International Research Journal of Engineering and Technology (IRJET)

e-ISSN:2395-0056
Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN:2395-0072

International Research Journal of Engineering and Technology (IRJET)

e-ISSN:2395-0056
Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN:2395-0072
1*Lecturer, Metallurgy Engineering Department, Dr. S. & S. S. Ghandhy College of Engineering & Technology, Surat, Gujarat, 395001 and Ph.D. Student, Gujarat Technological University, Ahmedabad, Gujarat, 382424.
2Principal, Mahatma Gandhi Institute of Technical Education & Research Center, Navsari, Gujarat 396450. ***
Abstract - Nanomaterials have piqued researchers' interest in the last ten years because of their distinctive physical and chemical features, which have led to the creation of new construction materials with innovative uses. Compared to micron fillers, the use of silica nanoparticles greatly improved the breakdown strength and voltage endurance. The volume fraction and filler size of this composite are what give it its main advantages. One of the foremost alluded to and utilized cementitious nanosized materials is nano silica (nSiO2). The downsizing of a certain electronic gadget uses nanomaterials. The need for a more practical, less expensive approach to generating superior materials, particularly silicon dioxide, arises from the new technological era. This paper covers a literature review on the various techniques of production of a nanocomposite’s substance called nSiO2 and its technological applications, along with the conditional suitability of the process.
Key Words: Nanocomposites, Silica, Sol-gel, Polymerization,Ballmilling.
The findings of study demonstrate that adding nano silica particles to a modified polypropylene polymer binder increases its resistance to fatigue. In a number of disciplines, such as chemistry, physics, and materials research, metal oxides are crucial. NEMS, MEMS, sensors, piezoelectric devices, coatings for corrosion passivation, gas cells, and catalysts in technical applications are all made with oxides [1-5]. Oxide nanoparticles can display distinctive physiological and chemical characteristics as a result of their confined measurement and an excessive density of corner or area floor locations. Because of this, transition metallic oxides (TMO) are most frequently employedintherapidlyevolvingdisciplinesofelectronics, magnetics, solar cells, photocatalysis, and gas sensors [2]. SiO2 has established itself as a challenging study topic because of its exceptional physical and chemical features. Amorphous silicon dioxide is the most well-known crystalline form, followed by quartz, cristobalite, tridymite,stishovite,andcoesite.
Silicapowderhasbeenusedinthemechanicalindustryas wellasprecisestructuresforchemicalcatalysts,ceramics, and photo-electricity elements, among other things, because of its purity, shape, measurement, and
distribution. Due to its physicochemical advantages, the silica corpuscular powder's application fields are constantly expanding. The electrical spectra of the interband transition show more pronounced peaks in crystallineSiO2thaninamorphousSiO2,andthepowerof theabsorptioncomponentisabout1eVhigher[3,4]. The sensitizedmesoporoussilicawithacriflavinedyecouldbe advantageous for nanosensors and nanolasers. [5]. SiO2 canbeusedtoincreasethephotocatalyticreproductionof oxidematerialsandpolymersbecauseofitshighbandgap, which increases the catalyst's accessible surface area [6, 7]. Micro-injection molding, in-situ polymerization, coprecipitation,sol-gel,andmanyothersynthesistechniques are reportedly used for Nanocomposite materials [8, 9]. Theleachingprocess,achemical-basedmethod,isusedto create crystalline Nano silica powder. This crystalline nanosilicapowderisproducedandthenutilizedasafiller inthecreationofnanocomposite.Accordingtothearticle, different fillers like SiO2, TiO2, MoO3, etc., allow the electromagnetic behavior of ferrite magnetic materials to be significantly modified. [10] These fillers encourage the catalytic process for adjusting the electromagnetic behavior.
Silica is a micronutrient that is crucial for plant growth and production in agriculture and is regarded as an essential micronutrient. Additionally, it strengthens the leaf, shoot, and root as well as increases weather tolerance, and lessens the detrimental impacts of some poisonous components in the soil. Silica can sterilize and kill fungi during the germination process, which boosts germinationrates.Andfinally, nanosilica(0–100nm)can easilypassthroughthecellmembraneduetoitsnanoscale andhighsurfacearea.Asa result,utilizingnanomaterials, suchasnano-silica,isthoughttobeanewareaofresearch for soaking seeds. Natural or synthetic materials can be used to extract nano-silica. Several approaches, including the Stobr technique [11], sol-gel methods [12], and a water-in-oil nanoemulsion system, were used to create nanosilica[13].
However,onlyasmallnumberofinvestigationshavebeen done using the delayed gelation approach and freezedryingtechniques[14].
Thepropermethodofsolution,acomponentofsol-gel,has the benefit of being simple to use, requiring little processing, and allowing for the modification of the
© 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page947
International Research Journal of Engineering and Technology (IRJET)

e-ISSN:2395-0056
Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN:2395-0072
material's composition or the control of the purity and uniformity of the SiO2 properties. Easy solution strategy reports are mentioned in references [15-17]. However, it is necessary to generate vast amounts of SiO2 particles using a straightforward process and low-cost raw ingredients. In the present study, the SiO2 particles were producedusingcheaprawmaterialsandtheeasysolution method.
Composites are man-made multiphase materials. It means that the two phases were individually existing, and thentheywereputtogethertoforma compositematerial. Materials like phenolic, polystyrene, polyester, and vinyl were manufactured, but still, to obtain strength and rigidity, there is a need for reinforcement. Further, in 1930’s development of resins was a very important milestone achieved in the industry of composites, and thereafter, within five to eight years, the world observed the development of glass fiber and fiber reinforced polymer(FRP)[18,19].
As we know, composites are combinations of twophase/constituents; these two constituents are generally mixedatthemacroscopiclevelwiththeconditionthatthey are not soluble in each other. These two constituents are namely reinforcing phase and matrix. Matrix is the one in whichreinforcingphaseslikeflakes,fibers,orparticlesare embedded, and it is generally continuous. The reinforcing phase is also called the discontinuous phase. However, both phases may be interrupted in some composites,such as sandwich composites. Composites can be divided into polymer matrix composites (PMC), metal matrix composites (MMC), and ceramic matrix composites (CMC)based on the matrix. Examples of polymer matrix composites from this category include GFRP (glass fiber reinforced plastic), CFRP (carbon fiber reinforced plastic), and others. The composites are also categorized based on the distribution and shape of the reinforcing phase as particlesandfibers,whereasfurtherfibersareclassifiedas discontinuous (short fiber) and continuous (aligned or long). Advancement and utilization of modern composite (invention of resins with glass fibers) were started. Automobilebodies,boats,andaircraftarebeingstartedto form from this fiberglass. In 1938, other higherperformance resin systems like epoxies also became available.Thereafter,duringWorldWar2,theresearchers understood that fiber composites are transparent to radio frequencies, so this phenomenon can be helpful in planes/jets and avoid visibility, but before that, this was firstly implemented in commercial grade boat hulls. Now till1960’sresearchersunderstoodthatbecauseofthegood stiffness-to-weight ratios of carbon fiber, they found their application in a variety of fields like sports, boats, aerospace,automotive,andeveninconsumergoods.
Two or more particles are added together, fibers or particlesembeddedintothematrix,andthepreparationof thenewmaterialisnothingbutcompositematerial,which is the modern-day example of structural composite material. In the composite material, the matrix performs two supreme tasks, the first one is to bind the embedded fibers or particles, and the second is the distribution of force or stresses over embedded fibers. So based on the matrix, composite materials are get classified as shown in Figure1[20].
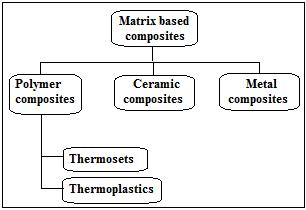
The use of composite materials in various structures has grown in popularity over the past few decades, particularly in aerospace structures and ship hulls. Airframes, etc. Composite materials are chosen over traditional because of their advantages, particularly stiffnessandstrength.Thisishowepoxyworks.Resinsare frequently used in various matrix components, including engineering, because of their superior mechanical as well asinsulatingqualities.
The uniform dispersal and scattering of filler particles inthepolymermatrixareessentialfortheenhancementof the mechanical characteristics of polymer matrix nanocomposites(PMNCs).Inotherwords,amajorissuein the production of PMNCs is inadequate nanoparticle dispersion and distribution in the polymer matrix. In a matrix, agglomeration normally declines as particle dispersion increases, i.e., more particle dispersion results in less agglomeration of particles. On the other side, the distributiondescribeshowevenlytheprimaryparticlesor theiragglomeratesarespreadthroughoutthevolume.[2123]
PEK is a Poly-(ether ketone) polymer that appears in the form of a semi-crystalline polymer. It is thermoplastic and has good thermo-electro-mechanical and chemical properties,renownedforitssuitableimpactenergy,fatigue electricity, and creep resistance. The glass transition temperature (Tg) of PEK is around 155-160°C. Because of this high glass transition temperature, it possesses good
International Research Journal of Engineering and Technology (IRJET)

e-ISSN:2395-0056
dimensional stability and can be used for wide temperatureranges likethespaceshuttle,spaceantennas, flexible electronics, robotic systems, and many more [2426].
One of the many techniques to increase the continuity between the hydrophobic polymer and hydrophilic nano silica is surface chemical treatment [27-29]. Utilizing a reactivemodifier,suchassilanecouplingagents,canresult inastronginterfaceandgooddispersion.Accordingtothis theory,thecouplingagent'sorganofunctionalgroupsmight be replaced by hydroxyl groups on the surface of nanoparticleswhilealsoreactingwithpolymerchains[30, 31].Duetoitssuperiorthermo-mechanicalstability,better resistance to chemical corrosion, and great tribological behavior, thermoplastic poly ether ether ketone has been usedmoreandmoreinindustrialapplications.
The qualities of poly ether ether ketone (PEEK), including its great mechanical and thermal strength, stability, and resistance against corrosive conditions because of chemicals, make it one of the most significant engineeringpolymers.PEEKhasbeenusedasabiomaterial for orthopedic, trauma, and spinal implants more frequently since 1987 [32, 33]. According to in vitro biocompatibilitytests,PEEKdoesnotcauseanymutagenic orcytotoxicactivities[34].PEEKhasevenbeenreferredto as a bioinert material because it doesn't harm human tissues in any way and doesn't release any ions or other compoundseither[35].
Thissectiondescribesthemostrecentdevelopmentsinthe mechanical milling of polymer matrix nanocomposites. An explorationofpotentialfuturecircumstancesthatcouldbe producedbytheuseofenhancedexperimentaltechniques. Preparation of composites with PEEK was a challenging job. PEEK has a high melting point as well as has low dissolving capacity in solvents. So the possibility of heat treatment and homogeneous liquid mixing process get deductedfromtheprocess.Nowonlyoptionthatremained was solid-state polymer processing (using methods like mechanical alloying and solid-state shear pulverization, which do away with the heat and solvent issues of conventional approaches and offer processing flexibility and simplicity) is one way to handle this issue. A widely accepted MA instrument and tried-and-tested method for dealing with materials with tiny microstructures in both metals and ceramics is the planetary high-energy ball milling process [36-39]. Initially ball mill process was employed in 1994 by Namboodri et al. [40] to generate a polyamide/BaTiO3 composite. In order to embrittle polymers and get rid of their viscoelastic properties, ball milling of polymers was modified below cryogenic temperature, which was produced bya cryogenic medium likeliquidnitrogen.This techniqueiscalledascryomilling
[36-38]. Cryomilling was employed in 1997 by Giri et al. [41]tocreateapolyethylene/Fenanocomposite.
Becauseverysmallquantitiesofprecursorpowdersare utilized, planetary ball mills are typically employed in mechanical alloying. As a result, this method is frequently usedinresearchlabs.Aplanetarymillincludesaturntable and four bowls. The turn disc rotates in the opposite direction from the bowls. The turn disc and bowl both rotate on their axes, creating centrifugal forces. The powdermixtureisfracturedandcoldweldedasaresultof the centrifugal forces acting on the balls and the powder mixture. The impact energy of the milling balls in normal orientations can be up to 40 times larger than the acceleration caused by gravity. A planetary ball millis a good alternative for high-speed/energy milling because of this[42].
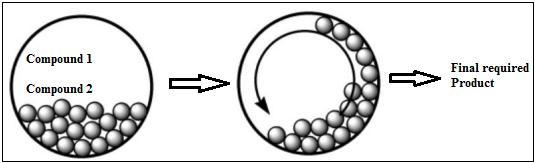
Ballprocessingcouldbeamechanicalhandlefrequently utilized to break up solid materials into minor pieces [4345]. Within the routine approach, the reactants are ordinarily broken and separated utilizing dissolvable atoms;inanycase,inballmilling,thereactantsarebroken using mechanical powers. Mechanochemistry may be a term that has as it was recently utilized [46]. In various surveyarticles[47-49],theutilizationofballmillingwithin the blend and responses of natural compounds have been examined. Figure 2 shows the process of synthesis of nanomaterial. Solvent-free ball processing is once in a while utilized within the amalgamation of natural compounds. Fig.2Ballmillingnanomaterialsynthesis
Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN:2395-0072 © 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page949
Sol-gel reactions, a well-known method for creating inorganic glasses and ceramic precursors, can be used to produce these materials at relatively low temperatures. Thefundamentaladvantageoftheprocessisthatceramics are treated gently under low pressure and temperature conditions. In recent years, the sol-gel technique has been widely used to create innovative organic-inorganic composite (hybrid) materials, referred to, respectively, as "ceramers"and"ormosils"or"ormocers"[50,51].Thesolgel reaction is used to improve the adherence of organic molecules, which are frequently polymeric and include
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN:2395-0072
functionalgroups,totheceramic-likephaseincomposites. This innovative reinforcement technique is very beneficial and may result in reinforcing particles within a polymer matrix. Additionally, because they are typically nanocomposites, these novel hybridsol-gel materials have the potential to offeruniquecombinationsof features that are not possible with other materials [52]. Numerous books [53–59] have provided thorough reviews of the researchontheuseofthesol-gelprocessinthecreationof organic/inorganic hybrid materials. The well-known twostep network-building sol-gel process consists of the hydrolysis of a metal alkoxide in the first stage and the polycondensation reaction in the second. Metal-organic alkoxides, in particular silica, are ofspecial interest in this method because they can create an oxide network in organicmatrices.
Thesol-gelprocessofalkoxysilanecanbeexplainedas hydrolysis(Eq.(1)-(3)):
Two methods are typically used among the numerous other synthetic procedures that can be used in the sol-gel process to create polymer/silica hybrid materials, including:
(i) In situ creation of an inorganic network with an organicpolymerthathasalreadybeenproduced[62].Itis necessary to determine the circumstances in which phase separation won't take place throughout the gel-forming and drying processes in order to produce optically clear materials. To lessen phase separation, covalent connections are frequently introduced between the inorganic and organic phases. The cosolvent that is utilized is the most crucial variable in regulating polymer solubility. THF, alcohol, andDMF are three regularly used solvents.
where,thealkylgroupisdenotedbyR.
Eq. (4) shows the nature of totally condensed silica after thecompletionofthesol-gelprocessofreaction:
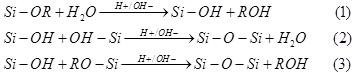
(ii) The stages of organic-inorganic synthesis can be differentiated based on the growth of organic and inorganic matter. Particle sizes and interactions between thedispersedandcontinuous phasesfrequentlyaffectthe characteristics of sol-gel nanocomposites. As previously discussed [54], hybrid materials can be broadly categorized into two fundamental types based on the natureofinterfacial contact. Hydrogenbondsandvander Waalsareexamplesofphysicalorweakphaseinteractions in C1 hybrids. Between the organic and inorganic phases of the hybrid in C2, there are powerful chemical connections[56].
TEOS, which is easy to purify and has a somewhat slow reactionrate,isthemostusedceramicprecursor[52].The kinetics of the hydrolysis and condensation reactions in the sol-gel process is influenced by a number of factors, includingthesilaneandwaterratio,temperature,thetype of solvent, the catalyst, and others. The sol-gel methodology beats the traditional blending method because it can accurately regulate the shape or surface featuresofthedevelopinginorganicphaseinthepolymer matrixbyadjustingthesereactionparameters.
To increase silicon's limited reactivity, catalysts that are acidic or basic are frequently utilized. It has been shown that basic catalysis frequently results in opaque composites with phase dimensions that are significantly larger than 100 nm and more typically in the micrometer range. It is impossible to classify these materials as nanocomposites. Alternately, when acid catalysis is used, transparent nanocomposites with unique morphologies and diameters below 100 nm are often produced. Therefore, the polymer/silica nanocomposites made by sol-gel techniques are commonly made using acidcatalysis[60,61].
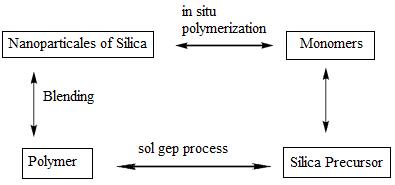
In situ polymerization has a variety of advantages. These include simple handling, quick processing, and enhanced performance of the finished items [63]. In situ polymerization typically consists of three sequential phases. The necessary surface modifiers are used to pretreat the nanoscale additives, and then the modified additives are disseminated into monomer (s). Bulk or solution polymerization then occurs. The nanocomposites are then created in the polymerization process. The dispersion and adhesion at the polymer and filler interfaces are without a doubt the most crucial elements that influence the characteristics of composites. When inorganic particles are premodifier by a coupling agent, theymaydisperseuniformlyinthepolymermatrices[64]. Figure3showsthemethodofpreparationofEpoxy/Sio2 nanocomposites[65].
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN:2395-0072
between the silica and the matrix for silicas treated with titanate or aluminate, the amino functional groups implementedcouldtakepartinthepolymerization,giving riseingraftpolymersonthesilicasurface[67].
Aprocessapproximatingbulkpolymerizationwasusedto polymerize the mixture at a high temperature in a nitrogen atmosphere after initial stirring to suspend the silica particles in the caproamide. The silica was premodifiedwithaminobutyricacidbeforetopolymerization. The experimental result showed that there is a uniform silicas dispersion takes place. The morphological study's findings demonstrated that the crystalline phase of these composites was unaffected by the presence of particles. The amount of filler and the manner in which it was dispersedallhadaneffectontheobservedrise,whichwas clearlyreinforcedbytheadditionoffiller.Bothcontainthe yieldpoint,identically.
It was observed that the latter parameters affected the results of compressive and tensile tests [66]. Literatures were observed to investigate the GPS treatment or APS treatment over 6/nano-SiO2 with in situ polymerization [67].TGAofsilicasseparatedfromthecompositesand an end group analysis of the composites demonstrated that functional silane treatment of nano-SiO2 prior to in situ polymerizations of nylon 6 did not result in a noticeably different level of reactivity of silicas' surface groups. However, mechanical testing has shown that it may concurrently increase the composites' strength and hardness. The addition of a flexible layer to the interface was mostly responsible for this. To study how interphase affects nanocomposites, nano silica (nano-SiO2) was prepared with coupling agents like aluminate, titanate, or silane.Whilethehydrogen-bondingconnectionsalongthe metaphase may have helped the interfacial interactions
Polyethylene terephthalate monomer mixed with organically modified silica nanoparticles was effectively used to create PET/silica nanocomposites in situ [68].Results revealed that the nanoparticles were evenly distributedthroughoutthepolymermatrix;theirinclusion could hasten crystallization and raise the melting temperature, but it had no discernible impact on the synthesisprocess.
Twodistincttypesofpolymer/silicananocompositeshave been produced by the free-radical polymerization of HEMA,eitherduringthepresenceofHEMA-functionalized SiO2 nanoparticles (T1) or with the concurrent in situ growth of the silica phase through the acid-catalyzed solgel polymerization of TEOS (T2). The particles in T1 systems had a traditional particle-matrix shape, although they preferred to group together. T2 systems had a finer morphology with a very open mass-fractal silicate structurethatwasthoughttobemolecularlybicontinuous withtheorganicphase[60].
Epoxy/silica is a well-researched nanocomposite system. As an organic matrix, epoxy resins offer great heat, moisture, and chemical resistance, as well as good adherence to a variety of substrates. They are unable to fully satisfy the demands of applications like epoxy molding compounds, nevertheless. They differ from inorganic materials in that they have low mechanical characteristics and a high CTE value. In order to reduce shrinkage during curing and CTE, enhance heat conductivity, and satisfy mechanical requirements, silica particlesarefrequentlyutilizedasreinforcementinepoxy matrix. Typically, the hardener is introduced after the nano-silica, and epoxy polymer have been combined together to produce the curing reaction. Table 1 includes some examples of this type of in situ polymerized epoxy/SiO2 nanocomposite, as well as a list of typical epoxy resins and hardeners for the production of such nanocomposites.
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN:2395-0072
UVlightstimulatesthepolymerizationprocess,enablinga quick conversion of the liquid monomer into a solid film with specialized physicochemical and mechanical properties. The UV light interacts with an appropriate photoinitiator to produce radical or cationic species, which trigger the curing reaction of reactive monomers and oligomers [69]. Because there are no solvents involved in the process, it is an environmentally friendly method. Energy is conserved because the substrate does notneedtobeheatedasinconventionalthermalcuring.A related technique called electron beam (EB) driven polymerizationtechnologyhasalsodrawnalotofinterest [70]. The formulation of UV-curable nanocomposites typically includes reactive dilutes (e.g.,HDDA) and/or oligomers (e.g.: epoxy acrylate). In order to ascertain the relative importance of these two characteristics on the mechanicalbehavior,[71]createdmodel-filledelastomers and varied the chemistry individually at the particle surface and the dispersion state. These samples were madeinaccordancewiththemethodcreatedbyFordetal. [72, 73], which involved photopolymerizing a concentrated dispersion of grafted silica particles in acrylate monomers. It has been demonstrated that using trialkoxysilane-modified nanosized silica in radiationcurable acrylate systems produces nanocomposite films thataremoreresistanttoabrasionandscratching.
The establishment of unique interactions at the interface between organic and inorganic components is a crucial component in the creation of nanocomposite systems since the interface is largely responsible for the creation andcharacteristicsofnanocomposites.Therefore,there is currently a lot of interest in the development of grafting techniques that can adjust the surface characteristics of mineral substrates. At the surface of the particles, linear polymerchainshavebeengraftedusingtwotypical
methods. The "grafting-to" technique is one method, and the"grafting-from"techniqueistheother.Thesurfacecan be improved by grafting polymers to inorganic particles like silica, however, nongrafted chains frequently contaminate the surface. Therefore, it is still difficult to distinguish between grafted and non-grafted chains. Strong resistance between grafted polymer chains also prohibits the attachment of further ones, which in turn restrictsgraftdensity.
The "grafting-from" method, also known as surfaceinitiated polymerization, for example, in situ polymerization with monomer expansion of polymer chainsfromencapsulatedinitiatorsonmineralsurfaces,in turn, gives rise to so-called "polymer brushes" or "hairy nanoparticles," seems to be a very flexible and promising
method. There are a variety of other beginning procedures, such as free radical polymerization [74-80], whichcombinesconventionalradicalpolymerizations.
In the present paper, literature related to the study of high-performance polymer matrix nanocomposite is reviewedindetail.Itisobservedthat aplanetaryballmill is useful for highly abrasive material and also gives fine and uniform particle size. The insoluble and thermally unstable polymer can be processed through polymerization techniques. For the preparation of polymer composites with high nanotube loading, the insitu process provides very good miscibility with almost any type of polymer. High chemical homogeneity can be achievedthrough theSol-Gel technique,anditoperates at low pressure and temperature. The sol-gel process can controlthemorphologyandsizeofparticles.Also,thesolgeltechniquecanproduceorganicandinorganichybrids.
[1] H. Gleiter, “Nanostructured materials: state of the art andperspectives”,J.NanostructuredMater.,6,1995,3–14.
[2]H.H.Kung,“TransitionMetalOxides:SurfaceChemistry and Catalysis”, 1st Edition - Elsevier: Amsterdam, April 1, 1989.
[3] M. Valden, X. Lai, D.W. Goodman, “Onset of Catalytic ActivityofGoldClustersonTitaniawiththeAppearanceof NonmetallicProperties”,J.Sci.,281,1998,1647.
[4] N. Millot, D. Aymes, F. Bernard, J.C. Niepc, A. Traverse, F.Bouree,B.L.Cheng,P.J.Perriat,“Hydrothermalsynthesis of nanostructured inorganic powders by a continuous process under supercritical conditions”, J. Phys Chem B, 2003,107,5740.
[5] A. Yelil Arasi, M. Hema, P. Tamilselvi, R. Anbarasan, “Synthesis and characterization of SiO2 nanoparticles by sol-gelprocess”,IndianJ.Sci.1(1),2012,6–10.
[6]S. Kumari, P.D. Sahare, M. Gupta, “Sensitization of mesoporous silica nanoparticles by laser grade dye acriflavin”, Journal of Advanced Materials Letters, VBRI Press,2012:172.DOI:10.5185amlett.2012.icnano
[7]M. Ali, B. Mohammad Ali, M. Naser, “Characterization and photocatalytic activity of SiO2-TiO2 mixed oxide nanoparticlespreparedbysol-gelmethod”,IndianJ.Chem. 49,2010,1593–1600.
[8]S. Elias, L. Panagiotis, K. Chritophoros, “Dye sensitized photo-electrochemical cell using a nanocomposite SiO2/Poly (Ethylene Glycol) thin film as electrolyte support characterization by time-resolved luminescence
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

and conductivit measurements”, J. Phys Chem, 105, 2001, 3486–3492.
[9] H. Shokrollahi, K. Janghorban, "Influence of additives on the magnetic properties", microstructure and densificationofMn–Znsoft ferrites,MaterialsScienceand Engineering:B,141,3,2007,91-107.
[10] Shifeng Yan, Jianxin Geng, Jianfeng Chen, Li Yin, YunchunZhou,LeijingLiu,EnleZhou,"AstudyofNiZnCuferrite/SiO2 nanocomposites with different ferrite contents synthesized by sol–gel method", Journal of MagnetismandMagneticMaterials,292,2005,304-309.
https://doi.org/10.1016/j.jmmm.2004.11.145.
[11]T. Gholami, M. Salavati-Niasari, M. Bazarganipour, E. Noori, “Synthesis and characterization of spherical silica nanoparticles by modified Stöber process assisted by organicligand”,SuperlatticesMicrostruct.61,2013,33-41.
[12] Le, V.H., Thuc, C.N.H. & Thuc, H.H. “Synthesis of silica nanoparticles from Vietnamese rice husk by sol–gel method”, Nanoscale Res Lett, 8, 2013, 58. https://doi.org/10.1186/1556-276X-8-58
[13] Sang-Bae Jung, Jung-Ho Kim, Hong-Ryul Kim, HyungHo Park, “Effect of solvent on the preparation of ambient pressure-dried SiO2 aerogel films”, Microelectronic Engineering,65,2003,113–122.
[14] Ping Lu, You-Lo Hsieh, “Cellulose nanocrystal-filled poly(acrylic acid) nanocomposite fibrous membranes”, Nanotechnology, 20(41), 2009, 415604. DOI:10.1088/0957-4484/20/41/415604.
[15]Panatarani C, Anggoro D and Faizal F, “Challenging and Development of Phosphors for Lighting Applications, AIP Conference Proceedings 1712, 020003 (2016); https://doi.org/10.1063/1.4941864
[16]PanataraniC,D.AnggoroandF.Faizal,“SolutionPhase SynthesisandPhotoluminescentPropertiesofNanocrystal LaPO4:Eu3+”, AIP Conference Proceedings - Bandung, Indonesia,16June2010.10.1063/1.3515567
[17]Panatarani C and Joni I M 2013 AIP Conf. Proc. 1554 109
[18] T.D. Ngo, “Introduction to Composite Materials”, 2020. doi:10.5772/intechopen.91285.
[19] Schatzberg, E.M, “Keynote lecture Materials and the developmentofaircraft:Wood-aluminium composites”,in Vermeeren,C.(ed.)AroundGlare:ANewAircraftMaterial inContext.Dordrecht:SpringerNetherlands,2002, 43–72. doi:10.1007/0-306-48385-8_7.
Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN:2395-0072 © 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page953
[20] Ibrahim, I. et al., ‘The Use of Polypropylene in Bamboo Fibre Composites and Their Mechanical Properties–AReview”,JournalofReinforcedPlasticsand Composites2015[Preprint].
[21]E. Petrovicova, “Structure and Properties of Polymer Nanocomposite Coatings Applied by the HVOF Process, Ph.D. dissertation”, Drexel University, Philadelphia, PA 1999,p.42-45
[22] Markad Kanif and Achchhe Lal. "Experimental investigation of shape memory polymer hybrid nanocomposites modified by carbon fiber reinforced multi-walled carbon nanotube (MWCNT)," Materials ResearchExpress8.10,2021,105015.
[23] Markad Kanif and Achchhe Lal. "Synthesis of the multiphase shape memory hybrid composites hybridized with functionalized MWCNT to improve mechanical and interfacial properties," Polymer-Plastics Technology and Materials61.6(2022):650-664.
[24] Tiwari Nilesh, and A. A. Shaikh. "Hybridization of carbon fiber composites with graphene nanoplatelets to enhance interfacial bonding and thermomechanical properties for shape memory applications." PolymerPlasticsTechnologyandMaterials,61.2,2022,161-175.
[25] Tiwari Nilesh and A. A. Shaikh. "Flexural analysis of thermally actuated fiber reinforced shape memory polymer composite," Advances in materials Research 8.4 (2019):337-359.
[26] A. M. Patki & R. K. Goyal, “Investigation of nonisothermal crystallization, dynamic mechanical and dielectric properties of poly(ether-ketone) matrix composites”, Polymer-Plastics Technology and Materials, 2020.DOI:10.1080/25740881.2020.1786583
[27]F.Hussain,M.Hojjati,M.Okamoto,R.E.Gorga,“Review article: polymer-matrix nanocomposites, processing, manufacturing, and application: an overview”, J. Compos. Mater.40(17),2006,1511–1575.
[28]L.S. Schadler, K.O. Laul, R.W. Smith, PetrovicovaE. “Microstructure and mechanical properties of thermally sprayed silica/nylon nanocomposites”, J. Therm. Spray Technol.6(4),1997,475.
[29]M.Q. Zhang, M.Z. Rong, K. Friedrich, Handbook of Organic–Inorganic Hybrid Materials and Nanocomposites, in: H.S. Nalwa (Ed.), Ameican Scientific Publishers, StevensonRanch,2003,113–150.
[30]A Guide to Silane Solutions, Dow Corning Company, 2005.
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN:2395-0072
[31]Y.Sun,Z.Zhang,C.P.Wong,“Studyonmono-dispersed nano-size silica by surface modification for underfill applications”,J.ColloidInterfaceSci.292,2005,436–444.
[32]S.M.Kurtz,J.N.Devine,“PEEKbiomaterialsintrauma, orthopedic, and spinal implants”, Biomaterials, 28, 2007, 4845–4869.doi:10.1016/j.biomaterials.2007.07.013.
[33]Monich Patricia R, Henriques Bruno, Novaes de Oliveira, Antonio P, Souza Júlio C.M, Fredel, Márcio C, “Mechanical and biological behavior of biomedical PEEK matrix composites: a focused review”, Materials Letters, 2016. S0167577X16314665–doi:10.1016/j.matlet.2016.09.005
[34]A.Katzer,H.Marquardt, J.Westendorf, J.VWening, G. von Foerster, “Polyetheretherketone-cytotoxicity and mutagenicity in vitro.”, Biomaterials. 23, 1749–1759. doi:10.1016/S0142-9612(01)00300-3.
[35]S.M. Kurtz, PEEK biomaterials handbook, 2011. https://doi.org/10.1016/C2010-0-66334-6
[36] Y.G. Zhu, Z.Q. Li, D. Zhang, T. Tanimoto, “PET/SiO2 nanocomposites prepared by cryomilling”, J. Polym. Sci., PartB,Polym.Phys.44,2006,1161–1167.
[37] Y.G. Zhu, Z.Q. Li, D. Zhang, T. Tanimoto, “ABS/iron nanocomposites prepared by cryomilling”, J. Appl. Polym. Sci.99,2006,501–505.
[38] Y.G. Zhu, Z.Q. Li, D. Zhang, T. Tanimoto, “Polyaniline/Iron nanocomposites prepared by cryomilling”, J. Polym. Sci., Part B, Polym. Phys. 44, 2006, 3157–3164.
[39]C. Suryanarayana, “Mechanical alloying and milling”, Prog.Mater.Sci.46,2001,1–184.
[40]S.L. Namboodri, H.Y. Zhou, A. Aning, R.G. Kander, “Formation of polymer–ceramic composite grain boundarycapacitorsbymechanicalalloying”,Polymer,35, 1994,4088.
[41]A.K. Giri, “Magnetic properties of iron–polyethylene nanocomposites prepared by high energy ball milling”, J. Appl.Phys.,81,1997,1348.
[42] Takacs Laszlo, "Self-sustaining reactions induced by ball milling", Progress in Materials Science, 47 (4), 2002, 355–414.doi:10.1016/S0079-6425(01)00002-0
[43]Mehdi Hedayati, Morsal Salehi, Rouhollah Bagheri, M. Panjepour, “Ball milling preparation and characterization of poly (ether ether ketone)/surface modified silica nanocomposites”, Powder Technology 207(1-3), 2011, 296-303.
[44] Yokoyama T, CC H, “Nanoparticle technology for the production of functional materials”, KONA Powder Part J 23,2005,7–17.https://doi.org/10.14356/kona.2005006.
[45] S.D Doke, C.M Patel, & V.N. Lad, “Improving Performance of the Synthesis of Silica Nanoparticles by Surfactant-incorporated Wet Attrition Milling”. Silicon 14, 2022, 913–922. https://doi.org/10.1007/s12633-02000871-x
[46] William Jones and Mark D. Eddleston. “Introductory Lecture: Mechanochemistry, a versatile synthesis strategy fornewmaterials”,FaradayDiscuss.,170,9.2014.
[47] Haowen Zou, Jiawei Zhao, Feng He, Zhong Zhong, Jinsheng Huang, Yulin Zheng, Yue Zhang, Yicheng Yang, Fang Yu, M. Asaad Bashir, Bin Gao, "Ball milling biochar iron oxide composites for the removal of chromium (Cr(VI)) from water: Performance and mechanisms",JournalofHazardousMaterials,413,2021, 125252.https://doi.org/10.1016/j.jhazmat.2021.125252.
[48] Majid Abdellahi, Hamed Bahmanpour, Maryam Bahmanpour, "The best conditions for minimizing the synthesis time of nanocomposites during high energy ball milling:Modelingandoptimizing",CeramicsInternational,
40, 7, Part A, 2014, 9675-9692. https://doi.org/10.1016/j.ceramint.2014.02.049.
[49]G. Pozo López, A.M. Condó, S.E. Urreta, S.P. Silvetti. “Synthesis of Fe/SiO2 and iron oxides/SiO2 nanocomposites by long-term ball milling”, Materials ResearchBulletin,49,January2014,237-244.
[50]Huang,H.H.;Orler,B.;Wilkes,G.L.Polym.Bull.1985, 14,557.
[51]Schmidt,H.J.Non-Cryst.Solids1985,73,681.
[52]Chen,Y.;Iroh,J.O.Chem.Mater.1999,11,1218.
[53]Novak,B.M.AdV.Mater.1993,5,422.
[54]Sanchez,C.;Ribot,R.New.J.Chem.1994,18,1007.
[55] Schubert, U.; Hu¨sing, N.; Lorenz, A. Chem. Mater. 1995,7,2010.
[56] Judeinstein, P.; Sanchez, C. J. Mater. Chem. 1996, 6, 511.
[57]Wen,J.Y.;Wilkes,G.L.Chem.Mater.1996,8,1667.
[58]Pomogailo,A.D.Russ.Chem.ReV.2000,69,53.
[59]Schottner,G.Chem.Mater.2001,13,3422.
2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page954

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 09 Issue: 09 | Sep 2022 www.irjet.net p-ISSN:2395-0072
[60] P. Hajji, L. David, J. F. Gerard, J. P. Pascault, G. Vigier, “Synthesis, structure, and morphology of polymer–silica hybrid nanocomposites based on hydroxyethyl methacrylate”,37,22,1999,3172-3187.
[61] Sain, P.K., Goyal, R.K., Prasad, Y.V.S.S., Sharma, K.B. and Bhargava, A.K., 2015. Few‐layer‐graphene/polycarbonatenanocompositesasdielectricand conducting material.Journal of Applied Polymer Science,132(34).
[62] Sanchez, C.; Julia´n, B.; Belleville, P.; Popall, M.,” Applications of hybrid organic–inorganic nanocomposites”,J. Journals of Materials Chemistry,15,2005, 3559-3592,DOIhttps://doi.org/10.1039/B509097K
[63] Yang, F.; Nelson, G. L.,” PMMA/silica nanocomposite studies: Synthesis and properties”,Journal of Applied Polymer Science,91(6), 2004, 3844-3850. DOI:10.1002/app.13573[64]Li,Y.;Yu, J.;Guo,Z.X.Polym. Int.2003,52,981.
[65] Zou, Hua; Wu, Shishan; Shen, Jian (2008). Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. , 108(9), 3893–3957.doi:10.1021/cr068035q
[66]Wen,J.Y.;Wilkes,G.L.Chem.Mater.1996,8,1667.
[67]Li,Y.;Yu,J.;Guo,Z.X.J.Appl.Polym.Sci.2002,84,827.
[68]Liu,W.T.;Tian,X.Y.;Cui,P.;Li,Y.;Zheng,K.;Yang,Y.J. Appl.Polym.Sci.2004,91,1229.
[69]Kang,S.;Hong,S.I.;Choe,C.R.;Park,M.;Rim,S.;Kim, J.Polymer2001,42,879.
[70]Liu,Y.L.;Li,S.H.J.Appl.Polym.Sci.2005,95,1237.
[71]Ragosta,G.;Abbate,M.;Musto,P.;Scarinzi,G.;Mascia, L.Polymer2005,46,10506.
[72] Preghenella, M.; Pegoretti, A.; Migliaresi, C. Polymer 2005,46,12065.
[73] Zhang, M. Q.; Rong, M. Z.; Yu, S. L.; Wetzel, B.; Friedrich,K.Macromol.Mater.Eng.2002,287,111.
[74] Zheng, Y. P.; Zheng, Y.; Ning, R. C. Mater. Lett. 2003, 57,2940.
[75] Sun, Y. Y.; Zhang, Z. Q.; Moon, K. S.; Wong, C. P. J. Polym.Sci.,PartB:Polym.Phys.2004,42,3849.
[76] Goyal, R.K., 2013. Cost-efficient high performance polyetheretherketone/expandedgraphitenanocomposites with high conductivity for EMI shielding
application.Materials Chemistry and Physics,142(1), pp.195-198.
[77] Sain, P.K., Goyal, R.K., Bhargava, A.K. and Prasad, Y.V.S.S., 2014. Thermal and dielectric behavior of flexible polycarbonate/lead zirconate titanate composite system.JournalofAppliedPolymerScience,131(4).
[78] Sain, P.K., Goyal, R.K., Bhargava, A.K. and Prasad, Y.V.S.S., 2013. Thermal and electronic behaviour of polycarbonate–copper nanocomposite system.Journal of PhysicsD:AppliedPhysics,46(45),p.455501.
[79]Goyal,R.K.andYadav,M.,2014.Thewearandfriction behavior of novel polytetrafluoroethylene/expanded graphitenanocompositesfortribologyapplication.Journal oftribology,136(2).
[80] Goyal, R.K., Namjoshi, A.H. and Joshi, B.B., 2011, November. Fabrication and properties of PVDF/BaTiO 3 nanocomposites for electronic applications. InInternational Conference on Nanoscience, Engineering andTechnology(ICONSET2011)(pp.642-645).IEEE.
2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page955
