1 minute read
Section 5: Validation of Bionector as a 360 Access Device
from Bionector Handbook
by VygonGroup
bionector®
Advertisement
Can Bionector be suitable for use up to 360 accesses ? bionector®
Introduction
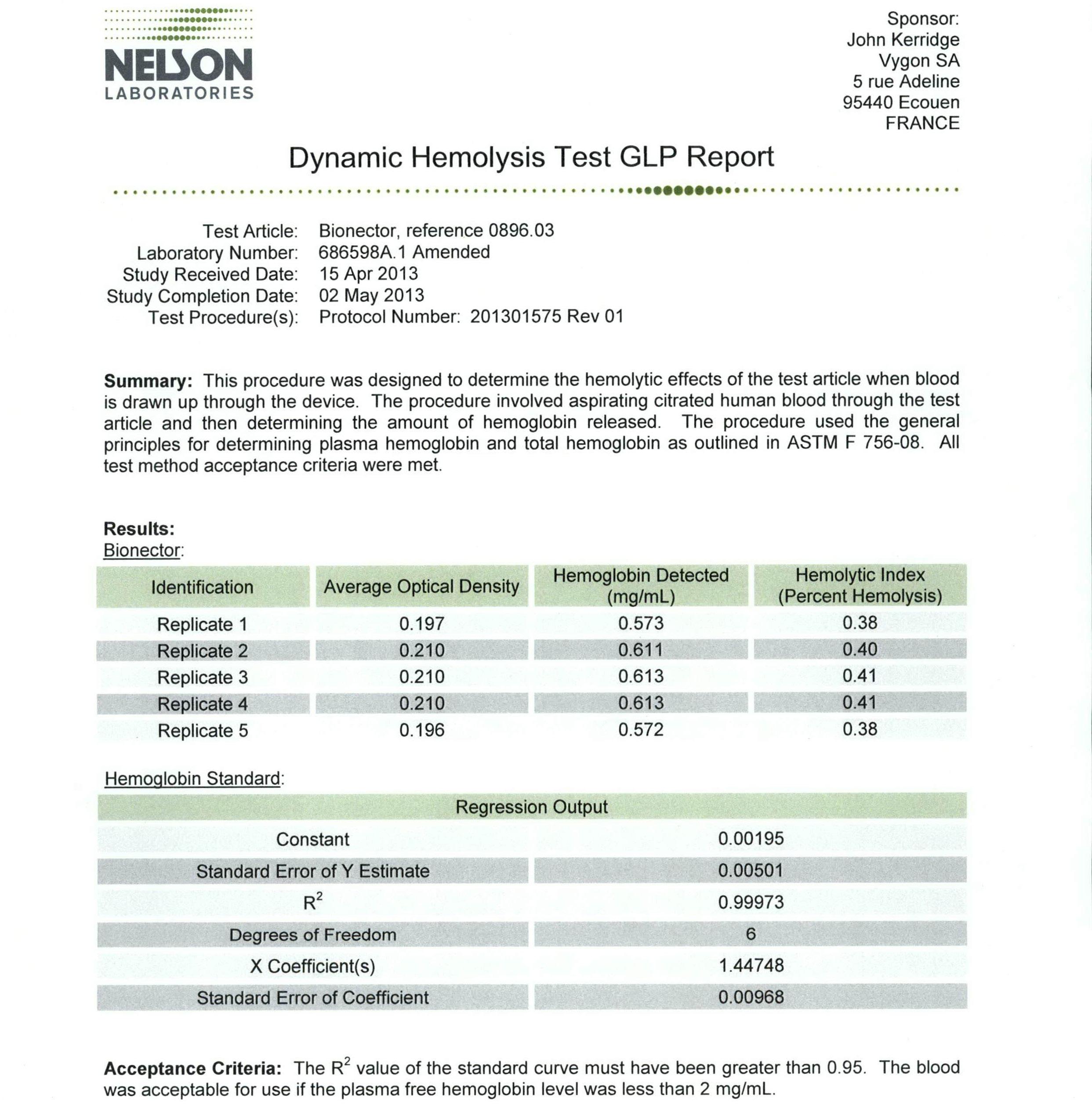
This report summarises the validation of Bionector as a 360 access device, with reference to the tests carried out and the results found. Bionector has been initially tested to validate the acceptable number of uses, and production batches are subjected to random sample testing to ensure continued compliance. Bionector is produced in such high volumes that the test machines run almost constantly performing these production sample tests.
Method
Testing equipment is used to simulate repeated access to the sample Bionector devices. Each sample Bionector is attached to the testing machine, then subjected to repeated accesses using a syringe barrel. Each sample is subjected to an initial 100 accesses before being checked for the following: • Leaks: The Bionector is connected to a pressurised system and checked for any visible leaks. Testing is carried out at 700 mbar of counterpressure. • Membrane deformation: The membrane should return to its original resting position and should show no signs of being deformed. • Membrane movement: The membrane must remain contained in the Bionector casing and should not be either partially or completely expelled from the device. • Presence of shavings: There should be no evidence of plastic or polyisoprene becoming detached from the Bionector, which could lead to the product failing to function correctly.
Bionector access simulation
The samples are then subjected to further repeated accesses and tested at fixed intervals until failure is reported, or until 360 accesses have been made with no reported failure.
Conclusion
The needleless I.V. access device Bionector has been tested and proven to be suitable for use up to 360 accesses. No leaks or shavings were found, and the membrane returned to its original resting position after each use.
Validation Reports
• Validation report 04227 for initial production batch testing. • Validation report 00294 testing Bionector to determine the maximum number of accesses. • Quality Control Document Ref C2083 testing membrane function after repeated accesses.