Slowly-evolving Ramicrusta textilis (Peyssonneliaceae, Rhodophyta) invasions – Is it a driver of net shifts in Caribbean coral reef ecosystem functions?
Edwin A. Hernández-Delgado, Ph.D.1,2
1Sociedad Ambiente Marino, PO Box 22158, San Juan, PR 00931-2158; 2University of Puerto Rico, Department of Biology, Center for Applied Tropical Ecology and Conservation, Coral Reef Research Group, PO Box 23360, San Juan, P.R. 00931-3360 coral_giac@yahoo.com
Héctor J. Ruiz-Torres, Ph.D.3 , Samuel E. Suleimán-Ramos, M.A 1
3HJR Reef Scaping, PO Box 1126, Hormigueros, PR 00660 astreoides@gmail.com ; 1Sociedad Ambiente Marino samuelsuleiman@gmail.com
BACKGROUND AND PROJECT RELEVANCE
The resistance of coral reefs to disturbance has significantly declined across a global scale as well as their ecosystem resilience. How to manage coral reefs locally in a globally changing world so that they retain or regain the critical ecosystem attributes of uninhabited reefs and still meet human needs is the central challenge facing reef conservation today (Knowlton and Jackson, 2008). One key concern has been that coral reef research, monitoring and traditional global-scale assessments have largely focused on producing detailed descriptions of reef decline, and continue to pay insufficient attention to the underlying processes causing degradation (Hooper et al. 2005, 2012;Hughes et al. 2010; Brook et al. 2013) and to the A lot is known about the ecological consequences of reef decline, but mechanisms involved in such processes, particularly in those that slowly evolve over very large temporal scales still remain unknown. For example, biodiversity loss has been shown to have paramount consequences in ecosystem productivity and sustainability (Tillman 1996, 1999; Hooper et al. 2005). Further, species loss can accelerate change in ecosystem processes (Hooper et al. 2012) Based on Tillman (1999), we argue that lower levels of available limiting resources (i.e., open space for colonization) at higher diversity (in the case of a coral reef: high coral species richness and percent living cover) are predicted to decrease the susceptibility of an ecosystem to invasion. In other words, a highly diverse, coral-dominated reef ecosystem should be resilient and highly resistant to invasions, supporting the diversity-invasibility hypothesis (Stachowicz et al. 1999, 2002; Levine 2000; Bruno et al. 2003; Davies et al. 2007). But when there is a significant coral reef biodiversity loss (i.e., as a result of a slow but chronic degradation), an alternate resilient low-diversity stable state may establish, which can facilitate invasibility by a sort of opportunist species, often ephemeral or persistent non-reef building taxa. Species loss tends to become a stable state, increasing the resilience of remnant populations (i.e., opportunist invasive taxa), with a highly persistent composition (Pimm 1984). In this sense any competitive dominant invasive species can exclude other primary space holders, such as corals (Paine 1966; Dayton 1971;
Power et al. 1996), resulting in permanent alternate states (Knowlton 1992, 2004). This may explain the potential long-term effects of pervasive algal invasions on coral reefs. Marine biodiversity loss is increasingly impairing the ocean's capacity to provide food, maintain water quality, and recover from perturbations (Worm et al., 2006). It is paramount to harness new theoretical insights and empirical information on why some reefs degrade and others do not. We argue that climate changerelated factors (i.e., sea surface warming) may operate in combination with other local scale (but still pervasive) factors such as declining water quality pulses and long-term fishing impacts to unfold very slow regime shifts in coral reef ecosystems. These can in turn trigger very slow transitions in ecosystem’s dynamic responses across multiple biological scales, from coral colonies to ecosystem processes. One such interaction might be the slow, but yet aggressive invasion and dominance of Staghorn coral (Acropora cervicornis) monospecific biotopes by encrusting red algae Ramicrusta textilis (Peyssonneliaceae, Rhodophyta).
There is still a need to determine how climate change can affect human-altered ecosystems, such as coral reefs, increasing the likelihood of slow transitions that could slowly cascade and eventually spread regionally or globally. We could argue that the escalating impact of multiple anthropogenic drivers on ecosystems and climate may very soon reach levels that in the geological past have triggered long-lasting global responses (Barnosky et al. 2012; Harnik et al. 2012). Therefore, the potential long-lasting impacts of anthropogenic-driven change could be significant. There is evidence suggesting that we may have already passed unrecognized global tipping points, and a slow trajectory to a new regime could have already begun (Hughes et al. 2010). Factors such as habitat fragmentation, loss of biodiversity, emergent diseases, or any population explosion, have been often regarded as the causes (or proximate drivers) of long-term regime shifts, when these phenomena are in fact the responses of ecosystems to larger-scale anthropogenic drivers, such as climate change and overharvesting (Hooper et al. 2012; Brook et al. 2013). The distinction between drivers and responses is important, because one is cause and the other effect, and management interventions work best when they tackle causes rather than symptoms (Hughes 2013b). But this is often a highly complex task due to the major lack of long-term data in coral reef ecosystems. Yet there is still a need to address questions such as how do feedback interactions influence threshold dynamics, how can tipping points be detected or anticipated, how do pulse disturbances contribute to regime shifts, or how can slowly evolving regime shifts be identified. In this project we propose to address the ecological impacts of the still largely unknown slowly-evolving phenomena of the coral reef invasion by the red encrusting algae R. textilis
Coral reefs persist in an accretion-erosion balance influenced by multiple local human threats (i.e., water quality decline, sedimentation, fishing) and by ocean acidification, sea surface warming, massive coral bleaching, disease outbreaks, and mass coral mortality events. The increased interaction of these factors has made coral reef management a major challenge. Endangered Species Act (ESA)-listed Staghorn coral (A. cervicornis) is a highly threatened keystone ecosystem engineer species in Caribbean coral reefs, is largely responsible for shallow-water reef accretion, with calcium carbonate (CaCO3) accumulation rates of up to 1.4 kg/m2/yr (Tunnicliffe 1983), largely propagates by fragmentation (Gilmore and Hall 1976; Tunnicliffe 1981), and constitute a critical nursery ground for multiple species. However, it is highly vulnerable to long-term, slowly-evolving environmental changes associated to these factors and to impacts by algal overgrowth. Several species of red encrusting macroalgae in the family Peyssonneliaceae are known to overgrow corals (James et al. 1988, Antonius and Ballesteros 1998; Velarque et al. 2000; Bruckner et al. 2008; Pueschel and Saunders 2009; Ballantine et al. 2011; Ruíz 2012; Ballantine and Ruíz 2013). One particular species, R textilis has successfully invaded and proliferated across Caribbean coral reefs, and has been reported to overgrow many coral species and other benthic organisms in the Caribbean (Eckrich et al. 2010, Eckrich and Engel 2013), including A. cervicornis. It can occupy very large extensions of reef bottom, creating a nearly closed canopy of calcareous layers preventing sunlight to reach the bottom, coral tissue regeneration and coral larval settlement, largely contributing to reef resilience decline. This has created a novel, seldom documented Ramicrusta-dominated reef
biotope. Surprisingly, direct effects of R. textilis proliferation on multiple coral reef ecosystem processes, including net reef accretion (coral growth vs. bioerosion rates), coral tissue regeneration ability, coral demographic dynamics, coral and fish recruitment dynamics, and the maintenance of reef fish community structure have not received any attention yet. Understanding these processes is critical to develop a conceptual model of impacts by a novel coral reef invasive algal species, and to understand altered coastal reef ecosystem dynamics and its impact on important ecosystem services.
The dynamics of coral reefs is characterized by a combination of positive and negative feedback mechanisms. A reef might follow a positive or negative feedback mechanism depending on whether herbivory intensity is sufficiently high (top-down mechanism) (Hay 1984; Hughes et al. 1987, 2007), or whether land-based source pollution (LBSP) and eutrophication are properly controlled (bottom-up mechanism) (Cloern 2001) that macroalgal blooms are prevented from occurring. Grazing rates could also be influenced by algal species composition (Lewis 1985). Depending on the interactions of top-down and bottom-up feedback mechanisms, as well as on algal species composition, reef communities might follow different trajectories (Littler et al. 2006). Therefore, macroalgal blooms often occur in systems characterized by rapid algal growth (i.e., from LBSP, low- grazing rates, or a combination of both) and by abundant open areas available for macroalgal settlement, such as reefs where recent large-scale coral mortality has occurred (Hughes et al. 2007; Mumby and Steneck 2008) However, R. textilis growth seems to be very slow, but still highly pervasive across very large spatial scales, and does not seem to adjust to any of the rapidly-evolving positive or negative feedback mechanisms that may explain typical macroalgal blooms. Any factor that could potentially lead to coral death or could reduce levels of herbivory will leave more substrate open for macroalgal colonization or make the effects of even low-level nutrient enrichment more severe (Szmant 2002). We argue that low herbivory levels due to the long-term decline of Long-spine urchin, Diadema antillarum (Lessios 1988) and to the long-term effects of artisanal fishing impacts (Hawkins and Roberts 2004), in combination, with widespread coral mortality (Hughes et al 1994; Miller et al. 2009; Hernández-Pacheco et al. 2011), and the long termdecline in coral recruitment and altered trajectories of coral recruit assemblages in recent time (Edmunds and Elahi 2007; Hernández-Delgado et al. 2014a; Edmunds 2015), might have facilitated the conditions for slow, but progressive invasions by R. textilis This suggests that a key target to manage coral reef resilience should be reducing open substrate areas prone to macroalgal colonization by either increasing percent living coral cover, increasing herbivore guilds, increasing herbivory rates, reducing LBSP, or a combination of any of the above. But does R. textilis respond to any of these factors still remains unknown.
The long-term decline in Caribbean-wide Acroporid and Faviid coral populations, local extinctions and/or recovery processes can be significantly influenced by variation in demography, recruitment rates and life-history traits (Hughes & Tanner 2000; Edmunds and Elahi 2007; Edmunds 2015) Age-related life tables can be used to document these changes through time on individual cohorts by studying birth and mortality rates. However, for modular organisms, such as corals, genetic and environmental factors can produce largely variable population growth rates, which can result in a very poor correlation between age and the probabilities of birth and mortality (Hughes 1984). But size can more accurately predict the fate of a coral cohort than age (Connell 1973; Hughes and Jackson, 1980; Highsmith 1982; Hughes et al. 1992). This type of analysis can be a powerful tool to predict the fate of wild, cultured and restored Acroporid coral populations.
A size-staged matrix population model will allow testing the effects of increased fecundity, increased mean colony size, growth rate, survivorship of individuals, recruitment, re-sheeting,
fragmentation, fragment reattachment, and colony shrinking (i.e., fragmentation, disease outbreak, other epizootics, predation) by examining their effects on the population growth rates. A sensitivity analysis of the growth rates to disturbance in life history parameters can be calculated from the stage-specific reproductive values and the equilibrium population structure (Caswell 2001). Elasticity analysis of the size-staged transitions (Horvitz et al. 1997; Hughes and Tanner 2000) will allow identify which are the most important transitions in terms of their relative effect on population growth rates. In the case of A. cervicornis impacted by R. textilis overgrowth, this would provide paramount information regarding critical minimum colony sizes, survival and growth rates necessary to survive under wild conditions.
Project relevance
The proposed project will have major relevance addressing key national, regional and local priorities identified by NOAA and PRDNER for coral reef conservation and ESA-listed Acroporid coral recovery (NMFS, 2015). It will first address key management priorities recently identified by PRDNER aimed at fostering the recovery of coral reefs and achieving a stronger community-based participation in management-oriented activities. This project will provide critical timely information to advance the primary goal of NOAA’s Coral Reef Conservation Program (CRCP) “to protect, conserve, and restore coral reef resources by maintaining healthy ecosystem function”, as identified in the document entitled “NOAA Coral Reef Conservation Program Roadmap for the Future: A Plan for Developing CRCP Direction Through 2015” (2008). Its outcomes will also be consistent with goals and objectives identified in the “NOAA Coral Reef Conservation Program Goals & Objectives 2010-2015” (2009), including the general goal of supporting “monitoring efforts to: Document the status of reef species of ecological and economic importance; Track and assess changes in reef communities in response to environmental stressors or human activities; and Evaluate the effectiveness of specific management strategies and identify actions for future adaptive responses”.
This project will also conduct applied research that will directly contribute to improved management, will include prompt mechanisms to disseminate information to resource managers in addition to peer-reviewed publications, and will be in compliance with listed jurisdictional coral reef management priorities
This project will address specific issue areas identified through the P.R. LAS for Coral Reef Conservation 2011-2015, including:
Issue Area A: Objective 3 (A2.4): Establish water quality monitoring stations in coral reef ecosystem areas and add water quality monitoring components to established coral monitoring sites around P.R.
Issue Area B: Objective 2 (B1.3): Search for and identify management tools that could be applied to fisheries and related ecosystem protection and management in Puerto Rico
Issue Area D: Manage for climate change and diseases emanating from increase in storm frequency and impact, water temperature and air pollution and promote recovery of reefs from previous events. Goal 8 (D1 modified): Promote recovery of reefs from natural stressors, atmospheric phenomena and invasive species. Objective 1 (DI.1): Identify areas of high diversity and live coral coverage for additional protection and expand existing protected areas to include these areas. Objective 2 (DI.2): Support more research on coral diseases and on the relationship of bleaching to disease; support more research on coral resistance to bleaching/disease and resilience following global, regional and local stressors and on possible effects of climate change on coral reefs and other ecosystems.
This project will also provide critical baseline information to parameterize coral population models to guide future Acroporid corals restoration in the future. It will also provide baseline data to parameterize models to address ecosystem-level responses under variable environmental (water
quality) scenarios and under variable fishing impact scenarios. Modeling products will provide the basis to guide large-scale management of future coral reef rehabilitation efforts, as well a theoretical guidance for testing future rehabilitation efforts across other Caribbean reefs. It will finally strengthen community-based participatory roles into coral reef management, by maintaining its continuously successful formula of integrating community volunteers with SAM members, academic researchers, and reef managers following a transformative, hands-on educational model that can be used as model to other Caribbean Islands.
PROJECT DESCRIPTION AND OBJECTIVES
We propose to address the impact of R. textilis on several key ecosystem processes within Ramicrusta-dominated (experimental) and outside (control) plots, across mid-shelf Arrecifes Los Corchos system, off Culebra Island, PR. Our objectives include addressing:
1. Coral tissue regeneration – Assess during Year 1 living tissue regeneration rates in tagged outplanted A. cervicornis colonies showing partial tissue mortality. Small tissue lesions will be experimentally induced in a set of experimentally transplanted fragments in contact with R. textilis and compared to unaltered fragments in contact with the algae, as well as to control fragments (no contact- with lesions, and unaltered)
2. Demographic impacts on A. cervicornis – Address over a period of two years the demographic impacts of R. textilis on A. cervicornis populations and project population trends under different tissue regeneration and algal invasion scenarios Demographic impacts will be addressed first by using data from the tissue regeneration analysis during Year 1, and during Year 2 by setting triplicate experimental fragment transplanting plots in contact with the algae under different population proportion of contact (25%, 50%, 100% contact), in comparison to controls (no direct contact). Population dynamics will be addressed at time t=0 and 1 Populations will be projected into the future by analyzing responses to different levels of responses to tissue lesions and to algal overgrowth.
3 Coral recruitment rates –Assess coral recruitment rates during two years on multiple replicate 1 m 2 plots at six month intervals over Ramicrusta-dominated (experimental) and over open substrates (control).
4. Net reef accretion – Compare skeletal extension and calcification rates in living A. cervicornis colonies vs. bioerosion rates in dead A. cervicornis fragments, between experimental and control plots, during Year 2. We propose to use outplanted living and dead A. cervicornis colonies obtained from existing coral farms.
5 Algal growth and turnover rates – Assess during Year 2 R. textilis algal growth rates and turnover rates by measuring its linear extension and its natural ability to recolonize artificially opened reef bottom.
6. R. textilis impacts on algal biodiversity and productivity – Assess during Year 1 R. textilis impacts on coral reef algal biodiversity comparing R. textilis dominated bottoms and other open bottoms.
7. Fish community structure – Assess spatio-temporal dynamics in fish community structure within and outside Ramicrusta-dominated bottoms, including juvenile assemblages and herbivore guilds, at six month intervals during two years.
8 Water quality below the R. textilis canopy – Water quality parameters (i.e., temperature, light, dissolved oxygen concentration, pH, salinity, conductivity, turbidity, ammonium) will be addressed below and above Ramicrusta canopies.
This approach will provide crucial baseline information for coral reef managers and decision
makers regarding a novel management challenge across the Wider Caribbean region. This would have multiple implications regarding the long-term fate of A. cervicornis wild populations and the long-term success of future coral propagation and reef rehabilitation efforts across the region. It will also provide basic information to understand the influence of R. textilis biotopes on coral reef fish assemblages and herbivory processes across reef spatial scales.
METHODOLOGY
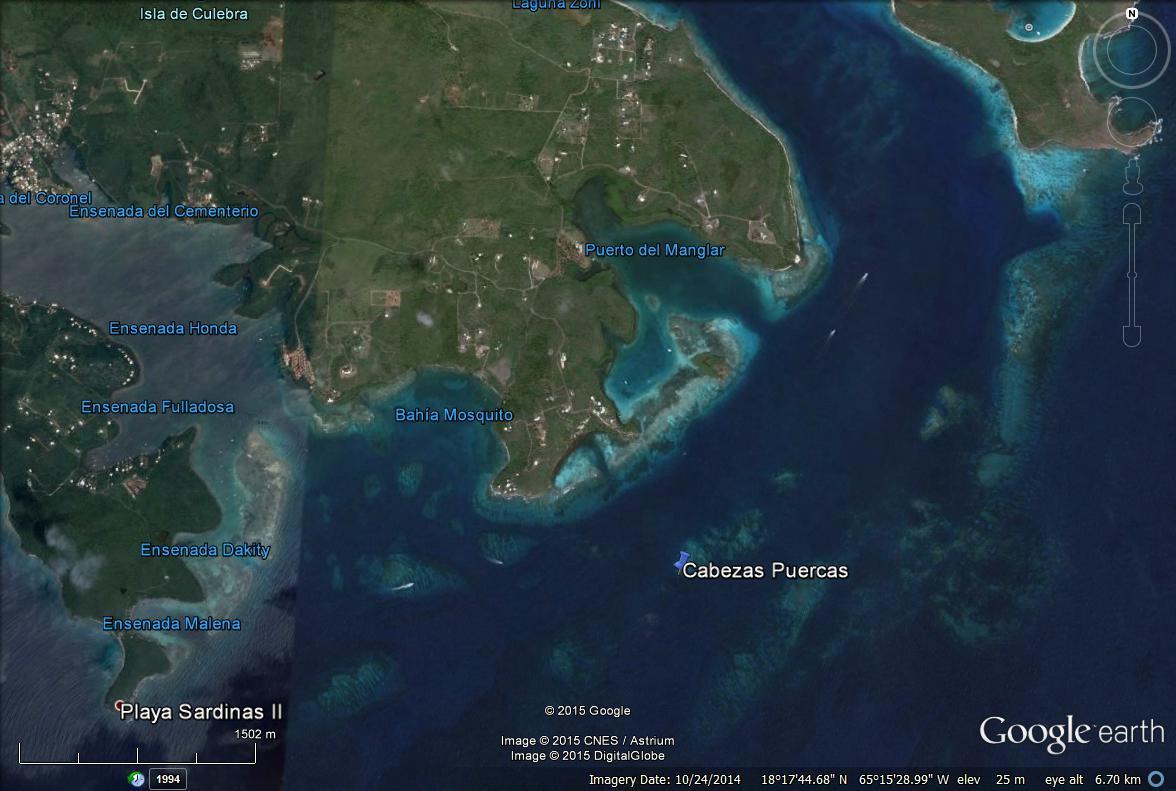
Study site – The proposed project will be conducted at the westernmost limits of Arrecifes Los Corchos system, off southeastern Culebra Island, PR (18°17.148’N, 65°15.134W) All field experiments and monitoring efforts will be conducted at depths ranging between 5 and 7 m (Site Map 1).
Task #1: Coral tissue regeneration – Living tissue regeneration rates in tagged out-planted A. cervicornis colonies showing partial tissue mortality will be addressed through a field experiment during Year 1. All coral out-plants, as well as dead colony pieces, will be obtained from existing coral farms operated by Sociedad Ambiente Marino (SAM) in Culebra. This will be achieved by setting up a factorial experiment with two treatment levels (within Ramicrusta-dominated bottom, control outside on adjacent open bottoms), and two injury conditions (artificial lesion, unblemished colonies). A total of three replicate plots of 20 out-planted fragments (15-20 cm each) will be established as described above for each combination of treatments and injury conditions. Artificial tissue lesions will be produced in situ following Bak and Van Es, 1980), using and air pick and will affect approximately 20% of the colony surface by eliminating only tissue and leaving intact coral calices (Hernández-Delgado, 2000). Colony survival and extension rates will be determined at 0, 1, and 2 weeks, and then at 1, 3, 6, 9, and 12 months by direct fragment measurements of multiple parameters following Hernández-Delgado et al. (2014b). Fragment survival, growth, branch production, branchiness index (# harvestable branches >6 cm), % mortality, causes of mortality, predator density (snails, fireworm, damelfish), disease prevalence and partial tissue mortality will be monitored for each permanently marked fragment/plot. Data will be tested using a multivariate two-way permutational analysis of variance (PERMANOVA) in PRIMER-e v6.1.16) + PERMANOVA 1.06 Statistical Package (Clarke and Warwick, 2001; Anderson et al., 2008), with time and treatment as main variables. Data will also be used to parameterize size-staged population models to test hypothesis regarding demographic impacts of tissue lesions and R. textilis impacts on A. cervicornis population trends (described below).
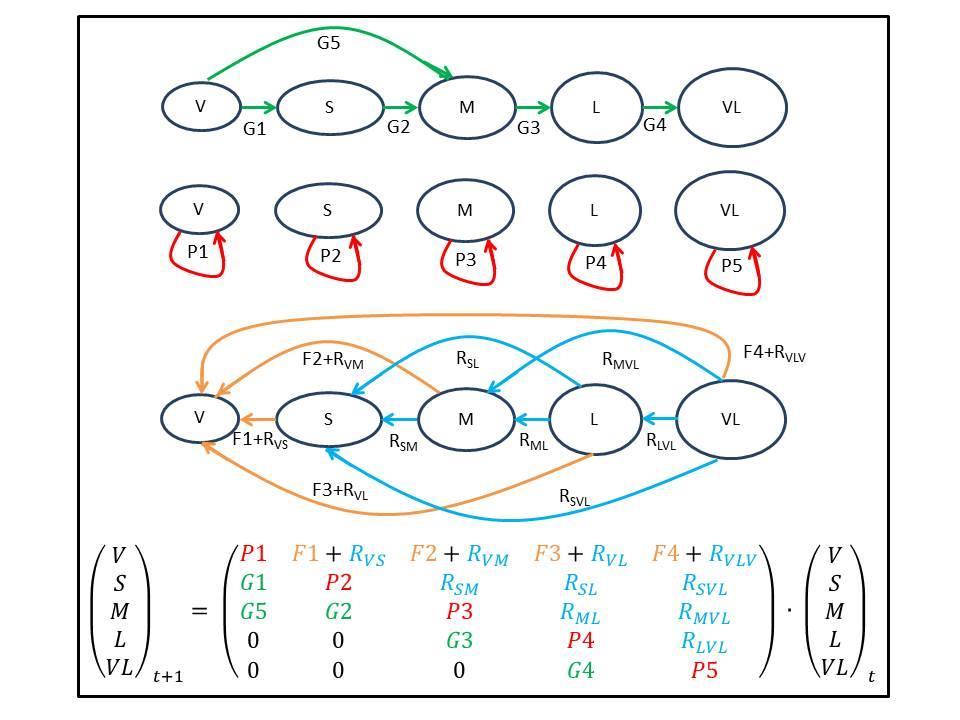
Task #2: Demographic impacts on A. cervicornis – We will address over a period of two years the demographic impacts of R. textilis on A. cervicornis populations and project population trends under different tissue regeneration and algal invasion scenarios. First, demographic impacts will be addressed first by using data from the tissue regeneration analysis during Year 1 (Task #1). Individual size-staged population projection matrices will be constructed for each cohort under each combination of treatment levels (within Ramicrusta-dominated bottom, control outside on adjacent open bottoms) and injury conditions (artificial lesion, unblemished colonies). Populations of A. cervicornis will be sub-divided into five size classes based on total colony length (cm): very small [V] (<10 cm), small [S] (10-25 cm), medium [M] (25-50 cm), large [L] (50-100 cm), and very large [VL] (>100 cm). The number of colonies in each of the life cycle stages at time t+1 equals what is expressed in Figure 1. The contribution of each life cycle stage at time t to all others at t+1 is contained in the 5 x 5 matrix that projects the population vector between t and t+1 P1 to P5 are the probabilities of very small, small, medium, large, and very large colonies, respectively, of remaining alive in the same size category for one year (Eq. 1 in Figure 1). The Gs represent growth transitions. For example, G1 is the probability that a very small colony at time t will survive and grow into a small colony at time t+1. G2 is the probability that a small colony at time t will survive and grow into a medium colony at time t+1, and so on. The Rs are size retrogressions (i.e. a colony that becomes smaller by losing tissue through partial mortality or by physical fragmentation). RVS is
the probability that a small colony at time t retrogress to a very small one at time t+1. RVM is the probability that a medium colony at time t retrogress to a very small one at time t+1, and so on. Artificial tissue lesions will be treated as colony retrogressions immediately after time=0. Therefore, they will be addressed during the following census. The Fs represent sexual recruitment and will be considered only if we document sexual recruits (“crusts”) during time t+1, but given the type of experiment, Fs will end up being eliminated from Eq. 1 as no sexual recruitment will be expected to occur. Assessed colonies within each plot will be tagged, mapped and photographed to help individual identification in the future.
We will calculate the real dominant eigenvalue and its corresponding right and left eigenvectors to obtain the asymptotic population growth rate (λ), the stable stage distribution (w), the reproductive value vector (v) and the sensitivity and elasticity matrices for every census period matrix. The 95% confidence intervals for the population growth rates will be calculated using a bootstrap analysis. Demographic transitions will be calculated from this sample, a transition matrix will be constructed and the λ calculated. The entire procedure will be repeated 1000 times and asymmetric 95% confidence limits will be determined from the 2.5th and 97.5th percentiles of the data using R version 3.1.3 (R Development Core Team 2015).
To analyze the effect of coral colony tissue lesions on the viability of A. cervicornis populations, each experimental and control population will be projected 100 years into the future, one thousand times with five different annual partial coral mortality event probabilities; 0%, 10% (1/10 yr), 20% (1/5 yr), 33% (1/3 yr), 50% (1/2 yr), and 100% (annual) using Mathcad Plus 6.0 MathSoft, Inc. The stochastic population growth rate, λs, will be calculated for each of the 1,000 trajectories by taking the arithmetic mean of logs of the λs (let r(t) = log(λ), then log(λs) = 1 ��∑ ��(��) �� 1 ��=0 . The number of colonies remaining alive after 100 years of stochastic population projection from an initial population of 1000 colonies will also be recorded to address the long-term ecological fate of the population. Similar projection analysis will be made simulating variable frequencies of partial colony mortality by simulating different hurricane frequencies: 0%, 5% (1/20 yr), 10% (1/10 yr), and 20% (1/5 yr), 33% (1/3 yr), as described above. Impacts will be simulated based on our previous data from coral farms (Hernández-Delgado et al. 2014b) and on the available literature for Acroporid corals (i.e., Knowlton et al. 1988; Edmunds and Witman 1991; Lirman and Fong 1997; Lirman 2003). This approach will provide a baseline analysis for reef managers and decision makers regarding the potential impacts of different environmental and climate change scenarios on A.cervicornis population trends in the presence or absence of R. textilis.
A similar process will be repeated during Year 2 to address the demographic impacts of different R. textilis algal invasion scenarios on A. cervicornis population dynamics and project population trends into the future. Triplicate experimental fragment out-planting plots in contact with the algae under different treatments of population proportion of contact (25%, 50%, 100% contact), in comparison to a control (no direct contact). A total of 20 replicate colonies will be out-planted per treatment and monitored at 0, 6, and 12 month intervals. Colony extension rates will be determined at 0, 6 and 12 month intervals by direct fragment measurements of multiple parameters following HernándezDelgado et al. (2014b), as described above. Data will be tested using a multivariate two-way permutational analysis of variance (PERMANOVA), with time (0, 6, 12 months) and treatment (experimental, control) as main variables
A two-way PERMANOVA will be used to test differences in results using time and treatment as main variables. Population dynamics will be addressed at time t=0, 1 as described above.
Populations will be projected into the future as described by analyzing A. cervicornis demographic responses to different levels of algal overgrowth (0, 25, 50, 100% contact), and by simulating different hurricane frequencies as described above. This will allow assessing the combined impacts of R. textilis under different levels of invasion, in combination with different hurricane frequencies.
FIGURE 1. Conceptual size-staged matrix model of Acropora cervicornis population dynamics across five size stages (described in Task #2). During each time interval corals may: A) grow (G); B) stay the same size (P); or C) contribute to smaller size classes as a result of sexual recruitment (F), by partial reduction in colony size due to shrinking, physical or physiological fragmentation (R). Transitional probabilities will be: (growth) 0 G 1; (Remain) 0 P 1; (shrinking due to partial mortality or asexual reproduction) 0 R 1; (sexual reproduction) 0 F 1.
Task #3: Coral recruitment rates – Coral recruitment rates will be assessed during two years comparing Ramicrusta-dominated (experimental) and open substrates (control). Briefly, five replicate fixed 1 m2 quadrats will be randomly established within each of the triplicate plots over Ramicrusta-dominated substrates. A similar design will be used to assess recruitment rates over adjacent open substrates. Data will be collected by a combination of direct counting and highresolution digital images at 0, 6, 12, 18 and 24 month time intervals. Data will be tested using a two-way PERMANOVA with time and treatment as main variables.
Task #4: Determine net reef accretion – Net reef accretion at the scale of A. cervicornis biotopes will be assessed during Year 2 by comparing skeletal extension and calcification rates in living A. cervicornis colonies vs. bioerosion rates in dead A. cervicornis fragments, between experimental and control plots. Triplicate experimental plots of 20 colonies each will be out-planted in direct contact with R. textilis. Triplicate control plots of 20 colonies each will be out-planted to adjacent open reef bottom with no direct contact with the algae. Out-plants will be secured using a combination of rebars, fishing line, plastic-covered wire, and tagged to aid identification in the field. Each plot will measure approximately 1.5 x 1.5 m and will consist of a square PVC horizontal frame fixed to the bottom, with four fishing lines and with 5 suspended colonies each line (approximately 15-20 cm-long each fragment). Colonies within experimental plots will be fixed in direct contact with R. textilis surface. Those in control plots will be suspended at approximately 70 cm off the adjacent substrate. Colony extension rates will be determined at 0, 6 and 12 month intervals by direct fragment measurements of multiple parameters following Hernández-Delgado et al. (2014b), as described above. Data will be tested using a two-way PERMANOVA, with time and treatment as main variables.
A similar design will be used to assess coral biorosion rates. Briefly, replicate 5-cm long fragments
of dead A. cervicornis will be collected from coral farms. Triplicate sets of 20 dead fragments will be out-planted below the Ramicrusta canopy. Another triplicate set will be out-planted to the adjacent open bottom. Out-planted coral pieces will be fixed in four arrays of 5 pieces per plot, attached to a fishing line with plastic-covered wire and rebars. All fragments will be dried at 60ºC/24 h, measured (length, circumference), and pre-weighted in the laboratory before setting up the experiment. Coral pieces will be tagged to aid identification in the field. A total of 10 replicate fragments will be collected per plot after six months. The remaining 10 pieces will be collected after one year. All fragments will be dried and re-weighted. Pieces will also be cut in half using a rock saw and examined under the microscope. Pieces will be photographed and images analyzed for identification of bioeroding taxa (i.e., Clionid sponges, Bivalves). Pieces will also be measured (total length, circumference) to address any loss of skeletal material at the end of the experiment. Bioeroded surface of inner skeleton will be determined using the tracing analysis function of the image analysis software Coral Point Count with Excel extension (CPCE, v. 4.1) (Kohler & Gill 2006). Data will be tested using a two-way PERMANOVA with time and treatment as main variables.
Net reef accretion will be addressed at 6 and 12 month intervals by determining the difference between net skeletal growth (by estimated CaCO3 weight gain) of living colonies and net skeletal weight loss due to bioerosion. Skeletal weight gain will be estimated by determining total colony length and mean circumference. Triplicate measures of circumference will be obtained from each colony during each monitoring effort. CaCO3 weight will be calculated by transforming skeletal mean circumference to radius, then calculating the area of a circle and the volume of a cylinder. Net CaCO3 weight (g) will be obtained by multiplying the colony volume by a conversion factor of 2.49. The same formula can be applied to net CaCO3 loss in bioeroded coral pieces, though direct weight loss will also be calculated. Data will be tested using a two-way PERMANOVA with time and treatment as main variables.
Task #5: Algal growth and turnover rates – Ramicrusta textilis algal growth rates and turnover rates will be assessed during Year 2 by measuring its linear extension and its natural ability to recolonize artificially opened reef bottom. Fixed triplicate 10 m long transects will be established along the edge of Ramicrusta-dominated bottoms. Five non-overlapping 30 x 20 cm quadrats will be permanently fixed at 2 m haphazard intervals where R. textilis horizontal extension will be determined using high-resolution digital images and using the tracing analysis function of CPCE, v. 4.1. Vertical extension will be determined by establishing 3 pins within each quadrat to determine vertical extension. Data will be monitored at 0, 6, and 12 month intervals. Results will be tested using a using a one-way PERMANOVA with time (0, 6, 12 months) as main variables.
Algal turnover rates will also be assessed during Year 2 by artificially removing fragments of algae and assessing its benthic recolonization ability. Four different treatments will be used in the experiment, including: artificially opened substrates with dead A. cervicornis framework, artificially opened substrates without dead A. cervicornis framework, opened bare substrate (open control), and control substrates with R. textilis (algal control). Five quadrats/plot and triplicate plots will be established per treatment. Algal recolonization will be analyzed at 0, 6, and 12 month intervals high-resolution digital images and using the tracing analysis function of CPCE, v. 4.1. (Kohler and Gill 2006) and tested using a two-way PERMANOVA with time (0, 6, 12 months) and treatment as main variables.
Task #6: R. textilis impacts on algal biodiversity and productivity – We will assess during Year 1 R. textilis impacts on coral reef algal biodiversity. Briefly, 6 replicate 20m-long transects will be
evaluated comparing algal assemblages below and above R. textilis dominated bottoms with other 6 replicate transects over open bottoms. Data regarding algal assemblages will be obtained using four replicate 0.25m2 quadrats and a combination of methods, including collection for laboratory identification. Four 0.25m2 (0.61m x 0.42m) quadrats will be divided into four 1/16(0.0625) m2 subquadrats, and will be randomly placed along transects. Each quadrat will be labeled with number for identification reference. Photographs of each 0.0625 m2 quadrat will be taken at 0, 6, and 12 month intervals with high-resolution digital camera (Cannon 7D) in a underwater housing (Sea & Sea MDX 7D) with a strobe. Percent cover of the algal elements recognizable from the digital photographs will be calculated by overlaying each photo with 100 (randomly stratified points) using Coral Point Count with an Excel extension (CPCe, v4.1) (Kohler & Gill, 2006). Data will also include percent cover of individual algal species, species diversity index (H’n) (Shannon and Weaver 1948) and evenness (J’n) (Pielou 1966a,b). Results will be tested using a two-way PERMANOVA with time and treatment as main variables. Data will also be used to test algal assemblage spatio-temporal impacts on fish community assemblages (described below).
To address algal productivity, macroalgae within each quadrat will be harvested by hand and with a paint scraper. The algae then will be transported to the laboratory, identified and species comparisons made (Goldberg and Kendrick 2004). Algae assemblages will be characterized by species richness (average number of species in 0.25m2), density (number of individuals 0.25m2), biomass (g wet weight/0.25m2). Data will also include percent cover of individual algal species, H’n and J’n. Results will be tested using a two-way PERMANOVA with time (0, 6, 12 months) and treatment as main variables. Data will also be used to test algal assemblage spatio-temporal impacts on fish community assemblages (described below).
Task #7: Fish community structure – We propose to assess the spatio-temporal dynamics in fish community structure within and outside Ramicrusta-dominated bottoms, including juvenile assemblages and herbivore guilds, at six month intervals during two years. Fish counts will be conducted following on six replicate fixed experimental (Ramicrusta-dominated bottoms) and six replicate control plots (no algal dominance) through 15 min periods of time/count within 177 m 2 stationary circular transects (radius 7.5 m) on fixed stations per site, following Bohnsack and Bannerot (1986). Fish abundance and estimated fork lengths will be determined and used to estimate biomass at the lowest taxa possible. Data will include abundance and biomass of individual species (following Bohnsack and Harper 1988; Bohnsack 1996), fishery target species, fish functional groups and families, H’n, and J’n Data on juvenile fish individuals will be extracted from fish counts, as well as data on herbivore guilds. Diadema antillarum density will also be documented within each circular transect. We will also estimate reef structural complexity on a scale of 0-5, progressing by increments of half a unit from 0, representing somewhere totally flat and featureless bottom, to 5, representing extreme complexity (Hawkins and Roberts, 2004). Although semi-quantitative, this scale provides a rapid means of assessing complexity and has been used successfully in previous studies (Polunin and Roberts, 1993; Hawkins et al., 1999). Benthic community composition will be obtained by means of point counts along a 12 m-long transect and at fixed 0.5 m haphazard intervals (n=24 points/transect). This will provide data on percent cover of different benthic categories by species or functional group (i.e., corals, sponges, macroalgae, fiamentous algae, crustose coralline algae, R. textilis, Lobophora variegata, Halimeda spp., cyanobacteria, open substrate).
Data will be analyzed by means of a two-way PERMANOVA test using time and treatment as main variables. Also, we will use the multivariate routines DIVERSE and TAXDTEST in PRIMER-e Statistical Package44 to test taxonomic diversity and distinctness over both treatment levels and temporal scales. The BEST and LINKTREE routines44 will also be used to link potential environmental drivers (benthic community composition, reef structural complexity, algal assemblages, water quality data described below) to observed fish assemblage patterns. Principal Component Ordination (PCO) will be used to test hypotheses regarding fish community structure
indicator species44 . Also RELATE routine will be used to test fish community multivariate correlations with different environmental variables. This approach will allow testing multiple hypotheses regarding impacts of benthic dominance of R. textilis on fish communities which will provide crucial information for managers.
Task #8: Water quality below the R. textilis canopy – Water quality parameters will be addressed during a period of two years below and above Ramicrusta canopies This will include continued records of temperature using Hobo temp V2 data loggers (Onset Computers, Co.). Also, data will be obtained during each visit to study sties from triplicate measurements at triplicate sampling points located below the R. textilis canopy, from triplicate points above the canopy, and from triplicate adjacent points over open reef bottom. Data will include irradiance Hobo Pendant (Onset Computers, Co.), pH, salinity, and conductivity, and dissolved oxygen concentration using aYSI85 data logger (YSI Corp.), turbidity using a portable turbidimeter, chlorophyll-a using a digital fluorometer (Turner Designs, Co.), and ammonium using a Smart V2 portable spectrophotometer (Lamotte Co.). Irradiance will be determined at 5 second intervals for a continuous period of 3 min. Below-canopy parameter lectures will be obtained through small holes drilled through the canopy.
Environmental data will be tested following a two-way multivariate PERMANOVA design with time and treatment (below canopy, above canopy, adjacent open bottom) as main variables. A similar two-way PERMANOVA test will be made with Season and treatment as main variables. Water quality parameters will also be tested multivariate BEST procedure in PRIMER-e Statistical Package (Clarke and Warwick, 2001) to link potential environmental drivers to observed biological parameters. A similar process will also be followed for linking potential environmental drivers to observed assemblage patterns of coral data, fish community data, and benthic community data. Multivariate classification and regression linkage trees (LINKTREE) will be used to explain biological patterns by environmental variables (Clarke and Warwick, 2001). Resemblance matrices of environmental and biological data will be correlated following the multivariate routine RELATE (Clarke and Warwick, 2001). Outcomes of these analyses will provide important data for managers and decision-makers to address potential impacts by micro-scale changes in water quality parameters.
FACILITIES
The main facility that will be used during this project will be the Culebra Island Field Station operated in Culebra Island by SAM. This is a small wooden house located in Dewey, Culebra. It has two rooms and one bathroom, and can accommodate about 8 people. It has also storage facilities for maintaining SCUBA tanks, diving gears, and all materials necessary to carry out the project. Also, a 26.5’ vessel will be provided by SAM to complete all field work. Additional equipment such as two fully-equipped laptop computers and high-resolution digital cameras (2) necessary for data collection and analysis will be provided by the P.I.s. All SCUBA equipment will also be provided by SAM. Laboratory facilities will be shared by Dr. Elvira Cuevas, Director of the Center for Applied Tropical Ecology and Conservation (CATEC) at the University of Puerto Rico, Río Piedras.
ANTICIPATED BENEFITS
The proposed methodological approaches will provide responses to multiple coral reef management-oriented questions regarding the biological consequences of R. textilis invasion of A. cervicornis biotopes. A key novel component of this project will be the generation of demographic data and modeling products regarding the interaction of R. textilis and A. cervicornis tissue regeneration ability. Also, demographic modeling will address the coral population response to
different levels of contact with R. textilis. This has never been done before. Modeling will provide a significant tool to managers and decision-makers regarding A. cervicornis conservation and restoration efforts as it will allow projecting A. cervicornis population trends over the next 100 years by simulating different environmental scenarios (i.e., different coral disease frequencies, different levels of R. textilis impacts, different hurricane impact frequencies). This project will also provide for the first time quantitative accounts regarding R. textilis population dynamics which can provide preliminary information to parameterize models to project populations into the future and help elucidate potential management strategies. Further this project will provide the first ever approach to document the impact of R. textilis on the coral reef native algal community dynamics and its effect on algal productivity dynamics. This will provide critical information regarding transitional reef trophic dynamics that can be fundamental for developing coral reef benthic and fish community recovery strategies in the future.
Our project will also provide a novel look at the impact of R. textilis dominance on several ecological processes which are fundamental for sustaining reef ecosystem resilience and ecological functions, including reef accretion (coral skeletal extension vs. bioerosion), coral recruitment, and the maintenance of fish assemblages. Addressing such impacts may shear new light into reef biodiversity-invasibility theory, as well as into the relationship between reef biodiversity and productivity. It is paramount for reef science to address the theoretical basis of the slowly-evolving, yet pervasive changes that are occurring through most of the wider Caribbean region. This will require a combination of inter-disciplinary approaches lead by a combination of a dynamic research team such as the one assembled by SAM. All information produced will be initially timely used by PRDNER and NOAA for the revision and development of future multi-year strategic management, funding and research plans. Both agencies have strongly supported and endorsed this project (see attached letters). It will also be used by the academia for developing novel areas of applied ecological and eco-physiological research.
Other anticipated benefits will help elucidate feedback mechanisms regarding the interaction of R. textilis with coral reef conservation and restoration efforts, which will allow implementing basic conceptual and mathematical models to address such impacts on larger spatial and temporal scales in the future. This approach will further provide a set of new or enhanced tools implemented to improve management preparedness and response to guide future efforts aimed at the recovery of fishing impacts, managing emerging local needs, and implementing adaptive approaches to manage climate change impacts. This project will further address several emergent issues important for managers and decision-makers at NOAA and PRDNER regarding climate change impacts on transitional coral reef ecosystems, community-based integration, and the development of conceptual models to address future multi-disciplinary management challenges. This information will be useful for the entire Caribbean region and will provide important timely and multi-disciplinary information to shape future management strategies for Acroporid.
The project will have direct measurable impact now through several outreach/educational activities (described in the Data sharing section below), and in the future through several peerreviewed publications. Project’s outcomes, products and lessons learned will be mainly used by DNER and NOAA managers and decision makers. But information will also be available to a wide array of scientists, managers, NGOs, interested stakeholders and the general public through multiple dissemination efforts and media.
PROJECT LEADERSHIP AND PERSONNEL
The implementing organization for the project will be the Sociedad Ambiente Marino, Inc. (SAM). SAM was registered 14 years ago in the Commonwealth Department of State as a nonprofit, non-governmental organization. Also, it later got registered as a Federal exempt 501(c)3 organization. SAM has already previous experience administering grants from Federal agencies such as the NOAA-Coral Restoration Center/The Nature Conservancy, the U.S. Fish and Wildlife Service Coastal Program, UPR Sea Grant College Program, and from several other private sources. Specific tasks of Co-P.I.s and project collaborators are listed below:
Edwin A. Hernández-Delgado, Ph.D. – (P.I. 787-449-0566) Coral Reef Ecologist. He will be the project Chief Scientist, and will be in charge of coordinating all of the scientific data collection, statistical analyses, population modeling and results interpretation. He will also coordinate technical training and supervision of research assistants, and will coordinate technical aspects of progress and final reports.
Héctor J. Ruíz-Torres, Ph.D – (P.I. 787-691-7410) Algal Ecologist He will be in charge of coordinating all benthic assessments and studies regarding R. textilis impacts on algal community dynamics and productivity. Will assist in data processing, analysis, interpretation and report drafting.
Samuel E. Suleimán-Ramos, M.A. – (P.I. 939-642-7264) Science Educator, SCUBA diving instructor, President Sociedad Ambiente Marino, and coral farmer. He will be the Project Administrator, and coordinator of all project’s personnel and volunteer research assistants. He will coordinate all coral outplanting efforts, will address all logistics of field work and will assist in data collection and processing. He will coordinate all aspects of grant administration and reporting to Sea Grant
Research assistants. – Several graduate, undergraduate and professional scientists volunteer research assistants will participate in data collection, out-planted coral monitoring, coral recruitment and fish monitoring efforts of the project..
DATA SHARING PLAN
The proposed data sharing plan will be in compliance with NOAA Data Sharing Policy for Grants and Cooperative Agreements – Procedural Directive, Version 2.0
Outreach/education – A minimum of five annual educational seminars will be presented to the general public regarding the outcomes of the project, including at least an annual Sea Grant meeting, and at least an annual meeting with the PRDNER and NOAA-Restoration Center key management personnel to share project’s outcomes, lessons learned, and management-oriented recommendations. Results will also be disseminated using a variety of digital platforms (i.e., http://sampr.org, http://catec.upr.edu), other social media, mass media, etc.
Scientific presentations – Data will also be shared with the local, national and international scientific communities through technical presentations in scientific meetings to discuss results and lessons learned with peers.
Peer-reviewed publications – Data will be widely disseminated with the global scientific community through peer-reviewed publications. Several manuscripts will be produced from this project, preliminary subdivided by the different topics addressed.
Data sharing with users – All data will be accompanied with metadata files and deposited at the UPR’s Center for Applied Tropical Ecology and Conservation (CATEC) data bank (currently under development). Data will be accessed after manuscripts have been published through agreements with the project’s P.I.’s. However, through the project, both SAM’s web page (currently under reconstruction) and CATEC’s web page will exhibit general information about the project, including general information for the general public. In order to comply with the Information Quality Act, data made available to the public by the grantee will be accompanied by the following statement: “These environmental data have not been formally disseminated by NOAA, and do not represent and should not be construed to represent any agency determination, view, or policy”
Sea Grant Funds
Personnel: All personnel will dedicate 6.67% of their time. P.I. (EAH) will receive a $7,600 compensation estimated at $300/day for 16 full days of field work, and at $200/day for 14 full days of data analyses and report drafting. Co-P.I. (HJR) will receive $2,600 at $250/day for 4 full days of field work, and at $200/day for 8 full days of laboratory work and data analyses. Co-P.I. (SSR) will receive $4,800 at $300/day for 16 full days of field work. A field technician (Bernard J. Rosado) assisting in fish censuses will receive $400 at $200/day for 2 days of field work. A second technician (to be named) will assist in multiple field tasks and will receive $2,600 at $200/day for a maximum of 13 days of field work. This will totalize $18,000.
Fringe benefits: None.
Permanent equipment: None
Expendable supplies and equipment: A total of $1,743 will be used for SCUBA tank air fills ($7/tank x 249 tanks). Also, $1,145 will be used in expendable supplies (i.e., underwater paper, underwater slates, coral tags, PVC supplies, fishing lines, plastic-covered wire, masonry nails, ammonium reagents, and other miscellaneous materials. These materials and services will be fundamental to complete the project. This will totalize $2,888.
Travel: This project involves extensive field work at Culebra Island. A total of $16,112 of travel funds is requested as follows: $1,909 for per diem expenses ($23/day x 83 person/days) for project’s personnel during field work (following standard UPR rates), and $6,600 for lodging in Culebra at $79.52/person/day for 83 rental days. This estimate is based on the lowest standard rates available in Culebra. Also, $7,200 will be spent in vessel time at $450/day for 16 days, and $403 will be spent to cover 50% of the mileage of roundtrips between San Juan and Fajardo for one vehicle at 50% of 90 miles/trip x $0.56/mile x 16 trips. All of these expenses will be absolutely critical to carry out the project due to the remote location of the study site and to the lack of meaningful low-cost alternatives for lodging and the total lack of hardware storage facilities in Culebra Island
Other costs: None
Indirect costs: None
Match funds:
Personnel: All personnel will donate in kind a similar amount of their time. P.I. (EAH) will donate $7,600 at $300/day for 16 full days of field work, and at $200/day for 14 full days of data analyses and report drafting. Co-P.I. (HJR) will donate $2,600 at $250/day for 4 full days of field work, and at $200/day for 8 full days of laboratory work and data analyses. Co-P.I. (SSR) will donate $4,800 at $300/day for 16 full days of field work. A field technician (Bernard J. Rosado) assisting in fish censuses will donate $400 at $200/day for 2 days of field work. A second technician (to be named) will assist in multiple field tasks and will donate $2,600 at $200/day for a maximum of 13 days of field work. In addition, two volunteers will participate entirely in kind $6,400 at $200/day each one for 16 days of field work. Time donations will cover all time spent in the complex logistics of planning and traveling for field work to Culebra. This will totalize $24,400
Fringe benefits: None.
Permanent equipment: A total of $14,400 of equipment use will be donated in kind by SAM as follows: $4,000 for 16 days of use of two computers for data processing at $125/day each one, $4,000 for 16 days of use of two high-definition underwater digital cameras for data collection at $125/day each one, and $6,400 for water quality equipment use (turbiditimeter, spectrophotometer, YSI logger, fluorometer) at $100/instrument/day x 4 instruments x 16 days.
Expendable supplies and equipment: A total of $5,976 of equipment use will be donated in kind by SAM as follows: $2,241 for the use of 249 SCUBA tanks at $9/tank/day, $3,735 for SCUBA gear
use for 83 diver/days (both based on standard daily rental rates in Culebra), These will be provided by the grantee in order to complete project’s objectives.
Travel: A total of $403 will be donated in kind to cover 50% of the mileage of roundtrips between San Juan and Fajardo for one vehicle at 50% of 90 miles/trip x $0.56/mile x 16 trips
Other costs: A total of $500 will be donated in kind by the grantee to cover miscellaneous expenses during the development of outreach and educational seminars and other activities.
BUDGET JUSTIFICATION – YEAR 2
Sea Grant Funds
Personnel: All personnel will dedicate 6.82% of their time. P.I. (EAH) will receive a $7,600 compensation estimated at $300/day for 16 full days of field work, and at $200/day for 14 full days of data analyses and report drafting. Co-P.I. (HJR) will receive $2,600 at $250/day for 4 full days of field work, and at $200/day for 8 full days of laboratory work and data analyses. Co-P.I. (SSR) will receive $4,800 at $300/day for 16 full days of field work. A field technician (Bernard J. Rosado) assisting in fish censuses will receive $600 at $200/day for 3 days of field work. A second technician (to be named) will assist in multiple field tasks and will receive $2,600 at $200/day for a maximum of 13 days of field work. This will totalize $18,200
Fringe benefits: None.
Permanent equipment: None
Expendable supplies and equipment: A total of $1,764 will be used for SCUBA tank air fills ($7/tank x 252 tanks). Also, $901 will be used in expendable supplies (i.e., underwater paper, underwater slates, coral tags, PVC supplies, fishing lines, plastic-covered wire, masonry nails, ammonium reagents, and other miscellaneous materials. These materials and services will be fundamental to complete the project. This will totalize $2,665.
Travel: This project involves extensive field work at Culebra Island. A total of $16,135 of travel funds is requested as follows: $1,932 for per diem expenses ($23/day x 84 person/days) for project’s personnel during field work (following standard UPR rates), and $6,600 for lodging in Culebra at $78.57/person/day for 84 rental days. This estimate is based on the lowest standard rates available in Culebra. Also, $7,200 will be spent in vessel time at $450/day for 16 days, and $403 will be spent to cover 50% of the mileage of roundtrips between San Juan and Fajardo for one vehicle at 50% of 90 miles/trip x $0.56/mile x 16 trips. All of these expenses will be absolutely critical to carry out the project due to the remote location of the study site and to the lack of meaningful low-cost alternatives for lodging and the total lack of hardware storage facilities in Culebra Island.
Other costs: None
Indirect costs: None
Match funds:
Personnel: All personnel will donate in kind a similar amount of their time. P.I. (EAH) will donate $7,600 at $300/day for 16 full days of field work, and at $200/day for 14 full days of data analyses and report drafting. Co-P.I. (HJR) will donate $2,600 at $250/day for 4 full days of field work, and at $200/day for 8 full days of laboratory work and data analyses. Co-P.I. (SSR) will donate $4,800 at $300/day for 16 full days of field work. A field technician (Bernard J. Rosado) assisting in fish censuses will donate $600 at $200/day for 3 days of field work. A second technician (to be named)
will assist in multiple field tasks and will donate $2,600 at $200/day for a maximum of 16 days of field work. In addition, two volunteers will participate entirely in kind $6,400 at $200/day each one for 13 days of field work. Time donations will cover all time spent in the complex logistics of planning and traveling for field work to Culebra. This will totalize $24,600
Fringe benefits: None.
Permanent equipment: A total of $14,400 of equipment use will be donated in kind by SAM as follows: $4,000 for 16 days of use of two computers for data processing at $125/day each one, $4,000 for 16 days of use of two high-definition underwater digital cameras for data collection at $125/day each one, and $6,400 for water quality equipment use (turbiditimeter, spectrophotometer, YSI logger, fluorometer) at $100/instrument/day x 4 instruments x 16 days.
Expendable supplies and equipment: A total of $6,048 of equipment use will be donated in kind by SAM as follows: $2,268 for the use of 252 SCUBA tanks at $9/tank/day, $3,780 for SCUBA gear use for 84 diver/days (both based on standard daily rental rates in Culebra), These will be provided by the grantee in order to complete project’s objectives.
Travel: A total of $403 will be donated in kind to cover 50% of the mileage of roundtrips between San Juan and Fajardo for one vehicle at 50% of 90 miles/trip x $0.56/mile x 16 trips
Other costs: A total of $500 will be donated in kind by the grantee to cover miscellaneous expenses during the development of outreach and educational seminars and other activities.
BUDGET JUSTIFICATION – CUMULATIVE*
Sea Grant Funds
Personnel: All personnel will dedicate a mean 6.75% of their time. P.I. (EAH) will receive a $15,200 compensation estimated at $300/day for 32 full days of field work, and at $200/day for 28 full days of data analyses and report drafting. Co-P.I. (HJR) will receive $5,200 at $250/day for 8 full days of field work, and at $200/day for 16 full days of laboratory work and data analyses. CoP.I. (SSR) will receive $9,600 at $300/day for 32 full days of field work. A field technician (Bernard J. Rosado) assisting in fish censuses will receive $1,000 at $200/day for 5 days of field work. A second technician (to be named) will assist in multiple field tasks and will receive $5,200 at $200/day for a maximum of 26 days of field work. This will totalize $36,200
Fringe benefits: None.
Permanent equipment: None.
Expendable supplies and equipment: A total of $3,507 will be used for SCUBA tank air fills ($7/tank x 501 tanks). Also, $2,046 will be used in expendable supplies This will totalize $2,665
Travel: total of $32,247 of travel funds is requested as follows: $3,841 for per diem expenses ($23/day x 167 person/days) for project’s personnel during field work (following standard UPR rates), and $13,200 for lodging in Culebra at a mean of $77.55/person/day for 167 rental days This estimate is based on the lowest standard rates available in Culebra. Also, $14,400 will be spent in vessel time at $450/day for 32 days, and $803 will be spent to cover 50% of the mileage of roundtrips between San Juan and Fajardo for one vehicle at 50% of 90 miles/trip x $0.56/mile x 32 trips.
Other costs: None
Indirect costs: None
Match funds:
Personnel: All personnel will donate in kind a similar amount of their time. P.I. (EAH) will donate $15,200 at $300/day for 32 full days of field work, and at $200/day for 28 full days of data analyses and report drafting. Co-P.I. (HJR) will donate $5,200 at $250/day for 8 full days of field work, and at $200/day for 8 full days of laboratory work and data analyses. Co-P.I. (SSR) will donate $9,600
at $300/day for 32 full days of field work. A field technician (Bernard J. Rosado) assisting in fish censuses will donate $1,000 at $200/day for 5 days of field work. A second technician (to be named) will assist in multiple field tasks and will donate $5,200 at $200/day for a maximum of 26 days of field work. In addition, two volunteers will participate entirely in kind $12,800 at $200/day each one for 32days of field work. Time donations will cover all time spent in the complex logistics of planning and traveling for field work to Culebra. This will totalize $49,000
Fringe benefits: None.
Permanent equipment: A total of $28,800 of equipment use will be donated in kind by SAM as follows: $8,000 for 32 days of use of two computers for data processing at $125/day each one, $8,000 for 32 days of use of two high-definition underwater digital cameras for data collection at $125/day each one, and $12,800 for water quality equipment use (turbiditimeter, spectrophotometer, YSI logger, fluorometer) at $100/instrument/day x 4 instruments x 32 days.
Expendable supplies and equipment: A total of $12,024 of equipment use will be donated in kind by SAM as follows: $4,509 for the use of 501 SCUBA tanks at $9/tank/day, $7,515 for SCUBA gear use for 167 diver/days (both based on standard daily rental rates in Culebra), These will be provided by the grantee in order to complete project’s objectives.
Travel: A total of $806 will be donated in kind to cover 50% of the mileage of roundtrips between San Juan and Fajardo for one vehicle at 50% of 90 miles/trip x $0.56/mile x 16 trips
Other costs: A total of $1,000 will be donated in kind by the grantee to cover miscellaneous expenses during the development of outreach and educational seminars and other activities.
*Detailed calculations and justifications are provided in Years 1 and 2 budget justifications.
REFERENCES
Anderson, M.J., R.N. Gorley, & K.R.Clarke. 2008 PERMANOVA + for PRIMER: Guide to Software and Statistical Methods. PRIMER-E: Plymouth, U.K.
Antonius, A., & E. Ballesteros. 1998. Epizoism: a new threat to coral health in Caribbean reefs. Rev. Biol. Trop. 46:145–156.
Bak, R.P., & Y. Steward-Van Es. 1980. Regeneration of superficial damage in the scleractinian corals Agaricia agaricites f. purpurea and Porites astreoides Bull. Mar. Sci. 30:883-887.
Ballantine, D.L., A. Athanasiadis, & H. Ruiz. 2011. Notes on the benthic marine algae of Puerto Rico. X. Additions to the flora. Bot. Mar 54:293-302.
Ballantine, D.L., & H. Ruiz. 2013. A unique red algal reef formation in Puerto Rico. Coral Reefs doi:10.1007/s00338-013-1016-2.
Barnosky, A.D., E.A. Hadly. J. Bascompte, E.L. Berlow, J.H. Brown, M. Fortelius, W.M. Getz et al. 2012. Approaching a state shift in Earth/'s biosphere. Nature 486:52-58.
Bohnsack JA (1996). Biomass calculation update. Fishes added or estimated after NOAA Technical Memorandum NMFS-SEFC-215. 2 pp Unpubl. MS.
Bohnsack JA, Bannerot SP (1986). A stationary census technique for quantitatively assessing community structure of coral reef fishes. NOAA Technical Report NMFS 41. 15 pp.
Bohnsack JA, Harper DE (1988). Length-weight relationships of selected marine reef fishes from the Southeastern United States and the Caribbean. NOAA Technical Memorandum NMFSSEFC-215. 31pp.
Brook, B.W., E.C. Ellis, M.P. Perring, A.W. Mackay, & Blomqvist, L. (2013). Does the terrestrial biosphere have planetary tipping points?. Trends in ecology & evolution, 28(7), 396-401.
Bruckner AW, Bruckner RJ, Hill R (2008). Improving restoration approaches for Acropora palmata: lessons from the Fortuna Reefer grounding in Puerto Rico. Proc 11th Int Coral Reef Symp 2:1199-1203.
Bruno, J.F., J.J. Stachowicz, & M.D. Bertness. 2003. Inclusion of facilitation into ecological theory. TREE 18:119-125.
Clarke, K.R., & R.M. Warwick 2001. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation. 2nd Ed. PRIMER-E, Ltd., Plymouth Marine Laboratory, UK.
Cloern, J.C. 2001. Our evolving conceptual model of the coastal eutrophication problem. Mar. Ecol. Progr. Ser. 210:223-253.
Davies, K.F., S. Harrison, H.D. Safford, & J.H. Viers. 2007. Productivity alters the scale dependence of the diversity-invasibility relationship. Ecology 88:1940-1947.
Dayton, P.K. 1971. Competition, disturbance, and community organization: the provision and subsequent utilization of space in a rocky intertidal community. Ecol. Monogr. 41:351-389.
Eckrich, C E , & M.S.Engel. 2013. Coral overgrowth by an encrusting red alga (Ramicrusta sp.): a threat to Caribbean reefs? Coral Reefs 32:81-84.
Eckrich, C.E., M.S. Engel, & R.B.J. Peachey. 2010. Crustose, calcareous algal bloom (Ramicrusta sp.) overgrowing scleractinian corals, gorgonians, a hydrocoral, sponges, and other algae in Lac Bay, Bonaire, Dutch Caribbean. Coral Reefs 30:131.
Edmunds, P.J. 2015. A quarter-century demographic analysis of the Caribbean coral, Orbicella annularis, and projections of population size over the next century. Limnol. Oceanogr. 00:1-16 (in press).
Edmunds, P.J., & J.D. Witman. 1991. Effect of Hurricane Hugo on the primary framework of a reef along the south shore of St. John, US Virgin Islands. Mar. Ecol. Progr. Ser 78:201-204.
Edmunds, P.J., & R. Elahi. 2007. The demographics of a 15-year decline in cover of the Caribbean
reef coral Montastraea annularis Ecol. Mongr. 77:3-18.
Gilmore, M.D., & B.R. Hall. 1976. Life history, growth habits, and constructional roles of Acropora cervicornis in the patch reef environment. Journal of Sedimentary Research 46:519-522.
Goldberg, N.A. & G.A. Kendrick. 2004. Effects of island groups, depth, and exposure to ocean waves on subtidal macroalgal assemblages in the Recherche Archipelago, Western Australia. Phycol. Soc. Amer. 40:631-641.
Harnik, P.G., H.K. Lotze, S.C. Anderson, Z.V. Finkel, S.Finnegan, D.R. Lindberg, L. Hsiang Liow, R. Lockwood, C.R. McClain, J.L. McGuire, A. O’Dea, J.M. Pandolfi, C. Simpson, & D.P. Tittensor. 2012. Extinctions in ancient and modern seas. TREE 27: 608-617.
Hawkins, J.P., C.M. Roberts, T. Van'T Hof, K. De Meyer, J. Tratalos, & C. Aldam. 1999. Effects of recreational scuba diving on Caribbean coral and fish communities. Conserv. Biol. 13:888-897.
Hawkins, J.P., & C.M. Roberts. 2004. Effects of artisanal fishing on Caribbean coral reefs. Conservation Biology. 18:215-226.
Hay, M.E. 1984. Patterns of fish and urchin grazing on Caribbean coral reefs: Are previous results typical? Ecology 65:446-454.
Hernández-Delgado, E.A. 2000. Effects of anthropogenic stress gradients in the structure of coral reef epibenthic and fish communities. Ph.D. Dissertation, Department of Biology, University of Puerto Rico, San Juan, P.R. 330 pp.
Hernández-Delgado, E.A., C.M. González-Ramos, & P.J. Alejandro-Camis. 2014a. Large-scale coral recruitment patterns in Mona Island, Puerto Rico: Evidence of shifting coral community trajectory after massive bleaching and mortality Rev. Biol. Trop. 62 (Suppl. 3):49-64.
Hernández-Delgado, E.A., A.E. Mercado-Molina, P.J. Alejandro-Camis, F. Candelas-Sánchez, J.S. Fonseca-Miranda, C.M. González-Ramos, R. Guzmán-Rodríguez, P. Mège, A.A. MontañezAcuña, I. Olivo-Maldonado, A. Otaño-Cruz, & S.E. Suleimán-Ramos. 2014b. Communitybased coral reef rehabilitation in a changing climate: Lessons learned from hurricanes, extreme rainfall, and changing land use impacts. Open J. Ecol. 4:918-944.
Hernández-Pacheco, R., E.A. Hernández-Delgado, & A.M. Sabat. 2011. Demographics of bleaching in the Caribbean reef-building coral Montastraea annularis. Ecosphere 2(1):art9. 113.
Hooper, D.U., F.S. Chapin III, J.J. Ewel, A. Hector, P. Inchausti, S Lavorel, J.H. Lawton, D.M. Lodge, M. Loreau, S. Naeem, B. Schmid, H. Setälä, A.J. Symstad, J.Vandermeer, & D.A.Wardle. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75:3-35.
Hooper, D.U., E.C. Adair, B.J. Cardinale, J.E.K. Byrnes, B.A. Hungate, K.L. Matulich, A. Gonzalez, J.E. Duffy, L. Gamfeldt, & M.I. O’Connor. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486:105-108.
Hughes, T.P., D.C. Reed, & M.J. Boyle. 1987. Herbivory on coral reefs: community structure following mass mortalities of sea urchins. J.Exp. Mar. Biol. Ecol. 113:39-59.
Hughes, T.P, M.J. Rodrigues, D.R. Bellwood, D. Ceccarelli, O. Hoegh-Guldberg, L. McCook, N. Moltschaniwskyj, M.S. Pratchett, R.S. Steneck, & B. Willis. 2007. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Current Biol. 17:360-365.
Hughes, T.P., N.A. Graham, J.B. Jackson, P.J. Mumby, & R.S. Steneck. 2010. Rising to the challenge of sustaining coral reef resilience. TREE 25, 633-642.
Hughes, T.P., S. Carpenter, J. Rockström, M. Scheffer, B. Walker. 2013. Multiscale regime shifts and planetary boundaries. TREE 28:389-395.
Knowlton, N. 1992. Thresholds and multiple stable states in coral reef community dynamics. Am. Zool. 32:674-682.
Knowlton, N. 2004. Multiple “stable” states and the conservation of marine ecosystems. Progr. Oceanogr. 60:387-396.
Knowlton, N., J.C. Lang, & B.D. Keller. 1988. Fates of staghorn coral isolates on hurricanedamaged reefs in Jamaica: the role of predators. Proc. 6th Int. Coral Reef Symp. 2:83-88.
Knowlton, N., & J.B.C. Jackson. 2008. Shifting baselines, local impacts, and global change on coral reefs. PLoS Biology 6.2:e54, 215-220.
Kohler, K.E. & S.M. Gill. 2006. Coral Point Count with Excel estensions (CPCe): A visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comp. & Geosci. 32:1259-1269.
Lessios, H.A., B.D. Kessing, & J.S. Pearse. 2001. Population structure and speciation in tropical seas: global phylogeography of the sea urchin Diadema Evolution 55:955-975.
Levine, J. M. (2000). Species diversity and biological invasions: relating local process to community pattern. Science, 288(5467), 852-854.
Lewis S. 1985. Herbivore abundance and grazing intensity on a Caribbean coral reef. J. Exp. Mar. Biol. Ecol. 87:215-228.
Lirman, D. 2003. A simulation model of the population dynamics of the branching coral Acropora palmata Effects of storm intensity and frequency. Ecological Modelling, 161(3), 169-182.
Lirman, D., & P. Fong. 1997. Patterns of damage to the branching coral Acropora palmata following Hurricane Andrew: damage and survivorship of hurricane-generated asexual recruits. J. Coast. Res. 13:67-72.
Littler, M.M., D.S. Littler, & B.L. Brooks. 2006. Harmful algae on tropical coral reefs: Bottom-up eutrophication and top-down herbivory. Harmful Algae 5:565-585.
Mumby, P.J., & R.S. Steneck. 2008. Coral reef management and conservation in light of rapidly evolving ecological paradigms. TREE 23:555-563.
National Marine Fisheries Service. 2015. Recovery Plan for Elkhorn (Acropora palmata) and Staghorn (A. cervicornis) Corals. Prepared by the Acropora Recovery Team for the National Marine Fisheries Service, Silver Spring, Maryland.
Paine, R.T. 1966. Food web complexity and species diversity. Am. Nat. 100:65-75.
Pimm, S.L. 1984. The complexity and stability of ecosystems. Nature 307:321-326.
Polunin, N.V.C., & C.M. Roberts. 1993. Greater biomass and value of target coral-reef fishes in two small Caribbean marine reserves. Mar. Ecol. Progr. Ser. 100:167-176
Power, M.E., D. Tilman, J.A. Estes, B.A. Menge, W.J. Bond, L.S. Mills, G. Daily, J.C. Castilla, J. Lubchenco, & R.T. Paine. 1996. Challenges in the quest for keystones. BioScience 46:609-620.
Pueschel, C M., & G.W. Saunders. 2009. Ramicrusta textilis sp. nov. (Peyssonneliaceae, Rhodophyta), an anatomically complex Caribbean alga that overgrows coral. Phycologia 48:480-491.
Ruíz-Torres, H.J. 2012. Spatial and temporal variations in the relative abundances of coral reef algae in Southwest Puerto Rico. Ph.D. Dissertation, Department of Marine Sciences, University of Puerto Rico, Mayagüez, P.R. 121 pp.
Stachowicz, J.J., H. Fried, R.W. Osman, & R.B. Whitlatch. 2002. Reconciling pattern and process in marine bioinvasions: how important is diversity in determining community invasibility. Ecology 83:2575-2590.
Stachowicz, J.J., R.B. Whitlatch, & R.W. Osman. 1999. Species diversity and invasion resistance in
a marine ecosystem. Science 286:1577-1579.
Szmant, A.M. 2002. Nutrient enrichment on coral reefs: Is it a major cause of coral reef decline? Estuaries 25:743-766.
Tilman, D. 1996. Biodiversity: population versus ecosystem stability. Ecology 77:350-363.
Tilman, D. 1999. The ecological consequences of changes in biodiversity: A search for general principles. Ecology 80:1455-1474.
Tunnicliffe, V. 1981. Breakage and propagation of the stony coral Acropora cervicornis. Proc. Natl. Acad. Sci 78:2427-2431
Tunnicliffe, V. 1983. Caribbean staghorn coral populations: pre-Hurricane Allen conditions in Discovery Bay, Jamaica. Bull. Mar. Sci. 33:132-151.
Verlaque, M., E. Ballesteros, & A. Antonius. 2000. Metapeyssonnelia corallepida sp. nov. (Peyssonneliaceae, Rhodophyta), an Atlantic encrusting red alga overgrowing corals. Bota. Mar. 43:191-200.
Worm, B., E.B. Barbier, N. Beaumont, J.E. Duffy, C. Folke, B.S. Halpern, J.B.C. Jackson, H.K. Lotze, F. Micheli, S.R. Palumbi, E. Sala, K.A. Selkoe, J.J. Stachowicz, & R, Watson. 2006. Impacts of biodiversity loss on ocean ecosystem services. Science 314:787-790.
SITE MAP 1. Proposed study site at the western limits of Arrecife Los Corchos system, Culebra Island, P.R.
PROJECT MILESTONE CHART*
Specific task –2016-2017
Month
Coral
Demography tissue regreneration x
Deliverables
Database, mgmt. recommendations, report; Population model parametrization
Preliminary database, model outputs, mgmt. recommendations, report
Algal contact experiments 2 None yet, just setting up the baseline experiments
Algal dynamics and biodiversity 2 1 1 Database, mgmt. recommendations, report
Algal growth and recolonization rates 1 None yet, just setting up the baseline experiments
Coral recruit counts 1
Database, mgmt. recommendations, report Fish/benthic monitoring
Benthic monitoring
Database, mgmt. recommendations, report
Database, mgmt. recommendations, report WQ
Database, mgmt. recommendations, report Outreach/educ
Web page, reports, presentations, press, peer reviewed papers, seminars Specific task –
Month
Algal contact experiments
Demography contact experiments
Algal growth and recolonization rates
Coral recruit counts
Deliverables
Database, mgmt. recommendations, report; Population model parametrization
Preliminary database, model outputs, mgmt. recommendations, report
mgmt. recommendations, report
Database, mgmt. recommendations, report
Database, mgmt. recommendations, report
monitoring
Benthic monitoring 1 1 1
WQ monitoring 1 1 1 1 1 1 1
Outreach/educ x x x x x x x x x x x x
Database, mgmt. recommendations, report
Database, mgmt. recommendations, report
Web page, reports, presentations, press, peer reviewed papers, seminars
*=Numbers in charts represent number of field days per task (some task being overlapped with others)
LIST OF CURRENT OR PENDING RESEARCH SUPPORT
Current support
Sea Grant College Program, Omnibus Proposal Program – Ecosystem-level impacts of community-based coral reef rehabilitation in light of rapidly evolving ecological paradigms (20152016). (Co-investigator with Samuel E. Suleimán-Ramos ($59,994).
NOAA Coral Reef Conservation Program – Addressing land based sources of pollution and restoring reefs through coral farming in Culebra, Puerto Rico (2014-2015). Co-investigator with Roberto Viqueira (Protectores de Cuencas, Inc.) and Paul Sturm (Ridge to Reefs, Inc.) ($69,704).
Pending support
NOAA, Habitat Blueprint Program – Building resiliency in the Puerto Rico Northeast Reserves by reducing recreational impacts, addressing land-based sources of pollution (LBSPs), and restoring coral reef habitats (2015-2017). Co-Investigator with Roberto Viqueira-Ríos and Samuel E. Suleimán-Ramos ($468,979).
University of Puerto Rico, Office of the President, Seed Money Program – Actual status of Endangered Species Act (ESA)-listed corals across the Mona Island shelf, Puerto Rico (2015). (Principal Investigator) ($14,046),
University of Puerto Rico, Office of the President, Seed Money Program – Conservation and rehabilitation of Endangered Species Act (ESA)-listed coral species in Puerto Rico: A modeling approach (2015-2017). (Principal Investigator) ($363,572).
University of Puerto Rico, Center for Applied Tropical Ecology and Conservation, Coral Reef Research Group, PO Box 23360, San Juan, PR 00931-3360
http://upr.academia.edu/EdwinHernandez, http://catec.upr.edu edwin.hernandezdelgado@gmail.com; edwin.hernandez13@upr.edu
Education
B.Sc. – Marine Biology, University of Puerto Rico-Humacao (1988)
M.Sc. – Environmental Microbiology, University of Puerto Rico-Río Piedras (1992)
Ph.D. – Tropical Biology (Coral Reef Ecology), University of Puerto Rico-Río Piedras (2000)
Post-Doc. – Diseased Coral Molecular Microbiology, University of Puerto Rico-Río Piedras (2004-2005)
Appointments
Position, Institution
Research Fellow, Center for Applied Tropical Ecology and Conservation, UPR-RP, 2007-present
Affiliate Researcher/Lecturer, Department of Biology, UPR-RP, 2005-present
Lecturer, Department of Biology, UPR-RP, 2004-present
Post-Doctoral Researcher, PR-NSF-EPSCoR Program, Resource Center for Science & Engineering, UPR, 2004-05
Contractor, Environmental Defense, Miami, FL, 2003-2004
Contractor, Culebra Conservation and Development Authority, Culebra,PR, 2003-2004
Assistant Professor. Department of Biology, UPR-RP, 2001-2003
Contractor, U.S. Coral Reef Initiative, Dept. Natural and Environmental Resources, San Juan, PR, 2000-2003
Research Associate/Lecturer. Department of Biology, UPR-RP, 1999-2000
Lecturer, Universidad del Turabo, Department of Science and Technology, Gurabo, PR, 1997-2000
Publications since 2014
Ramos-Scharrón, C., D. Torres-Pulliza, & E.A. Hernández-Delgado 2015. Watershed- and island-scale land cover changes in Puerto Rico (1930s-2004) and their potential effects on coral reef ecosystems. Science of the Total Environment 506-507:241-251.
Hernández Delgado E.A., A. Montañez-Acuña, A. Otaño-Cruz, & S.E. Suleimán-Ramos. 2014. Bomb-cratered coral reefs in Puerto Rico, the untold story about a novel habitat: From reef destruction to community-based ecological rehabilitation. Rev. Biol. Trop. 62 (Suppl. 3):183-200. http://dx.doi.org/10.15517/rbt.v62i0.15913
Hernández-Delgado, E.A., C.M. González-Ramos, & P.J. Alejandro-Camis. 2014. Large-scale coral recruitment patterns in Mona Island, Puerto Rico: Evidence of shifting coral community trajectory after massive bleaching and mortality. Rev. Biol. Trop. 62 (Suppl. 3):49-64. http://dx.doi.org/10.15517/rbt.v62i0.15901
Schärer-Umpierre, M., D. Mateos-Molina, R. Appeldoorn, I. Bejarano, E.A. Hernández-Delgado, R. Nemeth, M. Nemeth, M. ValdésPizzini, & T. Smith. 2014. Marine managed areas and associated fisheries in the US Caribbean. Adv. Mar. Biol. 69:129-152. http://dx.doi.org/10.1016/B978-0-12-800214-8.00004-9
Hernández-Delgado, E.A., M. Shivlani, & A.M. Sabat. 2014. Ecosystem-based and community-based model integration to designate no-take marine protected areas: A case study from Puerto Rico Natural Resources 5(10):538-560. http://dx.doi.org/10.4236/nr.2014.510049
Hernández-Delgado, E.A., A.E. Mercado-Molina, P.J. Alejandro-Camis, F. Candelas-Sánchez, J.S. Fonseca-Miranda, C.M. GonzálezRamos, R. Guzmán-Rodríguez, P. Mège, A.A. Montañez-Acuña, I. Olivo-Maldonado, A. Otaño-Cruz, & S.E. Suleimán-Ramos. 2014. Community-based coral reef rehabilitation in a changing climate: Lessons learned from hurricanes, extreme rainfall, and changing land use impacts. Open J. Ecol. 4(14):918-944. http://dx.doi.org/10.4236/oje.2014.414077
Hernández-Delgado, E.A., & S.E. Suleimán-Ramos. 2014. E.S.A. coral species listing: A roadblock to community-based engagement in coral reef conservation and rehabilitation across the U.S. Caribbean? Reef Encounter 29(1):11-15. http://www.sgmeet.com/isrs/membership/files/reef-encounter-29-1-feb2014.pdf#page=11 Díaz-Ortega, G., & E.A. Hernández-Delgado. 2014. Land-based source pollution in a climate of change: A roadblock to the conservation and recovery of Elkhorn coral Acropora palmata (Lamarck 1816). Natural Resources 5(10):561-581. http://dx.doi.org/10.4236/nr.2014.510050
PO Box 22158 San Juan PR 00931,
EDUCATION
- University of Phoenix, Guaynabo, PR
- M.B.A., Technology Management
- University of Puerto Rico, San Juan, PR
- B.A., Secondary Education, Mayor: Biology
- Minors: Generals Sciences, Education in Technology, Psychology, Recreation and Military Sciences.
APPOINTMENTS
1999 - Present - Sociedad Ambiente Marino - Founder, Director Direct and coordinate of conservation oriented and research activities. Proposal development for projects and community services. Budget and personnel administrator. Train volunteer for: Coastal Clean-ups, Coral Surveys, Fish Population Surveys, Coral farming, Reef Rehabilitation, Environmental mitigation and other activities for the conservation of marine environment.
1994 – 1997 - Morale Welfare and Recreation (MWR) Sabana Seca Naval Base - SCUBA Instructor Coordinate and certify SCUBA Courses
1992 - Present - Ambiente Marino, Video Production - Founder Underwater Professional Video and Photo maker
1990-Present - University of Puerto Rico, San Juan, PR - Educational Production Director Director and coordinator audiovisuals, graphics, reproductions services and activities. Evaluate, select and contract personnel to perform audiovisuals, graphics and reproductions services. Train personnel in the use and performance of the software, computer, network and technology.
CERTIFICATIONS
2001 REEF Fish survey instructor, The Reef Environmental Education Foundation - REEF 2000 RECON instructor, The Ocean Conservancy (formerly Center for Marine Conservation) 1994 PADI Open Water SCUBA Instructor, (OWSI)
1985 Auxiliary Emergency Medical Technical, University of Puerto Rico, Medical Science Campus
1976 Coast Guard Auxiliary Boat Certification
Publications since 2011
Hernández Delgado E.A., A. Montañez-Acuña, A. Otaño-Cruz, & S.E. Suleimán-Ramos. 2014. Bomb-cratered coral reefs in Puerto Rico, the untold story about a novel habitat: From reef destruction to community-based ecological rehabilitation. Rev. Biol. Trop. 62 (Suppl. 3):183-200. http://dx.doi.org/10.15517/rbt.v62i0.15913
Hernández-Delgado, E.A., A.E. Mercado-Molina, P.J. Alejandro-Camis, F. Candelas-Sánchez, J.S. Fonseca-Miranda, C.M. GonzálezRamos, R. Guzmán-Rodríguez, P. Mège, A.A. Montañez-Acuña, I. Olivo-Maldonado, A. Otaño-Cruz, & S.E. Suleimán-Ramos 2014. Community-based coral reef rehabilitation in a changing climate: Lessons learned from hurricanes, extreme rainfall, and changing land use impacts. Open J. Ecol. 4(14):918-944. http://dx.doi.org/10.4236/oje.2014.414077
Hernández-Delgado, E.A., & S.E. Suleimán-Ramos 2014. E.S.A. coral species listing: A roadblock to community-based engagement in coral reef conservation and rehabilitation across the U.S. Caribbean? Reef Encounter 29(1):11-15. http://www.sgmeet.com/isrs/membership/files/reef-encounter-29-1-feb2014.pdf#page=11
Mercado Molina, A., E.A. Hernández Delgado, J.E. Rivera Rivera, M. Rivera Rivera, S.E. Suleimán Ramos, I. Olivo Maldonado, J.S. Fonseca Miranda, y E.A. Rodríguez Inoa, 2013. Protocolo para la propagación y la restauración de poblaciones del coral Cuerno de ciervo, Acropora cervicornis: Estrategias de bajo costo de la Sociedad Ambiente Marino. Tech. Rept. submitted to The Nature Conservancy, Alexandria, VA.
Hernández-Delgado, E.A., S. Suleimán, I. Olivo, J. Fonseca, y M.A. Lucking. 2011. Alternativas de baja tecnología para la rehabilitación de los arrecifes de coral. 178-186. En, J. Seguinot-Barbosa (ed.), Islas en Extinción: Impactos Ambientales en las Islas de Puerto Rico. Ediciones SM, Cataño, PR 255 pp.
Appendix 1 – Support letters