International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 06 | June 2022 www.irjet.net p ISSN: 2395 0072

International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 06 | June 2022 www.irjet.net p ISSN: 2395 0072
Dilip Mane1 and S. D Yadav2
1Rishikesh Dilip Mane: Student at rajarambapu institute of technology, Islampur, Maharashtra, India. 2Dr. S. D Yadav: Professor at rajarambapu institute of technology, Islampur, Maharashtra, India. ***
Abstract Electric vehicle batteries had become very privileged nowadays our world is moving towards a green environment. The lithium ion battery (Li IB) currently rules the EV market but the dark side of a lithium ion is not so popular, to make Li IB material needed nickel and cobalt which are the most toxic materials and those batteries also explodeasthetemperaturecrosses40 45degreesCelsius,with Li IB world is not heading towards a so called green revolution. To overcome the disadvantages of Li IB many researchersandscientistshavedevelopedalternativebatteries for Li IB. In this review paper, different battery types are discussed with their challenges and how to enhance the performance of electric vehicle batteries.
Key Words: Electric vehicle, green environment, lithium ion,explode,nickelandcobalt,temperature.
Theultimatumforbatteryandcleanenergyhaspursuedthe evolutionintheworldofelectricvehicles.Thelithium ionis ruling the battery market and is growing on a large scale, there is an extensive application of Li IB with further demand.All electricvehiclepresenttoday(theyear2022)is poweredbyaLi IB.However,manyquestionsariseaboutLi IBliketheyareharmfultoourenvironmentandexpensive becauseoftheirrarepresence.Whereas,Li IBusesharmful components such as nickel and cobalt which cannot be decomposedorrecycled.ToovercomemostproblemsofLi IB researchers and scientists are discovering different resources which could replace lithium ion batteries, the mostprominentandefficientreplacementcouldbesodium ionbatteries(NA IB)becauseofaffordability,thelong life cyclecanbeeasilyrecycledandsodiumisfoundinallover the world. Although NA IB also have major disadvantages like low energy density, NA IB is heftier than Li IB. Li IB haveUltra Fastchargingtechnologyupto5C,whichisnot thesameasthechargingtimeofNa IB.
Aswellasresearchersarealsoseeingenoughopportunityin lithium iron phosphate (LiFePO4) batteries to replace current lithium ion batteries. Lithium iron phosphate batterieshavemorelifecycleandaresaferthanlithium ion batteries. LiFePO4 is low cost, has stable thermal and chemical chemistry, good energy density, and no thermal. Lithium ionbatteriesareharmingourenvironmentthrough their toxic component and are too costly due to there is specific location for mining lithium and rare components.
But, lithium ion batteries are more efficient and more powerful. Whereas Na IB and lithium iron phosphate are goodfortheenvironmentandarelowcost,theyarenotas efficientaslithium ionbatteriesduetolessresearch.
Li IB are famously used in mobile phones, laptops, and computers. Li IB first started in the 1990s as a result of thoroughresearchandtheofferingofmanyscientistsand engineers.Intheendeavourofdevelopingandimprovingthe performanceofLi IBwiththeincreasingdemandforenergy storage,forelectricvehicles,escalateresearchisrequiredfor betterandnon toxicLi IBwhichwillimproveperformances, includingspecificenergy,lifecycle,andprotectionfromheat. TherearemanyfurtherimprovementstobemadeinLi IB forsuperiorperformance.
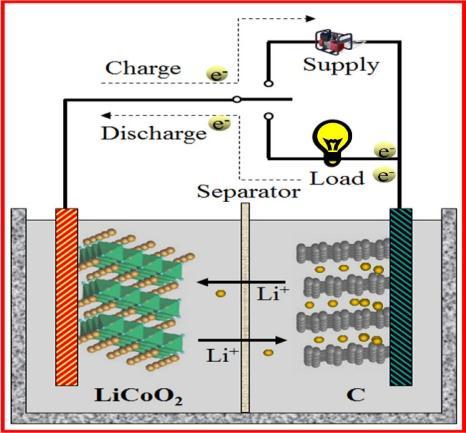
TheLi IBismadebyconnectingLithium ioncellsparallelor in series figure 2.1 show the detailed construction of a Lithium ion battery. Multiple battery cells can be put together.ALi IBhavecathodeandanodeastheirpositive andnegativeelectroderespectively.Bothanodeandcathode aredifferentiatedbyseparator[1].
Li IBiscurrentlybeingproducedinmillionpermonthandit replacestheheavynickel cadmiumandnickel metalhydride batteriesin,suchasmobilesandcomputers[10].
It is important to note that when Li IB is fully charged, combustible lithium negative materials pose the greatest safetyhazards[9].
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 06 | June 2022 www.irjet.net p ISSN: 2395 0072
When24 voltlifecycletestsweredoneontencellsheldin seriesat4CandatadischargerateofC/2thenithadminor degradationinAmp hourscapacityfor1000cycles[8].
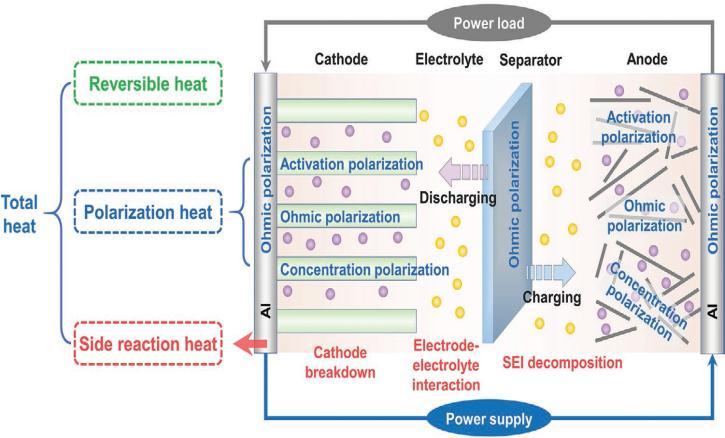
Thechallengeistoevolveanewbatterytosupplanttoday’s Li IB. The most famous lithium phosphate battery has minimum capacity. The challenge is to make minimum carbonelectrodematerials[1].
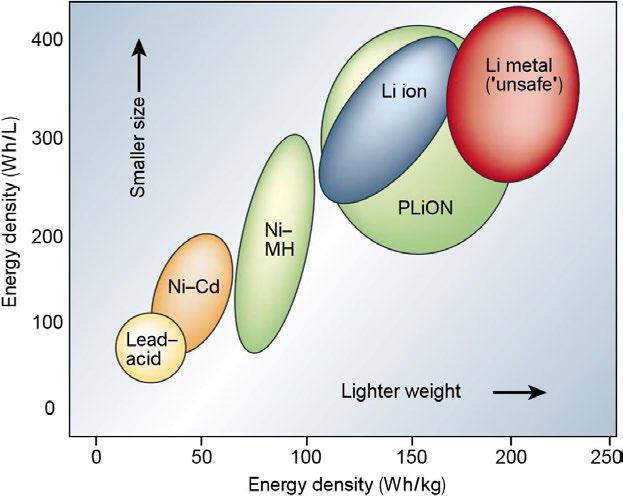
ThecostofaLi IBpackforEVsisRs.50,000.ThepriceofLi IBisexpensiveandalsotheperformanceofLi IBdegradesat a highertempaswell,chargingatminimumtempisnot a safedecision.So,tosafeguardthecircuitsprotectionisused and these protective circuits add weight which minimizes theenergydensity.Figure2.3theenergydensitiesofvarious batteries and neatly shows the advantages of Li IB over others. Li IB has more energy density but they have disadvantages of low recharge ability and risk of fire or explosion[1].
its chemistry is similar to a Li IB, Na IB has influenced considerable people. In the last 10 years, though extraordinaryeffortsweremadetoencouragemakingNa IB, notableresearchhasbeenmade,andotherdevelopmentis required for energy enhancing, power density, and more cyclelife[3].
Figure2.3(Comparisonofvariousbatteries)[1]
Na-IBis famousfor its ampleresourcesat minimal cost, Na IBispromisingsubsequenttechnologyelectricitygarage structuresforlarge-scalepackages,which include smart gridsandminimum-velocityelectric-poweredautomobiles Li-IBhadcausedmanytocombustweknowthatbeingsafe should be the first priority. The main cause of the fire is temperature and heat as Lithium-ion batteries cannot toleratetemperature above 40 degrees Celsius whereas, Na-IBcanstaycalmtill60degreesCelsius[2].
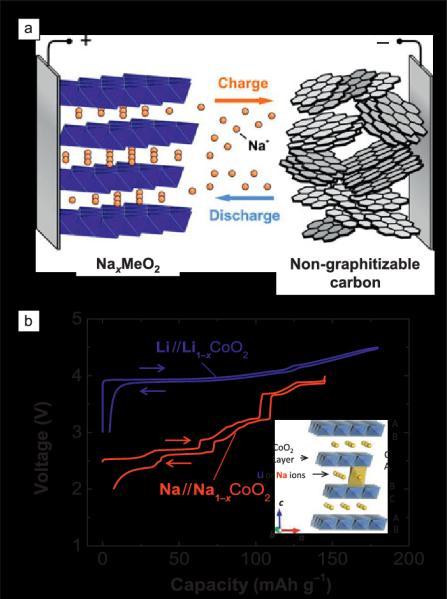
Having a huge abundance and minimal price of Na IB, it additionally has comparable electrochemistry to Li IB. Figure3.1showstheconstructionofaSodiumbatterywhere
The cathode material of a Na IB determines the energy densityofabattery,developingcathodematerialsisthekey challengeformakingmoreenergyinNa IB.Manysubstances had given brilliant storage capacity because of their acceptablestructure.ThesodiumstorageforSodium ionis indistinguishablefromthatofLi IB[3].
As shown in figure 3.2 construction of Na IB consists of heaped transition metals with Na as ‘+’ whereas carbon being‘ ’.Whenchargingionsmovesfrom‘+’to‘ ’[5]
(Na IBwithaheapedtransitionhavingmetaloxideas‘+’ andcarbonaceousmaterialas‘ ’)[5]
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056
Volume: 09 Issue: 06 | June 2022 www.irjet.net p ISSN: 2395 0072
More existence and minimal cost of Na have made him a goodexchangetoLi IB.ScientisthasgivenusmanySodium chemistries which were suitable for Na IB. Some Sodium substances with the same structure as Lithium are electrochemically active where the latter has not. In the futurescientistsshouldworkon‘ ’materialsofNa IB.The concernofanelectronandadditionalsupplementsonsolid electrolyte interface heaped formation can be determined properly.[5]
Nowadays popular battery is Lithium iron phosphate (LiFePO4)whereLithiumPhosphateisusedasaGraphite carbon electrode withcathode material andmetal substrateasananode.Lithiumironphosphatebatteriesare popular in vehicles due to factors such as minimumcost, safer,minimumtoxicity,andmorelife.LiFePO4ispopularin vehicles[6].
Suitable for EVs, HEVs, Bicycles, and heavy tools, ithas become a very promising choice amongphosphate basedcathodematerials.It has a minimal cost,is less hazardous, and environmentalfriendliness setitapart. Lithiumironphosphatebatteryhasgreatthermalandcyclic stability.Duetotheseproperties,itattractsgreaterattention asanewcathodeelectrodematerialforlithium ionbatteries [13].
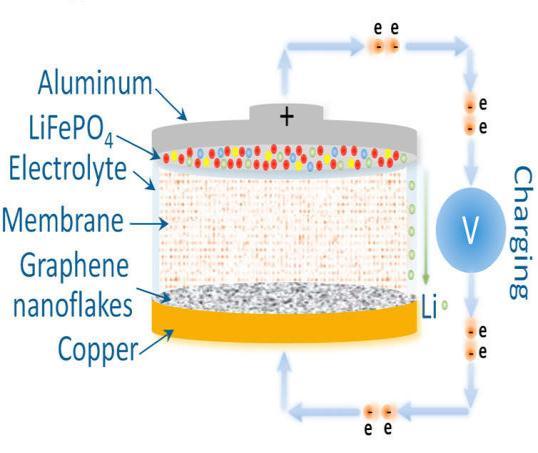
Figure 4.1 shows the advanced Li IB with graphene Nano flakeink anode and iron phosphatelithiumcathode. The batteryisdesignedtostabilizetheconfigurationofthecell and subdue the initial irreversible behaviour of the anodeoverafewcycles[6].
lithium ion battery that is lithium phosphate has zero maintenance.
Lithiumironphosphatebatteriesarethermallystableand their chemicalproperties are likely to be widely usedin thefuture.Hencethecircuitdesignersdesignacircuitwitha battery model which predicts performance with high precisionto controlusageand enhance safety during use [17]
Lithium iron phosphate batteries have a considerable reversible capacity of up to 3.5 Voltage and because of a small volume change of (6.8%), they have long cycle life. Because of their low inherent electron conductivity and minimumionscatteringrateofLithiumtheydonotreachup totheirtheoreticalcapacity.
LiFePO4 batteries are also distributed as a crystal olivine constructionasshowninFig4.2
Figure4.1(ConstructionofLiFePO4)[6]
LithiumphosphateisextremelysafeascomparedtoLi IB, alsoimproveschargeefficiency,andhasalongerlifespan, andbeginlightweightlithiumphosphateisbecominganew optionforelectricvehicles.Theyhavetheultimateedgeona
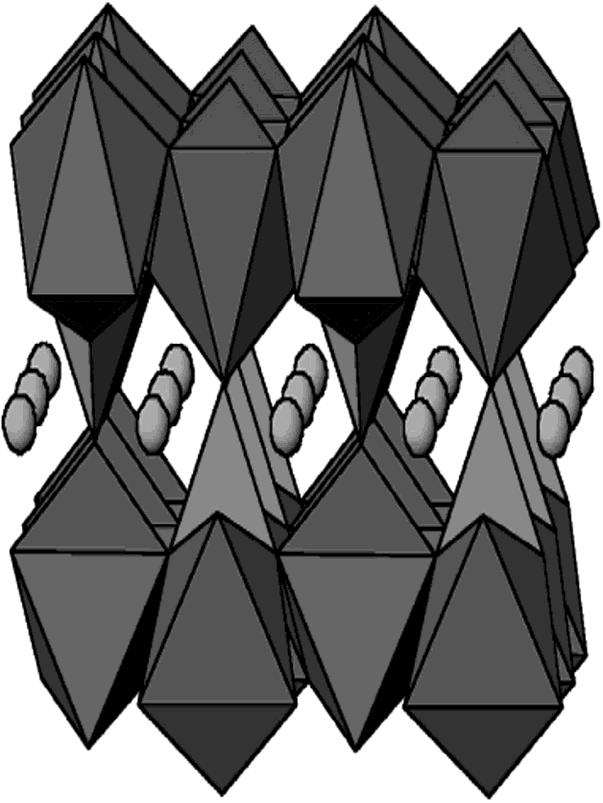
Fig4.2(3 DcrystalstructureofLiFePO4)[13]
Not all chemistry allows fast charging of Li IB because it impairsbatteryfunctionand boosts its continuousmechanism. But for the commercialization of EVs, quick charging methods will become a major thing. Reducing the timing for battery charging is an important factor[16].
NMCisacombinationofnickel,manganese,andcobaltthis batteryhashighspecificenergy.Thehigh capacitybatteries aretoday’sevolvingmovement.Formakingagoodbattery design, some characteristics should be learned and considered. Different types ofbatterycapacity at various
2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal

International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 06 | June 2022 www.irjet.net p ISSN: 2395 0072
temperatureshavebeenperformed.Throughthesetestdata, the electrical characteristics ofvariousparameters, such ascapacitance data, internal resistance data, OCVSOC characteristicrelationshipcurve,and temperature data These results can be used to compare different data. Its estimation of battery state, and enhance the standard of development[11].
However,NMCbatteriesarehazardoustoourenvironment becauseoftheircomponentusedforcathodematerial[12].
In NMCs the possibility ofstable high voltages cannot be reachedbybalancingthecathodeelectrolyte,accessingthis highvoltagebyoptimizingthecrystallographicorientation ofthecathodeisimportant.[14]
ThecapacityoftheNMCcell decreaseswiththeclock and decreasesrapidlyathighstoragetemp.Athighertempand moreSOC,accelerationresistanceincreasesovertimeandit isdeterminedbythecurrentpulsetest.[15]
LiNaFePO4isanotherpromisingmaterialthatcouldreplace Li IB,whereasitisnotpossibletomixlithiumwithsodium buttodoithastogothroughthesolvothermalprocess,the compoundofcathodematerialswascombinedwiththeeasy solvothermalmethod.Sodiumdopinghadanegligibleresult of replaced particles that gives a promise for its great electrochemicalperformance.
TheLi0⋅99Na0⋅01FePO4upgradesLithiumconductivityand manifestswiththefinestelectrochemicalperformance[7].
In the current situation for the development of new generation electric vehicle batteries, with improved performance and less harmful effect on the environment, there is a need of noticing the challenges of batteries accessibleinourpresentmarketi.e.,Li IB.Byaddingsome materials which are similar to Li IB according to their chemical structure and properties. It can reduce major harmfuleffectsandmakethemenvironmentfriendly.
The challenges of Li IB and Na IB is the use of certain materials which improve the performance of batteries. by studying various parts of the battery their functions and required properties deeply i.e., anode, cathode, and electrolytematerialswhicharemajorcomponentsinbattery and total battery performance are dependent on these components, some challenges may get reduced. Many researchershavefoundseveralmaterialsforanode cathode and electrolytes suitable for batteries to get the best performance.
Li IB degrades at high temperatures and it is not safe to chargefasteratlowtemperaturesalsoinLi IB,lowcarbon
electrode materials are difficult to develop. And in Na IB developmentofcathodematerialsisdifficulttocreatehigh energy. As Na IB and Li IB have the same working properties,bothmaygivesimilaroutput.
There are many more challenges related to batteries of lithiumandsodiumionshenceinsteadofusingonlylithium or sodium, adding other materials with them helps to improve battery performance. Such as now day lithium phosphatebatteriesaremorepopularnowadaywhichare lower in cost and safer to use than lithium ion batteries. Theirweightislessandmaintenancerequirementsareless.
[1] Da Deng, “Li ion batteries: basics, progress, and challenges,” Energy Science and Engineering, 2015, 3(5):385 418.
[2] ChaoYang,SenXin,LiqiangMai,“MaterialsDesignfor High Safety Sodium Ion Battery,” Advance Energy Material,2020,2000974.
[3] YongjinFangandZhongxueChen,“RecentAdvancesin Sodium IonBatteryMaterials,”ElectrochemicalEnergy Reviews,2018.
[4] Monica Sawicki and Leon L. Shaw, “Advances and challengesofNa IBaspost lithium ionbatteries,”RSC Advances,2015,5,53129.
[5] JusefHassoun,FrancescoBonaccorso,MarcoAgostini, MarcoAngelucci,MariaGraziaBetti,RobertoCingolani, MauroGemmi,CarloMariani,StefaniaPanero,Vittorio Pellegrini, and Bruno Scrosati, “A lithium ion battery based on a graphene nanoflakes ink anode and a lithium iron phosphate cathode,” arXiv: 1403.2161, 2014.
[6] YanLiu,WenchaoQin,DengkeZhang,LiweiFeng,Lei Wu,“EffectofNa+insitudopingonLiFePO4/Ccathode materialforlithium ionbatteries,”ElsevierB.V,2020.
[7] AndewBurkeandMarshallMiller,“Lifecycletestingof lithium batteries for fast charging and second use applications,”EVS27InternationalBattery,Hybridand FuelCellElectricVehicleSymposium,2013.
[8] KaiLiu,YayuanLiu,DingchangLin,AllenPei,YiCui,Liu, “Materialsforlithium ionbatterysafety,”Sci.Adv.;4: eaas9820,2018.
[9] BrunoScrosati,“Recentadvancesinlithiumionbattery materials,”ElectrochimicaActa45,2000,2461 2466.
[10] Ruifeng Zhang, Bizhong Xia, Baohua Li, Yongzhi Lai, WeiweiZheng,HuawenWang,WeiWangandMingwang Wang,“StudyontheCharacteristicsofaHighCapacity
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056
Volume: 09 Issue: 06 | June 2022 www.irjet.net p ISSN: 2395 0072
Nickel Manganese Cobalt Oxide (NMC) Lithium Ion Battery,”AnExperimentalInvestigation.Energies,11, 2275,2018.
[11] AntonellaAccard,Giovanni Dotelli,MarcoLuigiMusa and Ezio Spessa, “Life Cycle Assessment of an NMC Battery for Application to Electric Light Duty CommercialVehiclesandComparisonwithaSodium Nickel Chloride Battery,” Applied Science, 11, 1160, 2021.
[12] T.V.S.L.Satyavani,A.Srinivas Kumar,P.S.V. Subba Rao, “Methodsofsynthesisandperformanceimprovement oflithiumironphosphateforhighrateLi ionbatteries,” EngineeringScienceandTechnology,anInternational Journal,Volume19,Issue1,Pages178 188,2016.
[13] Nathan D. Phillip, Andrew S. Westover,Claus Danieland,GabrielM.Veith, “StructuralDegradationof HighVoltageLithiumNickelManganeseCobaltOxide (NMC) Cathodes in Solid State Batteries and ImplicationsforNextGenerationEnergyStorage,”ACS AppliedEnergyMaterials,2020,3,2,1768 1774.
[14] Julius Schmitt, Arpit Maheshwari, Michael Vetter, “Impedencechangeandcapacityfadeoflithiumnickel manganese cobalt oxide based batteries during calendaraging,”JournalofPowerSources,Volume353, Pages183 194,2017.
[15] DAnsean,MGonalez,J.C.Viera,V.M.Garcia,C.Blanco, M.Valledor, “Fast charging technique for high power lithiumironphosphatebatteries:Acyclelifeanalysis,” Journal of Power Sources, Volume 239, Pages 9 15, 2013.
[16] W.Y.Low, J.A.Aziz, N.R.N.Idris, R.Saidur, “Electrical modeltopredictcurrent voltagebehavioursoflithium ferro phosphate batteries using a transient response correctionmethod,”JournalofPowerSources,Volume 221,Pages201 209,2013.
“Rishikesh D. Mane is Student in RIT college pursuing Automobile Engineeringandskilledindesign”

“Dr S. D Yadav is experienced professorwithhistoryofworking inhighereducationandheisalso have completed Ph.D. in mechanicalengineering”

2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal
