Report
Project Title: Genetic structure and diversity of bottlenose dolphin, Tursiops truncatus, off Puerto Rico
Principal Invetsigator: Grisel Rodriguez
Co-Principal Investigator: Nikolaos Schizas, PhD
Associate Investigator: Richard Appeldoorn, PhD
Methods used:
The distribution of bottlenose dolphins was determined by conducting boat-based surveys in both coastal and offshore waters of Puerto Rico. Surveys included skin and fecal sampling, photo identification of dorsal fins and analysis of dolphin behavior. Collection permits were approved.
Genetic analysis
Skin samples collected were analyzed to determine genetic diversity (mitochondrial DNA) and sex determination (nuclear DNA). Five samples of stranded Tursiops of known sex (after the autopsy examination) were used as controls, to determine efficacy of the process.
Extraction
DNA was isolated using a commercial DNA extraction kit and the manufacturers’ instructions (DNeasy Tissue kit, Qiagen Inc., Valencia, California).
Sex determination
Gender was determined with a pattern of DNA bands amplified with the primers Zfx/Zfy and Sry (Bérubé and Palsbøll 1996) Reactions consisted of 2 µl template DNA, a 5 mm MgCl2, 0.2 mM dNTPs, 0.2 µM of primers. The amplification profile was 7 min at 95 C, then 37 cycles of 30 s at 94 °C, 30 s at 47 °C, and 30 s at 72 °C. A final 2 min elongation was performed at 72 °C, and reactions were then held at 4 °C. Amplification products were visualized using gel electrophoresis in a 1%.
MtDNA
The primers MtCRf and MtCRr (Hoezel et al. 1998) were used to amplify the control region of mitochondrial DNA. This region should provide enough genetic variability to distinguish between coastal and pelagic ecotypes. PCR conditions were 100µM dNTP, 1.5 mM MgCL2, 10 mM Tris HCL pH 8.0, 50 mM KCl, 200 nM of primer and 0.02 µl of Taq Polymerase. PCR profile was 4 min at 95°C, 35 cycles of 45 sec at 94°C, 1.5 min at 50 °C, 1.5 min at 72°C and 8 min at 72 °C.
Results and findings
The grant period of November 1, 2015 – April 15. 2016 was dedicated to sample analysis. A total of fifty (50) more samples from stranded dolphins were obtained from the Caribbean Stranding Network. This samples go back from 1937-2006.
We performed more analysis on the samples collected during in both field seasons with different primers to determine the presence of inshore and offshore ecotypes on the samples. The DNA amplified patterns determined unequivocally the gender of 20 dolphins (13 males and 7 female), which were sampled during the summer 2014 (Figure 1).
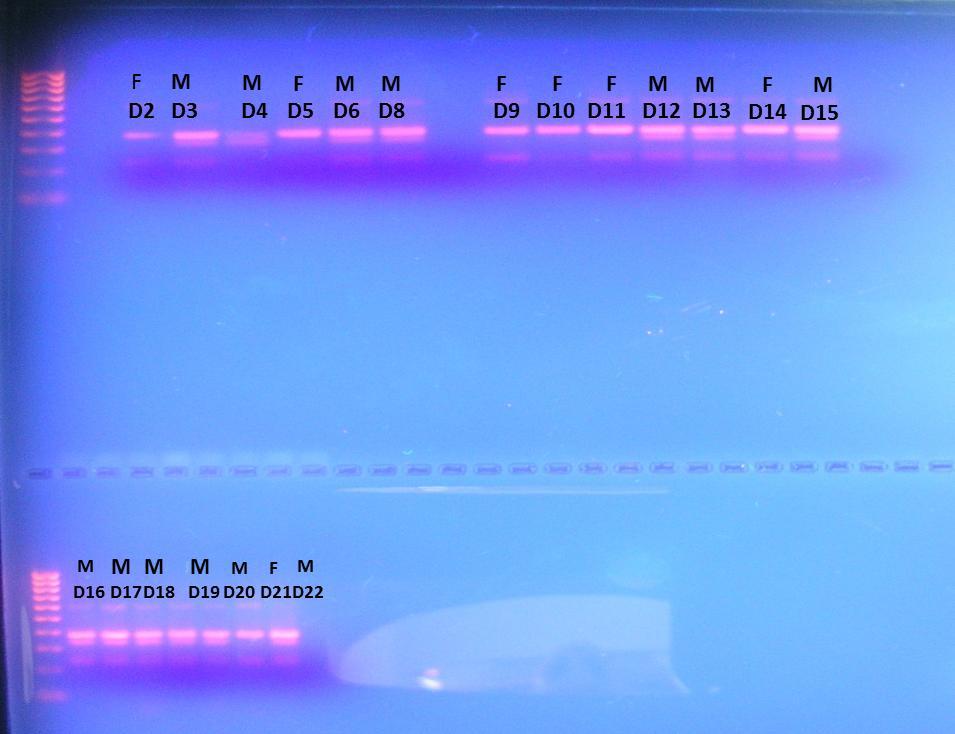
Figure 1. Sex determination using amplified bands of DNA for male (M) and female (F) using the Zfx/Zfy and Sry primers
Objectives (List them and indicate whether accomplished or not and why)
The differentiation between coastal and offshore bottlenose dolphin ecotypes
Skin sample biopsy- This task was initiated in summer 2014. PI Rodriguez contacted Dr. Keith Mullin from the NOAA Mississippi Laboratory and he agreed to send the biopsy sampler, Ms. Carrie Sinclair, from NOAA Mississippi Laboratory for two weeks. We were able to sample 10 dolphins during her visit (summer 2014). PI Rodriguez participated in the Mote Marine Laboratory Bottlenose Biopsy Training Workshop (April 13-April 18, 2015). A new regulation from NOAA established that any new biopsy sampler needs a mentor before a collection permit can be granted. To complete the biopsy sampling of the dolphins we contracted the services of Aaron Barleycorn from the Chicago Zoological Society and trainer of the attended workshop. The biopsy sampling took place from October 19 to October 30 2015 with 19 samples collected from Añasco, Cabo Rojo, Lajas and Ponce.
Fecal sampling- The task of getting fecal sampling from live individuals has not been successful The main reason is that the weather conditions during dolphin encounter have not being ideal for a diver to get in the water or even to be able to spot the feces as the material is liquefied and in
rough seas is hard to spot. We will use the two collected samples from dead animals to explore the possibility of using the fecal material as a source for dolphin DNA in addition to surveying the microbial communities of the feces.
Photo identification of dorsal fins- the database was completed and the results will be publish on a peer reviewed publication.
Future Plans
November-May 2017
• Sample analysis-this task includes the last step of the analysis which is the sequencing and the SNP’s analysis.
• Publish findings on photoidentification and distribution
• Finish DNA analysis, and work on the publications
• Dissertation
Other products (Include copies of guides, websites, maps and CDs) www.mamiferosmarinospr.com- A blog with information on marine mammal sightings and on information about the research itself was established for the Puerto Rico community. PI Rodriguez is the owner and author of the blog.
Before the list of students supported, indicated below, please provide information for the following metrics table.
-List students supported
During the period October 1 2015 to September 30, 2016 no assistantships and or students worked on the project.
-List presentations, technical reports and special awards. Oral presentation at the RIEMMCCA: Network of Aquatic Mammal Specialists of Central America and the Caribbean December 12, 2015 San Francisco California: Rodriguez-Ferrer G., Schizas N. and Apeldoorn R. Genetic structure and diversity of the bottlenose dolphin Tursiops truncatus, preliminary results.
Poster presentation A poster, Rodriguez-Ferrer G., Schizas N. and Apeldoorn R. Genetic structure and diversity of the bottlenose dolphin Tursiops truncatus with preliminary results of
the survey were presented and the Caricoos General Assembly, Club Nautico, San Juan, March 20, 2015.
-List PI’s supported
In addition to the students indicated above, we provide a breakdown of time and effort attributed to PI’s, Co-PI’s and associates (% time dedicated to project and amounts paid SG/Match during the report period, partial or full year)
