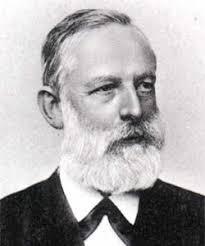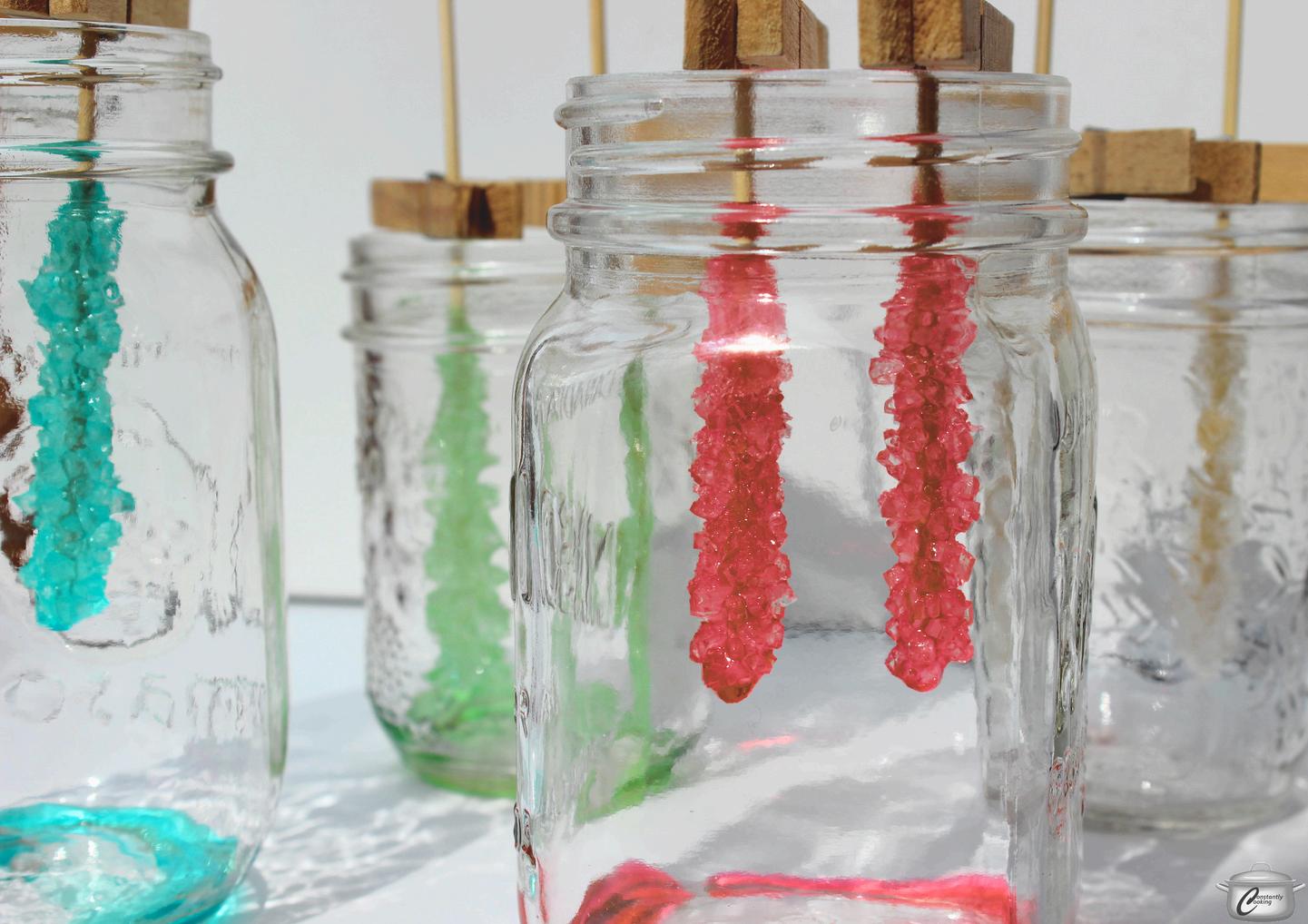VOL. 3, 2025-2026
Pioneer of the Periodic Law
He independently discovered the periodic relationship of elements.
Visualized Atomic Volume Trends
He plotted atomic volume vs. atomic weight, revealing periodic patterns.
Advanced Atomic Theory
He contributed to understanding atomic weights and their relationship to element properties.
CMS MAGAZINE - THIRD SCIENTIST:
OCTOBER 2025
AUTHORS
Ella
MillieLee
SoominLim
HahjinKim
DESIGNERS
DahyeLim
PhilipIm
ErinKang
Science
&Curiosity
Puzzles
Chemistryisoftensaddledwitha reputation as a difficult subject, reserved only for scientists in white lab coats In reality, it permeates every aspect of our lives.Itispresentinthefoodwe eat, the air we breathe, and the materials we use daily At its heart, chemistry helps us understand the fundamental components of matter and the interactions that allow substances to transform, combine, and create the world around us. This magazine is designed to make chemistry engaging and accessible for everyone, regardless of prior knowledge.
Our goal is to show that chemistry is not only relevant but also enjoyable to explore In thisinauguralissue,wespotlight Julius Lothar Meyer, a figure often overshadowed in the history of chemistry While Dmitri Mendeleev is widely celebrated for developing the periodic table, Meyer independently formulated strikingly similar ideas at the same time. This magazine is designed to make chemistry engaging and accessible for everyone, regardless of prior knowledge.
Hisresearchontherelationships between atomic weights and elemental properties provided crucial insights that shaped the periodic system we rely on today.Withouthiscontributions, the patterns and connections of theperiodictablemightnothave come into focus so clearly. We also introduce Science in the Home, a section dedicated to simple, safe experiments using everyday materials such as lemon juice, baking soda, and salt
Why?
Julius Lothar Meyer was a 19th-century German chemist whose work significantly advanced the development of the periodic table. Working contemporaneously with Dmitri Mendeleev, Meyer helped establish the systematic organization of the elements and provided crucial theoretical insights that shaped modern chemical knowledge (Science History Institute, n.d.).
His most important contribution was the classification of elements by atomic weight, through which he demonstrated that chemical properties recur at regular intervals—a phenomenon now known as periodicity. In 1870, Meyer published a graph plotting atomic volume against atomic mass, offering one of the earliest and clearest visual proofs of periodic trends. This work provided strong empirical evidence that elements form part of a natural order (Britannica, 2025).
While Mendeleev is often credited for predicting undiscovered elements, Meyer’s emphasis on rigorous, data-driven analysis was equally vital. His cautious empiricism complemented Mendeleev’s more intuitive approach, and together their work secured the acceptance of the periodic system (Science History Institute, n.d.). Meyer’s recognition of atomic volume as linked to atomic mass also anticipated later developments in atomic theory and quantum chemistry (LibreTexts, 2023). His legacy lies not only in co-developing the periodic table but also in his enduring influence on the theoretical foundations of chemistry.

Meyer's approach to understanding elemental properties differed markedly from the speculative tendencies that characterised -nineteenth-century chemistry. Rather than relying solely on theoretical frameworks, he meticulously compiled and analysed experimental data concerning atomic weights and physical properties of known elements. His systematic methodology involved creating detailed tables and graphs that revealed relationships previously obscured by the sheer volume of disparate information. Through this painstaking process, Meyer began to discern recurring patterns in properties such as atomic volume and valency that suggested an underlying order to the seemingly chaotic array of chemical elements (Guharay, 2021)
Unlike many of his contemporaries who focused primarily on chemical reactivity, Meyer recognised that physical properties offered equally valuable insights into elemental behaviour. His groundbreaking work on atomic volumes demonstrated that when elements were arranged according to increasing atomic weight, their atomic volumes exhibited a periodic variation This discovery revealed that elements with similar chemical properties occupied analogous positions in the periodic sequence, suggesting that atomic weight served as a fundamental organising principle for the entire system of elements. (Lagowiski, 2006) His graphical representation of atomic volume versus atomic weight produced a curve with distinct peaks and valleys, providing visual evidence for the periodic nature of elemental properties.

The theoretical implications of Meyer's discoveries extended far beyond mere classification. His recognition of periodic trends suggested that elemental properties were not arbitrary characteristics but rather manifestations of deeper structural principles governing atomic architecture This insight challenged prevailing notions about the fundamental nature of matter and pointed toward the existence of underlying patterns that could predict the properties of undiscovered elements. Meyer's work demonstrated that systematic analysis of empirical data could reveal natural laws governing chemical behaviour, establishing a methodology that would influence generations of chemists.
Fig Meyer’s First table of elements
LET'S TALK ABOUT ELEMENTS
MEET HELIUM, CARBON, AND XENON!
NOBLE GAS
The chemical element helium has the atomic number 2 and the symbol He It is the first monatomic gas in the periodic table's noble gas and is colorless, odorless, non-toxic, and inert Among all the elements, it has the lowest boiling point and no melting point at normal pressures.
The Periodic Table is a collection of the chemical elements arranged in order of atomic number There are 118 known elements, with 92 of those elements found in nature. They are sorted based on their atomic weight, symbols, density, etc
NONMETAL
With the atomic number 6 and the symbol C, carbon is a chemical element It is nonmetallic and tetravalent, which means that because its valence shell has four electrons, its atoms can form up to four covalent bonds It is a member of the periodic table's group 14 Approximately 0.025 percent of the Earth's crust is composed of carbon
NOBLE GAS
With the atomic number 54 and the symbol Xe, Xenon is a chemical element. Traces of this noble gas, which is thick, colorless, and odorless, can be found in the Earth's atmosphere
CARBON
XENON
HOW TO OBTAIN: Xenon can be extracted from liquefied air- it is rare but can be found in the atmosphere at a concentration of about 1 part per 11 5 million
DETAILS: The radioactive isotope Xenon-135, which can be obtained by beta decay of Iodine135 (which is formed by nuclear fission)- is used to absorb neutrons in nuclear reactors
QUITE RANDOM: The metallic solid state of xenon is sky blue, wheras Ionized xenon gas is blue-violet, while the usual gas and liquid are colorless
Approximately one-fifth of your body is composed of carbon. All life forms are composed of carbon, which produces a vast array of different chemicals, including sugars and fats Carbon is the building block of all living things By weight, carbon makes up around 19% of the human body It can also be inorganic; it forms large portions of the non-living world, like as rocks and minerals, by combining with oxygen and other chemicals
HELIUM
Helium is the second most prevalent element and accounts for almost 24% of the universe's mass!
The Greek word for sun, helios, is where the name "helium" originates! Because Helium atoms are so light, they can defy gravity on Earth
The radioactive decay of uranium and thorium produces the helium that is purchased in cylinders, similar to the ones you use to inflate your balloons!
Strainer or paper towel HOMEMADE
PLASTIC (CASEIN FROM MILK)
What you need:
Warm milk
Vinegar
Spoon
What to do:
1. Heat the milk (not boiling).
2. Add vinegar and stir, clumps will form.
3. Strain the lumps and press them together into a shape.
4. Let dry for a few days, it turns into plastic! Chemistry: Acid causes casein protein in milk to precipitate out, a primitive plastic!
ROCK CANDY CRYSTAL (SUPERSATURATED SOLUTIONS)
Homemade Plastic (Casein):
Milk contains casein, a protein suspended in liquid. Adding vinegar (acid) lowers the pH of the milk.
The acid causes casein to denature and clump togeth called precipitation.
The clumps can be molded and left to dry, forming a hard, plasticlike material.
⟶ This is how people used to make early plastics before petroleumbased ones—casein plastic!
Rock Candy Crystal Chemistry:
Heat dissolves more sugar in water than usual, creating a supersaturated lution.
the solution cools, it becomes unstable and ready to form crystals.
gar molecules stick to a string or stick (called nucleation), starting stal growth.
er time, more sugar builds up in an ordered pattern, forming large stals.
This process is an example of crystallization from a supersaturated solution.
Science Buddies. (n.d.). Milk into plastic. Retrieved from Donovan and L, A. (2025) Antoine Lavoisier | Biography, Discoveries, & Facts.
Lagowski, J. (n.d.). Lothar Meyer. In Encyclopædia Britannica. Retrieved from Science History Institute (2024) Antoine-Laurent Lavoisier | Science History Institute.
Lagowiski,J.“LotharMeyer.”Encyclopædia Britannica,EncyclopædiaBritannica,inc , www britannica com/biography/Lothar-Meyer
SpringerNature(Boeck&Rocke,Eds ) (2022) LotharMeyer:Moderntheoriesandpathwaysto periodicity(ClassicTextsintheSciences). BirkhäuserCham.https://doi.org/10.1007/978-3030-78342-6
RoyalSocietyofChemistry.(n.d.).Developmentofthe periodictable/History:JuliusLotharMeyer Retrieved fromDonovanandL,A (2025b)AntoineLavoisier| Biography,Discoveries,&Facts
EncyclopædiaBritannica (2024,June27) Lothar Meyer EncyclopædiaBritannica,Inc Retrievedfrom ThecreationofthePeriodicTable|Chem13News Magazine(2021)
Lagowiski,J “LotharMeyer ”Encyclopædia Britannica,EncyclopædiaBritannica,inc , www.britannica.com/biography/Lothar-Meyer.
Boeck,G (2019) JuliusLothar(von)Meyer(18301895)andthePeriodicSystem Substantia,3(2), 13-25 https://doiorg/1013128/Substantia-503
Developmentoftheperiodictable(nodateb). https://periodic-table rsc org/history/about? utm source