International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 06 | Jun 2022 www.irjet.net p ISSN: 2395 0072

International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 06 | Jun 2022 www.irjet.net p ISSN: 2395 0072
Visibility of the headlamp outer lens is commonly affected by haziness, fogginess, fading, or white patches caused by the depositionofvolatileorganiccompounds,oxidation,chemical attack, water vapors, flying debris, and dirt. In the present research, two wheelerheadlampwithglassfadingissueswere identified and potential preventions have been discussed. Many of the headlamp outer lens is fabricated using glass materialandouterlensglassfadingisgenerallycausedbythe deposition of lower molecular weight moieties and lower temperaturethermallyunstablecompounds.Forthisattempt, two wheeler headlamp assembly was dismantled and characterized for possible root cause analysis and failure prevention. Different parts of the headlamp assembly such as reflector paint, bulb shield paint, dust cap, and housing were analyzed for thermal and morphological analysis. Thermal stability of the components was analyzed using thermogravimetric analysis (TGA). Morphological analysis was characterized by using scanning electron microscopy (SEM) and optical microscopy to evaluate the elemental alternationafter depositionontotheouterlens.Inhousepetri dish thermal box set up was prepared to evaluate the release of the lower temperature volatile organic compounds (VOC). The bulb shield paint system consists of filers, extenders, biocides, and lower molecular moieties for properties enhancement. The liberation of the lower molecular weight components and their deposition onto the inside layer of the glassouterlensresultedintheglassfadingissue.Thefailurein the headlamp components was identified and remedies have been implemented for the failure prevention in future headlamp parts.
bythelensprovidethedrivermorevisibilityonroadwhile drivingatnightandlowsunlightconditions[3] Majorityof headlamp outer lens are fabricated using glass and polycarbonate materials. When light emits through the headlamplensitpassesthroughthetwodifferentsurfaces first one is the own lens glass and the second one is the headlightouterlenswhichisgenerallymadeupofglass[4]. Inadditiontotheseadvantages,theouterlens'transparency isacriticalaspectinpreventingaccidentsduringthenight, rainy,andfoggyseasons.Glassmaterialishavingexcellent transparencyandcanwithstandhigherthermalstabilitybut possess lower impact strength. In the recent years, researchers have developed the alternate material for the glass outer lens. There are severe concerns of glass outer lens as when impacted with sudden load glass material shatteredandcancauseseriousinjurytothedrivers[5 7] In comparisonwithpolycarbonatematerial,glassmaterialhas goodUVstabilityandscratchresistanceandhencesomeof two wheelermakershaverequirementsforglassouterlens material [8]. Polycarbonate polymers is having excellent impactstrength,scratchresistance,recyclability,lowercost, betterprocessabilityandlightweight[9 10].
Accelerated change in technology, construction, and manufacturing sectors lead to an increase in ambient temperature, emissions, toxic gas pollution, and climatic circumstances [11]. Headlamp visibility and transparency canbeimpactedbymanyfactors.Thesefactorsincludeas water vapor stuck into the headlamp assembly, dirt deposition onto inside or outside layer of outer lens, fog accumulationduringextremeweatheringconditions,white patchesduetosuddenloadorimpact,orfadingissuedueto depositionofgasesorchemicalsglassmaterial[12 13].
The recent developments in the automotive sectors, industrial sectors, and building sectors have gained the researchersattentiontowardsthedroversafetyconcerns. Electricvehicles,driverlesscars,hydrogenfuelcellsarethe recentadvancementsintheworldwideautomotivesector[1
2] Headlamp is one of the most important features in automotiveindustry.Two wheelervehicleheadlampconsist of many different parts such as outer lens, housings, dust caps,reflector,bulbshield,andcasings.Thelightilluminated
The outside surface of the lenses can be damaged by a variety of environmental factors as well as manual interference,whereastheinsidesurfaceofthelensescanbe chemically attacked due to the release and deposition of volatileorganiccompounds(VOC)[14 15].Intheglassouter lens,glassfadingandhazinessaremajorlyobservedfailures duetothedepositionoflowermolecularweightcompounds onto the inside layer of the headlamp outer lamp which resulted in the reduction in the transparency [16] On the otherhand,dirtandmudparticlesaccumulatedontheouter lenses,reducingthevisibilityandtransparencyoftheouter lens material [17]. The volatile organic compounds (VOC) depositionontotheinsidesurfaceoftheouterlensismajor
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 06 | Jun 2022 www.irjet.net p ISSN: 2395 0072
concernsduetoreleaseanddepositionoflowermolecular weight compounds from the other plastic parts like dust caps,reflectors,shieldpant,andhousingsetc.Headlampin onconditiongeneratetheheatmorethan120 ℃which is responsibleforthereleaseofvolatilespresentintheplastic orpaintsystem[18].
In the present work, we have found out the possible root causes for glass fading issues observed in the headlamp. Figure 1 showsthephotographsofglassfadedouterlens, reflector paint and bulb shied paint Minda industries are leading original equipment manufacturer of automotive components. The glass fading issue was observed in the headlamp outer lens and several experiments were implemented to find out the root causes for the failure prevention. In order to avoid this kind of glass fading we havecarefullyobservedtheindividualheadlampcomponent more over polymeric material, which has possibility to release the volatile gases Table 1 shows the different components of the headlamp assembly along with fabrication material. We have done the petri dish thermal boxtestoftheindividualheadlampcomponenttoobserve anyreleaseofgasesfromtheplasticpartsmountedontothe backsideofthelens.Differentialscanningcalorimetry(DSC) and thermogravimetric analysis (TGA) has been done in ordertoconfirmthegasesreleasedfromchildpartslike.We have also run the SEM EDS analysis to check the ash recoveredfromtheTGAanalysisforbetterunderstandingon thefillerside.
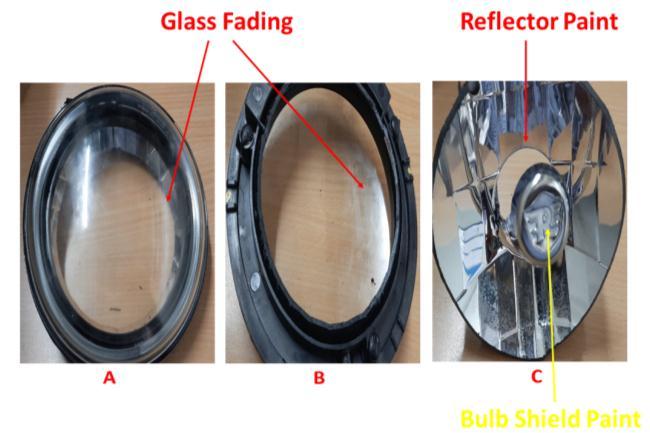
Table 1: Components of headlamp and their fabrication materials.
Two wheeler headlamps obtained from Minda Rinder IndustriesPune,India.Theobtainedheadlampwascomplete assemblywithglassfadingissue.Isopropylalcohol(IPA)was used to clean the sample pans of DSC, TGA and optical microscopyplatformwaspurchasedfromLobaChemicals, India.Nitrilehandgloveswereusedtoeliminatethedirect contactwithsampleswerepurchasedformSwatiScientific, India.
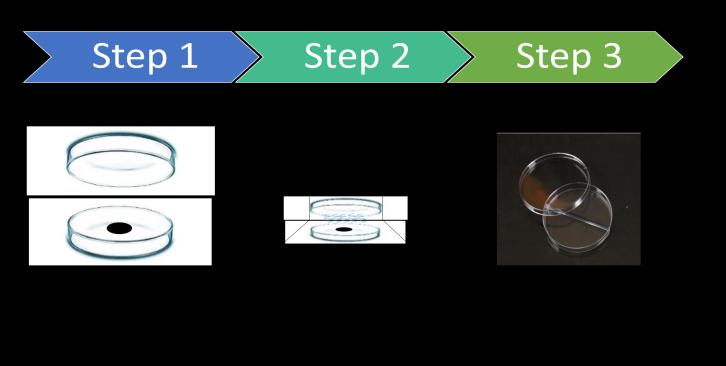
Themainaimoftheexperimentistocheckwhethertheglass fadingcausedbytheindividualpartsornumberofpartsare releasing gases due to heating. Because glass fading can reducetheglarewithreducingthevisibilityoftheouterlens. Thecompleteheadlampassemblywasdismantledintoouter lens, dust caps, housings, reflector, and bulb shield. The dismantled components were characterized for petri dish thermalboxtestat180℃for4h.Inthepetridishtest,itwas observed that bulb shield paint system releasing some volatilecompoundsanddepositedontotheinsidelayerof the petri dish. The schematic representation of the bulb shieldpaintpetridishsampleisshownin Figure 2. Inorder to confirm the volatile organic compounds releasing from the bulb shield paint, we have carried out thermal and morphological characterization using thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), scanningelectronmicroscopy(SEM)andopticalmicroscopy.
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 06 | Jun 2022 www.irjet.net p ISSN: 2395 0072
Secondheatingfrom40°Cto300°Cisothermalfor1min. Sometimes during first heating, some erroneous peaks appear resembling to the releasing of volatile compounds due to moisture absorbed by polymers or any other low molecularweightcomponentspresentsinthepolymers.In thesecondheatingcycle,thesepeaksweregotdiminished
Optical microscope analysis is employed to check the morphologicalbehaviorbulbshieldpaintsampleafterthe petri dish thermal box test. Optical microscopy is a nondestructive testing used to analyses the surface morphology. The optical microscope, also referred to as a lightmicroscope,isatypeofmicroscopethatcommonlyuses visible light and a system of lenses to generate magnified imagesofsmallobjects. LeicaDM2700modelhasbeenused foranalysingthepetridishtocheckthedepositedlayer.
Thermogravimetricanalysisisappliedtocheckanykindof volatile organic compound release (VOC). Thermogravimetricanalysis(TGA)isdestructivetechnique used to determine a material's thermal stabilityand its fraction of volatile components by monitoring the weight changethatoccursasasampleisheatedataconstantrate. The thermogravimetric analysis of bulb shield paint and reflectorpaintwasobtainedwithTAmakeinstrumentTGA 55(SerialNumber:791).WehaveruntheTGAinpresence ofnitrogenpurging. TestParameters:Heatingfrom25°Cto 200 °C with the heating rate of 20 °C/min. The test was maintainedat200℃for1husingisothermalmode.
DSC is used to analyze the thermal properties of the bulb shield paint and reflector paint. Also, release of any low temperaturevolatilesoranyotherregrindmixwiththebase material was analyzed using DSC analysis. Differential ScanningCalorimetry(DSC)isathermalanalysistechnique inwhich the quantification of heat flow is measured as a function of time or as a function of time at a given temperature.DSCofbulbshieldpaintandreflectorpaintis obtainedwithTAmakeinstrumentDSC25(SerialNo1152). WehavecarriedoutDSCanalysisinthepresenceofnitrogen atmosphere. Test parameters: First heating from 40 °C to 300°Cisothermal for 3 min coolingfrom 300 °Cto 40°C.
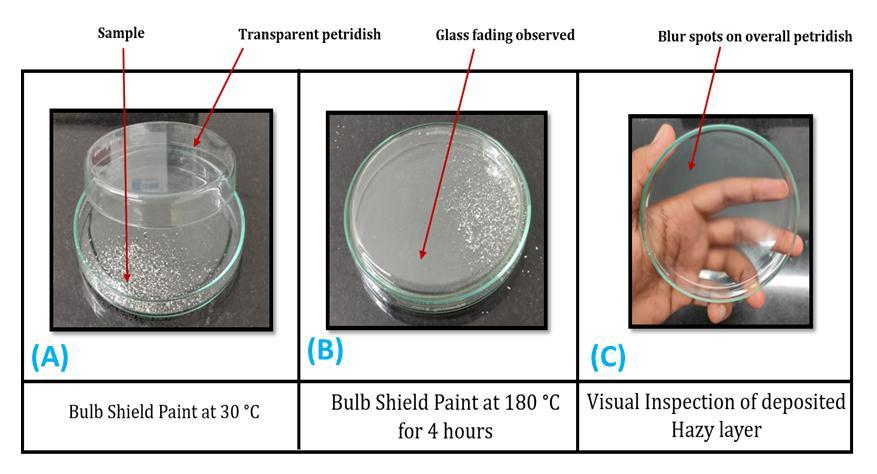
Thispetridishthermalboxtestishousemadesetup.Inthis testtwopetridishwereusedfortheanalysisalongwiththe thermal box. Bulb shield paint and reflector paint was scratchedoutformthesurfaceandkeptintothefirstpetri dishandcoveredwithsecondpetridish.Thispetridishwas thenkeptinsidethethermalboxandtestwascarriedat180 ℃for4h.Visualinspectionwascarriedouttoobservethe deposition of any lower temperature volatile organic compounds. All the components of the headlamps were analyzedusingpetridishthermalboxtest.
Scanning Electron Microscope is highly sophisticated analytical technique often use to analyze the die/package cracks,fracturesurfaces,bondfailure,physicaldefectswhich is all related to the surface morphology. SEM analysis is a powerful analytical tool, which uses a focused beam of electronstoproducecomplex,highmagnificationimagesofa sample’ssurfacetopography.Howsmallthesizeofelectron beam shot into the specimen can get is the key to high resolutionofSEM.Metalsamplecandirectlyplaceontothe mounting stab. Both sided carbon tape is used to fix the sample with the sample holding stab. Polymeric samples cannotusedirectlyaspolymersarenonconductivematerial whichaccumulatethestaticelectricfieldandstartscharging. Topreventthechargingofthesampleultrathincoatingof gold, silver, platinum, chromium is done onto the sample, which is called sputter coating. We have used the latest model of JEOL make SEM (IT 200) (Serial Number: MP1040003050305) which has a unique feature of low vacuummodewhichpreventsthepolymericsamplesfrom chargingevenifyouareusingwithoutsputtercoating.Both sidedcarbontapeisusedtofixthesampleontothesample holdingstab.Sampleiscarefullyblownouttoremoveany dirt and dust particles, Height is measured to avoid any breakdown issue and successfully mounted onto the specimenchamberstage.
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 06 | Jun 2022 www.irjet.net p ISSN: 2395 0072

Morphological analysis of the paint coated bulb shield samples and petri dish samples were carried out using opticalmicrocopy.Opticalmicroscopytestwasemployedto check the surface roughness of the petri dish after the heating and VOC deposition. Along with the petri dish, paintedbulbshiedsurfacewasalsocapturedusingoptical microscopy.Theglassfadinginheadlampismajorlydueto depositionoftheVOContoinsidesurfaceoftheheadlamp.It wasdifficulttoputtheheadlampforopticalmicroscopytest hence we have taken some of the scratch out some of the paintedmaterialandkeptitintopetridish.Further,thepetri dishwasheatedabout180℃for4h.Thelowertemperature VOCgotdepositedandtestedforopticalmicroscopy.Figure 3(C)and(D)demonstratestheopticalmicroscopyimagesof the petri dish after heating at 180℃. It can be seen from Figure3(C),surfaceroughnesswithsomeblackmarksare observed which signifies that some gases were released during heating process and got deposited onto the inside surface of the glass headlamp and fading was observed. Figure3(d)showsthemicrographsofthepetridishsample after heating process with 5 g of sample and checked the gasesdepositionontothepetridishlayer.Itwasobserved that with highest amount of material the gas released at lower temperature got deposited and formed the uniform layerontotheinsidesurfaceofthepetridishandfadingwas noticed. Figure 3 (B) displays the petri dish under the microscopelens.FurtherthisVOCreleasedwasconfirmed byTGAandDSCanalysis.
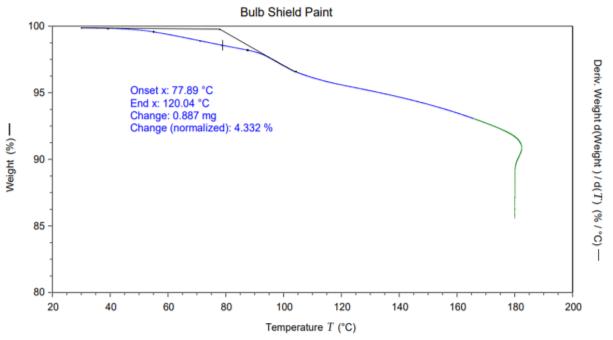
Thermalstabilityofthereflectorpaintandbulbshieldpaint wasdetermineusingthermogravimetricanalysis.Asfrom thepetridish,itwasnoticedthatsomegasesweregetting deposited after heating above the 150 ℃. In order to the check the temperature at which thermal degradation occurred, we have analyzed dust cap and paint samples. Figure4showstheweightlossgraphofbulbshieldpainted
sample. It can be seen form the figure that bulb shielded paintsampleshowstwostepthermaldegradation.Thevery firststepthermaldegradationstartedataround80 ℃and ended at 120 ℃ with weight loss nearly 9 %. TGA was carriedoutatisothermalconditionat180℃for3h.Itcanbe seen from the results that some low molecular weight compoundsgotreleasedinthegiventemperaturerange(80 120 ℃) and got deposited onto the inside surface of glass headlamp. This deposited VOC results in the reduction in transparencyduetothefadingmechanism.Thevisibilityof theglassheadlampcanreduceifdepositionofanyvolatile organiccompoundtakeplaceontothetransparentsurfaces. Headlampisclosedassemblyandevaporationofanyvolatile compoundsmaydepositsontotheinsideoftheouterlens. Figure5displaysthereflectorpaintsample.Itcanbeseen form the graph that 0.085 % weight loss observed in the isothermal conditions which is negligible in the tested temperaturerange.Thisreflectorpaintwasalsoheatedup to180℃for3hatisothermalconditions.Thisconcludesthat volatile organic compounds got released from bulb shield paintanddepositedontotheinsidesurfaceoftheouterlens. Thisdepositionresultsintheglassfadingandreductionin thetransparencyoftheheadlampouterlens.
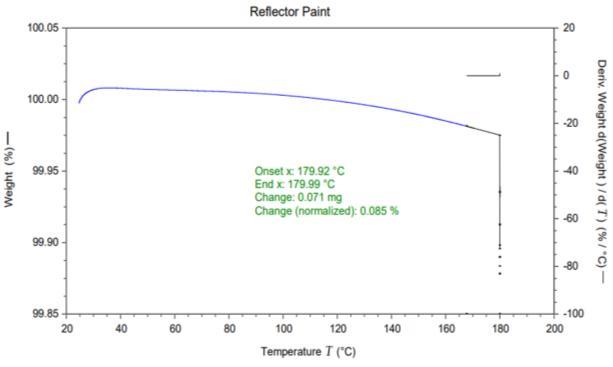
Figure 5: TGA thermograph of reflector paint sample
Differentialscanningcalorimeterwasemployedtoevaluate the thermal properties of the bulb shield paint material. Polymeric coated paint resin generally comes in the
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 06 | Jun 2022 www.irjet.net p ISSN: 2395 0072
thermoset category which upon curing mechanism got crosslinkednetworkanddoesnotchangeitssizeandshape. FromtheTGA andoptical resultsitconfirmsthat some of lower molecular weight compound got evaporated and depositedontotheinsidesurfaceofouterlens. IntheDSC analysis we have observed that no exothermic and endothermic peaks were present which signifies that no meltingandcrystallizationwereobservedduringtheheating and cooling cycle. During the first heating cycle the slight transitionintheheatflowcurvewasobservedat58°C,98°C and 113 °C temperature. This can be attributed to the differentpaintlayersofthecoatedmaterials.Inthesecond heatingcycle(redlinecurve),notransitionswereobserved around58°C,98°Cand113°Ctemperaturewhichclarifies thatvolatilecompoundsgotreleasedduringthefirstheating cycleonly.Thisconfirmsthatvolatilegotdepositedontothe insidelayeroftheheadlampouterlensandresultedinthe glassfadingissue.
depositionand black spots were observed onto the inside layerofthesecondpetridish.Thistestresultedthatfading oftheglasssurfaceiscausedbythedepositionofthevolatile organic compound. This glass fading also resulted in the reductionintheheadlampvisibility.
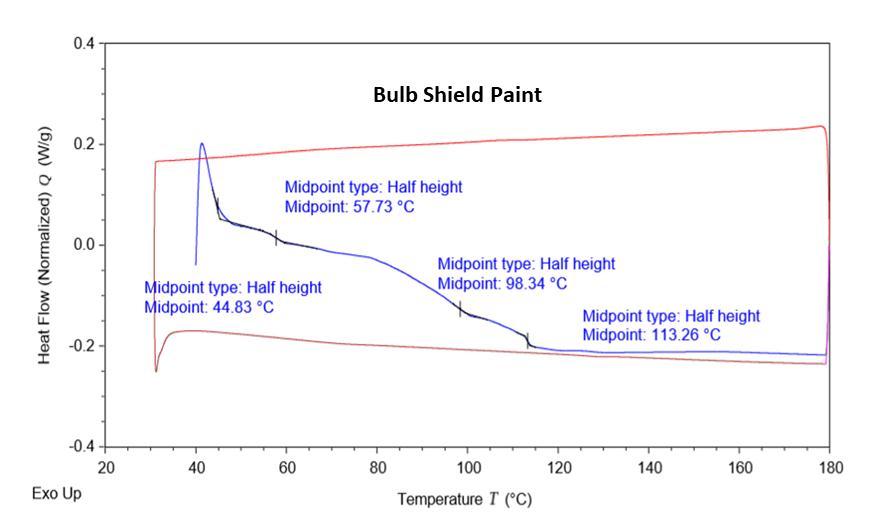
Figure7showsthepetridishesbeforeandafterthethermal boxtest.Twopetridisheswereusedfortheanalysis.Bulb shieldpaintsamplewasscratchedandputintothefirstpetri dish.Thesecondpetridishwasusedtocoverthefirstpetri dishasshowninFigure7(A).Thesetwo petridisheswere keptintheheatingboxandtheboxtemperaturewasraised to180℃andkeptitfor4handthisisshownasFigure7(B). During the heating cycle, it can be seen that some of the lowermolecularweightcompoundsgotdepositedontothe inside surface of the second petri dish. This id due to the liberationandevaporationofthevolatileorganiccompound (VOC).Itisconfirmedformthepetridishthermalboxtest thatintemperaturerangeof30 180℃,someoftheVOCgot releasedandasthesystemisenclosed,itgotdepositedonto theinsidelayerofthepetridish.Coatingpaintconsistofthe numberofadditiveslikefillers,antifoamingagents,biocides, wettingagents,extenders.Impurityoftheseadditivesmay resultinthereleaseoflowermolecularweightsubstances. In the Figure 7 (C), it can be seen that after cooling also
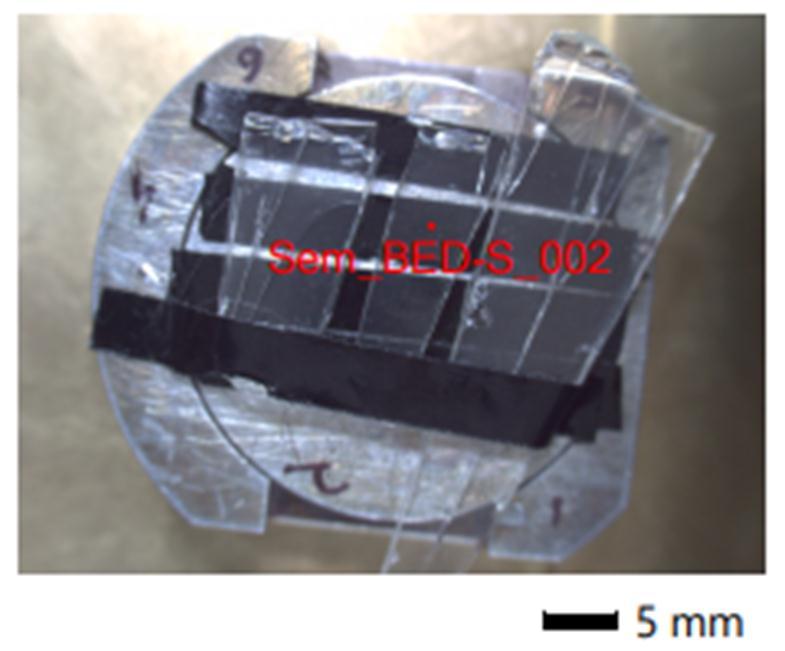
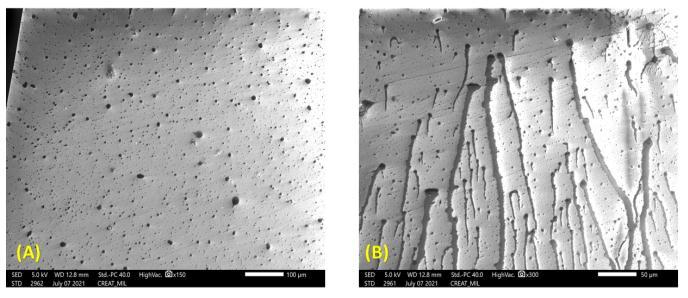
The received two wheeler headlamp assembly part were difficulttoplaceintheSEMassembly.Forthisattempt,we havecarriedouttheheatedpetridishtest.Thebulbshield paintsamplewerescratchedandputintothepetridish.The petridishwascoveredbythesecondpetridishandheated at 180 ℃ for 4 h. The volatile organic compounds got depositedontocoveredpetridishmaterialandcutintothe smallpiecesfortheSEMcharacterization. Figure 8 and 9 represents the petri dish small pieces placed for the characterizationanddepositedmicrographsofthereleased gases.Itcanbeseenfrom Figure 8 thatvolatilecompounds onto the petri dish sample have been placed on the SEM sample holder. Figure 9 (A and B) shows that samples at differentmagnificationandcanbeeasilyseenthatparticles afterreleasinggotdepositedontothepetridishsurface.The sameobservationwasobservedintheheadlampassembly. Figure 9 (A) shows the small black dots of the deposited volatilecompoundwhereasathighermagnificationitcanbe seenthat(Figure9B)glasssurfacegotcompletelycovered withthegassurface.
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 06 | Jun 2022 www.irjet.net p ISSN: 2395 0072
everynewconsignmenttoavoidanyfurthercomplications intheheadlampvisibility.
Inthisstudy,differentcharacterizationmethodsareusedto findoutrootcausefortheglassfadingissueobservedonto theglassheadlampouterlens.Fortheinitialscreening,we have used the optical microscopy images to confirm the deposition of volatile gases. Also, for the fine tuning and finalconfirmation,wehaveusedthermogravimetricanalysis (TGA)anddifferentialscanningcalorimetry(DSC)analysis. TGA analysis conformed that there was weight loss in the temperature range of 80 120 ℃ This low temperature decomposition attributed to the release of the volatile organic compounds. In the DSC curve also, irregular heat flow curve was noticed around 58 °C, 98 °C and 113 °C temperature range. This irregular heat flow pattern was absent in the second heating cycle which confirms the releaseofthevolatilesubstancestakesplaceduringthefirst heatingcycleonly.Theopticalimagesofthedepositedlayer ofgasesreleasedduringthepetridishtestofpaintedbulb shieldconfirmsthereisareleaseofsomevolatiles,whichis beingdepositedontotheinternalsurfaceofheadlightlens. ThesamethingwehaveobservedfromtheTGAanalysis.In the petri dish test at 180 ℃ for 4 h, we have noticed that some of the particles present in the paint sample got evaporatedanddepositedontothepetridishsample. TGA and DSC analysis concluded that weight loss and irregularities in the heat flow was observed nearly temperature range of 80 120 ℃. SEM analysis concluded that there were some morphological changes in the paint samplealongwiththenon uniformityinthepaintlayer.
Headlamp is one of the most fabricated products of the MindaIndustries.Earlierfabricatedproductswerefailedto qualifybasicstandardsoftheheadlampvisibilityduetothe thermaldecompositionofvolatileorganiccomponents.This liberatedVOCswerethendepositedontotheinsidelayerof theouterlensandcausedtheglassfadingissue.Inorderto resolvethisissue,weasaresearchanddevelopmentteam, havedetachedallthepartsfromtheheadlampassemblyand tested for the thermal, structural and morphological analysis.Duringthethermalcharacterizationofbulbshield paint, we have identified that there are several lower temperaturevolatileorganiccomponentsarepresentinthe systemwhichcausingtheglassfadingissue. Coatingpaint shouldhavepreparedwith purifiedadditivestoavoidthe release of low molecular weight compound. Coating resin and hardener system should have prepared in optimized ratiotoavoidpartial crosslinkingofresin. Afterrectifying the issue, we have asked the paint vendor to change the formulationofthepaintmaterialwithvirgingradesofthe filler and additives material. Also, aske them check the impuritiespresentintheadditivescompound.Wehavealso set and followed some primary characterization steps for
The authors would like to thank Minda Rinder plant for cooperation throughout the characterization and analysis process.Theauthorsalsothankfultothemanagementteam ofMindaIndustriesLimitedforthemotivation.
[1]J.A.Sanguesa,V.Torres Sanz,P.Garrido,F.J.Martinez, andJ.M.Marquez Barja,“Areviewonelectricvehicles: Technologies and challenges,” Smart Cities, vol. 4(1), Mar. 2021, pp. 372 404, doi:10.3390/smartcities.4010022
[2] Y. Miao, P. Hynan, A. Von Jouanne, and A. Yokochi, "CurrentLi ionbatterytechnologiesinelectricvehicles and opportunities for advancements,"Energies, vol.12(6) Mar.2019, pp.1074, https://doi.org/10.3390/en12061074
[3] D. Heitbrink, C. Schwarz, and W. Wang, “Simulation of Automotive Headlights for Human Factors Research,” IMAGE,June.2017.
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 06 | Jun 2022 www.irjet.net p ISSN: 2395 0072
[4] Muttart, Jeffrey, Wade D. Bartlett, Chris D. Kauderer, Grant L. Johnston, Matthew RE Romoser, Jan Unarski, and Daniel Barshinger, "Determining when an object enters the headlight beam pattern of a vehicle, ” SAE, April. 2013, pp. 01 0787, http://dx.doi.org/10.4271/2013 01 0787.
[5]C.Macías,O.Meza,andE.Pérez,“Relaxationofresidual stressesinplasticcoverlenseswithapplicationsinthe injection molding process,” Engineering Failure Analysis, vol. 57, Nov. 2015, pp.490 498, https://doi.org/10.1016/j.engfailanal.2015.07.026
[6]C.D.May,andR.J.Watling,“Thedevelopmentofanalytical and interpretational protocols to facilitate the provenanceestablishmentofpolycarbonateheadlamp lensmaterial,”.JournalofForensicSciences,vol.56,Jan. 2011, pp.S47 S57, https://doi.org/10.1111/j.1556 4029.2010.01590.
[7]B. T. Anthony, “Lexan polycarbonate for automotive forward lighting,” Materials & Design, vol. 6(6),Dec 1985, pp.293 302, https://doi.org/10.1016/0261 3069(85)90011 1
[8] C. Tuchinda, S. Srivannaboon, and H.W Lim, “Photoprotection by window glass, automobile glass, and sunglasses,” Journal of the American Academy of Dermatology, vol.54(5), May.2006, pp.845 854, https://doi.org/10.1016/j.jaad.2005.11.1082
[9]M.E.Nichols,andC.A.Peters,“Theeffectofweatheringon the fracture energy of hardcoats over polycarbonate,”Polymerdegradationandstability,vol. 75(3), Jan 2002, pp.439 446, https://doi.org/10.1016/S0141 3910(01)00244 0
[10]C. Seubert,K.Nietering,M.Nichols,R. Wykoff,andS. Bollin, “An overview of the scratch resistance of automotive coatings: exterior clearcoats and polycarbonatehardcoats,”Coatings,vol.2(4),Nov.2012, pp.221 234,https://doi.org/10.3390/coatings2040221
[11]Y.M.Yusoff,M.K.Omar,M.D.K.Zaman,andS.Samad, “Doallelementsofgreenintellectualcapitalcontribute toward business sustainability? Evidence from the Malaysian context using the Partial Least Squares method,”Journal of Cleaner Production,vol. 234, Oct. 2019,pp.626 637.
[12] D. Kyriacos, “Polycarbonates,” In Brydson's Plastics Materials, Jan.2017, pp. 457 485. Butterworth Heinemann, https://doi.org/10.1016/j.jclepro.2019.06.153
[13]M.D.Pustode,C.S.,Singh,R.Verma,T.Kochi,H.Barge,S. Gouda, and A. Dutta, “Root Cause Analysis and Mitigation of White Patch Formation in Automotive
value:
Headlamp,”Journal of Failure Analysis and Prevention,vol. 21(2), Apr.2021, pp.387 397, https://doi.org/10.1007/s11668 021 01120 y
[14]P.SarkarandandA.K.Bhowmick,“Sustainablerubbers and rubber additives,”Journal of Applied Polymer Science,vol.135 (24), June.2018, p.45701, https://doi.org/10.1002/app.45701
[15]M.J.SamideandG.D.Smith,“Analysisandquantitation of volatile organic compounds emitted from plastics usedinmuseumconstructionbyevolvedgasanalysis gas chromatography mass spectrometry, “Journal of Chromatography A, vol.1426, Dec. 2015 pp.201 208, https://doi.org/10.1016/j.chroma.2015.11.066
[16]M.Barletta,M.Puopolo,G.Rubino,V.Tagliaferri,andS. Vesco, “Hard transparent coatings on thermoplastic polycarbonate,” Progress in Organic Coatings,vol.90, Jan.2016, pp.178 186, https://doi.org/10.1016/j.porgcoat.2015.10.014
[17]H.Yahyaei,M.Mohseni,andS.Bastani,“UsingTaguchi experimentaldesigntorevealtheimpactofparameters affecting the abrasion resistance of sol gel based UV curablenanocompositefilmsonpolycarbonate,“Journal ofsol gelscienceandtechnology,vol.59(1),July.2011, pp.95 105,https://doi.org/10.1007/s10971 011 2466 z
[18]M.D.Pustode,C.S.,Singh,R.Verma,T.Kochi,H.Barge,S. Gouda, and A. Dutta, “Root Cause Analysis and Mitigation of White Patch Formation in Automotive Headlamp,”Journal of Failure Analysis and Prevention,vol.21(2),Apr.2021,pp.387 397
Mr. Ikhlas Chandkoti is senior executiveinUNOMindaIndustries. He is a B.Tech. in Polymer Engineering and working on the severalprojectsregardingthermal managementinelectricvehicles.

Dr.AmolNaikwadiisresearchlead in UNO Minda Industries. He has completed his Ph.D. Tech. in Polymer Engineering from Institute of Chemical Technology Mumbai.Hisexpertise is polymer synthesis, polymer thermal management,polymercomposites, energystorage.

International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056
Volume: 09 Issue: 06 | Jun 2022 www.irjet.net p ISSN: 2395 0072
Dr.ManojMaliisprinciplescientist in UNO Minda Industries. He has completed his Ph.D. Tech. in Polymer Engineering from Institute of Chemical Technology Mumbai.Hisexpertiseispolymer blendsandalloys,failureanalysis and prevention, polymer thermal management,andcomposites.


Mr. Srikanth T.S. is general managerinUNOMindaIndustries. HeisanElectronicsEngineer Have extensively worked on software development,test,deploymentand support.Provenskillsspanacross Delivery management, evaluating existing processes for improving effectiveness and efficiency, Outsourcing solutions & Contract Management.
2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal
