International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072

International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
Department of Chemistry, DBS (PG) College, Dehradun Uttarakhand, India 248001.
Abstract: AlesscosteffectivemethodbasedonMn(II)catalyzedperiodateoxidationofo toluidineforthedetermination of Mn (II) in aqueous / mixed media in nanogram has been developed. The main reaction product is 4 methyl 1,2 benzoquinone. The progress of reaction in acetone water, medium, was followed by monitoring the increase in the absorbance of reaction intermediate. The reaction is found to be the first order with respect to catalyst, substrate and oxidanteach.Differenttypesofcalibrationcurvesweredevelopedandtestedsuccessfullyfornanogramdeterminationof manganese(II)inaqueous/mixedmedium.Themethodisbetterintermsofcostofanalysisandeaseofdeterminationas wellasinvolvementofeasilyavailableequipmentsandfacilities.
Keywords:Mn(II),periodateion,o toluidine,nanogramestimation
Introduction:
Keeping in view the reported less cost effective methods involving costly instrumentation not available at all places of researches/ scientific institutions/ testing laboratories in India, an attempt has been made to work out the best suitable conditionsleadingtothekineticspectrophotometricestimationofMn(II)innanogramswhileitcatalyzestheo toluidine (o TOL) periodate or p toluidine (p Tol) periodate redox systems in acetone water medium. While p Tol periodate systemhasbeenstudiedatabsorptionmaxima490nm,theo TOL periodatesystemhasbeenusedforestimationofMn (II)attwoabsorptionmaximaofreactionmixturesi.e.475nmand525nm.
The conditions worked out for estimation of Mn(II):
Three methods were developed for the estimation of Mn (II) in various ranges of concentration. In method- I, following are the finally worked out conditions for running the kinetic sets for the purpose of determination of Mn (II) in mixed (acetone water)medium based upontheperiodateoxidation ofo TOL:[o TOL] =0.001M; [Nal04]=0.01M;Acetone= 10%(v/v);pH5.5; λmax=525nm;Temp.35±0.1°C.Thereactionwasfoundtofollowsecondorderkineticswithorder being one in each reactant. The method developed was fit for estimation of Mn(II) in the range 52.0 ng/ml to 109.8 ng/ml
In method II,theMn(II)estimationcouldbeachievedinsamemediumbutintherange 109.8 ng/ml to 2635.2 ng/ml by making use of periodate oxidation of o TOL. The conditions worked out and found suitable are: [o TOL] = 0.0005 M; [NaIO4]=0.005M;Acetone=5%(v/v);pH6.5; λmax=475nm;Temp.35±0.1°C. Third method forestimationofMn(II)insamemediumintherange 0.5 ng/ml to 48.0 ng/ml wasdevelopedbyusingp TOL periodate redox system. This method was worked out under following conditions: [p TOL] = 0.001 M; [NalO4] = 0.02M;Acetone=15%(v/v);pH8.0;λmax =490nm;Temp.35±0.1°C.Thereactionhasalsobeenfoundtobefirstorder w.r.t.periodateandp TOL.
Adefinitevolumeofstocksolutionofo TOLorp TOLinacetonewasmixedwithcalculatedvolumeofthestocksolutionof Mn(II),acetone and water andstirred a little with the helpofthe pipette. This mixtureand stock solutionofNaIO4 were thenclampedinathermostatat35±0.1°C.After30minutes,arequiredamountoftheperiodatesolutionwasaddedtothe mixture and stirred to start the reaction. All additions were made in amounts calculated for maintaining the concentrations of different reagents as mentioned above. Different sets were prepared in a similar manner varying the [ Mn(II)]. Aliquots were withdrawn from the reaction mixture after repeated intervals of 0.5 or 1 or 5 minutes and the absorbancewasrecordedondoublebeamspectrophotometer.Theabsorbancevstimeplotswerethenmadefordifferent sets.Theinitialrates[(dA/dt)i]wereevaluatedafter0.5or1or5minutefromthestartofthereactionbyapplyingplane
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
mirror method on the absorbance vs time plots. The pseudo first order rate constants (k1) were found by Guggenheim's method.
Usingthemethodofleastsquares,linearcalibrationcurveswereobtained.FormethodI,type'A',type'B',type'C',type'D', type 'E' and type 'F' plots were obtained in terms of A10 or A20 or A30 or A40 or initial rate or k1 vs [Mn (II)] plots respectively (where A10, A20, A30and A40 are the absorbance values after 10, 20, 30 and 40 minutes from the start of reaction respectively) for the oxidation of o TOI studied at absorption maxima 525 nm. In method II, type 'A', type 'B', type'C',type'D',type'E',type'F'andtype'G'plotswereobtainedintermsofA2 orA4 orA6 orA8 orA10 orinitialrateork1 vs[Mn(II)]plotsrespectively(whereA2 orA4 orA6 orA8 orA10 aretheabsorbancevaluesafter2,4,6,8and10minutes from the start of reaction respectively) for the oxidation of o TOI studied atabsorption maxima 475 nm. In method III, type'A',type'B',type'C',type'D',type'E'andtype'F'plotswereobtainedintermsofA2 orA4 orA6 orA8orinitialrateor k1 vs[Mn(II)]plotsrespectively
The range of [Mn(II)] in which the Beer's law is obeyed, molar absorptivity, Sandell's sensitivity, correlation coefficient and the coefficient of determination, value of 't' (at 0.05 significance level), relative standard deviation and % error for various calibration curves are given in table. For the method I, involving the oxidation of o TOL that was studied at absorption maxima 525 nm, the characteristics of calibration curves were evaluated in the form of equations of straight lineasfollows:
A10 =2.44x10 2 +6.87x10 4 [Mn(II)] (1)
A20 =5.735x10 2 +9.11x10 4 [Mn(II)] (2
A30 =8.81x10 2+1.05x10 3 [Mn(II)] (3)
A40=11.81x10 2 +1.1x10 3 [Mn(II)] (4)
(dA/dt)i1.75x10 2+3.86x10 3 [Mn(II)] (5)
k1 =1.196x10 4+2.67x10 6 [Mn(II)] (6)
ForthemethodII,involvingtheoxidationofo TOLthatwas studiedatabsorptionmaxima475nm,thecharacteristicsofcalibrationcurveswereevaluatedintheformofequationsof straightlineasfollows:
A2=2.67x10 2 +3.147x10 4 [Mn(II)] (7)
A4 =6.854x10 2 +3.724x10 4 [Mn(II)] (8)
A6 =10.24x10 2+4.171x10 4 [Mn(II)] (9)
A8 =11.86x10 2 +4.45x10 4 [Mn(II)] (10)
A10 =12.32x 10 2 +5.48x10 4 [Mn(II)] (11)
(dA/dt)i= 2.2x10 3 +1.83x10 4 [Mn(II)] (12)
k1=7.36x10 4 +4.1x10 6 [Mn(II)] (13) Similarly,forthemethod III,involvingtheoxidationofp TOL that was studied at absorption maxima 490 nm, the characteristics of calibration curves were evaluated in the form of equationsofstraightlineasfollows:
A₂=8.55x10 2 +3.67x10 3 [Mn(II)] (14)
A4 = 11.75x10 2 +6.2x10 3[Mn(II)] (15)
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056
Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
A6 =15.61x10 2 +7.6x10 3 [Mn(II)] (16)
A8 =18.84x10 2+9.2x10 3 [Mn(II)] (17)
(dA/dt)i= 8.3x10 3+7.1x10 3 [Mn(II)] (18)
k1 =3.88x10 4 +3.44x10 5 [Mn(II)] (19)
Inequation5,12and18,thevaluesofinterceptsandslopeareinabsorbanceunits.min 1 andabsorbanceunitmin 1.ng 1.ml respectively.Theseareinsec 1 andsec 1.ng 1.mlrespectivelyforequation6,13and19.The[Mn(II)]areinng/ml.
The method I is not applicable in presence of 2,3 dimethylaniline, o anisidine, o phenetidine and 3 chloro 2 methylaniline, while method II and III are not suitable in presence of N ethylaniline, o ethylaniline, p ethylaniline, p toluidine, o chloroaniline, p chloroaniline, m anisidine, p anisidine, N,N diethyl aniline and 2,4 diethylaniline as these aromaticaminesinterfereinthesemethodsbygettingoxidizedbyperiodateionandthereactionmixtureshowingλmaxin therangeinwhichitinfluencestheabsorbanceintheproposedestimationmethods.Mostoftheotheraromaticamines/ anilinesdonotinterfereinthesemethods.
ThemethodmaybeusedinpresenceoftheionslikeNa+ ,K+ NO2 ,ClO4 2 ,NO3 andSO4 2 astheydonotinterfereinpresent case.However,themetalslikeAg,As,B,Co,Cd,Cr,Cu,Fe,Hg,Mo,Ni,Pb,Sb,Se,U,andZnareexpectedtointerferein this method. Therefore, a pretreatment is required for separating/ precipitating/ masking these ions before undertaking the proposedmethod.Forthispurpose,H2Smaybepassedinpresenceof0.3MH+ solution,followedbyfiltrationandboiling off H2S. After it, a dilute alkaline solution of α nitroso β naphthol should be added and again the solution should be filtered. Thereafter, the solution should be neutralized and the present method be applied. Fe may be removed by precipitation using basic formate method. In absence of the above given interferrants, the proposed method may successfullybeusedforthedeterminationofnanogramquantitiesofMn(II)inwatersamples.
[Mn(II)]maybedeterminedinaqueoussolutionsandwatersamplesbymixingthe samplewithcalculatedquantityofo TOL or p TOL and acetone and starting the reaction by adding NaIO4. followed by noting the absorbance of reaction mixture at different desired times as described above, or evaluating initial rate in terms of (dA/dt)iat a desired time by plane mirror method or evaluating k1 by Guggenheim's method as discussed above. After it, different calibration curves maybeusedfordeterminationof[Mn(II)]inng/ml.
The proposed method was tested for many water samples containing known amounts of Mn(II) in the range of the detection limits reported above. The results were found to be reproducible with reasonable standard deviation and low rangeoferrorsascalculatedfromsixdeterminations(table 1,2and3).
Method I : The value of slope of the calibration curves, molar absorptivity, and Sandell's sensitivity (table 1) indicated thatthesensitivityofthemethodisgood.Achangeinabsorbanceby0.001unitisexpectedonchangingtheconcentration ofMn(II)by0.909 1.456ng/ml.Further,achangeinconcentrationby1.0ng/mlwillchangetherateofreactionby3.860x 10 3 absorbanceunits/minute.Inaddition,thevalueofk1 willchangeby2.67x10 5 in1secondonchanging[Mn(II)]by1 ng/ml. The detection limits (52 ng/ml to 109.8 ng/ml) are also considerably low and these are good for the trace determination of Mn(II). The correlation coefficient (r) is in the range 0.988 to 0.999 which indicates the high precision involved in the determination and almost perfect correlation of the data. The value of coefficient of determination (r2) suggeststhat 97.6%to99.8%changein the value of A10 or A20or A30 or A40or (dA/dt)i ork1 iscaused byMn(II)andthe
© 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page819

International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
rest2.4%to0.2%istheeffectofunknownfactors.Thevalueof't'ascalculatedforthecalibrationcurves,areintherange 9.93to22.17whicharemuchhigherthanthetabulatedcriticalvalueat5%significancelevelor1%significancelevel.This suggeststhattherearelessthan1%chancesoferrorindrawingconclusions.Thestandarddeviationiswithinreasonable limits.Percentagerecoveryonthebasisofsixparalleldeterminationsis99.23%to99.99%.Molarabsorptivityis37760to 60500 L. mol 1.cm 1 for the curves ‘A’, ‘B’, ‘C’ and ‘D’ respectively. It is clear that curves ‘A’, ‘B’, ‘C’ and ‘D’ are the useful calibrationcurves.Curve‘E’and‘F’arealsosignificantasthechangeinrateorrateconstantvaluesisreasonable.
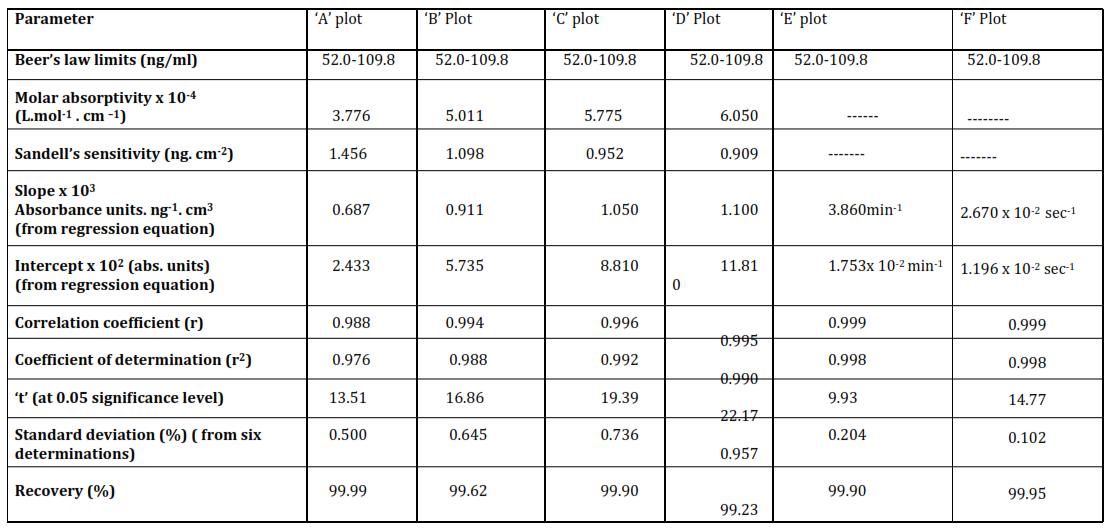
Method II : The value of slope of the calibration curves, molar absorptivity, and Sandell's sensitivity (table 2) indicated thatthesensitivityofthemethodisgood.Achangeinabsorbanceby0.001unitisexpectedonchangingtheconcentration ofMn(II)by1.826 3.178ng/ml.Further,achangeinconcentrationby1.0ng/mlwillchangetherateofreactionby1.83x 10 4 absorbanceunits/minute.Inaddition,thevalueofk1 willchangeby4.1x10 6 in1secondonchanging[Mn(II)]by1 ng/ml.Thedetectionlimits(109.8ng/mlto2635.2ng/ml)aregoodforthetracedeterminationofMn(II)Actually,method I and method II can be coupled for determination of Mn(II) in the range 52.0 ng/ ml to 2635.2 ng/ml that shows a wide rangeofdetermination.Thecorrelationcoefficient(r)isintherange0.996to0.999indicateshighprecisioninvolved.The valueofcoefficientofdetermination(r2)suggeststhat99.2%to99.8%changeinthevalueofA2 orA4 orA6.orA8 orA10 or (dA/dt)i ork1iscausedbyMn(II)andtherest0.8%to0.2%istheeffectofunknownfactors.Thevalueoftascalculatedfor the calibration curves, are in the range 6.530 to 7.610 which are much higher than the tabulated critical value at 1% significance level. This suggests that there are less than 1% chances of error in drawing conclusions. The standard deviationandpercentagerecoveryalongwiththehighvalueofmolarabsorptivitysuggestthatcurves‘A’,‘B’,‘C’‘D’,‘E’,‘F’ and'G' aretheusefulcalibrationcurves.
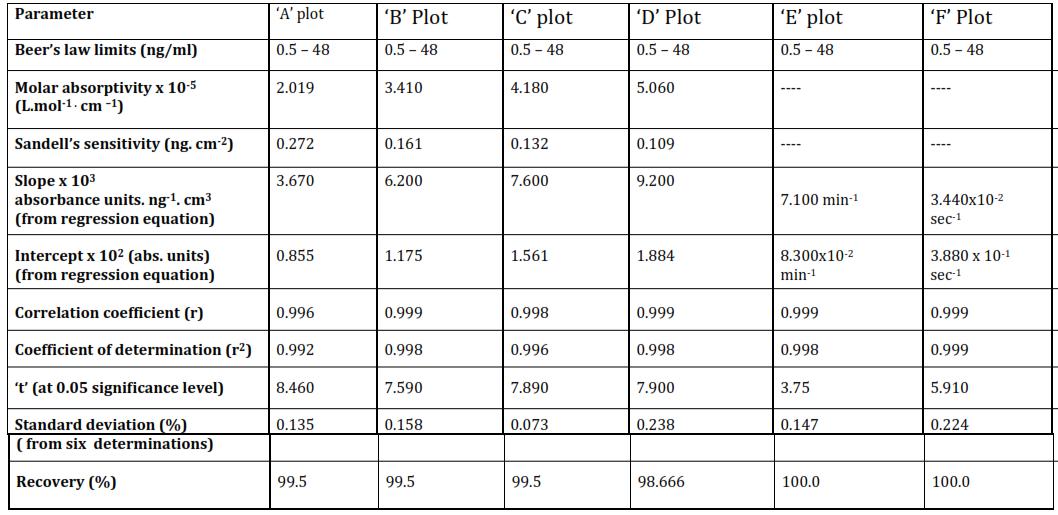
Method III: Variouscharacteristicsofthecalibrationcurves(table 3)indicatereasonablesensitivity,molarabsorptivity, percentage recovery, and correlation the range of [Mn(II)] 0.5 ng/ml to 48 ng/ml. Although the limits of applicability of Beer's law are not as wide as in case of Method I or II, the method is very well suited to estimation of Mn(II) in trace amounts.
A comparison of the proposed method with twelve reported methods is shown in table 4. It suggests that the proposed methods are better than a few reported methods based on the periodate oxidation of aromatic However, the simplicity involvedintheprocedureandthelowcostofdeterminationgoinfavoroftheproposedmethods.TheproposedmethodsI toIII offer better and wider range of concentration for the preparation of calibrationcurves. Muchlowerconcentrations canbedetectedwithreasonablygoodsensitivity.
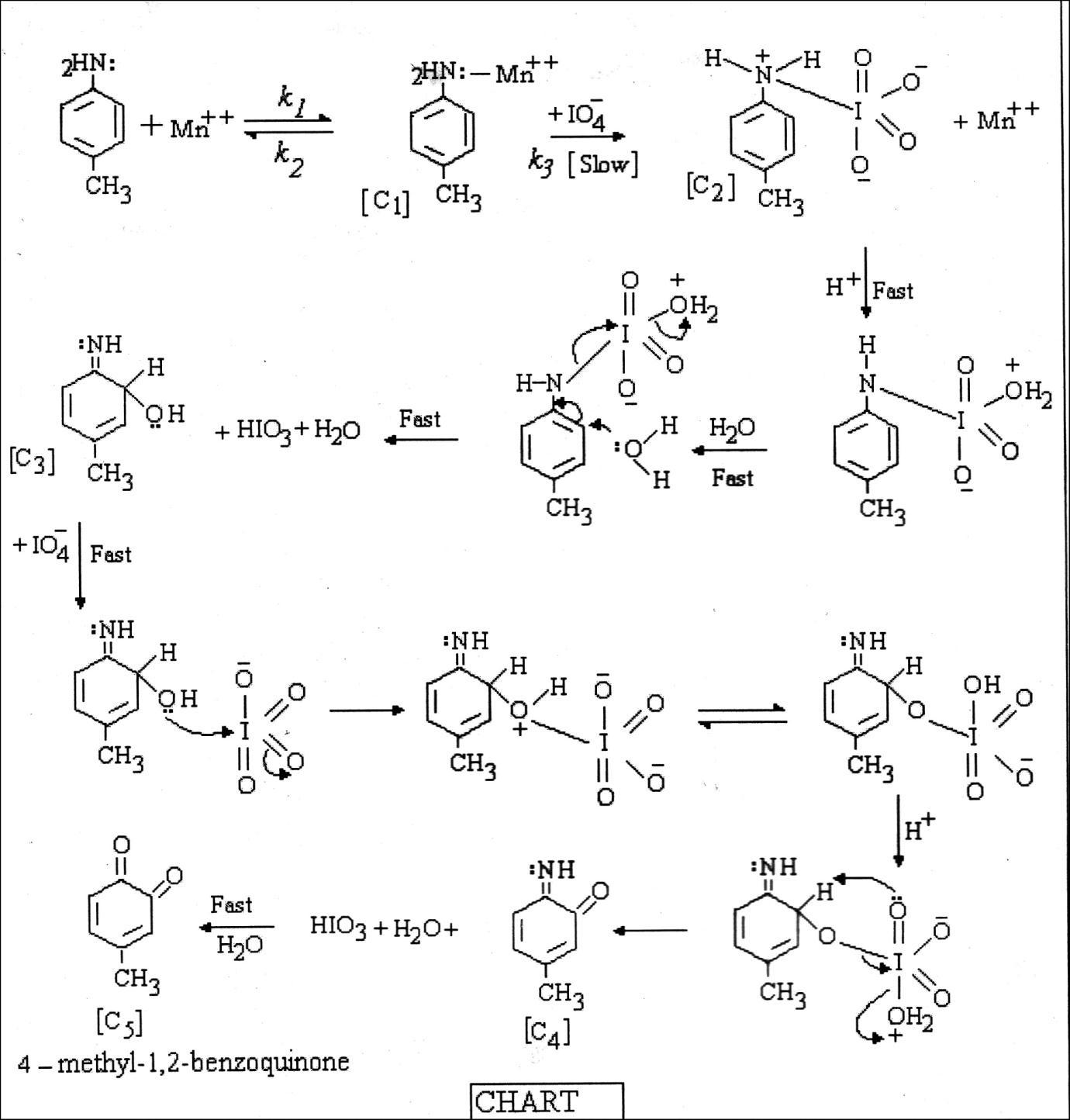
The redox process involved in the method, may be mechanistically similar to our earlier report related to the Mn(II) catalyzedperiodateoxidationof4 chloro 2 methylaniline,2,3dimethylanilineando toluidine.Ithasalsobeengiveninthe chart.
The methods developed by us are cost effective and involve use of simple equipments and chemicals that are generally expectedtobeavailableatsmallcentersofresearchorlaboratories.TherangeinwhichBeer’slawisbeingobeyed,molar absorptivity, Sandell’s sensitivity, detection limits, reproducibility of results are good enough to make these methods. Thesemethodsarebetter thansomeof thepreviouslyreportedmethodsintermsofcharacteristicsof calibrationcurves and the ease of the procedure involved. Further these methods are simple and less time consuming comparison to the other available methods for estimation of Mn(II) in aqueous /mixed media, as no pretreatment of the samples etc. are involved expect in cases where some rare in interferrants are present as already discussed. In general, the proposed methodsarefairlysuitableforestimationofMn(II)atnanogramlevel.
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
Table 1
Characteristics of various types of calibration curves for the proposed method [o TOL] x 103 = 1.0 M; [NaIO4] x 102 = 1.0 M; pH = 5.5; Acetone = 10.0% (v/v); Temp. = 35 ± 0.1°C; λmax = 525 nm.
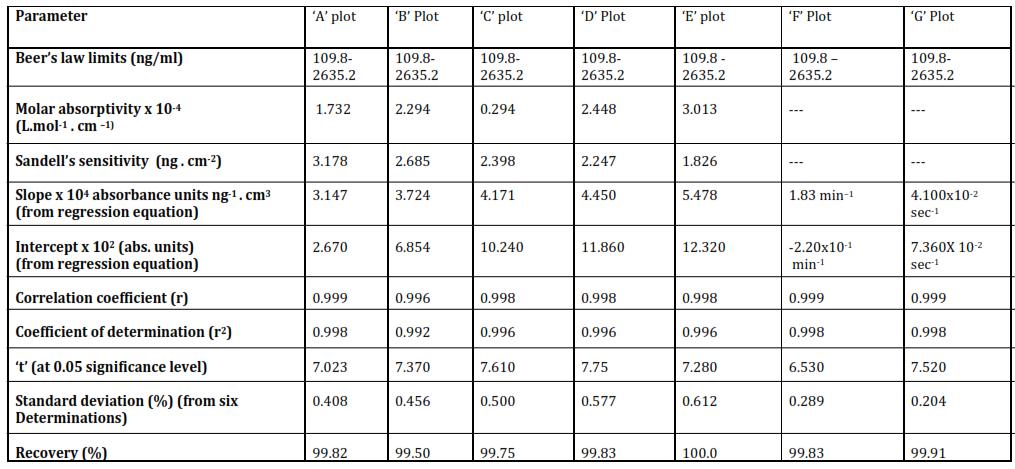
Table – 2
Characteristics of various types of calibration curves for the proposed method [o TOL] x 104 = 5.0 M; [NaIO4] x 103 = 5.0 M; pH = 6.5; Acetone = 5.0% (v/v); Temp = 35 ± 0.10 C; λmax = 475 nm
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
Table 3
Characteristics of various types of calibration curves for the proposed method [p TOL] x 103 = 1.0 M; [NaIO4] x 102 = 1.0 m; pH = 8.0; Acetone = 15.0% (v/v); Temp. = 35 ± 0.1°C; λmax = 490 nm.
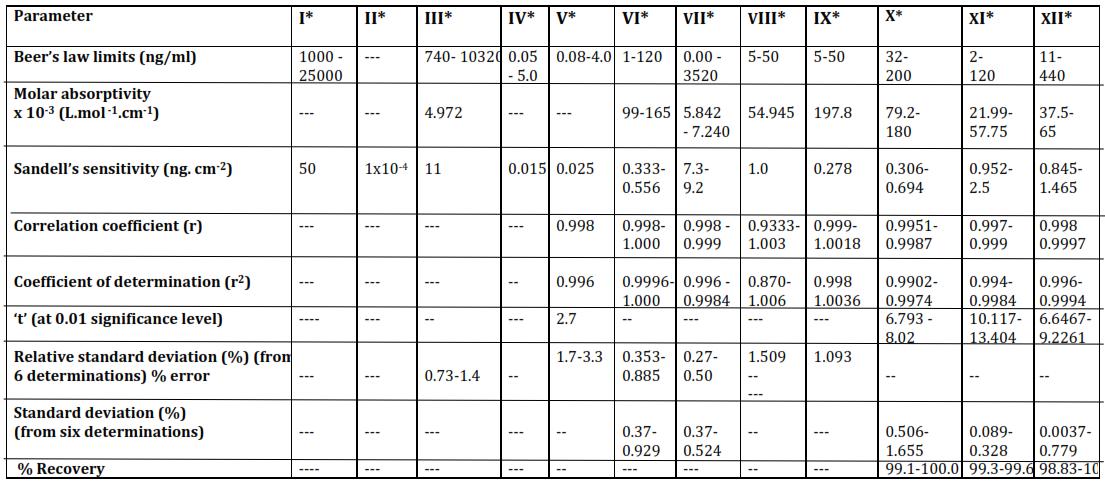
[I,II,III…XII arethenumbersassignedtomethodsforwhichthereferencesaregivenattheendofthetable.]
*whilemethodIispersulphate;methodIIisbasedonperiodateoxidationofp phenetidine;IIIisbasedoncomplexationof Mn(II )withp methylacetoacetanilide;MethodIVandVarealsononaromaticamineoxidationbasedones;MethodVIto XII arebasedonMn(II) catalyzedperiodateoxidationofm ansidine,m chloroaniline,2,4 dimethylaniline,p phenetidine,2,3 dimethylaniline,2,5 dimethylanilineand5 chloro,2methylanlinerespectively.
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
References:
1.KaushikR.D.,Shashi,DeviS.andSinghR.P.,Simplekinetic-spectrophotometricmethodforthedeterminationofMn(II) inwater,AsianJ.Chem.,2004,16,837.
2. Dolmanova I.F., Poddubienko V.P. and Peshkova V.M., Determination of manganese by kinetic method using the oxidationofp-phenetidinebypotassiumperiodate,Zh.Anal.Khim.,1970,25,2146.
3.WeiQ.,YanL.G.,ChangG.H.andOuQ.Y.,Kinetic spectrophotometricdeterminationoftracemanganese(II)withdahlia violetinnonionicmicroemulsionmedium,Talenta,2003,59,253
4. Biswas P.D. and De K., Extractive spectrophotometric determination of Mn(II) using 4-(methyl-phenyl)- 3oxobutanamide,J.IndianChem.Soc.,2003,80,195.
5. Mutaftchiev K.L., Catalytic spectrophotometric determination of manganese in some medicinal plants and their infusions,Turkish.J.Chem.,2003,27,619.
6. Mutaftchiev K.L., Determination of manganese in some medicinal plants and their water extracts, Chemical Papers Chemickezvesti.,2002,56,194.
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
7. Mutaftchiev K.L., Kinetic determination of nanogram levels of manganese(II) by naphthol blue black potassium periodate 1,10 phenanthrolinesystem,MikrochimicaActa,2001,36,79 82.
8.SuL.,LiJ.,MaH.andTaoG.,Determinationoftraceamountsofmanganeseinnaturalwatersbyflowinjectionstopped flowcatalytickineticspectrophotometry,AnalyticaChimicaActa,2004,522,281.
9. Kaushik R.D., Chaubey A.K. and Garg P.K., Kinetics and mechanism of periodate oxidation of p phenetidine, Asian J.Chem.,2003,15,1655.
10.KaushikR.D.andJoshiR.,Kineticsandmechanismofperiodateoxidationofm toluidineinacetone watermedium, AsianJ.Chem.,1997,9,527.
11. Kaushik R.D., Singh R.P. and Shashi, A kinetic mechanistic study of periodate oxidation of p chloroaniline, Asian J. Chem.,2003,15,1485.
12. Kaushik R.D., Kumar V., Arya R.K. and Singh D., Periodate oxidation of o toluidine in acetone water medium A kineticandmechanisticstudy,AsianJ.Chem.,2000,12,1123.
13.Kaushik R.D., JoshiR. andSinghD., Periodate oxidationofaromaticamines studieson the role of substituents and linearfreeenergyrelationships,AsianJ.Chem.,1998,10,567.
14. Kaushik R.D., Singh D., Joshi R. and Kumar S., Kinetics of periodate oxidation of aromatic amines studies on the kineticparametersandisokineticrelationshipforfewanilines,AsianJ.Chem.,1998,10,573.
15.PavolvaV.K.,SevchenkoY.S.andYatsimiriskiiK.B.,KineticsandMechanismofoxidationreactionofdiethylanilinewith periodate,Zh.Fiz.Khim.1970,44,658.
16. Kaushik R.D., Kumari R., Kumar T. and Singh P., Periodate oxidation of N,N dimethylaniline and N,N diethylaniline, AsianJ.Chem.,2010,22,7959.
17. Kaushik R.D., Amrita, Dubey M. and Singh R.P., Periodate oxidation of p bromoaniline in acetone water medium A kinetic mechanisticstudy,AsianJ.Chem.,2004,16,831.
18.KaushikR.D.,KumarD.,KumarAnujandKumarAjay.,Manganese(II)catalyzedoxidationof2,4 xylidinebyperiodate Akinetic mechanisticstudy,J.IndianChem.Soc.,2010,87,811.
19. Kaushik R.D., Kaur M., Malik R. and, Kumar A., Studies on Manganese(II) catalyzed oxidation of N methylaniline by periodateion,Int.J.Chem.Sci.,2010,8,1379.
20.KaushikR.D.,KumarA.,KumarT.andSinghP.,Manganese(II) catalyzedperiodateoxidationofp-toluidine-Akineticmechanisticstudy,React.Kinet.Mech.Cat.,2010,101,13;DOI10.1007/s11144-010 0214-y.
21.KaushikR.D.,Shashi,AmritaandDeviS.,KineticsandmechanismofMn(II) catalyzedperiodateoxidationof4-chloro2-methylaniline,AsianJ.Chem.,2004,16,818.
22. Kaushik R.D., Sundriyal P., Tyagi P., Singh P. and Singh J., Mn II catalyzed periodate oxidation of N, N-diethyl-mtoluidine AkineticandMechanisticstudy,Int.J.Chem.Sci.,InPress
23.BrittonH.T.S..Hydrogenions,D.VonNostrandCo.,1956,354.
24.MeitesL.,HandbookofAnalyticalChemistry,McGraw-HillbookCo.,INC,NewYork,1963,3-4.
25.VogelA.I.,ATextBookofQuantitativeInorganicAnalysis,LongmannsGreen,London,1961
26.KemmerF.N.,TheNelcoWaterHandbook,McGraw-HillCo.,Singapore,Internationaledition,1988,7.32.
27. Kaushik R.D., Amrita, Singh R.P. and Devi S., Kinetic method for microgram determination of Mn(II) based on its catalyticeffectonperiodateoxidationofmchloroaniline,J.Curr.Sci.,2004,5,341.
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
28.Kaushik R.D.,ChaubeyA.K.and Singh R.P.,Indian J. Environ.Ecoplan.,A newkinetic spectro photometric methodfor determinationofMn(II)inmicrogramsinwater,2003,7,29.
R.D.Kaushiketal/Int.J.ChemTechRes.2014,6(5),pp2695 2703.2703
29. Kaushik R.D., Amrita and Devi S., An improved method for nanogram determination of Mn(II) in aqueous medium, J. Curr.Sci.,2003,3,197.
30. Kaushik R.D., Devi S, Shashi and Amrita, Periodate oxidation of 2,3 dimethylaniline determination of Mn (II) in nanogramsinaqueousmedium,IndianJ.Environ.Ecoplan.2004,8,253.
31.BartkusP.andNauekaitisA.,Nauchn.Konf.Khim.Anal.Pribalt.Resp.BSSP(TesizyDokl.),1974,190.
32.RubioS.,HensA.G.andValcarcelM.,Analyst,1984,109,717.
33.KolotyrkinaI.V.,ShpigunL.K.,ZolotovY.A.andTsysinG.I.,Analyst,1991,116,707.
34.R.D.Kaushik,SurekhaKannaujia&Shashi,J.Natur.Phys.Sci.,18(1)(2004),115 124.
35.R.D.Kaushik,PrabhaSingh&SurekhaKannaujia,J.Natur,Phys.Sci.,19(1)(2005), 19 27.
DrSurekhaKannaujia
AssociateProfessor DepartmentofChemistry DBS(PG)College,Dehradun, Uttarakhand,INDIA. Shehasateachingexperienceofmorethanfifteenyears.
DrRadheyShyam

AssociateProfessor DepartmentofChemistry DBS(PG)College,Dehradun, Uttarakhand,INDIA. Hehasateachingexperienceofmorethanfifteen years.
