International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN: 2395 0072
Classification and Identification of Flavonoids from plant kingdom as Acacia species
Hassan .E . Elkhidr (1), Tayseir .M. Ahmed (2)
Shendi university ,faculty of science and technology ,department of chemistry ,shendi ,Sudan Red sea University , Department of chemical engineering, Port Sudan , Sudan
Abstract
Flavonoids, a group of natural substances with variable phenolic structures, are found in fruits, vegetables, grains, bark, roots, stems, flowers, tea and wine. These natural products are well known for their beneficialeffectsonhealthandeffortsarebeingmadeto isolate the ingredients so called flavonoids. Flavonoids are now considered asanindispensablecomponent ina variety of nutraceutical (are products, nutrition are also used as medicine), pharmaceutical, medicinal and cosmetic applications. This is attributed to their anti oxidative, anti inflammatory, anti mutagenic and anti carcinogenic properties coupled with their capacity to modulatekeycellularenzymefunction. Inthisstudywetrytoclassifytheflavonoidsfromhigher plant specially Acacia species. Various sub groups of flavonoids are classified according to the substitution pattern of ring C. Flavonoids classified into: flavones, flavanols, flavanones, chalcones, aurones, isoflavonoids andanthocyanins.Thechemicalstructureofthealltypes of flavonoids can be deduced on the basis of its Infra red(IR),Ultra violet (UV), proton nuclear magnetic resonance(1HNMR)Spectrophotometer.
Key words: Acacia species, Flavonoids, Spectrophotometer
I. Introduction
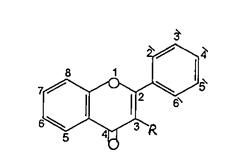
The flavonoids are poly phenolic compounds their conjugatesfromaverylargegroupsofnaturalproducts, they are found in many plants tissues, where they are present inside the cells or on the surfaces of different plant organs. Flavonoids possessing 15 carbon atoms; twobenzeneringsjoinedbyalinearthreecarbonchain. The chemical structures of this class of compounds are based on a C6 C3 C6 skeleton. They differ in the saturation of the hetero aromatic ring C, in the
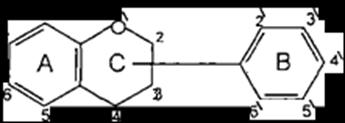
placement of the aromatic ring B at the position C2 orC3 ofringC(Figure1).
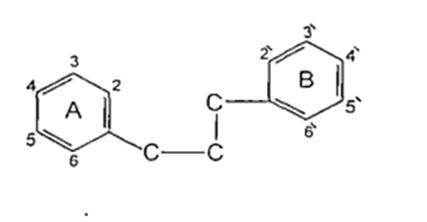
Flavonoids constitute one of the most characteristic classesofcompoundsinhigherplantandtheyarewidely distributedthroughtheplantkingdom1.Manyflavonoids arc easily recognized as flower pigments in most angiosperum families (flowering plants) and they are usually known as plant pigments. However their occurrenceisnotrestrictedtoflowerbutincludeallpart oftheplant2.Thechemicalstructureofflavonoidsarc Based on a C15 skelton with achromance ring bearing a secondaromaticringBinposition2,3to43(Figure2).

(Figure1):theskeletonoftheflavonoids
(Figure2):skeltonwithachromanceringbearinga secondaromaticringBinposition2,3
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN: 2395 0072
Infewcases,thesix memberedhetrocyelicringCoccurs in an isomeric open form or is replaced by a five memberedring,givingaurones 4 (Figure3)
(Figure3): thestructureofaurones(fivemembered ringsinCring)
1.1Classification of Flavonoid
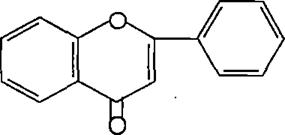
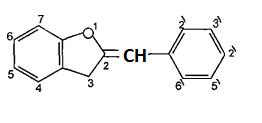
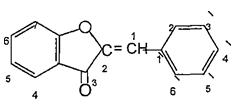
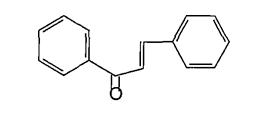
Varioussubgroupsofflavonoidsareclassifiedaccording tothesubstitutionpatternsofringC.Boththeoxidation state of the heterocyclic ring and the position of ring B are important in the classification. The flavonoids are classified into: flavones, flavonols , flvanones ,chalcones, aurones,isoflvonoids,andanthocyanins5.(Figures4 9)
(Figure7):ThestructureofChalcones
(Figure8):ThestructureofAurones
(Figure4):thestructureofFlavones
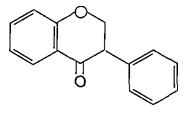
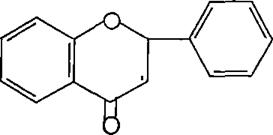
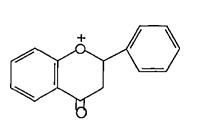
(Figure5):thestructureofFlavanols
(Figure9):ThestructureofIsoflavonoids
(Figure6):ThestructureofFlavanones
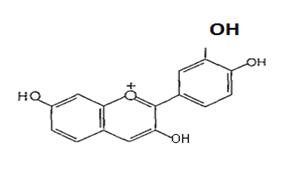
(Figure10):thestructureofAnthracyanins. Theflavones are generally found in herbaceous families’e.glabiatice,unbelliferae, compositae. Apigenin ( Figure 11) and luteolin (Figure12)areexamples.
(Figure11): ThestructureofApigenin
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN: 2395 0072
(Figure16):thestructureofflavannucleus
(Figure12):Thestructureof Luteolin Flavonolsaregenerallyfoundinwoodyangiosperum. Quercitol (Figure13) and kampherol ( Figure14) are examples.
(Figure17):ThewholestructureofCyaniding. In contrast to the large number of isoflavones encountered in nature, isoflavones can be distinguished from flavones and isoflavanones by UV and N NIR spectroscopy 7 ,anexampleisshownin (Figure18).
(Figure13):ThestructureofQuercitol
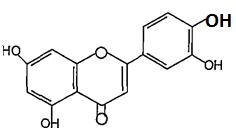
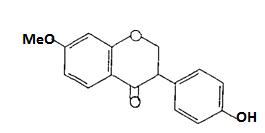
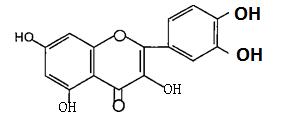
(Figure14):Thestructureofkampherol Flavanones are characterized by the absence of the double bond located between C2 and C3. Flavanones can be dehydrogenated to yield flavones or undergo hydroxylationatposition 3toyielddihydroflavonols(3 hydroxyf1avanones); an example of flavanone is naringenin(Figure15)6
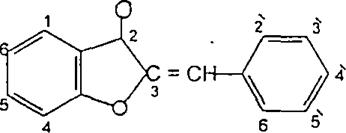
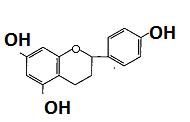
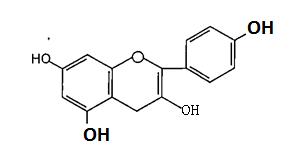
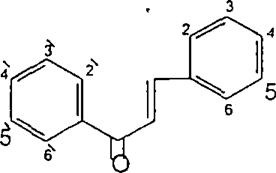
(Figure18):Thestructureofpadmakastein Chalcones are open chain flavonoid in which the two aromatic rings are joined by a three carbon α andβ, unsaturated carbonyl. Fundamentally they can be considered as derivatives of phenyl ketones shown below8 (Figure19).
(Figure15):ThestructureofNaringenin. Anthocyanin are very similar to the flavan nucleus (Figure16), the difference is that the oxygen which is citedattheI.positioninflavoneinnowapositivecharge in anthocyanins the change is delocalized over whole structure. Cyaniding (Figure17)inanexampleofclass.
(Figure19): Thederivativesofphenylketones. The aurones are based on the 2 benzylidenecoumaranone or 2 benzyliden 3 (2Hbenzofuranonesystem)asshownin(Figure20).6
(Figure20):The structureofor2 benzyliden3(2H benzofuranonesystem).
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN: 2395 0072
Most of the flavonoids (Flavone, flavonols anal anthocyanins) bear ring B in position 2 of the Heterocyclic ring, but is iso flavonoids ring B occupies position3.AgroupofchromanederivativewithringBin position 4 (4 phenyI coumarins) are termed neo flavonoids they are illustrated in ( Figure 21 ). The neo flavonoids as well as the isoflavonoids are regarded as abnormalflavonoids 6 .
Table(1)illustratesthecolorreactionofflavonoids. Table (1) The color properties of flavonoid in UV Vis light
Visible colour Colour in UV light Flavonoid presentAlone With ammonia
Orange red mauve
Dull orange, red or mauve fluorescent yellow cerise or pink
Bright yellow Dark brown or black
Blue Blue Anthocyanidin 3 glycoside most anthocyanidin 3,5 di glycosides
(Figure21)ThestructureofNeoflavonoids.
Flavonoid compounds occur in all parts of plant: roots, stem,leave,flower,fruit,seed,woodandbark.However, some kinds of flavonoids are more characteristic of certaintissues6
1.2 The Flavones
Alargenumberofnaturallyoccurringflavonesisknown now. The difference between flavones and flavonols residues in the presence o f a 3 hydroxy substituent in case of flavonols6, and this effects their U V absorption, chromatographic mobility and color reactions and it is possibletodistinguishsimpleflavonesonthesebasic.
There are only two common on flavones apiginin and luteolinas weshowedthatin (Figure11,12).FlavoneshaveB ringattachedatC 2and thecarbonyl function isα, β unsaturated. The parentun substituted flavone (Figure 4) produced apparently by an biosynthetic pathway, occurs in farina on species of primula and the closely related Dionysia9 . 2' hydroxyflavone and 5, 2' diydroxy flavone have been detected in the secretion of the glandular ce1ls of primulaflorinaeflavones10 .
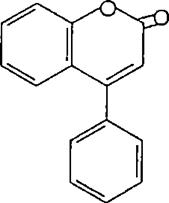
A pigenin and leuteolin free and as glycoside are the most widely occurring flavones. The A ring of the great majority of flavones is derived from phloroglcinol and the B ring is oxygenated in the 4' or 3', 4'. Or 3', 4', 5 positions as expected from their established acetate shikimate biosyntheticorigin7.Inthesurveyoftwelve
Very pale yellow
Dark brown orblack 6 hydroxylated flavonoid and flavone, some chalcone glycoside Dark and or brightorange Mstchalcone Bright yellow or yellow green
Brightorange orred Aurones
Dark brown Brightorange yellow Most flavonol glycosides, bidflavanyl and usually substitution flavonols
Vivid yellow ,green, dark brown
Most isoflavones and flavonols
None Dark mauve Faintmauve Most isoflavnes andflavonols Faintblue Intenseblue 5 deoxy isoflavones and 7 8 dihydroflavone Dark mauve Pale yellow or yellow green
Flavanones flavanol 7 glycosides
highly specialized herbaceous families, harbone and Williams9 foundthat6 hydroxyleutolinispresentinthe majority of plant, as a lead constituents, accompanied occasionally by its 6 methyI and 6, 4' dimethy1 ether. Theabilityofangiospermstohydroxylflavonesinthe6 position apparently arose relatively late in evaluation anytime. Strobochrysin Figure22) (6 metliylchrysin) occur in heartwood of of pinus strobes and other pine species11
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN: 2395 0072
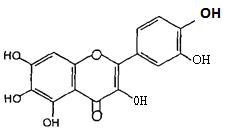
(Figure22):ThestructureofStrochrysin.
Ithasbeenencounteredinthefernlonchitistisserantii12 5 hdroxy7, 4' dimethoxy 6 methyl flavone and 6,8 dimethyl derivatives (eucalyptin) have been isolated from heart wood of eucalyptus trolliana (myrtace) and 5, 4' dihydroxy 7 methoxy 6 6 dimethy1 flavone (sideroxylin)fromEsideroxiam12 1.3 The
Flavonols
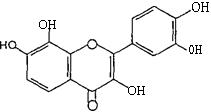
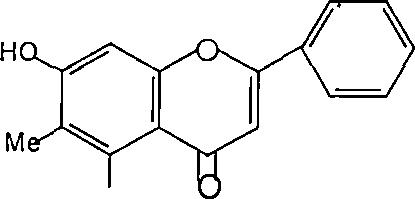
Sinceflavonol(I,R=OH)aresimplyflavone(I,R=H)in which the 3 position is substituted by a hydroxyl is shownin(Figure23).
leadsalt18’ 19. about30chromatogramsobtainedfrom2 dimensiona1 paper chromatography yield sufficient quantities either of a pure flavonoid for UV spectral analysis, or of partially purified product for one dimensionalpaperchromatographyseparation. Lists ofRtvaluesareavailablefor different developing system 20’21,22,23 The yellow flower pigment gossypetin (Figure 24) is readily distinguished by its low Rf values.
Thedullblackquercetagetin (Figure25), has all most the same Rf value in range as solvents.
(Figure24):Thestructureof gossypetin
(Figure23):R=OH,flavonolandR=H,Flavone.
Bothclassesofpigments(flavonolorflavone)havesofar been considered together, 13,14,15. This practice is justified in much of their chemistry, analysis, synthesis, reactions have a common theoretical basis. However, it is apparent that this simple difference in structure is of considerable biosynthetic, physiological, phytogenic, chemo systematic, pharmacological and analytical significance6
Preliminary information on flavonol, present in plant extract can be obtained by two dimensional paper chromatography16 . Column chromatography though of inferior separation efficiency is the method of choice whenlargerquantitiesofflavonolarerequired17
Of the various thin layers chromatography (TLC) proceduresthatcanbeusedsilicaimpregnatedwithlead acetate is of interest since it is based on the earlier isolationprocedureforflavonol by precipitation oftheir
(Figure25):ThestructureofQuercetagetin
But can be distinguished in saturated aIcoholic sodium acetate and drying at room temperature, within 40 minutes, gossypetin (23) gives a blue grey color, white auercetagenin(Figure25)remainsyellow24
Thetwoisomersalsohayedifferentspectralproperties, gossypetin (Figure24) has λ max 262,278,341 and 386nm; quercetaetin(Figure25) λmax9.272and 365 25. Gas liquid chromatography of flavonol methyl ether on lowloadedcolumnsisfeasible.
However, higher retention times are related to number of hydroxyls in ring B26. Gas liquid chromatography is particularly useful when coupled with mass spectra analysis27. Paper chromatography in borate does not rapidly separate flavonols from their glycosides. But relative motilities may even reveal structural features27 lt must be remembered, however, that flavonols are oxidized very quickly at alkaline PHS28. Fractional
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN: 2395 0072
sublimation in vacuo may sometimes prove useful29. Co four reaction are important and they will even distinguish between gossypetin (Figure24) and queractagetin(Figure25),twoisomerswhichareusually difficulttoseparatechromatographically 30 Flavonols are widely distributed in plants as co pi amendstoanthocyaninsinpetalsandinleavesofhigher plants. they occur most frequently in glycosidic combinations. The most common flavonoIs are: kaempterol(Figure14)andquerctin6 (Figure26).
on the paper, followed by spraying with 2N HCL on chromatogramsoriginally runinwater,mauvespotwill appear. Some flavanoncs beer prenyl side chains examples are preny lated flavanones artocarpesin and its oxydihydro cartocarpesin as we showed below in (Figure28) 32
(Figure26):Thestructureofquerctin
Flavonols are more susceptible to oxidation than other flavonoidssothatchemicalmethodsofdeterminationof structurerelyonoxidants.Thereactionoffiavonolswith diazonium compounds give, as by products, hydroxyl phenyl azo derivatives via displacement of the two phenyl group6 Flavonolsdisplayarrangeof colour when chromatographed on paper and viewed in UV light.Flavonols substituted in 3 positin appear as dark spotwhilederivativeswhichdonothaveafreehydroxyl atC 5aregenerallydistinguishedbyintensefluorescent colour6
1.4 The Flavanones
Theflavanones asweshowedbeforein(figure6)which areisomericwithchalcones(figure7)areobtainedfrom thelatter byacid oralkaline catalyzedringcolor ofring C. They cannot be detected during chromatographic survey unless chromatographic sprays are employed. It istrue thatsome flavanonesgive brightyellow green or lightbluecoloursonpaperwhenviewedinUVlightwith thehelpofammoniavapour7 .Animportantcolortestin alcoholicsolutionisreductionwithMgpowderand Conc. HCl. Only flavanones among the flavonoids give intense cherry red colours31. This procedure can be applied to paper chromatography or thin layer chromatography plates by spraying first with alcoholic solutionofsodiumborohydride (Ca.1%) andthenlater sprayingwithethanolicaluminiumchloride31 .Asuitable procedure for detecting 3 hydroxy flavanone or (di hydro flavonols) on paper is by use of zinc dust spread
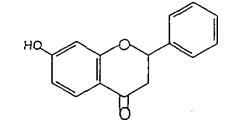
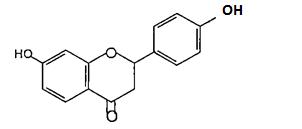
(Figure28):thestructureofArtocarpesinandits Oxydihydrocarpesin.
The naturally occurring fJavanones will be treated according to B ring hydroxy lation pattern. Flavanone lacking B ring hydroxyl groups are examplified by 7 hydroxy flavanones (Figure29) which was isolated from two legumes 33 Flavanones having one B ring hydroxyl groupareexemplifiedby7,4' di1iydroxyflavanone(31) .FlavanoneshavingtwoB ringhydroxylsareexamplified bybutin(31) 6
(Figure29):Thestructureof7 hydroxylflavanones.
(Figure30):thestrucureof7,4' dihydroxyflavanone
(Figure31):ThestructureofButin.
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN: 2395 0072
In alkaline solutions some flavanones undergo ring opening to the corresponding chalcone , such a change will be a cambanied by large bathochromic shifts in the UVspectrum.
1.5 Chalcones
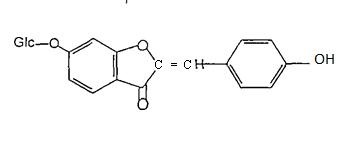
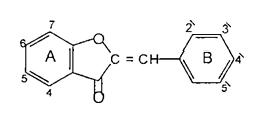
Chalcones are described as open chain flavonoids in which the two aromatic rings are joined by a three carbon u, b unsaturated carbonyl. It should be noted thatthe numberingoftheposition ofsubstitution in the chalcone nucleus is reversed from that in most other flavonolds.TheA ringisnumbered2' 6'andB ring2 6asshownbelow(32)6 .
(Figure(33)Thestructureof2 benzylidine coumaranone
Aurones having one B ring hydroxy I as hispidol (34) and its 6 O glucoside ( Figure35) occur in glycinemax seedlings38
(Figure34):ThestructureofHispidol
(Figure32): ThestructureofChacones. Chalcones are yellow phenolic pigments which give intense deep brown UV colour when chromatographed on paper; on fuming the paper with ammonia, the color may change to a rich deep red, though few chalcones failtorespondinthisway.Fundamentallychalconescan beconsideredasderivativesofphenylstyrylketones19 Naturally occurring chalcones are all hydroxylated to greater or lesser extent6. The parent compound chalcone( Figure 32) itself is not known as natural product6 , Chalcones play ecological role in nature in relation to plant color. These brightly yellow colored compounds are found in many plant organs, but most conspicuonsly in flower. Chalcones are synthesized 34’35 utilizing aryl methyl ketones and aryl aldehydes as synthons. This will allow Perkin condensation with strongbases(e.g.NaOEt).
1.6 Aurones
Aurones havea limitedoccurrenceandwerediscovered in 1943 front flowers of Coreopsis grandiflora 36’37 .Auroneslikechalconesappearonpaperchromatograms as yellow sptots in daylight. In the UV, they are very different, the color of aurones is intense bright yellow, changingwithammoniatobrightorange red.Auroncs are based on the 2 benzlidine coumaranone or 2benzylidine 3(2H) benzofuranone system as showed in (Figure33).
All the natural glycosides of aurones have been synthesizedbycondensationofthe Appropriately glycosidated components in acetic anhydride39
(Figure35)Thestructureof 6 Oglycosidesaurone.
1.7 Isoflavonoids
Isoflavonoidsdifferfromotherflavonoidsintheposition oftheB ringwhichisattachedat3 position. Skeletalstructuresofthevariousclassesofisoflavonoids showedbeforein(Figure10).
Many classes of isoflavonoids are known: isoflavones, isoflavanones, retonoids, pterocarpans, and coumestans40 .
Isoflavans, 3 aryI 4 hydroxycoumarins,chromones and hydroxyl and de hydro variants of ptetrocarpans and retonoidshavealsobeenreported7
1.7.1 Isoflavones
The isoflavones are very similar to flavones except that theB ringisattachedto3 positionofC ringratherthan the2 positionofflavones.Thereare manyexamplesof this class differing from each other is the nature of
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN: 2395 0072
substituentgroupsandtheir location such as genistiendaidzeinandbaptigenin41 43 Methods of preparation of isoflavones deserve a special attention since they often serve as key intermediates in synthesisofisoflavansandpterocarpans.The3 phenyl benzopyrane ring system of isoflavones can be formed from C14 , or C15 , compounds . by ring closure of benzyl phenyl ketones and Oxidative transformation of chalcones or by Joining C7 an d C8, units as in the an amineacylationmethod 7
1.8
Anthocyanins
AI2tliocyanins are very similar to flavan nucleus, the dÎ ffereliceisthattheoxygencitedinl positioninflavanis no iv carrying positive charge in the anthocyanins also thereisadoublebondintheC ringofanthocyanins.The basic skeletonofanthocyanins shown in (Figure9) and flavansareshownbelowin(Figure35)
1.10 Flavonoids of acacia species
The genus Acacia has been classified into section45 , and this classification depends on morphological characteristicoffoliageandinflorescence.
PhytochemicaI survey of the heartwood flavonoids of Acacia specics collected from Australia gave a number offlavonoidswithsimilarhydroxylationpattern46
The heartwood of Acacia melanoxylon gave flavonoid possessingthe3',4',7,8 hydroxylationpatternwhichis characteristicofagreatnumberingofAcaciaspecies.
The heartwoodalso gave apairofdiastereomeric Leucoanthocyanidins 47’48 .
7, 8, 3', 4' tetrahydoxydihydroflavonol (Figure36) was found to occur in lequminoseae and especially in Acacia species6 .
(Figure35):ThestructureofFlavan.
Theanthocyaninsarewater solublepigmenswhichare largely responsible for the attractive colors of lowers, leaves fruits and wines. Apart from their biological role, they are important aesthetically and economically since their stability is of significance in the marketability of plantproducts7
Anthocyaninsoccurnotonlyasmonomers,butaspartof much larger system in loose association with or chemically bonded to other components. This has led to a desire to characterize anthocyanins as they actually exist in plant material using methods of extraction and examination designed to cause least interference or alterationinstructure7 .
Anthocyanin are normally found as the aglycones (without glucose). They may al so occur as glycosides withglucose.
1.9 Flavonoid Glycoside
Flavonoid glycosides are generally soluble in water and alcohol but insoluble in organic solvents. They also dissolve in alkalis giving a yellow solution, which becomes colorless on addition of acid44 . Flavonoids are presentinplantsboundtosugara glycosides and any flavonoid a glycone may occur in a singleplantinseveralglycosidescombinations1,2 .
(Figure36): 7,8,3',4' tetrahydoxydihydroflavono 1.7. Conclusions
• The flavonoids are poly phenolic compounds. Flavonoids cane be obtained from plant as Natural products(organiccompounds).
.Flavonoids arc easily recognized as flower pigments in mostangiosperumfamilies(floweringplants).
• The flavonoids are classified into: flavones, flavonols , flvanones ,chalcones, aurones, isoflvonoids , and anthocyanins.
•phytochemicalscreeningof plantextractsgiveyellow colourevidenceofpresenceofflavonoids.
•Spectroscopic data give strong evidence of the formationofflavonoidsanditsderivativesmaterial.
ACKNOWLEDGENT
The authors would like to acknowledge of College of Graduate.
References:
1. Philip , M., "The chemotaxonomy of the Plants", EdwardArnold,London1st,Edward(1976).
2. Harbone , J. B., "phytochemical methods "Chapman, andHall,London(1969).
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

3.http ://www.friedi.com/bcrbs/phytochem/fIavonoids.html.
4. Mastumura , F., "Toxicity of Insecticides", plenum Prcss,York(1985).
5.Pterson,J.andDwyer,J.J AmDietetAssoc,98(1998).
6.Harbone,J.B.,Mabry,T.J., HelgaMabry "The F!avonoids"partI,Academicpress,NewYork(I975).
7. Harbone, J. B. and Williams, C. A., phytochemistry, 10, 2727(1971).
8. Kumar, A. A., Pande, C. S. and Kaul, R. K., lithium J. Chem.,4,640(1966).
9.Bouillant, M. L.,Wollen Weber, E. and Chopin. J. C. r., Acad.Sci. 273,1629(1971).
10.Harbone, J. B., "Comparative Biochemistry of Flavonoids",AcademicPress, YorkandLondon(1967).
11.Lebreton,P.,Jay,M.andVoirin,B., Chim.Analy .,Paris. 49,375(1967).
12.Hillis,W,EandIosi,K.,phytochemistry, 4,451(1965).
13. Venkalarmurn, K.,Forisch. Chan. Org. Naturs 17, 1 4(1959).
14. Geissman, T. A., "The he chemistry of flavonoid compound",Pergamanpress,Oxford(1902).
15.Dean, F. M., "Naturally occcurring oxygen ring compounds",Butterworth,London(1963).
16.Simon, J. P. and Goodull, D. W., Aust .J. Bol.. 16, 89(1968).
17.HarboneJ.B.,MabryT.J.andl4.."ThefJavonoid"part one,ClaepmanandHall,London(1975).
18.Pitteni,G.P.,J.Chroma,43,539(1069).
19.Kumar, A. R., Punde, C. S. and KauI, R. K., Indian, J. Chem.,4,640(1966).
20.Egger,K.,J.Chromat,5,74(196I).
21.Wong.E.,J.Chromat.,9.449(1962).
22. Bianki, G. B., Fizoil,Rost., 11, 544 Chem.Absa.,61.. 61,7359(1964).
23.Harbone,J.B.,"Comparativechemistryofflavonoids" AcademicPress.London,((1967)
24.Pellizzari,E.D.,Chuany,C.M.,Kue.,J.andWilliams,E. B.,J.Chromat.,40,285(1969).
25.Harbone, J. B., "Phytochmicalmetthods", Chapmpand andHall,London(1984).
26.Furuga,T., J.Chromat, 19, 607,(1965).
27.Paris, R. and Faugeras, G.Ann,pharmy,France,81,29(1960).
28.Kolasek, Z. 8nd 1nngmur. F., Veda avyzkum, V., Prumystu Kozeddnm, 6, 7,. J. Chemabst. 57, 13937 ( l 962).
29.Sim,Y.K., J.ChemSoc.,(C)976(1967)
30.Harbone,J.B., "Phytochmistry",, 8,177(1969).
31.Koppen,B.ll.,J.C/iiomatoy.8,604 i965).
32.Imamura, H.,Korosu,K. andTakahashi, T.,NipponMokuzaiGakkaishi,13,295(1967).
33. Knekt, P., Jarvinen R. Sappaen, R., Heliavara M., Teppo! Pukkala, E., Aroma A.”Dietary flavonoid and the risk of lung cancer and other malignant neoplasm AmericanJournalofEpidemiology.,164,223(l997).
34. Lunardi, L.,Guzela, M., et al., J. Antimicrobial Agent and Chromatography, 47, 1449 (2003). Pitteni, G. P., J. Chroma,43,530[1969).
35.Correa, R., NI. A. S.. Pereria, D., Button. L. Santos, V., Cechinal Filho, A. R. S., Santos, and R. J. Nunes., J. Arch. Pharma.33J,332 (2001).
36.Giessman,T. A. andHeaton,C.D.,J.Am.Soc.,65,677 (I943).
37.Harbone,J.B.,"Phytochmicalmetthods",Chapmpand andHall,London(1969).
38.Wong,E.Phytochemistry,5,463(1966).
39.Asen,S. and Plimer,J.R., Phytochemistry, 11,2601(1972).
40.Farkas,L.,Palles. L., "In progress in the chemistry of organic natural products".,spring verlage,New York, 25, 163,(1967).
41.Murakami,T.Nishikawa, Y. and Ando, T., Chem,pharm.Bull.,Tokyo,5,688(1960).
42.Farkas,L., Varady,J. and Gottegen,A.,Chem.Ber., 46,1865(1963).
43. Francis,C.M., Millington,A.S. and Bailey, E.T., Aust.J.ORGIC.Res.,18,47(1967).
44. Harbone, J. B., "Phytochmistry", Chapman and Hall, London(1973).
45.Frakas, I.and Pollos,I., Forischer.Chem.Org. Natures.,25,105,(1967).
46. Clark Lewis, J. W. and Proter, L. J., Australianjournal ofchemistry,25,1943(1972).
47.King,F.EandClark Lewis, J.,Chem.Soc.3384(1955).
48.Clark Lewis, J.W. and Mortimer J. ,Chem.Soc.,4104(1960).
Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN: 2395 0072 © 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page3361
