International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072

International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
1Sherly Hazel Monica E R A, Dept. of civil engineering, Malabar college of engineering, Kerala, India 2Prof. M.A Chinnamma, Dept. of civil engineering, Malabar college of engineering, Kerala, India 3Assistant prof Anitha.K , Dept. of civil engineering, Malabar college of engineering, Kerala, India
Abstract As the industrial areas are the most prone areas that contributes to the air pollutants now a days, it is necessary to reduce the outcomes of air pollutions from thess areas by using some methods. Inspite of this scenario, I have selected one of the most polluted industrialareaofKeralathat is Edayar, Eloor belt in Ernakulam district forcarryingoutthe study. As several industries are situated in this area and almost all industries here contribute towards air pollution which are more acidic in nature. So, in this study the impregnated activated carbon produced from coconut shellis adopted as a medium to absorb all kinds of air pollutants present in the air particularly carbon dioxide,Sulphur dioxide, oxides of nitrogen etc which are the most serious polluting gases that would harm both the environment and the human being as well. And also in this study we present the adsorption capacity of impregnated activated carbon (ac) preparedfrom coconut shells at various optimum temperatures of25°C,35°C ,45°C and/or high concentration of impregnation(~20mol%) which were examined by me and there corresponding characteristics have been plotted by carrying out the the test in the company Activated Carbon Production Limited(ACPL)also known as Indo German chemicals ltd in Edayar, Eloor belt, with the use of a fixed bed column adsorption system designed and developed intheindustryand also the kinetic and thermodynamic parameters of the coconut shell impregnated activated carbon was analyzed in detail for acidic gases like carbon dioxide, Sulphur dioxide, oxides of nitrogen and the results were interrupted. Research on the adsorption of impregnated activated carbon (ac) produced from coconut shelladsorbentshasgainedsignificant interest due to their low cost, low regeneration energy, and ecofriendly characteristics. This would help us in future to meet out the pollution problem at economical way and also there by preparing a filter medium of appropriate quality to absorb the air pollutants and thereby saving the universe and also the future generation from this serious problem.
Key Words: Air pollution, coconut shell, Impregnated activated carbon, carbon dioxide, Sulphur dioxide, oxides of nitrogen.
As days proceed pollution is increasing day by day in the caseofairandwater.Airpollutionhasbecomemoreserious than water pollution as the control on air is very less
compared to water and we inhale air continuously throughout our life. Air gets contaminated due to several reasons, two of the most prominent ones being chemical factories and vehicles. At present the air has started been pollutedbymicroorganismslikevirusandbacteriaaswell. There are several ways by which the air gets polluted. Chemicalsandrelatedindustriesarethemainsourceofair pollution.Theothersourcesaremotorvehiclesrunningon fossil fuelsand natural gas.Theincreased populationalso pollutes the air by several means by generating different kindsofpollutants.Exceptthetreeshavingchlorophylwhich produceoxygenduringdaytimebyphotosynthesisallother livingentitiespollutetheairbycarbondioxideandconsume oxygen.Ifwehavetosurviveonearth forlong,weshould havetohavestrictcontrolontheemissionofpollutantsto theair
Theaimofthepresentprojectistoprovidesolutiontothe pollutingindustriesbyprovidingthemsuitablefilterssothat theoutgoingairispurifiedfrompollutants.Forthepurpose of this study the most polluted area of Kerala, namely Edayar Eloor area of Kochi is selected where in several pollutants are let out by several industries freely into the atmosphere.Ifthistrendcontinuesoxygenparlourswillbe requiredforgettingpureairinthisarea.Otherwise,wehave to fix suitable carbon filters at the exhaust from which pollutantsarecomingoutasemissionsfromtheindustry.
Asfaraspollutionfromthevehiclesisconcerned,vehicles give out carbon dioxide, carbon monoxide and unburnt hydrocarbonsintotheair.Theout letoftheexhaustofthe engineshouldbeattachedtoafilterwhichcanremovethese pollutants so that the air coming out will be pure. Now severalthousandsofvehiclesarepollutingtheatmosphere andinsomeoftheIndiancitiesithasbecomeaseriousissue. Soevenintheabsenceofpollutingindustriestheaircanget polluted due to large number of vehicles. Such a situation willbecomeimminentincitieslikeKochi.
OfthegasesemittedbyvehiclesCOisverypoisonous.Itisas poisonousascyanide.COwillgetattachedtohemoglobinof blood and stop the oxygen supply to the cells. CO forms a permanent bondwiththe ironofthehemoglobinandwill stopactingastheoxygencarrierfromlungstoheart.Thus theaffectedpersonwilldieimmediatelyduetothelackof oxygen in the cells. The other pollutant given out by the
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
vehicles is CO2. Even though CO2 as such is not a poison, beingheavierthanairitaccumulatesonthesurfaceofearth. It gets filled in drainages and unused wells and cuts off oxygensupply.Thisleadstothedeathofpersonsenteringto such pits. Its concentration in the air increases the atmospheric temperature as it is a polar molecule and absorbs infrared radiations entering the air. Unburnt hydrocarbonswillgodirectlytothelungsandgetssettled there and thus reduces oxygen absorption capacity of the lungs. By constant inhalation of this, the total oxygen availabilityintheblooddecreasestoagreatextentandwill leadtoseveraldiseases.
Pollutantsfromvehiclesincludeoxidesofcarbon,sulphur and unburned hydrocarbons. Pollutants from factories include a wide spectrum of chemicals depending on the factoryitself.Thesecanincludeoxidesofnitrogen,sulphur etc. Almost all of these oxides are acidic in nature. Yet anothertype ofpollutantsisammonia Hydrogensulphide andmercaptans(thiols)etc...Almostallofthesepollutants areharmfultolivingbeings.Satisfactorysolutiontopurify the air from pollution is the very need of the hour. Some citieslikeNewDelhihavealreadycomeundertheclutchesof pollutionmakingthelifeunbearablefortheinhabitants.This canhappenforourcityalsointhenearfuture.Eventhough someattemptsweremadetocurtailtheproblemofpollution by different agencies none of them have really succeeded andapermanentsolutionwasnotyetarrivedat.Theaimof theprojectistoapproachtheproblemofpollutionandsolve it. Activated carbon has been identified as the material to fight against air pollution. The reason for selecting this materialwasduetothefactthatcarboncanbeactivatedand impregnatedwithawidevarietyofmaterialstocurtailthe pollution wherein the pollutants never attack the carbon (thebase)whereasmanybasematerialslikezeolitesreact withthepollutantsandthusgetsdegradedbyitself.Carbon has three allotropes and out of this amorphous carbon is selectedforthepurposeduetoitseasyavailabilityandlow cost. The 3 forms of amorphous carbons that are easily available are (i) wood carbon (ii) coal carbon and (iii) coconut carbon. Of the three, coconut carbon is the best suited for air purification. Being a natural material, its disposalisnotaseriesproblem.Theprojectwascarriedout inACPLBinanipuram,whichisoneoftheearliestactivated coconutcarbonmanufacturingcompanyinthecountry.
1. To provide a solution for industrial air pollution by letting the polluted exhaust from the industries through a suitable filters so that the outgoing air is purifiedfrompollutants.
2. To investigate the adsorption efficiency of chemically treatedactivatedcarbonproducedfromcoconutshell forvariouspollutinggaseslikecarbondioxide,Sulphur dioxide,oxidesofnitrogenetc.
3. Tounderstandthesuitabilityofvarioustemperaturefor the adsorption of various pollutant gases on to the chemically treated activated carbon produced from coconutshell.
4. As far as pollution from the vehicles is concerned, vehiclesgiveoutcarbondioxide,carbonmonoxideand unburnt hydrocarbons into the air. The out let of the exhaust of the engine should be attached to a filter whichcanremovethesepollutantslikeCO2andunburnt hydrocarbonssothattheaircomingoutwillbepure.
5. Thepresentstudyaimstoinvestigatetheadsorptionof carbon dioxide, Sulphur dioxide, oxides of nitrogen using chemically treated activated carbon utilizing specificallyacidic
1.The study should focus on the selection of reusable chemicalcompoundforthechemicalactivationofactivated carbon. That the process may involve a complex recovery andrecycleoftheactivatingagentthatgeneratesproblems intherecoveryanddisposalofadsorbedmaterials.
2.Using impregnated activated carbon completely eliminationofpollutionfromindustrialareasandvehiclesis tobeachievedorsuitablerecommendationsistobemade.
Olivares Marin M et al (2011) Theadsorptionprocessof carbondioxide,Sulphurdioxide,oxidesofnitrogen,carbon monoxideonasolidadsorbent,canbeeasilyexploitedfor several applications aimed to these approaches. Several effectivemethodsofcarboncaptureandstorage(CCS),such as adsorption, membrane separation and cryogenic separation, have been proposed to reduce the amount of emittedCO2intheatmosphere.
S. Sumathi, et al (2009) and A. Arami Niya, et al (2019) Activatedcarbonhasbeenknownasthemosteffectiveand useful adsorbents for the removal of pollutants from pollutedgasandliquidstreams.Thisisduetotheproperties ofactivatedcarbonswhichhavealargeactivesurfacearea whichcanprovidehighadsorptioncapacity,welldeveloped porous structures and good mechanical properties. In addition,activatedcarbonismostwidelyusedsincemostof its chemical (e.g. surface groups) and physical properties (e.g.surfaceareaandporesizedistribution)canbedesigned andadjustedaccordingtotherequiredapplication.Besides, the adsorption on activated carbon appears to be most common techniques because of its simplicity of operation sincethesorbentsmaterialcanbemadehighlyefficient,easy tohandleandinsomecasestheycanberegenerated.
El Shafey et al, (2016) Physical adsorption on activated carbons has been widely used for the applications of
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
separationandpurificationofgasesandadsorptionbased gasstoragesystems.Todesignanddevelopthesesystems,it is important to determine the adsorption isotherms and isostericheatofadsorptionoftheadsorbateadsorbentpairs. Inrecentyears,considerableattentionhasbeenfocusedon removalofpollutantsbyusingadsorbentsderivedfromlow cost agro wastes. Adsorption processes are generally performedusingactivatedcarbonandpolymericadsorbents. HealsorevealedthatactivatedcarboncancaptureCO2and SO2 because it consists of a large surface area per unit volume and submicroscopic pores, in which contaminant adsorption occurs. Moreover, activated carbon is stable under acidic and basic conditions. It is also cost effective becauseitcanberegeneratedandthussuitablefororganic compound removal. Considering cost effectiveness in activated carbon production, researchers developed differentprecursorsfromabundantwastematerials,suchas palmshells,seamango,cocoapodshells,andricehusks.For instance,successfullyproducedactivatedcarbonfromrice husksandutilizedittoremoveCO2andSO2generatedfrom industrialactivitiesaswell.
Coconut shell (CS) is selected for activated carbon preparation.CSwascollectedfromthelocalcommunityin Kerala. The materials were cleaned with distilled water several times to remove dust and impurities. CS samples werelaterdriedintheovenat110oCfor24htoremoveany surfacemoistureandwerethengroundtoadesiredsize.The proximateandultimateanalysiswerecarriedouttoevaluate thevolatilesandfixedcarboncontentsaswellastoquantify theelementalcomposition,respectively.Coconutshell(CS) isselectedforactivatedcarbonpreparation.CSwascollected from the local community in Kerala. The materials were cleaned with distilled water several times to remove dust andimpurities.CSsampleswerelaterdriedinthe ovenat 110oC for 24h to remove any surface moisture and were thengroundtoadesiredsize.Theproximateandultimate analysiswerecarriedouttoevaluatethevolatilesandfixed carbon contents as well as to quantify the elemental composition,respectively.
Carboncanbeactivatedintwowaysoneofthemainis
Coconutshell(CS)wereloadedintoastainlesssteelreactor, which was heated up by an electrical tube furnace. In the initial stage, the reactor was heated up to 300oC and was keptatthistemperaturefor 30minutes.Thetemperature was later ramped up to about 800oC. At this rate, CS was
completelypyrolyzed.Waterwastheninjectedattheflow rateof120ml/hrtothereactortoactivatethesamples.The reaction between steam and carbon was taken place and porewasgenerated.Aftercompletingtheactivationprocess, thereactorwascooldown,thesampleswastakenoutand washedusingdistilledwater.
Fig 1: Flowdiagramofpreparationofsteamactivated activatedcarbon.
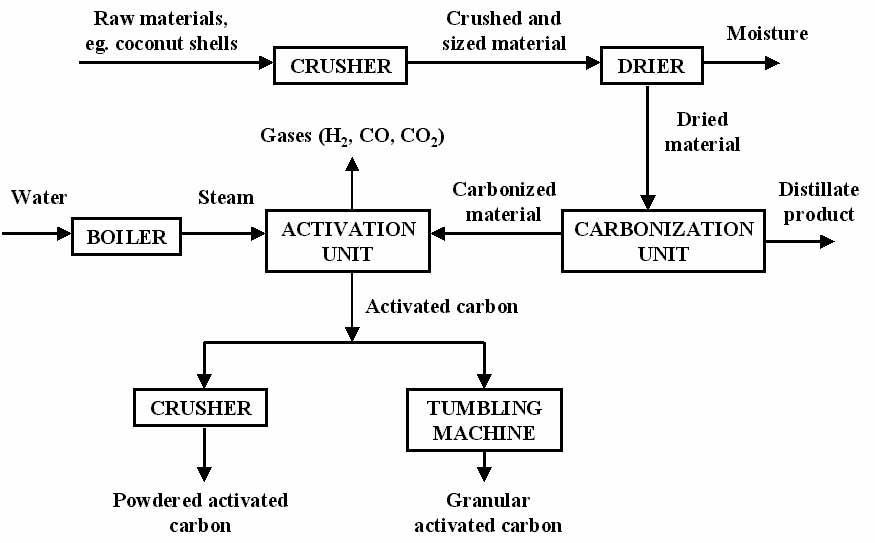
Theuseofsteamforactivationcanbeappliedtovirtuallyall raw materials. A variety of methods have been developed but all of these share the same basic principle of initial carbonizationat500 600degreesCfollowedbyactivation withsteamat800 975degreesC.Sincetheoverallreaction (converting carbon to carbon dioxide) is exothermic it is possible to utilize this energy and have a self sustaining process.
C+H2O(steam) >CO+H2( 31Kcal)
CO+½O2 >CO2(+67Kcal)
H2+½O2 >H2O(steam)(+58Kcal)
C+O2 >CO2(+94Kcal)
Rawmaterialisintroducedthroughahopperontopofthe retortandfallsundergravitythroughacentralducttowards theactivationzone.Astherawmaterialmovesslowlydown theretortthetemperatureincreasesto800 9750Candfull carbonizationtakesplace.Theactivationzone,atthebottom of the retort, covers only a small part of the total area availableanditisherethatsteamactivationtakesplace.Air isbledintothefurnacetoconverttheproductgases,COand H2 into CO2 and steam which, because of the exothermic nature of this reaction, reheats the firebricks on the downside of the retort, enabling the process to be self supporting.Every15minutesorso,thesteaminjectionpoint isalternatedtoutilizethe“insitu”heatingprovidedbythe product gas combustion. The degree of activation (or quality)oftheproductisdeterminedbytheresidencetime intheactivationzone.Theresultingproductisintheformof
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
1”to3”piecesandrequiresfurtherprocessingbeforebeing suitable for its various end uses. This entails a series of crushingandscreeningoperationstoproducespecificmesh ranges. Certain products may undergo further processing such as drying, acid washing or chemical impregnation to satisfyparticularrequirements.
The best activated carbon from physical and chemical activation were loaded with 5wt%,10wt% ,15wt% and 20wt% of selected different materials (NaOH, KOH and K2CO3)toenhancetheselectiveadsorptioncapacityofthe adsorbent. The selection of 5wt% concentration for all materialswasbasedonthepreliminarystudy.Initially,the solutions of materials were prepared in a beaker and a required amount of activated carbon was added into the solution.Themixturewasleftfor24hatroomtemperature, thentheexcesssolutionwasfilteredoutandthesolidmass was dried at 70 80 °C in oven for overnight. The dried samplewasthenplacedinthesamestainlesssteelreactor andwasheatedupto750oCandwasleftthereactoratthis temperaturefor1hundertheflowofnitrogengasattherate of200ml/min.After1h,thereactortemperaturewascool downtoroomtemperature,andtheproductwastakenout andstoredinadesiccator.
TheschemeoftheexperimentalsetupisshowninFigbelow. A fixed bed reactor set up for gaseous adsorption experiments was designed and built at the Active char ProductsPvtLtd,Edayar,Ernakulam.ForthistheActivated CoconutCarbonwasusedtoeliminatecontaminantspresent inairinapollutedindustrialarea.Columnwaspackedwith ActivatedCarbonandcontaminatedairwaspassedthrough it under normal conditions. As a typical contaminant SO2, CO2,NOandCOwaschosen.Concentrationoftheinletand outletweremeasuredusinggasdetectors.A1000ppmSO2, CO2, and NO in Nitrogen was used for the purpose. Experiment was conducted by passing this gas mixture throughthecarbonpackedingascolumnofheight4cmand diameter2cm.Itwasfoundthatthegasmixturecomingout from the column was free from SO2. The passing of gas mixturethroughthecolumnwascontinueduntiltheoutlet showedaconcentrationof50ppmofSO2,CO2,NO.

TABLE 1: Thedetailsofexperimentalsetupareshownin thetablebelow:
height of the carbon column
The flow rate of CO2 and N2 mixture are controlled by a mass flowcontrollers,maxflow600ml/minandametering valve coupled with a mass flow meter. The fixed bed adsorption of CO2 from CO2/N2 mixtures on activated carbon was studied. The single component adsorption equilibrium of CO2 and N2 were measured at feed concentration of 15mol%, temperature of 25 C, 35 C and 45 Candfeedflowrateof50ml/min.Theinletandoutlet gasesconcentrationsareanalysedwithGasChromatograph. Theadsorptionprocesswascontinueduptothesaturation point where the outlet concentration of CO2 reached the inletconcentrationofCO2.
Thecommercialactivatedcarbonofsize8x30arecompared withACimpregnatedwithNaOH,K2CO3,KOH,FeCl2etcat different concentration of 10mol%, 15mol% and 20mol% are used. The experiment is conducted at different temperatureof25°C,35°Cand45°Cwithaconstantflowof 50ml/minmixtureof15mol%CO2,SO2etcand85mol%N2. Priortotheadsorptionprocess,thesamplematerialswere weighed using a thermal gravimetric analyser (EXSTAR TG/DTA 6300) under a vacuum condition, to ensure that excessmoisturehadbeenentirelyremoved. Inasimilarway gas adsorbed AC is also weighed. The amount of gas adsorbedonadsorbents(S mol/gram)ata certain time (t sec)ataconstanttemperatureandinletconcentrationcan bedeterminedby
CO2AdsorptionCapacity=[wt(mg) w0(mg)]/w0(g)
Where,wtandworepresentsmassofadsorbentattimet andoriginalmassofadsorbent.
Theadsorptioncapacityisreportedasthenumberofmolof CO2 adsorbed per kg of adsorbent (mol/kg) and it can be converted to mg/g by multiplying by 44(CO2 molecular weight).
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056 Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072


Theaimofthepresentprojectistoprovidesolutiontothe pollutingindustriesbyprovidingthemsuitablefilterssothat the outgoing air is purified from pollutants. Activated Coconut Carbon was found to be a very good material for adsorbingcommonpollutantspresentinindustrialarea.The fixed bedadsorptionofgaseousfromCO2orSO2orNOand N2 mixtures on activated carbon was studied. The single componentadsorptionequilibriumofCO2(SO2,NO,CO)and N2weremeasuredat25 C,35 Cand45 C. Fig -2 4x30size,impregnatedcoconutshellactivated carbon. Table 2: ConsolidatedlabreportofCSACsamples Parameters of Adsorbent Test
4 15%K 2CO3 Im CSAC
4x30 11.5 15. 03 0.610 850 862
4x30 12.0 1 20. 02 0.624 780 785 6 5%KO H Im CSAC
5 20% K2CO 3Im CSAC
7 10%K OH Im CSAC
8 15%K OH Im CSAC
9 20%K OH Im CSAC
10 10.01 %Cu Im CSAC
11 10%N aOH Im CSAC
12 15%N aOH Im CSAC
13 20%N aOH Im CSAC
14 10%H 2SO4 Im CSAC
15 20%H 2SO4 Im CSAC
16 10.02 %HCL Im CSAC
17 10%K MnO4 Im CSAC
4x30 12.2 4.9 8 0.528 1035 1051
4x30 11.6 10. 04 0.580 975 980
4x30 12.4 15. 01 0.610 861 870
4x30 11.8 20. 06 0.630 790 800
4x30 12.3 12. 01 0.595 960 948
4x30 12.6 10. 04 0.560 970 950
4x30 11.2 15. 01 0.590 850 870
4x30 12.6 20. 04 0.630 800 790
4x30 11.2 10. 03 0.575 979 965
4x30 11.2 10. 03 0.574 978 965
4x30 11.4 12 0.560 1040 1048
4x30 12.0 3 11. 6 0.590 980 950
International Research Journal of Engineering and Technology (IRJET)

e ISSN: 2395 0056
Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
Coconut Shell can be used as the perfect raw material to prepare activated carbon with high surface area for CO2 (SO2,NOetc)adsorptionrate.Amongthepreparedactivated carbons,CSproducestheactivatedcarbonwithhighsurface area(1128m2/g)usingphysicalactivationtechniques.The bestofphysicalandchemicalactivatedcarbonwereloaded withdifferentalkalitofurtherimprovetheiradsorption.
10%K2 CO3 lmCSAC
15%K2 CO3lm CSAC
20% K2CO3 lm CSAC
4x3 0 10.5 47 68.23 57.683 5.469 54.691
4x3 0 10.6 9 69.11 58.42 5.465 54.649
4x3 0 10.8 1 73.12 62.31 5.764 57.641
5%KO HCSAC 4x3 0 11.0 4 96.023 84.983 7.698 76.977
10% KOH CSAC
4x3 0 11.2 1 97.68 86.47 7.714 77.136
15%KO HCSAC 4x3 0 11.4 6 100.97 89.51 7.811 78.106
20%KO HCSAC 4x3 0 11.7 3 104.64 92.91 7.921 79.207
5%NA OH CSAC
10%NA OH CSAC
4x3 0 11.0 04 88.012 77.008 6.998 69.982
4x3 0 11.0 87 89.21 78.123 7.046 70.464
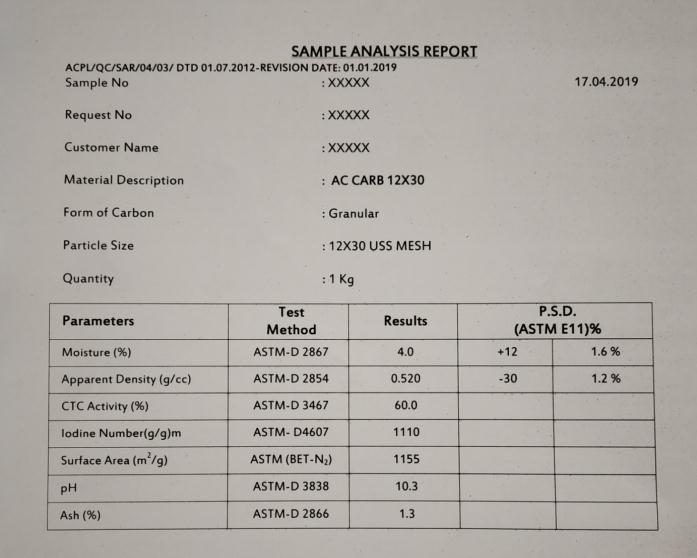
Fig 3 CertificateofAnalysisofCarbonusedisbeing attachedherewith.
4.2 Comparison of Adsorption capacity of samples at 25°C, 35°C and 45°C
Adsorption capacity of CSAC impregnated with different materials at different concentrations of 5%,10%,15% and 20% at temperatures25°C,35°Cand 45°C are tabulated in tablesbelow:
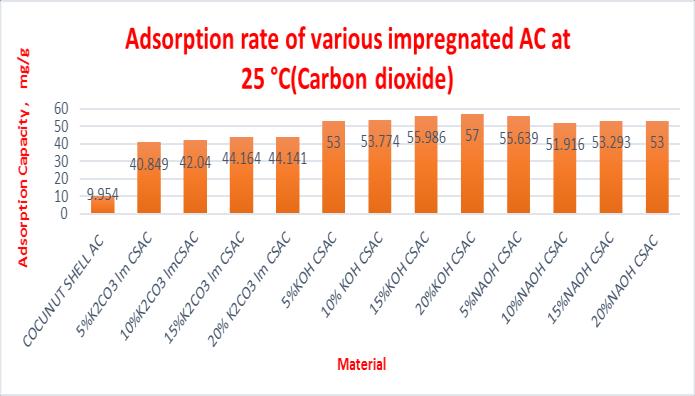
Table 3 Adsorptioncapacityofsamplesat25°C(Carbon dioxide)
Comparison of adsorbtion capacity of impregnated activated carbon at 25° C and flow rate of 50ml/min
Materia ls part icle size
Mass ofAC in bed
Massof AC afterad sorpt ion
Massof adsorb ate,C O2
COCUN UT SHELL AC
5%K2C O3lm CSAC
Mass/ gram Adsorp tion Capacit y, mg/g
4x3 0 10.9 8 22.457 11.477 1.045 10.453
15% NAOH CSAC
20%NA OH CSAC
4x3 0 11.2 33 91.56 80.327 7.151 71.510
4x3 0 11.3 2 93.16 81.84 7.230 72.297
4x3 0 10.3 4 67.11 56.77 5.490 54.903
Factor value:
At 25°C maximum adsorption capacity of CSAC & impregnatedCSACwithK2CO3,NaOHandKOHarefoundto be10.453,57.641,72.297&79.207mg/gforaconcentration of20mol%impregnation.
International Research Journal of Engineering and Technology (IRJET)

e ISSN: 2395 0056
Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
Table 4: Adsorptioncapacityofsamplesat35°C (Carbondioxide).
Comparison of adsorbtion capacity of impregnatedactivated carbon at 35 °C and flow rate of 50ml/min
Materi als particle size Mass ofAC in bed
COCU NUT SHELL AC
5%K2 CO3 lm CSAC
10%K 2CO3 lmCSA C
15%K 2CO3 lm CSAC
20% K2CO 3 lm CSAC
5%KO H CSAC
10% KOHC SAC
15%K OH CSAC
20%K OH CSAC
5%NA OH CSAC
10%N AOH CSAC
15% NAOH CSAC
Mas s of AC afte rad sor pt ion
Mass of adsor bate, CO2
Mass/g ram Adsorpti on Capacity , mg/g
4x30 10.9 22.0 1 11.11 1.019 10.193
20%N AOH CSAC
4x30 11.3 3 76.8 9 65.56 5.786 57.864
4x30 10.2 1 58.2 31 48.02 1 4.703 47.033
4x30 10.5 2 61.8 9 51.37 4.469 48.831
At 35°C maximum adsorption capacity of CSAC & impregnatedCSACwith K2CO3,NaOHandKOH arefound to be 10.193, 50.970, 57.864 & 62.619 mg/g for a concentrationof20mol%impregnation.Similartestcanbe donefor45 °C
4x30 10.6 9 63.6 5 52.97 4.960 49.597
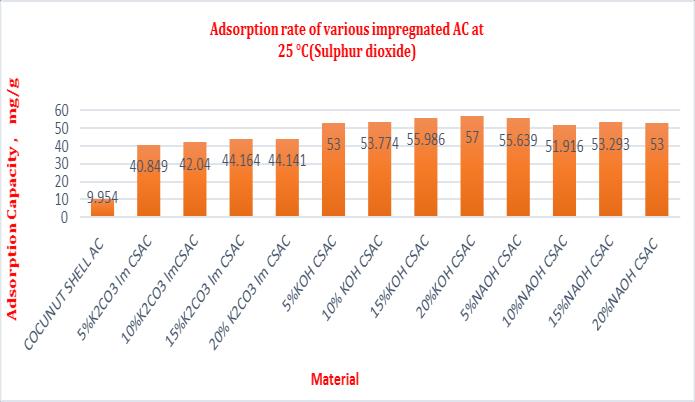
Table- 5 Adsorptioncapacityofsamplesat25°C(Sulphur dioxide)
4x30 10.8 6 66.2 13 55.35 3 5.097 50.970
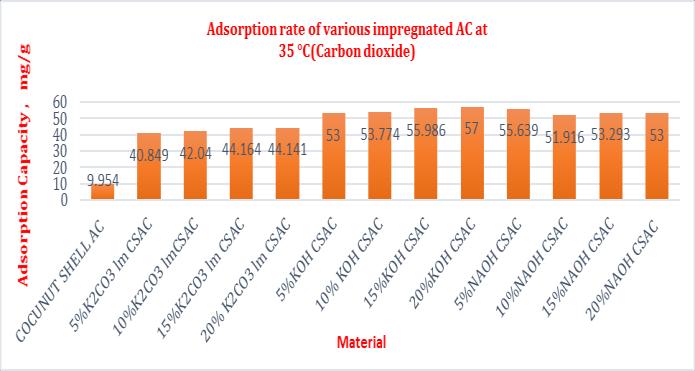
Comparison of adsorbtion capacity of impregnated activated carbon at 25° C and flow rate of 50ml/min
4x30 11.4 5 79.2 1 67.76 5.918 59.179
4x30 11.2 1 81.1 2 69.91 6.236 62.364
4x30 11.5 1 83.2 1 71.7 6.229 62.294
4x30 11.7 2 85.1 73.39 6.262 62.619
4x30 11.2 5 72.1 2 60.87 5.411 54.107
4x30 11.0 7 72.5 3 61.46 5.552 55.519
4x30 11.3 1 74.9 8 63.67 5.630 56.295
Material s parti cle size
Mas s of AC in bed
Mas s of AC after adso rpt ion
Ma ss of ads orb ate, S O2
Mass/ gram Adsorpti on Capacity , mg/g
COCUN UT SHELL AC
5%K2C O3 lm CSAC
10%K2 CO3 lmCSAC
15%K2 CO3 lm CSAC
4x30 10.9 8 27.5 46 16. 566 1.509 15.09
4x30 10.3 4 72.3 3 61. 99 5.995 59.95
4x30 10.5 47 73.1 3 62. 583 5.933 59.33
4x30 10.6 9 74.1 2 63. 43 5.934 59.34
2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

20% K2CO3 lmCSAC
4x30 10.8 1 78.2 2 67. 41 6.236 62.36
5%KOH CSAC 4x30 11.0 4 101. 020 89. 98 8.151 81.51
10% KOH CSAC
4x30 11.2 1 102. 98 91. 77 8.186 81.86
15%KO HCSAC 4x30 11.4 6 105. 13 93. 67 8.174 81.74
20%KO HCSAC 4x30 11.7 3 110. 01 98. 28 8.379 83.79
5%NAO HCSAC 4x30 11.0 04 93.0 14 82. 01 7.453 74.53
10%NA OH CSAC
15% NAOH CSAC
20%NA OH CSAC
4x30 11.0 87 95.6 7 84. 583 7.629 76.29
4x30 11.2 33 97.4 5 86. 217 7.675 76.75
4x30 11.3 2 99.0 1 87. 69 7.746 77.46
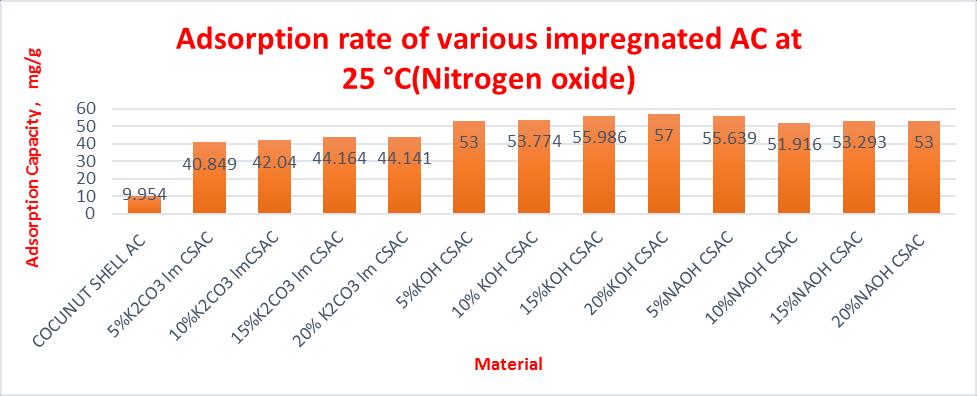
Table 6 Adsorptioncapacityofsamplesat25°C (Nitrogenoxide).
Comparison of adsorbtion capacity of impregnated activated carbon at 25° C and flow rate of 50ml/min Materia ls partic le size
Mass of AC in bed
COCUN UT SHELL AC
5%K2C O3 lm CSAC
10%K2 CO3 lmCSAC
15%K2 CO3 lm CSAC
20% K2CO3 lm CSAC
Mass of AC after adsorp tion
Mass of adsorb ate , NO
Mas s/gr am
Ads orpti on Capa city, mg/ g
4x30 10.98 24.567 13.587 1.23 7 12.3 7
4x30 10.34 70.121 59.781 5.78 2 57.8 2
4x30 10.54 7 71.01 60.463 5.73 3 57.3 3
4x30 10.69 72.12 61.43 5.74 6 57.4 6
4x30 10.81 75.14 64.33 5.95 1 59.5 1
5%KOH CSAC 4x30 11.04 99.043 88.003 7.97 1 79.7 1
10% KOH CSAC
4x30 11.21 100.78 89.57 7.99 0 79.9 0
15%KO HCSAC 4x30 11.46 104.07 92.61 8.08 1 80.8 1
20%KO HCSAC 4x30 11.73 107.96 96.23 8.20 4 82.0 4
5%NAO HCSAC 4x30 11.00 4 92.112 81.108 7.37 1 73.7 1
At 25°C maximum adsorption capacity of CSAC & impregnatedCSACwithK2CO3,NaOHandKOHarefoundto be15.09,62.36,77.46&83.79mg/gforaconcentrationof 20mol%impregnation.Similardatasarecomputedfor35 °C and45°Candfoundmaximumtobeabsorbedfor25 °C.
10%NA OH CSAC
15% NAOH CSAC
20%NA OH CSAC
4x30 11.08 7 95.51 84.423 7.61 5 76.1 5
4x30 11.23 3 94.56 83.327 7.41 8 74.1 8
4x30 11.32 97.16 85.84 7.58 3 75.8 3
Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072 © 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page382
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
regeneratedat100 150°Cwhilezeoliteusuallycannotgain their initial adsorption capacity. CO2 SO2 and NO uptake decreaseswithtemperatureduetotheexothermicnatureof CO2,SO2andNOadsorption.
Thusactivatedcarbonfiltersarethesolutiontoabsorbthe smell and other pollutants present in the air. On impregnating, activated coconut carbon with suitable chemicals,allthepollutantscanbeeffectivelyremoved.
At 25°C maximum adsorption capacity of CSAC & impregnatedCSACwithK2CO3,NaOHandKOHarefoundto be12.37,59.51,75.83&82.04mg/gfora concentrationof 20mol%impregnation.Similardatasarecomputedfor35 °C and45°Candfoundmaximumtobeabsorbedfor25 °C.
Airisincreasinglygettingpolluteddaybyday.Itseemsthat thereisnoendtoit.Unlesscontrolledatthisstageitselfour city will become a place where from nobody escapes. The capitalcityofDelhiisonlyanindicationtothis.Thenextcity canbeKochi.Apossiblesolutiontothisisgiventousbythe Creatorhimselfandthatis ActivatedCoconutCarbon was found to be a very good material for adsorbing common pollutantspresentinindustrialarea.Ithasgainedsignificant interestduetotheirlowcost,lowregenerationenergy,and ecofriendlycharacteristics.Thecurrentstudywasfocused onthesystematicdevelopmentofCSACusingdifferenttypes ofchemicalcompoundsandadsorptionconditions.
Thefixed bedadsorptionofCO2,SO2andNOfromCO2,SO2 andNO/N2mixturesonactivatedcarbonwasstudied.The single component adsorption equilibrium of CO2,SO2 and NO and N2 were measured at 25 , 35 and 45 . Accordingtotheexperimentaldataresults,itwasconfirmed thatthemicroporediffusionisthecontrollingstepforCO2, SO2 and NO adsorption on the microporous activated carbon. From the study it is clear that the adsorption capacity of activated carbon increases with decrease in temperature and also with increase in concentration of impregnation.
Overall, the coconut shell derived ACs showed the best adsorptioncapacityof79.207mg/g,83.79mg/g,82.04mg/g (at 20 mol % CO2, SO2 and NO in N2 and at decreasing temperature of 25°C). KOH activated carbon with higher surface area and porosity can be considered as the best optionforCO2,SO2andNOcaptureatatmosphericpressure andlowtemperatureof25°CforCO2,SO2andNO.
Thecapacityofactivatedcarbontoadsorbthepollutantsis usually around 10% by weight of the plane carbon. The reason for selecting this material was due to the fact that coconutshells are easilyavailableinKerala andrelatively cheap. However, activated carbon adsorbents are fully
Making filters of activated carbon impregnated with 20% KOHforacidicvapourslikeCO2,SO2andNO.Thesefilters can be fixed at the outlet of the factories polluting the atmosphere and also be used in vehicles at the outlets of enginesandbeforethecarburetor
[1] Abechi S.E., et al (2013) “Preparation and characterizationofactivatedcarbonfrompalmkernel shell by chemical activation”, Research Journal of ChemicalScience,ISSN2231 606XVol.3(7),54 61
[2] Brunetti A, Scura F, Barbieri G and Drioli E (2010) “Membrane technology for CO2 separation” J. of MembraneSci.359115 125
[3] Caglayan BS, Aksoylu AE. (2013) “CO2 adsorption on chemically modified activated carbon”. J Hazard Mater;252e253:19e28
[4] S. Choi, J.H. Drese, C.W. Jones, (2009) Adsorbent materials for carbon dioxide capture from large anthropogenicpointsources,ChemSusChem2796 854.
[5] Chiang Yu Chun,Cheng YuYehandChih HsienWeng, (2019) . “Carbon Dioxide Adsorption on Porous and Functionalized Activated Carbon Fibers Appl”. Sci.10.3390/app9101977
[6] ChungKL,ShinSL,LainCJ,ChengCW,KuenSLand Meng D L (2007) “Application of MCM 41 for dyes removalfromwastewater”J.ofHazardousMaterials147 997 1005
[7] Dantas TLP, Luna FMT, Silva Jr IJ, Torres AEB, de AzevedoDCS,RodriguesAE,etal.(2011).“Modelingof thefixed bedadsorptionofcarbondioxideandacarbon dioxide nitrogen mixture on zeolite 13x.” Brazilian Journal of Chemical Engineering.;28(3): 533544. DOI: 10.1590/S0104 66322011000300018