14 Late-breaking data

12
16 IVC stenting US FDA approves Gore stent following positive data 3

14 Late-breaking data

12
16 IVC stenting US FDA approves Gore stent following positive data 3

trial shows reduced stroke risk with carotid stenting versus medical therapy alone
The US National Institutes of Health (NIH)-funded CREST-2 study has found that, for people with high-grade asymptomatic carotid artery stenosis who have not experienced recent stroke symptoms, a carotid artery stenting (CAS) procedure—combined with intensive medical therapy—significantly lowered stroke and death rates compared with medical therapy alone. However, the more traditional “gold standard” approach of carotid endarterectomy (CEA) did not show the same benefit.
These first “game-changing” results outlining four-year outcomes were presented at the 2025 VEITHsymposium (18–22 November, New York, USA) by CREST-2 co-principal investigator (PI) Brajesh K Lal (University of Maryland, Baltimore, USA). Earlier the same day, the data were delivered at the 2025 Society of Vascular and Interventional Neurology (SVIN) annual meeting (19–22 November, Orlando, USA) by James Meschia (Mayo Clinic, Jacksonville, USA). The trial results were also published in the New England Journal of Medicine
Lal outlined how the study’s two simultaneously running randomised controlled trials (RCTs) comparing CAS plus medical therapy to medical management alone, and CEA plus medical therapy to medical management alone, enrolled 2,485 patients from 155 sites across five countries.
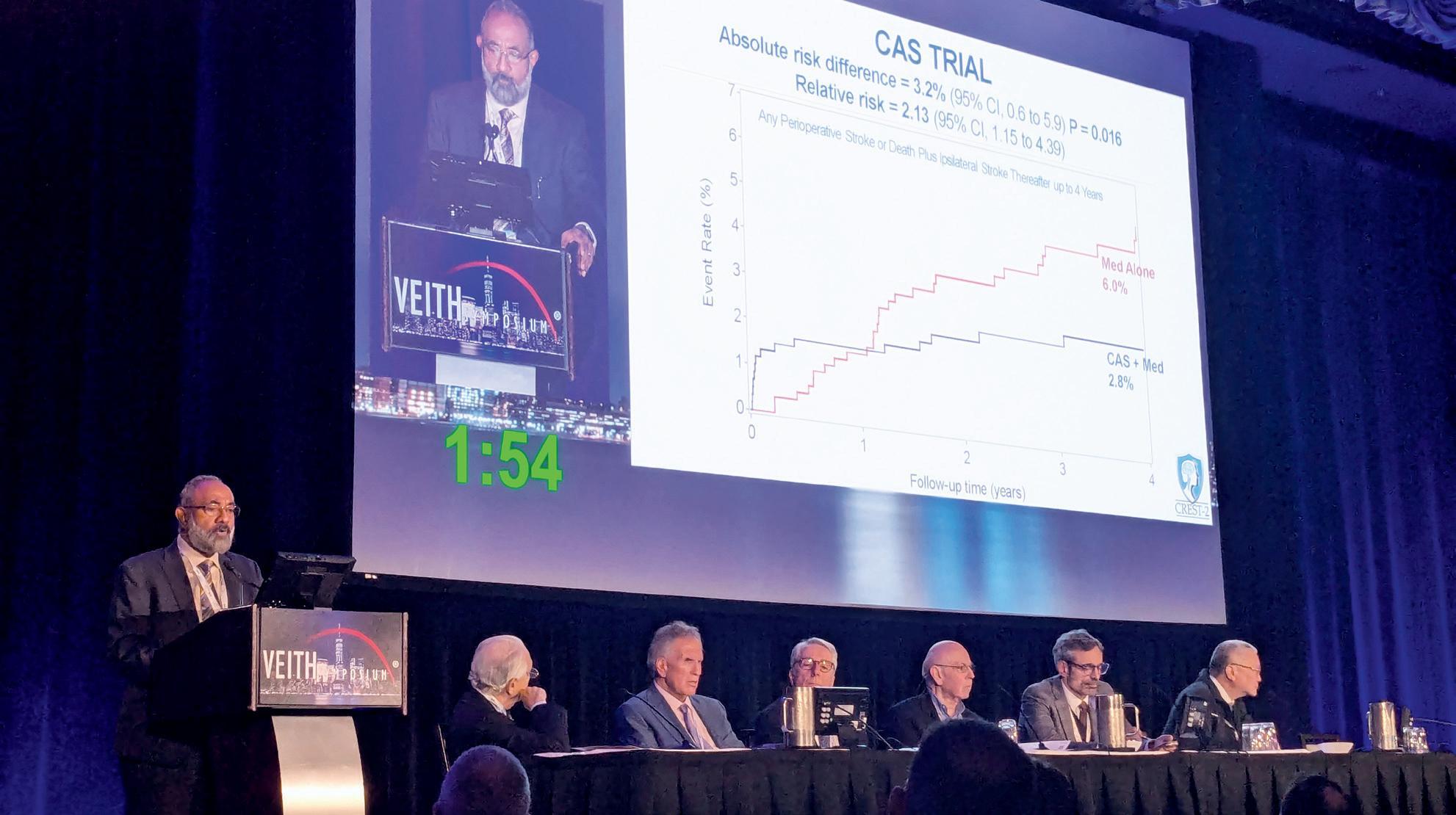
In the CAS trial, stroke and death rates out to four years were 6% in patients who were assigned medical management and 2.8% when CAS was added, Lal told VEITH 2025. “The absolute risk difference of 3.2% in favour of CAS was statistically significant,” he said.
In the CEA trial, stroke and death out to four years was 5.3% in the medical therapy group and 3.7% when CEA was added, Lal continued. “The absolute risk difference was still in favour of CEA; however, it did not reach significance.”
Lal explained: “The patterns of differences in the CAS and CEA trials were mirrored in the 44-day periprocedural period; however, in the post-procedural period starting 45 days out to four years, both CAS and CEA performed better in terms of preventing stroke and death compared to their respective medical management groups.”
Concluding, Lal emphasised how the absolute difference favouring CAS was significant, noting “31 people with highgrade asymptomatic carotid stenosis needed to be treated to prevent a primary event at four years in the trial”.
Thomas Brott (Mayo Clinic College of Medicine, Jacksonville, USA), co-PI, followed Lal at the VEITH 2025 podium to tackle whether the CREST-2 evidence is conclusive
In one generation, since ACAS, we’ve gone from medical risk of 11% to a risk of 6%, which is remarkable.”
Thomas Brott

STORM-PE finds mechanical thrombectomy superior to anticoagulation
THE USE OF MECHANICAL thrombectomy, specifically computerassisted vacuum thrombectomy (CAVT) using the 16Fr Lightning Flash system (Penumbra), with anticoagulation achieves superior reduction in right heart strain compared to anticoagulation therapy alone in patients with acute intermediate-high-risk pulmonary embolism (PE). This is according to data recently published in Circulation from the STORM-PE randomised controlled trial (RCT).
“These findings mark a pivotal step in advancing care for PE, providing the strongest evidence to date that advanced therapy with CAVT can rapidly and safely improve recovery of the right heart compared to conventional anticoagulation therapy,” said co-global principal investigator (PI) Robert Lookstein (Icahn School of Medicine at Mount Sinai, New York, USA) in a press release announcing the results. Lookstein had presented the data for the first time at the 2025 Transcatheter Cardiovascular Therapeutics (TCT) conference (25–28 October, San Francisco, USA).
“STORM-PE supports the role of CAVT as a more effective therapeutic option for intermediate-high-risk patients and will evolve the paradigm of care by delivering rapid relief with a comparable safety profile to anticoagulation alone,” Lookstein continued.
The trial enrolled 100 patients across 22 international sites. Patients treated with CAVT demonstrated a greater reduction in right-to-left ventricular (RV/LV) diameter ratio within 48 hours (mean reduction 0.52 vs. 0.24; p<0.001) and nearly 80% of patients had positive treatment effect with CAVT, which was significantly greater than the patients who
Continued on page 5
The last few months of 2025 saw the presentation of two trials that provide welcome answers to some of the most pressing questions in the management of asymptomatic carotid disease and pulmonary embolism (PE), respectively. We here share our initial thoughts on what is now known, and what data are still to be sought, following the publication of CREST-2 and STORM-PE, both of which feature on the cover of this first issue of Vascular News for 2026.
Ross Milner on CREST-2
We have waited for years for the final answer to how we should treat carotid artery bifurcation disease. We now have the answers following the publication of CREST-2. Or do we?
Randomised clinical trials are perceived as our best source of evidence for decision making for medical therapy and invasive procedures. It is important to remember that CREST-2 is obviously a randomised trial, but it is not a direct comparison of carotid endarterectomy (CEA) and stenting. And it was the best medical management that has ever been offered to a patient with carotid artery bifurcation disease. There are many more important issues that limit the applicability of the trial results to a patient that is sitting in your office with a high-grade carotid stenosis. Anatomic limitations such as thrombus, calcification, and location of the bifurcation are not part of the randomisation scheme and clearly impact our decisions when speaking to patients. Where do we go from here now that CREST-2 is complete and being questioned? I do not think we have the definitive answers that we wanted from this study. I do think that carotid bifurcation disease is a team sport. I am fortunate that I have been trained to medically manage, operate, and stent. I know that this is not true for everyone as individuals. It is true for most teams though. We need to avoid specialty-specific bias for the procedures we are trained to do and offer our patients the most suitable procedure for them. The CREST-2 data make me question the value of medical management alone as most patients will never have the resources provided in the trial. Therefore, intervention is still a viable option. In my opinion, instead of focusing too much on CREST-2 results alone, we should provide the most reasonable intervention for a given patient that
includes a complete evaluation of comorbid risks and anatomic findings seen on cross-sectional imaging.
Bart
Dolmatch on STORM-PE
The clouds surrounding whether or not to use mechanical thrombectomy to treat acute intermediatehigh-risk PE are starting to clear based on results from the STORM-PE trial. While it has been known for decades that most of these patients will improve with anticoagulation, catheters that remove or lyse PEs are now being widely used in conjunction with anticoagulation. Does it make sense to do this? Previous studies have shown that removal or lysis of PEs provides early benefit for patients with acute intermediate-high-risk PE, including relief of right ventricular dilation and strain, decreased pulmonary artery pressures, and improvement of tachycardia, tachypnoea, oxygen requirements, and reduced duration of intensive care unit (ICU) stay (and even avoidance of ICU stay). Improvement of haemodynamic and respiratory compromise can reduce the likelihood of clinical deterioration and may reduce mortality. But can PE thrombectomy afford benefit beyond the acute phase?
STORM-PE randomised acute intermediate-highrisk PE patients between anticoagulation, alone, and mechanical thrombectomy with anticoagulation. Both the acute benefits of pulmonary thrombectomy and subsequent benefits were demonstrated. Mechanical thrombectomy plus anticoagulation was as safe as anticoagulation, with no statistical difference in major adverse events (but with numerically lower major adverse events in the thrombectomy group).
While there are other ongoing PE-related trials that may provide more guidance regarding treatment of PE using mechanical thrombectomy, the STORM-PE trial is the first randomised, prospective trial that demonstrates significantly better acute and sustained benefit using mechanical thrombectomy with anticoagulation to treat acute intermediate-high-risk PE patients when compared to anticoagulation alone.


ROSS MILNER is chief of the Section of Vascular Surgery and Endovascular Therapy at University of Chicago Medicine (Chicago, USA).
BART DOLMATCH is an interventional radiologist at The Palo Alto Medical Foundation (Palo Alto, USA).

US editorial board: Ross Milner, Erin Murphy, Eric Secemsky and Bart Dolmatch
EU editorial board: Ian Loftus, Rob Morgan, Stephen Black and Nicholas Inston
Publisher: Stephen Greenhalgh | Editor: Jocelyn Hudson Jocelyn@bibamedical.com
Content solutions manager: Bryan Kay | Editorial contribution: Jamie Bell, Will Date and Éva Malpass
Design: Terry Hawes and David Reekie | Advertising: Nathalie Fortin Nathalie@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
Published by: BIBA News, which is a subsidiary of BIBA Medical Ltd BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788 BIBA Medical, North America, 155 North Wacker Drive, Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323
Printed by: Buxton Press. Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2026. All rights reserved.
n NEW GUIDELINES:

The European Society for Vascular Surgery (ESVS) has released updated clinical practice guidelines for the care of patients with descending thoracic and thoracoabdominal aortic pathologies. The document, which is currently available as an article in press in the society’s dedicated journal, provides a “comprehensive and fully updated revision” of recommendations published by the ESVS in 2017.
For more on this story go to page 4.
n DIALYSIS
ACCESS:
The interim results of a first-inhuman trial of a sirolimus-eluting covered stent graft—Solaris DE (Solaris Endovascular)—for the treatment of dialysis access dysfunction demonstrate the promise of the device in treating vascular access outflow stenosis, an investigator in the trial has commented. Results of the DEScover trial were presented at the 2025 Transcatheter Cardiovascular Therapeutics (TCT) meeting (25–28 October, San Francisco, USA).
For more on this story go to page 8.
n DIABETIC FOOT ULCER:
A US National Institutes of Health (NIH)-funded randomised controlled trial is due to commence this month (January) to assess the efficacy of continuous digital monitoring in reducing diabetic foot ulcer recurrence among high-risk patients. Led by Caitlin Hicks and colleagues at the Johns Hopkins University School of Medicine (Baltimore, USA), the WIREDUP study aims to enrol 400 patients with a diabetic foot ulcer that has healed within the past 12 months.

For more on this story go to page 15.
Scan the QR code to subscribe
If you have comments on this issue or suggestions for upcoming editions write to jocelyn@bibamedical.com
COVER STORY continued
Continued from page 1
or further study is needed.
“In one generation, since ACAS, we’ve gone from medical risk of 11% to a risk of 6%, which is remarkable, particularly in light [of the fact] that today our surveillance is via MRI [magnetic resonance imaging] in almost all instances,” he said.
“The trial shows CAS is effective,” Brott continued, with its stroke and death rate “one half of medicine alone”. As for CEA, he said, “there is a difference, but it did not reach statistical significance”.
So, Brott asked, are future studies needed?
“Yes of course,” he said, going on to list several areas in need of scrutiny, among them carotid plaque risk, patients with higher-risk features, carotid stent optimal design, flow reversal as well as the optimal regimen of medical therapy.
“The elephant in the room in one of these areas of further study is TCAR [transcarotid artery revascularisation]: no level-one evidence. But a randomised controlled trial showing a drop in risk that you would consider clinically significant over four years would require a sample size of 4,400 patients,” he added.
“Discoveries will happen, but rock-solid validation may not be feasible via RCTs in patients with asymptomatic carotid disease.”
POINT OF VIEW
Analysis: Medical management, trial design and plaque imaging in focus
For site PI Caitlin Hicks (Johns Hopkins University School of Medicine, Baltimore, USA), CREST-2 defied expectations, with strides in medical management over the past decade not borne out in the data. Hicks’ surprise was elevated by the fact that the medical management in CREST-2 was “pretty intense”. “Blood pressure targets were aggressive, LDL [low-density lipoprotein] targets were probably more aggressive than in real practice, and the patients even had a coach,” she tells Vascular News. “I really thought I was going to be told that, from now on, we need to start preaching medical management, except for in extreme circumstances, and that didn’t happen.”
Gustavo Oderich (Baylor College of Medicine, Houston, USA), meanwhile, remarks on the trial design. “One has to look at the results of the study in the context that carotid stenting was not compared to endarterectomy,” he comments to Vascular News “The study was basically two concurrent RCTs comparing different types of interventions—CEA or CAS—to medical therapy. No one can say that carotid stenting is superior, or inferior for that matter, to endarterectomy. Therefore, centres participating in both trials had a selection bias into which patients investigators perceived ideally suited for stenting or CEA. Conclusions that CAS was superior to CEA based on this trial are incorrect. You would have had to randomise in the same arm.”
According to Peter Schneider (University of California San Francisco, San Francisco, USA), the results of CREST-2 that have been published thus far do not answer what he dubs the “big unknown” in carotid disease, namely how medical management works when used only in patients with critical lesions. “We have lots and lots of data showing that
SWHSI-2: Negative pressure “not the one-size-fits-all treatment it had been perceived to be”

Keith Jones
Vascular Society of Great Britain and Ireland (VSGBI) president Keith Jones (Camberley, UK) shares his thoughts on the SWHSI-2 trial results (see Vascular News 108, page 20).
THE SWHSI-2 TRIAL RESULTS looking at surgical wound healing by secondary intention (SWHSI) in the UK were first presented by Ian Chetter (Hull York Medical School, University of Hull, Hull, UK) at the 2024 VSGBI annual scientific meeting (VSASM; 27–29 November, Brighton, UK). I, sat in the audience that day, was very impressed by the strength of the trial message but also very disappointed in the outcomes for these wounds that the trial highlights. The trial, which was published in The Lancet last summer, has been presented at other events, and it is interesting to see how it has been received.1 The authors explain that the majority of the 686 participants come
from within vascular surgery and that the majority have diabetes and so it became a vascular surgery diabetic foot wound trial. Why this is interesting to me is that I chaired a debate at a multidisciplinary diabetic foot meeting between Chetter and a diabetologist who runs a foot clinic with the title ‘Negative pressure wound therapy does not add value in the management of diabetic foot wounds’.
As both chair and as a vascular surgeon with a significant interest in the management of vascular diabetic foot disease I expected only one outcome. As the results of this multicentre randomised controlled trial were presented, I felt the audience would be impressed by the quality of the trial itself with large
medical management can cause stabilisation of plaque and sometimes even regression of plaque, but we don’t have any data on patients that already have a critical, bulky, quite well-developed atherosclerotic lesion of the carotid bifurcation,” he notes. “That’s the part that’s lacking, since many of the patients had lesions that would not have otherwise been severe enough to quality for repair outside of a clinical trial. And although the medical management arms of [CREST-2] were valiant efforts to look at that, there were many patients in there who I believe probably were not in that range of the critical bulky carotid bifurcation plaque.”
Schneider highlights the fact that further data from CREST-2 are forthcoming, which he believes will show “that there were a lot of lesions that were more moderate rather than critical”.
For Schneider, beyond CREST-2 having shown a benefit for intervention despite medical management delivered with an “aggressiveness” that would be near impossible to replicate in the real world, the “broader, more important benefit” of CREST-2 is “the idea that actually carotid stenosis is important, it’s not negligible”.
He elaborates: “We should look for it and, when we find it, the most likely outcome should be to institute best medical management, as aggressively as we can, with the highest quality we can deliver. And, ultimately, that’s how CREST-2 is going to save lives.”
Looking ahead, Schneider envisions a future defined by more individualised carotid treatment. “If we could personalise the treatment to the patient’s problem a little bit better, this would really enhance the whole system,” he says, suggesting that imaging-based plaque analysis “may be the missing piece, the holy grail, if it immediately directs us toward the ability to assess the risk of the plaque”.
patient numbers screened (1,895) and recruited (686), and the maintenance of those numbers through the 12 months of follow-up. I expected disappointment in reflection upon the poor outcomes of this wound cohort, with 42% unhealed at 12 months, 10% having amputations and 10% dying, which—as shocking as these outcomes are—I feel is a highlight for the trial since it clearly shows we must do better in the management of these wounds, potentially by improving the quality/extent of the debridement. Yet despite the clarity of the trial results, showing that negative wound pressure did not aid or speed up wound healing, the diabetic foot meeting audience still felt that negative pressure wound therapy (NPWT) added value to the management of diabetic foot wounds. This was a surprise since the results of the trial are clear on cost-effectiveness and very
I do expect negative pressure budgets to be cut, based on the trial evidence, but I do hope there is a next stage, where the research shows what does work.”
clearly imply that the findings do not support the use of NPWT to augment SWHSI healing. However, that vote did highlight the challenge of stopping the use of negative pressure, which we have utilised for more than 20 years, without evidenced protocols.
I would hope, however, that all who are interested in healing vascular diabetic foot wounds take the time to read the publication and, on reflecting on the results, see that we must get better at healing this wound group, whether that relates to the technique and quality of debridement, the enhancement of vascularity or the topical adjuncts used. What I see as a real strength of the trial is that it highlights the need for ongoing research in this area, to show how we enhance the wound healing of this group, because negative pressure is not the one-size-fits-all treatment it had been perceived to be.
I do expect negative pressure budgets to be cut, based on the trial evidence, but I do hope there is a next stage, where the research shows what does work. But for the moment I would congratulate the authors on an excellent trial and its challenging results.
References: 1. Arundel C, Mandelfield L, Fairhurst C, et al. Negative pressure wound therapy versus usual care in patients with surgical wound healing by secondary intention in the UK (SWHSI-2): an open-label, multicentre, parallelgroup, randomised controlled trial. The Lancet. 2025; 405(10490): 1689–1699.
Keith G Jones is a consultant vascular surgeon at Frimley Health NHS Foundation Trust in Camberley, UK.
The European Society for Vascular Surgery (ESVS) has released updated clinical practice guidelines for the care of patients with descending thoracic and thoracoabdominal aortic pathologies. The document, which is currently available as an article in press in the society’s dedicated journal, provides a “comprehensive and fully updated revision” of recommendations published by the ESVS in 2017.
AUTHORS ANDERS WANHAINEN (UPPSALA University, Uppsala, Sweden and Umeå University,
Umeå, Sweden) and colleagues write in the European Journal of Vascular and Endovascular Surgery (EJVES) that they have issued 129 recommendations, including 42 graded Class I, across several key topics: acute thoracic aortic syndrome, chronic type B aortic dissection, descending thoracic and thoracoabdominal aortic aneurysms, ruptured descending thoracic and thoracoabdominal aortic aneurysms, and blunt aortic injury.

Anders
Wanhainen and colleagues note that every section of the 2017 guidelines has been either revised or rewritten “to reflect the rapid technological advances in the field over the past decade”.
The authors write that, as a whole, the 2026 guidelines emphasise an endovascular-first paradigm wherever anatomy permits, reflecting the central role of fenestrated and branched techniques in contemporary practice. They also underline the importance of multidisciplinary care, promoting centralised treatment in high-volume aortic centres and structured team-based decision making.
Furthermore, the authors note that quality assurance is reinforced through recommendations on registry participation, standardised outcome reporting, and continuous quality improvement.
An international panel of experts has released a series of consensus statements on the management and follow-up of acute type B intramural haematoma (IMH) and penetrating aortic ulcer (PAU), with findings recently published as an article in press in the European Journal of Vascular and Endovascular Surgery (EJVES).
KEY FINDINGS INCLUDE A clearer definition of complications, the weakened significance of certain morphological criteria as sole indicators for thoracic endovascular aortic repair (TEVAR), and the importance of strict imaging follow-up in the early period to assess for highrisk features. In addition, the panel provides specific recommendations regarding the planning and technical aspects of TEVAR.
In order to develop the consensus document, a team of researchers employed a modified Delphi consensus process involving multiple rounds of anonymous questionnaires. The team—consisting of principal investigator Michele Piazza, first author Francesco Squizzato (both University of Padua, Padua, Italy), and three external facilitators including Mario D’Oria (University Hospital of Cattinara, Trieste, Italy)—sent surveys to international experts practicing in high-volume aortic centres worldwide.
Piazza, Squizzato and colleagues specify in their EJVES paper that a series of statements—prompted and refined from currently available guidelines or the best available evidence on the standard of care— were voted on using a four-point Linkert scale in a three-round Delphi
process. Statements achieving grade A (full agreement 75%) or B (overall agreement 80%, full disagreement <5%) were included as expert recommendations.
A total of 83 experts from across Europe, North America, Latin America, Asia, and Oceania were included in the final analysis.
The authors state that 25 statements achieved a consensus, with 18 (72%) receiving a grade B strength and seven (28%) a grade A strength. They note that most statements (97%) had a high consistency classified as grade I or II.
In EJVES, the panel of international experts summarise that they agreed on the indication for TEVAR for complicated IMH and PAU, defined by rupture or refractory pain/hypertension. They add that uncomplicated IMH and PAU should be managed conservatively and followed with serial computed tomography (CT) imaging during the acute phase.
Furthermore, the experts agreed that high-risk uncomplicated IMH is identified by increased haematoma thickness, new onset or increased size of ulcer-like projections, or transition to aortic dissection, while high-risk uncomplicated PAU is defined by new associated haematoma, PAU width/
A greater focus is placed on patientcentred care, Wanhainen and colleagues add, with an emphasis on incorporating patient perspectives, shared decision making, and tailoring interventions to life expectancy and functional status.
The document closes with a section on unresolved issues regarding the management of thoracic and thoracoabdominal aortic diseases, with the authors highlighting a lack of high-quality evidence in the field as a “fundamental challenge”.
“The field urgently awaits data from well-designed prospective registries and RCTs [randomised controlled trials],” Wanhainen and colleagues write, pointing out that several such initiatives are already underway.
The authors continue: “In the meantime, the 2026 guidelines aim to mitigate this uncertainty by emphasising individualised, multidisciplinary care in experienced high-volume aortic centres, supported by structured SDM [shared decision making] with patients.” The authors also encourage active participation in national and international registries “to expand the collective evidence base and improve future guideline development”.
depth increase, or total aortic diameter increase.
The authors continue that the expert panel agreed uncomplicated high-risk IMH and PAU may be considered for TEVAR. In performing TEVAR, they add, a proximal sealing length >20mm in a site free from haematoma should be achieved, eventually extending in zone 2, with a 0–10% oversize, and that patency of the left subclavian artery should be maintained.
In the discussion of their findings, the authors stress that currently available guidelines on the management of IMH and PAU are characterised by a low level of evidence and lack of consistency on important clinical aspects, such as definitions of complications, characterisation of high-risk features, indications for TEVAR, and technical aspects of the surgical treatment.
While the authors state that the international expert panel achieved a consensus on several of these unsolved topics, they underscore a lack of consensus on the timing of
Regardless of the timing of TEVAR, the expert panel were in agreement that a postoperative CT angiogram should be obtained within three to seven days to assess for technical success and potential complications, such as dissection at the level of the proximal or distal landing site.
The panel of experts advise that certain limitations of the present study should be taken into account when interpreting the results. “Delphi studies reflect the opinions and practice patterns of selected experts and cannot be considered as a substitute for traditional scientific literature or guidelines,” they state.
Furthermore, the authors emphasise that different opinions and lack of agreement “do not necessarily reflect an incorrect practice, but may be the result of different available options, institutional setting, local regulations, and geographical habits, in topics where there is a low level of evidence”.
Overall, the authors conclude that the resulting consensus statements
While it is well established that complicated patients should undergo urgent/emergent TEVAR, the situation is more nuanced in uncomplicated, high-risk IMH/PAU.”
endovascular treatment. “While it is well established that complicated patients should undergo urgent/ emergent TEVAR,” the authors write, “the situation is more nuanced in uncomplicated, high-risk IMH/PAU.”
They note that clinical guidelines do not mention the suggested timing of intervention in this clinical setting.
from the Delphi process offer “valuable insights” for current clinical practice and “may be considered for updated guidelines”.
Closing their paper, Piazza, Squizzato and colleagues write that additional research is warranted to further investigate the topics of recommendations.
Continued from page 1
received anticoagulation alone (78.3% vs. 51.9%; p=0.011), reflecting rapid haemodynamic recovery.
“What’s particularly compelling is that a significantly greater portion of patients treated with CAVT achieved normalisation of RV/LV ratio within 48 hours—a critical indicator of right heart recovery—without an increase in complications,” added Rachel Rosovsky (Massachusetts General Hospital and Harvard Medical School, Boston, USA), co-global PI. “These findings represent a meaningful advancement in optimising early interventions in patients with intermediate-highrisk PE, and we look forward to exploring how changes in RV/LV ratio correlate with other clinical and functional outcomes.”
The rate of major adverse events (MAE) within seven days—including a composite of PE-related mortality, recurrent PE, clinical deterioration requiring rescue therapy, and major bleeding—was comparable between groups (4.3% [2/47] with CAVT vs. 7.5% [4/53] with anticoagulation alone).
in pulmonary artery obstruction when compared to anticoagulation alone.
“The latest STORM-PE findings demonstrate substantial improvements in both clinical and functional outcomes, with a significantly higher proportion of patients treated with CAVT plus anticoagulation returning to normalisation within 48 hours compared to anticoagulation alone—a remarkable result,” said Rosovsky. “STORM-PE highlights the critical importance of early intervention in patients with intermediate-high-risk PE and provides the strongest evidence to date that advanced therapy with CAVT offers superior efficacy compared to the current standard treatment of anticoagulation alone. These breakthrough results not only advance the field but also have the potential to meaningfully improve patient care and recovery.”


“For the first time, we have prospective, levelone evidence demonstrating that CAVT with anticoagulation is superior to anticoagulation alone,” said James F Benenati, chief medical officer (CMO) at Penumbra. “Combined with Penumbra’s strong prospective data from STRIKE-PE, this randomised evidence from the STORM-PE trial will play a critical role in advancing PE care and supporting the inclusion of CAVT in future treatment guidelines.”
The most recent data from the STORM-PE RCT were shared during a late-breaking clinical trials session at the 2025 Vascular Interventional Advances (VIVA) conference (2–5 November, Las Vegas, USA).
Among the findings, presented by Rosovsky, were that CAVT demonstrated functional assessment improvements, earlier physiological recovery and greater reduction
In the trial, patients treated with CAVT plus anticoagulation experienced significantly lowered thrombus burden at 48 hours, with a 2.7 times larger reduction in refined modified Miller score (42.1% vs. 15.6% relative reduction; p<0.001), Rosovsky reported. They also saw early physiological recovery with significantly lower heart rate (80.0bpm vs. 86.4bpm; p=0.022) and less tachycardia (heart rate >100, 2.2% vs. 20%; p=0.008), reduced supplemental oxygen requirements (0.5L/min vs. 1.4L/min; p=0.027), and a lower National Early Warning Score 2 (NEWS2) risk of clinical deterioration (1.8 vs. 2.7; p=0.034) at 48 hours. CAVT-treated patients experienced 2.2 times greater likelihood of progressing towards recovery of functional status, based on post-venous thromboembolism functional status (PVFS) from pre-PE event to discharge (p=0.032); as well as significantly longer distance walked during the 90-day six-minute walk test (472m vs. 376m; p=0.019). Additionally, Rosovsky said, CAVT patients near normalised by 90 days, walking 94% of their predicted walk distance versus 75% among the patents treated with anticoagulation only (p=0.022). Safety rates through 90 days were comparable, she added. “STORM-PE is the first RCT to report the results of mechanical thrombectomy with anticoagulation versus anticoagulation alone. The CAVT arm demonstrated superior reduction in RV dilatation and a similar safety profile to the anticoagulation arm,” Rosovsky told VIVA 2025. “These promising results reinforce the role of mechanical thrombectomy, specifically CAVT, as an effective treatment strategy in patients with acute intermediate-high-risk PE.”
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has outlined moves to deepen collaboration with the USA on medical technology regulation, with initiatives to accelerate innovation, strengthen patient safety, and reduce transatlantic barriers to market access.
SPEAKING AT THE 2025 ADVANCED MEDICAL Technology Association (AdvaMed) conference (5–8 October, San Diego, USA), MHRA chief executive Lawrence Tallon highlighted the agency’s commitment to advancing global regulatory harmonisation and its strong partnership with the US Food and Drug Administration (FDA) during his fireside chat with Michelle Tarver, director of the FDA Center for Devices and Radiological Health (CDRH).
Tallon says: “We continue to work in close collaboration, and are taking steps forward in the relationship between FDA and MHRA to strengthen regulatory alignment and reciprocity. We share an ambition to accelerate joint initiatives, enhance policy development, and identify and work
together on strategic opportunities more effectively.”
Tallon also emphasises that the UK’s medtech regulatory reforms will support earlier and safer patient access to innovative technologies, drawing parallels with the FDA’s Total Product Life Cycle Advisory Program (TAP) with opportunities for deeper transatlantic collaboration.
“The US and UK share a common goal— ensuring patients benefit quickly and safely from the latest medical innovations. With US-based thought leadership inputting to our new National AI Commission, and new reliance frameworks for FDA approvals, we are laying the foundations for a truly global, innovation-ready regulatory environment,” adds Tallon.
At VIVA 2025, John Moriarty (UCLA Health, Los Angeles, USA) delivered one-year functional and quality-of-life (QoL) outcomes from the STRIKE-PE study, a singlearm, prospective, multicentre, ongoing trial looking at computer-assisted vacuum thrombectomy (CAVT) patients treated using the 16Fr Lightning Flash system (Penumbra).
RESULTS FROM THE TRIAL show that mean right-to-left ventricular (RV/LV) ratio decreased from 1.34 at baseline to 0.95 at 48 hours—a 27.3% decrease (p<0.001), he said. The composite major adverse event (MAE) rate was 0.8% (two patients). All functional outcomes were improved at one year: median Borg dyspnoea scale at rest improved, decreasing from four at baseline to zero (p<0.001), mean six-minute walk test distance increased from 222.5m at discharge to 370.7m (p<0.001), and the New York Heart Association (NYHA) classification distribution recovered to that before the pulmonary embolism (PE) event. Multiple QoL measures showed gains—notably, mean total PEmb-QoL improved, decreasing from 43.7 at baseline to 14.2 at one year (p<0.001), a continued improvement from the 90day result (p<0.001). “These findings demonstrate that treatment with CAVT using the 16F catheter system enhances patient outcomes and potentially reduces the long-term burden of PE-related sequelae,” he concluded.
New MHRA AI commission with US expert input
The MHRA’s new National Commission on the Regulation of AI in Healthcare brings together leading voices from across the UK and internationally, alongside representation from global tech organisations, including Google and Microsoft.
The Commission will shape recommendations on regulating artificial intelligence (AI)-driven medical technologies, contributing to international alignment and accelerating safe access to AI in healthcare and across the UK’s National Health Service (NHS).
The MHRA confirms that planned international reliance routes will allow medical devices approved by trusted regulators, including the FDA, to gain faster access to the UK market. This includes products cleared through the 510(k), de novo, and premarket approval (PMA) pathways, with a proportionate approach balancing rapid access with robust patient safeguards.
The medtech regulatory reforms in the UK are intended to enter legislation in 2026 and open new reliance routes from 2027.
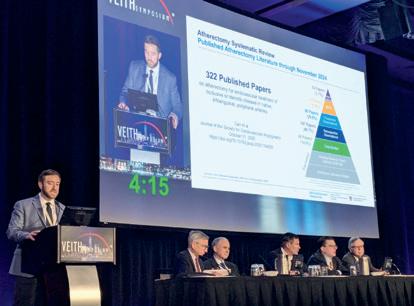
A systematic review and meta-analysis of more than 300 original investigations on atherectomy in peripheral arterial disease (PAD) highlights “overall favourable” clinical outcomes. This and other findings from what researchers say is the first investigation to provide a “comprehensive” overview of the atherectomy literature were recently published in the Journal of the Society for Cardiovascular Angiography & Interventions (JSCAI) and presented at the 2025 VEITHsymposium (18–22 November, New York, USA).
“ALTHOUGH ATHERECTOMY for peripheral interventions has been studied for over 35 years, recent criticisms suggest it lacks supportive evidence,” the researchers, led by co-first authors Jeffrey Carr (Christus Health Heart and Vascular Institute, Tyler, USA), Ralf Langhoff (Humboldt University Berlin, Berlin, Germany) and Eric Secemsky (Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, USA) write. As a result, Carr and colleagues set out to provide an “intentionally broad overview” of both the quality of the “extensive” published literature on atherectomy and the procedure’s clinical outcomes. The research team conducted a systematic review of original research published in MEDLINE, Embase, and PubMed through November 2024, identifying both prospective and retrospective studies on atherectomy for infrainguinal peripheral artery interventions. They note that while
case studies and meta-analyses were included in the systematic review, these study types were excluded from the quantitative meta-analysis.
Carr and colleagues specify that study design, device class, patient and lesion characteristics, provisional stenting, distal embolisation, and 12-month outcomes—namely patency, major amputation, target lesion revascularisation, and mortality—were captured.
In JSCAI, the authors share that their systematic review included 322 published atherectomy papers comprising 121 directional, 44 laser, 30 orbital, 72 rotational, and 55 mixed atherectomy classes. They note that the designs were meta-analyses in 3.7% (12 papers), randomised controlled trials in 5.9% (19 papers), prospective observational studies in 29.8% (96 papers), retrospective observational studies in 45.7% (147 papers), and case studies in 14.9% (48 papers). Additionally, they state that adjunctive
therapies were used in 91.5% and 29.2% were included in a comparator arm.
Carr and colleagues report that, among 190 papers included in their meta-analysis, the 12-month patency, target lesion revascularisation, major amputation, and mortality rates were 75.4% (51 studies), 15.6% (67 studies), 1.7% (71 studies), and 2.8% (63 studies), respectively. Furthermore, they report that the distal embolisation rate was 2.2% (159 studies) and the provisional stenting rate, 9.3% (131 studies). “Considerable heterogeneity was observed,” the authors write.
up data beyond the five-year mark. In addition, they accept their review is restricted in its ability to compare classes of atherectomy devices across studies and is “unable to provide firm conclusions” regarding the outcomes of atherectomy as a standard procedure, given that the majority of included studies used adjunctive therapies, among other drawbacks.
Acknowledging some limitations of their paper, Carr and colleagues recognise that a lack of data prevented the assessment of longer-term follow-
As atherectomy devices continue to evolve, it is important to support newer technology with trials, registries, and other means of evidence generation.”
Black women with claudication had the highest rate of progression to chronic limbthreatening ischaemia (CLTI) after lower extremity revascularisation at 180 days, new data from a Medicare-linked Vascular Quality Initiative (VQI) procedural registry show. THE RESULTS WERE DEMONSTRATED in a national cohort study of VQI-Vascular Implant Surveillance and Interventional Outcomes Network (VQI-VISION) data exploring the impact of intersectional identity among patients with claudication on progression to CLTI, amputation and mortality following revascularisation. The data were recently published in JAMA Surgery
The study—led by Olamide Alabi (Emory University School of Medicine, Atlanta, USA)—lays out how among 10,012 adults with claudication, the rate of symptom progression from claudication to CLTI was 5.85% at 180 days. Median progression was 5.4% among men and 6.5% among women, Alabi and colleagues report. Ethnoracial differences in median percentage disease progression among men were detected—7.2% in Black, 8.8% in Hispanic and 5.2% in white men progressing to
CLTI within 180 days after their index revascularisation. Median progression at one year was lowest among white men (9.2%) and white women (10.1%), and highest among Black men (13.7%) and Black women (18.7%).
On univariate Cox regression analysis, the risk of progression to CLTI within 180 days after index revascularisation for claudication did not significantly differ when comparing Black to white men or when comparing Hispanic to white men, Alabi et al find. There were no significant differences between Hispanic and white women, but Black women were “twice as likely to progress to CLTI within 180 days after index [lower extremity revascularisation] for claudication [than white women],” they report, with a hazard ratio (HR) of 2.06 (95% confidence interval [CI], 1.49–2.84;
Carr and colleagues summarise in their conclusion that the present analysis highlights low rates of amputation, mortality, provisional stenting, and distal embolisation with atherectomy and demonstrates an absence of safety signals and efficacy rates that are within accepted standards. They remark that the investigation “supports the use of atherectomy in appropriately selected patients as part of the endovascular treatment algorithm for [PAD]”.
Looking ahead, the authors suggest future trials should address the impact of plaque modification with atherectomy in the context of evolving drug delivery solutions with drugcoated balloons, drug-eluting stents, and bioabsorbable devices.
“As atherectomy devices continue to evolve, it is important to support newer technology with trials, registries, and other means of evidence generation,” the authors suggest, considering future research in this space. They also highlight the importance of evaluating the cost-effectiveness and accretive value of atherectomy outcomes with longer-term studies.
Carr and colleagues note in their JSCAI paper that this work was supported by Medtronic.
p<0.001).
“Our study took the examination of progression to CLTI among patients with claudication a step further to better understand intersectional ethnoracial and gender identity and its association with progression to CLTI,” the authors write in JAMA Surgery. “We found that Black women with claudication had the highest risk for CLTI progression at both 180 days and at one year. Vascular clinicians’ awareness of intersectional disparities in claudication outcomes will augment their ability to appropriately counsel patients regarding their individualised risks for adverse outcomes.”

Concluding, Alabi and colleagues point to a “lack of adherence” to established appropriate use criteria (AUC), overutilisation and/or early revascularisation among those with claudication as potentially perpetuating “disparate care”. “Given the preponderance of evidence and societal guidelines describing the value of exercise therapy as first-line treatment for claudication and established poor equitable application of [guidelinedirected medical therapy], there is a critical need to develop evaluative metrics at the payor and policy level that ensure that all patients with claudication receive the equitable care they deserve,” they note.



Readers can now save an additional 10% off the Early Bird prices using the code CX26VASCULARNEWS

PROGRAMME HIGHLIGHTS:
▪ Peripheral arterial & chronic limb-threatening ischaemia: Podium first updates from BEST-CLI and BASIL trials
▪ CX Aortic Live: Full day of live and edited cases

Vascular access: Debates, cases & podium firsts
▪ Venous: Consensus on pulmonary embolus management
▪ Carotid & acute Stroke: Debate: ECST-2 and CREST-2 will influence my clinical practice
▪ Vascular trauma: Evolving clinical practice using experience from vascular injuries in recent conflicts


consistent physiologic maturation without the need for flow diversion”
One-year results of the VENOS-1 first-in-human study, investigating the use of the Velocity (Venova Medical) percutaneous arteriovenous fistula (pAVF) system, have been published in the Journal of Endovascular Therapy (JET).
Robert Shahverdyan (Vascular Institute, Hamburg, Germany) and colleagues write that the results of the 10-patient, prospective, single-arm study demonstrate that an implantbased pAVF can consistently achieve physiologic maturation without the need for flow diversion from the deep venous system.
The study was designed to evaluate the safety and feasibility of the Velocity pAVF system, enrolling patients who were haemodialysis-dependent with a central venous catheter and had anatomy suitable for a surgical brachial-cephalic AVF.
The device creates an AVF in the proximal forearm between the proximal radial artery and cubital perforating vein with an endovascular implant, allowing for upper-arm cannulation of the cephalic vein for patients requiring haemodialysis, the JET paper details.
To test its technical feasibility and safety, investigators assessed the device against a primary effectiveness outcome of procedural success and primary safety outcomes of serious adverse device events and major reinterventions at six weeks. Secondary outcomes of the study included physiologic maturation, defined as brachial artery flow ≥500mL/ min and venous outflow diameter ≥5mm, reintervention rate, and functional use.
Brachial artery flow volume, which the study investigators describe as a key predictor of maturation and cannulation, increased progressively from 744mL/min at day one to 977mL/ min at day 42. The study team reports a corresponding increase in upper-arm cephalic vein diameter from 3.4mm at baseline to 6.2mm at six weeks.
The composite outcome measure of physiologic maturation was achieved in all subjects, with a median time to achieving physiologic maturation of 18 days.
In the subsequent follow-up, one patient exited the study early due to catheter-associated sepsis at day 65 that colonised the implant, which was subsequently removed; however, there were no infections in the remainder of subjects who were followed up through study termination.
All patients met the primary effectiveness endpoint, with intraprocedural duplex ultrasound used to confirm outflow from the proximal radial artery with no signs of arterialised blood flow into the deep venous system in any of the cases.

Four subjects had split venous outflow through both basilic and cephalic veins and underwent ligation of the medial basilic vein to direct the flow toward the cephalic vein to facilitate inline cannulation, the investigators report, whilst nine patients achieved functional maturation with two-needle cannulation of the upper-arm cephalic vein, without the need for special training on cannulation technique.
The interim results of a first-in-human trial of a sirolimus-eluting covered stent graft— Solaris DE (Solaris Endovascular)—for the treatment of dialysis access dysfunction demonstrate the promise of the device in treating vascular access outflow stenosis, an investigator in the trial has commented.
RESULTS OF THE DESCOVER TRIAL, evaluating the safety and efficacy of the Solaris drug-eluting endovascular stent graft in patients with arteriovenous fistulas (AVF) and arteriovenous grafts (AVG), were presented at the 2025 Transcatheter Cardiovascular Therapeutics (TCT) meeting (25–28 October, San Francisco, USA) by Leonardo Harduin (State University of Rio de Janeiro, Rio de Janeiro, Brazil), who described the device as opening a “new avenue” in dialysis access treatment.
Balloon angioplasty is the current standard of care for the treatment of vascular access stenosis, but recurrent stenosis up to one year after treatment remains high among these patients, Harduin described in his presentation. Stent grafts have been considered as one option to address this issue, he noted, though such technologies have been held back by unfavourable rates of target lesion primary patency (TLPP).
“The reason for stent graft failure is neointernal hyperplasia that occurs mainly on the edge of the device and sometimes in the middle of the stent graft,” Harduin detailed. The Solaris DE covered stent is built with an impermeable electrospinning PTFE membrane to limit cellular migration, allied with the sirolimus coating to block neointimal
hyperplasia and restenosis.
Animal studies of Solaris DE have, to date, shown the persistence of the sirolimus on the outer edge of the device, he said, with one study comparing it to a conventional stent graft finding no instances of neointimal hyperplasia at 60 days following Solaris DE implantation, compared to 4.9mm3 with the conventional device.
Investigators therefore established the DEScover trial, a phase II, first-in-human, feasibility trial, to evaluate the safety and effectiveness of the Solaris DE in the venous outflow compared to percutaneous
These interim analyses confirm the safety of Solaris drug-eluting covered stent in humans.”
transluminal angioplasty (PTA) for treatment of de novo or restenotic lesions of native AVF and for graft-vein stenosis of AVG. Patients were eligible for inclusion if they had AVF/AVG with clinical function and a haemodynamically significant
“The pAVF access provided sustained clinical use over the duration of follow-up,” Shahverdyan and colleagues report. “There was a single balloon angioplasty of the venous end of the implant at 224 days in one subject, otherwise all other subjects have had uninterrupted functional use of the pAVFs throughout follow-up. The total number of reinterventions to maintain or restore access patency for all subjects was two over the course of the study, for an average of 0.2 reinterventions-per-patient-year. The access circuit primary patency at one year was 80% and cumulative patency was 90%.”
Though the implant-based pAVF reached the threshold for physiologic maturation, over the ensuing 12 months, brachial artery flow volume plateaued, never exceeding 1,400ml/min, they note. The radial artery remained patent through the duration of the study for all subjects, with the flow in the radial artery retrograde or bidirectional in all patients, with no instances of thrombosis or occlusion. No instances of hand ischaemia or high-output pAVF were reported in any patient during follow-up.
“The results of this first-in-human study demonstrate the effectiveness of an implant-based approach for creating pAVFs for haemodialysis vascular access,” the study’s authors write in their discussion of the findings.
restenosis in the outflow circuit.
Patients in the AVF cohort were randomised 1:1 after successful predilatation with a high-pressure balloon to receive either Solaris DE or PTA. The AVG cohort was a single-arm group, with patients only receiving the covered stent. The primary endpoints were TLPP rates at six months and freedom from any serious adverse events at 30 days.

Harduin reported that 65 patients completed the six-month follow-up, with 19 patients in the AVG group, 22 patients in the AVF group who received Solaris DE, and 24 patients in the AVF group who underwent standard PTA treatment. Hypertension was less frequent in the PTA group, and brachiocephalic fistulas with cephalic arch stenosis and de novo lesions were more similarly proportioned in both groups.
Regarding the results, Harduin detailed that there was 100% freedom from adverse events affecting the access or venous outflow circuit in all groups at 30 days after the index procedure. Reporting an overall TLPP rate of 95% at six months across both groups, Harduin detailed that there was 100% TLPP at six months for patients receiving Solaris DE in the AVG cohort, with a TLPP rate of 91% at six months for AVF patients who received the device. This compared to a 60% rate of TLPP for patients who underwent PTA treatment.
“These interim analyses confirm the safety of the Solaris drug-eluting covered stent in humans,” Harduin said of the results in the concluding remarks of his presentation at TCT 2025. “These early results suggest it may open a new avenue in dialysis access treatment.”

Scan to learn more or visit laminatemedical.com/vasQ

EU: The VasQ device is intended for use as subcutaneous arteriovenous conduit support for vascular access.
US: VasQ is intended for use as an external support for upper extremity arteriovenous fistulas created for vascular access by means of vascular surgery.
Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions and adverse events available at https://laminatemedical.com/eIFU.



Despite original ambitions to become a journalist, the loss of a close relative to pulmonary embolism (PE) saw Marianne Brodmann (Graz, Austria) redirect her focus towards the medical world. Now the head of the Division of Angiology at the Medical University of Graz, Brodmann talks bioresorbable scaffold technologies, CREST-2, and artificial intelligence (AI) in this interview with Vascular News on her life and career in the vascular field thus far.
Why did you choose to pursue a career in vascular medicine?
A career in vascular medicine was not my first choice. I originally wanted to become a journalist, but my beloved aunt—who was my rock during childhood—died from a pulmonary embolism (PE) and that led me towards vascular medicine/angiology. During medical school, I received a grant that allowed me to work in Minneapolis with Kurt Amplatz, which is how I got involved in the endovascular field of vascular medicine specifically.
Who were your career mentors and what was the best advice that they gave you?
The most important mentor I had was Professor Gerhard Stark, who trained me in the endovascular field, gave me the opportunity to do basic research in an animal lab, and was always there to answer questions and provide guidance. He told me: never give up, you will find a solution.
You have been involved in numerous studies on endovascular technologies for the treatment of peripheral arterial disease (PAD). What has been the most important development in this field over the course of your career so far?
The development of the field from plain balloon angioplasty and bare metal stenting to the array of techniques that are now available has been significant. In addition, the importance of how we should approach our endovascular procedures has changed dramatically over the years, with vessel preparation, attention to recoil, and drug deployment all now crucial considerations in current practice.
What do you see as the next frontier in endovascular technologies for PAD?
We need to focus on leaving nothing permanent behind when it comes to endovascular technologies for PAD, especially in patients with chronic limb-threatening ischaemia (CLTI). With this in mind, I think bioresorbable scaffold technologies are the future.
If you leave something behind in a vessel in a patient with CLTI, for example a permanent scaffold, you always need to have in mind that the patient might stop taking their medication. This is especially important to consider when managing a patient with CLTI who is old and particularly sick.
The situation is different in claudicants. Claudicants know that they need to continue to take their medication, their antithrombotic
treatment, and that they need to go to the doctor if something is running out, but that’s not the case in CLTI. In a patient with CLTI who has very diseased arteries, you have the dangers of reobstruction, rethrombosis, and then you really get into trouble because if something is permanent in this anatomy, it’s an issue. There might then be a case that requires a bypass, but there is not a landing zone available because there is a scaffold in place.
Therefore, I think if you have something that is in the vessel only for a certain time, maybe six months or 12 months, and then it goes away, it’s much easier to keep the vessel open. This is what is most important from a patient’s perspective and also from a practical perspective.
In addition, bioresorbable scaffolds are associated with less restenosis than nonresorbable scaffolds.
What does the landscape of bioresorbable scaffold technologies for PAD look like at present?
There are currently several bioresorbable scaffold technologies out there at various stages of development, with the field only set to grow in the coming years.
Of course, there is the Esprit below-the-knee (BTK) resorbable scaffold system from Abbott that has been assessed in the LIFE-BTK trial.
Most recently, three-year data on this CEmark and US Food and Drug Administration (FDA)-approved device were presented at the 2025 Vascular Interventional Advances (VIVA) conference (2–5 November, Las Vegas, USA; see page 14), which showed prolonged efficacy and safety in a population of patients with CLTI. Esprit scaffold registries are currently underway in Europe to provide further data on this technology.
The ELITE-BTK pivotal trial is ongoing, which is designed to assess R3 Vascular’s Magnitude drug-eluting bioresorbable scaffold. The company announced in April of last year that the first patient has been treated in this trial, and so we eagerly await the findings.
The Motiv bioresorbable scaffold, from Reva Medical, is another interesting technology in this space. This device has been CE-mark approved and has been granted FDA Breakthrough Device designation.
The Motiv is currently being assessed in the recently completed MOTIV BTK randomised controlled trial.
The VITAL-IT 1 study is another one to look out for. This will assess StentIt’s resorbable fibrillated scaffold. This device is not metallic, unlike those listed above, but is
CURRENT APPOINTMENTS
Head of the Division of Angiology, Internal Medicine
Department, Medical University of Graz (Graz, Austria)
EDUCATION (SELECTED)
1996–1998: Training as subspecialist in angiology (vascular medicine), Medical University of Graz
1990–1996: Training as a medical specialist, Medical University of Graz
1983–1984: Medical school, University of Graz
SOCIETY POSITIONS (SELECTED)
Fellow of the European Society of Cardiology
Past president of the European Society of Vascular Medicine
Past pesident of the Austrian Society of Angiology
instead built from microfibres. The first patient was enrolled in June of last year.
I think there will be a big focus on innovation in the field of bioresorbables in the near future.
What do you think is the most important vascular research paper to have been published in the last 12 months?
Without a doubt the most important paper to have been published in the last year is CREST-2 (see page 1). Published in the New England Journal of Medicine last November, the results of this trial will help us to move forward with carotid treatment at an updated, modern, and adequate level.
For a very, very long time, we would have discussions about how to properly manage patients with asymptomatic carotid stenosis, and more often than not the answer would be that open surgery was the only viable option. In many countries, other surgical options are not available, especially not in Europe. They are done in the USA, but not in Europe. Patients with high-degree carotid stenosis who are asymptomatic will at some point become symptomatic. You want to avoid this progression and therefore I think if we can offer patients a non-invasive procedure, like stenting, this will be really helpful for all of us. We waited for such a long time for these data, and it is great to see answers to our questions after not having adequate information to share with patients up until now.
The results of CREST-2 are certainly going to influence my day-to-day practice, because I and indeed all my colleagues see a lot of asymptomatic patients. We find them in several ways, whether by duplex ultrasound, under general conditions, or even sometimes by accident when they go for an examination for something unrelated to carotid disease. When these patients are identified, the only option is to just provide medication, and that’s it. Then, inevitably, the patients have to live with the constant fear that at any point they might have a stroke.
It’s not only about us, it’s also about the patient’s fear. These results will make a big difference to patients.
You have held several senior society positions, including president of the European Society of Vascular Medicine and president the Austrian Society of Angiology. How did these roles influence your vascular medicine practice?
I served as president of the European Society of Vascular Medicine from 2017–2018 and was recently awarded an honorary fellowship
from this society. Slightly earlier in my career, I served as president of the Austrian Society of Angiology.
These experiences helped me to have an overview of what is done in other fields, other countries, and other institutions, in order to optimise the way we do things in vascular medicine. They also helped me to build connections to improve my level of work and science. Furthermore, I got to know so many people. I have made a lot of friends and formed good relationships while involved with these societies.
One of your main interests is the training of young angiologists. How do you anticipate training will change in the age of AI?
AI will be an important factor in the future, but if you have to treat patients with your own hands as we need to do in the endovascular field, technical skills will remain essential to good clinical practice.
AI might help with guidance, but at the end of the day a physician needs to treat the patient
with their own hands and skills.
What are the biggest challenges currently facing vascular surgery and what do you think can be done to address these?
I think the ongoing ‘battle’ between open versus endovascular methods in vascular surgery is one of the biggest challenges we face. Ideally, we need algorithms to help guide us towards the best approach for each individual patient, situation, and set of resources.
Could you outline one of your most memorable cases?
A PE thrombectomy case from just last year has stayed with me. One of my teachers at medical school had a PE, and the case is particularly memorable because I can distinctly remember bringing her from haemodynamic instability one day, and then two days later she was going to the local coffee shop in our hospital area with her husband.
“We need to focus on leaving nothing permanent behind when it comes to endovascular technologies for PAD.”
These are situations and memories you will always have, and they are worth everything.
What advice would you give to someone looking to start a career in medicine?
I would advise anyone looking to start a career in medicine to be patient but determined, because sometimes it takes some time to get the result you want. But it is one of the best jobs you can have. You work with great people, and you can help people and their loved ones.
Consider carefully which medical subspecialty you might be interested in pursuing and don’t be afraid to switch if you discover it is not the way you want to go.
What are your hobbies and interests outside of medicine?
In my spare time I like to work on my nephew’s farm, which was previously my parents’ farm, and I enjoy gardening, with the best time of the year to do this being early spring, in my opinion. I also enjoy hiking and reading books.
The surge in endovascular procedures, radiation risks and radiation mitigation strategies

Patrick Muck
Patrick Muck (Cincinnati, USA) examines some of the latest technologies available for radiation protection in the vascular space amid a “meteoric” rise in endovascular procedures.
OVER THE PAST DECADE, THE adoption of endovascular techniques has accelerated dramatically, driven by technological innovations, demographic shifts, and healthcare economics. However, this proliferation comes with challenges, particularly the heavy reliance on fluoroscopy for real-time imaging, which exposes healthcare providers to ionising radiation.
Globally, arterial procedures dominate endovascular volumes, comprising over 60% of hospital caseloads. Endovascular aneurysm repair (EVAR) for abdominal aortic aneurysms (AAAs) exemplifies this, with procedure volumes rising 25% from 2011 to 2016. In 2023–2024, lower limb revascularisation—primarily angioplasty and stenting for peripheral arterial disease (PAD)—saw a 45% increase, driven by below-the-knee therapies approved in early 2024. Peripheral vascular interventions (PVI) for PAD trended toward complexity, with tibial PVI usage up 1% annually and atherectomy 1.6% from 2011–2020, a pattern persisting into 2024. Officebased labs (OBLs) reported a dramatic uptick since 2008, performing complex arterial cases with lower costs. Carotid stenting procedures continue to increase as well.
Venous procedures, though less voluminous than arterial, have seen robust expansion, focusing on chronic venous disease (CVD) affecting 30% of adults. Demographic factors, such as obesity and sedentary lifestyles, exacerbate venous issues, with projections estimating a 15% annual rise in CVD prevalence through 2030. Venous interventions have democratised treatment for lower extremity venous disease, affecting over 30 million Americans alone. The peripheral vascular devices market, heavily reliant on venous tools, is poised to expand from US$13.51 billion in 2025 to US$21 billion by 2032, at a compound annual growth rate (CAGR) of 6.5%. Training logs show venous cases rising from 25 to 43 per fellow annually (2007–2024), a statistically significant jump (p<0.001). In the UK, National Vascular Registry data indicate wait times for venous interventions stretched to 86 days in
2023, yet volumes grew amid rising CVD referrals. Venous procedures benefit from outpatient feasibility, with ambulatory surgical centre (ASC) volumes up 27% in 2023. Despite shorter fluoroscopy times than arterial counterparts, venous interventions still contribute to cumulative staff exposure. Radiation mitigation spans procedural techniques, shielding, and innovative technologies. Protection starts with avoidance. As procedure volumes increase so do the occupational risks, prompting European Society for Vascular Surgery (ESVS) guidelines for ALARA, or As Low As Reasonably Achievable, strategies. The ALARA principle calls for reducing radiation to the patient, operator, and staff. Time, distance, and shielding are the cornerstones. Basic strategies include collimation and pulsed fluoroscopy. Personal protection with lead aprons, thyroid shields, and ceiling-suspended screens reduce scatter by 70–90%.
Digital subtraction angiography (DSA) should be used judiciously during peripheral procedures. Personal dosimetry monitors should be worn by all operators and staff. Advanced systems such as Fiber Optic RealShape (FORS; Philips) use light-emitting guidewires for fluoroscopy-free navigation. Electromagnetic tracking and image fusion systems from Cydar Medical and Centerline Biomedical eliminate 20–40% of live fluoroscopy needs. Traditional lead aprons reduce exposure by 70–90% but cause musculoskeletal strain and orthopaedic concerns. Recently, scatter control and barrier protection systems have been developed to dramatically reduce radiation exposure to team members. Radiaction and EggNest target scatter control whereas Protego and Rampart

offer barrier protection. Radiaction and Protego are more aimed for cardiac procedures, while EggNest Complete and Rampart are more applicable to the peripheral vascular space and will be highlighted for this readership.
The EggNest Complete system (Egg Medical) is an integrated radiation protection system designed to integrate into the existing workflow of any fluoroscopy suite. Its modular design allows for easy integration for multiple vascular and cardiovascular case types. The retractable shielding and unique modular design allow for full lateral and bi-plane use as well.
The EggNest Complete system is comprised of carbon fibre and nonlead equivalent panels above the table, flex shielding below the table, and a large ceiling-mounted shield. The patented shielding easily integrates into existing rooms and offers total team protection without taking up valuable floor space in crowded surgical suites. A 2025 real-world study presented at the Transcatheter Cardiovascular Therapeutics (TCT) meeting (25–28 October; San Francisco, USA) showed
A new era of barrier protection has arrived and it’s our generation’s duty to protect patients and the entire team.”
that the EggNest Complete total operator dose was incredibly low at 0.16mRem, meaning that the operator could do 31,000 cases in a year before reaching their annual allowable dose limit.
The EggNest portfolio also includes the EggNest Protect, which is similar to the EggNest Complete, but without the ceiling-mounted shield. Clinical data presented at the 2025 Western Vascular Society (WVS) annual meeting (14–17 September; Ojai, USA) by Brant Ullery (Providence St Joseph Health, Portland, USA) utilising the EggNest Protect (without the ceiling-mounted shield) demonstrated a 97.4% reduction in radiation exposure across 27 complex multivessel aortic cases for every person in the

procedure room.
The Guardian is Rampart’s latest innovation in their suite of radiation safety solutions and represents a paradigm shift in occupational protection, offering comprehensive, total room protection via modular, ceiling- or floor-mounted panels that block scatter radiation at its source. Unlike traditional lead aprons, Guardian deploys transparent, lightweight shields engineered from proprietary composite materials that are completely lead-free. In a recent randomised controlled trial, the Rampart system demonstrated greater than 99% attenuation, a 20-fold reduction in total body radiation when compared to standard lead aprons and shields.
Guardian integrates seamlessly with C-arms, allowing 360-degree rotation without repositioning. In a 2025 study of peripheral vascular cases, Guardian reduced effective doses from 2–5mRem to under 0.1mRem per procedure, addressing head and extremity exposure where lead aprons provide limited protection. Real-world data from high-volume centres show 40% fewer orthopaedic complaints among users, as the system eliminates apron weight (up to 20lbs). Cost-effectiveness stems from durability—shields last 10+ years—and reduced absenteeism.
In recognition of this new era in radiation safety devices, societies such as the Society for Cardiovascular Angiography & Interventions (SCAI) and the Society for Vascular Surgery (SVS) have recently come together to write a consensus document to be published in Q1 that further discusses the role of Enhanced Radiation Protection Devices (ERPDs).
At my institution, the last five EVARs with the Guardian have recorded a combined exposure of less than 2.1mRem—less than one receives on a coast-to-coast flight in the USA. In fact, the exposures were so low, in two of the EVARs, the entire team was lead apron free.
The meteoric rise of endovascular procedures—arterial and venous alike—ushers in an era of precise, patient-friendly care, yet underscores the imperative for radiation stewardship. A new era of barrier protection has arrived and it’s our generation’s duty to protect patients and the entire team. As endovascular volumes grow 7% yearly, prioritising these barrier technologies safeguards the workforce. Protego and Radiaction are efficacious for the cardiology space, whereas Rampart’s Guardian and EggNest Complete are incredibly effective in the peripheral vascular space. Rampart’s Guardian and EggNest Complete redefine mitigation, blending efficacy, ergonomics, and equity to combat radiation’s hidden toll.
References: available online.
Patrick E Muck is chief of vascular surgery at the Good Samaritan Hospital in Cincinnati, USA.
The author declared no relevant disclosures.




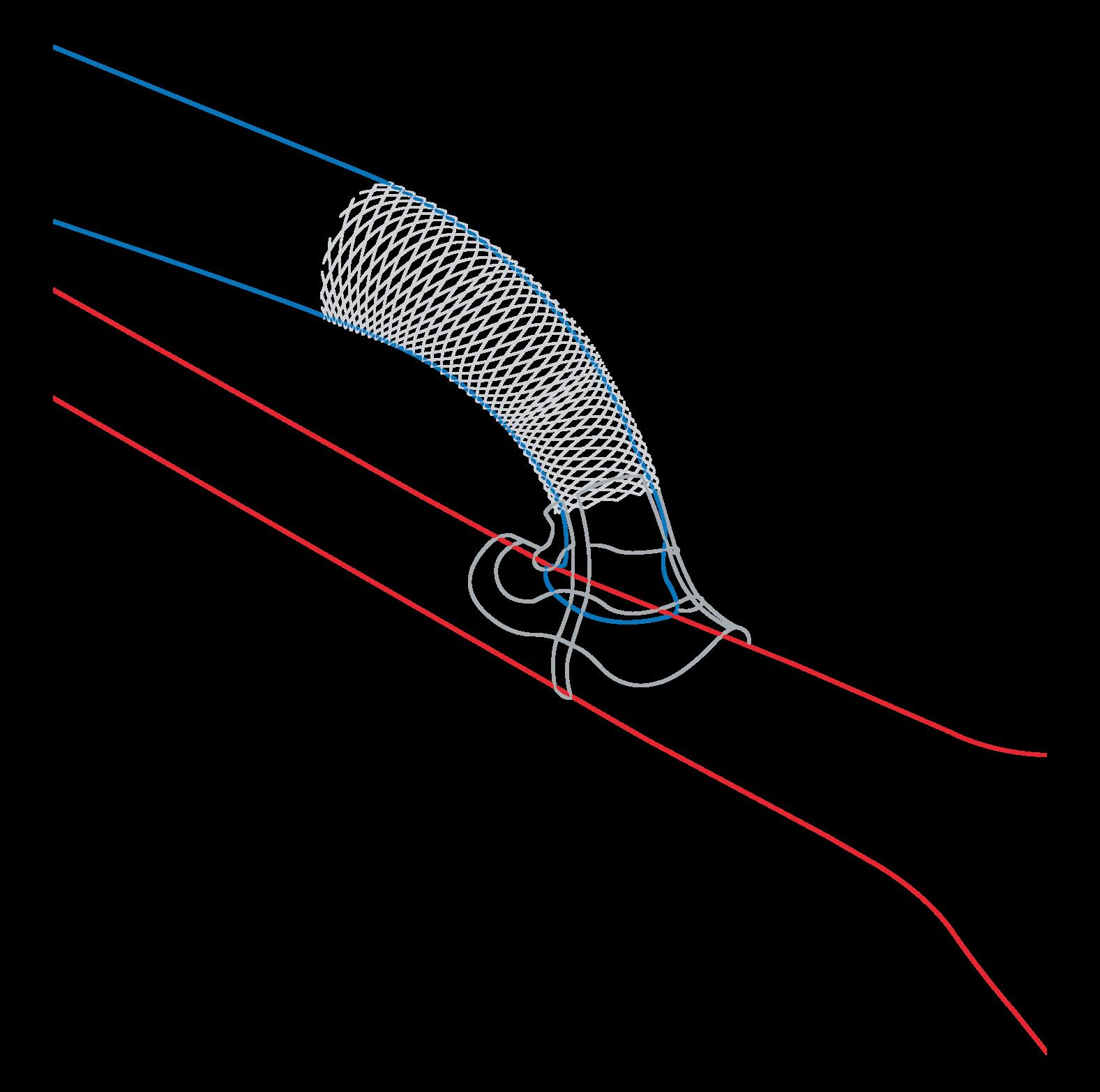
“Excellent” one-year outcomes from ROADSTER 3 show TCAR is “safe and effective”
One-year outcomes in the ROADSTER 3 post-approval study to evaluate the safety and effectiveness of transcarotid artery revascularisation (TCAR) using the Enroute stent and neuroprotection system (Boston Scientific) in standardsurgical-risk patients revealed a 1.5% intention-to-treat rate of composite major adverse events (1.3% per protocol).
DELIVERING THE RESULTS at the 2025 Vascular Interventional Advances (VIVA) conference (2–5 November, Las Vegas, USA), Meghan Dermody (Penn Medicine/Lancaster General Hospital, Lancaster, USA) also reported an intention-to-treat ipsilateral stroke rate between 31–365 days of 0.6% (0.7% per protocol).
The results were drawn from patients enrolled between September 2022 and
Three-year

June 2024—344 intention to treat and 219 per protocol—at 48 US sites. The primary endpoint was the hierarchical composite incidence of major adverse events defined as stroke, death or myocardial infarction within 30 days and ipsilateral stroke from 31 to 365 days post procedure. The rate of stroke/ death/myocardial infarction (MI) at 30 days was 0.9% (0.6% per protocol), with a 30-day stroke rate of 0.9% (0.6% per protocol). “ROADSTER 3 is the first independently adjudicated, prospective study evaluating TCAR in a standard-surgical-risk population,” said Dermody, who first presented 30-day outcomes from the study at VIVA 2024. “Results demonstrate that TCAR is safe and effective, with excellent clinical outcomes.”
The 30-day outcomes represented the lowest reported in a standard-surgicalrisk population, she added.
Three-year data from the LIFE-BTK randomised controlled trial, presented at VIVA 2025, demonstrate the prolonged efficacy and safety of the Esprit BTK drug-eluting resorbable scaffold (DRS; Abbott) in chronic limb-threatening ischaemia (CLTI).
SAHIL PARIKH (COLUMBIA UNIVERSITY IRVING MEDICAL CENTER, New York, USA) reported that, at three years, Esprit BTK demonstrated a 33% relative improvement in the composite primary efficacy endpoint of primary patency and limb salvage compared to percutaneous transluminal angioplasty (PTA; 59.5% vs. 44.8%; p=0.0025 log rank test).
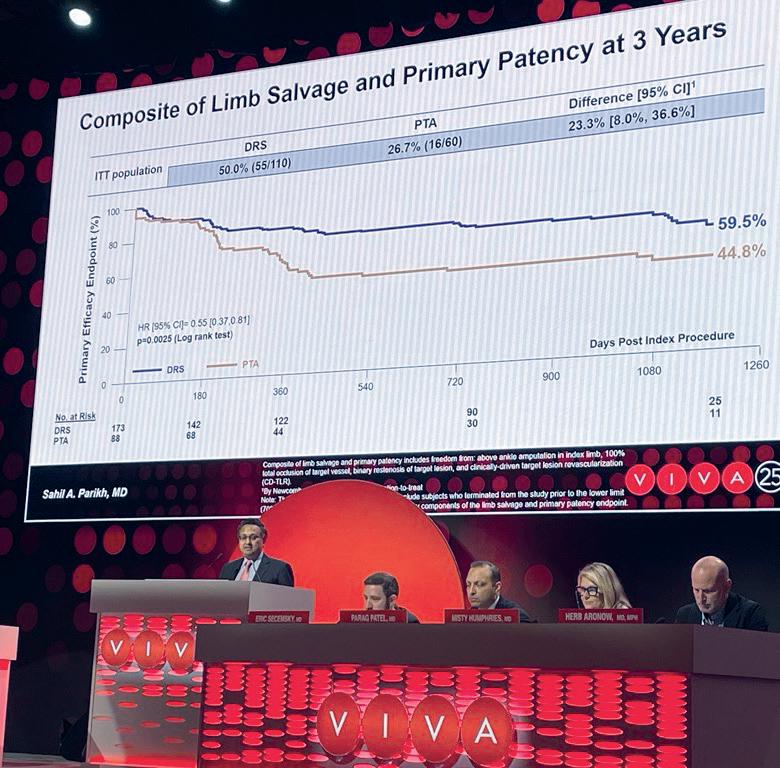
Parikh added that the Esprit BTK was also associated with significantly lower rates of binary restenosis (38% vs. 49%; p=0.018 log rank test), and lower clinically driven target lesion revascularisation (CD-TLR) rates, though the latter did not reach statistical significance (10.2% vs. 18.4%; p=0.052 log rank test).
Furthermore, the presenter shared that safety outcomes, the composite of freedom from major adverse limb events at three years, and perioperative death at 30 days were similar between the two arms (90.8% vs. 94.2%; p=0.23 log rank test).
Parikh also outlined the findings of subgroup analyses. These showed that the efficacy of the Esprit BTK was consistent across most patient and lesion subgroups; however, significantly greater benefit was observed in patients with RutherfordBecker class 4 disease and those not requiring inflow lesion treatment. Additional trends favouring the Esprit BTK emerged in patients with none or mild calcification, mixed wound aetiology, ‘Other’ race, and in those treated outside the USA.
“These findings support DRS as a durable, effective endovascular treatment option for long-term vessel preparation and reduced need for reintervention in CLTI,” Parikh concluded.
Thirty-day results from the PERFORMANCE III study of integrated embolic protection (IEP) in transcarotid artery revascularisation (TCAR) demonstrate “very low” 30-day event rates. Sean Lyden (Cleveland Clinic, Cleveland, USA) shared this finding at VIVA 2025.
THE PROSPECTIVE, MULTICENTRE PERFORMANCE III STUDY evaluated the safety and effectiveness of the Neuroguard IEP system (Contego Medical), 70cm carotid stent, and the Neuroguard IEP embolic protection system for direct TCAR in 146 patients across 26 clinical sites. The Neuroguard IEP embolic protection system includes both a direct access kit and a blood flow reversal system.
“The 30-day event rates in the PERFORMANCE III study are the lowest reported in a prospective, multicentre carotid artery revascularisation study,” said Lyden, who is co-national principal investigator of the trial.
In the PERFORMANCE III study, the 30-day stroke rate was 0% per the intention-to-treat analysis, with zero myocardial infarctions, zero cranial nerve injuries, zero neurological deaths, and zero stent thromboses reported. The primary endpoint of major adverse events through 30 days was 0.69% (intention to treat) and 0% (per protocol), which Lyden noted was significantly lower than the predefined study performance goal.
Additional data were shared at the VEITHsymposium (18–22 November, New York, USA).
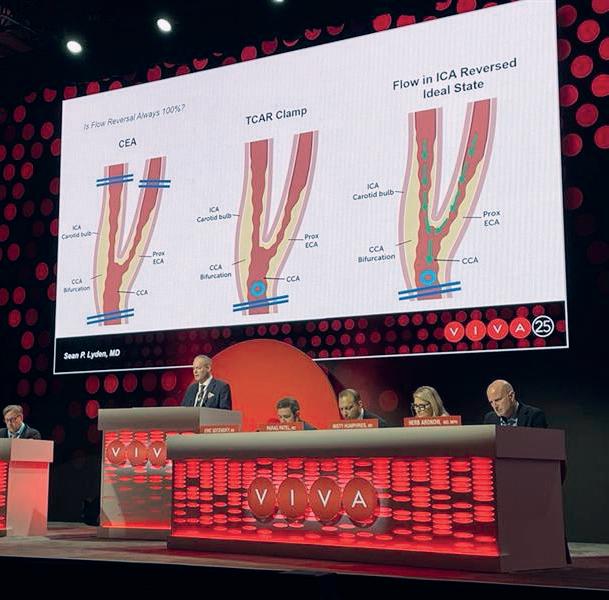
Two-year outcomes from the IN.PACT Admiral drug-coated balloon (DCB; Medtronic) French quality of life (QoL) registry were recently presented by Yann Gouëffic (Groupe Hospitalier Paris Saint Joseph, Paris, France) at the 2025 Paris Vascular Insights (PVI) Course (11–13 December, Paris, France).
THE STUDY EVALUATED 80 patients at eight sites in France with de novo obstructive lesions in lower limb peripheral arteries. The study objectives were to evaluate QoL, pain, and walking capacity through 24 months following treatment with the IN.PACT Admiral.
These real world-data demonstrated statistically significant improvements in QoL (EQ-5D-5L) and walking ability (Walking Impairment Questionnaire) from baseline to 12 months, with the greatest improvements noted in patients with the worst baseline QoL scores.
Strong clinical outcomes were also observed, including high rates of freedom from target vessel revascularisation (84.4%), freedom from major amputation (98.8%), and freedom from all-cause
mortality (84.5%) through 24 months. Notably, the study included a high percentage of patients with severe disease—27.5% were Rutherford 5 at baseline.

“We need to look at the totality of evidence”: VIVA panellists examine SWEDEPAD findings
A focused session at the 2025 Vascular Interventional Advances (VIVA) conference (2–5 November, Las Vegas, USA) homed in on the latest findings from the SWEDEPAD registry-based randomised controlled trials (RCTs), with panellists discussing the results with reference to the wider evidence base on drugcoated device use in peripheral arterial disease (PAD).
JOAKIM NORDANSTIG (University of Gothenburg, Gothenburg, Sweden), who joined the session remotely, opened proceedings with a summary of the SWEDEPAD results. He reiterated that drug-coated balloons and stents were not associated with reduced risk of amputation or improved quality of life compared with uncoated devices in the SWEDEPAD 1 and 2 trials of patients with chronic limb-threatening ischaemia (CLTI) and intermittent claudication, respectively. He added that higher five-year mortality with drug-coated devices in patients with intermittent claudication was noted.
Subsequently, Eric Secemsky (Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, USA) took to the podium to consider the SWEDEPAD findings in the context of real-world data pointing to the contrary.
“Obviously, we have to take SWEDEPAD seriously because it’s a
prospective trial,” Secemsky remarked, before stressing that clinicians “need to be thoughtful of all the other evidence we have to support the safety of paclitaxel”. Secemsky pointed to he and his and colleagues’ recently published and US Food and Drug Administration (FDA)-commissioned SAFE-PAD study among several others that demonstrate the safety of paclitaxel-coated devices.
Secemsky also questioned the relative importance of a mortality signal to the patient population in question. He highlighted data showing that, on average, patients would accept a device offering a reduction in twoyear clinically driven target vessel revascularisation (CD-TVR) risk from 30% to 10% and a reduction in fiveyear CD-TVR risk from 40% to 30% if the five-year mortality risk increase was less than or equal to 4.6%.
On this note, Secemsky warned against a repeat of the reaction to the Katsanos et al meta-analysis from
2018, which saw a cutback in the use of paclitaxel devices. “It’s really critical for us to not get stuck where we were seven years ago,” he said, stating that “real patient harm” resulted from the Journal of the American Heart Association (JAHA)-published paper.
During a panel discussion following the two presentations, session comoderator Joshua Beckman (UT Southwestern Medical Center, Dallas, USA) asked Nordanstig for his explanation of the mortality finding in SWEDEPAD 2.
“That is the million-dollar question,” Nordanstig replied, noting he was “very surprised” when he first saw the results. Nordanstig recalled that the SWEDEPAD team published an unplanned interim analysis in 2020— in response to the paclitaxel safety discussion that arose following the publication of the Katsanos et al paper—which did not identify a mortality signal. He remarked that the signal in SWEDEPAD 2, however, is “hard to ignore” and that it would have been “impossible” not to raise concerns.
But Nordanstig was clear that discussion around SWEDEPAD should move away from the mortality signal and recentre around the trial’s primary findings. He professed to being “very concerned” that the SWEDEPAD team did not observe effectiveness of drug-coated devices for reducing limb amputations in SWEDEPAD 1. “In our dataset, it looks like we are postponing reinterventions rather than preventing them,” he said.
A US National Institutes of Health (NIH)-funded randomised controlled trial is due to commence this month (January) to assess the efficacy of continuous digital monitoring in reducing diabetic foot ulcer recurrence among high-risk patients.
LED BY CAITLIN HICKS AND COLLEAGUES at the Johns Hopkins University School of Medicine (Baltimore, USA), the WIREDUP study aims to enrol 400 patients with a diabetic foot ulcer that has healed within the past 12 months.
In the trial, this high-risk group of patients will be randomised to receive either a diabetic shoe with a custom insole—the current standard of care—or a diabetic shoe with a custom insole that features pressure and temperature sensor technology connected to a patient-facing application.
In this latter group, Hicks explains, “the patients have an app on their phone, which will send them alerts if they’re having excessive onloading, meaning too much pressure in one area of the foot for sustained periods of time”. This information will then be sent through an escalation pathway to promptly resolve the issue.
The primary outcome of the study is time to ulcer recurrence, with Hicks hypothesising that the digital monitoring technology “should be able to delay or reduce ulcer recurrence” in high-risk patients and catch reulceration earlier when it does occur. The team also plans to collect patient-reported health and digital technology utilisation outcomes, as well as cost-effectiveness data.
At the centre of the trial are the sensory insoles and digital health platform set to be used in the intervention arm, both of which have been provided by Canada-based company Orpyx Medical Technologies. “The tech is widely accepted in Canada, but Orpyx needs a clinical efficacy trial to get into the US market”, Hicks says, underlining the rationale behind the WIREDUP trial.
The technology in question has existed for over a decade and has been through several iterations, with Hicks describing the current version as “very slick”. “The insoles don’t have to be plugged in, they all have insole batteries”, she says.
[An app will send patients] alerts if they’re having excessive onloading, meaning too much pressure in one area of the foot for sustained periods of time.”
Secemsky focused on postponing reinterventions. “I wouldn’t minimise what postponing intervention means to patients,” he said. “In the USA, most of these patients are of low socioeconomic status, they don’t want to take time off work, so part of what we do is postpone.”
“The patient needs to be first, and [postponing reinterventions] is a very patient-centric endpoint,” Secemsky stressed. Nordanstig agreed that “there is value in postponing interventions”, but also expressed concern that this did not translate to better limb salvage rates
The patient needs to be first, and [postponing reinterventions] is a very patientcentric endpoint.”
Eric Secemsky
in SWEDEPAD 1.
Later in the discussion, Beckman asked Nordanstig for his reaction to the “total dataset” on the efficacy and safety of paclitaxel.
“We need to be careful, and we need to look at the totality of evidence,” Nordanstig responded, before noting that the SWEDEPAD team is “planning further scrutiny of the evidence”.

Hicks specifies that the trial will involve nurses employed by Orpyx to review the data from the platform—including insole wear patterns, adherence, and temperature and pressure patterns—on a weekly basis and “reach out to the patient to help them troubleshoot if things aren’t going as planned”.
The trial received NIH funding in September last year. “We’d been waiting on funding for a while”, Hicks shares, citing delays arising from the US government shutdown in the early stages of the process. “We have been in a feasibility run-in phase for a while, where we are trying to identify patients and keep track of people who might be interested in participating in this.”
Alongside her team at Johns Hopkins and the Orpyx collaboration, Hicks also acknowledges the involvement of researchers at the University of Southern California (USC; Los Angeles, USA). “It’s a bi-costal trial”, she says, noting that several other sites are also set to join the project. Furthermore, Hicks underlines the multidisciplinary nature of the trial, with podiatrist David Armstrong heading up recruitment at USC.
Considering the wider context of the trial, Hicks comments that digital health technology in the diabetes space is in an “exciting” phase, while acknowledging a somewhat crowded market. “There are a lot of different products out there, and I think what’s important now is to try and weed through what’s available, and to find options that are really going to help patients in a meaningful way.”
Earlier this month (January), Gore announced US Food and Drug Administration (FDA) approval of the Gore Viabahn Fortegra venous stent, which the company notes is the first device for the treatment of deep venous disease in the inferior vena cava (IVC), iliac and iliofemoral veins. The approval is supported by late-breaking clinical data presented at The VEINS 2025 (1–2 November, Las Vegas, USA).
PREVIOUSLY KNOWN AS THE Gore Viafort vascular stent, the Fortegra venous stent is specifically engineered to treat patients with deep venous disease. It consists of an open-structure, self-expanding wirewound nitinol frame and an expanded polytetrafluoroethylene (ePTFE) polymer lattice.
According to Gore, this technology helps provide an “optimal” balance and “unique” combination of allowing the stent to conform to the natural anatomy while providing compression resistance throughout the entire device.
Gore received Breakthrough Device designation from the FDA for the Fortegra venous stent. This programme helps expedite the development and FDA review of medical devices that
offer more effective treatment for lifethreatening or irreversibly debilitating diseases or conditions.
The international clinical trial was the first prospective trial of its kind to include IVC, iliac and iliofemoral veins, Gore shares in a press release.
As revealed by Stephen Black (King’s College London, London, UK) at The VEINS 2025, the Fortegra venous stent was demonstrated to be both safe and effective for its indicated use in 89 patients treated with deep venous disease.
The study included a patient population with extensive disease burden: all were treated for thrombotic disease (acute, subacute and postthrombotic syndrome), 94.3% of patients had lesions that span three
In November 2025, enVVeno Medical announced that it received an “unfavourable” decision from the US Food and Drug Administration (FDA) in response to its supervisory appeal of the “not-approvable” letter it received on 19 August in response to its premarket approval (PMA) application for the VenoValve device, a surgical replacement venous valve for treating severe deep chronic venous insufficiency (CVI).
THE SUPERVISORY APPEAL UPHELD the review staff verdict in the not-approvable letter that the VenoValve did not meet the standard of reasonable assurance of safety and effectiveness, enVVeno reported.
“Although the appeal decision was not the result we are looking for, it did provide valuable insight into the criteria that would be necessary for approval of enVVe, our next-generation transcatheterbased replacement venous valve,” said Robert Berman, enVVeno Medical’s chief executive officer (CEO). “EnVVe should have a different safety profile than an open surgical device and is ready for human testing. Assuming that we can reach alignment with
Extensive disease burden
12 83.4% months of patients
Despite the study consisting of patients with extensive disease burden, 12-month primary patency was achieved in 83.4% of patients
vessel regions (IVC plus bilateral iliofemoral veins) and 68.5% required stents that extended below the inguinal ligament into the common femoral vein.
Despite the study consisting of patients with extensive disease burden, 12-month primary patency was achieved in 83.4% of patients. Further, results demonstrated 96.5%, 88.9%
The Fortegra venous stent represents a significant advancement in the treatment of patients with the most difficult-totreat venous obstructive pathology.”
Kush Desai
the agency on achievable endpoints for enVVe, it makes sense to turn our attention and devote our resources to enVVe.”
Meanwhile, data on the VenoValve device continued apace at the podium during the autumn.
At the 39th European Society for Vascular Surgery (ESVS) annual meeting (23–26 September, Istanbul, Türkiye), SAVVE trial principal investigator Matthew Smeds (Saint Louis University, Saint Louis, USA) shared a new finding that the VenoValve’s impact on ulcer area reduction and symptoms is dependent upon ulcer location.
Smeds outlined that the US pivotal study of the device assessed 61 ulcers in 43 of the 75 patients enrolled in SAVVE. The presenter specified that, of these 43 patients, 65% had ulceration above the malleolus and 35% had ulceration below. He noted that average starting ulcer size was larger in the above-malleolus location as

and 89.8% primary patency in the IVC, left iliofemoral and right iliofemoral vessel regions, respectively. There were no stent embolisations/migrations, fractures, vascular injuries or clinically significant pulmonary embolisms through 12 months. There were also no device-related deaths or major bleeding through 30 days. Overall, the study met its 12-month composite efficacy and safety primary endpoint.
“The Fortegra venous stent represents a significant advancement in the treatment of patients with the most difficult-to-treat venous obstructive pathology; occlusion of the inferior vena cava, iliac veins and inflow femoral veins,” commented Kush Desai (Northwestern University, Chicago, USA), national primary investigator for the Fortegra clinical trial, in Gore’s press release announcing the FDA approval. “Patients will benefit from a device that is designed specifically for this disease and its unique anatomic and physiologic challenges, including preservation of optimal flow dynamics through iliocaval confluence and side branch preservation.”
compared to the below-malleolus location. Smeds stated that, when stratified by location, average ulcer size reduction was 12cm in ulcers above the malleolus, while the corresponding figure was 3.3cm in those below the malleolus. In terms of ulcer duration, Smeds revealed that patients who had had an ulcer for less than a year at baseline had a more significant reduction in ulcer size.

Smeds concluded that the VenoValve device appears to have a positive effect on ulcer healing, particularly in patients with ulcers of less than one-year duration. He added that the impact of ulcer healing as well as patient-reported symptoms and quality of life is more substantial in patients with ulcers above the ankle and that this occurs within one month of device placement.
EnVVe should have a different safety profile than an open surgical device and is ready for human testing.”
Robert Berman
Similarly, at the 2025 annual meeting of the Western Vascular Society (WVS; 14–17 September, Ojai, USA), data were presented by general surgery resident Adam Surti (Cedars-Sinai Medical Center, Los Angeles, USA) showing that, among 44 patients in the SAVVE trial classified as having C6 disease, 31 had ulcers for more than one year. At two-year follow-up, 35 C6 patients remained in the study, and, for patients with ulcer duration greater than one year, ulcer area reduction was 1.8cm2 at one year and 0.3cm2 at two years. Ulcers located above the malleolus showed the most significant healing, with area decreasing from 12 to 3.8cm2. The ulcer recurrence rate at two-year follow-up was 7%, notably lower than the 15–44% recurrence rates reported in literature, Surti noted.



ARISE III pivotal study of Gore ascending stent graft for acute type A dissection begins enrolment
Gore recently announced the first implantation of the investigational Gore ascending stent graft in the ARISE III trial for the treatment of an acute type A dissection.
The implant was performed at Emory University (Atlanta, USA) by Brad Leshnower, site principal investigator, and Yazan Duwayri, study investigator.
Eric Roselli (Cleveland Clinic, Cleveland, USA), national principal investigator, stated: “Ascending aortic dissection is a surgical emergency that presents serious risk to a complex group of patients and can be challenging to treat.”
While open repair is the current standard, there are many patients who are not candidates for open surgery or are at high risk for surgery. Gore details that medical management of type A dissections carries a rapidly escalating risk, with mortality increasing 0.5–1% per hour for the first two days, reaching 60% inhospital mortality.
Gore notes that ARISE III builds on the company’s ARISE early feasibility study and ARISE II pivotal trial, which is currently enrolling at hospitals across the USA. Together, these trials aim to develop an endovascular treatment option for various ascending aorta pathologies as an alternative to open surgery or medical management.
First patient treated with A3-Shield system in PRINCIPIIS FIH trial
Angiolutions has announced that the first patient has been successfully treated with its investigational A3Shield system in the PRINCIPIIS FIH trial, a first-in-human (FIH) study evaluating the device’s safety and effectiveness.
The trial is being conducted at several centres in Tashkent, Uzbekistan, and is designed to evaluate the device—an implant that stops abdominal aortic aneurysm (AAA) growth by locally modulating aortic pulse waves—as an early interventional option for patients with small AAAs.

Investigational Gore ascending stent graft
“The ARISE III study is designed to evaluate an endovascular option as a potential solution for high-risk patients with acute type A dissection. This first implant in the pivotal trial is a vital step on the path toward expanding options for more patients,” Roselli added.
Leshnower remarked that the ascending aorta “presents a challenging environment for the use of endovascular devices due to the high blood flow rates and significant motion and angulation”.
“The procedure went well, and the patient is recovering,” he reported. “Having a catheter-based solution where the patient does not have to be placed on cardiopulmonary bypass or circulatory arrest could change how we think about treating patients—and ultimately broaden treatment options for complex cases.”
The ARISE III trial is a prospective, multicentre, non-randomised pivotal study approved by the US Food and Drug Administration (FDA) to assess the safety and effectiveness of the Gore ascending stent graft in patients with acute type A aortic dissections who are considered high risk for conventional surgery.
The study will enrol up to 112 patients at multiple centres across the USA. Each enrolled patient will be closely monitored throughout the study, with follow-up assessments conducted for up to five years to evaluate safety and effectiveness outcomes over the long term.
ViTAA Medical announces US FDA 510(k) clearance for aortic planning platform ViTAA Medical recently announced that the US Food and Drug Administration (FDA) has granted 510(k) clearance for AiORTA Plan, which the company describes as a “fully automated, hyper-precise aortic surgery planning solution”.
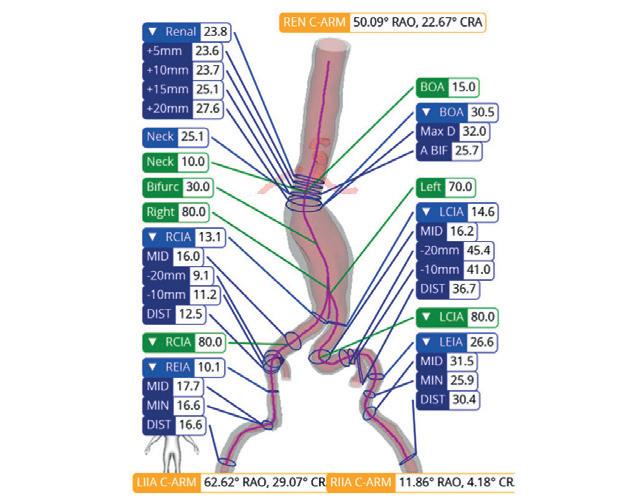
The tool aims to automate key preoperative measurements and streamline aortic case preparation by generating a complete aortic plan in minutes in a web-based system, producing automated aneurysm segmentation, centerline extraction, precise diameter and angulation measurements, and instant volumetric modelling.
Frank J Veith (New York University Medical Center, New York, USA), who performed the first endovascular aneurysm repair (EVAR) of an abdominal aortic aneurysm (AAA) in the USA three decades ago, hailed the approval as a milestone. “This is an important advance,” he said. “AiORTA Plan can streamline how physicians prepare for complex aortic procedures, and as the broader AiORTA platform matures with Maps and Watch in clinical evaluation, it has the potential to transform individualised care by providing critical insight.”
InterVene receives US FDA 510(k) clearance for Recana thrombectomy system
InterVene recently shared it has received 510(k) clearance from the US Food and Drug Administration (FDA) for the Recana thrombectomy catheter system, which is designed for the treatment of venous in-stent restenosis and native vessel obstructions.
“The first procedure in PRINCIPIIS-FIH is a major milestone for Angiolutions and for patients living with small AAAs,” said Uwe Raaz, chief executive officer of Angiolutions. “For too long, these patients have had to accept a ‘watch-and-wait’ approach in a progressive, life-threatening disease. With the A3-Shield system, we aim to open a new therapeutic window where we can intervene earlier and intercept the disease in a relatively benign state.”
“Treating the first patient in PRINCIPIIS-FIH is an important step toward offering an earlier, less invasive option to patients with AAAs who are currently managed by surveillance alone,” said Hubert Schelzig (University Hospital Düsseldorf, Düsseldorf, Germany). “By targeting a root biomechanical driver of AAA progression, the A3-Shield system aims to reduce the risk of future rupture and limit the need for complex aortic repair. The data from PRINCIPIIS-FIH will help us determine how this concept can be translated into routine clinical practice.”
Terumo Aortic launches RapidLink pivotal IDE study in USA and Europe
Terumo Aortic has announced enrolment of the first patient in the RapidLink pivotal investigational device exemption (IDE) study in the USA and Europe.
The study is designed to evaluate the safety and effectiveness of the RapidLink device for the repair or replacement of supra-aortic vessels during open surgical repair of aortic disease involving the thoracic aorta.
The other elements of ViTAA’s broader AiORTA platform—which also includes AiORTA Maps and AiORTA Watch—are currently in multicentre clinical studies. Maps is a diagnostic aid that aims to produce patientspecific vessel strength analysis, while Watch looks to provide longitudinal monitoring for early complication detection.
“Regulatory clearance of AiORTA Plan validates our approach of combining automation with precision medicine,” said Randy Moore (University of Calgary, Calgary, Canada), a vascular surgeon and chief medical officer (CMO) at ViTAA Medical. “As we continue our international clinical studies for AiORTA Maps and AiORTA Watch, we’re focused on delivering solutions that help physicians see beyond outdated size guidelines and gain insights into vessel behaviour that can inform decision-making throughout the patient journey.”
“This FDA clearance is a foundational milestone for ViTAA,” said Mitchel Benovoy, ViTAA Medical chief executive officer (CEO). “AiORTA Plan brings hyper-precision, efficiency, and extreme speed to the front line of the aortic workflow. It’s the first step in our platform strategy: from preoperative planning with AiORTA Plan to critical vessel-integrity insights and outcome predictions with AiORTA Maps, and long-term postoperative endoleak surveillance and risk stratification with AiORTA Watch—both currently in clinical studies. Our vision is end-to-end, patient-specific vascular care at every stage of the disease.”
Recana is a fully integrated system featuring a debulking catheter, introducer and collection sheaths, and nitinol collection baskets designed to simplify challenging venous procedures for physicians and improve clinical outcomes for their patients.
“The long-term consequences of venous obstructions and occlusions can be devastating for patients,” said William Marston (University of North Carolina, Chapel Hill, USA). “Based on my preclinical experience, I believe the Recana system represents a promising treatment option that overcomes key limitations of traditional recanalisation methods.”
InterVene shares that it designed Recana’s integrated system of complementary components to enable single-session treatment, reduce the need for additional devices, and improve procedural efficiency. Its stainless-steel helical coring element has a sharpened bevelled edge to clear tough, residual venous obstructions and occlusions, with a spiral nose cone to assist with crossing. Nitinol collection baskets deploy from the lower extremities to capture thrombotic material, streamlining the procedure workflow.
“The Recana system marks an important advancement in the treatment of venous stent obstruction, a common outcome following deep venous stent placement, particularly in patients with thrombotic pathology,” said Kush Desai (Northwestern Medicine, Chicago, USA). “This innovative solution has the potential to transform the standard of care for this challenging condition, ultimately improving patient outcomes and quality of life.”

Akura Medical closes series C financing
Akura Medical has secured a US$53 million first close in series C financing, with the funds to be used to support development activities for the Katana thrombectomy system and the NavIQ quantification software, alongside completion of enrolment in the QUADRA-PE clinical trial and regulatory submissions.
The financing round was led by Qatar Investment Authority (QIA) with participation by current investors. Part of the funds will be used to set up a joint venture in Qatar.
“Akura Medical is advancing a differentiated portfolio of venous thrombectomy technologies aimed at improving procedural precision and patient outcomes. The recent introduction of our NavIQ quantification software represents the next step toward intelligent, data-driven thrombectomy,” said Amr Salahieh, chair and acting chief executive officer (CEO) of Akura Medical. “This new capital enables us to accelerate product development and continue building the foundation for long-term growth. We appreciate QIA’s confidence and partnership as we deliver on our vision.”
Akura Medical’s thrombectomy portfolio includes both the flagship Katana system and the NavIQ procedural planning software in development. The Katana system is an intelligent thrombectomy system designed to remove diverse clot types, optimise catheter delivery and provide supplementary intraoperative feedback.
The system leverages high-velocity saline jets that are engineered to effectively break up clots independent of morphology and prevent catheter clogging for procedural efficiency. The system also incorporates sensors that provide real-time pulmonary artery pressure data to provide insights into
27–30 January
Leipzig Interventional Course (LINC) Leipzig, Germany hmpglobalevents.com/linc
28 February–4 March 38th annual meeting of the American Venous Forum (AVF) Denver, USA venousforum.org/education/ annual-meeting
procedure progress.
NavIQ is a quantification software that is designed to convert a computed tomography (CT) angiogram into a 3D model of the pulmonary vasculature. The model is intended for use in anatomical visualisation of the arteries and clots to allow for pre-procedural planning. Additionally, NavIQ is designed to provide clot characterisation data to assist physicians with treatment prioritisation.
Akura Medical is a Shifamed portfolio company.
Pathfinder Medical appoints Lorenzo Patrone as chief medical officer
Pathfinder Medical recently announced the appointment of interventional radiologist Lorenzo Patrone (Azienda USL Toscana Centro, Florence, Italy) as its chief medical officer (CMO).
Patrone has been collaborating with Pathfinder since 2019 as lead clinical consultant. A press release states that he has played a pivotal role in the development and validation of the company’s ePath catheter system, which is designed to enable precise, minimally invasive vascular connections.
During his time with Pathfinder, Patrone has performed the first clinical cases using the company’s CE-marked first-generation ePath device to treat lower limb chronic total occlusions (CTOs). He has also led Pathfinder’s preclinical endovascular arteriovenous fistula (endoAVF) studies and will now serve as an investigator in the upcoming first-in-human study for wrist-level endoAVF creation.
Sorin Popa, chief executive officer (CEO) of Pathfinder Medical, said: “Lorenzo has been a driving force behind the clinical progress of our technology. His extensive endovascular experience, creativity and passion for improving patient outcomes make him an exceptional addition to our leadership team as we advance toward commercialisation.”
Pathfinder notes in its press release that Patrone brings over a decade of international experience as a consultant vascular and interventional radiologist, currently at Azienda USL Toscana
8–10 March 29th European Vascular Course (EVC) Maastricht, the Netherlands vascular-course.com
19–21 March IMEndo Forum Rome, Italy imendoforum.com
Centro. He has published widely on endovascular innovation, served as assistant editor for the Journal of Endovascular Therapy, and is active in global vascular education having founded the Find Your Algorithm (FYA) Congress.
Patrone commented: “Creating reliable, minimally invasive vascular access has long been one of the holy grails in interventional medicine. Having worked closely with Pathfinder for over six years, I’ve seen the transformative potential of its technology and am excited to help guide its journey from concept to clinical adoption.”
InspireMD names Peter Soukas as chief medical officer InspireMD, developer of the CGuard Prime carotid stent system for the prevention of stroke, has announced the appointment of Peter Soukas (The Miriam Hospital and Brown University, Providence, USA) as chief medical officer (CMO).
“We are thrilled to have Dr Soukas join InspireMD to help lead our clinical and medical organisation with strategy and oversight at this pivotal time for the company,” said Marvin Slosman, chief executive officer (CEO) of InspireMD. “His expertise and clinical practice focus on carotid intervention and peripheral vascular disease offer valuable insights as we launch CGuard Prime into the US market. Dr Soukas has been a strong supporter of our work advancing our next-generation CGuard Prime stent platform to transform patient care through best-in-class clinical outcomes. This transformational time for carotid intervention requires us to build a team to lead the field, and Dr Soukas will be a tremendous contributor to our work ahead.”
the American College of Cardiology, the Society of Vascular Medicine, the Society of Coronary Angiography and Interventions, and the American College of Physicians. In addition to his role at InspireMD, Soukas will be maintaining his clinical practice.
Soukas has served as the site principal investigator on more than 24 trials in carotid stenting and the site principal investigator on more than 150 endovascular trials. In addition, he has delivered over 300 invited lectures, and is a prolific researcher, publishing dozens of peer-reviewed papers. He is board-certified in cardiovascular disease, interventional cardiology, vascular medicine, and endovascular medicine, and is certified in noninvasive vascular lab interpretation.

“I’m honoured to join InspireMD as chief medical officer. As a physician, I’ve witnessed firsthand the devastating impact of stroke and the transformative difference that innovations like CGuard Prime can make for patients,” said Soukas. “Physicians depend on the partnership with industry to advance new innovation and the team at InspireMD is an incredible example of how this ecosystem works well. My commitment is to guide the company with oversight of clinical and medical topics that build on success to date while establishing greater awareness of this breakthrough stent platform.”
Biocoat announces formation of Surmodics Services & Technologies division
Biocoat has formed Surmodics Services & Technologies, a newly named division that will carry forward the portion of Biocoat’s business remaining after merging with Surmodics and divesting certain Biocoat assets in connection with the transaction.
8–10 April
A press release notes that Soukas brings significant experience and credentials in the field of vascular intervention to InspireMD. He serves as the director of vascular medicine and the peripheral vascular interventional laboratory at the Miriam Hospital and is an associate professor of medicine at the Warren Alpert Medical School of Brown University, where he also directs the vascular and endovascular medicine fellowship programme, which he founded. He is a fellow of
CLI-C Global Venice, Italy cli-courses.com/events/ cli-c-global-2026
21–23 April
Charing Cross (CX) Symposium London, UK cxsymposium.com
27–29 April Advanced Vascular Conference (AVC) Frankfurt, Germany avc-vasc.com
10–13 June Vascular Annual Meeting (VAM) Boston, USA vascular.org/vam-2026
A press release details that Surmodics Services & Technologies represents the legacy Biocoat business operations that were not divested. This division will now operate as part of the Surmodics organisation under its new name. Based in Horsham, USA, Surmodics Services & Technologies will continue delivering an array of advanced hydrophilic and other coating technologies as well as specialised services to medical device manufacturers worldwide.
25–26 June
British Society of Endovascular Therapy (BSET) annual meeting South Gloucestershire, UK bset.co.uk
25–27 June
26th annual meeting of the European Venous Forum (EVF) Versailles, France europeanvenousforum.org