Mississippi State University Mississippi University Scholars Junction Scholars
Theses and Dissertations Theses and Dissertations
5-15-2022
Stress response and recovery of Atlantic Tarpon (Megalops
Stress response and recovery Atlantic (Megalops Atlanticus) to catch-and-release angling Atlanticus) catch-and-release angling
Laura B. Horowitz lbh270@msstate.edu
Follow this and additional works at: https://scholarsjunction.msstate.edu/td
Recommended Citation Recommended Citation Horowitz, Laura B., "Stress response and recovery of Atlantic Tarpon (Megalops Atlanticus) to catch-andrelease angling" (2022). ThesesandDissertations. 5498. https://scholarsjunction.msstate.edu/td/5498
This Graduate Thesis - Open Access is brought to you for free and open access by the Theses and Dissertations at Scholars Junction. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of Scholars Junction. For more information, please contact scholcomm@msstate.libanswers.com
Stress response and recovery of Atlantic Tarpon (Megalops Atlanticus) to catch-and-release angling
By TITLE PAGE
Laura B. Horowitz
Approved by:
Sandra B. Correa (Major Professor)
Peter J. Allen
J. Wesley Neal
Kevin M. Hunt (Graduate Coordinator)
L Wes Burger (Dean, College of Forest Resources)
A Thesis
Submitted to the Faculty of Mississippi State University in Partial Fulfillment of the Requirements for the Degree of Master of Science in Wildlife, Fisheries, and Aquaculture in the Department of Wildlife, Fisheries, and Aquaculture
Mississippi State, Mississippi
May 2022
Copyright by COPYRIGHT PAGE
Laura B. Horowitz
Name: Laura B. Horowitz
Date of Degree: May 13, 2022
Institution: Mississippi State University
ABSTRACT
Major Field: Wildlife, Fisheries, and Aquaculture
Major Professor: Sandra B. Correa
Title of Study: Stress response and recovery of Atlantic Tarpon (Megalops Atlanticus) to catch-and-release angling
Pages in Study: 88
Candidate for Degree of Master of Science
Atlantic Tarpon Megalops atlanticus support a catch-and-release fisheries in the United States and other territories such as Puerto Rico. Survival of angled fish is imperative to catchand-release fishing yet numerous factors have yet to be looked at over a timescale to determine if laboratory and wild Puerto Rico tarpon experience increased stress and risk of mortality. To evaluate stress in simulated catch-and-release angling, laboratory trials were conducted over a 24-hour time scale with 2 varying intensities of simulated angling and various physiological parameters were explored. Blood samples concluded that simulating angling only had an interaction effect in time and fishing intensity in the factor of osmolality. In field studies, tarpon studied physiologically gave inconclusive results due to lack of a time scale. Field studies tracking mortality were completed by acoustic telemetry of angled fish in the San Juan Lagoon network. Studies resulted in a mortality that ranges from 4.5-20.5%.
DEDICATION
I would like to thank my mother Marcia Horowitz and father Joshua Horowitz for their constant support and encouragement throughout my academic history, for always seeing me to my fullest potential and for helping me through the times I struggled the most. You always see me for only my best. Without you, I would not be who I am today. I would also like to thank KaDee, Brian and Maggie Jay for following me through my advanced academic career. You all gave me a home away from home with constant love and support that I know will follow me wherever I go, although Pineapple Island is the key to it all, Super Laura’s superpowers come from you. Thank you to my boyfriend, Matthew Mullins for the endless hours you have given me of love and encouragement and never letting me give up on anything. You’re the best friend, adventure buddy, workout partner, home chef, everything and anything a girl could ask for. In addition I would like to thank Jessica, Nathan, Ethan, Parker, Emmet, Rosie and Sunshine Berglund for following me throughout my academic experience and making Mississippi feel like home. With our adventures, you made time fly by and this is something I will be forever blessed to have made new friends. I would also like to thank my friends Gabbie D'Amore and Alexandria Tanase, even though we are far apart I know support is only one call away, in addition thank you Karold Coronado-Franco for always making me see the best in myself our adventures are my favorite memories. With the love of everyone mentioned, I see what I am capable of and how lucky I am.
ACKNOWLEDGEMENTS
Funding for this research was provided by Sea Grant (award #: 2020-2021-007), Mississippi Agricultural and Forestry Experiment Station and US Department of Agriculture (USDA) Agricultural Research Service (award #: 58-6066-5-042); USDA National Institute of Food and Agriculture (grant #: 1005154) funding for P.J.A., and Puerto Rico Department of Natural Resources for permitting use of the San Juan lagoon system. Specifically for Chapter 1, we thank Mack Fondren and Troy Lindsey of the Mississippi State University South Farm Aquaculture Facility for their assistance during experiments and with daily tank care; Karold Franco Coronado, Abby Vaughn, Manuel Coffill Rivera, Jacob Moreland, Rachel Tillman, Yvanna Mendez Paez, Alicia Santiago and Amanda Daulong for tank care and sampling assistance and Angel Muntaner, Lucas Valdivieso, Yorman Sierra, Gustavo Rodriguez, Miguel Muntaner and Francisco (Chiki) Prieto. For Chapter 2, we thank our partner agency Caribbean fishing adventures and the owner Angel Muntaner for working with us throughout the extent of the study and allowing us to join charters when our boats failed. Fishing guides at Caribbean Fishing Adventures: Lucas Valdivieso, Yorman Sierra, Gustavo Rodriguez, Miguel Muntaner and Francisco (Chiki) Prieto for taking us along on trips and teaching us about tarpon fishing. In addition, Karold Coronado-Franco for assisting in mapping fish location and data analysis. Lastly, thank you to all my committee members for allowing me this opportunity and providing feedback to produce this work.
LIST OF TABLES
Table 1.1 Average water quality parameters and standard error for experimental laboratory design........................................................................................................21
Table 2.1 Summary statistics of categorical data recorded during the fishing experience ........64
Table 2.2 Logistic regression models for mortality. 65
LIST OF FIGURES
Figure 1.1 Mean ± standard error plasma cortisol concentration (ng/mL) of simulated angled fish in laboratory settings (n=67)..................................................................22
Figure 1.2 Cortisol concentration (ng/mL) of wild-caught fish (n=42) as a function of total length (cm). ........................................................................................................23
Figure 1.3 Cortisol concentration (ng/mL) of wild-caught fish (n=42) as a function of fishing time as the total fishing experience (being caught and handled). ..................24
Figure 1.4 Glucose concentration (mg/dL) of simulated angled fish in laboratory settings (n=67) in combined trial runs over a 24 hour time period. ........................................25
Figure 1.5 Glucose concentration (mg/dL) of wild-caught fish (n=43) as a function of total length of the fish in cm.......................................................................................26
Figure 1.6 Glucose concentration (mg/dL) of wild-caught fish (n=43) as a function of fishing time as the total fishing experience (being caught and handled). ..................27
Figure 1.7 Lactate concentration (mmoles) of simulated angled fish in laboratory settings (n=67) in combined trial runs over a 24-hour time period................28
Figure 1.8 Lactate concentration (mmoles) of wild-caught fish (n=43) as a function of total length of the fish in cm.......................................................................................29
Figure 1.9 Lactate concentration (mmoles) of wild-caught fish (n=43) as a function of fishing time as the total fishing experience (being caught and handled). ..................30
Figure 1.10 Osmolality (Osmol/kg) of simulated angled fish in laboratory settings (n=67) in combined trial runs over a 24-hour time period.....................................................31
Figure 1.11 Osmolality (mmol/kg) of wild-caught fish (n=43) as a function of total length of the fish in cm.
Figure 1.12 Osmolality (Osmol/kg) of wild-caught fish (n=43) as a function of fishing time as the total fishing experience (being caught and handled).
Figure 1.13 pH of simulated angled fish in laboratory settings (n=67) in combined trial runs over a 24-hour time period.
32
33
34
Figure 1.14 Hematocrit (%) of simulated angled fish in laboratory settings (n=67) in combined trial runs over a 24-hour time period.........................................................35
Figure 1.15 Air breathing counts over a 5-minute time period for various time trials before and after inducing stress of laboratory fish (n=60).........................................36
Figure 2.1 Map of the study area.................................................................................................66
Figure 2.2 Data collection form for an angling event and short-term telemetry assessment. .................................................................................................................67
Figure 2.3 Telemetry tag picture along with labeled design comprised of the anchor, leader wire, crimps and acoustic tag...........................................................................68
Figure 2.4 Manual tracking telemetry sites within the study area and passive receiver locations......................................................................................................................69
Figure 2.5 Examples of fish movement and status classification as alive, dead, or censored based on rules..............................................................................................70
Figure 2.6 Passive receiver deployment picture along with labeled design comprised of PVC pole, receiver, rope and concrete stand..............................................................71
Figure 2.7 Rod action of caught tarpon by Caribbean Fishing Adventures (n=93; 1 missing data point). ....................................................................................................72
Figure 2.8 Location of where the hook was set on tarpon caught by Caribbean Fishing Adventures (n=92; 2 missing data points)..................................................................73
Figure 2.9 Bleeding experienced by tarpon due to the fishing exposure of fish caught by Caribbean Fishing Adventures (n=93; 1 missing data point).....................................74
Figure 2.10 Fish holding during photographs taken with clients of Caribbean Fishing Adventures (n=94)......................................................................................................75
Figure 2.11 Body size (total length in cm) of tarpon caught in Puerto Rico (n=94) as a function of fishing time expressed as the total fishing experience (being caught and handled). 76
Figure 2.12 Body size (total length in cm) of tarpon caught in Puerto Rico (n=94) as a function of angling time expressed as the total time the fish was on the line. 77
Figure 2.13 Body size (total length in cm) of tarpon caught in Puerto Rico (n=93; 1 missing data point) as a function of air exposure expressed as the total time the fish was out of water.............................................................................................78
Figure 2.14 Map of study site in the San Juan lagoon system with fish distribution from the study period (May-July 2021). Colors indicate different individual tarpon when caught and their individual movement through the lagoon over the extent of the study. .....................................................................................................79
Figure 2.15 Predicted probability of mortality of tarpon in response to (a) body size (total length) (b) fish time (time the fish was on the line) (c) handling time (time the fish was out of the water) length.
Figure 2.16 Predicted probability of mortality of tarpon contrasting individuals with and without hook removal.
Figure 2.17 Predicted probability of mortality of tarpon contrasting individuals with differing types of positions the fish was held in photography.
80
80
81
CHAPTER I
EXAMINATION OF THE PHYSIOLOGICAL IMPACT OF SIMULATED AND WILD STUDIES OF CATCH-AND-RELEASE FISHING ON ATLANTIC TARPON
Abstract
Catch-and-release fishing aims to promote the sustainable use of fish populations, yet negative physiological impacts and mortality may be an unintended result. Atlantic Tarpon (Megalops atlanticus; hereafter “tarpon”), support an important catch-and-release sport fishery within their range; however, little is known of the potential physiological impacts of angling or the response of tarpon to this acute stressor. Therefore, wild juvenile tarpon from the northern Gulf of Mississippi were collected and held in a controlled setting to determine the time course of physiological response to simulated angling, and compared with wild tarpon sampled as a part of catch-and-release sport fishing in Puerto Rico. In the laboratory, tarpon were stressed using 2 intensities of simulated angling (n=72; Low: 2-min chase, 1-min air exposure; High: 6-min chase, 2-min air exposure), and blood chemistry parameters indicative of stress and air breathing frequency were monitored during pre-angling (baseline) and at 0.25, 1, 4, 8 and 24 hours post angling. Although results for cortisol were inconclusive, secondary indicators of physiological stress including glucose, lactate, osmolality, hematocrit, pH and air breathing frequency followed a time course of an acute stress response and a recovery period. The osmolality response was of greater magnitude following the high-intensity stressor compared to the low-intensity stressor. Larger tarpon angled in Puerto Rico (n=43) were sampled immediately following capture and
had comparatively low values of secondary stress responses, possibly due to the shorter duration (generally < 0.25 hr) of post-stressor sampling. These results demonstrated that the process of angling and handling induces a short-term physiological stress response in tarpon, similar to many other species, although additional research is needed to determine whether there are longer-term implications to health and survival.
Introduction
Harvest-oriented fisheries result in 100% mortality of harvested fish; thus, harvest must be restricted using size limits, bag/trip limits, quotas, and seasonal closures to promote the sustainability of the fisheries (Bartholomew and Bohnsack 2005). In contrast, catch-and-release fishing is designed to allow a fish to be angled, released, and possibly caught again in the future
As a management tool, catch-and-release fishing is particularly beneficial for conserving broodstock (Cooke et al. 2002), protecting long-lived species (Wegner et al. 2021), and species that receive intense fishing pressure (e.g., Hutt et al. 2008). This approach is intended to impart sustainability to a fishery (Pollock and Pine 2007) but requires that fish are as physiologically fit after release compared to pre-capture. In other words, released fish must survive the angling experience and be able to defend and support themselves and reproduce successfully (Orr 2009).
The process of angling is not without injurious effect. There are numerous potential stressors that may act individually or synergistically to affect fish health. These repercussions vary by duration (and magnitude of the stressors Meka and McCormick 2005) and are influenced by gear type (Sass et al. 2018), environmental conditions (Suski et al. 2006), and post-capture handling and release practices (Guindon 2011). Physical wounds from catch-and-release fishing may involve direct tissue damage in the mouth, digestive tract, and body, which in turn may decrease mobility, therefore impacting physiological performance (Cooke et al. 2002). Wounds
to the body, organs and spine can occur from the fish having to support its weight out of the water when held by anglers (Gould and Grace 2009). Physiological impacts may include disturbances in cardiac output and functionality, respiratory challenges, and diminished shortand long-term immune responses (Cooke et al. 2002), all of which may lead to behavioral changes. Reduced physiological performance can affect individual health through diminished stressor response, predator avoidance and immune capacity, potentially leading to delayed mortality. These factors may impact individual and correspondingly population-level fitness (Cooke et al. 2002; Brownscombe et al. 2017).
The physiological stress response of fish is similar to many other vertebrates (Wendelaar Bonga 1997; Davis 2006). In teleost fishes, stressors induce primary responses such as cortisol release. In turn, primary responses produce secondary responses, including increased heart rate, permeability of gill epithelia, and increased circulating glucose levels, mobilizing energy to respond to a stressor but leading to greater hydromineral fluxes and depletion of stored energy substrates (Wendelaar Bonga 1997). These variables can be used to quantify intensity and duration of the stressor (Davis 2006), which are important for understanding implications to health and survival. While acute responses to stressors can be beneficial and extend normal adaptive ability, chronic exposure may result in decreased performance or survival (Davis 2006)
Identifying this stressor threshold is key to guiding fisheries management
Controlled experiments in a laboratory setting are commonly used to determine the physiological impact of fishing, stress response of the fish, and delayed mortality rate. Experiments can range from catch-and-release studies (Campbell et al. 2010) to simulated commercial net trawls (Davis 2007) designed to measure stress responses and recovery at discrete time intervals (Wedemeyer and Wydoski 2008; Conde-Sierira et al. 2018). For example,
marine fishes, including Walleye Pollock (Gadus chalcogrammus), Coho Salmon (Oncorhynchus kisutch), Rock Sole (Lepidopsetta bilineata) and Pacific Halibut (Hippoglossus stenolepis) were chased in tanks with motorized nets to simulate the fishing experience and measure fishing mortality (Wedemeyer and Wydoski 2008). Wild trout species and Arctic Grayling (Thymallus arcticus) were angled at select study sites for set durations, representing average times of an angling experience, and held in holding pens to extract blood to measure stress parameters over 72 hours (Wedemeyer and Wydoski 2008). Hatchery reared Senegalese
Sole (Solea senegalensis) were chased with dip nets for 5 minutes at various timepoints throughout 1 day and/or for multiple days, and then blood was drawn to test for physiological stress (Conde-Sierira et al. 2018). Paddlefish (Polyodon spathula) were chased for 1 hour with some treatments having air exposure (in dip nets for 30 seconds) to simulate a fishing experience and determine fishing stressors (Barton et al. 1998).
Yet in any study, researchers must be cautious not to induce procedural stress unrelated to the angling experience. For example, drawing blood from fish can be a stressful process, and researchers must minimize induced stress from handling during blood sampling (Acerete et al. 2004). Likewise, wild fish can be studied; yet moving wild fish to aquaculture settings can increase stress due to confinement (Woodward and Strange 1987). When fish husbandry and experiments are conducted with care and consideration to potential confounding effects, controlled environments allow for the use of standardized stressors such as chasing methods and aerial exposure times (Barton et al. 1998).
Physiological responses to stressors associated with catch-and-release angling have been examined in a wide variety of species. For example, Largemouth Bass (Micropterus salmoides) and Smallmouth Bass (M dolomieu) are 2 of the most targeted freshwater sportfish in the United
States (White et al. 2008). In these species, maladaptive responses to catch-and-release fishing have been correlated to the degree of exhaustion (Kieffer et al. 1995), air exposure (Cooke et al. 2002), water temperature, and dissolved oxygen (Suski et al. 2006; Keretz et al. 2018) Survival decreases as water reaches maximum temperature tolerance and minimum dissolved oxygen thresholds are reached (Keretz et al. 2018). Conversely, rapid capture, minimal handling, and rapid release can minimize immediate and latent mortality (Keretz et al. 2018).
In large migratory and pelagic fishes, blood chemistry stress-response variables may not be as effective at quantifying physiological impacts of stressors. This is due to the homeostatic disruptions caused by high anaerobic muscular activity (Skomal 2007). Understanding the physiology of marine fish, especially in larger species, is often challenged by the difficulty of obtaining unstressed specimens that can be handled adequately in captivity (Skomal 2007). For this reason, an acclimation period for wild fish should be implemented to allow confinement stressors to equilibrate. Further, stress response variables are often species-specific (Skomal 2007; Fanouraki et al. 2011). For example, blood glucose, lactate and osmolality increased following capture in Atlantic Sharpnose Shark (Rhizoprionodon terraenovae) (Hoffmayer and Parsons 2001), whereas blood lactate and glycogen were more responsive in Skipjack Tuna (Katsuwonus pelamis) and Yellowfin Tuna (Thunnus albacares) (Barrett and Connor 1962; Skomal 2007). There have been relatively few studies conducted on large marine species despite their popularity and importance as sport fishes.
Atlantic Tarpon (Megalops atlanticus) are a popular marine inshore sport fish species distributed in the Atlantic Ocean (Virginia to Florida coast), Bermuda, the Gulf of Mexico, and the Caribbean Sea to Brazil (Wade 1962). In the United States, the tarpon fishery generates millions of dollars in revenue (Anyanwu et al. 2009). For example, in Florida's Caloosahatchee
River and Charlotte Harbor region tarpon fishing generates > $65 million annually (Felder 2011).
Within these fisheries, varying regulations control the legal harvest of tarpon. In Mississippi, anglers can keep 1 legal-size tarpon per angler per day (Graham et al 2021). In Alabama, there is a harvest tag system which requires the purchase of a tag to allow for taking a tarpon, yet there is no limit to the tags an angler can purchase (Graham et al 2021). In Louisiana, there are no management regulations for tarpon (Graham et al. 2021). In Puerto Rico, past fishing pressure and harvest caused a population decline of tarpon (Zerbi et al. 1999); with up to 10,189 kg harvested annually from 2000 - 2003 (Guerrero Pérez et al. 2013). As a result, in 2004, the Puerto Rico Department of Natural and Environmental Resources (DNER) imposed harvest prohibition on tarpon to protect stocks for local and tourist sport fishing to ensure the sustainability of the resource (Guerrero Pérez et al. 2013; Luo et al. 2020).
Tarpon have been studied for tagging efficiency (Zerbi et al. 1999), movement (Griffin et al. 2018), conservation genetics (Seyoum et al. 2008, Blandon et al. 2003) and ageing (Elmo 2020). However, few studies have examined physiological effects of angling on tarpon. In a simulated angling study on Atlantic Tarpon, blood lactate, glucose, hematocrit and electrolytes increased following a stressor; however, duration of angling and intensity of angling stressors were not examined (Guindon 2011). Therefore, the purpose of the present study is to 1) characterize the physiological response of tarpon to simulated angling stressors, 2) evaluate responses to different stressor intensities, and 3) determine the time duration of post-angling recovery over a 24-hour period. Results are compared to physiological responses of wild tarpon from catch-and-release fishing in Puerto Rico.
Methods
Fish collection and laboratory setting
Wild tarpon (n=50) were collected from the northern Gulf of Mexico at Ocean Springs, Mississippi, from August-November 2021. Tarpon were captured using cast nets deployed into estuarine inlets and coastal marshes. Fish were held in holding tanks at the Gulf Coast Research Laboratory until November, and then transferred to the South Farm Aquaculture Facility at Mississippi State University. Smaller tarpon (< 205 mm total length (TL); n=25) were placed into an 800-L tank recirculating system, whereas larger tarpon (> 205 mm TL; n=25) were placed into a 3,500-L tank; separation of size classes was used to minimize competition and enhance acclimation of tarpon. The recirculating systems were composed of a holding tank, sump tank, biofilter, ultraviolet sterilizer, aeration via forced air and water circulation pump.
Fish were transitioned from a diet of live fish to prepared fish after approximately 1 month. Fish immediately consumed live feed consisting of Guppy Poecilia reticulata, but required approximately 5 weeks to fully transition to prepared fish (mix of cut pieces of catfish
Ictalurus spp. and sunfish Lepomis spp.). Fish were fed to satiation daily throughout the study.
After 4.5 months, small and large fish were placed together in the larger holding tank.
Acclimation conditions consisted of mean ± standard error (SE) water temperature of 22.7°C ± 0.2°C, dissolved oxygen of 82.5 ± 1.1% saturation or 10.8 ± 0.9 mg/L, and salinity of 19.4 ppt ± 0.1 ppt. Dissolved oxygen and salinity measurements were conducted using a YSI 85 (YSI, Inc., Yellow Springs, Ohio), pH using a pH10A (YSI, Inc.) and ammonia/nitrite using colorimetric assays (DR/850, Hach Company, Loveland, Colorado). Experiments began after 5 months of acclimation to tank systems.
Simulated angling experiment
Two standardized stressor treatments were applied: 1) low fishing intensity (2-min chase, 1-min air exposure), and 2) high fishing intensity (6-min chase, 2-min air exposure). Each trial consisted of 3 tanks with 6 fish per tank per treatment. The experiment was repeated 1 month later for a total of 6 tanks per treatment (36 fish; 6 time-series replicates per treatment). Tanks were 800-L with a diameter of 127 cm and a water height of 61 cm. Tanks used in experimental trials were partially submerged in 2 flow-through raceways to maintain homogenous temperature.
Fish were randomly selected from the holding tank and assigned to treatments using a complete randomized design. Total length ranged 191-395 mm (mean ± SE = 292 ± 9 mm) in the first trial and 209-399 mm (302 ± 9 mm) in the second trial. Feeding was suspended 48 hours prior to experiments to minimize stress. Following transfer to the treatment tanks, fish were allowed to acclimate for 24 hours. The top of each tank was continuously covered with foam insulation to prevent escape and avoid visual stimuli by researchers. Oxygen was supplied by forced air via air stones. Water temperature (mean ± SE) averaged 22.8 ± 0.2 C °, dissolved oxygen averaged 7.11 ± 0.07 mg/L, and salinity averaged 18.5 ± 0.4 ppt (Table 1). Water quality was monitored daily to ensure minimal variation among tanks.
Covers were removed during each experimental procedure and immediately replaced between sampling periods to reduce external stimuli. Chasing and air exposure were accomplished using 1 dip net (0.6-cm-mesh, 40.6 x 40.6 cm mouth frame). The net was moved vigorously in each tank to chase fish and produce the simulated angling effect for each allotted treatment time. Immediately following chasing, all fish were captured in the net and held out of the water for the allotted treatment air exposure time. Fish were then immediately returned to the
water. Specific protocols for fish sampling are presented below. Signs of fish impairment, shortterm mortality, and delayed mortality were monitored daily for 30 days following the experimental trial, with no signs of significant injury or mortality detected.
Blood collection
Blood was collected from 1 experimental fish per tank per sampling period. The time series sampled was 0 (before simulated angling), 0.25, 1, 4, 8, and 24 hours (after simulated angling). To collect blood, each fish was removed from the tank and placed in an anesthetic bath (200 mg/L tricaine methanesulfonate (MS-222) at treatment conditions; Popovic et al. 2012).
Blood (~1-mL) was collected from the caudal vasculature or via cardiac puncture (Clark et al. 2011) using sodium heparin-coated 1- or 3-mL syringes (20-gauge needle). After blood collection, the fish was transferred to a recovery tank (same dimensions as the experimental tank) until visible signs of recovery occurred (i.e., movement of caudal fin and resumed swimming) and subsequently transferred to the original holding tank.
Once a blood sample was collected, it was immediately placed on ice in a 1-mL snap cap vial. Whole blood pH was measured with a pH meter (Accumet Basic AB15 pH Meter, Fisher Scientific) and microelectrode (#13620850, Fisher Scientific) using a temperature-controlled water bath (WB10, Polyscience) set to the same temperature as the experimental tanks. Immediately afterwards, hematocrit (Hct) was measured in hematocrit tubes (heparinized microhematocrit capillary tube) after centrifugation for 5 minutes at 6,000 x g. Remaining blood was centrifuged for 3 minutes at 5,000 x g and plasma was aspirated into a new vial, flash-frozen in liquid nitrogen and subsequently stored at -80°C.
To determine if fish exhibited stress when moved into experimental tanks, 6 fish from the original holding tank were collected 1 month after the 2nd trial (April) and processed identically
to experimental fish. These fish were collected in April to avoid any impact on experimental fish. Furthermore, these fish were used as a baseline control to compare with Time 0 fish in each treatment to assure experimental fish did not experience elevated stress levels due to being in different tanks. The size range of fish in the control group was 229- 352 mm (TL; mean ± SE = 348 ± 21 mm).
Air breathing
The frequency of air-breathing was quantified by visual observation over a 5-min period in each experimental tank. The number of air breaths was divided by the number of fish in the tank at the time of the count to provide a per fish rate of breathing. Air-breathing was measured at 0 minutes (immediately before chasing stressor), and at 0.08 (immediately after chasing to 5 min), 2, 5, and 23 hours post-angling stressor.
Blood analyses
When thawing blood plasma, it was noted that a high frequency of plasma samples had presumably fibrinogen clots which were removed prior to analytical tests. Commercial kits were used in blood plasma analyses to measure cortisol (EA65; Oxford Biomedical Research, Oxford, Michigan), glucose (DIGL-100; BioAssay Systems), and lactate (A-108L; Biomedical Research Service Center, Buffalo, New York). Osmolality was measured using a vapor pressure osmometer (Vapro 5520; Wescor, Logan, Utah). Methods were similar to those used by Dinken et al. (2020).
Stress response of angled wild tarpon
The stress response curves of lactate, cortisol, glucose, and osmolality from the simulated fishing treatments were compared to values from wild fish caught from chartered sport fishing
trips in Puerto Rico. Caribbean Fishing Adventures, located in San Juan, Puerto Rico, hosted the researchers on fishing charters with clients to observe their fishing experience. Trips ranged from 4 to 6 hours and were available 3 times per day (morning, afternoon, and night). Fishing was conducted between May 2021 to July 2021. Tarpon were angled by guides or clients, lifted from the water via a hand-hold on the jaw, and handled briefly in the air for pictures and blood collection. Blood was collected from the caudal artery or gill arch without anesthesia. Syringes (5-mL) were used, and plasma was centrifuged immediately after blood collection. Once blood draw was completed, fish were immediately released. Blood was immediately placed on ice in a cooler, and subsequently stored at -20 °C until transportation to Mississippi State University, where it was stored at -80 °C until processing. In total, blood samples were collected from 43 fish in the San Juan lagoon system, where fish ranged between 43-125 cm in total length (Mean ± SE: 78 ± 3 cm). Due to limited equipment, pH and Hct were not measured in fish collected in Puerto Rico. In addition, air breathing was not able to be measured for Puerto Rico fish due to release after capture.
Statistical Analyses
All statistical analyses were conducted using R statistical software 64-bit version 3.6.2.
Data from laboratory studies were analyzed for outliers using Dixon's Q tests with a maximum of 1 outlier removed from a treatment group. Comparisons between control (fish from the 3,500-L holding tank) and experimental fish at Time 0 (low and high angling intensity groups) were conducted for each blood parameter with a one-way analysis of variance (ANOVA). To evaluate if there was any effect from using the same fish for both trials (i.e., February and March), differences in blood parameters between trial runs were tested via a three-way ANOVA to determine the effects of treatment group (i.e., low and high) and time (i.e., 0, 0.25, 1, 4, 8, and 24
hours). No differences in any of the measurement variables were detected, therefore samples from separate months were pooled into a two-way ANOVA with factors of stressor intensity and time Before implementing ANOVA models, residuals were assessed by quantile comparison plots, histograms, and Shapiro Wilk's test of normality and Levene's test for homogeneity of variance. If any assumptions were violated, data were logarithmically, square root or inversely transformed. If the transformation was ineffective, the ANOVA proceeded with caution as it is a robust analysis (Oliver-Rodríguez and Wang 2015). If an ANOVA was significant, differences among groups were tested using a Tukey’s post hoc test. Normality was assessed similarly for fish sampled in the field in Puerto Rico, and linear regressions were conducted to assess relationships between physiological parameters and body size or duration of angling (i.e., fight time and handling time) For all statistical tests, a significance level of α = 0.05 was used. Graphs to compare laboratory fish were created in SigmaPlot.
Results
Simulated angling consisted of rapid chasing, impact with the net and tank walls, and rapid changes in direction. Fish exhibited evasive jumping behavior in response to angling stressors, similar to observed jumping behavior during angling of wild fish. Further, some evidence of light abrasions and scale loss was observed. However, no mortality occurred during simulated angling or during the 30-day post-stressor monitoring period. In 2 instances, blood was unable to be collected from a fish.
Control fish were statistically indistinguishable from Time 0 low and high stressor intensity treatments for all blood parameters except glucose, which was lower in control fish (control average of 66.1 mg/dL compared to the experimental fish of 119.2 mg/dL in low treatment group and 106.3 mg/dL in high treatment group; p < 0.05). There was no interaction
between stressor intensity and time except for plasma osmolality (df=5, f=2.497, p < 0.041).
There were no main effects of stressor intensity (p > 0.05); however, all variables measured had a main effect of time (p < 0.05).
Cortisol concentration was greater at Time 0 and 0.25 hours compared to 24 hours post stressor (Fig. 1). In wild caught fish from Puerto Rico, there was not a linear relationship between cortisol and total length or time duration of angling (Fig. 2 and 3). Glucose concentration peaked at 4 hours and returned to baseline by 8 hours post-stressor (Fig. 4). In wild caught fish from Puerto Rico, fish total length or time duration of angling did not impact glucose concentration (Fig. 5 and 6). Lactate increased at Time 0.25, 1 and 4 hours and returned to baseline by 8-hours post stressor (Fig. 7) In wild caught fish from Puerto Rico, fish total length or time duration of angling did not impact lactate concentration (Fig. 8 and 9) There was an interactive effect of angling intensity*time and an effect of time on plasma osmolality (Fig. 10).
The high angling intensity resulted in a greater, but non-significant, stress response compared to the low angling intensity. In the low angling intensity treatment, plasma osmolality was elevated at 0.25 hours post-stressor but was indistinguishable from Time 0 by 1- hour post-stressor. In the high angling intensity treatment, plasma osmolality was elevated at 0.25- hours post-stressor but was indistinguishable from Time 0 hour by 4 hours post-stressor. In wild caught fish from Puerto Rico, there was no relationship between total length of fish or time fished impacting osmolality (Fig. 11 and 12). Blood pH decreased at Time 0.25 and 1 hour compared to Time 0 and returned to baseline 4- hours post-stressor (Fig. 13). There was a decline in Hct with time, with decreased Hct at Time 8 and 24 hours compared to Time 0, 0.25 and 1 hours post-stressor (Fig. 14). Air breathing frequency increased approximately 400% and 700% immediately following the low
and high intensity treatments (Time 0.08 hours) compared to Time 0, and returned to baseline by 2- hours post stressor (Fig. 15).
Discussion
This is the first study to characterize the time course of angling stress response in tarpon, investigate the response to differing magnitudes of angling stressors, and investigate stress responses of wild tarpon angled from Puerto Rico. The physiological response of fish to an acute stressor typically consists of an increase in primary stress responses, which mobilize secondary stress responses to provide energy substrates to respond to the stressor, followed by a return to homeostatic initial or baseline conditions. Cortisol is a well-established primary indicator of stress in fish, as large differences in the corticosteroid responses to stress are dependent on magnitude of stress and species dependent (Barton 2000). Glucose, lactate, osmolality, pH and Hct are all secondary indicators of stress; these parameters occur over a slower timescale and allow for a greater understanding of how the organism is responding to a stressor (Sopinka et al. 2016). Based on this established relationship, blood was analyzed for physiological indicators of stress, including cortisol, glucose, lactate, osmolality, pH, and hematocrit (Hct) (Wendelaar Bonga 1997).
Although cortisol is the primary stress hormone in teleosts (Vijayan et al. 2010; Ellis et al. 2012), less is known in primitive fishes (Youson 2007). In this study cortisol did not respond in a classical manner, with no apparent response to the stressor. Guindon (2011) also found cortisol did not respond to an acute stressor in tarpon. A related Elopiformes species, Bonefish (Albula spp.), showed no cortisol response following strenuous exercise and varying levels of dissolved oxygen (Shultz et al. 2011). Because tarpon are descendants of a primitive lineage of teleost fish (Ault and Luo 2013), it is possible that cortisol is not the primary stress hormone in
this group, or that these fish have a minimal cortisol response to a stressor, such as with some other groups of primitive fishes. For example, sturgeons Acipenseridae (Haukenes et al. 2008), gars Lepisosteidae, and bowfin Amia calva (Davis and Parker 1986) have reduced or no detectable cortisol responses. Therefore, it is quite possible that tarpon do not have a predictable cortisol response as a primary indicator of stress. Guindon (2011) reported that larger tarpon had significantly lower cortisol responses compared to smaller tarpon. It is possible that fish size is not a significant proxy for cortisol concentration whereas fish age is. In the Silver Catfish, Rhamdia quelen, there were large differences in the stress response of fish that were of different ages even though they were the same size (Barcellos et al. 2012). Age could, in this case, be more influential in stress response than fish size as tarpon can live a maximum age of at least 55 years (Crabtree et al. 1995).
Secondary stress response indicators displayed a time-series effect that followed the classical pattern. Plasma glucose increased in response to the simulated angling stressor, peaking at 4 hours and then returning to baseline concentrations by 8 hours. These findings are supported by Guindon (2011), who reported angling tarpon for 15 minutes increased glucose concentrations from 87 mg/dL to 94 mg/dL. Glucose did not respond differentially to the intensity of the angling experience. Increases in glucose may suggest utilization of metabolic energy reserves (Hemre et al. 2002), which may increase the individual tarpon's energy expenditure. As tarpon were noted to have rapid jumping behavior in both laboratory and field settings, the need for increased energy to be drawn to the muscles would support glucose increases within the body (Driedzic and Hochachka 1976).
Control fish displayed significantly lower glucose than experimental fish at Time 0; therefore, some level of pre-treatment stress may have been present in experimental fish Further,
average glucose concentration at Time 0 was 113 mg/dL in the controlled experiment compared to 81 mg/dL for angled Puerto Rico fish, which was closer to the value of 87 mg/dL in tarpon reported by Wells et al. (2003). Many factors could account for this observed variability. One possible explanation is that tarpon have a varying glucose response. Cortisol activates glucocorticoid receptors which can alter glucose levels (Vijayan et al. 2010). Glucose responses in salmonids differ to some extent depending on the stressor and species, with concentrations of glucose potentially remaining elevated for extended periods of time (> 72 hours) (Wedemeyer and Wydoski 2008). In the present study, experimental fish were stressed for a maximum of 8 minutes in total (chasing and air exposure), so with longer stress periods, results might change. Lastly, increased concentration of glucose could be related to fish size as this has been determined to be an influential factor in species such as wild Rainbow Trout Oncorhynchus mykiss (Meka and McCormick 2005). In the current laboratory study glucose was averaged at 112.7 mg/dL compared to wild-caught fish with a glucose concentration of 81.0 mg/dL; wildcaught fish were larger in size compared to laboratory fish indicating that these trends may be opposite for species such as tarpon.
Lactate also displayed predictable temporal response to a stressor, peaking at 1 hour and exhibiting return to baseline by 8 hours after simulated angling No influence of angling intensity was detected. Lactate is produced in conditions of anerobic respiration to provide energy to the individual (Allen and Holm 2008). Many studies have reported that lactate continues to build after exercise ceases (Wood et al. 1983; Wells et al. 2007). Furthermore, lactic acid in fish species using burst swimming is rapidly accumulated and then is released in excess after 5-10 minutes (Wood 1991). Since tarpon use burst swimming to leap out of water when stressed, it is intuitive that lactate would increase rapidly with exertion and then decrease rapidly to a base
level following cessation of exertion. Guidon (2011) reported that lactate was the physiological characteristic with the greatest response to stress in Atlantic Tarpon. In the congener Pacific Tarpon (Megalops cyprinoides), lactate levels followed a similar pattern following exertion, with recovery within 4 hours (Wells et al. 2007). Although no effect of intensity was found, the duration of chase and air exposure has been shown to affect lactate in other species (e.g., Australasian Snapper Pagrus auratus; McArley and Herbert 2014).
Blood plasma osmolality was the only stress response variable measured displaying both a temporal response and an intensity response. Osmolality peaked at 0.25 hours before returning to baseline (statistically similar at 4 hours), with significantly greater response to the high intensity treatment Osmolality had not previously been studied in tarpon or other bonefish in a simulated fishing scenario. Results from this study are in line with those found for many species, including White Sturgeon Acipenser transmontanus and Elephant Fish, which, when exposed to increasing intensities of simulated fishing and air exposure, the response of osmolality increased (McLean et al. 2016; Martins et al. 2018).
Blood pH declined immediately following simulated angling, but recovered by 4-hours post-stressor; no influence of angling intensity was detected. pH is an important metabolic indicator and is involved in maintaining homeostasis (Aoi and Marunaka 2014.). Increasing lactate, as noted above, causes a decrease in blood pH (Aoi and Marunaka 2014). In addition, other factors such as metabolic and respiratory acidosis cause a reduction in blood pH (Lopez et al. 2002). Low blood pH in fishes can increase disease susceptibility, impair osmoregulatory and ion-regulation, inhibit hormone production/activity, create genetic damage to future generations of brood, and increase susceptibility of toxic substances (Fritz 1980). pH has not been previously examined in Atlantic Tarpon, but Pacific Tarpon have shown a similar response (Wells et al.
2007). In that study, intensity did influence pH, which also recovered following a 4-hour recovery period (Wells et al. 2007). For Bonefish, blood pH was negatively correlated with fight time and fish length, but lactate was not the sole cause for pH decline (Brownscombe et al. 2015). In Rainbow Trout, exercising fish for 10 minutes with the addition of air exposure for 1 minute significantly altered acid base relationships when comparing stressed fish to those that were only exercised (Ferguson and Tufts 1992). This suggests that the additional stress of air exposure may be detrimental.
Hematocrit (Hct) exhibited a steady decline following the angling stressor with no effect of stressor intensity, but had not recovered to baseline level at the end of the 24-hour assessment period. Hct measures the proportion of red blood cells in the blood which influences oxygen carrying capacity (Gallaugher et al. 1995). Aerobic stress due to exercise is linked to increases in Hct percentages (Gallaugher et al. 1995), yet this was not the case in the current study. In previous tarpon studies, Hct was measured but did not have any significant effects in terms of fight time, handling time, size, or angling treatment (Guindon 2011). The mean Hct percentage for tarpon previously studied was 46.9% (Guindon 2011), whereas this study’s angled tarpon had a mean value of 35.1%. Tarpon naturally have a high resting Hct percentage of 37.6% (Wells et al. 2003), yet our results are lower compared to previous studies in tarpon. Hct has been shown to decrease due to factors such as confinement, induced stress, and lack of food (Affonso et al. 2002), which might explain the observed results since tarpon were not fed 48 hours before experimental simulations. Decreased Hct percentages have been a factor of starvation (Larsson and Lewander 1973), but the specific timeframe where this occurs is currently unknown. As the laboratory fish were well fed throughout their holding period, determining whether the starvation
factor impacts the Hct following the angling-stressor in the current study will have to be further investigated.
Although angling intensity did not influence tarpon Hct, exertion level did affect Bonefish Hct, with a response only detected for the greater level of exertion (4 minutes stressed) compared to low level exertion (1 minute stressed), and no return to base levels during the 4hour post stress observation period (Suski et al. 2007). Under harsh water quality conditions, Tambaqui (Colossoma macropomum) exhibited a decreased Hct percentage at 96 hours post stressor (Affonso et al. 2002). It is currently unknown how long it takes tarpon to fully recover to a resting Hct percentage.
Air-breathing increased 3 to 7-fold immediately following both low and high angling intensity stressors, respectively, and returned baseline within 2 hours. Tarpon are facultative air breathers (Geiger et al 2000; Seymour et al 2008), and air breathing can aid in recovery from oxygen debt by restoring physiological parameters back to baseline levels (Wells et al. 2003). Tarpon increase air breathing frequency in non-optimal environments and due to exercise (Wells et al. 2003). In this study, the increase in air breathing was presumably also beneficial for recovery of other physiological parameters such as lactate (Wells et al. 2003, 2007), pH (Gonzalez et al. 2001) and hematocrit (Wells et al. 2003). In Pacific Tarpon, the air breathing organ has been shown to help prolong aerobic activity in events that require a high energy demand, but it is gill breathing that is used to recover from oxygen debt (Wells et al. 2007). Although not documented in Puerto Rico fish, air breathing frequency may differ in adult tarpon (Clark et al. 2007).
Due to the nature of charter angling and field research, time series analysis of wild angler-caught tarpon in Puerto Rico was not feasible. Physiological stress variables were not
related to fish size or time of fishing experience. This may be due to the lack of significant variability in size range or fight and handling time (O’Toole et al. 2010), as most angled tarpon were smaller juveniles and were captured quickly (average about 5 minutes; maximum of 24 minutes). Further, sampling was conducted immediately upon landing, which may not have allowed sufficient time for physiological effects to manifest. Bower et al. (2016) found that Bluefinned Mahseer (Tor putitora) physiological factors had pronounced effects the longer the fight time continued.
Conclusion
Following an acute induced stressor (angling and air exposure), the stress response in tarpon was characterized by temporary changes in the secondary stress indicators of osmolality, glucose, lactate and pH. These physiological variables returned back to baseline by the end of the 24-hour observation period. Osmolality was the only physiological parameter to respond to stressor intensity, but other parameters displayed patterns suggestive of an intensity effect and it is possible that greater sample sizes or longer stress times might yield more conclusive differences. Airbreathing also proved to be a useful tool in visually determining stress of tarpon exposed to fishing experiences. This study provides insight into how wild tarpon react to and recover from the fishing experience. This information can be used to refine catch-and-release protocols to minimize the response and recovery times of osmolality, glucose, lactate and pH.
Table 1.1 Average water quality parameters and standard error for experimental laboratory design.
Trial runs of February (n=24) and March (n=23) of 2021 at South Farm Aquaculture Facility
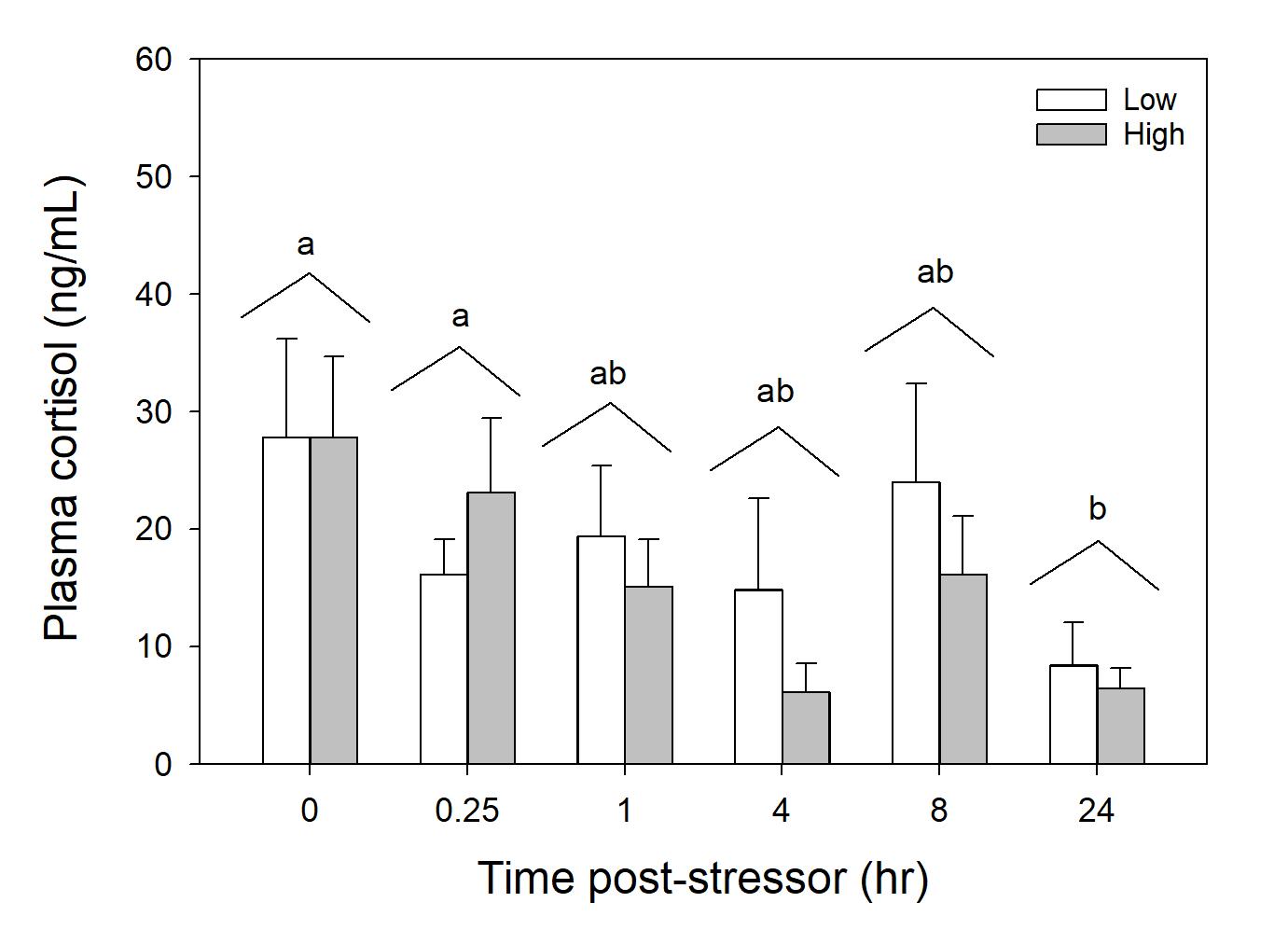
Figure 1.1 Mean ± standard error plasma cortisol concentration (ng/mL) of simulated angled fish in laboratory settings (n=67).
Combined trial runs (February and March) over a 24-hour response period. Three outliers were removed from the low treatments of Times 0.25, 4, and 8. Furthermore, blood was unable to be obtained from a high intensity treatment in Time 4 and low intensity treatment of Time 4. Standard error bars are accounted for in each individual time slot per treatment group. Different letters indicate statistical significance.
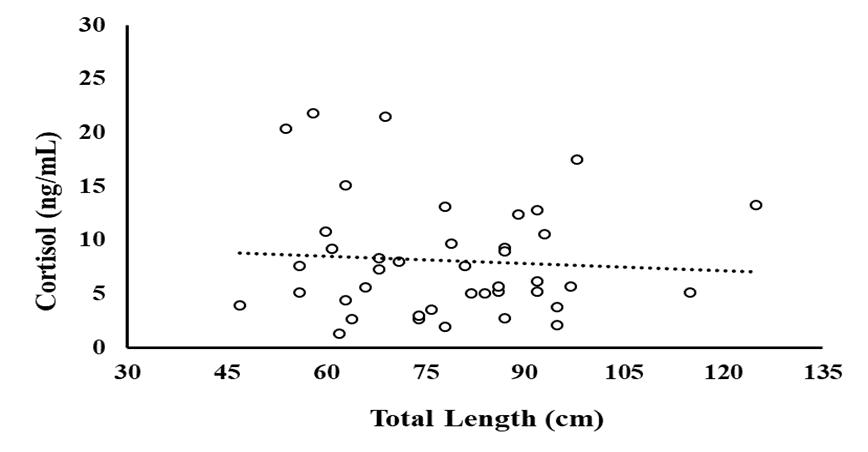
Figure 1.2 Cortisol concentration (ng/mL) of wild-caught fish (n=42) as a function of total length (cm).
One outlier was censured. Dots represent individual fish with a regression of Cortisol Concentration = -0.07254 (Total Body Length) + 13.787. The adjusted R square value = 0.030 and the p-value = 0.139

Figure 1.3 Cortisol concentration (ng/mL) of wild-caught fish (n=42) as a function of fishing time as the total fishing experience (being caught and handled).
One outlier was censured. Dots represent individual fish with a regression of Cortisol Concentration = -0.279(Fishing minutes) + 10.145. The adjusted R square value is 0.014 and the p-value = 0.213

Figure 1.4 Glucose concentration (mg/dL) of simulated angled fish in laboratory settings (n=67) in combined trial runs over a 24 hour time period.
Blood was unable to be obtained from a high treatment in Time 4 and low treatment of Time 4. Standard error bars are accounted for in each individual time slot per treatment group. Different letters indicate statistical significance.

Figure 1.5 Glucose concentration (mg/dL) of wild-caught fish (n=43) as a function of total length of the fish in cm.
Dots represent individual fish with a regression of Glucose = -0.101(Total Length)+ 88.855. The adjusted R square value = -0.0149 and the p-value = 0.540.
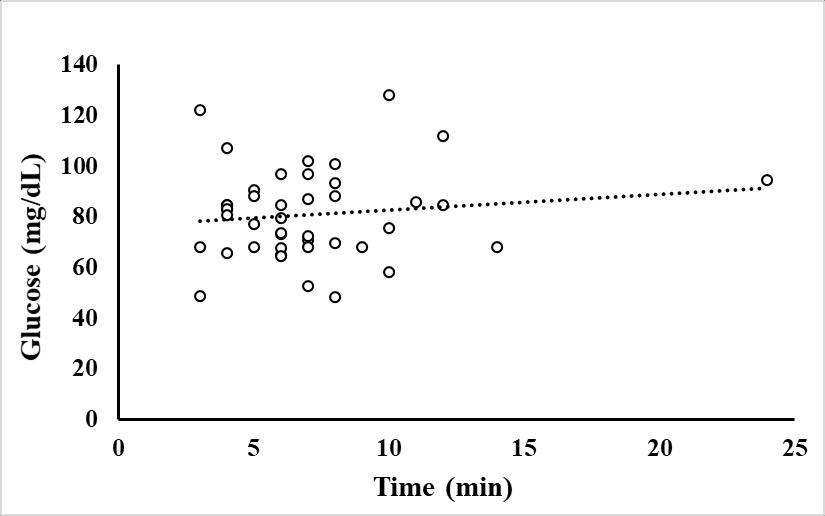
Figure 1.6 Glucose concentration (mg/dL) of wild-caught fish (n=43) as a function of fishing time as the total fishing experience (being caught and handled).
Dots represent individual fish with a regression of Glucose = 0.612(Fishing minutes) + 76.639. The adjusted R square value = -0.008 and the p-value = 0.415.

Figure 1.7 Lactate concentration (mmoles) of simulated angled fish in laboratory settings (n=67) in combined trial runs over a 24-hour time period.
Blood was unable to be obtained from a high treatment in Time 4 and low treatment of Time 4. Standard error bars are accounted for in each individual time slot per treatment group. Letters indicate statistical significance.

Figure 1.8 Lactate concentration (mmoles) of wild-caught fish (n=43) as a function of total length of the fish in cm.
Dots represent individual fish with a regression of Lactate = -3.858(Total Length) - 5846.820. The adjusted R square value = -0.0232 and the p-value = 0.827.

Figure 1.9 Lactate concentration (mmoles) of wild-caught fish (n=43) as a function of fishing time as the total fishing experience (being caught and handled).
Dots represent individual fish with a regression of Lactate = 40.790(Fishing minutes) + 5256.870. The adjusted R square value = -0.018 and the p-value = 0.611
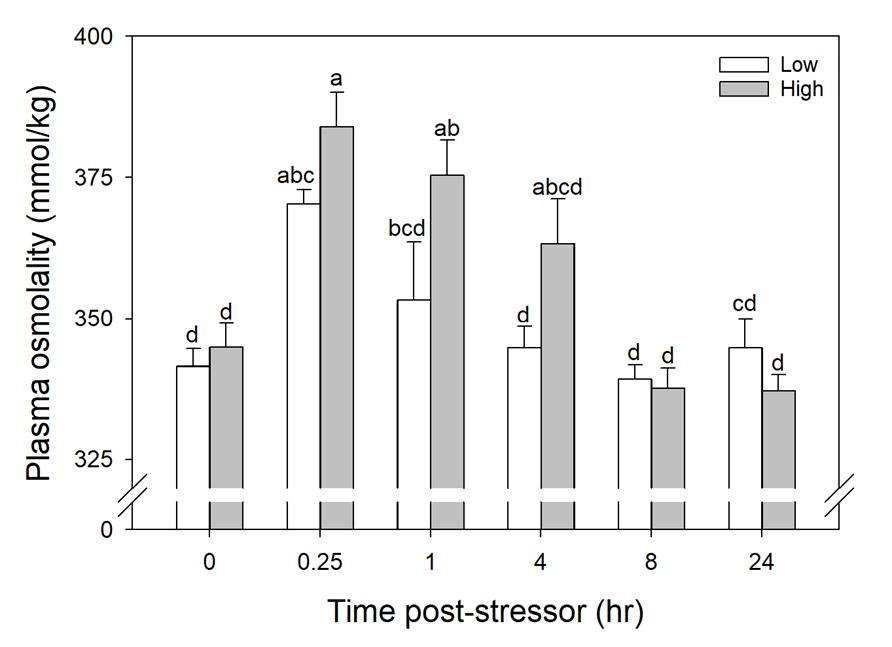
Figure 1.10 Osmolality (Osmol/kg) of simulated angled fish in laboratory settings (n=67) in combined trial runs over a 24-hour time period.
Blood was unable to be obtained from a high treatment in Time 4 and low treatment of Time 4. Standard error bars are accounted for in each individual time slot per treatment group. Letters indicate statistical significance.
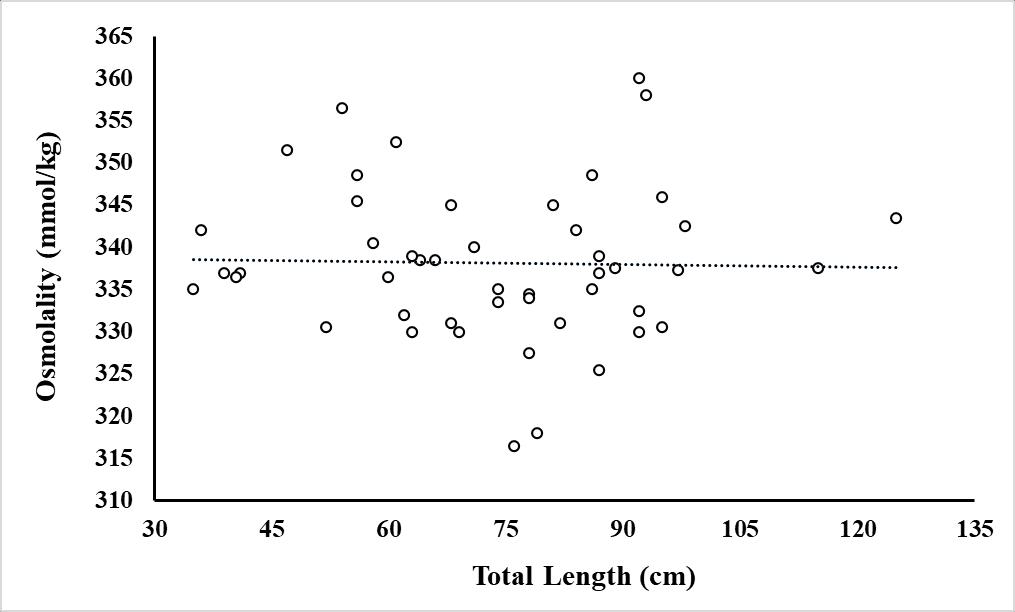
Figure 1.11 Osmolality (mmol/kg) of wild-caught fish (n=43) as a function of total length of the fish in cm.
Dots represent individual fish with a regression of Osmolality = -0.0276(Total Length) + 340.360. The adjusted R square value = -0.022 and the p-value = 0.756.
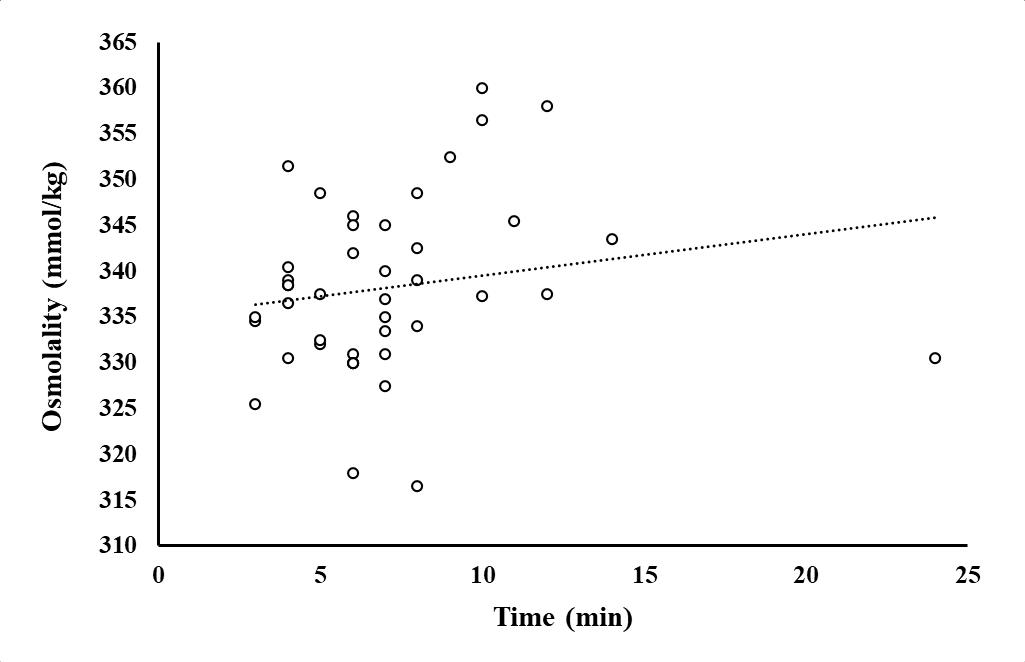
Figure 1.12 Osmolality (Osmol/kg) of wild-caught fish (n=43) as a function of fishing time as the total fishing experience (being caught and handled).
Dots represent individual fish with a regression of Osmolality = 0.453(Fishing minutes) + 334.992. The adjusted R square value = -0.0220 and the p-value = 0.756.

Figure 1.13 pH of simulated angled fish in laboratory settings (n=67) in combined trial runs over a 24-hour time period.
Blood was unable to be obtained from a high treatment in Time 4 and low treatment of Time 4. Standard error bars are accounted for in each individual time slot per treatment group. Letters indicate statistical significance.
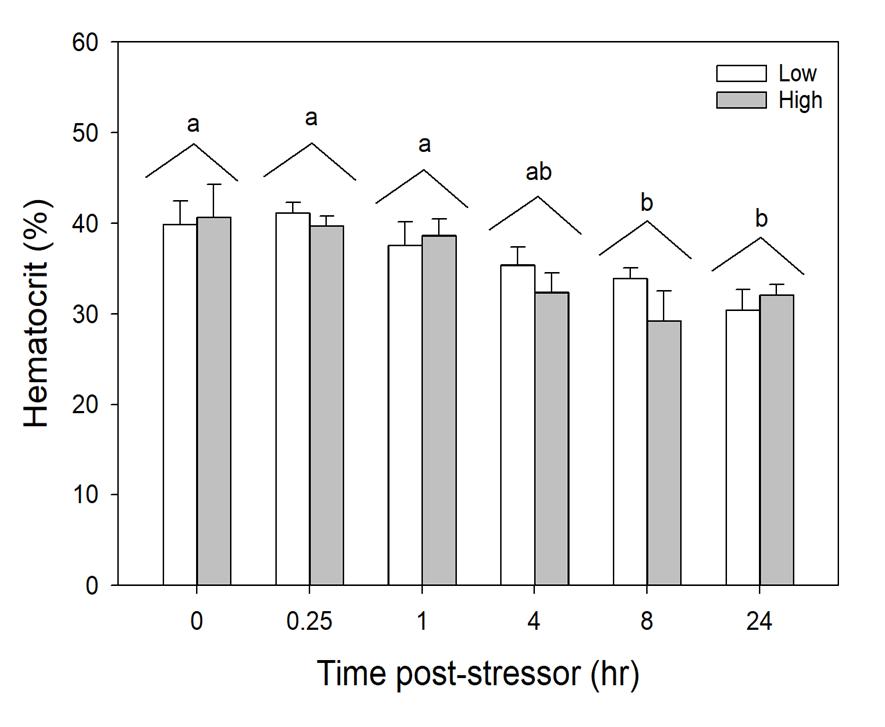
Figure 1.14 Hematocrit (%) of simulated angled fish in laboratory settings (n=67) in combined trial runs over a 24-hour time period.
Blood was unable to be obtained from a high treatment in Time 4 and low Treatment of time 4. Standard error bars are accounted for in each individual time slot per treatment group. Letters indicate statistical significance
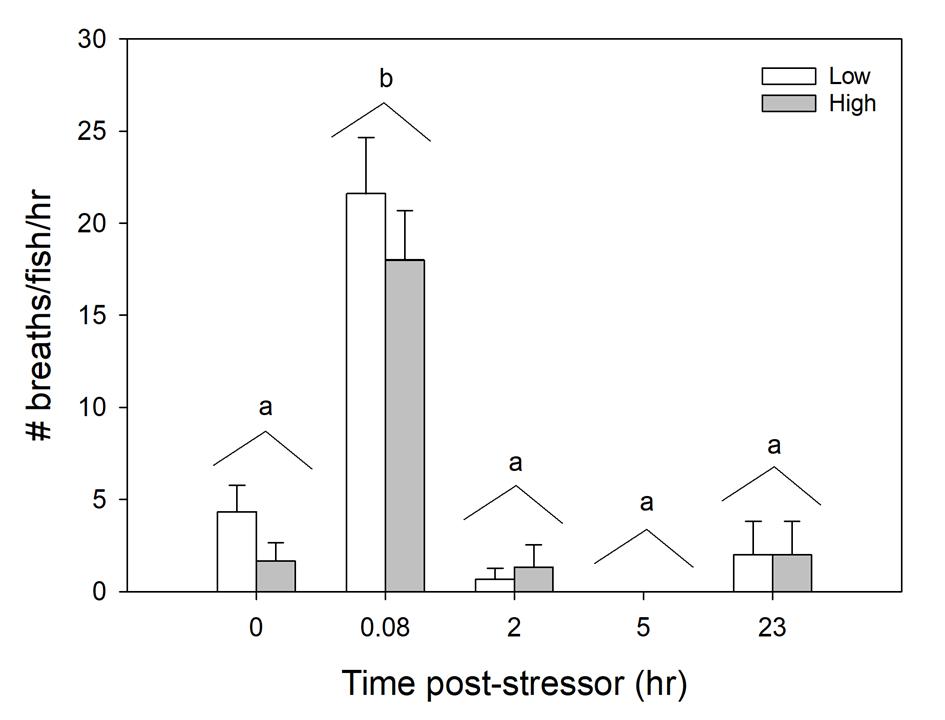
Figure 1.15 Air breathing counts over a 5-minute time period for various time trials before and after inducing stress of laboratory fish (n=60).
Standard error bars are accounted for in each individual time slot per treatment group. Time 5 fish exhibited no air breathes. Letters indicate statistical significance
References
Acerete, L., Balasch, J.C., Espinosa, E., Josa, A. and Tort, L. 2004. Physiological responses in Eurasian perch (Perca fluviatilis, L.) subjected to stress by transport and handling. Aquaculture. 237(1-4): 167-178.
Affonso, E.G., Polez, V.L.P., Corrêa, C.F., Mazon, A.D.F., Araujo, M.R.R., Moraes, G. and Rantin, F.T. 2002. Blood parameters and metabolites in the teleost fish Colossoma macropomum exposed to sulfide or hypoxia. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 133(3):375-382.
Allen, S.E. and Holm, J.L. 2008. Lactate: physiology and clinical utility. Journal of Veterinary Emergency and Critical Care. 18(2):123-132.
Anyanwu, P. E., Kusemiju, K., Okoro, C. B., Ayo-Olalusi, C. I., Ayorinde, A. O., Fagbenro, O. A. and Olufayo, M. 2009. Potential of Tarpon (Megalops atlanticus) for sport fisheries and ecotourism development in Nigeria. Akure, Nigeria: FISON.
Aoi, W. and Marunaka, Y. 2014. Importance of pH homeostasis in metabolic health and diseases: crucial role of membrane proton transport. BioMed research international. 2014.
Ault, J.S. and Luo, J. 2013. A reliable game fish weight estimation model for Atlantic tarpon (Megalops atlanticus). Fisheries research. 139:110-117.
Barcellos, L.J.G., Kreutz, L.C., Koakoski, G., Oliveira, T.A., da Rosa, J.G.S. and Fagundes, M. 2012. Fish age, instead of weight and size, as a determining factor for time course differences in cortisol response to stress. Physiology & behavior. 107(3):397-400.
Bartholomew, A. and Bohnsack, J.A. 2005. A review of catch-and-release angling mortality with implications for no-take reserves. Reviews in Fish Biology and Fisheries. 15(1): 129-154.
Barton, B.A. 2000. Salmonid fishes differ in their cortisol and glucose responses to handling and transport stress. North American Journal of Aquaculture. 62(1): 12-18.
Barton, B.A., Rahn, A.B., Feist, G., Bollig, H. and Schreck, C.B. 1998. Physiological stress responses of the freshwater chondrostean paddlefish (Polyodon spathula) to acute physical disturbances. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology.120(2): 355-363.
Barrett I. and Connor A.R. 1962. Blood lactate in yellowfin tuna, Neothunnus macropterus, and skipjack, Katsuwonus pelamis, following capture and tagging. Inter-American Tropical Tuna Commission Bulletin 6:231–280
Blandon, I.R., De León, F.J.G., Ward, R., Van Den Bussche, R.A. and Needleman, D.S. 2003. Studies in conservation genetics of tarpon (Megalops atlanticus) V. Isolation and characterization of microsatellite loci. Molecular Ecology Notes. 3(4): 632-634.
Bower, S.D., Danylchuk, A.J., Raghavan, R., Clark‐Danylchuk, S.E., Pinder, A.C. and Cooke, S.J. 2016. Rapid assessment of the physiological impacts caused by catch‐and‐release angling on blue‐finned mahseer (Tor sp.) of the Cauvery River, India. Fisheries Management and Ecology. 23(3-4): 208-217.
Brownscombe, J.W., Danylchuk, A.J., Chapman, J.M., Gutowsky, L.F. and Cooke, S.J., 2017. Best practices for catch-and-release recreational fisheries–angling tools and tactics. Fisheries Research. 186: 693-705.
Brownscombe, J.W., Griffin, L.P., Gagne, T., Haak, C.R., Cooke, S.J. and Danylchuk, A.J.2015. Physiological stress and reflex impairment of recreationally angled bonefish in Puerto Rico. Environmental Biology of Fishes. 98(11):2287-2295.
Campbell, M.D., Patino, R., Tolan, J., Strauss, R. and Diamond, S.L. 2010. Sublethal effects of catch-and-release fishing: measuring capture stress, fish impairment, and predation risk using a condition index. ICES Journal of Marine Science. 67(3): 513-521.
Clark, T.D., Donaldson, M.R., Drenner, S.M., Hinch, S.G., Patterson, D.A., Hills, J., Ives, V., Carter, J.J., Cooke, S.J. and Farrell, A.P. 2011. The efficacy of field techniques for obtaining and storing blood samples from fishes. Journal of fish biology. 79(5):13221333.
Clark, T.D., Seymour, R.S., Christian, K., Wells, R.M.G., Baldwin, J. and Farrell, A.P. 2007. Changes in cardiac output during swimming and aquatic hypoxia in the air-breathing Pacific tarpon. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 148(3):562-571.
Crabtree, R.E., Cyr, E.C. and Dean, J.M.1995. Age and growth of tarpon, Megalops atlanticus, from South Florida waters. Fishery Bulletin, (4).
Cooke, S. J., Schreer, J.F, Wahl, D.H., and Philipp, D.P. 2002 Physiological impacts of catchand-release angling practices on largemouth bass and smallmouth bass. Pages 489-512 in D. P. Philipp and M. S. Ridgeway, editors. Black Bass: Ecology, Conservation, and Management. American Fisheries Society Symposium 31. Bethesda, Maryland.
Davis, K.B. 2006. Management of physiological stress in finfish aquaculture. North American Journal of Aquaculture. 68(2): 116-121.
Davis, M.W. 2007. Simulated fishing experiments for predicting delayed mortality rates using reflex impairment in restrained fish. ICES Journal of Marine Science. 64: 1535–1542.
Davis, K.B. and Parker, N.C. 1986. Plasma corticosteroid stress response of fourteen species of warmwater fish to transportation. Transactions of the American Fisheries Society. 115(3): 495-499.
Dinken, C.P., Keretz, K.R., Schramm Jr, H.L., Petrie‐Hanson, L., Wes Schilling, M. and Allen, P.J. 2020. Changes in Physiology and Stress Responses of Pellet‐Reared Largemouth Bass Fed Live‐Forage Diets. North American Journal of Aquaculture. 82(1): 3-23.
Driedzic, W.R. and Hochachka, P.W. 1976. Control of energy metabolism in fish white muscle. American Journal of Physiology-Legacy Content. 230(3): 579-582.
Ellis, T., Yildiz, H.Y., López-Olmeda, J., Spedicato, M.T., Tort, L., Øverli, Ø. and Martins, C.I. 2012. Cortisol and finfish welfare. Fish physiology and biochemistry. 38(1):163-188.
Elmo, G.M. 2020. Early life history of tarpon (Megalops atlanticus) in South Carolina estuaries: Assessment of juvenile recruitment and validity of aging and back-calculation methods (Doctoral dissertation, Coastal Carolina University).
Fanouraki, E., Mylonas, C.C., Papandroulakis, N. and Pavlidis, M. 2011. Species specificity in the magnitude and duration of the acute stress response in Mediterranean marine fish in culture. General and comparative endocrinology. 173(2): 313-322.
Felder T. 2011. The economic impact of recreational tarpon fishing in the Caloosahatchee River and Charlotte Harbor region of Florida. The Everglades Foundation. Available from: https://www.bonefishtarpontrust.org/downloads/researchreports/stories/Caloosahatchee%20Final%20Report%20wExSum.pdf
Ferguson, R.A. and Tufts, B.L. 1992. Physiological effects of brief air exposure in exhaustively exercised Rainbow trout (Oncorhynchus mykiss): implications for "catch and release" fisheries. Canadian journal of fisheries and aquatic sciences. 49(6):1157-1162.
Fritz, E.S. 1980. Potential impacts of low pH on fish and fish populations. Fish and Wildlife Service, US Department of the Interior. No. 2
Gallaugher, P., Thorarensen, H. and Farrell, A.P. 1995. Hematocrit in oxygen transport and swimming in rainbow trout (Oncorhynchus mykiss). Respiration physiology 102(2-3): 279-292.
Geiger, S.P., Torres, J.J. and Crabtree, R.E. 2000. Air breathing and gill ventilation frequencies in juvenile tarpon, Megalops atlanticus: responses to changes in dissolved oxygen, temperature, hydrogen sulfide, and pH. Environmental Biology of Fishes. 59(2):181-190.
Gonzalez, R.J., Milligan, L., Pagnotta, A. and McDonald, D.G. 2001. Effect of air breathing on acid-base and ion regulation after exhaustive exercise and during low pH exposure in the bowfin, Amia calva. Physiological and Biochemical Zoology 74(4): 502-509.
Gould, A. and Grace, B., 2009. Injuries to barramundi Lates calcarifer resulting from lipgripping devices in the laboratory. N. Am J. Fish. Manag. 29, 1418–1424.
Griffin, L.P., Brownscombe, J.W., Adams, A.J., Boucek, R.E., Finn, J.T., Heithaus, M.R., Rehage, J.S., Cooke, S.J. and Danylchuk, A.J.. 2018. Keeping up with the Silver King: using cooperative acoustic telemetry networks to quantify the movements of Atlantic tarpon (Megalops atlanticus) in the coastal waters of the southeastern United States. Fisheries Research 205: 65-76.
Guerrero Pérez, C. R., García, M.A., Lilyestrom, C., Rodríguez, G., and Rodríguez, Y. 2013. Puerto Rico marine recreational statistics program, final report 2009-2013. Federal Aid in Sport Fish Restoration Project F-42. Department of Natural and Environmental Resources, San Juan.
Guindon, K.Y. 2011. Evaluating lethal and sub-lethal effects of catch-and-release angling in Florida's Central Gulf Coast recreational Atlantic Tarpon (Megalops atlanticus) Fishery. University of South Florida.
Graham, P.M., Franks, J.S., Anderson, E.J., Leaf, R.T. and Tilley, J.D. 2021. Age and growth of early life stage Atlantic Tarpon Megalops atlanticus from the northcentral Gulf of Mexico. Journal of Fish Biology.
Haukenes, A.H., Barton, B.A. and Bollig, H. 2008. Cortisol responses of pallid sturgeon and yellow perch following challenge with lipopolysaccharide. Journal of Fish Biology. 72(3): 780-784.
Hemre, G.I., Mommsen, T.P. and Krogdahl, Å. 2002. Carbohydrates in fish nutrition: effects on growth, glucose metabolism and hepatic enzymes. Aquaculture nutrition. 8(3): 175-194.
Hoffmayer, E.R. and Parsons, G.R. 2001. The physiological response to capture and handling stress in the Atlantic sharpnose shark, Rhizoprionodon terraenovae. Fish Physiology and Biochemistry. 25(4): 277-285.
Keretz, K.R., Dinken, C.P., Allen, P.J., Colvin, M.E. and Schramm Jr, H.L. 2018. The effect of water temperature, angling time, and dissolved oxygen on the survival of Largemouth Bass subjected to simulated angling and tournament handling procedures. North American Journal of Fisheries Management. 38(3): 606-622.
Kieffer, J.D., Kubacki, M.R., Phelan, F.J.S., Philipp, D.P. and Tufts, B.L.1995. Effects of catch‐and‐release angling on nesting male smallmouth bass. Transactions of the American Fisheries Society. 124(1): 70-76.
Larsson, Å. and Lewander, K. 1973. Metabolic effects of starvation in the eel Anguilla anguilla L. Comparative Biochemistry and Physiology. Part A. Molecular and Integrative Physiology.
Luo J, Ault J S , Ungar B T , Smith, S.G., Larkin, M.F., Davidson, T.N., Bryan, D.R., Farmer, N.A., Holt, S.A., Alford, A.S., Adams, A.J., Humston, R., Marton, A.S., Mangum, D., Kleppinger, R., Requejo, A., and Robertson, J. 2020. Migrations and movements of Atlantic tarpon revealed by two decades of satellite tagging. Fish Fish. 21:290–318.
Martins, C.L., Walker, T.I. and Reina, R.D. 2018. Stress-related physiological changes and postrelease survival of elephant fish (Callorhinchus milii) after longlining, gillnetting, angling and handling in a controlled setting. Fisheries Research. 204:116-124.
McArley, T.J. and Herbert, N.A. 2014. Mortality, physiological stress and reflex impairment in sub-legal Pagrus auratus exposed to simulated angling. Journal of Experimental Marine Biology and Ecology. 461:61-72.
McLean, M.F., Hanson, K.C., Cooke, S.J., Hinch, S.G., Patterson, D.A., Nettles, T.L., Litvak, M.K. and Crossin, G.T. 2016. Physiological stress response, reflex impairment and delayed mortality of white sturgeon Acipenser transmontanus exposed to simulated fisheries stressors. Conservation physiology. 4(1):cow031.
Meka, J.M. and McCormick, S.D. 2005. Physiological response of wild rainbow trout to angling: impact of angling duration, fish size, body condition, and temperature. Fisheries Research. 72(2-3): 311-322.
Oliver-Rodríguez J.C. and Wang X.T. 2015. Non-parametric three-way mixed ANOVA with aligned rank tests. Br J Math Stat Psychol. 68(1):23-42.
O'Toole, A.C., Danylchuk, A.J., Suski, C.D. and Cooke, S.J. 2010. Consequences of catch-andrelease angling on the physiological status, injury, and immediate mortality of great barracuda (Sphyraena barracuda) in The Bahamas. ICES Journal of Marine Science. 67(8): 1667-1675.
Pollock, K. H. and W. F. Pine. 2007. The design and analysis of field studies to estimate catchand-release mortality. Fisheries Management and Ecology 14:123-130.
Popovic, N., Strunjak‐Perovic, I., Coz‐Rakovac, R., Barisic, J., Jadan, M., Persin Berakovic, A. and Sauerborn Klobucar, R. 2012. Tricaine methane‐sulfonate (MS‐222) application in fish anaesthesia. Journal of Applied Ichthyology. 28(4): 553-564.
Sass, G.G., Gaeta, J.W., Allen, M.S., Suski, C.D. and Shaw, S.L. 2018. Effects of catch-andrelease angling on a largemouth bass (Micropterus salmoides) population in a north temperate lake, 2001–2005. Fisheries Research. 204:95-102.
Seymour, R.S., Wegner, N.C. and Graham, J.B. 2008. Body size and the air-breathing organ of the Atlantic tarpon Megalops atlanticus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 150(3):282-287.
Seyoum, S., Tringali, M.D. and Higham, M. 2008. Development of 15 polymorphic microsatellite markers in the Atlantic tarpon (Megalops atlanticus) for capture–recapture studies. Molecular ecology resources. 8(1): 126-128.
Shultz, A.D., Murchie, K.J., Griffith, C., Cooke, S.J., Danylchuk, A.J., Goldberg, T.L. and Suski, C.D. 2011. Impacts of dissolved oxygen on the behavior and physiology of bonefish: implications for live-release angling tournaments. Journal of Experimental Marine Biology and Ecology. 402(1-2):19-26.
Skomal, G.B. 2007. Evaluating the physiological and physical consequences of capture on post‐release survivorship in large pelagic fishes. Fisheries Management and Ecology. 14(2): 81-89.
Sopinka, N.M., Donaldson, M.R., O’Connor, C.M., Suski, C.D. and Cooke, S.J. 2016. Stress indicators in fish. In Fish physiology 35: 405-462.
Suski, C.D., Cooke, S.J., Danylchuk, A.J., O'Connor, C.M., Gravel, M.A., Redpath, T., Hanson, K.C., Gingerich, A.J., Murchie, K.J., Danylchuk, S.E. and Koppelman, J.B. 2007. Physiological disturbance and recovery dynamics of bonefish (Albula vulpes), a tropical marine fish, in response to variable exercise and exposure to air. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 148(3): 664673.
Suski, C.D., Killen, S.S., Kieffer, J.D., and Tufts, B.L. 2006. The influence of environmental temperature and oxygen concentration on the recovery of largemouth bass from exercise: implications for live–release angling tournaments. Journal of Fish Biology. 68(1): 120136.
Youson, J. H. 2007. Peripheral endocrine glands. II. The adrenal glands and the corpuscles of Stannius. Fish Physiology Series, Primitive Fishes. 26: 457-513.
Vijayan, M.M., Aluru, N. and Leatherland, J.F. 2010. Stress response and the role of cortisol. Fish diseases and disorders. 2:182-201.
Wade, R. A. 1962. The biology of the tarpon, Megalops atlanticus, and the ox-eye, Megalops cyprinoides, with emphasis on larval development. Bulletin of Marine Science of the Gulf and Caribbean. 12(4):545–622.
Wedemeyer, G.A. and Wydoski, R.S. 2008. Physiological response of some economically important freshwater salmonids to catch-and-release fishing. North American Journal of Fisheries Management. 28(5):1587-1596.
Wells, R.M., Baldwin, J., Seymour, R.S., Baudinette, R.V., Christian, K. and Bennett, M.B.2003. Oxygen transport capacity in the air-breathing fish, Megalops cyprinoides: compensations for strenuous exercise. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 134(1):45-53.
Wells, R.M., Baldwin, J., Seymour, R.S., Christian, K.A. and Farrell, A.P. 2007. Air breathing minimizes post‐exercise lactate load in the tropical Pacific tarpon, Megalops cyprinoides Broussonet 1782 but oxygen debt is repaid by aquatic breathing. Journal of Fish Biology. 71(6):1649-1661.
Wendelaar Bonga, S.E. 1997. The stress response in fish. Physiological reviews. 77(3): 591-625.
White, A.J., Schreer, J.F. and Cooke, S.J. 2008. Behavioral and physiological responses of the congeneric largemouth (Micropterus salmoides) and smallmouth bass (M. dolomieu) to various exercise and air exposure durations. Fisheries Research. 89(1): 9-16.
Wood, C.M., Turner, J.D. and Graham, M.S. 1983. Why do fish die after severe exercise? Journal of Fish Biology. 22(2):189-201.
Wood, C.M. 1991. Acid–base and ion balance, metabolism, and their interactions, after exhaustive exercise in fish J. Exp. Biol. 160:285-308.
Woodward, C.C. and Strange, R.J. 1987. Physiological stress responses in wild and hatchery‐reared rainbow trout. Transactions of the American Fisheries Society. 116(4): 574-579.
Zerbi, A., Aliaume, C. and Miller, J.M. 1999. A comparison between two tagging techniques with notes on juvenile tarpon ecology in Puerto Rico. Bulletin of marine science. 64(1): 9-19.
CHAPTER II
CATCH-AND-RELEASE FISHING AND ITS EFFECTS ON TARPON (MEGALOPS
ATLANTICUS) IN PUERTO RICO
Abstract
Atlantic tarpon (Megalops atlanticus) is a popular inshore sport fish in Puerto Rico, and pursuit of this species by local tourists and island visitors contributes to the economy. This species is managed as a no-take fishery, which aims to preserve populations by catching and releasing fish that would otherwise be subjected to harvest and removal from the population. This approach assumes minimal mortality or reduced fitness of released fish, yet the process of angling can produce many sub-lethal side effects or direct mortality. In this study, charter angling for tarpon in the San Juan Lagoon system in Puerto Rico was examined to determine post-release mortality and contributing factors. Angled fish were tagged with external acoustic telemetry tags and relocated periodically to determine fate after release. Post-release mortality was at least 4.5% (confirmed mortality) and at most 20.5% (confirmed and classified mortalities). Confidence around confirmed mortality ranges from 0-10.8% whereas combined confirmed and classified mortality ranges from 8.3-32.6%. Some tag loss was observed, which could have artificially elevated mortality estimates. Logistic regression failed to relate characteristics of the angling process to mortality risk due to low sample size; however, important contributing factors are discussed and compared to the literature. Hook design, gear action, landing procedures, and air exposure were key areas of potential improvement.
Recommendations to minimize fish harm during angling include use of heavier action gear to
reduce fight time, a circle hook requirement for live bait to reduce deep hooking, maintaining fish in the water during landing and photography using a cradle, and limiting air exposure to 2 minutes or less if fish are removed from the water.
Introduction
Catch-and-release regulations, which require fish to be released following capture, are becoming more commonplace in recreational fisheries management (e.g., Isermann and Paukert 2010). The goal of catch-and-release angling is that captured fish survive to be caught again, ensuring the sustainability of the stock (Pollock and Pine 2007). This requires that fish are as ecologically fit after release compared to pre-capture, which is measured by how well an individual can defend and support itself and reproduce successfully (Orr 2009). As a management tool, catch-and-release fishing is particularly beneficial for species that protect nests and need older and larger fish to conserve the broodstock (Cooke et al. 2000). Protecting breeding fish through catch-and-release angling can positively affect multiple population parameters, including age, total length, abundance, stock-recruitment, and stock composition (Aas et al. 2002). Catch-and-release has also been used to protect long-lived species (Wegner et al. 2021), and species that receive intense fishing pressure (e.g., Hutt et al. 2008). Furthermore, catch-and-release angling is necessary for implementation of size and bag limits to restrict harvest (Bartholomew and Bohnsack 2005). Lastly, catch-and-release fishing is an ideology that influences anglers' mentality to avoid killing fish during angling and creates an ethical component within the practice of sport fishing (Aas et al. 2002).
Despite the intent of catch-and-release practices, angling can cause acute stress and physical damage to fish, with potential lethal or sub-lethal consequences (Mazeaud et al. 1977; Skomal 2006). Catch-and-release fishing has been shown to increase broodstock predation and
nest abandonment of species such as Largemouth Bass (Micropterus salmoides) and Smallmouth Bass (M. dolomieu) (Phillip et al. 1997). Such behaviors have direct adverse effects on population health and fitness. Visible wounds created by catch-and-release fishing include hooking wounds, physical deformities from repetitive catching and previous hooking and handling injury, bacterial/fungal infections, and can lead to immediate mortality (Meka 2004).
Physiologically, fish can experience respiratory issues and compromised immune systems leading to behavioral changes (Cooke et al. 2002; Wilson et al. 2014). Other physiological impairments include damage to the reflex response, increased risk of predation, and decreased individual fitness (Campbell et al. 2010; Brownscombe et al. 2017). Increasingly, research raises ethical concerns about animal welfare and the morality of releasing fish that are possibly injured during catch-and-release fishing (Arlinghaus and Schwab 2011). These negative impacts justify questioning whether catch-and-release fishing is an effective tool in fisheries management.
Recreational fishing can promote tourism and increase economic revenue of locations with access to desirable fishing opportunities as long as adequate fisheries management is established to ensure the long-term stability of populations. In the case of ocean jurisdictions, such as Puerto Rico and other Caribbean islands, the revenue generated from the recreational fishing industry can be substantial. Fishing revenue includes purchasing fishing gear and boats, charter/guided trips, and trip-related expenditures like lodging and food (Garcia-Moliner et al. 2002). In Puerto Rico, Atlantic Tarpon (Megalops atlanticus; hereafter, "tarpon") is a popular inshore sport fish, and this popularity derives from their large size, powerful fight when hooked, and accessibility, as they are mainly fished inshore and in calm coastal waters (Guidon 2011).
Pursuit of this species by local tourists and island visitors contributes to the economy (GarciaMoliner et al. 2002).
Increasing fishing pressure by promoting tourism can affect the abundance and structure of fish populations. Population decline has been reported in Puerto Rico due to increasing fishing pressure and harvest (Zerbi et al. 1999). Between 2000 and 2003, up to 10,189 kg of tarpon were harvested annually (Guerrero Pérez et al. 2013). In 2004, the Puerto Rico Department of Natural and Environmental Resources (DNER) imposed a harvest prohibition on tarpon to protect stocks for sport fishing by locals and tourists (Guerrero Pérez et al. 2013). Currently, tarpon angling is only catch-and-release in Puerto Rico by law. However, data on catch, angling effort, and postrelease mortality are limited (Guerrero Pérez et al. 2013).
Research from southern Florida on hooking mortality of tarpon reported that hook location and swimming condition when released affects survival, but angling duration, handling time, fish length and bait/hook type did not (Guindon 2011). Tarpon are a widespread species (Winemiller and Dailey 2002) and results may differ for fish angled in other locations, as different environmental parameters (i.e., water quality, salinity, temperature, and water chemistry; Leichter et al. 2006) and angling techniques (i.e., gear, bait type, and angler ethics; Brownscombe et al. 2017) likely alter tarpon's response to the angling experience.
Acoustic telemetry is often used to determine post-release mortality (Prince et al. 2002; FFWCC 2013). Red Snapper (Lutjanus campechanus) tagged with external acoustic transmitters demonstrated that fishing mortality occurred within a 72-hour time period following release (Eberts and Somers 2017). Acoustic telemetry was also used for Bonefish (Albula vulpes), where fish were tracked for a minimum of 48 hours and up to 24 days (Danylchuk et al. 2007). Results suggested that fishing-induced mortality often occurs within minutes of fish being released (Danylchuk et al. 2007). Furthermore, in Bonefish, 43% of 30 fish succumbed to fishing-induced predation within a two-week timespan (Moxham et al. 2019).
The current study used acoustic telemetry to assess how catch-and-release fishing affects tarpon survival in Puerto Rico lagoons. Specific research objectives were to 1) evaluate the status (dead or alive) of fish with acoustic telemetry, and 2) determine which characteristics of the angling experience influence mortality/survivability of tarpon to help improve fishery management practices. This information can be used to decrease the impact catch-and-release fishing has on the tarpon fishery.
Methods
Study Site
In San Juan, Puerto Rico, there are 4 interconnected lagoons (San José, Los Corozos, La Torrecilla, and Piñones) and associated canals (e.g., Canal Suárez), all of which are commonly used by anglers for recreational fishing (Fig. 2.1). This system was selected because it is a relatively closed system and is frequently identified as one of the world's top tarpon fishing destinations by the angling media (Suescun 2019). The San Juan lagoon system is considered an urban forest due to its proximity to the city and extensive mangrove and subtropical forests covering 21,658 hectares (Brandeis et al. 2014). For this reason, it serves as an important economic and ecological area for San Juan and Puerto Rico in general (Brandeis et al. 2014).
These lagoons empty into the Atlantic Ocean through the narrow passage of Boca de Cangrejos in Piñones (Fig. 1). Tidal cycles influence water quality and salinity, as does rainfall input via feeder streams and canals. Lagoons are surrounded by mangrove (red Rhizophora mangle, black Avicennia germinans, and white Laguncularia racemosa) forests (Pool et al. 1977). Mangroves are an essential coastal ecosystem as they serve as feeding grounds, nurseries, and shelter for fish communities (Vaslet et al. 2010). By providing shelter and habitat, mangroves become a refuge for both predatory and prey fish. Mangroves also enhance fish
biomass by providing effluxes of detritus and nutrients that can increase the productivity of a region and the nutrient supply, which affects the food chain (Mumby 2004). For this reason, since 1970, all mangroves in Puerto Rico have been protected by the Puerto Rico Department of Natural and Environmental Resources (Martinuzzi et al 2009). Despite their vital importance to coastal fisheries and the protections afforded to the mangroves, human activity has greatly influenced the lagoons. Due to dredging, landfills, and other recreational activities, the hydrological characteristics of the estuary have considerably changed (Villanueva et al. 2000).
Hydrological characteristics that have been altered by urbanization in the San Juan Lagoon system include disconnection from San Juan Bay, sediment denitrification and fluxes of ammonium, nitrate, and phosphorus nutrients (Pérez-Villalona et al. 2015).
Angling Characteristics
This research was conducted in partnership with Caribbean Fishing Adventures, which is a private, locally owned fishing charter, located in San Juan, PR, specializing in San Juan lagoon tarpon fishing. Caribbean Fishing Adventures hosted the researchers on fishing charters with clients to observe the fishing experience. Trips ranged from 4 to 6 hours and were available 3 times per day (morning, afternoon, and night). Fishing was conducted for this research from May to July of 2021. Most fishing locations were selected for a depth of 1.8-2.4 m using the boat's depth finder.
Information about the fishing experience was recorded from hookset until landing (Fig. 2). To bring the fish into the boat, the Caribbean Fishing Adventures guide would grab the fish by the lower jaw (hand lipped). Fish were pulled into the boat over the gunwale or bow and on to the deck. For photography, fish were generally supported using one hand gripping the jaw and the other supporting the abdomen. Once clients were done handling and taking pictures of their
captured fish, the fish was handed over to the researchers. Researchers measured total length (mm), fork length (m), and recorded any visible signs of injury. Time of air exposure was recorded. In total, 94 Atlantic Tarpon were captured, and fishing experiences recorded. Of those, 49 were externally tagged with acoustic transmitters.
Transmitter Implantation
Acoustic tag assemblies consisted of a Sonotronics (CT-82-2-E) transmitter attached to a titanium anchor (45L mm x 14W mm x 1.3 thickness mm, 47 mm - Wildlife Computers) with a stainless-steel leader (42.18 kg test 0.005 cm diameter) and crimp sleeves (double barrel size 7, 0.135 cm diameter) (Fig. 3). The length of the leader was adjusted to fish size so that the transmitter did not reach to or interfere with the dorsal fin. Tag assemblies were attached to the fish’s left side, 2 scale rows below the insert of the dorsal fin. Using forceps, 2-4 scales were removed from the location of tag insertion to facilitate implantation of the anchor. The tag anchor was inserted with a tag pole ([comprised of AZ-TAGPOLE-004 - Swobbit Tagging Pole
16.51 cm length, AZ-DARTBUSH-001 - Dart Bushing fits 1.91 cm pole and AZ-DARTAPP-
011 - Dart Applicator 1/4-28] – Wildlife Computers) within the scale removal zone through the musculature and between the pterygiophores (Wildlife Computers 2021). Fish were immediately released back to the site of capture.
Telemetry
Relocation efforts were conducted once per week by using a Sonotronics Manual Tracking Kit (MANTRAK; contains USR-14 receiver, DH-4 directional hydrophone, TH-2 towed omnidirectional hydrophone, and accessories). Prior to the study, researchers established 28 listening stations, each selected to optimize lagoon coverage by utilizing a maximum line of sight distance of no more than 1 km between listening zones due to gear effectiveness (Fig. 4).
During each telemetry period, the boat was maneuvered to each listening station and the omnidirectional hydrophone was lowered into the water and all frequencies were scanned. When a tagged fish was located, approximate location was determined using the directional hydrophone. Because Atlantic Tarpon are highly mobile (e.g., Duffing Romero et al. 2021), the lagoon system is large and complex, and time to conduct telemetry was limited, exact locations using triangulation were not determined. Instead, the protracted telemetry period was used to confirm whether a fish was non-mobile and presumed dead, or mobile and alive. In this study, post-release fate was assessed by relocating fish weekly for up to 9 weeks. The post-release evaluation period was set at 5 days, so to classify a fish as alive, movement must be detected after the 5-day observation period.
Classification rules were developed to interpret fish status based on movement. Examples of the movements described by these rules and the resulting fish status classification can be found in Fig. 5. Rules were based on previous studies that inferred fish status by classifying and grouping patterns of telemetry data from receivers (Heupel and Simpfendorfer 2002; VillegasRíos et al. 2020; Weinz et al. 2020). For a fish to be considered alive, it must meet all 3 of the following criteria:
1. fish was relocated at 2 or more different listening stations, and
2. observed movement occurred at least 5 days after release (indicating it was alive through 5 days), and
3. listening stations cannot have overlapping fields of detection (i.e., > 1000 m).
For a fish to be censored for analyses (i.e., unknown status), it must meet any of the following criteria:
1. the fish was found just once (indicating it could have left the system, experienced tag failure, etc.), or
2. fish had only 2 detections, with the first detection occurring within the 5-day observation period, or
3. fish had only 2 detections in the same location or overlapping fields of detection immediately following the 5-day evaluation period on consecutive tracking days and then disappeared.
For a fish to be considered dead, it must meet any of the following criteria:
1. during all detections after day 5 with a minimum of 3 detections, fish was detected in the same area (within the 1 km receiver range), or
2. researchers recovered the dead fish, or
3. researchers received reports of a fish being dead within the 5 day evaluation period, and it was relocated in the reported location for 2 or more tracking periods.
Two passive Submersible Ultrasonic Receivers (SUR, Sonotronics) were placed at the mouth of the lagoon system to determine if fish exited to the Atlantic Ocean. Before deployment, passive receiver bodies (excluding the transducer) were wrapped in black plastic and electrical tape to reduce biofouling. Mounts were constructed of PVC poles (5.08 cm diameter x 1.22 m height) inserted in concrete bases; PVC was spray painted black for concealment. Receivers were attached to PVC mounts with 550 paracord and nylon cable ties (Fig. 6). Once deployed, SUR mounts were tethered to dock pylons to prevent lateral movement.
Data Analysis
SurSoftCDPA software (Sonotronics) was used to download and analyze passive receiver data. Detections from these receivers were combined with active telemetry data for the
assessment of fish status. Combined fish location data were plotted in ArcMap (10.8.1) to analyze fish movement for determination of fish status. Satellite base-maps were retrieved from the ArcGIS website (ESRI - https://www.esri.com/en-us/arcgis/products/arcgisplatform/services/basemaps). Confidence intervals for mortality were based on methods used by Wilde (2002) to determine confidence of status around fish using acoustic tracking.
Summary statistics and linear regression analyses of the fishing experience included all fish caught during the study (n=94). Linear regression assessed whether fish size influenced the duration of the fight, handling, and total fishing experience. Logistic regression modeling was used with the telemetered fish (n=49) to determine if post-release mortality was influenced by 1) the angling experience (i.e., fight, handling, and total fishing experience time) and their interaction with fish size (i.e., total length), and 2) the landing experience (i.e., hooking location or the type of photography an angler used) and the interaction with fish size (i.e., total length). In all cases, quantitative data were log-transformed to correct for normality. Analysis to see if fishing experience influenced mortality was completed by using logistic regressions. Model fit was visually assessed on fitted versus residual plots and implementation of the deviance test. Models were implemented in R (64 Bit 3.6.2 packages RCMDR and emmeans). All tests used a significance level of alpha = 0.05.
Results
Fishing Experience
Fishing experience was evaluated for n=93 angled tarpon that ranged from 47 to 200 (mean = 88) cm in total length (Table 1). Fight time ranged from 1 to 41 minutes, but was skewed towards shorter times with a mean (± SD) of 5 (± 7) minutes. Observed jumps ranged from 0 to 14 (mean ± SD = 3 ± 2). Time of air exposure ranged from 1 to 11 minutes (mean ±
SD = 5 ± 3 minutes), and included time required for angler photography and researcher data collection. Researcher involvement lasted a maximum of 5 minutes; thus, the air exposure time may have been slightly longer than in a typical fishing experience. The total fishing experience ranged between 3 to 46 minutes and was the sum of fight time and air exposure.
Caribbean Fishing Adventures primarily used light action rods, live bait, and circle hooks for chartered fishing trips (Fig. 7). With circle hooks, most fish were caught in either the corner of the mouth or upper jaw (Fig. 8). Most fish (90%) were released after the hooks were removed; however, when hooking occurred in the throat or deeper, lines were cut leaving the hook in place to reduce injury. Little to no bleeding was observed among angled fish (Fig. 9). Following hook removal, clients could take photographs with the fish and choose the angle they wanted to hold the fish (Fig. 10). Horizontal photography was the most popular and encouraged by the fishing company to better grasp the fish. This practice had the client support the fish’s body weight.
Larger fish were often photographed lying on the deck.
Fish size positively influenced the time of the total fishing experience (adjusted R2 = 0.615, p < 0.0001; Fig. 11). This effect was due to both to the effect of fish size on fight time (adjusted R2 = 0.637, p < 0.0001; Fig. 12), and its effect on handling time (adjusted R2 = 0.0826, p < 0.0001; Fig. 13).
Post-Release Mortality
Tagged tarpon moved considerably between lagoons, suggesting that the classification rules based on movement are valid (Fig. 14). Three fish were detected at the lagoon entrance, but no fish exited to the Atlantic Ocean. Of the 49 tagged fish, 5 were censored from further analyses due to insufficient data to classify a fish as alive or dead. Of the remaining 44 fish, 35 were classified as alive based on observed movement following the 5-day evaluation period (79.5%).
Conversely, 9 fish were classified as dead based on lack of movement (20.5%). However, a fish classified as dead could also represent a shed tag. Caribbean Fishing Adventures reported that at least 2 tarpon have been recaptured with scarring consistent with tags being shed, and 1 fish that was recaptured with the tether broken and tag missing. If shedding occurred soon after tagging and the fish survived, these fish would have been misclassified as dead. One fish died soon after angling and the transmitter was retrieved, and another fish was reported dead and relocated consistently in the area reported. This yields a confirmed mortality of 4.5% (CI = 0-10.8%) and a potential mortality of up to 20.5% (8.3-32.6%), although it is likely that true post-release mortality was somewhere in between these values.
When comparing how the angling experience and air exposure of tarpon influenced mortality, logistic regression models did not detect significant effects (p > 0.05), indicating that these factors did not influence tarpon mortality when accounting for total length (Table 2, Fig. 15). In addition, if the hook stayed present, as well as the position the fish was held in for photography, were not influential (p > 0.05) to mortality when considering total length (Table 2, Fig. 16-17).
Discussion
This study was the first to investigate catch-and-release mortality within the Atlantic Tarpon’s tropical range. Mortality within 5 days of angling was at least 4.5% and at most 20.5% based on confirmed and classified mortalities, respectively. The 2 confirmed mortalities (1 was recollected, 1 was reported) occurred within 24-hour of release. This suggests that post-release mortality occurs quickly, and the 5-day evaluation period was adequate to assess the effect of angling. It is likely that some classified mortalities represented tag shedding instead of fish death during the 5-day evaluation period, as the loss rate of external transmitters can be substantial and
has been reported to be as high as 100% in some studies (see review by Jepsen et al 2015).
Caribbean Fishing Adventures reported recapturing 2 tarpon that had scarring consistent with tag loss, and a third with the tether in place but transmitter missing, lending credence to this assertion. This study did not collect data on tag retention, and tag loss that occurred after the initial classification period would not affect results.
The range of post-release mortality from this study was comparable to one estimate from Florida, where Guindon (2011) reported a 13% mortality rate (95% confidence interval: 6-21%) in angled tarpon (N=82). That study described predation on released tarpon by sharks to be a major vector of mortality. In comparison, this study’s angled tarpon (n=44) reported a 4.5-20.5% mortality rate (95% confidence interval: 0-32.6%). The current study did not observe predation on tarpon and predatory shark species are not common in the lagoon complex. Another study from Florida (n=27) reported lower mortality (3.7%) of angled tarpon, and the authors attributed this low rate to angling practices, including style and size of hook, use of heavy gear and aggressive fishing techniques to bring the fish to the boat as quickly as possible, and releasing the tarpon by not taking it out of the water (Edwards 1998). Angling in the current study utilized circle hooks, which reduced deep hooking. However, light action spinning rods were the primary gear, which should prolong fight time and increase the time of physical and physiological stress. Likewise, the Puerto Rico charter guides typically remove tarpon from the water for landing and photography, inducing additional stress and air exposure.
With a relatively low sample size of 44 telemetered tarpon and only 9 possible mortalities, logistic regression models were unable to link specific fishing practices to mortality. However, certain factors have been identified in the literature that reduce mortality during the angling process. Hooking location can affect fishing mortality in tarpon (Guindon 2011) and
other species (e.g., Rainbow Trout Oncorhynchus mykiss, Meka 2004). Potentially lethal hooking locations can include the eye, gills, and esophagus (Ostrand et al. 2005). For example, Derbio (Trachynotus ovatus) hooked internally had an 85% mortality rate (Alós et al. 2008). Similarly, 95% and 75% of Spotted Seatrout (Cynoscion nebulosus) died when hooked in the esophagus and gills, respectively, compared to only 10% when hooked in the bony mouth (James et al. 2007). The use of circle hooks in the current study contributed to the prevalence of non-lethal hooking locations (Fig. 8; Cooke et al. 2003). Most (79) tarpon were hooked in the corner of the mouth or in the upper or lower jaw, with few (14) fish hooked in other locations (i.e., outer head or body, inner oral cavity, or esophagus).
Most (n=85) of the fish had the hook removed prior to release. Only hooks that were too deep to safely remove were left in the fish by cutting the line. When fish have deeply ingested hooks, it is often more beneficial to leave the hook in the individual as attempting to remove the hook often results in injuries and increased mortality (Butcher et al. 2007). In White Seabass (Atractoscion nobilis), for example, leaving deeply embedded hooks increased survival rates, and 39% of hooks that were deeply ingested could pass through the organism's system (Aalbers et al. 2004). When looking at simulated angling in Sand Whiting (Sillago ciliate), fish that ingested a hook that was not removed experienced 23% mortality, but surviving fish were able to feed, and some fish passed ingested hooks with limited long-term physiological impacts documented (McGrath et al. 2009).
Hooking location can determine whether bleeding occurs, especially when the hook penetrates soft or highly vascularized tissues such as the gills and esophagus. Although some bleeding was observed in this study, 71 individuals experienced no bleeding with only 2 individuals experiencing heavy bleeding; 1 of which was confirmed dead based on available
tracking information. Previous studies have noted that bleeding intensity with hooking location causes increased mortality (e.g., Schisler and Bergersen 1996). Conversely, hooking location did correlate to bleeding intensity but not mortality in Artic Grayling (Casselman 2005).
Roth et al. (2018) asserted that longer fight times may cause physiological disturbances that can lead to greater risk of post-release mortality. Increased fight time in Shortfin Mako Shark (Isurus oxyrinchus) did increase physiological stress factors such as lactate and glucose but did not impact survival (French et al. 2015). For Brook Trout (Salvelinus fontinalis), mortality was independent of fight time (Kerr et al. 2017). However, Guindon (2011) reported that the average fight time of tarpon was 23.7 minutes for fish that survived and 16.5 minutes for fish that experienced mortality, which is contrary to Roth et al.’s (2018) hypothesis.
The mean fight time in the current study (5 ± 7 min) was 3-5 times less than the Guindon (2011) study, despite angling primarily using light action gear. Rod action relates to how easily and where along the shaft a rod bends when tension is applied to the tip (Shelton 2006). Many studies previously have noted the action of the gear used but did not relate it to fishing mortality.
For example, researchers mainly used medium-heavy action rods for Permit (Trachinotus falcatus; Holder et al. 2020) and Lemon Sharks (Negaprion bevirostris; Danylchuk et al. 2014); heavy action rods were suggested to be used for Walleye (Sander vitreus) to promote faster hooking and minimize injury to the individual (Jones 2005). Catching the fish faster reduces the intensity of the fight and stress to the fish, but fishing charters may prefer a longer experience for clients. Lowering the action creates more exciting and challenging experiences for customers.
The disparity in fight time between Guindon (2011) and the current study was largely due to fish size. Although larger tarpon are captured in the San Juan lagoon system, the fishery is largely supported by smaller juvenile fish. Tarpon evaluated during the current study averaged
88 cm total length (± 27 cm), whereas fish in the Florida evaluation averaged 160.8 cm for fish that survived and 146.5 cm for fish that experienced mortality (Guindon 2011). The lack of a relationship between fish size and post-release mortality reported by Guindon (2011) was further supported by a meta-analysis of the catch-and-release literature (Bartholomew and Bohnsack 2005). This is a surprising relationship, because larger fish tend to have greater fight times and presumable more angling-induced stress response. Fish size was positively correlated with fight time and handling time in the current study, and other studies support this finding. For example, Lemon Shark fight time was positively correlated to total size (Danylchuk et al. 2014), and both fight and handling/air exposure times increased with size for Black Bream (Spondyliosoma cantharus) angling (Pinder et al. 2017). For Brook Trout, fight time was not correlated to total length (Kerr et al. 2017), suggesting that the relationship of fight time and length is most likely species dependent.
Tarpon can be acrobatic and leap from the water during angling in an attempt to dislodge the hook (Kokomoor 2010). Reports of tarpon jumping as high as 3 m and into watercraft are common (Pinckney 1888). In a confined mangrove environment like the San Juan lagoon system, jumping behavior has potential to injure via impact with mangroves and other obstacles, or with the water surface, but this has not been empirically established (Luo and Ault 2012). In this study, tarpon averaged 3 (± 2) jumps during angling experience. Schlenker et al. (2016) observed that jumping by White Marlin (Kajikia albida) increases fish exhaustion and adds additional stress to the individual (Schlenker et al. 2016). Further, jumping by angled Rainbow Trout correlated with increased risk of injury as jumping often led to deeper hook wounds and entanglement of the fish in the line (Meka 2004). It is likely that jumping behavior at the very least adds to the stress response of the angling experience.
Landing was a relatively stressful process, particularly for larger tarpon, which were dragged by the lower jaw over the edge of the gunwale or bow and into the floor or deck of the vessel. In the evaluation by Edwards (1998), tarpon were supported in the water, thus eliminating air exposure and related injury, and survival rates were high at 96.3%. This raises the question of whether tarpon survive better when left in the water to prevent air-exposure and the effects of gravity outside a liquid medium. Water supports the weight of a fish, and removal from the water can cause injury to internal organs (Grubich 2004). Holding large fish by the mandible can lead to bone breaks and separated tongue (Danylchuk et al. 2008), vertebral separation (Gould and Grace 2009; Frawley 2015), and unexpected fish movement can lead to accidental drops and impact injuries. Air exposure time in this study averaged at 5 (± 3) minutes, compared to 2.2 minutes for fish that survived and 3.2 minutes for fish that experienced mortality in Guindon (2011). Increased air exposure has been correlated to increased stress in fish (Ferguson and Tufts 1992; Brownscombe et al. 2017), and increased amount of time required for the fish to recover from the fishing experience (Cooke et al. 2001; Brownscombe et al. 2017). Whereas evidence suggests that air exposure has adverse effects on the survivability of some species (Arlinghaus and Hallermann 2007), it might be advantageous to minimize air exposure.
Anglers like to share their fishing experience with others, especially through photography. Clients were encouraged to hold fish horizontally supported (n=54) when taking photographs; only a few fish (n=12) were supported vertically or on the deck, particularly for larger fish due to their heavy weight. All fish were gently revived and released back into the water after capture. Horizontally supporting and gently releasing the fish minimizes injury and stress (Frawley 2015). Practicing these efforts are now considered etiquette and part of safe handling practices of catch-and-release fishing. Some researchers recommend leaving the fish in
the water for hook removal and photography, minimizing air exposure and possible injury (Cooke and Sneddon 2007).
Although movement in this study was primarily used as a tool for determining fish fate, the data collected represent the first known attempt to quantify spatial use of tarpon in an enclosed coastal lagoon system. Previous studies have determined migratory movement of rod and reel caught adult tarpon along the East Coast (Griffin et al. 2018) and movement of juvenile tarpon in Brewers Bay, St. Thomas Virgin Islands caught on rod and reel (Duffing Romero et al. 2021). The uniqueness of the San Juan lagoon network is that tarpon, which are normally a migratory species, are contained within a semi-closed system, and can only enter and exit via a shallow opening that is about 35 m wide. It is unclear whether tarpon in the San Juan lagoon network are year-round residents or typically migrate out of the system during some part of the year. In this study, only 3 fish approached the exit to the Atlantic Ocean but did not leave for any significant duration within the 9-week observation period.
If fish are year-round residents, it raises concern about how continuous fishing pressure impacts individuals confined within a semi-closed system. Although fishing effort was not measured, it is substantial, with many charter outfits with client anglers and private recreational anglers using their personal watercraft daily. It was common during this study to be targeting tarpon within a small area with 5 or more other boats nearby. Further, telemetered fish from this study were caught multiple times by different fishing charters, as researchers received multiple reports of tagged fish being caught. While this indicates that some fish recover quickly and return to normal feeding behavior (Cooke et al. 2013), it also suggests that fish in this lagoon fishery are repeatedly exposed to the risk of injury and post-release mortality. In addition, there were 2 reports of fish captured that looked like the tag was pulled out and another that was
disconnected from the anchor line. This indicates that tag shedding occurred, but the timing and effect is currently unknown. Further, this research occurred only during the summer. Although environmental conditions are relatively stable in Puerto Rico, some seasonal variation occurs and could influence seasonal results. Finally, this study assessed specific practices of a single fishing charter operator. The angling and handling techniques of other charter companies, freelancing captains, and independent anglers may differ (Brownscombe et al. 2017).
With optimal fishing and handling practices, catch-and-release fishing can be successful with minimal mortality (Brownscombe et al. 2017). Angler techniques can be altered to minimize catch-and-release fishing from having negative impacts (Bartholomew and Bohnsack 2005). Hook design, gear action, landing procedures, and air exposure were identified as areas where modifications could yield significant improvement in post-release survival. The use of heavier action rods and greater line test could reduce fight time, shortening the period of angling stress (Mohan et al. 2020). This may not be the best option to maximize the fishing experience, as anglers pursue tarpon for the fight. Circle hooks are well-researched to reduced deep hooking, and the Puerto Rico Department of Natural and Environmental Resources should consider requiring circle hooks when fishing with natural bait, like the circle hook regulations for many other high-value species elsewhere (see reviews by Cooke and Suski 2004; Keller et al. 2020).
Maintaining fish in the water during landing and photography would eliminate air exposure and injuries related to lifting fish from the support water provides (Edwards 1998). To control the fish in the water, a cradle could be used. Cradles are common for other long bodied species where catch and release angling is the norm (Casselman 2005). If the fish must be removed from the water, use of a cradle would provide support to the body and reduce potential for injury. Finally, when tarpon are exposed to air, the exposure time should be kept at 2 minutes or less
(Guindon 2011). Although most released tarpon survived the angling experience, fishing pressure in the San Juan lagoon system is intense and these recommendations could help reduce the risk to fish that are subjected to multiple angling events.
Table 2.1 Summary statistics of categorical data recorded during the fishing experience
of Fishing Experience (min)
- 14 Fish Size (cm)
± 27
- 200 (n=94; besides “Time of Air Exposure” and “Number of Observed Jumps” n=93). Fishing variables were recorded based on the experience of catching the fish. Values include average, standard deviation and range to depict data spread.
Table 2.2 Logistic regression models for mortality.
Fish size (total length) was examined in relation to total fishing experience, fight, and air exposure time. The Z-value indicates differences in the observed statistic and the hypothesized population (units of standard deviation). P-value indicates significance of the model in terms of 0.05. AIC (Akaike Information Criterion) indicate how well the model fits the data.
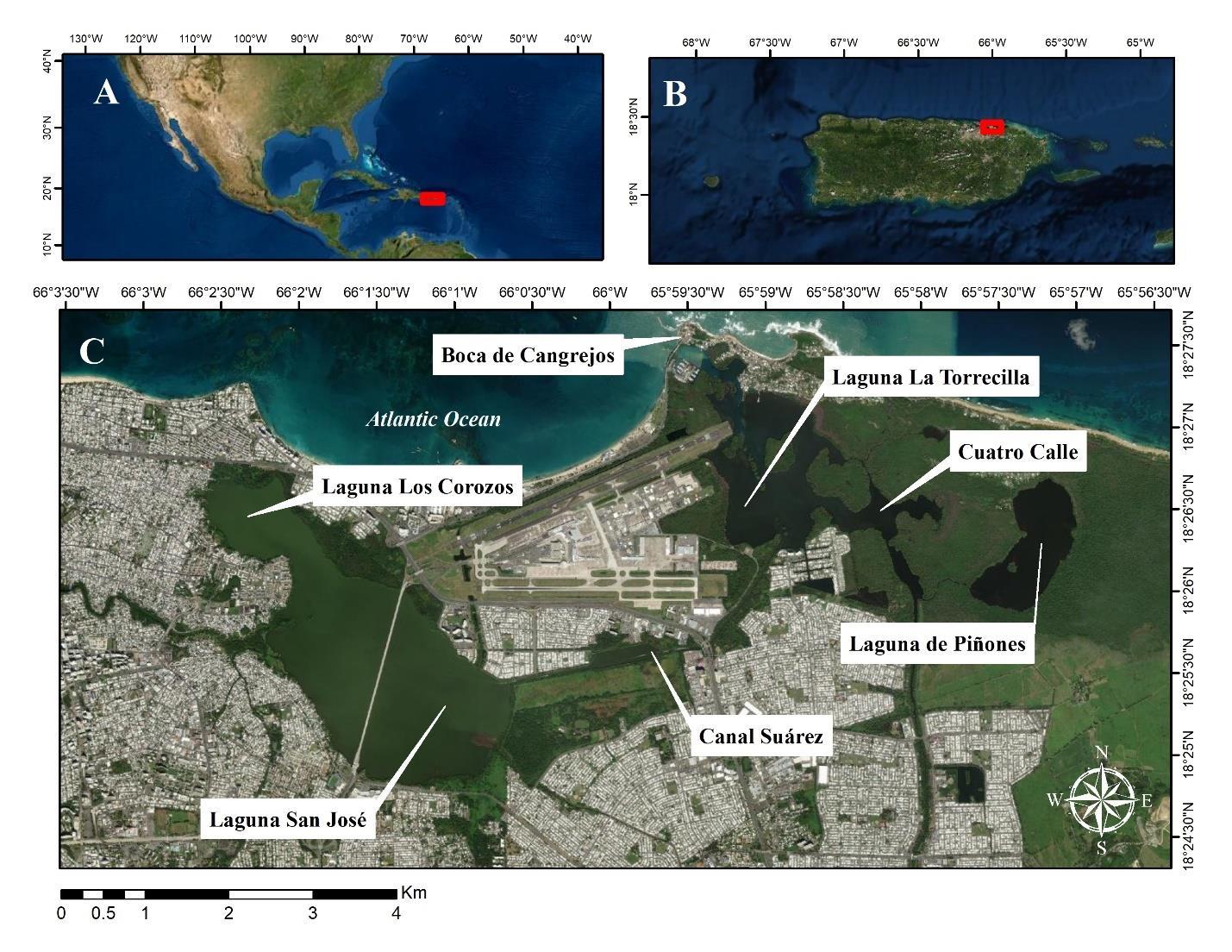
Figure 2.1 Map of the study area.
A) Study area in relation to the Southern region of the United States of America. B) Study area in relation to the territory of Puerto Rico. C) Study area in San Juan with other important natural features
Fish Size: TL _________ SL _________ Blood sample: ☐No ☐Yes – ID #:______________________
Transmitter number: _______
Release time:
Water temperature: Holding tank _________ Release site _________
Dissolved oxygen: Holding tank _________ Release site _________
NOTES:
Short-term Telemetry
Position North West Water Quality
1 h post-release
2 h post-release
3 h post-release
4 h post-release
5 h post-release
6 h post-release
_______ DO _______
_______ DO _______
_______ DO _______
_______ DO _______
_______ DO _______
DO _______
Considered short-term mortality? ☐No ☐Yes – Time of death: ______________
24-h assessment: ☐Alive ☐Dead Tag recovered? ☐No ☐Yes
NOTES:
Figure 2.2 Data collection form for an angling event and short-term telemetry assessment

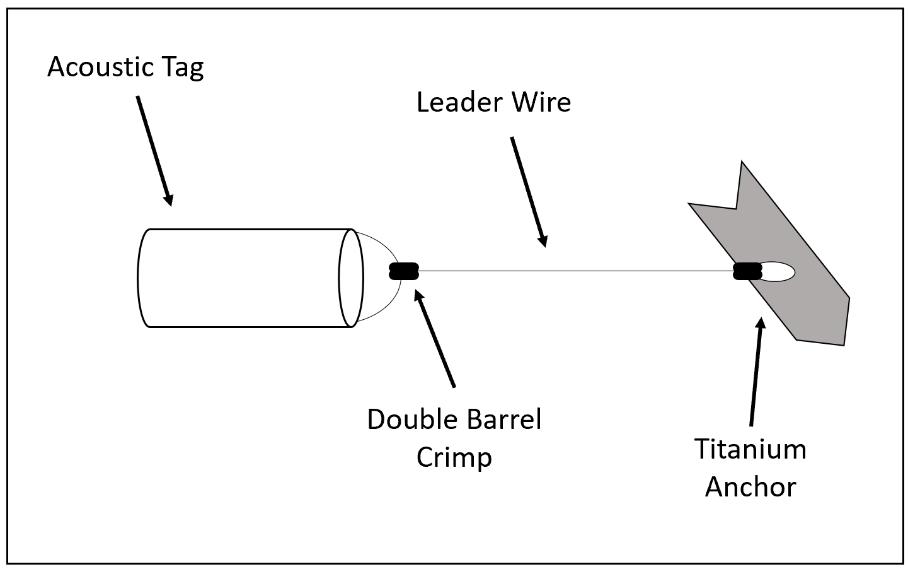
2.3 Telemetry tag picture along with labeled design comprised of the anchor, leader wire, crimps and acoustic tag.

2.4 Manual tracking telemetry sites within the study area and passive receiver locations.
A total of 28 manual tracking sites were selected based on access availability and gear range. In addition, 2 passive receiver locations are displayed along the entrance to the lagoon near the Atlantic Ocean.

Figure 2.5 Examples of fish movement and status classification as alive, dead, or censored based on rules.
Each color represents a different fish (n=3). Dots indicate where the fish was manually tracked and/or released from capture and the lines represent patterns of fish movement in between tracking locations. A) A comparative map of all 3 types of movement represented by panels B-D to gain a spatial perspective of movement. B) A representation of fish considered alive. C) A representation of fish considered dead. D) A representation of fish considered censored.
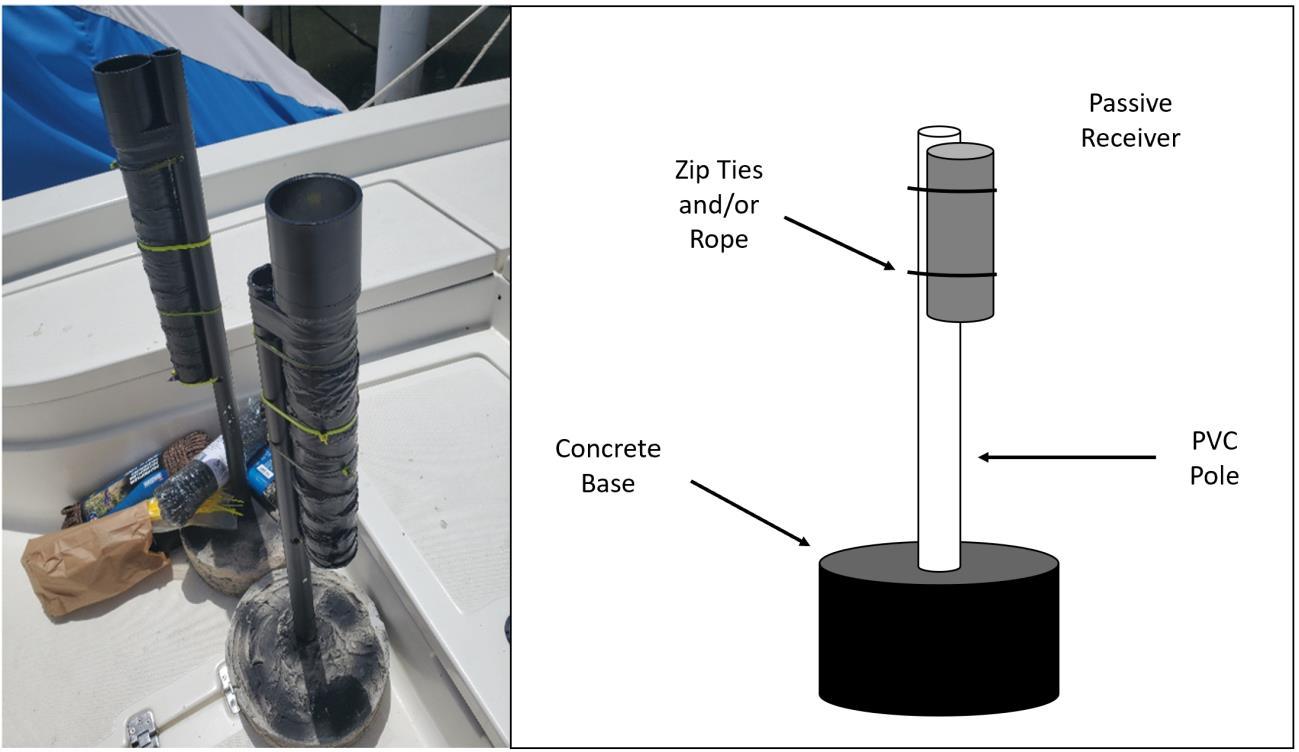
Figure 2.6 Passive receiver deployment picture along with labeled design comprised of PVC pole, receiver, rope and concrete stand.
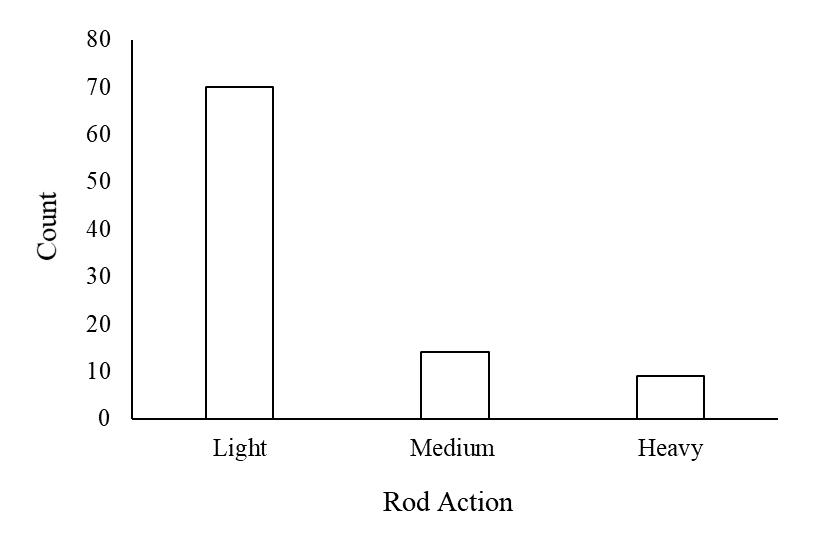
Figure 2.7 Rod action of caught tarpon by Caribbean Fishing Adventures (n=93; 1 missing data point).
Rod action is the amount of bend that a rod will allow for when a fish is caught on a line. Classification for rod bend was determined by the rod manufacturer.

Figure. 2.8 Location of where the hook was set on tarpon caught by Caribbean Fishing Adventures (n=92; 2 missing data points).

Figure 2.9 Bleeding experienced by tarpon due to the fishing exposure of fish caught by Caribbean Fishing Adventures (n=93; 1 missing data point).
Light and heavy bleeding was determined based on researcher judgement where light was little scratches and heavy included pooling of the blood.

Figure 2.10 Fish holding during photographs taken with clients of Caribbean Fishing Adventures (n=94).
Fish position and preference of whether a photograph was taken was determined by clients.

Figure 2.11 Body size (total length in cm) of tarpon caught in Puerto Rico (n=94) as a function of fishing time expressed as the total fishing experience (being caught and handled).
Dots represent individual fish with a trend line of Total Length = 0.230(Total Fishing Minutes)10.918. The adjusted R2 = 0.615, p < 0.0001).

Figure 2.12 Body size (total length in cm) of tarpon caught in Puerto Rico (n=94) as a function of angling time expressed as the total time the fish was on the line.
Dots represent individual fish with a trend line of Total Length = 0.209(Angling Time) -13.678. The adjusted R2 = 0.637, p < 0.0001).
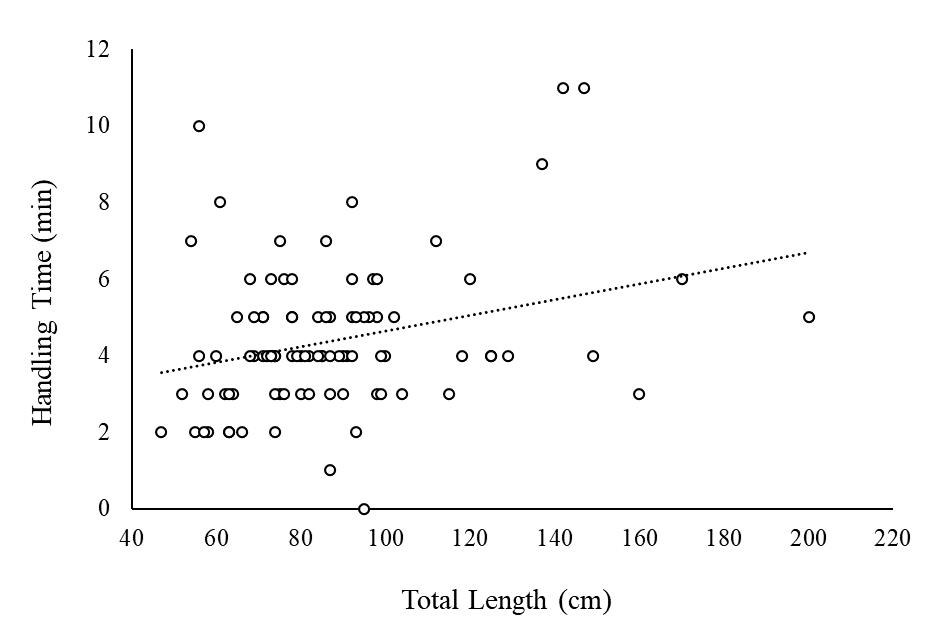
Figure 2.13 Body size (total length in cm) of tarpon caught in Puerto Rico (n=93; 1 missing data point) as a function of air exposure expressed as the total time the fish was out of water.
Dots represent individual fish with a trend line of Total Length = 0.021(Handling Time) -2.613. The adjusted R2 = 0.083, p < 0.0001

Figure 2.14 Map of study site in the San Juan lagoon system with fish distribution from the study period (May-July 2021). Colors indicate different individual tarpon when caught and their individual movement through the lagoon over the extent of the study.
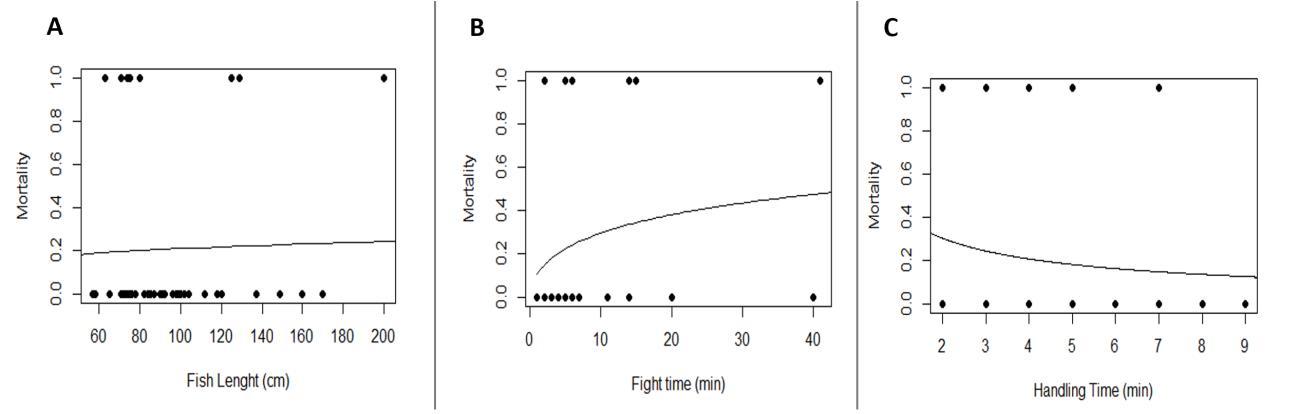
Figure 2.15 Predicted probability of mortality of tarpon in response to (a) body size (total length) (b) fish time (time the fish was on the line) (c) handling time (time the fish was out of the water) length.
There was not a significant effect body size or time on the likelihood of mortality. Lines represent the predicted response based on a logistic regression.

Figure 2.16 Predicted probability of mortality of tarpon contrasting individuals with and without hook removal.
There was not a significant effect of hook removal on the likelihood of mortality. Dots represent estimated marginal means of the probability of mortality; bars represent 95% C.I. Plots were generated with the R package emmeans.

Figure 2.17 Predicted probability of mortality of tarpon contrasting individuals with differing types of positions the fish was held in photography.
There was not a significant effect of how the fish was held on the likelihood of mortality. Dots represent estimated marginal means of the probability of mortality; bars represent 95% C.I. Plots were generated with the R package emmeans.
References
Aalbers, S.A., Stutzer, G.M. andDrawbridge, M.A. 2004. The effects of catch-and-release angling on the growth and survival of juvenile white seabass captured on offset circle and J-type hooks. North American Journal of Fisheries Management. 24: 793-800.
Aas, Ø., Thailing, C.E. and Ditton, R.B. 2002. Controversy over catch-and-release recreational fishing in Europe. Recreational Fisheries: Ecological, Economic and Social Evaluation. 15: 95-106.
Alós, J., Cerdà, M., Deudero, S. and Grau, A.M. 2008. Influence of hook size and type on short‐term mortality, hooking location and size selectivity in a Spanish recreational fishery. Journal of Applied Ichthyology. 24: 658-663.
Arlinghaus, R. and Hallermann, J. 2007. Effects of air exposure on mortality and growth of undersized pikeperch, Sander lucioperca, at low water temperatures with implications for catch‐and‐release fishing. Fisheries Management and Ecology. 14: 155-160.
Arlinghaus, R. and Schwab, A. 2011. Five ethical challenges to recreational fishing: what they are and what they mean. In American fisheries society symposium. 75: 219-234.
Bartholomew, A. and Bohnsack, J.A. 2005. A review of catch-and-release angling mortality with implications for no-take reserves. Reviews in Fish Biology and Fisheries. 15:129-154.
Brandeis, T.J., Escobedo, F.J., Staudhammer, C.L., Nowak, D.J. and Zipperer, W.C., 2014. San Juan Bay Estuary watershed urban forest inventory. Gen. Tech. Rep. SRS-GTR-190. Asheville, NC: USDA-Forest Service, Southern Research Station. 44: 1-44.
Brownscombe, J.W., Danylchuk, A.J., Chapman, J.M., Gutowsky, L.F. and Cooke, S.J. 2017. Best practices for catch-and-release recreational fisheries–angling tools and tactics. Fisheries Research. 186: 693-705.
Butcher, P.A., Broadhurst, M.K., Reynolds, D., Reid, D.D. and Gray, C.A. 2007. Release method and anatomical hook location: effects on short-term mortality of angler-caught Acanthopagrus australis and Argyrosomus japonicus. Diseases of Aquatic Organisms. 74: 17-26.
Casselman, S.J. 2005. Catch-and-release angling: a review with guidelines for proper fish handling practices. Fisheries Section, Fish and Wildlife Branch, Ontario Ministry of Natural Resources.
Cooke, S.J., Donaldson, M.R., O'connor, C.M., Raby, G.D., Arlinghaus, R., Danylchuk, A.J., Hanson, K.C., Hinch, S.G., Clark, T.D., Patterson, D.A. and Suski, C.D. 2013. The physiological consequences of catch‐and‐release angling: perspectives on experimental design, interpretation, extrapolation and relevance to stakeholders. Fisheries Management and Ecology. 20: 268-287.
Cooke, S.J., Philipp, D.P., Dunmall, K.M. and Schreer, J.F. 2001. The influence of terminal tackle on injury, handling time, and cardiac disturbance of rock bass. North American Journal of Fisheries Management, 21: 333-342.
Cooke, S.J., Philipp, D.P., Schreer, J.F. and McKinley, R.S. 2000. Locomotory impairment of nesting male largemouth bass following catch-and-release angling. North American Journal of Fisheries Management. 20: 968-977.
Cooke, S. J., Schreer, J. F., Wahl, D. H. and Philipp D. P. 2002. Physiological impacts of catchand-release angling practices on largemouth bass and smallmouth bass. Pages 489-512 in D. P. Philipp and M. S. Ridgeway, editors. Black Bass: Ecology, Conservation, and Management. American Fisheries Society Symposium 31. Bethesda, Maryland.
Cooke, S.J. and Sneddon, L.U. 2007. Animal welfare perspectives on recreational angling. Applied Animal Behaviour Science. 104: 176-198.
Cooke, S.J. and Suski, C.D. 2004. Are circle hooks an effective tool for conserving marine and freshwater recreational catch‐and‐release fisheries? Aquatic Conservation: Marine and Freshwater Ecosystems. 14: 299-326.
Cooke, S.J., Suski, C.D., Barthel, B.L., Ostrand, K.G., Tufts, B.L. and Philipp, D.P. 2003. Injury and mortality induced by four hook types on bluegill and pumpkinseed. North American Journal of Fisheries Management. 23: 883-893.
Campbell, M.D., Patino, R., Tolan, J., Strauss, R. and Diamond, S.L. 2010. Sublethal effects of catch-and-release fishing: measuring capture stress, fish impairment, and predation risk using a condition index. ICES Journal of Marine Science. 67: 513-521.
Danylchuk, A.J.,Adams, A., Cooke, S.J. and Suski C. D. 2008. An evaluation of the injury and short-term survival of Bonefish (Albula spp.) as influenced by a mechanical lip-gripping device used by recreational anglers. Fisheries Research 93:248-252.
Danylchuk, A.J., Danylchuk, S.E., Cooke, S.J., Goldberg, T.L., Koppelman, J.B. and Philipp, D.P. 2007. Post‐release mortality of bonefish, Albula vulpes, exposed to different handling practices during catch‐and‐release angling in Eleuthera, The Bahamas. Fisheries Management and Ecology. 14: 149-154.
Danylchuk, A.J., Suski, C.D., Mandelman, J.W., Murchie, K.J., Haak, C.R., Brooks, A.M. and Cooke, S.J. 2014. Hooking injury, physiological status and short-term mortality of juvenile lemon sharks (Negaprion bevirostris) following catch-and-release recreational angling. Conservation Physiology. 2: 1-10.
Duffing Romero, M. D., Matley, J. K., Luo J., L., Ault, J. S., Pittman, S. J. and Nemeth R. S. 2021. Movement patterns of juvenile Atlantic Tarpon (Megalops atlanticus) in Brewers Bay, St. Thomas, U. S. Virgin Islands. Animal Biotelemetry 9, 16 ( https://doi.org/10.1186/s40317-021-00239-x)
Eberts, R. L. and Somers, C. M. 2017. Venting and descending provide equivocal benefits for catch-and-release survival: Study design influences effectiveness more than barotrauma relief method. North American Journal of Fisheries Management. 37: 612-623.
Edwards, R.E. 1998. Survival and movement patterns of released tarpon (Megalops atlanticus). Gulf of Mexico Science. 16:1-7.
Ferguson, R.A. and Tufts, B.L.1992. Physiological effects of brief air exposure in exhaustively exercised rainbow trout (Oncorhynchus mykiss): implications for "catch and release" fisheries. Canadian journal of fisheries and aquatic sciences. 49: 1157-1162.
FFWCC (Florida Fish and Wildlife Conservation Commission). 2013. Summary report on the catch-and-release mortality study on tarpon in Boca Grande Pass, 2002–2004 (revised and updated September 11, 2013). Final Report. Tallahassee, Florida.
Frawley, J. 2015. Kissing fish: Rex Hunt, popular culture, sustainability and fishing practices. Journal of Australian Studies. 39: 307-325.
French, R.P., Lyle, J., Tracey, S., Currie, S. and Semmens, J.M. 2015. High survivorship after catch-and-release fishing suggests physiological resilience in the endothermic shortfin mako shark (Isurus oxyrinchus). Conservation Physiology. 3: 1-15.
Garcia-Moliner, G., I. Mateo, S. Maidment-Caseau, W.J. Tobias and B. Kojis. 2002. Recreational chartered fishing activity in the U.S. Caribbean. Pages 307-317 in R. LeRoy Creswell, editor. Proceedings of the 53rd Annual Gulf and Caribbean Fisheries Institute. Gulf and Caribbean Fisheries Institute.
Gould, A. and Grace, B. S. 2009. Injuries to Barramundi Lates calcarife resulting from lipgripping devices in the laboratory. North American Journal of Fisheries Management 29:1418-1424.
Griffin, L.P., Brownscombe, J.W., Adams, A.J., Boucek, R.E., Finn, J.T., Heithaus, M.R., Rehage, J.S., Cooke, S.J. and Danylchuk, A.J. 2018. Keeping up with the Silver King: using cooperative acoustic telemetry networks to quantify the movements of Atlantic tarpon (Megalops atlanticus) in the coastal waters of the southeastern United States. Fisheries Research. 205: 65-76.
Grubich, J.R. 2004. Lip gripping may harm fish. Saltwater Sportsman (March). 164-170.
Guerrero Pérez, C. R., M. A. García, C. Lilyestrom, G. Rodríguez and Y. Rodríguez. 2013. Puerto Rico marine recreational statistics program, final report 2009-2013. Federal Aid in Sport Fish Restoration Project F-42. Department of Natural and Environmental Resources, San Juan, Puerto Rico.
Guindon, K. Y. 2011. Evaluating lethal and sub-lethal effects of catch-and-release angling in Florida's central Gulf Coast recreational Atlantic Tarpon (Megalops atlanticus) fishery. Ph.D. dissertation. University of South Florida, Tampa.
Heupel, M.R. and Simpfendorfer, C.A. 2002. Estimation of mortality of juvenile blacktip sharks, Carcharhinus limbatus, within a nursery area using telemetry data. Canadian Journal of Fisheries and Aquatic Sciences. 59: 624-632.
Holder, P.E., Griffin, L.P., Adams, A.J., Danylchuk, A.J., Cooke, S.J., Brownscombe and J.W. 2020. Stress, predators, and survival: Exploring permit (Trachinotus falcatus) catch-andrelease fishing mortality in the Florida Keys. Journal of Experimental Marine Biology and Ecology. 524: 151289.
Hutt, C. P., Neal, J. W. and Lang, T. J. 2008. Stocking harvestable hybrid striped bass in an urban fishing program: angling success, angler satisfaction, and influence on Bluegill size structure. Pages 403-411 in R. T. Eades, J. W. Neal, T. J. Land, K. M. Hunt, and P. Pajak, editors. Urban and community fisheries programs: development, management, and evaluation. American Fisheries Society, Symposium 67, Bethesda, Maryland.
Isermann, D. A. and Paukert, C. P. 2010. Regulating harvest. Pages 185-212 in W. A. Hubert and M. C. Quist, editors. Inland fisheries management in North America, 3rd edition. American Fisheries Society, Bethesda, Maryland.
James, J.T., Stunz, G.W., McKee, D.A. and Vega, R.R. 2007. Catch-and-release mortality of spotted seatrout in Texas: effects of tournaments, seasonality, and anatomical hooking location. North American Journal of Fisheries Management. 27: 900-907.
Jepsen, N., Thorstad, E. B., Havn, T. and Lucas, M. C. 2015. The use of external electronic tags on fish: an evaluation of tag retention and tagging effects. Animal Biotelemetry 3, 49 (https://doi.org/10.1186/s40317-015-0086-z).
Jones, T.S. 2005. The influence of circle hooks on the capture efficiency and injury rate of walleyes. North American Journal of Fisheries Management. 25: 725-731.
Keller, B., Swimmer, Y. and Brown, C. 2020. Review on the effect of hook type on the catchability, hooking location, and post-capture mortality of the shortfin mako, Isurus oxyrinchus. PIFSC Working Paper WP-20-003. ( https://doi.org/10.25923/gx1p-m838).
Kerr, S.M., Ward, T.D., Lennox, R.J., Brownscombe, J.W., Chapman, J.M., Gutowsky, L.F., Logan, J.M., Twardek, W.M., Elvidge, C.K., Danylchuk, A.J. and Cooke, S.J., 2017. Influence of hook type and live bait on the hooking performance of inline spinners in the context of catch-and-release brook trout Salvelinus fontinalis fishing in lakes. Fisheries Research. 186: 642-647.
Kokomoor, K. 2010. The "Most Strenuous of Anglers' Sports Is Tarpon Fishing": The Silver King as Progressive Era Outdoor Sport. Journal of Sport History. 37: 347-364.
Leichter, J.J., Helmuth, B. and Fischer, A.M. 2006. Variation beneath the surface: quantifying complex thermal environments on coral reefs in the Caribbean, Bahamas and Florida. Journal of Marine Research. 64: 563-588.
Lopez, I., Aguilera‐Tejero, E., Felsenfeld, A.J., Estepa, J.C. and Rodriguez, M. 2002. Direct effect of acute metabolic and respiratory acidosis on parathyroid hormone secretion in the dog. Journal of Bone and Mineral Research. 17(9): 1691-1700.
Luo, J. and Ault, J.S. 2012. Vertical movement rates and habitat use of Atlantic tarpon. Marine Ecology Progress Series. 467: 167-180.
Martinuzzi, S., Gould, W.A., Lugo, A.E. and Medina, E. 2009. Conversion and recovery of Puerto Rican mangroves: 200 years of change. Forest Ecology and Management. 257:7584.
Mazeaud, M. M., F. Mazeaud and F. M. Donaldson. 1977. Primary and secondary effects of stress in fish: some new data with a general review. Transactions of the American Fisheries Society 106: 201-212.
McGrath, S.P., Butcher, P.A. and Broadhurst, M.K. 2009. Effects of salinity and anatomical hook location on the mortality and physiological response of angled‐and‐released sand whiting Sillago ciliata. Journal of Fish Biology. 74: 220-234.
Meka, J.M. 2004. The influence of hook type, angler experience, and fish size on injury rates and the duration of capture in an Alaskan catch-and-release rainbow trout fishery. North American Journal of Fisheries Management. 24:1309-1321.
Mohan, J. A., Jones, E. R., Hendon J. M., Falterman, B., Boswell, K. M., Hoffmayer, E. R. and Wells, R. J. D. 2020. Capture stress and post-release mortality of blacktip sharks in recreational charter fisheries of the Gulf of Mexico. Conservation Physiology 8(1):coaa041 (https://doi.org/10.1093/conphys/coaa041).
Moxham, E.J., Cowley, P.D., Bennett, R.H. and Von Brandis, R.G. 2019. Movement and predation: a catch-and-release study on the acoustic tracking of bonefish in the Indian Ocean. Environmental Biology of Fishes. 102: 365-381.
Mumby, P.J., Edwards, A.J., Arias-Gonzalez, J.E., Lindeman, K.C., Blackwell, P.G., Gall, A., Gorczynska, M.I., Harborne, A.R., Pescod, C.L., Renken, H. and Wabnitz, C.C. 2004. Mangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature. 427: 533-536.
Orr, H.A. 2009. Fitness and its role in evolutionary genetics. Nature Reviews Genetics. 10: 531539.
Ostrand, K., Siepker, M., Cooke, S., Bauer, W. F., and Wahl, D. H. 2005. Largemouth bass catch rates and injury associated with non-offset and offset circle hook configurations. Fisheries Research 74(1):306-311.
Pérez-Villalona, H., Cornwell, J.C., Ortiz-Zayas, J.R and Cuevas, E. 2015. Sediment denitrification and nutrient fluxes in the San José Lagoon, a tropical lagoon in the highly urbanized San Juan Bay Estuary, Puerto Rico. Estuaries and Coasts. 38: 2259-2278.
Philipp, D.P., Toline, C.A., Kubacki, M.F., Philipp, D.B. and Phelan, F.J. 1997. The impact of catch‐and‐release angling on the reproductive success of smallmouth bass and largemouth bass. North American Journal of Fisheries Management. 17(2): 557-567.
Pinckney, F.S. 1888. The Tarpon: Or "Silver King.". Anglers' Publishing Company.
Pinder, A.C., Velterop, R., Cooke, S.J. and Britton, J.R. 2017. Consequences of catch-andrelease angling for black bream Spondyliosoma cantharus, during the parental care period: implications for management. ICES Journal of Marine Science. 74: 254-262.
Pollock, K. H. and W. F. Pine. 2007. The design and analysis of field studies to estimate catchand-release mortality. Fisheries Management and Ecology 14: 123-130.
Pool, D.J., Snedaker, S.C. and Lugo, A.E. 1977. Structure of mangrove forests in Florida, Puerto Rico, Mexico, and Costa Rica. Biotropica. 9:195-212.
Prince, E. D., M. Ortiz, A. Venielos and D. S. Rosenthal. 2002. In-water conventional tagging techniques developed by the cooperative tagging center for large, highly migratory species. Pages 155-171 in J. A. Lucy and A. L. Studholme, editors. Catch and release in marine recreational fisheries. American Fisheries Society Symposium 30, Bethesda, Maryland.
Roth, C. J., Schill D. J. and M. C. Quist. 2018. Fight time and air exposure times of caught and released salmonids from the South Fork Snake River. Fisheries Research 201:38-43.
Schlenker, L.S., Latour, R.J., Brill, R.W. and Graves, J.E. 2016. Physiological stress and postrelease mortality of white marlin (Kajikia albida) caught in the United States recreational fishery. Conservation Physiology. 4: cov066.
Schisler, G.J. and Bergersen, E.P. 1996. Post release hooking mortality of rainbow trout caught on scented artificial baits. North American Journal of Fisheries Management, 16: 570578.
Shelton, M.D. 2006. Introduction to Fly Fishing. Introduction to Fly Fishing.
Skomal, G. 2006. The physiological effects of capture stress on post-release survivorship of sharks, tunas, and marlin. Ph.D. dissertation. Boston University, Massachusetts, 304 pp.
Suescun, A. 2019. Best tarpon fishing locations in the world [Internet]. Available from: https://www.saltwatersportsman.com/worlds-top-12-tarpon-destinations/
Vaslet, A., Bouchon-Navaro, Y., Charrier, G., Lousi, M. and Bouchon, C. 2010. Spatial patterns of mangrove shoreline fish communities in relation with environmental variables in Caribbean lagoons. Estuaries and Coasts. 33: 195–210.
Villanueva, E., Rivera-Herrera, L.J., Rivera-Colón, S., Tacher-Roffe, M., Perez, C.G. and OrtizGomez, C. 2000. Comprehensive conservation and management plan for the San Juan Bay Estuary. San Juan, Puerto Rico: U.S. Army Corps of Engineers. 1:420.
Villegas‐Ríos, D., Freitas, C., Moland, E., Thorbjørnsen, S.H. and Olsen, E.M. 2020. Inferring individual fate from aquatic acoustic telemetry data. Methods in Ecology and Evolution. 11:1186-1198.
Wegner, N. C., Portner, E. J., Nguyen, D. T., Bellquist, L., Nosal, A. P., Pribyl, A. L., Stierhoff, K. L., Fischer, P., Franke, K., Vetter, R. D., Hastings, P. A., Semmens, B. X. and Hyde, J. R. 2021. Post-release survival and prolonged sublethal effects of capture and barotrauma on deep-dwelling rockfishes (genus: Sebastes): implication for fish management and conservation. ICES Journal of Marine Science 78(9):3230-3244.
Weinz, A.A., Matley, J.K., Klinard, N.V., Fisk, A.T. and Colborne, S.F. 2020. Identification of predation events in wild fish using novel acoustic transmitters. Animal Biotelemetry. 8: 1-14.
Wilson, S.M., Raby, G.D., Burnett, N.J., Hinch, S.G. and Cooke, S.J. 2014. Looking beyond the mortality of bycatch: sublethal effects of incidental capture on marine animals. Biological Conservation. 171: 61-72.
Wilde, G.R. 2002. Estimation of catch and release fishing mortality and its sampling variance. 3rd World Recreational Fishing Conference. 83-85.
Winemiller, K.O. and Dailey, W.H. 2002. Life history strategies, population dynamics, and consequences for supplemental stocking of tarpon. Contributions in Marine Science. 35: 81-94.
Zerbi, A., Aliaume, C., and Joyeus., J.C. 2001. Growth of juvenile tarpon in Puerto Rican estuaries. ICES Journal of Marine Science 58(1):87-95.
