International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056


International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Varadaraj S1, Shreeprakash B1, B.M. Praveen2, Bharath K. Devendra2,3
1 Department of Mechanical Engineering, Srinivas University, Institute of Engineering & Technology, Mukka, Mangaluru, Karnataka, 574146, India.
2 Department of Chemistry, Srinivas University, Institute of Engineering & Technology, Mukka, Mangaluru, Karnataka, 574146, India.
3Department of Chemistry, M. S. Ramaiah College of Arts, Science and Commerce, MSR Nagar, MSRIT Post, Bengaluru, Karnataka, 560054, India. ***
Abstract - Foralongtime,environmentalprotectionofmetalliccomponentshasbecomeapressingconcernfortheengineering andmanufacturingindustries.Coatingtechnologyhasgainedprominencetomeettheneedsofindustrialdemands.Thecoatingis usedtoprotectthemetalsandensuretheproduct'sperformanceforalongtime.Amongothercoatingprocessessuchasthermal spray, spark plasma sintering, and chemical vapour deposition, the electrodeposition process has proven to be the most costeffectiveandsimple.ThepaperdiscussesthepropertiesofvariousNickelcompositeelectrodepositedProtectivecoatings.
Keywords:ProtectiveCoatings,Electrodeposition,Nickelcomposites.
Electrodepositionisoneofthemostsuccessfulmethodforcoatingmetalduetoitsuncomplicatedandconsistentprocesswhich has unique advantages in the microstructure and property modification of the depositions.The components, phases, and microstructures of the deposits can be regulated by modifying the electrodeposition parameters, such as the deposition potential,currentdensity,electrolytecomposition,PHvalue,temperature,etc[1].Duetoflawslikewear,corrosion,andfatigue, alotofmachinepartsfail.Thecharacteristicsliketribological,mechanical,andcorrosionresistanceareboostedbycoatingthe reinforcingmaterialonit[2].Therecommendedmethodisnickelplatingbecauseitisrenownedforitsconsistentplating thicknessontheplatedsurfaceandtheeasinesswithwhichintricatecomponentscanbecoated.Italsopossessesexceptional levelsof hardnessandcorrosion resistance[3].Theanti-wear qualitiesof the nickel coating have expanded theirrange of possibleusesfordies,tools,andworkingpartsforcarsandothervehicles.Withtheavailableone,thespectrumofalloysmight beexpandedforfunctionalpurposes.Bymodifyingthecompositecoatingonthematerials,itisfeasibletoavoidusingpricey heattreatmentproceduresonstandardalloys.[4].Theelectro-chemicalprocessisimportantinthefieldofnanotechnology.The co-depositionofcompositematerialparticlesdispersionandthepresenceoftheseparticlesinthecoatingareaccomplished usingelectrolytebathscontainingmicron/sub-micron-sizedparticles.Thetypeofcompositecoatingmaterialparticlesand theirinclusiondeterminethequalitiesofthecoatingmaterial.[5][6].
Electrodepositionprocesscanaccomplishthereductionofthedesiredmetal'scationsbeforetheyaredepositedonthesurfaceof aconductingsubstrate.Twoconductiveelectrodesareusedinthecoatingsetup,andtheyaresubmergedinanelectrolytebath. Theworking-cathodeorelectrodeisthoughttobeasingleterminalconnectedtothenegativeterminal.Onemoreisreferredto asacounter-electrodeoranodeandisattachedtothepositiveterminal.Thecationstraveltothecathode,aredischarged,and thendepositasametalliclayerwhenanexternalelectricfieldisintroduced.Electrodepositionistheidealtechniqueforachieving a smooth and ideal contact between the matrix metal and ceramic particles. [7][8]. Electrodeposition, often known as electroplating, isa technique for electrolytically depositing a coatingon a substratethat is immersedin an electrolyte. An aqueous medium at room temperature can be used for this. Aqueous solution electroplating refers to the method of electroplatingaqueoussolutionsatroomtemperature.usingafused-saltsolution,alsoknownaselectroplating,orinahightemperature fusion salt. One of the major drawbacks of electrodeposition is the inability to obtain consistency in coating thickness. Other limitations of the substrate include hydrogen ion assessment and essential surface preparation [9]. Electrodepositioniswidelyutilisedforaestheticcoatingsaswellascorrosionandwearresistance.Itisalsousedinspecialised applications including high temperature resistance, biomedical, and ceramic coating by increasing the electrode potential [10].Electrodepositioncanproducemetallicsystemswithallofthesedimensionalities,whilethreedimensionalsystemshavethe largestvarietyofapplications[11].Bothdirect(DC)andpulsecurrentcanbeusedintheelectrodepositionprocess(PC).The
Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN: 2395-0072 © 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page160
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN: 2395-0072
coatingcanbeappliedtothesubstrate'ssurfacetoimprovesolderability,lubricatingcharacteristics,electricalconductivity, corrosionresistance,wearandthermalresistance[12].Deeprecessesandirregularlyshapedgeometriescanbedepositedupon usingelectrodepositionwithlesssophisticatedplatingconditionsandequipment[13].
Compositecoatingisanewtypeofcoatingdevelopedbymodernexperts.Normally,severaltypesofcompositematerialsare useddependingonthematrix.Examplesofthesematerialscompositesincludeceramicmatrixcompositesandmetalmatrix composites. Composites include things like carbon matrix composites and polymer matrix composites [14]. Metal matrix compositecoatingisoneofthemostwidelyutilisedintheindustryduetoitsefficiency[15].MMCcoatings,whichcombinethe ceramicphase'shighhardnessandstrengthwiththematrix'ssoundtoughness,arefrequentlyutilisedforsurfacerepairand strengtheningofengineeringmetalcomponents.Theinclusionofreinforcingparticlescanbedoneintwoways.Duetothelow wetting capabilities of the ceramic and metallic phases, particle-matrix bonding is fundamentally difficult with external additionorexsitu.Theceramicreinforcingphaseofthecoatingiscreatedbyanin-situchemicalreactionbetweenelementsof the precursor material at a very high temperature, followed by in situ nucleation and growth.These hard particles are consequentlymorethermodynamicallystableandfinerthanhardparticlesinjectedfromtheoutside.Therearemultiplesorts and designs of composite coatings. Composite coatings of different forms, shapes, sizes, and quality can be produced by choosingtherightceramicandmatrixcoatingmaterials,procedures,andtechnologies[16].Al,Fe,Mg,Ti,Ni,BeandCoare someofthecommonlyusedmaterialsformetalmatrixcomposites[17.18].Metalmatrixcomposites'(MMC)propertiesare generally determined by their composition and structure [19]. To maximize coating characteristics, a homogeneous distributionandlargenumberofparticlesparticipatinginthemetalmatrixarerequired[20,21].Enhancedcoatingperformance isoftencausedbymodificationstothegrowthmodeorgrainsizeofthemetalmatrix.[21,22].Thesize,diversity,andshapeof theimplantedparticles,aswellascurrentdensity,pH,electrolyteagitation,andbathcomposition,arealldependentfactors thataffectparticleincorporation[23].Duetotheenormouspotentialfordevelopingmaterialswithspecialisedfeatures,MMCs havefounduseinavarietyoffields[13].TheNi-basedMMCcoatingisoneofthemostchallengingandinterestingmetalmatrix coatings[15].Manycompositecoatingshavebeenextensivelyelectrodeposited,includingNi-SiC,Al203,Tio2,Zro2,Si3N4,Mo,Cr, and others [8].Nickel-based composite coatings have opened up new possibilities because of their exceptional wear and corrosionresistanceaswellastheirhighhardness[15].Ontribologicalparametersincludingcoefficientoffriction,hardness, roughness,andwearrate,variousalloyingelementsandhardparticlesincorporatedinthenickelmatrixhaveavarietyof effects.Thesefactorscancausethevalueofcharacteristicstoriseorfall[24]
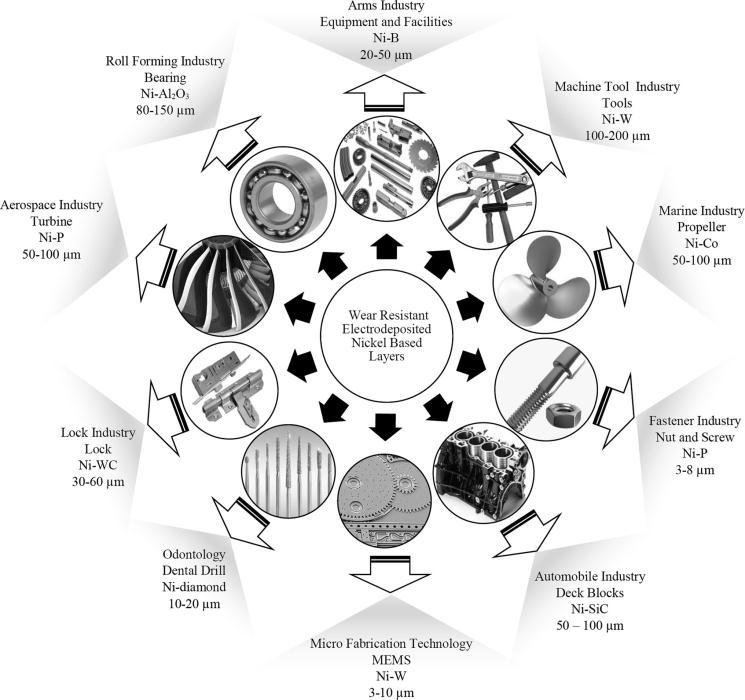
Fig -1:ApplicationsofElectrodepositedNickelcoating[24]
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN: 2395-0072
Numerous chemical, mechanical, and electrical processes require nickel and alloys including nickel phosphorous, nickel tungsten,nickelcobalt,andothers.Theimportanceofcompositecoatingswithsuperiorwearresistanceisrisingalongwiththe demandforlongerindustrialcomponentservicelives[25].TheapplicationsofnickelcompositecoatingsareshowninFig.1, whichrangefromthemaritimesectortothelockindustry.Theadditionoftheparticlesraisesthecoating'shardnessandwear resistancewhilealsoloweringitscoefficientoffriction.Amongotherengineeringcomponents,anickel-basedcoatingisapplied tocuttingtools,turbineblades,rollers,plungers,rollingmillrolls,extruders,pistonheads,androds[15].
Mohanreddyetal.[26]studiedtheelectrodepositionprocedureusedtomakeaNi-Si3N4 coating.Morecorrosionresistance wasseeninNi–Si3N4 nanocompositescontaining3g/LSi3N4nanoparticlesinthebathsolution.TheperformanceofNi-Si3N4 nanocompositespreparedinthepresenceofSDSwassubparincomparisontoNi-Si3N4nanocompositespreparedwithout SDS.Withouttheuseofasurfactant,Ni-Si3N4nanocompositeshavehighercorrosionresistanceandparticleinclusion.Inthe investigation,about9%ofthenanoparticlesweredepositedinthecoating.
DRashmietal.[27]investigatedtheelectrofabricationofnanostructuredNi-Fealloycoatingsonmildsteelusingasulphate bath.Forthecoating,thecompositionoftheelectrolytic bathandcurrentdensitywereadjusted.EnergydispersiveX-ray spectroscopyhasbeenusedtoidentifythecompositionoftheNi-Fealloy.Thecoatingsareuniqueinkind.Testsforcorrosion usingpotentiodynamicpolarisationandelectrochemicalimpedanceSpectroscopictechniquesindicatethat4Adm-2isthe lowestcorrosionrateinasolutionof3.5percentNaCl.TheVickershardnesstestandatomicforcemicroscopywereusedto measurethecoatings'hardnessandroughness,respectively.Thesurfacemorphologyofthecoatingswasexaminedusing scanningelectronmicroscopy.X-raydiffractionwasusedtocalculateandanalysethetexturecoefficient,phasestructure,and crystallitegrainsizeofthecoating.
RobertaLeeetal.[28]investigatedtheelectrodepositionofniobiumoxidefromavarietyofaqueousandnon-aqueoussolvent systems using sol-gel processing techniques. The process depends on protons and hydroxide ions being produced electrochemicallybyadjustingtheelectrochemicalpHofthesolution.Innon-aqueoussolutionscontainingtertiaryniobium alkoxides,two-electronreductionresultsinhydroxideions.Inaqueousalkalinesystemscontainingniobate,waterundergoes electrochemical oxidation, which results in a pH drop. Niobium oxide and mixed niobate are created as the niobate sol destabilisesandanelectrodecoatingforms.
BapuGretal.[29]studiedthedispersion-strengthenednickelwasgeneratedutilisingtheelectro-codepositionmethodusinga nickelfluoborateelectrolytecontainingvanadiumpentoxide(V2O5)particlesinsolution.Theeffectsofparticlesize,particle concentrationinthe bath,current density, pH, and temperatureonthevolume percent(vol.percent)integrationofV2O5 particlesinthecompositewereinvestigated.Investigationandcomparisonofthecomposite'scorrosionbehaviourina5% sodiumchloride(NaCl)solutionwithmildsteelandnickeldepositswerealsoconducted.ThepercentageofV2O5particlesin thecompositeincreasedwithrisingV2O5contentinthebathandrisingcurrentdensity.Thebath'soperationat6.0ampdm-2, pH3.0,and50"CproducedthebestincorporationofV2O5(12.6vol.percent).Accordingtocorrosiontesting,thecomposite prevents steel from corroding when there is NaCl present. In a NaCl solution with a pH of 3.0, the composites' corrosion resistanceisweakertothatofnickeldeposits,butinaNaClsolutionwithapHof6.5,theyoffercomparabledefence.
Thepreparationoftheelectrolyticbathwiththerightproportionisverymuchessentialfortheelectrodepositionquality.The nickelelectrodepositionmainlyconsistsofNickelsulphate,NickelChlorideand Boricacidinitselectrolyticsolution.The relatedparticlesiezinc,graphene,ironetcaretobeaddedwithrequiredproportion fornickelcompositecoatingalongwith theelectrolyticsolution.Theelectrolyticcompositiondataofthevariouscompositecoatingsaregiveninthetable.
SDS(SodiumDo-DecylSulphate),ananionicsurfactant,hasbeenshowntoboostthecoating'shardnessandimprove itsadherencetothematrix[30].Thecorrosionresistancehasincreasedandtheparticledistributionhasbecomemoreuniform duetotheadditionofmoreSDS,asurfactant,totheelectrolyticsolutionforNi-Alumina[31].Thecompositionandoperational parametersofvariousNickelcompositecoatingsarelistedinTable1.
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN: 2395-0072
Table1- Compositionandparametersofelectrolyticbath
Composite Coating NiSo4.6H2O (Nickel Sulpate) (gL-1)
NiCl2.6H20 (Nickel Chloride)(gL1)
H3 B03(gL1)
Particles (gL-1) pH Temperature (ₒC) Current Density (A dm-2)
Time (min) Reference
Nickel–graphene 85-100 12to15 25-35 0.2 3-4 45±5ₒC 5 60 [32]
NickelGraphene 330 35 40 0.1 3.5 51±1ₒC 5 55 [33]
NickelGraphene 300 50 40 0.5,0.25,0.1 4±2 45±5ₒC 5 50-60 [34]
NickelGraphene 26.26 56.81 18.54 0.1 3.8-4 40ₒC 5.66 50 [35]
NickelGraphene 110-115 18-25 35-45 0.2,0.4 3-4 50ₒC 5 30 [36]
NickelGraphene oxide
NickelReduced Graphene oxide
240 45 30 0.1(GO) 3.54.5 55-60 ₒC 6to10 65 [37]
250 35 35 4.37 3-4 55ₒC 3.5 12h [38]
NickelGraphene 300 35 40 0.05 4±1 50ₒC 0.15-4 1-3 hours [39]
NickelGraphene 95-110 15-20 30-40 0.2 3-4 50-55 ₒC 5 50 [40]
NickelVanadium pentoxide
280 5 40 5to50 1-5 30-70 ₒC 2to10 8h [29]
NickelSi3N4 27 57 19 3 3 40ₒC 5 60 [41]
AsperthevariousliteraturedatabeinglistedoutintheTable1itisunderstoodthathightemperatureistheprerequisitefor electrodepositing nickel composite coating. Temperature ranging from 50-60оC is required fornickel composite electrodeposition.ThePHistobemaintainedbetween3-4andanaveragecurrentdensityof5-6A/dm-2 istobemaintained duringtheprocess.Mostoftheresearchershavecarriedouttheelectrodepositionworkfor60mins.
Thecommonlymeasuredresultparametersoftheelectrodepositedcoatingsaresurfacemorphology,Corrosionpotential, Roughness,MicrohardnessandContactanglemeasurements.ThevariousresultsofNickelcompositesarelistedinTable2.
Coating Composition XRD /EDAX Analysis
Ni-Si3N4
Peaksdetectedfor Ni,Si3N4 not detecteddueto lower concentration
Corrosion Potential Microhardness SEM Analysis AFM Analysis Ref
Improvedcorrosion resistanceobserved withcurrentof 0.00812A/cm2 .
value:
Increased hardnessvalue observedwith about400HV
Moreuniform grainstructure isobserved
Hill-valleylike structurewith homogenous distributionofgrains [26]
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN: 2395-0072
Ni-Co-CeO2
AdditionofCeO2 influencedthe growthofthe crystallites
Corrosionresistace increasedwiththe additionofCeO2
Withthe additionofCeO2, thecomposite coating's microhardness enhanced..
Polyhedral crystalsto nodularcrystals havebeen observed NA [52]
Theresultsofthevariousnickelcompositeelectrodepositioncoatingshavebeenpresentedintable2.Itwasfoundfromthe literaturesthatmildsteelwasthemostcommonlyusedsubstrateanditiswidelyusedmaterialinindustries,andhenceitis pronetocorrosion.Themechanicalproperties,surfacecharacteristicsandcorrosionpotentialshavebeenlistedformostofthe varietiesofnickelcompositecoating.Researchershavediscoveredthebettersurfacepropertiesandcorrosionresistance whichcouldpavethepathforwidespreadusageintheindustrialandcommercialusage.
Tafel extrapolation method and AC impedance method are the most commonly used corrosion resistance measurements methods.Tafelextrapolationmethodusespotentialexcitationintheformofdirectcurrenttogivelargerandappreciable currentstomeasurethecorrosionrate,Sincethecurrentandpotentialarenonlinear,semilogarithmicplotsisobtainedcalled asTafelPlots.Utilizingalternatingcurrentpotentialexcitation,theACimpedancemeasuringapproachofferscrucialdetailson thecapacitive behaviourof thedoublelayerthatcontributestothecoatings'corrosionresistance.Itoffers detailsonthe polarityandquantityofchargespresentattheelectrodeandelectrolyte,whichareeasilycomprehendedbyexaminingthe impedancecurves.
AllofthestudiesdescribedthatinclusionofparticleslikeGraphene,Niobium,Vanadium,TitaniumOxide,Zincandboronwith nickelbyelectrodepositionhasprovedtoprovideenhancedcorrosionresistanceandmechanicalcharacteristics.Themajority oftheresearchershavenotfocusedonthelowerelectrolyteconcentrationoftheparticles,furtherthemechanicalproperties likemicrohardness,scratchresistance,roughness,profileandcontactanglearebarelyunexplored.
Nickelisincreasinglyusedinmanynewfields,puttingitincompetitionwithothermaterialsduetoitsspecialcombinationof qualities and traits and its many industrial applications. This has led to the development of innovative nickel processing methods. The review paper provides a comprehensive overview of the overall elements of electrodeposition of nickel composite coating. Nickel Composite coating have been vastly researched with various combination of the particles. The inclusionofnanoparticlesinthecoatingexhibitimprovedpropertiesintermsofroughness,microhardness,andcorrosion resistancewithanuniformdistributionoftheparticles.Theelectrodepositionoftheseparticlesatlowerconcentrationswith nickelanditsimpactoncorrosionandmechanicalpropertiesmustbeexploredinthecurrentstudy.Theresearcherscanstart workingontheanalysiswiththehelpofthispaper.
[1]Y.H.Ahmad,A.M.A.Mohamed,(2014).Electrodepositionofnanostructurednickel-ceramiccompositecoatings:areview,Int. J.Electrochem.Sc.91942-1963.
[2]RaghavendraCR,BasavarajappaS,SogaladI,(2016).ElectrodepositionofNi–Al2O3nanocompositecoatingandevaluation ofwearcharacteristics.IOPSci.https://doi.org/10.1088/1757-899x/149/1/012110
[3] Smrithi,S.P.,Kottam,N.,&Vergis,B.R.(2022).HeteroatommodifiedhybridcarbonquantumdotsderivedfromCucurbita pepoforthevisiblelightdrivenphotocatalyticdyedegradation. TopicsinCatalysis,1-12.https://doi.org/10.1007/s11244022-01581-x
[4] Casati, R., & Vedani, M. (2014). Metal matrix composites reinforced by nano-particles a review. Metals, 4(1), 65-83. https://doi.org/10.3390/met4010065
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN: 2395-0072
[5] Aruna, S. T., Bindu,C.N.,Selvi,V. E.,Grips, V. W., & Rajam,K. S.(2006).Synthesisand propertiesof electrodeposited Ni/ceria nanocomposite coatings. Surface and Coatings Technology, 200(24), 6871-6880. https://doi.org/10.1016/j.surfcoat.2005.10.035
[6]Zhou,Y.,Zhang,H.,&Qian,B.(2007).Frictionandwearpropertiesoftheco-depositedNi–SiCnanocomposite coating. Appliedsurfacescience, 253(20),8335-8339.https://doi.org/10.1016/j.apsusc.2007.04.047
[7]Yang,Y.,&Cheng,Y.F.(2013).MechanisticaspectsofelectrodepositionofNi–Co–SiCcompositenano-coatingoncarbon steel. ElectrochimicaActa, 109,638-644.https://doi.org/10.1016/j.surfcoat.2005.10.035
[8]Torabinejad,V.,Rouhaghdam,A.S.,Aliofkhazraei,M.,&Allahyarzadeh,M.H.(2016).ElectrodepositionofNi–FeandNi–Fe(nano Al2O3) multilayer coatings. Journal of Alloys and Compounds, 657, 526-536. https://doi.org/10.1016/j.jallcom.2015.10.154
[9]Davis, J. R. (Ed.). (2001). Surface engineering for corrosion and wear resistance. ASM international. https://doi.org/10.31399/asm.tb.secwr.9781627083157
[10]Fotovvati,B.,Namdari,N.,&Dehghanghadikolaei,A.(2019).Oncoatingtechniquesforsurfaceprotection:Areview. Journal ofManufacturingandMaterialsprocessing, 3(1),28.https://doi.org/10.3390/jmmp3010028
[11]Bicelli,L.P.,Bozzini,B.,Mele,C.,&D’Urzo,L.(2008).Areviewofnanostructuralaspectsofmetalelectrodeposition. Int.J. Electrochem.Sci, 3(4),356-408.https://doi.org/10.1149/1.1869976
[12] Chandrasekar, M. S., &Pushpavanam, M. (2008). Pulse and pulse reverse plating Conceptual, advantages and applications. ElectrochimicaActa, 53(8),3313-3322.https://doi.org/10.1016/j.electacta.2007.11.054
[13] Chaudhari, A. K., & Singh, V. B. (2018). A review of fundamental aspects, characterization and applications of electrodeposited nanocrystalline iron group metals, Ni-Fe alloy and oxide ceramics reinforced nanocomposite coatings. JournalofAlloysandCompounds, 751,194-214.https://doi.org/10.1016/j.jallcom.2018.04.090
[14]Park,S.J.,&Seo,M.K.(2011). Interfacescienceandcomposites (Vol.18).AcademicPress.https://doi.org/10.1016/b978-012-375049-5.00007-4
[15]Karmakar,R.,Maji,P.,&Ghosh,S.K.(2021).Areviewonthenickelbasedmetalmatrixcompositecoating. Metalsand MaterialsInternational, 27(7),2134-2145.https://doi.org/10.1007/s12540-020-00872-w
[16]Reddy,R.M.,Praveen,B.M.,&Alhadhrami,A.(2021).Characterization&electrochemicalbehaviorofDC&PCmethodsof electrodepositedNi-TiO2-CNTsnanocompositecoatings. PhysicaScripta,96(12),125719.https://doi.org/10.1088/14024896/ac33fa
[17]Smrithi,S.P.,Kottam,N.,Narula,A.,Madhu,G.M.,Mohammed,R.,&Agilan,R.(2022).Carbondotsdecoratedcadmium sulphideheterojunction-nanospheresfortheenhancedvisiblelightdrivenphotocatalyticdyedegradationandhydrogen generation. JournalofColloidandInterfaceScience, 627,956-968.https://doi.org/10.1016/j.jcis.2022.07.100
[18]Maji,P.,Ghosh,S.K.,Nath,R.K.,&Karmakar,R.(2020).Microstructural,mechanicalandwearcharacteristicsofaluminum matrix composites fabricated by friction stir processing. Journal of the Brazilian Society of Mechanical Sciences and Engineering, 42(4),1-24.https://doi.org/10.1007/s40430-020-02279-5
[19]Rathod,M.R.,Rajappa,S.K.,Minagalavar,R.L.,Praveen,B.M.,Bharath,D.K.,&A.A.Kittur.(2022).InvestigationofAfrican mangosteenleavesextractasanenvironment-friendlyinhibitorforlowcarbonsteelin0.5MH2SO4, InorganicChemistry Communications, 109488.https://doi.org/10.1016/j.inoche.2022.109488
[20]Garcia,I.,&Conde,A.(2003).G.langelaan,J.fransaer,JPCelis,Improvedcorrosionresistancethroughmicrostructural modifications induced by codepositing SiC-particles with electrolytic nickel. CorrosSci, 45, 11731189.https://doi.org/10.1016/s0010-938x(02)00220-2
[21]Bund,A.,&Thiemig,D.(2007).InfluenceofbathcompositionandpHontheelectrocodepositionofaluminananoparticles andnickel. SurfaceandCoatingsTechnology, 201(16-17),7092-7099.https://doi.org/10.1016/j.surfcoat.2007.01.010
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN: 2395-0072
[22]Zimmerman,A.F.,Clark,D.G.,Aust,K.T.,&Erb,U.(2002).PulseelectrodepositionofNi–SiCnanocomposite. Materials letters, 52(1-2),85-90https://doi.org/10.1016/s0167-577x(01)00371-8.
[23]Thiemig,D.,Lange,R.,&Bund,A.(2007).Influenceofpulseplatingparametersontheelectrocodepositionofmatrixmetal nanocomposites. ElectrochimicaActa, 52(25),7362-7371.https://doi.org/10.1016/j.electacta.2007.06.009
[24]Mahidashti,Z.,Aliofkhazraei,M.,&Lotfi,N.(2018).Reviewofnickel-basedelectrodepositedtribo-coatings. Transactionsof theIndianInstituteofMetals, 71(2),257-295.https://doi.org/10.1007/s12666-017-1175-x
[25] Lekka, M., Bonora, P. L., Lanzutti, A., Benoni, S., Caoduro, P., &Fedrizzi, L. (2012). Industrialization of Ni- SiC electrodeposition on copper moulds for steel continuous casting. La MetallurgiaItaliana.https://doi.org/10.1016/j.surfcoat.2012.03.016
[26]Mohan Reddy,R.,Praveen,B.M., Kumar, P.,& Venkatesha,T.V.(2017).Ni–Si3N4:Electrodeposition,propertiesand corrosion behavior. Surface Engineering and Applied Electrochemistry, 53(3), 258264.https://doi.org/10.3103/s1068375517030127
[27]Rashmi,D.,Pavithra,G.P.,Praveen,B.M.,&Devapal,D.(2020).DevelopmentofNanostructuredNi–FeAlloyCoatings; Characterisation and Corrosion Analysis. Surface Engineering and Applied Electrochemistry, 56(1), 4654.https://doi.org/10.3103/s1068375520010135
[28]RobertáLee,G.(1996).Studiesontheelectrochemicaldepositionofniobiumoxide. JournalofMaterialsChemistry, 6(2), 187-192.https://doi.org/10.1039/jm9960600187
[29] Bapu, G. R., & Yusuf, M. M. (1993). Electrodeposition of nickel-vanadium pentoxide composite and its corrosion behaviour. MaterialsChemistryandPhysics, 36(1-2),134-138.https://doi.org/10.1016/0254-0584(93)90020-m
[30]Devendra,B.K.,Praveen,B.M.,Tripathi,V.S.,Nagaraju,G.,Nagaraju,D.H.,&Nayana,K.O.(2021).HighlyCorrosion Resistant Platinum-Rhodium alloy coating and its photocatalytic activity. Inorganic Chemistry Communications, 134, 109065.https://doi.org/10.1016/j.inoche.2021.109065
[31]Sabri,M.,Sarabi,A.A.,&Kondelo,S.M.N.(2012).Theeffectofsodiumdodecylsulfatesurfactantontheelectrodeposition of Ni-alumina composite coatings. Materials Chemistry and Physics, 136(2-3), 566569.https://doi.org/10.1016/j.matchemphys.2012.07.027
[32]Yasin,G.,Arif,M.,Nizam,M.N.,Shakeel,M.,Khan,M.A.,Khan,W.Q.,&Zuo,Y.(2018).Effectofsurfactantconcentrationin electrolyteonthefabricationandpropertiesofnickel-graphenenanocompositecoatingsynthesizedbyelectrochemical co-deposition. RSCadvances, 8(36),20039-20047.https://doi.org/10.1039/c7ra13651j
[33] Devendra, B. K., Praveen, B. M., Tripathi, V. S., Kumar, H. P., &Chethana, K. R. (2022). The development of platinumrhodiumalloycoatingsonSS304usingapulse/directelectrodepositiontechniqueandtheirapplicationtoantibacterial activity. JournaloftheIndianChemicalSociety, 99(6),100466.https://doi.org/10.1016/j.jics.2022.100466
[34] Algul,H.,Tokur,M.,Ozcan,S.,Uysal,M.,Çetinkaya,T.,Akbulut,H.,&Alp,A.(2015).Theeffectofgraphenecontentand sliding speed on the wear mechanism of nickel–graphene nanocomposites. Applied Surface Science, 359, 340-348. https://doi.org/10.1016/j.apsusc.2015.10.139
[35]Siddaiah,A.,Kumar,P.,Henderson,A.,Misra,M.,&Menezes,P.L.(2019).Surfaceenergyandtribologyofelectrodeposited NiandNi–graphenecoatingsonsteel. Lubricants, 7(10),87.https://doi.org/10.3390/lubricants7100087
[36] Yasin, G., Khan, M. A., Arif, M., Shakeel, M., Hassan, T. M., Khan, W. Q., ...&Zuo, Y. (2018). Synthesis of spheres-like Ni/graphenenanocompositeasanefficientanti-corrosivecoating;effectofgraphenecontentonitsmorphologyand mechanicalproperties. JournalofAlloysandCompounds, 755,79-88.https://doi.org/10.1016/j.jallcom.2018.04.321
[37]Singh,S.,Samanta,S.,Das,A.K.,&Sahoo,R.R.(2018).TribologicalinvestigationofNi-grapheneoxidecompositecoating producedbypulsedelectrodeposition. SurfacesandInterfaces, 12,61-70.https://doi.org/10.1016/j.surfin.2018.05.001
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056 Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN: 2395-0072

[38]Devendra,B.K.,&PraveenBM.(2019).AReviewonNobleMetalsinControllingIntergranularStressCorrosion CrackinginBWRs. InternationalJournalofAppliedEngineeringandManagementLetters(IJAEML), 3 (1):53-59. https://zenodo.org/record/2653464.
[39]Ren,Z.,Meng,N.,Shehzad,K.,Xu,Y.,Qu,S.,Yu,B.,&Luo,J.K.(2015).Mechanicalpropertiesofnickel-graphenecomposites synthesized by electrochemical deposition. Nanotechnology, 26(6), 065706. https://doi.org/10.1088/09574484/26/6/065706
[40]Jabbar,A.,Yasin,G.,Khan,W.Q.,Anwar,M.Y.,Korai,R.M.,Nizam,M.N.,&Muhyodin,G.(2018).Electrochemicaldeposition of nickel graphene composite coatings: effect of deposition temperature on its surface morphology and corrosion resistance,RSCAdv.7(2017)31100–31109.https://doi.org/10.1039/c6ra28755g
[41]Reddy,R.M.,Praveen,B.M.,Chandrappa,K.G.,&Nayana,K.O.(2016).GenerationofNi–Si3N4nanocompositesbyDC,PC and PRC electrodeposition methods. Surface Engineering, 32(7), 501-507. https://doi.org/10.1080/02670844.2016.1148323
[42] Fratari, R. Q., & Robin, A. (2006). Production and characterization of electrolytic nickel–niobium composite coatings. SurfaceandCoatingsTechnology, 200(12-13),4082-4090.https://doi.org/10.1016/j.surfcoat.2005.02.168
[43]Kumar,C.P.,Venkatesha,T.V.,&Shabadi,R.(2013).PreparationandcorrosionbehaviorofNiandNi–graphenecomposite coatings. MaterialsResearchBulletin, 48(4),1477-1483.https://doi.org/10.1016/j.materresbull.2012.12.064
[44]Gavrila,M.,Millet,J.P.,Mazille,H.,Marchandise, D.,&Cuntz,J.M.(2000).Corrosionbehaviourofzinc–nickelcoatings, electrodeposited on steel. Surface and coatings technology, 123(2-3), 164-172.https://doi.org/10.1016/s02578972(99)00455-7
[45]Kumar,C.M.P.,Lakshmikanthan,A.,Chandrashekarappa,M.P.G.,Pimenov,D.Y.,&Giasin,K.(2021).Electrodeposition based preparation of Zn–Ni alloy and Zn–Ni–WC nano-composite coatings for corrosion-resistant applications. Coatings, 11(6),712.https://doi.org/10.3390/coatings11060712
[46]Parida,G.,Chaira,D.,Chopkar,M.,&Basu,A.(2011).SynthesisandcharacterizationofNi-TiO2compositecoatingsby electro-co-deposition. Surface and Coatings Technology, 205(21-22), 48714879.https://doi.org/10.1016/j.surfcoat.2011.04.102
[47]Chen,W.,&Gao,W.(2010).Sol-enhancedelectroplatingofnanostructuredNi–TiO2compositecoatings theeffectsofsol concentration on the mechanical and corrosion properties. ElectrochimicaActa, 55(22),68656871https://doi.org/10.1016/j.electacta.2010.05.079
[48]Gana,A.,BenTemam,H.,Lekmine,F.,Naoun,M.,&Herzallah,O.(2021).CharacterizationofelectrodepositedNi-MoS2 compositecoatingsundertheinfluenceofcurrentdensity. DigestJournalofNanomaterials&Biostructures(DJNB), 16(3). https://doi.org/10.15251/djnb
[49]Akhtar,K.,Khalid,H.,UlHaq,I.,Zubair,N.,UllahKhan,Z.,&Hussain,A.(2017).Tribologicalpropertiesofelectrodeposited Ni–Co3O4nanocompositecoatingonsteelsubstrate. JournalofTribology, 139(6).https://doi.org/10.1115/1.4036450
[50] Shakoor, R. A., Kahraman, R., Waware, U., Wang, Y., &Gao, W. (2014). Properties of electrodeposited Ni–B–Al2O3 compositecoatings. Materials&Design, 64,127-135.https://doi.org/10.1016/j.matdes.2014.07.026
[51]ManoharRathod,Rajappa,S.K.,Praveen,B.M.,&Bharath,D.K.(2021).InvestigationofDolichandraunguis-catileaves extractasacorrosioninhibitorformildsteelinacidmedium. CurrentResearchinGreen.AndSustainableChemistry 4, 100113.https://doi.org/10.1016/j.crgsc.2021.100113
[52]Srivastava,M.,Grips,V.W.,&Rajam,K.S.(2010).ElectrodepositionofNi–Cocompositescontainingnano-CeO2and theirstructure,properties. AppliedSurfaceScience, 257(3),717-722.https://doi.org/10.1016/j.apsusc.2010.07.046