

W E L C O M E W E L C O M E





W E L C O M E W E L C O M E





We are a local, family-owned, dairy cooperative committed to serving the needs of our members, community, and customers by marketing and selling high quality milk and dairy products.
With the growing number of individual questionnaires, United Dairymen of Arizona, has prepared this packet of information to meet our customer’s food safety and regulatory requirements.
The following information is based on current processes for the manufacture of milk products and is based on information we believe to be dependable. They are offered in good faith, but without guarantee, as the conditions and methods of use of our products are beyond our control. We kindly ask you to use the information in this packet in place of completing individual forms.
Thank you for choosing United Dairymen of Arizona,
Jessica Yang Director of Technical Services





We are a milk marketing cooperative owned by Arizona dairy families. Founded in 1960, the co-op merged with two local dairy associations to ensure an adequate supply of fresh milk and dairy products of the highest possible quality for customers.
Our milk processing plant in Tempe is the heart of UDA Since the start of the twenty-first century, we have developed a wide assortment of bulk milk powders including lactose and lactoferrin, butter, along with USDA-certified sweet cream and condensed skim milk
➢Code of Ethics and Business Conduct
UnitedDairymen ofArizona's Code is conducted at every level of the supplychain toensurewe adhereto high standards for the animals, environment, and our people UDA is committed to the highest ethical standards in the execution of safe and quality products
Food Safety and Quality
United Dairymen ofArizona has in place, implements, and maintains a permanent procedure(s) based on the HACCP principles. UDA can identify any hazards that must be prevented, eliminated, or reduced to acceptable levels. Based on a hazard analysis, UDA has established control measures, and limits at the critical control points (CCPs) and implemented effective monitoring procedures.
Since1960UDA has been headquartered, operated, and managed in Arizona Our passion is for our products and services to be recognized around the world
We are delighted to announce a significant development effective from October 1, 2023. We are now conducting business with you through our newly established entity, OneDairy, LLC, while the United Dairymen of Arizona cooperative (UDA) will continue to operate as it always has, focusing on cooperative functions.
OneDairy, LLC is dedicated to enhancing our professionalism in dairy ingredient trading, building upon UDA's substantial investments to align with a broader range of organizational objectives. OneDairy, LLC will oversee the marketing and transactions of all UDA products and will also engage in sourcing and partnerships with other manufacturers and cooperatives More information can be found https://onedairy com







2008 S. Hardy Drive | Tempe, AZ | 85282 | Plant # 04-15 www.uda.coop | 480-966-7211


This is to notify all buyers of UDA branded product(s) sold by United Dairymen of Arizona and or OneDairy, LLC that all such products are hereby guaranteed, as of the date of shipment or delivery, to:
1.Conform to the requirements of the Federal Food, Drug, and Cosmetic Act, as amended, and any substantially similar state or municipal laws or ordinances.
2.Be manufactured, packaged, stored, and shipped in conformity with the standards applicable to the food and dairy industry
3 Comply with the applicable ingredient specification requirements for the product being shipped
4.Ingredients and/or formulas will not change prior to notification of our customers.

Dave Kedzierski SVP, Business Transformation
is valid until January 31, 2027















2008S.Hardy Drive|Tempe,AZ |85282|Plant#04-15 www.uda.coop |480-966-7211

















































Corporate: United Dairymen of Arizona
Corporate: 404 W Broadway Rd. Tempe, AZ 85282
Manufacturing Site
Company Name: Address: Phone Fax
United Dairymen of Arizona 2008 S Hardy Drive Tempe, AZ 85282
480-966-7211
480-829-7491
Website www.uda.coop
Years in Business 66 years
Product(s) Raw Milk

Skim Milk
Condensed Skim Milk
Sweet Cream
Nonfat Dry Milk
Skimmed Milk Powder
Buttermilk Powder
Lactose
Lactoferrin
Milk Protein Concentrate 40%
Milk Protein Concentrate 70%
Unsalted Butter
Lactic Acid Unsalted Butter
Salted Butter
~300
Employees
Hours
Plant Size
Construction of Material
2 shifts day/7days a week
500,000 square feet
The property, buildings, and equipment are located, constructed, and designed to ensure food is manufactured in a safe, hygienic environment
UDA Warehouses Dry Storage: 3 Cold Storage: 1



United Dairymen of Arizona conducts its business in a manner that is protective of the environment, health, the safety of our employees, the communities surrounding our facilities, the public, and our customers.
UDA is also committed to the pursuit of sustainability by partnering with like-minded employees, suppliers, contractors, and customers to ensure Economic Growth, Social Responsibility, and Environmental Stewardship.
United Dairymen of Arizona requires our farmers/suppliers to participate in and adhere to The National Dairy FARM Animal Care Program guidelines, which provide best practices for the highest level of animal care in the dairy industry
United Dairymen of Arizona complies with all applicable laws and legislations for doing business in the USA and assists in the eradication of slavery and human trafficking from supply chains
United Dairymen of Arizona is an EEO/Affirmative Action employer. We prohibit unlawful discrimination against applicants or employees based on race, color, religion, sex, national origin, disability, veteran status, or any other status protected by law This prohibition includes unlawful harassment based on any of these protected classes
United Dairymen of Arizona is committed to the highest ethical standards in the execution of our duties and responsibilities These components are in place to establish anti-corruption measures, while also adhering to fair business practices and managing partner relationships.
United Dairymen of Arizona uses the Stewardship and Sustainability Framework for U.S. Dairy as a reference to track, measure, and communicate our commitment to environmental stewardship, social responsibility, and continuous improvement Feel free to request a copy of our Sustainability Reports
UDA continuously monitors compliance with child labor laws, antidiscrimination practices, health, and safety standards, working conditions, working hours, compensation, right to association, and freely chosen employment.



Arizona Department of Agriculture (AZDA) Food and Drug Administration (FDA)
United States Department of America (USDA)
Bioterrorism Act of 2002
USDA Plant Est No
FDA FEI No
FDA Food Facility Registration
FSMA Compliance
Comply with the FDA Bioterrorism Act of 2002
04-15
3002420102
United Dairymen of Arizona’s FDA Food Facility Registration Number is issued by Homeland Security We maintain compliance with the FDA by re-registering every 2 years as required All associated sites have been reregistered as of October 2024
United Dairymen of Arizona (UDA) has an established HACCP program and complies with all regulatory requirements for products manufactured at 2008 S. Hardy Dr. Tempe, AZ 85282.
As UDA operates under the Pasteurized Milk Ordinance (PMO), the compliance date for FSMA is September 17, 2018 The Preventive Controls have been reviewed and respective updates to our programs have been implemented Our HACCP team includes 5 Preventive Control Qualified Individuals (PCQI) to oversee our programs
Quality & Food Safety
GMP Certifications Warehouses
Lab Accreditation
(A2LA ISO/IEC 17025:2017)
Sustainability Rating Regulatory
SQF Food Safety
AIB GMP Certificate (Plant and Cold Storage)
CME Approved (Dry and Cold Storage)
All products produced by United Dairymen of Arizona are evaluated by an A2LA-accredited laboratory This laboratory is accredited in accordance with the recognized International Standard ISO/IEC 17025:2017 General requirements for the competence of testing and calibration laboratories This laboratory also meets the requirements of A2LA R204 – Specific Requirements – Food and Pharmaceutical Testing Laboratory Accreditation Program
This accreditation grants the United Dairymen of Arizona laboratory to perform both Biological and Chemical tests reported on the Certificate of Analysis.
EcoVadis, Platinum



The UDA Food Defense Program is designed to ensure safe products Our program puts measures in place to reduce the chances of the food supply becoming intentionally contaminated using a variety of chemicals, biological agents, or other harmful substances.
Our Food Fraud Mitigation Plan assures the authenticity of food by minimizing vulnerability to fraud and mitigating the consequences of food fraud The HACCP team conducts an annual food fraud vulnerability assessment to evaluate UDA’s food fraud mitigation plan and determine where improvements can be made
Other programs to support Food Defense and Fraud include Biosecurity, Bioterrorism registration, Crisis Management policy, HACCP, Recall, and Traceability program, Approved Supplier program, non-conforming supplied material, and Finished Product Warehouse Storage Control
UDA also has implemented security measures in place such as a gated facility, key card access, camera monitors, supply chain transparency - numbered seals on incoming and outgoing shipments, raw material specificationsproduct testing at raw, in-process, and finished stages, 24-hour employee presence, and documented training.
Annual audits are conducted using the AIB Food Security Evaluation to review the implementation and effectiveness of the program Should gaps be identified, corrective actions are performed
A traceability exercise/mock recall is held twice per year. The HACCP team initiates the traceability exercise/mock recall and includes products/ packaging and processing aids to evaluate the facility’s systems.
These programs comply with Federal and State Standards regarding the Public Health Security and Bioterrorism Act of 2002 with re-registration every 2 years.
United Dairymen of Arizona’s Crisis Management Team is a team of competent and trained individuals who have the authority to make decisions about the company during a crisis event.
All communication regarding inquiries, quality, and specifications for all milk products produced from our facilities is to go through your respected United Dairymen of Arizona Customer Service Representative and Sales Manager directly who will be your point of contact
In case of an emergency, please call the United Dairymen of Arizona main phone at 480-966-7211 which is available 24 hours, 7 days a week or email csops@udaz.org.



Allergen Status
Crustaceans or derivate
Eggs or derivate
Fish or derivate
Milk or derivate
Mollusks or derivate
Peanuts or derivate
Tree Nuts or derivate
Soy, Unrefined oil or derivate
Wheat/Gluten or derivate
Mustard or derivate
Sesame or derivate
Sulfites
MSG
Sunflower or derivate
Aflatoxin
Milk products produced at United Dairymen of Arizona are dairy products and therefore contain milk proteins that are an allergen. Employees are trained in allergen awareness. Product
Attestation of Animal Health
All raw milk, used in the production of all finished products at United Dairymen of Arizona, is regularly evaluated for M1 Aflatoxin and complies with the U S standard (0 50 µg/kg in raw milk) Additionally, the finished products are evaluated for aflatoxin twice per year as part of our periodic testing program.
The milk or the milk from which the dairy product was made originated from a country/zone recognized by the Office International des Epizooties (OIE) as foot and mouth disease-free (with or without vaccination).
The milk or the milk from which the dairy product was made originated from a country/zone which meets the OIE requirements for freedom from lumpy skin disease, and which is free from buffalo pox. The animals were clinically healthy at the time the milk was obtained. The products were processed in a foot and mouth disease-free country/zone
The milk or the milk from which the dairy product was made originated from a country/zone which meets OIE requirements for freedom from rinderpest (Code Article 2 1 4 2), and bovine brucellosis (Code Article 3 2 1 1), and bovine tuberculosis (Code Article 3 2 3 1), and which is free from Jembrana



Antibiotics All raw milk used in the manufacture of products has been screened and evaluated "Not Found" for drug residues according to the FDA PMOAppendix "N" (latest revision) This statement can also be found at the bottom of every Certificate of Analysis.
BSE-Free We hereby confirm that the dairy products manufactured at United Dairymen of Arizona are free from Bovine Spongiform Encephalopathy.
Bioengineered We hereby confirm that the dairy products manufactured at United (BE) Foods Dairymen of Arizona do not contain bioengineered materials.
Country of Origin Dairy products manufactured at United Dairymen of Arizona are 100% U.S.A. origin.
EU Compliance
United Dairymen of Arizona certifies that dairy products were produced from raw milk meeting the somatic cell and bacterial standard plate count requirements of the European Commission Council Directive 853/2004 Annex Ill, Section IX, Chapter I, Ill This compliance statement can also be found at the bottom of every Certificate of Analysis
Environmental Monitoring
Gluten-Free
United Dairymen of Arizona adheres to a rigorous environmental monitoring program to evaluate the effectiveness of its sanitation efforts. This includes testing for Salmonella, Listeria, Enterobacteriaceae, and, in specific areas, Cronobacter Testing frequency, designated zones, and sample quantities are monitored and documented Weekly management reviews are conducted to analyze results and perform root cause analyses for any counts exceeding established limits that require corrective action Additionally, Yeast and Mold levels are regularly monitored through air sampling
The United States Environmental Protection Agency, National Center for Environmental Assessment has surveyed the U S milk supply This study agrees with other global studies finding that the world milk supply, including the U.S., is approximately 0.8 picograms dioxin per gram from milk fat or lower. Recent European Union standards for dioxin in foods place a limit of 3 0 picograms dioxin per gram of fat (Total Dioxins (WHOPCDD / FTEQ) < 3 0 pg/g fat) in the product
Using the European Union standard as a guideline, United States dairy products fall well within these limits of concern
Dairy products manufactured by United Dairymen of Arizona are glutenfree These products are made from natural, bovine milk that originates in the United States of America



GMO (Genetically Modified Organisms)
To the best of our knowledge, GMOs (Genetically Modified Organisms) are not added in the manufacture of any United Dairymen of Arizona dairy products, liquid or powder. The milk used for manufacture was not sourced from cloned cattle or ionized material.
GRAS (Generally Recognized As Safe)
“GRAS" is an acronym for the phrase Generally Recognized as Safe. Under sections 201(s) and 409 of the Federal Food, Drug, and Cosmetic Act (the Act), any substance that is intentionally added to food is a food additive, that is subject to premarket review and approval by the FDA, unless the substance is generally recognized, among qualified experts, as having been adequately shown to be safe under the conditions of its intended use, or unless the use of the substance is otherwise excluded from the definition of a food additive.
Dairy products manufactured by United Dairymen of Arizona are derived from milk, which is the only ingredient used in our process unless otherwise stated. Therefore, each product carries the GRAS designation based on the natural origination of milk to the best of our knowledge.
Halal
Kosher Periodic Testing (incl Heavy Metals, Veterinary Drugs, & Pesticides)
Certified by IFANCA. Reference Halal Certificate for specific products. (GSO, BPJPH compliant)
Certified by the Orthodox Union. Reference Kosher Certificate for specific products.
Dairy products processed at United Dairymen of Arizona are evaluated using multiresidue methods which can detect a wide range of agricultural chemicals, including organochlorides, organophosphates, organonitrogens, melamine, heavy metals, and aflatoxins
Codex maximum limits for all agricultural chemicals or importing countries' specific limits, where provided by the appropriate authority, are used to determine the suitability of dairy products for human consumption in the global market
During the most recent screening, organophosphate compounds, pesticides, melamine, and heavy metals did not have detectable levels Historical results for dioxin & polychlorinated biphenyls (PCBs) were recorded to not have detectable levels.
Dairy products are not subjected to ethylene oxide; no, to the best of our knowledge, are any of the components of these products.
Hydrogenated oils (partially or completely) are the result of adding hydrogen to liquid (plant-based) oils which cause them to be solid. Dairy products manufactured by United Dairymen of Arizona do not contain any hydrogenated oils



Irradiation Dairy products are not subjected to ionizing radiation; no, to the best of our knowledge, are any of the components of these products.
Natural The Food and Drug Administration does not have a regulatory definition of “Natural” regarding label claims The FDA consistently holds to a policy it had outlined in 1993:
"FDA has not established a formal definition for the term 'natural', however, the agency has not objected to the use of the term on food labels provided it is used in a manner that is truthful and not misleading, and the product does not contain added color, artificial flavors, or synthetic substances. Use of the term 'natural' is not permitted in the ingredient list, except for the phrase 'natural flavorings'."
Dairy products consist only of milk with no other added ingredients or preservatives, so it would conform to the above policy statement by FDA and would be considered “Natural” unless otherwise stated.
No Added Ingredients or Preservatives
Dairy products are made from 100% Bovine Milk unless otherwise stated No additional ingredients or preservatives are added to any product, including but not limited to plasticizers, benzoyl peroxide, benzoic acid, antibiotics, and dicyandiamide. Please refer to the specification for more information
Melamine Dairy products are manufactured without the use of or in contact with Melamine no, to the best of our knowledge, are any of the components of these products.
Packaging (Food Grade)
Our suppliers have assured us that packaging material is suitable for its intended use; the use of the substance as components of articles that directly contact food shall comply with the applicable provisions of the Code of Federal Regulations, Title 21; Free of fungicide, preservatives, fumigants, pathogenic bacteria and will not leach chemicals in the amount above the established tolerance into the food product during the specified use of the article In addition, in compliance with CA Proposition 65, as amended.
(PFAS, PFOS, PFOA, BHT, BPA, GMO, NPE)
Our suppliers have assured us that they do not deliberately add Per- & Polyfluoroalkyl, Perflurooctane sulfonate, Perfluorooctanoic acid, Butylated Hydroxytoluene, Bis-Phenol A, Genetically Modified Organisms, or Nonyphenol Ethoxylate to the packaging material that we purchase from them, nor are such substances present, to the best of their knowledge, in any of the raw materials used in the manufacture of packaging materials



Pasteurization Statement (Heat Treatment)
All milk used in the production of United Dairymen of Arizona dairy products has been processed according to the Pasteurized Milk Ordinance (U S Department of Health and Human Services, Public Health Service, Food and Drug Administration) at a minimum temperature of 161ºF (72ºC) for a minimum time of 15 seconds.
Date Formatting United Dairymen of Arizona has updated our date formatting for Production/Expiration Production Dates and Expiration Dates We have stopped using the format MM/DD/YYYY and transitioned to DD/MM/YYYY to align with UDA, and export requirements.
This new format will apply to both the inkjet on the packaging as well as noted on any secondary labels, documentation. Due to formatting restrictions, our COA will show as DD/MM/YY. Additionally, expiry dates wil be based on the Production Date + 729 Days calculation unless otherwise stated *LF is 1095 or 730 Days See spec/COA for shelf life
The information below reflects the new format
DD/MM/YYYY
P 03/11/2022
E 01/11/2024
Prop 65 Dairy products are manufactured without the use of or in contact with chemicals listed on CA Proposition 65, to the best of our knowledge. United Dairymen of Arizona dairy products comply with local, state, and federal requirements as “Ready-To-Eat” foods.
According to the definition of the United States Department of Agriculture (USDA), Ready-To-Eat (RTE) foods refers to “food that is in a form that is edible without washing, cooking or heating by the consumer and that it is reasonably expected to be consumed in that form.”
In conjunction with RTE compliance, UDA has incorporated a pasteurization step in our process.
Pallet Wood pallets used for the shipping of our dairy products are in (Heat-Treated) compliance with the International Plant Protection Committee ISPM-15 guidelines for Heat-Treated Pallets
rBST-Free
(Artificial Growth Hormones)
Dairy products manufactured by United Dairymen of Arizona are made from cows that have not been treated with any artificial growth hormones (e g , recombinant bovine somatotropin (rBST) to the best of our knowledge



Sanitary Statement
(United States-MexicoCanada Agreement) Vegan/Vegetarian Sewage Sludge
Under the guidance of local and regulatory agencies including the United States Department of Agriculture (USDA), Agriculture Marketing Service (AMS), and Animal and Plant Health Inspection Service (APHIS) on sanitary and public health requirements; United Dairymen of Arizona certifies all milk and milk products have been manufactured under a U.S. safety system that is intended to ensure the safety of our products
Dairy products are not subjected to sewage sludge; no, to the best of our knowledge, are any of the components of these products
Sulfites are not added to any of our manufacturing processes In addition, sulfites are not commonly found to naturally occur in milk or milk products. Based on this information, we do not evaluate our products for sulfites.
Dairy products manufactured at United Dairymen of Arizona are 100% U.S.A. origin. *USMCA replaced the NAFTA Certificate of Origin.
Dairy products manufactured by United Dairymen of Arizona contain milk from cows
Vegan Vegetarian No Yes
Approved Suppliers (incl Warehouse 3PL) WADA (World Anti-Doping Agency)
No substances on the current World Anti-Doping Agency (WADA) prohibited list have been added to the dairy products manufactured by United Dairymen of Arizona nor do we bring anything on this list into the facility.
The approved suppliers are reviewed by the HACCP team annually.
Contracted warehouse storage facilities are required to be registered as food storage facility with the FDA according to the Bioterrorism Act of 2002 and have a third-party cert or be subjected to an internal audit by UDA

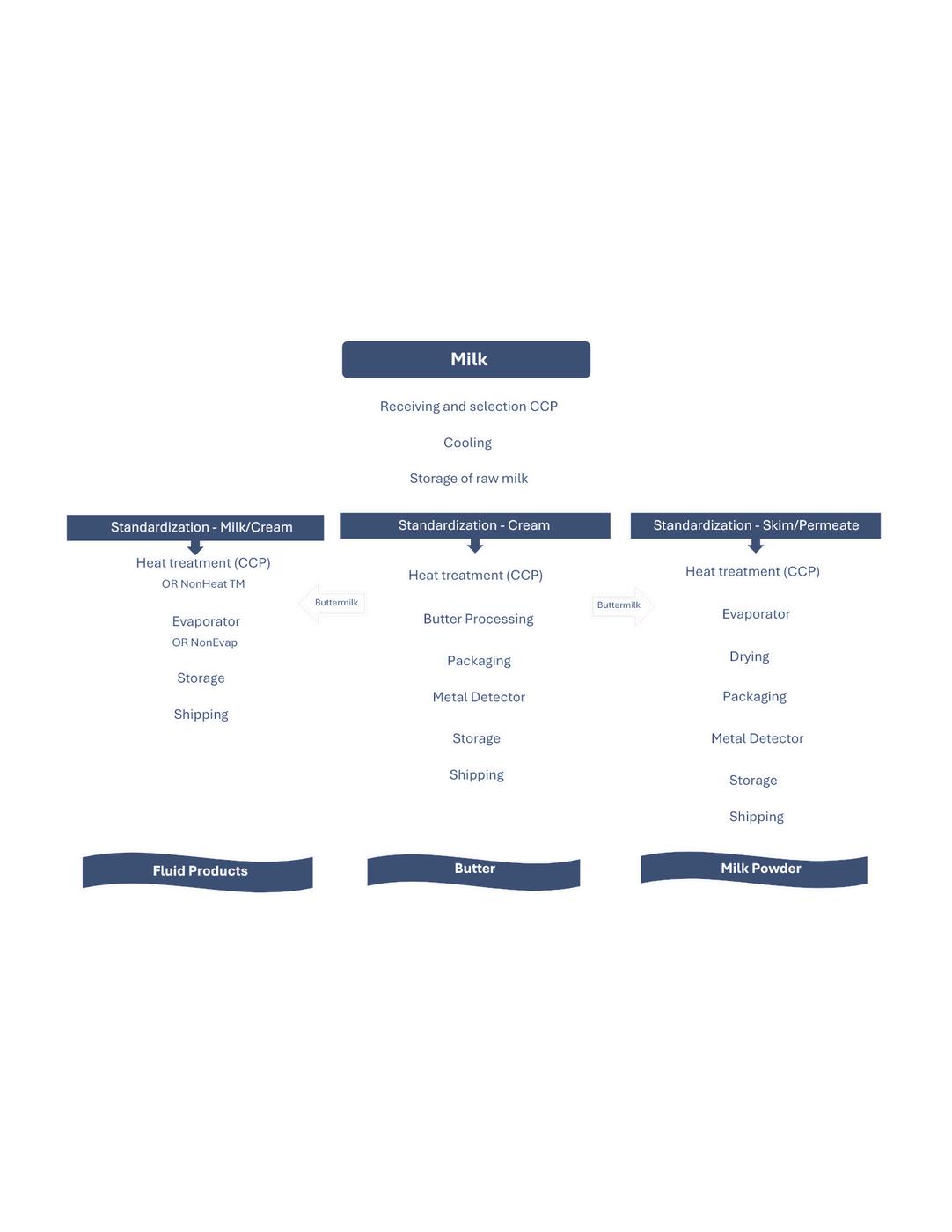
Fluid Milk Products
Butter - Unsalted, Salted, Lactic
Milk Powders - NFDM, SMP, MPC, Lactose, BMP

NFDM (LH, MH, HH)
Lot 16 155 Low Heat Plant #04-15 SL 001
Bag 001 P03/06/2016 E02/06/2018
16 = Year of manufacture
155 = Julian date of manufacture
Low Heat = Product Type
Plant #04-15 = Plant number
SL 001 = Sublot Number
Bag 001 = Bag Number
P03/06/2016 = Production Date
E02/06/2018 = Expiration Date




SMP (LH, MH, HH)
Lot 16 165 Low Heat Plant #04-15 SMP
25kg (55.115 lbs.) SL 034 Bag 267
P13/06/2016 E12/06/2018
16 = Year of manufacture
165 = Julian date of manufacture
Low Heat = Product Type
Plant #04-15 = Plant number
SMP = Product
25 kg (55 115 lbs ) = Package wt lbs /kg
SL 034 = Sublot Number
Bag 267 = Bag Number
P13/06/2016 = Production Date
E12/06/2018 = Expiration Date


MPC (Export)
Lot 16 165 MPC 70 Low Heat Plant #04-15 20kg (44.09 lbs.) SL 034 Bag
281 P13/06/2016 E12/06/2018
16 = Year of manufacture
165 = Julian date of manufacture
MPC 70 Low Heat = Product Type
Plant #04-15 = Plant number
20kg (44 09 lbs ) = Package wt lbs /kg
SL 034 = Sublot Number
Bag 281 = Bag Number
P13/06/2016 = Production Date
E12/06/2018 = Expiration Date



BMP


WPC
Lot 19 170 Plant #04-15 50LBS (22.68 kg) SL 001 Bag 001 P19/06/2019 E17/06/2020
19 = Year of manufacture
170 = Julian date of manufacture
Plant #04-15 = Plant number
50 lbs (22 68 kg) = Package wt in lbs /kg
SL 001 = Sublot Number
Bag 001 = Bag Number
P19/06/2019 = Production Date
E17/06/2020 = Expiration Date

Butter
Lot 14 203 001 Box 01 P 22/07/14 E 19/01/16 UNSALTED BUTTER
14 = Year of manufacture
203 = Julian date of manufacture
001 = Pallet number
Box 01 = Box number on pallet
P 22/07/14 = Production date
E 19/01/16 = Expiration date
Unsalted Butter = Product type

Lot 16 165 WP34 Plant #04-15 25kg (55.115 lbs.) SL 034 Bag 275 P13/06/2016 E12/06/2018
16 = Year of manufacture
165 = Julian date of manufacture
WP34 = Product
Plant #04-15 = Plant number
25 kg (55 115 lbs ) = Package wt in lbs /kg
SL 034 = Sublot Number
Bag 275 = Bag Number
P13/06/2016 = Production Date
E12/06/2018 = Expiration Date

Fluid Products (incl Condensed Skim, Cream, Milk)
Liquid milk products are stored in a shipping vessel that meets all appropriate federal, state, and local regulations. These products are manufactured and sold in bulk volumes only.
These products do not carry a lot number, but are traceable with the first particle of pasteurization (FPP) date and the silo it was stored in. Liquid products are further traceable by the tanker and PO number utilized for transport, when applicable.
Lactose
Lot 17 305 001 01 P01/11/2017 E31/10/2019
17 = Year of manufacture
305 = Julian date of manufacture
001 = Pallet Number
01 = Bag Number
P01/11/2017 = Production Date
E31/10/2019 = Expiration Date


CONFIDENTIALITY



With the growing number of individual questionnaires, United Dairymen of Arizona, has prepared this packet of information to meet our customer’s food safety and regulatory requirements We kindly ask you to use the answers to these questions in place of completing individual questionnaire forms
The following information is based on current processes for the manufacture of milk products and is based on information we believe to be dependable All documentation is considered proprietary and may be reviewed onsite
A
Organization/Personnel
Is an Organizational Chart available?
Are written job descriptions describing job tasks and training documented and available to staff?
Is training conducted to ensure compliance with Good Manufacturing Practices?
Are training, experience, and education adequate to perform job?
Is training documented?
Are personnel in manufacturing complaint with the following:
•
•

Eating, drinking, &smoking indesignated areasonly Wearing hair or beardrestraints
Wearing clean garments in a manner that protects against contamination
•
• No jewelry is allowed including plain metal bands Washinghands upon returning tomanufacturingareas
• Are personnel with illness and/or open wounds required to report such illness to management and be removed from manufacturing?
Are personnel with wounds required to cover them?
Are metal detectable bandages used?
Are Contract or Temporary employees qualified, approved, and trained?
Are visitors required to comply with personal hygiene requirements?
Does the company have a business contingency and/or emergency plan?
Building/Facilities/Warehouse/Pest Control
B Is the establishment located in an area that is free from environmental contaminants?
Is the outside area maintained in good repair?
Available on site during a plant tour Avai1able on site during a plant tour
Frequency: Employees are trained upon hire and at least annually



Is the facility of suitable size and construction for operations being performed and not to contaminate the product?
Is the facility maintained in a clean and good state of repair?
Are storage conditions appropriate to protect against contamination?
Are storage areas dry?
Are materials stored off the floor and 18 inches from the wall?
Is sufficient illumination available for personnel to perform tasks?
Is lighting protected or shatter proof
Are floors and walls intact?
Do restrooms open directly to production or storage?
Are Handwashing stations properly stocked and located throughout the facility?
Are there adequate changing facilities for employees?
Is water quality monitored?
Are backflow preventive devices fitted?
Is ventilation provided to remove dust and condensation?
Are drains constructed and located not to contaminate materials?
Is waste removed by a licensed contractor?
Are waste containers constructed of impermeable materials, closed when not in use, and located in a designated area not to contaminate materials?
Maintenance of grounds to prevent harborage of pests?
Are external doors and windows screened to prevent the entry of insects?
Is there a Third-Party Pest Control Program in place?

Frequency of visit: Weekly/Monthly site visits for interior, exterior bait stations and traps as well as insect light traps
Are operators trained and have valid applicator licenses?
Are traps located at key entry points?
Are potential pest harborage removed?
Are the results of the inspections kept on file?
Is a map of all bait and trap stations maintained?
Are pesticides stored on site? is fumigation performed?
Is the warehouse clean, dry, and well maintained?
Are products stacked in a way to prevent damage?
C
Is equipment of suitable size and construction material not to contaminate product?

Are food contact surfaces smooth, non-corrosive, and can with stand cleaning?
Are equipment linesdedicated? Equipment/Calibration/Maintenance



Are equipment and scares routinely calibrated and comply with NIST Standards?
Are records of calibration maintained?
Is a Preventative Maintenance Program in place to ensure equipment used for manufacturing products performed as designed?
Is a master list of equipment maintained specifying the type of maintenance?

Are Pre-Operational Inspections conducted prior to start up to ensure equipment is accounted for?
D. Is a Chemical Control Program implemented? Chemical Control Program
Are chemicals and lubricants that have the potential for food contact of food grade quality?
Are chemicals properly labeled and stored?
Is a master list maintained and reviewed?
Frequency: Magnets are evaluated quarterly, scales monthly (external), metal detectors once a year (external)
A preventative maintenance program for processing equipment as well as a work order system that documents work done on all equipment producing food grade products.

E
Is a cleaning program in place to ensure the cleanliness of the facility to food grade standards?
Has a Master Cleaning Schedule been implemented, followed, monitored, and documented?
Are chemical sanitizers used?
Is Environmental Monitoring conducted?
Are Micro Swabs/ ATP Swab test conducted?
Is Clean In Place equipment adequately cleaned?
Is compressed air used for cleaning?
Chemicals, not intended as an ingredient, are used, and stored in a manner not to contaminate the product

F. Supplier Approval Program
Is there a Supplier Approval Program in place?
Are specifications in place with approved suppliers? Are suppliers monitored to ensure compliance?
G.
Are SOPs in place for receiving incoming materials?
Are all containers inspected tor cleanliness, odor, contamination, pest, etc.?
Are incoming materials verified against product ordered, quantity, lot code, and seal numbers?
Are raw materials evaluated for identity & purity covered by the COA?
Reviewed by Quality Assurance Team




Are raw materials assigned a lot code to ensure traceability?

Are non-conforming materials labeled, quarantined, and rejected back to the vendor?
Are records maintained of all incoming materials?
H
Manufacturing/Control of Foreign Material
Is manufacturing a batch or continuous process?
How is a lot size defined?
Is the manufacturing process enclosed?
Are complete manufacturing SOPs specifying formulas, name of material used, equipment, sampling, and documentation available to appropriate staff?
Is in-process testing conducted?
Is product protected against foreign material?
Are Metal Detectors used? If so, what sensitivity?
What action is taken in metal detection fails?
Is a Glass and Brittle Plastics policy in place?

Are Food Grade Products manufactured under Good Manufacturing Practices (GMPs)? Is packaging material approved for food use? Are tamper-evident seals used on packaging?
I. Preventive Controls and HACCP
Are Food Grade Products manufactured under a Hazard Analysis Critical Control Point (HACCP) and Preventive Controls Plans?
Are applicable food safety hazards identified?
Does the plan include biological, chemical, physical, and radiological hazards?
Does the plan include allergens?
Is the HACCP Pian validated, monitored, and updated on a regular basis?
Does the HACCP Program have a HACCP Team?
Does the HACCP Team meet on a regular basis and conduct internal audits?
Is a member of the HACCP Team certified?
Continuous
Lots are defined by Julian date of date packaged
Manufacturing lines are equipped with strainer, magnet, and sifter and monitored per shift
Metal detectors are calibrated and vary in sensitivity based on the product
The line stopped, and the bag is sifted If suspect metal is found, an investigation is initiated.
A master list of glass and brittle plastic installations in production areas shall be maintained and inspected quarterly A glass or brittle plastic breakage plan has been in place and provides cleanup and product protection
Multiwall bags are heat sealed Exceptions are products packaged in bulk packaging are closed. Bulk trucks are sealed with seal numbers

The HACCP Plan with Flow Charts is available at the plant site
HACCP meetings are conducted monthly with a multi-disciplinary HACCP team

J.


Does the facility have an Allergen Control Program?
Does the facility use any of the major food allergens in product?

Are all raw materials reviewed for the presence of allergens?
Are employees trained on Allergen control? Allergen Control
K
Finished Product Control
Does Quality assure all testing and manufacturing records are reviewed before release?
Are non-conforming materials quarantined?

Non-conforming products are labeled as to their status Disposition of non-conforming products occurs in a timely manner and follows all company procedures If applicable, corrective actions are implemented
Is reworked used?
Is there an SOP for the investigation and corrective action of all non-conforming product?
Are records maintained?
Is an expiration date assigned to finish material?
Is First In First Out (FlFO) practiced?
Are retention samples maintained for the shelf life of the product?
Are Certificates of Analysis provided with each lot?
Is physical or chemical testing conducted?
Is microbiological testing conducted?
Is there a continuous improvement program?
Are shipping vehicles in a good state of repair & clean?
Do shipping vehicles have a documented inspection prior to loading?
Do food grade bulk vehicles require a wash certificate?
L. Recall/Traceability Program
Can the finished product be traced back to date & time of production and supplier?
Can the finished product be traced to the direct customer?
Are mock recalls conducted?
Has the facility had a Reportable FDA recall in the past 3 years?
Is a list of key contacts for the recall team maintained?
Are records maintained?
Records are maintained for 4 years
Retention samples are kept for 2 years
Refer to specification
Refer to specification

M Laboratory

Is routine testing conducted internal or external?
If internal, does the lab have adequate space and equipment for the required test?
Is the equipment calibrated?
Are calibrations documented?
Are containers adequately labeled to determine identity, dates, and expiration?
until
Internal, A2L2 Certified

N. Change Control



Is there an adequate system for controlling changes within processing, documents, and equipment?
Do changes receive the proper review and approval regarding potential effects?
Are changes communicated internally and are personnel trained?
0.Customer Complaints
Is a program implemented to investigate customer complaints, determine causes, corrective actions, trend analyses, and notification to customer?

Are customer complaints maintained on file?
P.
Complaints are thoroughly investigated, root cause determined, and if applicable, corrective/ preventative action is implemented

Are internal audits conducted covering all areas of operation and verify SOPs, polices, and procedures are being followed?
Are third party audits conducted? If so, to which standard/certification body? Audit
Food Defense
Q. Does the facility have a Food Defense Program?
Is the manufacturing site secured to prevent unauthorized entry?
Are visitors and guests restricted to areas and accompanied by authorized employees?
Are ingredients and finished products stored to prevent tampering?
Do incoming and outbound shipments require seal and seal numbers?
Weekly GMP audits conducted, monthly-internal audits (programs/documents)
The current SQF Global Standard to Food Safety Certificate is available Full audits are available upon receipt of NDA


SDS Name: Section 1: Dry Milk Powder IDENTIFICATION
Product Name: Powdered Milk Products
1.2 Synonyms:


Milk Protein Concentrate (MPC) – All Grades and Categories
Buttermilk Powder - All Grades and Categories
Non Fat Dry Milk (NFDM) - All Grades and Categories
Skim Milk Powder (SMP) - All Grades and Categories
Lactose Powder - All Grades and Categories
Whey Protein Concentrate (WPC) - All Grades and Categories
1.3 Name, Address, of Manufacturer: United Dairymen of Arizona 2008 S. Hardy Dr. Tempe, Arizona 85282
1.4 1.5 Emergency Phone Number: (480) 966-7211
Recommended uses: No use is specified.
Section 2:HAZARD IDENTIFICATION 2.1
Classification of Substance/Mixture: May form combustible dust if not cleaned adequately.
Principle Component: Milk & Milk By-Products
Percent by Weight: 94% - 96%
Unknown Acute Toxicity: No data available
Section 3:COMPOSITION/INFORMATION ON INGREDIENTS
3.1 Substance: Powdered Milk Products Name Milk, Hydrolyzed Product Identifier % Classification Powder 100 Comb. Dust
3.2 Mixtures: Not Applicable
Section 4:FIRST-AID MEASURES
4.1 4.2
Description of First-Aid Measures: None – Not Applicable
Most Important Symptoms/Effects, Acute and Delayed: Symptoms/Injuries: May cause irritation or allergy symptoms in people that have milk allergies.
Section 5:FIRE FIGHTING MEASURES
5.1
5.2
5.3
Extinguishing Media: Suitable…..Water Spray
Specific Hazard Arising from the Substance/Mixture: Dust may become combustible when mixed with air in enclosed areas – Keep out of areas where ignition, open flame, or sparks may occur. Use under adequate ventilation and dust prevention.
Special Protective Actions for Fire Fighters: None

Section 6:ACCIDENTAL RELEASE MEASURES


6.1 Personal Precautions, Protective Equipment and Emergency Procedures: Avoid generating dust; keep away from open flames, hot surfaces, and ignition sources. Environmental Precautions: Keep it out of the Dry Wells and Sewer. Methods and Materials for Containment and Cleaning Up: Containment…. Avoid Dust Production Clean Up…..Use Explosion proof vacuum, if possible, if sweeping has to be done avoid producing dust as much as possible. Wetting the powder is always an option to prevent airborne dust. Use only non-sparking tools. Clean spill or release immediately and dispose of as solid waste in accordance with local, state, and federal regulations. Be very careful, powder can be slippery whether it is wet or dry.
Section 7:Handling and Storage
7.1 Precautions for Safe Handling: Avoid dust production. Dust may become combustible when mixed with air in enclosed areas – Keep out of areas where ignition, open flame, or sparks may occur. Use Good Manufacturing Practices and Safety Procedures. Wash hands with mild soap and warm water before eating, drinking and at the end of shift.
Conditions for Safe Storage, including any Incompatibilities: Avoid producing dust – store at ambient conditions of < 125F and 80% RH Protect from moisture.
Products: None
Section 8:EXPOSURE CONTROLS/PERSONAL PROTECTION
8.1 Control Parameters
For substances listed in section 3 that are not listed here, there are no established exposure limits from the manufacturer, supplier, importer, or appropriate agency including: ACGIH (TLV), NIOSH (REL), or OSHA (PEL).
Appropriate Engineering Controls: Use under adequate ventilation and dust prevention. Personal Protective Equipment: Not Required
Section 9:Physical and Chemical Properties
9.1 Physical State
Appearance
Odor
Odor Threshold
Melting/Freezing Point
Boiling Point
Flash Point
Evaporation Rate
Flammability (solid, gas); Vapor Pressure/Density
Relative Density
Solubility
Partition Coefficient
Auto Ignition Temperature
Decomposition Temperature
Viscosity
Solid
Off-White powder
Slight dairy odor
Not Applicable
Not Applicable
Not Applicable
Not Applicable
Not Applicable
Not Applicable
Not Applicable
Not Applicable
Soluble
Not Applicable
Not Applicable
Not Applicable
Not Applicable



Section 10: STABILITY and REACTIVITY
Reactivity: Product is stable.
Chemical Stability: Stable under recommended handling and storage conditions (see section 7)
Possibility of Hazardous Reactions: Not Applicable
Conditions to Avoid: Extremely high temperatures and Ignition sources.
Incompatible Material: Not Applicable
Hazardous Decomposition Products: Not Applicable
Section 11:
11.1
Toxicological Information:
Acute Toxicity:
Skin Corrosion/Irritation:
Serious Eye Damage/Irritation:
Respirator or Skin Sensitization:
Germ Cell Mutagenicity: Carcinogenicity:
Reproductive Toxicity:
STOT Single/Repeated Exposure
Aspiration Hazard:
Section 12: ECOLOGICAL INFORMATION
Not Applicable
Not Applicable
Not Applicable
Not Applicable
Not Applicable
Not Applicable
Not Applicable
Not Applicable
Not Applicable
Toxicity: Persistence and Degradability: Not Applicable Not Applicable
Not Applicable
Not Applicable
Bioaccumulative Potential: Mobility in Soil: Other Adverse Effects: Not Applicable
Section 13: DISPOSAL CONSIDERATION
13.1 Disposal Method: Dispose of as solid waste in accordance with local, state, and federal regulations.
Section 14: TRANSPORT INFORMATION
14.1 14.2 In Accordance with DOT: In Accordance with IMDG: Not Regulated for transport Not Regulated for transport
Section 15: REGULATORY INFORMATION
15.1 Not Applicable
Section 16:
Revision Date: OTHER INFORMATION 06/10/2015
Other Information: This document has been prepared in accordance with the SDS requirements of the OSHA Hazard Communication Standard 29 CFR 1910.1200
UndercurrentU.S. Department of LaborOSHA requirements, themilk products(incl.liquid, powder, butter) that United Dairymen of Arizona produces are not considered hazardous. Additionally, under House Superfund Amendments of 1985, Subtitle C, Section 326, page 750, and Senate Superfund Improvement Act of 1985, the following substances are not treated as “Hazardous Chemicals” for communication standards or material safety standards. (A)Any food, food additive, color additive, drug,or cosmetic regulated bythe Food& Drug Administration.


This inf ormation pack e t is valid un til Januar y 31, 2027 C ONFIDENTIALITY NO TICE: This documen t is the property of Unit ed Dair ymen of Ariz ona and is f or the e x clusive use of the individual or en tity t o which it was sen t, and may c on t ain proprie t ar y , c onfiden tial, and/ or privileged inf ormation which c an change at an y time. Y ou are hereb y notified that an y dissemination, dis tribution, or c op ying of this documen t is prohibit ed and may be unlaw ful. If you have rec eived this documen t in error , please immediat ely dele t e all electronic or other c opies made. 2 0 0 8 S .
