International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072

International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
1Central Institute of Petrochemicals Engineering & Technology (CIPET) Hyderabad, Hyderabad 500051, Telangana, India.
2Department of Chemical Engineering, University College of Technology, Osmania University, Hyderabad 500007, Telangana, India.
3Department of Chemical Engineering, B V Raju Institute of Technology, Narsapur, Medak Dist. 502313, Telangana, India.
*To whom correspondence should be addressed: Kiran Kumar Vuba & Appala Naidu Uttaravalli ***
Abstract In the study, polymer nanocomposites were prepared by using polypropylene co polymer (PPCP), maleic anhydride grafted polypropylene (MAgPP) and multiwall carbonnanotubes(MWCNTs). Thethermal stability ofthe in house prepared nanocomposites was studied using thermogravimetricanalysis(TGA).FromtheTGAdata,itwas observed that the thermal stability of the nanocomposites increased significantly with the increase in MWCNTs loading in the nanocomposites. Thermal degradation kinetics was studied from the data obtained from TGA analysis. Coats Redfern (CR) method was used to evaluate the thermal degradationkineticparameterssuchasdegradationreaction order, activation energy and frequency factor of the nanocomposites using TGA data. From the kinetic study results, it was observedthat the thermal degradation process mostly followed 0.5 order. The estimated degradation activation energy of the nanocomposites was in the range of 120 355 kJ/mol. The observed activation energy was around 1.9 times higher in presence of 2 wt.% MWCNTs with respect tothenanocompositewithoutMWCNTs.Fromthestudy,itcan be concluded that the MWCNTs offered better thermal properties to the nanocomposites.
Key Words: Nanocomposites; MWCNTs; Thermal degradation;Kineticstudy,Activationenergy.
In recent years, carbon nanotubes (CNTs) filled polypropylene co polymer composites [1] have been considered as the most versatile and economic way to engineeramaterialwithspecificdesiredproperties.Wide application of this polymer can be found in areas such as auto mobile, electronic, food packaging, textile, transportationandotherindustrialsectors.Polypropylene Co polymer (PPCP) is polymer that has relatively good mechanicalpropertiesandlow cost.However,PPCPisalso well known for having low thermal stability or low heat distortiontemperature.ThisshortcomingofPPCPmakesits applicationslimited.Forexample,foodcontainermadefrom PPCPcouldnotbeusedinovenormicrowaveatarelatively
hightemperature,electronicpartsorsemiconductorsmade from PPCP has small range of operating temperature, and PPCP coating in subsea pipelines could not withstand a relatively high crude oil temperature in a long intended lifetime.
Multiwall carbon nanotubes (MWCNTs) have been reinforced in the composites to improve the thermal stability. The carbon nanotubes have a tendency to form agglomerates during synthesis because of van der Waals attraction between nanotubes. In the composites, these agglomerations decrease the surface area and disturb the formationnetworkstructurewhichisessentialtoimprove mechanicalpropertiesandthemaintaskofprocessingisto dissolvesuchagglomeratesasgoodaspossible.Therefore, uniform dispersion of the nanotubes [2] is required to realizethepotentialityofthenanotubesasreinforcingfillers. CommonmethodsforthepreparationofCNTfilledpolymer composites include in situ filling polymerization, solution mixing and melt blending. In situ polymerization and solutionmixingtechniqueshavemanylimitations,including that they may not be commercially viable and are environmentally contentious. Many properties such as electrical conductivity, mechanical strength and thermal stability are strongly affected by the network structure which can restrain the mobility of the polymer chains in polymer/CNT nanocomposites. The extent of property improvement in CNT filled polymer composites generally dependsonseveralfactorssuchasvolumefractionoffillers, dispersion of CNTs in polymer matrix, type of polymer employedandfabricationmethod,etc.
The thermal degradation of polymers is a phenomenon where the polymeric material at elevated temperature undergoes chemical changes. The study of thermal degradation of polymers is important for developing an efficient technology for polymer processing and in understanding the thermal degradation mechanism. The studyisalsoimportantinunderstandingtheirapplicability atelevatedtemperatureandstorage[3].Thermogravimetric analysis(TGA)isausefultechniquetoevaluatethethermal
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
decomposition behavior, melting, stability, and thermodynamicparametersofthepolymers.
Chaudhary et al. [4], have studied the kinetic and thermodynamic parameters of various polymers by thermogravimetric method. In the study, Coats Redfern method was used to estimate the kinetic parameters of various coordination polymers. The reported activation energy was in the range of 30 105 kJ/mol depending on types of polymer and for a reaction order of around 1.5. Burkanudeenetal.[5]havestudiedthethermaldegradation kinetics ofterpolymer resins.Freeman Carroll andSharp Wentworth methods were used to estimate the kinetic parameters, such as, activation energy, order of reaction, entropy and free energy of activation. The reported activationenergyandorderofreactionwere24kJ/moland 0.92respectively.
Vasconcelos et al. [6], have studied the decomposition kinetics of poly (ether ether ketone) polymer using TGA technique with multiple heating rates. Flynn Wall Ozawa and Coats Redfern models were used to estimate the degradation activation energy. Fatemeh et al. [7] have studied the thermal degradation kinetics of bisphenol A (BPA) basedepoxyresinandlignin basedepoxycomposites usingTGA FTIRanalysisundernon isothermalcondition.In thestudy,itisreportedthatlignin basedepoxycomposites are more thermally stable and offered higher degradation activation energies in comparison with BPA based epoxy resin.
Zhiming et al. [8] have studied the thermal degradation kineticsofpolypropyleneusingTGAanalysis.Inthestudy, thereporteddegradationreactionorderwas0.35,andthe reportedactivationenergiesofthepolymerwasintherange of125 140 kJ/mol. Qin etal.[9]have studied the thermal stabilityandthermaldegradationkineticsofpolypropylene (PP)/clay microcomposites and nanocomposites using thermogravimetricanalysis(TGA)withthehelpofKissinger method, Coats Redfern method, and Freeman Carroll method. In the study, it was reported that pure polypropylene followed first order and the composites followed zero order kinetics. The activation energy of the composites increased dramatically, and the reported activationenergiesareintherangeof110 570kJ/mol.
Zhou et al. [10] have studied the effects of multi walled carbonnanotubes(MWCNTs)sizeandloadingonthermal properties and thermal stability of polypropylene (PP) compositesusingthermogravimetricanalysis.Inthestudy,it wasreportedthatthedecompositiontemperatureincreased withincreasingoffillerloadinganditslength to diameter ratio. Rajesh Kumar et al. [11] have prepared the MWCNT/Maleic anhydride grafted polypropylene (MAgPP)/PP nanocomposites and estimated various propertiessuchasthermo mechanical(i.e.,Vicatsoftening temperature (VST), heat deflection temperature (HDT)),
Factor value:
thermal (i.e., melting temperature, crystallization temperature and thermal degradation temperature), and electromagneticinterference(EMI)shieldingproperties.In the study, it was reported that the thermo mechanical propertiesofthecompositesincreasedwiththeincreaseof MWCNTsloading.
Fromtheliterature,itisobservedthatmostofthestudies areavailableonthethermaldegradationkineticsofvarious polymer based composites such as polypropylene, poly (ether ether ketone,epoxyetc.However,tothebestofour knowledge, the literature pertaining to the thermal degradation kinetics of PPCP based nanocomposites in presenceofMWCNTandMAgPPisnotdisclosedintheopen literature.Therefore,theobjectiveofthepresentstudyisto study the thermal degradation behaviour of the in house preparednanocompositesandestimationofitsdegradation kineticparameters.
Polypropyleneco polymer(meltflowindex=9g/10minat 230 °C) was purchased from Reliance Polymers (Mumbai, Maharashtra,India).TheMWCNT PPmasterbatchcontains 20 wt.% of MWCNT procured from Hyperion Catalysis International (Cambridge, USA). Maleic anhydride grafted polypropylene(meltflowindex=110g/10minat190°C) was procured from Plus Advanced Technologies Private Limited(Gurugram,Haryana,India).
ThenanocompositepreparationprocessisshowninFigure 1. In the process, required amounts of pellets of PPCP, MWCNT PPmasterbatchalongwithMAgPPcompatibilizer were added into the extruder. The extrusion process was carried out at a temperature of 190 230 °C; and mixed throughtheactionofthetwocounter rotating bladesat a rotorspeedof80rpm.Afterthat,theextrudedproductwas cutinto pelletsand dried at roomtemperature.Following that,thepelletswerefedintoaninjectionmouldingmachine (Japan Steel Works, India) that was operated at a temperatureof190 210°C,speedof100rpmandholding pressure of 300 bar. For testing and characterization, the pelletsweremouldedintoconventionalshapesandcooledto room temperature. Table 1 shows the composition of the composites used in this study. A control sample, i.e., a polypropyleneco polymeralongwithMAgPPsample,was alsopreparedusingasimilarprocedure.
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056
Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
Weight (%)
Figure 1. Flowchartofprocessingandcharacterization of PPCP/MAgPP/MWCNTComposites.
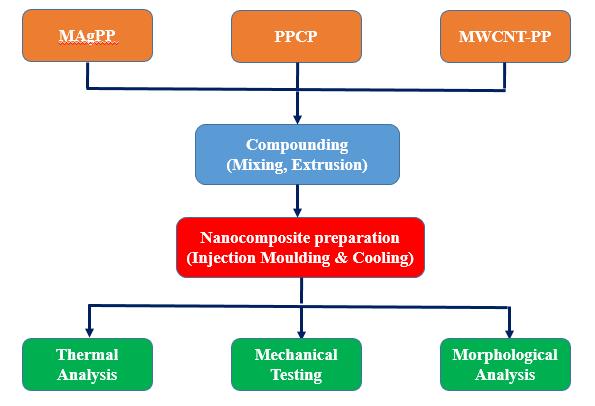
Table 1. ThechemicalcompositionofPPCPnanocomposites.
SampleNo. PPCP (wt.%) MAgPP (wt.%) MWCNT (wt.%)
NC 1 98.0 2.0 0.0
NC 2 97.5 2.0 0.5
NC 3 97.0 2.0 1.0
NC 4 96.5 2.0 1.5 NC 5 96.0 2.0 2.0
The thermal stability and degradation behavior of the nanocompositeswereestimatedusingthethermogravimetric analysis (TGA) (TGA/SDTA 851e Thermogravimetric analyzer, Mettler Toledo, Switzerland). The TGA measurementswererecordedinthetemperaturerangeof 30 600°Cataheatingrateof20°C/minunderthenitrogen environment.
Tostudythethermalstabilityanddegradationkineticsofthe in house prepared nanocomposite products, thermogravimetric analysis was carried out using a TGA instrument. The nanocomposite sample was taken in the samplepanandheateditfrom30°Cto600°Cataheating rate of 20 °C/min under the nitrogen environment. The obtainedTGA thermogramsofthenanocompositesamples areshowninFigure2.
90
70
50
110 NC-1 NC-2 NC-3 NC-4 NC-5
30
10
0 100 200 300 400 500 600 -10
Temperature(°C)
Figure 2. TGAthermogramsofPPCPnanocompositesat differentamountsofMWCNTs.
From the TGA thermograms, it is observed that the PPCP nanocompositewithoutMWCNTcontent(NC 1)isthermally stableuptoatemperatureofmorethan300°Cwithamass lossofbelow2wt.%.However,thethermalstabilityofthe nanocomposites increased significantly in presence of MWCNTs.ThenanocompositesNC 2,NC 3,NC 4andNC 5 arethermallystableuptoatemperatureofgreaterthan400 °Cwithamasslossofbelow2wt%.Itisalsoobservedfrom the thermograms that all the materials are decomposed almostcompletelyataround500°Cwitharesidualmassof around2wt.%.
3.2. Thermal degradation kinetics of in house prepared nanocomposites
Thermogravimetric techniques have been widely used to studythethermaldegradationkineticsofpolymermaterials. Differentmethods,suchas,Kissenger Akahira Sunoe(KAS) method, Flynn Wall Ozawa (FWO) method, Friedman method, Madhusudhanan Krishnan Ninan (MKN) method, andCoats Redfern(CR)methodhavebeenproposedbymany authors to estimate the kinetic parameters for thermal degradation of polymer materials. Among the various methods,CRandMKNmethodsareverypopulartoestimate thekineticparametersfromtheweightlossdataofmaterials for a fixed heating rate. In the present study, degradation kinetics of the in house prepared nanocomposites are evaluatedfromtheweightlossdataobtainedfromtheTGA thermogramsundernon isothermalcondition.TheTGAdata was coupled with Arrhenius equation to find the thermal degradationkinetics.Coats Redfernmethodhasbeenusedto evaluate the kinetic parameters with the help of the data obtainedfromtheTGAthermograms.
2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal

International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056
Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
ThelogarithmicformofArrheniusequationcanbewritten as:
loglog 2.303 E kA RT (1) where, k isdegradationrateconstant, T istemperature(K), A isfrequencyfactor(1/s), R isgasconstant,and E isactivation energy.
The rate expression for a non isothermal degradation reactionisexpressedas: / ..() ERT dx Aefx dt (2) where, x isthefractionalconversionofasampleattime t.
reciprocal of degradation temperature. The Coats Redfern plotsweredrawnforthein housepreparednanocomposites for different values of ‘n’ and the corresponding plots are shown in Figure 3. From Figure 3, it is noticed that the thermaldegradationofnanocompositesfollowedmostly0.5 order. The values of activation energy (E) and pre exponential factor (A) were calculated from the slope ( E/2.303R) and intercept log[(AR/aE)(1 (2RT/E))]oflinear equation for the best fit curve (higher R2 value). The estimated values of degradation reaction order, activation energyandpre exponentialfactorobtainedfromtheCoats RedfernplotsaretabulatedinTable2.
8.0
7.5
7.0
The fractional conversion of the material at a particular temperature xT was calculated using the following expression: iT T if
Y
WW x WW
(3)
where Wi, Wf and WT are initial weight, final weight and weightataparticulartemperaturerespectively.Finalweight wasconsideredbasedontheplateauregionofathermogram. If f(x) =(1 x)n (where n isorderofdegradationreaction)and constantheatingrate, a=dT/dt,thenaccordingtotheCoats Redfernmethod,theArrheniusequationcanbeexpressedby thefollowingrelations:
Y
6.5
6.0
5.5
n=0.0;R2=0.9938 n=0.5;R2=0.9956 n=1.0;R2=0.9777
0.13 0.14 0.15 0.16 0.17 0.18 5.0
1/Tx102(K-1)
8.0
7.5
7.0
6.5
6.0
5.5
(NC-2)
n=0.0;R2=0.9851 n=0.5;R2=0.9917 n=1.0;R2=0.9790
(NC-1) 0.125 0.130 0.135 0.140 0.145 0.150 5.0
1/Tx102(K-1)
2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal

International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

8.0
7.5
7.0
6.5
Y
6.0
5.5
(NC-3)
Y
Y
n=0.0;R2=0.9770 n=0.5;R2=0.9795 n=1.0;R2=0.9636
0.130 0.135 0.140 0.145 5.0
1/Tx102(K-1)
8.0
7.5
7.0
6.5
6.0
5.5
(NC-4)
Table 2. Kineticparametervaluesofdifferent nanocompositeproducts.
Sample No. Order, n R2 value E (kJ/mol) A (1/s) NC 1 0.5 0.9956 122.43 2.5094 NC 2 0.5 0.9917 269.91 1.1687×10 9 NC 3 0.5 0.9795 292.41 3.8102×10 11 NC 4 0.5 0.9863 319.81 5.0491×10 13 NC 5 0.5 0.9923 352.90 3.1214×10 15
n=0.0;R2=0.9749 n=0.5;R2=0.9863 n=1.0;R2=0.9817
0.130 0.135 0.140 0.145 5.0
1/Tx102(K-1)
(NC-5)
7.5
7.0
6.5
6.0
5.5
8.0 n=0.0;R2=0.9806 n=0.5;R2=0.9923 n=1.0;R2=0.9899
0.130 0.135 0.140 0.145 5.0
1/Tx102(K-1)
Figure 3. Coats RedfernplotsofdifferentPPCP nanocompositesatvarious n values.
From the data (Table 2), it is observed that the thermal degradationreactionofin housepreparednanocomposites followedaround0.5order.Furtheritisobservedfromthe data that the activation energy of the in house prepared nanocompositeinpresenceofMWCNTs(NC 2)ismorethan double with respect to the nanocomposite in absence of MWCNTs(NC 1).Theobservedactivationenergyisaround 1.9timeshigherinpresenceof2wt.%MWCNTswithrespect to the nanocomposite without MWCNTs. The increase in activationenergywiththeadditionofMWCNTsindicatesthat the thermal stability of the nanocomposites increased in presence of MWCNTs. The decrease in frequency factor values indicates that the rate of thermal degradation is slowed down with the increase ofMWCNTsloading in the nanocompositesi.e.,withtheincreaseofthermalstabilityof the nanocomposites.Inthestudy,the estimatedactivation energy of the in house prepared nanocomposites is in the rangeof120 355kJ/molfora0.5orderdegradationreaction.
In the study, polymer nanocomposites were prepared by usingpolypropylene co polymer (PPCP), maleicanhydride grafted polypropylene (MAgPP) and multiwall carbon nanotubes(MWCNTs).Thethermalstabilityofthein house prepared nanocomposites was studied using thermogravimetricanalysis(TGA).FromtheTGAdata,itwas observed that the thermal stability of the nanocomposites increasedsignificantlywiththeincreaseinMWCNTsloading in the nanocomposites. Thermal degradation kinetics was studied from the data obtained from TGA analysis. Coats Redfern (CR) method was used to evaluate the thermal degradationkineticparameterssuchasdegradationreaction order, activation energy and frequency factor of the nanocomposites using TGA data. From the kinetic study results,itwasobservedthatthethermaldegradationprocess mostly followed 0.5 order. The estimated degradation activationenergyofthenanocompositeswasintherangeof 120 355kJ/mol.Theobservedactivationenergywasaround 1.9timeshigherinpresenceof2wt.%MWCNTswithrespect tothenanocompositewithoutMWCNTs.Fromthestudy,it canbeconcludedthattheMWCNTsofferedbetterthermal propertiestothenanocomposites.
Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072 © 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page667
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 07 | July 2022 www.irjet.net p ISSN: 2395 0072
TheauthorsexpresstheirgratitudetoCIPETHyderabadfor the necessary support and facilities for the present study; and also grateful to University College of Technology, Osmania University, Hyderabad and B V Raju Institute of Technology(BVRIT),Narsapur,MedakDist.,Telanganafor providingnecessarysupportandfacilities.
[1] IijimaS.(1991)SynthesisofCarbonNanotubes.Nature, 354,56 58.
DOI:http://dx.doi.org/10.1038/354056a0
[2] Arrate H, Mercedes F, Juanjo P, María EM, Antxon S. Liquid state and solid state properties of nanotube/polypropylene nanocomposites elaborated via a simple procedure. Nanomater. 2013; 3(1):173 191.
DOI:https://doi.org/10.3390%2Fnano3010173
[3] P.Krzysztof,N.James,Thermaldegradationofpolymeric materials,1sted,RapraTechnologyLtd.,UK,2005.
[4] R.G. Chaudhary, P. Ali, N.V. Gandhare, J.A. Tanna, H.D. Juneja, Thermal decomposition kinetics of some transition metal coordination polymers of fumaroyl bis(paramethoxyphenylcarbamide) using DTG/DTA techniques.ArabianJ.Chem.2016.
DOI:http://dx.doi.org/10.1016/j.arabjc.2016.03.008
[5] A.R.Burkanudeen,M.A.R.Ahamed,R.S.Azarudeen,M.S. Begum,W.B.Gurnule,Thermaldegradationkineticsand antimicrobial studies of terpolymer resins, Arabian J. Chem.9(2016)296 305.
[6] G.D.C.Vasconcelos,R.L.Mazur,B.Ribeiro,E.C.Botelho, M.L. Costa, Evaluation of decomposition kinetics of poly(ether ether ketone) by thermogravimetric analysis,Mat.Res.17(1)(2014)227 235.
[7] F. Ferdosian, Z. Yuan, M. Anderson, C.C. Xu, Thermal performance and thermal decomposition kinetics of lignin based epoxy resins, J. Anal. Appl. Pyrol. 119 (2016)124 132.
[8] Zhiming G, Tsuyoshi K, Iwao A, Masahiro N. A kinetic studyofthermaldegradationofpolypropylene.Polymer DegradationandStability80(2003)269 274.
[9] QinH,ZhangS,ZhaoC,YangM.Zero orderkineticsof the thermal degradation of polypropylene/clay nanocomposites. Journal of Polymer Science: Part B: PolymerPhysics43(2005):3713 3719.
[10] Zhou T.Y, Tsui G.C.P, Liang J.Z, Zou S.Y, Tang C.Y, StankovicV.M.Thermalpropertiesandthermalstability of PP/MWCNT composites. Composites Part B 90 (2016):107 114.
[11] Rajesh Kumar B, Kiran Kumar V, Nagabhushan E, KrishnaC.E.Enhancedthermo mechanical,thermaland EMI shielding properties of MWNT/MAgPP/PP nanocompositespreparedbyextrusion.CompositesPart C:OpenAccess4(2021)100086.
© 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page668