International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072

International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
1PG Student, 1Department of Civil Engineering, Mar Athanasius College of Engineering, Kothamangalam, Kerala, India. ***
Abstract The most frequently using construction material on the planet is concrete. The environment is polluted by CO2 emissions from the making of Ordinary Portland cement. One of the materials that could be utilised to effectively replace concrete is alkaline activated slag concrete. Alkaline activated slag concrete (AASC) is an inorganic alumino silicate polymer consisting primarily of silicon, aluminium, and waste materials such as slag and fly ash. When compared to OPC, fly ash and slag are more environmentally efficient and sustainable less. With the increased deterioration of concrete, strength can no longer be use as the primary criterion for judging concrete quality. The concrete's strength and durability must both be proved. Dumped acidic effluents from companies have been identified. Acidic effluents from industries have been discovered to be dumped into the environment without being properly treated. Again, those acids may be natural in addition to inorganic acids. Organic acids are classified as susceptible acids, as opposed to inorganic acids, due to their partially dissociative nature. The mechanism of acid attack varies mainly depending on the type of acid and the properties of the calcium salt that can be formed. Traditional Portland Cement (OPC) concretes are not acid resistant. Additionally, as we move towards sustainable development, alkali activated or geopolymeric concrete has started to gain interest due to its miles of higher mechanical residence time and strength compared to standard concrete. This article evaluates the damage mechanisms of sulfuric, hydrochloric, citric, nitric, and acetic acids in alkali activated binders.
Key Words: Acid resistance, Durability, Fly Ash Geopolymerconcrete,Alkalineactivatedslagconcrete,Slag Geopolymerconcrete,Alkalineactivation.
Intoday'ssociety,concreteisoneoftheprincipalbuilding materials. Due to greenhouse gas emissions as from production of concrete, which are a vital factor in global warming, the industry has a significant impact on the environment. The fundamental elements of concrete are cement, water, fine aggregates, and coarse aggregates. However, the PC is regarded as the primary source of gas emissions in the production of concrete, accounting for between 74 and 81 percent of the CO2 emissions from typical concrete mixes [3]. Though many research studies havebeenperformedonAACasanalternativetoPCCafew decades ago, it has gained popularity as a construction
material.BecauseAACcanbemanufacturedwithouttheuse of PC, it can be considered green concrete. AAC has been shown to have superior mechanical properties while also limiting CO2 emissions. Furthermore, AAC emits less CO2 than PCC. AAC not only reduces CO2 emissions but also consumesasignificantamountofindustrialwastesuchas slagandflyash.AACismadeupofalumino silicatesources (suchasgroundgranulatedblastfurnaceslag(GGBFS),fly ash (FA), or silica fume (SF), alkali activators (such as silicates, hydroxides, or carbonates), water, and fine and coarseaggregates
Concreteisasuperiorbuildingmaterialthathasbeenwidely used in the production industry worldwide. Due to the growthofbusinesssportsinurbanareas,concretesystems are exposed to aggressive environments. Acid assault on concreteisahugelocationwhereinseveralresearcheshas taken area due the non stop deterioration of concrete systemsresultingfromthosecompetitivespeciesovertime. Concrete is located to be in disequilibrium with its surroundingsbecauseofitsalkalinenature.TheCa2+and OH ions must obtain described levels of attention for the hydratedcompoundstobestrong.Concretedeterioratesasa result of the hydrated compounds' hydrolytic decomposition,whichcausesdistortionofthecementmatrix asthepHofthesolutionactuallyreduces[5]
Acidsthatattackconcreteisorganicorinorganicinnature. Theattackoforganicacidsismorecomplexwithinthefood and agricultural industries. Corrosion causes to biological vitriol is one in all the fastest causes of concrete deterioration. Between 2002 and 2022, the us alone estimated $390 billion to repair existing wastewater infrastructurethankstobiocorrosion[6].Theentryofthose aggressive species can affect the porous network, the mechanicalpropertiesofthestructure.thesortofacid,its concentration,thepH,thesolubilityofthesaltsformedarea numberofthemostfactorsthatinfluencetheaggressiveness of those species. Both organic and inorganic acids are structurally destructive, but there are differences in their decompositionmechanisms.
Asofpresently,criticalamountofthinksaboutcanvasesis executed to upgrade concrete with modern creation substances emphasizing on maintainability, diminished carbon impressions, sturdiness and eco neighborliness. AntacidenactedorGeopolymerconcretehasstartedoutto
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
advantage intrigued as it's miles found to have higher mechanicalhomesandsturdinessassessingtoconventional concrete.Thecoversutilizedmustbeaffluentinsilicaand alumina with diminish calcium substance. Impact heater slag,coalinferredflyfierydebris,calcinedclaysandhome grownpozzolansareanumberofthefoliosfoundtodisplay fitting comes about[4]. But more prominent amount of thinks about canvases is ordinarily prescribed for forcing geopolymer concrete nearly since of its workability and putting time issues. In spite of the fact that geopolymer periodhaspositivedrawbacks,ithascapacitytobeamoo carbonimpressiontextureopportunitytotheconventional concrete. Thus, this paper exclusively portrays about acid mechanismonAASCindifferentenvironmentslikesulphuric acid,nitricacid,hydrochloricacid,aceticacidandcitricacid.
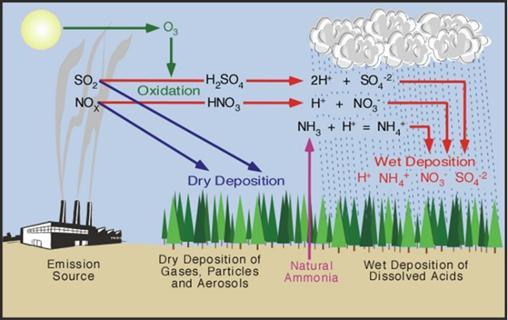
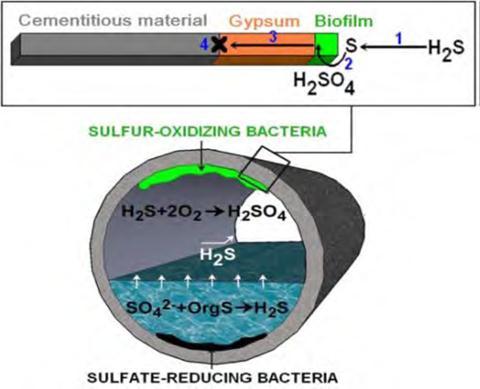
Acidattackisacommonoccurrenceinindustriesthatstilluse chemical acids for various processing. Acid discharge and improper disposal in such industries will result in rapid degradationofconcretecomponentsifacidsgetsincontact with them. One of the primary strong sources of concrete degradationissulphuricacid.Ingeneral,aerobicbacteriaina sewercollectionsystemreactwithhydrogensulphide(H2S) gas to produce sulphuric acid (H2SO4). These acids then permeate the concrete, causing leaching and, eventually, concretedisintegration.Theconcreteinsidebiogasreactors digesters are also prone to deterioration due to the production of organic acids and ammonium in the liquid phaseandH2SandCO2gasesinthegasphase.Thefertilizer manufacturingindustriesusesnitricacidandsulphuricacid intheproductionofammoniumnitrateandsuperphosphate fertilizers[2]. Industrial emissions of sulphur and nitrogen compounds, can be later converted to sulphuric and nitric acidsandleadtoacidprecipitationswithapHlevelranging from5.0to3.0.Acidicrainormististhenanotheraggressive agentforconcretestructures[8].
Mechanicalplantsandproductionlinesutilizingdiverse formsutilizeorcreateaassortmentofmanufacturedacids. Thecoincidentalspillageandspillageoftheseacidscantruly harm concrete structures, as the concentration of acids assaultingconcretecanreachdisturbingvalues.Otherthan the real generation of these acids, the fertilizer industry employments nitric corrosive (HNO3) and sulfuric acid(H2SO4) or phosphoric acid(H3PO4) within the generation of ammonium nitrate (NH4NO3)) and superphosphate(Ca(H2PO4)2),separately[6 10].Theglass industry employments hydrofluoric corrosive (HF) as a destructiveoperator.Essentially,numerousacidstakepartin metalhandlingunits.Inspecific,hydrochloriccorrosive(HCl) isbroadlyutilizedwithintherustexpulsionpreparewithin thesteelindustrytoexpeltherustlayersonthesteelsurface. Superphosphatefertilizersutilizedinagribusinessmayhave thenearnessoffreesulfuricandphosphoricacids.Sewage, beneathperfectconditions,canharmconcretesewerline.Fig 1showstheproductionofsulphuricacidinsewageunits.
In addition to sulphuric and nitric acids, industrial emissions of sulphur and nitrogen compounds may be oxidisedandtransformed.BecausethepHcostislow,rain containing those acids will be destructive to cementitious materials(pHapproximately3 5).Asaresult,acidrainwill beanadditionalcompetitivewayforconcretestructures[11] Figure 2 depicts the formation of sulphuricacid and nitric acid,whichresultsinacidrain.
Organicacidattacktakesplaceinindustrialapplicationsas well,butitismostcommonlycausedbyeffluentsdischarged intheagricultureandfoodindustries.Dependingonthetype of wastewater output, this effluent contains organic acids such as acetic, propionic, tartaric, oxalic, lactic, citric, and others[13]Theproductionoforganicacidsandammoniumin theliquidphase,aswellasH2SandCO2inthegaseousphase, canalsodamagetheconcreteinsidethecompostingpit
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

The first use of alkaline activated materials dates back to 1930,whenKuhlstudiedmixturesconsistingofGGBFSand analkalinesolutionofKOH.ThereactivityofGGBFSusing alkaline solutions of KOH and NaOH was studied by Chasseventin1937[14].Inaddition,GGBFSwasactivatedby PurdonwithNaOHsolutionin[15].
In1959,Gluskhovskywasthefirsttostudytheproductionof bindersusingafreeorweaklybasiccalciumaluminosilicate source (clay) with alkaline activators [16]He developed a new binder named ‘soil cement', soil referring to the appearance of crushed stone and cement referring to its adhesiveability.
In1980,additionallyGluskhovskyinvestigatedtheactivation ofGGBFS:(a)describingthehydrationmerchandisewhich consistedofcalciumsilicatehydratesandsodiumalumino silicatehydrates,and(b)reportingthatthealkali activated clay minerals fashioned aluminum silicate hydrates (zeolite)[20]
n 1984, Davidovits obtained a binder by mixing kaolinite, limestone, and dolomite with an alkaline solution. The resulting binder is called `geopolymer’ because of its polymericstructure(TimothyA.Aiken,JacekKwasny,Wei Sha,2021). In addition, it has released several brands for adhesivessuchasGeopolycemandPyrament.KyivNational UniversityorganizedtwointernationalconferencesonAAC in1994and1999[18].
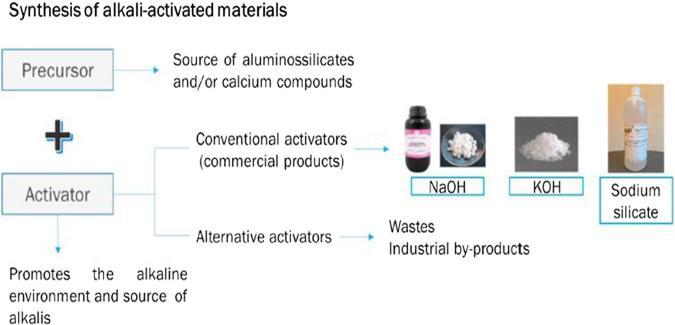
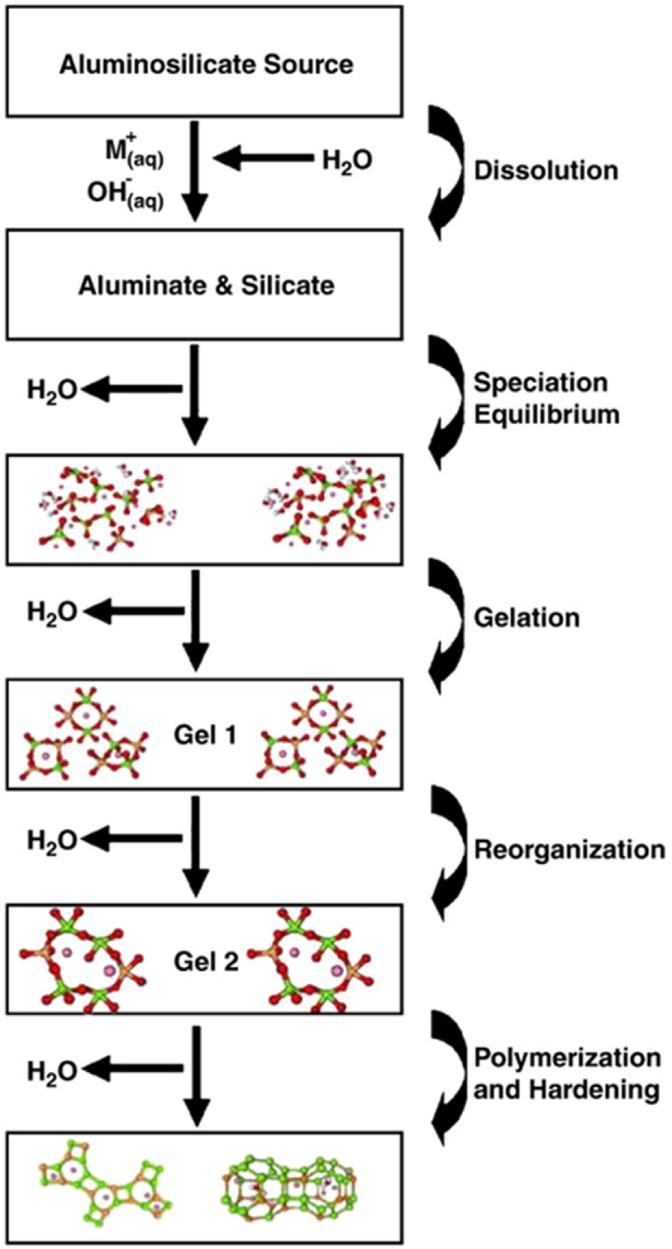
One or more sources of aluminosilicates and one or more alkalineactivatorsconstituteanalkalineactivatorsystem. The illustration in fig. 3 depicts the creation of alkali activatedmaterial.HighpHenvironmentsaredevelopedby activation solutions (e.g. hydroxide, silicate, carbonate or sulfate).Pre mixeddrymaterialsmadeofthealuminosilicate sourceandalkaliactivatorcanthenbecombinedwithwater andaggregatestoestablishmortarorconcrete.Alternately, the alkaline activator can be added to the aluminosilicate source separately. This wet binder is then combined with additional water (if necessary to dilute the lye concentration), gathered, and used to make mortar or concrete.Aluminosilicatesource,alkaliactivator,water,and aggregate can also be utilized to develop AASC.[13]. The Cementitiousalkali activatedsystemcomponentsareshown inFig.4.
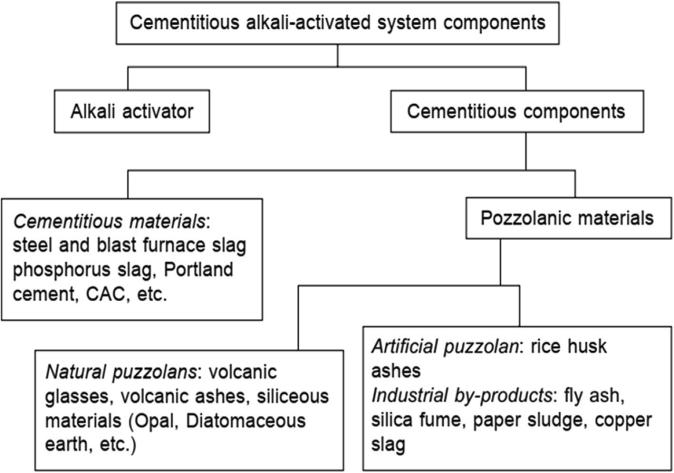
Fig 4: Cementitiousalkaliactivatedsystemcomponents
AASC are inorganic polymers that are amorphous rather thancrystallineinnature.Theyareconsideredtobeasubset ofthealkalineactivatedbindersystem.Alkalineactivationis the general term applied to the reaction of a solid aluminosilicate (known as `precursor') under alkaline conditions (produced by `alkaline activator'), to make a curing binder. on the combination of hydrated phases of alkaline earth aluminosilicate and/or alkaline earth aluminosilicate[17]. Geopolymerisation is an exothermic reaction in which a reaction occurs between silica (Si) alumina(Al)underalkalineconditions,whichthenproduces a three dimensional polymer chain linking Si O Al O. The processofcreatinggeopolymerisationincludesathree step reaction, which is Dissolved Coagulation, Coagulation, Condensation Polymerization[21]. The reaction rate is affected by condensation rather than dissolution. Ancient studies used geopolymers as fireproof coatings for cruise ships, heat resistant adhesives, thermal protection of wooden structures, and more. Geopolymers differ in their reactivity and availability. Figure 1 illustrates a simplified reactionmechanismforgeopolymerizationofgeopolymers. Thisfiguredepictstheconversionofasolidaluminosilicate toasyntheticalkalinealuminosilicate.
Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072 © 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal |
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
Na2SiO3ispreferredbecauseforNa2SiO3tobereactive,it must first be sterilized with NaOH[24]. The rate of the reactionisaffectedmorebythecondensationstepthanby thedissolution.
Potassiumionsrequireagreaterefforttopenetratethrough the paste due to its larger size than sodium ions and thereforesodium basedalkaliactivatorsarewidelyused[5]. Alkalineactivatorsaredifficulttohandleinlargequantities because they are viscous, corrosive, and hazardous. Solid NaOHiscorrosiveandformssodiumcarbonateoncontact withCO2.
Sodiumaluminosilicatehydrate(N A S H)gel,theprincipal response manufactured from the alkali activated aluminosilicates,differsofthealuminium changedcalcium silicatehydrate(C A S H)gelofPCpastes.Flyashandslag aredeterminedtobethemaximumcapacitysourcesofAASC manyofthewasteproducts.Highattentionofcalciumionin eleganceCFlyashprimarilybasedtotallygeopolymerscan bring about better compression power[16]. But using eleganceCflyashprimarilybasedtotallyprecursorsbecame determined because the causation of speedy setting. Gel compositioninlargepartimpactsthemechanicalpowerand durability.Thefundamentalhydrationproductisacalcium silicate hydrate (C A S H gel) with aluminium in its composition.TheshapeandcompositionofC A S Hgeland the presence of different secondary stages or compounds relyuponthekindandquantityofactivatorused,slagshape andcompositionandthecuringsituationswhereinthecloth hardens [9]. When slag became in part changed through Ca(OH)2, workability and compressive power became determinedtobereduced[23].
The AASC raw material should be rich in aluminum and silica. The materials must be amorphous in nature as the degreeofpolymerizationdependsonit.AASCprecursorscan be natural pozzolanic materials such as volcanic ash, diatomaceousearth,shale,zeolite,kaolinite,phonolite,etc. orman madematerialsincludingmaterialsfromindustrial oragriculturalwastesuchaslow calciumflyash,silicafume, brick powder, granulated kiln slag, bagasse ash, rice husk ash, red mud, fluorescent lamp waste, ceramic waste, etc. Theactivatorsusedinaluminosilicateprecursorsarealkali hydroxides, alkaline silicates, or a mixture of the two, to producehighalkalinity.Thecommonlyusedalkaliactivators aresodiumhydroxide(NaOH)/potassiumhydroxide(KOH) andsodiumsilicate(Na2SiO3)/potassiumsilicate(K2SiO3). The concentration and molarity of the solution affect the properties of the dough. The combination of NaOH and
Due to the hydrated slag formation of sodium calcium silicate, slag based geopolymer concrete has a higher expansion rate than fly ash based AASC. CASH gels are formed by alkaline activation of silica and calcium rich materialssuchasslagandNASHgelsareformedbyalkaline activationofsilicaandalumina richmaterialssuchasflyash andmetakaolin[24].Theincinerationofagriculturalwaste produceshullsandhuskashwhicharehighlyactivesilica richresidues.Chinaisthelargestricehuskcontributorwith an estimated production of about 80 tons. The one component geopolymer rice husk mixture activated with solidsodiumaluminatewasfoundtohavearelativelyhigh compressive strength (30 MPa)[14]. Although these ash sometimescontainlargeamountsofunburntcarbon,poor qualityricehuskash(whichloses40%onignition)hasbeen shown to be successfully used in single component geopolymers[9]. Vincent et al. [11]used alkali activated materialsobtainedfromasphaltfillersandfluorescentlamps to verify the suitability of their use for urban pavements. Because the fluorescent lamp waste is rich in SiO2, the flexuralstrengthofthecompositionsisincreasedunlikethat of the asphalt admixture waste containing CaO and MgO. IncreasingtheSiO2/Al2O3ratiohasamorepositiveeffect onstrengththanasphaltfillers.
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
Alkalineactivatedbinderscanbemanufacturedasonepart mixingsystemortwopartmixingsystem.Two partblends arecommonlyusedforprefabricatedconstructionworkby mostindustries,aschemicaltreatmentandhandlingregimes canbeeffectivelytightlycontrolled[14].Inatwo partmixing system, in addition to water, a solid aluminosilicate precursor and an alkaline activator solution are required. Figure2showsapartialmixingsystemthat,inadditionto water,requiresadrysolidmix.
strength increased as the healing time increased, but the increase in strength was negligible when the healing time lastedbeyond24hours.Inthecaseofgeopolymerconcrete madewithflyashandactivatedwithsodiumhydroxideand sodiumsilicatesolutions,theresultsshowedthattheseven daystrengthofthekiln curedsampleswasalmostsixtimes greaterthanthatofthecuredsamples[1].inthesurrounding air. They found that the strength of geopolymer concrete improvedathighertemperaturesandtheoptimumstrength was found to be 80°C for steam curing, while for water curing,strengthwasfound.Theintensityobtainedafter28 dayswaslowerthanthecharacteristicintensityduetopoor strengthdevelopment.atlowertemperature.Theslag based geopolymer was found to have lower strength at room temperature than the steam treated samples. However, curing at high temperature for a long time leads to degradationofthesampleduetothermalanalysisofSi O Al Osilicatebonding[14]
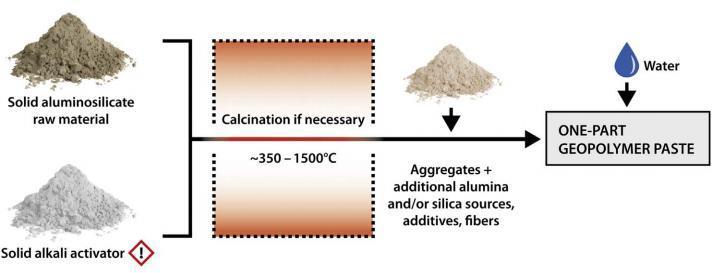
Fromfigure6,itcouldbereferredtothatthedryaggregate isreadythroughblendingastrongalkali activatorattheside ofastrongaluminosilicateprecursor.Alsotheguidanceof the dry aggregate can take vicinity without or with the calcinationstep.Theactivatorinaoneelementgeopolymer blend may be any substance that gives alkali cations, increases the pH of the response aggregate, and allows dissolution.Fortraditionalgeopolymers,alkalineactivators maybefocusedaqueousanswerofalkalihydroxide,silicate, carbonate, or sulphate [18].Normally, one element blend precursorsusedareflyash(eleganceF)inaggregatewith blastfurnaceslag.ClassCflyashisn'tdistinctlyadvocated because it reasons speedy putting because of excessive quantity of calcium content. In a few researches in which mechano chemical activation approach turned into used whereinflyashturnedintoball milledwithdrycombined activators are located to have excessive power, elevated resistance to moisture and great microstructure. One element aggregate is located to have problems associated withpoweri.e.theirpowerislocatedtobedecreasenotlike two elementmixtures[3].Ifproblemsassociatedwiththose mayberesolved,thenone elementblendwillbeabusiness fabricasit'smilesdry,itcouldbepackedintoluggageand transportedefficiently.
Theprocessofcreatinggeopolymerizationoftendependson the curing method. The curing method applied has a significantimpactonthethermalpropertiesoftheAASC,the microstructural characteristics and the strength development. Curing temperatures between 40C and 85C havebeenshowntobealmostperfectforgenotyping.Forfly ash geopolymers, temperature curing or furnace curing is usually applied. Nuruddin et al. [24]reported that for alkaline activated fly ash, curing temperature is critical to achieve higher strength and that test pieces subjected to highercuringtemperaturehavehighermechanicalstrength thansamplesatlowtemperature.Theyalsoobservedthat
value:
Concrete is a porous multi phase material. The porous solutionisalkaline(pHabout13)andhydrationtakesplace inthisporoussolution.Therefore,duetoitshighalkalinity, thenaturalbalanceofconcreteisdisturbedwhenitreacts withacids.Thecompoundspresentinthebinderaswellas theacid solubleaggregates(e.g.dolomiteorlimestone)are unstableinsolutionscontaininganexcessofhydrogenions (acid). The attack is a classic acid (HA)base (BOH) type reactiontoformsalt(BA)andwater.
HA(aq)+BOH(aq)→B+(aq)+A (aq)+H2O(aq)
TheproductofPortlandcementhydrationhasastrongacid reaction.Thechemicalstabilityofthematerialisgoverned by the chemical composition of the components of the hydratedcementpasteandtheirrelativeproportionsinthe substrate. Portlandite is the least stable of the cement hydrates;Itsdissolutionoccurswhentheconcentrationof calcium ions in the interstitial solution falls below 22 mmol/L.CSHgelsaremorestablewhentheconcentrationof calciumionsintheinterstitialsolutionisbetween22and2 mmol/L,thisalsodepends ontheCa/Simolarratioofthe CSH gel. When the concentration of calcium ions in the interstitialsolutionfallsbelow2mmol/L,theCSHgelalso becomes unstable; it undergoes calcification to eventually formsilicagel,whichisstableatlowcalciumconcentrations ininterstitialsolution(RamaswamyKPandMSanthanam, 2019).
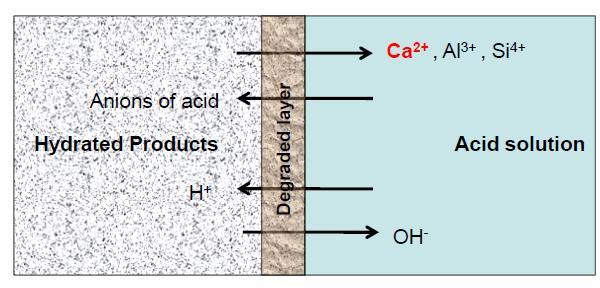
Thepenetrationofacidicionsintotheadhesive matrix by diffusion disturbs the stoichiometric balance and this phenomenonisschematicallyshowninFigure3.Dissociated cations from hydrates such as Ca2, Al3 are wash away towards acidic solution with alkaline ions to maintain chemicalbalance.Inthisprocess,thesecationssuchasCa2 andAl3canreactwiththeanionsoftheacidtoformsaltsof different solubility depending on the properties of the
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

acid[17]. The kinetics of the decomposition essentially dependsonthedifferentpropertiesofthesaltsthusformed.
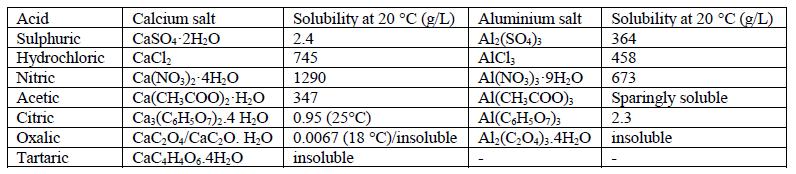
Thechemicalreactionswithinsidethepasteareessentially oftypes;thedissolutionofhydratedandanhydrousstages withinside the binder machine and the precipitation/leachingoflatestresponsemerchandisewhich arefashioned[6].Thesereactionscontinuefromoutofdoors to the interior, forming a degraded layer withinside the process.Thephysico mechanicalresidencesofthedegraded layer are weaker in comparison to the sound zone. If the goodsfashionedaresoluble,theyleachoutintotheanswer growing the porosity of the matrix. Sometimes, if merchandisefashionedaremuchlesssoluble,thosecanalso additionally precipitate withinside the matrix, exerting crystallisation stress ensuing withinside the formation of cracks.Thesecrackssimilarlylessenthemechanicalenergy anddecoratethediffusionofacidtotheinside.Eventually, thecompetitiveretailersattainthereinforcementensuing withinside the corrosion of strengthened structures. However,thosearesimplemechanismsofdegradation;the degradationisespeciallydependingonthekindofacidand the residences of the goods fashioned. Table 1 gives the solubilitydataofcalciumandaluminiumsaltsthatmaybe formedwhenacidreactswithcementpaste.
stays at the floor of the cement after the dissociation of calciumsilicates.Aluminiumhydroxide(Al(OH)3)andiron hydroxide(Fe(OH)3)precipitatewithinsidethelayerafter dissolutionofthealuminatesandalumino ferritesrelyingat thehydrogenionawarenessoftheanswer;ironhydroxide precipitates at a pH more than 1.zero and aluminium hydroxide precipitates at a pH more than 3.zero. More specifically, calcium hydroxide undergoes dissolution at a poresolutionpHof12.5,observedthroughettringiteatpH fee of 10.7. CSH gel turns into volatile and undergoes dissolutionatpHbetween~10.fiveand8.8(inkeepingwith special authors) observed through calcium aluminate and ferritehydratestages.Eventually,theCASHgelisobtained,if thepHisabove7.IfthepHisbetween1and6,amorphous silicagelcontainingAlandFe(SiO2.nH2O)willbeobtained asafinalproduct[5].Thesemicrostructuralchangesdueto hydratefusionleadtotheformationofthemineralpartition; thesechangesmanifestaschangesinmass,strength,elastic modulus,etc.andtheintegrityofthematrixisaffected.The lossofalkalinityofthesubstrateandthusincreasedporosity duetoattack(duetoosmosisofhydrates)eventuallyleads tocorrosionofthereinforcementswhentheentirethickness oftheconcretecoatingcardboardisattacked.However,this phenomenon is not straightforward, as the kinetics and mechanism of weathering degradation are affected by variousfactorsrelatedtothematerial,thecorrosivesolution andthetestmethod.
According to Alexander and Fourie (2011), Portlandite (Ca(OH)2) is the maximum reactive of the hydrates and absolutely dissociates, even as calcium silicates are much less reactive observed through calcium aluminates and calciumalumino ferrites.ThefactorswhichincludeCa,Na,K, Mg depart the matrix while Si, Al and Fe remain, the steadinessintheirbearingstagesconsiderablyrelyingatthe pH. A gel layer of particularly acid insoluble silica (SiO2)
Thesulphuricacidfirstreactswiththecalciumhydroxide foundinconcretetosupplygypsum(CaSO4.2H2O).Gypsum isn'talwaysagreatdealsolubleinacidanswerandstaysasa precipitate withinside the concrete floor consequently lowering the penetration of acid withinside the early publicity period. This gypsum produced reacts with the calcium aluminate hydrate gift withinside the cement to supplyettringite(3CaO.Al2O3.3CaSO4.32H2O),whichmay beverynegativetothehardenedconcrete.Thefull sizemass advantage of all mixes on publicity to sulphuric acid, particularlyontheearlyageofpublicity,becameattributed to the gypsum formation across the specimens. The interfacialtransitionzone(ITZ)withexcellentaggregatesof anAlkaliactivated(AA)patternafterimmersioninsulphuric acidconfirmedcomparableshapeinmorphologyfromthat earlierthanimmersion,indicatingthatAApasteremainedin trueshapebeneathneathsulphuricacidattack[15].Inthe takealookatperformedviawayofmeansofGuetal.2018, demarcatesthepreliminaryscanningelectronmicroscopy (SEM)evaluationofAAflyashmatrixwhichincludesadense shapeofsodiumaluminosilicatehydrate(N A S H)earlier than immersion in which the atomic ratio of silicon and aluminium became 1.5. When N A S H is dissolved in reaction with sulfuric acid, the atomic ratio of silicon and aluminum increases to 2.3. For higher concentrations of sulfuricacid,itwasfoundtodissolvetheNASHgel,thereby reducingthecompressivestrengthofgeopolymerconcrete.
Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072 © 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page300
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
Reju,2019foundthatthedegradationprogressedinwardas theexposuretimeincreased.Thevariabledepthvariationof thespecimenbeforeandafteracidetchingisanindicatorof phenolphthaleinthathasbeensprayedontothefreshlycut surface. Areas in pink indicate areas unaffected by acid exposure.
Unlike other acids, specimens immersed in sulfuric acid (concentration0.5M)hadacolorlesssurfaceindicatingthat they were completely decomposed by acid attack. The fly ash basedgeopolymer(FGP)uponvisualinspectionshowed nocolorchangeorsaltdepositiononthespecimen,whilethe slag basedgeopolymer(SGP)showedcolorchange(Athira Ajay, K P Ramaswamy, Anu V Thomas, 2020). The mass reductionoftheFGPsampleswaslessthanthatoftheOPC, indicatingthatthegeopolymersamplesaremoreresistantto acids than the others[13]. No mass loss was observed for SGPsamplesexposedto0.25Macid.TheFGPsampleswere foundtobemoreresistanttosulfuricacidattackduetothe betterstructuralstructureobtainedafterhightemperature hardening.
Citricacidissaidtobethemostactiveoftheorganicacids andshedsthicknessrapidly.Thehigh concentrationused, the poly acidity of citric acid and the non protective characteroftheprecipitateformedappeartohaveincreased theaggressivenessofcitricacid.AccordingtoKoeingetal. the greater harmful effect could also be due to the acid bufferingactionoforganicacids.AccordingtoRamaswamy and Santhanam, the decomposition kinetics of citric acid strongly depends on its concentration. A large amount of whitesaltwasprecipitatedonthesamplessoakedincitric acid and confirmed as calcium citrate tetrahydrate (Ca3(C6H5O7)2.4H2O) by X ray diffraction study (RamaswamyKPandMSanthanam,2019).Thissalthasless solubilityandveryhighmolarvolumethanportlanditeand CSH gels and has therefore been shown to be harmful to adhesivesubstrates.
InthehavealookatwiththeaidofusingReju,2019,non stop publicity of FGP specimens in citric acid did now no longerdisplayanystructuraldisintegration.Severecracking ofSGPpastespecimenshavebeendeterminedbecauseofthe formationoflowsolublelooselyadheredwhiteprecipitate. SGPspecimensfromdeterminethreearediscoveredtohave decrease charge of deterioration as a purple shade is discovered closer to the core. However, effects from the microstructuralevaluationconfirmedthat,FGPspecimens hasahigheroverallperformanceinresistingcitricacidasno expansive merchandise have been fashioned not like OPC andSGP.
Nitric acid attack is a typical acid corrosion that causes a reduction in the volume of the corroded layer due to the
leaching of the highly soluble nitrate calcium salts. Blast furnace fly ash geopolymers corrode when immersed in nitricacidsolutionforaperiodofonemonth.Thisisdueto ion exchange reactions between the skeleton charge compensating cations (i.e. sodium, potassium or calcium) andtheHionspresentinsolution.Itcouldalsobeduetothe electrophilicattackoftheSi O Almacromolecularbondsby acidicprotons,whichcausestheejectionofthetetrahedral aluminum from the aluminosilicate framework. It is observed that all mixtures exhibit mass loss due to progressiveleachingofthehydratedphasessinceleachingis predominantduringnitricattack.
The worst case state of affairs from the above discern is while OPC specimens are subjected to hydrochloric acid because it reacts with calcium compounds (along with PortlanditeandCSHgel)maintotheformationofcalcium chloride, which has extraordinarily excessive solubility in solution. Geopolymeric mine waste binders have low acid resistance overall performance while as compared to geopolymeric binders primarily based totally on metakaolin[9]howeveruniversalgeopolymerbindershad higherresistancewhileascomparedtoOPC.
Aceticacidsaredeterminedspecificallyinwastewatersand it's miles determined to be competitive in terms of acid attack. The corrosion system is speedy similar to that of robust acids inclusive of sulphuric acid however lesser competitive in comparison to citric acid at equal concentrations. These acids produce soluble calcium salts with the aid of using dissolution of calcium hydroxide in concrete. In the case of OPC specimens, orange yellow coloration turned into discovered at the specimens with none precipitation of salts. No precipitation or extrade in thicknessturnedintodiscoveredforgeopolymerspecimens evenin0.5Mor0.25Maceticacidsolutions[13].
The FGP samples showed superior acid resistance performancerelatedtotheweightloss(0.8%)inthemortar samplesexposedto0.5Maceticacid.Themasslossforthe SGPsamplewasdetectediscurrentlyhigherthanthatofthe FGPsample.InSGPconcrete,thelowerCHcontentandC/S ratiointhehydrationproductsandtheformationofsilicagel (alumino)duringdecalizationhinderedthepenetrationof acids and contributed to the potential greatest acid resistanceofSGPconcrete.Acidpenetrationisalsohindered due to the finer pore structure of SGP concrete. [18]when evaluating the degradation kinetics of alkaline activated concreteexposedtoorganicacidattackdemonstratedthat the residual strength decreased as the Ca content of the binderincreased.Theorganicacidsstudiedwereamixture ofacetic,lacticandpropionicacidswithasolutionpHof3.
The resistance decreases continuously with time, for all samples immersed in organic acids, with the exception of AAFA (alkaline reactive fly ash). Acid exposed alkaline
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
activated fly ash samples showed a steady increase in intensity over time. Reju, 2019 also observed improved performance of sneak ash geopolymer when exposed to aceticacidsolution.
Acid assault is a complicated phenomenon bobbing up in creation industries global as it's miles chargeable for the deteriorationofconcreteinacidicenvironmentsensuingin untimely degradation in phrases of microstructural alterationoflevelsmanifestingwithinsidetheshapeofmass changes, weakening of mechanical properties, boom in porosity because of calcium leaching etc. This complex mechanismofdecaythroughnumerousacidsremainsnow no longer nicely understood and similarly explanation is importantonthisregard.Currently,therearen'tanycodes or requirements to be had for comparing resistance of substances to acid assault. Further research is vital to research and make clear the mechanisms of deterioration andalterationkineticsinthosesituationsviawayofmeans ofgrowingdependabletakealookattechniquesandthus,to layout substances that carry out properly in those environments.
Bothnaturalacidsinadditiontoinorganicacidsfluctuateof theirmechanismofdecayofconcrete.Thechargeofdecayis located to be depending on the kind of acid, awareness of acid, chemical composition of binders and solubility and traits of the salts shaped. Based at the aggressiveness of acids, sulphuric acid and citric acid are located to be the maximum competitive from maximum of the studies. On publicity to sulphuric and citric acid, expansive salts with excessive molar extent have been shaped inflicting fast degradation. Fly ash primarily based totally geopolymer confirmedincrediblysteppedforwardoverallperformance towardssulphuric,aceticandcitricacidassault.Nitricacid assaultonmetakaolingeopolymerdidnownolongerhave intense degradation on assessment to different waste binders used. Fly ash slag primarily based totally geopolymer composites cured in ambient situations had similaroverallperformancetothatofflyashprimarilybased totally geopolymers cured at excessive temperature. The additions of small quantities of slag to fly ash primarily based totally geopolymers also are located to beautify its compressivepoweronpublicitytoacids.
Theimprovedenlargementofcityregionsandproliferation ofindustrieshavecausedthebigscaletechnologyofacidic media. This has caused extreme degradation of concrete systemsmadewithOPCwhilstitreceivesintouchwiththe acidicsolutions.Asofnow,bignumbersofresearchesare performedinchangingcementinconcretewithnewbinders emphasisingonsustainability,decreasedcarbonfootprints, sturdiness and eco friendliness. Reinforcing the usage of supplementary cementitious materials (SCMs) as alkali activatedbinderscouldbeahigheranswerinpresentinga sustainable concrete (100% substitute of cement), and
stepped forward resistance towards harsh competitive environments. From maximum of the studies, it's miles obtrusive that sturdiness overall performance of geopolymersarelocatedtobesuperior.Thusalkaliactivated binder structures can provide a sustainable and sturdy opportunity to Portland cement primarily based totally structuresforpublicityincompetitiveacidicenvironments.
Althoughreferredtoasaweakacid,aceticacidcancausea rapidincreaseinthedepthofweatheringofthesample,and thestabilityofvariousbindersystemsinthisacidicmedium isoftennotstudiedindetail.Thedevelopmentofacidattack mechanismandpredictionofdegradationdepthinacertain time, study of the effect of acid mixture and the effect of curingpatternonreactionratearesomeadditionalstudies. Supplements need to be considered to understand the broader aspects of acid attack and its degradation mechanisms.
[1] Gu L, Bennett T, and Visintin P, 2018 “Evaluation of accelerated degradation test methods for cementitious compositessubjecttosulphuricacidattack;applicationto conventional and alkali activated concretes”. Cement and ConcreteComposites87187 204.
[2]ProvisJ.L,2018,”Alkali activatedmaterials”,Cementand ConcreteResearch11440 48.
[3] Reju R.M,2019,” Acid resistance of geopolymer composites”, M.Tech Thesis TKM College of Engineering KollamIndia1 123
[4]RamaswamyKPandSanthanamM,2017,”Durabilityof cementitiousmaterialsinacidicenvironments:evaluationof degradation kinetics”, 14th Int. Conf. on Durability of BuildingMaterialsandComponents(DBMC)(Universityof GhentBelgium)pp1 14.
[5] Bertron A and Duchesne J ,2013,” Performance of Cement Based Materials in Aggressive Aqueous Environments”,RILEMState of the ArtReport,vol10,edM Alexander et al.(RILEM,TC 211 PAE: Springer)pp131 173.
[6] Dogangun A, Karaca Z, Durmus A and Sezen H ,2009 ,”CauseofDamageandFailuresinSiloStructures”,Journalof PerformanceofConstructedFacilities23(2)65 71.
[7]StephanD,FirdousR,NoëlJandDjoboY,2018,Natural pozzolan based geopolymers: A review on mechanical, microstructuralanddurabilitycharacteristics”,Construction andBuildingMaterials,1901251 1263.
[8] Ma C, Awang A Z and Omar W, 2018, Structural and material performance of geopolymer concrete: a review”, ConcreteandBuildingMaterials18690 102
[9]LodeiroG,PalomoA,Fernández JiménezAandMacphee DE,2011,”CompatibilitystudiesbetweenN A S HandC A S Hgels:study in theternarydiagramNa2O CaO Al2O3 SiO2 H2O,”CementandConcreteResearch41923 931.
[10] Ma C, Awang A Z and Omar W, 2018, Yliniemi J, Kinnunen, P and Illikainen M, 2018, ”One part alkali activated materials: a review,” Cement and Concrete Research10321 34.
[11] Vincent M, Criado M and Garcia Ten J, 2018 ,”Alkali activated materials obtained from asphalt fillers and fluorescentlampwastes,”JournalofCleanerProduction215 343 353.
[12]NurruddinMF,HarunaS,MohammedBSandSha΄aban I. G ,2018,” Methods of curing geopolymer concrete: a review,”Int.JournalofAdvancedandAppliedSciences5(1) 31 36.
[13]RamaswamyK P,BertronAand SanthanamM, 2017, “Additional insights on the influencing factors and mechanismofdegradationduetoacidattack:specialcaseof acids forming soluble salts,” Int. Conf. on Advances in Construction Materials and Systems (ICACMS) (Chennai, India)vol4pp279 290.
[14] Gu L, Bennett T and Visintin P, 2019,”Sulphuric acid exposure of conventional concrete and alkali activated concrete:Assessmentoftestmethodologies”,Construction andBuildingMaterials197681 692.
[15]KoenigA,HerrmannA,OvermannSandDehnF,2017, ”Resistanceofalkaliactivatedbinderstoorganicacidattack: assessmentofevaluationcriteriaanddamagemechanisms”, ConcreteandBuildingMaterials151405 413.
[16]RamaswamyKPandMSanthanam,2019,”Degradation kinetics of cement based materials in citric acid”, Recent AdvancesinStructuralEngineering(Singapore:Springer)vol 1pp891 905.
[17] Provis J L and Van Deventer J S, 2009,” Geopolymers Structure, processing, properties and industrial applications”,WoodheadPublishingLimitedandCRCPress LLC(UK)pp1 6.
[18] A.Z. Warid Wazien, 2016,” Strength and Density of GeopolymerMortarCuredatAmbientTemperatureforUse asRepairMaterial”,MaterialsScienceandEngineering133 (2016)012042.
[19] Timothy A. Aiken. Jacek Kwasny. Wei Sha, 2020, “Resistanceofflyashgeopolymerbinderstoorganicacids”, MaterialsandStructures(2020)53:115.
[20]T.Bakharev,2005,”Resistanceofgeopolymermaterials to acid attack”, Cement and Concrete Research 35 (2005) 658 670.
[21] Peng Zhanga, Yuanxun Zhenga, Kejin Wangb, Jinping Zhanga,2018,”A Review on Properties of Fresh and HardenedGeopolymerMortar”,CompositesPartB(2018), doi:10.1016/j.compositesb.2018.06.031.
[22] Timothy A. Aiken, Jacek Kwasny, Wei Sha,2021, ”Performance of cementless binders produced from industrial waste products in strong acid”. Cleaner EngineeringandTechnology2(2021)100035.
[23]AthiraAjay,KPRamaswamy,AnuVThomas,2020,”A criticalreviewonthedurabilityofgeopolymercompositesin acidicenvironment,”EarthandEnvironmentalScience491 (2020)012044.
[24]AliAllahverdi,FrantiŠekŠkvára,2001,”Nitricacidattack On hardened paste of geopolymeric cements”, Ceramics Silikáty45(4)143 149(2001).
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056 Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072 © 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal
