International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 08 | Aug 2022 www.irjet.net p-ISSN: 2395-0072

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 08 | Aug 2022 www.irjet.net p-ISSN: 2395-0072
Pranoti Deshpande1
1Student, Dept. of Biochemical Engineering and biotechnology, KIT’s college of engineering (Autonomous), Kolhapur, Maharashtra, India. ***
Abstract - The foundation of downstream processing remains chromatography, especially at the process scale. There are several causes for this, but crucial ones include the superior scalability, robustness, and selectivity that process chromatography provides in comparison to its competitors. For the purpose of removing contaminants and capturing antibodies, chromatography selection is crucial. In this minireview, we attempt to summarize different types of chromatography for monoclonal antibody purification.
Key Words: Downstream process, purification, chromatography, mAbs, biopharmaceuticals.
Therearehundredsofmonoclonalantibodies(mAbs)either inuseorbeingdeveloped.DuetothepopularityofmAbsas therapeutics, several businesses have many antibodies. Today, monoclonal antibodies are recognized as a critical treatment approach for a variety of disorders. Companies have steadily increased the total number of mAbs under clinicaldevelopmentthroughouttime.Anappealingstrategy for solving the diseases is to use monoclonal antibodies (mAbs),whichmaybemadetotargetcellsonlywhenthey arespecificallydesiredandcauseawiderangeofreactions when bound. These substances have the ability to either directlykillcellsbydeliveringpoisonoussubstancestothe targetortoorchestratecelldeathinotherways,suchasby activatingimmunesystemcomponents,inhibitingreceptors, or scavenging growth factors. [1] The most common categoryofrecombinantproteintreatmentsisestablishedto be monoclonal antibodies (mAbs). They are often very soluble,exhibithighlevelsofexpressionincellculture,and are comparatively stable after processing. It is generally knownthatmammaliancellculturescanproduceantibodies. Currently, mammalian cells make up around 70% of recombinant therapeutic proteins, with Chinese hamster ovary(CHO)cellsbeingthemostcommonexpressionhost. In addition to purity and process capacity, downstream processdevelopmentplacesastrongemphasisonyieldand productivity.[2]Themostimportantstepinthedevelopment of biopharmaceuticals is the efficient recovery and purificationofmAbsfromthecellculturemedium.Product stabilityisoneofthemostcrucialcharacteristicsthatmust bepreservedthroughouttheprocess.Thehighestpriority shouldbegiventomaintainingproductqualityandboosting purity while overcoming multiple obstacles. To minimize
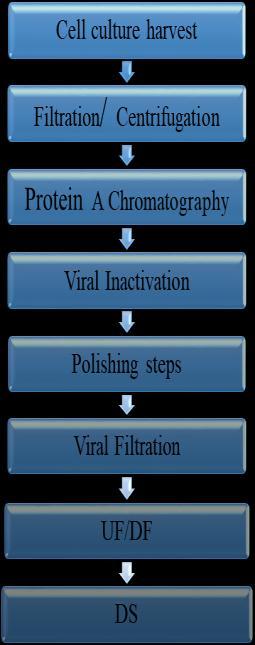
materiallossandtoreducethepotentialofcontamination, each step of downstream processing should be handled carefully. [3] Removing the majority of the cell culture's insolublecomponentsfromtheproductstreamisthefirst step in recovering secreted antibodies. Whole cells, cell fragments,colloids,andotherkindsofcontaminantsmake upthesecomponents.Harvestsfrombioreactorsareusually clarifiedbycentrifugationand/orfilteringthroughanumber ofdepthfiltersUtilizingcontinuousdisc-stackcentrifugation alongwithdepthfilteringisoneapproachthattheindustry prefers to use for completing this initial separation. Followingadepthfilter,afilterwithanabsoluteporesize rating (usually 0.45 m or 0.2 m) is used to assure the removalofsolidparticles(andbacteriainthecaseofthe0.2 m filter) from the cell culture harvest supernatant. These initial recovery methods are designed to eliminate the majorityofparticlesfromcellbrothinordertoreducethe workloadforthefollowingpurificationsteps.[4][5]Dueto itsgreatresolution,chromatographyisacrucialandoften used separation and purification method in the biopharmaceutical sector. Chromatography separates biomoleculesbymakinguseoftheirphysicalandchemical distinctions. After that, a succession of chromatography procedures,beginningwiththecapturestage,areappliedto theclarifiedcrop.Capturechromatography,atechniquethat involvesbindingandeluting,guaranteesbothadecreasein harvest volume and the safety of the final product by eliminating the majority of contaminants, including potentially harmful proteases. Ion exchange, hydrophobic interaction,andmultimodalchromatographyarefrequently utilizedaspolishingprocessesforthemonoclonalantibody purificationaftercapturingwithProteinA.[6]
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 08 | Aug 2022 www.irjet.net p-ISSN: 2395-0072
-1: ProcessflowformAbpurification
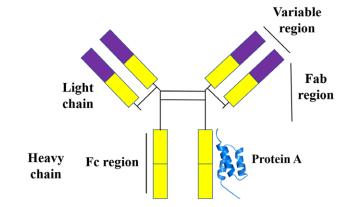
A native or recombinant proteic ligand derived from Staphyloccocus aureus or Escherichia coli, respectively, is combinedwithanaturalorartificialbasematrixinProteinA affinity chromatography. Five homologous domains of Protein A E, D, A, B, and C can bind to the Fc moiety of immunoglobulin G. (IgG). Because of its strong binding affinity and the high levels of purity that may be attained, Protein A affinity chromatography is a well-known procedure in the pharmaceutical sector. The target antibodies are loaded onto the immobilized Protein A support at neutral pH as part of the purification process, facilitating the interaction of the ligands. Following the separationofthe proteinfromcontaminantslikehostcell proteins(HCP),themobilephase'spHisloweredtofacilitate theproduct'sdesorption.Theresinisthenregeneratedand putthroughaclean-in-placetechnique.[7][8]
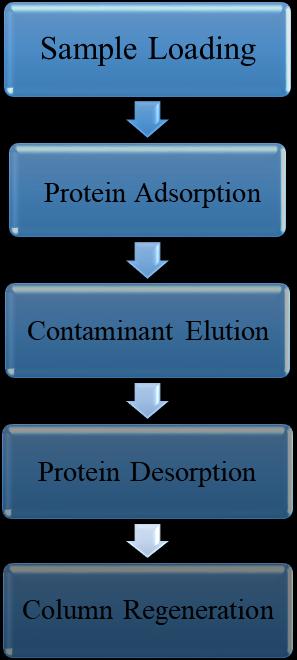
Protein A chromatography is the most expensive stage, making it a suitable place to start. The monoclonal antibody's interaction with immobilized Protein A is the foundation of this affinity chromatography technique. Hydrophobicinteractionsarepredominantlyresponsiblefor thebindingbutionicandhydrogenbondsalsoplayarole. Dependingontheantibodysubclass,thepHrangeof6–9is wheretheantibodybindstoProteinA,andthesaltcontent ofthebindingbuffercanaffectthis.ApHbufferwithalow rangebetween2and4.5ischosentoelutethebinding.Since theantibodyelutionoccursatalowpHlevel,thismethodis alsoutilizedtoinactivateviruses.[9][10][11][12]ProteinA purificationisfocusedandeffective.However,therearestill anumberofproblemsthatneedtoberesolved,suchasthe inadequate removal of contaminants including host cell proteins,DNA,aggregates,etc.Moreover,theimpactofwash buffers on protein. The physicochemical properties of antibodiesarestillnotwellunderstoodafterpurification.A newmonoclonalantibodypurificationmethodthatenhances the physicochemical characteristics of the antibodies by simply adding a basic buffer wash step following the traditionalproteinApurification'scapturestage.[13][14]In comparison to packed columns, Protein A membrane adsorbers and monoliths are more productive because of theirlowbedheightsandhighoperationalflowrates.These gadgets might serve as a model for future increases in protein A productivity even though they are not now practicableforlarge-scaleproduction.[15][16][17]
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 08 | Aug 2022 www.irjet.net p-ISSN: 2395-0072
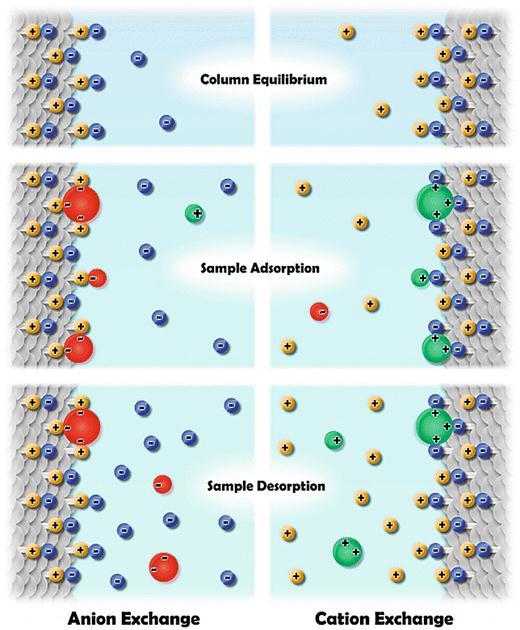
The protein's pI value is used in ion-exchange chromatographytoachieveseparation.Apositivelycharged protein is drawn to a negatively charged solid support in cation-exchange chromatography, whereas a negatively chargedproteinisdrawntoapositivelychargedsupportin anion-exchange chromatography. Elution is often accomplishedbychangingthebuffer'ssaltorpHcontentina stepwise or gradient fashion. Ion-exchange is a quick and affordable approach for purifying antibodies, and it's frequently employed as a polishing step after Protein-A affinity chromatography.[3][18]. For the purification of monoclonalantibodies,cationexchangechromatographyis commonly used in bind-and-elute mode. However, it was discoveredthatbind-and-eluteconditionswereinsufficient foreliminatingappreciableamountsofantibodyaggregate. Thedesiredproductflowsthroughastationaryphaseandis subsequentlyretainedbyamediuminpositivepurification, also known as the retention mode. Protein A affinity and cationexchangechromatographyisemployedinretention modeina broadplatformmAbpurificationprocedure.On theotherhand,theflow-throughmodeisfrequentlyutilized withanionexchangechromatography.Giventhattheflowis continuous,theoperationtechniqueknownasflow-through chromatography (FTC) is regarded as an effective way to separate two components. The desired product is flowed throughamediuminthenegativepurificationprocess,also knownastheflow-throughmode,whilecontaminantsare trappedbyastationaryphase.Targetproteinsareremoved from the chromatography column in FTC without being adsorbed, whereas contaminants are tightly attached. In contrast,tobindandelute,highercolumnloadingsareoften attainableintheflow-throughmode.[11][19][20][21][22]
If applicable, purification using the flow-through mode providesmanybenefitsoverpositivepurificationintermsof the quantity and size of buffers needed, workload, and processingtime.[23]
2.3
Multimodalormixed-modechromatographicmedia(MMC) that demonstrate several binding interactions are more tasteful substitutes for a sequence of processes that each involve a single interaction. MMC is a chromatographic techniquethatutilizesmanysortsofinteractionsbetween thestationaryphaseandthemobilephase,wherevarious solutes are present. [24] In multimodal or mixed mode chromatography, the chromatography ligand interacts selectively with the analyte molecule through a variety of interactions. These contacts can be ionic hydrophobic, hydrogenbonding-based,orevenVanderWaalsinteractions. Inthedevelopmentofmixed-modechromatography,ligands arecrucial.Theadsorbentcanoffersalttolerantqualities, improved separation, and high binding capabilities with which a wide range of medicines can be purified if the hydrophobic and ionic moieties in the mixed mode ligand arebalancedproperly.Electrostaticrepulsionbetweenthe analyteandresinligandcanresultinselectiveelutioninthe caseofhydrophobicchargeinductionchromatographywith the choice of an adequate pKa value. [18] [25]. MMC has selectivitiesandspecificitiesthataredistinctfromthoseof conventional ligands, giving it a versatility that makes it possibletoaddressavarietyofdifficultpurificationissues. [26][27].
Someofthecrucialcomponentsofaplatformdownstream processformAbswerediscussedinthisreview.Inaddition to this, several other technologies are still utilized during mAb purification. The development of chromatographic processes is a crucial step in the manufacturing of
biopharmaceuticals.Itusuallyrequiresalotofresourcesand time,whichaffectshowlongittakestolaunchaproduct.
IwanttothankMr.Nikhil,Mr.Mayur,Mr.Sufiyan,andMrs. Saeema’am,whohelpedmetocompletethismanuscript.
[1] “The treatment of cancer remains a formi,” 2007. [Online]. Available: www.nature.com/reviews/drugdisc
[2] B. Kelley, “Industrialization of mAb production technology: The bioprocessing industry at a crossroads,” mAbs,vol.1, no.5.LandesBioscience, pp.443–452,2009.doi:10.4161/mabs.1.5.9448.
[3] E. Burak Erkal, D. Baş, M. Köprülü, M. Korkmaz, D. Demirhan, and Ö. Can, “Monoklonal Antikor ÜretimleriİçinAltAkımProsesleri.”
[4] M.Iammarino,J.Nti-gyabaah,M.Chandler,D.Roush, andK.Göklen,“ImpactofCellDensityandViability,” Bioprocess Int.,no.November,pp.38–50,2007.
[5] D.J.RoushandY.Lu,“Advancesinprimaryrecovery: Centrifugation and membrane technology,” Biotechnol. Prog.,vol.24,no.3,pp.488–495,2008, doi:10.1021/bp070414x.
[6] J. H. Chon and G. Zarbis-Papastoitsis, “Advances in the production and downstream processing of antibodies,” New Biotechnology, vol. 28, no. 5. pp. 458–463,Sep.2011.doi:10.1016/j.nbt.2011.03.015.
[7] M.Grom,M.Kozorog,S.Caserman,A.Pohar,andB. Likozar, “Protein A affinity chromatography of Chinese hamster ovary (CHO) cell culture broths containingbiopharmaceuticalmonoclonalantibody (mAb): Experiments and mechanistic transport, bindingandequilibriummodeling,” J. Chromatogr. B Anal. Technol. Biomed. Life Sci.,vol.1083,pp.44–56, Apr.2018,doi:10.1016/j.jchromb.2018.02.032.
[8] S.Ghose,M.Allen,B.Hubbard,C.Brooks,andS.M. Cramer,“Antibodyvariableregioninteractionswith protein A: Implications for the development of genericpurificationprocesses,” Biotechnol. Bioeng., vol. 92, no. 6, pp. 665–673, 2005, doi: 10.1002/bit.20729.
[9] A.R.Mazzer,X.Perraud,J.Halley,J.O’Hara,andD.G. Bracewell, “Protein A chromatography increases monoclonal antibody aggregation rate during subsequent low pH virus inactivation hold,” J.
Chromatogr. A,vol.1415,pp.83–90,Oct.2015,doi: 10.1016/j.chroma.2015.08.068.
[10] A. M. Ramos-de-la-Peña, J. González-Valdez, and O. Aguilar,“ProteinAchromatography:Challengesand progress in the purification of monoclonal antibodies,” Journal of Separation Science,vol.42,no. 9.Wiley-VCHVerlag,pp.1816–1827,May01,2019. doi:10.1002/jssc.201800963.
[11] S. Hober, K. Nord, and M. Linhult, “Protein A chromatographyforantibodypurification,” Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences,vol.848,no.1.pp.40–47, Mar. 15, 2007. doi: 10.1016/j.jchromb.2006.09.030.
[12] A.A.Shukla,B.Hubbard,T.Tressel,S.Guhan,andD. Low, “Downstream processing of monoclonal antibodies-Application of platform approaches,” Journal of ChromatographyB:AnalyticalTechnologies in the Biomedical and Life Sciences,vol.848,no.1.pp. 28–39, Mar. 15, 2007. doi: 10.1016/j.jchromb.2006.09.026.
[13] Y.Imura et al., “Washing withalkalinesolutions in protein A purification improves physicochemical propertiesofmonoclonalantibodies,” Sci. Rep.,vol. 11, no. 1, Dec. 2021, doi: 10.1038/s41598-02181366-6.
[14] A. A. Shukla and P. Hinckley, “Host Cell Protein Clearance During Protein A Chromatography: Development of an Improved Column Wash Step,” 2008,doi:10.1021/bp.50.
[15] G.R.BoltonandK.K.Mehta,“Theroleofmorethan 40 years of improvement in protein A chromatography in the growth of the therapeutic antibodyindustry,” Biotechnology Progress,vol.32, no.5.JohnWileyandSonsInc.,pp.1193–1202,Sep. 01,2016.doi:10.1002/btpr.2324.
[16] A. S. Rathore, D. Kumar, and N. Kateja, “Recent developments in chromatographic purification of biopharmaceuticals,” Biotechnology Letters,vol.40, no. 6. Springer Netherlands, pp. 895–905, Jun. 01, 2018.doi:10.1007/s10529-018-2552-1.
[17] C. Brämer et al., “Membrane adsorber for the fast purificationofamonoclonalantibodyusingproteina chromatography,” Membranes (Basel).,vol.9,no.12, Dec.2019,doi:10.3390/membranes9120159.
[18] R.O’Kennedy,C.Murphy,andT.Devine,“Technology advancements in antibody purification,” Antib. Technol. J.,vol.Volume6,pp.17–32,Aug.2016,doi: 10.2147/anti.s64762.
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056 Volume: 09 Issue: 08 | Aug 2022 www.irjet.net p-ISSN: 2395-0072 © 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page328

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056 Volume: 09 Issue: 08 | Aug 2022 www.irjet.net p-ISSN: 2395-0072
[19] J. T. Way, “Ion chromatography Ion exchange chromatography,” Order A J. Theory Ordered Sets Its Appl.,pp.1–5,1970.
[20] A.Goyon et al.,“Determinationofisoelectricpoints and relative charge variants of 23 therapeutic monoclonal antibodies,” J. Chromatogr. B Anal. Technol. Biomed. Life Sci.,vol.1065–1066,pp.119–128,Oct.2017,doi:10.1016/j.jchromb.2017.09.033
[21] N. Yoshimoto, S. Hasegawa, and S. Yamamoto, “A methodfordesigningflow-throughchromatography processes,” MATEC Web Conf., vol. 268, p. 01004, 2019,doi:10.1051/matecconf/201926801004.
[22] Y.Du,A.Walsh,R.Ehrick,W.Xu,K.May,andH.Liu, “Chromatographic analysis of the acidic and basic species of recombinant monoclonal antibodies,” mAbs, vol. 4, no. 5. pp. 578–585, Sep. 2012. doi: 10.4161/mabs.21328.
[23] T. Yamada, K. Yamamoto, T. Ishihara, and S. Ohta, “Purification of monoclonal antibodies entirely in flow-throughmode,” J. Chromatogr. B Anal. Technol. Biomed. Life Sci.,vol.1061–1062,pp.110–116,Sep. 2017,doi:10.1016/j.jchromb.2017.07.002.
[24] M. Toueille, A. Uzel, J. F. Depoisier, and R. Gantier, “Designing new monoclonal antibody purification processes using mixed-mode chromatography sorbents,” J. Chromatogr. B Anal. Technol. Biomed. Life Sci.,vol.879,no.13–14,pp.836–843,Apr.2011, doi:10.1016/j.jchromb.2011.02.047.
[25] V. Halan, S. Maity, R. Bhambure, and A. S. Rathore, “Multimodal Chromatography for Purification of Biotherapeutics–AReview,” Curr. Protein Pept. Sci., vol. 20, no. 1, pp. 4–13, Nov. 2018, doi: 10.2174/1389203718666171020103559.
[26] I. F. Pinto, M. R. Aires-Barros, and A. M. Azevedo, “Multimodal chromatography: debottlenecking the downstreamprocessingofmonoclonalantibodies,” Pharm. Bioprocess., vol. 3, no. 3, pp. 263–279, Jun. 2015,doi:10.4155/pbp.15.7.
[27] K. Kallberg, H. O. Johansson, and L. Bulow, “Multimodal chromatography: An efficient tool in downstream processing of proteins,” Biotechnol. J., vol. 7, no. 12, pp. 1485–1495, 2012, doi: 10.1002/biot.201200074.
2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page329
