equipped to handle both emergency and routine care.

cGMP Practicing, ISO-Certified
In compliance with cGMP and ISO 7 (Class 10,000 Grade C) standards, our cell therapy lab uses positive pressure clean rooms for the creation of each personalized treatment. All processes in our clean room involve the use of non-reusable sterile materials to avoid contamination.
Health License Registration Immunocine operates under the following health licenses issued by the Mexican Government: 18-TR-23-005-0005, 18-TR-23-005-0003, 18-TR-23-005-0004.
Our team of specialists have a vast array of experience in Cancer, Immunology and Cell Therapy. With a complete Medical Staff, Cell Therapy Team and Patient Support, we work seamlessly to provide the best patient experience possible.



Matthew Halpert, Ph.D
CEO
After earning his doctorate in Microbiology and Immunology, Dr. Halpert spent 10 years at Baylor College of Medicine in Houston as a Cancer Immunologist. While at BCM, he founded Diakonos Oncology, which is currently studying its revolutionary Dendritic Cell Treatment in multiple FDA clinical trials. In 2021, he left Baylor College of Medicine to found the Immunocine Cancer Center, while continuing his work at Diakonos Oncology.
Dr. Stephen Noga is a leading expert in immunotherapy for solid tumors, combining decades of clinical, academic, and leadership experience in oncology. With an M.D. from Johns Hopkins and a Ph.D. in experimental pathology, he’s helped shape the field through groundbreaking research, over 200 publications, and key roles in major cancer organizations. As a consultant, he brings deep scientific insight and a relentless commitment to advancing innovative cancer treatments.
Luis Ferbeyre, MD
Chief Medical Officer, Oncologist
Dr. Ferbeyre received his Medical Degree from the Medical University of Havana. After receiving his MD, Dr. Ferbeyre pursued Oncology with his thesis focused on Mixed Tumors within the Salivary Glands. Dr. Ferbeyre has published additional research on the genetic and molecular basis of cancer with 31 published peer-reviewed articles and has participated in multiple clinical trials.




Director of Cell Therapy
Susana received her Undergraduate Degree in Pharmaceutical Science and her Master’s Degree in Clinical Biomedicine from the University of the Americas Puebla (UDLAP). She received an additional Master’s Degree in Bioethics from Anahuac University (Cancun). For the last 10 years, Susana has taught Molecular Biology, Genomics and Cell Physiology at Anahuac University (Cancun) and the University of Quintana Roo.
Interventional Radiologist
Dr. Viramontes received his Medical Degree from the National Autonomous University of Mexico (UNAM). With a focus on Radiology, he received his Board Certification from the Mexican Council of Radiology and Imaging. He has published 11 peerreviewed papers regarding Radiology and Cancer. In addition, he is a professor at the Medical School of Anahuac (Cancun).
Medical
Dr. Angulo earned her degree at Universidad Autónoma de Ciudad Juarez in Chihuahua, Mexico. She has been practicing emergency, critical and primary care in the Cancun area for years. She’s committed to improving the standard doctorpatient relationship by gaining a more thorough understanding of each and every one of her patients. She operates on the premise that a patient is not their cancer, but so much more.
Dr. Antonio López Rivera is a passionate advocate for wholepatient oncology, blending clinical expertise with a deep commitment to personalized, science-driven care. With a background in emergency medicine and global health, he brings a meticulous, patient-first approach to treatment evaluation. At Immunocine, he helps bridge innovation and compassion to advance the future of precision cancer care.
The entire cost of the Immunocine Dendritic Cell Treatment (IDCT) protocol includes everything related to the medical treatment and intra-Cancun transportation. Each treatment is personalized to every patient and takes roughly a week to develop in our cGMP laboratory. Without cutting any corners, our team has optimized the protocol to make it as efficient as possible.
A variety of cancers are amenable to this immunotherapy protocol, even if previously told “Immunotherapy will not work for them.”
Objective and Targeted Immunological Responses are seen in approximately 90% of patients. Top side effects include fatigue, swollen lymph nodes, low grade fever, tenderness, and tumor associated pain and sensation. Examples of cancers that have responded to the level of NED – melanoma, bladder, renal, tonsillar, squamous cell carcinoma, pancreatic, colorectal, prostate, head and neck, ovarian, breast. Examples of cancer that have responded to the level of stable (>1year) – neuroendocrine, triple negative breast, ependymoma, chordoma, desmoplastic small-round-cell, peritoneal carcinomatosis. And the list continues to grow!
Approximate cost is $120,000 USD, and includes all activities within the purview of this medical treatment. See below.
Travel and lodging are not included.
Medical Personnel
Oncologist
Radiologist
General
Internist
Hematologist
Angiologist
Phlebotomists
Scientific Personnel
Scientists
Cell Therapy
Biologists
Cellular Analytics Expert
Cancer Immunotherapist
Procedures
Nurses US-Guided Injections of personalized vaccine
Facilities
cGMP ISO-7 Laboratory
Procedure Rooms
Private, Full-Service Hospital
Imaging Centers
Clinics
Support Team
Ongoing and Remote
Medical Support Team
Ex: Integrative Oncologist, Functional Medicine Consultant, Nurse Navigator
Continued Data Review
Coordination With Home Medical*
Scans and Imaging
Biopses
Pathology
Central Line
Aphaeresis
Blood Draws
Microbubbling of Tumor*
Overnight Hospital Stay
Data Analysis
Radiology Reports
Pathology Reports
Dendritic Cell Qualification
Immune System Analysis
Blood Lab work
Data Reports
Patient Concierges
Transportation Advisory Board
Vaccine Development Coordination
Reagents
Centrifuges
Incubators
Tissue Hoods
Media and Cytokines
QC Analysis
mRNA Isolation
mRNA Amplification
Lysate Preparation
Sterility
Miscroscopy
Flow Cytometry
Storage of Boost
Dendritic Cells
Etc.
Medications
Neupogen
Pegylated IFNα
Symptom Management
We partner with physicians regularly to provide the Immunocine Dendritic Cell Treatment protocol to their cancer patients.


If your patient meets the minimum requirements and is interested in seeing how our treatment could work for their specific case, please submit their information on our submission form or call us directly at +1 (888) 575-2572.

Our team loves discussing our treatment with potential patients. We will schedule a call to learn about their diagnosis and answer initial questions. Prior to the call, we’ll share additional info for them to review with their physician.

After confirming their eligibility, we will perform a complimentary medical evaluation. This includes a questionnaire and review of their medical records. Once complete, they can be approved for treatment.



