International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 06 | Jun 2022 www.irjet.net p ISSN: 2395 0072

International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 06 | Jun 2022 www.irjet.net p ISSN: 2395 0072
Abstract This article serves as an explanation to the application of Carbon Nanotubes(CNTs)inwaterpurification. The article is concerned with identifying the role that nanotechnology plays in water treatment and the specifics of watertransportinCNT basedmembranes. Thearticleanalyzes the fabrication of CNT membranes and their effectiveness in water permeability, water desalination and otherforms of water purifi cation. Functionalized membranes were also analyzed by breaking down the process whereby PEG is added to carboxylic CNTs in a reaction catalyzedbysulphuricacid,with such structure being found to have increased mechanical properties. AcomparisonbetweenCNTbasedmembranesand otherconventionalmembranesusedinwater purificationwere formed.Thiscomparisonwasmadebyconsideringtheefficiencyof the membranesinwaterpermeability,saltrejection,aswellasthe overallphysicalandmechanical properties of the membranes. CNTbasedmembraneswerefoundtoperform better than the conventional membranes in most categories,makingthemthe most costeffectiveandusefulamongthemembraneswithroom forfurtherimprovement.
Inthemodernworld,nanomaterialsaremanufacturedandused for a variety of different purpose. Each purpose that a nanomaterialcanservedependsonthestructureandphysical propertiesthatthematerialmaypossessasaresultofexistingin thenanoscale.Animportantpurposefornanomaterialsthathas emergedinthepastfewyearsiswaterpurification.Thisisafield thatisarequirementconsideringthedepletionoftheavailabilityof safedrinkingwateracrosstheworld.Duetotheriseinsealevel andevaporationcausedbyglobalwarmingandclimatechange, fresh water sources are facing rapid salination and increased forms of pollution. Without effective water purification technology,thisisresultinginalargernumberofpeoplegetting less access to potable water, especially in less economically developedcountrieswhereismostneeded.Nanotechnologyis beingtestedanddevelopedinordertoalleviatethisproblem.A widerangeofnanomaterialsthatperformusefultasks suchas adsorption, ultrafiltration, reverse osmosis, ion exchange and electrolysis have been developed. However, their cost of production and energy they require make them difficult to implementatalargescaleandmakethemavailabletolow income areas. Also some processes such as the adsorption techniquesfailtodesalinatewater, andthereforecannot deal
withtheissue. Someoftheseprocesses,suchasthemembrane technologies,seemtobeperformingthetaskofdesalinationwith increasing promise. Although they have not yet been made commercially viable, they utilize deionization in order to properlyreducethesaltcontentinwater.Scientistsarelooking to incorporate carbon nanotubes (CNTs) into themembrane technologies, using them as robust pores for water decontamination proper ties. Since CNTs have self cleaning properties,itmakesthemmoreusefulandsuitableforseparating andrejectingsaltionsandpermittingwatertoflowthroughthe interiorofthenanotubes.ThefunctionalityoftheseCNTswillbe examined,andtheireffectivenesswill be compared to other conventionalmembranes.[2]
In recent times, the general use of nanomaterials in water purificationhasrisenasnanotech nologycontinuestogrowand develop.Thesenanomaterialsareeffectiveinthedesalinationof water, as well as in other forms of water purification. Other nanomaterials, in particular nanoscale metals, have the propertiesofreactivity,adsorption,aswellashydrophilicand hydrophobicinteractionswhichcanbeusedtocontainandremove impurities from drinking water. An example of this is the structureofsilvernanoparticleswhichhavetheproperties of killingbacteria,virusesandfungithatresideinbodiesofwater. Thismakesiteasierofothernanomaterialswithadsorptionand filteringpropertiestocollectthedeadpollu tantsandremove them from the body of water without the fear of them reproducing and multiplying. Other nanomaterials such as titanium nanoparticles have the ability to induce reduction reactionsthattransformthestructureofharmfulbacteriaand virusesintoinactive andnon toxicsubstances. Sincebacteria, virusesandfungileadtothespreadofwater bornediseasessuchas cholera and bilharzia, rendering these species inert could save countless lives in poverty stricken areas. In relation towater desalination, most nanomaterials have been found to have increased surface porosity. This property improves salt rejection, and some metal nanoparticles play a large part in removinginorganicmaterialsfromthesurfaceofwaterbodies. Other nanomaterials that play an important role in the desalination of water include graphene and CNTs. These materialshaveenhancedadsorptioncapabilitiesthatareuseful inthedesalinationprocess. Thereare,however,limitationsto usingnanomate rialsinwaterdesalination. Theselimitations nanomaterials also become unusable after the initial run, requiringthemanufactureoffreshparticles,whichisalongand costlyprocess.Thecostoftheactualnanomaterialsthemselves
International Research Journal of Engineering and Technology (IRJET)

e ISSN: 2395 0056
Volume: 09 Issue: 06 | Jun 2022 www.irjet.net p ISSN: 2395 0072
also proves to be a serious limitation in their use in water purification. Most of the nanomaterials used are heavy expensivemetalssuchasgold,silverandtitaniumduetotheir inertproperties. Processingthemintonanomaterialsisalsoan expensive process because oftheirhightensilestrengthand meltingpoints. Developingthemostcost effective method of manufacturing these nanomaterials and using them to desalinatewaterintheleastexpensivewayisthegoalofmany scientistsinvolvedinwaterpurification.[2]
TherearetwotypesofCNTmembranesforwaterpurification SingleWalledNanotubeMembranes SWNT andMultiWalled Nanotube Membranes W N T We can classify the water pollutantsinthreedifferenttypes:Organicpollutantssuchas Industrial waste, pesticides, chlorinated compounds, pharmaceuticals, Inorganic pollu tants such as soil erosion, metals,nitrates,phosphatesandMicroorganismssuchasanimal excrementand sewage.[6]
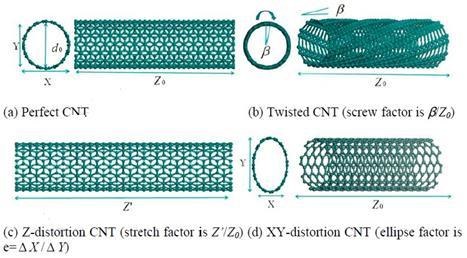
Thebehaviorofwaterflowingthroughdifferentconfigurationsof CNTshasbeenstudiedtounderstandhowtheseconfigurations influenceontheflowofwater.Thesechangeontheconfiguration oftheCNTstructureproducesasignificantchangeonthediffusion coefficientathighandlowtemperatureswhenthedistortionison theZaxis,OntheotherhandifthedistortionisatXYplaneoritis causedforalocaldefectthediffusioncoefficientchangescannot be ignored.[3]
Fig. -2: CVDfabricationprocess[1]
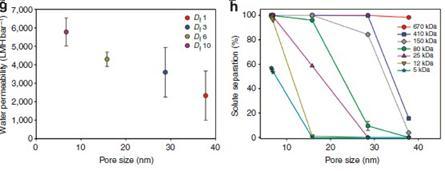
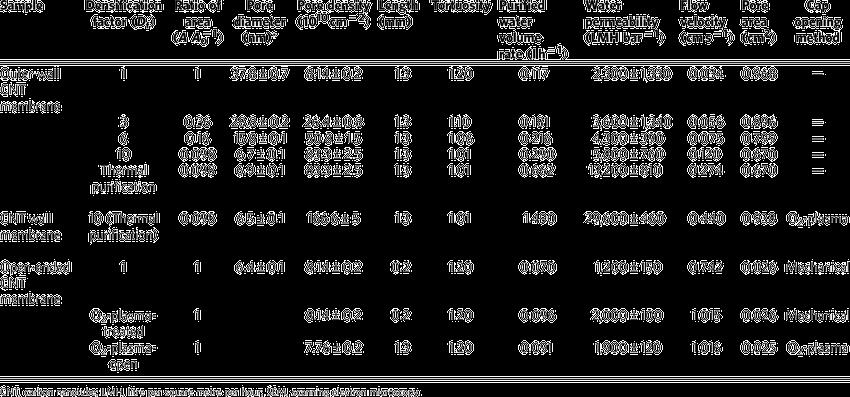
VerticalalignedCNTweresynthesizedusingwaterassistedCVD. Themicrostructureofthe CNT was characterized using SEM images.Theinnerstructure,innerdiameterandwallnumberof theCNTswerestatisticallymeasured,andtheporesoftheopen endedmembrane wereobservedfromtransmissionelectron microscopy(TEM)imagesrecordedusingaJEOLJEM 3000Fhigh resolutiontransmissionelectronmicroscopeatanacceleration voltageof300kV.Thefollowingresultswere obtained.[5]
Fig. 3: Performanceoftheouterwallmembrane.[5]
Fig. 1: DistorsionofCNTinZanXYaxis[3]
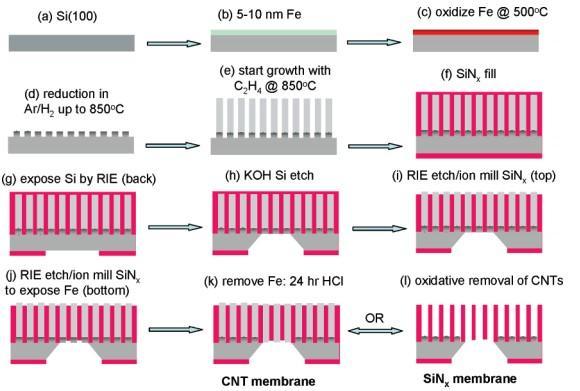
The preferred process is CVD because of the quality of nanotubesthatcanbeobtained.
Factor value: 7.529 | ISO 9001:2008 Certified
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 06 | Jun 2022 www.irjet.net p ISSN: 2395 0072
Fig. 4:CharacteristicsoftheCNTmembranes.[5]
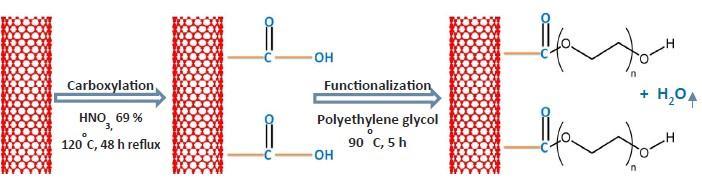
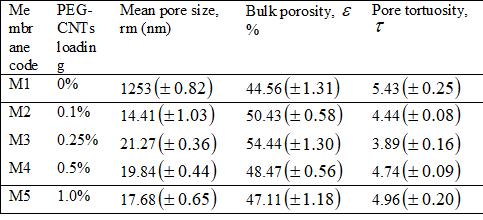
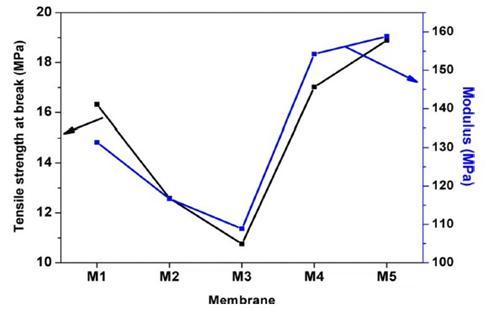
TofunctionalizecarboxylicCNTs(COOH CNT)withPEG,10gof PEGweremeltedinaroundbottomflaskonahotplateat90C and 1 g of COOH CNTs was then added. The mixture was allowedtostirabout10min,followedbyadditionoffewdropsof sulfuricacid(H2SO4)asacatalyst.Then,thereactionproceeded for 5 h under nitrogen atmosphere followed by cooling at ambient temperature. The resulting mixture was repeatedly washedandprecipitatedwithpetroleumetheruntilnomorePEG couldbeobservedinthesuper natantsolution.Afterthat,the precipitated PEG CNTs were decanted withacetoneasa final washingstepbeforedryingovernightundervacuumat85C. The obtainedresultsrevealthattheconcentrationofPEG CNTsinthe castingsolutiondecreasedthetensilestrengthandthemodulus ofthemembranesuntilaspecificloading(0 025wt%PEG CNTs)butafterthisloadingthemechanicalpropertieswere increaseddramatically.[4]
Fig. 6: Differenttypesofmembranessynthesized.[4]
Fig. -7: TensileStrengthandYoungModulus.[4]
Fig. 5: PreparationofPEGfuntionalizedCNT.[4]
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

Volume: 09 Issue: 06 | Jun 2022 www.irjet.net p ISSN: 2395 0072
membranecurrentlyyieldthesameratesofsaltrejection,theCNT membranescanbemodifiedtoyieldahigherrateatareasonable costofproduction.Asidefromthedifferencesintheefficiencyof themembranesintheprocessofwaterdesalination,therearealso numerous physical differences betweenCNTbasedmembranes andtheotherconventionalmembranes.Intermsofmaterialsused toproducethemembranes,CNTsaremadeoutofcarbonarranged intubularform,whileROandNFmembranesaremanufactured fromorganicpolymerssuchaspolyamide,UFmembranesare made from polysulfone and acrylic, and MF membranes are manufacturedusingpolypropeneandpolyurethane.Intermsof thickness,CNTsareusually2 6umthick,whichiswithinthe nanoscale. The thickest membranes are the UF membranes whichrangefrom150300um,whilethethinnestmembranesare theROmembraneswithathicknessof0.10.2um.Intermsofself cleaningcapability,theCNTshavefullcapabilitywhiletheother membranesrequirefunctionalization.[2]
1.ForSWNT Membranes higher water permeability, purified water volume and flow velocity were obtained with smaller pore sizes.
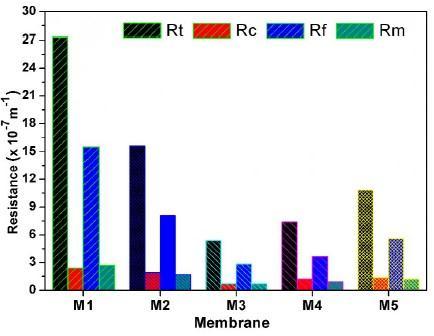
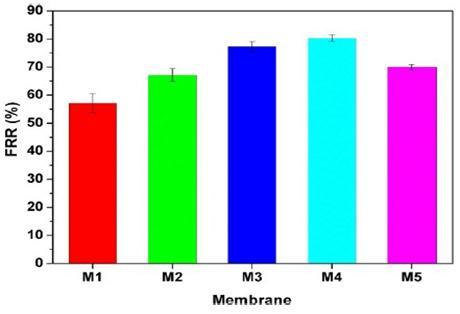
2.ForaPEG CNTloadingfrom0.25%propertiessuchastensile strength, flux recovery ratio and filtration resistances start growing.
Fig.9FiltrationResistances.[4]
MembranetechnologiesachievedusingCNTshasbeenfoundto performmoreefficientlyintheprocessofwaterdesalinationas comparedtootherconventionalmembranessuchasre verse osmosis (RO), nanofiltration (NF), ultrafiltration (UF) and microfiltration(MF).Thisisbecauseofanumberofreasonsthat have been well researched by scientists specializing in the manufacture of membranes for water purification. The first reasonisthatCNTsarehollowandhydrophobic,allowingpolar water molecules to flow easier within them. The other membranesdonothavethehydrophobicinterior,whileothers arehydrophobictoalesserextentascomparedtoCNTs.Secondly, CNTshavebetterwaterpermeabilitythantheothermembranes, namely the RO membranes which have the highest level of permeability among the other membranes. The water permeabilityamongarmchairCNTswasfoundtobefourtimes higherthaninROmembranes.CNTsalsohaveahigherlevelofsalt rejection amongthemembranes. However,theNFmembrane performssimilarlytotheCNTsinthissection.ACNTmembrane withanincreasedporediametercouldtheoreticallyretainmore solute particles that make up a salt, and it is possible to manufacture.SuchanalterationintheporediameterofaCNT membraneresultsina%removalofsaltsdissolved,aratethatis muchhigherthanthatofROmembranes. WhileCNTsandRO
3. Thisenhancedinflowpropertiesareabigsteptowardsthe improvementofmembranes efficiency, howeverthe theoretical valuesarestillfarfrombeingreached.Moreresearchinthisfieldand newfabrication techniquesandimprovementsonthecurrent techniques will be a key factor on the development of this technology.
[1] OlgicaBakajin,AleksandrNoy,FrancescoFornasiero,CostasP. Grigoropoulos,JasonK. Holt,JungBinIn,SangilKim,and HyungGyuPark.Chapter11 nanofluidiccarbonnanotube membranes: Applications for water purification and desalination. In Anita Street, Richard Sustich, Jeremiah Duncan, and Nora Savage, editors, Nanotechnology ApplicationsforCleanWater(SecondEdition),Microand NanoTechnologies,pages173 188. William Andrew Publishing,Oxford,secondeditionedition,2014.
[2] RaselDas,Md.EaqubAli,SharifahBeeAbdHamid,Seeram Ramakrishna, and Zaira Za man Chowdhury. Carbon nanotube membranes for water purification: A bright future in water desalination. Desalination, 336(SupplementC):97 109,2014.
[3] JiameiFeng,PeirongChen,DongqinZheng,andWeirong Zhong. Transport diffusion in deformed carbon nanotubes. Physica A: Statistical Mechanics and its Applications,493:155 161, 2018.
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056

[4] ArsalanKhalid,AhmedAbdelKarim,MuatazAliAtieh, SaqibJaved,andGordonMcKay.PEG CNTsnanocomposite PSUmembranesforwastewatertreatmentbymem brane bioreactor. Separation and Purification Technology, 190:165 176,jan 2018.
[5] ByeonghoLee,YoungbinBaek,MinwooLee,DaeHong Jeong,HongH Lee,Jeyong Yoon,andYongHyupKim.A carbonnanotubewallmembraneforwatertreatment.
[6] Neeta Pandey, S. K. Shukla, and N. B. Singh. Water purificationbypolymernanocom posites:anoverview. Nanocomposites,3(2):47 66,apr2017.
Volume: 09 Issue: 06 | Jun 2022 www.irjet.net p ISSN: 2395 0072 © 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal