









As I reflect on my time as dean of the University of Maryland School of Pharmacy, I continue to be filled with pride — pride in our legacy, our forward momentum, and, most of all, in the extraordinary people who make up our community. Each day, I see faculty, staff, students, alumni, preceptors, and partners working with determination and vision to advance pharmacy practice, research, and education — not only here in Maryland, but across the globe.
We are living in a time of complex and difficult realities for higher education. Fiscal constraints are a challenge for nearly every institution, including ours. Yet, what sets us apart is the strength of our community: the way we support one another, innovate boldly, and stay focused on our mission. In the face of uncertainty, we remain united by a clear purpose: to globally engage and lead education, pharmacy practice, scientific research, and pharmapreneurial initiatives to collaboratively and equitably improve the health of society.
In this issue of Capsule magazine, you’ll see just how intentionally we are living out that mission. You’ll read about our work in substance use prevention and treatment through state-funded educational initiatives. You’ll learn about our contributions to pediatric patient care, including the development of a new tool to help improve the taste of medications, an innovation with the potential to significantly improve adherence and outcomes for our youngest patients. We also spotlight the groundbreaking work of our Computer-Aided Drug Design Center, whose long-term impact is a testament to the power of sustained, visionary research, and to the value of investing in talent and ideas that shape the future of therapeutics.
Throughout the pages of this magazine, you'll meet people who are making a difference — whether it’s our preceptors guiding the next generation of pharmacists, our alumni and donors expanding our reach and impact, or our students and faculty pushing boundaries in classrooms, clinics, and laboratories. These stories remind us that progress is never made alone. It’s built through collaboration, shared purpose, and a steadfast commitment to excellence.
Thank you for being part of our community. Together, we will continue to grow, adapt, and lead, always guided by the mission, vision, and core values that define the University of Maryland School of Pharmacy.
With warm regards,
Sarah L.J. Michel, PhD Dean and Professor of Pharmaceutical Sciences
OUR MISSION:
The University of Maryland School of Pharmacy globally engages and leads education, pharmacy practice, scientific research, and pharmapreneurial initiatives to collaboratively and equitably improve the health of society.
OUR VISION:
The University of Maryland School of Pharmacy is internationally recognized for:
• empowering learners and graduating visionary leaders
• pioneering social impact and business innovation through pharmapreneurship
• listening to all voices to equitably enrich the lives of our internal and external communities
• positively influencing the delivery of convenient and affordable health care
• leveraging pharmaceutical expertise and relentlessly collaborating to solve scientific, clinical, and social problems that matter to all citizens of the world
OUR VALUES:
We embrace a culture that embodies the University of Maryland, Baltimore’s core values:
• Respect and Integrity: We value each other and hold ourselves accountable for acting ethically and transparently using compassion and empathy.
• Well-Being and Sustainability: We care about the welfare of our people, planet, communities, and University.
• Equity and Justice: We embrace and are committed to diversity, and we value inclusive and just communities. We oppose discrimination in all its forms.
• Innovation and Discovery: We imagine and explore new and improved ways to accomplish our mission of education, research, clinical care, and service.
University of Maryland School of Pharmacy Alumni Magazine
Becky Ceraul, Capsule Editor
Assistant Dean, Communications and Marketing, School of Pharmacy
Chris Zang, Editor
Julie Bower, Director, Design Services University of Maryland, Baltimore Office of Communications and Public Affairs
Special thanks to the following contributors: School of Pharmacy
Ken Boyden, JD, EdD
Associate Dean, Development and Alumni Affairs
Pamela Carder
Senior Web Content Specialist/Writer Communications and Marketing
Greer Griffith, MS
Executive Director, Development and Alumni Affairs
Tae Kerney
Videographer/Photographer, Communications and Marketing
Liz Myers, MBA
Lead Marketing Specialist, Communications and Marketing
Student Government Association
UniverSity of maryland, Baltimore office of commUnicationS and PUBlic affairS
Emily Bleiweis, Senior Media and Public Relations Specialist
Lou Cortina, Director, Editorial Services
Joanne Morrison, Senior Director, Marketing and Public Relations
We welcome your comments, news, and suggestions for articles. Send your ideas to Becky Ceraul at the University of Maryland School of Pharmacy, 20 N. Pine St., Room N302, Baltimore, MD 21201. Email: rceraul@rx.umaryland.edu; Telephone: 410-706-1690; Fax: 410-706-4012.






By going above and beyond, three University of Maryland School of Pharmacy (UMSOP) employees have earned recognition in recent months as Employees of the Month at the University of Maryland, Baltimore (UMB).
Erica Chaffin, MBA, started the trend in November 2024 for her work as director of human resources at UMSOP.
William J. Cooper, MBA, senior associate dean for administration and finance, nominated Chaffin for the award, saying she is “a go-to person for other schools and even UMB Human Resources on ways to approach complex issues and functions. A handful of her protocols and processes have been copied and used by other UMB schools and central administration.”
Added UMSOP Dean Sarah L.J. Michel, PhD, “She really is a calm voice who is always able to figure out how to solve what we like to call opportunities, not challenges. She is always available, and her colleagues in the School view her as a really wonderful person to go to with small things, big things, or whatever the issue is.”
Chaffin, who first worked for UMSOP from 2005 to 2013 and then returned in 2018 after a job in UMB’s Office of Central Administration Support Services, handles human resources tasks such as hirings, terminations, benefits, learning and development, and much more.
Chaffin says her five-year detour to the UMB job gave her great insight into dealing with Universitywide issues.
“In this office, we do not only handle negative aspects of disciplinary action or policy, we help people with their benefits and we help people with their leave administration,” she said. “We just make sure that the employees’ overall experience coming in the door and while they’re here is enjoyable.”
Two months after Chaffin, Thomas “Joe” Howarth, PhD, MS, was named UMB’s January 2025 Employee of the Month as the lead training specialist for the PATIENTS Program. He was recognized for fostering collaboration, advancing health equity, and building meaningful relationships within and beyond UMB.
One of his most significant contributions has been the development and launch of the PATIENTS Professors Academy, a free annual five-week online program that brings together patients, caregivers, government representatives, researchers, pharmaceutical representatives, and more to learn from each other on how to conduct patient-centered and community-engaged health research.
“Joe has an incredible ability to connect with people from all walks of life, making everyone feel seen, heard, and valued,” said C. Daniel Mullins, PhD, executive director of the PATIENTS Program.
Howarth was thrilled by the UMB award, which comes with a plaque, letter of commendation, and a $250 bonus. “Obviously, this is an incredible honor,” said Howarth, who joined the PATIENTS Program in 2022 with the mission to advance patient-centered, community-engaged research. “It is without any competition the best job I’ve ever had, and I think it comes down to the culture of this University and the culture of this team.”
A month later Donna Dortch was UMB’s February 2025 Employee of the Month. Her job title at UMSOP is associate director of records and registration. But Dortch’s job duties extend far beyond merely tracking students’ academic performance and making sure they are properly signed up for classes each semester.
“I’m also involved in the academic hearings process, and I’ve worked on several Schoolwide projects,” said Dortch, who has worked at UMSOP since 2016. “I also help with all our office’s events and work
Continued on page 3
Continued from page 2 with the UMB registrar and SIMS [school information management systems] team.
“I do audits for graduating students, making sure they are on track to graduate. It’s hard to put my job into just a couple of words, because it covers a big area.”
Shannon Tucker, PhD, MS, assistant dean of instructional design and technology who wrote the Employee of the Month award nomination, pointed out how Dortch had worked to modernize
UMSOP’s processes.
Added JuliAna Brammer, MBA, director of records and registration, “Our office knows how valuable Donna is and all of the things she does for our students, coordinating with multiple offices on campus to make sure that our students get what they need, when they need it.”
Congratulations to all three of the School of Pharmacy’s recent UMB Employees of the Month.

and Learning (SoTL).
Mandee Booth, PharmD, BCIDP, assistant professor in the Department of Practice, Sciences, and Health Outcomes
Research at the University of Maryland School of Pharmacy (UMSOP), has been awarded a 2025–2026 Elkins Fellowship for the Scholarship of Teaching
The Elkins SoTL Fellowship, administered by the University System of Maryland’s (USM) Kirwan Center for Academic Innovation, supports faculty-led research projects that aim to enhance student learning and contribute to institutional improvement. Booth’s selection followed a competitive application process open to faculty across USM’s 12 institutions.
Her fellowship project focuses on applying Transparency in Learning and Teaching (TILT) strategies to pharmacy experiential education, particularly the Advanced Pharmacy Practice Experience (APPE) rotations. The project seeks to better prepare students for real-world pharmacy practice by clarifying learning objectives and expectations during clinical rotations.
“I’ve been interested in the scholarship of teaching and learning since transitioning to academia in 2022,” says Booth. Before that,
Booth worked full time as an infectious diseases clinical pharmacy specialist at the University of Maryland Medical Center, where she continues to practice and precept students on rotation. “During a UMSOP PharmD curriculum retreat in December, I learned about the TILT framework, and I saw potential to apply it in a new way — specifically within experiential education, which hasn’t been explored widely in the literature,” she says.
The TILT model emphasizes transparency by structuring assignments and learning activities around three core elements: purpose, task, and success. Booth’s project will begin by surveying UMSOP’s APPE preceptors to identify barriers students face during clinical rotations. These insights will inform redesigned teaching strategies within her own elective rotation on infectious diseases, followed by pre- and post-rotation student surveys to measure the impact on student preparedness and professional identity formation.
The fellowship officially began in April 2025 with a virtual kickoff meeting, where Booth and 10 other fellows presented their project ideas. Throughout the 2025-2026 academic year, the fellows will meet regularly to offer peer feedback. They will present final findings in May 2026.
“There’s already a strong sense of collaboration and innovation among the cohort,” Booth says. “Some of the other projects involve using AI to create new forms of simulated learning, which I am excited to learn from. This fellowship offers a wonderful opportunity for both mentorship and growth.”

Andrew Coop, PhD, professor in the Department of Pharmaceutical Sciences (PSC) at the University of Maryland School of Pharmacy (UMSOP) and associate dean for students, has been named chair of the Maryland Task Force on the Responsible Use of Natural Psychedelic Substances.
The task force, established through legislation signed into law by Gov. Wes Moore in 2024, aims to explore pathways for the safe, accessible, and equitable use of natural psychedelics while addressing their status as federally illegal substances.
Coop, a nationally recognized medicinal chemist, brings decades of expertise to the role. His career has been dedicated to studying the pharmacology of drugs with abuse liability, specifically the design and synthesis of new opioid analgesics with reduced tolerance, and the development of novel antidepressants. Coop also has testified as an expert witness in federal criminal trials, at the U.S. Congress, and at the Maryland General Assembly, providing critical insights into the pharmacological effects of psychoactive substances.
“Natural psychedelics have shown great potential for treating a range of conditions, from mental health disorders to chronic pain,” says Coop. “I’m honored to lead this task force as we work to create a blueprint for their responsible use in Maryland. Our work has the potential to improve lives while ensuring safety and access for all Marylanders.”
The 19-member state task force is a multidisciplinary group comprising experts in regulatory affairs, law, health care, patient advocacy, and social justice. Their charge includes examining:
• Existing laws and practices regarding natural psychedelics.
• Scientific evidence on their therapeutic benefits and potential risks.
• Barriers to access, such as licensing, insurance coverage, and zoning regulations.
• Policy changes needed to create a Maryland Natural Psychedelic Substance Access Program.
Coop emphasized the importance of education in the task
force’s mission. “A major focus will be educating providers, patients, law enforcement, lawmakers, and the general public about natural psychedelics,” he says. “Understanding their pharmacology and applications is key to ensuring their safe and effective use.”
Psychedelics are a class of psychoactive substances that produce changes in perception, mood, and cognitive processes. They can treat suicidality, addiction, post-traumatic stress disorder, anxiety, obsessive-compulsive disorder, chronic headache, chronic pain, eating disorder, and traumatic brain injury. The U.S. Food and Drug Administration has designated the psychedelic psilocybin as “breakthrough therapy,” and the U.S. Department of Defense is funding psychedelic research for the military and veterans.
At UMSOP, Coop teaches courses in the Doctor of Pharmacy program on pharmacology and chemistry, and a course in the MS in Medical Cannabis Science and Therapeutics program on medical psychedelics, bridging the gap between scientific research and clinical practice.
The task force’s recommendations are poised to set the stage for Maryland to become a national leader in the responsible use of natural psychedelics.
“This work is crucial,” Coop notes. “It’s about creating a system that serves everyone while addressing public health and social justice issues.”
“We are incredibly proud of Dr. Coop’s appointment as chair of this important state task force,” says Sarah L.J. Michel, PhD, dean of the School of Pharmacy and professor of PSC. “His decades of research in the area of medicinal chemistry and his deep understanding of psychedelic pharmacology uniquely positions him to lead this critical effort.
“The task force’s work will help shape evidence-based policies that balance the potential therapeutic benefits of natural psychedelics with public safety, accessibility, and equity. Dr. Coop’s leadership exemplifies our School’s commitment to advancing science, education, patient care, and public health.”


Joga Gobburu, PhD, MBA, professor in the Department of Practice, Sciences, and Health Outcomes Research (P-SHOR) at the University of Maryland School of Pharmacy (UMSOP), has been appointed as the School’s Gyi Endowed Memorial Professor in Pharmapreneurship. A nationally and internationally recognized leader in pharmacometric innovation and drug development, Gobburu exemplifies the entrepreneurial spirit that defines pharmapreneurship.
As founder and director of the School’s Center for Translational Medicine, Gobburu has pioneered novel approaches to drug therapy, regulatory science, and translational research, co-founding two companies — PumasAI, Inc., and VivPro Corp. — that support pharmaceutical scientists, health care providers, biotechnology and pharmaceutical companies, and regulatory agencies. Additionally, Gobburu created the School of Pharmacy’s MS in Pharmacometrics in 2012, the first 100 percent online master’s program at the University of Maryland, Baltimore (UMB). He also recently launched an MS in Artificial Intelligence for Drug Development.
“The Gyi Endowed Professorship is not just an honor — it’s a call to action to champion bold ideas, foster entrepreneurial thinking, and push the boundaries of what’s possible in health care,” says Gobburu. “I am deeply grateful to Dean Michel for entrusting me with this opportunity and for supporting a vision of innovation that empowers both our School and the future of health care.”
Terry Gyi, BSP ’83, PharmD ’06, and her daughter Rebecca GyiHovis, MD, established this endowed professorship in memory of their late husband and father Felix A. Khin-Maung-Gyi, BSP ’83, PharmD, MBA, chair and founder of Chesapeake Research Review and one of the School of Pharmacy’s nine Founding Pharmapreneurs. Gobburu succeeds Magaly Rodriguez de Bittner, PharmD ’83, MS, FAPhA, FNAP.
Nicole Brandt, PharmD ’97, MBA, BCGP, FASCP, professor in P-SHOR and executive director of the School’s Peter Lamy Center on Drug Therapy and Aging, has been named the School’s Parke-Davis Chair in Geriatric Pharmacotherapy.
“I am humbled and honored to be named the Parke-Davis Chair
in Geriatric Pharmacotherapy,” says Brandt. “This recognition affirms the critical importance of advancing evidence-based medication use and safety in older adults and acknowledges the collective efforts of colleagues, mentors, and collaborators dedicated to this field. I am committed to upholding the legacy of this position and to furthering innovations in geriatric pharmacotherapy through research, education, and advocacy.”
Since joining the School of Pharmacy in 1999 after earning her PharmD in 1997, Brandt has been a transformative leader in geriatric pharmacotherapy. She has expanded geriatric training opportunities, including the geriatrics/palliative care pathway in our Doctor of Pharmacy (PharmD) program, an American Society of Health-System Pharmacists-accredited geriatrics residency, and a two-year postPharmD fellowship.
Brandt leads key initiatives at the MedStar Center for Successful Aging and played a pivotal role in securing Age-Friendly University distinction for UMB and the University of Maryland, Baltimore County. She is leading efforts with the American Society of Consultant Pharmacists in leveraging pharmacists as Age-Friendly Champions.
Brandt, who succeeds Linda Wastila, BSPharm, MSPH, PhD, as Parke-Davis Chair, is a recognized advocate for policy change at both the state and national levels. She has led projects addressing Medicare Part D Medication Therapy Management programs, highrisk medications, and medication stewardship. As an author of the 2012, 2015, 2019, and 2023 American Geriatrics Society (AGS) Beers Criteria, a past president and board chairman of the American Society of Consultant Pharmacists, and a co-chair of the COVID-19 Implementation Guide for Post-Acute and Long-Term Care, she has had a profound impact on the field. Additionally, she helped launch the Elder Care Medicine Network, focused on patient-centered outcomes research in medication use among older adults.
Her outstanding contributions to geriatric education earned her AGS’s 2019 Dennis W. Jahnigen Memorial Award and the 2022 Clinician of the Year Award from the Foundation for Post-Acute and Long-Term Care Medicine.

The University of Maryland School of Pharmacy (UMSOP) has been designated an ACT Community Pharmacy Center of Excellence by the American Association of Colleges of Pharmacy (AACP).
The recognition from the Academia-Community Transformation (ACT) Pharmacy Collaborative and AACP, the country’s leading higher education pharmacy organization, reflects the School’s ongoing commitment to advancing community pharmacy through teaching, service, scholarship, leadership, and partnerships.
The 17 Centers of Excellence in this inaugural cohort will serve as ambassadors for pharmacy academia through convenings with pharmacy and health care industry leaders, uniting to mobilize and amplify community pharmacy practice transformation efforts.
“Community pharmacy is at the heart of the pharmacy profession and what most people associate with pharmacists,” said Sarah L.J. Michel, PhD, dean of the School and professor in the Department of Pharmaceutical Sciences. “UMSOP’s faculty, in partnership with organizations in Baltimore and surrounding communities, are strongly invested in our communities and the health of our patients. The School’s community pharmacy faculty are exemplars of these efforts and work closely with our students and residents to nurture their interest in this vital area of pharmacy and assist with practice transformation. I congratulate everyone involved in our community pharmacy efforts on this much-deserved recognition.”
To qualify for the Center of Excellence designation, the School of Pharmacy had to demonstrate its efforts and expertise across a spectrum of community pharmacy activities such as curriculum,
community service, research, advocacy, and patient care.
• UMSOP partners with Safeway Pharmacy to offer a one-year PGY-1 community-based pharmacy residency program to develop community pharmacist practitioners with diverse patient care, leadership, and education skills.
• The School was heavily involved in the University of Maryland, Baltimore’s COVID-19 vaccination efforts and annually offers an on-campus clinic for various vaccinations and COVID-19 boosters.
• The Center for Innovative Pharmacy Solutions (CIPS) is the leader in delivering health care and business solutions that address critical health problems. The P3 (Patients, Pharmacists, Partnerships) Program represents the clinical initiative arm of CIPS that pairs pharmacists with patients through their employer to deliver effective medication therapy management solutions.
• UMSOP faculty, staff, and residents are actively involved in community pharmacy-based research and scholarship.
• The Office for Continuing Education offers various certificate programs, such as pharmacy-based immunization delivery and medication therapy management.
“Our community pharmacies play such a vital role in our health care system,” said Deanna Tran, PharmD ’11, BCACP, FAPhA, associate professor in the Department of Practice, Sciences, and Health Outcomes Research (P-SHOR) and the School’s ACT Champion. “We are excited to continue working within our communities and in close collaboration with our community pharmacy partners to uplift community pharmacy, transform practice, and improve the health outcomes of our patients. We are deeply honored that UMSOP was chosen to represent the inaugural cohort. Thank you to the many faculty, staff, alumni, and students involved in our community pharmacy efforts at UMSOP.”
“Community pharmacy continues to be a valuable resource, and the University of Maryland School of Pharmacy has a long history of faculty, staff, students, preceptors, and alumni helping patients stay healthy in the community pharmacy space,” said Cherokee Layson-Wolf, PharmD ’00, BCACP, FAPhA, professor of P-SHOR and PGY-1 community pharmacy residency program director. “The community pharmacist is the most accessible health care provider, and through initiatives such as the ACT Center of Excellence, we are able to highlight the importance of community pharmacists and champion their efforts.”

The Maryland Poison Center (MPC), a key service of the University of Maryland School of Pharmacy, has extended its coverage to encompass all 23 Maryland counties, Baltimore City, and the District of Columbia. Previously, Prince George’s and Montgomery counties, along with the District of Columbia, were serviced by the National Capital Poison Center. This expansion represents a significant milestone in the delivery of comprehensive poison control services and is being led by the Maryland Department of Health and the District of Columbia Department of Health (DC Health).
“The Maryland Department of Health is proud to support the School of Pharmacy’s expansion of Maryland Poison Center services to every jurisdiction in our state,” said Nilesh Kalyanaraman, MD, FACP, former deputy secretary for public health services at the Maryland Department of Health. “This expanded coverage gives Marylanders closer proximity to poison control education and event reporting and helps eliminate gaps in state, local, and regional incident response preparedness.”
Ayanna Bennett, MD, MSPH, FAAP, director of DC Health, emphasized the importance of accessible emergency resources, stating, “We remain committed to ensuring all residents have access to life-saving resources and expert guidance when faced with a poisoning emergency. This expansion marks a vital step in strengthening regional public health services, improving response times, and enhancing collaboration across jurisdictions. We look forward to working alongside the Maryland Poison Center to continue protecting and educating our communities.”
Since 1972, the MPC has served as an essential resource, providing 24/7 expert guidance to Marylanders through the help line, 1-800-222-1222. Callers receive immediate assistance from experienced health care professionals — including pharmacists, nurses, and medical experts with a combined 250 years of experience at the Maryland Poison Center — helping to address poisoning emergencies and provide critical information on treatment and prevention.
“We are thrilled to bring the talents of our extremely dedicated staff to Prince George’s and Montgomery counties and the District of Columbia,” said Angel Bivens, BS Pharm, MBA, CSPI, managing director of the MPC. “Whether it’s our pharmacists and nurses providing expert and compassionate care to callers or our public educator creating programs and materials to provide awareness and poison safety tips, our new communities are in good hands.”
“Maryland emergency medical service clinicians in EMS, Eds, and primary care across Maryland have relied on the Maryland Poison Center for just-in-time, evidence-based clinical guidance when a poison exposure has occurred,” said Cyndy Wright-Johnson, MSN, RN, director of EMS for Children at Maryland Institute for Emergency Medical Services Systems. “Having immediate access to experienced health care professionals in poisoning situations is a crucial part of providing the best care to our communities. The expansion to include Montgomery and Prince George’s counties in the population of citizens, visitors, and clinicians served by the same Maryland Poison Center enhances the support in emergencies and increases the distribution of poison prevention educational materials.”
Sarah L.J. Michel, PhD, dean of the School of Pharmacy and professor of the Department of Pharmaceutical Sciences, emphasized the significance of this expansion. “This is an exciting development for public health throughout the region,” said Michel. “Expanding our service coverage area ensures that every community member has access to the expertise and resources of the Maryland Poison Center. This step forward in integration streamlines communications and improves the efficiency of the entire system, benefiting all residents.”
With this expansion, the MPC not only increases its geographic coverage but also streamlines data collection and health care communication across the region. The reorganization of services ensures that health care providers can access up-to-date, reliable information and fosters new opportunities for collaboration and response to poisoning incidents.
In addition to the 1-800 help line, the MPC is utilizing a digital companion tool, PoisonHelp.org, to provide individuals with valuable information on poisons, symptoms, and treatments. This userfriendly online resource complements the center’s phone services.
This regional expansion will enhance the MPC’s ability to respond to poisoning emergencies, improve public health outcomes, and ensure that critical data is available to health care providers and emergency responders when needed most.
“We are excited to provide a coordinated, regional approach to poisoning management and education,” said Joshua King, MD, FAACT, FACMT, medical director of the MPC. “Our goal is for this change to be completely seamless for all residents of Maryland and the District of Columbia, with access to the same expert care in times of need.”
The University of Maryland School of Pharmacy has launched a new Master of Science in Artificial Intelligence for Drug Development (MS in AIDD). The program’s goal is to equip students with a comprehensive understanding of AI’s applications and opportunities in pharmaceuticals.
“The role of artificial intelligence [AI] in drug development is transformative, enhancing the capabilities of the pharmaceutical industry and researchers,” says Joga Gobburu, PhD, MBA, professor in the Department of Practice, Sciences, and Health Outcomes Research and director of the School’s Center for Translational Medicine, who oversees the new academic program. “Our exploration of AI’s growing influence inspired us to develop this program to equip students with the skills to leverage AI’s potential, driving innovation in drug development.”
“The University of Maryland School of Pharmacy is very excited to offer the MS in AIDD degree,” says Sarah L.J. Michel, PhD, dean of the School and professor in the Department of Pharmaceutical Sciences. “The features of the new program distinctly emphasize interdisciplinary research and interprofessional education, a strategic focus of the University of Maryland, Baltimore. The program uniquely integrates cutting-edge AI technologies with
pharmaceutical sciences.”
Gobburu, who is also the Gyi Endowed Memorial Professor in Pharmapreneurship, adds that the degree is designed to prepare graduates for diverse career paths in the pharmaceutical industry, academia, regulatory agencies, and AI-driven health care startups.
The key focus of the program, which begins in fall 2025, will be on AI-enabled predictive analytics. Gobburu explains: “The question is no longer whether AI will transform health care, but how we can harness its immense potential to elevate what matters most — saving lives. We’ve flipped the narrative by focusing on producing health care professionals with hands-on expertise in applied AI, equipping them to solve real-world challenges. Instead of teaching AI as a stand-alone technique, we are building a future workforce that seamlessly integrates AI into health care, driving innovation and impact where it’s needed most.”
Coursework will develop students’ practical skills through hands-on experiences with AI tools, programming, and methodologies. “Students will complete application-oriented exercises in natural language processing, data analytics, and machine learning geared toward predictive analytics, which are crucial to the future of innovative drug development,” says Gobburu.
Throughout the program, students also will explore ethical and societal implications of AI in drug development, including privacy, bias, and transparency.
The program’s eight graduate-level required courses cover the principles of drug development, AI and machine learning (ML) methodology, and applications to drug development strategy, pharmacovigilance, precision medicine, and clinical trial optimization. Additional coursework will allow students to discover the applications of AI/ML to other areas of drug development, regulatory policies, and ethics.
The MS in AIDD program is 100 percent online and follows case-based teaching. Instruction includes lectures, active learning discussion boards, and individual and group assignments.
“Students from a variety of disciplines will be able to join us from wherever they are in the world via an internet connection,” says Gobburu. “The MS in AIDD was designed for professionals who want to transition into decision-making data scientists in pharmaceutical, biotechnology, or government institutions,” he adds.
The following University of Maryland School of Pharmacy faculty members received promotions.
• Catherine Cooke, PharmD, MS ’18, BCPS, PAHM – research associate professor in the Department of Practice, Sciences, and Health Outcomes Research (P-SHOR) to research professor.
• Alison Duffy, PharmD, BCOP, FNAP – associate professor of P-SHOR to professor.
• Natalie D. Eddington, PhD ’89, FAAPS, FCP – dean and professor in the Department of Pharmaceutical Sciences (PSC) to dean emeritus.
• Steven Fletcher, PhD – associate professor of PSC to professor with tenure.
• Mojdeh Heavner, PharmD ’08, BCCCP, FCCM, FCCP – associate professor of P-SHOR to professor.
• Amy Ives, PharmD, BCPS – assistant professor of P-SHOR to associate professor.
• Jason Noel, PharmD, MPA, BCPP – associate professor of P-SHOR to associate professor emeritus.
• James Trovato, PharmD, MBA, FASHP – professor of P-SHOR to professor emeritus.
• Fengtian Xue, PhD – associate professor of PSC to professor with tenure.
• Wenbo Yu, PhD – research assistant professor of PSC to research associate professor.
Cynthia Boyle, PharmD ’96, FAPhA, FNAP, FASCP, has been elected president of the American Institute of the History of Pharmacy.
Nicole Brandt, PharmD ’97, MBA, BCGP, FASCP, was named the Doctor of Pharmacy’s Class of 2025 Faculty Preceptor of the Year.
Katharine Briggs, PhD, Marc Taraban, PhD, and Bruce Yu, PhD, received a U.S. patent for “Detection of Pharmaceutical Product Freezing History Using Water Proton NMR.”
Becky Ceraul graduated from the University of Maryland, Baltimore’s (UMB) President’s University Leadership Program as a member of its sixth cohort.
Andrew Coop, PhD, Cherokee Layson-Wolf, PharmD ’00, BCACP, FAPhA, and Shannon Tucker, PhD, MS, are co-recipients of the Best Poster Award from the American Association of Colleges of Pharmacy’s (AACP) Administrative Services Section. Coop also has been named vice chair of AACP’s Research and Graduate Affairs Committee.
Daniel Deredge, PhD, and Jill Morgan, PharmD, BCPS, BCPPS, FNAP, have been voted the School of Pharmacy’s AACP Teachers of the Year.
Bethany DiPaula, PharmD ’95, BCPP, FASHP, Megan Ehret, PharmD, MS, BCPP, and Raymond Love, PharmD ’77, BCPP, FASHP, were named Fellows of the American Association of Psychiatric Pharmacists.
Natalie D. Eddington, PhD ’89, FAAPS, FCP, received the Maryland Pharmacists Association’s (MPhA) Honorary President Award.
Oacia Fair, MS, and Troy Parker, MBA, have been elected to the UMB Staff Senate as a full voting senator and an alternate senator, respectively. Parker also graduated from UMB’s Emerging Leaders program.
Steven Fletcher, PhD, Alexander MacKerell Jr., PhD, and Paul Shapiro, PhD, were among the co-inventors of “Non-ATP/Catalytic Site p38 Mitogen Activated Protein Kinase Inhibitors,” which has been issued European patents in Norway, Spain, Switzerland, and the United Kingdom. Fletcher also has been voted the School of Pharmacy’s alternate delegate to AACP’s House of Delegates.
Joga Gobburu, PhD, MBA, was named the UMB Founders Week 2024 Entrepreneur of the Year. He was also the keynote speaker at the University of Florida College of Pharmacy’s Bodor Distinguished Lecture.
Mojdeh Heavner, PharmD ’08, BCCCP, FCCM, FCCP, received the Presidential Citation Award from the Society of Critical Care Medicine (SCCM) and was named chair of its Leadership, Empowerment, and Development Committee and chair-elect of its Clinical Pharmacy and Pharmacology Section.
Continued on page 10
Continued from page 9
Jace Jones, PhD, has been appointed PSC’s vice chair for education. He’s also been voted president-elect of the School of Pharmacy’s Faculty Assembly.
Kaitlin Landolf, PharmD, BCCCP, received SCCM’s Presidential Citation Award.
Cherokee Layson-Wolf, PharmD ’00, BCACP, FAPhA, and Magaly Rodriguez de Bittner, PharmD ’83, MS, FAPhA, FNAP, received the University of Maryland School of Pharmacy (UMSOP) Alumni Association’s 2024 Evander Frank Kelly Honored Alumnus Award. Layson-Wolf also received the 2025 University System of Maryland Regents Faculty Award for Excellence in Mentoring and was named the Doctor of Pharmacy’s Class of 2025 Teacher of the Year.
Lisa Lebovitz, JD, MS ’21, is a co-recipient of the AACP Rufus A. Lyman Award for the best paper published in the American Journal of Pharmaceutical Education in 2024. She is also a corecipient of the Collaborative Research Publication Award from AACP’s Assessment Special Interest Group and the Innovations in Administrative Practice Award from AACP’s Administrative Services Section.
Daniel Mansour, PharmD ’06, MS ’24, AGSF, BCGP, FASCP, received the Christian Pharmacists Fellowship International’s Advisor of the Year Award.
The School of Pharmacy’s Master of Science in Regulatory Science program has been selected as the recipient of the 2025 Program Achievement Award from AACP’s Graduate Education Special Interest Group.
Mary Lynn McPherson, PharmD ’86, PhD, BCPS, received the MD Anderson Palliative Medicine for Outstanding Contribution to the Field of Cancer Pain Management Award and the UMB Graduate Student Association’s Dr. Patricia Sokolove Outstanding Mentor Award.
Sarah L.J. Michel, PhD, received the 2024 Maryland Chemist of the Year Award from the Maryland Section of the American Chemical Society. She also has been named vice chair of the AACP Council of Deans Programming Committee.
Jill Morgan, PharmD, BCPS, BCPPS, FNAP, received UMSOP Alumni Association’s 2024 B. Olive Cole Honorary Alumnus Award. She also has been elected president of the MPhA Foundation.
C. Daniel Mullins, PhD, received AACP’s Paul R. Dawson Award for Excellence in Patient Care Research.
Kristine Parbuoni, PharmD ’05, BCPPS, has been appointed the School’s inaugural assistant dean of PharmD student life and student success and has been voted the School of Pharmacy’s delegate to AACP’s House of Delegates.
Emily Paterson, MPH, CHES, has been appointed to the board of directors of the Partnership for a Safer Maryland.
Kathleen “Katy” Pincus, PharmD ’09, BCPS, BCACP, CDCES, has been elected to MPhA’s Board of Trustees.
Scott Riley, PhD, was named the MS in Pharmaceutical Sciences program’s Class of 2025 Teacher of the Year and has been named co-chair of the Health and Life Sciences Hub at the Universities at Shady Grove.
Charmaine Rochester-Eyeguokan, PharmD, CDCES, BCACP, has been accepted to AACP’s Academic Leadership Fellows Program.
Jana Shen, PhD, MS, has been named editor-in-chief of the Journal of Computational Chemistry
Yan Shu, MD, PhD, has been appointed PSC’s vice chair of research.
Julia Slejko, PhD, has been named director of the School of Pharmacy’s Pharmaceutical Health Services Research Graduate Program.
Audra Stinchcomb, PhD, RPh, FAAPS, FAIMBE, has been named to the advisory board of RSC Pharmaceutics.
Zoe Welsh has received certification from Asana as a workflow specialist and graduated from UMB’s Emerging Leaders program.
Fengtian Xue, PhD, has been appointed the School of Pharmacy’s associate dean for research.

Computer modeling finds the most promising compounds and guides their transformation into drugs.
BY NALA ROGERS
When structural biologist David Weber, PhD, sends a new drug target to the University of Maryland School of Pharmacy’s Computer-Aided Drug Design (CADD) Center, he imagines it being swarmed by tiny spiders.
The “spiders” are molecules such as benzene, propane, and methanol, modeled in a computer system along with the protein of interest and the water it all floats in. These mini-molecules represent fragments of larger compounds, and each type can undergo a different suite of chemical interactions. As the fragments surround the protein, computer software maps how they cling to its surface, revealing places where a new drug might bind.




This fragment-based mapping is the key innovation of the SILCS method, one of the CADD Center’s signature achievements and the foundation of its thriving spinoff company, SilcsBio LLC. The resulting “FragMaps” can be used to quickly screen millions of compounds, revealing the ones most likely to make good starting points for drugs.
“A larger drug might not be able to wiggle into a groove or a specific place, but the fragments can,” says Weber. “That, I think, has been really powerful.”
Weber is a professor of biochemistry and molecular biology at the University of Maryland School of Medicine and director of its Center for Biomolecular Therapeutics. He has worked closely with the CADD Center’s director, Alexander MacKerell Jr., PhD, the Grollman-Glick Professor in the Department of Pharmaceutical Sciences (PSC) at the School of Pharmacy, since the 1990s, before the center itself coalesced out of MacKerell’s research lab around the year 2000. At first, Weber was skeptical that MacKerell’s simulations could capture the complexity of biomolecular interactions accurately enough to be useful. Now, he is a convert — one of many across the University System of Maryland and beyond.
In the 21st century, computational modeling has emerged as a crucial component of drug discovery, and the School of Pharmacy’s CADD Center has been instrumental in its rise. Scientists and pharmaceutical companies around the world rely on methods developed at the CADD Center to save them from endless trial and error in the lab. The CADD team consists of MacKerell; the center’s co-director Jana Shen, PhD, MS, professor of PSC; Wenbo Yu, PhD, a research associate professor; and approximately 20 postdoctoral fellows, graduate students, researchers, and staff.
The center has been continuously funded since its inception, reflecting its value to medical science and society. Currently, it has eight grants from the National Institutes of Health, totaling nearly $13 million, with additional support from the U.S. Food and Drug Administration and the Samuel Waxman Cancer Research Foundation.
Work at the CADD Center includes both developing computational methodology and applying that methodology to real-life projects. Each drug development project begins with a target: a protein with a key role in a disease such as melanoma, muscular dystrophy, traumatic brain injury, or inflammatory bowel disease. A collaborator sends a new target to the CADD Center’s scientists, and they throw it to the virtual spiders, hunting for molecules that can provide a handhold with which to steer the course of disease.
“[MacKerell] can take all those structures and do his computational magic, and say ‘these compounds are going to interact best with the site that we want it to interact with,’” says Paul Shapiro, PhD, professor in PSC and a biochemist studying protein kinases.
Shapiro’s collaborations with the CADD Center have led to dozens of promising drug candidates, including a drug in phase 2 clinical trials that is designed to help people with acute respiratory distress syndrome (ARDS), a deadly lung condition.
Once scientists like Shapiro have tested compounds from CADD’s initial list, they send the most promising ones back to the CADD Center for ideas on how to tweak them. For instance, the models might show an unused pocket at the interface between target and proto-drug and suggest adding a particular chemical group that can reach into that pocket and grab hold more tightly.
This back-and-forth between in vitro and in silico experiments can vastly speed up drug development. Shapiro’s ARDS drug, dubbed Gen-1124, took just five years to go from a starting point compound with weak affinity for the target to an investigational drug in humans. A typical timeline is more like 10 to 15 years, says Shapiro.



MacKerell hasn’t always conducted his experiments on computers. He worked with real enzymes as a graduate student at Rutgers University, earning his PhD in biochemistry in 1985 for work on how the human body breaks down alcohol. But over the next few years, first at the Karolinska Institute in Sweden and then at Harvard University and Swarthmore College, he embraced the potential of computational modeling. By the time he joined the faculty at the University of Maryland School of Pharmacy in 1993, he was one of the key figures shaping the field.
When asked to explain how it all works, MacKerell struggles to compress the complexity into mere words. The underlying models are called “force fields,” and they represent the energy between atoms.
“It’s a mathematical equation that describes the reality of the system,” says MacKerell. “You need to calculate the energy and the forces acting on a system to understand how molecules interact, which is the core of drug design.”
The applications go far beyond drug design, from materials science to chemical physics. One of MacKerell’s early papers on force fields has more than 16,000 citations because so many people have used the method.
For example, as a student, Yiling Nan, PhD, MS, used force fields developed at the CADD Center for petroleum engineering. While looking for surfactants that could help petroleum mix with water during extraction, she realized she could improve how the force fields modeled ions. Now she is working on that problem as a postdoctoral fellow in MacKerell’s lab. She wants to use her advances to improve how batteries function at different temperatures and to extend their life span by adjusting their mixture of cations, anions, and organic solvents. Better ion modeling also could help her find alternative solvents that reduce the risk of batteries catching fire.
In the late 2000s, MacKerell developed the SILCS method,
which translates the modeled data into intuitive maps and practical suggestions. SilcsBio was launched in 2012, and its customers now include approximately 30 pharmaceutical companies, including many of the world’s largest.
“What we do better than anybody else is give a really easy visualization to why certain molecules bind better than others to a given protein,” says Ken Malone, PhD, chief executive officer of SilcsBio. “The person doing drug design can see things that they couldn't otherwise see.”
Generating the initial SILCS FragMaps can be time-consuming, but once they exist, using them is fast and computationally cheap. That’s because FragMaps simplify the details of a target protein into just the crucial information about where and how different chemical groups can bind. Competing methods such as free energy perturbation (FEP) must start from scratch to simulate how the target interacts with each compound being screened. A virtual experiment that takes a day using FEP might take a minute with a SILCS FragMap, says MacKerell.
That efficiency makes it possible to tackle problems that would otherwise be out of reach. One such project is an add-on application for the SILCS software that will model how long a given molecule stays attached to a target and what happens when it breaks away. This is called dissociation kinetics, and it is a major reason why drugs fail.
“Some drugs have a very strong affinity but can also have a very fast off-rate. So they can fall off the target very easily,” says Yu. “We want them to bind tightly, but also we want the ligand to stay there for a long time.”
Affinity, or binding strength, is defined by the fraction of molecules that are bound to their targets in a mixture, not by how long a given molecule stays attached. It’s comparatively simple to calculate affinity because you only have to model two states: fully bound or floating free. To understand the kinetics, you also have to model all the intermediate states as you drag the molecule off its target, says Yu.


That’s why drug developers normally wait to tackle kinetics until they can measure it directly. The SILCS Kinetics application could provide crucial information like the off-rate much sooner, before years and fortunes have been invested.
To perform their digital wizardry, the CADD Center scientists need vast computational power. Enter Ronald Kasl, MBA, who maintains the center’s five high-performance computing clusters with hundreds of graphics processing units (GPUs) and thousands of central processing units (CPUs). It is not typical for a school of pharmacy to have its own supercomputing center, but MacKerell realized early in his career that he would need serious hardware to run his simulations in-house. He identified Kasl, a Linux devotee, as the person to build it for him.
“Someone with Ron’s expertise and talents is essential for our work,” says MacKerell.
Kasl built the CADD Center’s High-Performance Computing (HPC) systems gradually and with minimal resources, relocating them through a series of larger and larger rooms culminating in a state-ofthe-art data center in the basement of Pharmacy Hall. There, they run hot 24/7, roaring as they strain to keep up with demands. Icy air blasts down from ceiling vents to keep the servers cool.
Both the computing clusters and their cooling system have backup power, but once during a power outage, the backup failed. By the time Kasl arrived a few minutes later, he could feel heat radiating through the cinderblock wall as he rushed down the hallway outside. “It was like a sauna,” he says.
Even during normal operations, systems are breaking and needing to be pampered as they are pushed to the limit, a given in research computing environments. Kasl has a cardboard box full of hundreds of dead hard drives, casualties of boundary-breaking science.
Several floors up in Pharmacy Hall, Juliet Obi expresses and purifies a protein that the Dengue virus uses to replicate its genetic material. She is looking for a drug to attack this viral protein, preparing to test candidates identified through SILCS screening. The HPC clusters in the basement allow her to simulate chemical processes that would take several microseconds to play out in the real world. At conferences, she encounters envy from colleagues who are limited to much shorter simulations lasting picoseconds to nanoseconds.
“They’re like, ‘Your school has that computational power?’” she says. “I feel lucky to be here.”
Obi is a PhD candidate in Daniel Deredge’s lab, much of whose success Deredge attributes to the generosity and support of the CADD Center.
“Alex [MacKerell] actually eggs me on and pushes me to use the resources, rather than me having to ask for access or time,” says Deredge, PhD, an assistant professor in PSC.
That generosity is intrinsic to the philosophy of the ComputerAided Drug Design Center. The expertise of its scientists and the power of its computers are for the benefit of the department, and for people outside as well, says MacKerell. Kasl attributes this vision to MacKerell and, like the rest of the CADD team, he has embraced it.
“It’s unique that we have all these resources,” he says, “but it's also unique that we are sharing them.”

BY ROBYN FIESER
From mixing antibiotics with pudding to freezing cough syrup into Popsicles, parents will try just about anything to get their kids to take bad-tasting medicines. And for years, the medical community wasn’t far behind. Health care practitioners and even hospitals were relying on the same tips and techniques, none of which were ever really proven to taste better. It was all based on anecdote and assumptions about what might work to help children better tolerate their medication.


Jill Morgan, PharmD, BCPS, BCPPS, FNAP, professor and chair of the Department of Practice, Sciences, and Health Outcomes Research and a pediatric pharmacist at the University of Maryland School of Pharmacy (UMSOP), and Amy Kruger Howard, PharmD ’17, MS ’21, a pediatric clinical pharmacist at UMSOP, knew there had to be a better way.
Nearly 75 percent of active pharmaceutical ingredients are bitter, and while capsules and tablets are designed to avoid taste issues, that doesn’t help infants and younger children who can't swallow them. Liquid medicines are often the only option, but they taste awful. Worse still, many of the common tricks used to hide the flavor just don’t disguise it.
The problem isn’t just children’s heightened perception of bitter taste. Poor palatability is directly linked to non-adherence, meaning children are more likely to skip or refuse doses altogether, which can be dangerous or even life-threatening.
So Morgan and Howard created the Ew Meds List™ — a comprehensive, evidence-based guide to the worst-tasting liquid medicines, along with proven strategies to make them taste better. Instead of relying on guesswork, the list provides pharmacists and providers with real tools that help — from better-tasting drug alternatives to pharmacy flavoring recommendations and home-based masking interventions. No more trial and error. No more bribery. Just science, with a little creativity on the side.
Now in the hands of pharmacists and providers, the Ew Meds List is helping caregivers make informed choices and getting kids the treatment they need.
“This is actually a huge problem, with the majority of medicines not tasting good,” says Morgan. “And it’s a big issue for kids with chronic health issues who have to take medication every day.”
For decades, pediatric medicine has faced a persistent challenge. Despite approximately 25 percent of all medicines being prescribed for children, most of these drugs are formulated for adults. This disconnect means the medicines are not optimized for taste, dosage, or ease of administration. As a result, providers, nurses, and pharmacists find themselves in a tough spot when trying to help parents ensure their children take their medicine.
A clear example of this issue came up during Howard's fellowship in pediatric pharmacy under Morgan at the School of Pharmacy, when she encountered a young patient who needed IV antibiotics for a bone infection. After several days in the hospital, the child was discharged with a prescription for oral antibiotics to continue treatment at home. After just four or five days at home, the child spiked a fever, and it became evident that the infection was spreading again.
The problem? The child refused to take the bad-tasting antibiotics at home. Howard desperately tried to find ways to make the medicine more palatable and easier for the child to take. She ultimately tried using chocolate milk as a chaser and it worked. The child finished the course of antibiotics.
Unfortunately, this is a common situation — poor medicine adherence due to bad-tasting medicine can worsen a child’s condition, making them sicker and potentially prolonging their recovery. This longstanding issue is something many health care professionals have seen firsthand for years. After experiencing it repeatedly at their practice sites, Morgan and Howard decided it was time to act. They set to work on the Ew Meds List.
Morgan, Howard, and then-student Dafne Espinal Peña, PharmD ’24, took a direct and thorough approach. First, they surveyed health care prescribers to understand their knowledge of and practices around

bad-tasting oral liquid medicines and taste masking. They found that few were confident in identifying unpleasant-tasting medicines, and most didn’t educate patients on masking techniques. While many suggested mixing medicine with food or drink, this technique can reduce drug effectiveness. The survey results underscored the need for better education on taste masking to ensure both adherence and efficacy.
The team then reviewed medical literature in databases like PubMed and Embase, focusing on studies involving children and keywords like "bitter," "flavor," "masking," and "adherence." Their search, limited to human randomized controlled trials and systematic reviews published between 1980 and 2021, focused specifically on studies with numerical taste ratings, which were converted to a five-point Likert scale. Medicines with scores below 2.5 were added to the Ew Meds List, while those rated 2.5 or higher were considered better-tasting.
Only a small number of medicines have evidence-based, flavormasking recommendations supported by research. For the rest of the medicines on the list, Morgan and Howard examined the pharmacologic properties of commonly used drugs and categorized them based on their likely dominant flavor profile. These profiles were then paired with findings from food science research on how to effectively mask bitter, sour, salty, or sweet tastes.
For example, to mask the taste of amoxicillin, one of the most prescribed antibiotics in the world, the list recommends flavors such as berry, fruit, or vanilla. Similarly, for the bitter taste of prednisone, flavors like chocolate, mint, or wild cherry can help mask it, while salty medicines may be masked with flavors such as apricot, butterscotch, peach, or vanilla.
This approach helps make medicines more palatable for children by matching the right masking flavors to each medicine's taste. The list also includes a series of at-home practices to mask bad taste, such as giving something cold before the medicine to numb the taste buds like a Popsicle or using a sticky or sugary food to coat the tongue.
BY GWEN FARISS NEWMAN
The Ew Meds List is now available on Pyrls, a clinical drug information platform developed by Derek Borkowski, PharmD, for clinicians and student clinicians. Pyrls offers a mobile app and web-based tools that provide evidence-based pharmacotherapy resources, making it easier to access essential drug information in clinical settings. Borkowski met Morgan and Howard at a conference and was quickly interested in adding their Ew Meds List to the Pyrls website, which covers a wide range of specific drugs.
According to Borkowski, the Ew Meds List used evidence-based research to fill a big gap that had previously been addressed with anecdotal opinions. “The scientifically based analysis we’re able to provide to our users through the Ew Meds List is a first of its kind,”
he says, adding that the list has received positive feedback from users in practice settings who interact frequently with parents and pediatric patients. One community pharmacist shared with him how he used it to help a parent select a compatible and effective flavoring to add to their child's antibiotic.
Morgan and Howard’s innovative work is a shining example of UMSOP’s Pharmapreneurship® initiative in action, which merges entrepreneurship and pharmacy to tackle real-world health care and research challenges. By fostering a mindset of critical thinking and problem-solving, Pharmapreneurship encourages students, faculty, staff, and alumni to think outside the box and push the boundaries of what’s possible.
The initiative offers a pathway within the School’s Doctor of Pharmacy curriculum that gives students a unique opportunity to develop their entrepreneurial skills through a specialized curriculum focused on innovation, business, and creative problem-solving. There are also business pitching competitions, workshops, seminars, and even scholarships focused on empowering future pharmacists to lead change not just in the lab or the clinic, but in the broader health care system.
For Morgan and Howard, the Ew Meds List is more than just a tool. It represents a step toward a future where pediatric care is revolutionized by innovation. They have a vision: to not only raise awareness among prescribers about the taste issues with certain medicines but also to encourage changes in prescribing habits and provide better guidance for parents. They’re exploring ways to integrate taste-related alerts into pharmacy systems, so when a medication is prescribed, a flag would notify both prescribers and parents about potential palatability issues.
While this is still a “pie in the sky” idea, it represents the kind of forward-thinking approach that defines the University of Maryland School of Pharmacy — and it’s just the beginning of a new era in health care.
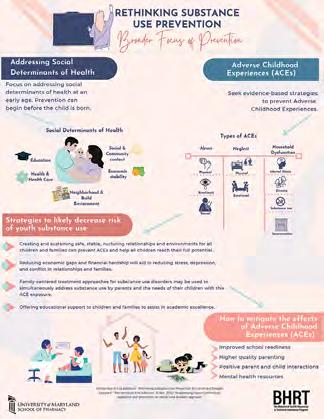
The Behavioral Health Resources and Technical Assistance Program drives data and substance solutions.

BY JOSEPH CANTLUPE
To help prevent and reduce substance use — especially among young people — communities, organizations, providers, and others in the health care field sometimes need that extra boost to understand how they identify those at risk and prevent negative outcomes.
With a precision population health approach, the University of Maryland School of Pharmacy’s Behavioral Health Resources and Technical Assistance (BHRT) Program provides that boost, using data-driven practices to understand and evaluate why people are misusing substances.


The questions are many. Are young people taking illicit opioids or misusing prescription medications? Are their interests outside school or what’s happening in their family life driving them to this? Are providers fully aware of the impact of drugs they prescribe? And should communities provide more specific education about certain types of drugs?
“We don’t just study these issues on paper, we step out from behind our computer screens and actively engage with public health departments and various stakeholders, working closely with them,” says BHRT Executive Director Fadia Shaya, PhD, MPH, a professor in the Department of Practice, Sciences, and Health Outcomes Research (P-SHOR) at the School of Pharmacy. “Patterns of drug use are heavily influenced by the environment, education level, and employment status of the population.”
In essence, BHRT uses innovation and technology to move from data to action, and Shaya’s team has sought to perfect the science of dissemination and implementation.
Working with federal and state governments, BHRT helps pharmacists, dentists, physicians, and other providers properly evaluate prescribed and over-the-counter medications to identify red flags for misuse. BHRT’s experts address substance use and behavioral health issues, as well as social determinants of health, across all 24 Maryland jurisdictions, with a mission to provide support and solutions.
“Our solutions are anchored in the profile and dynamics of a community,” adds Shaya, who was the University of Maryland, Baltimore (UMB) Founders Week Teacher of the Year in 2017 and now is a UMB Distinguished Professor and an MPower Professor. “A large part of what we do is stakeholder engagement to better understand the ecosystem, from providers, to regulators, to health systems, and payers.”
It's an approach that Lester Davis, vice president and chief of staff at CareFirst BlueCross BlueShield, applauds.
“Our mission is to serve the community and meet them where they are, providing services that match their needs, at the right place at the right time,” Davis says. “We spend a lot of time analyzing trends — both historical and current — to help guide and inform our
partnerships and investments. We are always happy to be engaged with thoughtful partners [like BHRT].”
BHRT offers technical guidance to assist communities in thwarting drug misuse or alcohol abuse. These actions range from providing one-page fact sheets on issues such as cannabis use to interactive epidemiological tables showing patterns of use. The fact sheets might include census data specific to the jurisdiction or other records that detail drug usage among groups in the jurisdiction’s neighborhoods.
The BHRT team of about 10 members also compiles complimentary educational outreach materials for providers to support prescribing decisions based on the latest evidence-based statistics. In addition, the team helps local health departments implement programs. Team members are adept at working with organizations to identify effective strategies, use focus groups, and help people more easily “visualize” reams of important data.
Thanks to its efforts, BHRT receives nearly $1 million in grants and contracts annually, according to Shaya.
“We work to understand the needs within communities by keeping our ear to the ground,” she says. “We assess these needs through quantitative and qualitative data collections and key informant interviews, which vary significantly from Baltimore City to Caroline County to Prince George’s County. We then collaborate with the jurisdictions to help them build capacity and understand the tools they need to engage with schools, families, and organizations.”
In its role as technical assistance provider for all the jurisdictions it serves, the BHRT team provides support for a variety of prevention programs. Jurisdictions across the state, assisted by BHRT in planning and strategic development, are currently implementing preventive education programs for youth and families, safe storage and disposal initiatives, naloxone distributions, the Maryland Opioid Academic Detailing Program, compliance checks and Responsible Beverage Service trainings, and increasing capacity and community mobilizing.
BHRT collaborates closely with the Maryland Department of Health’s Prescription Drug Monitoring Program, which offers free educational outreach for medical providers. It provides evidence-based

Shaya:
"Our solutions are anchored in the profile and dynamics of a community."

recommendations, especially for opioid prescribing, aiming to increase prescribers’ and pharmacists’ adherence to these recommendations for opioid safety. BHRT provides technical assistance and evaluation support to develop these strategies.
“I believe we have evidence showing that by addressing policyrelated factors, we can impact the entire community,” Shaya adds. “Providing answers, where people live, has significant policy implications.”
Ana Lazarides, director of Maryland’s Prescription Drug Monitoring Program, agrees, saying, “We strongly promote the use of the Prescription Drug Monitoring Program to help health care providers make informed clinical decisions when prescribing controlled substances. Whether it’s a pediatrician, prescriber, pharmacist, or dentist, we equip them with the knowledge and skills to recognize potential warning signs of substance use or misuse.
“We analyze our data and collaborate with the BHRT team to identify these warning signs. From this analysis, we educate providers on best practices, particularly in prescribing patterns and how we prescribe to different groups,” Lazarides says.
The BHRT team collaborates with the Prescription Drug Monitoring Program to develop various medical educational materials, as part of the Maryland Opioid Academic Detailing Program. Essentially, academic detailing helps “prescribers understand opioid misuse and engage more effectively with patients,” says Adrian Catwell, a health policy analyst and provider outreach coordinator for the state. One of their newer programs is a pediatrician working group that addresses opioid prescribing for the young population to “reduce unsafe or inappropriate” prescribing, he says.
Working with BHRT, “we paint a picture of what’s going on in the county, providing a snapshot with powerful illustrative tools,” says Catwell. “The goal is to change the mindset, encourage behavioral change, and help prescribers make informed clinical decisions.”
Communities often conduct surveys to evaluate drug issues within the population they serve. These surveys are detailed and provide answers that help teams address specific problems. For instance, a
survey on opioids may examine patients’ access to prescription and nonprescription opioids via friends, family members, and prescribers.
Nicole Sealfon, MPH, associate director of BHRT, explains that the team works closely with physicians, health departments, and community groups to develop data collection tools to measure outcomes and the impact of substance use prevention programs. In some instances, the planning may not be a good fit, so they readjust.
“We help organizations implement the plans, and if they hit a roadblock or the plan wasn’t a good fit, we make necessary adjustments,” she says. “We help providers, pharmacists, and others visualize data they have collected, displaying what’s happening in terms of needs assessment, support, or outcomes. We provide tools, tip sheets, and guidance documents. We’re not directly in the patient community, but are only one or two steps removed, depending on the grant.”
A crucial element of BHRT assignments is evaluating data in different communities to understand the impact of substance use and help officials carry out preventive measures.
One example is Project Upstream, part of a five-year initiative known as the Strategic Prevention Framework-Partnership for Success program, funded by the Substance Abuse and Mental Health Services Administration (SAMHSA). This grant program identified Carroll and Anne Arundel counties as the focus for implementing the grant activities.
BHRT facilitates the administrative component and guides the implementation of activities conducted by its partners. For example, the team has designed a comprehensive guidebook, and the data is inputted and analyzed. BHRT then translates that data into more digestible formats — such as creating various infographics in colorful and concise terms.
Carroll County, Md., like other areas of the country, is home to teens trying to find their way, with some struggling with mental health and substance use.
BHRT is collaborating with the Carroll County Health Department and the Boys and Girls Clubs of Carroll County to implement a Resiliency Program where the county offers structured programs to help middle and high school students avoid mental health struggles or other situations linked to alcohol, tobacco, and illegal drug use. Data shows that more than 48 percent of Carroll County middle school students reported their mental health “was sometimes, most of the time, or not always good the past 30 days,” and nearly 34 percent of high school students felt the same. BHRT created surveys to better understand the needs of the youths, and the county initiated extracurricular programs ranging from knitting to video games as a result.
“A few jurisdictions have youth-led initiatives that are similar to Carroll County in that they are learning skills, which are a benefit/ protective factor while they also gain peer and community support,” Sealfon says. For instance, “Montgomery County has had success growing their youth coalition.”
Similar infographic reports have been made for Anne Arundel County in Project Upstream, highlighting the impacts of substance use and the importance of preventive measures to reduce substance use. Under the SAMHSA grant, the county is implementing Strengthening Families, a prevention education program through which parents and youths attend weekly classes to enhance their skills to improve family dynamics.
All these projects, advocates say, continually evolve and are improved. For instance, the state Prescription Drug Monitoring Program continually examines and revamps its website to update providers and pharmacists, says Lazarides.
“One of the biggest challenges was ensuring that all prescribers knew the importance of how to use the Prescription Drug Monitoring Program,” she says. “We create training videos because providers have very limited time to do research and figure things out, so we make sure they get information as quickly as possible.”
Jill Morgan, PharmD, BCPS, BCPPS, FNAP, professor and chair of P-SHOR, says: "Any effort to enhance knowledge in population health and medication safety ultimately leads to better individual patient care. Fadia Shaya brings the passion, expertise, and skills needed to lead this work."
Whether it’s state, federal or local programs, the Behavioral Health Resources and Technical Assistance Program’s “work is very relevant in managing the optimal flow of medications so that we optimize health care,” Shaya says.
“It’s very important to have proper channels of dissemination of information, and efficient implementation that are scientifically sound,” she adds. “We have the tools and the technologies to reach people. Our next step is to leverage AI tools to accelerate the implementation and dissemination of prevention strategies.”




BY NALA ROGERS
When Ryan Pearson, PhD, associate professor in the Department of Pharmaceutical Sciences at the University of Maryland School of Pharmacy (UMSOP), started college he planned to be a physician. But he also was drawn to engineering and craved the freedom to pursue his own projects and ideas.
He found a mentor in Petr Král, PhD, MSc, a computational chemist at the University of Illinois Chicago (UIC). A turning point came when Král invited Pearson to a meeting about using lipidbased nanoparticles for drug delivery.
“It was fascinating. You can be an engineer, but you can also develop biomaterials and study them to affect human health,” says Pearson.
Nanoparticles can solve a wide range of drug formulation problems, from helping poorly soluble drugs to dissolve in the blood to preventing them from getting stuck in the wrong tissues and organs. They can even protect larger biologics such as gene therapies and monoclonal antibodies from being destroyed by the body’s defense systems.
Pearson carried his enthusiasm for nanoparticles through his PhD work at the UIC College of Pharmacy and a postdoctoral fellowship at the University of Michigan. In 2018 he joined the faculty at UMSOP, and in fall 2024 was named director of its Bio- and Nano-Technology Center (BNTC).
As BNTC director, Pearson is reaching out to scientists across the University System of Maryland, offering the center’s nanoparticle and drug delivery expertise and use of its cutting-edge instruments. He has developed seven new collaborations and is continuing to spread the word.
The collaborative approach Pearson is pursuing with the BNTC reflects that of UMSOP as a whole. The School welcomed Pearson with “open arms,” he says, and he attributes much of his success to that supportive environment.
“Everybody is so open and willing to collaborate. They want to help you succeed,” he says. “It's a beautiful thing to have all of these people working together in harmony to push each other's
research forward.”
Pearson believes that in addition to serving as vehicles for drug delivery, nanoparticles also can be used as therapeutic agents in and of themselves, a major area of research ongoing in his lab.
For instance, sepsis develops due to an uncontrolled immune response during infection and is often fatal, contributing to approximately one in five deaths worldwide. When researchers induce polymicrobial sepsis in mice, the mice die, even if they receive antibiotics. But Pearson has discovered that if he administers blank nanoparticles made from polylactic acid (PLA) in addition to antibiotics, half of the mice survive.
PLA is a polymer that naturally degrades in the body, with physicians using it for sutures and bone implants since the 1960s. While most new drugs must navigate years of safety testing and regulatory hurdles, a new use for PLA could be approved more quickly, illustrating a guiding principle behind Pearson’s work: simplicity.
Simple solutions have an “elegance,” he says, and therapies based on them can reach the clinic sooner than more intricate inventions. He believes many simple tools and therapies were abandoned too soon, before researchers understood fully how to use them.
“Most people will make something and then test the effect, and if it doesn't work, they'll abandon it,” he says. “What we want to understand are the causes for those failures.”
Pearson recently submitted collaborative grant proposals for the sepsis project to the National Institutes of Health (NIH), along with another proposal to use nanoparticles as a treatment for obesity. Both proposals received high marks from reviewers, putting them in the category that normally receives funding. His lab has been supported by an NIH R35 Maximizing Investigators’ Research Award since 2021.
Pearson is driven by the thought of the patients his research could someday help — patients like his mother, who passed away from breast cancer in 2009. That was just before he began graduate school, and it shaped who he became as a scientist.
“I'm motivated to push forward and to overcome challenges that we have in our research,” he says. “If you can save one person's life, then it's all worth it.”

Paris Barnes, MS ’24, is passionate about community advocacy. In her role as a senior training specialist with the PATIENTS Program at the University of Maryland School of Pharmacy (UMSOP), Barnes is part of a team whose vision is that patients and stakeholders are heard, inspired, and empowered to co-develop patient-centered outcomes research.
Citing the unfortunate case of Henrietta Lacks, who died in 1951 and whose cervical cells were used in research without her knowledge or permission, Barnes says that patients have not always been informed about the goals or outcomes of research. This includes how data is being disseminated or implemented.
“It is my goal that patients are heard, considered, and engaged in all parts of the research,” Barnes says. She works with a team that teaches the PATIENTS Program’s 10-Step Framework for continuous patient and community engagement. This framework equips patients, communities, and care providers to enhance health care research in their communities.
Barnes began her role with PATIENTS in December 2024, following a three-year stint as the health communications program specialist for the School’s Behavioral Health Resources and Technical Assistance Program. In that role she provided outreach, technical assistance, and data dissemination to local jurisdictions on substance use prevention programs. This kind of work is rewarding, she says: “I get to be part of directly impacting something that makes my community better.”
Clearly, supporting her community is a strong motivator for her. In March, Barnes was appointed by Baltimore Mayor Brandon Scott to serve on the city’s first Opioid Restitution Advisory Board.
BY CHRISTINE STUTZ
And her previous jobs have all been in public health: educating the community about substance use disorders and prevention, medical cannabis education, and helping increase enrollment and outreach efforts for the Affordable Care Act at HealthCare Access Maryland. And she earned an MS in Medical Cannabis Science and Therapeutics from UMSOP in 2024.
Barnes’ supervisor, Thomas “Joe” Howarth, PhD, MS, lead training specialist with the PATIENTS Program, praises her effectiveness. “Paris has immediately made an impact,” he says. “She has an incredible energy that has inspired us. I am excited for her to work with our newest cohort of the PATIENTS Professors Academy, a free, online training program that prepares participants to drive patient-centered health care research in their communities. I think she will make them very welcome and will create new ways to engage with our patient partners.”
In March, Barnes received the University of Maryland, Baltimore’s Community Service Award at its annual Employee Recognition and Service Awards. She was honored for her volunteer work at the Eagle Center in Westminster, Md., since 2021. The center is part of Eagle Restoration Ministries and works to empower families facing barriers by connecting them with resources and educational opportunities.
Married with a 5-year-old son, Barnes devotes about 20 hours a month to the Eagle Center, which includes attending meetings, conducting weekly outreach to schools, planning for the summer Camp Soar, and one week of camp counselor activities.
Howarth says Barnes goes above and beyond to use her skills to enhance the PATIENTS Program. “Paris has reignited our efforts to increase the accessibility of our public-facing documents,” he says. “This is important work, and she has given us new tools to use to increase access. She has led a major revamp of our biweekly newsletter to our patient partners, making it look more dynamic and providing more useful information.
“I am fortunate to work with someone like Paris, who can motivate me to be a better co-worker and supervisor,” Howarth says.

The American Society of Health-System Pharmacists-Student Society of HealthSystem Pharmacists hosted a pharmacy technician thank you letter-writing event in November 2024 as part of its Practice Advancement Initiative week.
Kappa Psi's Sigma Chapter hosted a Spice-A-Thon, a Universitywide wing-eating competition, in February 2025. All donations and proceeds supported Reach Out and Read, a national organization that promotes early childhood literacy by incorporating books into pediatric care.

Students from the School of Pharmacy chapter of the Academy of Managed Care Pharmacy attended the national organization’s Nexus 2024, Oct. 14-17 in Las Vegas, where they networked with professionals, explored innovations in managed care pharmacy, and gained valuable insights through educational sessions and workshops.
need this photo


assistant director of experiential learning; and Michelle Chang, PharmD ’11.
Pharmacy students from several student organizations — the American Pharmacists Association-Academy of Student Pharmacists, Rho Chi, Academy of Managed Care Pharmacy, and the Student Section of the Maryland Public Health Association — participated in a Research Roundtable in March 2025, an engaging event that brought together future pharmacists and faculty to share research, exchange ideas, and spark collaboration. From poster presentations to one-on-one discussions, it was a great opportunity to celebrate innovation and academic curiosity in pharmacy practice and science.



Students explored postgraduate opportunities at a Fellowship Symposium in November 2024 hosted by the School of Pharmacy’s Career Navigation program and the student chapters of the Academy of Managed Care Pharmacy and Industry Pharmacists Organization. Fellows and industry professionals shared their journeys, offered advice, and answered questions about fellowships and nontraditional career paths.



The following students in the PhD in Pharmaceutical Health Services Research (PHSR) program received the program’s Donald O. Fedder Memorial Fellowship:
• Yueh-Yi Chiang, BSPharm
• Udim Damachi, BPharm, MS
• Yu-Hua DFu, PharmD, MS
• Chia-Yun Hsu, BPharm, MS
• Hyung-Seok “John” Kim, MHS, BS
• Seyedeh Taraneh Mousavi, PharmD
• Nneka Okeke-Morgan, MHS
The following students in the PhD in PHSR program received the program’s Student Travel Scholarship:
• Jennifer Contreras, MPH
• Uzma Pathan, BSPharm, MPharm, MS
• Hoang Tien Tran, PharmD, MPH
Athanasios Chamzas, a graduate student in the Department of Pharmaceutical Sciences (PSC), has received a $43,200 fellowship from Oak Ridge Institute for Science and Education.
Megan Chang, a second-year student pharmacist, received a Maryland Pharmacists Association scholarship.
Jennifer Contreras, MPH, a student in the PhD in PHSR program, was selected as the 2024-2025 Takeda Patient-Centric Value Assessment Predoctoral Fellow.
Shruti Dharmaraj, a graduate student in PSC, has received a oneyear, $30,000 grant from the PhRMA Foundation for “MetaboliteBased Nanoparticles to Treat Allergic Airway Inflammation.”
The School of Pharmacy’s chapter of Christian Pharmacists Fellowship International received the Dr. Manasseh Heeralall Student Chapter of the Year award at the organization’s annual meeting in St. Simons Island, Ga., in May. This recognition was a reflection of the chapter’s commitment to outreach, discipleship, and Christ-centered fellowship. From health fairs and service projects to prayer nights and campus ministry, the students served with both compassion and clinical excellence.
Eposi Elonge, MS, a student in the PhD in PHSR program, has been accepted into the National Institutes of Health’s AIM-AHEAD All of Us training program.
Jiwon Oh, a fourth-year student pharmacist, was a 2025 summer intern for the Academy of Managed Care Pharmacy Foundation.
Rinky Parakra, Agbo-oma Uwakweh, and Madison Worth, students in the PhD in PSC program, received poster awards at the 2025 Frontiers in Chemistry and Biology Interface Symposium.
Roshni Patel, a student in the PhD in PSC program, received a presentation award at the American Association of Pharmaceutical Scientists’ Northeast Regional Discussion Group Annual Meeting.
Vanshika Patel, a student in the PhD in PSC program, received the Audience Choice Award at the Science in a Flash: The American Society for Biochemistry and Molecular Biology Flash Talk Communication Competition.
John Rizk, BSPharm, MSc, a student in the PhD in PHSR program, received the program’s Harris Zuckerman Scholarship Award.
Dominique Seo, MPH, a student in the PhD in PHSR program, has received a one-year, $50,000 grant from the Innovation and Value Initiative for “2024-2025 Health Equity Fellowship.”
Phuong Tran, BSPharm, MPH, a student in the PhD in PHSR program, received the program’s Arthur Schwartz Memorial Scholarship.

BY LYDIA LEVIS BLOCH
For Sharon Wilson, PharmD, BCPS, BCCCP, precepting goes beyond educating, affecting, and inspiring all those in her circle. A clinical pharmacist specialist II in surgery critical care at the University of Maryland Medical Center (UMMC), Wilson became a preceptor for the University of Maryland School of Pharmacy’s (UMSOP) Doctor of Pharmacy (PharmD) program in 1997, a year after joining UMMC.
Today, Wilson is director of the University of Maryland’s critical care pharmacy practice residency, a clinical pharmacy specialist at UMMC, as well as a UMSOP critical care rotation preceptor.
“Precepting gives the pharmacist a chance to be a role model and mentor to students in ways that will impact the course of the profession for years to come,” says Wilson, whose first name is pronounced Shah-ron. “More importantly, precepting is an opportunity to build on the foundation of future professionals who will be responsible for caring for patients and improving their lives.”
Wilson enjoys seeing students learn new skills and has a passion for care of critically ill patients. Precepting allows her to share that passion with her students.
According to Mojdeh Heavner, PharmD ’08, BCCCP, FCCM, FCCP, professor in the Department of Practice, Sciences, and Health Outcomes Research at UMSOP and assistant dean for experiential learning, “Preceptors have a tremendous influence on our PharmD students and their career trajectories.” Providing more than 30 percent of the curriculum, preceptors — pharmacists and other professionals who oversee the PharmD students on their rotations in a variety of settings — are vital to their training.
“A caring preceptor can positively impact students and open doors that they might not have known existed,” says Heavner, herself a former student of Wilson’s.
Jenny Ababio, PharmD ’24, a recent student of Wilson’s, agrees.
“I had two objectives, and the rotation allowed me to achieve them,” Ababio says. “First, I wanted to improve my communication skills, and second, better my teamwork skills, especially in the ICU setting.”
Ababio had envisioned a remote pharmacy career, but during her rotation with Wilson, she embraced the fast-paced environment of critical care and developed confidence in rapid decisionmaking. Her interest in patient care and in high-pressure situations increased. She now works as a pharmacist at a CVS in Queens, N.Y.
“Dr. Wilson created a supportive environment that helped me not only professionally but also personally,” says Ababio.
In addition to precepting, Wilson provides services to a 24-bed surgical ICU at UMMC and is director of the University of Maryland Residency and Fellowship Program’s postgraduate year 2 critical care pharmacy practice residency.
Wilson’s research interests include optimization of medication use in the ICU and pharmacokinetic/pharmacodynamic challenges in ICU patients. Wilson, who earned her PharmD degree from the University of Georgia School of Pharmacy in 1993, is a member of the American Society of Health-System Pharmacists and the Society of Critical Care Medicine, where she is the first non-physician vice chair of the Surgery Section’s Patient Safety Committee.
Wilson’s dedication to learning emanates beyond precepting, through her church’s student empowerment ministry, where she works with other members to provide resources that support the educational goals of the church’s students.
Heavner reminisces about her own rotation with Wilson at UMMC. “Dr. Wilson has a wealth of knowledge, is a calming presence in the hectic ICU environment, and is highly respected by professional colleagues. She genuinely cares about her mentees.”

BY MEREDITH LIDARD KLEEMAN
When Chintal Shah, PhD ’23, MS, BPharm, a health economist and pharmacoepidemiologist based in Rockville, Md., was undecided about pursuing a research career in academia or in the pharmaceutical industry, he conscientiously and judiciously considered the evidence. What one might call an evidence-based approach.
“We talk a lot about evidence-based medicine, or evidencebased decision-making, so I thought, why don’t I apply that to my life and my decisions as well?” Shah recalls. He already knew a bit about academic research from his time as a student in the University of Maryland School of Pharmacy’s PhD in Pharmaceutical Health Services Research (PHSR) program but was less familiar with industry research. “The only way I’ll know if that’s something I want to get into is if I do an internship,” Shah says.
At the end of the fourth year of his PhD studies, he landed an internship with Takeda Pharmaceuticals working on rare disease projects and went on to become a rare disease fellow with the company’s value and evidence generation team. Those experiences persuaded him to focus his career on industry. AstraZeneca Pharmaceuticals hired Shah as a senior scientist in oncology outcomes research after his 2023 School of Pharmacy graduation.
“Some individuals are disease-specific, but for me, I was more interested in the methods and the application of the methods to various disease areas,” Shah says. That curiosity about methods has led to research related to chronic obstructive pulmonary disease (COPD), hematology and rare diseases, and oncology.
Shah applied his interest in analytical methods in his PhD dissertation to quantify the economic burden of COPD in the United States. By contrasting data with previous research, Shah demonstrated that although the proportion of costs for pharmacotherapy has increased in the last 20 years, there has been
a decreased burden of inpatient hospitalizations. That’s in part due to the availability of medications that can manage the disease. That research was featured on the American Lung Association’s website.
When Shah was pursuing his bachelor’s degree at the University of Mumbai’s Principal K. M. Kundnani College of Pharmacy, he had never heard about the field of health economic and outcomes research (HEOR). At the time, it wasn't even part of the college’s curriculum. Shah’s sister, who was pursuing her PhD in medicinal chemistry at Virginia Commonwealth University, learned about it through a friend in the field, and thought it might be a good fit for her inquisitive brother.
Intrigued, Shah began researching the emerging field and decided to pursue a career working in HEOR. “I was, and still am, convinced that HEOR is the future of health care research,” he wrote in his application to the School of Pharmacy’s PhD in PHSR program. “A country’s prosperity depends not only on the financial well-being of its citizens, but also their physical well-being. To ensure this, it is imperative that we do research on real-world data.”
After completing his master’s degree in pharmaceutical outcomes and policy at the University of Florida, Shah was accepted to the School of Pharmacy.
“During his PhD, Chintal actively contributed to numerous research projects and published several manuscripts in peerreviewed journals,” says Zafar Zafari, PhD, MSc, associate professor in the Department of Practice, Sciences, and Health Outcomes Research. “Beyond his research achievements, Chintal excelled as a teaching assistant and later expanded his academic contributions by serving as a guest lecturer for the health economics course.”
Shah credits the School with nurturing and encouraging his innate curiosity. He often returns to campus to deliver guest lectures, and recently attended the School’s PATIENTS Professors Academy, which teaches the 10-Step Framework of patient-centered and community-engaged health research. “I still continue to be in touch with my professors, and to be affiliated with the School of Pharmacy because I had such a good experience,” Shah says.
The School of Pharmacy hosted its annual event on May 8 for members of the David Stewart Associates (DSA) Society, the School’s recognition society for leadership level giving by individuals. The DSA Society was founded in the mid-1980s in honor of David Stewart, the nation’s first professor of pharmacy and one of the University of Maryland School of Pharmacy’s (UMSOP) founders. Members of this prestigious group recognize the importance of sustained leadership giving to provide a solid base of private support and ensure the School’s continued prominence. Members make an annual gift to the School of $1,000 or more. More than half of the students attending UMSOP receive scholarship support thanks to the philanthropic contributions of individuals, including DSA Society members.




On May 15, the School of Pharmacy's student chapter of ISPOR — The Professional Society for Health Economics and Outcomes Research joined the Office of Development and Alumni Affairs and the Department of Practice, Sciences, and Health Outcomes Research (P-SHOR) in hosting an Alumni and Friends Reception at the 2025 ISPOR annual meeting in Montreal. The event brought together our vibrant community of alumni, faculty, and students for an evening of meaningful connection and celebration. Attendees also celebrated C. Daniel Mullins, PhD, professor of P-SHOR and executive director of the PATIENTS Program, who received ISPOR’s Avedis Donabedian Outcomes Research Lifetime Achievement Award, in recognition of his impactful three-decade career.


Stuart Cantor, PhD, is a scientific witness expert at Lemberg Law.
Susan Mercer, PhD, received a 2024 U.S. Excellence Award at Alexion Pharmaceuticals, Inc., recognizing top talent across the organization that goes above and beyond to make meaningful impacts on patient lives.
Adam Bress, PharmD, tenured associate professor, cardiovascular clinical pharmacist, and scientist in the University of Utah Population Health Sciences Department, was named one of the university’s 2024 Presidential Scholars.
Allen Tran, PharmD, started a new role as senior director of applications at UC San Diego Health.
Andrew Haines, PharmD, obtained professional certification in research skills from the American Society of Health-System Pharmacists.
Larren Suh, PharmD, started a new position as director of the 340B Program at Mount Sinai Health System.
Melissa Augustino, PharmD, associate director of pharmacy for quality, regulatory, and medication-use systems at the Hospital of the University of Pennsylvania, is a member at large on the board of the Pennsylvania Society of Health-System Pharmacists.
Mudit Verma, PharmD, obtained certifications in HIPAA and GCP for Clinical Trials with Investigational Drugs and Biologics from CITI Programs.
Cori Gray, PharmD, started a new position as director of Global Market Access and Pricing in Immunology at Sanofi.
Kathleen Kennedy, MS, founder of CannaWorkforce, and Jacquie Cohen Roth, MS, founder and CEO of CannabizMD LLC and the Tea Pad Foundation, are

partnering on a $194,000 grant from the Maryland Department of Labor to develop a comprehensive education program that prepares unemployed and underemployed individuals for careers in the state’s growing cannabis industry.
Michelle Wright, MS, has been appointed to the advisory board of Americans for Safe Access.
Shanetha Marble-Lewis, MS, and Codi Petersen, MS, have been appointed to the advisory board of Americans for Safe Access.
Kadi Nail, MS, has been appointed to the Oklahoma Medical Marijuana Advisory Council.
Sean Kim, PharmD, is director of practice and professional development at the Accreditation Council for Pharmacy Education.
The University of Maryland School of Pharmacy is pleased to re-launch RxIntersect, the School’s networking platform exclusively for alumni, faculty, staff, and current students.
Join your peers to connect with students, faculty, and other alumni as you navigate your professional journey.
Demystify careers and provide insight on what jobs are actually like.
Share what helped you achieve your career goals.
Provide students with real-world experiences, such as job shadowing and internship opportunities.
Create additional career access by providing résumé feedback and job referrals.
RxIntersect is where we come together as a community to support each other in a meaningful way. Visit rxintersect.umaryland.edu to update or complete your profile. We hope to see you there!

I hope this message finds you well! As an integral part of our University of Maryland School of Pharmacy (UMSOP) alumni community, I wanted to take a moment to highlight the important connection between your career experiences and the continued success of our School community.
Throughout your career, you’ve likely seen firsthand how valuable it is to build and nurture professional relationships. Whether it's finding new career opportunities, gaining industry insights, or simply receiving guidance during pivotal moments, the strength of your professional network has played a vital role in your success. That’s why I am including this message in Capsule magazine — to encourage you to stay connected with the School and your fellow alumni and consider mentoring current students through our exclusive online networking platform, RxIntersect.
By remaining engaged with your alma mater and fellow alumni, you not only support your own career growth but also contribute to the success of the next generation of UMSOP graduates. You have the unique opportunity to be a mentor, a resource, and a guiding force to those who may benefit from your expertise and experience.
I invite you to grow your professional network on RxIntersect (rxintersect.umaryland.edu) and take part in our alumni events, career development, and networking initiatives. It’s through these shared connections that we can all continue to grow and succeed in our professional paths. Together, we can create a powerful, interconnected community that supports one another in both personal and professional pursuits.
At the School of Pharmacy, we are excited to help you foster and grow these connections and continue building on the already prestigious reputation of the University of Maryland School of Pharmacy together.
Warmly,
Greer Griffith, MS
Executive Director, Office of Development and Alumni Affairs 410-706-5893 | ggriffith@rx.umaryland.edu
The University of Maryland School of Pharmacy honors the lives and memories of the following alumni who passed away between July 1, 2024, and Dec. 31, 2024. We are grateful to each of these alumni for the lasting impact that they made on the School community and the advances they achieved in education, research, or practice.
John E. Botzolakis, MS ’82, PhD ’85
Joseph W. Cavallaro, BSP ’50
Theodore D. Chung, PhD ’91
Mary W. Connelly, BSP ’51
Donald B. Elliott, BSP ’57
Jerome L. Fine, BSP ’56, PharmD ’96
Robert S. Goodwin, PhD ’83
Marta Hoffman, BSP ’60
David B. Knauer, BSP ’71
Melvin Lessing, BSP ’66
Robert M. Pilson Jr., BSP ’63
Gerald M. Rachanow, BSP ’65
Charles F. Trunk, BSP ’69
Mark S. Wienecke, BSP ’77
If you would like to make a memorial gift, please use the enclosed giving envelope or call 410-706-5893.

BY GWEN FARISS NEWMAN
Ritu Lal, PhD ’96, MS, is the CEO and co-founder of GEn1E Lifesciences Inc., a Palo Alto, Calif.-based biotech company focused on accelerating novel, next-generation immunomodulatory precision therapies for rare and inflammatory diseases.
Growing up in India, Lal watched her grandfather struggle with heart disease, diabetes, and depression. “He was always very sick,” she recalls, “and every day, it was medication after medication.” When her grandfather died at age 74, Lal was a high school student. His death both devastated and inspired her.
“Can you imagine spending 20 years of your life bedridden? To be alive but not healthy? I remember wondering, ‘Why aren’t these medicines helping? How can they be made less toxic, with fewer side effects?’ These experiences are what inspired me to go into the health sciences.”
Lal earned her Bachelor of Pharmacy degree from the University of Bombay in 1989 and an MS in Pharmaceutical Sciences from the University of Arizona. She then enrolled in the PhD in Pharmaceutical Sciences program at the University of Maryland School of Pharmacy (UMSOP), graduating in 1996. Seeking to bridge science with entrepreneurship, she earned an MS in Management from Stanford University’s Graduate School of Business in 2013.
“Sixteen years post-PhD, I decided to go back to school. I wanted to be a ‘Pharmapreneur’ — a pharmaceutical scientist, and also an entrepreneur,” Lal explains.
Lal’s career now blends those two worlds, bringing together key stakeholders who understand the urgency of delivering more effective therapeutics — faster, more efficiently, and with fewer side effects.
Lal has spent more than 20 years in biotech and at pharmaceutical companies, including Abbott Laboratories, HoffmannLa Roche, and XenoPort, Inc. She has taken multiple compounds from discovery through U.S. Food and Drug Administration (FDA)
and Japanese regulatory approval and has filed more than 15 Investigational New Drug applications with the FDA spanning neurology, inflammation, virology, pain, and oncology.
At GEn1E Lifesciences, Lal continues to partner closely with collaborators at the University of Maryland, Baltimore (UMB), including Paul Shapiro, PhD, professor in the Department of Pharmaceutical Sciences at UMSOP, and Jeffrey Hasday, MD, professor in the Department of Medicine at the University of Maryland School of Medicine, and UMB’s Office of Technology Transfer.
Shapiro and Hasday are researching innovative technologies and therapeutic approaches to fight inflammation that can cause fluid buildup in patients with acute respiratory distress syndrome (ARDS), a devastating condition with a 40 percent mortality rate, more than $25 billion in cost to the U.S. society, and no FDA approved treatment. Their research targeting p38alpha kinases — a class of enzymes linked to inflammation — aims to combat ARDS by reducing the deadly fluid buildup in patients’ lungs.
Lal credits her early student experiences at the School of Pharmacy — including cross-campus collaborations with the School of Medicine — for solidifying her belief in the power of partnerships and to lending support to the next generation of researchers, practitioners, and entrepreneurs whose focus is on improving the health of patients and communities.
Now, as an adjunct professor at UMSOP, Lal is on campus often — presenting to student entrepreneurs and working with faculty collaborators.
“I saw the incredible science coming out of UMB — the innovation, the data — and realized, ‘I have the experience and tools to add to it and help bring this to patients.’”
In addition to her scientific and business contributions, Lal and GEn1E Lifesciences have been ardent supporters of UMSOP through their philanthropy, support of students, and investment in the School’s mission and vision.
“Dr. Lal has played a vital role in the School’s continued growth and upward trajectory through generous donations, meaningful interprofessional collaborations with faculty, and a strong commitment to advancing student career development opportunities through internships," says Greer Griffith, MS, executive director of development at UMSOP.
“The University of Maryland School of Pharmacy is unlike any other in its collaborative endeavors,” says Lal, adding, “When I graduated, I already had a job. Today I am grateful and welcome the opportunity to give back.”

“A life lived for others is the only life worth living.”
— Albert Einstein
Every year I am so impressed with the extraordinary foresight and generosity of the University of Maryland School of Pharmacy’s community of alumni and friends. Our supporters have made transformational donations to support the School’s priorities through a diverse array of giving vehicles, such as donated real estate, publicly traded securities, closely held stock, donor advised funds, bequests, and trusts, among others.
2025 is a high-water mark for tax-wise benefits for charitable donations made from the individual retirement accounts (IRA) of retired donors via a qualified charitable distribution (QCD). This strategy is a direct transfer from an IRA to a nonprofit organization, such as the School of Pharmacy. And retirees can give more in 2025, according to the Internal Revenue Service. The transfer limit has increased to $108,000 in 2025. You must be 70.5 or older to qualify for a QCD.
The QCD tax break is often described as better than a tax deduction. While there’s no tax deduction for a QCD, the amount distributed is excluded from income, which is typically more beneficial than a deduction for most filers. When filing taxes, you must claim either the standard deduction or your itemized deductions (including charitable gifts), whichever is greater.
Another benefit of a QCD is that the transfer can offset your annual required minimum distribution (RMD), which in turn helps reduce your adjusted gross income, according to tax experts. Pre-tax IRA balances have grown in 2025 amid stock market highs, which can mean higher RMDs from an IRA for some retirees. Since 2023, most retirees must take RMDs from pre-tax retirement accounts starting at age 73.
Earlier this year, I received the following correspondence from a generous donor that illustrates the simple notification the School needs to receive when planned gifts are made. Notifications like this help ensure that the School fulfills the wishes of our IRA donors, knows who to thank, and alerts us to watch for the forthcoming fund transfer.
Dear Ken,
I have written to my IRA plan administrator to request a distribution of $XXX directly to the University of Maryland School of Pharmacy via the University of Maryland Baltimore Foundation as a charitable gift for the support of scholarships. I would appreciate a written confirmation when you have received the funds. I understand that I will not receive a federal income tax deduction for making this gift to the School. If you have any questions, please contact me. Thank you for your attention to this matter.
Sincerely,
Grateful Alumnus
So do well by doing good. Speak to your tax advisor or lawyer for guidance given your own tax circumstance, and please consider the University of Maryland School of Pharmacy when making your charitable donations this year and in the future.
Gratefully,
Ken Boyden, JD, EdD Associate Dean, Office of Development and Alumni Affairs
410-706-4415 |
kboyden@rx.umaryland.edu
to all the graduates of the School of Pharmacy’s PharmD, PhD, MS, and Graduate Certificate Classes of 2025!

