International Research Journal of Engineering and Technology (IRJET) e ISSN:2395 0056

Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN:2395 0072

International Research Journal of Engineering and Technology (IRJET) e ISSN:2395 0056

Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN:2395 0072

V.Ramya1 , S. Indhumathi2, M. A. Rajalakshmi3 ., J. Revathi4 E. Veeradharshini5 N. Sathyapriya6 1Department of Chemistry, Kamban college of arts and science. Tiruvannamalai
Nanoparticles are the spearheads of the rapidly expanding field of nanotechnology. Development of the green synthesis has gained extensive attention as a reliable, sustainable and eco friendly protocol for synthesizing a wide range of metal and metal oxide nanoparticles. The present study Green Synthesized Copper Oxide Nanoparticles using Albizia Amara Leaves. The synthesized copper oxide nanoparticles were characterized by Ultraviolet Vis spectroscopy (UV Vis), X ray Diffraction (XRD), Fourier Transform Infrared Spectroscopy (FT IR), Scanning Electron Microscope (SEM), Energy Dispersive X ray (EDX). The green chemistry approach used in the present work for the synthesize of copper oxide nanoparticles is simple, cost effective, and good alternative method.
Keywords: Albizia Amara Leaves Extract, Copper oxide nanoparticles, Characterization,
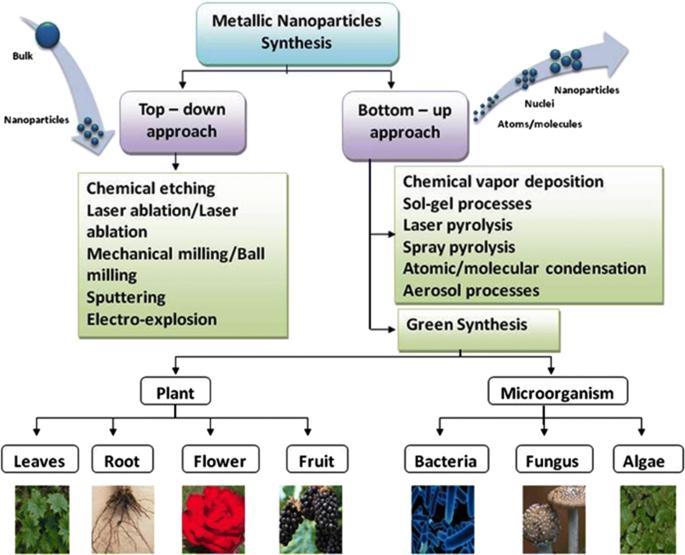
Nanotechnology can be defined as the manipulation of mater through certain chemical and physical process to create materialswithspecificproperties whichcanbeuseparticularapplication[1].Ananoparticlecanbedefinedasamicroscopic particle that has at least one dimension less than 100 nm in size [2]. Nanotechnology generally involves the application of extremelysmall particlesthatareusedacross all fieldofscience includingchemistry,biology,medicineandmaterial science [3 4].Nanoparticlesarethespearheadsoftherapidlyexpandingfieldofnanotechnology.Differenttypesofnanoparticleswith desiredshapeandsizehavebeenfabricatedusingvariousapproacheslikephysical,chemicalandbiological techniques[5].
Fig. 1. Schematic diagram of synthesis of nanoparticles
International Research Journal of Engineering and Technology (IRJET) e ISSN:2395 0056

Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN:2395 0072
The biological method which is represented as an alternative to chemical and physical methods, provides an environmentally friendly way of synthesizing nanoparticles. Moreover, this method does not require expensive, harmful and toxicchemicals.Metallicnanoparticleswithvariousshapes,sizescontentsandphysiochemicalpropertiescanbesynthesized thebiological methodactivelyusedinrecentyears.Traditional methodsareusedfrompastmanyyearsbut researcheshave provedthatthegreenmethodsaremoreeffectiveforthegenerationofNPswithadvantageoflesschancesoffailure,lowcost and ease of characterization.[6]. In the green synthesis method in which nanoparticles with biocompatibility are produced these agents are naturally present in the employed biological organism. Synthesis can be done in one step using biological organismsuchasBacteria,Actinobacteria,yeast,molds,algae,andplants(or)theirproducts.
Theplantsareconsideredtobemoresuitablecomparedtomicrobesforgreensynthesisofnanoparticlesastheyare non pathogenic and various pathways are thoroughly researched. The plants (or) plants extract, which act as reducing and capping agents for nanoparticle synthesis, are more advantageous over other biological process [7]. Because they eliminate theelaboratedprocessofculturingandmaintainingofthecellandcanalsobescaledupforlargescalenanoparticlesynthesis is preferred because it is cost effective, environment friendly, a single step method for bio synthesis process and safe for humantherapeuticuses.differentpartsofplantmaterialssuchasextracts.Fruit,fruitpeels,bark,root,leaves,andtubers[8]. Plantswhichhavegreatpotentialfordetoxification,reductionandaccumulationofmetals arepromisingfastandeconomical in removing metal borne pollutants. Metallic nanoparticles having various morphological characteristics can be produced intra cellularlyandextra cellularly.Withthematerialspresentintheplant extractsuchassugar, flavonoid,protein, enzyme, polymer,andgenieacidactingasareducingagenttakechargeinbioinductionofmetalionsintonanoparticles[9 13].
Metal and metal oxide nanomaterials prepared from earth abundant and inexpensive metals have attracted considerable attention because of their prospect as viable alternatives to the expensive metal based catalysts used in many conventional chemical processes[14]Nanomaterial’s exhibitactivitieswhichare differentfrom thoseofthecorresponding bulk materials because of their size and shape dependent physicochemical and optoelectronic properties [15]. The catalytic activity of nanomaterials represents a rich resource for chemical processes, employed both in industry and in academia. The great interest in catalysis using nanomaterials has prompted the synthesis and investigation of a diverse range of highly functionalized nanoparticles (NPs), including metal oxide nanostructures [16 20]. Some of the distinguish reported types of nanoparticles includes photochromatic nanoparticles, polymer coated nanoparticles, metal oxide nanoparticles, FeO, CuO, MgO,ZnO,FeNPs,AuNPs,AgNPs,PdNPs[21 23].
Among the various metals like Cu based nanomaterials which are cheap and environmentally friendly are especially attractiveinthiscontextduetothehighabundanceofCuinnatureandtheavailablesimpleandstraightforwardtechniques to synthesize these nanomaterials. The present green method for the synthesis of CuO nanoparticles is simple, mild, and environmentally friendly. Green synthesis of CuO nonoparticles could also be extended to fabricate other, industrially importantmetaloxides.
Inthepresentstudycopperoxidenanoparticles(CuONPs)weresynthesizedusing Albizia Amara LeavesExtract
All chemicals used were of analytical reagent without any further purification in addition to deionised water, copper chloridedihydrate(CuCl2.2H2O),Sodiumhydroxide(NaOH), hydrochloricacid(HCl)and ethanol. Albizia Amara Leaveswere collectedfromAshoknagarinChennai
TheAlbiziaAmaraLeaveswerecollectedfromAshokNagar,Chennai. Thefresh leaveswaswashedseveraltimeswithtap waterfollowedbydistilledwatertoremovethedustparticles. Thecleanandfreshsourcesaredriedinashadedplaceatroom temperaturefor10to15daysandthentheleaveswerepulverizedusingcommercialblender. The finepowderedwasstored
International Research Journal of Engineering and Technology (IRJET) e ISSN:2395 0056 Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN:2395 0072

at room temperature for further use. In a 250 ml of conical flask 10 gm of leaves powder were taken and to this 100 ml of double distilled water is added and it is heated at 80oC for 30 minutes. Then the solution was filtered using Whatman filter paperandkeptasideforfurtherprocess. TheobtainedextractinpalebrowncolourandadjustthepHat11byadding0.1Mof sodiumhydroxidesolution.
Ina250mlconicalflask,50mlofAlbiziaAmaraLeavesextractwastakenandtothis100mlof0.1MCuCl2.2H2Osolution is added slowly at room temperature under static conditions. The colour change of the reaction was observed and the time taken for the changes was noted. The solution colour changes immediately from pale brownish to yellowish grey colour indicating the formation of copper oxide nanoparticles (CuONP). Further the solution is centrifuged and precipitated is extractedanddriedinelectricalovenfor24hoursat100oC.Thedriedsamplekeptinmufflefurnacefor4hoursat500oC.the greensynthesisedCuONPsisformedatuniformparticlesizeandstoredforfurthercharacterisationanduses.
Synthesized CuO nanoparticles were subjected to UV Vis spectroscopy analysis, which confirms the formation of nanoparticlesintheinitialstage. TheCuOnanoparticlessynthesizedweresubjectedtoscanUV Visspectrophotometerinthe range190nm 800nmusingElicoSL210UVVISSpectrophotometer.
The plant extract and green synthesized CuO nanoparticles were characterized by FT IR spectrometer. The spectroscopic technique is based on the analysis of peaks at certain wave numbers. FT IR data indicates the presence of functionalgroupsintheplantextractandsynthesizednanoparticles. TheFT IRanalysiscarriedoutinthefrequencyrangeof 4000 400cm 1 usingPerkinElmerinstrument.
X ray diffractometer (lakjdf) was used to study the average particle size and crystalline nature of the synthesized adsorbents. ThediffractionpatternwasobtainedbyusingCuKαradiationwith wavelengthofλ=1.541Ao. Thescanning was donein2θvaluerangeof4o to80o at0.02min 1 andonesecondtimeconstant.
The SEM analysis provide the details about surface morphology, porosity and particle size distribution of the adsorbents. ThesurfacemorphologyofthesynthesizedCuOnanoparticleswasrecordedusingHitachiinstrument
EDX is an analytical technique used for the elemental analysis of a adsorbent and it depends on the interaction between known source of X ray excitation and the Adsorbent. The elemental composition of the adsorbent was determined withthehelpofelementalanalyser(CE 440elementalanalyser).
The Green approach for the formation of copper oxide nanoparticles using Albizia Amara Leaves extract was reported. FormationsofcopperoxidenanoparticlewereconfirmedbyUV visspectrophotometry.
International Research Journal of Engineering and Technology (IRJET) e ISSN:2395 0056 Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN:2395 0072

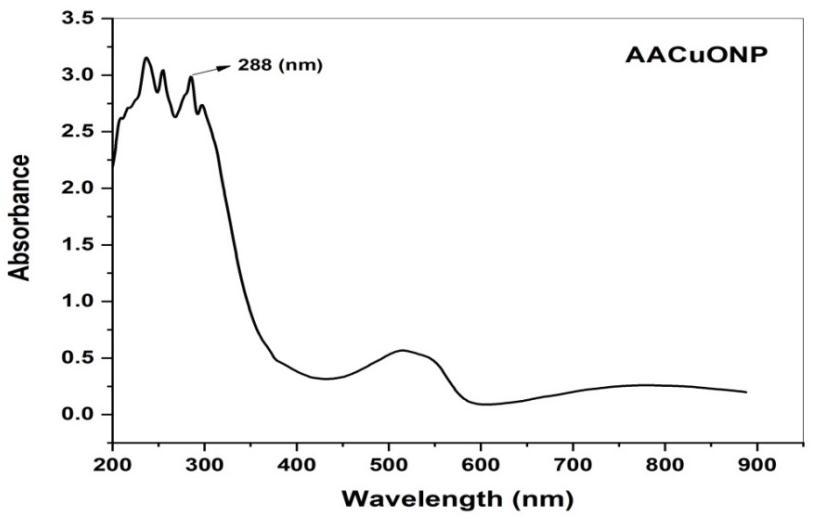
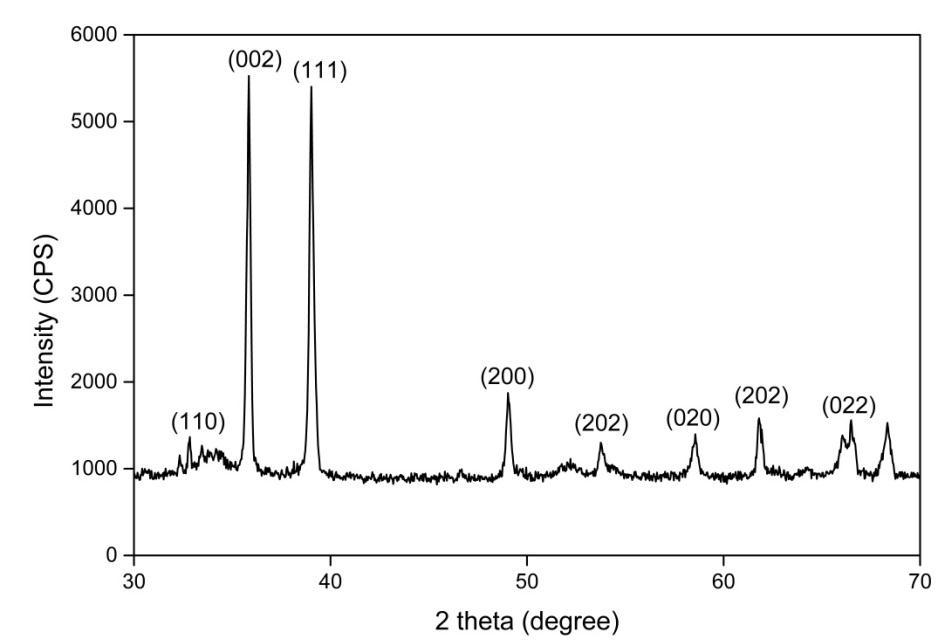
Fig 3. Shows the UV Vis absorption spectrum of copper oxide nanoparticle. The adsorption spectrum was recorded for the sample in the range of 200 800 nm. The spectrum showed the absorbance peak at 288 nm corresponding to the characteristicbandofcopperoxidenanoparticle[24].
The x ray diffraction (XRD) study was undertaken to Determine and confirm the crystalline structure of synthesized CuONPs.
Fig (3) Shows the appearance of diffraction pattern at 2θ= 33.3, 35.4, 38.8, 48.7, 58.3, 61.8, 66.28 and 68.0 which are assigned to the planes (110), (022), (111), (200), (202), (020), (202), (022) respectively of monoclinic phase CuONPs. No characteristic peak due to any impurity was observed in the diffraction grams Suggesting the formation of pure crystalline CuO.Theaveragesize ofthe CuOwascalculatedbyusingtheDebye SchererEquation(3)[25].Asharppeak at20=35.4and 38.8withthediffractionofthe(022)and(111)planeindicatesthatconfirmationofCuONPs.Theaveragecrystallitesizeinthe samplesofCuONPsisbelow21nm.
International Research Journal of Engineering and Technology (IRJET) e ISSN:2395 0056 Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN:2395 0072
D=0.9λ/βcosθ Eq.(3) Whereλisthewavelengthofthex rayradiation(0.154nm),θistheDiffractionangleandβisthefullwidthathalfmaximum. Thecrystallinesizehavebeen38.93nm
FTIR spectroscopy analysis also revealed the possible biomolecules and functional group responsible for capping or stabilizing of the synthesized CuONPs were expressed in fig (4,5). Taking the spectrum of leaves Extract as control the involvementofdifferentfunctionalgroupsofAlbiziaAmaraleavesextractinreducingandstabilizingprocessofnanoparticles synthesiswasevaluated.
Fig 4. FTIR spectrum of AAL extract
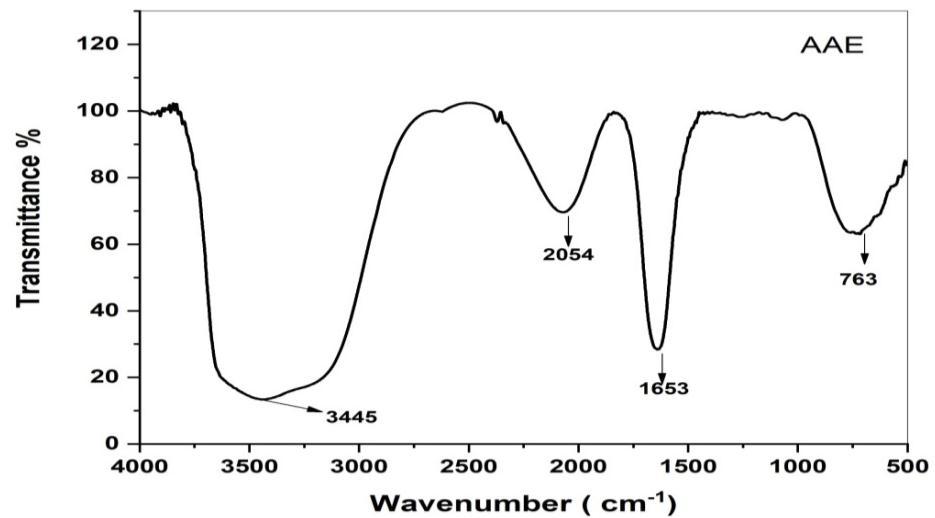
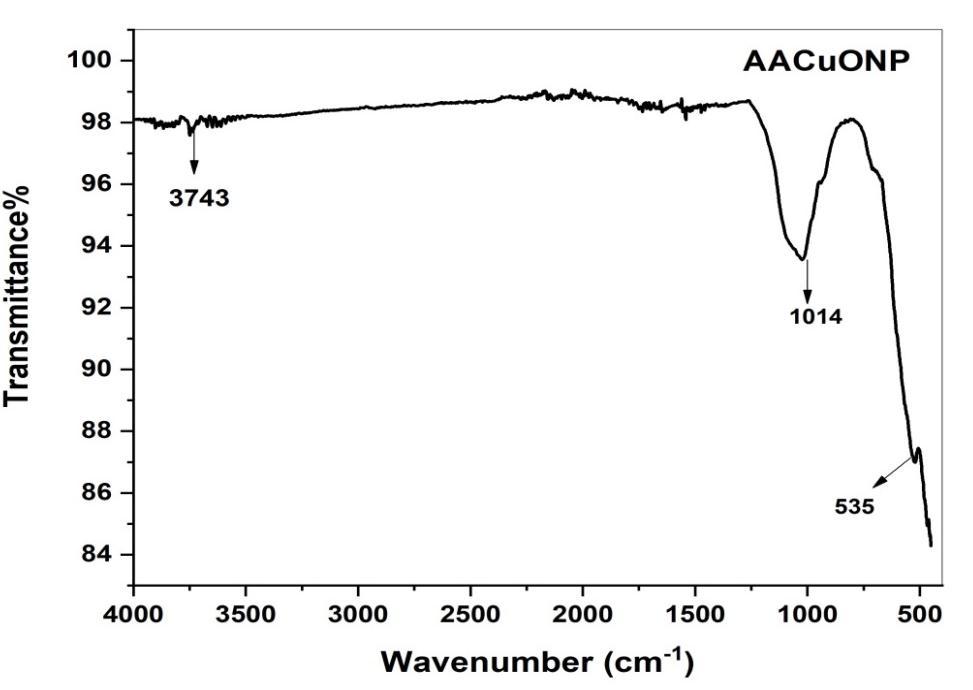
Fig 5 shows AA green synthesized CuO NPs
2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page

International Research Journal of Engineering and Technology (IRJET) e ISSN:2395 0056

Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN:2395 0072
Absorbancebandsat3248,2127,1362,and752cm 1 wereobservedinthespectrumofAlbiziaAmaraleavesextract.Abroad bandat3248cm 1 wasduetotheO Hstretchingofalcoholcompounds.Thepeaksat1827and1363cm 1 areContaining NH2 groupandC=Ogroupsofflavonoids[26 27].775cm 1 iscontainingC Hbonds.FTIRspectrumofCuONPforthepeakappeared at 3393, 1632, 1015, 709, and 562 cm 1. The peaks at 3393, 1632 and 1015 cm 1 corresponding to hydroxyl group ( OH) Stretching, hydroxyl ( OH) bending and C O stretching respectively. The Narrow bands at 535 confirm the formation of CuONPs.
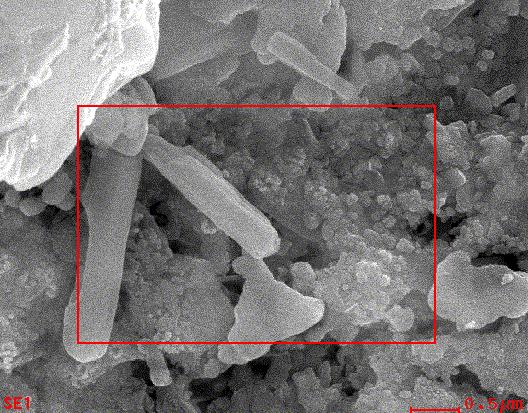
ThemorphologyofCuOnanoparticlesstudiedbySEManalysis.Fig(6)showathesurfacemorphologyofthecopperoxide nanoparticles was observed in the SEM image. It seems that the diameter of CuO nanoparticles range between 60 80 nm as calculatedbyimageJprogramme[28].
Fig 6. SEM image of Green synthesized copper oxide nanoparticles.

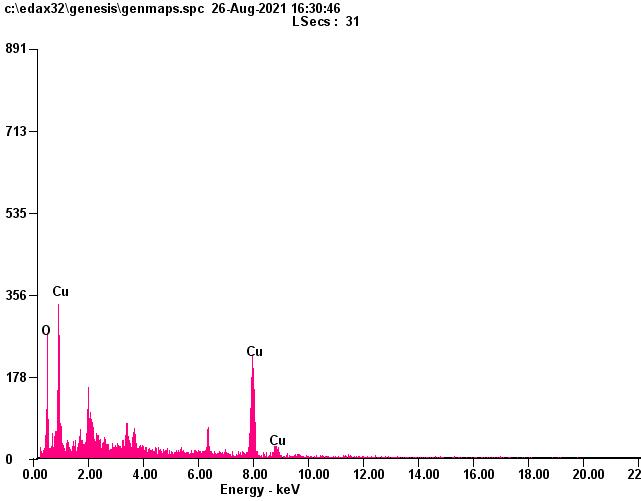
TheEnergyDispersive X ray(EDX)studywascarriedoutforthegreensynthesizedCuO nanoparticlestoknowaboutthe elementalcomposition.EDXconfirmthepresenceofCuandOsignalsofCuOnanoparticlesasshownintable1.Theelemental analysis of nanoparticles yields Cu 78.07% and 21.93% of oxygen which process that the produce nanoparticles is in its highestpurifiedform[29 30].
Table 1: EDX analysis for synthesized copper oxide nanoparticles.
International Research Journal of Engineering and Technology (IRJET) e ISSN:2395 0056

Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN:2395 0072
Fig 6. EDX spectrum of copper oxide nanoparticles
In this study, an eco friendly and convenient green method from copper chloride dihydrate solution using Albizia Amara leavesextractwasdeveloped.ThegreensynthesizedcopperoxidenanoparticleswereconfirmedbyUV vis,XRD,FT IR,SEM EDX CuO nanoparticles prepared from above mentioned route are expected to have more extensive applications such as reducing, stabilizing and efficient antimycobacterial agent, chemical sensor and semiconductor etc. This process is an economical method for the preparation of Nano crystalline CuO with respect to energy, time, simplicity and can be used for largescalesynthesisofcopperoxidenanoparticles
1. EnvironmentalProtectionAgency,“Nanotechnology white paper,”USEPA100/B 07/001,2007.
2. K.N.Thakkar,S.S.Mhatre,andR.Y.Parikh,“Biologicalsynthesisofmetallicnanoparticles,” Nanomedicine, vol,no. 2, 257 2622010
3. Chen,H.;Roco,M.C.;Li,X,Lin,Y. L.Trendsinnanotechnologypatents. Nat. Nanotechnol. 2008, 3,123 125.
4. Altikatoglu, M.; Attar, A.; Eric, F.; Cristache, C.M.; Isildak, I. Green synthesis of copper oxide nanoparticles using Ocimumbasilicumectractandtheirantibacterialactivity. Fresenius Environ. Bull. 2017, 25,7832 7837.
5. R.G.Chaudhuri,S.PariaCore/shellnanoparticles.Classesproperties,nanoparticlemechanism,characterizationand applications. Chem. Rev. 112 (4)(2012)2373 2433.
6. LeeJackson,dirtyOldLondon:TheVictorianFightAgainstFilth(2014)
7. JohnTarantino.“EnvironmentalIssues”. TheEnvironmentalBlog.Archivedfromtheoriginalon2011,12,10.
8. UdibaU.U.Gauje,B.,Ashade,N.O.,Ade Ajayi,F.A.okezieV.C.,AjiB.M.,andAgboun,T.DT.,2014.Anassessmentofthe heavy metal status of River Galma around Dakace industrial layout, Zaria, Nigeria,. Merit Research Journal Of Environmental Science and Toxicology 2(8):176 184
International Research Journal of Engineering and Technology (IRJET) e ISSN:2395 0056 Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN:2395 0072

9. M.N.Ahmed,R.N.Ram,Removalofbasicdyefromwastewaterusingsilicaasadsorbent, Environ. Pollut., 77(1992)79 85
10. W.A.Al Amrani,P.E.LimC.E.Seng,W.S.WanNgah,Bioregenerationofazodyes loadedmonoaminemodifiedsilicain batchsystem:Effectofparticlesizeandbiomassacclimationcondition, Chem Eng.j. 251 (20140175 182.
11. S.Mondal,Methodsofdyeremovalfromhouseeffluent Anoverview,Environ. Eng. Sci. 25 (2008)383 396
12. V, Larrechi MS, Callao MP (2007) kinetic and adsorption study of acid dye removal using activated carbon. Chemosphere 69:1151 1158.
13. GuptaVK,KumarR,NayakA,SalehTA,BarakatMA(2013)Adsorptiveremovalofdyesaqueoussolution ontocarbon nanotubes:areview. Adv Colloid Interface Sci 193 194:24 34.
14. Hassan,A.AandAbdulhussein,H.2015.MethylRedDyeRemovalfromAqueousSolutionbyAdsorptiononRiceHulls. Journal of Babylon University, 3:23
15. Mas Rosemal H. Mas Haris and Kathiresan Sathasivam (2009). The Removal of Methyl Red from Aqueous Solutions UsingBananaPseudostemFibers.
16. Laxmi,V.2014.Removalofmalachitegreendyefromwaterusingorangepeelasanadsorbent.MasterofTechnology Dissertation, National Institute of Technology,India.
17. Ferdous, J. Zainal Abedin, Hafizur Rahmanand M Ali Hossain.2014. Decolorization ofMethyl Red by Hog Plum Peel andSunfixRedbyBacterialStrains. International Journal of Chemical and Environmental Engineering 5(1):66 68.
18. NEILSEN LF., MOE D., KIRKEBY S., GARBARSCH C. Sirius red and acid fuchsin staining mechanisms. Biotechnic Histochem., 73,71,1998.
19. ZOBIR BIN HUSSEIN M., YAHAYA AH., SHAMSUL M., SALLEH HM., YAP T., KIU J. Acid fuchsin interleaved Mg Al layered double hydroxide for the formation of an organic inorganic hybrid nanocomposite. Mater. Letter, 58, 329, 2004.
20. Dash Bibek, “Competitive Adsorption of dyes (congo red, methylene blue, malachite green) on Activated Carbon”, 2011.
21. M. Q. Zhu, L. Zhu, J. J. Han, W. Wu, J.K. Hurst, A.D. Li, Spiropyranbased Photochromic polymer nanoparticles with opticallyswitchableluminescence J. Am. Chem.Soc. 128 (13)(2006)4303 4309.
22. K. Ulbrich, K. Hola, V. Subr, A. Bakandritsos, J. Tucek, R. Zboril, Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies, Chem Rev 116 (9) (2016) 5338 5431.
23. S. M. Dhoble, N.S. Kulkarni Investigation of in vitro and involve antifungal property of biological synthesized copper oxidenanoparticlesagainst rhizoctoniasolania phytopathogenofsoyabean(GlycinemaxL.Merrill) Int. J. Eng. Sci. 4 (5)(2018)17 30
24. Azhar,S. S,Liew A.G., Suhardy, D., Hafiz, K. F, Hatim,M. D. I., 2005, “Dye Removal from Aqueous Solution by using AdsorptiononTreatedSugarcaneBagasse”, American Journal of Applied Sciences 2 (11):1499 1503.
International Research Journal of Engineering and Technology (IRJET) e ISSN:2395 0056 Volume: 09 Issue: 05 | May 2022 www.irjet.net p ISSN:2395 0072

25. Long Bao Shi, Pei Fu Tang, Wei Zhang, Yan Peng Zhao, Li chang Zhang and Hao Zhang. Green synthesis of copper oxidenanoparticleusingcassiaauriculataleafextraxtandvitroevaluationoftheirbiocompatibilitywithrheumatoid arthritesmacrophages. Tropical Journal of Pharmaceutical Research.January2017; 16 (1):185 192
26. R Chowdhury, N. Barah and M. H. Rashid, Facile biopolymer assisted synthesis of hallow SnO2 babostructures and theirapplicationindyeremoval, ChemistrySelect, 2016, 1,4682 4689.
27. Kavitha R. Francisca P. Anxilia A. Biosynthesis characterization and antibacterial effect of plants mediated silver nanoparticlesfromAdenantheraPavoninaLeaves.JETIRFebruary2019,vol 6.(2)
28. D. Berra, S. Laouinia, B. Benhaouab, M. Ouahrania, D. Berrania and A. Rahald, “Green Synthesis of Copper Oxide nanoparticlesbyphoenixdactyliferaleavesextract”Digest Journal Nanoparticles and Biostructure.vol.13 1231 1238.
29. Maruthupandy, M., Zuo, Y., Chen, J. S., Song, J. M. Niu, H. L. Mao, C. J. Zhang, S. Y. Shen, Y. H. 2017. Synthesis of metal oxide nanoparticles ( CuO and ZnO Nps) via Biological template and their optical sensor application Applied Surface Science, 397:167 174.
30. S.M.YedukarC.B.Maurya,P.A.Mahanwar.Abiologicalapproachforthesynthesisofcopperoxidenanoparticlesby IxoraCoccineaLeafextract. Journal of Material and Environmental Science. 8 (4)(2007)1173 1178.