Acroporacervicornis
Carlos
Toledo-Herna ´ ndez 1*,ClaudiaPatricia Ruiz-Diaz 1* , JuanSebastian Ramı ´rez-Lugo 2,Marielys Torres-Dı ´ az 3 , Lisby Santiago-Paga ´ n 3,Andrea Bruno-Chardo ´ n 3 andLizM. Dı ´az-Va ´ zquez 3,4
1 SociedadAmbienteMarino(SAM),SanJuan,PuertoRico, 2 DepartmentofBiology,Universityof PuertoRico,SanJuan,PuertoRico, 3 DepartmentofChemistry,UniversityofPuertoRico,San Juan,PuertoRico, 4 NSF-CRESTCenterforInnovation,Research,andEducationinEnvironmental NanotechnologyUniversityofPuertoRico,SanJuan,PuertoRico
Worseningenvironmentalconditionsduetoclimatechangehaveprofoundly affectedthehealthofcoralreefsworldwide.Thus,understandinghowcorals respondto fluctuatingand/orextremelevelsoftemperatureandsolarirradiation willguidefutureprotectionandrestorationeffortsofthisvaluableecosystem. Herein,wepresentastudyoftheimmuneresponsesoftheendangeredcoral Acroporacervicornis toseasonal fluctuationsinwatertemperature(WT),light intensity(LI),andwaterdepth.Immuneresponseswereobservedbymeasuring theconcentrationofgreenandcyan fluorescentproteins(GFPandCyFP)and theactivityofphenoloxidase(PO),anenzymeinvolvedinthebiosynthesisofthe photoprotectiveproteinmelanin.Tostudytheseresponses,visuallyhealthy A. cervicornis fragmentswereplacedat8,and12mdepth,andGFP,CyPF,andPO activityweremeasuredat three-monthintervalso vera12-monthperiod. Seawatertemperatureandlightintensitywerealsomeasuredateachdepth duringthisperiod.Agenerallinearmixedmodelwasusedtodeterminethe effectsofseasonalvariationsofWT,LI,andwaterdepthontheimmuneproteins. GFP,CyFP,andPOactivityvariedsignificantlyacrosstime – allhigherinlate summer/earlyfallandlowerinlatewinter/earlyspring.Likewise,WTandLI significantlyaffectedGFP,CyFP,andPOactivity.Ontheotherhand,waterdepth onlyhadasignificanteffecton fluorescentproteinconcentrationsbutnotPO activity.Ourstudydemonstratesthatcoralscanmodulatethesekeyimmunerelatedproteinsthroughoutnaturalseasonal fluctuations.Thatis,increasingin monthsofhigherthermalandlightconditionswhiledecreasinginmonthswith mildthermalandlightconditions.Thephenotypicplasticityof A.cervicornis in
adaptingtoachangingenvironmentunderscorestheimportancethatinfuture studiestimeoftheyearshouldbeameaningfulconsiderationwhenevaluating theresponsesof A.cervicornis totheenvironment.
KEYWORDS climatechange, fluorescentproteins(FPs),phenoloxidase(PO)activity,coralimmunity, coralfarms
Introduction
Allcoralspossessconstitutiveimmunity,meaningabasallevel ofimmuneactivityrequiredtobalancetheorganism’sinternal biochemicalprocesses,promote,andprovideahealthy environmentfortheinteractionbetweenthecoralhostand microbestothrivewhileoptimizingtheallocationofresources intothedifferentlife-historictrait( Palmer,2018 ).However, constitutiveimmunitymustbeplastic,meaningthatitmusthave thecapacitytoadjustratherquicklytonaturalorseasonalvariations inenvironmentalconditions.Assessileorganisms,coralscan’t escapefromtheseharshenvironmentalconditionsbymigrating tositeswithbetterconditions.Instead,coralsrelyonasuiteof constituents,includingpigmentssuchas fluorescentproteins(FPs) andmelanin,toprotectthemselveswhenstressfulenvironmental conditionsthreatentheirinternalhomeostasis.
FPsandmelaninareubiquitousincoralsandserveasprimary constituentsofcorals’ innateimmunesystemduetotheirmultiple roles(Rothetal.,2010).FourFP-likeproteinshavebeenidentified incorals,green(GFP),cyan(CyFP),red(RFP),andyellow(Alieva etal.,2008).The firstthreearecommonincorals,whilethelastone israrelyobservedinreeforganisms(Alievaetal.,2008; Quicketal., 2018).Ingeneral,thesepigmentsarethoughttobeimmunologically competentduetotheirphotoprotectivepropertiesastheyscatter lightandabsorbpotentiallyharmfulhigh-energyradiation(Salih etal.,2000).FPsalsohavethecapacitytoquenchreactiveoxygen species(ROS),whichdramaticallyincreaseunderhighlevelsof abioticstressors,leadingtocoralbleaching(Palmeretal.,2009).
AsinthecaseofFPs,melaninprovidescoralswith photoprotectionbyscatteringandabsorbinghigh-energy radiationsuchasultravioletlight(UV)(Palmeretal.,2011 ). Melaniniscommonlyfoundinacoral’sepithelialcells,creatinga physicalbarriertopreventand/orreducetheharmfulimpactof radiation.Inaddition,melaninplaysavitalroleinthecoral’ s inflammatoryresponsessuchaswoundhealingandtheresponseto pathogens(Palmeretal.,2010).Insuchcases,melaninisstoredin granuleswithinmobilecellsknownasamoebocytes.Granular amoebocytesrushtoafflictedareasandreleasemelanintoseal theinjuryorencapsulatepathogensinthecaseofpathogen infection(Petesetal.,2003).
Inthisstudy,weinvestigatetheconstitutiveimmuneresponse of Acroporacervicornis toseasonalanddepth-relatedvariationsof seawatertemperatures(ST)andlightintensity(LI).Specifically,we measuredtheintrinsicmeanFPandphenoloxidase(PO)activity,
anenzymeresponsibleforactivatingthebiosynthesisofmelaninin A.cervicornis colonies,atfourmomentswithina12-monthsperiod tounderstandtheeffectofseasonalvariationofSTandLIonthe synthesisofphotoprotectivepigments.GiventhatSTandLIvary seasonally,withpeaksoccurringduringlatesummerinthe Caribbean,andthattheproductionofFPandMelaninareclosely linkedtothecoral’simmunesystemwherehighlevelsofthese proteinsindicateincreasedstressincorals(Mydlarzetal.,2010),we wouldexpectaseasonalresponseofCaribbeancorals’ innate immunesystemduetothesetemporallypredictablestressors. Meanwhile,providedtheimmuneconstituents ’ rolesareto protectcoralsfromheatandlightstressors,andthatthese environmentalfactorsvarywithdepths,wewouldexpectadepth responseoftheseinnateimmuneconstituents.Therefore,totest thishypothesis,weconductedaretro-transplantexperimentwhere werelocated A.cervicornis fragmentsfromtheiroriginaldepthstoa differentdepth,i.e.,from8mto12mand vice-versa,andmeasured thePO-activityandFPconcentration.
Weselected A.cervicornis asthestudysubjectbecausethiscoral ishighlysensitivetochangesinSTandLI.Hence,moderate changesinenvironmentalconditionsmayyielddetectablelevels ofvariationinFPconcentrationandPOactivity.Likewise,A. cervicornis wasoneofthemostconspicuouscoralsacrossthe Caribbeanbasin,coveringextensiveareasofreef-scape (O’Donnelletal.,2017).Sadly,itiscurrentlynearextinctiondue tobleaching-relatedevents,so larradiationandtemperature increases,anddiseases.Therefore,understandinghownatural fl uctuationsoftheseenvironmentalfactorsaffecttheinternal environmentofcoralsandhowtheseinternalchangesmayaffect viabilityiscrucialtofostertheconservationofthisimportantreefbuildingspecies.
Materialsandmethods
Experimentaldesign
ToevaluatetheeffectsofSTandLIseasonalvariationonFPsand PO,weusedgenetsof A.cervicornis thatwehavebeennursingin farminglines(donorfarms)atwaterdepthsof8and12msince2016 atPuntaSoldado,CulebraPuertoRico.Fivemonthsbeforethestart oftheexperiment,weselected20healthy-lookinggenetsfromthe donorfarminglines,tengenetsperwaterdepth.Wedividedthem intothreefragments(i.e.,ramets),twoofthesebetween15-20cmin
lengthandthethirdoneof6cm(Figure1).Immediatelyafterward, thetwolargerametsweretaggedwithauniquenumber,andoneof themwasplacedinanewfarminglinesetatitsoriginalcollection depth(hereaftershallow-shallowcontrol[SS])ordeep-deepcontrol [DD]; Figure1).Thesetwogroupswillbeknownasthecontrol groups.Theotherlargerametwassetfromshallowtodeep(shallowdeep[SD])ordeeptoshallow(deep-shallow[DS], Figure1).These fragmentswillbeknownastreatmentgroups.Farminglineswhere therametsweresetwereconstructedbyhammeringtwo-rodbarsof 1.5minlengtheach,0.5mintothesandybottom,2.5mapart,and connectingthemwith fishlines.Thefarmswerevisitedmonthlyfor thenext fivemonthstocheckforfragmentbleachinganddisease signs.Suchsignswereneverobservedduringthisperiod,suggesting thatbythestartoftheexperiment,allcoralfragmentswerefully acclimatedtotheenvironmentalconditions.The6cm-longramets wereplacedinnumberedconicalcentrifugetubes,frozeninliquid nitrogen,andtransportedtothelaboratory,wheretheywerestoredin a-80°Cfreezeruntilfurtheranalyses.These6cmfragmentswere subsequentlyanalyzedasdescribedbelowandunderstoodto representbaselinelevelsofFPconcentrationandPOactivity. Tissuefragmentsof4-6cminlengthfromcontrol(SSandDD) andtreatment(SDandDS)coloniesweresubsequentlycollectedon June09,2018(Summer2018),Nov02,2018(Fall2018),andMay18, 2019(Spring2019)followingthesameproceduresasexplained earlierforimmuneanalyses.Farmswerevisitedmonthlyfor maintenancethroughoutthestudy,asdescribedin Ruiz-Diaz etal.(2022)
Tissueextractionandtotal proteinquantification
Eachfrozencoralfragmentwasindividuallyairbrushedto separatesofttissuefromtheskeletonusingcoldphosphate
extractionbuffer(50mMphosphatebuffer,pH7.8with0.05mM dithiothreitol)andseparatelycollectedintosteriletubesonice.The crudeextractswerethenhomogenizedusingaFisherScientific PowerGen125TissueHomogenizerwithamediumsawtoothtip for60secondsontheiceatlowpower.Thehomogenizedtissue slurrywascentrifugedat4°Cat4,000RPMfor5minutes.The supernatantwasaliquotedandusedfreshorstoredat-20°C.The coralfragmentswereprocessedusingamodifiedBradfordassayto quantifythetotalproteinconcentration.Tomeasurethetotal proteinconcentration,5 mLofeachextractedsamplewereplated intriplicatein96-wellplatesalongwithabuffer-onlycontrol.Then 250 mLofBradfordreagentwasaddedandlefttoincubatefor5 minutesatroomtemperature.Absorbancewasrecordedat595nm usingaScientificMultiskanGOspectrophotometer.Asetofeight proteinstandardswaspreparedbyserialdilutionusingbovine serumalbuminata200mg/mLconcentrationasastocksolutionto preparethecalibrationcurve.Theconcentrationsforeachofthe standardsolutionswereasindicated:0.063,0.125,0.250,0.500, 0.750,1.000,1.500,and2.000mg/mL.
Biochemicalexaminationof fluorescentproteins
Meanintrinsicprotein fluorescence(RFU/mgoftotalproteins, hereaftermeanintrinsicFP)incoraltissueswasestimatedthrough thespectralemissionoftheseproteinsfollowing Palmeretal.(2009) withslightmodifications.TomeasurethemeanFP,30µLofthe extractedsampleswasplacedinablack96-wellplate,alongwitha controlsample(buffer-onlywell).Triplicatesofeachsamplewere excitedusinganInfinite200ProMultimodeMicroplateReaderat 280,410,470,and520nm,andtheemissionspectraweremeasured from450nmto750nmat5nmintervals.Eachsample’srelative fluorescenceandabsorbancewerenormalizedtototalprotein
concentrationdeterminedusingBradfordReagentandmeasuring absorbanceat595nm,usingBovineSerumAlbumin(BSA)to generatethemean fluorescencespectrapermgoftotalprotein.
Biochemicalexaminationofphenoloxidase enzymaticactivity
POactivitywasquanti fi edfromfreshtissueextractsand processedas Palmeretal.(2011) describedwithslight modifications.POactivitywasdeterminedbyadding20µLofthe freshlyextractedcoralsampletoa96-wellplate,inadditionto40µL of50mMphosphatebuffer,pH7.8,and25µlofdouble-distilled water(ddH2O).Afterincubatingatroomtemperaturefor20min, 30µLofa10mMdopaminehydrochloridesolutionwasaddedasa substrate.Thechangeinabsorbanceofthereactionwasmonitored every5minfor45minusinganInfinite200ProMultimode MicroplateReaderat490nm.Thechangeinabsorbanceforthe linearportionofthereactioncurvewasusedtodeterminePO activity.Controlreactionsunderthesamecondition,butwith70µL of50mMphosphatebuffer,pH7.8,andnosubstratewererunin parallelasacontrol.POactivitywasnormalizedtototalprotein concentrationineachsample.Thetotalproteinconcentrationof theextractedcoralsamplewasdeterminedasdescribedabove.
Environmentalmeasurements
Seawatertemperature(ST)andlightintensity(LI)weremeasured usingHoboPendanttemperature/lightdatalogger64k-UA-002-64 (OnsetCompany)attachedtooneoftherodsoftheexperimental nurserylinesateachdepth.Thedeviceswereprogrammedtorecord onedatumeveryhourfor30-40days,afterwhichtheywereretrieved andsubstitutedbynewonesuntiltheendofthestudy.Forthe statisticalanalysis,temperaturerecordswereaveragedbysummingall thetemperaturerecordsbetweenthecollectionoftissuedividedbythe numberofdaysbetweenthelastandprevioustissuecollection.ForLI, onlythe firsttendaysofrecordsfromeachmonthbetweentissue collectionwereused,asalgaeandsedimentscoveredthedevicesensor, potentiallyinterferingwiththedetectionoflightafterday10ofthe devicebeingdeployed.Therefore,LIrecordsafterthe10th daywerenot reliable.Thesedatawereusedtorunthegenerallinearmixedmodels.
Statisticalanalyses
Todeterminesigni fi cantdifferencesbetweenSTandLI, betweenwaterdepths(i.e.,8and12m)twoOne-wayrepeated ANOVAweredonewithdepthastheindependentvariableandST, andSImeasuredatfourdistincttimeintervals,(spring2018, summer2018,fall2018andspring2019)asthedependent variable. Post-hoc analyseswereperformedtodetermine differencesbetweenperiods.
OurstatisticalanalysisalsoconsistsofrepeatedmeasuresofFPs (includinggreenandcyanFP)andPOactivitytakeninaseasonalbasedfashion,i.e.,June09,2018,Nov02,2018,andMay18,2019.
Fortheseanalyses,weuseddatafromgenets(controlDDandSS) andramets(experimentalSDandDS)fragmentsof A.cervicornis, setattwodepths,i.e.,8&12mdepths.Exploratoryanalysesofthese datasuggestedalinearrelationshipbetweenmeanintrinsicFPsand POactivityacrossthemonitoringperiods,treatments(i.e.,depths), ST,andLI.Therefore,toexplorehowthesefactorsexplainthe observedvariationinmeanintrinsicFPsandPOactivityacross time,wedevelopedagenerallinearmixedmodel(GLMM)with fixedandrandomeffectsdescribedasfollows.Fixedeffects:(1) treatmentswithfourlevels,i.e.,controlshallowanddeepand experimentalshallowanddeepgroups;(2)ST,(3)LI,and(4) monitoringperiodwithfourlevels,i.e.,spring2018,summer2018, fall2018andspring2019.Therandomeffectonlyconsistedof rametsfragments.Todeterminetheeffectsofthenaturalvariation (seasonal)ofSTandLIontheimmune-relatedproteins,weranthe model(onerunperimmune-relatedprotein)consideringonlythe resultsfromcontrolcolonies,i.e.,DDandSScolonies.Weuseddata fromcontrolandexperimentalcoloniestodeterminetheeffectsof STandLIbetweendepthsontheimmune-relatedproteins.We performedthreeadditionalruns(oneperimmune-relatedprotein), yet,inthiscase,weonlyuseddatafromthesummerof2018tothe springof2019sincetheexperimentalgrouphadnodatainthe springof2018.Thestructureofthemodelwasspeci fi edby backwardstepwiseanalysis,excludingthosenon-signi fi cant effects.The fi nalmodelcomponents(i.e.,mostparsimonious) wereselectedbyperforminganAkaikeInformationCriterion (AIC)comparingnestedmodelsthatonlydifferedonthe componentsofinterest.Theseanalysesinvolvedchoosingthe moresaturatedmodelonlyifthereductionintheresidualsumof squareswasstatisticallysignificantcomparedtothesimplermodels.
Results
Environmentalvariables
STattheshallowfarmswasstatisticallydifferentfrom temperaturesatthedeep-waterfarms( Figure2 ; Table1 ). However,thetemperatureatthetwodepthsexhibitedasimilar temporalpatternofvariation,increasingfromspringtofall2018, whiledecreasingfromfall2018tospring2019.Variationsin temperatureacrosstimeper iodswerealsostatistically significant(Table1).
Meanwhile,LIwassignificantlyhigherattheshallowfarms thanatthedeepfarms( Figure2 ).Likewise,LIalsovaried significantlyacrossmonitoringperiods,yetinaslightlydifferent mannerthanthetemperatureatbothdepths,decreasingfrom springtosummer2018,reachingitshighestvaluesinfall2018, anddecreasingagaininspring2019.
Temporalvariationinintrinsicmean fluorescentproteins
Controlgroups(SSandDD)re mainedvisuallyhealthy throughoutthestudyperiods,yetdetectableandvaryinglevelsof
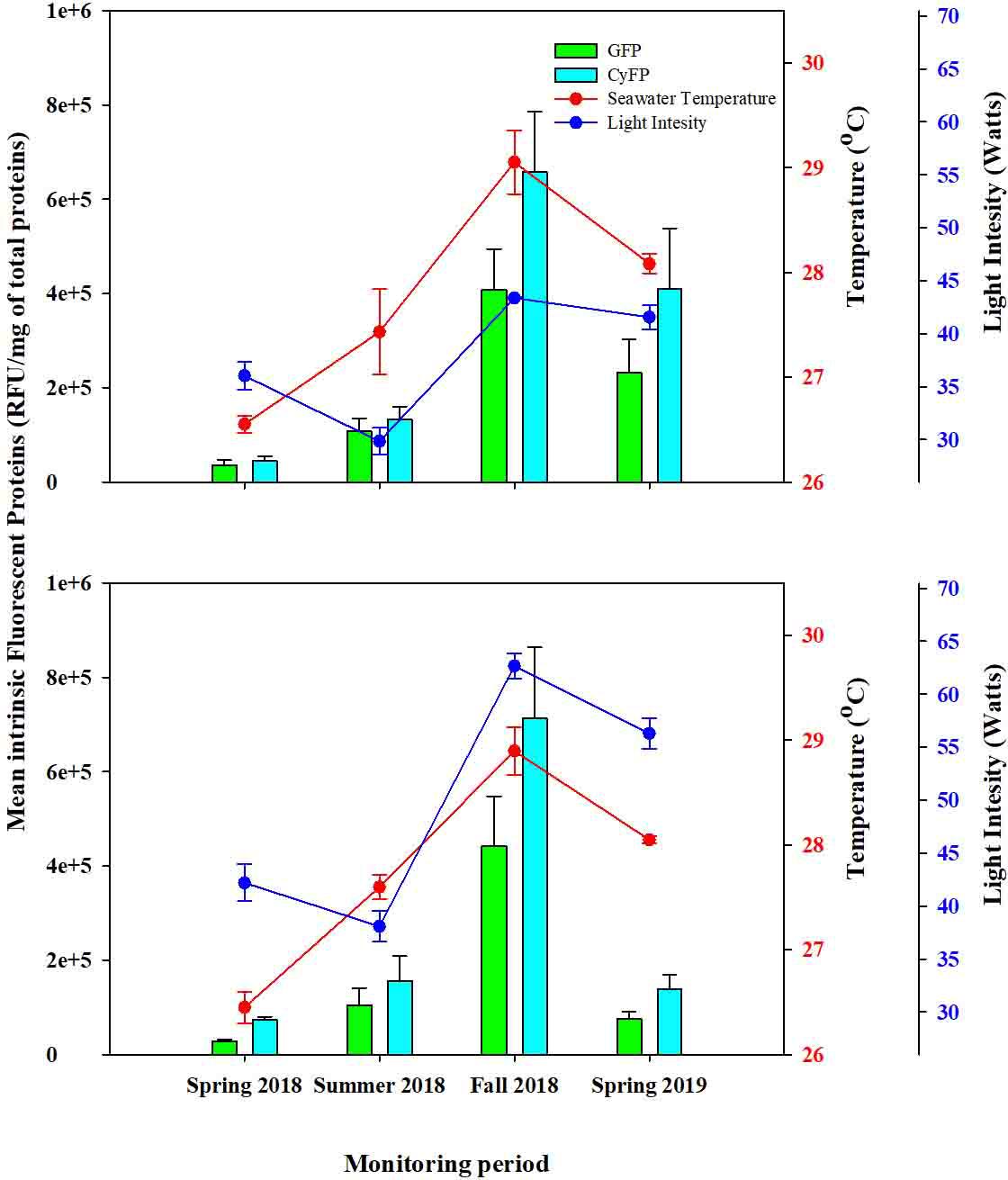
FIGURE2 ComparisonbetweenGreenFluorescent(GFP)andCyanFluorescentProtein(CyFP)forcontrolcoloniesacrosssurveyperiods. (A) Controlcolonies fromdeepwaterfarms(DD)and (B) controlcoloniesfromshallowwaterfarms(SS).Redandbluedotsrepresentseawatertemperatureandlight intensityrespectively;GreenbarsrepresentGFPandCyanbarsrepresentCyFP;whiskersrepresentstandarderror.
TABLE1Two-WayRepeatedANOVAanalysesforwatersurfacetemperature(ST)andlightintensity(LI).
GFPandCyFPproteinsandPOactivitywereobservedinevery colonyduringeachmonitoringperiod.However,levelsofthese immune-relatedproteinsvariedinmagnitudeacrossperiods.For instance,GFPinbothcontrolgroupssteadilyincreasedfromspring tosummerandfurtherinfall2018,whiledecreasingfromfall2018 tospring2019(Figure2).Thesedifferencesamongmonitoring periodswerestatisticallysignifi cantinbothgroups( Table2). Furthermore,seasonal fluctuationsinSTandLIalsoshoweda strongcorrespondencetotheconcentrationsofGFP,forboth controlgroups(F=3.89;P=0.013; Figure2; Table2).Thatis, increasesinSTandLIfromthespringtosummerof2018 correspondedtoincreasesinGFP,withthehighestpeakofST andLImirroringthehighestpeakofGFP.Consistently,the
observeddecreasesinSTandLIfromFall2018toSpring2019 correspondedtoadecreaseinGFPdetection.Theseeffectswerealso statisticallysignificant(ST:F=7.531,P=0.008;LI:F=7.527,P= 0.008; Table2)forbothcontrolgroups.
AsimilartrendwasobservedwhenconsideringCyFPintheDD andSScolonies,withdetectionlevelssteadilyincreasingfromspringto summerandthentofall2018whiledecreasingfromfall2018tospring 2019(Figure2).DifferencesinCyFPamongmonitoringperiodswere statisticallysignificant(F=3.048,P=0.036; Figure2; Table2).Seasonal fluctuationofSTandLIalso fluctuateacrosstimeinasimilarwayas CyFP fluctuates(Figure2; Table2),withthehighestpeakofSTandLI coincidingwiththehighestofCyFPandlowestpeaksofSTandLIalso coincidingwiththelowestpeakofCyFP.STandLIhadasignificant
effectonCyFPinbothexperimentalgroups(ST:F=5.995,P=0.018; LI:F=5.416,P=0.024; Table2).
Temporalvariationin phenoloxidaseactivity
Theactivityofthephenoloxidaseenzyme(PO)exhibiteda slightlydifferentpatternof fluctuationthanFPsinthecontrol groups.POactivityfromDDcoloniesincreasedfromspring2018to summerandfall2018andthendecreasedfromfalltospring2019 (Figure3).However,POactivityinSScoloniesdecreasedfrom spring2018andthenincreasedfromsummertofall2018,and decreasedfromfall2018tospring2019(Figure3).Thesedifferences amongmonitoringperiodswerestatisticaldifferencesforboth controlgroups( F=22.854,P=0.000 ; Table2 ).Meanwhile, seasonal fl uctuationsofSTandLIalsoshowedasimilar fluctuationasthePOactivity(Table2);increasingfromspringto summer2018,withthehighestpeakcoincidingwiththehighest peakofSTandLI(Figure3),andasSTandLIdecreasedfromFall 2018toSpring2019,sodoesPOactivity.STandLIhadasignificant effectonPOactivityinbothcontrolgroups(ST:F=5.098,P= 0.028,LI:F=4.474,P=0.039; Table2).
Depthrelatedvariationinintrinsicmean fluorescentproteins
GFPconcentrationfromDDcoloniesshowedsigni fi cant differencescomparedtoSScolonies( F=7.323,P=0.009 ,
Table2).Specifically,DDcoloniesexhibitedsignificantlyhigher GFPinthespring,andsummerandSSexhibitedsignificantly higherGFPinthefallof2018andspringof2019(Figure2A).
Adepth-relatedimpactinGFPwasalsoobservedbetweenthe controlandexperimentalgroups.Forinstance,GFPwashigherin DScoloniescomparedtoDDcoloniesinthesummerof2018.By thefallof2018,detectionlevelsinDSincreasedthreefoldwith respecttoDDcolonies.However,inspring2019,levelsofGFPin DDcoloniesalmosttripledcomparedtoDScolonies(Figure4). Thesedifferenceswerestatisticallysignificant(Table3).Asimilar detectionpatternwasalsoobservedwhencomparingDDandSD colonies.However,differencesinGFPdetectionlevelsshowno statisticaldifferences.
GFPlevelsbetweenSSandSD/DScoloniesexhibitedaslightly differentpattern.Forinstance,GFPlevelsfromSDcoloniesdouble thatofSSduringthesummerof2018.However,bythefallof2018, bothgroupsincreased.Nonetheless,SSdoubledtheconcentrationof SD.Whereasbyspring2019,bothgroupsdecreasedinGFPlevels. However,SDexhibitedhigherdetectionlevelsthanSScolonies ( Figure4 ).DifferencesbetweenSSandSDwerestatistically significant(Table3).Asimilardetectionpatternwasalsoobserved betweenSSandDScolonies,althoughdifferencesinGFPlevelsshow nostatisticaldifferences.
CyFPalsovariedbetweendepthsinthecontrolandexperimental colonies,consistentwithpreviousanalyses.Forexample,thedata revealedstatisticaldifferencesinCyFPbetweenDDandSScolonies (F=4.688,P=0.034),withDDcoloniesexhibitinglowermeanCyFP thanSScoloniesinallmonitoringperiodsexceptfromSpring2019 (Figure4; Table3).Meanwhile,CyFPinDScoloniesalmostdoubled thatofDDcoloniesinthesummerof2018.However,bythefallof
FIGURE3 ComparisonofPhenoloxidase(PO)activityovertimeandbetween, (A) Controlcoloniesfromdeepfarms(DD)and (B) controlcoloniesfromshallow farms.Redandbluecirclesrepresentwatertemperatureandlightintensity.Whiskersrepresentstandarderror.
TABLE2FixedeffectsresultfromtheGeneralLinearMixedModel(GLMM)analysesforGreenFluorescentProtein(GFP),CyanFluorescentProtein (CyFP),andPhenoloxidase(PO)activityfromcontrolcolonies fittedbymaximumlikelihood t-test usingSatterthwaite.
ModelVariable(FixedEffects)Estimatetvaluep-value GFP
Intercept552.0992.87.08E-03
Treatments:SS-158.064-2.7069.09E-03
Temperature-20.057-2.7478.15E-03
LignthIntensity-0.079-2.7448.23E-03
MonitoringPeriod:Summer201811.5262.984.31E-03
MonitoringPeriod:Fall201849.5762.8985.42E-03
MonitoringPeriod:Spring201934.3092.8576.06E-03
TreatmentsSS:Temperature5.8862.7059.12E-03
CyFP
Intercept473.1472.4691.67E-02
Treatments:SS-122.891-2.1653.48E-02
Temperature-17.135-2.4151.91E-02
LignthIntensity-0.066-2.3272.37E-02
MonitoringPeriod:Summer201810.1792.7089.10E-03
MonitoringPeriod:Fall201842.9852.5861.24E-02
MonitoringPeriod:Spring201929.7392.5491.14E-02
TreatmentsSS:Temperature4.5942.1733.42E-02
PO
Intercept32.172.2972.55E-02
Treatments:SS0.0710.7844.37E-01
Temperature-1.189-2.2582.80E-02
LignthIntensity-0.002-2.1153.90E-02
MonitoringPeriod:Summer20181.2022.4251.87E-02
MonitoringPeriod:Fall20183.6262.7298.56E-03
MonitoringPeriod:Spring20191.7992.0894.14E-02 p-valuerepresentslevelsofsignificance;Deep-deepcontrolcolonies(DD);Shallow-Shallowcontrolcolonies(SS).
2018,CyFPfromDDcoloniesnearlyquadrupledcomparedtothe previousmonitoringperiod,whereasDScoloniesdecreasedbyhalf. Bycontrast,inspring2019,CyFPconcentrationinDDcolonies decreasedbyhalf,whileDScoloniesincreasedbyhalfwithrespectto fall2018.CyFPconcentrationbetweenDDandDScolonieswas staticallysignificant(F=2.98,P=0.042; Table3).Asimilarpattern wasalsoobservedbetweenDDandSDcolonies,althoughdifferences inCyFPconcentrationshownostatisticaldifferences.CyFP concentrationbetweenSSandSD/DScoloniesbehavedsimilarly. Inthesummerof2018,SDcoloniesdoubledtheconcentrationof CyFPcomparedtoSScolonies.Bythefallof2018,CyFPinboth groupsincreased;however,CyFPconcentrationinSScolonies increasedbyorderof five,whileSDcoloniesincreasedbytwo.By spring2019,bothgroupsshowedCyFP,similartospring2018.
DifferencesacrosstimebetweenSSandSDwerestatistically significant(F=2.98,P=0.042; Table3).VariationinCyFP betweenSSandDSshowedsimilarincrease/decreasepatterns. Nonetheless,thesevariationswerenotstaticallysignificant.
Depth-relatedvariationinphenoloxidase activity
Meanwhile,theeffectofdepthonthePOactivitywaslessthan clear,asnostatisticaldifferenceswereobservedbetweencontrol colonies,andbetweencontrolandexperimentalcolonies(Figures3, 5A , B; Tables2, 3).Instead,theobserved fl uctuationsofPO activitieswererelatedtoseasonalityandWTandLIvariation.
FIGURE4
ComparisonbetweenGreenFluorescent(GFP),andCyan FluorescentProteins(CyFP)concentrationsfromexperimental coloniesacrosssurveyperiods. (A) Experimentalcoloniesfromdeep watersfarms(SD)and (B) experimentalcoloniesfromshallow watersfarms(DS).Redandbluedotsrepresentwatertemperature andlightintensityrespectively;GreenbarsrepresentGFP,andCyan barsrepresentCyFP.Whiskersrepresentstandarderror.
Discussion
Inthisstudy,weseektounderstandhowconstitutiveimmuneassociatedproteinsi.e.,green,andcyan fluorescentproteinsand phenoloxidaseenzymefrom A.cervicornis respondtoseasonal variationsinseawatertemperatures,lightintensity,andwater depth.OurresultsshowedapersistentdetectionofGFPand CyFPandPOinallcolonies,eventhoughnovisualsignsof physiologicalstresssuchasbleachingwereobserved.The presenceoftheseproteinswasrecordedregardlessofthe monitoringperiod,waterdepth,orcoralindividualsconfirming thecapacityof A.cervicornis tomaintainacertainlevelof constitutiveimmunity.Inaddition,ourresultsclearlyshowthat thelevelsofconstitutiveimmunityin Acervicornis aresubjectedto the fluctuationofenvironmentalfactors.Inourcase,weobserveda closecorrespondencebetweenseasonalvariationsintemperature lightintensityand,intrinsicmeanFPs,andPOactivity.Thatis,an increaseanddecreaseintemperatureandlightintensityledto synchronizedincreasesanddecreasesinintrinsicmeansofGFPand CyFP,andPOactivity.Thispatternwasobservedinallcoloniesi.e., DD,SS,DS,andDScolonies,butthemagnitudesofthechanges werebetterobservedinSSandDDcolonies.Forexample,our resultsshowthatwhenseawatertemperatureincreasedby2.5C°
andlightintensityby30Watt/m2 fromspringtofall2018,the concentrationsofGPFandCyFPincreasenearly11and15times respectively,whereasPOactivityincreasedfourtimes.Similarly, whenseawatertemperatureandlightintensitydecreased10Cand4 Watt/m2 fromfall2018tospring2019,theconcentrationsofGFP decreasedbyhalfwhencomparedtofall2018,whereasCyFPand POdecreased5timeswhencomparedtofall2018.Furthermore,the factthatSD(clonefragmentsofSScolonies)andDS(clone fragmentsofDD)respondedaccordingtotheirdepthsof settlementandnottotheirgeneticorigin,stronglysupportsthe conclusionthatindeedenvironmentalfactorsexertagreatinfluence intheconstituteimmunityof A.cervicornis corals.Fromthecoral’ s perspective,ourstudyshowsthat A.cervicornis hasthecapacityto acclimatetoseasonalchanges.
Previousstudieshavealsoreportedsimilarpatternsof variationsincoral’simmuneproteinsinresponsetoseasonal fl uctuationsinseawatertemperatureandsolarradiation.For instance, VandeWateretal.(2015, 2016) observedthatGPF concentrationandPOactivityfromthescleractiniancoral A. millepora increasedduringsummer ’ swarmerandbrighter months. RothandDeheyn(2013) alsodetectedincreasesinFP concentrationsin A.yongei coralafterincreasingSTandLI. Whereas Rothetal.(2010) showedthatcoralsincreaseor decreasetheconcentrationofGFPinresponsetoincreaseor decreaselightintensity.Meanwhile, Palmeretal.(2011) ,and Palmer(2018) reachedsimilarconclusionsworkingwiththe phenoloxidasepathsofthreeCaribbeancoralsi.e., Orbicela faveolate, Stephanocoenia interseptaand Poritesastreoides,and thePacificcoral Poritescylindrica
IncontrasttothestrongseasonalresponseinFPsandPOto variationintemperatureandlightintensity,theresultsofthe transplantexperimentindicateaweakresponsetochangesin depth.ThiswasparticularlyevidentwithPOactivity.For instance,equivalentlevelsofPOactivitywererecordedbetween coloniessetatdepthandshallowzones(DDandSS)andcolonies transplantedfromshallowtodeepanddeeptoshallowzones(SD andDSrespectively).Overall ,theseresultssuggestthat phenoloxidaseseemstobelesssensitivetowardlightintensity thantheFPssystem.Furthermore,theseresultsmayreflectthe differentrolesFPsandPOplayswithinthe A.cervicornis immune arsenal,withFPsmainlyreactingtowardenvironmentalstressorsas quencherofreactiveoxygenspeciesduringthermalstressandsun screening(Salihetal.,2000),andPOprimarilyrespondingtoward infectiousdiseases,andtissuerepairing(Palmeretal.,2011; Palmer andBaird,2018).However,sinceweonlymeasuredthePOactivity, whichisanintermediatestepinthemelanizationprocess,is uncertainifmelanin(the fi nalproductofthemelanization process)willshowasimilarpatternofvariationtothatobserved inPOactivityorwillminorthepatternsobservedinFPstoward changesindepth.
Ourdataalsoshowdifferentialphysiologicalresponsesamong individualstowardcyclicanddepth-relatedchangesinenvironmental conditions.Ingeneral,allindividualsdisplayedsimilarvariationinFP andPOthroughtime,butthemagnitudesoftheresponsevaried
TABLE3FixedeffectsresultfromtheGeneralLinearMixedModel(GLMM)analysesforGreenFluorescentProtein(GFP),CyanFluorescentProtein (CyFP),andPhenoloxidase(PO)activityfromcontrolcolonies fittedbymaximumlikelihood t-test usingSatterthwaite, p-value representslevelsof significance;Deep-shallowexperimentalcolonies(DS);Shallow-deepexperimentalcolonies(SD).
ModelVariable(FixedEffects)Estimatetvaluep-valu
Intercept743.52.411.81E-02
Treatments:SS-5.5582.737.77E-03
Treatments:SD-1.1140.476.40E-01
Treatments:DS-5.4752.688.91E-03
Temperature27.482.451.65E-02
LignthIntensity4.3632.669.34E-03
MonitoringPeriod:Fall201846.742.421.75E-02
MonitoringPeriod:Spring201924.362.551.27E-02
Intercept675.42.441.70E-02
Treatments:SS4.8522.6331.01E-02
Treatments:SD2.4620.1029.19E-01
Treatments:DS4.5232.4651.59E-02
Temperature25.012.4821.52E-02
LignthIntensity3.9262.6689.24E-03
MonitoringPeriod:Fall201842.172.4361.71E-02
MonitoringPeriod:Spring201921.722.5311.33E-02 PO
Intercept5.0384.61.39E-05
Treatments:SS4.8522.6335.11E-01
Treatments:SD2.4620.1025.68E-01
Treatments:DS4.5232.4653.18E-01
Temperature25.012.4822.64E-01
LignthIntensity3.9262.6681.07E-01
MonitoringPeriod:Fall201842.172.4362.64E-07
MonitoringPeriod:Spring20191.7992.0898.12E-02
betweencolonies.Severalfactorsarelikelycontributingtothese phenotypicvariations,includinggeneticheritages(Yetskoetal., 2020 ),environmentalmemoryoriginatedthroughepigenetic modifications(Hackerrottetal.,2021),or/andholobiont-related factors(Marangonetal.,2021).Thus,futureinvestigationshouldbe devotedtodeeperourunderstatingofthatmatters.
Thisstudyshowsthat,intheabsenceofsevereenvironmental disturbanceorabnormalenvironmentalstresses,constitutivelevels ofimmunityvaryconcomitantwithseasonalenvironmentalcycles, increasinginmonthsofhigherthermalandlightstressing condition,e.g.,latespring-earlyfall,decreasinginseasonswith lowerthermalandlightstressconditions,e.g.,latefall-earlyspring.
Yet,the fluctuatingimmuneresponsesalsosuggestaresource constraint,whereresourcesareallocatedintoimmune constituentsasdemandedbyenvironmentalpressures.However, giventheoptimalresourceallocationparadigmusedinlife-history theory(Stearns,1992 ),allocatingresourcesintoimmunitywill compromiseothervitalfunctionssuchasgrowthand reproduction.Consequently,coralsmuststrivetoutilizeresources optimallytomaximizetheir fitness.Thisparadigmhasbeen,also discussedfromthegrowthperspective.Forinstance, Ruiz-Diaz etal.(2022) comparedthegrowthrateofseveralhundredfragments of Acervicornis throughseasonalchangesintemperatureandlight intensityanddemonstratedthatthegrowthratewashigherduring
FIGURE5
ComparisonofPhenoloxidase(PO)activityacrosssurveyperiodsand, (A) Experimentalcoloniesfromdeepfarms(SD)and (B) experimentalcolonies fromshallowfarms(DS).Redandbluecirclesrepresentwatertemperatureandlightintensity.Whiskersrepresentstandarderror.
thewintermonthsthanduringthesummermonths.Toexplain theseseasonalgrowthpatterns,theauthorsarguedthatgrowthin thewinterseasonishigherbecauseseawatertemperature(ST)and lightintensity(LI),aremildincomparisontosummer,and thereforetheinvestmentofresourcesintogrowthwashigherthan intoimmunity.Otherstudieshavealsoreachedsimilarconclusions (Palmeretal.,2010; Palmeretal.,2011; Sheridanetal.,2014; Vande Wateretal.,2016).Thatis,thelevelofresourceallocationinto immunitycompetencyisdetrimentaltotheallocationofresources intogrowth.
Giventheclosecorrespondencebetweenimmuneconstitutive andenvironmentalfactorsandacknowledgingtheclimatechange scenario,wherebywintersarebecomingwarmerandsummers hotter,weshouldobserveanincreaseinthebaselineofthis constitutiveimmunityofcorals,concomitantwithareductionof growthorreproduction.Foracoralspeciessuchas A.cervicornis thatdependsomuchongrowthandbranchfracturingtoproduce newcolonies,thiscouldhaveadevastatingeffectbecausewemay observeareductioningrowthandbranchfracturingtocreatenew individualsandbecausegrowthandmortalityarecloselycorrelated thenewlyformedcoloniesshouldhavehighermortality.
FPsandPOinthecoralliteraturehavebeenmostlydiscussedin thecontextofdiseasesorenvironmentalchallenges.However,our
studyisoneofthefewaimedtoinvestigatethebaseline fluctuations oftheseimmune-relatedproteinsundernormalenvironmental conditions.Inthatregard,our fi ndingsaresigni fi cantto understandinghowcoralsnaturallyutilizetheseimmune-related proteins.Therefore,futurestudiesdevotedtounderstandingthe immuneresponseofcoralsthroughFPsandPOchallengesshould considerthenatural fluctuationoftheseimmune-relatedproteins, asthesamplingperiodmayinfluencethe finalinterpretationof theresults.
Dataavailabilitystatement
Therawdatasupportingtheconclusionsofthisarticlewillbe madeavailablebytheauthors,withoutunduereservation.
Authorcontributions
C-THandCPR-Dworkedequallyindevelopingthismanuscript, i.e.,datacollection(fieldandlaboratory),analysis,andmanuscript draft.JSR-LandLMD-Vequallyworkedinthelabwork,analysisof data,anddraftingofthemanuscript.MT-D,LS-PandAB-Cequally
workedinlabworkandanalysisofdata.Allauthorscontributedto thearticleandapprovedthesubmittedversion.
Funding
ThisprojectwasconductedwiththesupportoftheUniversity ofPuertoRicoSeaGrantCollegeProgram(NOAAGrantnumber NA14OAR4170068),NSF-CREST,grantnumberHRD-1736093; NIH-RISE:5R25GM061151-19.andUSDepartmentofEducation, MSEIP-P120A210035.
Acknowledgments
WewouldliketoextendourgratitudetoDr.A.Sabatforcritical reviewofthemanuscript.,P.Go mez,J.L.Sanchez,andother membersofSAMfortheir fieldworkhelp.
References
Alieva,N.O.,Konzen,K.A.,Field,S.F.,Memeshkevitch,E.A.,Hunt,M.E.,BeltranRamirez,V.,etal.(2008).Diversityandevolutionofcoral fluorescentproteins. PloS One 3(7),e2680.doi: 10.1371/journal.pone.0002680
Hackerrott,S.,Martell,H.A.,andEirin-Lopez,J.M.(2021).Coralenvironmental memory:causes,mechanisms,andconsequencesforfuturereefs. TrendsEcol. Evolution. 36,1011–1016.doi: 10.1016/j.tree.2021.06.014
Marangon,E.,Laffy,P.W.,Bourne,D.G.,andWebster,N.S.(2021).Microbiomemediatedmechanismscontributingtotheenvironmentaltoleranceofreefinvertebrate species. Mar.Biol.168,89.doi: 10.1007/s00227-021-03893-0
Mydlarz,L.D.,McGinty,E.S.,andHarvell,C.D.(2010).Whatarethephysiological andimmunologicalresponsesofcoraltoclimatewarminganddisease? J.Exp.Biol. 213 (6),934–945.doi: 10.1242/jeb.037580
O’Donnell,K.E.,Lohr,K.E.,Bartels,E.,andPatterson,J.T.(2017).Evaluationof staghorncoral(Acroporacervicornis,lamarck1816)productiontechniquesinan ocean-basednurseryconsideringcoralgenotype. J.Exp.Mar.Bio.Ecol 487,53–58. doi: 10.1016/j.jembe.2016.11.013
Palmer,C.(2018).Warmerwateraffectstheimmunityofatolerantreefcoral. Front. Mar.Sci. 5.doi: 10.3389/fmars.2018.00253
Palmer,C.V.,andBaird,A.H.(2018).Coraltumor-likegrowthanomaliesinduce andimmuneresponseandreducefecundity. Dis.Aquat.Organisms. 130,77–81.doi: 10.3354/dao03258
Palmer,C.V.,Bythell,J.C.,andWillis,B.L.(2010).Levelsofimmunityparameters underpinbleachinganddiseasesusceptibilityofreefcorals. FASEBJ. 24,1935–1946. doi: 10.1096/fj.09-152447
Palmer,C.V.,Bythell,J.C.,andWillis,B.L.(2011).Acomparativestudyof phenoloxidaseactivityindiseasedandbleachedcoloniesofthecoral Acropora millepora Dev.Comp.Immunol. 35,1096–1099.doi: 10.1016/j.dci.2011.04.001
Palmer,C.,McGinty,E.S.,Cummings,D.J.,Smith,S.,Bartels,R.,andMydlarz,L.D. (2011).Patternofcoralecologicalimmunology:variationintheresponsesofCaribbean coralstoelevatedtemperatureandpathogenelicitor. J.Exp.Biol. 214,4240–4249.doi: 10.1242/jeb.061267
Palmer,C.V.,Modi,C.K.,andMydlarz,L.D.(2009).Coral fluorescentproteinsas antioxidants. PloSOne 4(10),e7298.doi: 10.1371/journal.pone.0007298
Conflictofinterest
Theauthorsdeclarethattheresearchwasconductedinthe absenceofanycommercialor financialrelationshipsthatcouldbe construedasapotentialconflictofinterest.
Publisher
’snote
Allclaimsexpressedinthisarticlearesolelythoseof theauthorsanddonotnecessarilyrepresentthoseof theiraffi liatedorganizations,orthoseofthepublisher, theeditorsandthereview ers.Anyproductthatmay beevaluatedinthisarticle,orclaimthatmaybemade byitsmanufacturer,isnotguaranteedorendorsedby thepublisher.
Petes,L.E.,Harvell,C.D.,Peters,E.C.,Webb,M.A.H.,andMullen,K.M.(2003). PathogenscompromisereproductionandinducemelanizationinCaribbeanseafans. Mar.Ecol.Prog.Ser. 264,161–171.doi: 10.3354/meps264167
Quick,C.,D’Angelo,C.,andWiedenmann,J.(2018).Trade-offsassociatedwith photoprotectivegreen fluorescentproteinexpressionaspotentialdriversofbalancing selectionforcolorpolymorphisminreefcorals. Front.Mar.Sci 5.doi: 10.3389/ fmars.2018.00011
Roth,M.S.,andDeheyn,M.S.(2013).Effectofcoldstressandheatstressoncoral fluorescenceinreef-buildingcorals. Sci.Rep. 3,1421.doi: 10.1038/srep01421
Roth,M.,Lazt,M.,Georicke,R.,andDeheyn,D.(2010).Green fluorescentprotein regulationinthecoralacroporayoungerduringphoto-acclimation. J.Exp.Biol. 213, 3644–3655.doi: 10.1242/jeb.040881
Ruiz-Diaz,C.P.,Toledo-Hernandez,C.,andSanchez,J.L.(2022).Theeffectsof depth-relatedenvironmentalfactorsandsourceofcollectiononlifehistorytraitsin Acroporacervicornis raisedinnurseries. WaterJ. 14,212.doi: 10.3390/w14020212
Salih,A.,Larkum,A.,Cox,G.,Kuhl,M.,andHoegh-Gulderg,O.(2000).Fluorescent pigmentsincoralsarephotoprotective. Nature 408,850–853.doi: 10.1038/35048564 Sheridan,C.,Grosjean,PH.,Leblud,J.,Palmer,C.V.,Kusmaro,A.,andEackhout,I. (2014).Sedimentationrapidlyinducesanimmuneresponseanddepletesenergyin hardcoral. CoralReef 33,1067–1076.doi: 10.1007/s00338-014-1202-x
Stearns,S.C.(1992). Theevolutionoflifehistories (Oxford,UK:OxfordUniversity Press).doi: 10.1038/srep17639
VandeWater,J.A.J.M.,Lamb,J.B.,Heron,S.F.,vanOppen,M.J.H.,andWill,B. L.(2016).Temporalpatternsininnateimmunityparametersinreef-buildingcoralsand linkageswithlocalclimaticconditions. Ecosphere 7(11),e01505.doi: 10.1002/esc2.1505
VandeWater,J.A.J.M.,Lamb,J.B.,vanOppen,,.M.J.H.,Willis,B.L.,andBourne, D.G.(2015).Comparativeimmuneresponsesofcoraltostressorsassociatedwith offshorereef-basedtouristplatforms. ConervPhysiol. 3(1),cov032.doi: 10.1093/ conphys/cov032
Yetsko,K.,Ross,M.,Bellantuono,A.,Mersels,D.,Rodrıguez-Lanetty,M.,andGilg, M.R.(2020).Geneticdifferencesinthermaltoleranceamongcoloniesofthreatened coral Acroporacervicornis:potentialforadaptationtoincreasingtemperature. Mar. Ecol.Prog.Ser. 646,45–68.doi: 10.3354/meps13407