International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN:2395-0072

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN:2395-0072
Dept. of Civil Engineering, P G programme in Environmental Engineering, Bapuji Instituteof Engineering and technology, Davangere – 577 004, Karnataka India. ***
Thoughindustriesencourageeconomyofthecountry,thecontaminationproducedby themhastobemeasuredin ordertoprotecttheecologicalsystemfromdegradation.Thecurrentworkhasbeenpointedfortheremovalandreduction of chemical oxygen demand (COD) and total dissolved solids (TDS) of the distillery industries liquid waste using natural adsorbentindividually.About2g,4g,8gand10gofBagasseand commercialactivatedcarbonwereusedseparatelyandin combinations. The batch studies reported that maximum removal COD, BOD, Chloride and Sulphate of 91.4%, 98.5%, 98.4%and91.4%wereremovedusing10gofBagassefor1day.ForBagassehasbeenreportedthatthedosageof10ghas resultedinfourvariablesandthestatisticalappraisalrevealeda positivecorrelationbetweendecreaseandthetime.
Key words: Bagasse,liquidwaste,biomethanated,CODandBOD
The chief sources of water pollution are domestic, industrial, farming, thermal and radioactive liquid waste (Gaur 1997).Surfacewateroriginatesindirectcontactwiththe atmosphere,periodical streams,gulliesandsurfacegutters. So, their presence exchanges of dissolved and atmospheric gases while the liquid wastes are introduced through water passages. The industries, which promote much to water contamination, are chiefly industries like pulp and paper, distilleries, oil refineries, pharmaceutical, textile, paint industry, dairy, and flour mills etc. (Kudesia 1994). Liquid waste entered from distilleries is extremelycolored and toxic to aquatic species in receiving waters. Contamination of water sourceswithcolor,CODandTDSisnotdesired,astheyareaestheticallyoffensive.TheBSW isalsopreventingreoxygenation in entering waters by controlling off sunlight infiltration. Along with these also most of the liquid wastes as coloring substances are toxic to aquatic species. Existing industrial technology for BSW liquid waste treatment such as progressive oxidation techniques, electro-chemical reduction, etc. may be effective for the removal of COD and TDS but theirprimarilyandworkingcostsareveryhigh.\
Industrialliquidwastemanagementisoneofthechiefsignificantecologicalproblemstoday’sworldmanufacturing and processing industries generate a variety of liquid waste pollutants treatment is problematic to switch and costly (Kadam,2012).Additionally,therequirementforwaterandtheproductionofliquidwasteisenhancingquickly,whilethe discharge of liquid waste is quiet a main issue faced by number of production industriesmightbethedifficultyand massivevolumeofliquidwastewithverysmallspacefortreatmentanddischarge(Chopra,2012).
Thedevelopmentofadsorbentfromproductionandmanufacturingindustryby-productis a probable exploration area since; it is commercially activated carbon is costly. BagasseFly ash is by-product from the Energy production industryinthesugarfactory.UsageofBagasseFlyashasanadsorbentfortheliquidwastetreatmentisevidencedtobean operativeandgoodphenomenon(Kumar,etal.,2014andKushwaha,etal.,2010).
BagasseFlyashisa solidwastetothe ecologicallyaffectscreate enormousissuein sugarindustry. Theattempt of paying of this by-product as an adsorbent for treatment for liquid waste, bagasse fly ash may be best solution for the ecology and perfect exercise of liquid waste to resources transformation. A variety of researches appraised so far on adsorption treatment of liquid waste have been enlightened on either to appraise the efficiency of the treatment. However, in the current work, adsorption kinetics is estimated during treatment of distillery spent wash (DSW). Therefore, the opinion that the work is to appraise the physical chemical variables of the DSW and its treatment using activatedBagasseFlayash.
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

The DSW was collected from a Distillery industry. The physico-chemical variables likeTemperature, pH and electrical conductivity (EC), Total Solids, Nitrate, Phosphate, COD, BOD, Chloride and Sulphate measured as per the standard methods for the estimation ofwater and waste-water (APHA, 1998). Sample collected for a period of three month(DSW-1,DSW-2andDSW-3)are1st,2nd and3rd monthofsampling, correspondingly.ButCPCBrefersthemaximum permissiblelimitofDSWtobedischarged.Eachvalueofthevariabledescribedasaveragevalue±standarddeviationatn= 3.
Batchtypeexperiments:About100mLofthesamplewastakeninseparateconical flasks.Accurately2,4,8and10g of Bagasse and commercial activated carbon were added individually and in varied combination. After mixture of adsorbents the flasks were kept for 30 minutes to 1 hour, then filtrate will be separated. The separated filtrate was subjectedtophysicalandchemicalvariablesdetermination.
Statisticalanalysis:Thepercentagereductionofvariablesattainedinthestudybyvarioustreatmentswas exposed to statistical appraise. The regression equation has been consequent and the correlation co-efficient (r) has been calculated.Thecorrelationcoefficientrepresentstherelationbetweenthedosageoftheagentsandthe%removalofCOD, Nitrate,Phosphate,BOD,ChlorideandSulphate(PalanisamyandManoharan1994).
The physical - chemical variables of DSW were determined and the analytical values were presented in terms of temp.,pH,EC,Cl-,BOD,COD, nitrate,PO43- andSO42-sulphates. This estimationwas conducted for period of threemonths andtheaveragevaluesarepresentedinTable1.
Variables DSW-1 DSW-2 DSW-3 Average Standard pH 3.98±0.03 3.94±0.02 4.16±0.06 4.03 5.5–9.0 EC(mS/cm) 42.6±0.50 46.8±0.58 51.5±1.04 46.97Temp(oC) 45.7±0.70 48.2±0.26 48.6±0.51 47.50 <50C BOD(mg/L) 37662±52.78 42880±40.6 35781±52.6 38774.33 30 COD(mg/L) 126682±25.21 138209±20.8 167872±77.56 144254.33 250 BOD/COD 0.297 0.310 0.213 0.27Nitrate(N-NO3 -),(mg/L) 2.50±0.82 4.02±0.23 5.71±0.82 4.08 10 Phosphate(PO43-),(mg/L) 18.77±0.71 23.45±0.92 16.18±1.41 19.47 5 Chloride(Cl-)(mg/L) 5982±62.18 4852±62.49 76001±35.16 28945.00 1000 Sulphate(SO42-),(mg/L) 4425±18.31 4918±35.78 6014±38.46 5119.00 -
The relation of BOD and COD is a good index for the percentage of degradability of organic substance by the microorganismsandtheconditionofbio-chemicalprocessinliquidwastetreatmentunit.Hence,therelationshipbetween CODandBODinDSWwasappraised andtheanalyticalvaluesweregiveninTable 1.Thisrelationbetweenthevariables indicatedpositiveandlinearwithawell-definedoutline.
The average values of temperature 45.60C and pH 4.06 were recorded in raw DSW which are out of the CPCB distillery liquid waste discharging limits < 50C and 5.5 to 9, correspondingly. The enhancing in liquid waste temperature will reflect on the ecology by changing in the chemical reaction and biological phenomenon in water body and soil (Saranraj,2012).
The average DSW organic substance COD and BOD showed found to be 144254.33 mg/L and 38774.33 mg/L correspondingly. Thesevalues areextremely exceededthepermissiblelimit for landdisposal level set byCPCB distillery effluent(Table-1).ThedisposalofeffluentwithsuchhighloadsofBODandCODintothestream andlandcancreatetoxic
Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN:2395-0072 © 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page1278
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

conditionsbyimmediatedepletionofoxygenwhichdisturbsthewaterchemistryandbiologicalcommunities(Zaher,etal., 2014andFigaro,etal.,2006).
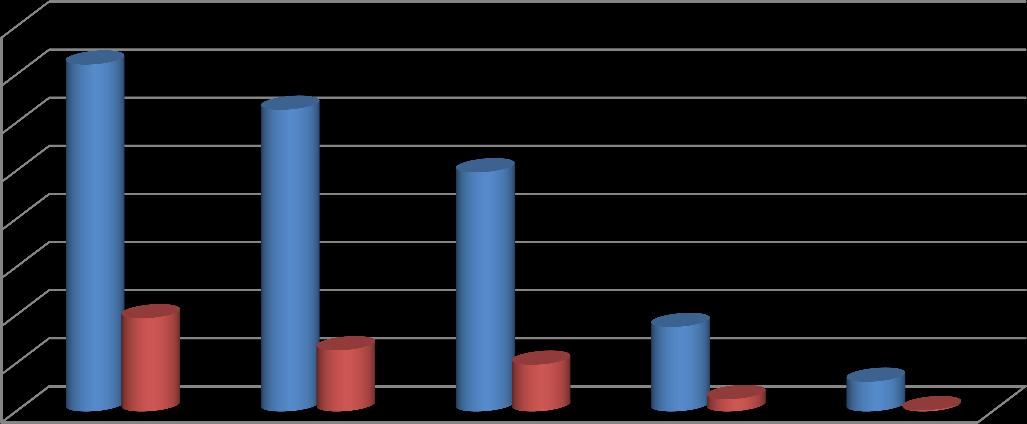
Thecontacttimeisoneof thesignificantvariablesthat shouldbe measured in batch adsorption labstudies.The contact time between the contaminants and adsorbent plays a supreme significance in adsorption treatment of liquid waste.Fig1toFig2representsthe effectofcontacttimeonreductionofCODandBOD.Theadsorptionsofcontaminants arefoundtoincreasewithincreaseincontacttime.Thelabsetupindicatedthatafter24hoursat adsorbentdosageof10 grams/200ml, COD and BOD reduction were found to be 12364 mg/L (91.4%) and 564 ppm (98.5%) correspondingly. ThesametrendswerenoticedinotherpollutantslikeNitrateandChloride(Fig3toFig6).
160000 140000 120000 100000 80000 60000 40000 20000 0
1g 2g 4g 5g 10g 2 hr 4 hr 8 hr 12hr 1 day
Fig 1 Removal efficiency of COD
45000 40000 35000 30000 25000 20000 15000 10000 5000 0
1g 2g 4g 5g 10g 2 hr 4 hr 8 hr 12hr 1 day
Fig 2 Removal efficiency of BOD
Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN:2395-0072 © 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page1279
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN:2395-0072
1g 2g 4g 5g 10g 30000 25000 20000 15000 10000 5000 0 2 hr 4 hr 8 hr 12hr 1 day
6000 1g 2g 4g 5g 10g 5000 4000 3000 2000 1000 0 2 hr 4 hr 8 hr 12hr 1 day
Adsorption equilibrium is generally described through isotherms, which are the quantity of adsorbate on the adsorbentasautilityofitscontentatconstanttemperature.Theamountadsorbedisnearlyconstantlynormalizedbythe weightoftheadsorbenttopermitcomparisonofdifferentmaterials.Variousarithmeticalrelationshipshavebeenusedto describethedynamicequilibriumdistributionofadsorbatebetweentheadsorbentandbulkliquidphases.
ThepHvaluechangedfrom4to10undertheexperimentalsetupofanadsorbentdoseof10gin100mL,contact time 24h and initial COD and BOD content (Simmi Goel, 2011). This might be accredited to the result of the activating agent of adsorbent which increased thepositive ion on the surface on the adsorbent and extends adsorption process at acidicmedia fornegativelychargedDSW.Moreover,theadsorptivecapacityofBagasseandcommercial activatedcarbon was subjective chiefly by the contact between the adsorbent and DSW in solution media either theH+/ OH- (Mane, et al., 2007andGupta,etal.,2003)
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN:2395-0072
The current study appraised the effectiveness of Bagasse and commercial activated carbon for the treatment of DSW. The batch studies reported that maximum removal COD, BOD, Chloride and Sulphate of 91.4%, 98.5%, 98.4% and 91.4%corresponding were obtainedatoptimum operatingvariablesof24hoursof contactwhentreatedwitha Bagasse andcommercialactivatedcarbondoseof10g/mLatpH4.Thestatisticalappraisalrevealedapositivecorrelationbetween decrease and the time. Hence it can be concluded that Bagasse and commercial activated carbon could be a practicable alternativeforthetreatmentofBSW.
The authors are gratefully acknowledged to the Department of Civil Engineering,EnvironmentalEngineering Lab,BIETforprovidingexperimentalfacilities.
APHA,StandardMethodsfortheExaminationofWaterandWastewater,Stand.Methods.(1998)541.
Chopra, K. V. A. 2012. Fertigation effect of distillery effluent on agronomical practices of Trigonella foenum-graecum L. (Fenugreek),Environ.Monit.Assess.,1207–1219.doi:10.1007/s10661-011-2033-7.
Figaro, S., S. Louisy-Louis, J. Lambert, J.J. Ehrhardt, a. Ouensanga, S. 2006. Gaspard, Adsorption studies of recalcitrant compounds of molasses spentwash on activated carbons, Water Res. 40, pp. 3456–3466. doi:10.1016/j.watres.2006.07.037.
Gaur,G.1997.WaterPollutionandItsManagement.SarupandSons,NewDelhi.
Gupta, V. K., C.K. Jain, I. Ali, M. Sharma, V.K. Saini. 2003. Removal of cadmium andnickel from wastewater using bagasseflyash asugarindustrywaste,WaterRes.37,pp.4038–4044.doi:10.1016/S0043-1354(03)00292-6.
Kadam, A., K. Upadhyay. 2012. Wastewater treatment of alcohol distillery, Jr. Ind. Pollut. Control.28,pp.1–4.
Kudesia,V.P.1994.IndustrialPollution.PragatiPrakashan,Meerut,pp:8-26.
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Kumar, A., B. Prasad, I.M. Mishra. 2014. Adsorption of acrylonitrile from aqueous solution using bagasse fly ash, J. Water
ProcessEng.2,pp.129–133.doi:10.1016/j.jwpe.2014.05.003.
Kushwaha, J. P., V.C. Srivastava, I.D. Mall. 2010. Treatment of dairy wastewater by commercial activated carbon and bagasseflyash:Parametric,kineticandequilibrium modelling, disposal studies,Bioresour. Technol. 101,pp.3474–3483. doi:10.1016/j.biortech.2010.01.002.
Mane,V.S.,I.D.Mall,V.C.2007.Srivastava,Useofbagasseflyashasanadsorbentfortheremovalofbrilliantgreendyefrom aqueoussolution,Dye.Pigment.73,pp.269–278.doi:10.1016/j.dyepig.2005.12.006.
Saranraj, P., D. Stella. 2012. Effect of bacterial isolates on reduction of physico – chemical characteristics in sugar mill effluent,Int.J.Pharm.Biol.Arch.3(2012)1121–1128.
Simmi Goel, 2011. Isotherm studies for cod removal and devolorization of distillery waste byactivated carbons, Asian JournalofEnvironmentalScience,6(1),58–61.
Zaher, Z., G. Hammam, Correlation between biochemical oxygen demand and chemical oxygen demand for various wastewatertreatmentplantsinEgypttoobtainthebiodegradabilityindices,Int.J.Sci.BasicAppl.Res.3(2014)42–48.
Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN:2395-0072 © 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page1282