International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 08 | Aug 2022 www.irjet.net p-ISSN:2395-0072

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 08 | Aug 2022 www.irjet.net p-ISSN:2395-0072
1,2,3B.E Student, SSM College Of Engineering Kashmir, India 4B.E Graduate, SSM College Of Engineering Kashmir, India 4,5Associate Professor, SSM College Of Engineering Kashmir, India*** -
Abstract- As water is precious resource for sustaining life on earth, hence quality of water must be highly maintained so that water is fit for drinking purposes and the aquatic life residing inside water body possess no risk on their survival. During winter in Kashmir-India, the surface of earth gets covered by white patches that we call it snow, which creates an immense problem for the life on earth due to its increment in death rate. The roads covered by snow create risk for the people to walk over them and traffic movement is totally reduced until the roads get cleared. In order to get rid of from such a harsh condition, Govt. of Kashmir use rock-salt for melting ice (snow) from roads. When salt is applied over the Ice stricken roads, exothermic reaction takes place because of solvation process and the ice starts to melt. This salted water percolate over the surface of water and joins different water bodies particularly the river Jhelum and affects the water quality thereby. This study mainly focuses on the determination of the effects of different salts on the ice melting process and their individual effects on the water quality parameters. Different tests were conducted on the virgin water sample and on salt-applied water samples to evaluate the respective effects
because positive and negative ions of salt disturb the networkofhydrogenbonding.Asaresult,freezingpoint of the solution is reduced; this concept is called as “freezingpointdepression”.
When salt is added to ice (snow), during its initial decomposition, heat is absorbed irrespective of the hydrationprocesswhichisanexothermicreaction.Icein contactwithsaltywatermeltswhichleadstoanincrease in the amount of liquid water which further help in dissolvingmoresalt,hence,moreicetomelt.Ifmoresalt concentration is dissolved, freezing point drops to a greatextent.Ifthetemperatureisbelow0°F,saltfailsto melt ice; hence, required amount of salt is used to melt ice(snow)fromtheroads.
KeyWords: Snow, Common Salt, Rock Salt, Calcium Acetate Hydrate, Potassium Acetate, Hardness, Alkalinity.
DuringwinterinKashmir,India –duetosnowfall,roads get blocked causing a severe threat to life as the movement is restricted. People face immense problem which lead to an increase in mortality rate. In order to overcome such a circumstance, Govt. of Kashmir use rock-saltoverthesnowcoveringroads,sothaticemelts, roads get cleared and the problem faced is reduced. Since, salt makes it stiff for a water molecule to stick on one another, hence, in water salt is a salute and it will break into its elements. When salt is added to ice, temperature drops from freezing point or 0°C to -21°C
Now, the salted water after completion of melting process of snow from roads percolates over the surface of earth, joins sideway drains, streams and finally large waterbodiesmainlyriverJheluminKashmir.Duetothis salted water, the water quality parameters and the aquatic life of river Jhelum get dramatically changed. If the concentration of salt inside water body increases, there is a substantial change in the water quality parameters because of which water becomes unfit for drinkingpurposesashighsaltintakeleadstohighblood pressure which in turn cause heart diseases, kidney failures andotherhealthrisk problems. Alsocausealife risk threat to various aquatic organisms, even some die. Hence,inordertogetthebetterof,anewsaltisanalyzed formeltingiceduring winterinKashmirwhichdoesnot disturbthewaterqualityparametersandalsonotprone a threat to aquatic life thereof. Mostly, the salt suited bestformeltingice.
Various tests were performed on virgin water sample collected from river Jhelum in Kashmir at room temperatureof25°Csoastodeterminethewaterquality parametersandthereadingsduringtestprocedurewere recorded. After test on virgin water sample, 850ml of
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 08 | Aug 2022 www.irjet.net p-ISSN:2395-0072
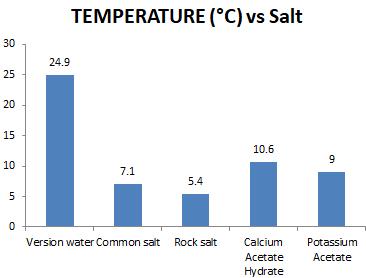
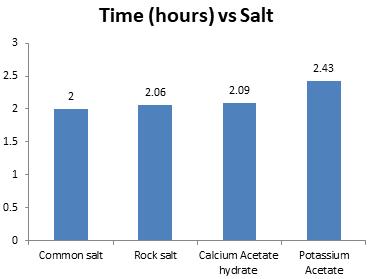
virginwatersamplewastakeninfourdifferenttreysof2 litres capacity each. The treys with virgin water sample werekeptinafreezerforabout13hourssoastofreeze thewatersample.Now,thetemperatureoftheiceblocks was measured using digital thermometer and was equal to -5°C. After this, 100g of each salt was weighed and applied to separate ice blocks within the trey. Different salts as SODIUM CHLORIDE, ROCK SALT, CALCIUM ACETATE HYDRATE and POTASSIUM ACETATE took differenttimetomelticeblocksofsamevolumeandalso thetemperatureofsaltedwaterwasrecordedsoonafter theiceblocksgotfullymelted.Againthesametestswere preceded on different salted water treys as were performedonvirginwatersample.
Fig-1: Frozeniceanddifferentsaltsappliedonice

Fig-2: Rocksalt


International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056 Volume: 09 Issue: 08 | Aug 2022 www.irjet.net p-ISSN:2395-0072
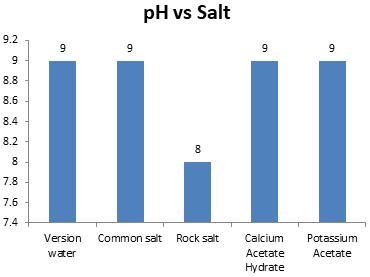

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 08 | Aug 2022 www.irjet.net p-ISSN:2395-0072
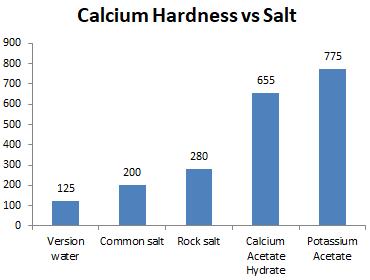
Chart-5: Effectonhardness(ppm)
Chart-6: Effectonfluoridecontent(ppm)
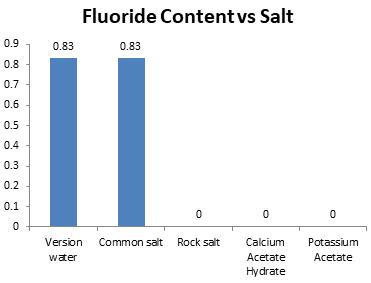
Chart-8: Effectoniron(ppm)
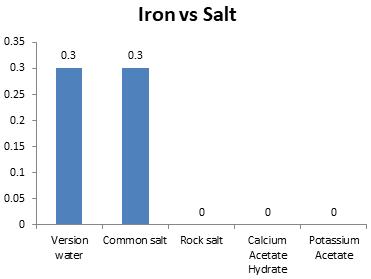
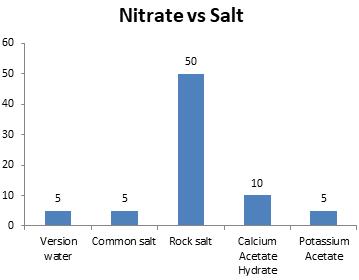
Chart-9: Effectonnitrate(ppm)
The conclusions drawn from the study and recommendationsarejotteddownas:
Temperature of ice blocks was measured and foundequalto-5°C.
Calciumhardnessofwater should bewithin the range of 200 to 400 mg/l but as the tests were performed on salted ice water, NaCl and Rock salt maintained the required range of calcium hardness.
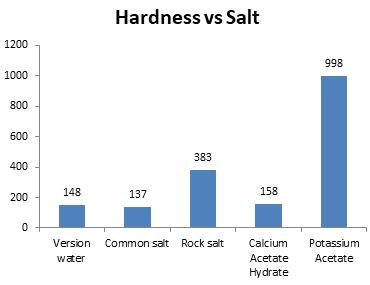
Thehardnessofwaterwhenappliedbydifferent saltsdisplacesfromthenormalrangeof0to180
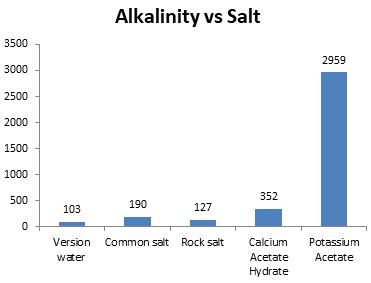
Chart-7:Effectonalkalinity(ppm)
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 08 | Aug 2022 www.irjet.net p-ISSN:2395-0072
ppm but NaCl and Calcium Acetate Hydrate sustainedtherequiredvalueofwaterhardness.
Fluoride content of water should be within the scope of 0.7 to 1.2 ppm. It was only NaCl salt whichkeptinexistencetherequiredrange.
stormwater.pca.state.mn.us/index.php/Environmental_i mpacts_of_road_salt_and_other_de-icing_chemicals.
Safe value of alkalinity of water is 20 to 200 ppm. Asdifferentsalts were applied to meltice, NaCl and Rock salt sustained the esteemed range.
[4] Gigliotti, Nicholas, et al. A Framework for Assessing Impacts of Road Salt on Groundwater Supplies in Massachusetts. Boston Project Center Worcester Polytechnic Institute, 2015, pp. 1–94, A Framework for AssessingImpactsofRoadSalton GroundwaterSupplies inMassachusetts.
Ironbestsuitedforwateris0.3ppm.Whensalts wereappliedtomelticeblocks,itwasonlyNaCl saltwhichkeptintherangeof0.3ppm.
[5] Johnson. M.R. (2008). Impacts to marine fisheries habitat from nonfishing activities in the Northeastern United States. Gloucester, MA: U.S. Dept. of Commerice, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Northeast Regional Office.
Nitratelevel of watershouldbe withinthelimit of3to10ppm.Amongallthesaltsusedtomelt ice, NaCl, Calcium Acetate Hydrate and Potassium acetate maintained the required level.
Thus,ingeneral, itwas onlyNaCl saltthatmustbeused tomelticeinKashmirduringwinterbecauseofitsability to melt snow at a faster rate and also it mostly helps to maintain the water quality parameters and does not affecttheaquaticlifethereof.
Thestudycanbecarriedforwardto:-
1. Check the effects of above road salts on irrigationwaterandcropproducts.
2. Determine the effects of the road salts on strengthandserviceabilityofroads
[1] Albers, Tregan. Best Practices for Winter MaintenanceRoadwayDeicerApplicationsintheStateof Nebraska. University of Nebraska, 2015, pp. 1–73, Best Practices for Winter Maintenance Roadway Deicer ApplicationsintheState ofNebraska.
[2] Environmental Canada, Congress, Ministry of the Environment. “Priority Substances List Assessment Report for Road Salts.” Priority Substances List AssessmentReportforRoadSalts,vol.40,ser.63,Health Canada,1999,pp.9139.63.
[3] “Environmental Impacts of Road Salt and Other DeIcing Chemicals.” Environmental Impacts of Road Salt and Other De-Icing Chemicals, Minnesota Stormwater Manual (MSM), 2000,
[6] Maine Road Salt Risk Assessment Project. University ofMaine(UoM),2009,MaineRoadSaltRiskAssessment Project. Mineau,
[7]Pierre,andLorna J.Brownlee.“RoadSaltsandBirds: an Assessment of the Risk with Particular Emphasis on Winter Finch Mortality.” Wildlife Society Bulletin, vol. 33, no. 3, 2005, pp. 835–841., doi:10.2193/0091 7648(2005)33[835:rsabaa]2.0.co;2.
[8] Montoliu, Almudena, et al. “A Novel in Vitro Tissue Culture Approach to Study Salt Stress Responses in Citrus.”PlantGrowth Regulation,vol.59,no.2,2009,pp. 179–187.,doi:10.1007/s10725-009-9401-0.
[9] Rosfjord, Catherine H., et al. “Anthropogenically DrivenChangesinChloride ComplicateInterpretationof Base Cation Trends in Lakes Recovering from Acidic Deposition.” Environmental Science & Technology, vol. 41, no. 22, 2007, pp. 7688–7693., doi:10.1021/es062334f.