International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072

International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
1Western New Mexico University, Deming, New Mexico
2Early College High School, Deming, New Mexico ***
Abstract: Southern New Mexico encompasses a large portion of the Chihuahuan Desert that is located in the United States. Thedeserthostsavarietyoffloraandfauna. Orthopteransareanintegralpartoftheecosystem,andcan alsobevectorsfor diseases. Brachystola magna Girard (Orthoptera: Romaleidae), commonly known as Plains Lubber Grasshopper are large, polyphagous insects that emerge after a two year diapause. Besides being a food source for many species, they can also be infected with parasites such as Wolbachia pipientis and Oxyspirura petrowi Wolbachia pipientis is commonly known as the “male killing bacteria” and is changing the sex ratio of insect populations. Oxyspirura petrowi is a nematode that causes “eyeworm” diseaseinNorthernBobwhite Quails (Colinus virginianus) and isdecimating the quail populationin NewMexico, OklahomaandTexas.Sixty nine B. magna,14malesand55females,wererandomlycapturedbetweenJulyandNovemberof 2021 in the northern section of the Chihuahua Desert at the base of the Florida Mountains. Thirty seven specimens were tested for W. pipientis Hertig (Rickettsiates: Ehrlichiaceae) Thirty percent tested positive for W. pipientis. Fifty five were testedfor O petrowi Skrjabin(Spirurida:Thelaziidae). Forty ninepercenttestedpositivemostofthembeingfemales.
Keywords: Wolbachia pipientis, Oxyspirura petrowi, Brachystola magna, Colinus virginianus Plains Lubbers Grasshoppers, EyewormDisease,NorthernBobwhiteQuail
Brachystola magna, commonly known as the Plains Lubber Grasshopper, inhabits the plains region of the United States and Mexico.They populateseveraltypesofprairies,including desertprairies(Capinera,Scott&Walker,2004).Beingomnivores, they feed on herbaceous plants, grasses, feces, detritus, and insects including those of their own species (Wyoming AgriculturalExperimentStation,1982). Oxyspirura petrowi,aheteroxenousparasiticnematode,isdecimatingthepopulations ofbirdsintheOrderGalliformes especially C. viginianus. Thequails’populations,whichhaveanimportanteconomicimpact onthecentralplainsofOklahoma,Texas,andNewMexico,havebeendecliningatarateof4%annuallyfordecades(Houston & Easton, 2021; Sauer, et al., 2013). The United States Department of Agriculture (USDA) identified B. magna as an intermediatehost,andtheNorthernBobwhiteQuails(C virginianus)asadefinitivehost ofthe O. petrowi parasite(Brust,et al., 2014; Dunham, et al., 2016). The Northern Bobwhite quails have been especially vulnerable to the eyeworm disease thoughotherspeciesintheOrderGalliformescanbeinfected. Thelifecycleofthenematodeispoorlyunderstood;however,it is known that two hosts are needed, an intermediate host and a definitive host (Kistler, et al., 2016). Additionally, there is paucity of information on the grasshopper as well (Bright, Bernays, & Moran, 1994). In 2016, 174 B. magna were collected from the plains in Mitchell County, Texas. Thirty seven percent of the specimens were infected with third stage O. petrowi larvae Oxyspirura petrowi eggswerefoundinthefecesofboththe B. magna and C. virginianus (Blanchard,etal.,2019). Brachystola magna, a large flightless grasshopper with a reduced pink tagmen spotted in black (Richman, Lightfoot, Sutherland,&Ferguson,1993),emergesafteratwo yeardiapause. Thenymphalperiodlastsforapproximately45daysinthe wild. The maturation period before oviposition is around 23 days (Wyoming Experiment Station, 1982). Being a hemi metabolicinsect,itgoesthroughfiveinstarsbeforereachingadulthood Brachystola magna emergencestendtohaveahigher populationlevelthengrasshopperswithaone yeardiapause(Burleson,1974).Thegrasshopperisbivariant,flightless(Joern, 1981) and cryptic in rocky, gravelly soil with wild grasses, which it prefers (Jacques, 1953). Female B. magna have been
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p-ISSN: 2395-0072
documented jumping 35.6 centimeters in one hop, and males up to 2.7 meters. The females are strikingly larger than the males,somewithamassof4.5grams(WyomingAgriculturalExperimentStation,1982).
Populations of these grasshoppers can be problematic. They have been observed in Oklahoma defoliating cotton and sunflower crops creating a negative economic impact in the region (Coppock, 1962). Internal temperature regulation is important for insects. Brachystola magna regulates its internal temperatures within a tolerable parameter by varying behaviorsbetweenposturingandselectionofmicrohabitats.Selectionofmicrohabitatwasshowntobemoreimportantthan posturing (Joern, 1981). The feeding habits of B. magna have been of particular interest, due to their population numbers upon emergence. Once thought to be oligophagous (Burleson, 1974), it is now known that B. magna feeds on a variety of plants,soil,detritus,feces,plusliveanddeadinsectsincluding thoseoftheirownspecies(WyomingAgriculturalExperiment StationBulletin,1982). Beingthelargestgrasshopperontheplains, B. magna readilysubjugatessmallerspecies.Femalesfeed more often than males because of their size difference (Bright, Bernays, & Moran, 1994). Brachystola magna can also have a positiveeconomicimpact.Theyareknownfortheirpalatability,probablyduetoits toitsfeedinghabits.Theycontain64.7% unsaturatedfats,plus theyarehighinpotassium,magnesium,calciumandarelowinsodium(Monter Miranda, etal.,2018). Alsoofpositiveeconomicimportance,thechitosanfilmsfrom B. magna haveproventobeexcellentsausagecasingthatresist deteriorationovertime(Tirado Gallegos,etal.,2021).
Oxyspirura petrowi, a heteroxenous parasitic nematode, infects all organs of the visual and the olfactory systems of avian species (Dunham,etal.,2016).Thenematodehasbeen foundinmanywildavian species,buthasparticularlydecimatedthe NorthernBobwhitepopulationsofTexas,NewMexicoandOklahoma.Theoccurrenceof Oxyspirura petrowi infectionhasbeen increasinginquailpopulationsinrecentyears(Dunham,etal.,2016;Kalyanasundaram,etal.,2019).
Wolbachia pipientis is a member of a classification of bacteria that infects arthropods and nematodes plus cause parthenogenesis and cytoplasmic incompatibility. These microorganisms are abundant in insects and form a monophyletic group,thealphadivisionofProteobacteria(Stouthamer,Breeuwer,Luck,&Werren,1993).Thesebacterialendosymbiontsare cytoplasmicallyinherited AlphaProteobacteriaaltershostchromosomalbehaviorbymanipulatingtheearlymitoticdivisions of the egg and sperm. Cytoplasmic incompatibility bacteria interfere with the paternal chromosomes, and parthenogenesis bacteria prevent segregation of the chromosomes in the egg (Stouthamer, Breeuwer, Luck, & Werren, 1993). Wolbachia pipientis infestation uses these methods among others to manipulate the reproduction of arthropods causing cytoplasmic incompatibility, feminization, parthenogenesis, sterilization, and male killing which decreases the number of progeny and skewsthemale/femaleratiosinarthropodpopulations(Fukui,etal.,2015;Rech,etal.,2020). Wolbachia pipientis isknown to be a master manipulator of insect reproductive systems in order to enhance its own spread and survival (Weeks & Breeuwer, 2001). Insect hosts attempt to produce an even sex ratio, however, Wolbachia’s manipulation of insect gametes leads to increased female progeny which results in uneven sex ratios (Stouthamer, 2001). In some species, this has changed matingbehaviors.Usually,themaleinsects competefor femalemates.But,in Acraea encedon,a Ugandanbutterfly,therehas beenareversalofmatingbehavior. Now,thefemalescompeteforthemales (Jiggins,Hurst,&Majerus,2000). Extremeratio reductions,likethatoftheonecited,canleadtotheextinctionofaspeciesorrestricthabitats(Stouthamer,2001).Thisstudy investigatestheinteractionbetween Wolbachia pipientis, Brachystola magna and Oxyspirura petrowi
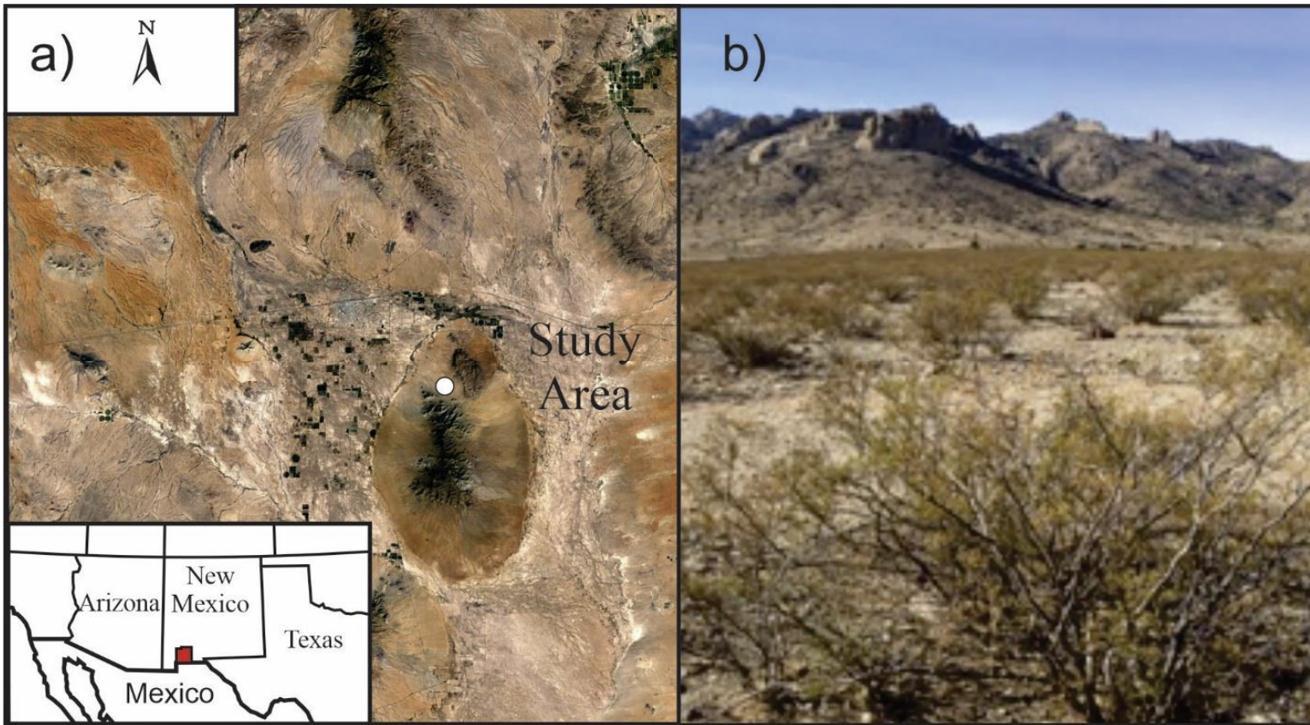
Brachystola magna specimens were collected from a five hectare area (~32.17ᵒN, 107.63ᵒW) at an elevation of ~14,000 meters from the bajada on the north side of the Florida Mountains near Deming, New Mexico. The Florida Mountains are an inactive fault block range comprised of Paleozoic limestone and dolomite rocks that extend north south for 38.6 kilometers (Clemons,1998).
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
1.
The B. magna emergencebeganinJulyof2021. ThespecimenswerecollectedbetweenJulyandNovemberofthatyear.Many species of flora and fauna inhabit the area. Creosote bushes, Larrea tridentate (Coville) are a major component of the area, comprising 98% of the vegetation. They are an invasive species from Argentina arriving approximately 1000 years ago. The bushes are high in nordihydroguaiaretic acid which serves as a repellent for B. magna. Wild grasses, Joshua trees, Yucca brevifolia (Engelm), honey mesquite trees, Prosopis glandulosa (Fabaceae) and cacti in the genus Opuntia are interspersed betweenthecreosotebushes(Dodson,2012;Salisbury&Ross,1992) Thehightemperaturesfortheregionaveragefrom35ᵒ C to19.44ᵒC betweenJulyandNovember. Thelowsarebetween19.44ᵒCand2.22ᵒC. Theaverage rainfall peryearisless than25.4centimeters(NOAA,2021).
METHODS: Oxyspirura petrowi examination Brachystola magna specimens were kept at 4ᵒ C after capture, and then thawedfordissection.Dissectionproceededunderadissectingmicroscope.Dissectingscissorswereusedtocutthespecimens fromtheventralposteriortotheventralanterior.Thespecimenswereeviscerated,andtheinternalorganswereexaminedfor nematodelarva.Larvawereremovedandcounted.Finally,theprocuticleswereinspectedforeggs.Allresultswererecorded.
Wolbachia and Insect DNA extraction and PCR protocols. Two millimeters (mm) were removed from the specimen’s posteriorabdomen.Theabdominalsegmentwasthenplacedina1.5milliliters(mL)microfugetubewith200microliters(µL) of lysis buffer. The abdominal segment was macerated for 1 minute. Eight hundred µL of lysis buffer was added to the microfuge tube then vortexed. The tube was placed in a 99°C water bath for 5 minutes. After heating, the tube was opened brieflytoreleasepressurethencentrifugedfor8minutesat10,000rpm.Anothermicrofugetubewasobtainedand400µLof the supernatant and put into the new tube. Forty µL of 5.0 M NaCl was added and placed on ice for 5 minutes. Tubes were placedinthecentrifugeattherpm’sandtimeaspreviouslystated.Anothercleanmicrofugetubewasobtainedand300µLof supernatant was transferred. Four hundred microliters of isopropanol was added and then centrifuged at 10,000 rpm for 8 minutes.Thesupernatantwascarefullypouredoutandthemouthoftubewastappedlightlytoremovemostoftheliquid.The pelletwasairdriedfor10minutes.Two hundredµLofTE/RNasewasadded.Thepelletwasdisturbedbypipetting andthen tube was centrifuged at 10,000 rpm for 1 minute. The DNA was frozen until PCR amplification. PCR amplification was done withaBio Radthermocyclert100;PuReTaq™Ready To Go™PCRbeadswereused.TheDNAwasthawed.Twentymicroliters ofprimerwasaddedtothePCRbeadalongwith5µLofextractedDNA.Primerfor16SrDNAwasusedtoidentify W. pipientis, andprimerfortheCytochromeCoxidasegenewasusedtoidentifyinsectDNA. PCRcyclesincluded95degreesfor2minutes, 30cyclesof:94degreesfor 30seconds,55degreesfor 45seconds,72degreesfor 1minute,then72degreesfor10minutes, and finally left at 4 degrees for the rest of the allotted time. One point two percent agarose electrophoresis gels were run at
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056 Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072

150V for 30 minutes. SYBR safe green loading dye was used with lithium bromide buffer. An EDVOTEK TruBlu2 DNA illuminator was used to view the DNA. Wolbachia pipientis DNA is identified at 438 kilo basepairs (kbp) and insect DNA is identifiedat708kbp.
RESULTS: Sixty nine B. magna were randomly captured during their emergence season, 14 males and 55 females. The sex ratioshowsaskewof3.9femalesforeachmaleasopposedtoanormal1:1ratio,whichindicatedthepresenceof W. pipientis Thirty sevenofthespecimensweretestedfor W. pipientis Thirtypercentof B. magna testedwerepositivefor Wolbachia Of thatsample,eighty twopercentofthe W. pipientis infectedspecimenswerefemale(table1).

Table1.W.pipientis&O.petrowiInfectionRates

Specimens W. pipientis Infection W. pipientis & O. petrowi Infection
TotalInfected 11 7 Male 2 0 Female 9 7 Specimens Eggs Larva Total 55 26 27 Male 7 0 3 Female 48 26 24
Table2.NumberofSpecimensInfectedwithEggsandLarva
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
Thesespecimenswerealsotestedfor O. petrowi;sevenspecimenstestedpositiveforbothparasites. Oxyspirura petrowi DNA isidentifiedat218kbp.Allofthespecimensthattestedpositiveforbothparasiteswerefemales.
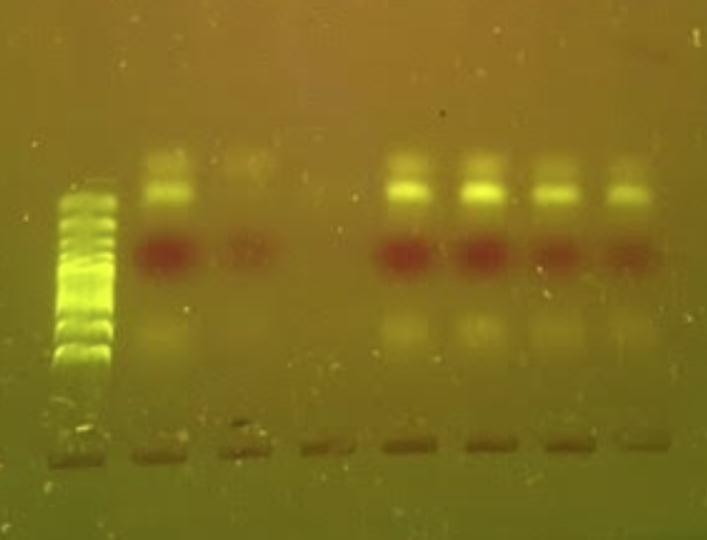
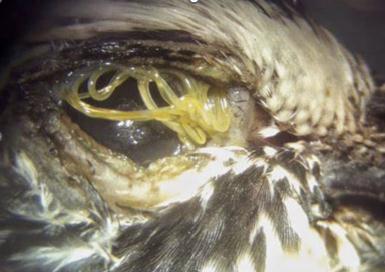
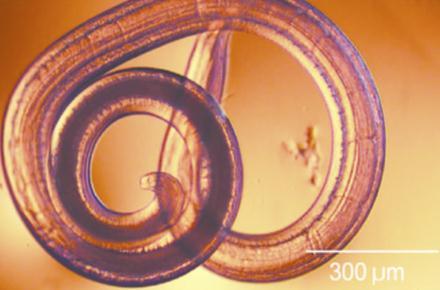
Fifty five B. magna weretestedfor O. petrowi. Ofthosespecimens,7malesand48femaleswerefoundtocontaineitherlarva, eggsorboth(table2).Noneofthemale B. magna contained O. petrowi eggs. Threecontainedlarva.However,those3werenot infectedwith W. pipientis Twenty sixoutof48females,or54%contained O. petrowi eggs,and24ofthe48,or50%contained larva. Therefore, 44% of the females tested contained both larva and eggs. All of the larva (figure 2) were found in the abdominalentrails(figure2).Theeggswerefoundattachedtotheperi cuticalofthe B. magna specimens Theelectrophoresis gel(figure3)showsthedoubleinfectionofspecimens54,35,47,63,and70. Fourteenofthe B. magna werenottesteddueto anerrorinextractingtheDNA.
Figure4 (a) Brachystola magna,(b) Wolbachia pipientis,(c) Oxyspirura petrowi,(d) Colinus virginanus infectedwithadvancedeyewormdisease




Oxyspirura petrowi and W pipientis are both endosymbiont parasites. Oxyspirura petrowi requires an intermediate and a definitive host, W. pipientis does not. The relationship between the two parasites, and their hosts is poorly understood. Our results imply a direct correlation between the rising W. pipientis infection rate, and the increase in O. petrowi infections that aredecimatingtheNorthernBobwhiteQuail(C. virginianus).
We propose that the cycle of infection between B. magna and O. petrowi is exasperated by the increasing infection rates of insectsby W. pipientis. Brachystola magna becomeinfected W. pipientis throughverticalandhorizontaltransferthusskewing thesexratiooftheinsects.Thegrasshoppersarepronetoinfectionviahorizontaltransferduetotheirfeedinghabits.Females B. magna are larger than males and therefore consume an increased amount of biomass strengthening the probability that theywillconsume O. petrowi eggsthroughcannibalismandengulfingfeces.NorthernBobwhitequailhaveahigherprobability ofconsumingfemale B. magna simplybecausetherearemorefemalesdueto W. pipientis infection,plusthefemalesarelarger andmorevisible. Colinus virginanus thanconsumethegrasshoppersandbecomeinfectedwiththenematode.Thecycleisself perpetuating. Much more research is needed to corroborate our findings. Brachystola magna will emerge again in 2023, allowingforanotheravenueofresearch.Atthat time,weareplanningtocapturealargersampleofspecimens,testthemfor both W. pipientis and O. petrowi,andcomparetheresultstoourinitialfindings.
[1] Blanchard, K., Kalyanasundaram, A., Henry, C., Commons, K., Brym, M. S., Surles, J., &Kendall, R. (2019). Identification of eyeworm (Oxyspirura petrowi) and caecal worm (Aulonocephalus pennula) infections levels in Northern Bobwhite quail (Colinus (virginianus) of the rolling plains, TX using a mobile research laboratory: Implication for regional sourveillanc. Biomolecular Detection and Quantification, 17,1 5.
[2]Bright,K.,Bernays,E.,&Moran,V.(1994).Foragingpatternsanddietarymixinginthefieldbythegeneralistgrasshopper Brachystola magna (Orthoptera:Acrididae). Journal of Insect Behavior, 7(6),779 793.
[3]Brust,M.,Thurman,J.,Reuter,C.,Black,L.,Quartarone,R.,&Redford,A.(2014). Grasshoppers of the western United States, 4th edition. FortCollins:UnitedStatesDepartmentofAgricultureAnimalandPlantHealthInspectionServices.

International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
[4] Burleson, W. (1974). Two year life cycle in Brachystola magna (Orthoptera: Acrididae) with notes on rearing and food preference. Annals of the Entomological Society of America, 67(3),526 528.
[5] Capinera, J., Scott, R., & Walker, T. (2004). Field Guide to Grasshoppers, Katydids and Crickets of the United States. Ithaca: CornellUniversityPress.
[6] Clemons, R. (1998). Geology of the Florida Mountains southwestern New Mexico. Socorro: New Mexico Institute of Mining andTechnology.
[7]Coppock,S.(1962). The grasshoppers of Oklahoma. Stillwater:OklahomaStateUniversity.
[8]Dodson,C.(2012). A Guide to Plants of the Northern Chihuahuan Desert. LasCruces:UniversityofNewMexicoPress.
[9]Dunham,N.,Bruno,A.,Almas,S.,Rollins,D.,Fedynich,A.,Presley,S.,&Kendall,R.(2016).Eyeworms(Oxyspirura petrowi) in the Northern Bobwhites (Colinus virginianus) from the rolling plains ecoregion of Texas and Oklahoma. Journal of Wildlife Diseases, 52(3),562 567.
[10] Fukui, T., Kawamoto, M., Shoji, K., Kiuchi, T., Sugano, S., Shimada, T., Katsuma, S. (2015). The endosymbiotic bacterium Wolbachia selectivelykillsmalehostsbytargetingthemasculinizinggene. PLOS Pathogens, 11(7).
[11] Houston, A., & Easton, J. (2021, December 16). The decline of the bobwhite quail populations during the 20th century Retrieved from Mossy Oak: https://www.mossyoak.com/our obsession/blogs/small game/the decline of bobwhite quail populations during the 20th century
[12]Jacques,R.(1953). The grasshoppers, cockroaches, and their allies. Dubuque:WilliamC.BrownCompany.
[13] Jiggins, F., Hurst, G., & Majerus, M. (2000). Sex ratio distorting Wolbachia causes sex role reversal in its butterfly host. Proceedings of the Royal Society of London, 267,69 73.
[14]Joern,A.(1981).Importanceofbehaviorandcolorationinthecontrolofbedytemperatureby Brachystola magna Girard (Orthoptera:Acrididae). Association d'acridologie, 10(3),117 130.
[15]Kalyanasundaram,A.,Brym,M.,Blanchard,K.,Henry,C.,Skinner,K.,Henry,B.,Dendall,R.(2019).Life cycleof Oxyspirura petrowi (Spirurida:Thelaziidae),aneyewormofthenorthernbobwhitequail(Colinus virginianus). Parasites & Vectors, 12,1 10.
[16] Kistler, W., Hock, S., Hernout, B., Brake, E., Williams, N., Downing, C., Kendall, R. (2016). Plains lubber grasshopper (Brachystola magna) as a potential intermediate host for Oxyspirura petrowi in northern bobwhites (Colinus virginianus). Cambridge University Press,1 8.
[17]Monter Miranda,J.,Zamudio Flores,P.,Tirado Gallegos,J.,Molina Corral,F.,Ochoa Reyes,E.,Rios Velasco,C.,Lopezdela pena, H. (2018). Nutritional characterization of fatty acids and minerals in Brachystola magna (Girard) during their development. Emirates Journal of Food and Agriculture, 30(5),389 395.
[18] NOAA. (2021, November 15). National Weather Service. Retrieved from Luna County, New Mexico: https://www.weather.gov/
[19] Rech, N., Darrow, A., Lopez, E., Mendoza, D., Nicoll, V., & Paulk, L. (2020). Prevalence of Wolbachia pipientis and Varroa destructor mitesinafricanizedbeesintheDeming,NewMexicoarea. International Journal of Science, Environment and Technology, 9(2),274 284.
[20]Richman,D.,Lightfoot,D.,Sutherland,C.,&Ferguson,D.(1993). A Manual of the Grasshoppers of New Mexico . LasCruces: NewMexicoStateUniversity.
[21]Salisbury,F.R.(1992). Plant Physiology, Fourth Edition. Belmont:WadsworthPublishingCompany.
[22]Stouthamer,R.(2001).MeettheHerodbug. Nature, 412,12 14.
[23] Stouthamer, R., Breeuwert, J., Luck, R., & Werren, J. (1993). Molecular identification of the microorganisms associated withparthenogenesis. Nature, 361,66 68.
[24] Tirado Gallegos, J., Zamudo Flores, P., Espino Diaz, M., Salgado Delgado, R., Vela Gutierrez, G., Hernandez Centeno, F., Ortega Ortega, A. (2021). Chitosan films obtained from Brachystola magna (Girard) and its evaluation on quality attributesinsausagesduringstorage. Molecules, 26(1782),1 19.
[25] Weeks, A., & Breeuwer, J. (2001). Wolbachia induced parthenogenesis in a genus of phytophagous mites. The Royal Society, 268(1482),2245 2251.
[26] Wyoming Agricultural Experiment Station. (1982). Plains Lubber Grasshopper Brachystola magna (Girard) Fact Sheet. Wyoming USDA, Entomology. Laramie: Wyoming Agricultural Experiment Station. Retrieved January Friday , 2022, from https://www.ars.usda.gov/ARSUserFiles/30320505/grasshopper/Extras/PDFs/Species%20Fact%20Sheets/PlainsL b.pdf
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056 Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072 © 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page2863
